Abstract
Cardiovascular manifestations are common in patients suffering axial spondyloarthritis and can result in substantial morbidity and disease burden. To give an overview of this important aspect of axial spondyloarthritis, we conducted a systematic literature search of all articles published between January 2000 and 25 May 2023 on cardiovascular manifestations. Using PubMed and SCOPUS, 123 out of 6792 articles were identified and included in this review. Non-radiographic axial spondyloarthritis seems to be underrepresented in studies; thus, more evidence for ankylosing spondylitis exists. All in all, we found some traditional risk factors that led to higher cardiovascular disease burden or major cardiovascular events. These specific risk factors seem to be more aggressive in patients with spondyloarthropathies and have a strong connection to high or long-standing disease activity. Since disease activity is a major driver of morbidity, diagnostic, therapeutic, and lifestyle interventions are crucial for better outcomes.
|
Key Points • Several studies on axial spondyloarthritis and associated cardiovascular diseases have been conducted in the last few years addressing risk stratification of these patients including artificial intelligence. • Recent data suggest distinct manifestations of cardiovascular disease entities among men and women which the treating physician needs to be aware of. • Rheumatologists need to screen axial spondyloarthritis patients for emerging cardiovascular disease and should aim at reducing traditional risk factors like hyperlipidemia, hypertension, and smoking as well as disease activity. |
Supplementary Information
The online version contains supplementary material available at 10.1007/s10067-023-06655-z.
Keywords: Atherosclerosis , Axial spondyloarthritis, Heart disease, Hyperlipidemia, Vascular disease
Introduction
Cardiovascular comorbidities are common in patients with radiographic and non-radiographic (r-, nr- resp.) axial spondyloarthritis (axSpA) and known for many years, yet many aspects of these comorbidities are still under investigation. Fortunately, almost 2.5-fold more articles were published in the time period 2010–2020 compared with the period before (2000–2010) according to our search strategy (see supplemental material). In addition, gender differences indeed are gaining more and more attention in the last years but data is still scarce, although gender differences could lead to distinct manifestation, course and comorbidities of axSpA [1].
While an association between disease and cardiovascular risk was not well defined until lately, addressing risk reduction is nowadays crucial in clinical practice.
Not only the concomitant inability to move or perform sports due to pain and stiffness seems to be the culprit for increased risk of cardiovascular disease (CVD), but also independent risk factors that accompany axSpA burden including environmental factors and gender differences.
For better addressing this topic, recommendations for management of cardiovascular (CV) risk in patients with rheumatic diseases have been proposed in 2016 by the European League Against Rheumatism (EULAR), although a specific recommendation for r-axSpA and nr-axSpA respectively is still lacking [2]. Since these two entities are distinct diseases with distinct characteristics, risk assessment should be individualized. Inhibitors of Cyclooxygenase (COX)-2 for example are known to increase risk of cardiovascular events in patients suffering pain from other than rheumatologic causes but seem to reduce risk in ankylosing spondylitis (AS) patients due to a decrease in inflammatory burden and therefore overall reduction of CV risk in this vulnerable population [3, 4].
A main factor of high risk is thought to be an earlier and faster genesis of atherosclerosis similar to that seen in patients suffering systemic lupus erythematosus (SLE). As for this population, patients with AS can benefit from traditional optimization of risk factors such as lipid-lowering agents and antihypertensive medications but disease-specific thresholds for risk assessment and treatment targets are yet to be developed. This is underlined by the fact that traditional CV risk algorithms are performing poorly in a retrospective AS cohort using machine learning. In this study, the best, but also modest, performance showed the Systematic Coronary Risk Evaluation (SCORE) and the Reynold’s risk score (RRS) compared to machine learning [5]. This was also shown before for rheumatoid arthritis (RA), psoriatic arthritis (PsA), and SLE [6].
Of note, not all classic CV endpoints are of major concern in patients suffering AS, but others do pose an even higher risk. For this reason, identification of “personal” risk factors is crucial for optimal treatment and the reduction of morbidity burden. This article summarizes current knowledge and therefore aims to help in terms of identification and management of CV manifestations in AS.
Methods
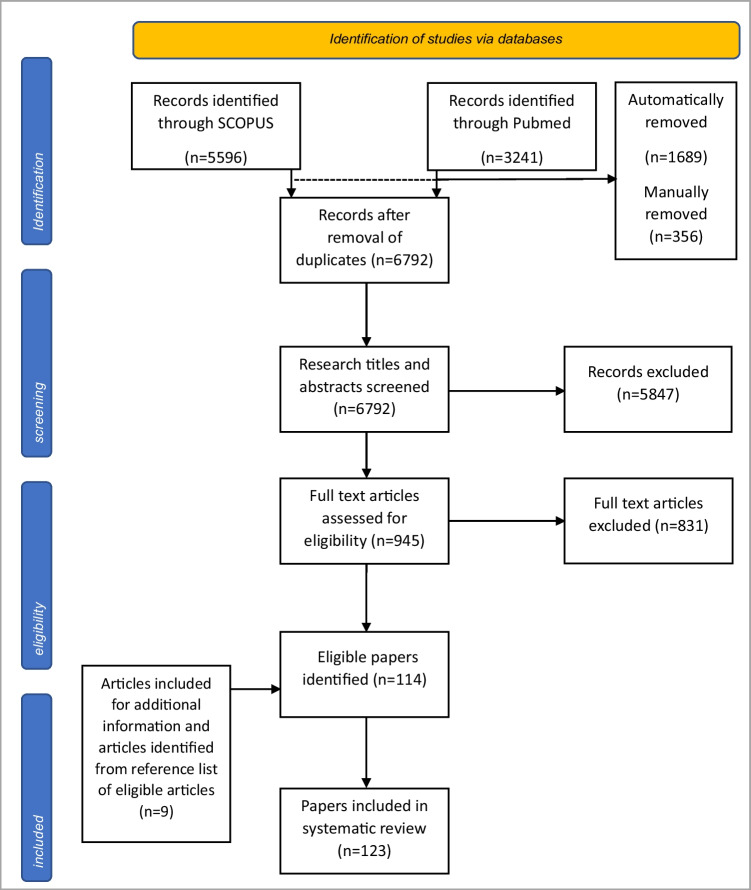
The extensive systematic literature search was conducted according to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement 2020 [7]. SCOPUS and PubMed were chosen to identify relevant literature. In total, 8837 papers were primarily identified, leaving 6792 after removal of duplicate articles. After screening of these 6792 papers, 945 were retrieved and assessed for eligibility of which 114 were included in this review. In addition, 9 articles were included for further information and articles from reference list of eligible articles. Duplicates were removed automatically as well as manually (Fig. 1). The literature search on either platforms was last updated on 25 May 2023. Meta-analysis, review articles, original articles, and case reports were included in this review. Papers published before 2000 were excluded as well as case reports with 5 or less cases.
Fig. 1.
Flow diagram of identification and inclusion of papers
Results
Epidemiology
The first 3 studies on cardiovascular mortality in ankylosing spondylitis were conducted in the late 1970s and early 1980s, respectively, showing similar results in terms of a higher risk ratio for CV mortality (about 1.3, n = 836) not including morbidity due to radiation therapy which was originally thought to be the main driver of increased mortality in those patients [8, 9]. In 2007, a register study from the Netherlands retrieved data from patients with inflammatory arthritis—comprising rheumatoid arthritis (RA) and AS—presented at a general practice. Patients aged 50–75 were stratified by age and sex and CVD prevalence compared to healthy controls were analyzed, resulting in an almost 1.6-fold increase in myocardial infarction and almost twofold taking cerebrovascular events into account [10]. A large study from a national insurance dataset in Taiwan identified 11,701 patients suffering AS and 58,505 controls showed similar results with significant differences in hypertension (HTN), ischemic heart disease (IHD), hyperlipidemia (HLP), congestive heart failure (CHF), and arrhythmias (ORs 1.87 [95% CI 1.75–1.99], 2.74 [95% CI 2.15–3.49], 1.46 [95% CI 1.35–1.57], 1.42 [95% CI 1.17–1.73], and 1.82 [95% CI 1.58–2.09] resp.) although this has to be interpreted with caution since relative risk strongly overrates clinical relevance when prevalence is low which was the case for IHD in this study due to a young collective. Interestingly, in this cohort, there was no sign for increased risk of stroke [11] which is contrary to more recent studies [12–14]. A recent meta-analyses including 40 studies showed similar data for hypertension (ORs 1.58 [95% CI 1.29–1.92]) and CVD (OR 1.42 [95% CI 0.999–2.03]) [15].
Although CVD is not the only cause of higher mortality in axSpA patients, this is the largest group accounting for 40% of cases followed by malignant disease (27%) according to a single-center study with 360 patients and a mean follow-up time of 31.9 years. Independent predictors of increased mortality were C-reactive protein (CRP), non-steroidal antirheumatic drugs (NSAIDs), and work disability with OR of 2.68 (95% CI 1.774–4.048), 4.35 (95% CI 1.753–10.771), and 3.65 (95% CI 1.400–9.506) resp. Diagnostic delay was not a significant risk factor probably due to lower disease and inflammatory burden and thus leading to longer time to diagnosis [16]. A recent meta-analysis showed an increased all-cause mortality RR 1.64 [95% CI 1.49–1.80] besides higher death rates from CV causes RR 1.35 (95% CI 1.01–1.81) in 6 and 3 studies respectively [17].
Of note, a Chinese study on life expectancy showed a mean loss of 7.0 years in male patients and 1.2 years in female patients who died between 1999 and 2008 compared to the general population. Although life expectancy of the general population could not be ascertained by the article [18], there are several studies regarding cardiovascular mortality with similar death rates [19], among other articles, one study with 166,920 patient years also identified male gender as independent risk factor for vascular death (HR 1.46 [95% CI 1.13–1.87]). Interestingly, in this study, lack of exposure to NSAIDs was also an independent risk factor in patients aged 65 year or older [20].
