Abstract
Background:
Ferroptosis is a unique form of regulated cell death that provided a new opportunity for cancer therapy. Ferroptosis suppressor protein 1 (FSP1) is a key regulator in the NAD(P)H/FSP1/CoQ10 antioxidant system, which sever as an oxide redox enzyme to scavenge harmful lipid hydroperoxides and escape from ferroptosis in cells. This study aimed to investigate the role of FSP1 on sorafenib-induced ferroptosis and disclosed the underlying mechanisms.
Methods:
Cell viability, malondialdehyde (MDA), glutathione (GSH), and lipid reactive oxygen species levels were assessed using indicated assay kits. The levels of FSP1 and glutathione peroxidase 4 (GPX4) in the patients with HCC were analyzed based on the database. Western blot and quantitative real-time PCR were performed to detect the protein and mRNA expression. Co-immunoprecipitation was applied to detect the interaction between proteins. Tumor xenograft experiments were used to evaluate whether overexpression of FSP1-inhibited sorafenib-induced ferroptosis in vivo.
Results:
We verified that sorafenib-induced ferroptosis in HCC. Furthermore, we found that sorafenib decreased the protein level of FSP1, and knockdown FSP1 rendered HCC cells susceptible to sorafenib-induced ferroptosis. Co-immunoprecipitation and ubiquitination assays showed that sorafenib accelerated the TRIM54-mediated FSP1 ubiquitination and degradation. Sorafenib-induced ferroptosis was abrogated by TRIM54 suppression. Mechanically, sorafenib-promoted TRIM54 ubiquitinated and degraded FSP1 by means of the ERK pathway. Moreover, FSP1 enhanced tumor development and decreased HCC cellular susceptibility to sorafenib in vivo.
Conclusions:
Sorafenib facilitated the TRIM54-mediated FSP1 ubiquitination through the ERK pathway, thereby inducing ferroptosis in HCC cells.
INTRODUCTION
HCC is a primary liver cancer characterized by a low survival rate and a poor prognosis.1 Heredity, chronic hepatitis B and HCV infection, aflatoxin exposure, smoking, alcohol abuse, and obesity are considered the major risk factors for developing HCC.2 The treatments for early-stage HCC mostly rely on surgical resection and liver transplantation.3 While most patients with advanced-stage HCC always lose the best time for surgery and resort to nonsurgical treatments including immunotherapy, chemotherapeutic treatment, and targeted therapy.4 Sorafenib is a first-line medication licensed by the Food and Drug Administration for the treatment of unresectable HCC.5 It is a multitargeted kinase inhibitor that impairs cancer cell proliferation and neoangiogenesis.6 However, most patients respond unsatisfactorily to sorafenib, which is a critical hindrance to achieving the expected anticancer efficacy.7 Therefore, expounding the mechanisms of sorafenib resistance and optimizing personalized therapy is of paramount importance in the progress of HCC therapy.
In 2012, Dixon et al8 found an unprecedented cell death form named ferroptosis. Emergence of ferroptosis has provided a new model of regulatory cell death. Mechanically, excessive iron-dependent lipid peroxide generation is the essential driver of ferroptosis.8,9 It is generally believed that the high proliferation rate of cancer cells due to their metabolic aberration is accompanied by an increase in lipid peroxidation.10 To alleviate oxidative stress, cancer cells depend on the antioxidant system to interfere with lipid peroxide generation and escape from death.11 Therefore, ferroptosis resistance becomes a considerable obstacle to the eradication of cancer cells.12 In the context of the imbalance between the antioxidant system and oxidative stress, dampening the antioxidant defense system and inducing cancer cell ferroptosis is a feasible cancer treatment strategy.13 Existing studies have illustrated that sorafenib-induced cancer cells ferroptosis.14 In HCC cells, system Xc− is implicated in ferroptosis and is possibly a promising target of sorafenib-induced ferroptosis15; however, the enigmatic underlying mechanisms are still ambiguous.
Apoptosis-inducing factor mitochondria associated 2 (AIFM2) located in the mitochondria of the cell and was involved in inducing cell apoptosis by means of the caspase-independent pathway.16 Currently, convincing studies suggested that AIFM2 played an essential role in the modulation of another cell death process ferroptosis, and was redefined as ferroptosis suppressor protein 1 (FSP1).17 FSP1 functions as an oxide redox enzyme that impedes the accumulation of lipid peroxides by decreasing coenzyme Q10 (CoQ) and conduces to lipophilic radical-trapping antioxidant generation.17,18 FSP1 is independent of the mitochondrial CoQ antioxidant system and cooperates with the glutathione (GSH)-based glutathione peroxidase 4 (GPX4) pathway to induce ferroptosis.17,18 Beyond that, FSP1-mediated lipid peroxidation by means of endosomal sorting complexes required for transport-III induced membrane repair mechanism and inhibited ferroptosis in a CoQ-independent pathway.19 FSP1 is an essential component of ferroptosis resistance; however, it is not clear whether FSP1 participates in sorafenib-induced ferroptosis.
The tripartite motif (TRIM) family belongs to the class of single protein RING-finger E3 ubiquitin ligases.20 The expression and activity of the TRIM family members are correlated with the development of cancers, TRIM family members bind to the different substrates determining their distinct cellular regulatory functions.21 Due to the frequent dysregulation of TRIM family members, the TRIM proteins sever as tumor promoters or tumor suppressors in various tumors.22,23 In this study, we found that sorafenib could downregulate FSP1 expression in HCC cells. As an E3 ligase, TRIM54 bound to FSP1 and promoted its ubiquitination degradation. Sorafenib promoted the interaction between TRIM54 and FSP1 through the ERK pathway, thereby inducing ferroptosis.
METHODS
Cell culture
Human HCC cell lines (HepG2 and Huh-7) were purchased from the Cell Bank of the Chinese Academy of Science (Shanghai, China). Cells were cultured in DMEM supplemented with 10% fetal bovine serum (Gibco, NY), 1% antibiotic/antimycotic solution, and maintained at 37°C with 5% CO2.
Cell transfection
The negative control siRNA (si-NC), 2 individual FSP1 siRNAs (si-FSP1#1, 5′-CCAAAUCAGUGGCUUCUAUTT-3′, si-FSP1#2, 5′-UUUCUUACUCGGUGACUUUTT-3′), and si-TRIM54 (5′-CCUGCUAGUGGAGAACAUUTT-3′) were obtained from GenePharma (Shanghai, China). The siRNA sequences were transfected in HCC cells using siLentFect Lipid Reagent (Bio-Rad, Hercules, CA). The plasmids were transiently transfected into HCC cells using jetPRIME transfection reagent (Polyplus Transfection, Bioparc, France). The pcDNA3.1 FSP1, LV-NC, and LV-FSP1 were purchased from GenePharma (Shanghai, China).
