Abstract
Hepatitis A virus (HAV) immunoassays use cell culture-derived HAV antigen to detect HAV-specific antibodies. The current method of production of HAV antigen in tissue culture is time-consuming and expensive. We previously expressed the HAV open reading frame in recombinant vaccinia viruses (rV-ORF). The recombinant HAV polyprotein was accurately processed and was assembled into subviral particles. These particles were bound by HAV-neutralizing antibodies and were able to elicit antibodies which were detected by commercial immunoassays. The present investigation compared the production of HAV antigen by standard tissue culture methods to the production of HAV antigen with the recombinant vaccinia virus system. In addition, HAV and rV-ORF antigens were assessed for their utility in diagnostic immunoassays. Serum or plasma samples from HAV antibody-positive and antibody-negative individuals were evaluated by immunoassay that used either HAV or rV-ORF antigen. All samples (86 of 86) in which HAV antibody was detected by a commercial enzyme-linked immunosorbent assay (ELISA) also tested positive by the recombinant antigen-based immunoassay (VacRIA). Similarly, all samples (50 of 50) that were HAV antibody negative also tested negative by the VacRIA. The lower limit of detection of HAV antibody was similar among immunoassays with either HAV or rV-ORF antigen. Thus, in the population studied, the sensitivity and specificity of the VacRIA were equivalent to those of the commercial ELISA. Since production of recombinant antigen is faster and less expensive than production of traditional HAV antigen, the development of diagnostic HAV antibody tests with recombinant HAV antigen appears warranted.
Hepatitis A virus (HAV) is the sole member of the hepatovirus genus within the picornavirus family (6, 19). With a few notable exceptions, the virus does not cause cytopathic effects in cell culture and grows slowly and only to a low titer (9, 11, 23). Thus, production of HAV antigen for vaccine use or for diagnostic testing purposes is slow and expensive. The virus contains a positive-sense, single-stranded RNA genome which encodes a single polyprotein of 2,227 amino acids (7, 8). The polyprotein is autocatalytically processed into three major structural proteins (VP0, VP1, and VP3; also referred to as 1AB, 1D, and 1C respectively) (for a review, see reference 34).
Each of the three viral structural proteins has been expressed as a recombinant protein in a variety of prokaryotic and eukaryotic expression systems (12, 13, 15, 16, 18, 20, 22, 26, 38); however, none of the individual recombinant proteins was able to elicit high-titer neutralizing antibody or to bind to neutralizing monoclonal antibodies. Although human antibodies binding to denatured HAV structural and nonstructural proteins have been documented (18, 24, 25, 30, 37), the diagnostic utility of these nonneutralizing antigens has not been extensively studied. In some experimentally infected primates, the antibody response to nonstructural proteins is short-lived (25, 37), and commercial HAV antibody tests do not appear to detect antibody directed against individual structural proteins (15, 38; for reviews, see references 29 and 39). Consequently, individual recombinant HAV antigens do not appear promising as an antigen source for routine diagnostic immunoassays.
These results, along with other data, have led to the understanding that the critical epitopes within the immunodominant neutralization antigenic site on HAV are defined conformationally and require assembly of the structural proteins into subviral particles (33, 35). HAV morphogenesis requires several steps, the first of which is the assembly of the promoter, a structure containing one copy each of VP0, VP1, and VP3 (1AB, 1C, and 1D, respectively). This structure has a sedimentation coefficient of 5S (2, 4, 27). Five promoters assemble into a pentamer (sedimentation coefficient of 14S), which contains most of the neutralization antigenic sites found on complete virions (35). Twelve pentamers form the viral empty capsid (70S), and this particle is antigenically indistinguishable from infectious virions (35). To become infectious, the empty capsid must encapsidate full-length, genomic RNA. The final infectious HAV particle (virion) has a sedimentation coefficient of 156S (2, 4, 8).
We previously expressed the entire HAV open reading frame in recombinant baculoviruses and vaccinia viruses (18, 26, 36, 38). We demonstrated that the polyprotein is accurately processed into structural proteins that assemble into pentamers and empty capsids (26, 35, 38). These particles contain the epitopes comprising the immunodominant HAV neutralization antigenic site and elicit HAV-neutralizing antibodies in experimental animals (35). The purpose of this study was to evaluate different cell culture systems to optimize recombinant HAV antigen production and to determine the suitability of this antigen for use in diagnostic immunoassays.
(This work was presented in part at the American Gastroenterological Association and American Association for the Study of Liver Diseases Annual Meeting, 11 May 1997, Washington, D.C.)
MATERIALS AND METHODS
Virus and cells.
