Abstract
Background:
There is a well-described mechanism of communication between the brain and gastrointestinal system in which both organs influence the function of the other. This bi-directional communication suggests that disease in either organ may affect function in the other.
Objective:
To assess whether the evidence supports gastrointestinal system inflammatory or degenerative pathophysiology as a characteristic of Alzheimer's disease (AD).
Methods:
A review of both rodent and human studies implicating gastrointestinal changes in AD was performed.
Results:
Numerous studies indicate that AD changes are not unique to the brain but also occur at various levels of the gastrointestinal tract involving both immune and neuronal changes. In addition, it appears that numerous conditions and diseases affecting regions of the tract may communicate to the brain to influence disease.
Conclusion:
Gastrointestinal changes represent a perhaps overlooked aspect of AD, representing a more system influence of this disease.
Keywords: microbiome, Alzheimer, amyloid, intestine, enteric neuron, inflammation
1. INTRODUCTION
In the 19th and early 20th centuries, an association of the brain with the gut was critically investigated by William Beaumont, Ivan Petrovich Pavlov, Walter Bradford Cannon, and Stewart Wolf (1). Later, the pivotal role of gut flora in modulating the gut-brain axis was recognized (2). The central nervous system (CNS), including the brain and spinal cord, bi-directionally communicates with the gastrointestinal (GI) tract, its enteric nervous system (ENS), and the gut microbiota via the sympathetic and parasympathetic nerves, the hypothalamic-pituitary-adrenal (HPA) axis, endocrine signaling, the immune system, metabolic signaling, and microbial metabolites (Figure 1). The ENS, along with sympathetic and parasympathetic nervous systems comprising the autonomic nervous system, is a part of the peripheral nervous system (3). This well-established connection is often called the brain-gut-microbiota axis (4, 5).
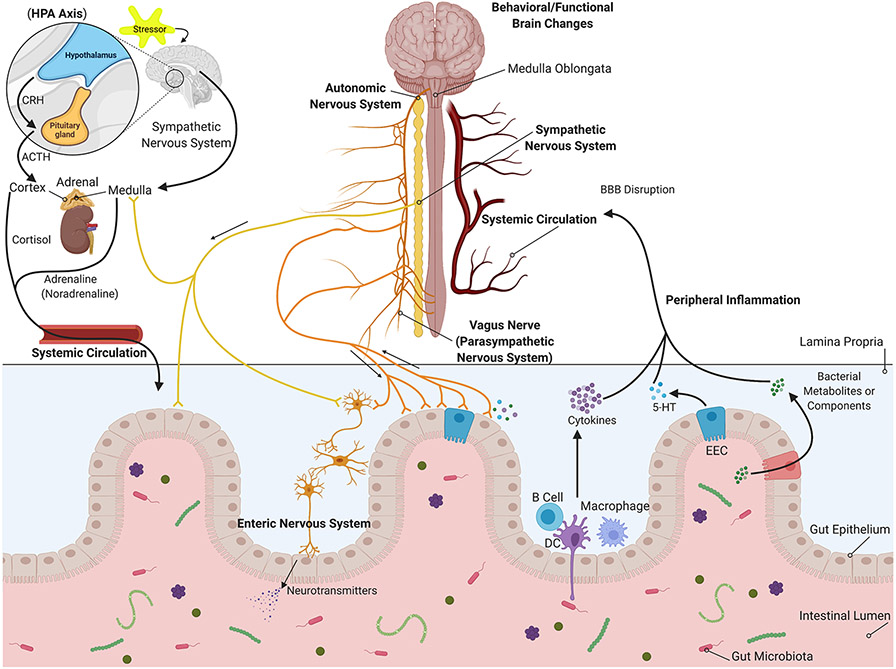
Fig. 1. Interactions between the Gastrointestinal Tract and Brain of Relevance in AD.
The autonomic nervous system innervates the gastrointestinal tract to directly influence the enteric cells, immune cells, and intestinal microbiome. In addition, circulating hormonal secretions can have similar influences on the intestine. In turn, intestine-derived secretions such as bacterial products or metabolites, immune cells, cytokines, neurotransmitters, or enteric hormones may influence nervous communication to the brain directly or travel via the vasculature to affect the brain through mechanisms perhaps involving disruption of the blood-brain barrier (BBB).
The release of neurotransmitters allows the ENS to regulate the gut immune system. The epithelial cells, which line the mucosal surfaces, absorb nutrients and provide a barrier between the mucosal immune system and luminal contents, such as digestive enzymes, toxins, and gut microbiota (6, 7). Bacterial metabolites and secreted cytokines and hormones from mucosal immune cells and enteroendocrine cells (EECs) in response to gut microbiota can reach the brain via the bloodstream and vagus nerve (6).
Lipopolysaccharide (LPS) as a typical Gram-negative bacterial endotoxin and peptidoglycan (PG) and lipoteichoic acids (LTA) from Gram-positive bacteria are considered pathogen-associated molecular patterns (PAMPs) (8). PAMPs along with damage-associated molecular patterns (DAMPs), endogenous danger signals released by damaged or dying cells, are recognized by various membrane-bound and cytosolic pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain-like receptors (NLRs), in host cells (8-10). At the intestinal mucosal surface, PAMPs and DAMPs can be distinguished by PRRs (TLRs and NLRs) on antigen sampling-dendritic cells (DCs), intestinal epithelial cells and other resident immune cells. TLRs-PAMPs activation leads to the expression of cytokines, chemokines, interferons, and antimicrobial molecules through induction of pathways, including the nuclear factor-κ B family of factors (NF-κB), members of the interferon regulatory factor family (e.g., IRF3/7), and activator protein 1 (AP1). NLRs-PAMPs activation results in the inflammasome assembly and release of proinflammatory cytokines and antimicrobial molecules (11-13). Innate immune response via TLR-dependent antimicrobial factor and proinflammatory cytokine production and IgA secretion of the adaptive immune system, triggered by DCs-B cells interaction, maintains homeostasis of the intestine, the first line of host defense (13). Serotonin (5-hydroxytryptamine, 5-HT), cholecystokinin, glucagon-like peptide-1, and peptide YY secreted by EECs and peripheral inflammatory factors (i.e., IL-1β/IL-1β receptors) activate afferent vagus fibers (parasympathetic nervous system) terminated in the medulla oblongata in the brainstem. ENS and afferent vagus nerve can also be stimulated by bacterial products and metabolites, like γ-aminobutyric acid (GABA), 5-HT, noradrenaline, dopamine, short-chain fatty acids (SCFAs; propionate, butyrate, and acetate), amino acids (i.e., tyramine and tryptophan), and LPS (LPS-TLR4), through related receptors (10, 14-16). Afferent signals are further transferred to the hypothalamus and higher forebrain regions involved in the integration of visceral sensory information, regulation of autonomic function, and behavioral manifestations. The efferent vagus nerve, known as the anti-inflammatory cholinergic pathway, suppresses proinflammatory cytokine production via secretion of acetylcholine and its binding to α 7 nicotinic acetylcholine receptors (α7nAChR) in macrophage, dendritic cells, and other immune cells in peripheral tissues. This mechanism is called the inflammatory reflex (10, 16). Moreover, cytokines, EECs-secreted neurotransmitters and hormones, and bacterial products, consisting of SCFAs, trimethylamines, amino acid derivatives, and vitamins, can travel to the brain via the bloodstream and exert effects on the blood-brain barrier (BBB) integrity and brain function (17). Unresolved leaky gut and disturbance of immune response lead to consistent TLRs (i.e., TLR4) and NLRs activation and consequently excessive production of proinflammatory cytokines, including elevated serum levels of TNF-α, IL-6, IL-1β, and IL-18, and enteric neuronal damage. This chronic systemic inflammation increases BBB permeability by upregulation of matrix metalloproteases and downregulation of tight junctions, and finally, neuroinflammation (10, 17).
The HPA axis, as a part of the brain limbic system, is an endocrine pathway of the brain-gut axis. Activation of the HPA axis, due to stressful stimuli, induces the secretion of corticotrophin-releasing hormone (CRH) from the hypothalamus paraventricular nucleus. CRH triggers the production of adrenocorticotropic hormone (ACTH) by the anterior pituitary gland. This response subsequently results in the release of the glucocorticoid stress hormone cortisol from the adrenal cortex to the bloodstream (18-20). Cortisol decreases inflammatory factors, such as IL-1, IL-6, and TNF-α at transcription levels and promotes the production of anti-inflammatory cytokines, including IL-10. Thus, it plays an indispensable role in gut physiology (14, 21). Cortisol secretion is also associated with proinflammatory responses, intestinal epithelial barrier disruption, gut inflammation, and alteration of microbial composition (18). Furthermore, stress-induced activation of the hypothalamus regulates the autonomic nervous system function through pontomedullary nuclei. Epinephrine and norepinephrine are secreted from the adrenal medulla in response to stress communicated via the sympathetic nervous system (21).
Because the brain communicates with the gut bi-directionally, CNS disorders may be affected by a dysfunctional GI tract, and one may precede the other (22). For instance, meta-analysis studies report a dramatic prevalence of GI dysfunction in children with autism spectrum disorder (ASD) compared to healthy controls (23). In addition, precedence of constipation before the development of motor symptoms and also accumulation of α-synuclein in the ENS as well as the dorsal motor nucleus of the vagus nerve, support the idea that Parkinson's disease (PD) pathological hallmarks may originate in the ENS by the mucosal invasion of a neurotropic pathogen that is later transmitted to the CNS (22, 24, 25). Furthermore, detection of amyloid precursor protein (APP) and amyloid-β (Aβ) in the ENS and GI tract of aged healthy and Alzheimer's disease (AD) individuals as well as in the ENS and intestinal epithelial cells of APP overexpressing transgenic mice suggest that AD may be a systemic disorder (26-29). Alzheimer's disease is a progressive neurodegenerative disease characterized by dementia. It is the most common form of dementia accounting for 60-80% of all cases (30). In addition to neurodegeneration, brains are characterized by atrophy, inflammatory changes, and protein Aβ and tau protein aggregates (30). However, based upon the fact that the intestine has a rich innervation of both the nervous and immune systems, well-known gut-brain communication mechanisms, and increasing evidence of intestinal pathophysiology during AD, it is likely that gastrointestinal dysfunction is an underappreciated aspect of AD. Below we review the evidence from human and rodent studies implicating changes in the gastrointestinal tract that may be contributing to the progression of AD (Table 1).
Table 1.