Immunoblotting and co-immunoprecipitation
Cells were harvested and lysed in RIPA lysis buffer (KeyGen BioTECH, Jiangsu, China), then protein was quantified using the Enhanced BCA Protein Assay Kit (KeyGen BioTECH, Jiangsu, China) and boiled in SDS-PAGE loading buffer. Cell protein lysates were resolved by SDS-PAGE, then transferred to nitrocellulose membrane and subsequently blocked with 5% skim milk. Add primary antibody and incubate for 8 hours. After being washed and incubated with secondary antibodies, the protein signals were detected by means of Chemistar High-sig ECL Western Blot Substrate (Tanon, Shanghai, China). For co-immunoprecipitation, 1 μg specific primary antibodies were added to cell protein lysates. After incubating at 4°C for 10 hours, 30 μL of the resuspended volume of Protein A + G-Agarose (Bioepitope, Minneapolis, MN) was added and rotated together with the sample at 4°C for 6 hours. Beads were boiled in a loading buffer, and the immunoprecipitated proteins were tested by Western blot.
Antibodies and reagents
Antibodies used in this study include anti-GAPDH (60004-1-Ig, Proteintech), anti-FSP1 (20886-1-AP, Proteintech), anti-GPX4 (ab125066, Abcam), anti-TFRC (A5865, ABclonal), anti-SLC7A11 (A2413, ABclonal), anti-TRIM54 (21074-1-AP, Proteintech), anti-ERK (#4695, Cell Signaling Technology), anti-ubiquitin (sc-9133, Santa Cruz Biotechnology), and horseradish peroxidase-conjugated secondary antibodies (VA001 and VA002, Vicmed). Reagents used in this study include Sorafenib (T0093L, Targetmol), Ferrostatin-1 (A4371, APE × BIO), Z-VAD-FMK (A1902, APE × BIO), Necrosulfonamide (B7731, APE × BIO), cycloheximide (C7698-1G, SIGMA), MG-132 (A2585, APE × BIO), and PD98059 (HY-12028, MedChemExpress).
Cell viability assay
Cell proliferation was gauged with the Cell Counting Kit-8 (CCK8) kit (APE × BIO). The optical density was measured at 450 nm.
Malondialdehyde and GSH assay
The relative malondialdehyde (MDA) concentration in cell or tumor lysates was determined by a MDA Colorimetric Assay Kit (E-BC-K025-M, Elabscience). The relative GSH concentration in cell or tumor lysates was measured using a Reduced GSH Colorimetric Assay Kit (E-BC-K030-M, Elabscience).
Lipid peroxidation assay C11-BODIPY
Post-treatment cells were incubated in 2 µM C11 BODIPY 581/591 (27086-1mg, Cayman) for 30 minutes. Then cells were harvested and resuspended in PBS containing 5% fetal bovine serum. Fluorescence intensity was detected by flow cytometry (Becton Dickinson, Franklin Lakes, NJ). For C11-BODIPY staining, cells were plated in coverslips. Cells were stained with 2 μM C11 BODIPY for 30 minutes. Images were acquired using microscopy (ZEISS AXIO Scope.A1 HAL100).
Quantitative real-time PCR
Total RNA was isolated with the TRIzol reagent (Takara, Dalian, China) and reverse transcribed using PrimeScript RT reagent Kit (Takara, Dalian, China). Quantitative real-time PCR was used in the BrightGreen Express 2 × qPCR MasterMix (Abm, Canada). The relative gene expression levels were normalized to GAPDH and calculated by means of the 2−ΔΔCT method.
Colony formation assay
Equal numbers of cells were seeded in a 6-well plate and cultured with medium for 14 days. Removed the medium, the cells were fixed and stained with 0.1% crystal violet (Vicmed, China).
Tumor xenograft experiments and immunohistochemistry
All mice experiments were strictly followed by means of the ARRIVE (Animals in Research: Reporting In Vivo Experiments) guidelines and approved by the ethics committee of Xuzhou Medical University. The female 6-week-old BALB/c nude mice were injected subcutaneously with indicated HepG2 cells (2 × 106 cells per mouse) and treated with or without sorafenib (10 mg/kg i.p., once every other day). Tumors were measured every 5 days and calculated the volume of the tumor was according to the formula (length × width2)/2. After 28 days, the neoplasms were harvested for the subsequent experiment. Tissue sections from the indicated nude mouse models and subjected to immunohistochemistry staining using anti-FSP1 antibodies. All mice were housed in a specific pathogen‐free animal facility with free access to water and food.
Statistical analysis
Statistical analysis was performed by SPSS 21.0 software (SPSS, USA), and images were acquired with GraphPad Prism 7.0 software (La Jolla). The significance of the differences between the groups was evaluated using Student t test or 1-way ANOVA, and the correlation analyses were evaluated using Pearson correlation analyses, with p < 0.05 defined as statistically significant (*p < 0.05, **p < 0.01, ***p < 0.001).
