Abstract
Hypertension is the leading cause of death worldwide, affecting 1.4 billion people. Treatment options include the widely used calcium channel blockers, among which amlodipine, a dihydropyridine, has unique characteristics that distinguish it from other drugs within this class. This review aims to provide an updated overview of the evidence supporting the use of amlodipine over the past 30 years and highlights its cardiovascular benefits in current hypertension management. Amlodipine has low renal clearance (7 mL/min/mg) and long half‐life (35–50 h) and duration of action, which allows it to sustain its anti‐hypertensive effect for more than 24 h following a single dose. Additionally, blood pressure (BP) control is maintained even when a dose has been missed, providing continuous protection in case of incidental noncompliance. It has proven to reduce BP variability and successfully lower BP. Amlodipine also controls BP in patients with a systolic/diastolic BP of 130/80 mm Hg or higher, diabetes, or chronic kidney disease without worsening glycemic or kidney function. Additionally, amlodipine is a wise choice for older adults due to its ability to control BP and protect against stroke and myocardial infarction. Side effects of amlodipine include edema, palpitations, dizziness, and flushing, which are more common with the higher dose of 10 mg. Amlodipine is cost effective and predicted to be cost saving when compared with usual care.
Keywords: amlodipine, blood pressure variability, calcium channel blockers, hypertension
1. INTRODUCTION
Hypertension is the leading cause of death worldwide, affecting 31.1% of the adult population (1.4 billion people). 1 It accounts for about half of all heart disease‐ and stroke‐related deaths worldwide. 2 The residual lifetime risk for developing hypertension is 90% for middle‐aged or older individuals. 3
In recent decades, hypertension management has seen the introduction of several new principles/technologies. Advances in information technology have revolutionized hypertension monitoring with the development of new devices. 4 Current global guidelines recommend treating hypertension based on 24‐h ambulatory blood pressure monitoring (ABPM) and home blood pressure (BP) measurements rather than measurement in the clinical setting. 5 , 6 , 7 Additionally, observational studies and clinical trials reported that short‐ and long‐term BP variability (BPV) are linked to hypertension‐mediated target organ damage, cardiovascular events, and mortality. 8 , 9
Most guidelines recommend angiotensin‐converting enzyme inhibitors (ACE‐I) or angiotensin receptor blockers (ARBs), calcium channel blockers (CCBs), and thiazide‐like diuretics as first‐line agents for hypertension management, but little differentiation is given to specific molecules within their respective class. 10 , 11 , 12 , 13 , 14 CCBs were first introduced for coronary heart disease but gained widespread recognition for their efficacy in hypertension. 15 These agents can be classified into dihydropyridines, phenylalkylamines, and benzothiazepines. 16 Among dihydropyridines, amlodipine has unique characteristics that set it apart from other drugs within this class. 15 This review aims to provide an updated overview of the evidence supporting the use of amlodipine over the past 30 years and highlights its cardiovascular benefits in current hypertension management.
2. HYPERTENSION EPIDEMIOLOGY
Hypertension becomes progressively more common with advancing age, with a prevalence of more than 60% in people aged 60 years or older. 11 A pooled analysis with more than 100 million participants showed that hypertension prevalence was highest throughout Central and Eastern Europe, Central Asia, Oceania, Southern Africa, and some countries in Latin America and the Caribbean. 17
A systolic/diastolic BP (SBP/DBP) of 130−139/80–89 mm Hg or higher, which has been defined as stage 1 hypertension in the 2017 American College of Cardiology/American Heart Association (AHA) BP guideline, is widespread among young adults and can be serious if not treated. 10 , 18 In China, a long‐term study with 21 441 participants showed that 65% of middle‐aged adults (35−59 years old) with stage 1 hypertension progressed to a SBP/DBP of 140/90 mm Hg or higher (stage 2 hypertension) within 15 years. 19 In this study, stage 1 hypertension was associated with 26.5% higher risk of cardiovascular deaths and 13.4% higher risk of cardiovascular events relative to normotension. 19 Furthermore, the AHA emphasizes that the benefits of treating stage 1 hypertension in patients with a low 10‐year atherosclerotic cardiovascular disease risk outweigh the risks. 18
3. GUIDELINES SUGGEST CCBS AS FIRST‐LINE TREATMENT, BUT CCBS ARE NOT ALL THE SAME
Although all CCBs share the same ability to interact with L‐type voltage‐dependent transmembrane calcium channels, they have distinctive pharmacokinetics and pharmacodynamics that influence their efficacy and safety profile. 16 Compared to other dihydropyridine agents, amlodipine has low renal clearance (7 mL/min/mg) and long half‐life (35−50 h). 20 , 21 Amlodipine has a high bioavailability (60%−80%) and sustains its antihypertensive effect for more than 24 h following a single oral dose, 22 which is beneficial for patient compliance. 15 In addition, BP control is maintained even when a dose has been missed, which is the most common form of noncompliance in the management of hypertension. 23 Following amlodipine discontinuation, BP usually returns to baseline over 1 week without any dangerous rebound elevations in BP. 22
