Abstract
Peanut (PN) allergy is a common life-threatening disease; however, our knowledge on the immunological mechanisms remains limited. Here, we describe the first mouse model of inhalation-driven peanut allergy. We administered PN flour intranasally to naïve wild-type mice twice a week for 4 weeks, followed by intraperitoneal challenge with PN extract. Exposure of mice to PN flour sensitized them without addition of adjuvants, and mice developed PN-specific IgE, IgG1, and IgG2a. After challenge, mice displayed lower body temperature and other clinical signs of anaphylaxis. This inhalation model is an ideal system to allow for future examination of immunological mechanisms critical for the development of PN allergy.
Keywords: Peanut, Allergy, IgE, Anaphylaxis, Inhalation, Food allergy
1. Introduction
Peanut (PN) allergy is a growing public health concern [1]. Among children in the United States, the incidence of PN allergy increased fivefold from 0.4% in 1997 [2] to 2.0% in a national survey taken in 2010 [3]. While PN allergy remains increasingly problematic, especially for the youngest in our society, our understanding of how the disease initiates after the immune system encounters PN remains unclear. The majority of PN-allergic children experience their first allergic reaction to PN upon first ingestion of PN [4]. Recent clinical trials have provided strong evidence that eating PN early in life allows the development of an oral tolerance that protects children from developing allergic responses to PN [5, 6]. Since PN is readily detectable in household dust [7, 8], we examined whether mice could be sensitized to PN via inhalation. Here, we demonstrate mice exposed to PN via the airways developed clinical PN allergy [9]. Specifically, we demonstrate a 4-week-long, twice-weekly inhalation mouse model to establish PN allergy (Fig. 1). Using this model, we documented the development of PN-specific IgE, IgG1, and IgG2a responses (Fig. 2) and clinical symptoms resembling PN allergy in humans (Fig. 3) [9].
Fig. 1.
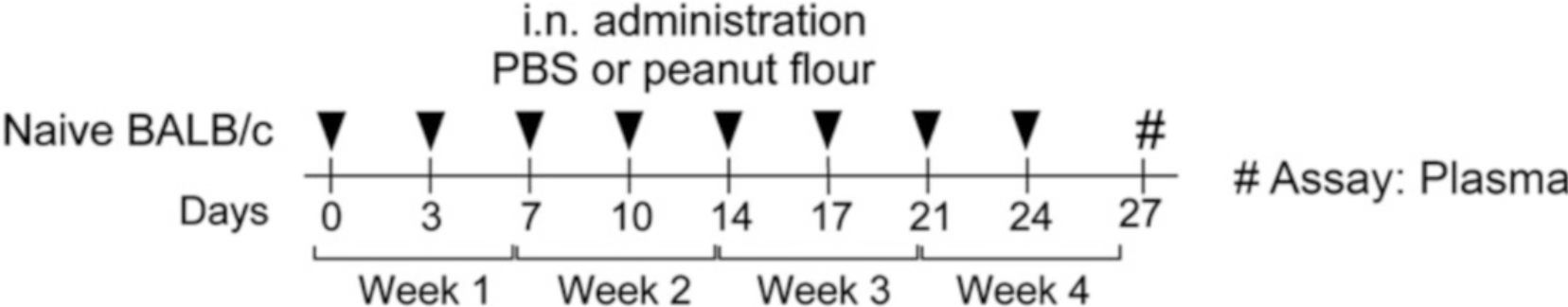
Timeline of experimental model. Mice are exposed to either PBS or PN flour intranasally twice/week for 4 weeks. On Day 27, mice are bled to obtain plasma for PN-specific antibody ELISA analysis. On Day 28 (not pictured), mice are intraperitoneally challenged with PN to induce an anaphylactic reaction (Reproduced from ref. 9 with permission from Elsevier)
