Abstract
Japanese encephalitis (JE) occurs in rural settings in southern and eastern Asia, where diagnostic facilities are limited. For the diagnosis of JE virus (JEV) infection, we developed a nitrocellulose membrane-based immunoglobulin M (IgM) capture dot enzyme immunoassay (MAC DOT) that is rapid, simple to use, requires no specialized equipment, and can distinguish JEV from dengue infection. In a prospective field study in southern Vietnam, 155 cerebrospinal fluid (CSF) and 341 serum samples were collected from 111 children and 83 adults with suspected encephalitis. The JEV MAC DOT, performed on site, was scored visually from negative to strongly positive by two observers, and the results were compared subsequently with those of the standard IgM capture enzyme-linked immunosorbent assay. For the 179 patients with adequate specimens, the MAC DOT correctly identified 59 of 60 JEV-positive patients and 118 of 119 JEV-negative patients (sensitivity [95% confidence intervals], 98.3% [92.1 to 99.9%]; specificity, 99.2% [95.9 to 100.0%]; positive predictive value, 0.98; negative predictive value, 0.99). The MAC DOT also correctly identified three patients with dengue encephalopathy. Admission specimens were positive for 73% of JE patients. Interobserver agreement for MAC DOT diagnosis was excellent (kappa = 0.94). The JEV MAC DOT is a simple and reliable rapid diagnostic test for JE in rural hospitals.
Japanese encephalitis virus (JEV) is the most common cause of viral encephalitis in the world, causing an estimated 45,000 cases and 10,000 deaths annually (24). Up to 50% of survivors are left with severe neurological sequelae. Most cases occur in southern and eastern Asia. JEV is a member of the genus Flavivirus (family Flaviviridae) that is transmitted between birds, pigs, and some other domestic animals by Culex mosquitos (16, 22). Humans are an incidental host, infected when living or passing in close proximity to this enzootic cycle. Hence, most infections of humans occur in rural tropical areas, where facilities for diagnosis are limited.
Even with the best laboratory facilities, JEV cannot usually be isolated from clinical specimens, probably because of low circulating viral numbers and the rapid development of neutralizing antibodies (2). The diagnosis is therefore usually made serologically (16). For many years, the hemagglutination inhibition test has been employed, but this has various practical limitations. Most importantly, it requires paired serum samples and cannot therefore give an early diagnosis (12). In the 1980s, an antibody capture radioimmunoassay was developed (6); this was soon replaced by simpler enzyme-linked immunosorbent assays (ELISAs) (3, 17). The immunoglobulin M (IgM) antibody capture ELISA (MAC ELISA) for serum and cerebrospinal fluid (CSF) has become the accepted standard for diagnosis of Japanese encephalitis (JE) (16). This assay is sensitive and specific; it is often positive for specimens collected on admission and distinguishes between JEV and the related dengue flaviviruses, which are serologically cross-reactive. However, because these ELISAs require sophisticated equipment, their use has been confined largely to a few academic or referral centers. Since most patients with JE are seen in rural hospitals with limited facilities, there is a need for a simple and reliable diagnostic test which is appropriate for such settings.
Recently, the diagnosis of dengue virus infection has been simplified with a modification of the dengue virus MAC ELISA: IgM capture antibody is dotted onto a nitrocellulose membrane, and the result of the assay is a color change visible to the naked eye (8). This dengue virus IgM dot enzyme immunoassay (MAC DOT) requires no specialized skills or equipment and has been validated both in the laboratory (8) and in multicenter field studies (19). It is becoming an accepted means of diagnosing dengue virus infections. We report here the development and field trial of a similar IgM dot enzyme immunoassay for JEV, which is able to distinguish between infection by dengue viruses and that by JEV.
MATERIALS AND METHODS
Virus antigen preparation.
Viral antigens were prepared by growing JEV (Nakayama strain) and dengue viruses (DEN 1 Hawaii, DEN 2 New Guinea C, DEN 3 H-87, and DEN 4 H-241) in C6/36 Aedes albopictus as described previously (8). Control antigens were prepared similarly from cell culture supernatants of mock-infected C6/36 cells. The antigen titer of each harvest was tested by dot enzyme immunoassay using pooled convalescent-phase patient serum as described previously (11). Supernatants giving a clear positive reaction at a dilution of 1:10,000 were pooled. For dengue virus antigens, a cocktail of equal volumes of all four serotypes was prepared.