Side effects of amlodipine include edema, palpitations, dizziness, and flushing, which are more common with the higher dose of 10 mg, 24 as well as, uncommonly, gum hypertrophy with an incidence around 2%, which is reversible on drug withdrawal. 25 , 26 It is worthwhile mentioning that amlodipine‐related edema is not secondary to salt retention; rather, it is due to dilatation of precapillary arterioles in the lower extremities, where the effects of increased hydrostatic pressure force fluid into the interstitial space. 27
Studies showed no differences in safety parameters between amlodipine and nifedipine gastrointestinal therapeutic system (GITS), and neither drug caused any serious or severe treatment‐related adverse events. 28 , 29
4. ROLE OF AMLODIPINE IN MILD TO MODERATE HYPERTENSION
Randomized studies indicate that amlodipine is superior to diltiazem 30 and hydrochlorothiazide 31 in reducing SBP and DBP in patients with mild or moderate hypertension. Amlodipine is also as effective as chlorthalidone in reducing mean BP in patients aged ≥50 years. 32 In Asians with mild to moderate hypertension, amlodipine titration from 5 to 10 mg/day significantly reduced SBP. 33
5. ROLE OF AMLODIPINE IN BPV, INCLUDING THE MORNING BP SURGE
BPV over 24 h is a significant and independent risk factor for cardiovascular morbidity and mortality. 34 , 35 There are substantial differences in the effect of different classes of antihypertensive drugs on short‐term 24‐h BPV. When patients were treated with combination drug therapy, regimens that included CCBs showed lower BPV than regimens that did not. 36 In a retrospective analysis of BPV data from five studies, amlodipine was superior to other classes of antihypertensive drugs in reducing BPV. 37 In a randomized, double‐blind, placebo‐controlled trial, amlodipine significantly decreased daytime, nighttime, and 24‐h SBP variability, whereas candesartan did not, as measured by ABPM after 3‐month treatment. 38 Many studies also showed the importance of long‐term visit‐to‐visit BPV on cardiovascular outcomes and mortality. 39 For example, in the randomized controlled Anglo‐Scandinavian Cardiac Outcomes Trial‐Blood Pressure Lowering Arm (ASCOT‐BPLA), the group assigned to amlodipine‐based treatment had a significantly lower BPV, as determined by SBP standard deviation and variation independent of the mean, than the atenolol group at all follow‐up visits, mainly because of lower within‐individual visit‐to‐visit variability. Within‐visit and ABPM variability in SBP were also lower in the amlodipine group than in the atenolol group. 40
The morning BP surge is linearly associated with risk of stroke 41 and can be reduced when antihypertensive drugs are taken in the evening compared with in the morning. 42 This reflects the fact that many drugs are not completely effective over a 24‐h dosing interval. Thus, long‐acting drugs are more likely to be effective in reducing the morning BP surge. Within the dihydropyridine class of CCBs, amlodipine is associated with significantly greater reductions in morning BP surge than nifedipine GITS. 43
6. ROLE OF AMLODIPINE IN STROKE PREVENTION
Many landmark trials have shown the benefit of amlodipine protection against stroke. For example, based on nine landmark trials, amlodipine reduced the incidence of stroke compared with placebo (by 40%), ACE‐I (by 18%), ARBs (by 16%), and diuretics or β‐blockers (by 14%). 44 In the ASCOT study, amlodipine‐based treatment reduced the relative risk of stroke by 23% compared with atenolol‐based treatment. 45 Amlodipine also showed better protection against stroke (16%) and myocardial infarction (MI, 17%) when compared with ARBs in a quantitative overview. 46 Based on a recent meta‐analysis that included 13 studies and more than 50 000 patients, amlodipine reduced the incidence of MI (13%) when compared to other antihypertensive drugs. 47
Several mechanisms have been proposed to explain the benefits of CCBs/amlodipine on stroke protection: (1) Amlodipine has a longer duration of action than almost all other antihypertensive drugs 21 ; (2) Carotid intima‐media thickness (IMT) is strongly correlated with cardiovascular events. CCBs, such as amlodipine, can reduce the progression of carotid IMT more than diuretics, β‐blockers, or ACE‐I, and this might contribute to CCBs superior protection against stroke 48 ; (3) CCBs can reduce inter‐individual BPV. In a meta‐analysis in which each class was compared with all other drug classes combined, CCBs and non‐loop diuretics reduced inter‐individual variation in SBP, whereas ACE‐I and ARBs had little effect and β‐blockers increased BPV; compared with placebo, CCBs reduced inter‐individual BPV more than all other classes of drugs (Figure 1). 49
FIGURE 1.