HAV HM-175 was propagated in BS-C-1 cells as described previously (30, 31, 38). This strain originated from the same strain used for the SmithKline Beecham HAV vaccine (3). Wild-type (WT) vaccinia virus and recombinant vaccinia virus expressing the HAV open reading frame (rV-ORF) were selected and propagated as described previously (38). Cells were maintained and vaccinia virus infections were carried out in BS-C-1 TK-143, HeLa, MRC-5, and Vero cells (38) and in Epstein-Barr virus (EBV)-transformed human B cells (40) as described previously.
Patients’ samples.
Serum or plasma samples were obtained from 25 well-characterized patients with acute hepatitis A (32), 10 healthy volunteers prior to and at various times following vaccination with an inactivated HAV vaccine (HAVRIX; SmithKline Beecham) (5), and 50 patients with chronic liver disease caused by hepatitis C virus (28). Acute hepatitis A was diagnosed by the presence of typical symptoms, biochemical evidence of acute hepatitis, and a positive immunoglobulin M (IgM) anti-HAV antibody test (32). This study was approved by the Institutional Review Board (Committee A) of the University of Iowa, and informed consent was obtained from all participants.
Antigen detection immunoassay.
A sandwich radioimmunoassay (RIA) was used to detect HAV antigen from either HAV- or vaccinia virus-infected cells as described previously (30, 32). High-titer anti-HAV polyclonal human serum was applied to a 96-well polyvinyl chloride microtiter plate for 4 h at 37°C. Following three washes of the wells, vaccinia virus- or HAV-infected cell lysates were applied to the wells and the plates were incubated overnight at 4°C. The plates were again washed, and 125I-labeled anti-HAV IgG was applied for 4 h at 4°C. The wells were washed, the contents were removed, and the wells were cut out and counted in a gamma counter. All samples were tested in duplicate or triplicate, and the results represent the average counts per minute per well.
Anti-HAV antibody detection.
A commercial enzyme-linked immunosorbent assay (ELISA) for HAV antibody detection (HAVAB; Abbott Laboratories, Abbott Park, Ill.) was used to test for the presence of anti-HAV antibodies. To evaluate the ability of recombinant HAV antigen to bind to anti-HAV antibodies in clinical samples, a sandwich RIA was used (VacRIA). Test or control sera were serially diluted in log10 increments in carbonate buffer prior to being applied in duplicate to 96-well polyvinyl chloride plates (4 h, 37°C) (32). The wells were washed, and lysates from equivalent numbers of either rV-ORF-infected cells or control HAV-infected BS-C-1 cell lysates (positive control) were applied overnight at 4°C. Equivalent numbers of cells infected with WT vaccinia virus and no antigen (phosphate-buffered saline [PBS]) were used as negative control antigen preparations. Wells were washed, 125I-labeled human polyclonal anti-HAV IgG was applied for 4 h at 4°C (31), and the counts per minute bound to the wells was determined in a gamma counter. Optimal dilutions of sera for HAV antibody detection ranged from 1:100 to 1:10,000 (data not shown). Unless otherwise noted, sera were diluted 1:100 since this is the dilution recommended by the manufacturer for use in the HAVAB assay.
A sample was considered positive if the P/N value was >2.1, where P is the counts per minute bound by the sample used to coat the well of a microtiter plate when rV-ORF was used as the antigen, and N is the counts per minute bound by the same sera when WT vaccinia virus or PBS was used as the control antigen source. This cutoff value was selected by determining the P/N values for the 50 samples that tested negative by the commercial test (HAVAB) and measuring the counts per minute bound when either WT vaccinia virus or PBS was used as the antigen source. The mean counts per minute and P/N value for each negative antigen was calculated, and a cutoff of 3.5 standard deviations above the mean counts per minute was selected to represent a positive result. When either WT vaccinia virus or PBS was used as the negative control, the P/N value of 2.1 was always greater than 3.5 standard deviations above the mean for the negative samples.
To characterize anti-HAV binding to both HAV and rV-ORF antigen, the World Health Organization (WHO) IgG reference preparation standardized for anti-HAV antibody content was used in these studies (for a review, see reference 39). This preparation has been widely used in the characterization of HAV antibodies (39) and contains a concentration of HAV antibody designated 100 IU/ml (14). The lower limit of detection and the relative sensitivity of both the HAVAB assay and the antibody detection RIA (by using rV-ORF and strain HM-175 as the HAV antigen) were determined with this anti-HAV IgG preparation.
Rate-zonal sedimentation in sucrose.
