Abstract
OBJECTIVES:
To determine the effectiveness and safety of ciprofol for sedating patients in ICUs who required mechanical ventilation (MV).
DESIGN:
A multicenter, single-blind, randomized, noninferiority trial.
SETTING:
Twenty-one centers across China from December 2020 to June 2021.
PATIENTS:
A total of 135 ICU patients 18 to 80 years old with endotracheal intubation and undergoing MV, who were expected to require sedation for 6–24 hours.
INTERVENTIONS:
One hundred thirty-five ICU patients were randomly allocated into ciprofol (n = 90) and propofol (n = 45) groups in a 2:1 ratio. Ciprofol or propofol were IV infused at loading doses of 0.1 mg/kg or 0.5 mg/kg, respectively, over 4 minutes ± 30 seconds depending on the physical condition of each patient. Ciprofol or propofol were then immediately administered at an initial maintenance dose of 0.3 mg/kg/hr or 1.5 mg/kg/hr, to achieve the target sedation range of Richmond Agitation-Sedation Scale (+1 to –2). Besides, continuous IV remifentanil analgesia was administered (loading dose: 0.5–1 μg/kg, maintenance dose: 0.02–0.15 μg/kg/min).
MEASUREMENTS AND MAIN RESULTS:
Of the 135 patients enrolled, 129 completed the study. The primary endpoint-sedation success rates of ciprofol and propofol groups were 97.7% versus 97.8% in the full analysis set (FAS) and were both 100% in per-protocol set (PPS). The noninferiority margin was set as 8% and confirmed with a lower limit of two-sided 95% CI for the inter-group difference of –5.98% and –4.32% in the FAS and PPS groups. Patients who received ciprofol had a longer recovery time (p = 0.003), but there were no differences in the remaining secondary endpoints (all p > 0.05). The occurrence rates of treatment-emergent adverse events (TEAEs) or drug-related TEAEs were not significantly different between the groups (all p > 0.05).
CONCLUSIONS:
Ciprofol was well tolerated, with a noninferior sedation profile to propofol in Chinese ICU patients undergoing MV for a period of 6–24 hours.
Keywords: ciprofol, intensive care unit, mechanical ventilation, noninferior, sedation
KEY POINTS.
Question: Is ciprofol safe and effective for sedation in Chinese ICU patients undergoing mechanical ventilation?
Findings: This phase 3 trial confirmed a noninferiority sedation profile for ciprofol compared with propofol regarding the sedation success rate of Chinese ICUs patients receiving mechanical ventilation for 6–24 hours, as indicated by safety profiles.
Meaning: Ciprofol is a potential suitable alternative sedative to propofol for Chinese patients in ICUs receiving mechanical ventilation.
At present, propofol, benzodiazepines, and dexmedetomidine are typical drugs used for sedation of patients in ICUs. Propofol produces a rapid onset of sedation and a quick recovery, with a dose-dependent sedation depth, which is especially beneficial in reducing intracranial pressure (1). However, propofol has a narrow therapeutic index (2) and is commonly administered at higher doses than currently recommended, leading to an intraoperative hypotension rate of 26% especially in elderly patients (3–5). In general, benzodiazepines have little impact on the circulation, but benzodiazepines accumulate due to a slow clearance and also prolong the necessary duration of mechanical ventilation (MV) (6, 7).
The 2,6-disubstituted phenol derivative ciprofol is an analog of propofol, with increased stereoselective effects adding to its anesthetic properties, mainly attributed to the incorporation of the cyclopropyl group (8). The therapeutic index of ciprofol is 1.5 times that of propofol and its potency is four- to five-fold higher (9). Dose normalized maximum plasma concentration (Cmax), time to reach Cmax, elimination half-life (t1/2), elimination rate constant, and mean residence time were similar, but plasma clearance and the volume of distribution and volume of distribution at steady state values were significantly lower after 4-hour infusions of ciprofol versus propofol (10). In addition, mean arterial pressure (MAP) reductions were lower when induced by ciprofol compared with propofol (11). Ciprofol showed good tolerance and comparable anesthesia/sedation in patients undergoing gastrointestinal endoscopy (12, 13), fiberoptic bronchoscopy (14), and general anesthesia (15, 16). A continuous IV infusion of ciprofol was well tolerated during 12-hour sedation in Chinese healthy subjects (10). In a phase 2 clinical trial, ciprofol produced a comparable sedation profile to propofol in ICU patients receiving MV within a 6–24 hours study period (17).
Therefore, this present multicenter, randomized, phase 3 trial was conducted with a noninferiority design, to confirm the ICU sedation profiles of ciprofol in a broader patient population.
