Abstract
BACKGROUND:
Severe traumatic brain injury (TBI) patients are at high risk for early aspiration and pneumonia. How pneumonia impacts neurological recovery after TBI is not well characterized. We hypothesized that, independent of the cerebral injury, pneumonia after TBI delays and worsens neurological recovery and cognitive outcomes.
METHODS:
Fifteen CD1 male mice were randomized to sham craniotomy or severe TBI (controlled cortical impact [CCI] − velocity 6 m/s, depth 1.0 mm) ± intratracheal lipopolysaccharide (LPS-2 mg/kg in 0.1 mL saline) as a pneumonia bioeffector. Neurological functional recovery by Garcia Neurologic Testing (GNT) and body weight loss were recorded daily for 14 days. On Days 6–14, animals underwent Morris Water Maze learning and memory testing with cued trials (platform visible), spatial learning trials (platform invisible, spatial cues present), and probe (memory) trials (platform removed, spatial clues present). Intergroup differences were assessed by the Kruskal-Wallis test with Bonferroni correction (p < 0.05).
RESULTS:
Weight loss was greatest in the CCI + LPS group (maximum 24% on Day 3 vs. 8% [Sham], 7% [CCI], both on Day 1). GNT was lowest in CCI + LPS during the first week. Morris Water Maze testing demonstrated greater spatial learning impairment in the CCI + LPS group vs. Sham or CCI counterparts. Cued learning and long-term memory were worse in CCI + LPS and CCI as compared to Sham.
CONCLUSION:
A pneumonia bioeffector insult after TBI worsens weight loss and mortality in a rodent model. Not only is spatial learning impaired, but animals are more debilitated and have worse neurologic performance. Understanding the adverse effects of pneumonia on TBI recovery is the first step d patients.
Keywords: Traumatic brain injury, Murine model, Lung infection, Morris Water Maze, Learning, Memory
Traumatic brain injury (TBI) is associated with a significant healthcare and individual burden. Approximately 16% of those hospitalized in the United States after injury have TBI as a primary or secondary diagnosis and represent nearly one third of all hospital deaths.1 Outcomes after TBI are worsened, moreover, when brain injury is accompanied by extracranial injury.2 Because many TBI patients also develop postinjury respiratory failure or require durable airway access for airway protection in light of neurologic compromise, respiratory infectious insults are unsurprisingly a leading cause of postinjury morbidity in patients who sustain TBI.3
Significant work in murine models of multiple trauma has demonstrated the synergistic effect of concomitant injuries on outcomes from each individual injury. For example, in brain-injured mice subjected to a concurrent bone fracture, the presence of the brain injury appears to accelerate callus formation in the healing bone,3–6 while the presence of the fracture appears to worsen cognitive and behavioral recovery from the brain injury.7 This additive effect has been demonstrated in animal models looking at the acute period following injury (48 hours),8 as well as in a longer-term evaluations of animal learning and memory using cognitive assessments such as the Morris Water Maze (MWM).7
The pulmonary system may be directly injured during the event that leads to the TBI or may sustain secondary injuries from either inflammation, infection or both. Interorgan effects have been well documented—isolated TBI itself can induce secondary acute lung injury in the absence of a unique and direct pulmonary blunt or penetrating injury.9,10 The effect of lung injury on outcomes after TBI, on the other hand, is less well understood. Early work suggests that a remote lung insult influences the leukocyte response to concomitant blunt cerebral injury in pial microvasculature and worsens systemic and neurologic effects of TBI. The focus of the current work was to explore whether a pulmonary insult after TBI worsens cognitive recovery, a process that appears related to inflammation.7,8
Given these findings, we sought to determine how the addition of a lung insult to TBI affected neurological outcomes in a longer-term study and with more advanced behavioral metrics. To evaluate this question, we used the same murine model of severe TBI with an associated lung infectious insult with LPS. We selected a 2-week time frame evaluating physiologic and neurologic outcomes but also the effects of the concurrent insult on animal memory and learning ability. We hypothesized that animals who underwent both TBI coupled with a pulmonary inflammatory insult would demonstrate: (1) significantly impaired physiologic parameters (weight loss, neurologic scores) and (2) worsened spatial learning and memory abilities than animals who sustained isolated TBI alone or were uninjured.
METHODS
Reagents
Lipopolysaccharides from Escherichia coli 0111:B4, bovine fluorescein isothiocyanate-labeled albumin, and 0.3% rhodamine 6G (RHD) were obtained from Sigma Aldrich (St. Louis, MO).
Experimental Design and Study Groups
The Institutional Animal Care and Use Committee of the University of Pennsylvania approved all animal procedures. Adult, male, 6-week- to 8-week-old CD1 mice (25–35 g) were obtained from Charles River Laboratories (Wilmington, MA), housed with a 12-hour light/dark photoperiod, and given ad libitum access to food and water. LPS (lipopolysaccharide) was used to represent the inflammatory component of pneumonia in select animal groups. Fifteen (15) animals were randomized into three groups: (1) Sham (sham craniotomy, no controlled cortical impact [CCI], no intratracheal LPS), (2) CCI (CCI, no intratracheal LPS), and (3) CCI + LPS (CCI followed by 60 μg intratracheal LPS).
Cerebral Injury and Pulmonary Insult
Following a period of colony acclimatization, animals underwent intraperitoneal anesthesia (ketamine 100 mg/kg, xylazine 10 mg/kg, and acepromazine 1 mg/kg, KXA) and placed prone in a stereotactic frame. Subcutaneous bupivacaine (0.5 mg/mL) was injected prior to scalp incision. Once the skull was exposed, a hand-held trephine was used to make a 4-mm left-sided circular craniotomy between lambda and bregma sutures. All animals including Sham underwent craniotomy without durotomy to this point. In CCI and CCI + LPS animals, a CCI device (AMS 201; AmScien Instruments, Richmond, VA) was subsequently used to impart a validated cerebral injury consistent with severe TBI7,11,12 as previously described. Briefly, the CCI device uses a 3-mm impact tip moving at 6 m/s to depress the dural surface by 1 mm at the center of the craniotomy. The scalp was closed once hemostasis was achieved.