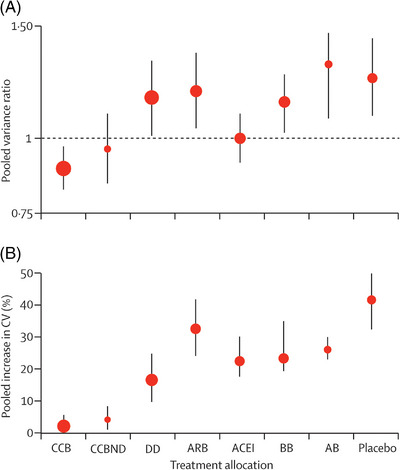
Meta‐analysis of antihypertensive drug effects on long‐term BPV. (A) Change in group variation in SBP at follow‐up compared with baseline as variance ratio. (B) Percentage increase in coefficient of variation. Error bars represent the 95% confidence intervals. A is plotted on a logarithmic scale. The apparent increase in variance ratio and coefficient of variation (CV) from baseline to follow‐up was mainly a consequence of the requirement in many trials for narrow ranges of BP at randomization, which tended to lead to an increase in group standard deviation on follow‐up; however, this effect applied almost equally to all drug classes. AB, α−1 blocker; ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin‐2‐receptor blocker; BB, β‐blocker; CCB, calcium channel blocker; CCBND, non‐dihydropyridine calcium channel blocker; DD, non‐loop diuretic drug. Reprinted with permission from Webb. 49
7. AMLODIPINE IN SPECIFIC POPULATIONS
7.1. Patients with angina pectoris
About 47% of the risk of developing ischemic heart disease is attributable to hypertension. 2 Both the European Society of Cardiology and the AHA recommend CCBs alone, or in combination with a β‐blocker, as first‐line treatment in the management of stable ischemic heart disease. 10 , 50 A prospective, double‐blind study showed that amlodipine reduced the incidence of repeat percutaneous transluminal coronary angioplasty and clinical complications when used 2 weeks before, and continued for 4 months after, angioplasty. 51 In an open‐label, randomized study of patients with hypertension and type 2 diabetes, amlodipine therapy resulted in a significantly greater reduction of IMT compared with ARB therapy, suggesting that amlodipine has an inhibitory effect on early atherosclerotic process. 52 Furthermore, in a prospective, randomized study, amlodipine was associated with fewer hospitalizations for unstable angina (33% reduction) and coronary revascularization (43% reduction), regardless of the use of β‐blockers, nitrates, or lipid‐lowering therapy. 53 Interestingly, in the ASCOT trial, amlodipine was reported to act synergistically with atorvastatin in preventing coronary outcomes. 54 There have been other reports of synergy between these two drugs, 55 and a molecular mechanism has been proposed for this interaction. 56 Thus, amlodipine may be a reasonable choice to prevent the progression of atherosclerotic vascular disease.
7.2. Patients with diabetes mellitus
The 2023 American Diabetic Association guideline recommends CCBs as first‐line therapy for patients with diabetes without albuminuria and coronary artery disease. It also notes that in the absence of albuminuria, ACE‐Is and ARBs do not seem to offer superior cardiovascular protection when compared to CCBs and diuretics. 57 It is important to point out that β‐blockers and diuretics worsen insulin resistance and deteriorate lipoprotein metabolism, whereas ACE‐Is, CCBs, and α‐blockers are neutral. 58 A database analysis showed that morning home BPV was lower in patients with type 2 diabetes treated with CCBs than that in those treated with ARBs and/or ACE‐Is. Treatment with CCBs was significantly correlated with decreased home BPV independent of other potential cofactors. 59 A systematic review and meta‐analysis showed that the risk of stroke in patients with diabetes treated with amlodipine was decreased when compared with other treatments (diuretics, β‐blockers, α‐blockers, ACE‐Is, or ARBs). 60 In patients with diabetes who had not responded sufficiently to amlodipine 5 mg, a retrospective analysis showed that up‐titrating amlodipine to 10 mg daily resulted in a clinically relevant and statistically significant reduction in SBP and DBP. 61
7.3. Patients with chronic kidney disease
BP lowering is an effective strategy for preventing cardiovascular events in patients with moderately reduced estimated glomerular filtration rate (eGFR). 62 The long‐term follow‐up of the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) showed that patients with kidney dysfunction treated with amlodipine retained higher mean levels of eGFR compared with chlorthalidone in year 4. 63 In a retrospective post hoc analysis of patients enrolled in the ASCOT and ALLHAT trials, systolic BPV was consistently lower in patients treated with amlodipine compared with other antihypertensives (chlorthalidone or lisinopril), regardless of eGFR. 64 Furthermore, in a real‐world‐evidence study, while showing kidney protection similar to other CCBs, amlodipine showed higher potency by triggering greater BP reduction at lower doses. 65 Amlodipine is also recommended for lowering BP in patients on hemodialysis. One small, double blind, placebo‐controlled study showed that hemodialysis patients randomized to amlodipine 10 mg had a significantly reduced risk of the secondary composite endpoint of mortality, cardiac events, need for coronary angioplasty or coronary bypass surgery, ischemic stroke, and peripheral vascular disease than those who were randomized to placebo. 66
7.4. Older persons with hypertension
In persons 65 years or older, diuretics and CCBs are preferred for patients with isolated systolic hypertension 67 and marked BPV.68 CCBs have an advantage over diuretics because they do not affect electrolytes. 31 , 69 Furthermore, in a small, double‐blind study, amlodipine was significantly more efficacious than hydrochlorothiazide for reducing 24‐h, daytime and nighttime DBP in patients 65 years or older. 70 In a phase 4, randomized trial, 80% of the patients (≥60 years) treated with amlodipine achieved sitting SBP values of ≤150 mm Hg compared with 54% in the hydrochlorothiazide group. 71 Nocturnal hypertension seems to be associated with increased cardiovascular risk. 72 In a randomized, double‐blind trial in patients 60 years or older, amlodipine reduced SBP to a significantly greater extent than nicardipine as assessed by 24‐h ABPM, particularly during the nocturnal period. 73 Amlodipine tolerability was rated as good or excellent by most patients (≥65 years of age, 89.2%; ≥75 years, 87.2%). 74 In a randomized, placebo‐controlled trial in Chinese patients (60−80 years), intensive lowering of SBP to 110−130 mm Hg with amlodipine and/or olmesartan was associated with significantly lower incidence of cardiovascular events than standard lowering (130−150 mm Hg), with no difference in safety outcomes. 75 Similarly, in the randomized, open‐label Systolic Blood Pressure Intervention Trial (SPRINT), patients (28% were ≥75 years) were randomized to an SBP target of ≤120 mm Hg (intensive) or ≤140 mm Hg (standard). With amlodipine as the preferred CCB, the intensive group had a lower rate of cardiovascular events (MI, other acute coronary syndromes, stroke, heart failure, or death from cardiovascular causes) but a higher incidence of adverse events. 76 These results led to the updated recommendation for older adults to be treated to a BP goal of < 130/80 mm Hg if they can tolerate therapy. 10 Without untoward effects on electrolytes, amlodipine offers an advantage for older adults, because there is no requirement for follow‐up laboratory monitoring.