RESULTS
Sorafenib downregulates FSP1 protein levels in HCC cells
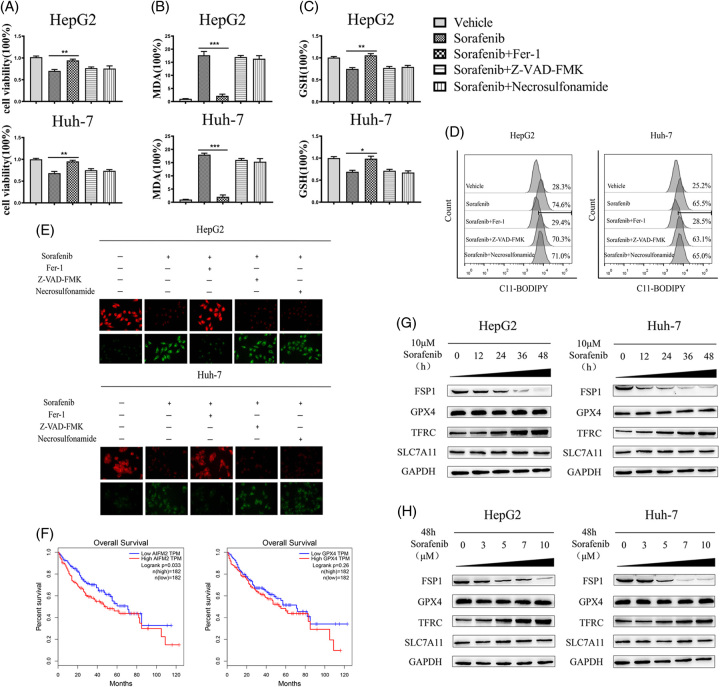
Sorafenib has been reported to promote HCC cell ferroptosis in prior investigations.24 We first measured the effect of various inhibitors on the cell survival of sorafenib-treated HCC cells to confirm the type of cell death. Our results revealed that Ferrostatin-1 (a ferroptosis inhibitor) but not Z-VAD-FMK (an apoptosis inhibitor) or necrosulfonamide (a necroptosis inhibitor) impaired sorafenib-induced HCC cell death (Figure 1A). The amassing of lipid peroxide and the depletion of GSH were characteristic events during ferroptosis.8 We then examined the characteristic manifestations of the ferroptosis process. On sorafenib treatment, lipid peroxidation product MDA levels increased and GSH levels decreased. As expected, the typical series of ferroptotic events were reversed by ferrostatin-1 rather than Z-VAD-FMK and necrosulfonamide (Figure 1B, C). In addition, we used C11-BODIPY 581/591 (a lipid peroxidation fluorescent probe) to treat HCC cells and evaluate the intracellular lipid reactive oxygen species content. Flow cytometry results revealed that the maximum emission fluorescence migrated from 590 nm (nonoxidized state) to 510 nm (oxidized state) under the induction of sorafenib (Figure 1D). Meanwhile, C11-BODIPY fluorescence staining indicated that the red fluorescence (nonoxidized lipids) became weaker, and the green fluorescence (oxidized lipids) became stronger in the cells of the sorafenib-treated group (Figure 1E). Hence, sorafenib facilitated the generation of lipid reactive oxygen, and the sorafenib-induced ferroptotic events were only reversed by Fer-1 in HCC cells (Figure 1D, E). These assays all further confirmed that the mode of sorafenib-induced HCC cell death was ferroptosis.
FIGURE 1.
Sorafenib downregulates FSP1 in HCC cells. (A–E) HCC cells were treated with sorafenib (10 μM), with or without the indicated inhibitors (ferrostatin-1, 1 μM; Z-VAD-FMK, 10 μM; necrosulfonamide, 10 μM) for 24 hours, then cell viability (A); MDA levels (B); GSH levels (C); C11-BODIPY flow cytometry analysis (D); and C11-BODIPY stain (× 400 magnification) (E) were assayed. (F) The Kaplan-Meier survival curves for patients with HCC were generated from the TCGA database based on FSP1 and GPX4 expression levels. (G and H) HCC cells were treated with 10 μM sorafenib at 0, 12, 24, 36, 48 hours or treated with 0, 3, 5, 7, 10 μM sorafenib at 48 hours. The levels of proteins were detected by western blot analysis. The data are the means ± SDs of the 3 independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001. Abbreviations: FSP1, ferroptosis suppressor protein 1; GPX4, glutathione peroxidase 4; GSH, glutathione; MDA, malondialdehyde; SLC7A11, solute carrier family 7 member 11; TFRC, transferrin receptor.
FSP1, a GSH-independent ferroptosis suppressor, functions as an oxidoreductase in the nonmitochondrial CoQ antioxidant system.17,18 We further analyzed the levels of FSP1 and GPX4 in the patients with HCC (http://gepia.cancer-pku.cn/index.html) and found that the high level of FSP1 was associated with poor overall survival (Figure 1F). Then, we tested the protein levels of FSP1 and several proteins associated with ferroptosis including GPX4, transferrin receptor (TFRC), and solute carrier family 7 member 11 (SLC7A11) following sorafenib treatment. The protein level of TFRC was markedly upregulated which accord with the preceding study.25 The expression of GPX4 and SLC7A11 had no significant changes. Notably, sorafenib decreased FSP1 expression at the protein levels in time-dependent and dose-dependent manners (Figure 1G, H). Thus, these indicated that sorafenib attenuated FSP1 expression in time‐dependent and dose‐dependent manners in human HCC cells.
The expression of FSP1 affects sorafenib-induced ferroptosis
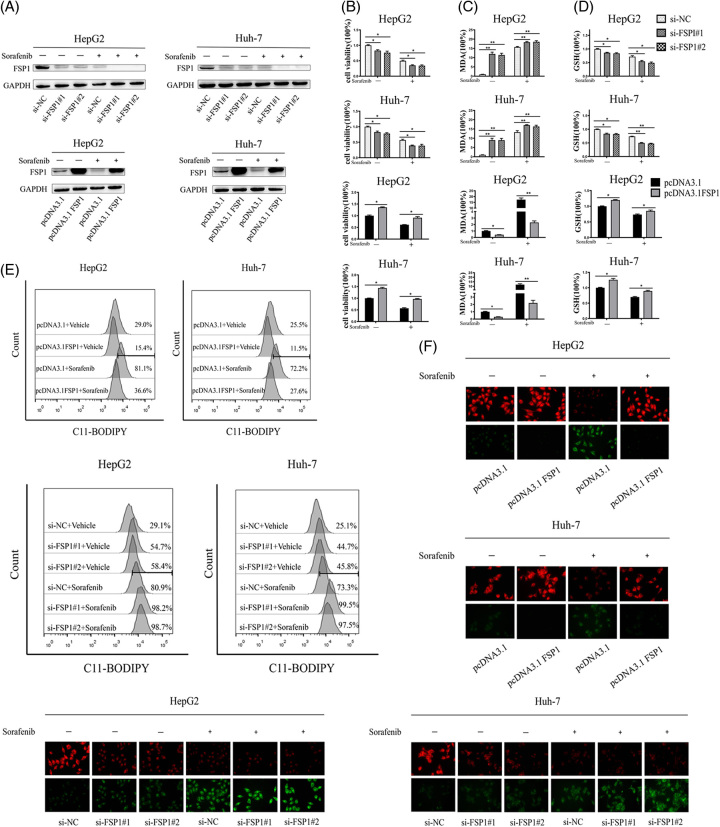
Next, we further investigate whether FSP1 was engaged in sorafenib-induced ferroptosis. Based on the above experiments, the expression of FSP1 was significantly downregulated under the condition of 10 μM sorafenib treated for 36 hours, and thus subsequent experiments were performed with this treatment condition. Western blot assay was conducted to determine the interference and overexpression efficiency of FSP1 in HCC cells (Figure 2A). Subsequently, we found that overexpression of FSP1 impeded ferroptosis, and partially reversed the sorafenib-induced ferroptosis (Figure 2B–F). In contrast, HCC cells were more sensitive to sorafenib-induced ferroptosis when FSP1 was suppression (Figure 2B–F). Thus, a high level of FSP1 restrained sorafenib-induced ferroptosis, and silencing FSP1 enhanced the cellular sensitivity to ferroptosis triggered by sorafenib. Together, it implied that the expression of FSP1 affected sorafenib-induced ferroptosis and sorafenib-promoted ferroptosis by downregulating FSP1 in HCC cells.