Under the same anesthetic period, animals in the CCI + LPS group were then placed supine with upper limbs and neck extended to allow creation of a tracheotomy through which LPS was instilled. A total of 0.1 mL of LPS (0.6 mg/mL, total: 60 μg) was instilled into the tracheotomy which was covered by soft tissue followed by skin closure. As early preliminary observations demonstrated that animals receiving this dose of LPS were distinctly suffering greater mortality from dehydration, a 1-mL bolus of subcutaneous normal saline was administered immediately after intratracheal instillation as previously described.13 Sham and CCI animals did not undergo tracheotomy but were similarly fluid resuscitated. All animals also received 0.25 mL of subcutaneous normal saline every 12 hours through the end of experiments.
Body Weight Loss and Neurological Recovery
Body weight loss and neurologic recovery were assessed prior to injury and every 12 hours starting 6 hours after injury. Body weight loss is used as a surrogate for how animals are recovering to fulfill activities of daily living. Neurologic testing was performed using the Garcia Neurologic Test (GNT), which is an 18-point rodent score assessing motor, sensory, reflex, and balance abilities.
The Morris Water Maze—Assessing Learning and Memory
Based on our laboratory’s established protocol and prior studies,14 starting on Day 6 after craniotomy, animals were exposed to daily trials of learning and memory using a MWM. The 100-cm diameter, 50-cm deep MWM pool is black (base and sides) and was filled with 21°C tap water and fitted with a 23.5-cm high black cylindrical platform which was positioned at various locations. Animals underwent three types of swimming trials to assess learning and memory: (1) cued learning trials (Days 6–8 postinjury), (2) spatial learning trials (Days 9–13 postinjury), and (3) probe trials (Days 10–14 postinjury) as described below (Fig. 1). All MWM trials were conducted and scored by a single operator and were performed at approximately the same time of day on all experiment days. Mice were allowed at least a 10-minute rest period between trials. During this period, they were warmed by an overhead heating lamp. All animal maze exercises were video-recorded and analyzed using a commercial video-tracking system (Ethovision, Noldus, Leesburg, VA) mounted above the pool.
Figure 1.
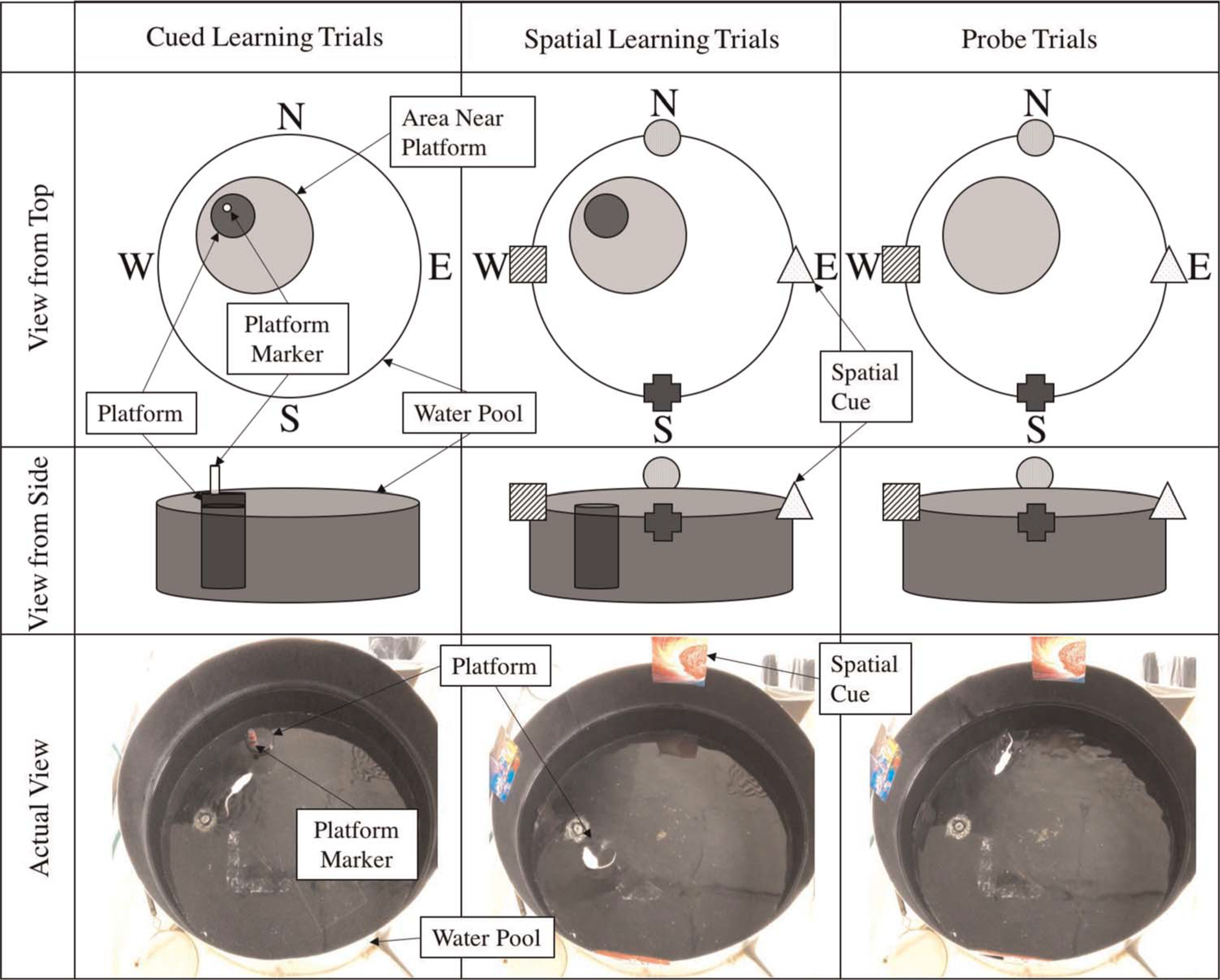
Schematic of MWM setup for cued learning, spatial learning, and probe trials. The platform is elevated above the water for cued learning trials, and there are no visual clues. The platform is submerged below the water level for spatial learning trials and spatial cues are provided. The platform is removed but the spatial cues remain in place for the probe trials.