Fig. 2.
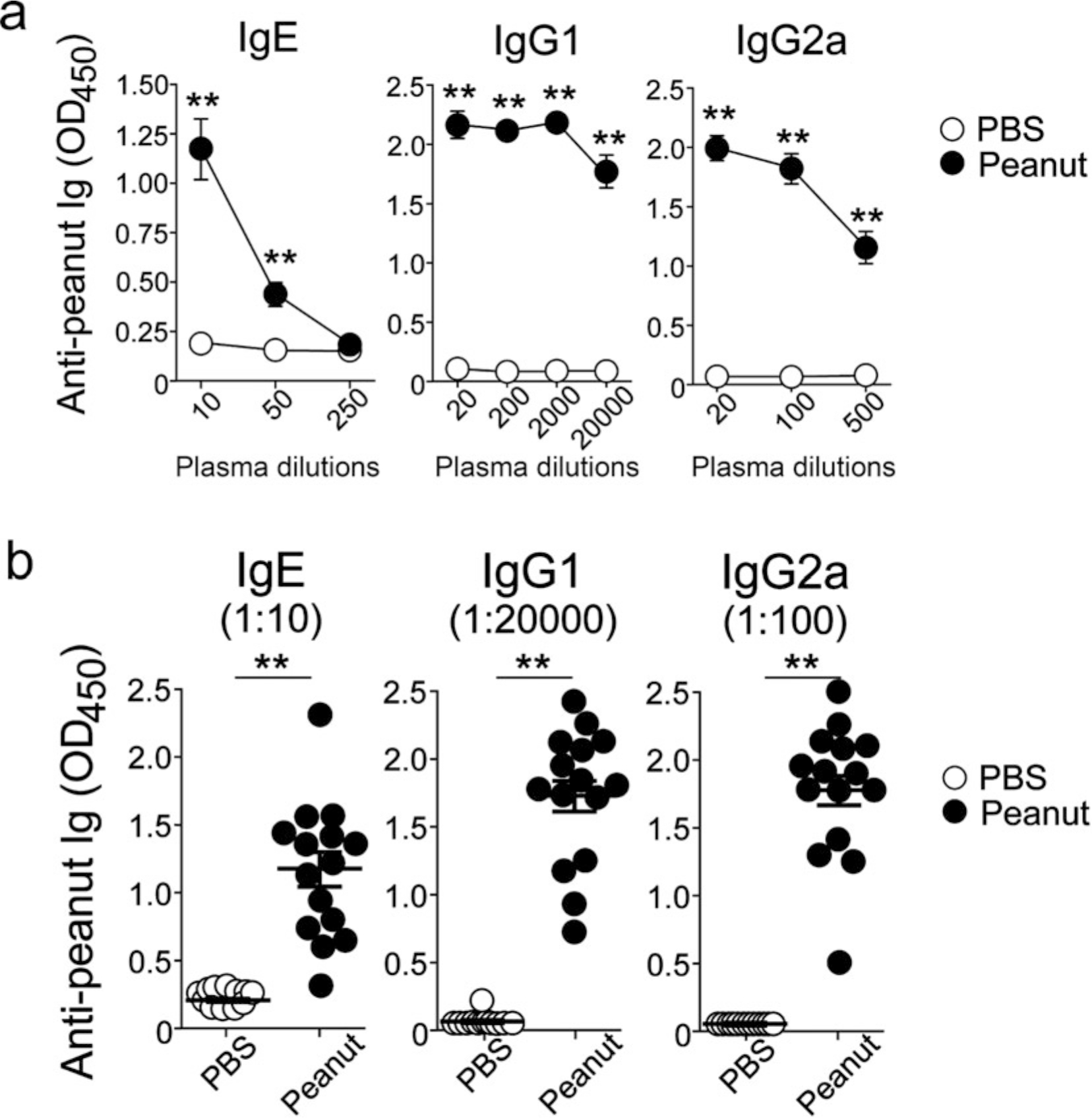
Inhalation of PN stimulates PN-specific antibody responses. (a) Titers of anti-PN antibodies in plasma were determined on day 27 by ELISA. **P < 0.01 compared to mice exposed to PBS. (b) Levels of anti-PN antibodies in each mouse are shown. Data are a pool from three experiments and are presented as mean ± SEM (n = 12–15 in each group) (Reproduced from ref. 9 with permission from Elsevier)
Fig. 3.
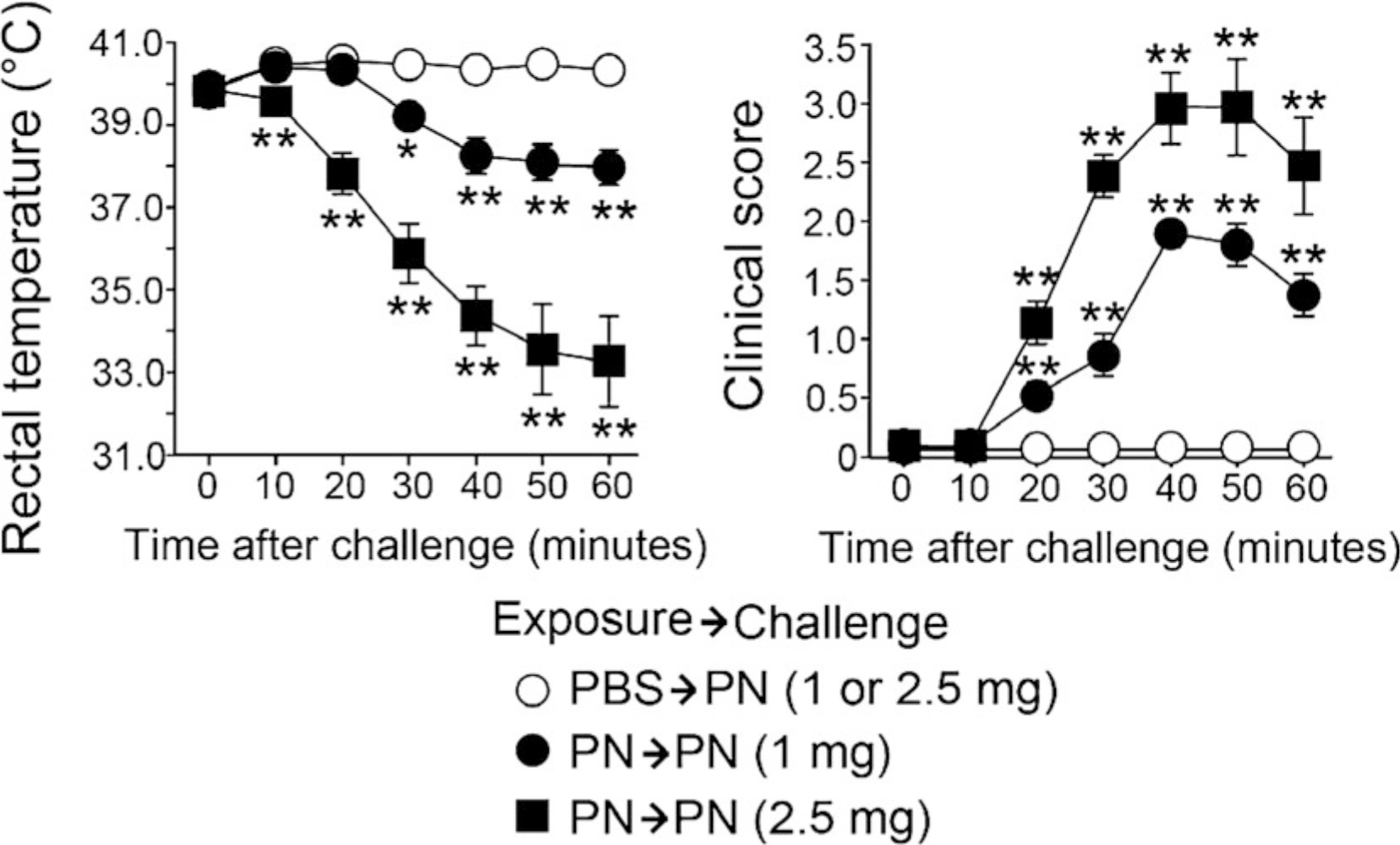
Challenge with PN stimulates anaphylactic reaction in PN-sensitized, but not PBS-sensitized, mice. Changes in rectal temperature (left) and clinical scores (right) in mice challenged by intraperitoneal injection of CPE are shown. Data are presented as mean ± SEM (n = 9–15 in each group) and are a pool of three experiments. *P < 0.05 and **P < 0.01 compared with mice exposed to PBS (Reproduced from ref. [9] with permission from Elsevier)
Due to the proposed link between eating PN and becoming sensitized to PN, many models of PN allergy are oral [10–12]. In addition, a majority of PN allergy mouse models require the use of mucosal adjuvants, such as cholera toxin and staphylococcal enterotoxin B [10–14]. To remove the adjuvant requirement from these models, mouse models that genetically inhibited Toll-like receptor 4 [15] and mice that expressed a disinhibited form of the IL-4 receptor were created [16]. These different manipulations, whether by adjuvant or genetics, make it difficult to elucidate the immunologic mechanisms involved in the initiation of peanut allergy. Moreover, data strongly support early oral exposure leads to tolerance, not sensitization, underscoring the importance of examining non-oral routes of sensitization [5, 6]. An adjuvant-free skin model of PN sensitization has been described, a finding consistent with the growing clinical evidence that demonstrates an association between atopic dermatitis and PN allergy in children [17]. Given environmental exposure to PN can occur three ways: eating (oral), touching (skin), or breathing (airway inhalation), we asked whether mice could become allergic to PN through the airways. Thus, we have described the first mouse model of inhalation-driven peanut allergy. This model is an ideal system for dissecting the immunological mechanisms that lead to the development of PN allergy and could be modified to test other food allergens in future studies.