7.5. Amlodipine in Asian and Black patients
7.5.1. Asian patients
The prevalence of hypertension in China has increased in the past 30 years, mainly due to lifestyle changes and increased life expectancy. BP control rates are generally low, owing to poor awareness and treatment adherence. 77 Asian countries/regions (except in South Korea and Taiwan) show a higher prevalence of uncontrolled hypertension, even though Asians generally derive greater benefits from stringent BP control compared to Western countries. 78 Morning and nocturnal hypertension are more common in Asians compared with Europeans, 12 possibly due to high salt sensitivity in Asians. 79
In Asia, CCBs have emerged as the preferred choice among antihypertensive medications. 80 The Asian Pacific Heart Association's Writing Committee reviewed randomized controlled trials that were conducted in the Eastern Asian region and showed that monotherapy with CCBs, mainly amlodipine, was more efficacious in lowering BP when compared with other classes of antihypertensive. 81 The study also found that CCBs provided superior protection against stroke and that agents such as amlodipine also provided similar protection against MI. 81 In a randomized, double‐blind trial in Asian patients, amlodipine was more effective than nifedipine GITS in attenuating the consequent BP rise over the next 48 h following controlled withdrawal at steady state. Interestingly, in this patient population, only four of 109 patients (3.7%) experienced edema when treated with amlodipine 5 mg daily, increased to 10 mg daily after 6 weeks, if needed. 28
7.5.2. Black patients
CCBs, like amlodipine, or thiazide‐type diuretics are recommended as initial therapy for Black patients, including those with diabetes. 13 In Nigerian patients with mild, moderate, and severe hypertension treated with amlodipine 5 mg once daily for 2 weeks and increased to 10 mg once daily for 10 weeks, amlodipine was well‐tolerated and laboratory tests, including plasma lipids done at the start and end of the trial, remained unchanged. 82 Another small, randomized study in Nigerian patients showed that amlodipine reduced SBP and DBP significantly more than hydrochlorothiazide. 31 Furthermore, a randomized trial showed that amlodipine plus either hydrochlorothiazide or perindopril was more effective in lowering BP than perindopril plus hydrochlorothiazide in Black patients. 83 Another study in Nigerian patients showed that a regimen of amlodipine to which hydrochlorothiazide was subsequently added provided superior BP control when compared with a regimen of hydrochlorothiazide to which amlodipine was subsequently added or when treatment started with the amlodipine‐hydrochlorothiazide combination therapy. The sudden initial decrease in BP with the amlodipine‐hydrochlorothiazide combination may increase release of vasoconstrictors, which may lead to increased SBP. 84
Amlodipine seems equally effective in lowering BP in three broad ethnic groups (Africans, Europeans, and South‐Asians), 85 remaining a viable first‐line option.
8. PHARMACOECONOMIC CONSIDERATIONS
The results of a pharmacoeconomic review of amlodipine indicated that amlodipine was cost effective and predicted to be cost saving when compared with usual care. Amlodipine resulted in fewer hospitalizations and surgical procedures in the short and long term at a modest cost increase compared with standard care. 86 Another important consideration is the absence of electrolyte abnormalities required to monitor with amlodipine, 31 , 69 limiting the need for patients to return to the health system. By contrast, potential changes in kidney function and development of hyperkalemia often require patients to have laboratory tests within weeks of starting drugs that target the renin‐angiotensin‐aldosterone system. 11
9. CONCLUSIONS
This review highlights data emphasizing that amlodipine is effective in managing hypertension in patients with SBP/DBP of 130/80 mm Hg or higher, including those at low risk of cardiovascular disease and those with established cardiovascular diseases. Mean BP and BPV control are crucial in reducing the risk of cardiovascular events. Amlodipine not only provides 24‐h BP control but also effectively reduces BPV, thereby protecting against stroke and MI. Furthermore, amlodipine effectively controls BP in different ethnic groups, older adults, patients with diabetes, and patients with chronic kidney disease without worsening glycemic and kidney function.
AUTHOR CONTRIBUITIONS
All authors contributed to critical revision of the manuscript and gave final approval.
CONFLICT OF INTEREST STATEMENT
Jiguang Wang has received financial grants from Omron and has been paid as a speaker for Novartis, Omron, Servier, and Viatris. Peter Sever has received research grants from Pfizer, Servier, and Amgen; has worked as a paid consultant for Pfizer and Amgen; and has been a paid speaker for Pfizer, Amgen, and Viatris. All other authors report no potential conflicts of interest.
ACKNOWLEDGMENTS
The authors acknowledge Luminology Scientific Communications for support with this manuscript.