FIGURE 2.
The expression of FSP1 affects sorafenib-induced ferroptosis. (A–F) The specified plasmids or short interfering RNAs were transiently transfected into HCC cells, which were subsequently treated with sorafenib or without sorafenib (10 μM, 36 h). The levels of the indicated proteins (A); cell viability (B); MDA levels (C); GSH levels (D); C11-BODIPY flow cytometry analysis (E); and C11-BODIPY stain (× 400 magnification) (F) were assayed. The data are the means ± SD of the 3 independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001. Abbreviation: FSP1, ferroptosis suppressor protein 1.
Sorafenib accelerates FSP1 ubiquitination degradation by promoting the interaction between TRIM54 and FSP1
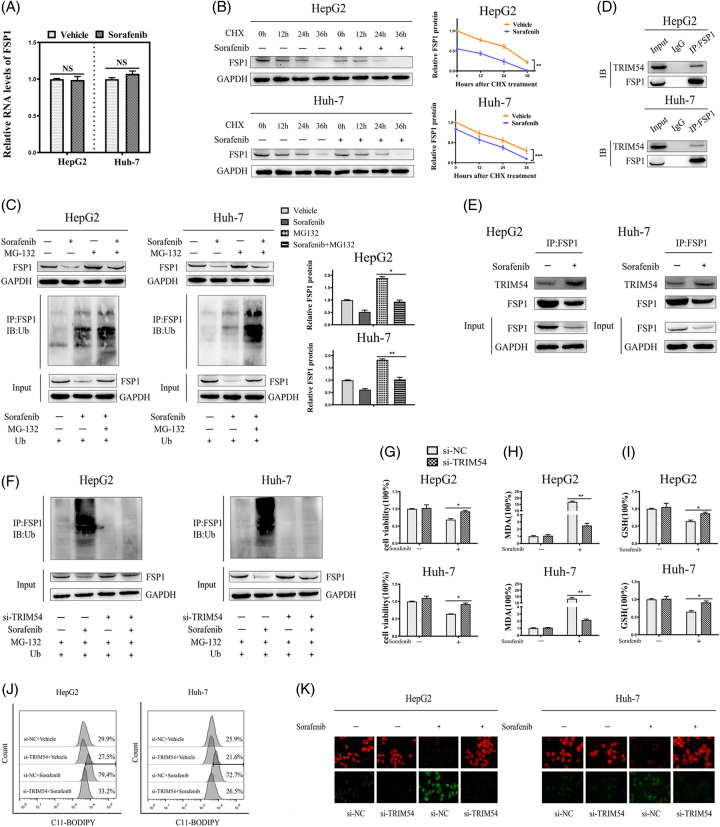
To get a further understanding of the mechanism underlying sorafenib reducing the level of FSP1, we detected the mRNA levels of FSP1 in the sorafenib-treated group and control group. We found no significant differences in the mRNA levels of FSP1 between the 2 groups (Figure 3A), which hinted that the reduction of FSP1 might be regulated at the post-transcriptional level rather than the transcriptional level. Next, we used cycloheximide to inhibit protein synthesis and then observed that sorafenib treatment significantly reduced the half-life of FSP1 (Figure 3B). As shown in Figure 3C, MG132 (a classic 26S proteasome inhibitor) restored FSP1 protein levels that had been decreased by sorafenib, and the ubiquitination assays indicated that sorafenib reduced the FSP1 level by inducing the ubiquitination of FSP1 (Figure 3C). These results demonstrated that sorafenib regulated FSP1 expression through the ubiquitination system.
FIGURE 3.
Sorafenib accelerates FSP1 ubiquitination degradation by promoting the interaction between TRIM54 and FSP1. (A) HCC cells were treated with vehicle or sorafenib (10 μΜ, 36 h), and the mRNA levels of FSP1 were assayed by RT‐qPCR. (B) Effect of sorafenib (10 μΜ, 36 h) on FSP1 stability in HCC cells treated with cycloheximide (30 μg/mL) at 0, 12, 24, 36 hours. (C) HCC cells were transient transfected with Ub plasmid, then treated with sorafenib (10 µM 36 h) and MG132 (100 μM 4 h). Lysates from HCC cells were immunoprecipitated with an anti-FSP1 antibody. The protein expression and ubiquitination degree were determined by western blotting. (D) The co-immunoprecipitation (Co-IP) assay was used to determine the interaction of FSP1 with TRIM54 in FSP1-overexpressing HCC cells. (E) The Co-IP assay was used to determine the interaction of FSP1 with TRIM54 in FSP1-overexpressing HCC cells treated with or without sorafenib (10 μM, 36 h). (F) HCC cells were cotransfected with Ub plasmid and si-NC or Ub plasmid and si-TRIM54, then treated with or without sorafenib (10 μM, 36 h). HCC cells were treated with MG132 (100 μM) for 4 hours before harvest. The levels of proteins and ubiquitination were assayed by western blot. (G–K) HCC cells were transfected with si-NC or si-TRIM54 for 24 hours, then treated with or without sorafenib (10 μM, 36 h); cell viability (G); MDA levels (H); GSH levels (I); C11-BODIPY flow cytometry analysis (J); and C11-BODIPY stain (×400 magnification) (K) were assayed. The data are the means ± SD of the 3 independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001. Abbreviations: FSP1, ferroptosis suppressor protein 1; GSH, glutathione; MDA, malondialdehyde; TRIM, tripartite motif.
Next, we used the public bioinformatics databases uniport and hitpredict to predict that TRIM54 might bind to FSP1. TRIM54, belonging to the TRIM family, functions as an E3 ubiquitin ligase because it harbors a RING-finger domain.26 We speculated that TRIM54 functions as an actual E3 ubiquitin ligase for the ubiquitination of FSP1. To confirm the relationship between TRIM54 and FSP1, we did co-immunoprecipitation assays in FSP1-overexpression HCC cells and detected an interaction between TRIM54 and FSP1 (Figure 3D). Moreover, the interaction of FSP1 and TRIM54 was markedly intensified by sorafenib (Figure 3E). Knockdown of TRIM54 significantly reduced the ubiquitination of FSP1 in sorafenib-treated HCC cells (Figure 3F). Additionally, sorafenib-induced classical ferroptotic events were abolished in TRIM54-knockdown cells (Figure 3G–K). Collectively, these data demonstrated that TRIM54-mediated ubiquitination of FSP1 was essential for sorafenib-induced ferroptosis, in detail, sorafenib degraded FSP1 by promoting the interaction between the TRIM54 and FSP1, thereby inducing ferroptosis.