Cued Learning Trials
Animals underwent four cued trials per day on Days 6 to 8 postinjury to establish platform arrival as a goal. The purpose of the cued trials was to introduce animals to the pool of water and to teach them that the platform was the goal of the maze. For cued trials, the platform was randomly placed in one of four quadrants of the pool (northwest, northeast, southwest, southeast). No visual cues were available for these trials. The water level was 5 mm below the top surface of the platform, so the platform was exposed and visible, and a colorful marker was placed on the platform itself to improve its visibility to the swimming animal. Animals were placed in the pool, facing outward, at different positions in relation to the location of the platform (location and orientation with respect to platform varied by trial). Performance parameters were collected including time to reach the platform and the region around the platform (a predefined circular area around the platform), total distance traveled, average velocity, and swimming duration in each region. Animals were given 15 seconds to stand on the platform once they reached it, and then they were removed from the pool. Animals that did not reach the platform within 60 seconds were guided to the platform and allowed to stand on it for 15 seconds before being removed from the pool.
Spatial Learning Trials
Animals underwent four spatial learning trials per day conducted on Days 9 to 13 postinjury. The purpose of the spatial learning trials was to assess animals’ ability to use visual cues to navigate to their goal (the platform). The MWM setup was similar to that of the cued trials except that the platform remained in a fixed location for all trials (northwest) and the water level was raised so that the platform was submerged 1 cm below the water surface. The colorful platform marker was also removed so that the platform was now fully hidden from view of the swimming animals. Visual cues were displayed on the surrounding walls (north, south, east, and west) of the pool for animals to use to localize the hidden platform. Animals were then released into the maze facing outward from various points in relation to the platform so that they could not visually identify the platform before entering the maze. Animals were given 60 seconds to find the platform and allowed to stand on it for 15 seconds before being removed from the pool to develop spatial orientation. After 60 seconds, those that had not reached it were guided to the platform and again allowed to stand on it for 15 seconds before being removed from the pool. The same parameters were collected as were collected in the cued learning trials.
Probe Trials (Long-Term Memory)
The final set of trials was probe trials conducted daily on Days 10 to 14 postinjury to assess long-term memory of platform location. Probe trials were conducted 24 hours after spatial learning trials (and before the new spatial learning trial for the day on days in which both trials were conducted). The purpose of these trials was to assess animals’ long-term memory of platform location using visual cues. The setup of the MWM was identical to that of the spatial learning trials (spatial cues present) except that no platform was present. Animals were released from various positions along the perimeter of the pool but only given 30 seconds of swim time. The duration, frequency, and time to first reach the area where the platform had been, as well as the area surrounding prior platform location, were recorded. Once 30 seconds had elapsed, animals were removed from the pool. Probe trials were performed immediately preceding spatial learning trials for each day to test long-term (defined as 24 hours or greater) memory of platform location.
Statistical Analysis
Statistical analyses were performed using SPSS Statistics Version 25 (IBM, Chicago, IL). Data are mean values ± standard error of the mean (SEM) and were compared using Kruskal-Wallis analysis with Bonferroni correction; p < 0.05 was required for significance.
RESULTS
Mortality
The initial cohort was designed to equally include 15 animals in three groups. However, one CCI + LPS animal died on postinjury Day 4.
Body Weight Loss and Neurological Recovery
Body weight loss was significantly greater in the CCI + LPS than Sham group throughout the first week postinjury (Fig. 2). Weight loss was significantly greater in the CCI + LPS group than CCI alone group, on Days 3 and 5, with trends towards a significant difference on Days 2, 6, and 7. The maximum mean weight loss in the CCI + LPS group was 24% on Day 3 versus a maximum of 8% in Sham and 7% in CCI, peaking instead on Day 1 after injury. From Day 8 onward, there were no significant differences in body weight loss between any of the groups. There were no significant differences in weight loss between CCI and Sham groups on any day postinjury (Fig. 2).
Figure 2.
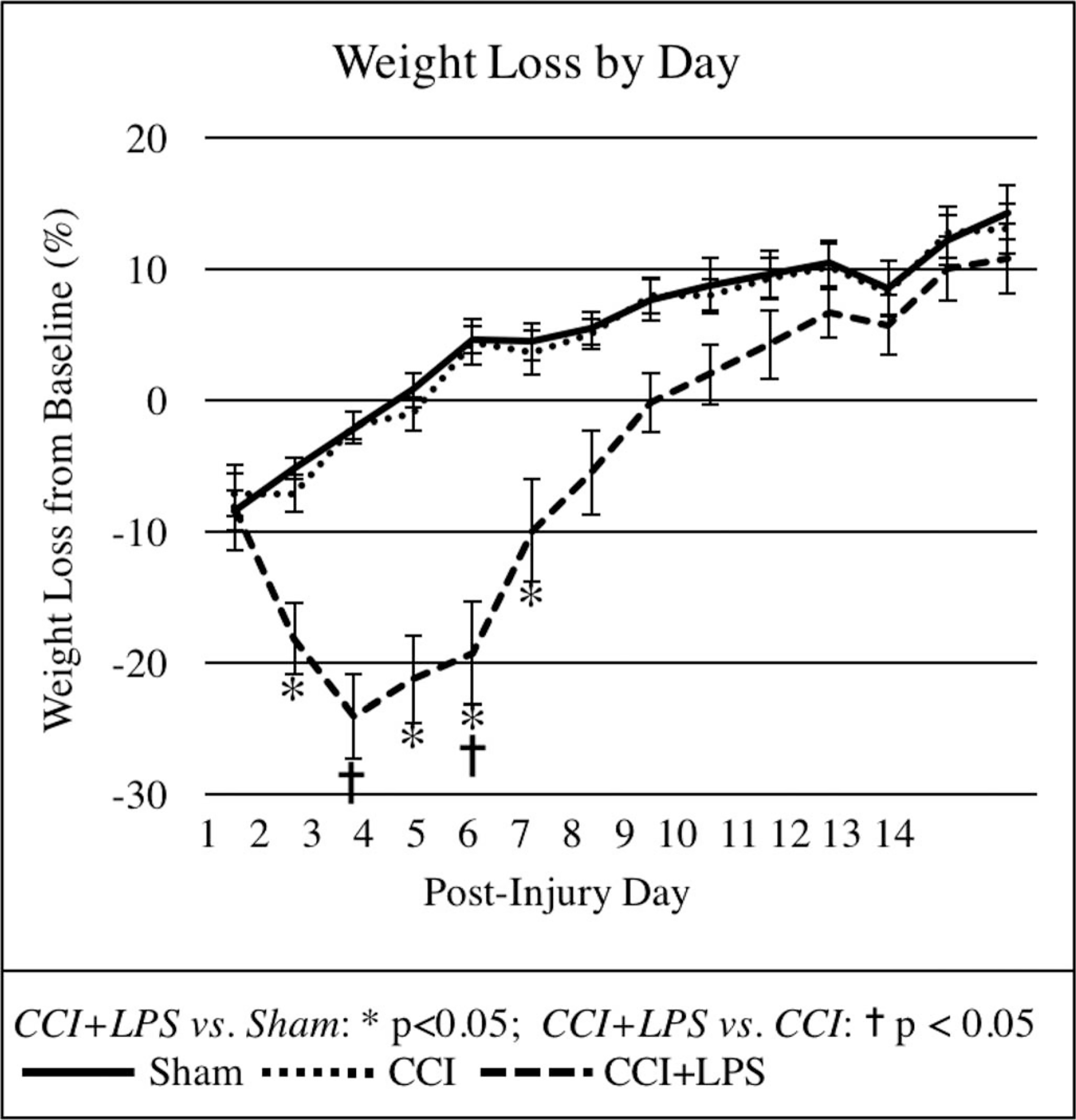
Body weight loss was significantly greater in CCI + LPS animals than in CCI or Sham animals for up to one-week postinjury.