Gastrointestinal-associated inflammatory changes that may contribute to AD.
| GI Tract Regions |
Inflammatory Changes | References |
|---|---|---|
| Oral Cavity | Invasion of periodontal pathogens or their harmful products directly into the brain via systemic circulation or peripheral nerves correlates with inflammation and neurodegeneration in humans. | (45-53) |
| Periodontitis induces systemic inflammation, potentially resulting in neuronal injury via a compromised blood-brain barrier (BBB) in rodent models. | (57-59) | |
| Stomach | Stomach infection of mice with H. pylori alters eating behavior, brain cytokine levels and increases gliosis. | (83, 85) |
| 88% of AD patients and 46.7% of controls are H. pylori-positive. H. pylori eradication significantly improves cognitive performance. | (89) | |
| Small Intestine | Human AD intestines have increased Aβ and CD68 immunoreactivity compared to controls. | (190) |
| APP/PS1 mouse model has increased ileum Aβ and CD68 immunoreactivity and elevated luminal IgA compared to wild-type mice. | (190) | |
| Large Intestine | IBD-like disease induced by 2,4,6-trinitrobenzene sulfonic acid (TNBS) results in microglial activation and elevation of TNF-α levels in the hippocampus of adult male rats. | (114) |
| Colonic inflammation induced by 2 bouts of 2% DSS administration led to Aβ plaque load increase in the hippocampus and temporal cortices and decreased microglial CD68 immunoreactivity in AppNL-G-F mice. | (115) | |
| Induction of colonic inflammation is associated with alteration in hippocampal neurogenesis and memory dysfunction in IBD mouse models. | (116-118) | |
| Human IBD patients are at higher risk of dementia with an average onset of 7 years younger compared to controls. | (122) | |
| Bacteroides colonization increased intestinal inflammation and exacerbated Aβ deposition in the Tg2576 mouse model of AD. | (153) | |
| A diet containing wheat amylase trypsin inhibitor in an AD mouse mice induced dysbiosis, increased intestinal myeloid immune cell subsets, and potentiated pathological hallmarks of AD. | (136) | |
| Gut dysbiosis elevated bloodstream/brain LPS in human AD brains. | (52, 53, 161, 162) | |
| Antibiotic treatment-induced dysbiosis altered circulating levels of cytokines/chemokines and reduced Aβ plaques in the APPSWE/PS1ΔE9 mouse model of AD. | (157) | |
| The altered ratio of Firmicutes/Bacteroidetes correlated with intestinal proinflammatory cytokine changes and increased intestinal permeability in the AppNLG-F mouse AD model. | (137) |
2. Periodontitis and Oral Cavity Changes that may Communicate to the Brain in AD
Periodontitis is a chronic inflammatory gum disease caused by the host response to primarily Gram-negative anaerobic bacteria such as Treponema denticola, Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, and Tannerella forsythia. The host response leads to a gingival inflammatory condition, alveolar bone loss, damage to the supporting tissue of the tooth, and consequently causing tooth loss (31-34). Various studies have suggested periodontitis is a risk factor for AD (35-38). Chen et al., for instance, demonstrated that periodontitis is linked with a surge of approximately 1.7-fold in the risk of development of AD (39). Another study by Kamer et al. showed that clinical measures of periodontitis in healthy elderly with normal cognitive function are associated with the degree of amyloid plaque accumulation estimated by [11C]PIB-PET (37). Also, a recent study based on the National Health and Nutrition Examination Survey (NHANES) database illustrated that mild to severe periodontitis patients showed some degree of dementia compared to the healthy group (40). A few reports have also demonstrated a substantial decline in the number of hippocampal neurons and an increase in astrogliosis and microgliosis correlated with periodontitis-associated tooth loss (41, 42). The oral cavity in periodontitis provides a reservoir of inflammatory markers (34). Studies suggest that systemic inflammation exacerbates neurodegeneration via microglial activation and production of proinflammatory factors, thereby accelerating AD progression (43, 44). Periodontal pathogen ability to invade the brain and the effects of pathogen-stimulated systemic inflammation on the brain are two plausible mechanisms behind this mouth-brain cross talk.
The pathophysiological pathway between periodontitis and AD may involve bacteria from dental plaque biofilms reaching the brain via systemic circulation or peripheral nerves (45). The periodontal pathogens and their harmful products may leak from the compromised barriers of the oral cavity, resulting in transient systemic bacteremia (46). Gram-negative bacterial infections in the brain have been linked to late-onset sporadic AD (47). In a case study, Treponema bacteria (Gram-negative spirochetes), a common periodontal pathogen, were found in higher numbers in brain abscesses of AD patients than in cognitively normal patients demonstrating their ability to invade brain tissue (48). In the vicinity of the trigeminal nerve, pons, and hippocampus, proteins of two variants of Treponema were found, indicating that the pathogen might have migrated through the trigeminal nerve into the brain (48, 49). Analogous to this hypothesis, previous studies have also demonstrated the presence of spirochetes, mainly Treponema, with higher occurrence in AD patient brains compared to cognitively normal individuals using molecular techniques and dark field microscopy (48, 50, 51). Moreover, LPS, the chief membrane constituent of Gram-negative bacteria, can be found in increased amounts in the brains of AD patients compared to healthy controls (52). In this context, Zhan et al. have shown co-localization of LPS with amyloid plaques and around vessels of AD patient brains (53). Also, systemic administration of LPS can activate microglia and stimulate the production of proinflammatory factors (54). In addition, there is ample evidence that daily oral activities such as brushing, flossing or chewing (55, 56) can elevate circulating periodontal pathogens and their toxic byproducts, potentially elevating proinflammatory factors in systemic circulation that could contribute to the progression of dementia (45).
Besides the invasion of periodontal pathogens or products directly into the brain, another potential mechanism connecting periodontitis and AD is the induction of a systemic inflammatory response that potentially results in neuronal injury via a compromised BBB (57-59). Although the BBB is an important physiological barrier that maintains the homeostasis of the central nervous system, the brain can respond to pathological stimuli by altering BBB permeability (60). Reports suggest that proinflammatory factors, especially IL-6, IL-1, TNF- α and C-reactive protein (CRP), may transmigrate across the BBB via specific processes, particularly through the fenestrated capillaries in circumventricular organs (61-63), thereby altering neuroinflammatory cascades leading to synaptic dysfunction and neuronal death (62, 64-66). A study on post-mortem plaques by Doung et al. revealed that serum amyloid P and CRP are colocalized in neuronal tau protein aggregates and extracellular amyloid deposits in AD (67). Considering the role of chronic inflammation, as is evident in AD studies, it is rationale to believe that chronic systemic exposure to periodontal pathogens and subsequent production of proinflammatory factors may accelerate pathophysiology leading to dementia (34, 68, 69).
Rodent studies of periodontitis-associated oral infection support the notion that oral infection communicates to the brain to alter AD progression. For example, oral administration of the periodontal pathogen, Porphyromonas gingivalis, or its product gingipain, to C57BL/6 mice results in elevated brain levels of cytokines, degenerating hippocampal neurons, elevated Aβ, and increased p-tau levels (70). A similar study of P. gingivalis oral infection of C57BL/6 mice also demonstrated elevated brain cytokines and reduced memory performance (71). Finally, periodontitis induced by P. gingivalis in the hAP-J20 AD transgenic line resulted in impaired memory, elevated brain cytokines, increased Aβ levels, and higher brain levels of LPS (72).
Although bacterial products or systemic immune communication to the brain are likely mechanisms of mouth-brain connection in periodontitis-exacerbated AD (Figure 2), several other possibilities may explain the relationship between these diseases that are worth exploring. For instance, changes in nutritional habits (73), lower levels of dietary vitamins and antioxidants and high unsaturated fats (74, 75), and impaired masticating ability have also been linked to cognitive dysfunction (76).
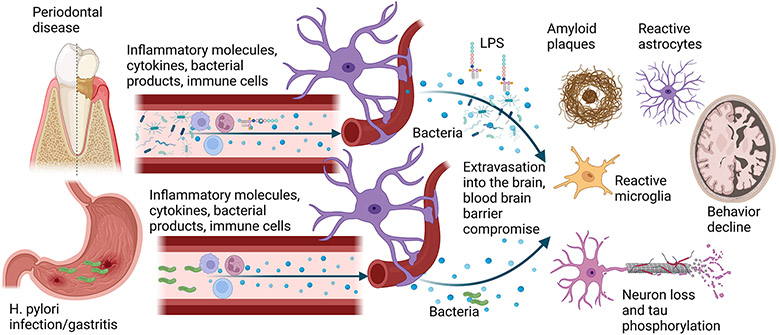
Fig. 2. Possible mechanisms of brain and stomach communication to the brain to potentiate AD pathophysiology.
Periodontitis, characterized by chronic gingival inflammation and bone and tooth loss, increases the risk for AD and dementia. Periodontitis is also positively correlated with increased amyloid plaque load, gliosis, and neuron loss. Increased immune cell activation and circulating cytokines associated with periodontitis may infiltrate through the blood-brain barrier to directly influence disease pathophysiology. Similarly, periodontal pathogens and their components, such as LPS, have been reported to be elevated in AD brains. Rodent studies of oral pathogen infection support the human disease findings by stimulating increased brain cytokines and LPS, memory dysfunction, neuron loss, elevated Aβ, and increased phospho-tau levels. In addition to the inflammatory contributions of changing oral health to the brain in AD, several studies demonstrate that the peripheral inflammatory changes associated with gastric infection and gastritis also induce AD-like changes in rodents, including elevated brain cytokines and altered behavior. This notion is supported by human studies demonstrating an increased association of H. pylori infection with AD patients compared to controls and improved behavior with eradication therapy.
3. Stomach Infection and Inflammatory Changes that may Influence AD Progression
Stomach-associated inflammation may also communicate to the brain to affect AD progression. For instance, Helicobacter pylori (H. pylori) is a Gram-negative bacteria capable of infecting the stomach to increase the risk of gastric diseases, including peptic ulcers (77-79). H. pylori infection is characterized by a chronic inflammatory state responsible for increased production of inflammatory mediators and immune cells, potentially leading to dysfunction of the enteric nervous system. A recent study has suggested that H. pylori infection may be a causative factor for dysfunction of the gut-brain axis in AD (80-82). For instance, H. pylori can trigger an inflammatory response through changes in eating behavior and altered levels of circulating stomach hormones and brain cytokines, which persist even after eradication therapy (83). Another study indicated that H. pylori-infected C57BL/6 mice had reduced exploratory behavior in an open field chamber compared to sham-treated mice suggesting infection-associated memory impairment. In addition to behavioral alteration, H. pylori-infected mice have shown a significant reduction in two hippocampal myelination genes compared to control mice, also demonstrating a communication to the brain (84).