Membrane preparation.
Rabbit anti-human IgM μ chain (A425; Dakopatts, Copenhagen, Denmark) was spotted onto nitrocellulose membranes (MFS, Pleasanton, Calif.; pore size, 0.45 μm) in 5-μl drops at a 1:250 dilution, and the membranes were allowed to dry in air. Unbound sites were then blocked with phosphate-buffered saline containing 5% nonfat skim milk (PBS-SM), and the membranes were washed in distilled water and air dried before storage at 4°C.
Clinical evaluation.
This study was conducted at the Centre for Tropical Diseases, Ho Chi Minh City, Vietnam, an infectious disease referral hospital for southern Vietnam. In this area, both JEV and dengue virus infections are endemic. The study protocol was approved by the hospital’s scientific and ethical committee, and consent for entry was obtained from the patients’ relatives.
Between March 1996 and August 1997, children and adults with suspected encephalitis were investigated as part of a prospective clinicopathological study of central nervous system infections. Encephalitis was suspected in patients with a fever and clouding of consciousness (Glasgow coma scale of ≤14 [23]), convulsions, or focal neurological signs with no other obvious cause. CSF was collected on admission, and, where possible, 7 days later. Serum was collected on admission, at 7 days, and at discharge. Samples were divided into two aliquots and stored at −70°C before assay.
MAC DOT.
Anti-JEV and anti-dengue virus IgM antibodies were assessed in CSF and sera by using an approach similar to that of the dengue virus MAC DOT test (Venture Technologies, Sarawak, Malaysia) described previously (9) but with samples assessed for JEV and dengue virus antibodies in parallel. MAC DOT membranes were incubated for 2 h at ambient room temperature (25 to 30°C) with serum (diluted 1:100 in PBS-SM) or CSF (diluted 1:5). Following this, JEV or dengue virus antigen was added for overnight incubation at 4°C. The membranes were then incubated with monoclonal antibodies reactive to JEV (MV12/1/C2-2) or dengue (MF4/5/A5/C3-3/D4) for 1 h at room temperature, followed by rabbit anti-mouse immunoglobulin conjugated with horseradish peroxidase (Dakopatts) at a dilution of 1:1,000 for 1 h at room temperature. Between each step, the membranes were washed three times in phosphate-buffered saline for 5 min. Bound antibody was visualized by using a chromogenic substrate of 4-chloro-1-naphthol and hydrogen peroxide. The reaction was stopped after 30 min by washing with distilled water, and the membranes were air dried. All samples producing a blue circle were considered to contain detectable levels of JEV or dengue virus IgM. Color intensities were classified as described below. Negative and positive controls for JEV and dengue virus were included in each run. Depending on the number of samples to be tested, up to 20 samples could be studied simultaneously. This test was developed by one of us (M.J.C.) in a form that is now available commercially (Venture Technologies).
MAC ELISA.
Anti-JEV and anti-dengue virus IgM antibodies were measured in CSF and sera by using a double sandwich capture ELISA, as described previously (17). For single serum and CSF specimens, 40 U of anti-JEV IgM (with anti-JEV IgM greater than anti-dengue virus IgM), or for paired samples an increase from less than 15 U to 30 U or more, was considered evidence of JEV infection (17). Dengue virus infection was diagnosed if the dengue virus IgM titer was greater than the JEV IgM titer (21). Elevated CSF IgM was considered diagnostic of central nervous system infection (6, 7). Patients with negative paired serum samples or a single negative convalescent-phase sample were considered to be not infected with a flavivirus. Patients who had only an acute-phase sample that was negative were classed as having no serological diagnosis.
