Abstract
The authors evaluated the efficacy, safety, and characteristics of patients who respond well to standard dose triple combination therapy including chlorthalidone 25 mg with telmisartan 80 mg plus amlodipine 5 mg in hypertensive patients. This is a multicenter, double‐blind, active‐controlled, phase 3, randomized trial. Patients are randomized to triple combination (telmisartan 40 mg/amlodipine 5 mg/chlorthalidone 12.5 mg, TEL/AML/CHTD group) or dual combination (telmisartan 40 mg/amlodipine 5 mg, TEL/AML group) treatment and then dose up titration to TEL 80/AML5/CHTD25mg and TEL80/AML5, respectively. The primary endpoint is the change of mean sitting systolic blood pressure (MSSBP) at week 8. A Target BP achievement rate, a response rate, and the safety endpoints are also evaluated. Total 374 patients (mean age = 60.9 ± 10.7 years, male = 78.3%) were randomized to the study. The baseline MSSBPs/diastolic BPs were 149.9 ± 12.2/88.5 ± 10.4 mm Hg. After 8 weeks treatment, the change of MSSBPs at week 8 are −19.1 ± 14.9 mm Hg (TEL/AML/CHTD) and −11.4 ± 14.7 mm Hg (TEL/AML) (p < .0001). The achievement rates of target BP (53.8% vs. 37.8%, p = .0017) and responder rate (54.8% vs. 35.6%, p = .0001) at week 8 were significantly higher in TEL/AML/CHTD. There are no serious adverse event and no one discontinued medication due to adverse event. Among the TEL 80/AML5/CHTD25mg treatment group, patients of female or age ≥ 65 years old showed higher rate of target BP achievement than relatively young male. (61.4 vs. 46.8%, p = .042) Our study showed standard dose triple combination of telmisartan 80 mg/amlodipine 5 mg/chlorthalidone 25 mg is efficacious and safe in treatment of primary hypertension. Target BP achievement with triple therapy would be facilitated in female or old age.
Keywords: amlodipine, blood pressure, chlorthalidone, telmisartan, triple combination
1. INTRODUCTION
Cardiovascular diseases (CVDs) are the leading cause of death and about 17.9 million people died from CVDs in 2019, representing 32% of all global deaths. 1 Hypertension is one of the strongest risk factors among the major modifiable risk factors for CVD. 2 The American and European guidelines on the management of arterial hypertension emphasize intensive blood pressure (BP) lowering below 130/80 mm Hg for most hypertensive patients. 3 , 4 However, the BP control rate remains low worldwide and only 40%−50% of treated hypertensive patients achieve the targets. 5 The BP control rate of hypertensive patients (among prevalent) is 48% in Korea 6 Even with dual combination therapy, about 25%–30% of hypertensive patients fail to achieve target BP level and require three or more antihypertensive agents. 7 , 8 The proportion of uncontrolled patients will increase further by latest guidelines. 5
European guidelines recommend angiotensin converting enzyme inhibitor (ACEi)/angiotensin receptor blocker (ARB), a dihydropyridine calcium channel blocker (CCB) and a thiazide/thiazide like diuretics as triple drug combinations. 4 It has been known that chlorthalidone, a thiazide like diuretic produces smooth BP control throughout the diurnal cycle, 9 and more potent with a longer duration of action than hydrochlorothiazide. 10
Because of the concern about diuretic side effects, low dose combination therapy is preferred to reduce dose dependent diuretic side effects. 11
The objectives of the study were to evaluate the efficacy, safety, and characteristics of patients who respond well to standard dose triple combination therapy including chlorthalidone 25 mg with telmisartan 80 mg plus amlodipine 5 mg in hypertensive patients who fail to achieve target BP with ARB plus CCB dual combination therapy.
2. METHODS
2.1. Study design
This is a multicenter, double‐blind, active‐controlled, phase 3, randomized trial with two treatment arms to evaluate the safety and efficacy of single‐pill triple drug combination compared with single‐pill dual drug combination in patients with primary hypertension who fail to achieve target BP after 4 weeks treatment of telmisartan 40 mg/amlodipine 5 mg combination. The detail of design is shown in Figure 1. In the run‐in period, the study patients discontinue previously prescribed antihypertensive medication and take telmisartan 40 mg/amlodipine 5 mg once a day for 4 weeks. The patients of 140 mm Hg ≤ mean sitting systolic BP < 200 mm Hg, or the patients of diabetes mellitus or chronic kidney disease, 130 mm Hg ≤ mean sitting systolic BP < 200 mm Hg after 4 weeks are randomized. There are two treatment arms, one is triple (telmisartan 40 mg/amlodipine 5 mg/chlorthalidone 12.5 mg, TEL/AML/CHTD group) combination drug and the other is dual (telmisartan 40 mg/amlodipine 5 mg, TEL/AML group) combination drug treatment once a day for 2 weeks after randomization. In the dose up‐titration step, the patients who randomized to TEL/AML/CHTD group take telmisartan 80 mg/amlodipine 5 mg/chlorthalidone 25 mg, and the patients who randomized to TEL/AML group take telmisartan 80 mg/amlodipine 5 mg once a day for next 6 weeks.
FIGURE 1.
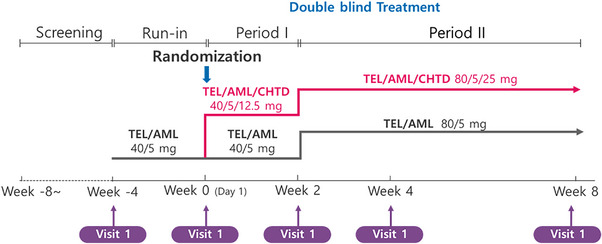
Flowchart of the study design. This is a randomized, double‐blind, active‐controlled, multicenter phase 3 trial to evaluate the safety and efficacy of telmisartan/amlodipine/chlorthalidone triple combination treatment in patients with primary hypertension inappropriately controlled on telmisartan/amlodipine dual combination treatment. TEL/AML/CHTD, telmisartan/amlodipine/chlorthalidone; TEL/AML, telmisartan/amlodipine.