MATERIALS AND METHODS
Design of the Study and Participants
Considering the regional differences in ICU patients, this phase 3 trial was initially planned to be carried out in 27 research centers across China, in order to control bias and ensure the objectivity of the research data. Due to the medical complexity and variable conditions for ICU patients, the strict inclusion/exclusion criteria, and the single-blind setting, this phase 3 trial was eventually conducted in 21 centers from December 2020 to June 2021. A total of 135 ICU patients who received MV were randomly allocated into ciprofol (n = 90) and propofol (n = 45) groups in a 2:1 ratio. Detailed methods of randomization and masking are presented in Appendix 1 (http://links.lww.com/PCC/C387). The entire trial included the screening period (–day 1), the drug administration period, and the follow-up period (0–24 hr after the end of drug administration). The full details of the study protocols have previously been reported (18). The overall administration duration of drugs (including the loading and maintenance doses administration times) was required to be greater than or equal to 6 hours ± 30 minutes and less than or equal to 24 hours ± 30 minutes for a target sedation depth of +1 to –2 using the Richmond Agitation-Sedation Scale (RASS) (19). Inclusion criteria were: patients 18–80 years old with endotracheal intubation and undergoing MV; expected sedation time 6 to 24 hours; and a sedation target RASS of +1 to –2. Further details of inclusion and exclusion criteria are given in Appendix 2 (http://links.lww.com/PCC/C387).
The Ethical Committee of The First Affiliated Hospital of Sun Yat-sen University (Approval No. 2020-057-03; title: “A multicenter, randomized, single-blind, propofol-controlled phase 3 trial to evaluate intravenous administration of ciprofol emulsion injection for sedation in ICU patients undergoing MV” on September 4, 2020), and all other hospitals participating in the trial approved the protocols, which were registered at ClinicalTrials.gov (NCT04620031). The procedures used in this study adhered to the tenets of the Declaration of Helsinki. All participating patients or legal representative signed informed consent forms.
Study Procedure
During the intervention period, a continuous IV infusion of remifentanil analgesia was (loading dose: 0.5–1 μg/kg, maintenance dose: 0.02–0.15 μg/kg/min) prior to ciprofol or propofol administration, if required. The Critical Care Pain Observation Tool (CPOT) was used to rate a patients’ degree of pain based on clinical observations (20). If CPOT was greater than or equal to 3, dose adjustment of remifentanil was considered when appropriate. Even with a CPOT score less than 3, if some patients complained of pain or at the investigator’s discretion, the remifentanil dose was adjusted to produce the required degree of analgesia based on the patients’ vital signs.
During the drug administration period, ciprofol or propofol were IV infused at loading doses of 0.1 or 0.5 mg/kg, respectively, over 4 minutes ± 30 seconds depending on the physical condition of each patient. Ciprofol or propofol were then immediately administered at an initial maintenance dose of 0.3 or 1.5 mg/kg/hr, with a target sedation depth of RASS +1 to –2, based on the Pain, Agitation/sedation, Delirium, Immobility (rehabilitation/mobilization), and Sleep (disruption) guideline (21). Maintenance doses were adjusted up or down, if necessary, in order to achieve target sedation, with the adjustment dose ranges being 0.06–0.8 or 0.3–4 mg/kg/hr, respectively. Furthermore, a top-up dose of ciprofol (0.05 mg/kg) or propofol (0.25 mg/kg) could be administered within 30–60 seconds according to the physical condition of each patient. The interval between each top-up dose was greater than or equal to 2 minutes. If the target RASS range (+1 to –2) could not be maintained for greater than or equal to 30 minutes, even when the maximum maintenance dose prescribed for protocol had been administered, other sedative drugs were used for rescue therapy after the examination of laboratory indicators, vital signs, and a 12-lead electrocardiogram.
If necessary, the analgesic dose of remifentanil was adjusted in the range 0.02–0.15 μg/kg/min according to COPT during the drug administration period.
Assessment of Outcomes
The primary endpoint was the successful sedation rate, which had to meet the following two criteria: 1) The duration within the target RASS range (+1 to –2) should account for greater than or equal to 70.0% of the overall administration duration of the study drugs (22) and 2) No rescue therapy was administered.
The secondary endpoints were: 1) Mean time to sedation; 2) The mean sedation compliance rate; 3) Extubation time; 4) Recovery time; 5) The usage dosage of ciprofol, propofol, remifentanil, or rescue drug; and 6) Nursing score; eTable 1 (http://links.lww.com/PCC/C387) gives the full grading criteria. The detailed definitions of secondary endpoints are presented in Appendix 3 (http://links.lww.com/PCC/C387).
The safety indicators were: the rates of occurrence of adverse events (AEs), drug-related AEs, and serious AEs (SAE); vital signs; 12-lead electrocardiogram; results of physical examinations and laboratory tests (routine blood and urine, blood biochemistry, coagulation, blood gas analysis); and changes in triglyceride concentrations. Additional definition of AEs and SAEs are shown in Appendix 4 (http://links.lww.com/PCC/C387) and eTable 2 (http://links.lww.com/PCC/C387).
The exploratory indicators were the plasma concentrations of ciprofol and propofol. All plasma concentrations below the lower limit of quantification were scored as below the quantization limit and marked as 0 when calculating the mean plasma concentration.
Sample Size
This study was a noninferiority design and the primary endpoint was sedation success. Based on clinical judgment and previous ICU studies, the noninferiority margin was set at 8%. Assuming an ICU sedation success rate of 99% for both ciprofol and propofol, the required sample size by intention-to-treat analysis was estimated to be 120 cases under the condition that the type I error (false positive) was 0.025 (one-sided), the power was 80%, and the noninferiority margin was 8%. Considering a 10% dropout rate, it was estimated that 135 patients receiving MV should be enrolled and randomly assigned in a 2:1 ratio, with 90 patients in the ciprofol group and 45 in the propofol group.