Mean GNT scores were significantly impaired in the CCI + LPS group as compared with Sham up to 8 days postinjury (p < 0.05 except for Day 7) (Fig. 3). There was no significant difference in GNT scores between CCI and Sham groups or between CCI + LPS and CCI groups, however. On the last day of the experiment (Day 14), both CCI and Sham animals had regained maximal GNT scores (18 ± 0 points for each); this was not observed in CCI + LPS animals (17.0 ± 0.4, p = 0.027 vs. Sham or CCI).
Figure 3.
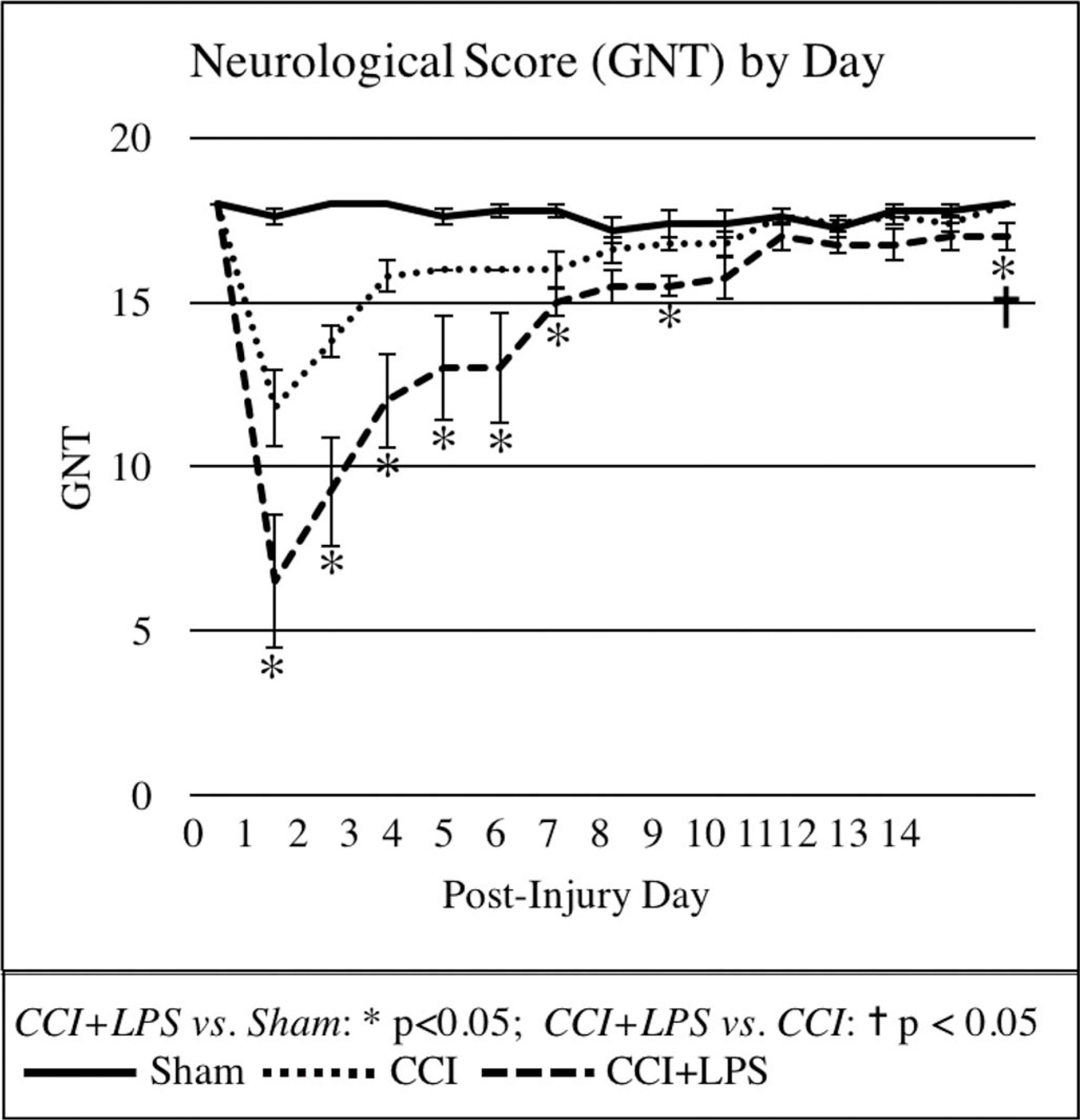
Neurologic scores were significantly worse in CCI + LPS animals than in CCI or Sham animals for up to 1 week postinjury.
Morris Water Maze Testing – Learning and Memory
In the cued learning trials, velocity was significantly lower in the CCI + LPS group (23.5 ± 0.9 cm/s) than in the CCI (28.1 ± 0.7 cm/s, p = 0.0005) or Sham groups (27.4 ± 0.9 cm/s, p = 0.007). However, the overall distance moved was similar between CCI and CCI + LPS groups (470 ± 40 cm and 390 ± 40 cm, respectively), and both head injury groups moved significantly greater distances than Sham (260 ± 30 cm, p = 0.0005 for Sham vs. CCI and p = 0.008 for Sham vs. LPS). Both brain-injured groups took longer than Sham to reach the platform in averaged trials over the 3 days of testing (Sham 12 ± 1 seconds vs. CCI 19 ± 2 seconds, p = 0.002 and vs. CCI + LPS 20 ± 2 seconds, p = 0.002).