HAV antigen produced by HAV infection of BS-C-1 cells and rV-ORF- and WT vaccinia virus-infected BS-C-1 cells were evaluated by rate-zonal centrifugation in 7.5 to 45% sucrose gradients. Lysates of 106 infected cells were layered onto the gradient and were fractionated as described previously (35, 38). Fractions were collected from the bottom of the gradient and were tested in duplicate for HAV antigen by RIA.
RESULTS
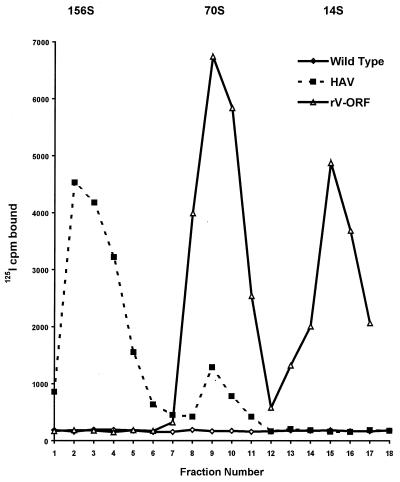
The standard method for the production of HAV antigen requires HAV infection of African green monkey kidney (AGMK) cells (BS-C-1 cells), fetal rhesus monkey kidney cells (FRhK-4 cells), or human cells (Vero or MR-C-5 cells) (3, 10, 11, 23, 31). Most of the antigenic material produced by these cell culture systems sediment at 156S and 70S. The 156S particles represent infectious virions, whereas the 70S particles represent empty capsids. Infection of a variety of cell types by the recombinant vaccinia virus (rV-ORF) produces a high concentration of 70S HAV particles and a significant amount of 14S HAV particles (Fig. 1) (35, 38). Sucrose gradient evaluation of the antigen used in these studies verified that the rV-ORF infection did not produce any infectious 156S particles (Fig. 1).
FIG. 1.
Cells (106) were infected with recombinant vaccinia virus expressing the HAV polyprotein (rV-ORF), HM-175 strain HAV, or WT vaccinia virus. The cells were lysed, and cell lysates were layered onto 7.5 to 45% sucrose gradients. The particles were separated by rate-zonal centrifugation. The fractions collected from the bottom of the gradient were tested for HAV antigen by RIA. Sedimentation markers (IgG, 7S; IgM, 19S) verified that the second antigen peak consisted of 14S pentamers (fractions 14 to 16) and that the first antigen peak (fractions 8 to 11) consisted of 70S empty capsids. HAV-infected cells contained 156S virions (fractions 2 to 4) and 70S empty capsids (fractions 9 and 10).
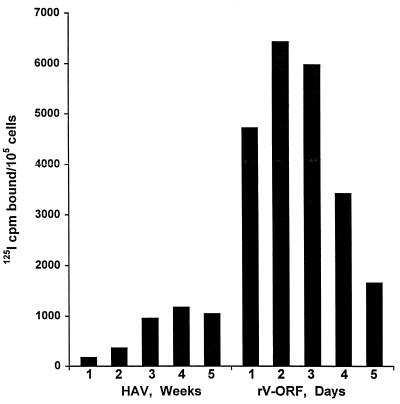
For HAV antigen production from infected tissue culture, 21 to 28 days is typically required to produce the maximum yield of antigen per cell. To compare HAV antigen produced by HAV infection and rV-ORF infection, we evaluated 105 BS-C-1 cells infected with either HM-175 HAV or rV-ORF at various times following infection (multiplicity of infection [MOI] = 1 for both infections; Fig. 2). Maximal HAV antigen was produced 3 weeks postinfection for HAV and 48 h postinfection for rV-ORF. The range of counts per minute bound within duplicate and triplicate assays was always <4% of the total counts per minute bound, and there was never an overlap in the values between the positive samples and the negative controls or the negative samples and the positive controls.
FIG. 2.
HAV antigen detection by RIA in cell lysates containing 105 BS-C-1 cells infected with either HAV HM-175 for 1 to 5 weeks or the recombinant vaccinia virus expressing the HAV polyprotein (rV-ORF) for 1 to 5 days (MOI = 1 for both HAV and vaccinia virus infections).
