Abstract
Background
New percutaneous techniques for the management of acute pulmonary embolism (PE) are emerging, but there is lack of data regarding the approach of mobile thrombus in the right chambers, with the added risk of potential thrombus dislodgement that may prevent from mechanical circulatory support devices to be implanted in unstable patients.
Case summary
We present the case of a 65-year-old male with cardiogenic shock of unknown aetiology, severe biventricular dysfunction, and large mobile thrombus in the right atrium. Mechanical circulatory support devices could not be implanted, and current thromboaspiration systems were either too small or not available at that time. However, the patient’s condition deteriorated rapidly with thrombus in transit, hence, a novel approach was required: using a deflectable 14 Fr sheath, directional thrombectomy was performed, achieving complete extraction of the thrombi and allowing for circulatory support with extracorporeal membrane oxygenation (ECMO) to be implanted with outstanding results and progressive weaning of all intensive care measures.
Discussion
Despite the growing interest in the development of percutaneous strategies for acute PE, there is no evidence-based guidelines regarding the treatment of mobile right heart thrombus. Even though some cases of percutaneous right heart thrombectomy have been reported, it is still a challenging scenario, given the potential risk of thrombus dislodgement and atrial perforation. We describe a novel technique of percutaneous directional thrombectomy in a patient with cardiogenic shock of unknown aetiology and large mobile thrombi in the right atrium as a bridge to ECMO proving to be a feasible alternative to treat thrombus in transit.
Keywords: Directional thrombectomy, Thromboaspiration, Right atrium thrombus, Right chambers, Cardiogenic shock, ECMO, Circulatory support, Case report
Learning points.
A patient who presented with cardiogenic shock and large mobile thrombus in the right atrium.
The presence of intracardiac thrombi (especially thrombus in transit) may preclude the use of certain intensive care measures such as electrical cardioversion or mechanical circulatory support devices given the high risk of thrombus embolization and further haemodynamical instability. Therefore, thrombectomy should be considered in these cases.
The devices that are currently available for percutaneous thrombectomy are the AngioVac system (AngioDynamics, Inc.; New York), the FlowTriever System (Inari Medical, CA) and the Indigo System (Penumbra, Inc., CA). So far, most of the cases that have been reported used the AngioVac system, however, it is not directional and may entail risk of right atrial suction and potential wall perforation. We describe an innovative approach of directional thrombectomy with good performance and excellent results.
Introduction
Large mobile intracardiac thrombi are difficult to manage medically due to high risk of embolization.1 In haemodynamically unstable patients, the presence of intracardiac thrombi may preclude the use of mechanical circulatory support devices, but when a life-threatening condition exists, direct thrombectomy might be the only way to provide the required support.
Even though several percutaneous thromboaspiration systems have been developed to achieve complete thrombus extraction, these are not directional and, consequently, full thrombi aspiration might be limited and could be associated to aspiration of large volumes of blood and potential mechanical complications such as wall perforation.2–5
Summary figure
Case presentation
We present the case of a 65-year-old male, former smoker with no other medical history, and a first-degree family history of sudden cardiac death and arrhythmias without a clear diagnosis. He was admitted to the Emergency Department hypotensive and tachypnoeic (BP 103/61 mmHg, HR 170 b.p.m., SpO2 94%), and his physical exam showed pulmonary crackles and lower limb lividness. He had increased venous lactate levels (6.6 mmol/L) and atrial fibrillation with rapid ventricular response refractory to medical treatment (i.v. amiodarone).
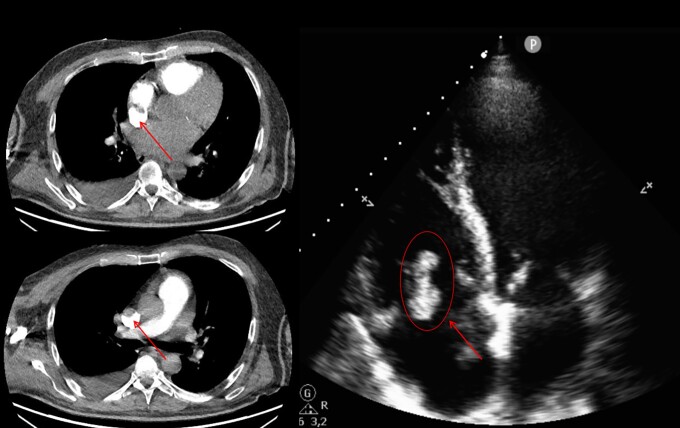
An urgent computed tomography allowed us to rule out acute pulmonary embolism (PE) but revealed the presence of a large thrombus in the right atrium (RA) extending to the superior vena cava, which was confirmed by a transthoracic echocardiogram along with severe biventricular dysfunction but no pericardial effusion nor valvulopathies (Figure 1. Supplementary material online, Video S1).