2. Materials
Prepare all solutions using ultrapure water (prepared by purifying deionized water to attain a sensitivity of 18 MΩ cm at 25 °C room temperature). Prepare and store all reagents at room temperature (unless otherwise specified). Follow all waste disposal regulations when disposing waste materials. We do not add sodium azide to any reagents.
2.1. PN Flour Suspension
-
Phosphate-buffered saline (PBS): 10 mM Na2HPO4, 1.8 mM KH2PO4, 137 mM NaCl, 2.7 mM KCl, pH 7.4. Combine 1.44 g of Na2HPO4, 0.24 g of KH2PO4, 8.0 g of NaCl, and 0.2 g of KCl in about 800 mL of water. After adjusting the pH to 7.4 with HCl, bring the final volume up to 1 L with water. PBS may be prepared as a 10× stock solution (14.4 g of Na2HPO4, 2.4 g of KH2PO4, 80.0 g of NaCl, and 2.0 g of KCl in 1 L of ultrapure water, pH 7.4). To prepare PBS using this 10× stock solution, dilute 5 mL of 10× PBS stock solution to a 50-mL conical tube and add water to a volume of 50 mL.
Mix and store at room temperature. Each control mouse will be exposed to 50 μL PBS via inhalation.
1.5-mL microcentrifuge tubes.
15-mL conical tubes.
Pipettes and tips.
PN flour suspension stock solution: 5 mg of PN flour/mL of PBS. Place a 15-mL conical tube into a 100-mL beaker to balance the tube for weighing PN flour. Place onto an analytical balance that measures to the thousandths place (three decimal places). Weigh 25 mg of partially defatted (12% fat), light roast PN flour into the 15-mL conical tube. Add 5 mL of PBS to make 5 mg/mL of stock PN flour solution (see Note 1).
PN flour suspension final solution: 2 μg of PN flour/μL of PBS. Measure out 300 μL of PBS into a 1.5-mL microcentrifuge tube. Vortex the PN flour stock solution for 30 s and pipette 200 μL of the PN flour stock solution into a microcentrifuge tube containing 300 μL of PBS (see Note 1). Vortex to ensure even mixture of PN flour particles in the 2 μg/μL final PN flour solution (see Note 2). Each PN-exposed mouse will inhale 50 μL of the 2 μg/μL final PN flour solution, which will deliver 100 μg of PN into the airways.
2.2. Exposing Mice to PN via Inhalation
Adult mice: 6–12 weeks of age preferred, BALB/c or C57BL/6 backgrounds.
Isoflurane vaporizer (see Note 3).
1.5-mL microcentrifuge tubes containing PBS or PN solution.
Pipettes and tips.
2.3. Crude PN Extract Suspension
PBS: pH 7.4. Prepared as described above (see Subheading 2.1).
Crude PN extract (CPE) stock solution: 50 mg of CPE/mL of PBS. Purchase CPE from Stallergenes Greer, Lenoir, NC, USA. Resuspend the CPE pellet with the requisite amount of PBS to obtain a 50 mg/mL solution, using the dry weight measurement found on the vial as a guide (see Note 4). Mix and store at 4 °C (see Note 5).
CPE Final Solution: 5 mg of CPE/mL of PBS. Conduct a 1:10 dilution of the CPE stock solution using PBS to generate the CPE final solution (see Note 6). Vortex to ensure even mixture of CPE in the final solution prior to use (see Note 7). During anaphylactic challenge on Day 28, each mouse will be injected intraperitoneally with 500 μL, which will deliver 2.5 mg of CPE.
2.4. Monitoring of Anaphylaxis in Mice
Electronic thermocouple thermometer with Type T input.
RET-3 rectal probe for mice.
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
0.5 M EDTA: pH 8.0. Place 186.1 g of disodium EDTA·2H2O and 800 mL of water in a 1-L beaker and stir on a magnetic stirrer. Adjust the pH to 8.0 with NaOH, and EDTA will dissolve completely. Bring the volume to 1 L with water.
High-binding ELISA 96-well microplates.
Coating buffer: 0.1 M carbonate–bicarbonate buffer solution, pH 9.5. Add about 800 mL of water to a glass beaker with a stir bar placed on a stir plate. Once water is added, start stirring. Weigh 8.40 g of NaHCO3 (sodium bicarbonate) and slowly transfer to the beaker, ensuring that NaHCO3 fully dissolves in the water. Weigh 3.56 g of Na2CO3 (sodium carbonate) and slowly transfer to the beaker, ensuring that Na2CO3 fully dissolves in the water (see Note 8). Adjust the pH to 9.5 if necessary. Add water to a volume of 1 L. Transfer to a bottle and store at 4 °C.
Coating antibody: Purified rat anti-mouse IgE heavy chain antibody, clone LO-ME-3. Store at 4 °C.
CPE: 2 μg of CPE/mL of the coating buffer (see item 3 above). Conduct a 1:25,000 dilution of the CPE stock solution (same stock as described above) using the coating buffer to generate a coating buffer containing CPE suitable for coating plates for IgG1 and IgG2a ELISA.
