Keywords: plum, myoglianin, Drosophila melanogaster, neuromuscular junction, synapse
Abstract
Alterations in the neuromuscular system underlie several neuromuscular diseases and play critical roles in the development of sarcopenia, the age-related loss of muscle mass and function. Mammalian Myostatin (MST) and GDF11, members of the TGF-β superfamily of growth factors, are powerful regulators of muscle size in both model organisms and humans. Myoglianin (MYO), the Drosophila homologue of MST and GDF11, is a strong inhibitor of synaptic function and structure at the neuromuscular junction in flies. Here, we identified Plum, a transmembrane cell surface protein, as a modulator of MYO function in the larval neuromuscular system. Reduction of Plum in the larval body-wall muscles abolishes the previously demonstrated positive effect of attenuated MYO signalling on both muscle size and neuromuscular junction structure and function. In addition, downregulation of Plum on its own results in decreased synaptic strength and body weight, classifying Plum as a (novel) regulator of neuromuscular function and body (muscle) size. These findings offer new insights into possible regulatory mechanisms behind ageing- and disease-related neuromuscular dysfunctions in humans and identify potential targets for therapeutic interventions.
1. Introduction
The neuromuscular system is composed of individual motor units, each consisting of a single motor neuron, a neuromuscular junction (NMJ), and muscle fibres innervated by the motor neuron. Diminished motor unit function and decreased muscle volume are hallmarks of several neuromuscular disorders [1] and of sarcopenia, the age-associated loss of skeletal muscle mass and function [2]. Gradual loss of skeletal muscle capacity has been reported in invertebrates, rodents and humans [3–5], with intrinsic mechanisms regulating age-related muscle dysfunction largely conserved across species [6]. Age-related muscle loss is accompanied by progressive modifications in the structure and function of the NMJ, the specialized synapse at the interface between the nervous and muscular system [1], with the resulting uncoupling of the excitation–contraction machinery [7,8]. In mammals, including humans, these modifications include changes in the branching pattern of the motor nerve terminal that contacts the myofibre, fragmented NMJ architecture, impaired synaptic neurotransmitter distribution, and decreased density of presynaptic active zone markers [9–13]. Functionally, aged mammalian NMJs exhibit increased failures in evoked release [14], changes in quantal release [15] and a slowing-down of axoplasmic transport of proteins [16]. Skeletal muscle and NMJ deficits are found in many motoneuronal and neuromuscular disorders, with impaired neurotransmission and muscle wasting characterizing amyotrophic lateral sclerosis (ALS) [17–19], myotonic and muscular dystrophies [20–24] and myasthenia gravis [25,26]. Whether muscle loss precedes or follows the changes in the function of the NMJ is currently unresolved, but animal studies suggest that NMJ remodelling plays a critical role in the progression of sarcopenia [27].
Drosophila melanogaster is a convenient and proven model system for studying various aspects of developmental regulation of muscle mass and control of NMJ function [28–30]. Drosophila larval glutamatergic NMJs share structural and functional similarities with mammalian junctions [31] and striated muscles in Drosophila resemble vertebrate skeletal muscles in structure, function, and protein composition [28]. Previously, we used this model system to investigate the role of Drosophila MYO, the muscle- and glia-expressed fly homologue of TGF-β growth factors Myostatin (MST) and GDF11 [32] in regulating synaptic function, muscle size and body weight [33]. MST (also known as GDF-8) is a circulating cytokine that serves as a powerful negative regulator of muscle mass in mammalian species [34,35]. In addition to its MST-like role as an inhibitor of larval weight and muscle size, muscle-derived MYO is a strong negative regulator of neurotransmission, synaptic morphology and the density of critical pre- and post-synaptic components [33].
Plum is a Drosophila transmembrane, immunoglobulin superfamily protein [36] and a distant homologue of Protogenin, Sidekick and Nope, mammalian regulators of developmental processes in nervous, muscle and epithelial tissues [37–39]. Protogenin was also associated with attention deficit hyperactivity disorder [40]. Sidekick regulates synaptic connections in the vertebrate retina [41], and Nope is a surface marker for human and murine liver cancers [42,43]. Recently, Plum was identified as a modulator of axon pruning in the Drosophila nervous system [36]. Plum genetically interacts and interferes with MYO function, likely by sequestrating MYO [36]. In this study, we examined the interactions between Plum and MYO in regulating larval muscles and NMJ physiology. We identified Plum as a novel modulator of MYO action on NMJ synaptic transmission and muscle size, and an independent regulator of synaptic strength and larval weight.
2. Results
2.1. Muscle-derived plum regulates NMJ synapse strength independently and by modulating MYO
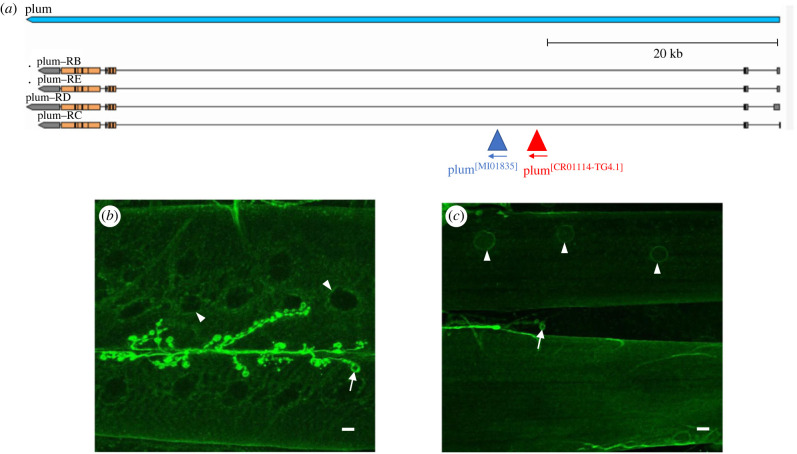
We previously showed that genetic attenuation of Myo specifically in the larval somatic muscle, using a microRNA construct to target the Myo transcript (genotype: Mef2-GAL4/UAS-miRNAmyo) [44], increases muscle size, NMJ synaptic transmission and locomotion by greater than 20% [33], defining MYO as a potent neuromuscular inhibitor in flies. Considering the expression of the Plum mammalian homologue Nope in developing skeletal muscles [37] and the microarray data indicating the expression of Plum in the body-wall muscles of WT and larvae expressing the human PAX-FKHR protein [45], we first investigated the possible expression of Plum in the larval somatic musculature. Using a previously published rabbit antibody to Plum [36], we visualized the expression of the muscular system of third instar larvae using immunofluorescence, but the result was inconclusive (data not shown). We therefore used a different strategy involving a protein trap (MI01835) that specifically introduced a GFP cassette (flanked with a splice donor and acceptor site) within an intron of the Plum transcriptional unit (figure 1a). The GFP cassette is spliced into the Plum protein, providing an alternative readout of protein expression. Importantly, the protein would also be expressed at physiologically normal levels as Plum expression is controlled from the endogenous promoter and enhancers. We detected strong expression of the Plum gene at the NMJ, particularly around the periphery of the bouton, typical of post-synaptic staining. We also observed weaker expression within muscle tissue (figure 1b). The muscle expression of Plum was also confirmed using a CRIMIC Gal4 line (CR01114-TG4.1) that also located to the same intron as the GFP protein trap (figure 1c). These data showed that the Plum protein could be detected post-synaptically at the NMJ and within the surrounding muscle tissue.
Figure 1.
Plum is expressed at the postsynaptic NMJ and within muscle. (a) Plum transcriptional unit (blue) on chromosome 3, showing four isoforms (Plum-RB,RC, RD and RE), modified from Flybase. Two independent insertion lines were used to detect expression of the Plum protein in 3rd Instar larvae. The mimic protein trap line MI01835 (red) and CRIMIC line CR01114-TG4.1 containing the GAL4 cassette (blue) insertion sites show that these tools detect all plum isoforms. (b) Protein trap clearly shows Plum expression at the neuromuscular junction (arrow) and perinuclear staining in muscle nuclei (arrowhead) as well as low level staining in muscle fibres. (c) CRIMIC line driving the expression of membrane localized CD8::GFP shows perinuclear staining (arrowheads) and staining at the NMJ. Note the staining is localized to the outer bouton membrane, indicating postsynaptic localization (arrows in b and c). Scale bars indicate 10 µm.
We examined the effect of overexpressing Myo and/or Plum in muscles. There was no significant electrophysiological differences observed when Myo, Plum or both were overexpressed in the muscle (electronic supplementary material, figure S1). We then assessed the impact of Plum downregulation on NMJ physiology, when expressed in the muscles. We analysed phenotypes in double knock-down Myo-Plum larvae (Mef2-GAL4/UAS-miRNAmyo/plumRNAi) and single knock-down Plum animals (Mef2-GAL4/UAS-plumRNAi).