Statistical Analysis
All calculations were conducted using the SAS Enterprise Guide (ver. 7.1; SPSS Inc., Cary, NC). Continuous variables are given as means ± sd or medians with ranges (minimum–maximum) and categorical variables as numbers and percentages. The definition of the analysis set is described in Appendix 5 (http://links.lww.com/PCC/C387).
The primary endpoint was the sedation success rate of ciprofol and propofol. The corresponding inter-group difference and 95% CIs were estimated based on the Newcombe-Wilson method. Noninferiority was concluded if the lower limit of the two-sided 95% CI of the inter-group difference was greater than –8% in the full analysis set (FAS) and per-protocol (PP) analysis. Hodges-Lehmann was employed to determine the median value of the inter-group difference and the corresponding 95% CI for the mean time to sedation and mean sedation compliance rate. The extubation time and recovery time between the two groups were compared with a log-rank test. Regarding the comparisons of other indicators, a t test or a rank-sum test was employed for continuous variables and Fisher test for categorical variables. A two-sided p value of less than 0.05 was deemed to be significant.
RESULTS
Enrollment and the Baseline Characteristics of Patients
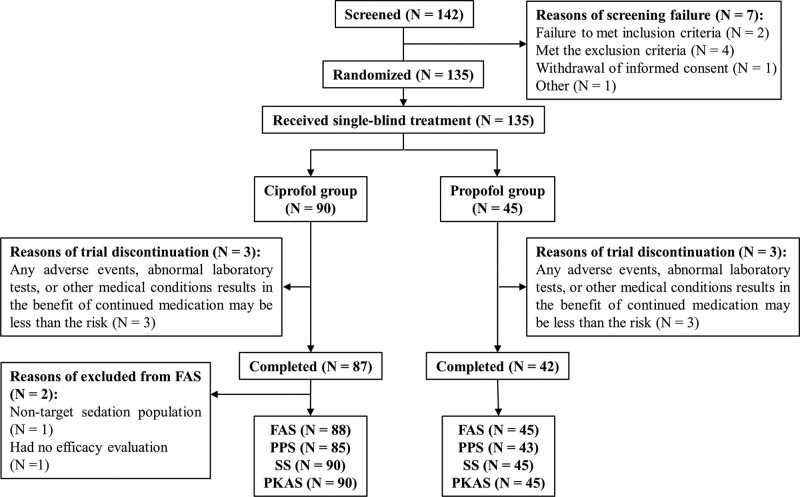
In total, 142 patients were screened, among which seven failed to meet the eligible criteria. Thus, 135 patients were enrolled in the trial, including 90 who received ciprofol and 45 propofol (Fig. 1). Of the 135 enrolled patients, 129 finished the trial. All 135 enrolled patients were included for analysis of safety and pharmacokinetics, of whom 133 and 128 were assigned to the FAS and PP set (PPS). Additional details about the enrollment in the analysis set are described in Appendix 6 (http://links.lww.com/PCC/C387). The demographic and baseline characteristics of the patients are listed in Table 1.
Figure 1.
Flow diagram of patient enrollment. FAS = full analysis set, PKAS = pharmacokinetic analysis set, PPS = per-protocol set, SS = safety set.
TABLE 1.
Demographic and Baseline Characteristics of ICU Patients in the Two Groups
| Variables | Ciprofol (n = 88) | Propofol (n = 45) |
|---|---|---|
| Age (yr) | 56.7 ± 12.7 | 52.2 ± 12.9 |
| Age range, yr, n (%) | ||
| < 65 | 60 (68.2) | 38 (84.4) |
| ≥ 65 | 28 (31.8) | 7 (15.6) |
| Gender, n (%) | ||
| Male | 58 (65.9) | 26 (57.8) |
| Female | 30 (34.1) | 19 (42.2) |
| Body mass index, kg/m2 | 23.6 ± 3.2 | 23.8 ± 3.3 |
| Admission source, n (%) | ||
| Surgery | 81 (92.0) | 42 (93.3) |
| Nonsurgery | 7 (8.0) | 3 (6.7) |
| Operation types (≥ 10%), n (%) | ||
| Exploratory laparotomy | 12 (13.6) | 1 (2.2) |
| Lymphadenectomy | 10 (11.4) | 4 (8.9) |
| Colectomy | 10 (11.4) | 1 (2.2) |
| Uvulopalatopharyngoplasty | 8 (9.1) | 6 (13.3) |
| Glasgow Coma Scale | 14.8 ± 0.5 | 15.0 ± 0.2 |
| Sepsis-related Organ Failure Assessment | 1.8 ± 1.6 | 1.1 ± 1.2 |
| Child-Pugh classification, n (%) | ||
| Grade A | 14 (15.9) | 6 (13.3) |
| Grade B | 8 (9.1) | 3 (6.7) |
| NE | 66 (75.0) | 36 (80.0) |
| Evaluation of renal function, n (%) | ||
| Normal (eGFR ≥ 90) | 71 (80.7) | 39 (86.7) |
| Mild (60 ≤ eGFR < 90) | 14 (15.9) | 6 (13.3) |
| Moderate (30 < eGFR < 60) | 2 (2.3) | 0 |
| Severe (eGFR ≤ 30) | 0 | 0 |
| NE | 1 (1.1) | 0 |
eGFR = estimated glomerular filtration rate, NE = not evaluated.