Wang JG, Palmer BF, Vogel Anderson K, Sever P. Amlodipine in the current management of hypertension. J Clin Hypertens. 2023;25:801–807. 10.1111/jch.14709
REFERENCES
- 1. Mills KT, Bundy JD, Kelly TN, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population‐based studies from 90 countries. Circulation. 2016;134(6):441‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lawes CM, Vander Hoorn S, Rodgers A. International Society of Hypertension. Global burden of blood‐pressure‐related disease. Lancet. 2008;371(9623):1513‐1518. 2001. [DOI] [PubMed] [Google Scholar]
- 3. Vasan RS, Enserro DM, Beiser AS, Xanthakis V. Lifetime risk of heart failure among participants in the Framingham Study. J Am Coll Cardiol. 2022;79(3):250‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kario K, Tomitani N, Nishizawa M, Harada N, Kanegae H, Hoshide S. Concept, study design, and baseline blood pressure control status of the nationwide prospective HI‐JAMP study using multisensor ABPM. Hypertens Res. 2023;46(2):357‐367. [DOI] [PubMed] [Google Scholar]
- 5. Kario K, Hoshide S, Mogi M. Digital hypertension 2023: concept, hypothesis, and new technology. Hypertens Res. 2022;45(10):1529‐1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hoshide S, Yamamoto K, Katsurada K, et al. Agreement regarding overcoming hypertension in the Asian Hypertension Society Network 2022. Hypertens Res. 2023;46(1):3‐8. [DOI] [PubMed] [Google Scholar]
- 7. Kario K, Hoshide S, Chia YC, et al. Guidance on ambulatory blood pressure monitoring: a statement from the HOPE Asia Network. J Clin Hypertens (Greenwich). 2021;23(3):411‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hastie CE, Jeemon P, Coleman H, et al. Long‐term and ultra long‐term blood pressure variability during follow‐up and mortality in 14,522 patients with hypertension. Hypertension. 2013;62(4):698‐705. [DOI] [PubMed] [Google Scholar]
- 9. Hsu PF, Cheng HM, Wu CH, et al. High short‐term blood pressure variability predicts long‐term cardiovascular mortality in untreated hypertensives but not in normotensives. Am J Hypertens. 2016;29(7):806‐813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: executive Summary: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269‐1324. [published correction appears in Hypertension. 2018;71(6):e136‐e139] [published correction appears in Hypertension. 2018;72(3):e33].29133354 [Google Scholar]
- 11. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021‐3104. [published correction appears in Eur Heart J. 2019;40(5):475]. [DOI] [PubMed] [Google Scholar]
- 12. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. J Hypertens. 2020;38(6):982‐1004. [DOI] [PubMed] [Google Scholar]
- 13. James PA, Oparil S, Carter BL, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507‐520. [published correction appears in JAMA. 2014;311(17):1809]. [DOI] [PubMed] [Google Scholar]
- 14. Joint Committee for Guideline Revision. 2018 Chinese Guidelines for Prevention and Treatment of Hypertension—a report of the Revision Committee of Chinese Guidelines for Prevention and Treatment of Hypertension. J Geriatr Cardiol. 2019;16(3):182‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fares H, DiNicolantonio JJ, O'Keefe JH, Lavie CJ. Amlodipine in hypertension: a first‐line agent with efficacy for improving blood pressure and patient outcomes. Open Heart. 2016;3(2):e000473. doi: 10.1136/openhrt-2016-000473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tocci G, Battistoni A, Passerini J, et al. Calcium channel blockers and hypertension. J Cardiovasc Pharmacol Ther. 2015;20(2):121‐130. [DOI] [PubMed] [Google Scholar]
- 17. NCD Risk Factor Collaboration (NCD‐RisC) . Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population‐representative studies with 104 million participants [published correction appears in Lancet. 2022;399(10324):520]. Lancet. 2021;398(10304):957‐980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jones DW, Whelton PK, Allen N, et al. Management of stage 1 hypertension in adults with a low 10‐year risk for cardiovascular disease: filling a guidance gap: a scientific statement from the American Heart Association. Hypertension. 2021;77(6):e58‐e67. [DOI] [PubMed] [Google Scholar]
- 19. Qi Y, Han X, Zhao D, et al. Long‐term cardiovascular risk associated with stage 1 hypertension defined by the 2017 ACC/AHA Hypertension Guideline. J Am Coll Cardiol. 2018;72(11):1201‐1210. [DOI] [PubMed] [Google Scholar]
- 20. Faulkner JK, McGibney D, Chasseaud LF, Perry JL, Taylor IW. The pharmacokinetics of amlodipine in healthy volunteers after single intravenous and oral doses and after 14 repeated oral doses given once daily. Br J Clin Pharmacol. 1986;22(1):21‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Zwieten PA, Pfaffendorf M. Similarities and differences between calcium antagonists: pharmacological aspects. J Hypertens Suppl. 1993;11(1):S3‐S11. [DOI] [PubMed] [Google Scholar]
- 22. Abernethy DR. Pharmacokinetics and pharmacodynamics of amlodipine. Cardiology. 1992;80(1):31‐36. [DOI] [PubMed] [Google Scholar]