2.2. End point
The primary endpoint is the change of mean sitting systolic BP at week 8. The secondary endpoints are the changes of mean sitting diastolic BP at week 8 and changes of means sitting systolic/diastolic BP at weeks 2 and 4. A Target BP achievement rate and a response rate are also evaluated. The definition of target BP achievement is mean sitting BP < 140/90 mm Hg. For the patients with diabetes mellitus or chronic kidney disease, target BP is mean sitting BP < 130/80 mm Hg. The definition of response to treatment is the reduction of mean sitting systolic BP ≥20 mm Hg and/or mean sitting diastolic BP≥10 mm Hg from baseline values.
The safety endpoints are evaluated with interview, physical examination, and laboratory data on all patients who have received the study drug at least once. Patients with self‐reported or observed adverse symptoms and sign are recorded according to treatment group and severity and encoded to a system‐organ class. The relation of symptoms and sign with the drug treatment are also evaluated. Laboratory abnormalities are followed up until normalization and recorded for safety analysis.
2.3. Study population
The target population is primary hypertensive patients with age between 19 and 80 years old, who currently taking antihypertensive medication (140 mm Hg ≤ mean sitting systolic BP < 200 mm Hg, or the patients of diabetes mellitus or chronic kidney disease, 130 mm Hg ≤ mean sitting systolic BP < 200 mm Hg) or stop the medication for at least 4 weeks (160 mm Hg ≤ mean sitting systolic BP < 200 mm Hg). The exclusion criteria are: (1) mean sitting diastolic BP ≥110mgHg or mean sitting systolic BP ≥200 mm Hg at screening or randomization; (2) variability of ≥20 mm Hg in systolic BP or ≥10 mm Hg in diastolic BP among three measurements, or differences of ≥20/10 mm Hg between both arm brachial values of systolic or diastolic BP; (3) secondary hypertension; (4) allergies or contraindications to study drugs; (5) uncontrolled diabetes mellitus (HbA1c > 9%); (6) history of New York Heart Association (NYHA) class III to IV heart failure, angina, myocardial infarction, cardiomyopathy, arrhythmia, or aortic stenosis requiring treatment within 6 months; (7) cerebral vascular disease within 6 months; (8) serious liver or renal dysfunction; (9) symptomatic hyperuricemia or gout; (10) galactose or lactose‐intolerance; (11) pregnancy or the possibility of pregnancy, or breast feeding; (12) unable to withhold current medication; (13) be prescribed other study drugs within 4 weeks; and (14) abnormal laboratory results (AST, ALT > 3ULN, Cr≥3.0 mg/dL, Creatinine clearance < 15 mL/min, K < 3.0 or > 5.5 mEq/L, Na < 132 mmol/L,).
2.4. Office BP measurements
Office BP is measured in the sitting position with the pressure cuff placed at either the right or left brachial area using a semi‐automated sphygmomanometer (HEM‐7080IC, Omron Healthcare Co, Kyoto, Japan). After a 5‐min rest, BPs are measured three times with a 2−3 min interval. We take the BP data from the arm with higher BP, and mean value of three measurements are used in the analysis. If the BP differences between both arms were more than 20 mm Hg, the patients fail to randomization.
3. STATISTICAL ANALYSIS
3.1. Determination of sample size
Sample size is calculated with 92% power to detect a superiority of TEL/AML/THD to TEL/AML in change of mean sitting systolic BP of 4.9 mm Hg with 13.0 mm Hg standard deviation at a two‐sided significance level of 5%. To satisfy these assumptions and allowing for a drop‐out rate of 15%, a total of 378 patients (189 for each treatment arm) are required for the trial.
3.2. Efficacy analysis
All efficacy analysis is performed on the full analysis which is a modified intention‐to‐treat set that includes patients receiving at least one dose of the study drug and having undergone at least one efficacy evaluation. Additional analysis also be performed on the per protocol set. Continuous variables are reported as mean ± standard deviation and categorical variables are as frequency and percentage. Intergroup comparison was analyzed with two‐sample t‐test, and baseline to 2‐, 4‐, and 8‐week differences were evaluated with paired t‐test for continuous variables. Chi‐square test or Fisher's exact test are used for comparison of categorical variables. Missing values are imputed using Last Observation Carried Forward method.
For evaluation the effect of confounding factors, logistic regressions analysis was done. Two‐sided values of p < .05 indicate statistical significance. All statistical analyses were performed with SAS version 9.4 (SAS Institute Inc.).
All adverse events are encoded to a system‐organ class according to the Medical Dictionary for Regulatory Activities (MedDRA), version 19.0.
4. RESULTS
4.1. Patients disposition and baseline characteristics
From December 24, 2015 to December 2, 2016, primary hypertensive patients were recruited from 35 university hospitals in 16 cities via outpatient departments.
A total 585 patients were screened, of which 381 patients were randomized and received at least one dose of the study drug. After randomization, seven patients were drop out for the protocol violation or taking medication that prohibited. Among the 374 patients, 186 patients were assigned to TEL/AML/CHTD group and 188 patients were assigned to TEL/AML group. The mean age of population is 61 years old and 293(78.2%) patients of population are males. The baseline mean sitting systolic/diastolic BPs are 149.9 ± 12.2/88.5 ± 10.4 mm Hg and the mean value of BMI is 26.5 ± 3.5 kg/m2. The mean duration of hypertension is 127.3 ± 99.6 months. Ninety (24.1%) patients have diabetes mellitus and 54, (14.4%) patients have chronic kidney disease defined by estimated glomerular filtration rate < 90 mL/min.
The Baseline characteristics of patients according to the groups are described in Table 1. There were no significant differences in baseline characteristics except eGFR (85.8 ± 28.5 vs. 94.7 ± 35.4 mL/min/1.732 m2, p = .007) and glucose level (6.3 ± 1.4 vs. 6.7 ± 1.7 mmol/L, p = .017) in TEL/AML/CHTD and TEL/AML group, respectively.