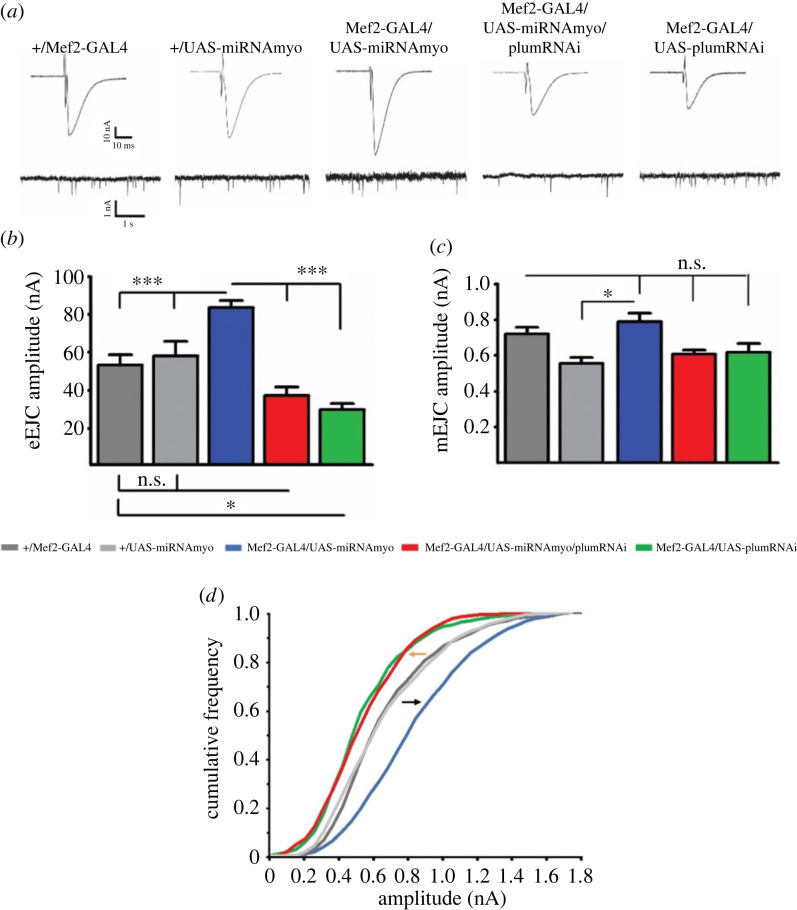
Body-wall muscles in developing larvae consist of bilaterally symmetrical hemi-segments composed of 30 multinucleated muscle fibres [46]. We focused our analyses on muscles 6 and 7, large myofibres innervated by two motoneurons forming a single, excitatory, glutamatergic NMJ. Contractions of these muscles are triggered by ‘non-spiking’ postsynaptic potentials that are graded in duration and amplitude, allowing for quantitative comparisons between genotypes [47]. The amplitude of these Ca2+-dependent, nerve-evoked postsynaptic excitatory junctional currents (eEJCs) reflects either the magnitude of presynaptic release or the postsynaptic sensitivity to neurotransmitter [47]. Muscle-specific reduction of MYO leads to dramatically increased evoked response [33]; simultaneous suppression of Plum, however, reversed the response to control (+ Mef2-GAL4) levels, with the downregulation of Plum alone further diminishing the evoked currents. Further examination of the data showed a statistically significant interaction between the effects of simultaneously reduced MYO and Plum relative to the control genotype (two-way ANOVA analysis in figure 2b). We then measured the amplitudes of spontaneous ‘miniature’ postsynaptic currents (mEJCs), also known as ‘quantal size’ [48]. The mEJCs represent postsynaptic responses to a single presynaptically released vesicle containing neurotransmitter and are a reliable indicator of the density of functional, NMJ, glutamate receptors [49]. While the mean mEJC amplitude showed only minor differences between the genotypes (figure 2a,c), the frequency distribution analysis revealed that Plum downregulation in either control or reduced MYO background caused a strong shift toward smaller ‘miniature’ currents (figure 2d). Taken together, our electrophysiological results imply that MYO and Plum affect NMJ physiology by controlling the density of the postsynaptic glutamate receptor field, with Plum having a modulatory effect on MYO and acting as an autonomous synaptic regulator.
Figure 2.
Plum is a regulator of neurotransmission at the larval NMJ. (a) Representative traces of evoked (top) and spontaneous (miniature) synaptic responses recorded from muscle 6. Histograms of evoked (b) and spontaneous (c) responses for given genotypes (control: +/Mef2-GAL4; Myo downregulation: Mef2-GAL4/UAS-miRNAmyo; Myo and Plum downregulation: Mef2-GAL4/UAS-miRNAmyo/plumRNAi; Plum downregulation: Mef2-GAL4/UAS-plumRNAi) (n = 7–16). Two-way ANOVA analysis: the interaction between Mef2-GAL4/UAS-miRNAmyo and Mef2-GAL4/UAS-miRNAmyo/plumRNAi is highly significant (p < 0.0001). (d) Cumulative frequency distribution diagram of mEJC amplitudes. Black arrow indicates shift toward higher amplitude ‘minis’, orange toward lower. For all paired analyses except between Mef2-GAL4/UAS-miRNAmyo/plumRNAi and Mef2-GAL4/UAS-plumRNAi and between +/Mef2-GAL4 and +/UAS-miRNAmyo, KS test, p < 0.0001 (n = 7–18 animals, ∼1500–2000 events measured per genotype). Bar graphs: error bars indicate SEM (one-way ANOVA + Tukey's post-test: *p < 0.05, ***p < 0.001, n.s. = not significant).
2.2. Plum modulates the action of MYO on synapse structure and receptor composition
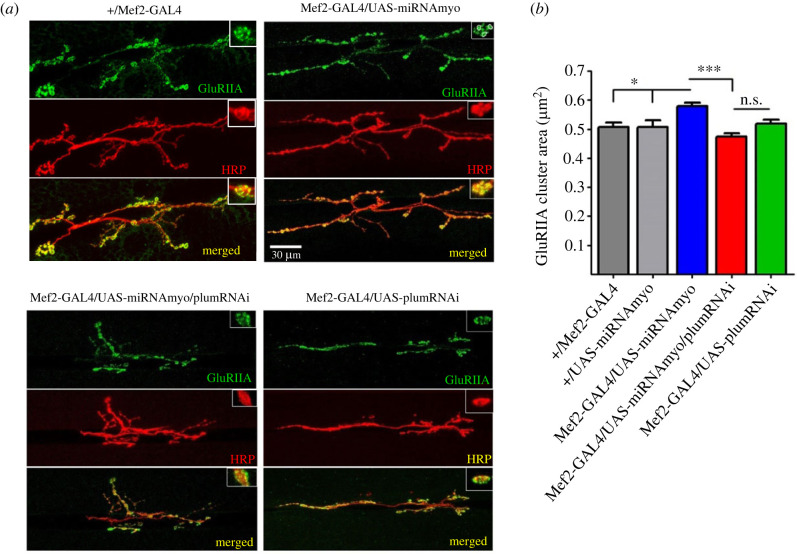
Postsynaptic receptors at the larval NMJ are AMPA-type tetrameric complexes formed by glutamate receptor (GluR) subunits IIC, IID and IIE, in addition to either subunit IIA or IIB [50,51]. Assemblies containing the IIA subunit (GluRIIA) are pharmacologically and biophysically distinct from the ones incorporating GluRIIB and carry the bulk of the ionic current at this synapse [48,49]. We have recently shown that elevated evoked and spontaneous synaptic currents in ‘low MYO’ larvae show corresponding increase in the density of GluRIIA receptors [33], in line with previously demonstrated correlation between GluRIIA levels and either evoked response [52] or quantal size [49]. We therefore used immunohistochemistry to measure the area of GluRIIA clusters in the NMJ boutons (figure 3a) of control and mutant animals. The GluRIIA cluster area is directly proportional to the number of functional GluRs measured electrophysiologically, and independent of changes in NMJ morphology [53–55].
Figure 3.
Downregulation of Plum reduces the size of GluRIIA clusters in reduced MYO background. (a) Representative confocal images of the 3rd instar larval 6/7 NMJs in denoted genotypes. Insets show synaptic varicosities (boutons), with individual GluRIIA clusters within boutons marked by circled areas. Anti-HRP antibody visualized the presynaptic (motoneuronal) membrane. (b) Quantification of GluRIIA cluster areas (n = 8–9). Bar graph: error bars indicate SEM (one-way ANOVA + Tukey's post-test: **p < 0.01, ***p < 0.001, n.s. = not significant).
Our results showed that Plum downregulation in the muscle led to significantly (approx. 20%) smaller GluRIIA clusters in animals with muscle-reduced Myo expression (figure 3a,b). This reduction was identical in magnitude to the increase in the density of IIA-type receptors upon Myo downregulation relative to the control genotype [33], demonstrating the reversal to wild-type receptor levels caused by reduced Plum. Unlike its effect on evoked response and distribution of mEJC amplitudes, muscle-specific Plum suppression alone was unable to further reduce the GluRIIA cluster area. The lack of perfect correlation between electrophysiological analyses and antibody staining probably occurred because the latter cannot distinguish between functional and non-functional glutamate receptors. Another possible explanation is that, in Mef2-GAL4/UAS-PlumRNAi larvae, there is a change in the receptors' biophysical properties resulting in reduced average single-channel conductance [56].