In a prospective cohort study with 6448 AS patients and stringent inclusion parameters, the overall hazard ratio (HR) for acute coronary syndrome (ACS), stroke, and venous thromboembolism (VTE) was 1.54 (95% CI 1.31–1.82), 1.25 (95% CI 1.06–1.48), and 1.53 (95% CI 1.25–1.87), respectively. Female patients showed more incident VTE than male patients (HR 1.82 [95% CI 1.28–2.58] vs. 1.41 [95% CI 1.10–1.80]) and ACS vice versa (HR 1.21 [95% CI 0.76–1.92] vs. 1.54 [95% CI 1.33–1.90]) [12]. A recent meta-analysis showed a similar risk especially for ischemic stroke (HR 1.46, 95% CI 1.23–1.68) [21].
Vascular pathologies in terms of aortitis as well as valvular pathologies and conduction abnormalities are also part of the spectrum of comorbidities [22] and are discussed below.
Traditional CV risk factors
Atherosclerosis
Subclinical atherosclerosis is a well-known manifestation of axSpA and a foundation for several CV morbidities. Recent data showed no significant difference in atherosclerotic burden between AS and nr-axSpA; hence, nr-axSpA, which are underrepresented in axSpA studies, have probably the same risk for generation of subclinical atherosclerosis. This finding was independent of CRP levels in this cross-sectional study with 806 patients including 21% nr-axSpA patients. Regardless of elevation of CRP, ASDAS and bath ankylosing spondylitis disease activity index (BASDAI) was associated with presence of carotid plaques [23]. These findings are supported by a study of 67 AS patients with low disease activity (BASDAI < 4) that did find no difference in intima-media thickness, plaques, and pulse wave velocity in AS and matched individuals that presented the same traditional risk factors [24]. Of note, 40% of patients had to be reclassified to high-risk patients after plaques were recognized in carotid ultrasound [23]. In contrast, CV events are indeed associated with persisting elevation of CRP through clinical visits [25]. Also, radiographic progression of the spine is accompanied by raised markers of subclinical atherosclerosis [26]. Women with high-risk SCORE showed higher plaque burden and disease activity compared to men in a cross-sectional Spanish study of 611 men and 301 women [27].
Blocking tumor necrosis factor (TNF)-a not only reduces disease activity but also seems to be protective of intima-media thickening according to studies that included more than 50% of patients treated with TNF-a blockade [28].
Alarmingly, computed coronary tomography showed significantly higher atherosclerotic lesions in young AS patients with a mean disease duration of 10 years compared to morbidity-matched controls [29].
A couple of biomarkers have been introduced as potential players in the pathogenesis of atherosclerosis in axSpA [30, 31]. Among these, interleukin-17 and its pathway, respectively, play an important role in pathogenesis of SPA but additionally in vasculitis and atherosclerosis. An extensive review on this topic has been published recently [32]
Hypertension
Surprisingly, there is little but conflicting data regarding the prevalence of hypertension in axSpA patients, yet, several studies showed higher risk for hypertension among AS patients [8,33] and a high risk of organ damage [34, 35]. In fact, longer disease duration—especially more than 5 years—and delay in diagnosis seem to be major risk factors for hypertension according to a large (n = 413, male = 77.2%) longitudinal cohort study from Shi et al. Additionally, recurrent rise in erythrocyte sedimentation rate (ESR) was also associated with hypertension highlighting the role of inflammation in developing hypertension. Interestingly, NSAIDs did not lead to a higher incidence of hypertension in these patients but salazopyrin (SZP) did [35].
An interesting retrospective cohort study conducted by Chou et al. showed that comorbidities, namely HTN and diabetes mellitus, did not only occur more often in the AS cohort but also had a higher impact on leading to the outcome measure ACS, especially when both risk factors combined were present [36]. Lately, a large cohort (n = 1111) with AS patients who are suffering uveitis were shown to have higher mortality rates after adjustment for age, sex, and comorbidities compared to AS patients without uveitis [37].
Furthermore, prevalence of hypertension seems to rise with increasing radiographic progression [38]. Another study showed an association between hypertension and longer disease duration as well as with pure axial involvement, but there was no association with ischemic heart disease, stroke, diabetes mellitus, or dyslipidemia [39].
Pathogenic mechanisms have been proposed in the last few years. Damage-associated molecular patterns acting as T-like receptor ligands are leading to activation of Th1 and Th17 cells. The activation might lead to the production of reactive oxygen species and interleukin-17 with the abovementioned involvement in vascular damage. Leptin, homocysteine production, and sodium retention also play a part in development of hypertension [40].
Hyperlipidemia
As with hypertension, the inflammatory state has an utmost influence on dyslipidemia. Hypertriglyceridemia and athrogenic index were significantly higher than in the control group [41]. Surprisingly, implementation of TNF-a-blockade prescription led to higher total cholesterol levels, high- and low-density lipoproteins, triglycerides, and higher atherogenic index [42] but this effect was not seen after a 2-year follow-up period [43]. A recent meta-analysis on lipids in axSpA showed highly significant reduction in high-density lipoprotein in axSpA patients compared to healthy controls [44]. Interestingly, levels of lipoprotein(a) which were believed to be almost exclusively genetically determined were shown to be decreased already after 6 weeks and 6 months of treatment with either MTX alone or combined with TNF-a-blockade or TNF-a-blockade monotherapy in a cohort comprising AS and PsA (n = 25, N = 37 resp.) despite the small case number [45].
Diabetes
Diabetes mellitus type II (DM2) in AS patients has a more deleterious effect on myocardial infarction, stroke, and all-cause mortality compared to patients suffering DM2 alone (HR 1.62 [95% CI 1.16–2.27], 2.27 [95% CI 1.78–2.88], and 1.34 [95% CI 1.09–1.66] resp.) [46].
Metabolic syndrome
AS patients have significantly higher prevalence of metabolic syndrome (MetS). MetS itself increased the 10-year CVD risk according to a small study with 63 men [47]. Furthermore, MetS is common in r-axSpA as a Spanish study showed (33% of patients). For identifying metabolic syndrome, arthrogenic index seems to be a potential prediction marker [48].
Heart disease
Several cardiac manifestations of SPA have been identified which are important reasons for progressive morbidity. There is evidence due to a present case–control study, that patients with AS and without cardiovascular risk factors have a higher prevalence for left ventricular systolic and diastolic dysfunction compared to the healthy control group. The second common cardiovascular risk factors based on echocardiography and ECG are left anterior fascicular block, left-axis deviation, and aortic valve insufficiency [49].
Aortic valve insufficiency is one of the most important cardiovascular involvements occurring in 18% of patients [50]. It has been proposed to be a result of chronic aortitis which involves the aortic root and leads to a dilatation and insufficiency [51, 52]. Especially for HLA-B27-positive male patients, an increased aortic root index—but no difference in the prevalence for aortic valve regurgitation compared with HLA-B27-negative patients—was found. Nevertheless, echocardiographic monitoring should be considered regardless of HLA-B27 status in male patients [53]. In general, echocardiography, cardiac magnetic resonance imaging (MRI), and computed tomography (CT) scans can be tools for follow-up [54]. To evaluate aortic regurgitation or conduction abnormalities, electrocardiogram and echocardiography are recommended as routine tools because their symptoms alone are not very specific and straightforward to interpret [55]. Searching for cardiovascular risk factors at least once every 5 years is also noted in the EULAR recommendation update 2015/2016 [2].
In addition, rheumatologists should be aware of cardiac rhythm disturbances in patients suffering SPA although there is contrary data on this topic. Based on a nationwide cohort study from Sweden from 2006 to 2012 including 6448 patients, the most common cardiac rhythm disturbance were atrioventricular (AV) blockades II–III, atrial fibrillation (AF), and aortic regurgitation (AR) compared to the general population with age- and sex-adjusted HR of 2.27 (95% CI 1.59–3.26), 1.35 (95% CI 1.16–1.57), and 1.93 (95% CI 1.28–2.91), respectively [52]. Furthermore, another study from South Korea with a similar sample size showed significant difference only for AF and AR (HR 2.55 [95% CI 1.49–4.37] and 1.20 [95% CI 1.04–1.39] resp.), but not for AV blockades in these multivariate analyses. Follow-up time was 6 years in the former study and thus 4 years shorter than the latter one with similar mean age of included patients [56]. In contrast, a study of 100 patients with long-standing disease (mean disease duration of 33 years) and older age (mean 54.9 years) showed no significant differences in conduction abnormalities and only a trend towards aortic and mitral regurgitation [57].
The fact that AS is an independent risk factor for AF especially in younger patients (< 40 years) was again recently shown. However, this risk could just be reproduced for male patients. Current TNF-a inhibitor therapy did increase the risk about 3 times that of patients without biological therapy in this study. Whether this is due to higher disease activity that made biologic therapy necessary or an effect of TNF-a is still a matter of debate [58]. Conversely, a biobank study from the UK did show that only women are at greater risk for AF (HR 1.53, 95% CI 1.13–2.07) [59].
Atrioventricular (AV) re-entry tachycardia occurs more often in AS patients; the pathogenesis can be found in inflammatory processes and fibromuscular proliferation [60]. Particularly, AS patients with symptoms like palpitation, dizziness, dyspnea, or syncope should undergo ECG and electrophysiological examinations to check for paroxysmal AV nodal re-entry tachycardia and Wolff-Parkinson-White syndrome [61].
The aim of several studies was to determine electrocardiographic parameters to identify serious life-threatening arrhythmias in SPA patients. T-peak to T-end interval and its relation to corrected QT time, obtained by Holter electrocardiogram, is a known marker for disrupted ventricular depolarization possibly leading to malignant ventricular arrhythmias and therefor aim of a cross-sectional study that examined 76 patients without comorbidities influencing the autonomous nervous system. Patients with AS are more likely to have disrupted ventricular depolarization compared to healthy controls [62, 63]. It is important to determine risk groups in the AS patients which are more likely to develop cardiac conduction disturbance. Especially patients with a higher ASDAS-CRP, a history of anterior uveitis, and longer duration of the disease measured by the age at diagnosis are suggested to have a higher risk [64, 65].