PBS: pH 7.4. Prepared as described above (see Subheading 2.1).
Wash buffer: PBS containing 0.05% Tween-20 (see Note 9). Store at room temperature.
Blocking buffer: PBS containing 1% (w/v) fraction V or molecular biology-grade bovine serum albumin (BSA). Store at 4 °C.
Assay diluent: PBS containing 1% (w/v) fraction V or molecular biology-grade BSA and 0.05% Tween-20 (see Note 9). Store at 4 °C.
Biotinylated CPE: BiotinTag™ Micro Biotinylation Kit or equivalent for labeling CPE with biotin (see Notes 10 and 11). Store at 4 °C.
Poly-HRP streptavidin reagent: Commercially available. Store at −20 °C.
Detection antibody for IgG1 ELISA: HRP-conjugated rat anti-mouse IgG1, Clone X56. Store at 4 °C.
Detection antibody for IgG2a ELISA: HRP-conjugated rat anti-mouse IgG2a, Clone R19–15. Store at 4 °C.
TMB (3,3ʹ,5,5ʹ-tetramethylbenzidine) substrate kit: Commercially available. Store at 4 °C.
Stop solution: 1 M HCl or 2 M H2SO4. Store at room temperature.
Microplate autoreader to measure absorbance at 450 nm.
3. Methods
3.1. Exposing Mice to PN via Inhalation: a 4-Week Model
Calculate the number of mice being exposed to either PBS alone (control) or PN flour suspension final solution. Based on this number, label two 1.5-mL microcentrifuge tubes as either PBS or PN. Measure out 500 μL of PBS into the PBS-labeled tube. Based on the instructions in Subheading 2.1 (item 6), make up 500 μL of PN final solution. Each mouse will be exposed via inhalation to 50 μL of either PBS or PN. Therefore, one tube of PBS can expose a cohort of up to ten control mice to PBS and one tube of PN final solution can expose up to ten additional mice to PN (see Note 12). If treating more mice, make additional tubes of PBS or PN final solution.
Place the first PBS mouse into the anesthesia chamber connected to an isoflurane vaporizer set up to deliver isoflurane. Establish the anesthesia conditions within the chamber by turning on the connected oxygen to flow at 2 L/min and set the vaporizer to 3.5%. Monitor the mouse as it experiences the effects of the isoflurane. Once the mouse stops moving and is breathing deeply, open the chamber, take out the mouse, and shut the chamber to minimize loss of anesthesia conditions within the chamber.
Lay the mouse in the prone position. Grab it with one hand at the top of the neck/base of the head, lifting the mouse to hold it upright, tilting the hand backwards to ensure the nose is upright.
Pipette 50 μL of PBS slowly, but deliberately onto the tip of the nose in a dropwise fashion, allowing the mouse to breath in the liquid. To facilitate the airway aspiration of the solution, hold the mouse upright for 30 sec before placing the mouse into its cage on its back to recover from anesthesia (see Note 13).
Place the second and any subsequent PBS mice one-by-one into the anesthesia chamber, making sure to monitor, take out, and treat the mice one at a time as described in steps 2–4.
Once treatment of the PBS cohort of mice is complete, move onto treating the PN cohort. Place the first PN mouse into the anesthesia chamber. As the mouse is falling asleep, mix the tube with PN solution to put the PN into suspension (see Note 14).
Once the first PN mouse stops moving and is breathing deeply, take the mouse out and quickly shut the chamber to maintain the anesthesia conditions.
Lay and grab the mouse as described in step 3.
Pull up 50 μL of PN solution, pipetting up and down to ensure an even suspension of PN is captured (see Note 15).
Pipette 50 μL of PN solution slowly, but deliberately onto the tip of the nose using the same technique as described for PBS in step 4.
Place the second and any subsequent PN mice one-by-one into the anesthesia chamber, making sure to monitor, take out, and treat the mice one at a time as described in steps 6–10.
Once treatment of the PN cohort of mice is finished, Day 0 of treatment is complete. Repeat the PBS and PN treatments as previously described on Days 3, 7, 10, 14, 17, 21, and 24 (Fig. 1) (see Note 16).
3.2. Biotinylation of CPE
Carry out a 1:10 dilution of the CPE stock solution using PBS to generate the CPE solution useful for labeling.
Mix 12.2 μL of 1:10 diluted CPE and 87.8 μL of 0.1 M sodium phosphate buffer, pH 7.2 from BiotinTag™ Micro Biotinylation Kit for a final CPE mixture volume of 100 μL.
To make the biotinylation solution, dissolve the contents of one vial of BAC-SulfoNHS from the kit with 30 μL of DMSO, and add 970 μL of 0.1 M sodium phosphate buffer from the kit for a final volume of 1 mL.
Mix 100 μL of CPE mixture with 100 μL of the biotinylation solution for a final reaction volume of 200 μL. Once mixed, allow to incubate for 30 min at room temperature with gentle stirring (see Note 10).
During incubation, vortex one of the micro-spin Sephadex G-50 columns provided by the kit to resuspend the resin. Loosen the cap 1/4 turn and snap off the bottom closure. Place the column in a 1.5-mL microcentrifuge tube with its cap cut off. Spin for 1 min at 700 × g.
After the spin, add 200 μL of PBS to the column. Spin again for 1 min at 700 × g. Repeat this step by adding 200 μL of PBS to the column and spinning for 1 min at 700 × g.
Cut off the caps and label four 1.5-mL microcentrifuge tubes used to elute biotinylated CPE. After the 30-min incubation is complete, place the column into a first tube and apply the biotinylation reaction mixture to the column, being careful not to disturb the resin.
Spin for 2 min at 700 × g to collect the first elution of purified bio-CPE. Put a cap onto the first tube and set aside. Put the column into a second tube and apply 200 μL of PBS to the column.
Spin for 2 min at 700 × g to collect the second elution of purified bio-CPE. Put a cap onto the second tube and set aside. Repeat steps described to elute bio-CPE from the column into the second tube with a third and fourth tube. After the column has been eluted into four tubes, determine protein concentration for each tube and pool the contents of tubes containing similar concentrations of bio-CPE (see Note 11). Store at 4 °C.