Our immuno-staining data confirm the negative effect of Plum downregulation on enhanced neurotransmission caused by muscle-specific knock-down of Myo.
2.3. The number of Brp puncta scales with NMJ size upon Plum and/or MYO attenuation
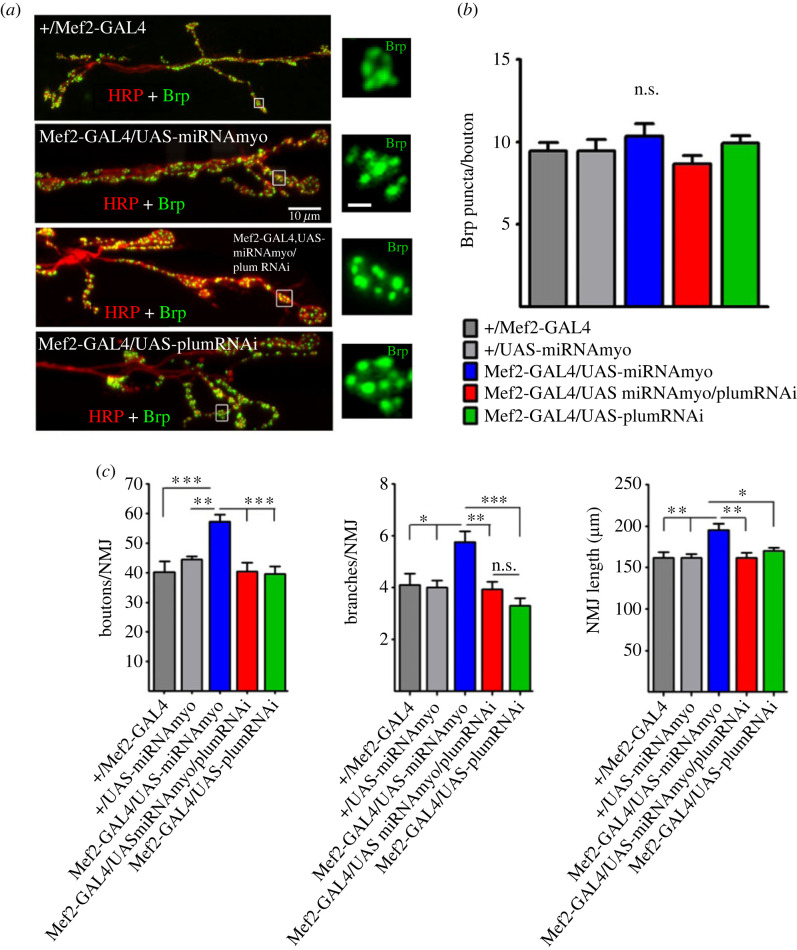
Bruchpilot (Brp) is a presynaptic marker at the larval NMJ and the Drosophila homologue of the vertebrate active zone protein ELKS [57]. Brp is required for function and structural integrity of synaptic active zones and is necessary for regulating evoked, but not spontaneous, release at the glutamatergic synapse of the NMJ [57].
We have previously shown that enhanced evoked response in reduced MYO background correlates with increased NMJ size [33]. These findings agree with the previously established positive correlation between the number of boutons and the strength of evoked signal transmission [52,58]. Here we show that the number of active zones per bouton (active zones marked by distinct Brp puncta) is not affected by Myo or Plum manipulations (figure 4a,b). The reversal of the (increased) amplitude of evoked synaptic responses in reduced MYO larvae upon Plum suppression (figure 2b) therefore cannot be explained by reduced number of Brp puncta, despite the previously demonstrated correlation between evoked response and Brp density [59]. Rather, reduced bouton number, NMJ branching, and NMJ length (figure 4c) are likely responsible for the physiological attenuation in the larvae suppressing both MYO and Plum. In addition, Plum probably exhibits a broader, non-Brp related, physiological effect because the morphological changes alone cannot explain the negative effect of Plum knock-down on synaptic strength in the control background (green bar in figure 2b). These findings implicate Plum as a modulator of synaptic strength in low MYO background via its impact on NMJ morphology.
Figure 4.
Number of Brp puncta and NMJ morphology. (a) Representative confocal images of Brp puncta in distal NMJ segments. Insets show the puncta in individual synaptic boutons (inset scale bar: 1 µm). (b) Mean number of Brp puncta per bouton (n = 7–9). (c) NMJ morphology: the number of boutons (left) and branches (middle) per 6/7 NMJ and NMJ length (right) (n = 9–17). All bar graphs: error bars indicate SEM (one-way ANOVA + Tukey's post-test: *p < 0.05, **p < 0.01, ***p < 0.001, n.s. = not significant).
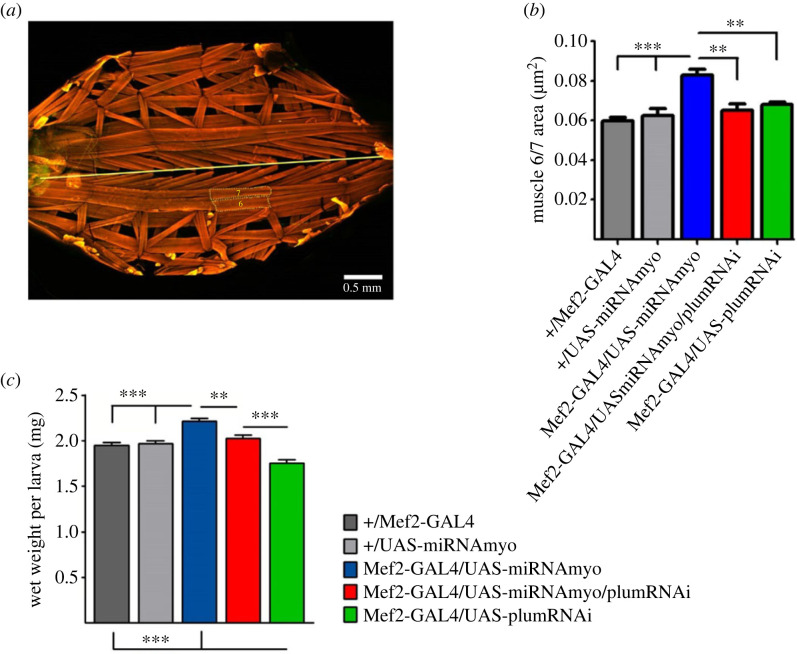
2.4. Knock-down of Plum abolishes the effect of reduced MYO on muscle size and body weight
Muscle-derived MYO negatively regulates larval weight and muscle size [33]. We examined the effect of Plum attenuation on the size of body-wall muscles 6 and 7 (figure 5a) and on total larval body weight in reduced MYO background. Plum suppression completely abolished the positive effect of Myo downregulation on the combined area of muscles 6 and 7 (figure 5b) and on larval wet weight (figure 5c), mirroring its effects on synaptic physiology (figure 2), synaptic composition (figure 3b) and NMJ morphology (figures 3a and 4c). The interaction between Myo and Plum downregulation was significant, indicating a combinatorial effect of these interventions in abolishing the positive effect of reduced MYO on muscle size and body weight (two-way ANOVA analysis in figure 5c,d). Furthermore, Plum has an independent effect on body mass, because Plum knock-down larvae exhibit significantly reduced wet weight (figure 5c, green bar).
Figure 5.
Plum regulates muscle size and larval weight. (a) Third instar larval preparation showing body wall muscles stained with phalloidin (anterior is on the left). Straight yellow line marks the midline. Dotted lines mark muscles 6 and 7. (b) Bar graphs compare mean combined area of muscles 6 and 7 (n = 6–14). (c) Total larval wet weight in indicated genotypes (n = 19–25). In both (b) and (c), two-way ANOVA analysis indicates statistically significant interactions between Mef2-GAL4/UAS-miRNAmyo and Mef2-GAL4/UAS-miRNAmyo/plumRNAi (p = 0.0134 and p = 0.0030, respectively). Error bars in the bar graphs indicate SEM (one-way ANOVA + Tukey's post-test: *p < 0.05, **p < 0.01, ***p < 0.001).
These experimental results identify Plum as a critical modulator of the action of MYO on the neuromuscular physiology, muscle size and weight, and consequently, a regulator of synaptic strength and body weight in developing D. melanogaster.
3. Discussion
Chemical transmission across the neuromuscular junction is critical for converting action potentials originating in the central nervous system into contractile activity in skeletal muscles. This present work uncovers a previously unknown role for the transmembrane protein Plum in regulating muscle size, muscle weight, GluRIIA receptor clustering and neurotransmission at the larval NMJ when MYO protein is reduced post-synaptically. Previous experiments identified a genetic interaction with MYO and Plum pre-synaptically in the central nervous system, as well as a non-cell autonomous effect from glial cells [36,60]. However, the post-synaptic effects of MYO and Plum had not been addressed.
