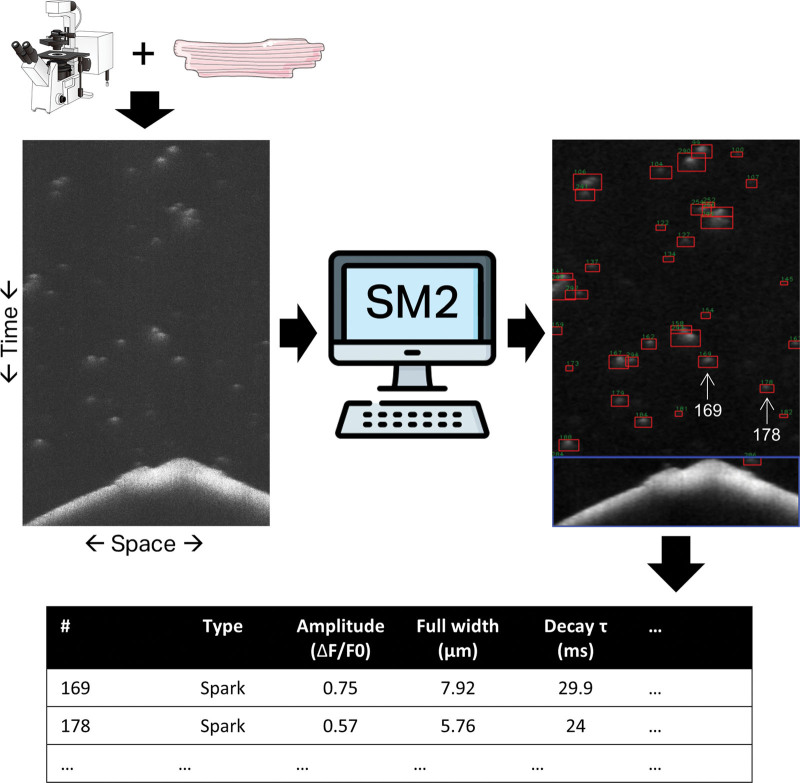
SparkMaster 2: A New Software for Automatic Analysis of Calcium Spark Data (p 450)
Tomek et al introduce a new, improved, free software for calcium imaging.
In heart cells, calcium release and uptake by the sarcoplasmic reticulum is tightly controlled to ensure coordinated contractions. Release events, known as sparks, vary in frequency and amplitude and, if excessive, can contribute to arrhythmia. Having accurate tools for analyzing sparks is thus valuable for assessing pathological calcium handling and drugs aimed at rectification. SparkMaster software, which measures the brightness of sparks captured by fluorescent imaging, is commonly used for such analysis but has limited accuracy, processing speed and customization. Tomek and colleagues have now developed SparkMaster2. With its new spark-detection algorithm, measuring both the brightness and size of sparks, as well as other improvements, SparkMaster2 identifies and distinguishes a large variety of release events, including clustered sparks, with great accuracy. It can also be customized and automated if controlled via Python scripting. The team tested SparkMaster2 in different rodent cardiomyocytes, at different calcium concentrations and with different imaging modalities showing its adaptability to various settings. Lastly, they showed SparkMaster2 outperformed the original software and human annotators when analyzing synthetic data, producing fewer false positives and negatives.
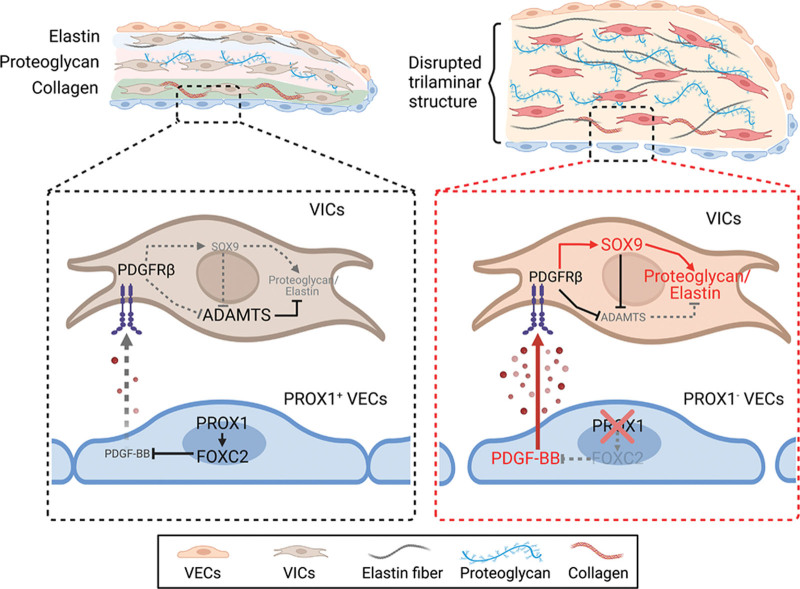
PROX1 Inhibits PDGF-B Expression to Prevent Myxomatous Degeneration of Heart Valves (p 463)
Blocking PDGF-B could be a strategy for treating valve disease, report Ho et al.
