Abstract
Background:
Obesity is associated with an increased risk of developing cirrhosis. However, body mass index (BMI) and waist-to-hip ratio (WHR) may not be indicative of body composition parameters that predispose to cirrhosis. Bioimpedance analysis (BIA) is a noninvasive cost-efficient method for more detailed estimation of body composition.
Methods:
We examined patients with cirrhosis who underwent BIA as part of enrollment into a prospective cohort study. We examined the correlation between BIA variables, BMI, and WHR. We performed sex-adjusted and race-adjusted and race-specific multivariable logistic regression analyses to examine the association between anthropometric variables and risk factors [NAFLD, alcohol-associated liver disease (ALD), and HCV].
Results:
We analyzed data from 348 cirrhosis patients; 23.3% were women; 48.3% were non-Hispanic White; 19.3% were Hispanic; and 30.7% were African American. The cirrhosis etiology was 21.8% NAFLD, 56.9% HCV mostly cured, and 11.5% ALD. Several BIA variables correlated well with BMI, and others showed modest correlations, but none correlated well with WHR. Higher body fat mass and basal metabolic rate were positively associated, while higher lean body mass, dry lean mass, total body water, or skeletal muscle mass were negatively associated with NAFLD. Associations between these BIA parameters and ALD-related cirrhosis were in the opposite direction. These associations of BIA variables were seen only in Hispanic and non-Hispanic White patients but not non-Hispanic Blacks. BIA variables were more predictive of cirrhosis etiology than BMI or WHR.
Conclusions:
Among patients with cirrhosis, several BIA-derived measurements indicative of body fat and muscle are associated with NAFLD and ALD etiology. BIA variables show stronger associations, as well as race/ethnicity-specific associations, with cirrhosis etiology than those of BMI or WHR.
INTRODUCTION
In the United States, HCC-related mortality has remarkably increased during the past 3 decades.1 Most HCC cases arise in people with cirrhosis. Obesity defined by high body mass index (BMI) or waist-to-hip ratio (WHR) is a major component of metabolic syndrome, which has been associated with an increased risk of developing NAFLD-related cirrhosis and HCC, as well as increased risk of progression and decompensation of HCV, HBV, and alcohol-associated liver disease (ALD).2–5 However, given that two third of adults in the United States have BMI >25, it is important to identify additional and more precise biomarkers of metabolic dysfunction that can refine risk stratification and prognosis among patients with chronic liver disease.
BMI and WHR have limitations as risk stratification markers. Specifically, they may not be indicative of body composition parameters, such as the amount and distribution of fat and muscle mass, which may predispose to advanced liver disease.6 For example, several studies described a significant correlation between NAFLD and low muscle mass and/or high visceral fat mass.7 However, these studies were conducted in mostly Asian cohorts,7 and therefore, there is little information on the associations between these detailed anthropometric measures and advanced liver disease in Western populations. Furthermore, no study has compared anthropometric measures other than BMI and waist circumference in adults with different etiologies of advanced liver disease (eg, NAFLD and HCV). Last, there are major racial/ethnic differences in the risk of developing cirrhosis and HCC among patients with HCV or NAFLD in the United States, with Hispanics having the highest risk, followed by non-Hispanic Whites and non-Hispanic Blacks. It remains unclear whether the association between anthropometric measurements and risk of cirrhosis differs among the major racial/ethnic groups.
Bioimpedance refers to the property of biological tissues to impede an alternating electrical current. Bioimpedance analysis (BIA) is a noninvasive, valid, and cost-efficient method for estimating body composition by measuring resistance and reactance.8 In BIA, a fixed, low-voltage, high-frequency alternating current is introduced into the body to assess the body electrical conductivity and resistance. The body is modeled as 5 cylindrical compartments: the trunk and the four limbs. The impedance is proportional to the height and inversely proportional to the cross-sectional area of each compartment. Impedance is used to estimate muscle, fat, and water. Several studies focused on BIA measures as a prognostic factor in cirrhosis,9–13 but none examined the other BIA-detailed parameters as risk determinants for developing advanced liver disease.14,15
The aim of this study is to examine the association between BIA-derived and traditional (BMI, WHR) anthropometric variables and the underlying etiological risk factors in a large prospective cohort of patients with cirrhosis. We examined these associations accounting for differences in race and sex.
METHODS
Study population
We examined patients with cirrhosis recruited from 2 sites (Baylor College of Medicine and Michael E. DeBakey VA Medical Center in Houston) of the Texas Hepatocellular Carcinoma Consortium (THCCC).16,17 Patients in these 2 sites underwent BIA during the baseline recruitment visit between December 2016 and March 2022. Patients were scheduled for 6- to 12-month follow-up visits as part of clinical care and followed until HCC diagnosis, liver transplantation, or death through October 2022. Potential participants were identified in the weekly screening of scheduled patients. During the clinic visit, the study coordinators verified eligibility criteria, presented the study informed consent, and collected study data and specimens. Among 1067 enrolled patients, most (n = 719) did not have BIA performed due to patient burden and coordinator availability, and 403 (37.77%) agreed to have BIA performed. Among those who agreed to have BIA, we did not perform BIA in 55 (5.15%) patients who could not remove their socks and had amputations or pacemakers. We used 2 different BIA machines: an Inbody 520 from December 2016 to March 2021 for 194 patients at the Michael E. DeBakey VA Medical Center and an Inbody 570 from May 2018 to March 2022 for 154 patients at the Baylor College of Medicine and the Michael E. DeBakey VA Medical Center. Both these machines employ direct segmental multifrequency BIA technology to measure body segments. The variable outputs from these 2 BIA models are listed in Supplemental Table S1 (http://links.lww.com/HC9/A509). The research was conducted in accordance with both the Declarations of Helsinki and Istanbul. The research was approved by the Baylor College of Medicine IRB, and written consent was given by all patients to conduct this research.
Study variables
Cirrhosis was defined as a diagnosis based on histopathology, radiology, elastography, or serum markers of hepatic fibrosis (Fibrosis-4, aspartate aminotransferase to platelet ratio index, or FibroTest/FibroSure). Patients with uncontrolled hepatic decompensation, primary nonhepatic cancer, or a history of HCC were excluded. We used a validated survey to ascertain alcohol use that classified alcohol use status as lifetime abstention (never), former light to moderate use, former heavy use, current light to moderate, and current heavy use, as defined by the Centers for Disease Control and Prevention. We defined ALD based on a combination of clinician-recorded diagnoses of ALD and patients’ self-report of former heavy (8 or more alcoholic beverages per week for women or 15 or more alcoholic beverages per week for men) or any current use of alcohol. The diagnosis of NAFLD requires documentation of hepatic steatosis on liver histology or imaging. Given that hepatic steatosis cannot be reliably ascertained in the clinical setting of liver cirrhosis, we defined NAFLD as the possible etiology of cirrhosis for patients without HCV (active/untreated or resolved HCV), HBV, ALD, or other clinician-documented etiologies (such as autoimmune hepatitis, primary biliary cholangitis, primary sclerosing cholangitis, hereditary hemochromatosis, Wilson’s disease, and a few patients who were classified as having cryptogenic cirrhosis). Most (>90%) patients classified as NAFLD under this definition also had a clinician-recorded diagnosis of NAFLD. We defined diabetes and hypertension based on the patient’s medical history or treatment with diabetes medications or antihypertensives (survey or electronic medical records). HCV status was defined as negative, positive, or resolved based on laboratory values of positive HCV RNA (active), positive HCV antibodies with negative RNA (resolved), or negative antibodies and HCV RNA.