Post-synaptic overexpression of Plum or Myo alone in muscle showed no significant effect on the physiology of the synapse (electronic supplementary material, figure S1). The current model of TGF-β signalling suggests that binding of the ligand by type I and Type II transmembrane receptor kinases are required for correct phosphorylation of R-Smad, forming a complex that translocates to the nucleus to initiate transcription of target genes (reviewed by [61]. Wang and colleagues suggest that Plum acts as a coreceptor to stabilize the type I and type II receptors [62]. This would explain why we do not observe any electrophysiological changes when Plum is overexpressed in the muscle, as plum is required to stabilize the complex, and there is enough endogenous Plum protein to do so. However, Wang et al. also show that when they use vCrz neurons as a model to study programmed cell death (PCD), TGF-β signalling is not epistatic to ecdysone signalling, which contrasts with signalling in the mushroom body neurons [36]. Thus, neuron-specific differences exist when addressing TGF-β signalling, although in vCrz neurons, Plum does still cooperate with myo to regulate PCD. Further work will be required to understand how Plum affects ecdysone signalling in vCrz neurons.
Post-synaptic downregulation of Myo and Plum, on the other hand, had a more noticeable effect. Myo downregulation resulted in a significant increase in bouton number, branching and NMJ length. Plum downregulation ameliorated the effects of Myo downregulation and reduced the increases below wild-type levels. Importantly, post-synaptic downregulation of Myo increased the number of GluRIIA puncta at the NMJ, which were significantly reduced when Plum was concomitantly downregulated. Thus, a homeostatic stabilization of NMJ architecture not only requires MYO and Plum pre-synaptically to ensure normal neuromuscular connectivity before metamorphosis [36], but also involves the interaction of MYO and Plum post-synaptically (this study).
This stabilizing effect is not unique and has also been observed between other secreted proteins and membrane-bound receptors. For example, the disruption of the interaction between the secreted extracellular matrix protein Tenectin and integrin receptor affected NMJ stability both pre-and post-synaptically, and overexpression of Tenectin in muscles not only restored but led to exceedingly high integrin levels [62]. Our data are consistent with the idea that proteins involved with NMJ architecture need to be correctly maintained both pre-synaptically and post-synaptically, and disruption of secreted extracellular molecules may affect NMJ morphology and consequently, NMJ function. Mammalian MYO has been shown to interact with extracellular matrix proteins such as laminin and the extracellular proteoglycan decorin [63,64], suggesting that the regulation of Myostatin by extracellular matrix proteins across the synaptic cleft plays a role in maintaining NMJ stability.
We also showed a significant role for Plum in regulating muscle mass and larval weight, specifically in the context of Myo downregulation. Given the role of MYO in regulating NMJ architecture and muscle mass, it is perhaps unsurprising that impairment of the NMJ, in many cases, leads to reduced muscle mass and function in both vertebrates and invertebrates [28,65]. Our work also addressed the consequences of Myo and Plum downregulation on neurotransmission, using electrophysiological measurements as a readout. Muscle-specific downregulation of Myo elicited an increased evoke response, which was attenuated by the concomitant downregulation of Plum. These data indicate that tight regulation of Myo and Plum is crucial in maintaining a stable NMJ, and Plum can counteract any potential influence on synaptic strength by deregulated Myo, post-synaptically. In addition to motor unit elimination [66], reduced motor axon conduction velocity [67], diminished motor cortex excitability [68,69], and modified activity of muscle-intrinsic factors [70], NMJ dysfunction is strongly correlated with decreased skeletal muscle size and strength under both healthy and pathological conditions [71]. For example, recent studies suggested that the malfunction of the NMJ plays a causative role in the onset of sarcopenia [72], and proposed NMJ stabilization as a way to delay its progression [73]. In dystrophic mdx mice, both pre- and post-synaptic abnormalities in the NMJ contribute to reduced muscle contractility [71] and therapeutic approaches that specifically target NMJs have been proposed for treating spinal muscular atrophy and, possibly, ALS [74].
Several mammalian proteins have identified roles in linking structural and functional properties of the NMJ and (skeletal) muscles, the most important being Agrin (no obvious homologue of Agrin is present in D. melanogaster). Agrin was identified as a marker for the diagnosis of sarcopenia [75] and implicated in the pathogenesis of sarcopenia caused by degeneration of the NMJ [76]. Degradation of Agrin results in structural changes in the NMJ and innervated muscles, consistent with the notion that impaired NMJ functionality plays a role in the onset of sarcopenia [72]. Agrin was first discovered as a neurotrophic factor sufficient for pre- and post-synaptic NMJ assembly and stabilization [77] and aggregation of the junctional acetylcholine receptors (AChRs) [78]. Agrin binding to transmembrane proteins such as dystroglycan and the extracellular matrix protein laminin is thought to stabilize the NMJ [79,80]. Reduced function and density of synaptic AChRs at the NMJ is a hallmark of ‘normal’ human [12] and rodent [81] ageing muscles and of several NMJ disorders characterized by skeletal muscle loss and weakness [82–84]. These findings underline the importance of investigating links between the NMJ and muscle function and searching for novel regulators of these processes. In the fly, there are several Plum paralogues that show high homology with Plum, and it is tempting to speculate that Plum may form a heterodimeric receptor with one or more of the paralogues to regulate MYO activity. TGF-β ligands are known to bind to different transmembrane receptors, with varying combinations of type I and type II forms [85].
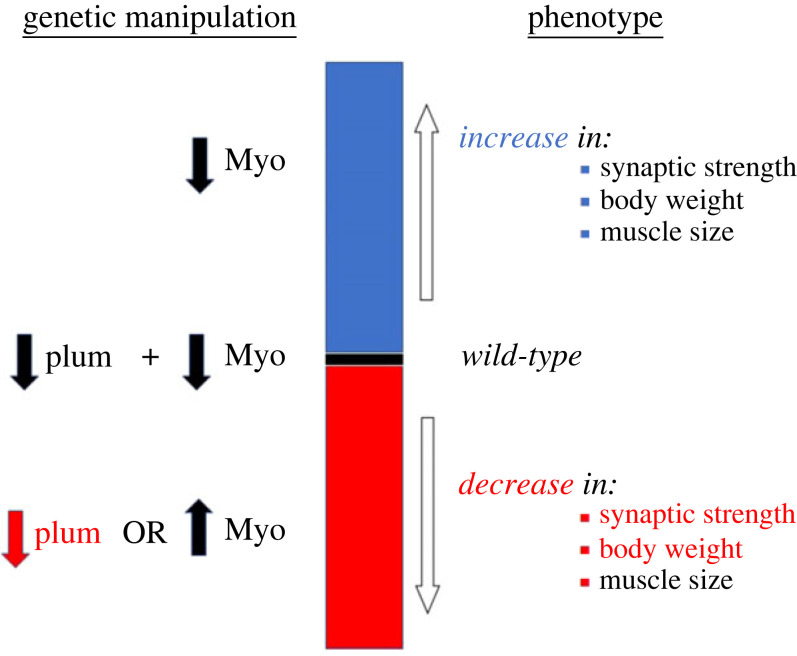
Just like Agrin, Drosophila MYO is a secreted ligand that regulates nervous system development [44] and NMJ synaptogenesis [33]). While MYO of glial origin governs remodelling of mushroom body neurons in developing animals [44], muscle-derived MYO functions as strong negative regulator of the size of larval somatic muscles and NMJ size and function [33,36]. In this study, we expanded our investigation of the role of MYO in the larval neuromuscular system by analysing its interaction with Plum, a trans-membrane protein recently found to modulate MYO signalling during mushroom body development [36]. The genetic interaction experiments indicate that Plum acts as a downstream effector of MYO possibly by sequestering it and thereby inhibiting its pruning-promoting effect on mushroom body neurons, with the reduction of endogenous Plum stimulating MYO-induced pruning [36]. In the fly brain, Plum expression in mushroom body neurons is required to prevent β-lobes from crossing the midline [60]. Overexpression of Plum within mushroom body neurons was sufficient to induce β-lobe retraction. However, the overexpression of Plum within muscle at the neuromuscular junction was not addressed. We did not see any significant effect of Plum overexpression in the muscle when looking at the gross morphology at the NMJ. The only significant effect observed with Plum overexpression in the muscle was when Myo was also overexpressed. The ‘low MYO’ animals used in our experiments have the Myo transcript levels reduced by approximately 60% [33], with the remaining circulating MYO possibly sequestered by Plum. Curiously, work from the O'Connor laboratory [86] did not find MYO functioning as a negative regulator of muscle size. Our work in this paper focuses on the genetic interaction of Plum and MYO, and although we do not directly address the specific function of MYO, we speculate that the differences seen from the O'Connor laboratory data may be due to differences in the genetic background of the flies used. De-sequestering this Plum-bound MYO could therefore reverse some of the effects of genetic MYO attenuation on NMJ function and muscle size. In agreement with this hypothesis, simultaneous suppression of MYO and Plum only in the muscle completely reversed the effect of reduced MYO on synaptic physiology, muscle size, and total body weight. In addition, Plum reduction alone (i.e. without concomitant downregulation of MYO) reduced the synaptic strength and body weight below control levels (figure 6), identifying muscle-derived Plum as a novel and independent target for manipulating neuromuscular function. It is important to note that MYO and Plum regulate physiological properties of the NMJ synapse by controlling the number of glutamate receptors, the functional analogues of ACh receptors in mammalian NMJs. Plum mutant alleles are viable and fertile, indicating that modulating plum levels alone is not detrimental to the development of the fly. Indeed, having little or no phenotypes when plum is deleted or overexpressed is desirable for therapeutic targeting, in order to modify MYO levels.