Figure 1.
Computed tomography angiography and transthoracic echocardiogram four-chamber view revealing the presence of a large thrombus (arrow) in the right atrium extending into the superior vena cava.
Within the following hour, the patient experienced multiorgan failure (glomerular filtration rate 25 mL/min/1.73 m2, prothrombin activity 21%, INR 3.26, ALT 4346 U/L, and AST 7826 U/L) and was started on mechanical ventilation, renal replacement treatment (RRT), and inotropes and vasopressor agents. However, a haemodynamically unstable tachycardia, refractory to medical treatment, aggravated his clinical status. The patient’s laboratory test results and their corresponding normal ranges can be found in the Supplementary material (see Supplementary material online, Table S1). Given the high risk of thrombus dislodgement, we had to dismiss procedures such as electrical cardioversion or circulatory support with veno-arterial extracorporeal membrane oxygenation (VA-ECMO) system—with the added risk of obstruction of the venous cannulae of the ECMO that the latter option presented. Thus, we considered performing emergent percutaneous thrombectomy and examined all potential strategies: Indigo System’s (Penumbra, Inc., CA) largest catheter at that time was 8 Fr, which was not big enough for the size of the thrombus, the FlowTriever System (Inari Medical, CA) had a wider range of catheter sizes, however, it would not be available on time and there was also a potential risk of atrial suction and wall perforation. Finally, the AngioVac system (AngioDynamics, Inc.; New York) was not available at our centre.
While studying alternative strategies, the patient’s condition deteriorated rapidly with severe haemodynamic instability (INTERMACS I). Considering that his chances of survival were extremely low at this point, we decided to perform directional thrombectomy (see Supplementary material online, Video S2): through a 14 Fr right femoral vein introducer, a 0.035″ J Wire and a 14 Fr deflectable sheath (Occlutech Int, Sweden) were advanced to the inferior vena cava-RA junction under fluoroscopy and transoesophageal echocardiography (TEE) guidance. The deflectable sheath was directed towards the thrombus and connected to a 50 mL Luer-lock syringe to perform manual aspirations. However, as the system kept clogging, we had to pull it out to remove the thrombus manually. Following a second run of manual aspiration, local tirofiban was administered at half its weight-corresponding dose and a final aspiration was performed, achieving complete extraction of the thrombus (Figure 2).
Figure 2.
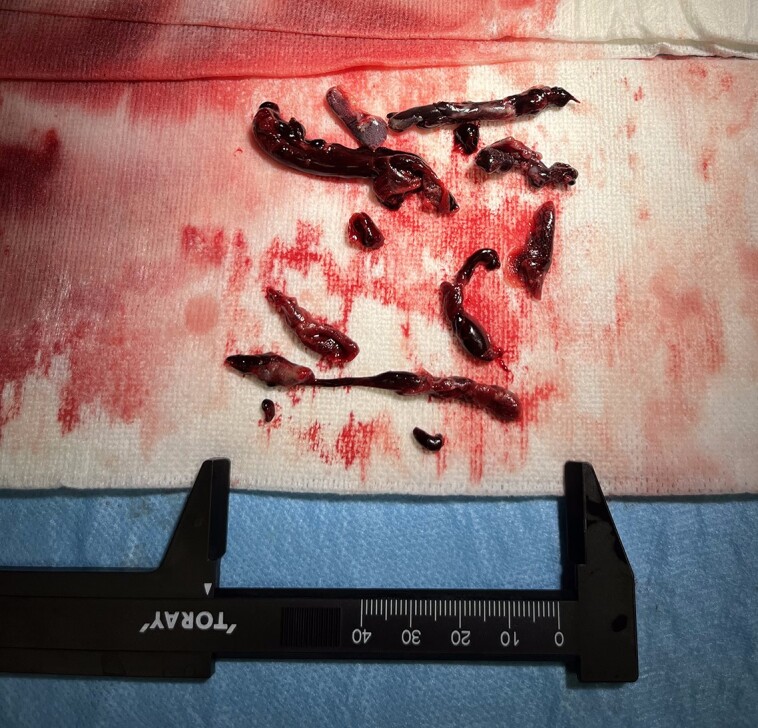
Thrombotic material extracted with percutaneous directional thrombectomy.
Once the absence of thrombus in both the right and left chambers was confirmed, VA-ECMO was implanted through a 25 Fr right femoral vein and 17 Fr right femoral artery vascular access, adding a distal perfusion cannula to avoid distal ischaemia.