Routine echocardiography seems to be crucial to find prognostic parameters for patients with axSpA. A depressed longitudinal strain in combination with a high-sensitive troponin I (hsTnI) ≥ 3.0 pg/ml can predict MACE in this population [66]. Generally, there is evidence that patients with axSpA have an impaired left ventricular longitudinal strain and a higher risk for diastolic dysfunction measured with the E/E′ ratio than the control groups after adjustment for confounding factors [67]. Diastolic dysfunction itself occurs more often in patients with a long-standing disease [57]. A difference exists when comparing patients with AS to nr-axSpA. The global longitudinal peak systolic strain as a marker for subclinical myocardial dysfunction is lower in patients with AS [68]. Another interesting study showed an association with endophtalmitis and new onset of myocardial infarction [69].
Macrovascular disease
An association of AS and inflammation of large vessels has been demonstrated in several case reports and articles [70–72]. Mainly, association with Takayasu arteriitis (TAK) is described in the literature. Large vessel involvement in patients suffering SPA leads to pronounced morbidity. Unfortunately, a specific therapy is not available at this moment [73]. Interestingly, one recent study found signs of axSpA and inflammatory bowel disease in 4 of 34 patients with formerly diagnosed TAK proposing screening for SPA whenever diagnosis of “primary” TAK is made [74]. Of note, in contrast to idiopathic or other secondary large vessel vasculitis, SPA-associated vasculitis seems to primarily become symptomatic in spring [73]. Additionally, high inflammatory markers and type IIb vascular involvement are associated with peripheral and axSpA [70, 75].
Two big Spanish cross-sectional projects including patients from 28 primary care centers showed significant association with peripheral artery disease, but patient collective was comprised of “inflammatory polyarthropathies” and “spondylopathies” after the respective ICD-10 code [76].
Impact of lifestyle
Changing lifestyle habits that have negative impact on axSpA and CV risk does pose an important role in attenuating disease manifestations of axSpA but also associated CVD. For this reason, EULAR has proposed recommendations for lifestyle behaviors in 2021 [77].
Especially, exercise is an important tool to improve disease-related outcomes; however, many patients do not frequently exercise due to different reasons including fatigue and tiredness [78], which reflects the lower peak oxygen uptake as a marker of cardiorespiratory fitness [79]. A single blinded randomized controlled trial (RCT) showed effect on arterial stiffness and pulse wave velocity (PWV) already after 12 weeks of combined endurance and strength training despite the small number of cases (n = 24) [80]. Furthermore, cardiorespiratory fitness was inversely correlated with arterial stiffness similar to the general population but independent from traditional risk factors [81]. High-intensity exercise for 3 months was shown to reduce AS disease activity scale (ASDAS) by 0.6 in 3 months according to another study conducted in 2020. In a retrospective study on 24 patients with limited spinal movement due to AS, two weeks of guided Yoga decreased systolic blood pressure and heart rate significantly [82].
Smoking is a major driver of inflammation and clinical activity among axSpA patients. Additionally, smoking can per se enhance physical inactivity via its negative effect on lung function [83, 84]. Nevertheless, the prevalence of smoking is much higher in 2 SPA cohorts compared with the UK population in 2018 (24–29% vs. 15%). In general, there are many negative aspects of smoking for axSpA but there are also many confounding factors that make calculation of smoking as an independent risk factor difficult [85]. No study found in our literature search evaluated smoking as an independent risk factor for CVD in axSpA.
There is no evidence of specific dietary regimens to help improve cardiovascular risk in axSpA. Yet, the Mediterranean diet has shown to be beneficial for both disease activity and cardiovascular health in the general population [86]. Therefore, the Mediterranean diet could be recommended to axSpA patients.
Impactof therapeutic interventions
While there is a lot of evidence which argues for an increased CV risk in SpA patients, there is much less robust data that could help to estimate the effect of anti-inflammatory therapy on CV outcome in this population. A list of included studies assessing interventions to lower CV risk is provided in Table 1.
Table 1.
Studies assessing cardiovascular outcomes after specific interventions
| Author, year of publication | Disease (n) classification criteria |
Study design | Females | Intervention | Outcome (p < 0.05) or (95% CI) |
|---|---|---|---|---|---|
| Sveaas SH et al. 2014 [80] |
axSpA (28) ASAS + BASDAI > 3.5 |
Single blinded randomized controlled pilot study | 50% |
Endurance and strength exercise 12w vs. usual treatment Aix, PVW: 2nd outcome measure |
AIx (%): − 5.3 (− 11.0, − 0.5). PVW (m/s): − 0.3 (− 0.7, 0.0) |
| Berg IJ et al. 2018 [81] | AS (118) | Cross-sectional cohort study | 36% | VO2peak via treadmill test |
AIx and PWV inversely associated with VO2peak CRP and ASDAS no association with VO2peak |
| Singh et al., 2021 [82] | AS (24) | Retrospective analysis | N/A | 2w Yoga retreat | Systolic bp↓ (% change 6.22 mmHg) |
| Syngle A et al. 2013 [89] |
AS (20) mNYc + BASDAI ≥ 4 |
Prospective, controlled, open-label | 25% | Spironolacton 2 mg/kg/day | FMD↑, nitrite↓, CRP↓, ESR↓ |
| Garg N et al. 2021 [90] |
AS (40) mNYc + BASDAI ≥ 4 |
RCT | 38% | Olmesartan 10 mg/day | FMD↑, EPC↑, nitrite↓, CRP↓, ESR↓, IL-6↓, TNF-a↓, VCAM-1 |
| Garg N et al. 2015 [88] |
AS (32) mNYc |
Single-blind, placebo-controlled, parallel study | 67% | Rosuvastatin 10 mg daily | FMD↑, IL-6↓, TNF-a↓ |
| Oza A et al. 2017 [91, 92] | AS | Incident user cohort study | 21% | Statin use | 37% reduction all-cause mortality |
| Tsai WC et al. 2015 [4] |
AS (10763) ICD-9-CM code |
Case–control study | 54,9% | NSAIDs exposure and risk of MACE |
OR 0.23; 95% CI 0.07 to 0.76 MPR < 80% for 6 months: OR 1.41; 95% CI 1.07 to 1.86 |
| Wu LC et al. 2016 [112] |
AS (4829) ± CAD ICD-9-CM code |
10-year population-based case–control study | ~ 44% |
High cumulative dose celecoxib (> 300 mg/day) High cumulative dose SZP (≥ 1000 mg/day) |
CAD risk OR 0.34; 95% CI, 0.13 0.89 CAD risk OR 0.63; 95% CI, 0.40–0.99 |
| Tam HW et al. [111] |
AS (1208) ICD-9-CM code |
10-year population-based retrospective cohort study | 40% |
High cumulative dose celecoxib (> 300 mg/day) High cumulative dose SZP (≥ 1000 mg/day) |
CVD risk HR = 0.39; 95% CI 0.20–0.77 HR = 0.72; 95% CI = 0.58–0.91 |
| Kiortsis DN et al. 2006 [104] | RA (50) + AS (32) | Prospective design |
48% AS (3%) |
IFX iv (6 months) ± NSAIDs, ± SZP, ± MTX |
TC ↑, TG ↑ HDL, LDL, TC/HDL ratio + TG/HDL ratio no change |
| Van Eijk et al. 2009 [102] |
AS (92) mNYc BASDAI ≥ 4 |
Prospective design | 36% | ETN (3 months) | ESR↓, CRP↓, SAA↓, TC↓, HDL↓, LDL↓, Trigl.↓, ApoA-I↓ |
| Syngle A et al. 2010 [96] |
AS (12) mNYc BASDAI ≥ 4 |
Prospective, uncontrolled, open-label | 20% |
IFX 5 mg/kg single iv + SZP + NSAIDs |
FMD↑, nitrite↓, CRP↓ |
| Mathieu S et al. 2010 [101] |
AS (34) mNYc |
Prospective design | N/A | IFX (59%), ETN (21%), ADA (21%) | CRP↓, ESR↓, Chol↑, HDL↑ |
| Angel K et al. 2012 [93] | RA + AS + PSA (55); AS (19) | Prospective, not randomized | 67% | 1 yr anti-TNF-a | PWV↓, cIMT↓, CRP↓, ESR↓, Calp↓ |
| Mathieu S et al. 2013 [99] |
AS (49) mNYc |
Prospective, not randomized | 39% | ETN (53%), ADA (35%), IFX (12%) |
CRP↓, ESR↓ PWV, AIP no change |
| Genre F et al. 2015 [100] |
AS (30) mNYc |
Prospective design | 30% | IFX single infusion |
E-selectin, VCAM-1 (120 m after admin) |
| Lee JL et al. 2018 [114] | RA (3167) + AS (561) + PsA (412) | Prospective National Cohort Study | 66.4% | Current anti-TNF-a therapy | HR 0.85, 95% CI 0.76–0.95 |
| Knyazeva LA et al. 2019 [95] |
AS (42) mNYc |
Prospective design | 33% | 2 yr GOL | cIMT↓,AIP↓, stiffness↓ |
| Vegh E et al. 2020 [43] | RA + AS (53); AS (17) | Prospective, not randomized | 21% |
1 yr anti-TNF-a ETN (37) or CZP (16) ± MTX (31) |
PWV↓, cIMT↓, CRP↓ |
| Min HK et al. 2020 [43] |
axSpA (238) mNYc or ASAS |
Prospective longitudinal cohort study | 26% |
TNF (132) vs. non-TNF (106) ADA (36%), IFX (33%), ETN (30%) |
TC↑ LDL-C, HDL-C, AIP no change |
| Kwon OC et al., 2022 [115] | axSpA (450) | Retrospective cohort study | 24% |
anti-TNF-a exposed (multivariate analysis) |
CVD risk HR 0.30, 95% CI 0.11–0.87 |
| Fakih O et al. 2023 [108] |
AS (22.929) ICD-10 code |
National cohort study | 55% | NSAIDs, csDMARDs, anti-TNF-a, anti-IL-17 |
NSAIDs: SHR 0.39; 95% CI 0.32–0.50 Anti-TNFa: SHR 0.61; 95% CI 0.46–0.80 |
Abbreviation:ADA, Adalimumab; AIP, atherogenic index of plasma; AIx, augmentation index; Apo-A, apolipoprotein A; AS, ankylosing spondylitis; ASAS, Assessment of SpondyloArthritis International Society; ASDAS, Ankylosing Spondylitis Disease Activity Score; axSpA, axial spondylarthritis; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASMI, Bath Ankylosing Spondylitis Metrology Index; BP, blood pressure; Calp, Calprotectin; CVD, cardiovascular disease; cIMT, carotid intima media thickness; CZP, Certolizumab; CI, confidence interval; csDMARDS, conventional synthetic disease modifying antirheumatic drug; CAD, coronary artery disease; CRP, C-reactive protein; EPC, endothelial progenitor cells; ESR, erythrocyte sedimentation rate; ETN, Etanercept; FBG, fasting blood glucose; GOL, Golimumab; HR, hazard ratio; HDL, high density lipoprotein; FMD, low-mediated dilation; MACE, major adverse cardiac event; IFX, Infliximab; IL, interleukin; LDL-C, low density lipoprotein; MPR, medication possession rate; MTX, Methotrexat; mNYc, modified New York criteria; NSAIDs, non steroidal anti inflammatory drugs; OR, odds ratio; VO2peak, peak oxygen uptake; PsA, psoriatic arthritis; PWV, pulse wave velocity; RA, rheumatoid arthritis; SZP, Salazopyrin; SHR, subhazard ratio; TC, total cholesterol; TG, triglycerides; anti-TNFa, Tumor necrosis factor-alpha inhibitors; VCAM-1, vascular cell adhesion molecule-1
As a first step, however, before a specific anti-rheumatic treatment is prescribed, the importance of assessing the CV risk in SpA patients should be emphasized and—if appropriate—interventions as those recommended for the general population should be considered. This is also emphasized in the 2015/2016 updated EULAR CV risk management recommendation, which clearly state that rheumatologists are responsible for CV risk management in patients with inflammatory joint disorders, including those with AS [2]. Beside anti-inflammatory treatment, the key risk factors cholesterol, blood pressure, cigarette smoking, diabetes, and adiposity should deserve attention also in axSpA patients [87]. In this context, it is of interest that small studies suggest positive effects of statins [88], spironolactone [89], and angiotensin receptor blockers in AS patients [90]. In a mixed population, rosuvastatin was associated with regression of carotid plaques [91] and UK data shows a reduced mortality in AS patients using statins [92].