More specific to AD, H. pylori infection and gastritis have been reported to influence the brain and gene expression in multiple studies to induce AD-like changes. For example, stomach infection of C57BL/6 mice with H. pylori induces subsequent gliosis (85). Stimulating a human gastric cell line with the H. pylori peptide Hp (2-20) results in upregulation of multiple AD-related genes, including APP, APOE, and PSEN1/2 and multiple inflammation pathways genes (86). An H. pylori filtrate induced tau hyperphosphorylation in mouse neuroblastoma N2a cells in a glycogen synthase kinase-3β (GSK-3β) dependent manner (87). However, H. pylori infection of rats produced no significant change in tau phosphorylation or cognitive performance (88). Finally, in a study comparing 50 AD and 30 age-matched anemic control individuals, 88% of the AD patients and 46.7% of the controls were H. pylori-positive. Therapeutic intervention to eradicate H. pylori significantly improved cognitive performance only in individuals with successfully eradicated infection (89).
In a fashion not necessarily associated with H. pylori infection and gastritis, there is evidence of disrupted stomach-brain interaction, in general. It is known, for example, that select stomach peptide hormones have a reported role in cognition and dementia. Treatment of 5XFAD transgenic AD mice with MK-0677, an agonist of the receptor for the stomach hormone ghrelin, was sufficient to reduce Aβ deposition, gliosis, and neuron/synaptic loss (90). In addition, serum levels of acetylated, functional ghrelin are significantly elevated in mild cognitive impairment compared to control individuals correlating with deficits in memory and language (91).
A better understanding of stomach-brain communication disruption with or without H pylori infection in AD should bring new insight into understanding the pathophysiology of AD and allow exploration of new therapeutic agents, perhaps via targeting host-pathogen interactions (Figure 2).
4. Small Intestine Manifestations of AD and their Potential Contribution to Brain Changes
Several reports also support the possibility of AD manifestation in the small intestine. For example, it appears that the levels of the amyloid precursor protein (APP) and Aβ peptide are upregulated in the absorptive epithelium following high-fat feeding in rodents (92, 93). Indeed C57BL/6 mouse consumption of a diet high in saturated fat results in elevated enterocyte Aβ levels and circulating Aβ (94). This feeding results in co-localization of Aβ with chylomicrons in the enterocytes suggesting that Aβ may be produced in response to a need for lipid handling in the epithelium (95). It is not clear what the outcome would be from increased enterocyte Aβ production. However, it is not unreasonable to expect that Aβ could be secreted from these cells apically into the intestinal lumen for fecal excretion or basally into the vasculature for transport to other organs such as the brain (Figure 3).
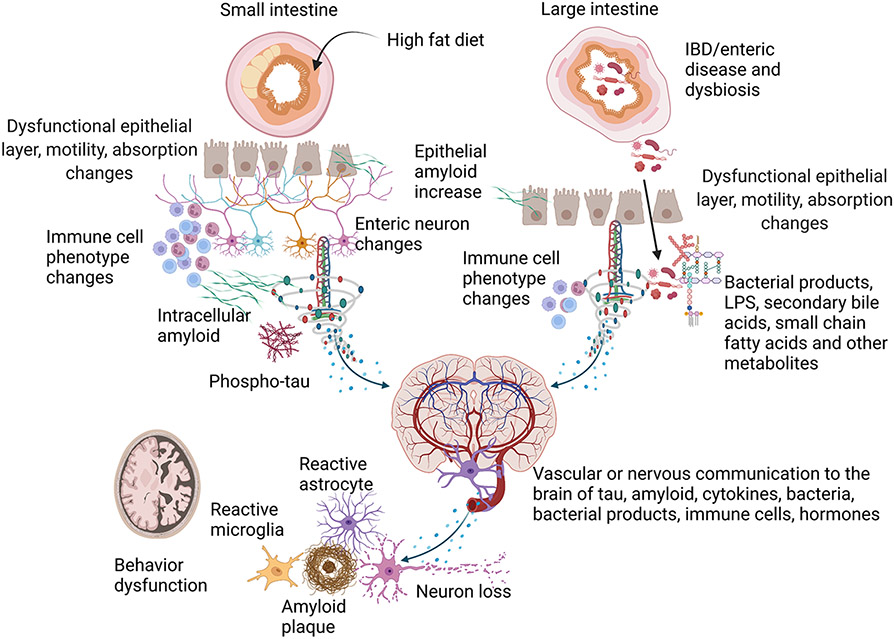
Fig. 3. Potential contributions of the small and large intestine to AD pathophysiology.
Several studies validate disease-related changes in the small intestines of AD patients and rodent models characterized by Aβ and tau pathology and inflammatory changes. For example, small intestine epithelial cells express APP in response to dietary changes such as a high-fat diet and can secrete Aβ peptide possibly into the intestinal connective tissue and the circulation. In agreement with this, enteric neurons in rodent models and AD patient small intestines have demonstrated intracellular Aβ, immune cell changes, neuron expression differences, and phospho-tau increases. Different rodent models of AD have also identified intestinal motility and permeability changes compared to controls. Although the intestine-related dysfunction represents a disease phenotype, it is possible that enteric-derived Aβ, phospho-tau, activated immune cells, enteric hormones, or cytokines may communicate to the brain to influence disease via the circulation or direct neuronal innervation. Similar contributions to disease may exist for the large intestine. For example, intestinal inflammatory changes such as those associated with IBD may provide a driving force for exacerbating bacterial translocation through a disrupted epithelial layer and immune cell activation and enteric neuron dysfunction that communicates to the brain in AD. Rodent IBD studies validate that intestinal immune and functional compromise and an impaired epithelial barrier communicate to the brain to exacerbate gliosis, cytokine elevations, and increased plaque load using AD mouse models. Age, diet, environment, or disease-associated changes in the intestinal microbiome, largely localized to the large intestine, have also been characterized in human AD patients and rodent models. This dysbiosis correlates with cognitive changes and peripheral inflammatory states. Disease-modifying, unique bacteria or their components and secretory products such as LPS, small chain fatty acids, and secondary bile acids have been examined in human and rodent models to identify therapeutic targets. It is clear from rodent studies that manipulation of the intestinal microbiome is sufficient to alter brain changes such as behavior, gliosis, inflammatory changes, and Aβ levels in rodent models.
Independent of the epithelial production of Aβ, the GI tract enteric neurons may also be a source of AD-related histopathology. One study examining enteric neurons from AD and elderly controls demonstrated no differences in tangle pathology using the ALZ-50 antibody (96). However, an additional comparison of AD and age-matched control intestines showed not only Aβ but also increased CD68 immunoreactivity in AD patients suggesting that an amyloid-associated inflammatory process may be occurring in the small intestines (28). A similar study also reported intraneuronal Aβ immunoreactivity in AD enteric neurons (27). These human changes were recapitulated in the APP/PS1 model, which demonstrated a similar increase in ileum Aβ and CD68 immunoreactivity and elevated luminal IgA and fecal Aβ compared to wild-type mice (28). Regardless of the source of intestinal Aβ, a recent study demonstrated that oligomeric Aβ injected directly into the mouse GI tract results in a prion-like spread from the intestine to the brain via the enteric neurons and the vagal nerve as well as neuromuscular uncoupling in the intestines (97). These studies support the idea that there may be inflammatory and degenerative changes in the intestines in AD directly related to changes occurring in the brain (Figure 3).
In addition to Aβ accumulation in the intestines, additional neuronal dysfunction exists. For example, a 2017 study utilizing APP/PS1 mice revealed that Aβ plaque and phospho-tau increases in the brain correlated with no overall change in enteric neuron number but a selective decrease in nitrergic and cholinergic neurons (98). In addition, kidney and brain expressed protein (KIBRA) has been identified as a modulator of the postsynaptic cytoskeleton (99). Variants of the KIBRA protein that act as structural components of neurons have been linked to memory-related functions of the mammalian brain (100). A control-matched study of neurons from AD patients found overexpression of KIBRA and underexpression of three KIBRA binding partners in the hippocampus, posterior cingulate, and temporal cortex (101). Interestingly, the GABAergic neural subtype (GAD67+) had increased expression of KIBRA in the jejunal myenteric nerve plexus of APP/PS1 mice compared to wild-type controls. These data indicate that dysregulation of neuronal proteins is not unique to the brain (102).
Intestinal dysfunction is also reported based upon the intestinal evidence of amyloid and inflammatory changes in both humans and mouse models of AD. An age/sex-matched study of the APP/PS1 and AppNL-G-F mouse models of AD examined motility 30 minutes after administration of non-absorbable dextran (103). Motility of the AppNL-G-F mice intestines was decreased compared to the wild-type and APP/PS1 groups. However, the APP/PS1 mice had increased gut permeability compared to the other groups. Although robust Aβ plaque accumulation within the mucosa or submucosa of the mouse intestines was not observed, the changes corresponded with Aβ accumulation in the brains of the mice further established the importance of the gut-brain axis in Alzheimer's disease (Figure 3).
5. Inflammatory Disease and Possible Large Intestine Contributions to AD Progression
Changes in the large intestine may also contribute to a pathologic crosstalk response that may affect the progression of AD. The imbalance between extreme and deficient apoptosis or anti-inflammatory and proinflammatory immune responses develops into GI tract diseases, including chronic intestinal inflammation (104). Inflammatory bowel disease (IBD), including Crohn's disease and ulcerative colitis, is a chronic relapsing inflammatory disease with increasing incidence and prevalence. It develops in young adulthood and continues throughout life. Aged individuals with IBD consist of patients with elderly-onset at age ≥ 60 years and older patients diagnosed at younger ages. IBD is clinically characterized by diarrhea+/− blood, abdominal pain, weight loss, and fatigue (104-106). Behavioral manifestations, including anxiety, depression, and cognitive and memory dysfunctions, are also associated with IBD (107-109).
After bowel epithelial damage, commensal bacteria and microbial metabolites are translocated into the bowel wall, where intestinal immune cells, such as dendritic cells and macrophages, are activated via TLR signaling and secrete a large number of proinflammatory cytokines, including IL-1β, IL-6, IL-18, and TNF-α (104, 110, 111). Initiating inflammation in the gut may induce CNS changes, leading to altered brain function. For example, TNF-α is the main proinflammatory cytokine in IBD pathology and is targeted to treat the disease (112). In AD subjects, the increased serum levels of TNF-α due to systemic inflammation exacerbates cognitive impairment and neurodegeneration through activation of primed microglia (113). Aligning with these data, IBD-like disease induced by 2,4,6-trinitrobenzene sulfonic acid (TNBS) results in microglial activation and elevation of TNF-α levels in the hippocampus of adult male rats (114). Our recent study demonstrated that intestinal inflammation induced by 2% dextran sulfate sodium (DSS) could increase brain insoluble Aβ1-40/42 levels correlating with attenuated microglial phagocytic phenotype in the AppNL-G-F AD mouse model (115). Induction of colonic inflammation is also associated with alteration in hippocampal neurogenesis and memory dysfunction in IBD mouse models (116-118). TNBS and DSS-induced colonic inflammation also cause dysbiosis via increasing Gram-negative bacteria, such as Proteobacteria and decreasing Gram-positive bacteria, including Firmicutes at the phylum level (116, 118, 119). Reciprocally, the brain may contribute to IBD severity and pathogenesis. Chronic stress, stressful life events, and depression exacerbate IBD, likely, via the autonomic nervous system and the HPA axis, two pathways involved in mediating the stress response (21, 120, 121).