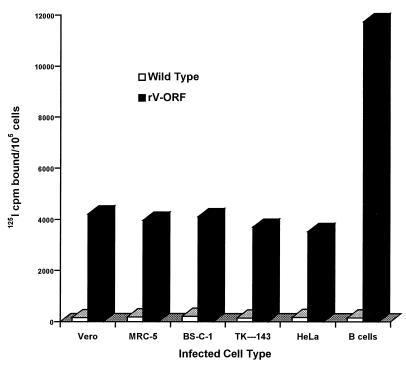
To determine if production of HAV antigen by rV-ORF could be enhanced by infecting different cell types with rV-ORF, we assessed HAV antigen production in WT vaccinia virus- and rV-ORF-infected Vero, MRC-5, BS-C-1, TK-143, HeLa, and human EBV-transformed B cells (MOI = 0.5; Fig. 3). Similar concentrations of HAV antigen were detected at 72 h postinfection for all cells except the human B cells, which consistently produced 2.8- to 4.7-fold more antigen than BS-C-1 cells. As demonstrated in Fig. 2, the level of HAV antigen production in BS-C-1 cells following infection with HM-175 strain HAV was three- to fivefold lower than that following rV-ORF infection of these cells. We attempted to grow HAV in this B-cell line; however, no antigen was detected by RIA. The evidence supporting HAV replication in these human B cells consisted of the detection of HAV RNA by RNA-DNA hybridization for two passes (33) (data not shown).
FIG. 3.
HAV antigen detection by RIA in 105 cells following 3 days of infection with WT vaccinia virus or the recombinant vaccinia virus expressing the HAV polyprotein (rV-ORF). Vero, MRC-5, BS-C-1, TK-143, and HeLa cells and an EBV-transformed human B-cell line were infected with rV-ORF or WT vaccinia virus (MOI = 0.5). Data represent the average counts per minute from triplicate determinations.
We tested multiple rV-ORF-infected cell lysates for antigen production, including TK-143 cells (n = 12 infections), HeLa cells (n = 8 infections), and BS-C-1 cells (n = 3 infections). The HAV antigen concentration per cell was determined by comparing the counts per minute bound in the antigen detection RIA. This revealed no more than a threefold difference between batches (data not shown).
To determine if the recombinant HAV antigen would perform similarly to cultivated HAV antigen in diagnostic immunoassays, 25 serum samples obtained from patients with biochemical evidence of hepatitis and a positive IgM anti-HAV antibody test were evaluated by both a commercial HAV antibody test (HAVAB) and VacRIA. All 25 samples positive by HAVAB were also positive by VacRIA, indicating that the HAVAB and VacRIA were equivalent in their abilities to detect human HAV antibodies following natural HAV infection. These sera were obtained between 2 weeks and 6 months after the development of jaundice from acute HAV infection (32).
Sixty-one plasma samples were obtained from 10 people prior to and following immunization with an inactivated HAV vaccine (HAVRIX). These samples were also evaluated by HAVAB and VacRIA. Fifteen samples were obtained prior to immunization, and all of these tested negative by both methods. The remaining 46 samples were obtained 6 to 28 weeks after immunization (immunization with 720 ELISA units [EU], with 720-EU boosts at 4 and 24 weeks), and each of these was positive by both methods, demonstrating that VacRIA and HAVAB were equally able to detect HAV antibodies in plasma following immunization.
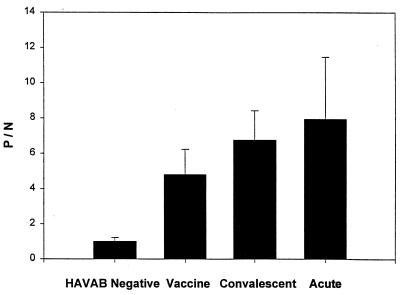
Plasma samples from 50 adult subjects with chronic hepatitis C (and unknown HAV antibody status) were also evaluated by HAVAB and VacRIA. Thirty-five samples tested negative by HAVAB, and all were negative by VacRIA. All 15 samples that were positive by HAVAB were also positive by VacRIA. The 30% seropositivity rate in this population is similar to that found in other U.S. studies (for a review, see reference 6), and the results demonstrate that VacRIA and HAVAB were equally able to detect convalescent-phase HAV antibodies in this group of patients. Figure 4 demonstrates the VacRIA results for HAVAB-negative samples and HAVAB-positive samples from vaccinees, individuals with convalescent-phase HAV infection, and individuals with acute HAV infection. For the three groups of subjects studied, there was complete agreement between the HAVAB and VacRIA. The relatively higher P/N values among acutely infected individuals and subjects with convalescent-phase HAV infection compared with the values for vaccinees are consistent with previous results (for a review, see reference 17).
FIG. 4.
Summary of the mean P/N values obtained by the recombinant HAV-based RIA. Results are presented for the 50 HAVAB-negative samples, 46 postvaccination samples (vaccine), 15 convalescent-phase samples from subjects with chronic hepatitis C virus infection, and 25 samples obtained from individuals with acute HAV infection. No HAVAB-negative samples had P/N values of >1.76, and no HAVAB-positive samples had P/N values of <3.16.