HCC was defined by the presence of a Liver Reporting & Data System (LI-RADS) LI-RAD 5 lesion during an imaging evaluation, a formal diagnosis by the site’s local multidisciplinary tumor board, or confirmation by biopsy. Subjects who were diagnosed with HCC within 30 days of their enrollment date were excluded from the analysis.
Statistical analysis
We converted all BIA-derived variables to standard form—variables were rescaled to have a mean of 0 and a SD of 1. This approach transformed BIA-derived variables into an identical scale making them simpler to compare and interpret. For variables with significant associations, we examined the tertile form of the variable. We then compared BIA-derived variables between groups of patients with cirrhosis defined based on the main 3 etiological risk factors (NAFLD, ALD, and HCV). We also compared these variables between patients with active HCV and cured HCV. We then compared these variables by age, sex, and major racial/ethnic groups (non-Hispanic Whites, non-Hispanic Blacks, and Hispanics) using the t test and ANOVA test. We performed multivariable logistic regression analyses to examine the association between BIA variables and each of the etiological risk factors adjusted for sex and race. We also conducted main analyses in a subgroup limited to patients without a history of hepatic decompensation.
RESULTS
We analyzed data from 348 patients with cirrhosis (Table 1). The mean age of patients was 62.7 years (SD, 8.5); 23.3% were women. The racial and ethnic distribution was 168 (48.3%) non-Hispanic White, 67 (19.3%) Hispanics, 107 (30.7%) non-Hispanic Blacks, and 6 patients (1.7%) belonging to other racial groups. Cirrhosis etiology at the time of enrollment was active HCV in 15 (4.3%), resolved HCV in 183 (52.6%), NAFLD in 76 (21.8%), ALD in 40 (11.5%), and other etiologies in 34 (9.8%) patients. During a follow-up, 24 patients developed HCC.
TABLE 1.
Characteristics of study population (N = 348)
| n (%) or Mean±SD | |
|---|---|
| Age | 62.73±8.54 |
| Age | |
| <55 | 45 (12.93) |
| 55 to <65 | 144 (41.38) |
| 65+ | 159 (45.69) |
| Sex | |
| Female | 81 (23.28) |
| Male | 267 (76.72) |
| Race and ethnicity | |
| NH-White | 168 (48.28) |
| NH-Black | 107 (30.75) |
| Hispanic | 67 (19.25) |
| Others | 6 (1.72) |
| BMI | 29.43±5.56 |
| WHR | 0.99±0.06 |
| MELD score | 9.05±2.73 |
| CPT score | 5.54±1.15 |
| Platelet counts | 151.13±72.09 |
| History of decompensation | |
| No | 295 (84.77) |
| Yes | 53 (15.23) |
Abbreviations: BMI, body mass index; CPT, Child-Pugh-Turcotte; MELD, Model for End-Stage Liver Disease; NH, non-Hispanic; WHR, waist-to-hip ratio.
BMI was slightly higher in patients with NAFLD than those without (30.50 vs. 29.07, respectively, p = 0.0347) and lower in those with ALD than those without (28.54 vs. 30.05, respectively, p = 0.0127). There were no significant differences in WHR according to HCV, ALD, NAFLD, or HCC status (Table 2).
TABLE 2.
Association between bioimpedance variables [standardized (mean = 0, SD = 1) variables] in subjects with cirrhosis and the presence of NAFLD-related, alcohol-associated liver disease (ALD)-related, and HCV-related cirrhosis
| NAFLD | ALD | HCV | ||||
|---|---|---|---|---|---|---|
| Bioimpedance variables | Crude OR (95% CI) | Adjusteda OR (95% CI) | Crude OR (95% CI) | Adjusteda OR (95% CI) | Crude OR (95% CI) | Adjusteda OR (95% CI) |
| Body fat mass (n = 348) | 1.35 (1.06–1.71) | 1.19 (0.91–1.55) | 0.69 (0.55–0.87) | 0.73 (0.57–0.93) | 0.74 (0.59–0.92) | 0.83 (0.65–1.06) |
| Lean body mass (n = 346) | 0.53 (0.41–0.69) | 1.14 (0.76–1.71) | 1.56 (1.24–1.97) | 0.98 (0.72–1.35) | 1.70 (1.35–2.14) | 1.00 (0.71–1.42) |
| Dry lean mass (n = 346) | 0.53 (0.41–0.69) | 1.10 (0.74–1.63) | 1.57 (1.24–1.97) | 1.01 (0.75–1.38) | 1.69 (1.34–2.13) | 0.99 (0.70–1.39) |
| Total body water (n = 348) | 0.54 (0.41–0.70) | 1.16 (0.77–1.74) | 1.56 (1.24–1.96) | 0.96 (0.70–1.32) | 1.70 (1.35–2.15) | 1.00 (0.71–1.42) |
| Lean body mass of right arm (n = 346) | 0.60 (0.41–0.90) | 1.08 (0.83–1.42) | 1.14 (0.92–1.42) | 0.84 (0.65–1.10) | 1.40 (1.05–1.87) | 0.98 (0.77–1.26) |
| Lean body mass of left arm (n = 346) | 0.52 (0.40–0.68) | 1.06 (0.72–1.56) | 1.42 (1.13–1.77) | 0.85 (0.62–1.15) | 1.76 (1.40–2.23) | 1.09 (0.78–1.52) |
| Lean body mass of trunk (n = 346) | 0.54 (0.41–0.71) | 1.09 (0.75–1.58) | 1.36 (1.09–1.70) | 0.82 (0.61–1.11) | 1.67 (1.33–2.11) | 1.02 (0.74–1.41) |
| Lean body mass of right leg (n = 346) | 0.56 (0.41–0.76) | 1.17 (0.85–1.60) | 1.45 (1.14–1.86) | 0.95 (0.72–1.26) | 1.68 (1.29–2.18) | 1.03 (0.77–1.38) |
| Lean body mass of left leg (n = 346) | 0.51 (0.38–0.68) | 1.04 (0.73–1.48) | 1.68 (1.30–2.15) | 1.13 (0.84–1.52) | 1.77 (1.37–2.27) | 1.07 (0.78–1.46) |
| Basal metabolic rate (n = 311) | 1.62 (1.23–2.12) | 1.50 (1.08–2.08) | 0.57 (0.45–0.72) | 0.61 (0.48–0.78) | 0.50 (0.39–0.64) | 0.54 (0.41–0.70) |
| Skeletal muscle mass (n = 154) | 0.56 (0.39–0.80) | 1.06 (0.58–1.94) | 1.43 (0.97–2.12) | 0.44 (0.20–0.93) | 1.30 (0.93–1.82) | 0.87 (0.48–1.58) |
| Visceral fat surface area (n = 154) | 1.25 (0.90–1.73) | 0.99 (0.68–1.43) | 0.53 (0.36–0.79) | 0.60 (0.39–0.92) | 0.85 (0.61–1.18) | 0.98 (0.68–1.40) |
| Body fat mass of right arm (n = 154) | 1.21 (0.88–1.67) | 1.06 (0.74–1.51) | 0.43 (0.24–0.77) | 0.50 (0.28–0.89) | 0.76 (0.53–1.10) | 0.83 (0.57–1.21) |
| Body fat mass of left arm (n = 154) | 1.20 (0.87–1.66) | 1.06 (0.74–1.50) | 0.44 (0.24–0.78) | 0.51 (0.29–0.91) | 0.75 (0.52–1.09) | 0.83 (0.57–1.21) |
| Body fat mass of right leg (n = 154) | 1.34 (0.97–1.85) | 1.08 (0.75–1.56) | 0.49 (0.30–0.79) | 0.57 (0.34–0.96) | 0.77 (0.54–1.09) | 0.88 (0.61–1.28) |
| Body fat mass of left leg (n = 154) | 1.33 (0.96–1.84) | 1.07 (0.74–1.54) | 0.48 (0.30–0.78) | 0.57 (0.34–0.96) | 0.78 (0.55–1.10) | 0.89 (0.61–1.29) |
| Body mass index | 1.29 (1.02–1.64) | 1.23 (0.94–1.60) | 0.75 (0.60–0.94) | 0.74 (0.58–0.94) | 0.82 (0.66–1.01) | 0.87 (0.68–1.10) |
| Waist-to-hip ratio | 0.94 (0.73–1.22) | 1.22 (0.90–1.65) | 0.98 (0.78–1.22) | 0.81 (0.63–1.03) | 1.01 (0.81–1.25) | 0.81 (0.62–1.06) |
Note: p<0.05.