Figure 6.
Model illustrating the effects of MYO and Plum on synaptic strength, muscle size and body weight in Drosophila larvae. Model showing that Plum reduction abolishes the (positive) effect of reduced MYO on synaptic strength, total body weight and muscle size. Attenuation of Plum on its own, or MYO upregulation, negatively affect synaptic strength and larval weight, with Myo overexpression having an additional (negative) effect on muscle size.
Sequestration is a well-described mechanism for the regulation of TGF-β ligands, with specific components of the extracellular matrix known to segregate secreted cytokines to either inhibit TGF-β signalling or concentrate the ligands for future use [87]. Sequestration of TGF-β ligands was also demonstrated in Drosophila [88] and proposed to facilitate autocrine TGF-β signalling in the larval NMJ [89]. Although the molecular link between MYO and Plum has not yet been elucidated, the model of sequestration currently appears to be the best fit for our observations. Further studies are required to formulate a molecular mechanism and firmly establish this mode of ligand control in regulating the Drosophila neuromuscular system. Considering the important role of TGF-β ligands in the regulation of muscle mass and NMJ function in mammalian model species [90,91] and in humans [92,93], these findings can point to novel mechanism for therapeutic interventions in pathologies associated with ageing and neuromuscular disorders.
4. Material and methods
4.1. Fly stocks and husbandry
All stocks were maintained and all experiments were conducted at 25°C on a 12 h : 12 h light: dark cycle at constant humidity using standard sugar/yeast/agar (SYA) media (15 g l−1 agar, 50 g l−1 sugar, 100 g l−1 autolysed yeast, 100 g l−1 nipagin and 3 ml l−1 propionic acid) [94]. Third-instar wandering larvae used in the experiments were selected based on morphological (larval spiracles and mouth-hook) and behavioural criteria (location outside of the food). Tissue-specific expression was achieved with the GAL4-UAS system [GAL4-dependent upstream activator sequence] [95]. Drosophila stocks used were: UAS-miRNAmyo [44], a gift from T. Awasaki from Tzumin Lee laboratory at Janelia Farm; Mef2-GAL4 (Bloomington Stock Center BDSC #27390); UAS-PlumRNAi (Vienna Drosophila Resource Centre #101135) and UAS-PlumFL were a gift from Oren Schuldiner laboratory at Weitzman Institute of Technology. The Mef2-GAL4/UAS-miRNAmyo/ UAS-PlumRNAi line was created using standard Drosophila crossing schemes. wDah was the ‘wild-type’ strain used in all experiments. The white Dahomey (wDah) stock was derived by incorporation of the w1118 mutation into the outbred Dahomey background by back-crossing. The plum protein trap line (plum[MI01835-GFSTF-1]) and plum CRIMIC line (plum[CR01114-TG4.1]) were obtained from Bloomington (BDSC stocks #60520 and #81177 respectively). UAS-IVS-CD8::GFP was a generous gift from Nic Tapon (BDSC#32185).
4.2. NMJ electrophysiology
Recordings were performed as described previously [96]. Two-electrode voltage clamp (TEVC) recordings using sharp electrodes were made from ventral longitudinal muscle 6 in abdominal segments 2–4 of wandering third instar larvae. NMJ recordings were performed using pClamp 10, an Axoclamp 900A amplifier and Digidata 1440A (Molecular Devices, USA) in haemolymph-like 3 (HL-3) solution: 70 mM NaCl, 5 mM KCl, 20 mM MgCl2, 10 mM NaHCO3, 115 mM sucrose, 5 mM trehalose, 5 mM HEPES and 2 mM CaCl2. Recording electrodes (10–30 MΩ) were filled with 3 M KCl. Miniature excitatory junctional currents (mEJCs) were recorded in the presence of 0.5 µM tetrodotoxin (Tocris, UK). All synaptic responses were recorded from muscles with input resistances ≥4 MΩ and resting potentials more negative than −60 mV at 25°C as differences in recording temperature cause changes in glutamate receptor kinetics and amplitudes [97]. Holding potentials were −60 mV. Mean single eEJC amplitudes (stimulus: 0.1 ms, 1–5 V) are based on the mean peak eEJC amplitude in response to ten presynaptic stimuli (recorded at 0.2 Hz). Nerve stimulation was performed with an isolated stimulator (DS2A, Digitimer). The data were digitized at 10 kHz and for miniature recordings, 200 s recordings were analysed to obtain mean mEJC amplitudes and frequency values. mEJC and eEJC recordings were off-line low-pass filtered at 500 Hz and 1 kHz, respectively. Materials were purchased from Sigma-Aldrich (UK) unless otherwise stated.
4.3. Immunocytochemistry and confocal microscopy
Immunocytochemistry and confocal microscopy were performed as described previously [33] using Zeiss 700 inverted confocal microscope and Olympus FV-1000 inverted confocal microscope. All neuromuscular junction (NMJ) images and analyses were from NMJs on larval ventral longitudinal muscles 6 and 7 (hemi-segments A3-A4). For glutamate receptor (GluRIIA) and Brp staining, 3rd instar larval preparations were dissected in modified HL-3 solution and fixed for 30 min in Bouin's fixative. Mouse monoclonal anti-GluRIIA (8B4D2) and anti-Brp (nc82) antibodies were obtained from the University of Iowa Developmental Studies Hybridoma Bank (Iowa City, USA) and used at 1 : 100 and 1 : 20, respectively. AlexaFluor-conjugated goat anti-mouse secondary antibodies were used at 1 : 200. AlexaFluor-conjugated 488 goat anti-rabbit polyclonal GFP (Cat.no #A21311) was used at 1 : 500 to visualize the plum protein in larval muscles and at the NMJ. TRITC-labelled anti-horseradish peroxidase (HRP) antibody (staining neuronal membranes) was used at 1 : 100. To visualize larval muscles, phalloidin was added to fresh larval preparations fixed for 30 min with 4% paraformaldehyde. Measurements of the postsynaptic glutamate receptors and quantifications of the Brp puncta were made from maximum intensity Z-projections of confocal image stacks using ImageJ (NIH, Bethesda MD). The postsynaptic receptor fields were measured by drawing a circle around individual GluRIIA clusters in type Ib synaptic boutons. NMJ branches, defined as an extension of the presynaptic motor neuron that included at least 3 boutons, were counted manually. NMJ length, defined as the longest end-to-end length across a NMJ, was measured using automated length measurement tool in ImageJ software.
4.4. Statistical analyses
Statistical analyses were performed using GraphPad Prism 5 software. For comparisons between two or more groups, a one-way ANOVA followed by a Tukey-Kramer test was used. In all instances, p < 0.05 is considered statistically significant (*p < 0.05; **p < 0.01; ***p < 0.001). Values are reported as the mean ± SEM. A 2-way ANOVA test was used to perform interaction calculations. The Kolmogorov–Smirnov (KS) test was used to analyse the cumulative distribution of mEJC amplitudes.
Acknowledgements
We would like to thank Nicolas Tapon (The Francis Crick Institute, UK) for fly stocks, Oren Schuldiner (Weizmann Institute of Science, Israel) for Plum lines and the Bloomington Drosophila Stock Center (Indiana, USA) for reagents.
Ethics
This work did not require ethical approval from a human subject or animal welfare committee.
Data accessibility
The data are provided in electronic supplementary material [98].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors' contributions
V.K.S.: formal analysis, investigation, writing—original draft, writing—review and editing; A.S.: formal analysis, investigation; N.S.W.: formal analysis, investigation, writing—review and editing; J.G.S.: formal analysis, investigation; J.R.S.: formal analysis, funding acquisition, investigation, resources, supervision, writing—review and editing; L.P.: funding acquisition, project administration, supervision, writing—review and editing; H.A.: conceptualization, formal analysis, funding acquisition, investigation, project administration, supervision, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
The authors declare no competing or financial interests.
Funding
This work was funded by the Max Planck Society and a Wellcome Trust Strategic Award to L.P., and by an MRC grant to J.R.S. and J.G.S.