In an open pilot study using samples from 20 patients with suspected encephalitis (data not shown), the MAC DOT and MAC ELISA results were compared to allow MAC DOT color intensities to be scored as follows: negative, no color change; very weakly positive, barely visible blue circle; weakly positive, circle visible but fainter than that of the positive control; positive, circle visible to a degree approximately equal to that of the positive control; strongly positive, stronger color than that of positive control. Only reactions greater than or equal to that of the positive control (i.e., scored positive or strongly positive) were considered diagnostic of infection. Where there was a reaction in both the JEV and dengue virus wells, the stronger reaction was considered diagnostic. After the pilot study, samples were tested independently in two centers (by the MAC DOT in Vietnam and the MAC ELISA in Thailand), blinded to the patient details and with no discussion of results until the study was completed. The MAC DOT result was scored independently by two observers, and if the observers disagreed, a third deciding opinion was sought. For comparisons with the MAC ELISA, only the final agreed MAC DOT result was used.
Statistical analysis.
Interobserver agreement for the MAC DOT results was assessed by using the kappa statistic as a measure of agreement. Values of kappa ranging from 0 (no agreement) to 1.00 (perfect agreement) were interpreted as follows: 0.0 to 0.2, poor; 0.21 to 0.40, fair; 0.41 to 0.60, moderate; 0.61 to 0.80, good; 0.81 to 1.00, very good (1).
RESULTS
In total, 496 samples (155 CSF and 341 serum samples) from 194 patients (111 children and 83 adults) with suspected encephalitis were assessed. The serological diagnoses given by the MAC ELISA were JEV infection (60 patients), dengue virus infection (3 patients), and no flavivirus infection (116 patients). No serological diagnosis could be made for the 15 patients from whom only an acute-phase sample was available, either because they died (n = 3) or because they left hospital soon after admission (e.g., children with febrile convulsions).
Serology.
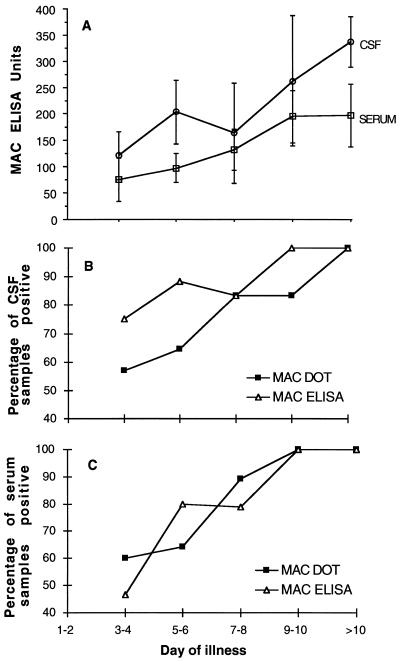
The MAC DOT correctly identified 43 of 52 positive CSF samples (sensitivity [95% confidence intervals], 83% [71 to 91%]; specificity, 99% [95 to 100%]; positive predictive value [PPV], 0.98; negative predictive value [NPV], 0.92) and 121 of 133 positive serum samples (sensitivity, 91% [85 to 95%]; specificity, 95% [92 to 98%]; PPV, 0.92; NPV, 0.94) (Table 1). The nine positive CSF samples misclassified by the MAC DOT consisted of seven scored as weakly positive and two scored as negative. The 12 positive serum samples misclassified (i.e., the false negatives) consisted of 8 scored as weakly positive and 4 scored as very weakly positive (Fig. 1). In most cases where the CSF and serum samples were falsely negative, the samples were collected early in the course of the disease and subsequent samples were positive. Combining the CSF and serum results, the MAC DOT correctly identified 59 of 60 JEV-positive patients and 118 of 119 JEV-negative patients (sensitivity [95% confidence intervals], 98.3% [92.1 to 99.9%]; specificity, 99.2% [95.9 to 100%]; PPV, 0.98; NPV, 0.99). The MAC DOT also identified three dengue virus-positive patients correctly (two with elevated IgM in serum only, the third with elevated IgM in serum and CSF). The MAC DOT misdiagnosed three (1.5%) patients, one false negative for JEV infection, one false positive for JEV infection, and one false positive for dengue virus infection. The patient with false-negative results was scored by MAC DOT as weakly positive for JEV in both CSF and serum samples, but the MAC ELISA results were positive (86 U of IgM in CSF and 129 U of IgM in serum). One patient with a measurement of 34 U of anti-JEV IgM in serum by the MAC ELISA was scored as positive for JEV infection by the MAC DOT. The patient with a false-positive dengue virus infection diagnosis had 25 U of anti-dengue virus IgM in serum but was scored as dengue virus positive by the MAC DOT. Of the JEV-positive patients, 45 (75%) were diagnosed by the MAC DOT with their first serum sample, and 29 (63%) of 46 CSF samples collected at admission were positive. Equivalent figures for the MAC ELISA were 45 (75%) for serum samples and 37 (80%) for CSF samples. The relationship between duration of disease and test positivity is shown in Fig. 2. By day 4, 57% of CSF samples and 60% of serum samples were positive by the MAC DOT.