TABLE 1.
Baseline characteristics and week‐8 FU laboratory data.
| TEL/AML/CHTD (n = 186) | TEL/AML (n = 188) | p | |||
|---|---|---|---|---|---|
| Age (years) | 61.5 ± 10.6 | 60.3 ± 10.8 | NS | ||
| Male, n(%) | 144(77.4) | 149(79.3) | NS | ||
| BMI (kg/m2) | 26.2 ± 3.5 | 26.7 ± 3.5 | NS | ||
| Hypertension duration (months) | 138.1 ± 101.2 | 116.7 ± 97.1 | NS | ||
| Diabetes Mellitus, n(%) | 39(21.0) | 51(27.1) | NS | ||
| Dyslipidemia, n(%) | 85(45.5) | 99(52.9) | NS | ||
| Chronic kidney disease, n(%) | 26(14.0) | 28(14.9) | NS | ||
| Pulse pressure (mm Hg) | 73.6 ± 10.2 | 73.5 ± 10.5 | NS | ||
| Heart rate (BPM) | 73.4 ± 10.4 | 73.6 ± 10.5 | NS | ||
| eGFR (mL/min/1.732m2) | 85.8 ± 28.5 | 94.7 ± 35.4 | .007 | ||
| Glucose (mmol/L) | 6.3 ± 1.4 | 6.7 ± 1.7 | .017 | ||
| Creatinine (μmol/L) | 71.3 ± 19.3 | 68.4 ± 19.3 | NS | ||
| Total cholesterol (mmol/L) | 4.8 ± 0.9 | 4.7 ± 1.1 | NS | ||
| LDL‐cholesterol (mmol/L) | 2.9 ± 8.3 | 2.8 ± 0.9 | NS | ||
| HDL‐cholesterol (mmol/L) | 1.3 ± 0.3 | 1.3 ± 0.4 | NS | ||
| Triglyceride (mmol/L) | 145.03 ± 80.62 | 177.78 ± 137.53 | .005 | ||
| Sodium (mmol/L) | 140.8 ± 2.1 | 140.3 ± 2.1 | NS | ||
| Potassium (mmol/L) | 4.35 ± 0.38 | 4.34 ± 0.39 | NS | ||
| Uric acid (μmol/L) | 341.2 ± 89.7 | 336.8 ± 94.4 | NS |
| FU at week 8 | |||||
|---|---|---|---|---|---|
| eGFR (mL/min/1.732m2) | 80.4 ± 28.1 | 93.5 ± 35.8 | <.0001 | ||
| Glucose (mmol/L) | 6.4 ± 1.4 | 6.4 ± 1.3 | .834 | ||
| Creatinine (μmol/L) | 75.9 ± 22.0 | 68.7 ± 19.6 | .002 | ||
| Total cholesterol (mmol/L) | 4.7 ± 1.0 | 4.6 ± 1.0 | .462 | ||
| LDL‐cholesterol (mmol/L) | 2.9 ± 0.9 | 2.8 ± 0.9 | .277 | ||
| HDL‐cholesterol (mmol/L) | 1.3 ± 0.3 | 1.3 ± 0.4 | .596 | ||
| Triglyceride (mmol/L) | 169.2 ± 129.2 | 162.3 ± 110.0 | .600 | ||
| Sodium (mmol/L) | 139.4 ± 2.5 | 140.4 ± 2.1 | <.0001 | ||
| Sodium change from baseline (mmol/L) | −1.39 ± 2.50 | 0.16 ± 2.03 | <.05 | ||
| Potassium (mmol/L) | 4.10 ± 0.43 | 4.32 ± 0.37 | <.0001 | ||
| Uric acid (μmol/L) | 414.7 ± 116.3 | 346.9 ± 92.6 | <.0001 |
| p | |||||
|---|---|---|---|---|---|
| Changes from Baseline to week 8 | Inter‐group | Intra‐group (TEL/AML/CHTD and TEL/AML, respectively) | |||
| eGFR (mL/min/1.732m2) | −5.36 ± 12.21 | −2.18 ± 12.82 | .0171 | <.0001 | .0222 |
| Creatinine (μmol/L) | 4.75 ± 9.49 | 0.70 ± 8.45 | <.0001 | <.0001 | .2609 |
| Sodium (mmol/L) | −1.39 ± 2.50 | 0.16 ± 2.03 | <.0001 | <.0001 | .2895 |
| Potassium (mmol/L) | −0.27 ± 0.43 | −0.01 ± 0.35 | <.0001 | <.0001 | .6328 |
| Uric acid (μmol/L) | 73.08 ± 69.67 | 9.88 ± 53.01 | <.0001 | <.0001 | .0125 |
| Patients with abnormal range of laboratory data, n(%) | |||||
|---|---|---|---|---|---|
| eGFR (mL/min/1.732m2) | 125(71.4) | 150(55.2) | .0015 | ||
| Creatinine (μmol/L) | 30(17.1) | 22(12.0) | .1693 | ||
| Sodium (mmol/L) | 7(4.0) | 2(1.1) | .0790 | ||
| Potassium (mmol/L) | 11(6.3) | 4(2.2) | .0529 | ||
| Uric acid (μmol/L) | 66(37.7) | 27(14.8) | <.0001 | ||
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; HDL, high density lipoprotein; LDL, low density lipoprotein; TEL/AML, telmisartan/amlodipine; TEL/AML/CHTD, telmisartan/amlodipine/chlorthalidone.
4.2. Efficacy results
Table 2 shows baseline and week 2−8 MSSBP, MSDBP and BP changes from baseline to week 8.
TABLE 2.