References
- 1.Gonzalez-Freire M, de Cabo R, Studenski SA, Ferrucci L. 2014. The Neuromuscular junction: aging at the crossroad between nerves and muscle. Front. Aging Neurosci. 6, 208. ( 10.3389/fnagi.2014.00208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cruz-Jentoft AJ, et al. 2010. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 39, 412-423. ( 10.1093/ageing/afq034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eddinger TJ, Moss RL, Cassens RG. 1985. Fiber number and type composition in extensor digitorum longus, soleus, and diaphragm muscles with aging in Fisher 344 rats. J. Histochem. Cytochem. 33, 1033-1041. ( 10.1177/33.10.2931475) [DOI] [PubMed] [Google Scholar]
- 4.Herndon LA, Schmeissner PJ, Dudaronek JM, Brown PA, Listner KM, Sakano Y, Paupard MC, Hall DH, Driscoll M. 2002. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature 419, 808-814. ( 10.1038/nature01135) [DOI] [PubMed] [Google Scholar]
- 5.Young A, Stokes M, Crowe M. 1985. The size and strength of the quadriceps muscles of old and young men. Clin. Physiol. 5, 145-154. ( 10.1111/j.1475-097X.1985.tb00590.x) [DOI] [PubMed] [Google Scholar]
- 6.Demontis F, Piccirillo R, Goldberg AL, Perrimon N. 2013. Mechanisms of skeletal muscle aging: insights from Drosophila and mammalian models. Dis. Model Mech. 6, 1339-1352. ( 10.1242/dmm.012559) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delbono O. 2000. Regulation of excitation contraction coupling by insulin-like growth factor-1 in aging skeletal muscle. J. Nutr. Health Aging 4, 162-164. [PubMed] [Google Scholar]
- 8.Delbono O, O'Rourke KS, Ettinger WH. 1995. Excitation-calcium release uncoupling in aged single human skeletal muscle fibers. J. Membr. Biol. 148, 211-222. ( 10.1007/BF00235039) [DOI] [PubMed] [Google Scholar]
- 9.Barker D, Ip MC. 1966. Sprouting and degeneration of mammalian motor axons in normal and de-afferentated skeletal muscle. Proc. R. Soc. Lond. B 163, 538-554. ( 10.1098/rspb.1966.0008) [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Mizushige T, Nishimune H. 2012. Active zone density is conserved during synaptic growth but impaired in aged mice. J. Comp. Neurol. 520, 434-452. ( 10.1002/cne.22764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishimune H, Badawi Y, Mori S, Shigemoto K. 2016. Dual-color STED microscopy reveals a sandwich structure of Bassoon and Piccolo in active zones of adult and aged mice. Sci. Rep. 6, 27935. ( 10.1038/srep27935) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oda K. 1984. Age changes of motor innervation and acetylcholine receptor distribution on human skeletal muscle fibres. J. Neurol. Sci. 66, 327-338. ( 10.1016/0022-510X(84)90021-2) [DOI] [PubMed] [Google Scholar]
- 13.Wokke JH, Jennekens FG, van den Oord CJ, Veldman H, Smit LM, Leppink GJ. 1990. Morphological changes in the human end plate with age. J. Neurol. Sci. 95, 291-310. ( 10.1016/0022-510X(90)90076-Y) [DOI] [PubMed] [Google Scholar]
- 14.Lee KM, Chand KK, Hammond LA, Lavidis NA, Noakes PG. 2017. Functional decline at the aging neuromuscular junction is associated with altered laminin-alpha4 expression. Aging (Albany NY) 9, 880-899. ( 10.18632/aging.101198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly SS, Robbins N. 1983. Progression of age changes in synaptic transmission at mouse neuromuscular junctions. The Journal of physiology 343, 375-383. ( 10.1113/jphysiol.1983.sp014898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutmann E, Hanzlikova V. 1973. Basic mechanisms of aging in the neuromuscular system. Mech. Ageing Dev. 1, 327-349. ( 10.1016/0047-6374(72)90078-4) [DOI] [PubMed] [Google Scholar]
- 17.Cappello V, Francolini M. 2017. Neuromuscular junction dismantling in amyotrophic lateral sclerosis. Int. J. Mol. Sci. 18, 2092. ( 10.3390/ijms18102092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chand KK, Lee KM, Lee JD, Qiu H, Willis EF, Lavidis NA, Hilliard MA, Noakes PG. 2018. Defects in synaptic transmission at the neuromuscular junction precede motor deficits in a TDP-43(Q331K) transgenic mouse model of amyotrophic lateral sclerosis. FASEB J. 32, 2676-2689. ( 10.1096/fj.201700835R) [DOI] [PubMed] [Google Scholar]
- 19.Pansarasa O, Rossi D, Berardinelli A, Cereda C. 2014. Amyotrophic lateral sclerosis and skeletal muscle: an update. Mol. Neurobiol. 49, 984-990. ( 10.1007/s12035-013-8578-4) [DOI] [PubMed] [Google Scholar]
- 20.Bombelli F, Lispi L, Porrini SC, Giacanelli M, Terracciano C, Massa R, Petrucci A. 2016. Neuromuscular transmission abnormalities in myotonic dystrophy type 1: a neurophysiological study. Clin. Neurol. Neurosurg. 150, 84-88. ( 10.1016/j.clineuro.2016.08.020) [DOI] [PubMed] [Google Scholar]
- 21.Malatesta M. 2012. Skeletal muscle features in myotonic dystrophy and sarcopenia: do similar nuclear mechanisms lead to skeletal muscle wasting? Eur. J. Histochem. 56, e36. ( 10.4081/ejh.2012.e36) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mateos-Aierdi AJ, Goicoechea M, Aiastui A, Fernandez-Torron R, Garcia-Puga M, Matheu A, Lopez de Munain A. 2015. Muscle wasting in myotonic dystrophies: a model of premature aging. Front. Aging. Neurosci. 7, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudolf R, Khan MM, Labeit S, Deschenes MR. 2014. Degeneration of neuromuscular junction in age and dystrophy. Front. Aging Neurosci. 6, 99. ( 10.3389/fnagi.2014.00099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin J, Tajrishi MM, Ogura Y, Kumar A. 2013. Wasting mechanisms in muscular dystrophy. Int. J. Biochem. Cell Biol. 45, 2266-2279. ( 10.1016/j.biocel.2013.05.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fambrough DM, Drachman DB, Satyamurti S. 1973. Neuromuscular junction in myasthenia gravis: decreased acetylcholine receptors. Science 182, 293-295. ( 10.1126/science.182.4109.293) [DOI] [PubMed] [Google Scholar]
- 26.Shigemoto K, Kubo S, Mori S, Yamada S, Akiyoshi T, Miyazaki T. 2010. Muscle weakness and neuromuscular junctions in aging and disease. Geriatr. Gerontol. Int. 10, S137-S147. ( 10.1111/j.1447-0594.2010.00608.x) [DOI] [PubMed] [Google Scholar]
- 27.Ezaki T, Oki S, Matsuda Y, Desaki J. 2000. Age changes of neuromuscular junctions in the extensor digitorum longus muscle of spontaneous thymoma BUF/Mna rats. A scanning and transmission electron microscopic study. Virchows Arch. 437, 388-395. ( 10.1007/s004280000237) [DOI] [PubMed] [Google Scholar]
- 28.Augustin H, Partridge L. 2009. Invertebrate models of age-related muscle degeneration. Biochimica et biophysica acta 1790, 1084-1094. ( 10.1016/j.bbagen.2009.06.011) [DOI] [PubMed] [Google Scholar]
- 29.Kreipke RE, Kwon YV, Shcherbata HR, Ruohola-Baker H. 2017. Drosophila melanogaster as a model of muscle degeneration disorders. Curr. Top. Dev. Biol. 121, 83-109. ( 10.1016/bs.ctdb.2016.07.003) [DOI] [PubMed] [Google Scholar]
- 30.Plantie E, Migocka-Patrzalek M, Daczewska M, Jagla K. 2015. Model organisms in the fight against muscular dystrophy: lessons from drosophila and Zebrafish. Molecules 20, 6237-6253. ( 10.3390/molecules20046237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Budnik V. 1996. Synapse maturation and structural plasticity at Drosophila neuromuscular junctions. Curr. Opin. Neurobiol. 6, 858-867. ( 10.1016/S0959-4388(96)80038-9) [DOI] [PubMed] [Google Scholar]
- 32.Lo PCH, Frasch M. 1999. Sequence and expression of myoglianin, a novel Drosophila gene of the TGF-β superfamily. Mech. Dev. 86, 171-175. ( 10.1016/S0925-4773(99)00108-2) [DOI] [PubMed] [Google Scholar]
- 33.Augustin H, et al. 2017. Myostatin-like proteins regulate synaptic function and neuronal morphology. Development 144, 2445-2455. ( 10.1242/dev.152975) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carnac G, Vernus B, Bonnieu A. 2007. Myostatin in the pathophysiology of skeletal muscle. Curr. Genom. 8, 415-422. ( 10.2174/138920207783591672) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McPherron AC, Lawler AM, Lee SJ. 1997. Regulation of skeletal muscle mass in mice by a new TGF- β superfamily member. Nature 387, 83-90. ( 10.1038/387083a0) [DOI] [PubMed] [Google Scholar]