A recent population-based cohort study reported that IBD patients are at higher risk of dementia with an average onset of 7 years younger compared to controls (122). It is plausible that IBD-associated disruption of intestinal epithelial integrity promotes the dissemination of dysbiotic bacterial neuroinflammatory metabolites, neurotransmitters, and neuromodulators to the CNS via the gut-brain axis (123). It is also feasible that Aβ production in the brains of IBD patients is upregulated in response to the bacterial extravasation since numerous studies have reported the antimicrobial property of Aβ (124-126). Further investigations are required to elucidate the mechanism involved (Figure 3).
6. Gastrointestinal Dysbiosis may Contribute to Intestine-derived Exacerbation of AD.
Based upon their clear role in mediating gut-brain interactions, it is important to specifically consider the possible contribution of intestinal bacterial changes to the progression of AD (127). Trillions of microbes residing in the intestine maintain a symbiotic relationship with the gut mucosa and impart metabolic, immunological, and gut protective functions in the host. Firmicutes, such as Clostridium and Lactobacillus species, and Bacteroidetes, including Bacteroides or Prevotella species, are the dominant gut bacteria phyla in humans as well as in mice, followed by the phyla Actinobacteria and Proteobacteria (128, 129). Alteration in the balance of these phyla has been associated with several diseases, including intestinal and extra-intestinal diseases such as metabolic syndrome, cardiovascular disease, and obesity (130-132). It is well known that intestinal barrier function and its integrity largely depend on gut microbiota. The normal aging process has been shown to increase intestinal epithelium and BBB permeability, disrupting tight junction protein complexes to allow microbial translocation (133-135). Altered gut microbiota induced by a diet containing wheat amylase trypsin inhibitor has been shown to increase intestinal myeloid immune cell subsets and potentiate pathological hallmarks of AD (136). Our recent study showed increased intestinal inflammation and intestinal permeability correlated with intestinal microbiome changes in a mouse model of AD (137).
Gut dysbiosis is the term used to describe an imbalance in the composition and metabolic capacity of gut microbiota that increases the risk of chronic and degenerative diseases (138-140). Emerging evidence from several preclinical and clinical studies has indicated a role for gut dysbiosis in the pathophysiology of AD (137, 141-145) (Figure 3). Numerous studies suggest an association of gut dysbiosis and peripheral inflammation with brain amyloidosis in cognitively impaired individuals (146-148). A recent report showed that AD patients with higher systemic inflammation, assessed by circulating inflammatory markers, were associated with higher pathogenic bacterial load during midlife and correlated with greater cognitive decline in older age (149). Bacterial 16S ribosomal RNA (rRNA) gene sequencing analysis performed using DNA isolated from fecal samples of humans with AD showed decreased microbial richness and diversity in AD patients compared to controls which correlated with cerebrospinal fluid (CSF) biomarkers of AD pathology (150).
A recent report published from a Chinese population showed depletion of SCFA-producing Firmicutes and enriched inflammation-promoting Proteobacteria in fecal samples of AD patients correlating with the severity of AD (151). Another recent study showed altered gut microbiota composition at different taxonomic levels, including changes in Bacteroides (genus), Actinobacteria (phylum), Ruminococcus (genus), Lachnospiraceae (family), and Selenomonadales (order), in fecal samples of AD patients compared to gender and age-matched controls suggesting a role in AD pathophysiology (152). Moreover, Bacteroides colonization increases intestinal inflammation and exacerbates Aβ deposition in the Tg2576 model of AD (153).
In 2004, Sudo and colleagues reported the involvement of postnatal microbial colonization (the exposure to microbes at an early developmental stage) in the programming of the HPA axis via studying the intensity of stress response in germ-free (GF) mice compared to specific pathogen-free and gnotobiotic mice. Colonization of Bifidobacterium infantis reversed the exaggerated HPA stress response in GF mice (154). Subsequent studies performed using GF mice have also demonstrated the importance of gut microbiota in brain development, learning, and memory functions (155). An increased anxiety-like behavior in GF mice is associated with changes in the production of neurotrophic factors, hormones, and the expression of their receptors. These findings imply that the presence or absence of conventional intestinal microbiota influences the development of behavior (156). GF APP transgenic mice show a drastic reduction in Aβ deposition in the brain compared to APP mice with intact intestinal microbiota. Another study showed that antibiotic treatment alters peripherally circulating cytokine/chemokine composition associated with a reduction in Aβ plaques in APPSWE/PS1ΔE9 mice (157). Colonization of GF APP transgenic mice with microbiota from conventionally-raised APP transgenic mice increased cerebral Aβ pathology supporting the hypothesis that gut microbes may be involved in the progression of cerebral Aβ amyloidosis (143).
In addition to dysbiosis, intestinal expression of human APP was also detected in the 5xFAD mouse model of AD (142). Another study detected the emergence of intestinal absorption impairment, dysbiosis, peripheral inflammation, and vascular Aβ deposition in the intestinal epithelial barrier before the onset of cerebral Aβ aggregation in the Tg2576 transgenic AD mouse model versus age-matched wild type controls. Intestinal epithelial Aβ deposition in AD patients compared to non-AD individuals was reported in the same study (158). In addition, gut microbiota produces amyloid fibrils (e.g., curli in Escherichia coli), mediated by prion-like peptide repeats and involved in the formation of bacterial biofilms (159). Bacterial and CNS misfolded amyloids share similarities in their tertiary structure that may permit, through molecular mimicry, cross-seeding of amyloids (160).
Bacterial metabolites, such as LPS, SCFAs, trimethylamine N-oxide (TMAO), and secondary bile acids (BAs) are also implicated in AD pathogenesis (Figure 3). Bacterial products such as LPS secreted by gut bacteria could influence AD pathophysiology via binding to the microglial receptors, TLRs and CD14. Through activating the downstream NF-κΒ signaling pathway, LPS leaked from intestinal bacteria could stimulate an inflammatory response in the brain (47, 161, 162). Bacterial endotoxin LPS-induced inflammation has been proposed as an important factor in the generation of Aβ42 (163, 164) and was recently reviewed by Bulgart et al. (165). SCFAs disrupted protein-protein interactions required for conversion of Aβ1-40/42 peptides into soluble neurotoxic aggregates in an in vitro study (166). Higher TMAO levels of CSF in patients with mild cognitive impairment and AD compared to cognitively unimpaired individuals were associated with biomarkers of AD pathology (p-tau and p-tau/Aβ42) and neuronal degeneration (167). Aligning with this data, age-related elevation in circulating TMAO levels in APP/PS1 mice was correlated with AD cognitive decline and pathological processes. Treating animals with 3,3-Dimethyl-1-butanol (DMB) decreased plasma TMAO levels and subsequently reduced levels of circulating clusterin and neuroinflammatory cytokines, β-secretase, Aβ1-42, and β-secretase-cleaved C-terminal fragment (βCTF) in the hippocampus. Altered long-term potentiation and reduced cognitive deficit of APP/PS1 mice were also detected in this study (168). Elevated serum levels of BAs have been linked to increased BBB permeability via disruption of tight junctions in vitro and in vivo (169). Primary BAs are derived from cholesterol in the liver and secreted from the gallbladder into the small intestine following a meal. Intestinal microbiota converts primary BAs to secondary BAs by bile salt hydrolysis and bile acid 7α-dehydroxylation (170, 171). Dramatic reductions in serum levels of primary BAs (cholic acid; CA) and elevated bacterially produced secondary BAs (deoxycholic acid; DCA and its glycine and taurine conjugated forms) were observed in serum samples of AD patients compared to cognitively normal older adults. The increased DCA:CA ratio was significantly associated with cognitive decline in a large multicenter study (172). Metabolomics analysis of primary and secondary BAs of post-mortem brain samples showed differences in taurine transport, BA synthesis, and cholesterol metabolism in AD vs. cognitively normal individuals. The presence of some BAs in brain tissues suggested their gut microbial origin and transportation to the brain (173). Altogether, these data indicate a contribution of gut dysfunction in AD etiology.
Based on this strong evidence of the involvement of gut dysbiosis in AD, numerous microbiome-targeted therapies have been attempted in animal models of AD. Approaches include the use of antibacterial drugs such as doxycycline and rifampin and gingipain inhibitors such as COR286, COR271 and COR388 (174-176), antiviral drugs such as valacyclovir (177), probiotics (137, 166, 178-181), prebiotics (182), and fecal transplantation (183). Taken together, this suggests some ability to alter the gut microbiota to provide benefits during disease. Some probiotic-based therapies have been proposed and tested in AD patients and have demonstrated effectiveness at changing behavior, including anxiety, depression, stress, and memory (184-188). It has been suggested that the mechanism of probiotic beneficial effects are mainly through the restoration of disrupted intestinal barriers, stimulation of the mucosal immune system, and prevention of growth of pathogenic microorganisms (189). Moreover, a recent clinical trial assessing the safety and feasibility of an oral-fecal microbiota transplant (FMT) intervention for AD (NCT03998423) with results pending.
The results of both preclinical and clinical studies demonstrate a solid link between altered gut microbiota composition and amyloid formation that associates with cognitive decline in AD. Moreover, interventions targeting dysbiosis appear capable of providing benefits against disease. Further work will form the basis for developing novel microbiome-based therapies to prevent or slow down the progression of the disease.
CONCLUSION
Rodent and human studies examining various gastrointestinal tract regions indicate that AD changes are not limited to the brain. Several histologic, biochemical, metabolic, and immune changes identified from AD intestines and mouse models support this idea. (Fig. 1). Future work will need to better define the temporal relationship between disease-associated dysfunction in both humans and AD models across these organs to define a relationship between gastrointestinal and brain changes in AD. More importantly, an assessment of intestinal immune, nervous, and endocrine function will need to be defined for AD. Increased effort to quantify histopathology, immune changes, and neuron dysfunction in the gastrointestinal tract of diseased individuals or rodent models is needed. Finally, specific interventions to test the causality of correlative relationships between the intestine and brain changes in AD are needed, which may call for increased specialization in gastrointestinal neuroscience.
ACKNOWLEDGMENTS
Figures were created with Biorender.com.