Changes of MSSBP and MSDBP‐ITT population.
| TEL/AML/CHTD (n = 186) | TEL/AML (n = 188) | Difference between groups | p | ||
|---|---|---|---|---|---|
| Mean systolic BP (mm Hg) | Baseline | 150.1 ± 12.0 | 149.7 ± 12.5 | .790 | |
| 2 week | 137.7 ± 14.6 | 143.0 ± 13.8 | <.0001 | ||
| 4 week | 131.6 ± 13.6 | 138.2 ± 14.2 | <.0001 | ||
| 8 week | 131.3 ± 13.6 | 138.6 ± 16.0 | <.0001 | ||
| Change from Baseline – 2 week | −12.5 ± 14.0 | −6.5 ± 11.3 | −5.8 ± 1.3 | <.0001 | |
| Change from Baseline – 4 week | −18.9 ± 13.4 | −11.1 ± 12.9 | −7.3 ± 1.3 | <.0001 | |
| Change from Baseline – 8 week | −19.1 ± 14.9 | −11.4 ± 14.7 | −7.5 ± 1.5 | <.0001 | |
| Mean diastolic BP, mm Hg | Baseline | 88.2 ± 10.2 | 88.7 ± 10.7 | .696 | |
| 2 week | 83.8 ± 10.2 | 86.1 ± 10.4 | .036 | ||
| 4 week | 80.2 ± 8.6 | 82.3 ± 10.7 | .046 | ||
| 8 week | 79.9 ± 9.8 | 82.8 ± 11.2 | .010 | ||
| change from Baseline – 2 week | −4.3 ± 8.2 | −2.3 ± 7.2 | −2.2 ± 0.7 | .0040 | |
| Change from Baseline – 4 week | −7.9 ± 8.7 | −5.6 ± 7.9 | −2.4 ± 0.8 | .0027 | |
| Change from Baseline – 8 week | −8.4 ± 8.6 | −5.5 ± 9.1 | −2.9 ± 0.9 | .0011 |
Abbreviations: MSDBP, mean sitting diastolic blood pressure; MSSBP, mean sitting systolic blood pressure; TEL/AML, telmisartan/amlodipine; TEL/AML/CHTD, telmisartan/amlodipine/chlorthalidone.
The mean sitting systolic BPs in baseline are 150.1 ± 12.0 mm Hg in TEL/AML/CHTD group and 149.7 ± 12.5 mm Hg in TEL/AML group (p = .790). The mean sitting systolic BP after 8 weeks treatments are 131.3 ± 13.6 mm Hg (TEL/AML/CHTD) and 138.6 ± 16.0 mm Hg (TEL/AML) (p < .0001). The change of mean sitting systolic BPs from baseline to week 8 are −19.1 ± 14.9 mm Hg (TEL/AML/CHTD) and −11.4 ± 14.7 mm Hg (TEL/AML) (p < .0001). The mean sitting systolic BP after 2 and 4‐week treatment are 137.7 ± 14.6 mm Hg, 131.6 ± 13.6 mm Hg in TEL/AML/CHTD group and 143.0 ± 13.8 mm Hg, 138.2 ± 14.2 mm Hg (TEL/AML) (p < .0001 for both week 2 and week 4). The change of mean sitting systolic BPs from baseline to week 2, 4 are −12.5 ± 14.0 mm Hg, −18.9 ± 13.4 mm Hg in TEL/AML/CHTD group and −6.5 ± 11.3 mm Hg, −11.1 ± 12.9 mm Hg in TEL/AML group (p < .0001 for both week 2 and 4) (Table 2, Figure 2A).
FIGURE 2.
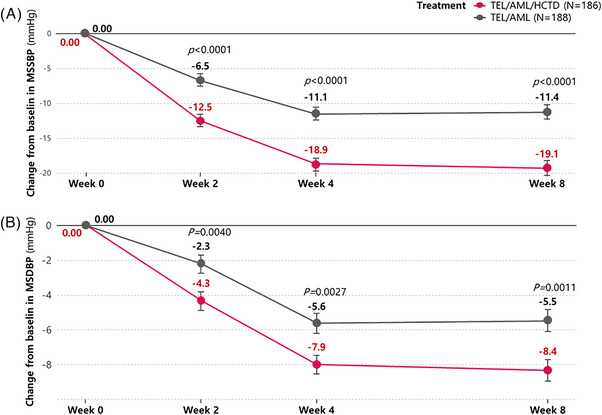
Line graphs of changes in MSSBP and MSDBP from baseline to visit time according to time sequences. The changes of MSSBPs (A) and MSDBP (B) from baseline to week 2, 4, and 8 are significantly greater in TEL/AML/CHTD group (red line) than TEL/AML group (black line). MSSBP, mean sitting systolic blood pressure; MSDBP, mean sitting diastolic blood pressure; TEL/AML/CHTD, telmisartan/amlodipine/chlorthalidone; TEL/AML, telmisartan/amlodipine.
The mean sitting diastolic BPs at in baseline are 88.2 ± 10.2 mm Hg in TEL/AML/CHTD group and 88.7 ± 10.7 mm Hg in TEL/AML group (p = .696). The mean sitting diastolic BP after 8 weeks treatments are 79.9 ± 9.8 mm Hg (TEL/AML/CHTD) and 82.8 ± 11.2 mm Hg (TEL/AML) (p = .010). The change of mean sitting diastolic BPs from baseline to week 8 are −8.4 ± 8.6 mm Hg (TEL/AML/CHTD) and −5.5 ± 9.1 mm Hg (TEL/AML) (p = .0011). The mean sitting diastolic BP after 2 and 4‐week treatment are 83.8 ± 10.2 mm Hg, 80.2 ± 8.6 mm Hg in TEL/AML/CHTD group and 86.1 ± 10.4 mm Hg, 82.3 ± 10.7 mm Hg (TEL/AML) (p = .036 for week 2 and p = .046 for week 4). The change of mean sitting diastolic BPs from baseline to week 2, 4 are −4.3 ± 8.2 mm Hg, −7.9 ± 8.7 mm Hg in TEL/AML/CHTD group and −2.3 ± 7.2 mm Hg, −5.6 ± 7.9 mm Hg in TEL/AML group (p = .0040 for week 2 and p = .0027 for week 4) (Table 2, Figure 2B).