- 36.Yu XM, Gutman I, Mosca TJ, Iram T, Ozkan E, Garcia KC, Luo L, Schuldiner O. 2013. Plum, an immunoglobulin superfamily protein, regulates axon pruning by facilitating TGF- β signaling. Neuron 78, 456-468. ( 10.1016/j.neuron.2013.03.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salbaum JM, Kappen C. 2000. Cloning and expression of nope, a new mouse gene of the immunoglobulin superfamily related to guidance receptors. Genomics 64, 15-23. ( 10.1006/geno.2000.6114) [DOI] [PubMed] [Google Scholar]
- 38.Takahashi KF, et al. 2010. Protogenin, a new member of the immunoglobulin superfamily, is implicated in the development of the mouse lower first molar. BMC Dev. Biol. 10, 115. ( 10.1186/1471-213X-10-115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong YH, Lu AC, Wang YC, Cheng HC, Chang C, Chen PH, Yu JY, Fann MJ. 2010. Protogenin defines a transition stage during embryonic neurogenesis and prevents precocious neuronal differentiation. J. Neurosci. 30, 4428-4439. ( 10.1523/JNEUROSCI.0473-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wigg KG, Feng Y, Crosbie J, Tannock R, Kennedy JL, Ickowicz A, Malone M, Schachar R, Barr CL. 2008. Association of ADHD and the Protogenin gene in the chromosome 15q21.3 reading disabilities linkage region. Genes Brain Behav. 7, 877-886. ( 10.1111/j.1601-183X.2008.00425.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamagata M, Sanes JR. 2008. Dscam and Sidekick proteins direct lamina-specific synaptic connections in the vertebrate lamina. Neuron 451, 465-469. [DOI] [PubMed] [Google Scholar]
- 42.Marquardt JU, Quasdorff M, Varnholt H, Curth HM, Mesghenna S, Protzer U, Goeser T, Nierhoff D. 2011. Neighbor of Punc E11, a novel oncofetal marker for hepatocellular carcinoma. Int. J. Cancer 128, 2353-2363. ( 10.1002/ijc.25567) [DOI] [PubMed] [Google Scholar]
- 43.Xiang Y, Yang T, Pang BY, Zhu Y, Liu YN. 2016. The progress and prospects of putative biomarkers for liver cancer stem cells in hepatocellular carcinoma. Stem Cells Int. 2016, 7614971. ( 10.1155/2016/7614971) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Awasaki T, Huang Y, O'Connor MB, Lee T. 2011. Glia instruct developmental neuronal remodeling through TGF- β signaling. Nature Neurosci. 14, 821-823. ( 10.1038/nn.2833) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Papatheodorou I, et al. 2020. Expression Atlas update: from tissues to single cells. Nucleic Acids Res. 48, D77-D83. ( 10.1093/nar/gkaa339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruiz-Canada C, Budnik V. 2006. Introduction on the use of the Drosophila embryonic/larval neuromuscular junction as a model system to study synapse development and function, and a brief summary of pathfinding and target recognition. Int. Rev. Neurobiol. 75, 1-31. ( 10.1016/S0074-7742(06)75001-2) [DOI] [PubMed] [Google Scholar]
- 47.Peron S, Zordan MA, Magnabosco A, Reggiani C, Megighian A. 2009. From action potential to contraction: neural control and excitation-contraction coupling in larval muscles of Drosophila. Comparative biochemistry and physiology Part A. Mol. Integr. Physiol. 154, 173-183. ( 10.1016/j.cbpa.2009.04.626) [DOI] [PubMed] [Google Scholar]
- 48.Petersen SA, Fetter RD, Noordermeer JN, Goodman CS, DiAntonio A. 1997. Genetic analysis of glutamate receptors in Drosophila reveals a retrograde signal regulating presynaptic transmitter release. Neuron 19, 1237-1248. ( 10.1016/S0896-6273(00)80415-8) [DOI] [PubMed] [Google Scholar]
- 49.DiAntonio A, Petersen SA, Heckmann M, Goodman CS. 1999. Glutamate receptor expression regulates quantal size and quantal content at the Drosophila neuromuscular junction. J. Neurosci. 19, 3023-3032. ( 10.1523/JNEUROSCI.19-08-03023.1999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Featherstone DE, Rushton E, Rohrbough J, Liebl F, Karr J, Sheng Q, Rodesch CK, Broadie K. 2005. An essential Drosophila glutamate receptor subunit that functions in both central neuropil and neuromuscular junction. J. Neurosci. 25, 3199-3208. ( 10.1523/JNEUROSCI.4201-04.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qin G, Schwarz T, Kittel RJ, Schmid A, Rasse TM, Kappei D, Ponimaskin E, Heckmann M, Sigrist SJ. 2005. Four different subunits are essential for expressing the synaptic glutamate receptor at neuromuscular junctions of Drosophila. J. Neurosci. 25, 3209-3218. ( 10.1523/JNEUROSCI.4194-04.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sigrist SJ, Thiel PR, Reiff DF, Schuster CM. 2002. The postsynaptic glutamate receptor subunit DGluR-IIA mediates long-term plasticity in Drosophila. J. Neurosci. 22, 7362-7372. ( 10.1523/JNEUROSCI.22-17-07362.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Featherstone DE, Rushton E, Broadie K. 2002. Developmental regulation of glutamate receptor field size by nonvesicular glutamate release. Nat. Neurosci. 5, 141-146. ( 10.1038/nn789) [DOI] [PubMed] [Google Scholar]
- 54.Liebl FL, Featherstone DE. 2008. Identification and investigation of Drosophila postsynaptic density homologs. Bioinform. Biol. Insights 2, 369-381. ( 10.4137/BBI.S2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rasse TM, et al. 2005. Glutamate receptor dynamics organizing synapse formation in vivo. Nat. Neurosci. 8, 898-905. ( 10.1038/nn1484) [DOI] [PubMed] [Google Scholar]
- 56.Chen K, Merino C, Sigrist SJ, Featherstone DE. 2005. The 4.1 protein coracle mediates subunit-selective anchoring of Drosophila glutamate receptors to the postsynaptic actin cytoskeleton. J. Neurosci. 25, 6667-6675. ( 10.1523/JNEUROSCI.1527-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wagh DA, et al. 2006. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron 49, 833-844. ( 10.1016/j.neuron.2006.02.008) [DOI] [PubMed] [Google Scholar]
- 58.Sigrist SJ, Reiff DF, Thiel PR, Steinert JR, Schuster CM. 2003. Experience-dependent strengthening of Drosophila neuromuscular junctions. J. Neurosci. 23, 6546-6556. ( 10.1523/JNEUROSCI.23-16-06546.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liebl FL, McKeown C, Yao Y, Hing HK. 2010. Mutations in Wnt2 alter presynaptic motor neuron morphology and presynaptic protein localization at the Drosophila neuromuscular junction. PLoS ONE 5, e12778. ( 10.1371/journal.pone.0012778) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marmor-Kollet N, Gutman I, Issman-Zecharya N, Schuldiner O. 2019. Glial derived TGF-β instructs axon midline stopping. Front. Mol. Neurosci. 12, 1-11. ( 10.3389/fnmol.2019.00232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Upadhyay A, Moss-Taylor L, Kim M-J, Ghosh AC, O'Connor MB. 2017. TGF-β Family signalling in Drosophila. Cold Spring Harb. Prospect. Biol. 9, a022152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Z, Lee G, Vuong R, Park JH. 2019. Two factor specification of apoptosis: TGF-β signalling acts cooperatively with ecdysone signalling to induce cell- and stage-specific apoptosis of larval neurons during metamorphosis in Drosophila melanogaster. Apoptosis 24, 972-989. ( 10.1007/s10495-019-01574-4) [DOI] [PubMed] [Google Scholar]
- 63.Yasaka N, Suzuki K, Kishioka Y, Wakamatsu J, Nishimura T. 2013. Laminin binds to myostatin and attenuates its signalling. Anim. Sci. J. 84, 663-668. ( 10.1111/asj.12052) [DOI] [PubMed] [Google Scholar]
- 64.Miura T, Kishioka Y, Wakamatsu J, Hattori A, Hennebry A, Berry CJ, Sharma M, Kambadur R, Nishimura T. 2006. Decorin binds myostatin and modulates its activity to muscle cells. Biochem. Biophys. Res.Commun. 340, 675-680. ( 10.1016/j.bbrc.2005.12.060) [DOI] [PubMed] [Google Scholar]
- 65.Tintignac LA, Brenner HR, Ruegg MA. 2015. Mechanisms regulating neuromuscular junction development and function and causes of muscle wasting. Physiol. Rev. 95, 809-852. ( 10.1152/physrev.00033.2014) [DOI] [PubMed] [Google Scholar]