In the US, roughly eight percent of people aged 65-and-over suffer heart valve degeneration, rising to thirteen percent in those over 75. While surgery for valve replacements are possible, non-invasive options are desired. To that end, more knowledge of valve pathology is needed. Valves consist of extra cellular matrix (ECM) proteins and valve interstitial cells covered with a layer of endothelial cells (VECs). Some VECs produce the transcription factor PROX1, which regulates valve development in both veins and lymph vessels. Ho and colleagues hypothesized PROX1 might perform a similar role in the heart and, sure enough, its loss from mouse VECs during development caused abnormal heart valve thickening and myxomatous degeneration (floppiness or prolapse). The animals’ VECs also displayed decreased production of transcription factor FOXC2 and increased production of growth factor PDGF-B, which the team showed drove the overproduction of ECM proteins. Furthermore, excessive PDGF-B expression was evident in valve samples from patients with heart valve disease. Pharmacological inhibition of PDGF-B could ameliorate the valve defects seen in Prox1-lacking mice, suggesting such inhibition might be a valid strategy for non-invasive valve treatments in patients.
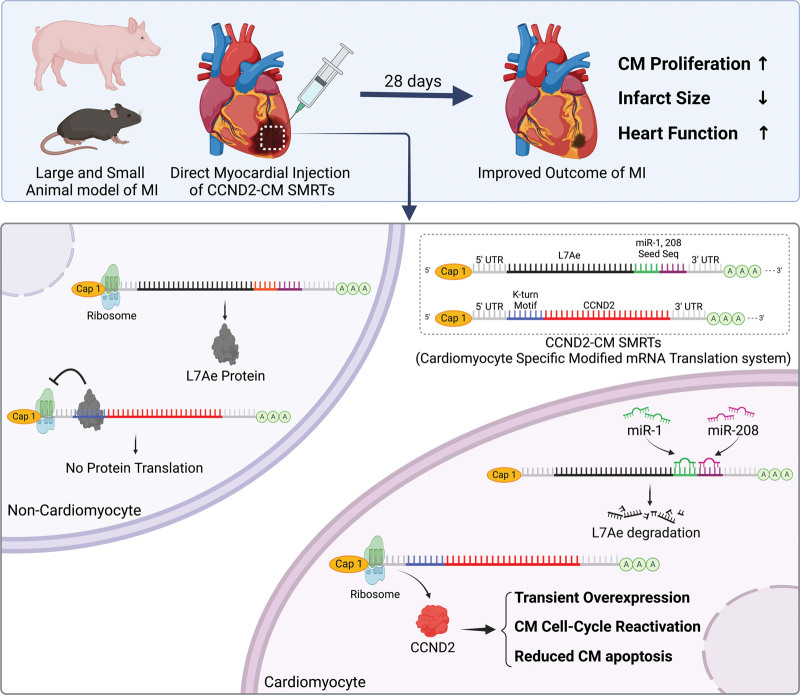
CCND2 Modified mRNA Activates Cell Cycle of Cardiomyocytes in Hearts with Myocardial Infarction in Mice and Pigs (p 484)
ModRNAs offer a transient, potentially safe way to promote heart repair, say Sun et al.
Adult cardiomyocytes (CMs) are mostly post mitotic with fewer than one percent of cells being replaced each year. Consequently, damage caused by a myocardial infarction (MI) results in a permanent scar that often leads to heart failure. Boosting cell cycle regulatory protein CCND2 may be one way to induce CM proliferation, minimize scarring and restore heart function. Indeed, it can improve heart performance after MI in preclinical models. Clinical translation, however, requires a safe way to temporarily increase CCND2 in patient hearts. While viral vectors can have undesirable off-target effects or genomic integration events, modRNAs—like those used for COVID vaccines—are considered relatively safe. Sun and colleagues used the Specific Modified mRNA Translation System (SMRTS) to build modRNAs that ensure CCND2 expression exclusively in CMs. Intracardial injection of these modRNAs in mice after MI led to increased CM proliferation, reduced fibrosis, improved heart function and increased survival compared with that seen in controls. Similar results were obtained in pigs, confirming applicability to large mammals. Importantly, expression of CCND2 and proliferation of CMs was short term in treated animals and did not cause arrhythmia, thus paving the way for clinical testing.