- 23. Leenen FH, Fourney A, Notman G, Tanner J. Persistence of anti‐hypertensive effect after ‘missed doses’ of calcium antagonist with long (amlodipine) vs short (diltiazem) elimination half‐life. Br J Clin Pharmacol. 1996;41(2):83‐88. [DOI] [PubMed] [Google Scholar]
- 24. NORVASC® (amlodipine besylate; ). Pfizer, Inc; 2019. [Google Scholar]
- 25. Madi M, Shetty SR, Babu SG, Achalli S. Amlodipine‐induced gingival hyperplasia ‐ a case report and review. West Indian Med J. 2015;64(3):279‐282. doi: 10.7727/wimj.2014.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jorgensen MG. Prevalence of amlodipine‐related gingival hyperplasia. J Periodontol. 1997;68(7):676‐678. doi: 10.1902/jop.1997.68.7.676 [DOI] [PubMed] [Google Scholar]
- 27. Sica D. Calcium channel blocker‐related peripheral edema: can it be resolved? J Clin Hypertens (Greenwich). 2003;5(4):291‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ongtengco I, Morales D, Sanderson J, et al. Persistence of the antihypertensive efficacy of amlodipine and nifedipine GITS after two ‘missed doses’: a randomised, double‐blind comparative trial in Asian patients. J Hum Hypertens. 2002;16(11):805‐813. [DOI] [PubMed] [Google Scholar]
- 29. Xu SK, Huang QF, Zeng WF, Sheng CS, Li Y, Wang JG. A randomized multicenter study on ambulatory blood pressure and arterial stiffness in patients treated with valsartan/amlodipine or nifedipine GITS. J Clin Hypertens (Greenwich). 2019;21(2):252‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Horwitz LD, Weinberger HD, Clegg L. Comparison of amlodipine and long‐acting diltiazem in the treatment of mild or moderate hypertension. Am J Hypertens. 1997;10(11):1263‐1269. [DOI] [PubMed] [Google Scholar]
- 31. Nwachukwu DC, Eze AA, Nwachukwu NZ, et al. Monotherapy with amlodipine or hydrochlorothiazide in patients with mild to moderate hypertension: comparison of their efficacy and effects on electrolytes. Malawi Med J. 2017;29(2):108‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grimm RH Jr, Black H, Rowen R, et al. Amlodipine versus chlorthalidone versus placebo in the treatment of stage I isolated systolic hypertension. Am J Hypertens. 2002;15(1):31‐36. [DOI] [PubMed] [Google Scholar]
- 33. Kario K, Robbins J, Jeffers BW. Titration of amlodipine to higher doses: a comparison of Asian and Western experience. Vasc Health Risk Manag. 2013;9:695‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Parati G, Schumacher H. Blood pressure variability over 24 h: prognostic implications and treatment perspectives. An assessment using the smoothness index with telmisartan‐amlodipine monotherapy and combination. Hypertens Res. 2014;37(3):187‐193. [DOI] [PubMed] [Google Scholar]
- 35. Hansen TW, Thijs L, Li Y, et al. Prognostic value of reading‐to‐reading blood pressure variability over 24 hours in 8938 subjects from 11 populations. Hypertension. 2010;55(4):1049‐1057. [published correction appears in Hypertension. 2010;55(6):e27]. [DOI] [PubMed] [Google Scholar]
- 36. de la SierraA, Mateu A, Gorostidi M, Vinyoles E, Segura J, Ruilope LM. Antihypertensive therapy and short‐term blood pressure variability. J Hypertens. 2021;39(2):349‐355. [DOI] [PubMed] [Google Scholar]
- 37. Wang JG, Yan P, Jeffers BW. Effects of amlodipine and other classes of antihypertensive drugs on long‐term blood pressure variability: evidence from randomized controlled trials. J Am Soc Hypertens. 2014;8(5):340‐349. [DOI] [PubMed] [Google Scholar]
- 38. Zhang Y, Agnoletti D, Safar ME, Blacher J. Effect of antihypertensive agents on blood pressure variability: the Natrilix SR versus candesartan and amlodipine in the reduction of systolic blood pressure in hypertensive patients (X‐CELLENT) study. Hypertension. 2011;58(2):155‐160. [DOI] [PubMed] [Google Scholar]
- 39. Stevens SL, Wood S, Koshiaris C, et al. Blood pressure variability and cardiovascular disease: systematic review and meta‐analysis. BMJ. 2016;354:i4098. doi: 10.1136/bmj.i4098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rothwell PM, Howard SC, Dolan E, et al. Effects of beta blockers and calcium‐channel blockers on within‐individual variability in blood pressure and risk of stroke. Lancet Neurol. 2010;9(5):469‐480. [DOI] [PubMed] [Google Scholar]
- 41. Hoshide S, Kario K. Morning surge in blood pressure and stroke events in a large modern ambulatory blood pressure monitoring cohort: results of the JAMP study. Hypertension. 2021;78(3):894‐896. [DOI] [PubMed] [Google Scholar]
- 42. Hosomi N, Sueda Y, Masugata H, et al. The optimal timing of antihypertensive medication administration for morning hypertension in patients with cerebral infarction. Hypertens Res. 2012;35(7):720‐724. [DOI] [PubMed] [Google Scholar]
- 43. Ferrucci A, Marcheselli A, Strano S, et al. 24‐Hour blood pressure profiles in patients with hypertension treated with amlodipine or nifedipine GITS. Clin Drug Invest. 1997;13(1):67‐72. [Google Scholar]
- 44. Messerli FH, Staessen JA. Amlodipine better than lisinopril? How one randomized clinical trial ended fallacies from observational studies. Hypertension. 2006;48(3):359‐361. [published correction appears in Hypertension. 2006;48(4):e24]. [DOI] [PubMed] [Google Scholar]