FUNDING
This work was supported by NIH awards 1R01AG042819, R01AG048993, P20GM103442, P20GM113123, RF1AG069378, RF1AG072727, AARF-17-533143, AARF-21-850265, and U54GM128729.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Aziz Q, Thompson DG. Brain-gut axis in health and disease. Gastroenterology. 1998;114(3):559–78. [DOI] [PubMed] [Google Scholar]
- 2.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nature reviews Gastroenterology & hepatology. 2009;6(5):306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waxenbaum JA, Varacallo M. Anatomy, autonomic nervous system. StatPearls [Internet]: StatPearls Publishing; 2019. [PubMed] [Google Scholar]
- 4.Dinan TG, Cryan JF. Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. The Journal of physiology. 2017;595(2):489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furness JB, Callaghan BP, Rivera LR, Cho HJ. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Advances in experimental medicine and biology. 2014;817:39–71. [DOI] [PubMed] [Google Scholar]
- 6.Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nature reviews Microbiology. 2012;10(11):735–42. [DOI] [PubMed] [Google Scholar]
- 7.Turner JR. Intestinal mucosal barrier function in health and disease. Nature reviews Immunology. 2009;9(11):799–809. [DOI] [PubMed] [Google Scholar]
- 8.Dickson K, Lehmann C. Inflammatory Response to Different Toxins in Experimental Sepsis Models. Int J Mol Sci. 2019;20(18). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roh JS, Sohn DH. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018;18(4):e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pavlov VA, Tracey KJ. The vagus nerve and the inflammatory reflex--linking immunity and metabolism. Nat Rev Endocrinol. 2012;8(12):743–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Günther J, Seyfert HM. The first line of defence: insights into mechanisms and relevance of phagocytosis in epithelial cells. Semin Immunopathol. 2018;40(6):555–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Kivit S, Tobin MC, Forsyth CB, Keshavarzian A, Landay AL. Regulation of Intestinal Immune Responses through TLR Activation: Implications for Pro- and Prebiotics. Front Immunol. 2014;5:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duerkop BA, Vaishnava S, Hooper LV. Immune responses to the microbiota at the intestinal mucosal surface. Immunity. 2009;31(3):368–76. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Santana A, Diaz Heijtz R. Bacterial Peptidoglycans from Microbiota in Neurodevelopment and Behavior. Trends Mol Med. 2020;26(8):729–43. [DOI] [PubMed] [Google Scholar]
- 15.Morais LH, Schreiber HLt, Mazmanian SK. The gut microbiota-brain axis in behaviour and brain disorders. Nature reviews Microbiology. 2021;19(4):241–55. [DOI] [PubMed] [Google Scholar]
- 16.Bonaz B, Bazin T, Pellissier S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front Neurosci. 2018;12:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parker A, Fonseca S, Carding SR. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes. 2020;11(2):135–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oligschlaeger Y, Yadati T, Houben T, Condello Oliván CM, Shiri-Sverdlov R. Inflammatory Bowel Disease: A Stressed "Gut/Feeling". Cells. 2019;8(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brzozowski B, Mazur-Bialy A, Pajdo R, Kwiecien S, Bilski J, Zwolinska-Wcislo M, et al. Mechanisms by which Stress Affects the Experimental and Clinical Inflammatory Bowel Disease (IBD): Role of Brain-Gut Axis. Curr Neuropharmacol. 2016;14(8):892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jedema HP, Grace AA. Corticotropin-releasing hormone directly activates noradrenergic neurons of the locus ceruleus recorded in vitro. J Neurosci. 2004;24(43):9703–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mawdsley JE, Rampton DS. Psychological stress in IBD: new insights into pathogenic and therapeutic implications. Gut. 2005;54(10):1481–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kujawska M, Jodynis-Liebert J. What is the Evidence That Parkinson's Disease is a Prion Disorder, Which Originates in the Gut? Int J Mol Sci. 2018;19(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McElhanon BO, McCracken C, Karpen S, Sharp WG. Gastrointestinal symptoms in autism spectrum disorder: a meta-analysis. Pediatrics. 2014;133(5):872–83. [DOI] [PubMed] [Google Scholar]
- 24.Braak H, Rub U, Gai WP, Del Tredici K. Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. Journal of neural transmission (Vienna, Austria : 1996). 2003;110(5):517–36. [DOI] [PubMed] [Google Scholar]
- 25.Klingelhoefer L, Reichmann H. Pathogenesis of Parkinson disease--the gut-brain axis and environmental factors. Nature reviews Neurology. 2015;11(11):625–36. [DOI] [PubMed] [Google Scholar]
- 26.Cabal A, Alonso-Cortina V, Gonzalez-Vazquez LO, Naves FJ, Del Valle ME, Vega JA. beta-Amyloid precursor protein (beta APP) in human gut with special reference to the enteric nervous system. Brain research bulletin. 1995;38(5):417–23. [DOI] [PubMed] [Google Scholar]
- 27.Joachim CL, Mori H, Selkoe DJ. Amyloid beta-protein deposition in tissues other than brain in Alzheimer's disease. Nature. 1989;341(6239):226–30. [DOI] [PubMed] [Google Scholar]
- 28.Puig KL, Lutz BM, Urquhart SA, Rebel AA, Zhou X, Manocha GD, et al. Overexpression of mutant amyloid-beta protein precursor and presenilin 1 modulates enteric nervous system. Journal of Alzheimer's disease : JAD. 2015;44(4):1263–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Semar S, Klotz M, Letiembre M, Van Ginneken C, Braun A, Jost V, et al. Changes of the enteric nervous system in amyloid-beta protein precursor transgenic mice correlate with disease progression. Journal of Alzheimer's disease : JAD. 2013;36(1):7–20. [DOI] [PubMed] [Google Scholar]
- 30.2021 Alzheimer's disease facts and figures. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2021;17(3):327–406. [DOI] [PubMed] [Google Scholar]
- 31.Page RC, Eke PI. Case Definitions for Use in Population-Based Surveillance of Periodontitis. Journal of periodontology. 2007;78 Suppl 7S:1387–99. [DOI] [PubMed] [Google Scholar]
- 32.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366(9499):1809–20. [DOI] [PubMed] [Google Scholar]
- 33.Singhrao SK, Harding A, Poole S, Kesavalu L, Crean S. Porphyromonas gingivalis Periodontal Infection and Its Putative Links with Alzheimer's Disease. Mediators of inflammation. 2015;2015:137357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tonsekar PP, Jiang SS, Yue G. Periodontal disease, tooth loss and dementia: Is there a link? A systematic review. Gerodontology. 2017;34(2):151–63. [DOI] [PubMed] [Google Scholar]
- 35.Kamer AR, Craig RG, Pirraglia E, Dasanayake AP, Norman RG, Boylan RJ, et al. TNF-alpha and antibodies to periodontal bacteria discriminate between Alzheimer's disease patients and normal subjects. Journal of neuroimmunology. 2009;216(1-2):92–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamer AR, Dasanayake AP, Craig RG, Glodzik-Sobanska L, Bry M, de Leon MJ. Alzheimer's disease and peripheral infections: the possible contribution from periodontal infections, model and hypothesis. Journal of Alzheimer's disease : JAD. 2008;13(4):437–49. [DOI] [PubMed] [Google Scholar]
- 37.Kamer AR, Pirraglia E, Tsui W, Rusinek H, Vallabhajosula S, Mosconi L, et al. Periodontal disease associates with higher brain amyloid load in normal elderly. Neurobiology of aging. 2015;36(2):627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kubota T, Maruyama S, Abe D, Tomita T, Morozumi T, Nakasone N, et al. Amyloid beta (A4) precursor protein expression in human periodontitis-affected gingival tissues. Archives of oral biology. 2014;59(6):586–94. [DOI] [PubMed] [Google Scholar]
- 39.Chen CK, Wu YT, Chang YC. Association between chronic periodontitis and the risk of Alzheimer's disease: a retrospective, population-based, matched-cohort study. Alzheimer's research & therapy. 2017;9(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sung CE, Huang RY, Cheng WC, Kao TW, Chen WL. Association between periodontitis and cognitive impairment: Analysis of national health and nutrition examination survey (NHANES) III. Journal of clinical periodontology. 2019;46(8):790–8. [DOI] [PubMed] [Google Scholar]
- 41.Onozuka M, Watanabe K, Nagasaki S, Jiang Y, Ozono S, Nishiyama K, et al. Impairment of spatial memory and changes in astroglial responsiveness following loss of molar teeth in aged SAMP8 mice. Behavioural brain research. 2000;108(2):145–55. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe K, Ozono S, Nishiyama K, Saito S, Tonosaki K, Fujita M, et al. The molarless condition in aged SAMP8 mice attenuates hippocampal Fos induction linked to water maze performance. Behavioural brain research. 2002;128(1):19–25. [DOI] [PubMed] [Google Scholar]
- 43.Holmes C. Review: systemic inflammation and Alzheimer's disease. Neuropathology and applied neurobiology. 2013;39(1):51–68. [DOI] [PubMed] [Google Scholar]
- 44.Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nature reviews Immunology. 2007;7(2):161–7. [DOI] [PubMed] [Google Scholar]
- 45.Pazos P, Leira Y, Dominguez C, Pias-Peleteiro JM, Blanco J, Aldrey JM. Association between periodontal disease and dementia: A literature review. Neurologia. 2018;33(9):602–13. [DOI] [PubMed] [Google Scholar]
- 46.Wilson W, Taubert KA, Gewitz M, Lockhart PB, Baddour LM, Levison M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Journal of the American Dental Association. 2008;139 Suppl:3S–24S. [DOI] [PubMed] [Google Scholar]
- 47.Itzhaki RF, Wozniak MA, Appelt DM, Balin BJ. Infiltration of the brain by pathogens causes Alzheimer's disease. Neurobiology of aging. 2004;25(5):619–27. [DOI] [PubMed] [Google Scholar]
- 48.Riviere GR, Riviere KH, Smith KS. Molecular and immunological evidence of oral Treponema in the human brain and their association with Alzheimer's disease. Oral microbiology and immunology. 2002;17(2):113–8. [DOI] [PubMed] [Google Scholar]
- 49.Ganesh P, Karthikeyan R, Muthukumaraswamy A, Anand J. A Potential Role of Periodontal Inflammation in Alzheimer's Disease: A Review. Oral health & preventive dentistry. 2017;15(1):7–12. [DOI] [PubMed] [Google Scholar]
- 50.Miklossy J. Alzheimer's disease--a spirochetosis? Neuroreport. 1993;4(7):841–8. [PubMed] [Google Scholar]