There is some evidence that anti-rheumatic treatment in axSpA patients might also yield some beneficial effects regarding CV outcomes. Some—mostly small- and short-term—studies, often in a mixed population, have found favorable effects of biologic disease-modifying anti-rheumatic drugs (DMARDs) on surrogate markers of CV risk such as markers of endothelial function or arterial stiffness [93–97], while others did not [98, 99]. TNF inhibitors seem to reduce biomarkers of endothelial cell activation in AS patients [100]. However, it is not fully clear whether the improvement of vascular function will finally translate into an improved CV outcome.
In addition, several studies investigated the effect of TNF inhibitors on the lipid profile in axSpA patients [43, 101–104]. In summary, besides a reduction of markers of inflammation, most of these studies found an increase in total cholesterol and HDL levels; however, total cholesterol to HDL ratio was stable or improved and the lipid profile was found to be less pro-atherogenic.
One of the cornerstones of therapy in axSpA patients are NSAIDs. It is well established that NSAIDs are associated with an increased number of CV events in the general population [105, 106]. For axSpA patients, this association is much less clear, as there are reports that suggest a possible beneficial effect of NSAIDs with regard to CV events in axSpA patients [4]. In a recent systematic review of observational studies, Karmacharya et al. did not find an increased CV risk for NSAIDs as well as cyclooxygenase 2 (COX2) inhibitors in AS patients. For the whole group, the risk of a CV event showed an RR of 0.96 (95% CI 0.51–1.81). The risk of a cerebrovascular accident was significantly lower in NSAID users (RR 0.58 [95% CI 0.37–0.93]). COX2 inhibition use was associated with a reduced risk of all CV events (RR 0.48 [95% CI 0.33–0.70]) [107].
Additionally, a recent national cohort study (n = 22,929) from France showed a significant reduction of MACE in 8-year cumulative incidence after treatment with NSAIDs und TNF inhibitors, but not after IL17-inhibition or treatment with conventional DMARDS [108]. However, there are also data which demonstrate an increased risk of new-onset hypertension in AS patients with continuous use of NSAIDs (HR 1.12 [95% CI 1.04–1.20]). Given these findings, there is ongoing debate about the place of NSAIDs in the context of AS and CV risk [109, 110].
One Asian retrospective cohort study found a borderline reduced CV event rate with sulfasalazine in AS patients (HR 0.65 [95% CI 0.43–0.998]) [111]. In contrast, in a population-based case–control study from Taiwan, sulfasalazine was—again borderline–negatively associated with the development of coronary artery disease (OR 0.63 [95% CI, 0.40–0.99]) [112].
Chan et al. assessed the impact of TNF inhibitors on the increased CV risk in SpA patients. The authors used a cohort of SpA patients (including 19.4% with psoriatic arthritis; overall 67.9% fulfilling AS criteria) and a matched cohort of patients with non-specific back pain. As expected, SpA patients had a higher risk of MACE (HR 1.70 [95% CI 1.29–2.26]) and cerebrovascular events (HR 1.50 [95% CI 1.08–2.07]). Of interest, SpA patients receiving treatment with TNF inhibitors (n = 649) had a reduced risk of MACE (HR 0.37 [95% CI 0.17–0.80]) and cerebrovascular events (HR 0.21 [95%CI 0.06–0.78]) compared with SpA patients without this treatment. Of note, there was no association between CV risk and synthetic DMARD use [113]. In a study using the Australian Rheumatology Association Database, which included patients with RA, PsA, and AS, the use of TNF inhibitors was associated with a reduced risk of CV events (HR 0.85 [95% CI 0.76–0.95]) and there was no difference between RA patients (the largest group) and AS patients (HR 1.14 [95% CI 0.96–1.36]). Of note, patients who had stopped biological DMARDs did not show this reduction of CV events (HR 0.96 [95% CI 0.83–1.11]) [114]. In a Korean retrospective cohort study, the authors found a reduced risk of CV events in patients receiving TNF inhibitors in unadjusted analysis and after adjustment for traditional CV risk factors, but not after further adjustment in different statistical models [115].
At present, there is an intense discussion on safety issues of JAK inhibitors, which also play a role in the treatment of axSpA. As discussed previously [116], a randomized trial in RA patients found an increased CV risk for tofacitinib as compared to etanercept or adalimumab [117]. This has been reflected in the 2022 updated EULAR RA recommendations, which caution the use of JAK inhibitors in RA patients with increased CV risk [118]. In contrast, this restriction has not been incorporated in the 2022 EULAR axSpA recommendations [119]. A recent meta-analysis comprising 19 RCTs investigating the effect of upadacitinib (UPA) on lipids and cardiovascular events showed no increase in cardiovascular events but an increase in LDL-C and HDL-C leaving the ratio unchanged although follow-up was only 52 weeks [120]. While more data are expected to finally clarify the role of JAK inhibitors with regard to CV risk, a prudent approach seems advisable when JAK inhibitors are considered for SpA patients at increased CV risk [121].
To date, no data with CV outcome have been published for IL-23 inhibitors.
Discussion
Our review gives a comprehensive overview on cardiovascular manifestations in patients suffering axSpA. While some traditional risk factors do not occur more often in axSpA, others are driving morbidity even more compared to the general population. On the one hand, small sample numbers in studies especially in nr-axSpA make substantial risk stratification difficult. On the other hand, estimating the CV risk for these patients seems to be of utmost importance. For that reason, a lot of effort was put into developing risk-identifying parameters or algorithms to supersede old traditional scoring methods that are known to perform poorly in axSpA patients. This is most likely explainable with a different pathomechanism leading to the same or even higher CV risk compared to patients with similar risk but without axSpA. Some studies used machine learning methods to find prediction models with variable success which have not yet been incorporated into formal recommendations [5]. To some extent, ultrasound of carotid arteries, echocardiography, and coronary CT scans can identify patients at risk similar to medical checkups performed in the general population potentially leading to stricter optimization of modifiable risk factors. Also gender-specific differences and characteristics of comorbidities should be taken into account. However, many of the cited studies on CV risk in axSpA have small sample sizes and inhomogeneous collectives. This does not only account for population age but also gender, which per se affects the underlying CV risk. Many studies included patients with axSpA, but also with similar inflammatory arthropathies. This limits the specificity of the results of these studies. In addition, the classification as well as our view of axSpA has changed over time. This is probably best reflected by the introduction of the concept of non-radiographic axSpA. It is likely that these patients behave similar, but not identical as compared to classical AS.
Of note, involvement of potentially life-threatening manifestations of CVD in axSpA patients with sudden occurrence should be paid particular attention to. Therefore, some articles have been published regarding this topic mentioned above.
Since inflammatory burden is strongly connected to CV risk, lowering especially axial disease activity is crucial for also reducing major cardiovascular endpoints and mortality. The best evidence for reduction of disease and inflammatory burden has been shown for TNF inhibitors, although other conventional DMARDs showed at least a small effect. Regarding inhibition of Janus kinases, the last word has not yet been spoken; whether this substance group is intrinsically increasing CV risk or risk is just reduced by other biological therapies as shown for RA leading to a relatively increased risk compared to other therapies is still under debate [121]. For this reason, rheumatologists and other prescribing specialists are encouraged to exercise particular diligence when choosing Janus kinase inhibitors for therapy in patients already at risk [118].