Adjusted for sex and race.
There was a strong correlation between calculated BMI and BIA-measured body fat mass (total, each arm, and each leg) with correlation coefficients ranging between 0.81 and 0.93 and a modest correlation between calculated BMI and BIA-measured lean body mass with coefficients between 0.28 and 0.39 (Figure 1 and Supplemental Table S2, http://links.lww.com/HC9/A509). In contrast, WHR had weak or no correlation with BIA measurements (Figure 1 and Supplemental Table S2, http://links.lww.com/HC9/A509). Therefore, examining BIA variables could yield different associations with cirrhosis etiology than those of WHR.
FIGURE 1.
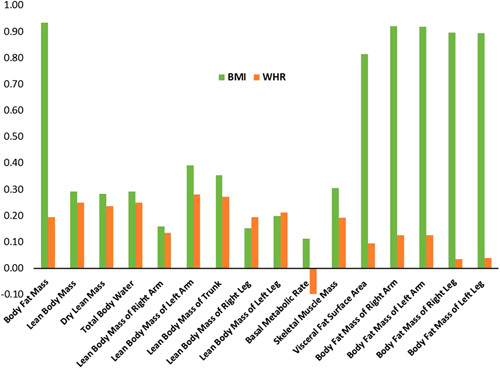
Correlation between bioimpedance analysis measurements, body mass index (BMI), and waist-to-hip ratio (WHR).
There were significant associations between sex and WHR, as well as most BIA variables. WHR, skeletal muscle mass, lean body mass, and dry lean mass were higher in men than women, whereas body fat mass and visceral fat area were higher in women (Supplemental Table S3, http://links.lww.com/HC9/A509). While there were no significant differences in BMI or WHR among the different racial groups, significant differences were detected for BIA variables. Compared with non-Hispanic Whites, Hispanics had lower BIA-measured lean body mass (overall, trunk, each arm, and each leg) and lower dry lean mass and skeletal muscle mass but higher body fat mass overall or in legs or arms (Supplemental Table S4, http://links.lww.com/HC9/A509). Conversely, non-Hispanic Blacks had higher lean body mass (overall, trunk, both arms, and both legs), dry lean mass, and skeletal muscle mass than non-Hispanic Whites. There were no significant associations between most BIA variables and age. Therefore, we adjusted the main analysis for sex and race/ethnicity and also presented sex-specific and race-specific associations.
Of the 16 BIA variables examined, higher body fat mass and higher basal metabolic rate were positively associated with NAFLD-related cirrhosis, whereas higher lean body mass (both arms, both legs, and trunk), higher dry lean mass, higher total body water, and higher skeletal muscle mass were negatively associated with NAFLD. However, after adjusting for sex and race, only the association with higher basal metabolic rate and NAFLD remained statistically significant (Table 2). Table 2 displays the ORs of BIA-derived measurements as continuous variables that are standardized or rescaled to have a mean of 0 and a SD of 1. For example, the adjusted OR associated with basal metabolic rate was 1.50 (95% CI: 1.08–2.08); this can be interpreted as each increase of 1 SD in basal metabolic rate is associated 50% increase in the odds of having NAFLD-related cirrhosis relative to having another cirrhosis etiology. Examining BIA variables in tertiles confirmed the association but also showed significant sex-adjusted and race-adjusted associations between total body fat mass in addition to basal metabolic rate and NAFLD. Among patients with cirrhosis, total body fat mass in the second and third tertiles was associated with an increase in the risk of having NAFLD with sex-adjusted and race-adjusted ORs of 3.55 (95% CI: 1.02–12.42) and 1.30 (95% CI: 0.40–4.23), respectively, compared with the lowest tertile. Race-specific associations in non-Hispanic Whites showed significant positive associations with total body fat mass and basal metabolic rate, and negative associations with lean body mass (total and in each leg), dry lean mass, and total body water (Table 3). Among Hispanics, NAFLD-related cirrhosis was positively associated with basal metabolic rate and negatively with lean body mass. There were no significant associations between BMI, WHR, or any BIA variables and NAFLD cirrhosis among non-Hispanic Black patients with cirrhosis. For example, among non-Hispanic Whites, the OR for the highest tertile group was 2.90 (95% CI: 1.18–7.13), and this is interpreted as individuals in the highest tertile of BIA-measured body fat mass had about 2.9 times the odd of having NAFLD-related cirrhosis compared with those in the lowest tertile. In contrast, there was no significant difference observed among non-Hispanic Blacks and a smaller difference among Hispanics.
TABLE 3.