Sorafenib promotes the interaction between FSP1 and TRIM54 by means of the ERK pathway
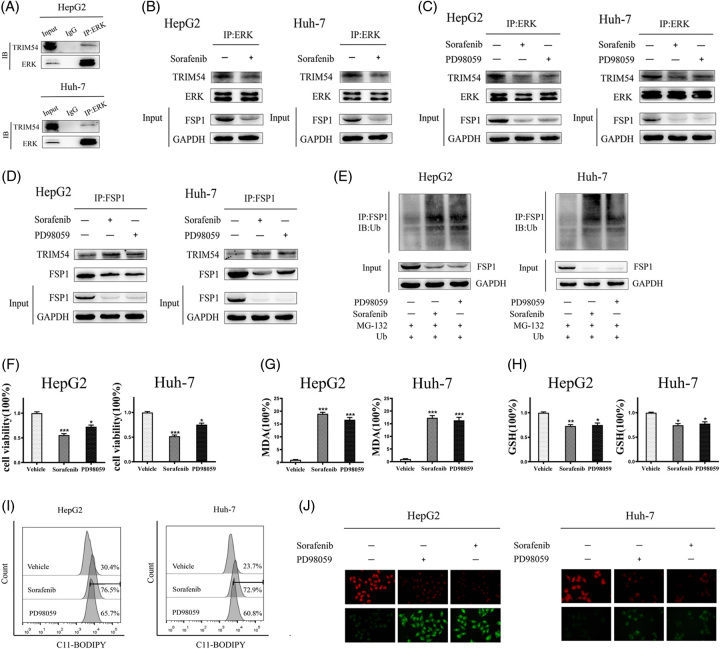
Sorafenib is a multiple-target kinase inhibitor, targeting the Ras/Raf/MEK/ERK signaling pathways to eradicate cancer cells.27 Since the MAPK/ERK signaling pathway is an integral part of the modulation of cellular transformation and carcinogenesis, its activated state is interwoven with more than half of human HCC cases.28 Hence, we assumed that sorafenib may regulate TRIM54 through the ERK pathway. Co-immunoprecipitation analyses demonstrated an interaction between ERK and TRIM54 (Figure 4A), and sorafenib significantly interfered with this interaction (Figure 4B). It was tempting to infer that ERK bound and modified TRIM54 and the modified TRIM54 failed to spark FSP1 ubiquitination. To determine whether sorafenib regulates the ubiquitinase activity of TRIM54 through the ERK pathway, we treated HCC cells with PD98059 (an ERK inhibitor). Similar to sorafenib, PD98059 inhibited the interaction of ERK and TRIM54 (Figure 4C). Besides, PD98059 promoted the interaction of TRIM54 and FSP1, then facilitated the ubiquitination of FSP1 (Figure 4D, E). These results illustrated that sorafenib regulated the interaction between TRIM54 and FSP1 through the ERK kinase pathway and promoted the ubiquitination of FSP1.
FIGURE 4.
Sorafenib promotes the interaction between FSP1 and TRIM54 by means of the ERK pathway. (A) The co-immunoprecipitation assay was used to determine the interaction of ERK with TRIM54 in HCC cells. (B) The interaction between ERK and TRIM54 in HCC cells treated with or without sorafenib (10 μM, 36 h). (C) The interaction between ERK and TRIM54 in HCC cells treated with vehicle, sorafenib (10 μM), or PD98059 (10 μM) for 36 hours. (D) The interaction between FSP1 and TRIM54 in FSP1-overexpression HCC cells treated with vehicle, sorafenib (10 μM), or PD98059 (10 μM) for 36 hours. (E) HCC cells were transient transfected with Ub plasmid for 12 hours, then treated with vehicle, sorafenib (10 μM), or PD98059 (10 μM) for 36 hours. HCC cells were treated with MG132 (100 μM) for 4 hours before harvest. The levels of proteins and ubiquitination were assayed by western blot. (F–J) HCC cells were treated with vehicle, sorafenib (10 μM) or PD98059 (10 μM) for 36 hours, cell viability (F); MDA levels (G); GSH levels (H); C11-BODIPY flow cytometry analysis (I); and C11-BODIPY stain (× 400 magnification) (J) were assayed. The data are the means ± SD of the 3 independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001. Abbreviations: FSP1, ferroptosis suppressor protein 1; TRIM, tripartite motif.
We further examined the effect of PD98059 on ferroptosis and found that PD98059 induced cell death, elevated MDA levels, and dropped GSH levels (Figure 4F–H). The lipid ROS assay also displayed that PD98059 promoted the manufacture of lipid ROS (Figure 4I, J). The results of these ferroptosis-related events tended to imply that PD98059, like sorafenib, decoyed ferroptosis in HCC cells. Altogether, we concluded that sorafenib-induced ferroptosis by promoting the interaction between FSP1 and TRIM54 through the ERK kinase pathway.
FSP1 promoted tumor growth and enhanced the resistance to sorafenib-induced ferroptosis in vivo
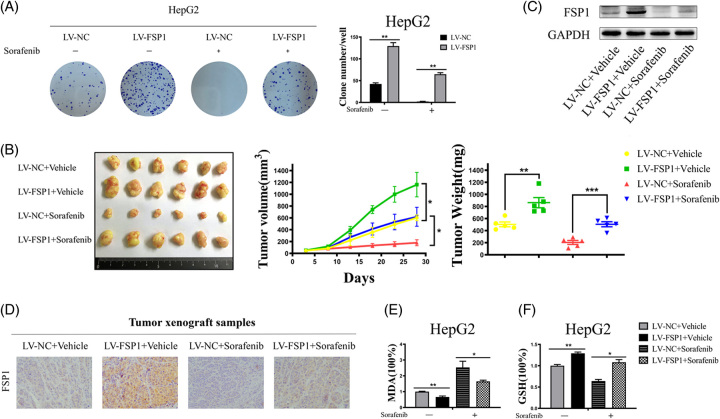
To determine whether the level of FSP1 modulates sorafenib sensitivity in HCC cells, we stably upregulated FSP1 in HCC cells by a lentiviral vector. As expected, we observed that FSP1-overexpressing promoted cell proliferation and significantly impaired sorafenib-induced cell death by colony formation assays (Figure 5A). To evaluate whether overexpression of FSP1 inhibited sorafenib-induced ferroptosis in vivo, FSP1 stably overexpressed HCC cells were injected subcutaneously into nude mice, and the tumors were removed from the mice after 28 days. Visually, the tumor volumes of the LV-FSP1 group were larger than the LV-NC group in both sorafenib-treated and control groups (Figure 5B). We recorded the volume of tumors every 5 days, and the results showed that FSP1 promoted the growth of tumors (Figure 5B). In addition, in the sorafenib treatment group, FSP1 reduced the sensitivity of the tumors to sorafenib and contributed to the growth of tumors (Figure 5B). Tumor tissues were taken for western blot assay and immunohistochemistry analysis to verify the expression levels of FSP1 in xenografted tumors. The results showed that the expression of FSP1 was decreased after sorafenib treatment, which was following the in vitro results (Figure 5C and D). Moreover, FSP1 downregulated MDA levels and increased GSH levels in tumor tissues (Figure 5E and F). In conclusion, upregulating FSP1 promoted tumor growth and enhanced the resistance to sorafenib-induced ferroptosis in HCC.