Immediately after starting circulatory support inotropes could be reduced and peripheral lactate levels started to decrease. Levosimendan infusion was administered to facilitate support withdrawal. At Day +4, his neurological response was normal, VA-ECMO could be withdrawn at Day +5 with significant improvement on left ventricular ejection fraction (LVEF 35–40%) and complete recovery of right ventricular function. Then, progressive weaning of inotropes, RRT, and mechanical ventilation was achieved, and he was discharged from the Cardiovascular Intensive Care Unit (CICU) at Day +12. At Day +16, biventricular function was normal (see Supplementary material online, Video S3) and study was completed, as usual, with a cardiac MRI, revealing signs of myocarditis (patchy and multifocal fibrosis in an intramyocardial distribution without oedema and restored ventricular function). Additionally, a complete haematology exam including coagulation tests was performed, with findings compatible with acute liver injury. Full haematology study can be found in the Supplementary material (see Supplementary material online, Table S2).
Even though the patient had tested positive for COVID-19 at Day +24 (which had been negative during his whole hospitalization), he experienced a favourable recovery with conventional analgesics and could be discharged from the hospital at Day +30 with no neurological deficits, signs of heart failure, renal impairment, vascular complications, or any other sequelae. At 1-year follow-up, the patient remains asymptomatic, under oral anticoagulation and antihypertensive treatment with hydrochlorothiazide. A new cardiac MRI showed persistent patchy and multifocal intramyocardial fibrosis with normal biventricular function.
Discussion
Despite the growing interest in the development of percutaneous strategies for acute PE, there are no evidence-based guidelines regarding the treatment of mobile right heart thrombus, which entail high risk of embolization and mortality. While the mortality rate in patients with right heart thromboembolism without treatment has been reported as nearly 100%, it still reaches 23.8% with surgical embolectomy and 11.3% after thrombolysis.1 Furthermore, pharmacologic thrombolysis in unstable patients is associated with high risk of major bleeding (9.5%) and intracranial haemorrhage (2%).6 In this regard, even though a less invasive percutaneous approach for acute PE is emerging, the insertion of interventional equipment in patients with right heart thrombus may dislodge thrombotic material towards the pulmonary arteries, causing further haemodynamic instability. Still, some cases of RA percutaneous thrombectomy have been published with promising results.
The vast majority of these cases used the AngioVac system (AngioDynamics, Inc.; New York), a 22 Fr catheter with a balloon-actuated funnel-shaped distal tip that connects to a built-in filter, a centrifugal pump, and a reinfusion cannula to aspirate the thrombus en bloc.3 The preliminary data showed minimal complication rates, but it is technically challenging and may increase the risk of vascular complications given the need of two large-bore cannulae. Also, there is risk of RA perforation and even though it is the largest catheter available, its lumen can still be occluded by large thrombus.2,4,5 Other possible strategies include the FlowTriever System (Inari Medical, CA), a 16–24 Fr catheter with a large-bore syringe for aspirational thrombectomy and self-expanding nitinol discs to disrupt the thrombus, and the Indigo System (Penumbra, Inc., CA), which has developed larger catheters and can be easily manipulated through the pulmonary branches. However, its use on the RA has not yet been reported.
On the other hand, directional thrombectomy may avoid the risk of RA suction and potential perforation. To our knowledge, only one case of thrombus aspiration using a deflectable sheath has been previously published during an ablation of atrial fibrillation in which the trans-septal deflectable sheath was pulled out to the RA and connected to a syringe to manually aspirate the thrombus.7
Intracardiac thrombi can be seen in several clinical settings such as ischaemic heart disease, myocarditis, autoimmune diseases, cancer, and haematologic disorders. In this sense, myocarditis is an inflammatory disease of the heart secondary to infections (mostly viral), autoimmune diseases, or exposure to drugs, among others. Its clinical presentation may vary from asymptomatic, mild symptoms or life-threatening conditions such as heart failure, arrhythmias, or cardiogenic shock. In this entity, especially those related to autoimmune diseases or certain infections such as SARS-CoV-2, it is not uncommon to develop intracardiac thrombus, predominantly in right heart chambers.8–10 Albeit controversial, in the treatment of severe cardiogenic shock with organ failure such as the one presented in this case, the presence of right heart thrombus precluded us from implanting circulatory support devices given the high risk of thrombus dislodgement and in-hospital mortality risk.11
We describe this innovative technique of emergent directional thrombectomy as a bridge to VA-ECMO as a feasible alternative to treat RA thrombus, which was crucial to reach haemodynamic stability and progressive weaning of all intensive care measures with excellent results.
Supplementary Material
Contributor Information
Sara Blasco-Turrión, Interventional Cardiology Unit, University Clinical Hospital of Valladolid, Avda Ramón y Cajal, 3, Valladolid 47003, Spain.
María Plaza-Martín, Cardiovascular Intensive Care Unit, University Clinical Hospital of Valladolid, Avda Ramón y Cajal, 3, Valladolid 47003, Spain.