Association between bioimpedance variables in subjects with cirrhosis and the presence of NAFLD by race/ethnicity
| Crude OR (95% CI) | |||
|---|---|---|---|
| Non-Hispanic Whites | Non-Hispanic Blacks | Hispanics | |
| Body fat mass | n = 168 | n = 107 | n = 67 |
| Continuous | 1.01 (1.00–1.02) | 0.97 (0.92–1.01) | 1.00 (0.99–1.02) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 3.38 (1.38–8.25) | 1.03 (0.14–7.75) | 1.44 (0.44–4.76) |
| Highest tertile | 2.90 (1.18–7.13) | 0.49 (0.04–5.61) | 1.32 (0.41–4.31) |
| Lean body mass | n = 168 | n = 105 | n = 67 |
| Continuous | 0.98 (0.97–1.00) | 1.02 (0.98–1.06) | 0.97 (0.94–0.99) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 0.49 (0.22–1.08) | 0.50 (0.04–5.79) | 0.16 (0.04–0.58) |
| Highest tertile | 0.36 (0.16–0.83) | 0.97 (0.13–7.30) | 0.11 (0.03–0.43) |
| Dry lean mass | n = 168 | n = 105 | n = 67 |
| Continuous | 0.93 (0.88–0.99) | 1.08 (0.92–1.26) | 0.88 (0.81–0.96) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 0.79 (0.36–1.71) | 0.47 (0.04–5.45) | 0.16 (0.04–0.58) |
| Highest tertile | 0.40 (0.17–0.95) | 1.03 (0.14–7.77) | 0.11 (0.03–0.43) |
| Total body water | n = 168 | n = 107 | n = 67 |
| Continuous | 0.98 (0.96–1.00) | 1.03 (0.97–1.09) | 0.95 (0.93–0.98) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 0.64 (0.29–1.39) | 0.49 (0.04–5.61) | 0.19 (0.05–0.69) |
| Highest tertile | 0.41 (0.18–0.94) | 0.94 (0.13–7.09) | 0.12 (0.03–0.46) |
| Lean body mass of right arm | n = 168 | n = 105 | n = 67 |
| Continuous | 0.96 (0.87–1.06) | 0.93 (0.63–1.38) | 0.83 (0.67–1.03) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 0.83 (0.38–1.81) | 1.00 (0.13–7.53) | 0.13 (0.03–0.49) |
| Highest tertile | 0.56 (0.24–1.28) | 0.49 (0.04–5.61) | 0.14 (0.04–0.52) |
| Lean body mass of left arm | n = 168 | n = 105 | n = 67 |
| Continuous | 0.82 (0.68–0.99) | 0.96 (0.55–1.67) | 0.66 (0.50–0.88) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 0.77 (0.35–1.67) | 0.30 (0.03–3.08) | 0.08 (0.02–0.33) |
| Highest tertile | 0.47 (0.20–1.08) | 0.32 (0.03–3.27) | 0.10 (0.03–0.42) |
| Lean body mass of trunk | n = 168 | n = 105 | n = 67 |
| Continuous | 0.97 (0.94–1.00) | 0.98 (0.90–1.07) | 0.94 (0.90–0.99) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 0.83 (0.38–1.82) | 0.30 (0.03–3.08) | 0.13 (0.03–0.49) |
| Highest tertile | 0.43 (0.19–1.00) | 0.32 (0.03–3.27) | 0.14 (0.04–0.52) |
| Lean body mass of right leg | n = 168 | n = 105 | n = 67 |
| Continuous | 0.95 (0.88–1.02) | 1.19 (0.93–1.53) | 0.81 (0.70–0.93) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 0.37 (0.17–0.85) | NA | 0.20 (0.06–0.72) |
| Highest tertile | 0.37 (0.17–0.85) | 1.55 (0.24–9.88) | 0.13 (0.04–0.50) |
| Lean body mass of left leg | n = 168 | n = 105 | n = 67 |
| Continuous | 0.91 (0.83–1.00) | 1.14 (0.88–1.48) | 0.80 (0.69–0.92) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 0.37 (0.17–0.85) | 0.46 (0.04–5.29) | 0.16 (0.04–0.58) |
| Highest tertile | 0.37 (0.17–0.85) | 0.97 (0.13–7.31) | 0.11 (0.03–0.43) |
| Basal metabolic rate | n = 150 | n = 92 | n = 63 |
| Continuous | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 4.21 (1.69–10.45) | 0.97 (0.06–16.19) | 6.40 (1.65–24.77) |
| Highest tertile | 1.77 (0.69–4.58) | 3.11 (0.30–31.68) | 1.00 (0.28–3.61) |
| Skeletal muscle mass | n = 84 | n = 26 | n = 41 |
| Continuous | 0.97 (0.94–1.01) | 1.01 (0.93–1.10) | 0.94 (0.89–0.99) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 1.33 (0.47–3.81) | NA | 0.33 (0.05–2.11) |
| Highest tertile | 0.31 (0.10–1.01) | 2.67 (0.19–36.76) | 0.10 (0.02–0.65) |
| Visceral fat surface area | n = 84 | n = 26 | n = 41 |
| Continuous | 1.05 (0.96–1.14) | 0.71 (0.50–1.02) | 1.04 (0.89–1.21) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 1.12 (0.37–3.43) | 0.31 (0.02–4.41) | 1.93 (0.39–9.60) |
| Highest tertile | 2.14 (0.75–6.14) | NA | 1.29 (0.29–5.77) |
| Body fat mass of right arm | n = 84 | n = 26 | n = 41 |
| Continuous | 1.08 (0.97–1.20) | 0.39 (0.14–1.10) | 0.93 (0.79–1.10) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 1.49 (0.50–4.41) | 0.33 (0.02–4.55) | 1.71 (0.37–7.92) |
| Highest tertile | 1.96 (0.66–5.86) | NA | 1.37 (0.29–6.53) |
| Body fat mass of left arm | n = 84 | n = 26 | n = 41 |
| Continuous | 1.08 (0.97–1.20) | 0.36 (0.12–1.10) | 0.93 (0.79–1.11) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 1.68 (0.55–5.08) | 0.44 (0.03–5.93) | 1.35 (0.29–6.18) |
| Highest tertile | 2.37 (0.78–7.20) | NA | 1.20 (0.26–5.59) |
| Body fat mass of right leg | n = 84 | n = 26 | n = 41 |
| Continuous | 1.08 (0.97–1.22) | 0.57 (0.30–1.10) | 1.03 (0.85–1.24) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 1.62 (0.53–4.94) | 0.50 (0.04–6.86) | 1.40 (0.30–6.62) |
| Highest tertile | 2.88 (0.95–8.72) | NA | 2.75 (0.58–12.98) |
| Body fat mass of left leg | n = 84 | n = 26 | n = 41 |
| Continuous | 1.08 (0.97–1.22) | 0.53 (0.26–1.08) | 1.03 (0.85–1.24) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 1.62 (0.53–4.94) | 0.50 (0.04–6.86) | 1.60 (0.35–7.40) |
| Highest tertile | 2.88 (0.95–8.72) | NA | 2.50 (0.52–11.93) |
| Body mass index | n = 168 | n = 107 | n = 67 |
| Continuous | 1.05 (0.99–1.12) | 0.94 (0.78–1.14) | 0.99 (0.91–1.08) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 2.72 (1.16–6.39) | 0.97 (0.13–7.30) | 0.76 (0.23–2.46) |
| Highest tertile | 2.19 (0.93–5.18) | 0.47 (0.04–5.45) | 1.09 (0.34–3.51) |
| Waist-to-hip ratio | n = 155 | n = 103 | n = 61 |
| Continuous | 0.26 (<0.001–66.33) | 188.00 (<0.001–>999) | 0.19 (<0.001–978.42) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 1.05 (0.46–2.40) | 1.94 (0.17–22.44) | 0.94 (0.28–3.14) |
| Highest tertile | 0.99 (0.42–2.35) | 2.13 (0.18–24.67) | 0.98 (0.27–3.52) |
Note: p<0.05.
Abbreviation: NA, not available; OR, Odds Ratio.
The associations between BIA parameters and ALD-related cirrhosis were generally in the opposite direction to those of NAFLD-related cirrhosis (Table 3). Sex-adjusted and race-adjusted models showed that higher body fat mass, higher basal metabolic rate, higher skeletal muscle mass, higher visceral fat surface area, higher body fat mass of both arms, and legs were less likely to be associated with ALD (Table 2). For example, an increase in 1 SD in body fat mass of the right arm was associated with 50% lower odds of having ALD (adjusted OR = 0.50, 95% CI: 0.28–0.89). Race-specific associations showed stronger associations between BIA-related variables and ALD in non-Hispanic Whites but weaker associations among Hispanics and no significant associations among non-Hispanic Blacks (Table 4).
TABLE 4.