FIGURE 5.
FSP1 promoted tumor growth and enhanced the resistance to sorafenib-induced ferroptosis in vivo. (A) Colony formations were performed with vehicle or sorafenib (5 μM, 24 h) treated cells. The clones were counted after stained. (B) The subcutaneously implanted tumor samples were obtained by establishing animal models (n = 6/group). Collected the data and protracted the weight and volume growth curves of tumor xenograft samples. (C) Western blot analyses of the expression levels of FSP1 in tumor xenograft samples. (D) Representative images of FSP1 immunohistochemical staining with FSP1 antibodies in indicated groups (×400 magnification). (E and F) The relative levels of MDA and GSH of indicated tumor xenograft samples. The data are the means ± SD of the 3 independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001. Abbreviation: FSP1, ferroptosis suppressor protein 1.
DISCUSSION
HCC remains a serious global health issue, particularly in China where the incidence and mortality rate of HCC is among the highest in the world.29 Despite remarkable progress in the HCC treatment, HCC has a long latency period and most patients are already in the advanced stages at the time of diagnosis, which causes a high mortality rate.30 Since 2008, sorafenib has been used as a first-line oral chemotherapy drug for HCC.31 Sorafenib is a multikinase inhibitor that dampens cell proliferation by antagonizing the serine/threonine kinase-associated signal pathway and inhibits tumor angiogenesis by targeting a series of specific tyrosine kinases.31 Although sorafenib can prolong the survivability of patients with advanced HCC, the primary and acquired sorafenib resistance and potential side effects restrict its survival benefit.32 The emergence of potential therapeutic targets has provided several combination therapies for HCC management, which has alleviated the limitation of sorafenib monotherapy.33 Each patient with HCC responds differently to sorafenib, and the reasons for this variation are unclear. Either way, an in-depth understanding of the mechanisms of sorafenib-induced cell death is necessary to take full advantage of its pharmacological potential to tailor individualized HCC treatment regimens.
Two key pathways regulating ferroptosis have been elucidated: the transporter-regulated (extrinsic) pathway and the enzyme-dependent (intrinsic) pathway.34 System Xc−, which is found on the cell membrane and facilitates cystine and glutamate transport, is a critical transporter in the ferroptosis process. Through the System Xc−, cystine enters the cell and contributes to the synthesis of GSH, thus preventing the cancer cells from ferroptosis.34 In addition, cancer cells depend on the endogenous antioxidant system to sustain redox equilibrium. The dysregulation of a variety of redox-active enzymes in this system disrupts the original homeostasis and leads to ferroptosis.35 Concerning the ferroptosis inhibition systems, there are 3 pathways have been demonstrated: cyst(e)ine/GSH/GPX4 system, NAD(P)H/FSP1/CoQ10 system, and GCH1/BH4/DHFR system.36 Thereinto, the cyst(e)ine/GSH/GPX4 system was the first system to be reported and has been considered a mainstay to prevent ferroptosis for many years. The function of the GPX4 as a key component in this system is to reduce hydroperoxides to their corresponding substances, thereby restricting lipid peroxidation.37 Consequently, the levels and/or activities of intracellular GPX4 are directly related to ferroptotic cell death. In HCC, GPX4 inhibitor RSL3 rendered HCC cells susceptible to sorafenib-induced ferroptosis.38 Ferroptosis-related gene FSP1 has attracted considerable interest in recent years, and it has been identified to be a novel target for predicting cancer prognosis and targeted therapy.39 Similar to GPX4, FSP1 acts as a peroxidase, and we can also control ferroptosis by regulating FSP1 in theory. Here, we found that sorafenib treatment reduced FSP1 protein levels, indicating that sorafenib-induced ferroptosis may be related to FSP1 in HCC cells. As respected, our results showed that knockdown of FSP1 promoted sorafenib-induced ferroptosis, and overexpression of FSP1-inhibited ferroptosis, proving that sorafenib-induced ferroptosis by downregulating FSP1.
Ubiquitination is an important type of post-translational modification and is involved in the modulation of most cellular biological activities.40 Currently, evidence has accumulated that ubiquitination modifications play a crucial role in the process of ferroptosis in various cancer cells.41 Bufotalin sensitizes ferroptosis by accelerating the ubiquitination of GPX4 in non–small cell lung cancer cells.42 Tribbles homolog 2 induces liver cancer cell ferroptosis through βTrCP-mediated TFRC ubiquitination and degradation.43 Our data displayed that MG-132 rescues sorafenib-induced FSP1 downregulation and sorafenib-promoted ubiquitination of FSP1, which indicated that sorafenib downregulates FSP1 by means of the ubiquitination pathway. An increasing number of the TRIM family protein has been reported to correlate with the development of tumors.20 Noteworthy, the TRIM family members are also engaged in ferroptosis regulation, for example, TRIM46 bound to GPX4 and facilitated GPX4 ubiquitination to decoy ferroptosis.44 Our results showed that TRIM54 interacted with FSP1 and promoted FSP1 degradation. In the absence of TRIM54, sorafenib failed to downregulate FSP1 and induce ferroptosis in HCC cells. Therefore, we deduced that sorafenib-induced HCC cells ferroptosis by means of TRIM54-mediated FSP1 ubiquitination. TRIM54 belongs to the TRIM family, previous studies have focused on the pathological processes of TRIM54 and have not been addressed in the areas of ferroptosis.45 Anyhow, the E3 ubiquitin ligase function of TRIM54 is undisputed,46 TRIM54 directly mediated the ubiquitination of FSP1 hinting that TRIM54 is indeed involved in the regulation of ferroptosis in HCC. The detailed biological role of TRIM54 in the carcinogenesis process remains to be further investigated.