TABLE 1.
Comparison of JEV results by MAC DOT and MAC ELISA for 496 samples
| Sample type and MAC DOT results | MAC ELISA results (no. of samples)
|
||
|---|---|---|---|
| JEV positive | JEV negative | Total no. of samples | |
| CSF | |||
| JEV positive | 43 | 1 | 44 |
| JEV negative | 9 | 102 | 111 |
| Total | 52 | 103 | 155 |
| Serum | |||
| JEV positive | 121 | 10 | 131 |
| JEV negative | 12 | 198 | 210 |
| Total | 133 | 208 | 341 |
FIG. 1.
Comparison of JEV MAC DOT test score and JEV IgM measured by MAC ELISA. The broken line is the cutoff for a positive MAC ELISA (40 U).
FIG. 2.
MAC ELISA units (A) and proportion (percentage) of CSF (B) and serum (C) samples positive by MAC DOT and MAC ELISA, by day of illness in patients with JE.
Interobserver agreement.
The MAC DOT results scored by the two observers are compared in Table 2. Interobserver agreement for all samples was very good, with a kappa value of 0.83; it was slightly better for CSF samples (kappa = 0.89) than for serum samples (kappa = 0.80). When the MAC DOT scores were considered with regard to diagnosis of JEV infection (scores of negative, very weakly positive, and weakly positive = negative for infection; scores of positive and strongly positive = positive for infection), the interobserver agreement was even better, with a kappa value of 0.94.
TABLE 2.
Interobserver agreement of MAC DOT results for 496 samples
| Observer 1 results | Observer 2 results (no. of samples)
|
Total no. of samples | ||||
|---|---|---|---|---|---|---|
| Negative | Very weakly positive | Weakly positive | Positive | Strongly positive | ||
| Negative | 198 | 15 | 3 | 0 | 0 | 216 |
| Very weakly positive | 12 | 37 | 6 | 0 | 0 | 55 |
| Weakly positive | 3 | 2 | 32 | 7 | 0 | 44 |
| Positive | 0 | 1 | 6 | 88 | 5 | 100 |
| Strongly positive | 0 | 0 | 0 | 1 | 80 | 81 |
| Total | 213 | 55 | 47 | 96 | 85 | 496 |
DISCUSSION
In the last 50 years, the epidemiology of JEV has changed. While mass vaccination campaigns have been associated with a decrease in the number of encephalitis cases in Japan, Taiwan, and South Korea, the geographic area affected by the virus has expanded to include the Indian subcontinent, China, Southeast Asia, and the Western Pacific region (22). The reasons for this expansion are incompletely understood, but increasing irrigation and animal husbandry favoring breeding of the Culex mosquito vector are thought to be important. Approximately 2.8 billion people live in this vast geographical area, and JE is likely to remain an important public health problem into the 21st century. Two epidemiological patterns are recognized: in temperate climates, JE occurs in epidemics, while in tropical climates, the disease is endemic with sporadic cases throughout the year. Estimates of the annual incidence of JE range from 35,000 to 50,000 (13, 15), but the true number is unknown, because most areas where JEV occurs lack diagnostic facilities. Although there is currently no effective antiviral treatment, the importance of diagnosing Japanese encephalitis extends beyond epidemiological interest. A correct diagnosis focuses the physician’s attention on the specific complications of JE such as hyponatremia, convulsions, and raised intracranial pressure (14, 16, 18) and avoids continued investigation and possibly inappropriate treatment of other central nervous system infections. In some countries, vaccination campaigns can be mobilized and targeted towards areas where patients with JE originate; in others, vaccination will not be introduced without good epidemiological support.