Conclusion
CVD is a frequent comorbidity in axSpA patients and patients with axSpA have a higher mortality because of CVD, which is the most frequent cause followed by malignant diseases. While aiming for low disease activity or—at best—remission as one of the core competencies of rheumatologists, management of axSpA patients should also include CV risk assessment. Recognizing patients at risk, especially early in the disease course, offers the possibility of timely intervention and, in turn, reduced CV morbidity and mortality later in the disease course.
More studies are needed to detect patients at risk early and prevent major disease burden. Especially, studies on major cardiovascular endpoints after pharmaceutical interventions and studies on gender differences are urgently needed to improve care of these vulnerable patients since respective data is scarce.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
HP and RH performed the literature search. BA, HP, and RH screened the obtained articles for eligibility. The obtained articles were divided into equal shares between BA, HP, RH, and VV for further synopses. RH prepared the table, the figure, and supplementary material. RH oversaw all aspects of the article. All authors contributed to editing of the manuscript and approval of the final version. All co-authors take full responsibility for accuracy and integrity of any part of the work.
Funding
Open access funding provided by Johannes Kepler University Linz.
Declarations
Disclosures
None.
Footnotes
Part of the Topical Collection entitled ‘Cardiovascular Issues in Rheumatic Diseases’
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
No part of this review is neither copied nor published elsewhere in any languages.
References
- 1.Stovall R, Van Der Horst-Bruinsma IE, Liu S-H, Rusman T, Gensler LS. Sexual dimorphism in the prevalence, manifestation and outcomes of axial spondyloarthritis. Nat Rev Rheumatol. 2022;18(11):657–669. doi: 10.1038/s41584-022-00833-0. [DOI] [PubMed] [Google Scholar]
- 2.Agca R, Heslinga SC, Rollefstad S, Heslinga M, McInnes IB, Peters MJ, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. 2017;76(1):17–28. doi: 10.1136/annrheumdis-2016-209775. [DOI] [PubMed] [Google Scholar]
- 3.Essers I, Stolwijk C, Boonen A, De Bruin ML, Bazelier MT, de Vries F, et al. Ankylosing spondylitis and risk of ischaemic heart disease: a population-based cohort study. Ann Rheum Dis. 2016;75(1):203–209. doi: 10.1136/annrheumdis-2014-206147. [DOI] [PubMed] [Google Scholar]
- 4.Tsai WC, Ou TT, Yen JH, Wu CC, Tung YC. Long-term frequent use of non-steroidal anti-inflammatory drugs might protect patients with ankylosing spondylitis from cardiovascular diseases: a nationwide case-control study. PLoS One. 2015;10(5):e0126347. doi: 10.1371/journal.pone.0126347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navarini L, Caso F, Costa L, Currado D, Stola L, Perrotta F, et al. Cardiovascular risk prediction in ankylosing spondylitis: from traditional scores to machine learning assessment. Rheumatol Ther. 2020;7(4):867–882. doi: 10.1007/s40744-020-00233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colaco K, Ocampo V, Ayala AP, Harvey P, Gladman DD, Piguet V, et al. Predictive utility of cardiovascular risk prediction algorithms in inflammatory rheumatic diseases: a systematic review. J Rheumatol. 2020;47(6):928–938. doi: 10.3899/jrheum.190261. [DOI] [PubMed] [Google Scholar]
- 7.Page MJ, Mckenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, The PRISMA, et al. statement: an updated guideline for reporting systematic reviews. BMJ. 2020;2021:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peters MJ, van der Horst-Bruinsma IE, Dijkmans BA, Nurmohamed MT. Cardiovascular risk profile of patients with spondylarthropathies, particularly ankylosing spondylitis and psoriatic arthritis. Semin Arthritis Rheum. 2004;34(3):585–592. doi: 10.1016/j.semarthrit.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Brown WM, Doll R. Mortality from cancer and other causes after radiotherapy for ankylosing spondylitis. Br Med J. 1965;2(5474):1327–1332. doi: 10.1136/bmj.2.5474.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters MJL, Nielen MMJ, Raterman HG, Verheij RA, Schellevis FG, Nurmohamed MT. Increased cardiovascular disease in patients with inflammatory arthritis in primary care: a cross-sectional observation. J Rheumatol. 2009;36(9):1866–1868. doi: 10.3899/jrheum.090010. [DOI] [PubMed] [Google Scholar]
- 11.Kang JH, Chen YH, Lin HC. Comorbidity profiles among patients with ankylosing spondylitis: a nationwide population-based study. Ann Rheum Dis. 2010;69(6):1165–1168. doi: 10.1136/ard.2009.116178. [DOI] [PubMed] [Google Scholar]
- 12.Bengtsson K, Forsblad-d’Elia H, Lie E, Klingberg E, Dehlin M, Exarchou S, et al. Are ankylosing spondylitis, psoriatic arthritis and undifferentiated spondyloarthritis associated with an increased risk of cardiovascular events? A prospective nationwide population-based cohort study. Arthritis Res Ther. 2017;19(1):102. doi: 10.1186/s13075-017-1315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JH, Choi IA. Cardiovascular morbidity and mortality in patients with spondyloarthritis: a meta-analysis. Int J Rheum Dis. 2021;24(4):477–486. doi: 10.1111/1756-185X.13970. [DOI] [PubMed] [Google Scholar]
- 14.Lin CW, Huang YP, Chiu YH, Ho YT, Pan SL. Increased risk of ischemic stroke in young patients with ankylosing spondylitis: a population-based longitudinal follow-up study. PLoS One. 2014;9(4):e94027. doi: 10.1371/journal.pone.0094027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao SS, Robertson S, Reich T, Harrison NL, Moots RJ, Goodson NJ. Prevalence and impact of comorbidities in axial spondyloarthritis: systematic review and meta-analysis. Rheumatology. 2020;59(4):iv47–iv57. doi: 10.1093/rheumatology/keaa246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bakland G, Gran JT, Nossent JC. Increased mortality in ankylosing spondylitis is related to disease activity. Ann Rheum Dis. 2011;70(11):1921–1925. doi: 10.1136/ard.2011.151191. [DOI] [PubMed] [Google Scholar]
- 17.Chaudhary H, Bohra N, Syed K, Donato A, Murad MH, Karmacharya P. All-cause and cause-specific mortality in psoriatic arthritis and ankylosing spondylitis: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2023;75(5):1052–1065. doi: 10.1002/acr.24820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mok CC, Kwok CL, Ho LY, Chan PT, Yip SF. Life expectancy, standardized mortality ratios, and causes of death in six rheumatic diseases in Hong Kong. China Arthritis Rheum. 2011;63(5):1182–1189. doi: 10.1002/art.30277. [DOI] [PubMed] [Google Scholar]
- 19.Exarchou S, Lie E, Lindström U, Askling J, Forsblad-d’Elia H, Turesson C, et al. Mortality in ankylosing spondylitis: results from a nationwide population-based study. Ann Rheum Dis. 2016;75(8):1466–72. doi: 10.1136/annrheumdis-2015-207688. [DOI] [PubMed] [Google Scholar]
- 20.Haroon NN, Paterson JM, Li P, Inman RD, Haroon N. Patients with ankylosing spondylitis have increased cardiovascular and cerebrovascular mortality: a population-based study. Ann Intern Med. 2015;163(6):409–416. doi: 10.7326/M14-2470. [DOI] [PubMed] [Google Scholar]
- 21.Bhagavathula AS, Bentley BL, Woolf B, Dissanayaka TD, Rahmani J. Increased risk of stroke among patients with ankylosing spondylitis: a systematic review and meta-analysis. Reumatol Clin. 2023;19(3):136–142. doi: 10.1016/j.reumae.2023.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Lee KS, Kronbichler A, Eisenhut M, Lee KH, Shin JI. Cardiovascular involvement in systemic rheumatic diseases: an integrated view for the treating physicians. Autoimmun Rev. 2018;17(3):201–214. doi: 10.1016/j.autrev.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 23.González Mazón I, Rueda-Gotor J, Ferraz-Amaro I, Genre F, Corrales A, Calvo Rio V, et al. Subclinical atherosclerotic disease in ankylosing spondylitis and non-radiographic axial spondyloarthritis. A multicenter study on 806 patients. Semin Arthritis Rheum. 2021;51(2):395–403. doi: 10.1016/j.semarthrit.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Arida A, Protogerou AD, Konstantonis G, Konsta M, Delicha EM, Kitas GD, et al. Subclinical atherosclerosis is not accelerated in patients with ankylosing spondylitis with low disease activity: new data and metaanalysis of published studies. J Rheumatol. 2015;42(11):2098–2105. doi: 10.3899/jrheum.150316. [DOI] [PubMed] [Google Scholar]
- 25.Navarini L, Currado D, Marino A, Di Donato S, Biaggi A, Caso F, et al. Persistence of C-reactive protein increased levels and high disease activity are predictors of cardiovascular disease in patients with axial spondyloarthritis. Sci Rep. 2022;12(1):7498. doi: 10.1038/s41598-022-11640-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aicha BT, Ahmed F, Seif B, Ines M, Leila R, Selma B, et al. Spinal radiographic progression is correlated with preclinical atherosclerosis in spondyloarthritis. J Back Musculoskelet Rehabil. 2023;36(3):701–708. doi: 10.3233/BMR-220141. [DOI] [PubMed] [Google Scholar]