In the spatial learning trials, both brain-injured groups traveled longer distances than Sham (CCI 430 ± 40 cm, p < 0.001 and CCI + LPS 400 ± 40 cm, p < 0.001 vs. Sham 200 ± 20 cm). Sham and CCI + LPS animals had similar swimming velocities, both significantly slower than CCI (Sham 24.6 ± 0.6 cm/s and CCI + LPS 25.0 ± 0.7 cm/s vs. CCI 28.6 ± 0.5 cm/s, and p < 0.001 for both comparisons). Brain-injured animals took longer to reach the target platform than did Sham animals (CCI 16 ± 1 seconds and CCI + LPS 15 ± 1 seconds vs. Sham 9.6 ± 0.9 seconds, p < 0.001 vs. CCI and p = 0.004 vs. CCI + LPS). Additionally, CCI + LPS animals took significantly longer than CCI alone animals, who also took significantly longer than Sham animals to reach the area surrounding the platform (9 ± 1 seconds for CCI + LPS, 5.7 ± 0.6 seconds for CCI, and 3.2 ± 0.3 seconds for Sham; CCI + LPS vs. Sham p < 0.001, CCI + LPS vs. CCI p = 0.002, and CCI vs. Sham p = 0.008). Looking at the pattern of learning over time, moreover, we found that over the course of the experiment, even though all three groups initially took similar times to reach the area around the platform, by the end of the spatial learning trials, CCI + LPS animals took longer than CCI or Sham animals to achieve the same goal (Fig. 4), indicating a reduced learning ability.
Figure 4.
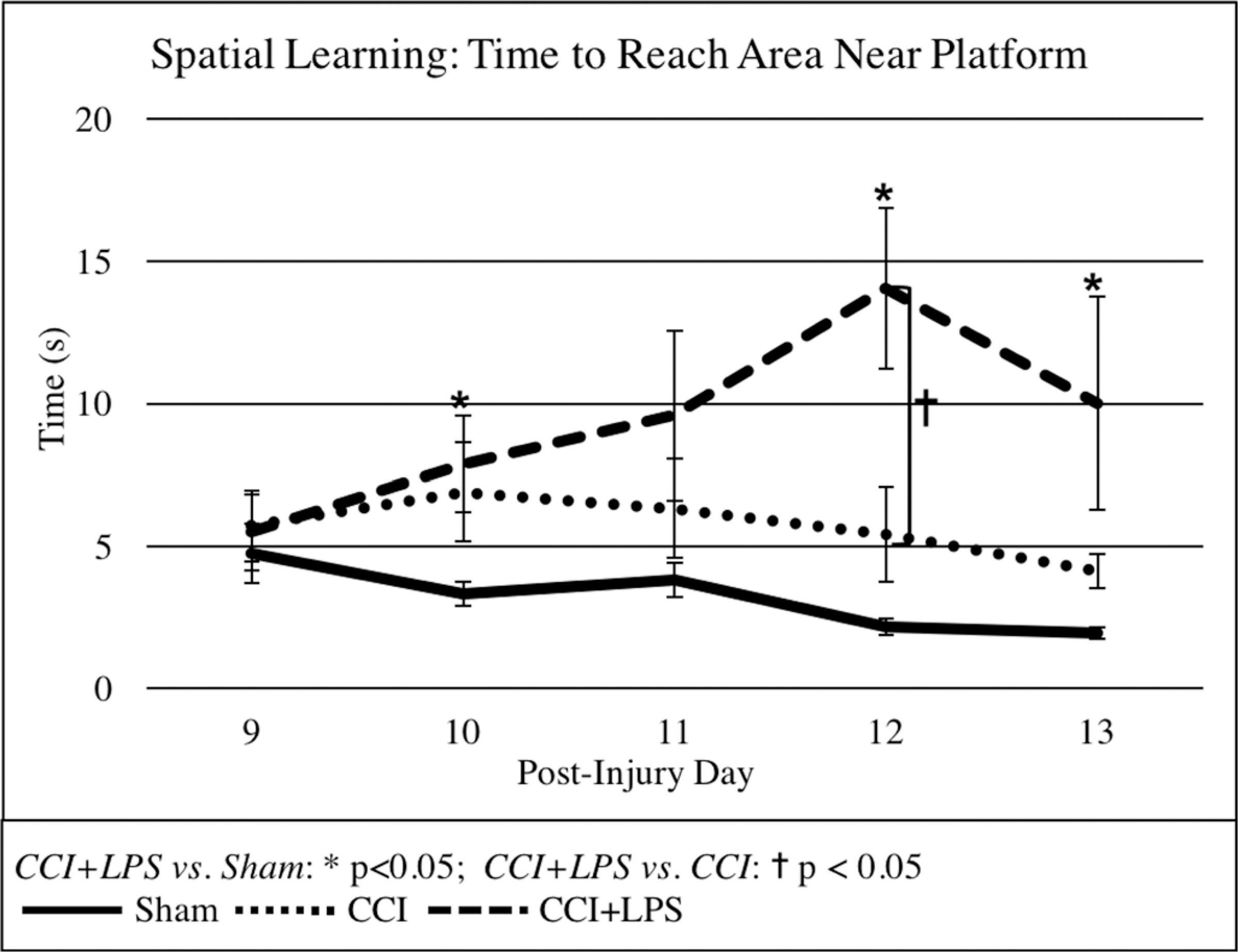
Although all groups initially take a similar amount of time to reach the area around the platform on spatial learning trials, by the end of the trials, CCI + LPS animals take significantly longer to reach the same area than do both CCI and Sham animals.
Probe trials showed no differences in velocity or distance traveled between any of the groups. CCI + LPS animals took longer than Sham to reach the target platform (CCI + LPS 13 ± 2 seconds vs. Sham 6 ± 1 seconds, p = 0.024) and both brain-injured groups took longer to reach the area around the platform than did Sham animals (Sham 2.4 ± 0.4 seconds vs. CCI 7 ± 1 seconds, p < 0.001 and vs. CCI + LPS 6.1 ± 1 seconds p = 0.001) (Fig. 5). CCI + LPS animals spent less time overall in the surrounding area where the platform had been (CCI + LPS 4.9 ± 0.6 seconds vs. Sham 9.1 ± 0.5 seconds, p < 0.001); CCI alone animals also spent less time in the area around the platform (CCI 5.0 ± 0.6 seconds, p < 0.001 vs. Sham). These results indicating that both head-injured groups demonstrated similar deficiencies in memory as compared to Sham animals.