The diagnosis of JE has advanced considerably in the last 20 years. The MAC ELISA overcame many of the problems associated with hemagglutination inhibition tests, namely, the need for paired serum samples, acetone extraction of serum, serial dilutions, and the lack of specificity among secondary flaviviruses infections. The MAC ELISA, scored by visual examination, has been assessed in the field and has performed well (5). However, since it uses 96-well plates, the MAC ELISA is more appropriate for investigating large numbers of samples in diagnostic centers rather than individual patients in small rural hospitals. Field diagnosis of flavivirus infections advanced in the 1990s with the development of nitrocellulose membrane-based enzyme immunoassays. In the first dot enzyme immunoassays, viral antigen from JEV, dengue virus, or other viruses was dotted onto a nitrocellulose membrane (9, 10, 11). Since these were not antibody isotype specific, they were appropriate for serological surveys rather than for diagnosis of acute infection. With modification to an IgM isotype capture form, clinical diagnosis became possible. The dengue virus MAC DOT has proved reliable in diverse field settings in Southeast Asia, the South Pacific, and South America (8, 19), with sensitivity and specificity around 90%. It has recently been modified to a more rapid colloidal gold-based immunochromatographic format (25). In the present study, we have shown the JEV MAC DOT to be equally reliable. Combining the serum and CSF test results, the MAC DOT correctly identified 59 of 60 patients infected with JEV, as detected by the MAC ELISA, with sensitivity and specificity over 98% and excellent interobserver agreement. Overall, 75% of infected patients were identified by the admission specimen results and the sensitivity and specificity of the test for individual serum samples were greater than 90%. For CSF samples, the sensitivity was lower (83%), but our initial cutoff may have been too high. When a diagnosis of infection was based on an anti-JEV reaction score of weakly positive, positive, or strongly positive, 50 of 52 JEV-positive CSF samples and 100 of 103 JEV-negative CSF samples (sensitivity [95% confidence intervals], 96% [88 to 99%]; specificity, 97% [92 to 99%]; PPV, 0.94; NPV, 0.98) were correctly identified. A priori this seems a reasonable approach, since any IgM antibody in the CSF is likely to be diagnostic (7).
The majority of infections with JEV do not lead to encephalitis. Estimates of the ratio of apparent to inapparent infections range from 1:25 to 1:300 (22). Asymptomatic infections are associated with elevated IgM in the serum only. The presence of IgM in the CSF indicates active antibody production by the central nervous system in response to JEV infection (7). Sensitivity and specificity are both greater than 95% after the seventh day after admission (16). Samples taken earlier may be negative, and hence it is recommended that serum and CSF antibodies be measured upon admission and on day 7 (16). Most patients infected with JEV will be identified by MAC DOT and MAC ELISA; however, a subgroup of patients die soon after hospital admission, before producing an antibody response (4). This may have been the case for three patients in this study who died without a serological diagnosis. Virus isolation may be positive for these patients, especially from postmortem brain tissue (20), but unfortunately such tissue was not available.
Many of the areas which are endemic for JEV are also endemic for dengue viruses. In the past, all encephalopathic patients with antibody reactive to JEV were assumed to have JE, but neurological manifestations of dengue virus infections are gaining increasing recognition (21, 22). In this study, the MAC DOT and the MAC ELISA identified three patients with higher antibody levels to dengue viruses than to JEV, including one with antibody in the CSF. Only by measuring antibody levels to JEV and to dengue virus in parallel can the two be distinguished. Further use of the JEV MAC DOT may help, not only in the identification of patients with JE but also in the identification of patients with neurological manifestations of dengue virus infection.