Data are presented as the mean ± sd or numbers with percentages.
Primary Endpoint
In the FAS, there were 86 (97.7%) and 44 (97.8%) patients who achieved successful sedation at the desired level for more than 70% of the study period in the ciprofol and propofol groups, respectively, without any rescue therapy being administered. In the PPS, the success rates were both 100% in the two groups. The median difference and corresponding 95% CI of the sedation success rate between the two groups were –0.05% (–5.98% to 9.44%) and 0.00% (–4.32% to 8.20%) in the FAS and PPS. The lower limit of two-sided 95% CIs were both greater than –8% in the FAS and PPS, indicating that ciprofol was noninferior to propofol regarding its sedation profile.
Secondary Endpoints
The mean time to sedation and the mean sedation compliance rates were 57.5 versus 57.7 min and 95.9% versus 96.2% in the ciprofol and propofol groups, respectively, but no significant differences were found (Table 2). It is worth noting that the recovery time of patients in the ciprofol group was significantly longer than in the propofol group (4.8 vs 1.6 min; p = 0.003), but there were no significant differences in the extubation time, usage of the study drugs or the nursing score (all p > 0.05). In addition, the remifentanil dose was not a significant difference in patients receiving ciprofol and propofol, with the inter-group difference being 0.00 (95% CI, –0.44% to 0.54%).
TABLE 2.
Summary of Secondary Endpoints in ICU Patients in the Two Groups
| Variables | Ciprofol (n = 88) | Propofol (n = 45) | Median of Difference, % (95% CI) | p |
|---|---|---|---|---|
| Mean time to sedation (min) | 57.5 ± 6.4 | 57.7 ± 6.7 | 0.00 (0.00–0.00) | NA |
| Mean sedation compliance rate (%) | 95.9 ± 10.6 | 96.2 ± 11.2 | 0.00 (0.00–0.00) | NA |
| Extubation time | ||||
| From intubation or admitted to ICU (hr) | 17.5 ± 9.8 | 16.4 ± 5.2 | 0.724 | |
| From the drug withdrawal (hr) | 1.1 ± 1.0 | 1.2 ± 1.1 | 0.718 | |
| Recovery time (min) | 4.8 ± 9.8 | 1.6 ± 2.8 | 0.003 | |
| Usage of study drugs | ||||
| Duration of loading dose (min) | 4.0 ± 0.4 | 4.0 ± 0.1 | 0.266 | |
| Numbers of top-up dose (times) | 0.0 ± 0.2 | 0.1 ± 0.6 | 0556 | |
| Number of patients with ≥ 1 top-up dose, n (%) | 2 (2.3) | 1 (2.2) | 1.000 | |
| Duration of maintenance administration (hr) | 9.8 ± 4.7 | 9.7 ± 4.7 | 0.968 | |
| Number of dose adjustments during maintenance (times) | 0.6 ± 1.2 | 0.6 ± 1.1 | 0.722 | |
| Number of patients with ≥ 1 dose adjustment, n (%) | 29 (33.0) | 17 (37.8) | 0.700 | |
| Overall administration duration of study drugs (hr) | 9.8 ± 4.7 | 9.8 ± 4.7 | 0.964 | |
| Remifentanil dose/min per body weight (μg/kg/min) | 0.04 ± 0.03 | 0.04 ± 0.03 | 0.00 (–0.44 to 0.54) | NA |
| Nursing scores (range, 0–12) | 5.0 ± 1.5 | 5.2 ± 1.2 | 0.090 |
NA = not applicable.
Data are presented as the mean ± sd or numbers with percentages.
Safety
A total of 57 patients (63.3%) given ciprofol experienced 146 AEs, 145 of which were treatment-emergent AEs (TEAEs). Thirty-four patients (75.6%) in the propofol group exhibited 108 AEs, of which 107 were TEAEs. Most TEAEs were grade 1 or 2 in severity. SAEs were exhibited by three patients (3.3%) and two patients (4.4%) given ciprofol or propofol, respectively (Table 3). All SAEs were not associated with ciprofol or propofol administration, but the vast majority of them were associated with the patients’ primary disease. Of note, one patient experienced serious respiratory depression (lasted for 2 min), that may be related to the initiation of remifentanil at 3 hours after the end of ciprofol administration. Twenty-two patients (24.4%) in the ciprofol group and 16 (35.6%) in the propofol group exhibited drug-related TEAEs, among which hypotension was the most common, occurring in 18 (20.0%) and 14 (31.1%) patients, respectively. No significant differences were found in the occurrence of AEs, TEAEs, SAEs, and drug-related TEAEs in the two groups (all p > 0.05). The durations of drug-related hypotension, bradycardia, and respiratory depression appeared to be shorter in the ciprofol group, but statistical significance was not reached (all p > 0.05). The severity of majority drug-related TEAEs were grade 1 or 2, but two patients (2.2%) and two patients (4.4%) in the ciprofol and propofol groups experienced grade 3 drug-related hypotension (2.2% vs 4.4%) and allergic dermatitis (1.1% vs 0) (Table 3).
