Abstract
The extraction of Rhizostoma pulmo discarded off the Goa coast, India resulted in obtaining remarkably higher yield of 47% (based on lyophilized weight) type I collagen. The amino acid composition showed presence of glycine, hydroxyproline and proline and other amino acids in consistent with mammalian collagens. Interestingly, it also possessed essential amino acid tryptophan that makes this collagen superior than other commercially available collagen products. The dose and time response anti-oxidant activity (DPPH assay) of jellyfish collagen showed increase in the percentage activity with its efficiency better than marine fish collagen. In the dose response, IC50 was found to be 11.0 mg/ml. The percentage DPPH activity gradually increased from 47.58 to 81.11% with time 1–8 h, respectively at concentration of 7 mg/ml of jellyfish collagen. It was noteworthy to observe that the anti-oxidant activity remained 80% even after 24 h of analysis. The EDX analysis showed presence of minerals like Cl, Na, Mg, K, Cu, Fe, Zn etc. essential for healthy bones. The mass assisted laser desorption ionization-time of flight mass spectrometric (MALDI-TOF MS) data showed several precursor peaks of different peptides which has been presented here for the first time. The finding showed higher production of tryptophan containing anti-oxidant collagen that will certainly enhance its benefit in neurotransmission and cognitive function.
Keywords: Amino acids, Anti-oxidant, Collagen, Jellyfish, Rhizostoma pulmo, Tryptophan
Introduction
Collagen is the most abundant protein found in the animal kingdom and the demand for collagen based products has kept on increasing globally. It is estimated that the collagen market size will grow to USD 5.3 billion by 2026 (https://www.globenewswire.com). The growing number of scientific publications and patents shows exciting evidences of its applications in various areas of healthcare, neutraceuticals, cosmetics, wound healing, medical devices etc. (Addadet al. 2011; Leoneet al. 2015). Currently, the needed demand is fulfilled from the bones, hides, and hooves of cattle and pigs (Mokrejs et al. 2009). However, due to religious and cultural constraints they have been forbidden by many communities. Recently, marine organisms have been considered as a promising source of collagen. It is abundantly found in fish and in particular jellyfish have high content of protein of which 80–90% is collagen (Šimat et al. 2020). Owing to its excellent bioactivity, good biocompatibility, good penetrability and non-irritation of the body, collagen and hydrolyzed collage peptides have become the most important components and reliable alternative for neutraceuticals (Leone et al. 2015) and in other healthcare applications such as wound care, regeneration medicine, cell culture etc. (Sumiyoshi et al. 2021; Nudelman et al. 2019; Pustlauk et al. 2015; Xu et al. 2021). Jellyfish Rhopilema esculentum have become a popular food in several Asian countries due to its high nutritional value and pharmacological activity (Eun et al. 2006). It is also known that, jellyfish collagen carries less risk of prion and viral contamination than the usual sources.
Rhizostoma pulmo (Scyphomedusae, Barrel Jellyfish) has been edible jellyfish in most of the Asian countries. Aggregation of this jellyfish in Indian coastal waters was evident from many electronic media reports as well as scientific publications (Baliarsingh et al. 2020). In the recent times, with the increase in the ocean warming conditions, occurrence of the blooms of R. pulmo and Chryosoara caliparea along the Goa coast (West coast of India) have become continuous process. There are no significant scientific reports on R. pulmo from Indian waters. Thus, our study aimed at investigating R. pulmo found along Goa coast for value added product collagen. Interestingly, our study revealed that collagen from this species contains essential amino acid tryptophan, a neurotransmitter which is limiting in currently marketed collagen derived from bovine and porcine. Further, anti-oxidant activity (DPPH assay) in the dose and time response suggested its efficiency better than commercially available marine fish collagen in India. Additionally, EDX and mass spectrometric studies of the collagen has been presented here for the first time. The analysis of commercial marine collagen has also been highlighted for comparison. The amino acid composition of collagen extracted from Indian species R. pulmo makes it very potent which will be suitable to develop neuroprotective anti-oxidant.
Materials and methods
Collection of jellyfish Rhizostoma pulmo
Jellyfishes Rhizostoma pulmo were collected from Caranzalem beach, Dona Paula, Goa during post-monsoon (November and December 2021). The local fisherman community helped in the collection of jellyfishes caught during fish catch. Thereafter, the jellyfishes were transported to laboratory and washed thoroughly with tap water followed by distilled water to remove sand and any debris. The jellyfishes were stored at − 20 °C until further process.