- 27.Ferraz-Amaro I, Genre F, Blanco R, Corrales A, Mazón IG, Portilla V, et al. Sex differences in cardiovascular and disease-related features in axial spondyloarthritis. A multicenter study of 912 patients. Semin Arthritis Rheum. 2023;60:152198. doi: 10.1016/j.semarthrit.2023.152198. [DOI] [PubMed] [Google Scholar]
- 28.Yuan Y, Yang J, Zhang X, Han R, Chen M, Hu X, et al. Carotid intima-media thickness in patients with ankylosing spondylitis: a systematic review and updated meta-analysis. J Atheroscler Thromb. 2019;26(3):260–271. doi: 10.5551/jat.45294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozdowska P, Wardziak Ł, Kruk M, Kępka C, Kowalik I, Szwed H, et al. Increased prevalence of subclinical coronary atherosclerosis in young patients with ankylosing spondylitis. Pol Arch Intern Med. 2018;128(7–8):455–461. doi: 10.20452/pamw.4300. [DOI] [PubMed] [Google Scholar]
- 30.Genre F, Rueda-Gotor J, Remuzgo-Martínez S, Pulito-Cueto V, Corrales A, Mijares V, et al. Omentin: a biomarker of cardiovascular risk in individuals with axial spondyloarthritis. Sci Rep. 2020;10(1):9636. doi: 10.1038/s41598-020-66816-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonek K, Kuca-Warnawin E, Kornatka A, Zielińska A, Wisłowska M, Kontny E, et al. Associations of IL-18 with altered cardiovascular risk profile in psoriatic arthritis and ankylosing spondylitis. J Clin Med. 2022;11(3):766. doi: 10.3390/jcm11030766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robert M, Miossec P, Hot A. The Th17 pathway in vascular inflammation: culprit or consort? Front Immunol. 2022;13:888763. doi: 10.3389/fimmu.2022.888763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han C, Robinson DW, Jr, Hackett MV, Paramore LC, Fraeman KH, Bala MV. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol. 2006;33(11):2167–2172. [PubMed] [Google Scholar]
- 34.Ullensvang G, Kringeland E, Ikdahl E, Provan S, Berg IJ, Rollefstad S, et al. Cardiovascular organ damage in relation to hypertension status in patients with ankylosing spondylitis. Blood Press. 2023;32(1):2205956. doi: 10.1080/08037051.2023.2205956. [DOI] [PubMed] [Google Scholar]
- 35.Shi LH, Lam SH, So H, Chan CY, Li TK, Szeto CC, et al. Inflammation is associated with incident hypertension in patients with axial spondyloarthritis: a longitudinal cohort study. Clin Exp Hypertens. 2023;45(1):2205056. doi: 10.1080/10641963.2023.2205056. [DOI] [PubMed] [Google Scholar]
- 36.Chou CH, Lin MC, Peng CL, Wu YC, Sung FC, Kao CH, et al. A nationwide population-based retrospective cohort study: increased risk of acute coronary syndrome in patients with ankylosing spondylitis. Scand J Rheumatol. 2014;43(2):132–136. doi: 10.3109/03009742.2013.822097. [DOI] [PubMed] [Google Scholar]
- 37.Feng KM, Chien WC, Chen YH, Sun CA, Chung CH, Chen JT, et al. Increased risk of acute coronary syndrome in ankylosing spondylitis patients with uveitis: a population-based cohort study. Front Immunol. 2022;13:890543. doi: 10.3389/fimmu.2022.890543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen CH, Chen HA, Liao HT, Chou CT, Chen CH. Association of blood pressure and hypertension with radiographic damage among the patients with ankyloing spondylitis. Medicine. 2022;101(38):e30811. doi: 10.1097/MD.0000000000030811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Derakhshan MH, Goodson NJ, Packham JC, Sengupta R, Molto A, Marzo-Ortega H, et al. Increased risk of hypertension associated with spondyloarthritis disease duration: results from the ASAS-COMOSPA study. J Rheumatol. 2019;46(7):701–709. doi: 10.3899/jrheum.180538. [DOI] [PubMed] [Google Scholar]
- 40.Bartoloni E, Alunno A, Gerli R. Hypertension as a cardiovascular risk factor in autoimmune rheumatic diseases. Nat Rev Cardiol. 2018;15(1):33–44. doi: 10.1038/nrcardio.2017.118. [DOI] [PubMed] [Google Scholar]
- 41.Ozdowska P, Kowalik I, Sadowski K, Szwed H, Głuszko P, Rupiński R, et al. Patterns of dyslipidemia in young patients with seronegative spondyloarthropathies without cardiovascular diseases. Reumatologia. 2021;59(5):285–291. doi: 10.5114/reum.2021.110610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hassan S, Milman U, Feld J, Eder L, Lavi I, Cohen S, et al. Effects of anti-TNF-α treatment on lipid profile in rheumatic diseases: an analytical cohort study. Arthritis Res Ther. 2016;18(1):261. doi: 10.1186/s13075-016-1148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Min HK, Lee J, Ju JH, Park SH, Kwok SK. Impact of TNF-α inhibitor on lipid profile and atherogenic index of plasma in axial spondyloarthritis: 2-year follow-up data from the Catholic Axial Spondyloarthritis COhort (CASCO) Clin Rheumatol. 2020;39(2):471–477. doi: 10.1007/s10067-019-04767-z. [DOI] [PubMed] [Google Scholar]
- 44.Masi AT, Fessler SL, Brezka ML, Wang Y, Donohue SE (2023) Systematic review and meta-analysis of individual serum lipids and analysis of lipid ratios in ankylosing spondylitis and healthy control cohorts: significantly lower mean HDL-cholesterol level in ankylosing spondylitis cohorts. Clin Exp Rheumatol. 10.55563/clinexprheumatol/gtcard [DOI] [PubMed]
- 45.Kwon OC, Han K, Chun J, Kim R, Hong SW, Kim JH, et al. Effects of immune-mediated inflammatory diseases on cardiovascular diseases in patients with type 2 diabetes: a nationwide population-based study. Sci Rep. 2022;12(1):11548. doi: 10.1038/s41598-022-15436-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Papadakis JA, Sidiropoulos PI, Karvounaris SA, Vrentzos GE, Spanakis EK, Ganotakis ES, et al. High prevalence of metabolic syndrome and cardiovascular risk factors in men with ankylosing spondylitis on anti-TNFalpha treatment: correlation with disease activity. Clin Exp Rheumatol. 2009;27(2):292–298. [PubMed] [Google Scholar]
- 47.Hokstad I, Greco D, Deyab G, Fagerland MW, Agewall S, Hjeltnes G, et al. Effects of antirheumatic treatment on cell cholesterol efflux and loading capacity of serum lipoproteins in spondylarthropathies. J Clin Med. 2022;11(24):7330. doi: 10.3390/jcm11247330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slouma M, Ben Ali K, Kharrat L, Zouaoui C, Ouertani H, Gharsallah I. Athrogenic indexes: useful markers for predicting metabolic syndrome in axial spondyloarthritis. Clin Investig Arterioscler. 2022;34(5):261–268. doi: 10.1016/j.arteri.2022.03.005. [DOI] [PubMed] [Google Scholar]
- 49.Almasi S, Farahani B, Samiei N, Rezaei Y, Mahmoodi H, Qorbani M. Echocardiographic and electrocardiographic findings in patients with ankylosing spondylitis without cardiovascular risk factors. J Tehran Heart Cent. 2020;15(2):43–49. doi: 10.18502/jthc.v15i2.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gartshteyn Y, Bhave N, Joseph MS, Askanase A, Bernstein E. Inflammatory and thrombotic valvulopathies in autoimmune disease. Heart. 2023;109(8):583–588. doi: 10.1136/heartjnl-2021-319603. [DOI] [PubMed] [Google Scholar]
- 51.Ozkan Y. Cardiac involvement in ankylosing spondylitis. J Clin Med Res. 2016;8(6):427–430. doi: 10.14740/jocmr2488w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bengtsson K, Forsblad-d’Elia H, Lie E, Klingberg E, Dehlin M, Exarchou S, et al. Risk of cardiac rhythm disturbances and aortic regurgitation in different spondyloarthritis subtypes in comparison with general population: a register-based study from Sweden. Ann Rheum Dis. 2018;77(4):541–8. doi: 10.1136/annrheumdis-2017-212189. [DOI] [PubMed] [Google Scholar]
- 53.Baniaamam M, Heslinga SC, Konings TC, Handoko ML, Kamp O, van Halm VP, et al. Aortic root diameter is associated with HLA-B27: identifying the patient with ankylosing spondylitis at risk for aortic valve regurgitation. Rheumatol Int. 2022;42(4):683–688. doi: 10.1007/s00296-021-05040-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chetrit M, Khan MA, Kapadia S. State of the art management of aortic valve disease in ankylosing spondylitis. Curr Rheumatol Rep. 2020;22(6):23. doi: 10.1007/s11926-020-00898-4. [DOI] [PubMed] [Google Scholar]
- 55.Klingberg E, Sveälv BG, Täng MS, Bech-Hanssen O, Forsblad-d’Elia H, Bergfeldt L. Aortic regurgitation is common in ankylosing spondylitis: time for routine echocardiography evaluation? Am J Med. 2015;128(11):1244–50.e1. doi: 10.1016/j.amjmed.2015.04.032. [DOI] [PubMed] [Google Scholar]
- 56.Min HK, Kim HR, Lee SH, Park S, Park M, Hong YS et al (2022) Increased risks of aortic regurgitation and atrial fibrillation in radiographic axial spondyloarthritis patients: a 10-year nationwide cohort study. Ther Adv Musculoskelet Dis 14:1759720X221088094 [DOI] [PMC free article] [PubMed]