The achievement rates of target BP after 8 weeks treatment is 53.8% in TEL/AML/CHTD group and 37.8% in TEL/AML group (p = .0017) The achievement rates of target BP after 2 and 4‐week treatment are 38.5%, 51.6% in TEL/AML/CHTD group and 18.0%, 29.8% in TEL/AML group (p < .0001 for both week 2 and 4) (Figure 3A). After 8 weeks treatment, the responder rates by definition of mean sitting systolic BP reduced by more than 20 mm Hg “OR” mean sitting diastolic BP reduced by more than 10 mm Hg from the corresponding baseline value are 54.8% in TEL/AML/CHTD group and 35.6% in TEL/AML group (p < .0001). After 2 and 4‐week treatment, responder rates by above definition are 39.6%, 54.8% in TEL/AML/CHTD group and 22.4%, 36.7% in TEL/AML group (p = .0003 for both week 2 and 4) (Figure 3B). After 8 weeks treatment, the responder rates by definition of mean sitting systolic BP reduced by more than 20 mm Hg “AND” mean sitting diastolic BP reduced by more than 10 mm Hg from the corresponding baseline value are 32.3% in TEL/AML/CHTD group and 16.5% in TEL/AML group (p = .0003). After 2 and 4‐week treatment, responder rates by above definition are 17.0%, 30.7% in TEL/AML/CHTD group and 6.0%, 13.3% in TEL/AML group (p = .0008 for week 2 and p < .0001 for week 4).
FIGURE 3.
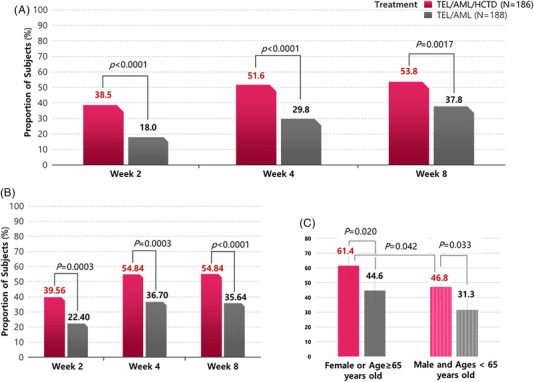
Bar graphs of the achievement rates of target BP (A, C) and responder rate (B). (A): Achievement rates of target BP after 2, 4 and 8 weeks are significantly higher in TEL/AML/CHTD group than TEL/AML group. (B): Responder rates by definition of MSSBP reduced by more than 20 mm Hg “OR” MSDBP reduced by more than 10 mm Hg from the corresponding baseline value are significantly higher in TEL/AML/CHTD group than TEL/AML group after 2, 4, and 8 weeks treatment. (C) Achievement rates of target BP after 8 weeks are significantly high in female or age over 65 years old among in patients with TEL/AML/CHTD treatment. BP, blood pressure; MSSBP, mean sitting systolic BP; MSDBP, mean sitting diastolic BP; TEL/AML, telmisartan/amlodipine; TEL/AML/CHTD, telmisartan/amlodipine/chlorthalidone.
4.3. Safety results
Total 48(12.6%) patients are reported to showed treatment emergent adverse event. Among them, 30(15.6%) patients are in TEL/AML/CHTD group and 18(9.5%) patients are in TEL/AML group (p = .0728). Treatment related adverse events are shown in 20(10.4%) patients of TEL/AML/CHTD group and 10 (5.3%) patients of TEL/AML group (p = .0633). “Dizziness” is the most frequently reported symptoms in both groups (11(5.7%) patients in TEL/AML/CHTD group, 4(2.1%) patients in TEL/AML group). Most of the cases are categorized as “mild”. “Moderate” adverse events are occurred in 6(3.1%) patients in TEL/AML/CHTD group and four (2.1%) patients in TEL/AML group. Serious adverse events are reported in 1(0.5%) patient of TEL/AML/CHTD groups (gastric ulcer) and 1(0.5%) patients of TEL/AML group (pulmonary mass). Events are revealed as not related with study medication. There are no serious adverse event and no one discontinued the medication due to adverse event (Table 3).
TABLE 3.
Compliance rate and summary of adverse events—Safety Population.
| Compliance rate | Total (n = 381) | TEL/AML/CHTD, (n = 192) | TEL/AML, (n = 189) | p |
|---|---|---|---|---|
| Baseline – weeks 2 | 98.0 ± 5.8 | 97.9 ± 5.2 | 98.1 ± 6.3 | NS |
| Week 2 – weeks 4 | 98.8 ± 4.7 | 98.8 ± 5.3 | 98.7 ± 4.0 | NS |
| Week 4 – weeks 8 | 98.7 ± 4.7 | 98.7 ± 4.6 | 98.7 ± 4.7 | NS |
| Total period | 98.2 ± 4.2 | 98.0 ± 4.5 | 98.4 ± 3.8 | NS |
| Adverse events | Total (n = 381) | TEL/AML/CHTD (n = 192) | TEL/AML (n = 189) | p |
|---|---|---|---|---|
| TEAE, n(%) | 48(12.6) | 30(15.6) | 18(9.5) | .0728 |
| Treatment related AEs, n(%) | 30(7.9) | 20(10.4) | 10(5.3) | .0633 |
| SAEs, n(%) | 2(0.5) | 1 (0.5) | 1 (0.5) | 1.000 |
| Treatment‐related SAEs, n(%) | 0(0) | 0(0) | 0(0) | |
| Discontinued due to AE | 0(0) | 0(0) | 0(0) |
Abbreviations: AEs, TEAEs which have the relationship with IP such as ‘Certainly’, ‘Probably’, ‘Possibly’, ‘Unlikely’ or ‘Unassessable/Unclassifiable; TEAE, Treatment emergent adverse event; SAE, Serious adverse event Treatment‐Related; TEL/AML, telmisartan/amlodipine; TEL/AML/CHTD, telmisartan/amlodipine/chlorthalidone.