Extraction of collagen
Jellyfish tissue (~ 5 kg, wet weight) was washed with 0.1 M NaOH (2.5 L) and stirred for 6 h. This step was repeated thrice. The pre-treated tissue was then stirred with 0.5 M acetic acid (5 L) at lower temperature (4 °C) as used by Miura and Kimura (1985) with modifications. The process was done for 3 h under the influence of ultra-sonication. This process resulted into the acid-solubilized collagen. Further, acid-soluble collagen was incubated with 1% pepsin at 4 °C in 0.5 M acetic acid for 3 h again assisted with ultra-sonication. The viscous solution was centrifuged at 15000×g 1 h and dialyzed against 0.02 M Na2HPO4 in order to inactivate the pepsin. The dialysis step was repeated several times using cellulose membrane dialysis tubing (molecular weight cut off 14,000 Da) and the resultant precipitate was re-dissolved in 0.5 M acetic acid. The pepsin-solubilized collagen was then precipitated by addition of NaCl to a final concentration of 1.0 M NaCl, the majority of the collagen was precipitated at 0.9 M NaCl. The precipitated collagen was collected and again dissolved in 0.5 M acetic acid. It was dialyzed against 0.1 M acetic acid, 0.05 M acetic acid and 0.025 M acetic acid sequentially. After dialysis, the pepsin hydrolyzed collagen was collected, lyophilized and stored at 2 °C.
Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS- PAGE)
SDS- PAGE analysis was performed on the pepsin-solubilized collagen (JFC) as previously described by Weber & Osborn with some modifications (Weber and Osborn 1969). The dried collagen powder was dissolved in phosphate buffer solution (pH 7.4). The mixture was centrifuged at 10,000g for 5 min at room temperature to remove undissolved matter. Equal parts of the supernatant and sample buffer was mixed and heated at 90 °C for 5 min. This was loaded on the gel (10% resolving, 4% stacking) and the run was maintained at 85 V for the initial 30 min after which the voltage was increased to and maintained at 95 V till the end of the run. The gel was stained overnight using 0.1% Coomassie Blue R-250. The desstaining of the gel was carried out using solution of acetic acid, methanol and distilled water and the bands were viewed using syngen Gbox gel documentation system. Molecular weight estimation was carried out by comparing the bands with Novex E-Page See-Blue prestained standard protein marker.
UV–VIS and FT-IR spectroscopy
Wavelength λmax of collagen was recorded using UV–Visible spectrophotometer (Shimadzu UV-2401PC, Japan). Collagen sample was dissolved in 0.5 M acetic acid at a ratio of 1:1 (w/v). The Fourier transform-Infrared spectrophotometer (FT-IR) spectra of the collagen were performed on IR Affinity-1 (Shimadzu, Japan) using DRS interface. Collagen samples were finely powdered with KBr. The FTIR spectra were recorded in the range 700–4000 cm−1 wavenumber.
Amino acid analysis
Amino acid composition of the jellyfish collagen (JFC) and commercial marine collagen (MC) were studied by adopting high performance liquid chromatography (HPLC) technique using Agilent 1260 infinity. The detector used was diode array detector with detection at 338 nm and 262 nm. The column used for amino acid separation was Advance bio AAA column, Agilent Technologies (particle size: 2.7 µm, Inner diameter: 3 mm, length 100 mm) maintained at a temperature of 40 °C. Mobile phase A was 10 mM Na2HPO4 and 10 mM Na2B4O7, pH 8.2 and mobile phase B was acetonitrile:methanol:water (45:45:10, v:v:v). The flow rate was 1.5 ml min−1. Norvaline purchased from fluka chemie was used as internal standard. It consists of 18 amino acids, l-aspartic acid (Asp), l-serine (Ser), l-glutamic acid (Glu), l-glutamine (Gln), glycine (Gly), l-histidine (His), l-asparagine (Asn), l-arginine (Arg), l-threonine (Thr), l-alanine (Ala), l-tyrosine (Tyr), l-valine (Val), l-methionine (Met), l-isoleucine (Ile), l-tryptophan (Trp), l-leucine (Leu), l-phenylalanine (Phe) and l-lysine (Lys) with > 98% purity. Collagen samples were digested using 6N HCl for 24 h at 110 °C. After 24 h of hydrolysis, the mixture was neutralized using 6 N NaOH followed by centrifugation at 5000 rpm for 10 min. Further, the supernatant was collected and evaporated to dryness using a rotary evaporator. After drying, the sample was reconstituted in 2 ml of milliQ water and subjected to HPLC system for amino acid analysis.