3.3. ELISA for PN-Specific Antibodies
On Day 27 (Fig. 1), label the number of 1.5-mL microcentrifuge tubes necessary to collect plasma from each mouse.
Measure out 100 μL of 0.5 M EDTA into each of the labeled tubes.
Lightly anesthetize a first PBS mouse by isoflurane inhalation using technique described in Subheading 3.1, making sure to take out the mouse a few seconds after they stop moving (see Note 17).
Using a 100-μL glass capillary tube, retroorbitally bleed the first PBS mouse.
After collecting 100 μL of blood, dispense the content of the full glass capillary tube into tube labeled for the first PBS mouse. Gently mix the blood with EDTA to prevent clotting of the sample.
Repeat steps 3–5 with each of the remaining mice.
Once blood collection is completed, centrifuge the tubes at 1500 × g for 5 min at room temperature.
Label new tubes for each sample, and once centrifugation is complete, collect supernatants from each tube. The supernatant is the plasma being analyzed by ELISA for PN-specific antibodies.
Freeze at −20 °C until Ig ELISA analysis of plasma samples is conducted (see Note 18).
Coat the wells of the ELISA microplate used to examine plasma levels of PN-specific IgE with 50 μL/well of the coating antibody diluted in the coating buffer at 5 μg/mL. Seal the plate with microplate seal.
Coat the wells of the ELISA microplates used to examine plasma levels of PN-specific IgG1 and PN-specific IgG2a with 50 μL/well of CPE diluted in the coating buffer at 2 μg/mL. Seal the plate.
Incubate the plates overnight at 4 °C.
Wash three times by filling the wells with the wash buffer, decanting, and tapping the plates on absorbent paper each time. The wash steps of this protocol can be carried out with a microplate washer or washing by hand with a squeeze bottle.
Block plates with 200 μL/well of the blocking buffer. Seal the plates and incubate for 1.5 h at room temperature.
During blocking incubation time, make the following dilution series for the PN-specific IgE ELISA by diluting plasma samples with the assay diluent in 1.5-mL microcentrifuge tubes: 1:10, 1:50, 1:250, 1:500 (see Note 19). Each plasma sample will have one set of these dilutions.
During blocking incubation time, make the following dilution series for the PN-specific IgG1 ELISA by diluting plasma samples with the assay diluent in 1.5-mL microcentrifuge tubes: 1:20, 1:200, 1:2000, and 1:20,000 (see Note 20). Each plasma sample will have one set of these dilutions.
During blocking incubation time, make the following dilution series for the PN-specific IgG2a ELISA by diluting plasma samples with the assay diluent in 1.5-mL microcentrifuge tubes: 1:20, 1:50, 1:100, and 1:500 (see Note 21). Each plasma sample will have one set of these dilutions.
After the blocking step is completed, wash as described in step 13.
For IgE ELISA, add 100 μL/well in duplicate for each plasma dilution (1:10, 1:50, 1:250, 1:500), using each column of the microplate/mouse (see Note 22). Running four dilutions in duplicate is eight samples down the microplate. After adding plasma dilutions, seal the plate and incubate for 2 h at room temperature.
For IgG1 ELISA, add 100 μL/well in duplicate for each plasma dilution (1:20, 1:200, 1:2000, 1:20,000), using each column of the microplate/mouse. Running four dilutions in duplicate is eight samples down the microplate. After adding plasma dilutions, seal the plate and incubate for 1 h at room temperature.
For IgG2a ELISA, add 100 μL/well in duplicate for each plasma dilution (1:20, 1:50, 1:100, 1:500), using each column of the microplate/mouse. Running four dilutions in duplicate is eight samples down the microplate. After adding plasma dilutions, seal the plate and incubate for 1 h at room temperature.
After plasma sample incubation is complete for both IgG1 and IgG2a ELISAs, wash as described in step 13.
Dilute IgG1 or IgG2a detection antibody at 1:1000 with the assay diluent. Add 100 μL/well of the diluted detection antibody. Seal each plate and incubate for 1 h at room temperature.
After plasma sample incubation is complete for IgE ELISA, wash as described in step 13.
Dilute biotinylated CPE to 1:2000 with the assay diluent for IgE ELISA. Add 100 μL/well of diluted biotinylated CPE. Seal the plate and incubate for 1 h at room temperature.
After the incubation with the detection antibody is complete for both IgG1 and IgG2a ELISAs (see Note 23), wash five times with the wash buffer.
Prepare the substrate solution from a TMB substrate kit following the kit instructions. Briefly, prepare a 1:1 mix of two solutions in the substrate kit.
Add 100 μL/well of the working substrate solution to IgG1 and IgG2a plates. Seal the plate and incubate at room temperature for 15 min in the dark. Turn on and set up the microplate autoreader.
After substrate incubation, add 100 μL/well of the stop solution. Read absorbance at 450 nm within 30 min of stopping reaction on a microplate autoreader (Fig. 2).
After incubation with biotinylated CPE is complete for IgE ELISA, wash five times with the wash buffer.
Dilute the poly-HRP streptavidin reagent 1:5000 with the assay diluent for IgE ELISA. Add 100 μL/well of the diluted poly-HRP streptavidin. Seal the plate and incubate for 30 min at room temperature.
After poly-HRP streptavidin incubation is finished, wash five times with the wash buffer.
Prepare the substrate solution as described in step 27.
Add 100 μL/well of working substrate solution to IgE plate. Seal the plate and incubate at room temperature for 30 min in the dark. Turn on and set up the microplate autoreader.
After substrate incubation, add 100 μL/well of the stop solution. Read absorbance at 450 nm within 30 min of stopping reaction on a microplate autoreader (Fig. 2).
3.4. Inducing and Monitoring Anaphylaxis to PN in Mice
On Day 28, prepare CPE final solution as directed above (see Note 24).
With a 1-mL syringe (see Note 7), pull up 500 μL of CPE final solution. Set the filled syringe aside.
Repeat step 2, filling the remaining syringes with CPE final solution.
Connect the RET-3 rectal probe to the electronic thermocouple thermometer. Turn on the thermometer. It should read the ambient temperature in the room.