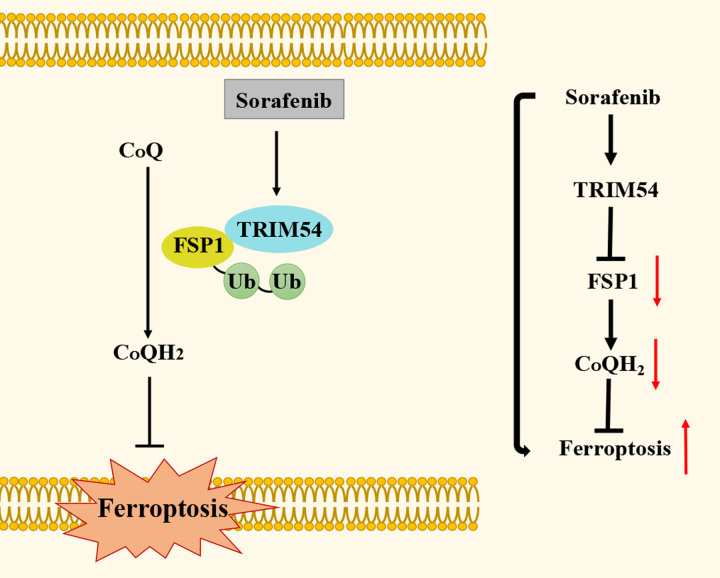
During the systemic treatment of cancer, chemotherapy tends to aggravate the oxidative stress in cancer cells.47 This may be one reason why sorafenib can induce ferroptosis. In this study, it was certain that sorafenib enhanced the ubiquitination of FSP1 and induced ferroptosis by means of the ERK kinase pathway in HCC cells. It is no surprise that ERK has an intersection with lipid peroxidation control. A previous study revealed that ADP Ribosylation Factor 6 serves as a downstream of the Kras/ERK signaling pathway in pancreatic cancer.48 Furthermore, suppression of ADP Ribosylation Factor 6 increased cellular sensitivity to RSL3-induced ferroptosis.49 Based on our data, the detailed molecular mechanism between ERK and TRIM54 is needed to further investigation. It is also possible that there exist additional regulatory pathways between sorafenib and TRIM54. Moreover, FSP1 accelerated tumor growth and enhanced cellular resistance to sorafenib-induced ferroptosis. In summary, we demonstrated that sorafenib-promoted TRIM54-mediated FSP1 ubiquitination by means of the ERK pathway, thereby triggering HCC cells ferroptosis (Figure 6). The specific pharmacological mechanism of sorafenib will provide new opportunities for developing ferroptosis-related cancer treatment.
FIGURE 6.
The model diagram to present how sorafenib-induced ferroptosis by facilitating FSP1 degradation. Abbreviations: FSP1, ferroptosis suppressor protein 1; TRIM, tripartite motif.
Footnotes
Abbreviations: AIFM2, apoptosis-inducing factor mitochondria associated 2; co-IP, co-immunoprecipitation; CoQ, coenzyme Q10; FSP1, ferroptosis suppressor protein 1; GPX4, glutathione peroxidase 4; GSH, glutathione; MDA, malondialdehyde; si-NC, negative control siRNA; SLC7A11, solute carrier family 7 member 11; TFRC, transferrin receptor; TRIM, tripartite motif.
Man-ru Liu and Ce Shi contributed equally to this work.
Contributor Information
Man-ru Liu, Email: 775327102@qq.com.
Ce Shi, Email: shice99@126.com.
Qiu-ya Song, Email: 1115792740@qq.com.
Meng-jie Kang, Email: 1296470418@qq.com.
Xin Jiang, Email: 1056249140@qq.com.
Hui Liu, Email: lh13812406087@163.com.
Dong-sheng Pei, Email: dspei@xzhmu.edu.cn.
CONFLICTS OF INTEREST
The authors have no conflicts to report.
REFERENCES
- 1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. [DOI] [PubMed] [Google Scholar]
- 2. Caruso S, O’Brien DR, Cleary SP, Roberts LR, Zucman-Rossi J. Genetics of hepatocellular carcinoma: approaches to explore molecular diversity. Hepatology. 2021;73(Suppl 1):14–26. [DOI] [PubMed] [Google Scholar]
- 3. Yao F, Deng Y, Zhao Y, Mei Y, Zhang Y, Liu X, et al. A targetable LIFR-NF-κB-LCN2 axis controls liver tumorigenesis and vulnerability to ferroptosis. Nat Commun. 2021;12:7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380:1450–62. [DOI] [PubMed] [Google Scholar]
- 5. Furuse J. Sorafenib for the treatment of unresectable hepatocellular carcinoma. Biologics. 2008;2:779–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cervello M, McCubrey JA, Cusimano A, Lampiasi N, Azzolina A, Montalto G. Targeted therapy for hepatocellular carcinoma: novel agents on the horizon. Oncotarget. 2012;3:236–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lai HY, Tsai HH, Yen CJ, Hung LY, Yang CC, Ho CH, et al. Metformin resensitizes sorafenib-resistant HCC cells through AMPK-dependent autophagy activation. Front Cell Dev Biol. 2021;8:596655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22:266–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martínez-Reyes I, Chandel NS. Cancer metabolism: looking forward. Nat Rev Cancer. 2021;21:669–80. [DOI] [PubMed] [Google Scholar]
- 11. Tang Z, Huang Z, Huang Y, Chen Y, Huang M, Liu H, et al. Ferroptosis: the silver lining of cancer therapy. Front Cell Dev Biol. 2021;9:765859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Su Y, Zhao B, Zhou L, Zhang Z, Shen Y, Lv H, et al. Ferroptosis, a novel pharmacological mechanism of anti-cancer drugs. Cancer Lett. 2020;483:127–36. [DOI] [PubMed] [Google Scholar]
- 13. Rodriguez R, Schreiber SL, Conrad M. Persister cancer cells: iron addiction and vulnerability to ferroptosis. Mol Cell. 2022;82:728–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lachaier E, Louandre C, Godin C, Saidak Z, Baert M, Diouf M, et al. Sorafenib induces ferroptosis in human cancer cell lines originating from different solid tumors. Anticancer Res. 2014;34:6417–22. [PubMed] [Google Scholar]
- 15. Dixon SJ, Patel DN, Welsch M, Skouta R, Lee ED, Hayano M, et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife. 2014;3:e02523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu M, Xu LG, Li X, Zhai Z, Shu HB. AMID, an apoptosis-inducing factor-homologous mitochondrion-associated protein, induces caspase-independent apoptosis. J Biol Chem. 2002;277:25617–23. [DOI] [PubMed] [Google Scholar]
- 17. Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:693–8. [DOI] [PubMed] [Google Scholar]
- 18. Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dai E, Zhang W, Cong D, Kang R, Wang J, Tang D. AIFM2 blocks ferroptosis independent of ubiquinol metabolism. Biochem Biophys Res Commun. 2020;523:966–71. [DOI] [PubMed] [Google Scholar]
- 20. Jaworska AM, Wlodarczyk NA, Mackiewicz A, Czerwinska P. The role of TRIM family proteins in the regulation of cancer stem cell self-renewal. Stem Cells. 2020;38:165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu W, Zhao Y, Wang G, Feng S, Ge X, Ye W, et al. TRIM22 inhibits osteosarcoma progression through destabilizing NRF2 and thus activation of ROS/AMPK/mTOR/autophagy signaling. Redox Biol. 2022;53:102344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao G, Liu C, Wen X, Luan G, Xie L, Guo X. The translational values of TRIM family in pan-cancers: from functions and mechanisms to clinics. Pharmacol Ther. 2021;227:107881. [DOI] [PubMed] [Google Scholar]