Proline and hydroxyproline analysis by colorimetric
Proline assay was carried out according to Bates et al. with some modifications (Bates et al. 1973). Protein sample was treated with equal amounts of manually and freshly prepared ninhydrin reagent and glacial acetic acid. The mixture was incubated in a boiling water bath for an hour until a bright red colour was formed. Double the amount of toluene was then added to this red solution and vortexed. After this the toluene layer was carefully pipetted out and the absorbance measured at 520 nm. The proline concentration was calculated using the formula:
Hydroxyproline determination was done using a technique previously reported by Edwards & O'Brien Jr. with slight modifications (Edwards and O'Brien 1980). Sample was treated with 5N HCl and autoclaved at 120 °C and 15 psi for 15 min. After this hydrolysis the sample was neutralized by adding 5N NaOH. The neutralized mixture was centrifuged and the suspension carefully transferred to a clean vial. To this, 625 µL of chloramine-T solution was added and left to incubate at room temperature for 20 min. This was followed by the addition of 625 µL of Ehrlich’s solution and incubated in the water bath for 20 min. Immediately after the incubation the tubes containing samples were immersed in an ice bath to cease the reaction. This was followed by checking the absorbance at 560 nm using UV spectrophotometer. Then hydroxyproline concentration was then calculated using the formula
Energy dispersive X-Ray (EDX)
Elemental content of JFC and MC were analyzed by using energy dispersive instrument. The sample was gold coated prior to elemental analysis using SPI sputter coater module. Quantitative estimates of elemental composition (weight%) were determined using Oxford Instruments AZtec software.
Antioxidant activity
Antioxidant activity was studied by conducting 2,2-Diphenyl-1-picrylhydrazyl (DPPH) assay to check, if collagen can inhibit the free radical activity of DPPH by following the method described by Wu et al. with slight modifications (Wu et al. 2003). Collagen was dissolved in 0.5 M acetic acid to get 5 mg/ml solution which was mixed with 0.3 mM solution of 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH, SIGMA Life Science) in methanol. Collagen free radical mixture was incubated and allowed to react for 30 min. Thereafter, optical density (OD) was recorded on UV–Vis spectrophotometer at 517 nm. The OD was also recorded for the blank without collagen sample. All the readings were recorded in triplicates. Similar procedure was followed to check anti-oxidant activities of standards kojic acid (Sigma Life Sciences) and ascorbic acid at different concentrations. The standard curve was plotted to calculate IC50. Percentage free radical scavenging activity of collagen was derived using the formula:
where Ab is the absorbance of blank and As is the absorbance of the sample at 517 nm.
Statistical analysis
All the anti-oxidant measurements were made in triplicate. The results were subject to variance analysis using single factor analysis of variance (ANOVA). Percentage anti-oxidant activity was expressed as mean ± SD (standard deviations).
Chemical and reagents
Glacial acetic acid (99–100%) was purchased from Merck KGaA (Darmstadt, Germany). Ethylene diamine tetra acetic acid (EDTA), sodium chloride, sodium dihydrogen phosphate and disodium hydrogen phosphate (Na2HPO4) were purchased from HIMEDIA (Maharashtra, India). β-Mercaptoethanol, 2,2-Diphenyl-1-picrylhydrazyl (DPPH), Ehrlich’s solution and α-glucosidase activity assay kit were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Coomassie Billiant Blue was purchased from Thermo Fisher Scientific (Waltham, Massachusetts, United States). E-PAGE See Blue Prestained protein marker was purchased from (Waltham, Massachusetts, United States). Acarbose (95% pure) was purchased from Sisco Research Laboratories Pvt Ltd Mumbai, Maharashtra. L-Ascorbic acid AR, ACS was purchased from SDFCL Sd Fine Chem Limited (Chennai, Tamil Nadu, India). Chloramine-T and L-proline standard was purchased from Thomas Baker (Chemicals) Pvt. Ltd. (TBCPL), Mumbai, India. Hydroxyproline standard was purchased from Loba Chemie Pvt Ltd, Mumbai, India. To carry out comparative anti-oxidant studies a commercially available fish collagen i.e. Marine collagen (MC) was purchased from Amar Aquatic (Amar Polyfills Pvt. Ltd., Gujarat, India).