In summary, the JEV MAC DOT is a rapid and reliable diagnostic test for JE. The only electrical equipment it requires is a refrigerator. Its simplicity and long storage life (12 months at 4°C) make it appropriate for use in rural hospitals. Since it is now commercially produced, it should become widely available and make a useful contribution to the diagnosis, management, and epidemiology of Japanese encephalitis.
ACKNOWLEDGMENTS
We are grateful to the Director and staff of the Centre for Tropical Diseases, in particular the doctors and nurses of the adult and pediatric intensive care units; Pham Thi Diep, Pham Thi Doan, Nguyen Thu Quyen, and Panor Srisongkram for laboratory support; Delia Bethell, Nicholas Day, Deborah House, Christopher Parry, and John Wain for helpful discussions; and Tio Phaik Hooi for preparing reagents. MAC DOT reagents were provided by Venture Technologies, UNIMAS Research Park, Universiti Malaysia Sarawak, Sarawak, Malaysia.
This work was funded by The Wellcome Trust of Great Britain.
REFERENCES
- 1.Altman D G. Practical statistics for medical research. Padstow, Cornwall, United Kingdom: TJ Press (Padstow) Ltd.; 1993. [Google Scholar]
- 2.Buescher E L, Scherer W F. Immunological studies of Japanese encephalitis virus in man. I. Antibody responses following overt infection of man. J Immunol. 1959;83:582–593. [PubMed] [Google Scholar]
- 3.Bundo K, Igarashi A. Antibody-capture ELISA for detection of immunoglobulin M antibodies in sera from Japanese encephalitis and dengue hemorrhagic fever patients. J Virol Methods. 1985;11:15–22. doi: 10.1016/0166-0934(85)90120-x. [DOI] [PubMed] [Google Scholar]
- 4.Burke D S, Lorsumrudee W, Leake C J, Hoke C H, Nisalak A, Laorakpongse T. Fatal outcome in Japanese encephalitis. Am J Trop Med Hyg. 1985;34:1203–1209. doi: 10.4269/ajtmh.1985.34.1203. [DOI] [PubMed] [Google Scholar]
- 5.Burke D S, Nisalak A, Hoke C H. Field trial of a Japanese encephalitis diagnostic kit. J Med Virol. 1986;18:41–49. doi: 10.1002/jmv.1890180106. [DOI] [PubMed] [Google Scholar]
- 6.Burke D S, Nisalak A, Ussery M A. Antibody capture immunoassay detection of Japanese encephalitis virus immunoglobulin M and G antibodies in cerebrospinal fluid. J Clin Microbiol. 1982;16:1034–1042. doi: 10.1128/jcm.16.6.1034-1042.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burke D S, Nisalak A, Ussery M A, Laorakpongse T, Chantavibul S. Kinetics of IgM and IgG responses to Japanese encephalitis virus in human serum and cerebro-spinal fluid. J Infect Dis. 1985;151:1093–1099. doi: 10.1093/infdis/151.6.1093. [DOI] [PubMed] [Google Scholar]
- 8.Cardosa M J, Baharudin F, Hamid S, Hooi T P, Nimmannitya S. A nitrocellulose membrane based IgM capture enzyme immunoassay for etiologic diagnosis of dengue virus infections. Clin Diagn Virol. 1995;3:343–350. doi: 10.1016/0928-0197(94)00049-z. [DOI] [PubMed] [Google Scholar]
- 9.Cardosa M J, Hah F-L, Choo B-H, Padmanathan S. Screening of pig sera for antibodies to Japanese encephalitis virus using a dot enzyme immunoassay and IgM capture ELISA: comparison with the haemagglutination inhibition and plaque reduction neutralization tests. Southeast Asian J Trop Med Public Health. 1993;24:472–6. [PubMed] [Google Scholar]
- 10.Cardosa M J, Tan H S, Ismail Z, Tio P H. Use of a dot enzyme immunoassay for determination of antibodies to a multianalyte panel: applications for rapid screening of population for antibody profiles. Trop Biomed. 1991;8:151–156. [Google Scholar]