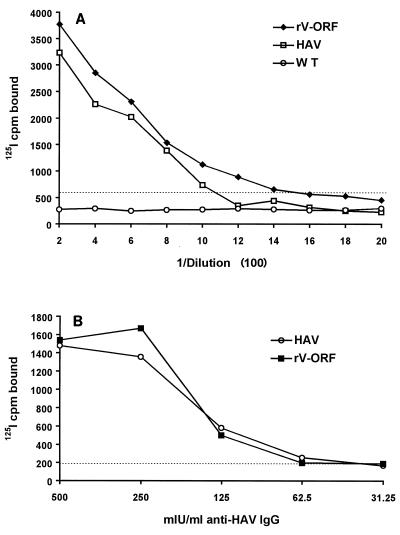
To compare the sensitivity of anti-HAV antibody binding to HAV antigen produced by HAV-infected BS-C-1 cells with that of the binding of anti-HAV antibody to rV-ORF-produced HAV antigen, sera obtained from four individuals 5 to 7 months following acute hepatitis A were evaluated. Twofold dilutions of these sera were applied to antigen-coated wells (initial dilution, 1:200). Figure 5A demonstrates that both HAV HM-175 antigen and rV-ORF antigen were similarly able to detect anti-HAV (correlation coefficient, 0.904), and the limits of detection between the two assays were very similar. Similar results were obtained with all four serum samples (data not shown).
FIG. 5.
(A) Serum obtained 5 months following acute hepatitis A infection from one patient was serially diluted and applied to wells coated with antigen produced by either HAV HM-175 infection, recombinant vaccinia virus infection (rV-ORF), or WT vaccinia virus infection. The RIA cutoff for this experiment was determined with both the HAV antigen and the rV-ORF antigen, and the dotted line demonstrates the counts per minute equal to a P/N value of 2.1 for the HAV antigen (which was higher than that for the rV-ORF antigen). Data represent the average counts per minute determined in triplicate. (B) The WHO anti-HAV IgG reference preparation was diluted with PBS, and various concentrations of IgG were applied to the wells of a 96-well microtiter plate in triplicate. HAV antigen or rV-ORF antigen was added, and the average counts per minute per well was determined. The cutoff for this assay is shown by the dotted line. By using the cutoff recommended by the manufacturer, the sensitivity of the HAVAB assay was 90 mlU/ml.
To directly compare the relative sensitivity of HM-175 HAV antigen to that of rV-ORF antigen, serial dilutions of the WHO IgG anti-HAV reference preparation (500, 250, 125, 62.5, and 31.25 mlU/ml) were used to coat the wells of a microtiter plate. Cell lysates containing equivalent concentrations of either the HAV HM-175 antigen or the rV-ORF antigen were subsequently applied to the wells, and the counts per minute of 125I bound per well was determined. Although the slopes of the curves are slightly different (Fig. 5B), the calculated cutoffs (levels of sensitivity) were comparable for these two antigen sources (67 mlU/ml for HAV and 55 mlU/ml for rV-ORF). By comparison, the limit of detection for HAVAB was 90 mIU/ml.
DISCUSSION
The data presented here demonstrate that HAV particles produced by recombinant vaccinia viruses appear to be equivalent to traditional HAV antigen in HAV antibody detection assays. Since this recombinant antigen appears to be antigenically indistinguishable from cell culture-derived HAV and may have practical use in diagnostic assays, we compared antigen production by the traditional cell culture method and with the rV-ORF system. We investigated various cell lines to determine which cells were optimal for HAV antigen production. Recombinant vaccinia virus infection of nonadherent, EBV-transformed human B cells did not produce as much of a cytopathic effect as it did in adherent cells, and the amount of HAV antigen produced per cell was markedly enhanced (Fig. 3). This is similar to results described by others (1), who reported high vaccinia virus titers and recombinant protein expression in human T and B lymphocytes. The HAV antigen yield per cell ranged from 4- to 15-fold higher for the rV-ORF-infected cells compared to that by the standard HAV cell culture method (Fig. 2 and 3). Additionally, the use of rV-ORF shortened the production time from 3 weeks to 72 h.