- 23. Zhang L, Afolabi LO, Wan X, Li Y, Chen L. Emerging roles of tripartite motif-containing family proteins (TRIMs) in eliminating misfolded proteins. Front Cell Dev Biol. 2020;8:802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Louandre C, Ezzoukhry Z, Godin C, Barbare JC, Mazière JC, Chauffert B, et al. Iron-dependent cell death of hepatocellular carcinoma cells exposed to sorafenib. Int J Cancer. 2013;133:1732–42. [DOI] [PubMed] [Google Scholar]
- 25. Bai T, Lei P, Zhou H, Liang R, Zhu R, Wang W, et al. Sigma-1 receptor protects against ferroptosis in hepatocellular carcinoma cells. J Cell Mol Med. 2019;23:7349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hatakeyama S. TRIM family proteins: roles in autophagy, immunity, and carcinogenesis. Trends Biochem Sci. 2017;42:297–311. [DOI] [PubMed] [Google Scholar]
- 27. Tang W, Chen Z, Zhang W, Cheng Y, Zhang B, Wu F, et al. The mechanisms of sorafenib resistance in hepatocellular carcinoma: theoretical basis and therapeutic aspects. Signal Transduct Target Ther. 2020;5:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moon H, Ro SW. MAPK/ERK signaling pathway in hepatocellular carcinoma. Cancers (Basel). 2021;13:3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [DOI] [PubMed] [Google Scholar]
- 30. Hilmi M, Neuzillet C, Calderaro J, Lafdil F, Pawlotsky JM, Rousseau B. Angiogenesis and immune checkpoint inhibitors as therapies for hepatocellular carcinoma: current knowledge and future research directions. J Immunother Cancer. 2019;7:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lai YL, Wang KH, Hsieh HP, Yen WC. Novel FLT3/AURK multikinase inhibitor is efficacious against sorafenib-refractory and sorafenib-resistant hepatocellular carcinoma. J Biomed Sci. 2022;29:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang H, Zhang W, Jiang L, Chen Y. Recent advances in systemic therapy for hepatocellular carcinoma. Biomark Res. 2022;10:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Song T, Lang M, Ren S, Gan L, Lu W. The past, present and future of conversion therapy for liver cancer. Am J Cancer Res. 2021;11:4711–24. [PMC free article] [PubMed] [Google Scholar]
- 34. Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31:107–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stockwell BR, Jiang X, Gu W. Emerging mechanisms and disease relevance of ferroptosis. Trends Cell Biol. 2020;30:478–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zheng J, Conrad M. The metabolic underpinnings of ferroptosis. Cell Metab. 2020;32:920–37. [DOI] [PubMed] [Google Scholar]
- 37. Ursini F, Maiorino M. Lipid peroxidation and ferroptosis: the role of GSH and GPx4. Free Radic Biol Med. 2020;152:175–85. [DOI] [PubMed] [Google Scholar]
- 38. Wang Q, Bin C, Xue Q, Gao Q, Huang A, Wang K, et al. GSTZ1 sensitizes hepatocellular carcinoma cells to sorafenib-induced ferroptosis via inhibition of NRF2/GPX4 axis. Cell Death Dis. 2021;12:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shi ZZ, Tao H, Fan ZW, Song SJ, Bai J. Prognostic and immunological role of key genes of ferroptosis in Pan-cancer. Front Cell Dev Biol. 2021;9:748925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cockram PE, Kist M, Prakash S, Chen SH, Wertz IE, Vucic D. Ubiquitination in the regulation of inflammatory cell death and cancer. Cell Death Differ. 2021;28:591–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang X, Wang Y, Li Z, Qin J, Wang P. Regulation of ferroptosis pathway by ubiquitination. Front Cell Dev Biol. 2021;9:699304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang W, Jiang B, Liu Y, Xu L, Wan M. Bufotalin induces ferroptosis in non-small cell lung cancer cells by facilitating the ubiquitination and degradation of GPX4. Free Radic Biol Med. 2022;180:75–84. [DOI] [PubMed] [Google Scholar]
- 43. Guo S, Chen Y, Xue X, Yang Y, Wang Y, Qiu S, et al. TRIB2 desensitizes ferroptosis via βTrCP-mediated TFRC ubiquitiantion in liver cancer cells. Cell Death Discov. 2021;7:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang J, Qiu Q, Wang H, Chen C, Luo D. TRIM46 contributes to high glucose-induced ferroptosis and cell growth inhibition in human retinal capillary endothelial cells by facilitating GPX4 ubiquitination. Exp Cell Res. 2021;407:112800. [DOI] [PubMed] [Google Scholar]
- 45. Liu J, Zhang C, Wang X, Hu W, Feng Z. Tumor suppressor p53 cross-talks with TRIM family proteins. Genes Dis. 2020;8:463–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fielitz J, Kim MS, Shelton JM, Latif S, Spencer JA, Glass DJ, et al. Myosin accumulation and striated muscle myopathy result from the loss of muscle RING finger 1 and 3. J Clin Invest. 2007;117:2486–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mbah NE, Lyssiotis CA. Metabolic regulation of ferroptosis in the tumor microenvironment. J Biol Chem. 2022;298:101617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liang C, Qin Y, Zhang B, Ji S, Shi S, Xu W, et al. ARF6, induced by mutant Kras, promotes proliferation and Warburg effect in pancreatic cancer. Cancer Lett. 2017;388:303–11. [DOI] [PubMed] [Google Scholar]
- 49. Ye Z, Hu Q, Zhuo Q, Zhu Y, Fan G, Liu M, et al. Abrogation of ARF6 promotes RSL3-induced ferroptosis and mitigates gemcitabine resistance in pancreatic cancer cells. Am J Cancer Res. 2020;10:1182–93. [PMC free article] [PubMed] [Google Scholar]