4.4. Laboratory data
After 8‐week treatment, there were significant changes of eGFR (−5.36 ± 12.21 vs. −2.18 ± 12.82, p = .0171), blood creatinine (4.75 ± 9.49 vs. 0.70 ± 8.45, p < .0001) sodium (−1.39 ± 2.50 vs. 0.16 ± 2.03, p < .001) and potassium (−0.27 ± 0.43 vs. −0.01 ± 0.35, p < .0001) levels in TEL/AML/CHTD group compared with in TEL/AML group. The blood serum uric acid level was significantly increased in TEL/AML/CHTD group compared with in TEL/AML group. (73.08 ± 69.67 vs. 9.88 ± 53.01, p < .0001) The percentage of patients who over the normal limit of each center's laboratory data were not different between group except uric acid level [66(37.7%) vs. 27(14.8%), p < .0001] (Table 1)
Patients who showed abnormal laboratory data after taking medications were normalized on followed‐up examinations.
4.5. Characteristics of patients who facilitate target BP achievement with TEL/AML/CHTD
The female patients or older age (65 ≥ years old) showed dominant target BP achievement rate with TEL/AML/CHTD treatment compared with male and young patients. (61.4 vs. 46.8, p = .0042) (Figure 3C, Table S1). However, the MSSBP/MSDBP changes from baseline at week 8 were not significantly different in TEL/AML/CHTD treatment group (−20.22 ± 16.16/−8.18 ± 8.40 vs. −17.73 ± 13.33/−8.71 ± 8.75, p = .286 for MSSBP and .688 for MSDBP). Even though female or older patients had significantly higher prevalence of DM and less BMI, the logistic regression analysis showed those factors were not affect target BP achievement with TEL/AML/CHTD treatment (Table S2).
5. DISCUSSION
The results of our study demonstrate that single pill standard dose of telmisartan 80 mg/amlodipine 5 mg/chlorthalidone 25 mg triple combination therapy is efficacious and safe after 8 weeks treatment in patients who failed to achieve target BP with 4‐week treatment of telmisartan 40 mg/amlodipine 5 mg dual combination.
Our study patients were relatively young, male dominant with grade 1 (by Korean and European criteria) hypertension at randomization after 4 weeks treatment with TEL 40 mg/AML 5 mg. The patients’ mean duration of hypertension was about 127 months.
Baseline characteristics and initial laboratory data were not significantly different except eGFR and glucose level between the groups. At 8 weeks of treatment, the MSSBP was significantly lower in TEL/AML/CHTD group than TEL/AML group. The primary endpoint, MSSBP change from baseline to 8 weeks treatment was significantly greater in TEL/AML/CHTD group than in TEL/AML group. Least squares mean (standard errors) of MSSBP changed value was −18.65 ± 1.04 mm Hg in TEL/AML/CHTD and −11.18 ± 1.04 mm Hg in TEL/AML group. The difference of changed value between groups was −7.48 ± 1.47 mm Hg (95% CI: −10.37, −4.58, p < .0001). The MSSBP lowering efficacy of TEL/AML/CHTD combination was superior to TEL/AML combination with statistical significance. This difference was the effect of chlorthalidone 25 mg in TEL/AML/CHTD. The secondary endpoint, MSSBP change from baseline to 2 weeks treatment was significantly greater in TEL/AML/CHTD group than in TEL/AML group. Least squares mean (standard errors) of MSSBP changed value was −12.57 ± 0.94 mm Hg in TEL/AML/CHTD and −6.86 ± 0.94 mm Hg in TEL/AML group. The difference of changed value between groups was −5.71 ± 1.33 mm Hg (p < .0001). After 2 weeks treatment, the MSSBP lowering efficacy of TEL/AML/CHTD combination was greater than TEL/AML combination due to the effect of chlorthalidone 12.5 mg in TEL/AML/CHTD. Likewise, other secondary endpoints showed significantly greater BP lowering efficacy of TEL/AML/CHTD than TEL/AML group. There were no differences in compliance between groups (98.0 vs. 98.4%, p = NS) (Table 3)
Hypertension is one of the strongest risk factors among the major modifiable risk factors for CVD. 2 The BP control rate remains low worldwide and only 40%−50% of treated hypertensive patients achieve the recommended targets. 5 , 6 Even when treated with a dual combination, almost 25%–30% of hypertensive patients fail to achieve target BP level and require three or more antihypertensive agents. 7 , 8 , 12
European guidelines recommend ACEi/ARB, a dihydropyridine CCB and a thiazide/thiazide like diuretics as triple drug combinations. 4 Thiazide and thiazide‐like diuretics are mandatory in triple combination therapy and has been the gold standard of antihypertensive therapy for primary hypertension. 13 , 14 , 15 However, despite the evidence of beneficial clinical data, thiazide and thiazide‐like diuretics’ use in real‐world practice has continued to decline. 16 In South Korea, diuretic prescription rate is 24.7% while ARB/ACE and CCB are 73.3% and 60.9%, respectively. 6 This might be related with several misconceptions and concerns about diuretic such as doubtful effect on outcome, tolerability and metabolic/electrolyte derangement. 17 , 18 , 19
Korean society of hypertension guidelines recommend diuretics in treatment of elderly hypertensive patients 20 and data from the National Health and Nutrition Examination Survey reveal that women were more likely to use diuretics than men (31.6% vs. 22.3%). 21 There is no proven antihypertensive medication more beneficial for older women, except thiazide diuretics, which reduce calcium excretion and prevent osteoporosis. 22 A study outcome of comparison between ACEi and diuretics for hypertension in the elderly that includes 3102 women, showed ACEi–based regimen benefit only for men. 23 Our study results show that compared with TEL/AML dual combination, TEL/AML/CHTD combination therapy is beneficial for target BP achievement, especially to elderly or women patients (Figure 3C, Tables S1 and S2).