Ignacio J Amat-Santos, Interventional Cardiology Unit, University Clinical Hospital of Valladolid, Avda Ramón y Cajal, 3, Valladolid 47003, Spain; Centro de Investigación Biomédica en Red, Enfermedades Cardiovasculares (CIBERCV), C. de Melchor Fernández Almagro, 3, Spain.
Lead author biography
 Sara Blasco Turrión graduated from Universidad Autónoma de Madrid in 2014, has completed a 5-year Cardiology residency programme and a 2-year fellowship programme on Interventional Cardiology, and is currently performing her clinical and research activities at University Clinic Hospital in Valladolid. Her training includes several master’s degrees focused on cardiac imaging and cardiac critical care, and her research is currently focused in interventional cardiology techniques such as percutaneous treatment of severe tricuspid regurgitation and transcatheter aortic valve replacement. Several publications have arisen from her research, including eight publications in JCR indexed journals and 35 presentations in national and international congresses.
Sara Blasco Turrión graduated from Universidad Autónoma de Madrid in 2014, has completed a 5-year Cardiology residency programme and a 2-year fellowship programme on Interventional Cardiology, and is currently performing her clinical and research activities at University Clinic Hospital in Valladolid. Her training includes several master’s degrees focused on cardiac imaging and cardiac critical care, and her research is currently focused in interventional cardiology techniques such as percutaneous treatment of severe tricuspid regurgitation and transcatheter aortic valve replacement. Several publications have arisen from her research, including eight publications in JCR indexed journals and 35 presentations in national and international congresses.
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports.
Consent: The authors have received informed consent from the patient to publish information and images in accordance with COPE guidelines.
Funding: The authors have no competing financial interest that could have influenced the present report.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
References
- 1. Rose PS, Punjabi NM, Pearse DB. Treatment of right heart thromboemboli. Chest 2002;121:806–814. [DOI] [PubMed] [Google Scholar]
- 2. Azpiroz-Franch MJ, Rello-Sabaté P, Oristrell-Santamaría G, González-Alujas T, Martí-Aguasca G, Soriano-Colomé T. Atrial thrombus aspiration through the AngioVac system: an alternative when surgery and anticoagulation are not an option. Rev Esp Cardiol 2021;74:626–628. [DOI] [PubMed] [Google Scholar]
- 3. Yu T, Yang EH, Ranade M. Angiovac aspiration of right atrial cardiac pacemaker lead-associated thrombus with concurrent PE under fluoroscopic and transesophageal echocardiographic guidance: a multidisciplinary collaboration for improved patient outcome. Clin Imaging 2022;81:33–36. [DOI] [PubMed] [Google Scholar]
- 4. Narang A, Mediratta A, Estrada JR, Rosenberg J, Decara JM, Howell MD, et al. Transcatheter therapy for a large mobile right atrial thrombus and massive pulmonary embolism. J Invasive Cardiol 2017;28:49–51. [PMC free article] [PubMed] [Google Scholar]
- 5. Patnaik S, Rammohan HS, Shah M, Garg S, Figueredo V, Janzer S, et al. Percutaneous embolectomy of serpentine thrombus from the right atrium: in a 51-year-old man. Tex Heart Inst J 2016;43:524–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chopard R, Behr J, Vidoni C, Ecarnot F, Meneveau N. An update on the management of acute high-risk pulmonary embolism. J Clin Med 2022;11:4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mansour MC, Blendea D, Barrett CD, Heist EK, Ruskin JN. Right atrial thrombus aspiration guided by intracardiac echocardiography during catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol 2009;2:20–22. [DOI] [PubMed] [Google Scholar]
- 8. Ammirati E, Frigerio M, Adler ED, Basso C, Birnie DH, Brambatti M, et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy: an expert consensus document. Circ Heart Fail 2020;13:e007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jiang XJ, Zhang WY. Myocarditis complicated by massive right ventricular thrombus and extensive pulmonary embolism: a case report. Front Surg 2022;9:924366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pereira IA, Teles-Silva C, Gonçalves ES, Da Mota TC, de Lurdes M, Sequeira L, et al. NASCER E CRESCER BIRTH AND GROWTH MEDICAL JOURNAL acute severe myocarditis with cardiac thrombus formation—a therapeutic challenge Miocardite aguda grave complicada com trombos intracardíacos-Um desafio terapêutico CASE REPORTS. Nasc e Cres Birth Grow Med J 2022;31:166–171. [Google Scholar]
- 11. Shaefi S, O’Gara B, Kociol RD, Joynt K, Mueller A, Nizamuddin J, et al. Effect of cardiogenic shock hospital volume on mortality in patients with cardiogenic shock. J Am Heart Assoc 2015;4:e001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.