- 51.Miklossy J, Kasas S, Zurn AD, McCall S, Yu S, McGeer PL. Persisting atypical and cystic forms of Borrelia burgdorferi and local inflammation in Lyme neuroborreliosis. Journal of neuroinflammation. 2008;5:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhan X, Stamova B, Jin LW, DeCarli C, Phinney B, Sharp FR. Gram-negative bacterial molecules associate with Alzheimer disease pathology. Neurology. 2016;87(22):2324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhan X, Stamova B, Sharp FR. Lipopolysaccharide Associates with Amyloid Plaques, Neurons and Oligodendrocytes in Alzheimer's Disease Brain: A Review. Frontiers in aging neuroscience. 2018;10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, et al. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19(10):1329–31. [DOI] [PubMed] [Google Scholar]
- 55.Forner L, Larsen T, Kilian M, Holmstrup P. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. Journal of clinical periodontology. 2006;33(6):401–7. [DOI] [PubMed] [Google Scholar]
- 56.Lucas VS, Gafan G, Dewhurst S, Roberts GJ. Prevalence, intensity and nature of bacteraemia after toothbrushing. Journal of dentistry. 2008;36(7):481–7. [DOI] [PubMed] [Google Scholar]
- 57.Hafezi-Moghadam A, Thomas KL, Wagner DD. ApoE deficiency leads to a progressive age-dependent blood-brain barrier leakage. American journal of physiology Cell physiology. 2007;292(4):C1256–62. [DOI] [PubMed] [Google Scholar]
- 58.Ranjan R, Abhinay A, Mishra M. Can oral microbial infections be a risk factor for neurodegeneration? A review of the literature. Neurology India. 2018;66(2):344–51. [DOI] [PubMed] [Google Scholar]
- 59.Singhrao SK, Chukkapalli S, Poole S, Velsko I, Crean SJ, Kesavalu L. Chronic Porphyromonas gingivalis infection accelerates the occurrence of age-related granules in ApoE(−)(/)(−) mice brains. Journal of oral microbiology. 2017;9(1):1270602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Habgood MD, Bye N, Dziegielewska KM, Ek CJ, Lane MA, Potter A, et al. Changes in blood-brain barrier permeability to large and small molecules following traumatic brain injury in mice. The European journal of neuroscience. 2007;25(1):231–8. [DOI] [PubMed] [Google Scholar]
- 61.Banks WA, Farr SA, Morley JE. Entry of blood-borne cytokines into the central nervous system: effects on cognitive processes. Neuroimmunomodulation. 2002;10(6):319–27. [DOI] [PubMed] [Google Scholar]
- 62.Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, van Swieten JC, et al. Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Archives of neurology. 2004;61(5):668–72. [DOI] [PubMed] [Google Scholar]
- 63.Perry VH. The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain, behavior, and immunity. 2004;18(5):407–13. [DOI] [PubMed] [Google Scholar]
- 64.Kamer AR, Craig RG, Dasanayake AP, Brys M, Glodzik-Sobanska L, de Leon MJ. Inflammation and Alzheimer's disease: possible role of periodontal diseases. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2008;4(4):242–50. [DOI] [PubMed] [Google Scholar]
- 65.Montgomery SL, Bowers WJ. Tumor necrosis factor-alpha and the roles it plays in homeostatic and degenerative processes within the central nervous system. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2012;7(1):42–59. [DOI] [PubMed] [Google Scholar]
- 66.Park KM, Bowers WJ. Tumor necrosis factor-alpha mediated signaling in neuronal homeostasis and dysfunction. Cellular signalling. 2010;22(7):977–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duong T, Nikolaeva M, Acton PJ. C-reactive protein-like immunoreactivity in the neurofibrillary tangles of Alzheimer's disease. Brain research. 1997;749(1):152–6. [DOI] [PubMed] [Google Scholar]
- 68.Akiyama H, Arai T, Kondo H, Tanno E, Haga C, Ikeda K. Cell mediators of inflammation in the Alzheimer disease brain. Alzheimer disease and associated disorders. 2000;14 Suppl 1:S47–53. [DOI] [PubMed] [Google Scholar]
- 69.McGeer PL, Rogers J, McGeer EG. Inflammation, anti-inflammatory agents and Alzheimer disease: the last 12 years. Journal of Alzheimer's disease : JAD. 2006;9(3 Suppl):271–6. [DOI] [PubMed] [Google Scholar]
- 70.Ilievski V, Zuchowska PK, Green SJ, Toth PT, Ragozzino ME, Le K, et al. Chronic oral application of a periodontal pathogen results in brain inflammation, neurodegeneration and amyloid beta production in wild type mice. PloS one. 2018;13(10):e0204941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ding Y, Ren J, Yu H, Yu W, Zhou Y. Porphyromonas gingivalis, a periodontitis causing bacterium, induces memory impairment and age-dependent neuroinflammation in mice. Immunity & ageing : I & A. 2018;15:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ishida N, Ishihara Y, Ishida K, Tada H, Funaki-Kato Y, Hagiwara M, et al. Periodontitis induced by bacterial infection exacerbates features of Alzheimer's disease in transgenic mice. NPJ aging and mechanisms of disease. 2017;3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Naorungroj S, Schoenbach VJ, Wruck L, Mosley TH, Gottesman RF, Alonso A, et al. Tooth loss, periodontal disease, and cognitive decline in the Atherosclerosis Risk in Communities (ARIC) study. Community dentistry and oral epidemiology. 2015;43(1):47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tucker KL, Maras J, Champagne C, Connell C, Goolsby S, Weber J, et al. A regional food-frequency questionnaire for the US Mississippi Delta. Public health nutrition. 2005;8(1):87–96. [PubMed] [Google Scholar]
- 75.Yu YH, Kuo HK, Lai YL. The association between serum folate levels and periodontal disease in older adults: data from the National Health and Nutrition Examination Survey 2001/02. Journal of the American Geriatrics Society. 2007;55(1):108–13. [DOI] [PubMed] [Google Scholar]
- 76.Lexomboon D, Trulsson M, Wardh I, Parker MG. Chewing ability and tooth loss: association with cognitive impairment in an elderly population study. Journal of the American Geriatrics Society. 2012;60(10):1951–6. [DOI] [PubMed] [Google Scholar]
- 77.Yeung CK, Fu KH, Yuen KY, Ng WF, Tsang TM, Branicki FJ, et al. Helicobacter pylori and associated duodenal ulcer. Archives of disease in childhood. 1990;65(11):1212–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tatsuta M, Ishikawa H, Iishi H, Okuda S, Yokota Y. Reduction of gastric ulcer recurrence after suppression of Helicobacter pylori by cefixime. Gut. 1990;31(9):973–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rauws EA, Tytgat GN. Cure of duodenal ulcer associated with eradication of Helicobacter pylori. Lancet. 1990;335(8700):1233–5. [DOI] [PubMed] [Google Scholar]
- 80.Doulberis M, Kotronis G, Gialamprinou D, Polyzos SA, Papaefthymiou A, Katsinelos P, et al. Alzheimer's disease and gastrointestinal microbiota; impact of Helicobacter pylori infection involvement. The International journal of neuroscience. 2020:1–13. [DOI] [PubMed] [Google Scholar]
- 81.Alvarez-Arellano L, Maldonado-Bernal C. Helicobacter pylori and neurological diseases: Married by the laws of inflammation. World journal of gastrointestinal pathophysiology. 2014;5(4):400–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Budzynski J, Klopocka M. Brain-gut axis in the pathogenesis of Helicobacter pylori infection. World journal of gastroenterology. 2014;20(18):5212–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bercik P, Verdú EF, Foster JA, Lu J, Scharringa A, Kean I, et al. Role of gut-brain axis in persistent abnormal feeding behavior in mice following eradication of Helicobacter pylori infection. American journal of physiology Regulatory, integrative and comparative physiology. 2009;296(3):R587–94. [DOI] [PubMed] [Google Scholar]
- 84.Burns M, Amaya A, Bodi C, Ge Z, Bakthavatchalu V, Ennis K, et al. Helicobacter pylori infection and low dietary iron alter behavior, induce iron deficiency anemia, and modulate hippocampal gene expression in female C57BL/6 mice. PloS one. 2017;12(3):e0173108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Albaret G, Sifré E, Floch P, Laye S, Aubert A, Dubus P, et al. Alzheimer's Disease and Helicobacter pylori Infection: Inflammation from Stomach to Brain? Journal of Alzheimer's disease : JAD. 2020;73(2):801–9. [DOI] [PubMed] [Google Scholar]
- 86.Contaldi F, Capuano F, Fulgione A, Aiese Cigliano R, Sanseverino W, Iannelli D, et al. The hypothesis that Helicobacter pylori predisposes to Alzheimer's disease is biologically plausible. Scientific reports. 2017;7(1):7817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang XL, Zeng J, Yang Y, Xiong Y, Zhang ZH, Qiu M, et al. Helicobacter pylori filtrate induces Alzheimer-like tau hyperphosphorylation by activating glycogen synthase kinase-3β. Journal of Alzheimer's disease : JAD. 2015;43(1):153–65. [DOI] [PubMed] [Google Scholar]
- 88.Zhou H, Guo Y, Li X, Liuyang ZY, Shentu YP, Jing XP, et al. Long-term Helicobacter pylori infection does not induce tauopathy and memory impairment in SD rats. Journal of Huazhong University of Science and Technology Medical sciences = Hua zhong ke ji da xue xue bao Yi xue Ying De wen ban = Huazhong keji daxue xuebao Yixue Yingdewen ban. 2017;37(6):823–7. [DOI] [PubMed] [Google Scholar]
- 89.Kountouras J, Boziki M, Gavalas E, Zavos C, Grigoriadis N, Deretzi G, et al. Eradication of Helicobacter pylori may be beneficial in the management of Alzheimer's disease. Journal of neurology. 2009;256(5):758–67. [DOI] [PubMed] [Google Scholar]
- 90.Jeong YO, Shin SJ, Park JY, Ku BK, Song JS, Kim JJ, et al. MK-0677, a Ghrelin Agonist, Alleviates Amyloid Beta-Related Pathology in 5XFAD Mice, an Animal Model of Alzheimer's Disease. Int J Mol Sci. 2018;19(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cao X, Zhu M, He Y, Chu W, Du Y, Du H. Increased Serum Acylated Ghrelin Levels in Patients with Mild Cognitive Impairment. Journal of Alzheimer's disease : JAD. 2018;61(2):545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Galloway S, Pallebage-Gamarallage MM, Takechi R, Jian L, Johnsen RD, Dhaliwal SS, et al. Synergistic effects of high fat feeding and apolipoprotein E deletion on enterocytic amyloid-beta abundance. Lipids in health and disease. 2008;7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Galloway S, Jian L, Johnsen R, Chew S, Mamo JC. beta-amyloid or its precursor protein is found in epithelial cells of the small intestine and is stimulated by high-fat feeding. The Journal of nutritional biochemistry. 2007;18(4):279–84. [DOI] [PubMed] [Google Scholar]