Because of the significant pharmacokinetic and pharmacodynamics differences 10 chlorthalidone is 1.5−2.0 times as potent as hydrochlorothiazide and shows smooth BP control throughout the diurnal cycle. 24 Those may be the cause for chlorthalidone's well‐documented benefits for reduced CV morbidity and mortality.
Most of the studies regarding combination therapy prefer lower dose of chlorthalidone as 3.125–12.5 mg combination in dual 25 or triple therapy. 26 , 27 , 28 , 29
Safety results of our study regarding triple combination therapy including chlorthalidone 25 mg showed that treatment emergent adverse event occurred in 30(15.6%) patients in TEL/AML/CHTD group and 18 (9.5%) patients in TEL/AML group. Treatment related adverse events occurred in 20(10.4%) patients of TEL/AML/CHTD group and 10(5.3%) patients of TEL/AML group. There were no significant differences in occurrence rate of adverse event between groups. Most of the cases are categorized as “mild” and all adverse events were not related with study medications. No one was discontinued the medication due to the adverse event. “Dizziness” was the most frequently reported symptoms in both groups. [11(5.7%) patients in TEL/AML/CHTD group, 4(2.1%) patients in TEL/AML group] Among total 15 patients who complaint dizziness, four patients called to research medical staff and revealed no significant hypotension on home BP measurement. The incidence of “dizziness” symptom was more frequent in TEL/AML/CHTD group than TEL/AML group. It has been known that dizziness is a common complication of chlorthalidone and even reported in lower dose as 6.25–12.5 mg of chlorthalidone in triple combination. 27 , 28 Dizziness symptoms also reported in the study with dual combination including chlorthalidone 25 mg. The study compare the efficacy between azilsartan medoxomil 80 mg/chlorthalidone 25 mg and azilsartan medoxomil 80 mg showed dizziness was more common (11.8% vs. 3.7%) in azilsartan medoxomil /chlorthalidone group. 30 Likewise, triple combination therapy including hydrochlorothiazide showed about 9.9% incidence rate of dizziness. 31 Serious adverse events are reported in 1(0.5%) patient of TEL/AML/CHTD groups (gastric ulcer in patient taking anticoagulant) and 1(0.5%) patient of TEL/AML group (pulmonary mass). Events were not related with study medication.
Follow‐up laboratory data showed that the changes from baseline to week 8 in blood sodium, potassium and uric acid levels was significantly different between groups. There were significant greater decrease in blood sodium (−1.39 ± 2.50 vs. 0.16 ± 2.03, p < .05) and potassium (−0.27 ± 0.43 vs. −0.01 ± 0.35, p < .05) levels and greater increase in blood uric acid level (73.08 ± 69.67 vs. 9.88 ± 53.01, p < .05) in TEL/AML/CHTD group than TEL/AML group. Those observed greater electrolyte and uric acid changes were characteristic findings occurred with thiazide and thiazide like diuretic therapy and no one showed serious electrolyte derangement.
6. CONCLUSIONS
From the study results, we concluded that standard dose triple combination of telmisartan 80 mg/amlodipine 5 mg/chlorthalidone 25 mg is efficacious and safe in treatment of primary hypertension. The BP lowering efficacy is superior to telmisartan 80 mg/amlodipine 5 mg dual combination and more pronounced in patients of elderly or women.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
Supporting information
Supplementary information
ACKNOWLEDGMENTS
Sponsorship for this study and article processing charges were covered by Yuhan Corporation. All authors had full access to all of the data in this study and take complete responsibility for the integrity of data and the accuracy of data analysis
Cho EJ, Kim MH, Kim Y‐H, et al. Efficacy and safety of standard dose triple combination of telmisartan 80 mg/amlodipine 5 mg/chlorthalidone 25 mg in primary hypertension: A randomized, double‐blind, active‐controlled, multicenter phase 3 trial. J Clin Hypertens. 2023;25:817–827. 10.1111/jch.14707
DATA AVAILABILITY STATEMENT
There is no shared data of this study.
REFERENCES
- 1. World Health Organization . Cardiovascular diseases (CVDs) Fact sheet. June 11, 2021 https://www.who.int/en/news‐room/fact‐sheets/detail/cardiovascular‐diseases‐(cvds)
- 2. GBD 2016 Risk Factors Collaborators . Global, regional, and national comparative risk assessment of 84 behavioral, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1345‐1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:2199‐2269. [DOI] [PubMed] [Google Scholar]
- 4. Williams B, Mancia G, Spiering W, et al, ESC Scientific Document Group . 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021‐3104. [DOI] [PubMed] [Google Scholar]
- 5. Redon J, Mourad JJ, Schmieder RE, Volpe M, Weiss TW. Why in 2016 are patients with hypertension not 100% controlled? A call to action. J Hypertens. 2016;34:1480‐1488. [DOI] [PubMed] [Google Scholar]
- 6. Kim HC, Lee H, Lee H, et al, the Korean Society of Hypertension–Hypertension Epidemiology Research Working Group . Korea hypertension fact sheet 2021: analysis of nationwide population‐based data with special focus on hypertension in women. Clin Hypertens. 2023;28:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mancia G, Laurent S, Agabiti‐Rosei E, et al, European Society of Hypertension . Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens. 2009;27:2121‐2158. [DOI] [PubMed] [Google Scholar]
- 8. Gradman AH, Basile JN, Carter BL, Co Bakris GL, American Society of Hypertension Writing Group . Combination therapy in hypertension. J Am Soc Hypertens. 2010;4:90‐98. [DOI] [PubMed] [Google Scholar]
- 9. Pareek AK, Messerli FH, Chandurkar NB, et al. Efficacy of low‐dose chlorthalidone and hydrochlorothiazide as assessed by 24‐h ambulatory blood pressure monitoring. J Am Coll Cardiol. 2016;67:379‐389. [DOI] [PubMed] [Google Scholar]