Mass spectrometry analysis
Collagen was added to the MS grade trypsin (Promega) such that the ratio is trypsin:collagen 1:20 (w/w). The digestion mixture was incubated at 37 °C overnight. 5 µL of solution (50% acetonitrile, 0.1% TFA) was added to the tube and gently agitated on a vortex at lowest setting. 0.5 µL of the digested collagen sample was spotted on MALDI plate followed by 0.5 µL of alpha-cyano-4-hydroxycinnamic acid matrix (10 mg/mL in 50% acetonitrile, 0.1% TFA). The spot was allowed to dry completely and plate was loaded into Voyager of mass spectrometer. The spectra was recorded on matrix-assisted laser desorption ionization/time of flight mass spectroscopy (MALDI-TOF) of Bruker Daltoncs model Ultraflex II Spectrometer, Germany, equipped with a pulsed Neodymium yttrium–aluminium garnet (Nd-YAG) smart beam solid state laser (337 nm), in reflectron positive-ion mode, using a 19-kV acceleration voltage). The instrument was calibrated using internal tryptic peaks of 842.5 and 2211.1 Da.
Result and discussion
Production of collagen from R. pulmo
The extraction of collagen from jellyfish (whole tissue, jellyfish size ~ 10–16 cm) was done by using 0.5 M acetic acid and 1% pepsin under the influence of ultra-sonication at lower temperature (10), to get pepsin-hydrolyzed collagen which resulted in collagen production upto 47% (lyophilized dry weight). The ultra-sonication process not only reduces the extraction timing but also increased the yield. So far, there is only one report of the collagen yield from R. pulmo (Tunisian Mediterranean coast) reporting pepsin (2–15 mg pepsin/mg of wet tissue) solubilized collagen obtained to be 0.83–3.15 and 2.61–10.3 (mg/g wet tissue) for umbrella and oral arms, respectively (Addad et al. 2011). The yields of collagen reported from mesoglea of Rhopilema esculentum with acid and 1% pepsin were 0.12% and 0.28% respectively (on a wet weight basis) (Cheng et al. 2017). From the same jellyfish, Falician and coworker reported 4.31% collagen yield (on a wet weight basis) using 1% pepsin (Felician et al. 2019). The production of acid solubilized collagen reported from C. mosaicus were found to be 14.61 ± 0.57 and 22.47 ± 1.25 mg/g dry weight (1.46% and 2.24% lyophilized dry weight) from umbrella and oral arm tissues, respectively (Rastian et al. 2018). The yield of collagen from Cyanea nozakii (Yellow sea) was found to be 13.0% (dry weight) (Zhang et al. 2014). So far, among the edible scyphomedusa, Nagai et al. reported 46.4% and 35.2% collagen (dry lyophilized weight) from exumbrella Stomolophus meleagris and Rhopilema asamushi, respectively (Nagai et al. 2000). From our investigation, it is revealed that R. pulmo is another scyphomedusa that produces highest collagen i.e. 47% (dry lyophilized weight).
Molecular weight of the collagen
SDS-PAGE was used to determine the molecular weight of the pepsin-solubilized jellyfish collagen (JFC). The gel showed two bands for α1 and α2 chain at ~ 92 and ~ 125 kDa, respectively and one band for β chain above 150 kDa suggestive of type-I collagen (Fig. 1a). The α chains are the major bands of the jellyfish collagens. The similar patterns were demonstrated from R. pulmo collagen (Addad et al. 2011; Widdowson et al. 2018). Widdowson et al. reported collagen with distinct α1 and α2 chains at ~ 105 and ~ 92 kDa and assessed biocompatibility of jellyfish collagen sponge over bovine collagen sponge. Their results revealed that, jellyfish collagen showed significant reduction in histopathology scores thereby increasing their biocompatibility with wound healing applications. Among other edible jellyfishes, R. esculentum and Catostylus mosaicus showed presence of type I collagen with identical band pattern (Rastian et al. 2018). In the case of commercial available marine collagen (MC) SDS-Page (Fig. 1b) showed broad band ranging between ~ 30–45 kDa for lower molecular weight collagen. This indicated that the MC to be collagen hydroxylate from fish as reported from other species (Chi et al. 2014; Nurilmala et al. 2020).