The standard method of HAV antigen production generates both 70S and 156S (infectious) particles. To ensure laboratory safety, each batch undergoes an inactivation process (usually with formalin) to inactivate infectious virus. Inactivation of HAV requires from 3 days to 1 week (3, 10) and must be evaluated for complete inactivation. Due to the very slow replication cycle of HAV, testing through three to five replication cycles requires an additional 9 to 15 weeks. On the other hand, vaccinia virus is more easily inactivated (21). In addition, it has a rapid replication cycle, thus markedly shortening the time required to inactivate and test for inactivation (estimates of 5 to 9 days). In addition, there is no risk of including infectious HAV in the antigen preparation, because full-length HAV RNA is never present.
In our laboratory infection with rV-ORF is less expensive than cultivation of HAV since media without fetal calf serum is added to rV-ORF-infected cells following viral attachment (38). For HAV infections, however, media containing 2% fetal calf serum is required to maintain the cells for the 3 to 4 weeks required for HAV replication (31). This requirement for fetal calf serum significantly increases the cost of production of HAV antigen. A summary of the advantages of rV-ORF over tissue culture-produced HAV is presented in Table 1.
TABLE 1.
Comparison of recombinant vaccinia virus expressing the HAV polyprotein (rV-ORF) and HAV HM-175 characteristics in cell culture
| Antigen | Infection time (days) | Antigen yield per cell | Inactivation and testing time (days) | Medium requirements postinfection | Antigen specificity | Purification | Stability |
|---|---|---|---|---|---|---|---|
| rV-ORF | 2–3 | 5- to 15-fold greater | 5–9 | No fetal calf serum | No difference | No difference | After fixation, no difference |
| HAV | 21–28 | NAa | 56–112 | Fetal calf serum (2%) | No difference | No difference | Prefixation, more stable |
NA, not applicable.
Our studies indicate that recombinant HAV antigen appears to be an acceptable HAV antigen for diagnostic testing purposes. Because of the many advantages that this method of production has over the standard method of cell culture-derived HAV antigen production, the possible uses of the recombinant vaccinia virus-produced antigen for diagnostic HAV antibody testing and vaccine production warrant further investigation.
ACKNOWLEDGMENTS
We thank James McLinden and American Biogenetic Sciences for helpful discussions and Naomi Erickson for assistance with manuscript preparation.
This work was supported by a Merit Review grant from the U.S. Department of Veterans Affairs (to J.T.S.) and a grant from American Biogenetic Sciences. Patient care was provided in part by the GCRC Program in NCRR (NIH grant RR0059).
REFERENCES
- 1.Alonso J M, Rodriguez J, Vinjuela E, Kroemer G, Martinez C. Highly efficient expression of proteins encoded by recombinant vaccinia virus in lyphocytes. Scand J Immunol. 1991;34:619–626. doi: 10.1111/j.1365-3083.1991.tb01585.x. [DOI] [PubMed] [Google Scholar]
- 2.Anderson D A, Ross B C. Morphogenesis of hepatitis A virus: isolation and characterization of subviral particles. J Virol. 1990;64:5284–5289. doi: 10.1128/jvi.64.11.5284-5289.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andre F E, Hepburn A, D’Hondt E. Inactivated candidate vaccines for hepatitis A. Prog Med Virol. 1990;37:72–95. [PubMed] [Google Scholar]
- 4.Borovec S V, Anderson D A. Synthesis and assembly of hepatitis A virus-specific proteins in BS-C-1 cells. J Virol. 1993;67:3095–3102. doi: 10.1128/jvi.67.6.3095-3102.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cederna, J. B., D. Klinzman, and J. T. Stapleton. Hepatitis A virus (HAV) specific cellular immune responses following immunization with an inactivated vaccine. Submitted for publication. [DOI] [PubMed]
- 6.Cederna J B, Stapleton J T. Hepatitis A virus. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: ASM Press; 1995. pp. 1025–1032. [Google Scholar]