- 45. Dahlöf B, Sever PS, Poulter NR, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo‐Scandinavian Cardiac Outcomes Trial–Blood Pressure Lowering Arm (ASCOT‐BPLA): a multicentre randomized controlled trial. Lancet. 2005;366(9489):895‐906. [DOI] [PubMed] [Google Scholar]
- 46. Wang JG, Li Y, Franklin SS, Safar M. Prevention of stroke and myocardial infarction by amlodipine and Angiotensin receptor blockers: a quantitative overview. Hypertension. 2007;50(1):181‐188. [DOI] [PubMed] [Google Scholar]
- 47. Iyengar SS, Mohan JC, Ray S, et al. Effect of amlodipine in stroke and myocardial infarction: a systematic review and meta‐analysis. Cardiol Ther. 2021;10(2):429‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang JG, Staessen JA, Li Y, et al. Carotid intima‐media thickness and antihypertensive treatment: a meta‐analysis of randomized controlled trials. Stroke. 2006;37(7):1933‐1940. [DOI] [PubMed] [Google Scholar]
- 49. Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive‐drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta‐analysis. Lancet. 2010;375(9718):906‐915. [DOI] [PubMed] [Google Scholar]
- 50. Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41(3):407‐477. [published correction appears in Eur Heart J. 2020;41(44):4242]. [DOI] [PubMed] [Google Scholar]
- 51. Jørgensen B, Simonsen S, Endresen K, et al. Restenosis and clinical outcome in patients treated with amlodipine after angioplasty: results from the Coronary AngioPlasty Amlodipine REStenosis Study (CAPARES). J Am Coll Cardiol. 2000;35(3):592‐599. [DOI] [PubMed] [Google Scholar]
- 52. Ikeda H, Minamikawa J, Nakamura Y, et al. Comparison of effects of amlodipine and angiotensin receptor blockers on the intima‐media thickness of carotid arterial wall (AAA study: amlodipine vs. ARB in atherosclerosis study). Diabetes Res Clin Pract. 2009;83(1):50‐53. [DOI] [PubMed] [Google Scholar]
- 53. Pitt B, Byington RP, Furberg CD, et al. Effect of amlodipine on the progression of atherosclerosis and the occurrence of clinical events. PREVENT Investigators. Circulation. 2000;102(13):1503‐1510. [DOI] [PubMed] [Google Scholar]
- 54. Sever P, Dahlöf B, Poulter N, et al. Potential synergy between lipid‐lowering and blood‐pressure‐lowering in the Anglo‐Scandinavian Cardiac Outcomes Trial. Eur Heart J. 2006;27(24):2982‐2988. [published correction appears in Eur Heart J. 2007;28(1):142]. [DOI] [PubMed] [Google Scholar]
- 55. Manisty C, Mayet J, Tapp RJ, et al. Atorvastatin treatment is associated with less augmentation of the carotid pressure waveform in hypertension: a substudy of the Anglo‐Scandinavian Cardiac Outcome Trial (ASCOT). Hypertension. 2009;54(5):1009‐1013. [DOI] [PubMed] [Google Scholar]
- 56. Clunn GF, Sever PS, Hughes AD. Calcium channel regulation in vascular smooth muscle cells: synergistic effects of statins and calcium channel blockers. Int J Cardiol. 2010;139(1):2‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. ElSayed NA, Aleppo G, Aroda VR, et al. 10. Cardiovascular disease and risk management: standards of care in diabetes—2023. Diabetes Care. 2023;46(1):S158‐S190. [published correction appears in Diabetes Care. 2023 Jan 26]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lithell HO. Effect of antihypertensive drugs on insulin, glucose, and lipid metabolism. Diabetes Care. 1991;14(3):203‐209. [DOI] [PubMed] [Google Scholar]
- 59. Ushigome E, Fukui M, Hamaguchi M, et al. Beneficial effect of calcium channel blockers on home blood pressure variability in the morning in patients with type 2 diabetes. J Diabetes Investig. 2013;4(4):399‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jeffers BW, Robbins J, Bhambri R, Wajsbrot D. A systematic review on the efficacy of amlodipine in the treatment of patients with hypertension with concomitant diabetes mellitus and/or renal dysfunction, when compared with other classes of antihypertensive medication. Am J Ther. 2015;22(5):322‐341. [DOI] [PubMed] [Google Scholar]
- 61. Jeffers BW, Bhambri R, Robbins J. Uptitrating amlodipine significantly reduces blood pressure in diabetic patients with hypertension: a retrospective, pooled analysis. Vasc Health Risk Manag. 2014;10:651‐659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Blood Pressure Lowering Treatment Trialists’ Collaboration , Ninomiya T, Perkovic V, et al. Blood pressure lowering and major cardiovascular events in people with and without chronic kidney disease: meta‐analysis of randomised controlled trials. BMJ. 2013;347:f5680. doi: 10.1136/bmj.f5680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rahman M, Ford CE, Cutler JA, et al. Long‐term renal and cardiovascular outcomes in Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) participants by baseline estimated GFR. Clin J Am Soc Nephrol. 2012;7(6):989‐1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jeffers BW, Zhou D. Relationship between visit‐to‐visit blood pressure variability (BPV) and kidney function in patients with hypertension. Kidney Blood Press Res. 2017;42(4):697‐707. [DOI] [PubMed] [Google Scholar]