Figure 5.
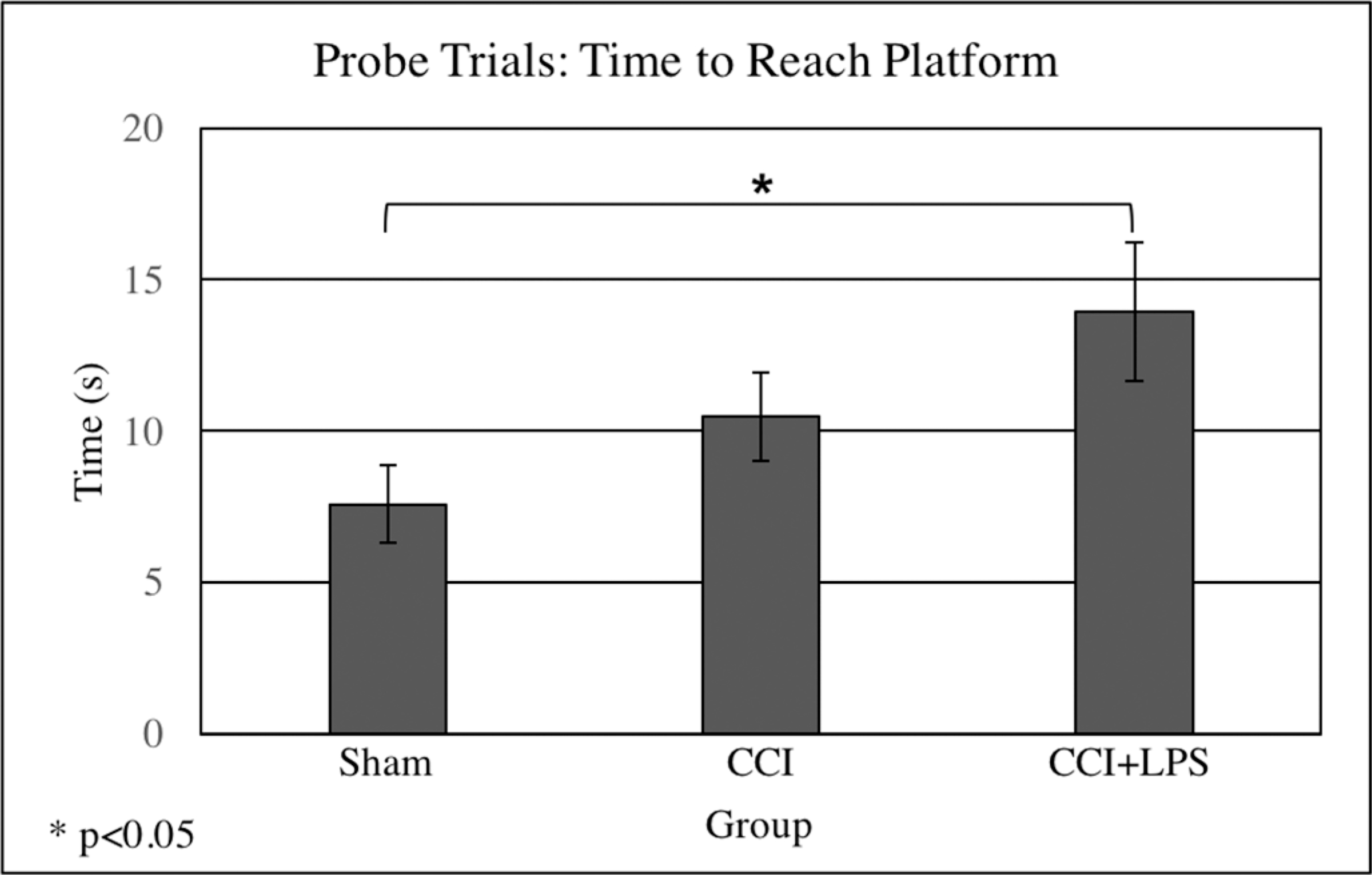
Probe trials demonstrated significant differences between Sham and CCI + LPS groups in time taken to reach the platform, with CCI + LPS animals taking significantly longer to reach the platform than Sham animals.
DISCUSSION
A lung infectious insult concurrent with a severe traumatic brain injury appears to worsen both physiologic and cognitive outcomes in mice. Weight loss and neurologic scores were significantly impaired in the LPS brain-injured cohort. The MWM testing showed important differences in the patterns of cognitive recovery, with CCI + LPS animals demonstrating impaired learning but not memory abilities as compared with the brain-injured counterparts that did not suffer a concurrent pulmonary infectious insult. Swimming velocity in CCI + LPS animals, however, was similar to that of Sham animals, indicating a neurologic alteration that extended beyond physiologic changes.
Traumatic brain injury is accompanied by acute and often long-term cognitive dysfunction. Some impairments are due to physical injury but others are related to secondary brain injuriants and offer the potential for mitigation. Secondary brain injury appears related to inflammation which was why LPS was appropriate to mimic infection-related inflammation while removing the other effects of a bacterial pneumonia. Since pulmonary injury, direct and indirect, impacts outcomes even in the absence of TBI, it is reasonable to ask how TBI and pulmonary inflammation interact. This experiment asks whether cognitive impairment after brain injury is worsened by pulmonary inflammation.
In rodent studies, weight loss is often used as a marker of the animal’s ability to complete activities of daily living.15 The CCI + LPS animals displayed significantly worsened weight loss in the first few days of the experiment, and these differences persisted to a week postinjury. Correlated with this weight loss was the decrement in neurologic scores noted in CCI + LPS as compared with Sham and CCI animals. In our previous work evaluating the early (e.g., 48 hours) effects of CCI + LPS, we saw the beginnings of this pattern, with CCI animals showing initial weight loss that improved by 48 hours but CCI + LPS animals demonstrating persistently worsened weight loss without evidence of improvement in the first 48 hours of the experiment.16 Systemic effects of LPS include reduced motivation for food or fluids and behavioral suppression,17 and so some of these findings may have been due to systemic effects of LPS. Animals still demonstrated markedly impaired neurologic scores during this first week, however, suggesting a worsening of their underlying brain injury. Importantly, the GNT scoring system used was designed as a model to evaluate outcomes after stroke (unilateral MCA occlusion)18 and evaluates unilateral impairment in strength, reflex, and balance. Systemic effects of LPS should lead to global functional changes, but not increased unilateral weakness. The addition of a lung injury to a TBI, therefore, did appear to worsen differences in both physiologic and neurologic outcomes in the current model.