Fig. 1.
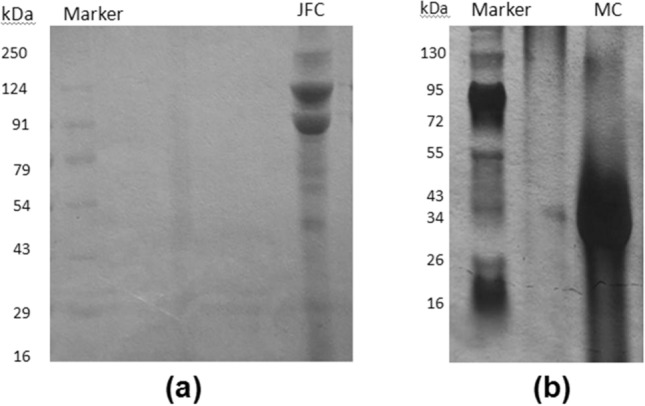
SDS page of a Jellyfish collagen (JFC); b Marine collagen (MC)
UV–Vis and FTIR spectroscopy
The UV absorption of proteins are mostly in the range of 180–230 nm due to π → π∗ transitions in the peptide bonds. However, the UV–Vis spectrum of the jellyfish collagen showed characteristic λmax at 275 nm which suggested that there would be presence of aromatic amino acids like tryptophan, tyrosine, and phenylalanine residues. The spectrum showed bands at 3278 and 2964 cm−1 observed for amide A and amide B, respectively. The amide I, II and III bands were found to occur at 1654, 1533 and 1240 cm−1, respectively as a result of N–H and C–H stretching. The spectrum showed the intact triple helical structure of collagen with the appearance of typical amide II band of collagen at 1533–1537 cm−1. Frequency at 1444 cm−1 corresponds to stretching vibration of cyclic proline’s C–N group. The CH2 groups in proline’s side chain contributing to wagging vibrations correspond to band position at 1334 cm−1.
Amino acid composition
The amino acid composition of 18 primary amino acids were analyzed using HPLC, while, proline and hydroxyproline were estimated using colorimetric standard curve. In HPLC analysis, JFC showed presence of 12 amino acids containing both essential and non-essential amino acids (Table 1). It showed highest percentage of glycine (29.34%) followed by glutamic acid (13.46%), aspartic acid (10.91%), alanine (10.38%), asparagine (6.80%) and tyrosine (1.77%) non-essential amino acids. The presence of hydroxyproline and proline were 4.82 g/100 g and 2.97 g/100 g, respectively. Among the essential amino acid, collagen contains arginine (5.63%), leucine (6.35%), lysine (4.62%), threonine (3.18%), valine (2.80%) and most importantly tryptophan (4.72%). For comparison, the amino acid analysis of marine collagen (MC) was also carried out. The HPLC analysis of MC showed presence of 16 amino acids with glycine (35.79%), glutamic acid (11.12%), aspartic acid (10.29%), alanine (9.57%), serine (3.22%), asparagine (1.94%), tyrosine (1.49%) and glutamine (0.17%) non-essential amino acids. The study revealed that the concentration of glycine in MC was higher while percentage of asparagine was lesser in MC with additional presence of glutamine and serine as compared in JFC. The essential amino acids in MC constituted arginine (8.17%), leucine (4.04%), lysine (4.38%), threonine (2.47%), valine (2.18%) and tyrosine (1.49%). Other amino acids like isoleucine, phenylalanine and methionine were detected < 2% with the absence of tryptophan as compared to JFC. It is reported that tryptophan is one such amino acid that help produce serotonin for neurotransmission and presence of which makes this jellyfish collagen very potent. The earlier research has shown that tryptophan is limiting amino acid in collagen produced by other sources such as collagen derived from fish skin of African catfish (Clarias gariepinus), Salmon (Salmo salar) and Baltic cod (Gadus morhua), waste freshwater carp fish (Cyprinus carpio) scales, Nile tilapia, jellyfish Rhopilema esculentum and calf-skin collagen (Tylingo et al. 2016; Zhuang et al. 2009). Even the widely consumed sources bovine and porcine collagen lacks tryptophan (Carvalho et al. 2018) and therefore considered as incomplete protein source according to the new current protein