- 65. Jadhav U, Mohanan PP, Almeida AF, et al. Effectiveness and effect on renal parameters of amlodipine vs. other dihydropyridine calcium channel blockers in patients with essential hypertension: retrospective observational study based on real‐world evidence from electronic medical records. Cardiol Ther. 2021;10(2):465‐480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tepel M, Hopfenmueller W, Scholze A, Maier A, Zidek W. Effect of amlodipine on cardiovascular events in hypertensive haemodialysis patients. Nephrol Dial Transplant. 2008;23(11):3605‐3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kaiser EA, Lotze U, Schäfer HH. Increasing complexity: which drug class to choose for treatment of hypertension in the elderly? Clin Interv Aging. 2014;9:459‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kario K, Wang JG, Chia YC, et al. The HOPE Asia network 2022 up‐date consensus statement on morning hypertension management. J Clin Hypertens (Greenwich). 2022;24(9):1112‐1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Khow KS, Lau SY, Li JY, Yong TY. Diuretic‐associated electrolyte disorders in the elderly: risk factors, impact, management and prevention. Curr Drug Saf. 2014;9(1):2‐15. [DOI] [PubMed] [Google Scholar]
- 70. Lacourcière Y, Poirier L, Lefebvre J, Archambault F, Cléroux J, Boileau G. Antihypertensive effects of amlodipine and hydrochlorothiazide in elderly patients with ambulatory hypertension. Am J Hypertens. 1995;8(1):1154‐1159. 12 pt. [DOI] [PubMed] [Google Scholar]
- 71. Calvo C, Gude F, Abellán J, et al. A comparative evaluation of amlodipine and hydrochlorothiazide as monotherapy in the treatment of isolated systolic hypertension in the elderly. Clin Drug Invest. 2000;19(5):317‐326. [Google Scholar]
- 72. Kario K. Sleep and nocturnal hypertension: genes, environment, and individual profiles. J Clin Hypertens (Greenwich). 2022;24(10):1263‐1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mounier‐Véhier C, Jaboureck O, Emeriau JP, Bernaud C, Clerson P, Carre A. Randomized, comparative, double‐blind study of amlodipine vs. nicardipine as a treatment of isolated systolic hypertension in the elderly. Fundam Clin Pharmacol. 2002;16(6):537‐544. [DOI] [PubMed] [Google Scholar]
- 74. Langdon C. Treatment of hypertension in patients >or = 65 years of age: experience with amlodipine. Clin Ther. 2000;22(12):1473‐1482. [DOI] [PubMed] [Google Scholar]
- 75. Zhang W, Zhang S, Deng Y, et al. Trial of intensive blood‐pressure control in older patients with hypertension. N Engl J Med. 2021;385(14):1268‐1279. [DOI] [PubMed] [Google Scholar]
- 76. SPRINT Research Group, Wright JT Jr, Williamson JD, et al. A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373(22):2103‐2116. [published correction appears in N Engl J Med. 2017;377(25):2506]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang JG, Zhang W, Li Y, Liu L. Hypertension in China: epidemiology and treatment initiatives. Nat Rev Cardiol. 2023;20(8):531‐545. doi: 10.1038/s41569-022-00829-z [DOI] [PubMed] [Google Scholar]
- 78. Kario K, Hoshide S, Mogi M. Uncontrolled hypertension: the greatest challenge and perspectives in Asia. Hypertens Res. 2022;45(12):1847‐1849. [DOI] [PubMed] [Google Scholar]
- 79. Li YY. α‐Adducin Gly460Trp gene mutation and essential hypertension in a Chinese population: a meta‐analysis including 10,960 subjects. PLoS ONE. 2012;7(1):e30214. doi: 10.1371/journal.pone.0030214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kario K, Chia YC, Siddique S, et al. Seven‐action approaches for the management of hypertension in Asia—The HOPE Asia network. J Clin Hypertens (Greenwich). 2022;24(3):213‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wang JG, Kario K, Lau T, et al. Use of dihydropyridine calcium channel blockers in the management of hypertension in Eastern Asians: a scientific statement from the Asian Pacific Heart Association. Hypertens Res. 2011;34(4):423‐430. [DOI] [PubMed] [Google Scholar]
- 82. Sowunmi A, Walker O, Salako LA. Amlodipine as monotherapy in hypertensive Africans: clinical efficacy and safety studies. Afr J Med Med Sci. 1996;25(3):213‐216. [PubMed] [Google Scholar]
- 83. Ojji DB, Mayosi B, Francis V, et al. Comparison of dual therapies for lowering blood pressure in Black Africans. N Engl J Med. 2019;380(25):2429‐2439. [DOI] [PubMed] [Google Scholar]
- 84. Iyalomhe GB, Omogbai EK, Isah AO, Iyalomhe OO, Dada FL, Iyalomhe SI. Efficacy of initiating therapy with amlodipine and hydrochlorothiazide or their combination in hypertensive Nigerians. Clin Exp Hypertens. 2013;35(8):620‐627. [DOI] [PubMed] [Google Scholar]
- 85. Gupta AK, Poulter NR, Dobson J, et al. Ethnic differences in blood pressure response to first and second‐line antihypertensive therapies in patients randomized in the ASCOT Trial. Am J Hypertens. 2010;23(9):1023‐1030. [DOI] [PubMed] [Google Scholar]
- 86. de Portu S, Mantovani LG. Amlodipine: a pharmacoeconomic review. J Med Econ. 2009;12(1):60‐68. [DOI] [PubMed] [Google Scholar]