Measure and record the rectal temperature for each mouse by inserting the probe into the anus of the mouse. Hold until temperature is stable. This is the zero (0)-min temperature reading. Normal rectal temperature for a mouse is between 37 and 40 °C [18]. When handling the mice, grab by the scruff of their neck, flip them over so their stomach faces upward, and curl the pinkie finger under a hind limb to stabilize the mouse.
Measure the clinical score for each mouse based on the following published criteria [19]: 0, no symptoms; 1, scratching of ear and mouth; 2, puffiness around eyes and mouth, pilar erection, labored breathing; 3, prolonged period of motionlessness; 4, severely reduced motility, tremors, severe respiratory distress; 5, death (see Note 25). Since this is the zero (0)-min time point, the clinical score is 0, no symptoms.
Using a clock with a second hand or digits as a guide, inject each mouse intraperitoneally with 500 μL of CPE final solution at the beginning of every minute (e.g., 9:00 AM, 9:01 AM, 9:02 AM, etc.) (see Note 26) until all mice have been injected. Record the time of each injection.
Ten minutes after the first injection (e.g., 9:10, 9:11, 9:12 AM, etc.), measure and record the rectal temperatures for each mouse beginning with the first mouse that was injected. Separated by 1 min, record the rectal temperature of each mouse in the experiment by order of injection. This is the 10-min temperature reading.
As the rectal temperature is being recorded, measure the clinical score for each mouse as described in step 6.
Repeat steps 8 and 9 for each time point (20, 30, 40, 50, and 60 min), recording the rectal temperatures and clinical scores (see Note 25) of each mouse in the experiment by order of injection (each separated by 1 min) (see Note 27). Anaphylaxis is observed through a significant drop in rectal temperature and presence of clinical symptoms (Fig. 3).
After the 60-min time point has been recorded, sacrifice the mice. If desired, retroorbital blood can be taken at this time, prior to sacrificing the mice, to measure mediators of anaphylaxis (e.g., MCPT-1, histamine) in the plasma with ELISA [9].
4. Notes
PN flour does not fully go into suspension. Therefore, we make a fresh PN stock solution in order to generate fresh final PN solutions each day we expose mice to PN. In this way, we ensure that each final PN solution is fresh and poised to deliver 100 μg of PN into the airways per 50 μL. Since PN flour is an inexpensive reagent, we have always made these solutions fresh on the days of PN exposure. Therefore, we are uncertain if using stored stock PN flour solution would be different than the fresh version.
500 μL of final PN flour solution will enable ten mice to be exposed to PN (50 μL/mouse). Scale as necessary to make more final PN flour solution to expose additional mice. To ensure we have enough of the final PN flour solution when we expose mice, we always make up twice as much as necessary.
Exposing mice to PN via inhalation is most optimally achieved by using an isoflurane vaporizer to put the mice under anesthesia. If the institution does not have one, a wide-mouth jar with an easily removable cover may be used as the chamber to anesthetize the mice, provided that the Institutional Animal Use and Care Committee of your institution approves this method. To put the mice initially under anesthesia in these conditions, place a cotton ball in the bottom of the jar and soak the cotton ball with 1 mL of isoflurane by pipetting the isoflurane directly onto the cotton. To maintain anesthesia conditions (normally done after treating 2–3 mice, depending on the size of the jar), pipette 500 μL of additional isoflurane onto the cotton ball. Repeat maintenance isoflurane every 2–3 mice treated as necessary until all mice are treated.
One vial of CPE we previously used had a dry weight measurement of 535 mg/vial. We resuspended the CPE pellet with 10.7 mL of PBS to make the CPE stock solution (535 mg divided by 50 mg/mL equals 10.7 mL).
During storage, CPE falls out of suspension. When it is taken out for use, vortex to thoroughly mix CPE before using it to generate CPE final solution.
Each mouse is intraperitoneally exposed to 500 μL of CPE final solution, which delivers 2.5 mg of CPE. While we normally deliver 2.5 mg of CPE/mouse to induce anaphylaxis, we have been successful in stimulating anaphylactic reactions by delivering 1.0 mg of CPE/mouse (Fig. 3). In order to deliver 1.0 mg of CPE, carry out a 1:25 dilution using PBS to generate a 2.0 mg of CPE/mL CPE final solution. At this concentration (2.0 mg/mL), 500 μL of CPE delivers 1.0 mg of CPE.
We use 1-mL syringes (tuberculin syringes or an allergist tray with 27 G × 1/2-in needles) to deliver injections. We transfer CPE final solution from the 15-mL conical tube we use to generate the final solution to 1.5-mL microcentrifuge tubes, so we can pull up the CPE final solution into the syringes.
Alternatively, the carbonate–bicarbonate coating buffer solution can be made from mixing the contents of commercially available pre-made carbonate–bicarbonate buffer packs with water. We have used buffer packs that make 500 mL of 0.2 M carbonate–bicarbonate coating buffer, pH 9.4.
Tween-20 is viscous and difficult to pipette in small amounts. When making wash buffer and assay diluent, we cut off the last 1/4 in of a 1000-μL pipette tip. This simple step makes pipetting Tween-20 much easier.
Use a 1.5-mL microcentrifuge tube for biotinylation reaction. Also, use a small stir bar made for microcentrifuge tubes on the stir plate. To stabilize a microcentrifuge tube on a stir plate, place the reaction tube in a microcentrifuge tube foam floating rack to keep it upright and stable during the reaction time.
Eluted fractions from tubes 1–3 typically have similar protein concentrations (measured using BCA assay), so we pool these fractions to make up our final bio-CPE reagent, and discard the fourth tube. Using this kit, we have generated a stable bio-CPE reagent (0.24 μg/mL) usable to conduct PN-specific IgE ELISA for around 12–18 months.
While each prepared tube of either PBS or PN can expose ten mice, we make an additional tube of whatever reagent we need (PBS or PN) if exposing 8–10 mice to either PBS or PN to ensure we have enough reagent to treat all mice.
Depending on how deeply the mice are under anesthesia, they may wake up before the 30 s of holding them is completed. In this case, as they are about to be awaken, put back into the cage, even if it is earlier than 30 s. The critical part is the mouse inhaling the reagent, which takes place within seconds of it touching the tip of the nose.