quality evaluation method protein digestibility-corrected amino acid score (PDCAAS) (Paul et al. 2019). The depletion of tryptophan in the diet decreases serotonin level in the brain thereby giving rise to several neurological disorders like decreased sleep time, lowered mood, depression, cognitive behavior, Alzheimer’s etc. (Jenkins et al. 2016). The research have indicated that strategy of administration of tryptophan-rich dietary proteins can enhance tryptophan availability to the brain and thus potentially enhanced serotonin synthesis that can lead to various improvements in health (Paul et al. 2019). Jellyfish collagen was also found to be enriched in non-essential amino acids i.e. glutamic acid and aspartic acid that serve as an important neurotransmitter at the synapses (Yamaguchi et al. 2016). Yamaguchi et al. had reported that combination of aspartic acid and glutamic acid exhibited synergistic effect on anti-proliferative activity against tumor cells. From the data, it was revealed that jellyfish collagen under this study has balanced essential and non-essential amino acids and thus, may be incorporated as protein mixture in the diet.
Table 1.
Amino acid composition of Jellyfish collagen (JFC) and marine collagen (MC)
| Amino Acids | JFC (%) | MC (%) |
|---|---|---|
| Aspartic acid | 10.91 | 10.29 |
| Glutamic acid | 13.46 | 11.12 |
| Asparagine | 6.80 | 1.94 |
| Serine | 0.00 | 3.22 |
| Glutamine | 0.00 | 0.17 |
| Histidine | 0.00 | 0.00 |
| Glycine | 29.34 | 35.79 |
| Threonine | 3.18 | 2.47 |
| Arginine | 5.63 | 8.17 |
| Alanine | 10.38 | 9.57 |
| Leucine | 6.35 | 4.04 |
| Lysine | 4.66 | 4.38 |
| Tyrosine | 1.77 | 1.49 |
| Valine | 2.80 | 2.18 |
| Methionine | 0.00 | 1.37 |
| Tryptophan | 4.72 | 0.00 |
| Phenylalanine | 0.00 | 1.93 |
| Isoleucine | 0.00 | 1.85 |
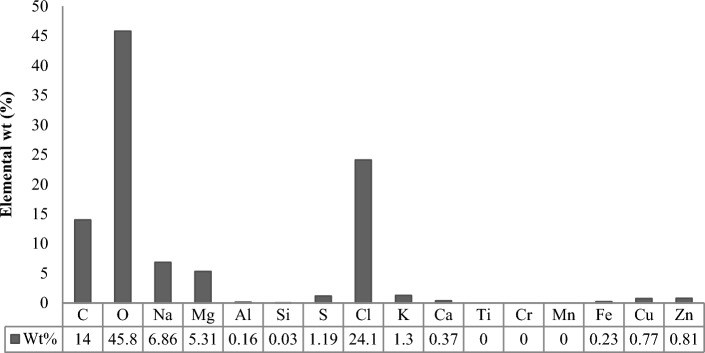
Elemental analysis
The inorganic mineral content was measured in dry jellyfish collagen powder (JFC) using EDX which constituted to 40–41% with higher concentration of chloride (Cl, 24.1%) followed by sodium (Na, 6.86%) and magnesium (Mg, 5.31%) (Fig. 2). The other minerals found were aluminum (Al, 0.16%), sulfur (S, 1.19%), potassium (K, 1.3%), calcium (Ca, 0.37%), copper (Cu, 0.77%), iron (Fe, 0.23%), zinc (Zn, 0.81) and silicon (Si, 0.03%). While, marine collagen (MC) showed only very few inorganic minerals, Zn (1.76%), Cu (0.5%), S (0.14%) and Pb (0.03%). Magnesium, calcium, Zinc and copper minerals are found to be very critical for maintaining healthy bones, detoxification, temperature and transmission of nerve impulses (Julian and Moran 2013). They are also involved in the interactions of more than 300 enzymes reactions. It is reported these minerals accumulation in jellyfish collagen may be have beneficial effect in osteoporosis (Marjan et al. 2015). The presence of elements such as Mg, Ca, Zn and Cu in R. pulmo makes this jellyfish very remarkable source of minerals binding collagen that may be beneficial in osteroporosis and bone regenerations. Khong et al. has reported elemental analysis of jellyfishes, A. hardenbergi, R. hispidum and R. esculentum (Khong et al. 2016). All these species have also shown to contain major elements Cl, Na, K, Mg along with other minor elements Ca, S, Si, Z and phosphorus (P). These elemental analysis data was found similar to that of JFC except for the absence of phosphorus.