Association between bioimpedance variables in subjects with cirrhosis and the presence of alcohol-associated liver disease–related cirrhosis by race/ethnicity
| Crude OR (95% CI) | |||
|---|---|---|---|
| Non-Hispanic Whites | Non-Hispanic Blacks | Hispanics | |
| Body fat mass | n = 168 | n = 107 | n = 67 |
| Continuous | 0.99 (0.98–1.00) | 0.98 (0.97–1.00) | 0.99 (0.97–1.01) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 0.60 (0.29–1.28) | 0.94 (0.37–2.40) | 0.82 (0.23–2.85) |
| Highest tertile | 0.38 (0.17–0.82) | 0.50 (0.19–1.30) | 0.93 (0.28–3.16) |
| Lean body mass | n = 168 | n = 105 | n = 67 |
| Continuous | 1.02 (1.00–1.03) | 1.00 (0.99–1.02) | 1.04 (1.01–1.06) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 1.47 (0.68–3.19) | 0.65 (0.25–1.71) | 5.33 (0.99–28.84) |
| Highest tertile | 2.27 (1.05–4.89) | 1.06 (0.42–2.70) | 14.44 (2.68–77.80) |
| Dry lean mass | n = 168 | n = 105 | n = 67 |
| Continuous | 1.08 (1.02–1.14) | 1.01 (0.94–1.09) | 1.16 (1.06–1.27) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 1.69 (0.78–3.67) | 0.67 (0.26–1.74) | 5.33 (0.99–28.84) |
| Highest tertile | 2.32 (1.06–5.05) | 1.06 (0.41–2.72) | 14.44 (2.68–77.80) |
| Total body water | n = 168 | n = 107 | n = 67 |
| Continuous | 1.03 (1.01–1.05) | 1.00 (0.98–1.03) | 1.05 (1.02–1.09) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 1.39 (0.64–3.01) | 0.79 (0.31–2.04) | 4.75 (0.88–25.64) |
| Highest tertile | 2.21 (1.02–4.77) | 1.01 (0.40–2.55) | 13.72 (2.54–74.13) |
| Lean body mass of right arm | n = 168 | n = 105 | n = 67 |
| Continuous | 1.02 (0.95–1.10) | 0.99 (0.87–1.14) | 1.09 (0.94–1.26) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 1.31 (0.61–2.79) | 0.63 (0.24–1.62) | 7.69 (1.45–40.91) |
| Highest tertile | 1.61 (0.75–3.45) | 0.56 (0.22–1.45) | 10.00 (1.87–53.48) |
| Lean body mass of left arm | n = 168 | n = 105 | n = 67 |
| Continuous | 1.24 (1.03–1.48) | 0.92 (0.73–1.17) | 1.56 (1.16–2.10) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 0.97 (0.46–2.07) | 0.53 (0.21–1.38) | 7.69 (1.45–40.91) |
| Highest tertile | 1.39 (0.65–2.94) | 0.66 (0.26–1.71) | 10.00 (1.87–53.48) |
| Lean body mass of trunk | n = 168 | n = 105 | n = 67 |
| Continuous | 1.03 (1.00–1.06) | 0.99 (0.95–1.03) | 1.06 (1.01–1.10) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 1.15 (0.53–2.47) | 0.48 (0.18–1.23) | 7.69 (1.45–40.91) |
| Highest tertile | 1.45 (0.69–3.07) | 0.52 (0.20–1.36) | 10.00 (1.87–53.48) |
| Lean body mass of right leg | n = 168 | n = 105 | n = 67 |
| Continuous | 1.05 (0.98–1.12) | 1.04 (0.93–1.16) | 1.27 (1.10–1.47) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 2.14 (0.98–4.63) | 1.00 (0.38–2.60) | 4.61 (1.05–20.30) |
| Highest tertile | 1.99 (0.92–4.31) | 1.42 (0.55–3.65) | 6.66 (1.53–29.08) |
| Lean body mass of left leg | n = 168 | n = 105 | n = 67 |
| Continuous | 1.13 (1.04–1.24) | 1.04 (0.93–1.16) | 1.28 (1.10–1.49) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 2.02 (0.92–4.41) | 1.02 (0.39–2.64) | 19.25 (2.21–168.01) |
| Highest tertile | 2.69 (1.23–5.87) | 1.20 (0.46–3.12) | 21.00 (2.39–184.51) |
| Basal metabolic rate | n = 150 | n = 92 | n = 63 |
| Continuous | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 0.14 (0.06–0.35) | 1.07 (0.39–2.93) | 0.08 (0.01–0.43) |
| Highest tertile | 0.34 (0.15–0.78) | 0.47 (0.16–1.34) | 0.38 (0.11–1.31) |
| Skeletal muscle mass | n = 84 | n = 26 | n = 41 |
| Continuous | 1.03 (0.99–1.07) | 0.98 (0.92–1.05) | 1.05 (0.99–1.11) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 1.25 (0.33–4.71) | 1.00 (0.14–7.10) | 0.92 (0.05–16.46) |
| Highest tertile | 2.56 (0.74–8.81) | 0.29 (0.02–3.52) | 4.80 (0.46–50.15) |
| Visceral fat surface area | n = 84 | n = 26 | n = 41 |
| Continuous | 0.87 (0.79–0.96) | 0.93 (0.78–1.12) | 0.88 (0.71–1.10) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 0.47 (0.15–1.53) | 0.67 (0.09–5.13) | NA |
| Highest tertile | 0.17 (0.04–0.70) | 0.15 (0.01–1.90) | 0.35 (0.05–2.31) |
| Body fat mass of right arm | n = 84 | n = 26 | n = 41 |
| Continuous | 0.78 (0.65–0.94) | 0.85 (0.60–1.20) | 0.90 (0.66–1.22) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 0.81 (0.27–2.44) | 0.25 (0.03–2.00) | NA |
| Highest tertile | 0.14 (0.03–0.74) | 0.14 (0.01–1.76) | 0.41 (0.06–2.77) |
| Body fat mass of left arm | n = 84 | n = 26 | n = 41 |
| Continuous | 0.79 (0.65–0.95) | 0.85 (0.60–1.21) | 0.91 (0.68–1.23) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 0.77 (0.25–2.32) | 0.36 (0.05–2.77) | 0.28 (0.03–3.11) |
| Highest tertile | 0.13 (0.03–0.67) | 0.18 (0.02–2.12) | 0.67 (0.09–4.80) |
| Body fat mass of right leg | n = 84 | n = 26 | n = 41 |
| Continuous | 0.81 (0.69–0.96) | 0.89 (0.65–1.20) | 0.85 (0.61–1.18) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 0.42 (0.13–1.37) | 1.20 (0.16–8.80) | NA |
| Highest tertile | 0.26 (0.07–0.95) | 0.25 (0.02–3.04) | 0.38 (0.06–2.54) |
| Body fat mass of left leg | n = 84 | n = 26 | n = 41 |
| Continuous | 0.81 (0.69–0.95) | 0.89 (0.65–1.21) | 0.84 (0.60–1.18) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 0.42 (0.13–1.37) | 1.20 (0.16–8.80) | NA |
| Highest tertile | 0.26 (0.07–0.95) | 0.25 (0.02–3.04) | 0.42 (0.06–2.77) |
| Body mass index | n = 168 | n = 107 | n = 67 |
| Continuous | 0.94 (0.89–1.00) | 0.94 (0.87–1.02) | 1.00 (0.91–1.09) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 0.80 (0.38–1.68) | 0.85 (0.33–2.15) | 1.96 (0.56–6.92) |
| Highest tertile | 0.44 (0.20–0.94) | 0.67 (0.26–1.73) | 1.62 (0.45–5.78) |
| Waist-to-hip ratio | n = 155 | n = 103 | n = 61 |
| Continuous | 1.68 (0.01–270.02) | 0.02 (<0.001–14.17) | 33.41 (0.005–>999.999) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 0.85 (0.39–1.83) | 0.72 (0.28–1.85) | 1.24 (0.34–4.50) |
| Highest tertile | 0.95 (0.43–2.09) | 0.94 (0.36–2.45) | 1.48 (0.39–5.71) |
Note: p<0.05.