TABLE 3.
Summary of Adverse Events in ICU Patients in the Two Groups
| Variables | Ciprofol (n = 88) | Propofol (n = 45) | p |
|---|---|---|---|
| Any adverse event, n (%) | 57 (63.3) | 34 (75.6) | 0.176 |
| Any TEAE, n (%) | 57 (63.3) | 34 (75.6) | 0.176 |
| Grade 1 | 45 (50.0) | 25 (55.6) | 0.587 |
| Grade 2 | 26 (28.9) | 16 (35.6) | 0.438 |
| ≥ Grade 3 | 9 (10.0) | 8 (17.8) | 0.271 |
| Drug-related TEAE, n (%) | 22 (24.4) | 16 (35.6) | 0.223 |
| Grade 1 | 13 (14.4) | 8 (17.8) | 0.622 |
| Grade 2 | 10 (11.1) | 6 (13.3) | 0.780 |
| ≥ Grade 3 | 2 (2.2) | 2 (4.4) | 0.601 |
| Hypotension | 2 (2.2) | 2 (4.4) | 0.601 |
| Allergic dermatitis | 1 (1.1) | 0 | NA |
| Any SAE, n (%) | 3 (3.3) | 2 (4.4) | 1.000 |
| Cerebral hemorrhage | 0 | 1 (2.2) | NA |
| Hyporeflexia | 1 (1.1) | 0 | NA |
| Postoperative bleeding | 0 | 1 (2.2) | NA |
| Upper gastrointestinal bleeding | 1 (1.1) | 0 | NA |
| Respiratory depression | 1 (1.1) | 0 | NA |
| Drug-related SAEs, n (%) | 0 | 0 | NA |
| TEAEs leading to discontinuation of the study drug administration, n (%) | 3 (3.3) | 3 (6.7) | 0.400 |
| Drug-related TEAE, termed by preferred term, n (%) | |||
| Hypotension | 18 (20.0) | 14 (31.1) | 0.198 |
| Hypertriglyceridemia | 3 (3.3) | 1 (2.2) | 1.000 |
| Bradycardia | 2 (2.2) | 1 (2.2) | 1.000 |
| Respiratory depression | 1 (1.1) | 2 (4.4) | 0.258 |
| Elevated hemobilirubin | 0 | 1 (1.1) | NA |
| Atrial fibrillation | 1 (1.1) | 0 | NA |
| Allergic dermatitis | 1 (1.1) | 0 | NA |
| Duration of drug-related TEAEs (min), median (range) | |||
| Hypotension | 90.0 (5.0–714.0) | 149.0 (15.0–10,899.0) | 0.299 |
| Bradycardia | 85.0 (50.0–120.0) | 151.0 (151.0–151.0) | 0.260 |
| Respiratory depression | 60.0 (60.0–60.0) | 108.0 (96.0–120.0) | 0.473 |
| Triglycerides concentration (mmol/L), mean ± sd | |||
| Baseline | 1.8 ± 2.1 | 1.2 ± 0.5 | 0.010 |
| Changes from baseline within 30 min after the end of drug administration | –0.41 ± 1.31 | –0.02 ± 0.43 | 0.058 |
| Changes from baseline to follow-upa | –0.41 ± 1.35 | 0.01 ± 0.53 | 0.048 |
NA = not applicable, SAE = serious adverse event, TEAE = treatment-emergent adverse event.
Within 0~24 hours after the end of drug administration.
Figure S1 (http://links.lww.com/PCC/C387) shows that the vital clinical signs in the two groups were stable after the study drug administration and the overall change trend was practically identical. The lowest systolic blood pressure occurred at 30 minutes after the initial administration of ciprofol or propofol, with a median (range) value of 121.0 mm Hg (78–184 mm Hg) versus 113.0 mm Hg (82–192 mm Hg). The decreased changes of triglyceride concentrations from baseline in patients given ciprofol was greater than those given propofol within 0–24 hours after drug administration ceased (p = 0.048) (Table 3).