- 94.Galloway S, Takechi R, Nesbit M, Pallebage-Gamarallage MM, Lam V, Mamo JCL. The differential effects of fatty acids on enterocytic abundance of amyloid-beta. Lipids in health and disease. 2019;18(1):209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Galloway S, Takechi R, Pallebage-Gamarallage MM, Dhaliwal SS, Mamo JC. Amyloid-beta colocalizes with apolipoprotein B in absorptive cells of the small intestine. Lipids in health and disease. 2009;8:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shankle WR, Landing BH, Ang SM, Chui H, Villarreal-Engelhardt G, Zarow C. Studies of the enteric nervous system in Alzheimer disease and other dementias of the elderly: enteric neurons in Alzheimer disease. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 1993;6(1):10–4. [PubMed] [Google Scholar]
- 97.Sun Y, Sommerville NR, Liu JYH, Ngan MP, Poon D, Ponomarev ED, et al. Intra-gastrointestinal amyloid-beta1-42 oligomers perturb enteric function and induce Alzheimer's disease pathology. The Journal of physiology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Han X, Tang S, Dong L, Song L, Dong Y, Wang Y, et al. Loss of nitrergic and cholinergic neurons in the enteric nervous system of APP/PS1 transgenic mouse model. Neuroscience letters. 2017;642:59–65. [DOI] [PubMed] [Google Scholar]
- 99.Kremerskothen J, Plaas C, Buther K, Finger I, Veltel S, Matanis T, et al. Characterization of KIBRA, a novel WW domain-containing protein. Biochemical and biophysical research communications. 2003;300(4):862–7. [DOI] [PubMed] [Google Scholar]
- 100.Johannsen S, Duning K, Pavenstädt H, Kremerskothen J, Boeckers TM. Temporal-spatial expression and novel biochemical properties of the memory-related protein KIBRA. Neuroscience. 2008;155(4):1165–73. [DOI] [PubMed] [Google Scholar]
- 101.Corneveaux JJ, Liang WS, Reiman EM, Webster JA, Myers AJ, Zismann VL, et al. Evidence for an association between KIBRA and late-onset Alzheimer's disease. Neurobiology of aging. 2010;31(6):901–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Feng J, Dong L, Zhang J, Han X, Tang S, Song L, et al. Unique expression pattern of KIBRA in the enteric nervous system of APP/PS1 mice. Neuroscience letters. 2018;675:41–7. [DOI] [PubMed] [Google Scholar]
- 103.Manocha GD, Floden AM, Miller NM, Smith AJ, Nagamoto-Combs K, Saito T, et al. Temporal progression of Alzheimer's disease in brains and intestines of transgenic mice. Neurobiology of aging. 2019;81:166–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Neurath MF. Cytokines in inflammatory bowel disease. Nature reviews Immunology. 2014;14(5):329–42. [DOI] [PubMed] [Google Scholar]
- 105.Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140(6):1785–94. [DOI] [PubMed] [Google Scholar]
- 106.Taleban S, Colombel JF, Mohler MJ, Fain MJ. Inflammatory bowel disease and the elderly: a review. Journal of Crohn's & colitis. 2015;9(6):507–15. [DOI] [PubMed] [Google Scholar]
- 107.Attree EA, Dancey CP, Keeling D, Wilson C. Cognitive function in people with chronic illness: inflammatory bowel disease and irritable bowel syndrome. Applied neuropsychology. 2003;10(2):96–104. [DOI] [PubMed] [Google Scholar]
- 108.Kurina LM, Goldacre MJ, Yeates D, Gill LE. Depression and anxiety in people with inflammatory bowel disease. Journal of epidemiology and community health. 2001;55(10):716–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Petruo VA, Zeißig S, Schmelz R, Hampe J, Beste C. Specific neurophysiological mechanisms underlie cognitive inflexibility in inflammatory bowel disease. Scientific reports. 2017;7(1):13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Current protocols in immunology. 2014;104:15.25.1–15.25.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ng SC, Benjamin JL, McCarthy NE, Hedin CR, Koutsoumpas A, Plamondon S, et al. Relationship between human intestinal dendritic cells, gut microbiota, and disease activity in Crohn's disease. Inflammatory bowel diseases. 2011;17(10):2027–37. [DOI] [PubMed] [Google Scholar]
- 112.Ślebioda TJ, Kmieć Z. Tumour necrosis factor superfamily members in the pathogenesis of inflammatory bowel disease. Mediators of inflammation. 2014;2014:325129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Holmes C, Cunningham C, Zotova E, Woolford J, Dean C, Kerr S, et al. Systemic inflammation and disease progression in Alzheimer disease. Neurology. 2009;73(10):768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Riazi K, Galic MA, Kuzmiski JB, Ho W, Sharkey KA, Pittman QJ. Microglial activation and TNFalpha production mediate altered CNS excitability following peripheral inflammation. Proc Natl Acad Sci U S A. 2008;105(44):17151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sohrabi M, Pecoraro HL, Combs CK. Gut Inflammation Induced by Dextran Sulfate Sodium Exacerbates Amyloid-β Plaque Deposition in the AppNL-G-F Mouse Model of Alzheimer's Disease. Journal of Alzheimer's disease : JAD. 2021;79(3):1235–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jang SE, Lim SM, Jeong JJ, Jang HM, Lee HJ, Han MJ, et al. Gastrointestinal inflammation by gut microbiota disturbance induces memory impairment in mice. Mucosal Immunol. 2018;11(2):369–79. [DOI] [PubMed] [Google Scholar]
- 117.Zonis S, Pechnick RN, Ljubimov VA, Mahgerefteh M, Wawrowsky K, Michelsen KS, et al. Chronic intestinal inflammation alters hippocampal neurogenesis. Journal of neuroinflammation. 2015;12:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Emge JR, Huynh K, Miller EN, Kaur M, Reardon C, Barrett KE, et al. Modulation of the microbiota-gut-brain axis by probiotics in a murine model of inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2016;310(11):G989–98. [DOI] [PubMed] [Google Scholar]
- 119.Wang SL, Shao BZ, Zhao SB, Chang X, Wang P, Miao CY, et al. Intestinal autophagy links psychosocial stress with gut microbiota to promote inflammatory bowel disease. Cell death & disease. 2019;10(6):391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sgambato D, Miranda A, Ranaldo R, Federico A, Romano M. The Role of Stress in Inflammatory Bowel Diseases. Current pharmaceutical design. 2017;23(27):3997–4002. [DOI] [PubMed] [Google Scholar]
- 121.Bernstein CN, Singh S, Graff LA, Walker JR, Miller N, Cheang M. A prospective population-based study of triggers of symptomatic flares in IBD. The American journal of gastroenterology. 2010;105(9):1994–2002. [DOI] [PubMed] [Google Scholar]
- 122.Zhang B, Wang HE, Bai YM, Tsai SJ, Su TP, Chen TJ, et al. Inflammatory bowel disease is associated with higher dementia risk: a nationwide longitudinal study. Gut. 2021;70(1):85–91. [DOI] [PubMed] [Google Scholar]
- 123.Sochocka M, Donskow-Lysoniewska K, Diniz BS, Kurpas D, Brzozowska E, Leszek J. The Gut Microbiome Alterations and Inflammation-Driven Pathogenesis of Alzheimer's Disease-a Critical Review. Molecular neurobiology. 2019;56(3):1841–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Moir RD, Lathe R, Tanzi RE. The antimicrobial protection hypothesis of Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2018;14(12):1602–14. [DOI] [PubMed] [Google Scholar]
- 125.Soscia SJ, Kirby JE, Washicosky KJ, Tucker SM, Ingelsson M, Hyman B, et al. The Alzheimer's disease-associated amyloid beta-protein is an antimicrobial peptide. PloS one. 2010;5(3):e9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kumar DK, Choi SH, Washicosky KJ, Eimer WA, Tucker S, Ghofrani J, et al. Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer's disease. Science translational medicine. 2016;8(340):340ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Annals of gastroenterology. 2015;28(2):203–9. [PMC free article] [PubMed] [Google Scholar]
- 128.Turner PV. The role of the gut microbiota on animal model reproducibility. Animal models and experimental medicine. 2018;1(2):109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sweeney TE, Morton JM. The human gut microbiome: a review of the effect of obesity and surgically induced weight loss. JAMA surgery. 2013;148(6):563–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tamboli CP, Neut C, Desreumaux P, Colombel JF. Dysbiosis in inflammatory bowel disease. Gut. 2004;53(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Durack J, Lynch SV. The gut microbiome: Relationships with disease and opportunities for therapy. The Journal of experimental medicine. 2019;216(1):20–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microbial ecology in health and disease. 2015;26:26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Erny D, Hrabe de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nature neuroscience. 2015;18(7):965–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Elahy M, Jackaman C, Mamo JC, Lam V, Dhaliwal SS, Giles C, et al. Blood-brain barrier dysfunction developed during normal aging is associated with inflammation and loss of tight junctions but not with leukocyte recruitment. Immunity & ageing : I & A. 2015;12:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jiang C, Li G, Huang P, Liu Z, Zhao B. The Gut Microbiota and Alzheimer's Disease. Journal of Alzheimer's disease : JAD. 2017;58(1):1–15. [DOI] [PubMed] [Google Scholar]
- 136.Dos Santos Guilherme M, Zevallos VF, Pesi A, Stoye NM, Nguyen VTT, Radyushkin K, et al. Dietary Wheat Amylase Trypsin Inhibitors Impact Alzheimer's Disease Pathology in 5xFAD Model Mice. Int J Mol Sci. 2020;21(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kaur H, Nagamoto-Combs K, Golovko S, Golovko MY, Klug MG, Combs CK. Probiotics ameliorate intestinal pathophysiology in a mouse model of Alzheimer's disease. Neurobiology of aging. 2020;92:114–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Weiss GA, Hennet T. Mechanisms and consequences of intestinal dysbiosis. Cellular and molecular life sciences : CMLS. 2017;74(16):2959–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nature reviews Microbiology. 2009;7(12):887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wilkins LJ, Monga M, Miller AW. Defining Dysbiosis for a Cluster of Chronic Diseases. Scientific reports. 2019;9(1):12918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dos Santos Guilherme M, Todorov H, Osterhof C, Mollerke A, Cub K, Hankeln T, et al. Impact of Acute and Chronic Amyloid-beta Peptide Exposure on Gut Microbial Commensals in the Mouse. Frontiers in microbiology. 2020;11:1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Brandscheid C, Schuck F, Reinhardt S, Schafer KH, Pietrzik CU, Grimm M, et al. Altered Gut Microbiome Composition and Tryptic Activity of the 5xFAD Alzheimer's Mouse Model. Journal of Alzheimer's disease : JAD. 2017;56(2):775–88. [DOI] [PubMed] [Google Scholar]