Abbreviation: NA, not available; OR, Odds Ratio.
Patients with cirrhosis and active or cured HCV had lower body fat mass, lower basal metabolic rate, higher total body water, and higher lean body mass (overall, each arm, each leg, and trunk) than patients without HCV, but these differences were mitigated after accounting for sex and race (Table 2). These associations were seen in non-Hispanic Whites and Hispanics, but there were no significant associations between BIA and HCV status among non-Hispanic Blacks (Table 5).
TABLE 5.
Association between bioimpedance variables in subjects with cirrhosis and the presence of HCV by race/ethnicity
| Crude OR (95% CI) | |||
|---|---|---|---|
| Non-Hispanic Whites | Non-Hispanic Blacks | Hispanics | |
| Body fat mass | n = 168 | n = 107 | n = 67 |
| Continuous | 0.99 (0.98–1.00) | 1.00 (0.98–1.02) | 0.99 (0.97–1.01) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 0.33 (0.15–0.72) | 0.75 (0.18–3.06) | 0.82 (0.23–2.85) |
| Highest tertile | 0.45 (0.21–0.96) | 0.63 (0.16–2.43) | 0.93 (0.28–3.16) |
| Lean body mass | n = 168 | n = 105 | n = 67 |
| Continuous | 1.02 (1.00–1.03) | 1.00 (0.98–1.03) | 1.02 (1.00–1.04) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 1.44 (0.68–3.07) | 4.74 (0.93–24.24) | 4.07 (0.93–17.84) |
| Highest tertile | 2.22 (1.04–4.74) | 1.84 (0.54–6.29) | 6.33 (1.45–27.73) |
| Dry lean mass | n = 168 | n = 105 | n = 67 |
| Continuous | 1.06 (1.01–1.13) | 1.00 (0.91–1.11) | 1.09 (1.00–1.18) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 1.22 (0.58–2.57) | 5.04 (0.99–25.70) | 4.07 (0.93–17.84) |
| Highest tertile | 1.94 (0.91–4.14) | 1.72 (0.50–5.90) | 6.33 (1.45–27.73) |
| Total body water | n = 168 | n = 107 | n = 67 |
| Continuous | 1.02 (1.00–1.04) | 1.00 (0.97–1.04) | 1.03 (1.00–1.06) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 1.36 (0.64–2.88) | 2.67 (0.63–11.31) | 3.60 (0.82–15.74) |
| Highest tertile | 2.16 (1.01–4.61) | 1.60 (0.46–5.61) | 6.00 (1.36–26.37) |
| Lean body mass of right arm | n = 168 | n = 105 | n = 67 |
| Continuous | 1.02 (0.95–1.10) | 1.19 (0.89–1.58) | 1.14 (0.97–1.34) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 1.39 (0.66–2.94) | 1.94 (0.51–7.33) | 4.07 (0.93–17.84) |
| Highest tertile | 1.85 (0.87–3.94) | 1.94 (0.51–7.33) | 6.33 (1.45–27.73) |
| Lean body mass of left arm | n = 168 | n = 105 | n = 67 |
| Continuous | 1.20 (1.01–1.43) | 1.20 (0.85–1.70) | 1.36 (1.04–1.78) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 1.30 (0.61–2.74) | 3.26 (0.79–13.50) | 7.69 (1.45–40.91) |
| Highest tertile | 2.00 (0.94–4.25) | 2.22 (0.60–8.22) | 10.00 (1.87–53.48) |
| Lean body mass of trunk | n = 168 | n = 105 | n = 67 |
| Continuous | 1.02 (0.99–1.05) | 1.03 (0.98–1.09) | 1.04 (1.00–1.09) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 1.33 (0.62–2.84) | 3.26 (0.79–13.50) | 4.07 (0.93–17.84) |
| Highest tertile | 1.90 (0.90–4.01) | 2.22 (0.60–8.22) | 6.33 (1.45–27.73) |
| Lean body mass of right leg | n = 168 | n = 105 | n = 67 |
| Continuous | 1.08 (1.00–1.15) | 0.97 (0.84–1.14) | 1.14 (1.00–1.29) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 1.95 (0.91–4.17) | 3.41 (0.64–18.25) | 2.71 (0.68–10.84) |
| Highest tertile | 2.60 (1.21–5.58) | 0.83 (0.25–2.77) | 4.75 (1.21–18.58) |
| Lean body mass of left leg | n = 168 | n = 105 | n = 67 |
| Continuous | 1.10 (1.01–1.19) | 0.99 (0.85–1.16) | 1.16 (1.01–1.32) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 1.67 (0.78–3.54) | 2.36 (0.54–10.30) | 2.40 (0.60–9.56) |
| Highest tertile | 1.92 (0.90–4.09) | 1.04 (0.30–3.60) | 4.50 (1.15–17.68) |
| Basal metabolic rate | n = 150 | n = 92 | n = 63 |
| Continuous | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 0.27 (0.12–0.61) | 1.04 (0.19–5.59) | 0.18 (0.04–0.71) |
| Highest tertile | 0.37 (0.17–0.84) | 0.32 (0.08–1.35) | 0.30 (0.08–1.08) |
| Skeletal muscle mass | n = 84 | n = 26 | n = 41 |
| Continuous | 1.02 (0.98–1.05) | 0.99 (0.93–1.05) | 1.00 (0.94–1.06) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 0.60 (0.19–1.90) | 1.00 (0.14–7.10) | 4.80 (0.46–50.14) |
| Highest tertile | 1.35 (0.46–3.96) | 0.50 (0.07–3.55) | 2.00 (0.16–25.11) |
| Visceral fat surface area | n = 84 | n = 26 | n = 41 |
| Continuous | 0.98 (0.90–1.07) | 1.10 (0.92–1.30) | 0.97 (0.80–1.18) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 1.15 (0.39–3.43) | 0.60 (0.08–4.40) | 1.65 (0.23–11.99) |
| Highest tertile | 0.66 (0.22–1.98) | 3.00 (0.35–25.87) | 0.85 (0.10–7.04) |
| Body fat mass of right arm | n = 84 | n = 26 | n = 41 |
| Continuous | 0.96 (0.85–1.07) | 1.10 (0.85–1.44) | 0.87 (0.63–1.19) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 1.10 (0.37–3.23) | 0.90 (0.13–6.08) | 1.38 (0.19–9.83) |
| Highest tertile | 0.76 (0.24–2.35) | 1.00 (0.13–7.57) | 1.00 (0.12–8.42) |
| Body fat mass of left arm | n = 84 | n = 26 | n = 41 |
| Continuous | 0.95 (0.85–1.07) | 1.12 (0.85–1.48) | 0.85 (0.61–1.18) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 1.04 (0.35–3.07) | 1.00 (0.16–6.42) | 0.61 (0.09–4.37) |
| Highest tertile | 0.68 (0.22–2.11) | 2.40 (0.30–19.04) | 0.67 (0.09–4.80) |
| Body fat mass of right leg | n = 84 | n = 26 | n = 41 |
| Continuous | 0.97 (0.86–1.09) | 1.14 (0.87–1.51) | 0.82 (0.60–1.13) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 0.86 (0.29–2.54) | 2.40 (0.30–19.04) | 1.22 (0.20–7.59) |
| Highest tertile | 0.62 (0.20–1.89) | 1.00 (0.16–6.42) | 0.26 (0.02–2.88) |
| Body fat mass of left leg | n = 84 | n = 26 | n = 41 |
| Continuous | 0.97 (0.86–1.09) | 1.16 (0.87–1.54) | 0.82 (0.59–1.14) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 0.86 (0.29–2.54) | 2.40 (0.30–19.04) | 1.10 (0.18–6.76) |
| Highest tertile | 0.62 (0.20–1.89) | 1.00 (0.16–6.42) | 0.28 (0.03–3.11) |
| Body mass index | n = 168 | n = 107 | n = 67 |
| Continuous | 0.98 (0.93–1.04) | 1.00 (0.90–1.11) | 0.98 (0.89–1.07) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 0.48 (0.22–1.02) | 1.03 (0.27–3.94) | 0.88 (0.25–3.03) |
| Highest tertile | 0.76 (0.36–1.58) | 1.03 (0.27–3.94) | 1.07 (0.32–3.63) |
| Waist-to-hip ratio | n = 155 | n = 103 | n = 61 |
| Continuous | 0.67 (0.004–104.50) | 0.08 (<0.001–608.39) | 7.76 (0.001–>999.999) |
| Lowest tertile | 1 | 1 | 1 |
| Middle tertile | 0.91 (0.42–1.94) | 0.48 (0.11–2.11) | 1.79 (0.51–6.34) |
| Highest tertile | 1.12 (0.51–2.48) | 0.54 (0.12–2.48) | 1.17 (0.30–4.59) |
Note: p<0.05.