- 57.Brunner F, Kunz A, Weber U, Kissling R. Ankylosing spondylitis and heart abnormalities: do cardiac conduction disorders, valve regurgitation and diastolic dysfunction occur more often in male patients with diagnosed ankylosing spondylitis for over 15 years than in the normal population? Clin Rheumatol. 2006;25(1):24–29. doi: 10.1007/s10067-005-1117-6. [DOI] [PubMed] [Google Scholar]
- 58.Moon I, Choi EK, Jung JH, Han KD, Choi YJ, Park J, et al. Ankylosing spondylitis: a novel risk factor for atrial fibrillation — a nationwide population-based study. Int J Cardiol. 2019;275:77–82. doi: 10.1016/j.ijcard.2018.10.024. [DOI] [PubMed] [Google Scholar]
- 59.Tilly MJ, Geurts S, Zhu F, Bos MM, Ikram MA, de Maat MPM, et al. Autoimmune diseases and new-onset atrial fibrillation: a UK Biobank study. Europace. 2023;25(3):804–811. doi: 10.1093/europace/euac244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gawałko M, Balsam P, Lodziński P, Grabowski M, Krzowski B, Opolski G, et al. Cardiac arrhythmias in autoimmune diseases. Circ J. 2020;84(5):685–694. doi: 10.1253/circj.CJ-19-0705. [DOI] [PubMed] [Google Scholar]
- 61.Ho HH, Yeh SJ, Tsai WP, Wang CM, Chen JY. Paroxysmal supraventricular tachycardia and Wolff-Parkinson-White syndrome in ankylosing spondylitis: a large cohort observation study and literature review. Semin Arthritis Rheum. 2012;42(3):246–253. doi: 10.1016/j.semarthrit.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 62.Candemir M, Candemir B, Ertürk A. Evaluation of cardiac autonomic nervous system in patients with ankylosing spondylitis using 12-lead electrocardiography and Holter monitoring. Clin Rheumatol. 2020;39(9):2631–2639. doi: 10.1007/s10067-020-05046-y. [DOI] [PubMed] [Google Scholar]
- 63.Acar G, Yorgun H, Inci MF, Akkoyun M, Bakan B, Nacar AB, et al. Evaluation of Tp-e interval and Tp-e/QT ratio in patients with ankylosing spondylitis. Mod Rheumatol. 2014;24(2):327–330. doi: 10.3109/14397595.2013.854072. [DOI] [PubMed] [Google Scholar]
- 64.Bengtsson K, Klingberg E, Deminger A, Wallberg H, Jacobsson LTH, Bergfeldt L, et al. Cardiac conduction disturbances in patients with ankylosing spondylitis: results from a 5-year follow-up cohort study. RMD Open. 2019;5(2):e001053. doi: 10.1136/rmdopen-2019-001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lange U, Stapfer G, Ditting T, Geiger H, Teichmann J, Müller-Ladner U, et al. Pathologic alterations of the heart and the kidney in patients with ankylosing spondylitis. Eur J Med Res. 2007;12(12):573–581. [PubMed] [Google Scholar]
- 66.Chen Y, Chan YH, Chung HY, Wu MZ, Yu YJ, Pi KL, et al. Cardiovascular events prediction by left ventricular longitudinal strain and serum high-sensitivity troponin I in patients with axial spondyloarthritis. Clin Rheumatol. 2020;39(11):3373–3382. doi: 10.1007/s10067-020-05112-5. [DOI] [PubMed] [Google Scholar]
- 67.Chen Y, Chung HY, Zhao CT, Wong A, Zhen Z, Tsang HH, et al. Left ventricular myocardial dysfunction and premature atherosclerosis in patients with axial spondyloarthritis. Rheumatology. 2015;54(2):292–301. doi: 10.1093/rheumatology/keu337. [DOI] [PubMed] [Google Scholar]
- 68.Emren SV, Gerçik O, Özdemir E, Solmaz D, Eren N, Şimşek E, et al. Evaluation of subclinical myocardial dysfunction using speckle tracking echocardiography in patients with radiographic and non-radiographic axial spondyloarthritis. Eur J Rheumatol. 2020;7(1):9–15. doi: 10.5152/eurjrheum.2019.19072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin TY, Lai YF, Chien WC, Chen YH, Chung CH, Chen JT, et al. Impact of endophthalmitis on the risk of acute myocardial infarction in ankylosing spondylitis patients: a population-based retrospective cohort study. J Clin Med. 2023;12(3):1211. doi: 10.3390/jcm12031211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gan FY, Fei YY, Li MT, Wang Q, Xu D, Hou Y, et al. The characteristics of patients having ankylosing spondylitis associated with Takayasu’s arteritis. Clin Rheumatol. 2014;33(3):355–358. doi: 10.1007/s10067-013-2444-7. [DOI] [PubMed] [Google Scholar]
- 71.Ernst D, Bearlecken N, Ernst Schmidt R, Witte T. Two subsets of large vessel vasculitis characterized by the absence or presence of spondyloarthritis or its associated diseases. Open Rheumatol J. 2016;10:101–108. doi: 10.2174/1874312901610010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ernst D, Baerlecken NT, Schmidt RE, Witte T. Large vessel vasculitis and spondyloarthritis: coincidence or associated diseases? Scand J Rheumatol. 2014;43(3):246–248. doi: 10.3109/03009742.2013.850737. [DOI] [PubMed] [Google Scholar]
- 73.Ernst D, Bearlecken N, Schmidt RE, Witte T. Two subsets of large vessel vasculitis characterized by the absence or presence of spondyloarthritis or its associated diseases. Open Rheumatol J. 2016;10:101–108. doi: 10.2174/1874312901610010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eshed I, Druyan A, Stern M, Bordavka M, Lidar M. The prevalence of sacroiliitis on abdominal MRI examinations of patients with Takayasu arteritis. Acta Radiol. 2022;63(3):387–392. doi: 10.1177/0284185121996270. [DOI] [PubMed] [Google Scholar]
- 75.Kwon OC, Lee SW, Park YB, Oh JS, Lee SH, Hong S, et al. Extravascular manifestations of Takayasu arteritis: focusing on the features shared with spondyloarthritis. Arthritis Res Ther. 2018;20(1):142. doi: 10.1186/s13075-018-1643-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Agüero F, González-Zobl G, Baena-Díez JM, Dégano IR, Garcia-Gil M, Alzamora MT, et al. Prevalence of lower extremity peripheral arterial disease in individuals with chronic immune mediated inflammatory disorders. Atherosclerosis. 2015;242(1):1–7. doi: 10.1016/j.atherosclerosis.2015.06.054. [DOI] [PubMed] [Google Scholar]
- 77.Gwinnutt JM, Wieczorek M, Balanescu A, Bischoff-Ferrari HA, Boonen A, Cavalli G, et al. 2021 EULAR recommendations regarding lifestyle behaviours and work participation to prevent progression of rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2023;82(1):48–56. doi: 10.1136/annrheumdis-2021-222020. [DOI] [PubMed] [Google Scholar]
- 78.Passalent LA, Soever LJ, O’Shea FD, Inman RD. Exercise in ankylosing spondylitis: discrepancies between recommendations and reality. J Rheumatol. 2010;37(4):835–841. doi: 10.3899/jrheum.090655. [DOI] [PubMed] [Google Scholar]
- 79.Halvorsen S, Vøllestad NK, Fongen C, Provan SA, Semb AG, Hagen KB, et al. Physical fitness in patients with ankylosing spondylitis: comparison with population controls. Phys Ther. 2012;92(2):298–309. doi: 10.2522/ptj.20110137. [DOI] [PubMed] [Google Scholar]
- 80.Sveaas SH, Berg IJ, Provan SA, Semb AG, Hagen KB, Vøllestad N, et al. Efficacy of high intensity exercise on disease activity and cardiovascular risk in active axial spondyloarthritis: a randomized controlled pilot study. PLoS One. 2014;9(9):e108688. doi: 10.1371/journal.pone.0108688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Berg IJ, Semb AG, Sveaas SH, Fongen C, van der Heijde D, Kvien TK, et al. Associations between cardiorespiratory fitness and arterial stiffness in ankylosing spondylitis: a cross-sectional study. J Rheumatol. 2018;45(11):1522–1525. doi: 10.3899/jrheum.170726. [DOI] [PubMed] [Google Scholar]
- 82.Singh J, Tekur P, Metri KG, Mohanty S, Singh A, Nagaratna R. Potential role of Yoga in the management of ankylosing spondylitis: a retrospective study. Ann Neurosci. 2021;28(1–2):74–78. doi: 10.1177/09727531211035335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaan U, Ferda O. Evaluation of clinical activity and functional impairment in smokers with ankylosing spondylitis. Rheumatol Int. 2005;25(5):357–360. doi: 10.1007/s00296-004-0451-3. [DOI] [PubMed] [Google Scholar]
- 84.Sakellariou GT, Anastasilakis AD, Kenanidis E, Potoupnis M, Tsiridis E, Savvidis M, et al. The effect of smoking on clinical and radiographic variables, and acute phase reactants in patients with ankylosing spondylitis. Rheumatol Int. 2015;35(12):2109–2114. doi: 10.1007/s00296-015-3381-3. [DOI] [PubMed] [Google Scholar]
- 85.Zhao SS, Goodson NJ, Robertson S, Gaffney K. Smoking in spondyloarthritis: unravelling the complexities. Rheumatology. 2020;59(7):1472–1481. doi: 10.1093/rheumatology/keaa093. [DOI] [PubMed] [Google Scholar]
- 86.Ciaffi J, Mitselman D, Mancarella L, Brusi V, Lisi L, Ruscitti P, et al. The effect of ketogenic diet on inflammatory arthritis and cardiovascular health in rheumatic conditions: a mini review. Front Med. 2021;8:792846. doi: 10.3389/fmed.2021.792846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. G Ital Cardiol. 2022;23(6 Suppl 1):e3–e115. doi: 10.1714/3808.37926. [DOI] [PubMed] [Google Scholar]
- 88.Garg N, Krishan P, Syngle A. Rosuvastatin improves endothelial dysfunction in ankylosing spondylitis. Clin Rheumatol. 2015;34(6):1065–1071. doi: 10.1007/s10067-015-2912-3. [DOI] [PubMed] [Google Scholar]
- 89.Syngle A, Vohra K, Khichi D, Garg N, Verma I, Kaur L. Spironolactone improves endothelial dysfunction in ankylosing spondylitis. Clin Rheumatol. 2013;32(7):1029–1036. doi: 10.1007/s10067-013-2233-3. [DOI] [PubMed] [Google Scholar]