- 10. Carter BL, Ernst ME, Cohen JD. Hydrochlorothiazide versus chlorthalidone: evidence supporting their interchangeability. Hypertension. 2004;43:4‐9. [DOI] [PubMed] [Google Scholar]
- 11. Braunwald E, Zipes DP, Libby P, et al. Heart Disease: A Textbook of Cardiovascular Medicine. 11th ed. WB Saunders; 2018. [Google Scholar]
- 12. Jamerson K, Weber MA, Bakris GL, et al, ACCOMPLISH Trial Investigators . Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high‐risk patients. N Engl J Med. 2008;359:2417‐2428. [DOI] [PubMed] [Google Scholar]
- 13. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group . The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002;288:2981‐2997. [DOI] [PubMed] [Google Scholar]
- 14. Wright JT Jr, Probstfield JL, Cushman CW, et al, ALLHAT Collaborative Research Group . ALLHAT findings revisited in the context of subsequent analyses, other trials, and meta‐analyses. Arch Intern Med. 2009;169:832‐842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. No authors listed . Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: the antihypertensive and lipid‐lowering treatment to prevent heart attack trial (ALLHAT). ALLHAT Collaborative Research Group. JAMA 2000;283:1967‐1975. [PubMed] [Google Scholar]
- 16. Manolio TA, Cutler JA, Furbert CD, et al. Trends in pharmacologic management of hypertension in the United States. Arch Intern Med. 1995;155:829‐837. [PubMed] [Google Scholar]
- 17. Moser M. Why are physicians not prescribing diuretics more frequently in the management of hypertension? JAMA. 1998;279:1813‐1816. [DOI] [PubMed] [Google Scholar]
- 18. Middeke M, Weisweiler P, Schwandt P, Holzgreve H. Serum lipoprotein during antihypertensive therapy with beta blockers and diuretics: a controlled long‐term comparative trial. Clin Cardiol. 1987;10:94‐98. [DOI] [PubMed] [Google Scholar]
- 19. Messerli FH, Nunez BD, Nunez MM, et al. Hypertension and sudden death: disparate effects of calcium entry blockers and diuretic therapy on cardiac dysrhythmias. Arch Intern Med. 1989;149:1263‐1267. [DOI] [PubMed] [Google Scholar]
- 20. Lee HY, Jinho Shin J, Kim G, et al. 2018 Korean Society of Hypertension Guidelines for the management of hypertension: part II‐diagnosis and treatment of hypertension. Clin Hypertens. 2019;25:20‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qiuping G, Burt VL, Paulose‐Ram R, Dillon CF. Gender differences in hypertension treatment, drug utilization patterns, and blood pressure control among US adults with hypertension: data from the National Health and Nutrition Examination Survey 1999‐2004. Am J Hypertens. 2008;21:789‐798. [DOI] [PubMed] [Google Scholar]
- 22. Puttnam R, Davis BR, Pressel SL, et al, Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Collaborative Research Group . Association of 3 different antihypertensive medications with hip and pelvic fracture risk in older adults: secondary analysis of a randomized clinical trial. JAMA Intern Med. 2017;177:67‐76. [DOI] [PubMed] [Google Scholar]
- 23. Wing LM, Reid CM, Ryan P, et al, Second Australian National Blood Pressure Study Group . A comparison of outcomes with angiotensin‐converting–enzyme inhibitors and diuretics for hypertension in the elderly. N Engl J Med. 2003;348:583‐592. [DOI] [PubMed] [Google Scholar]
- 24. Pareek A, Zawar SD, Salagre SB, Chandurkar NB, Karnik ND. Antihypertensive efficacy of metoprolol XL/low dose chlorthalidone (6.25 mg) combination: a randomized, comparative study in Indian patients with mild‐to‐moderate essential hypertension. Eur J Med Res. 2009;14:297‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pareek A, Basavanagowdappa H, Zawar S, Kumar A, Chandurkar N. A randomized, comparative study evaluating the efficacy and tolerability of losartan‐low dose chlorthalidone (6.25 mg) combination with losartan‐hydrochlorothiazide (12.5 mg) combination in Indian patients with mild‐to‐moderate essential hypertension. Expert Opin Pharmacother. 2009;10:1529‐1536. [DOI] [PubMed] [Google Scholar]
- 26. Sarkar G, Gaikwad VB, Sharma A, et al. Fixed‐dose combination of metoprolol, telmisartan, and chlorthalidone for essential hypertension in adults with stable coronary artery disease: phase III study. Adv Ther. 2022;39:923‐942. [DOI] [PubMed] [Google Scholar]
- 27. Hong SJ, Sung K, Lim S, et al, & On behalf of HM_APOLLO Investigator . Low‐dose triple antihypertensive combination therapy in patients with hypertension: a randomized, double‐blind, phase II study. Drug Des Devel Ther. 2020;14:5735‐5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Webster RR, Salam A, de Silva HA, et al, for the TRIUMPH Study Group . Fixed low‐dose triple combination antihypertensive medication vs usual care for blood pressure control in patients with mild to moderate hypertension in Sri Lanka: A randomized clinical trial. JAMA. 2018;320:566‐579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sung K, Sung JH, Cho EJ, et al. Efficacy and safety of low‐dose antihypertensive combination of amlodipine, telmisartan, and chlorthalidone: a randomized, double‐blind, parallel, phase II trial. J Clin Hypertens. 2022;24:1298‐1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sica D, Bakris GL, White WB, et al. Blood pressure‐lowering efficacy of the fixed‐dose combination of azilsartan medoxomil and chlorthalidone: a factorial study. J Clin Hypertens (Greenwich). 2012;14:284‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oparil S, Melino M, Lee J, Fernandez V, Heyrman R. Triple therapy with olmesartan medoxomil, amlodipine besylate, and hydrochlorothiazide in adult patients with hypertension: the TRINITY multicenter, randomized, double‐blind, 12‐week, parallel‐group study. Clin Ther. 2010;32:1252‐1269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information
Data Availability Statement
There is no shared data of this study.