Abbreviation: OR, Odds Ratio.
We have repeated the main analyses excluding 53 patients (15.2% of the study cohort) with any history of decompensation. The main findings did not change; data were not shown.
DISCUSSION
In this study of patients with cirrhosis, we examined the association between BIA measurements with the underlying etiology of cirrhosis, correlated the BIA findings with BMI and WHR, and evaluated these associations in different racial/ethnic groups. The major findings are given as follows: (1) several BIA variables are correlated with BMI, whereas others show modest correlations; (2) none of the BIA variables correlated well with WHR; (3) BIA-measured body fat variables were positively associated, whereas those of muscle mass were inversely associated with NAFLD, and the opposite associations were observed for ALD; (4) in general, BIA variables were more predictive of cirrhosis etiology than BMI or WHR; and (5) BIA variables’ associations with cirrhosis etiology were race/ethnic group-specific; they were seen only in Hispanic and non-Hispanic White patients but not in non-Hispanic Blacks.
BMI and WHR are the most commonly used measures of body fat in studies examining the risk and prognosis of liver disease, including NAFLD, cirrhosis, and HCC. However, the findings of these epidemiological studies, for example, those in HCC risk, were remarkably heterogeneous, with some showing a positive association with BMI and others showing no association or even an inverse association. Part of this inconsistency is likely related to BMI being a “distal” measure of body composition that does not reflect the key specific drivers of the relationship between body fat and liver disease. In the context of liver disease, we believe that WHR is a distal measure. It reflects variable proportions of subcutaneous and visceral obesity, as well as variable proportions of white and brown fat; these components have different etiological relevance to the formation or progression of liver disease. In addition, WHR is prone to random measurement errors (eg, placement of tape), which makes it unreliable. Therefore, examining more “proximal” relevant measures of body fat or muscle would reveal more consistent associations and serve as reliable measures of risk and prognosis of liver disease. In this study, BIA provides some advantages over BMI and WHR that could hold promise for both research and clinical care.
BIA offers a more detailed and nuanced understanding of the association between the amount and distribution of fat and muscle body components with the risk of liver disease than BMI or WHR. The modest correlation between BMI and several BIA parameters indicative of lean body mass and the lack of correlation with WHR suggest that BIA parameters reflect different aspects of body composition, and they could add to the predictive ability of BMI and WHR. In this study, BIA measures showed specific significant associations with the etiology of cirrhosis: a positive association with body fat mass and a negative association with lean body mass for NAFLD and opposite findings for ALD. Moreover, these associations had major sex-specific and race-specific differences that were not captured by BMI or WHR. The findings were also robust and persisted after excluding 53 patients with any history of decompensation.
Our study adds evidence to the importance of total body fat to the pathogenesis of NAFLD. Excess body fat is an established risk factor for the presence and severity of NAFLD. A cross-sectional study in The Netherlands used dual x-ray absorptiometry scans to measure body composition and concluded that a higher total fat mass was associated with a higher risk of NAFLD18 in both normal-weight and overweight populations. Another retrospective cohort study using BIA showed that higher total fat mass and lower skeletal muscle mass are associated with a higher NAFLD risk, especially in normal-weight individuals.19 Other studies also have shown that lower lean muscle mass was associated with higher NAFLD risk and severity.20
BIA needs to be further examined as a prognostic tool in patients with liver disease. Several investigations showed a correlation between the decrease in phase angle and muscle mass (a calculated value from BIA) and the development of hepatic encephalopathy in the course of liver cirrhosis.10,21,22 We explored the association between BIA measures and the risk of developing HCC in patients with cirrhosis, and while some weak associations can be seen, the number of newly developed HCC cases precluded a robust adequately powered analysis.
The study’s strengths included the large sample size with well-defined cirrhosis etiology and diverse racial/ethnic backgrounds, and uniform prospective BIA, BMI, and WHR measurements. However, the study was underpowered to examine the determinants of HCC due to the limited number of cases. BIA measurements have been shown in different studies to correlate well with those of computed tomography scans and dual x-ray absorptiometry.23,24 The indicators associated with BIA are affected by the patients’ hydration status and distribution of intracellular and extracellular water25,26; however, the multifrequency BIA analysis employed in our study is less influenced by overhydration.
Among patients with cirrhosis, several BIA-derived measurements indicative of body fat and muscle are associated with NAFLD and ALD etiology. BIA variables show stronger associations, as well as race/ethnicity-specific associations, with cirrhosis etiology than those of BMI or WHR. Therefore, BIA may offer a more precise tool to better understand the association between body composition and the development of chronic liver disease and cancer.
Supplementary Material
Footnotes
Abbreviations: ALD, alcohol-associated liver disease; BIA, bioimpedance analysis; BMI, body mass index; CPT, Child-Pugh-Turcotte; LI-RADS, Liver Reporting & Data System; MELD, Model for End-Stage Liver Disease; NA, not available; NH, non-Hispanic; THCCC, Texas Hepatocellular Carcinoma Consortium Cohort; WHR, waist-to-hip ratio.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.hepcommjournal.com.
Contributor Information
Hashem B. El-Serag, Email: hasheme@bcm.edu.
Ghida Akhdar, Email: ghida.akhdar@hotmail.com.
Aaron P. Thrift, Email: Aaron.Thrift@bcm.edu.
Michelle Luster, Email: Michelle.Luster@bcm.edu.
Saira Khaderi, Email: Saira.Khaderi@bcm.edu.