The PN flour will fall out of suspension during the time it takes to treat the PBS mice, so before pipetting PN final solution, hold the microcentrifuge tube containing the PN solution between the index finger and thumb and vigorously shake it for 5–10 s. This will put the PN back into suspension and ready to be pipetted.
Quickly pipetting up and down about five times will ensure an even suspension of PN captured within the 50 μL of PN solution about to be delivered to the mouse.
The 4-week-long, twice weekly model (Fig. 1) takes some planning to set up the dates. We do the PBS and PN exposures (Days 0, 3, 7, 10, 14, 17, 21, and 24) on a 4-weekly rotation of Tuesdays and Fridays. Using this rotation, Day 27, the day mice are bled to collect their plasma, falls on a Monday and Day 28, the day mice are challenged with PN to induce anaphylaxis, is on a Tuesday. Alternatively, Day 0 could be on a Friday, and using a Friday–Monday rotation, where Day 27 is on a Thursday and Day 28 on a Friday. Choosing to start Day 0 (the first day) on a Wednesday, Thursday, Saturday, and Sunday places PBS- and PN-exposure days on weekends. Choosing a Monday for Day 0 start would mean that the Day 27 bleed would fall on a Sunday.
When retroorbitally bleeding mice, if the mice undergo the effects of anesthesia for as long as necessary to expose mice to PBS or PN via inhalation, bleeding becomes more difficult as their blood pressure drops. To ensure successful bleeding, we mentally count to five from the moment the mouse stops moving.
Subheading 3.3, steps 10–12 should be done the day before collecting plasma (Day 26) if ELISA is to be done the same day as collection. Otherwise, plasma is incredibly stable, lasting for years when it is frozen at −20 °C.
To make up the dilution series for PN-specific IgE ELISA, we mix the following volumes for each plasma sample being analyzed: 1:10 is 30 μL of undiluted plasma + 270 μL of assay diluent; 1:50 is 60 μL of 1:10 dilution +240 μL of assay diluent; 1:250 is 60 μL of 1:50 dilution +240 μL of assay diluent; and 1:500 is 25 μL of 1:50 dilution +225 μL of assay diluent.
To make up the dilution series for PN-specific IgG1 ELISA, we mix the following volumes for each plasma sample being analyzed: 1:20 is 15 μL of undiluted plasma + 285 μL of assay diluent; 1:200 is 30 μL of 1:20 dilution + 270 μL of assay diluent; 1:2000 is 30 μL of 1:200 dilution + 270 μL of assay diluent; and 1:20,000 is 30 μL of 1:2000 dilution + 270 μL of assay diluent.
To make up the dilution series for PN-specific IgG2a ELISA, we mix the following volumes for each plasma sample being analyzed: 1:20 is 15 μL of undiluted plasma + 285 μL of assay diluent; 1:50 is 5 μL of undiluted plasma + 245 μL of assay diluent; 1:100 is 60 μL of 1:20 dilution + 240 μL of assay diluent; and 1:500 is 60 μL of 1:100 dilution + 240 μL of assay diluent.
Using each column of the microplate/mouse would allow testing of a maximum of 12 mice per plate. This gives flexibility to examine plasma from wild-type (WT) PBS, WT PN, along with knockout mouse models of interest [9] in a side-by-side comparison on the same plate.
The order the plasma dilutions were added to the plate will dictate which ELISA plate finishes first. We pipette IgE plasma dilutions first, follow with IgG1 plasma dilutions, and then finish with IgG2a. We suggest this order (the steps listed are in this order) because the plasma incubation time for IgE is 2 h, rather than the 1 h for both IgG ELISAs. Based on this timing, IgG1 will finish first, then IgG2a, and finally, IgE.
Each mouse will be injected intraperitoneally with 500 μL. Make sure in the calculations to account for extra doses due to the dead volume of the 1-mL syringes being used to carry out the injections. We make up additional 2 mL (four doses) to ensure satisfactory amounts of CPE for injections. Once the CPE final solution is made, transfer to 1.5-mL microcentrifuge tubes (see Note 7).
Using this model, clinical symptoms usually show up around 20–30 min in the mice that were sensitized to PN for 4 weeks, but not the PBS-treated mice. Symptoms start with 1, scratching of ear and mouth. This manifests in mice scratching their ears vigorously, beyond normal grooming. Around 30 min, mice enter into the 2–3 range and exhibit hair standing on its end (pilar erection), labored breathing, and motionlessness. To test, we pick up the mice and place them down into the cages. If the mice move slowly to the side of the cage, we score as a 2; if the mice move even slower or barely move, we score it as a 3. If the mice fail to move, are shaking, and even worse breathing, we give that mouse a score of 4. Normally, scores of 4 show up around 40 min post-injection. We have never had a mouse die using this method of anaphylactic challenge, so we have never scored mice as a 5.
Due to recording rectal temperatures every 10 min for the 1-h post-CPE injection anaphylactic period, injecting a different mouse at the beginning of each minute will allow for a maximum of 9 mice in the 1-h period of the anaphylactic challenge. Separating the mice 1-min apart allows for much easier temperature measurements. During times we have more than nine mice, but not enough to justify a second 1-h round (e.g., 12 mice), we have separated the mice by 30 s.
It is normal for mice to begin to recover and show an increase in rectal temperature and decrease in clinical score by 50–60 min post-injection. It does not happen in every mouse, but it occurs.
Acknowledgments
This work was supported by the following funding sources: NIH grant R01 AI71106 to Hirohito Kita at the Mayo Clinic, the Mayo Foundation, NIH T32 Training Grant in Allergic Diseases to Mayo Clinic (PI: Hirohito Kita), and University of Nebraska at Kearney start-up funds provided by the Department of Biology to J.J.D.