- 11.Cardosa M J, Tio P H. Dot enzyme immunoassay: an alternative diagnostic aid for dengue fever and dengue hemorrhagic fever. Bull W H O. 1991;69:741–745. [PMC free article] [PubMed] [Google Scholar]
- 12.Clark C H, Casals J. Techniques for hemagglutination inhibition with arthropod viruses. Am J Trop Med Hyg. 1958;7:561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- 13.Hennessy S, Zhengle L, Tsai T F, Strom B L, Chao-Min W, Hui-Lian L, Tai-Xiang W, Hong-Ji Y, Qi-Mau L, Karabatsos N, Bilker W B, Halstead S B. Effectiveness of live-attenuated Japanese encephalitis vaccine (SA14-14-2): a case control study. Lancet. 1996;347:1583–1586. doi: 10.1016/s0140-6736(96)91075-2. [DOI] [PubMed] [Google Scholar]
- 14.Hoke C H, Vaughn D W, Nisalak A, Intralawan P, Poolsuppasit S, Jongsawas V, Titsyakorn U, Johnson R T. Effect of high dose dexamethasone on the outcome of acute encephalitis due to Japanese encephalitis virus. J Infect Dis. 1992;165:631–637. doi: 10.1093/infdis/165.4.631. [DOI] [PubMed] [Google Scholar]
- 15.Igarashi A. Epidemiology and control of Japanese encephalitis. World Health Stat Q. 1992;45:299–305. [PubMed] [Google Scholar]
- 16.Innis B L. Japanese encephalitis. In: Porterfield J S, editor. Exotic viral infections. London, United Kingdom: Chapman & Hall; 1995. pp. 147–174. [Google Scholar]
- 17.Innis B L, Nisalak A, Nimmannitya S, Kusalerdchariya S, Chongswasdi V, Suntayakorn S, Puttisri P, Hoke C H. An enzyme-linked immunosorbent assay to characterize dengue infections where dengue and Japanese encephalitis co-circulate. Am J Trop Med Hyg. 1989;40:418–427. doi: 10.4269/ajtmh.1989.40.418. [DOI] [PubMed] [Google Scholar]
- 18.Kumar R, Mathur A, Kumar A, Sharma S, Chakraborty S, Chaturvedi U C. Clinical features and prognostic indicators of Japanese encephalitis in children in Lucknow (India) Indian J Med Res. 1990;91:321–327. [PubMed] [Google Scholar]
- 19.Lam S K, Fong M Y, Chungue E, Doraisingham S, Igarashi A, Khin M A, Kyaw Z T, Nisalak A, Roche C, Vaughn D W, Vordnam V. Multicentre evaluation of dengue IgM dot enzyme immunoassay. Clin Diagn Virol. 1996;7:93–98. doi: 10.1016/s0928-0197(96)00257-7. [DOI] [PubMed] [Google Scholar]
- 20.Leake C J, Burke D S, Nisalak A, Hoke C H. Isolation of Japanese encephalitis virus from clinical specimens using a continuous mosquito cell line. Am J Trop Med Hyg. 1986;35:1045–1050. doi: 10.4269/ajtmh.1986.35.1045. [DOI] [PubMed] [Google Scholar]
- 21.Lum L C S, Lam S K, Choy S, George R, Harun F. Dengue encephalitis: a true entity? Am J Trop Med Hyg. 1996;54:256–259. doi: 10.4269/ajtmh.1996.54.256. [DOI] [PubMed] [Google Scholar]
- 22.Solomon T. Viral encephalitis in Southeast Asia. Neurol Infect Epidemiol. 1997;2:191–199. [Google Scholar]
- 23.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;ii:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 24.Vaughn D W, Hoke C H. The epidemiology of Japanese encephalitis: prospects for prevention. Epidemiol Rev. 1992;14:197–221. doi: 10.1093/oxfordjournals.epirev.a036087. [DOI] [PubMed] [Google Scholar]
- 25.Vaughn D W, Nisalak A, Kalayanarooj S, Solomon T, Dung N M, Cuzzubbo A, Devine P L. Evaluation of a rapid immunochromatographic test for diagnosis of dengue virus infection. J Clin Microbiol. 1998;36:234–238. doi: 10.1128/jcm.36.1.234-238.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]