Our MWM protocol was based on previously used published studies7,11,14 and has demonstrated robustness in the past at differentiating between negative (Sham) and positive (CCI) control groups. The MWM protocol is highly reliable across multiple different configurations and species14,19 (e.g., humans,20 mice,7,11,21 rats22,23) and various tests and parameters can be performed and measured to assess different degrees of spatial, learning, and memory deficits. In particular, it is considered a good test for assessing hippocampal-related spatial navigation and reference memory abilities.14 CCI models of TBI typically result in particular injury to the hippocampus. Scheff et al.22 found both behavioral deficits on MWM and hippocampal and cortical immunohistochemical changes in a CCI model of moderate TBI; intriguingly, they found behavioral deficits on MWM in the absence of immunohistochemical changes in their CCI model of mild TBI. Moreover, behavioral deficits on MWM have been demonstrated in models of both hippocampal19 as well as cortical24 injury, although the former is considered more pronounced.24 These findings underlie the importance of various brain foci in the development of working and spatial learning and memory. Our specific model of CCI, moreover, has been shown to result in injury to and inflammatory cellular changes of the hippocampus on immunohistochemistry.7
We found that CCI + LPS and CCI animals showed similar levels of impairment as compared with Sham animals on several parameters noted in the MWM, consistent with the fact that brain injury was present in both these groups. In spatial learning trials, however, CCI + LPS animals took longer to reach the area around the platform than did CCI alone animals, indicating a direct effect on animal learning ability with the addition of LPS to a brain injury. Also, we found that over the course of the experiment, the differences between the time taken to reach the area around the platform became more pronounced between the CCI + LPS animals and the CCI or the Sham animals. Suto et al.7 recently showed that the time needed to reach the platform was increased in brain-injured mice that also suffered a bone fracture, supporting the concept that a remote injury affects animal learning following TBI. They also found that the distance needed to reach the platform was shortest in Sham animals in probe trials, indicating that memory ability was impaired by the additional bone injury—we did not observe this finding when utilizing a lung injury instead.
The current study is small and as such has a number of limitations. As an animal study, the generalizability of our results to a clinical human population is potentially limited. Additionally, our groups are small and so some real and relevant clinical differences between groups may not have been statistically apparent in our comparisons. Also, due to personnel constraints, the MWM observer was not blinded to animal groups, although we attempted to minimize bias in our findings by including only objective measures of performance in the MWM in our reported findings. Finally, an additional LPS alone group was not included and would have complemented the groups well. Nonetheless, Sham and CCI animals did not undergo tracheotomy or tracheal instillation of saline, as it is important to note that our study sought to evaluate the effects of a general lung inflammatory process on TBI recovery rather than the specific effects of LPS alone on the brain. Our preliminary experiments noted that tracheotomy and tracheal saline instillation did have inflammatory lung effects. For this reason, we chose not to expose our control animals (Sham and CCI) to tracheal manipulation to minimize additional inflammatory lung changes in these control groups. Intratracheal LPS was chosen to simplify and minimize the systemic insult to have a standard and non-overwhelming effect on post-TBI animals that were fragile. It is clear that this is not an equivalent surrogate for pneumonia in TBI patients. However, as a result we cannot comment on the cognitive effects of tracheal instillation of LPS in the absence of TBI. Of note, though, by 1 week postinjury, the systemic effects of LPS (previously described as increased lethargy, decreased body core temperature, and decreased oral intake25) appeared to have resolved in our CCI + LPS animals. By this point in the experiment they had recovered their weight loss to baseline levels and their neurologic scores were comparable to those of controls. We believe that this indicated that our subsequent findings of spatial learning and memory limitations in this group were more related to their brain injury in combination with LPS rather than due to LPS alone.
In conclusion, the current study demonstrates that animals who undergo combined brain and lung injury not only appear to have worsened physiologic and neurologic outcomes in the first week after injury, but also have impaired learning outcomes as compared with animals who suffer brain injury alone. Further study will be needed to better evaluate how a lung insult consistent with aspiration following TBI affects neurological recovery following TBI. Such brain-injured patients are common in trauma intensive care units and require careful and thoughtful management to maximize their physiologic and cognitive recovery.
Footnotes
The authors declare no conflicts of interest.
DISCLOSURE
Departments of Surgery at both Thomas Jefferson University Hospital and the University of Pennsylvania. Funding was used to supply materials, animals, and reagents for this study.
This work was a podium presentation at 32nd Annual Meeting of the Eastern Association for the Surgery of Trauma, Jan 15–19, 2019 in Austin, TX.
Contributor Information
Christina L. Jacovides, Division of Traumatology, Surgical Critical Care and Emergency Surgery, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania.
Syed Ahmed, Division of Traumatology, Surgical Critical Care and Emergency Surgery, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania.
Yujin Suto, Department of Emergency and Critical Care Medicine, Hachioji Medical Center, Tokyo Medical University, Tokyo, Japan.
Andrew J. Paris, Department of Medicine, University of Pennsylvania, Philadelphia, PA.
Ryan Leone, Division of Traumatology, Surgical Critical Care and Emergency Surgery, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania.
Jordan McCarry, Division of Traumatology, Surgical Critical Care and Emergency Surgery, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania.
Melpo Christofidou-Solomidou, Department of Medicine, Pulmonary, Allergy and Critical Care Division, University of Pennsylvania, Philadelphia, Pennsylvania.
Lewis J. Kaplan, Division of Traumatology, Surgical Critical Care and Emergency Surgery, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania.
Douglas H. Smith, Center for Brain Injury and Repair, Department of Neurosurgery, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania..
Daniel N. Holena, Division of Traumatology, Surgical Critical Care and Emergency Surgery, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania.
C. William Schwab, Division of Traumatology, Surgical Critical Care and Emergency Surgery, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania.