Plasma Concentrations
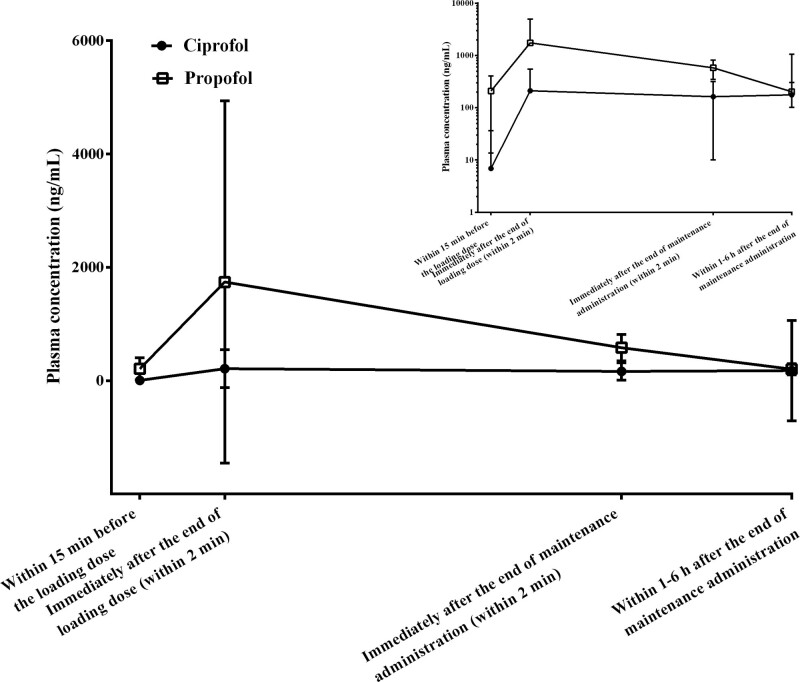
The change trend in plasma concentrations of ciprofol and propofol was basically the same, with ciprofol having a virtually identical terminal t1/2 to that of propofol (Fig. 2). The measured plasma concentrations of ciprofol and propofol were 7.3–210.6 and 190.8–1,741.7 ng/mL, respectively, when RASS had fallen to a range of +1 to –2.
Figure 2.
Mean plasma concentration-time plots (linear and semi-log) for ciprofol and propofol.
DISCUSSION
In the current study, a noninferiority design was adopted and the noninferiority margin was set at 8% ground based on clinical judgment and previous trials. We assumed that the success rate of propofol would be 99% based on the results of a previous phase 2 trial and other ciprofol trials (15, 17). Considering that the acceptable success rate of sedation should be greater than 80% in clinical practice and we referred to the noninferiority trial of dexmedetomidine versus propofol/midazolam for ICU sedation, the margin was set at less than 10% (23). Finally, the noninferior margin was conservatively set at 8%. The main finding was that an IV infusion of ciprofol in ICU wards to produce 6–24 hours sedation was noninferior to propofol, manifested by a lower limit of two-sided 95% CIs for the ciprofol-propofol successful sedation rate differences, being –5.98% and –4.32% (both > –8%) in the FAS and PPS. On the premise of not affecting the overall evaluation of efficacy outcomes, we adopted a 2:1 allocation ratio in this study to enroll more patients receiving ciprofol, so as to observe fully the drug safety profiles of ciprofol in ICU patients undergoing MV.
The remifentanil dose was not significantly different between patients receiving ciprofol and propofol, with the inter-group difference being 0.00 (95% CI, –0.44% to 0.54%), which was consistent with precedent ciprofol studies (15, 17). Regarding other secondary endpoints, only the recovery time for ciprofol was significantly longer than propofol (4.8 vs 1.6 min; p = 0.003). Several studies have reported delayed recovery from benzodiazepines and dexmedetomidine, due to a longer half-life, the presence of active metabolites, or complex dose relationships (24, 25). The terminal t1/2 and the metabolic pathways of ciprofol were found to be similar to propofol in previous studies (12, 26), and the extubation time was not significantly different between groups. In view of the baseline characteristics of enrolled patients, those admitted to the ciprofol group appeared to be elderly and had higher Sequential Organ Failure Assessment (SOFA) scores, which may explain the difference in recovery outcomes a finding that warrants further analysis.
Propofol-related hypertriglyceridemia has been frequently reported, mainly due to its formulation as a 10% oil-in-water lipid emulsion (27–29). In the present study, a significantly decreased triglyceride concentration was observed up to 24 hours after the end of ciprofol administration, but this may be clinically irrelevant and needs to be unequivocally established in subsequent studies.
The hemodynamic TEAEs observed, such as hypotension, respiratory depression, and bradycardia, have also been reported for other sedatives (30). The occurrence rate and duration of drug-related hemodynamic TEAEs appeared to be less in the ciprofol group, although these apparent differences did not reach statistical significance. Grade 3 drug-related hypotension (2.2% vs 4.4%) and allergic dermatitis (1.1% vs 0) occurred in two patients (2.2%) and 2 (4.4%) in the ciprofol and propofol groups, respectively, but all patients recovered or improved after discontinuation of drug administration. Of note, the vast majority of SAEs were associated with the patients’ primary disease, but one patient experienced serious respiratory depression (lasting for 2 min), which may have been related to the initiation of remifentanil 3 hours after the end of ciprofol administration. Ciprofol produced good hemodynamic profiles in former studies, such as less fluctuations in MAP, less prevalence of intraoperative hypotension, respiratory depression, and other intubation responses (11, 31–33). Generally speaking, ciprofol had a tolerable profile being a new choice for use in ICU sedation of Chinese patients receiving MV.