- 66.Piasecki M, Ireland A, Jones DA, McPhee JS. 2016. Age-dependent motor unit remodelling in human limb muscles. Biogerontology 17, 485-496. ( 10.1007/s10522-015-9627-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Metter EJ, Conwit R, Metter B, Pacheco T, Tobin J. 1998. The relationship of peripheral motor nerve conduction velocity to age-associated loss of grip strength. Aging (Milano) 10, 471-478. [DOI] [PubMed] [Google Scholar]
- 68.Clark BC, Mahato NK, Nakazawa M, Law TD, Thomas JS. 2014. The power of the mind: the cortex as a critical determinant of muscle strength/weakness. J. Neurophysiol. 112, 3219-3226. ( 10.1152/jn.00386.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oliviero A, Profice P, Tonali PA, Pilato F, Saturno E, Dileone M, Ranieri F, Di Lazzaro V. 2006. Effects of aging on motor cortex excitability. Neurosci. Res. 55, 74-77. ( 10.1016/j.neures.2006.02.002) [DOI] [PubMed] [Google Scholar]
- 70.Proctor DN, Balagopal P, Nair KS. 1998. Age-related sarcopenia in humans is associated with reduced synthetic rates of specific muscle proteins. J. Nutr. 128, 351S-355S. ( 10.1093/jn/128.2.351S) [DOI] [PubMed] [Google Scholar]
- 71.Pratt SJP, Valencia AP, Le GK, Shah SB, Lovering RM. 2015. Pre- and postsynaptic changes in the neuromuscular junction in dystrophic mice. Front. Physiol. 6, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Butikofer L, Zurlinden A, Bolliger MF, Kunz B, Sonderegger P. 2011. Destabilization of the neuromuscular junction by proteolytic cleavage of agrin results in precocious sarcopenia. FASEB J. 25, 4378-4393. ( 10.1096/fj.11-191262) [DOI] [PubMed] [Google Scholar]
- 73.Mori S, Koshi K, Shigemoto K. 2014. The important role of the neuromuscular junction in maintaining muscle mass and strength. J. Phys. Fitness Sports Med. 3, 111-114. ( 10.7600/jpfsm.3.111) [DOI] [Google Scholar]
- 74.Boido M, Vercelli A. 2016. Neuromuscular junctions as key contributors and therapeutic targets in spinal muscular atrophy. Front. Neuroanat. 10, 6. ( 10.3389/fnana.2016.00006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hettwer S, Dahinden P, Kucsera S, Farina C, Ahmed S, Fariello R, Drey M, Sieber CC, Vrijbloed JW. 2013. Elevated levels of a C-terminal agrin fragment identifies a new subset of sarcopenia patients. Exp. Gerontol. 48, 69-75. ( 10.1016/j.exger.2012.03.002) [DOI] [PubMed] [Google Scholar]
- 76.Drey M, Sieber CC, Bauer JM, Uter W, Dahinden P, Fariello RG, Vrijbloed JW, Fi ATIG. 2013. C-terminal Agrin fragment as a potential marker for sarcopenia caused by degeneration of the neuromuscular junction. Exp. Gerontol. 48, 76-80. ( 10.1016/j.exger.2012.05.021) [DOI] [PubMed] [Google Scholar]
- 77.Bezakova G, Ruegg MA. 2003. New insights into the roles of agrin. Nat. Rev. Mol. Cell Biol. 4, 295-308. ( 10.1038/nrm1074) [DOI] [PubMed] [Google Scholar]
- 78.Ferns MJ, Campanelli JT, Hoch W, Scheller RH, Hall Z. 1993. The ability of agrin to cluster AChRs depends on alternative splicing and on cell surface proteoglycans. Neuron 11, 491-502. ( 10.1016/0896-6273(93)90153-I) [DOI] [PubMed] [Google Scholar]
- 79.Gee SH, Montanaro F, Lindenbaum MH, Carbonetto S. 1994. Dystroglycan- α, a dystrophin-associated glycoprotein is a functional agrin receptor. Cell 77, 675-686. ( 10.1016/0092-8674(94)90052-3) [DOI] [PubMed] [Google Scholar]
- 80.Kammerer RA, Schulthess T, Landwehr R, Schumacher B, Lustig A, Yurchenco PD, Ruegg MA, Engel J, Denzer AJ. 1999. Interaction of agrin and laminin requires a coiled-coil conformation of the agrin-binding site within the laminin gamma1 chain. EMBO 18, 6762-6770. ( 10.1093/emboj/18.23.6762) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li Y, Lee Y, Thompson WJ. 2011. Changes in aging mouse neuromuscular junctions are explained by degeneration and regeneration of muscle fiber segments at the synapse. J. Neurosci. 31, 14910-14919. ( 10.1523/JNEUROSCI.3590-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kong L, Wang X, Choe DW, Polley M, Burnett BG, Bosch-Marce M, Griffin JW, Rich MM, Sumner CJ. 2009. Impaired synaptic vesicle release and immaturity of neuromuscular junctions in spinal muscular atrophy mice. J. Neurosci. 29, 842-851. ( 10.1523/JNEUROSCI.4434-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Querol L, Illa I. 2013. Myasthenia gravis and the neuromuscular junction. Curr. Opin. Neurol. 26, 459-465. ( 10.1097/WCO.0b013e328364c079) [DOI] [PubMed] [Google Scholar]
- 84.Xu R, Salpeter MM. 1997. Acetylcholine receptors in innervated muscles of dystrophic mdx mice degrade as after denervation. J. Neurosci. 17, 8194-8200. ( 10.1523/JNEUROSCI.17-21-08194.1997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nickel J, Dijke PT, Mueller TD. 2018. TGF-( co-receptor function and signalling. Acta Biochim. Biophys. Sin. 50, 12-36. ( 10.1093/abbs/gmx126) [DOI] [PubMed] [Google Scholar]
- 86.Upadhyay A, Peterson AJ, Kim M-J, O'Connor MB. 2020. Muscle-derived Myoglianain regulates Drosophila imaginal disc growth. Elife 2020, e51710. ( 10.7554/eLife.51710) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Horiguchi M, Ota M, Rifkin DB. 2012. Matrix control of transforming growth factor- β function. J. Biochem. 152, 321-329. ( 10.1093/jb/mvs089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Haerry TE. 2010. The interaction between two TGF- β type I receptors plays important roles in ligand binding, SMAD activation, and gradient formation. Mechan. Dev. 127, 358-370. ( 10.1016/j.mod.2010.04.001) [DOI] [PubMed] [Google Scholar]
- 89.Kim MJ, O'Connor MB. 2014. Anterograde Activin signaling regulates postsynaptic membrane potential and GluRIIA/B abundance at the Drosophila neuromuscular junction. PLoS ONE 9, e107443. ( 10.1371/journal.pone.0107443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fong SW, McLennan IS, McIntyre A, Reid J, Shennan KI, Bewick GS. 2010. TGF-beta2 alters the characteristics of the neuromuscular junction by regulating presynaptic quantal size. Proc. Natl Acad. Sci. USA 107, 13 515-13 519. ( 10.1073/pnas.1001695107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McLennan IS, Koishi K. 1994. Transforming growth factor- β-2 (TGF- β 2) is associated with mature rat neuromuscular junctions. Neurosci. Lett. 177, 151-154. ( 10.1016/0304-3940(94)90889-3) [DOI] [PubMed] [Google Scholar]
- 92.Chen JL, Colgan TD, Walton KL, Gregorevic P, Harrison CA. 2016. The TGF- β signalling network in muscle development, adaptation and disease. Adv. Exp. Med. Biol. 900, 97-131. ( 10.1007/978-3-319-27511-6_5) [DOI] [PubMed] [Google Scholar]
- 93.McLennan IS, Koishi K. 2002. The transforming growth factor-betas: multifaceted regulators of the development and maintenance of skeletal muscles, motoneurons and Schwann cells. Int. J. Dev. Biol. 46, 559-567. [PubMed] [Google Scholar]
- 94.Bass TM, Grandison RC, Wong R, Martinez P, Partridge L, Piper MD. 2007. Optimization of dietary restriction protocols in Drosophila. J. Gerontol. A Biol. Sci. Med. Sci. 62, 1071-1081. ( 10.1093/gerona/62.10.1071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brand AH, Perrimon N. 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401-415. ( 10.1242/dev.118.2.401) [DOI] [PubMed] [Google Scholar]
- 96.Robinson SW, Nugent ML, Dinsdale D, Steinert JR. 2014. Prion protein facilitates synaptic vesicle release by enhancing release probability. Human Mol. Genet. 23, 4851-4896. ( 10.1093/hmg/ddu171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Postlethwaite M, Hennig MH, Steinert JR, Graham BP, Forsythe ID. 2007. Acceleration of AMPA receptor kinetics underlies temperature-dependent changes in synaptic strength at the rat calyx of Held. J. Physiol. 579, 69-84. ( 10.1113/jphysiol.2006.123612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sahota VK, Stone A, Woodling NS, Spiers JG, Steinert JR, Partridge L, Augustin H. 2023. Plum modulates myoglianin and regulates synaptic function in D. melanogaster. Figshare. ( 10.6084/m9.figshare.c.6812307) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are provided in electronic supplementary material [98].