Jose L. Pascual, Division of Traumatology, Surgical Critical Care and Emergency Surgery, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania.
REFERENCES
- 1.Frieden TR, Houry D, Baldwin G. Report to Congress on Traumatic Brain Injury in the United States: Epidemiology and Rehabilitation. 2015.
- 2.Gross T, Schüepp M, Attenberger C, Pargger H, Amsler F. Outcome in polytraumatized patients with and without brain injury. Acta Anaesthesiol Scand. 2012;56(9):1163–1174. [DOI] [PubMed] [Google Scholar]
- 3.Hofman M, Koopmans G, Kobbe P, Poeze M, Andruszkow H, Brink PR, Pape HC. Improved fracture healing in patients with concomitant traumatic brain injury: proven or not? Mediators Inflamm. 2015;2015:204842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brady RD, Grills BL, Church JE, Walsh NC, McDonald AC, Agoston DV, Sun M, O’Brien TJ, Shultz SR, McDonald SJ. Closed head experimental traumatic brain injury increases size and bone volume of callus in mice with concomitant tibial fracture. Sci Rep. 2016;6:34491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bajwa NM, Kesavan C, Mohan S. Long-term consequences of traumatic brain injury in bone metabolism. Front Neurol. 2018;9:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Locher RJ, Lünnemann T, Garbe A, Schaser K, Schmidt-Bleek K, Duda G, Tsitsilonis S. Traumatic brain injury and bone healing: radiographic and biomechanical analyses of bone formation and stability in a combined murine trauma model. J Musculoskelet Neuronal Interact. 2015;15(4):309–315. [PMC free article] [PubMed] [Google Scholar]
- 7.Suto Y, Nagata K, Ahmed SM, et al. A concomitant bone fracture delays cognitive recovery from traumatic brain injury. J Trauma Acute Care Surg. 2018;85(2):275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suto Y, Nagata K, Ahmed SM, et al. Cerebral Edema and Neurological Recovery after Traumatic Brain Injury Are Worsened if Accompanied by a Concomitant Long Bone Fracture. J Neurotrauma. 2019;36:609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vermeij J-D, Aslami H, Fluiter K, Roelofs JJ, van den Bergh WM, Juffermans NP, Schultz MJ, Van der Sluijs K, van de Beek D, van Westerloo DJ. Traumatic brain injury in rats induces lung injury and systemic immune suppression. J Neurotrauma. 2013;30(24):2073–2079. [DOI] [PubMed] [Google Scholar]
- 10.Chen GS, Huang KF, Huang CC, Wang JY. Thaliporphine derivative improves acute lung injury after traumatic brain injury. Biomed Res Int. 2015;2015:729831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagata K, Suto Y, Cognetti J, Browne KD, Kumasaka K, Johnson VE, Kaplan L, Marks J, Smith DH, Pascual JL. Early low-anticoagulant desulfated heparin after traumatic brain injury: Reduced brain edema and leukocyte mobilization is associated with improved watermaze learning ability weeks after injury. J Trauma Acute Care Surg. 2018;84(5):727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith DH, Soares HD, Pierce JS, Perlman KG, Saatman KE, Meaney DF, Dixon CE, McIntosh TK. A model of parasagittal controlled cortical impact in the mouse: cognitive and histopathologic effects. J Neurotrauma. 1995;12(2):169–178. [DOI] [PubMed] [Google Scholar]
- 13.Lee JC, Kinniry PA, Arguiri E, et al. Dietary curcumin increases antioxidant defenses in lung, ameliorates radiation-induced pulmonary fibrosis, and improves survival in mice. Radiat Res. 2010;173(5):590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1(2):848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomez-Cabrera MC, Garcia-Valles R, Rodriguez-Mañas L, Garcia-Garcia FJ, Olaso-Gonzalez G, Salvador-Pascual A, Tarazona-Santabalbina FJ, Viña J. A New Frailty Score for Experimental Animals Based on the Clinical Phenotype: Inactivity as a Model of Frailty. J Gerontol A Biol Sci Med Sci. 2017;72(7):885–891. [DOI] [PubMed] [Google Scholar]
- 16.Jacovides CL, Ahmed SM, Suto Y, et al. Does a Lung Infection After Brain Injury Worsen Early Brain Inflammation and Subsequent Neurological Recovery? http://www.aast.org/AnnualMeeting/PastAbstracts.aspx. Accessed 02-10-2019.
- 17.Pardon MC. Lipopolysaccharide hyporesponsiveness: protective or damaging response to the brain? Romanian J Morphol Embryol Rev Roum Morphol Embryol. 2015;56(3):903–913. [PubMed] [Google Scholar]
- 18.Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26(4):627–634 discussion 635. [DOI] [PubMed] [Google Scholar]
- 19.Morris R Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11(1):47–60. [DOI] [PubMed] [Google Scholar]
- 20.Skelton RW, Ross SP, Nerad L, Livingstone SA. Human spatial navigation deficits after traumatic brain injury shown in the arena maze, a virtual Morris water maze. Brain Inj. 2006;20(2):189–203. [DOI] [PubMed] [Google Scholar]
- 21.Brody DL, Holtzman DM. Morris water maze search strategy analysis in PDAPP mice before and after experimental traumatic brain injury. Exp Neurol. 2006;197(2):330–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheff SW, Baldwin SA, Brown RW, Kraemer PJ. Morris water maze deficits in rats following traumatic brain injury: lateral controlled cortical impact. J Neurotrauma. 1997;14(9):615–627. [DOI] [PubMed] [Google Scholar]
- 23.Adelson PD, Fellows-Mayle W, Kochanek PM, Dixon CE. Morris water maze function and histologic characterization of two age-at-injury experimental models of controlled cortical impact in the immature rat. Childs Nerv Syst. 2013;29(1):43–53. [DOI] [PubMed] [Google Scholar]
- 24.Clausen F, Lewén A, Marklund N, Olsson Y, McArthur DL, Hillered L. Correlation of hippocampal morphological changes and Morris water maze performance after cortical contusion injury in rats. Neurosurgery. 2005;57(1):154–163; discussion 154–163. [DOI] [PubMed] [Google Scholar]
- 25.Catorce MN, Gevorkian G. LPS-induced murine neuroinflammation model: main features and suitability for pre-clinical assessment of nutraceuticals. Curr Neuropharmacol. 2016;14(2):155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]