Another concern for ICU sedation is the occurrence of delirium, as it could significantly increase the mortality of ICU patients and prolong the ICU stay (34). Delirium was monitored twice a day by using the Confusion Assessment Method for the ICU test in the former phase 2 trial, and negative results were reported (17). Nevertheless, the limitation of the current study is that the delirium was not set as an endpoint during the initial study design. Although delirium was added to the secondary endpoints in the study protocol, based on a reviewer’s helpful comment during the publication process (18), given that the enrollment of patients was completed earlier than the expected date, we ultimately did not evaluate delirium, which clearly warrants further studies in the near future. Other limitations included the small sample size, unbalanced baseline age and SOFA between groups, the high percentage of postoperative ICU patients, and the lack of baseline Acute Physiology and Chronic Health Evaluation II scores.
Additionally, the duration of sedation was short (6–24 hr) in the present study, but 6–24 hours sedation will meet the sedation demand of most ICU patients from a clinical perspective. The short-term sedation could also be used for drug evaluation of efficacy and safety profiles in ICU patients, as shown in the dexmedetomidine trial (35). Furthermore, an exploratory study to investigate the long-term sedation of ciprofol in ICU patients undergoing MV (up to 96 hr) has been carried out (NCT04669821).
CONCLUSIONS
A continuous infusion of ciprofol was found to be noninferior compared with a continuous infusion of propofol in critically ill MV patients for a period of 6–24 hours.
Supplementary Material
Footnotes
Dr. X. Liu disclosed work for hire; she is an employee of Haisco Pharmaceutical Group Co., Ltd. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Drs. Y. Liu and Guan contributed to the study conception and design. Material preparation, data collection, and analysis were performed by all authors. The first draft of the article was written by Drs. Y. Liu and Guan, and all authors commented on previous versions of the article. All authors read and approved the final version of the submitted article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
The study was funded by Haisco Pharmaceutical Group Co., Ltd.
Drs. Y. Liu, Peng, S. Liu, and X. Yu contributed equally to the article.
Trial registration: ClinicalTrials.gov; NCT04620031. Registered November 6, 2020.
REFERENCES
- 1.Wu M, Yin X, Chen M, et al. : Effects of propofol on intracranial pressure and prognosis in patients with severe brain diseases undergoing endotracheal suctioning. BMC Neurol 2020; 20:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sahinovic MM, Struys M, Absalom AR: Clinical pharmacokinetics and pharmacodynamics of propofol. Clin Pharmacokinet 2018; 57:1539–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farhan M, Hoda MQ, Ullah H: Prevention of hypotension associated with the induction dose of propofol: A randomized controlled trial comparing equipotent doses of phenylephrine and ephedrine. J Anaesthesiol Clin Pharmacol 2015; 31:526–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mirenda J, Broyles G: Propofol as used for sedation in the ICU. Chest 1995; 108:539–548 [DOI] [PubMed] [Google Scholar]
- 5.Phillips AT, Deiner S, Mo Lin H, et al. : Propofol use in the elderly population: Prevalence of overdose and association with 30-day mortality. Clin Ther 2015; 37:2676–2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carson SS, Kress JP, Rodgers JE, et al. : A randomized trial of intermittent lorazepam versus propofol with daily interruption in mechanically ventilated patients. Crit Care Med 2006; 34:1326–1332 [DOI] [PubMed] [Google Scholar]
- 7.Jakob SM, Ruokonen E, Grounds RM, et al. ; Dexmedetomidine for Long-Term Sedation Investigators: Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: Two randomized controlled trials. JAMA 2012; 307:1151–1160 [DOI] [PubMed] [Google Scholar]
- 8.Qin L, Ren L, Wan S, et al. : Design, synthesis, and evaluation of novel 2,6-disubstituted phenol derivatives as general anesthetics. J Med Chem 2017; 60:3606–3617 [DOI] [PubMed] [Google Scholar]
- 9.Liao J, Li M, Huang C, et al. : Pharmacodynamics and pharmacokinetics of HSK3486, a novel 2,6-disubstituted phenol derivative as a general anesthetic. Front Pharmacol 2022; 13:830791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu C, Ou X, Teng Y, et al. : Sedation effects produced by a ciprofol initial infusion or bolus dose followed by continuous maintenance infusion in healthy subjects: A phase 1 trial. Adv Ther 2021; 38:5484–5500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei A, Yang L, Ma S, et al. : A case report of ciprofol overdose during anesthesia/analgesia and literature review: Clinical presentation, blood pressure, and management. J Int Med Res 2022; 50:3000605221132466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teng Y, Ou M, Wang X, et al. : Efficacy and safety of ciprofol for the sedation/anesthesia in patients undergoing colonoscopy: Phase IIa and IIb multi-center clinical trials. Eur J Pharm Sci 2021; 164:105904. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Wang X, Liu J, et al. : Comparison of ciprofol (HSK3486) versus propofol for the induction of deep sedation during gastroscopy and colonoscopy procedures: A multi-centre, non-inferiority, randomized, controlled phase 3 clinical trial. Basic Clin Pharmacol Toxicol 2022; 131:138–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo Z, Tu H, Zhang X, et al. : Efficacy and safety of HSK3486 for anesthesia/sedation in patients undergoing fiberoptic bronchoscopy: A multicenter, double-blind, propofol-controlled, randomized, phase 3 study. CNS drugs 2022; 36:301–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang P, Dai M, Wang X, et al. : Efficacy and safety of HSK3486 vs. propofol for the induction and maintenance of general anaesthesia: A multicentre, single-blind, randomised, parallel-group, phase 3 clinical trial. Eur J Anaesthesiol 2023; 40:399–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Wang X, Liu J, et al. : Effects of ciprofol for the induction of general anesthesia in patients scheduled for elective surgery compared to propofol: A phase 3, multicenter, randomized, double-blind, comparative study. Eur Rev Med Pharmacol Sci 2022; 26:1607–1617 [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Yu X, Zhu D, et al. : Safety and efficacy of ciprofol vs. propofol for sedation in intensive care unit patients with mechanical ventilation: A multi-center, open label, randomized, phase 2 trial. Chin Med J (Engl) 2022; 135:1043–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Chen C, Liu N, et al. : Efficacy and safety of ciprofol sedation in ICU patients with mechanical ventilation: A clinical trial study protocol. Adv Ther 2021; 38:5412–5423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Society Of Critical Care Medicine Chinese Medical Association: Guidelines for analgesia and sedation treatment in intensive care unit of Chinese adults. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2018; 30:497–514 [DOI] [PubMed] [Google Scholar]
- 20.Li Q, Wan X, Gu C, et al. : Pain assessment using the critical-care pain observation tool in Chinese critically ill ventilated adults. J Pain Symptom Manage 2014; 48:975–982 [DOI] [PubMed] [Google Scholar]
- 21.Devlin JW, Skrobik Y, Gélinas C, et al. : Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med 2018; 46:e825–e873 [DOI] [PubMed] [Google Scholar]
- 22.Aitkenhead AR, Pepperman ML, Willatts SM, et al. : Comparison of propofol and midazolam for sedation in critically ill patients. Lancet 1989; 2:704–709 [DOI] [PubMed] [Google Scholar]
- 23.Ruokonen E, Parviainen I, Jakob SM, et al. ; "Dexmedetomidine for Continuous Sedation" Investigators: Dexmedetomidine versus propofol/midazolam for long-term sedation during mechanical ventilation. Intensive Care Med 2009; 35:282–290 [DOI] [PubMed] [Google Scholar]
- 24.Greenblatt DJ, Harmatz JS, Zhang Q, et al. : Slow accumulation and elimination of diazepam and its active metabolite with extended treatment in the elderly. J Clin Pharmacol 2021; 61:193–203 [DOI] [PubMed] [Google Scholar]
- 25.West N, Görges M, Poznikoff A, et al. : Association of dexmedetomidine with recovery room and hospital discharge times: A retrospective cohort analysis. Paediatr Anaesth 2021; 31:1170–1178 [DOI] [PubMed] [Google Scholar]
- 26.Bian Y, Zhang H, Ma S, et al. : Mass balance, pharmacokinetics and pharmacodynamics of intravenous HSK3486, a novel anaesthetic, administered to healthy subjects. Br J Clin Pharmacol 2021; 87:93–105 [DOI] [PubMed] [Google Scholar]
- 27.Coetzee A, Blaine EM, Labadarios D, et al. : Effect of 1% and 2% propofol on blood lipids during longterm sedation. S Afr Med J 2002; 92:911–916 [PubMed] [Google Scholar]
- 28.Devaud JC, Berger MM, Pannatier A, et al. : Hypertriglyceridemia: A potential side effect of propofol sedation in critical illness. Intensive Care Med 2012; 38:1990–1998 [DOI] [PubMed] [Google Scholar]
- 29.Corrado MJ, Kovacevic MP, Dube KM, et al. : The incidence of propofol-induced hypertriglyceridemia and identification of associated risk factors. Crit Care Explor 2020; 2:e0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buckley MS, Agarwal SK, MacLaren R, et al. : Adverse hemodynamic events associated with concomitant dexmedetomidine and propofol for sedation in mechanically ventilated ICU patients. J Intensive Care Med 2020; 35:1536–1545 [DOI] [PubMed] [Google Scholar]
- 31.Chen X, Guo P, Yang L, et al. : Comparison and clinical value of ciprofol and propofol in intraoperative adverse reactions, operation, resuscitation, and satisfaction of patients under painless gastroenteroscopy anesthesia. Contrast Media Mol Imaging 2022; 2022:9541060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding YY, Long YQ, Yang HT, et al. : Efficacy and safety of ciprofol for general anaesthesia induction in elderly patients undergoing major noncardiac surgery: A randomised controlled pilot trial. Eur J Anaesthesiol 2022; 39:960–963 [DOI] [PubMed] [Google Scholar]
- 33.Qin K, Qin WY, Ming SP, et al. : Effect of ciprofol on induction and maintenance of general anesthesia in patients undergoing kidney transplantation. Eur Rev Med Pharmacol Sci 2022; 26:5063–5071 [DOI] [PubMed] [Google Scholar]
- 34.Klein Klouwenberg PM, Zaal IJ, Spitoni C, et al. : The attributable mortality of delirium in critically ill patients: Prospective cohort study. BMJ 2014; 349:g6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venn RM, Bradshaw CJ, Spencer R, et al. : Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia 1999; 54:1136–1142 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.