Fig. 2.
EDX data of jellyfish collagen (JFC)
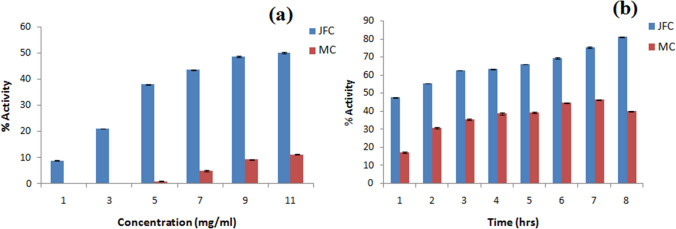
Anti-oxidant activity (DPPH assay)
The antioxidant activity was evaluated based on DPPH scavenging radical assay as dose and time response. In the dose response, 1.0–11.0 mg/ml concentration of jellyfish collagen (JFC) and for comparison, a neutraceutical product marine fish collagen (MC) was assessed. The dose response spectrophotometer reading was checked after 30 min and the percentage activity was calculated as given in Table 2. The study demonstrated that as the concentration of JFC increases, the activity increases and the 50% activity was observed at concentration of 11.0 mg/ml. In comparison, commercial product (MC) gave very less activity i.e. 11.29% at 11.0 mg/ml as depicted in the graph of percentage activity v/s concentration (mg/ml) (Fig. 3). JFC and MC at concentration 7 mg/ml were chosen to check changes in the percentage activity with time response (1–8 h). From the data (Table 2, Fig. 3a), it is observed that, with time there was increase in the activity of JFC from 47.58 to 81.11%. While, MC increased from 17.33 to 47.0% during 1 to 7 h, but there was drop in the activity i.e. 40.0% at 8 h (Fig. 3b). Interestingly, when the activity was checked at 24 h, the percentage activity of JFC remains 80%.
Table 2.
Dose and time response DPPH radical Scavenging Assays of jellyfish collagen (JFC) and Marine fish collagen (MC)
| Dose response DPPH radical Scavenging assays | Time response DPPH radical Scavenging assays | ||||
|---|---|---|---|---|---|
| Conc. (mg/mL) | % Activity@ JFC | % Activity@ MC | Time (h) | % Activity JFC* | % Activity MC* |
| 1 | 8.90 ± 0.15# | 0 | 1 | 47.58 ± 0.18 | 17.33 ± 0.32 |
| 3 | 21.05 ± 0.10 | 0 | 2 | 55.36 ± 0.20 | 30.90 ± 0.40 |
| 5 | 38.05 ± 0.14 | 1.0 ± 0.16 | 3 | 62.61 ± 0.16 | 35.51 ± 0.34 |
| 7 | 43.50 ± 0.15 | 5.0 ± 0.22 | 4 | 63.31 ± 0.19 | 38.69 ± 0.60 |
| 9 | 48.58 ± 0.24 | 9.27 ± 0.21 | 5 | 65.95 ± 0.21 | 39.36 ± 0.41 |
| 11 | 50.00 ± 0.26 | 11.29 ± 0.17 | 6 | 69.35 ± 0.35 | 44.62 ± 0.13 |
| 7 | 75.25 ± 0.25 | 46.39 ± 0.15 | |||
| 8 | 81.11 ± 0.26 | 40.00 ± 0.23 | |||
@activity measured after 30 min
*Sample concentration used was 7 mg/ml
#Values are mean ± standard deviation of three readings (P < 0.05)
Fig. 3.