- 7.Cohen J I, Ticehurst J R, Purcell R H, Buckler-White A, Baroudy B M. Complete nucleotide sequence of wild-type hepatitis A virus: comparison with different strains of hepatitis A virus and other picornaviruses. J Virol. 1987;61:50–59. doi: 10.1128/jvi.61.1.50-59.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coulepis A G, Locarnini S A, Westaway E G, Tannock G A, Gust I D. Biophysical and biochemical characterization of hepatitis A virus. Intervirology. 1982;18:107–127. doi: 10.1159/000149314. [DOI] [PubMed] [Google Scholar]
- 9.Daemer R J, Feinstone S M, Gust I D, Purcell R H. Propagation of human hepatitis A virus in African green monkey kidney cell culture: primary isolation and serial passage. Infect Immun. 1981;32:388–393. doi: 10.1128/iai.32.1.388-393.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellerbeck E F, Lewis J A, Nalin D, Gershman K, Miller W J, Armstrong M E, Davide J P, Rhoad A E, McGuire B, Calandra G, Provost P J, Midthun K. Safety profile and immunogenicity of an inactivated vaccine derived from an attenuated strain of hepatitis A. Vaccine. 1992;10:668–672. doi: 10.1016/0264-410x(92)90087-z. [DOI] [PubMed] [Google Scholar]
- 11.Flehmig B. Hepatitis A-virus in cell culture. I. Propagation of different hepatitis A virus isolates in a fetal rhesus monkey kidney cell line (Frhk-4) Med Microbiol Immunol. 1980;168:239–248. doi: 10.1007/BF02121807. [DOI] [PubMed] [Google Scholar]
- 12.Gauss-Muller V, Lottspeich F, Deinhardt F. Characterization of hepatitis A virus structural proteins. Virology. 1986;155:732–736. doi: 10.1016/0042-6822(86)90234-5. [DOI] [PubMed] [Google Scholar]
- 13.Gauss-Muller V, Zhou M Q, von der Helm K, Deinhardt F. Recombinant proteins VP1 and VP3 of hepatitis A virus prime for neutralizing response. J Med Virol. 1990;31:277–283. doi: 10.1002/jmv.1890310407. [DOI] [PubMed] [Google Scholar]
- 14.Gerety R J, Smallwood L A, Finlayson J S, Tabor E. Standardization of the antibody to hepatitis A virus (anti-HAV) content of immunoglobulin. Dev Biol Stand. 1983;54:411–416. [PubMed] [Google Scholar]
- 15.Johnston J M, Harmon S A, Binn L N, Richards O C, Ehrenfeld E, Summers D F. Antigenic and immunogenic properties of a hepatitis A virus capsid protein expressed in Escherichia coli. J Infect Dis. 1988;157:1203–1211. doi: 10.1093/infdis/157.6.1203. [DOI] [PubMed] [Google Scholar]
- 16.Karayiannis P, O’Rourke S, McGarvey M J, Luther S, Waters J, Goldin R, Thomas H C. A recombinant vaccinia virus expressing hepatitis A virus structural polypeptides: characterization and demonstration of protective immunogenicity. J Gen Virol. 1991;72:2167–2172. doi: 10.1099/0022-1317-72-9-2167. [DOI] [PubMed] [Google Scholar]
- 17.Lemon S M, Stapleton J T. Prevention of hepatitis A. In: Zuckerman A J, Thomas H C, editors. Viral hepatitis. London, United Kingdom: Churchill Livingstone; 1994. pp. 61–79. [Google Scholar]
- 18.McLinden J, Stapleton J, Rosen E. Anti-HAV neutralizing antibodies made to antigens produced by recombinant baculoviruses. In: Ginsberg H, Brown F, Lerner R A, Chanock R, editors. Vaccines 91: modern approaches to new vaccines. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1991. pp. 239–244. [Google Scholar]
- 19.Miller M J. Summary of current nomenclature, taxonomy, and classification of various microbial agents. Clin Infect Dis. 1993;16:597–615. doi: 10.1093/clind/16.5.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ostermayr R, von der Helm K, Gauss-Muller V, Winnacker E L, Deinhardt F. Expression of hepatitis A virus cDNA in Escherichia coli: antigenic VP1 recombinant protein. J Virol. 1987;61:3645–3647. doi: 10.1128/jvi.61.11.3645-3647.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paoletti E, Rosemond-Hornbeak H, Moss B. Two nucleic acid-dependent nucleoside triphosphate phosphohydrolases from vaccinia virus: purification and characterization. J Biol Chem. 1974;249:3273–3280. [PubMed] [Google Scholar]
- 22.Powdrill T F, Johnston J M. Immunologic priming with recombinant hepatitis A virus proteins produced in Escherichia coli. J Virol. 1991;65:2686–2690. doi: 10.1128/jvi.65.5.2686-2690.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Provost P J, Hilleman M R. Propagation of human hepatitis A virus in cell culture in vitro. Proc Soc Exp Biol Med. 1979;160:213–221. doi: 10.3181/00379727-160-40422. [DOI] [PubMed] [Google Scholar]