- 143.Harach T, Marungruang N, Duthilleul N, Cheatham V, Mc Coy KD, Frisoni G, et al. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Scientific reports. 2017;7:41802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shen L, Liu L, Ji HF. Alzheimer's Disease Histological and Behavioral Manifestations in Transgenic Mice Correlate with Specific Gut Microbiome State. Journal of Alzheimer's disease : JAD. 2017;56(1):385–90. [DOI] [PubMed] [Google Scholar]
- 145.Zhang L, Wang Y, Xiayu X, Shi C, Chen W, Song N, et al. Altered Gut Microbiota in a Mouse Model of Alzheimer's Disease. Journal of Alzheimer's disease : JAD. 2017;60(4):1241–57. [DOI] [PubMed] [Google Scholar]
- 146.Cattaneo A, Cattane N, Galluzzi S, Provasi S, Lopizzo N, Festari C, et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiology of aging. 2017;49:60–8. [DOI] [PubMed] [Google Scholar]
- 147.Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, Wesselingh S. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Molecular psychiatry. 2016;21(6):738–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Novotny M, Klimova B, Valis M. Microbiome and Cognitive Impairment: Can Any Diets Influence Learning Processes in a Positive Way? Frontiers in aging neuroscience. 2019;11:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Walker KA, Gottesman RF, Wu A, Knopman DS, Gross AL, Mosley TH Jr., et al. Systemic inflammation during midlife and cognitive change over 20 years: The ARIC Study. Neurology. 2019;92(11):e1256–e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, Johnson SC, et al. Gut microbiome alterations in Alzheimer's disease. Scientific reports. 2017;7(1):13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Liu P, Wu L, Peng G, Han Y, Tang R, Ge J, et al. Altered microbiomes distinguish Alzheimer's disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain, behavior, and immunity. 2019;80:633–43. [DOI] [PubMed] [Google Scholar]
- 152.Zhuang ZQ, Shen LL, Li WW, Fu X, Zeng F, Gui L, et al. Gut Microbiota is Altered in Patients with Alzheimer's Disease. Journal of Alzheimer's disease : JAD. 2018;63(4):1337–46. [DOI] [PubMed] [Google Scholar]
- 153.Cox LM, Schafer MJ, Sohn J, Vincentini J, Weiner HL, Ginsberg SD, et al. Calorie restriction slows age-related microbiota changes in an Alzheimer's disease model in female mice. Scientific reports. 2019;9(1):17904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. The Journal of physiology. 2004;558(Pt 1):263–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lu J, Synowiec S, Lu L, Yu Y, Bretherick T, Takada S, et al. Microbiota influence the development of the brain and behaviors in C57BL/6J mice. PloS one. 2018;13(8):e0201829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2011;23(3):255–64, e119. [DOI] [PubMed] [Google Scholar]
- 157.Minter MR, Zhang C, Leone V, Ringus DL, Zhang X, Oyler-Castrillo P, et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer's disease. Scientific reports. 2016;6:30028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Honarpisheh P, Reynolds CR, Blasco Conesa MP, Moruno Manchon JF, Putluri N, Bhattacharjee MB, et al. Dysregulated Gut Homeostasis Observed Prior to the Accumulation of the Brain Amyloid-β in Tg2576 Mice. Int J Mol Sci. 2020;21(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cherny I, Rockah L, Levy-Nissenbaum O, Gophna U, Ron EZ, Gazit E. The formation of Escherichia coli curli amyloid fibrils is mediated by prion-like peptide repeats. J Mol Biol. 2005;352(2):245–52. [DOI] [PubMed] [Google Scholar]
- 160.Friedland RP. Mechanisms of molecular mimicry involving the microbiota in neurodegeneration. Journal of Alzheimer's disease : JAD. 2015;45(2):349–62. [DOI] [PubMed] [Google Scholar]
- 161.Zhao Y, Jaber V, Lukiw WJ. Secretory Products of the Human GI Tract Microbiome and Their Potential Impact on Alzheimer's Disease (AD): Detection of Lipopolysaccharide (LPS) in AD Hippocampus. Frontiers in cellular and infection microbiology. 2017;7:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lukiw WJ. Bacteroides fragilis Lipopolysaccharide and Inflammatory Signaling in Alzheimer's Disease. Frontiers in microbiology. 2016;7:1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhao Y, Lukiw WJ. Microbiome-generated amyloid and potential impact on amyloidogenesis in Alzheimer's disease (AD). Journal of nature and science. 2015;1(7). [PMC free article] [PubMed] [Google Scholar]
- 164.Hill JM, Lukiw WJ. Microbial-generated amyloids and Alzheimer's disease (AD). Frontiers in aging neuroscience. 2015;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bulgart HR, Neczypor EW, Wold LE, Mackos AR. Microbial involvement in Alzheimer disease development and progression. Molecular neurodegeneration. 2020;15(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ho L, Ono K, Tsuji M, Mazzola P, Singh R, Pasinetti GM. Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer's disease-type beta-amyloid neuropathological mechanisms. Expert review of neurotherapeutics. 2018;18(1):83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Vogt NM, Romano KA, Darst BF, Engelman CD, Johnson SC, Carlsson CM, et al. The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer's disease. Alzheimer's research & therapy. 2018;10(1):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gao Q, Wang Y, Wang X, Fu S, Zhang X, Wang RT, et al. Decreased levels of circulating trimethylamine N-oxide alleviate cognitive and pathological deterioration in transgenic mice: a potential therapeutic approach for Alzheimer's disease. Aging (Albany NY). 2019;11(19):8642–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Quinn M, McMillin M, Galindo C, Frampton G, Pae HY, DeMorrow S. Bile acids permeabilize the blood brain barrier after bile duct ligation in rats via Rac1-dependent mechanisms. Dig Liver Dis. 2014;46(6):527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kiriyama Y, Nochi H. The Biosynthesis, Signaling, and Neurological Functions of Bile Acids. Biomolecules. 2019;9(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol. 2014;30(3):332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.MahmoudianDehkordi S, Arnold M, Nho K, Ahmad S, Jia W, Xie G, et al. Altered bile acid profile associates with cognitive impairment in Alzheimer's disease-An emerging role for gut microbiome. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2019;15(1):76–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Baloni P, Funk CC, Yan J, Yurkovich JT, Kueider-Paisley A, Nho K, et al. Metabolic Network Analysis Reveals Altered Bile Acid Synthesis and Metabolism in Alzheimer's Disease. Cell Rep Med. 2020;1(8):100138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Loeb MB, Molloy DW, Smieja M, Standish T, Goldsmith CH, Mahony J, et al. A randomized, controlled trial of doxycycline and rifampin for patients with Alzheimer's disease. Journal of the American Geriatrics Society. 2004;52(3):381–7. [DOI] [PubMed] [Google Scholar]
- 175.Iqbal UH, Zeng E, Pasinetti GM. The Use of Antimicrobial and Antiviral Drugs in Alzheimer's Disease. Int J Mol Sci. 2020;21(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Panza F, Lozupone M, Solfrizzi V, Watling M, Imbimbo BP. Time to test antibacterial therapy in Alzheimer's disease. Brain : a journal of neurology. 2019;142(10):2905–29. [DOI] [PubMed] [Google Scholar]
- 177.Wozniak MA, Itzhaki RF. Antiviral agents in Alzheimer's disease: hope for the future? Therapeutic advances in neurological disorders. 2010;3(3):141–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bonfili L, Cecarini V, Berardi S, Scarpona S, Suchodolski JS, Nasuti C, et al. Microbiota modulation counteracts Alzheimer's disease progression influencing neuronal proteolysis and gut hormones plasma levels. Scientific reports. 2017;7(1):2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kaur H, Golovko S, Golovko MY, Singh S, Darland DC, Combs CK. Effects of Probiotic Supplementation on Short Chain Fatty Acids in the AppNL-G-F Mouse Model of Alzheimer's Disease. Journal of Alzheimer's disease : JAD. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Athari Nik Azm S, Djazayeri A, Safa M, Azami K, Ahmadvand B, Sabbaghziarani F, et al. Lactobacilli and bifidobacteria ameliorate memory and learning deficits and oxidative stress in beta-amyloid (1-42) injected rats. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2018;43(7):718–26. [DOI] [PubMed] [Google Scholar]
- 181.Kaur H, Nookala S, Singh S, Mukundan S, Nagamoto-Combs K, Combs CK. Sex-Dependent Effects of Intestinal Microbiome Manipulation in a Mouse Model of Alzheimer's Disease. Cells. 2021;10(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chen D, Yang X, Yang J, Lai G, Yong T, Tang X, et al. Prebiotic Effect of Fructooligosaccharides from Morinda officinalis on Alzheimer's Disease in Rodent Models by Targeting the Microbiota-Gut-Brain Axis. Frontiers in aging neuroscience. 2017;9:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sun J, Xu J, Ling Y, Wang F, Gong T, Yang C, et al. Fecal microbiota transplantation alleviated Alzheimer's disease-like pathogenesis in APP/PS1 transgenic mice. Translational psychiatry. 2019;9(1):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Imbimbo BP, Lozupone M, Watling M, Panza F. Discontinued disease-modifying therapies for Alzheimer's disease: status and future perspectives. Expert opinion on investigational drugs. 2020:1–15. [DOI] [PubMed] [Google Scholar]
- 185.Hazan S. Rapid improvement in Alzheimer's disease symptoms following fecal microbiota transplantation: a case report. The Journal of international medical research. 2020;48(6):300060520925930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kobayashi Y, Kuhara T, Oki M, Xiao JZ. Effects of Bifidobacterium breve A1 on the cognitive function of older adults with memory complaints: a randomised, double-blind, placebo-controlled trial. Beneficial microbes. 2019;10(5):511–20. [DOI] [PubMed] [Google Scholar]
- 187.Pistollato F, Iglesias RC, Ruiz R, Aparicio S, Crespo J, Lopez LD, et al. Nutritional patterns associated with the maintenance of neurocognitive functions and the risk of dementia and Alzheimer's disease: A focus on human studies. Pharmacological research. 2018;131:32–43. [DOI] [PubMed] [Google Scholar]
- 188.Akbari E, Asemi Z, Daneshvar Kakhaki R, Bahmani F, Kouchaki E, Tamtaji OR, et al. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer's Disease: A Randomized, Double-Blind and Controlled Trial. Frontiers in aging neuroscience. 2016;8:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hemarajata P, Versalovic J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therapeutic advances in gastroenterology. 2013;6(1):39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]