Abeer Alsarraj, Email: alsarraj@bcm.edu.
Hao Duong, Email: hao.duong@bcm.edu.
Fasiha Kanwal, Email: kanwal@bcm.edu.
AUTHOR CONTRIBUTIONS
All authors wrote and reviewed the article.
FUNDING INFORMATION
This work was supported in part by the Cancer Prevention & Research Institute of Texas grant (RP150587) and the NCI (NCI P01 CA263025, NCI U01 CA230997, and R01CA186566), and in part by the Center for Gastrointestinal Development, Infection and Injury (NIDDK P30 DK 56338). Fasiha Kanwal and Hashem B. El-Serag are investigators at IQuESt (CIN 13-413), Michael E. DeBakey VA Medical Center, Houston, Texas.
CONFLICTS OF INTEREST
The authors have no conflicts to report.
REFERENCES
- 1. McGlynn KA, Petrick JL, El‐Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73(suppl 1):4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shin HS, Jun BG, Yi SW. Impact of diabetes, obesity, and dyslipidemia on the risk of hepatocellular carcinoma in patients with chronic liver diseases. Clin Mol Hepatol. 2022;28:773–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leslie J, Geh D, Elsharkawy AM, Mann DA, Vacca M. Metabolic dysfunction and cancer in HCV: shared pathways and mutual interactions. J Hepatol. 2022;77:219–236. [DOI] [PubMed] [Google Scholar]
- 4. Dhanasekaran R, Felsher DW. A tale of two complications of obesity: NASH and hepatocellular carcinoma. Hepatology. 2019;70:1056–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gupta A, Das A, Majumder K, Arora N, Mayo HG, Singh PP, et al. Obesity is independently associated with increased risk of hepatocellular cancer-related mortality: a systematic review and meta-analysis. Am J Clin Oncol. 2018;41:874–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. El-Serag HB, Kanwal F. Obesity and hepatocellular carcinoma: hype and reality. Hepatology. 2014;60:779–781. [DOI] [PubMed] [Google Scholar]
- 7. Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43(suppl 1):S99–S112. [DOI] [PubMed] [Google Scholar]
- 8. Grover I, Singh N, Gunjan D, Pandey RM, Chandra Sati H, Saraya A. Comparison of anthropometry, bioelectrical impedance, and dual-energy X-ray absorptiometry for body composition in cirrhosis. J Clin Exp Hepatol. 2022;12:467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pagano AP, Sicchieri JMF, Schiavoni IL, Barbeiro D, Manca CS, da Silva BR, et al. Phase angle as a severity indicator for liver diseases. Nutrition. 2020;70:110607. [DOI] [PubMed] [Google Scholar]
- 10. Belarmino G, Gonzalez MC, Torrinhas RS, Sala P, Andraus W, D’Albuquerque LAC, et al. Phase angle obtained by bioelectrical impedance analysis independently predicts mortality in patients with cirrhosis. World J Hepatol. 2017;9:401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Román E, Poca M, Amorós-Figueras G, Rosell-Ferrer J, Gely C, Nieto JC, et al. Phase angle by electrical bioimpedance is a predictive factor of hospitalisation, falls and mortality in patients with cirrhosis. Sci Rep. 2021;11:20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Okubo S, Shindoh J, Kobayashi Y, Umino R, Akabane M, Kojima K, et al. Adipose tissue distribution predicts prognosis of cirrhotic patients undergoing hepatectomy for hepatocellular carcinoma. Ann Surg Oncol. 2021;28:6738–6746. [DOI] [PubMed] [Google Scholar]
- 13. Nishikawa H, Enomoto H, Iwata Y, Nishimura T, Iijima H, Nishiguchi S. Clinical utility of bioimpedance analysis in liver cirrhosis. J Hepatobiliary Pancreat Sci. 2017;24:409–416. [DOI] [PubMed] [Google Scholar]
- 14. Nishida Y, Ide Y, Okada M, Otsuka T, Eguchi Y, Ozaki I, et al. Effects of home-based exercise and branched-chain amino acid supplementation on aerobic capacity and glycemic control in patients with cirrhosis. Hepatol Res. 2017;47:E193–E200. [DOI] [PubMed] [Google Scholar]
- 15. Nishikawa H, Enomoto H, Ishii A, Iwata Y, Miyamoto Y, Ishii N, et al. Comparison of prognostic impact between the Child-Pugh Score and skeletal muscle mass for patients with liver cirrhosis. Nutrients. 2017;9:595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kanwal F, Khaderi S, Singal AG, Marrero JA, Loo N, Asrani SK, et al. Risk factors for HCC in contemporary cohorts of patients with cirrhosis. Hepatology. 2023;77:997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. El-Serag HB, Kanwal F, Feng Z, Marrero JA, Khaderi S, Singal AG. Risk Factors for cirrhosis in contemporary hepatology practices-findings from the Texas Hepatocellular Carcinoma Consortium Cohort. Gastroenterology. 2020;159:376–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alferink LJM, Trajanoska K, Erler NS, Schoufour JD, Knegt RJ, Ikram MA, et al. Nonalcoholic fatty liver disease in the rotterdam study: about muscle mass, sarcopenia, fat mass, and fat distribution. J Bone Miner Res. 2019;34:1254–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ariya M, Koohpayeh F, Ghaemi A, Osati S, Davoodi SH, Razzaz JM, et al. Assessment of the association between body composition and risk of non-alcoholic fatty liver. PLoS One. 2021;16:e0249223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lu FB, Hu ED, Xu LM, Chen L, Wu JL, Li H, et al. The relationship between obesity and the severity of non-alcoholic fatty liver disease: systematic review and meta-analysis. Expert Rev Gastroenterol Hepatol. 2018;12:491–502. [DOI] [PubMed] [Google Scholar]
- 21. Ontanilla-Clavijo G, Ampuero J, Borreguero S, Rosell J, Romero-Gómez M. Usefulness of bioelectrical impedance analysis for monitoring patients with refractory ascites. Rev Esp Enferm Dig. 2019;111:223–227. [DOI] [PubMed] [Google Scholar]
- 22. Peres WA, Lento DF, Baluz K, Ramalho A. Phase angle as a nutritional evaluation tool in all stages of chronic liver disease. Nutr Hosp. 2012;27:2072–2078. [DOI] [PubMed] [Google Scholar]
- 23. Zopfs D, Theurich S, Große Hokamp N, Knuever J, Gerecht L, Borggrefe J, et al. Single-slice CT measurements allow for accurate assessment of sarcopenia and body composition. Eur Radiol. 2020;30:1701–1708. [DOI] [PubMed] [Google Scholar]
- 24. Achamrah N, Colange G, Delay J, Rimbert A, Folope V, Petit A, et al. Comparison of body composition assessment by DXA and BIA according to the body mass index: a retrospective study on 3655 measures. PLoS One. 2018;13:e0200465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dey DK, Bosaeus I. Comparison of bioelectrical impedance prediction equations for fat-free mass in a population-based sample of 75 y olds: the NORA study. Nutrition. 2003;19:858–864. [DOI] [PubMed] [Google Scholar]
- 26. Norman K, Wirth R, Neubauer M, Eckardt R, Stobäus N. The bioimpedance phase angle predicts low muscle strength, impaired quality of life, and increased mortality in old patients with cancer. J Am Med Dir Assoc. 2015;16:173.e17–173.e22. [DOI] [PubMed] [Google Scholar]