References
- 1.Togias A, Cooper SF, Acebal ML, Assa’ad A,Baker JR Jr, Beck LA, Block J, Byrd-Bredbenner C, Chan ES, Eichenfield LF, Fleischer DM, Fuchs GJ III, Furuta GT, Greenhawt MJ, Gupta RS, Habich M, Jones SM, Keaton K, Muraro A, Plaut M, Rosenwasser LJ, Rotrosen D, Sampson HA, Schneider LC, Sicherer SH, Sidbury R, Spergel J, Stukus DR, Venter C, Boyce JA (2017) Addendum guidelines for the prevention of peanut allergy in the United States: report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. J Allergy Clin Immunol 139(1):29–44. 10.1016/j.jaci.2016.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sicherer SH, Munoz-Furlong A, Sampson HA (2003) Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: a 5-year follow-up study. J Allergy Clin Immunol 112(6):1203–1207. 10.1016/s0091-6749(03)02026-8 [DOI] [PubMed] [Google Scholar]
- 3.Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, Holl JL (2011) The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics 128(1):e9–e17. 10.1542/peds.2011-0204 [DOI] [PubMed] [Google Scholar]
- 4.Sicherer SH, Burks AW, Sampson HA (1998) Clinical features of acute allergic reactions to peanut and tree nuts in children. Pediatrics 102(1):e6. [DOI] [PubMed] [Google Scholar]
- 5.Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, Brough HA, Phippard D, Basting M, Feeney M, Turcanu V, Sever ML, Gomez Lorenzo M, Plaut M, Lack G (2015) Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med 372(9):803–813. 10.1056/NEJMoa1414850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du Toit G, Sayre PH, Roberts G, Sever ML, Lawson K, Bahnson HT, Brough HA, Santos AF, Harris KM, Radulovic S, Basting M, Turcanu V, Plaut M, Lack G, Immune Tolerance Network L-OST (2016) Effect of avoidance on peanut allergy after early peanut consumption. N Engl J Med 374(15): 1435–1443. 10.1056/NEJMoa1514209 [DOI] [PubMed] [Google Scholar]
- 7.Trendelenburg V, Ahrens B, Wehrmann AK, Kalb B, Niggemann B, Beyer K (2013) Peanut allergen in house dust of eating area and bed— a risk factor for peanut sensitization? Allergy 68(11):1460–1462. 10.1111/all.12226 [DOI] [PubMed] [Google Scholar]
- 8.Brough HA, Santos AF, Makinson K, Penagos M, Stephens AC, Douiri A, Fox AT, Du Toit G, Turcanu V, Lack G (2013) Peanut protein in household dust is related to household peanut consumption and is biologically active. J Allergy Clin Immunol 132(3): 630–638. 10.1016/j.jaci.2013.02.034 [DOI] [PubMed] [Google Scholar]
- 9.Dolence JJ, Kobayashi T, Iijima K, Krempski J,Drake LY, Dent AL, Kita H (2018) Airway exposure initiates peanut allergy by involving the IL-1 pathway and T follicular helper cells in mice. J Allergy Clin Immunol 142(4):1144–1158. 10.1016/j.jaci.2017.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun J, Arias K, Alvarez D, Fattouh R, Walker T, Goncharova S, Kim B, Waserman S, Reed J, Coyle AJ, Jordana M (2007) Impact of CD40 ligand, B cells, and mast cells in peanut-induced anaphylactic responses. J Immunol 179(10):6696–6703 [DOI] [PubMed] [Google Scholar]
- 11.Chu DK, Llop-Guevara A, Walker TD, Flader K, Goncharova S, Boudreau JE, Moore CL, Seunghyun In T, Waserman S, Coyle AJ, Kolbeck R, Humbles AA, Jordana M (2013) IL-33, but not thymic stromal lymphopoietin or IL-25, is central to mite and peanut allergic sensitization. J Allergy Clin Immunol 131(1): 187–200. 10.1016/j.jaci.2012.08.002 [DOI] [PubMed] [Google Scholar]
- 12.Chu DK, Mohammed-Ali Z, Jimenez-Saiz R, Walker TD, Goncharova S, Llop-Guevara A, Kong J, Gordon ME, Barra NG, Gillgrass AE, Van Seggelen H, Khan WI, Ashkar AA, Bramson JL, Humbles AA, Kolbeck R, Waserman S, Jordana M (2014) T helper cell IL-4 drives intestinal Th2 priming to oral peanut antigen, under the control of OX40L and independent of innate-like lymphocytes. Mucosal Immunol 7(6):1395–1404. 10.1038/mi.2014.29 [DOI] [PubMed] [Google Scholar]
- 13.Ganeshan K, Neilsen CV, Hadsaitong A, Schleimer RP, Luo X, Bryce PJ (2009) Impairing oral tolerance promotes allergy and anaphylaxis: a new murine food allergy model. J Allergy Clin Immunol 123(1):231–238. 10.1016/j.jaci.2008.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forbes-Blom E, Camberis M, Prout M, Tang SC, Le Gros G (2012) Staphylococcal-derived superantigen enhances peanut induced Th2 responses in the skin. Clin Exp Allergy 42(2):305–314. 10.1111/j.1365-2222.2011.03861.x [DOI] [PubMed] [Google Scholar]
- 15.Berin MC, Zheng Y, Domaradzki M, Li XM, Sampson HA (2006) Role of TLR4 in allergic sensitization to food proteins in mice. Allergy 61(1):64–71. 10.1111/j.1398-9995.2006.01012.x [DOI] [PubMed] [Google Scholar]
- 16.Burton OT, Noval Rivas M, Zhou JS, Logsdon SL, Darling AR, Koleoglou KJ, Roers A, Houshyar H, Crackower MA, Chatila TA, Oettgen HC (2014) Immunoglobulin E signal inhibition during allergen ingestion leads to reversal of established food allergy and induction of regulatory T cells. Immunity 41(1):141–151. 10.1016/j.immuni.2014.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tordesillas L, Goswami R, Benede S, Grishina G, Dunkin D, Jarvinen KM, Maleki SJ, Sampson HA, Berin MC (2014) Skin exposure promotes a Th2-dependent sensitization to peanut allergens. J Clin Invest 124(11): 4965–4975. 10.1172/JCI75660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kind LS (1955) Fall in rectal temperature as an indication of anaphylactic shock in the mouse. J Immunol 74(5):387–390 [PubMed] [Google Scholar]
- 19.Smarr CB, Hsu CL, Byrne AJ, Miller SD, Bryce PJ (2011) Antigen-fixed leukocytes tolerize Th2 responses in mouse models of allergy. J Immunol 187(10):5090–5098. 10.4049/jimmunol.1100608 [DOI] [PMC free article] [PubMed] [Google Scholar]