- 24.Robertson, B. H., X.-Y. Jia, H. Tian, H. S. Margolis, D. F. Summers, and E. Ehrenfeld. 1992. Serological approaches to distinguish immune response to hepatitis A vaccine and natural infection. Vaccine 10(Suppl. 1):S106–S109. [DOI] [PubMed]
- 25.Robertson B H, Jia X-Y, Tian H, Margolis H S, Summers D F, Ehrenfeld E. Antibody response to nonstructural proteins of hepatitis A virus following infection. J Med Virol. 1993;40:76–82. doi: 10.1002/jmv.1890400115. [DOI] [PubMed] [Google Scholar]
- 26.Rosen E, Stapleton J T, McLinden J. Synthesis of immunogenic hepatitis A virus particles by recombinant baculoviruses. Vaccine. 1993;11:706–712. doi: 10.1016/0264-410x(93)90253-t. [DOI] [PubMed] [Google Scholar]
- 27.Ruchti F, Siegl G, Weitz M. Identification and characterization of incomplete hepatitis A virus particles. J Gen Virol. 1991;72:2159–2166. doi: 10.1099/0022-1317-72-9-2159. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt W N, Wu P, Han J-Q, Perino M J, LaBrecque D R, Stapleton J T. Distribution of hepatitis C virus (HCV) RNA in whole blood and blood cell fractions: plasma HCV RNA analysis underestimates circulating virus load. J Infect Dis. 1997;176:20–26. doi: 10.1086/514024. [DOI] [PubMed] [Google Scholar]
- 29.Stapleton J T. Passive imunization against hepatitis A. Vaccine. 1992;10:S45–S47. doi: 10.1016/0264-410x(92)90541-q. [DOI] [PubMed] [Google Scholar]
- 30.Stapleton J T, Frederick J, Meyer B. Hepatitis A virus attachment to cultured cell lines. J Infect Dis. 1991;164:1098–1103. doi: 10.1093/infdis/164.6.1098. [DOI] [PubMed] [Google Scholar]
- 31.Stapleton J T, Jansen R, Lemon S M. Neutralizing antibody to hepatitis A virus in immune serum gobulin and in the sera of human recipients of immune serum globulin. Gastroenterology. 1985;89:637–642. doi: 10.1016/0016-5085(85)90462-7. [DOI] [PubMed] [Google Scholar]
- 32.Stapleton J T, Lange D K, LeDuc J W, Binn L N, Jansen R W, Lemon S M. The role of secretory immunity in hepatitis A virus infection. J Infect Dis. 1991;163:7–11. doi: 10.1093/infdis/163.1.7. [DOI] [PubMed] [Google Scholar]
- 33.Stapleton J T, Lemon S M. Neutralization escape mutants define a dominant immunogenic neutralization site on hepatitis A virus. J Virol. 1987;61:491–498. doi: 10.1128/jvi.61.2.491-498.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stapleton J T, Lemon S M. New vaccines against hepatitis A. In: Levine M M, Woodrow G C, Kaper J B, Cobon G S, editors. New generation vaccines. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 571–585. [Google Scholar]
- 35.Stapleton J T, Raina V, Winokur P L, Walters K, Klinzman D, Rosen E, McLinden J H. Antigenic and immunogenic properties of recombinant hepatitis A virus 14S and 70S subviral particles. J Virol. 1993;67:1080–1085. doi: 10.1128/jvi.67.2.1080-1085.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stapleton J T, Rosen E, McLinden J. Detection of hepatitis A virus capsid proteins in insect cells infected with recombinant baculoviruses encoding the entire hepatitis A virus open reading frame. In: Hollinger F B, Lemon S M, Margolis H S, editors. Viral hepatitis and liver disease. Baltimore, Md: The Williams & Wilkins Co.; 1991. pp. 50–54. [Google Scholar]
- 37.Stewart D R, Morris T S, Purcell R H, Emerson S U. Detection of antibodies to the nonstructural 3C proteinase of hepatitis A virus. J Infect Dis. 1997;176:593–601. doi: 10.1086/514079. [DOI] [PubMed] [Google Scholar]
- 38.Winokur P L, McLinden J H, Stapleton J T. The hepatitis A virus polyprotein expressed by a recombinant vaccinia virus undergoes proteolytic processing and assembly into virus-like particles. J Virol. 1991;65:5029–5036. doi: 10.1128/jvi.65.9.5029-5036.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winokur P L, Stapleton J T. Immunoglobulin prophylaxis for hepatitis A. Clin Infect Dis. 1992;14:580–586. doi: 10.1093/clinids/14.2.580. [DOI] [PubMed] [Google Scholar]
- 40.Wunschmann S, Vallbracht A, Flehmig B, Winokur P, Klinzman D, Stapleton J T. Cytolytic T lymphocyte epitopes are present on hepatitis A virus structural proteins. In: Rizzeto M, editor. Viral hepatitis and liver disease, in press. 1998. [Google Scholar]