- 90.Garg N, Krishan P, Syngle A. Angiotensin-receptor blockade improves inflammation and endothelial dysfunction in ankylosing spondylitis: ARB-AS study. Int J Angiol. 2021;30(4):262–270. doi: 10.1055/s-0040-1722738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rollefstad S, Ikdahl E, Hisdal J, Olsen IC, Holme I, Hammer HB, et al. Rosuvastatin-induced carotid plaque regression in patients with inflammatory joint diseases: the rosuvastatin in rheumatoid arthritis, ankylosing spondylitis and other inflammatory joint diseases study. Arthritis Rheumatol. 2015;67(7):1718–1728. doi: 10.1002/art.39114. [DOI] [PubMed] [Google Scholar]
- 92.Oza A, Lu N, Schoenfeld SR, Fisher MC, Dubreuil M, Rai SK, et al. Survival benefit of statin use in ankylosing spondylitis: a general population-based cohort study. Ann Rheum Dis. 2017;76(10):1737–1742. doi: 10.1136/annrheumdis-2017-211253. [DOI] [PubMed] [Google Scholar]
- 93.Angel K, Provan SA, Fagerhol MK, Mowinckel P, Kvien TK, Atar D. Effect of 1-year anti-TNF-α therapy on aortic stiffness, carotid atherosclerosis, and calprotectin in inflammatory arthropathies: a controlled study. Am J Hypertens. 2012;25(6):644–650. doi: 10.1038/ajh.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Végh E, Kerekes G, Pusztai A, Hamar A, Szamosi S, Váncsa A, et al. Effects of 1-year anti-TNF-α therapy on vascular function in rheumatoid arthritis and ankylosing spondylitis. Rheumatol Int. 2020;40(3):427–436. doi: 10.1007/s00296-019-04497-0. [DOI] [PubMed] [Google Scholar]
- 95.Knyazeva LA, Damjanov N, Knyazeva LI, Meshcherina NS, Goryainov II, Stepchenko MA, et al. Effect of golimumab on endothelial vasomotor function and arterial stiffness in ankylosing spondylitis. Nauchno-Prakticheskaya Revmatologiya. 2019;57(3):312–317. [Google Scholar]
- 96.Syngle A, Vohra K, Sharma A, Kaur L. Endothelial dysfunction in ankylosing spondylitis improves after tumor necrosis factor-alpha blockade. Clin Rheumatol. 2010;29(7):763–770. doi: 10.1007/s10067-010-1402-x. [DOI] [PubMed] [Google Scholar]
- 97.Tam LS, Shang Q, Kun EW, Lee KL, Yip ML, Li M, et al. The effects of golimumab on subclinical atherosclerosis and arterial stiffness in ankylosing spondylitis-a randomized, placebo-controlled pilot trial. Rheumatology. 2014;53(6):1065–1074. doi: 10.1093/rheumatology/ket469. [DOI] [PubMed] [Google Scholar]
- 98.Angel K, Provan SA, Hammer HB, Mowinckel P, Kvien TK, Atar D. Changes in arterial stiffness during continued infliximab treatment in patients with inflammatory arthropathies. Fundam Clin Pharmacol. 2011;25(4):511–517. doi: 10.1111/j.1472-8206.2010.00872.x. [DOI] [PubMed] [Google Scholar]
- 99.Mathieu S, Pereira B, Couderc M, Rabois E, Dubost J, Soubrier M. No significant changes in arterial stiffness in patients with ankylosing spondylitis after tumour necrosis factor alpha blockade treatment for 6 and 12 months. Rheumatology. 2013;52(1):204–209. doi: 10.1093/rheumatology/kes272. [DOI] [PubMed] [Google Scholar]
- 100.Genre F, López-Mejías R, Miranda-Filloy JA, Ubilla B, Mijares V, Carnero-López B, et al. Anti-TNF-α therapy reduces endothelial cell activation in non-diabetic ankylosing spondylitis patients. Rheumatol Int. 2015;35(12):2069–2078. doi: 10.1007/s00296-015-3314-1. [DOI] [PubMed] [Google Scholar]
- 101.Mathieu S, Dubost JJ, Tournadre A, Malochet-Guinamand S, Ristori JM, Soubrier M. Effects of 14 weeks of TNF alpha blockade treatment on lipid profile in ankylosing spondylitis. Joint Bone Spine. 2010;77(1):50–52. doi: 10.1016/j.jbspin.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 102.van Eijk IC, de Vries MK, Levels JH, Peters MJ, Huizer EE, Dijkmans BA, et al. Improvement of lipid profile is accompanied by atheroprotective alterations in high-density lipoprotein composition upon tumor necrosis factor blockade: a prospective cohort study in ankylosing spondylitis. Arthritis Rheum. 2009;60(5):1324–1330. doi: 10.1002/art.24492. [DOI] [PubMed] [Google Scholar]
- 103.Ersozlu Bozkirli ED, Bozkirli E, Yucel AE. Effects of infliximab treatment in terms of cardiovascular risk and insulin resistance in ankylosing spondylitis patients. Mod Rheumatol. 2014;24(2):335–339. doi: 10.3109/14397595.2013.843752. [DOI] [PubMed] [Google Scholar]
- 104.Kiortsis DN, Mavridis AK, Filippatos TD, Vasakos S, Nikas SN, Drosos AA. Effects of infliximab treatment on lipoprotein profile in patients with rheumatoid arthritis and ankylosing spondylitis. J Rheumatol. 2006;33(5):921–923. [PubMed] [Google Scholar]
- 105.Bhala N, Emberson J, Merhi A, Abramson S, Arber N, Baron JA, et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382(9894):769–779. doi: 10.1016/S0140-6736(13)60900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Trelle S, Reichenbach S, Wandel S, Hildebrand P, Tschannen B, Villiger PM, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011;342:c7086. doi: 10.1136/bmj.c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Karmacharya P, Shahukhal R, Crowson CS, Murad MH, Davis JM, 3rd, Shrestha P, et al. Effects of therapies on cardiovascular events in ankylosing spondylitis: a systematic review and meta-analysis. Rheumatol Ther. 2020;7(4):993–1009. doi: 10.1007/s40744-020-00248-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fakih O, Desmarets M, Martin B, Prati C, Wendling D, Monnet E et al (2023) Impact of NSAIDs on 8-year cumulative incidence of major cardiovascular events in patients with ankylosing spondylitis: a nationwide study. Rheumatology (Oxford) kead072. 10.1093/rheumatology/kead072 [DOI] [PubMed]
- 109.Braun J, Baraliakos X, Westhoff T. Nonsteroidal anti-inflammatory drugs and cardiovascular risk - a matter of indication. Semin Arthritis Rheum. 2020;50(2):285–288. doi: 10.1016/j.semarthrit.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 110.So H, Tam LS. Nonsteroidal anti-inflammatory drugs and cardiovascular disease risk in spondyloarthritis-spectrum diseases. Curr Opin Rheumatol. 2022;34(4):203–208. doi: 10.1097/BOR.0000000000000881. [DOI] [PubMed] [Google Scholar]
- 111.Tam HW, Yeo KJ, Leong PY, Chen CH, Li YC, Ma CM, et al. Sulfasalazine might reduce risk of cardiovascular diseases in patients with ankylosing spondylitis: a nationwide population-based retrospective cohort study. Int J Rheum Dis. 2017;20(3):363–370. doi: 10.1111/1756-185X.12986. [DOI] [PubMed] [Google Scholar]
- 112.Wu LC, Leong PY, Yeo KJ, Li TY, Wang YH, Chiou JY, et al. Celecoxib and sulfasalazine had negative association with coronary artery diseases in patients with ankylosing spondylitis: a nation-wide, population-based case-control study. Medicine. 2016;95(36):e4792. doi: 10.1097/MD.0000000000004792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chan SCW, Teo CK, Li PH, Lau KK, Lau CS, Chung HY. Cardiovascular risk in patients with spondyloarthritis and association with anti-TNF drugs. Ther Adv Musculoskelet Dis. 2021;13:1759720x211032444. doi: 10.1177/1759720X211032444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee JL, Sinnathurai P, Buchbinder R, Hill C, Lassere M, March L. Biologics and cardiovascular events in inflammatory arthritis: a prospective national cohort study. Arthritis Res Ther. 2018;20(1):171. doi: 10.1186/s13075-018-1669-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kwon OC, Park MC. Effect of tumor necrosis factor inhibitors on risk of cardiovascular disease in patients with axial spondyloarthritis. Arthritis Res Ther. 2022;24(1):141. doi: 10.1186/s13075-022-02836-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Singh JA. Risks and benefits of Janus kinase inhibitors in rheumatoid arthritis - past, present, and future. N Engl J Med. 2022;386(4):387–389. doi: 10.1056/NEJMe2117663. [DOI] [PubMed] [Google Scholar]
- 117.Ytterberg SR, Bhatt DL, Mikuls TR, Koch GG, Fleischmann R, Rivas JL, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386(4):316–326. doi: 10.1056/NEJMoa2109927. [DOI] [PubMed] [Google Scholar]
- 118.Smolen JS, Landewé RBM, Bergstra SA, Kerschbaumer A, Sepriano A, Aletaha D, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis. 2023;82(1):3–18. doi: 10.1136/ard-2022-223356. [DOI] [PubMed] [Google Scholar]
- 119.Ramiro S, Nikiphorou E, Sepriano A, Ortolan A, Webers C, Baraliakos X, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023;82(1):19–34. doi: 10.1136/ard-2022-223296. [DOI] [PubMed] [Google Scholar]
- 120.Makris A, Barkas F, Sfikakis PP, Liberopoulos E, Agouridis AP. The effect of upadacitinib on lipid profile and cardiovascular events: a meta-analysis of randomized controlled trials. J Clin Med. 2022;11(23):6894. doi: 10.3390/jcm11236894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Evangelatos G, Fragoulis GE. JAK inhibitors, cardiovascular and thromboembolic events: what we know and what we would like to know. Clin Rheumatol. 2023;42(3):959–962. doi: 10.1007/s10067-022-06471-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.