DPPH radical scavenging assays of jellyfish collagen (JFC) and Marine fish collagen (MC); a Dose response, b Time response (P < 0.05)
De Domenico et al. reported anti-oxidant property of R. pulmo from Marina di Ginosa (Taranto, Italy) using ABTS assay (De Domenico et al. 2019). They extracted soluble and insoluble proteins which were sequentially hydrolysed using pepsin and collagenase and characterized different types protein based on their molecular weights. The anti-oxidant activity (AA) suggested that AA was found to be significantly higher for sub-fractions containing collagenase-hydrolysed jellyfish peptides with MW < 3 KDa followed by MW 3–10 KDa, as compared to proteins before collagenase digestion. The antioxidant activity of protein and phenolic contents of Mediterranean jellyfish R. pulmo was evaluated after thermal treatment at 100 °C (Leone et al. 2019). The study found that jellyfish R. pulmo showed the better performance after thermal treatment.
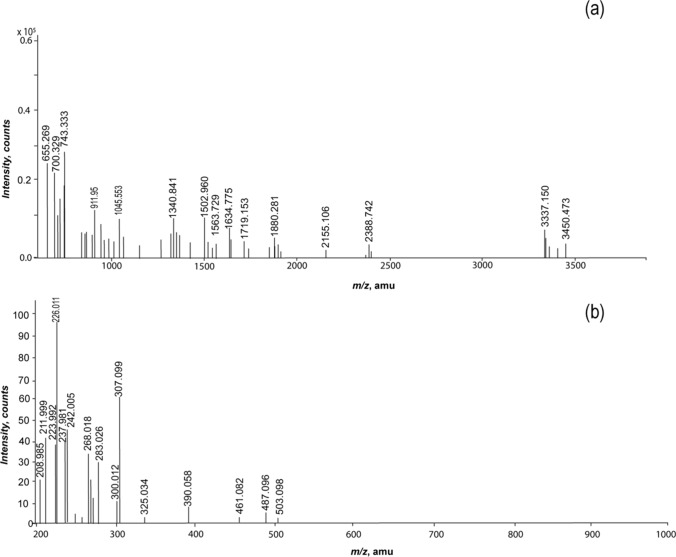
Mass spectrometry analysis
The molecular weight of the trypsin digested collagen peptide of JFC and MC were analyzed by MALDI-TOF MS (Bruker Daltonics, Germany) (Fig. 4a and b). The peptides for JFC were found in the molecular weight ranging below 4000 Da. The different MW distribution may be due to the result of different peptide sequences. Maximum numbers of cluster of peptides were observed in the mass range of 1–2 kDa and 3–4 kDa. Relatively lesser peptides were seen in the region below 1 kDa and 2–3 kDa. These peptides could be marker peptides that would contain 7–20 amino acid residues. The number of collagen peptide peaks also reflects remarkably good content of arginine and lysine residues in the jellyfish collagen. The finding of mass spectrometry was compared with the known data available. Further, MS/MS study of these precursor ions will be carried out in the future that will help in generating collagen sequence of jellyfish. In the case of MC, the mass spectrometric analysis (Fig. 4b) resulted in very few fragment ions for smaller peptides with peaks in the mass range m/z 200–500 basically for dipeptides to hexapapetides. The intense peaks observed were m/z 226 and m/z 307.
Fig. 4.
MALDI-TOF mass spectra of a Jellyfish collagen (JFC) and b Marine collagen (MC)
Conclusion
Jellyfish R. pulmo found along the coast of Goa, India was investigated for collagen. The extraction of jellyfish yielded 47% (% Yield dry weight) tryptophan-rich collagen in 0.5 M acetic acid and 1% pepsin assisted with ultra-sonication process at low temperature. The characterization of collagen using SDS-PAGE, FT-IR and amino acid analysis revealed that collagen is of type-I having both essential as well as non-essential amino acids. Interestingly, in the dose and time response anti-oxidant DPPH assays, jellyfish collagen showed steady increase in the percentage antioxidant activity with the increase in the concentration and time, respectively. The study also revealed that anti-oxidant activity was found to be better than the commercially available marine collagen. Infact, MC had presence of more amino acids like serine, glutamine, phenylalanine and isoleucine as compared to JFC. But, interestingly the presence of tryptophan in JFC makes it to be more effective anti-oxidant. The presence of important minerals along with tryptophan amino acid makes this jellyfish collagen as a superior special nutritional supplement that would have wider benefits in neuroprotection and cognitive. As compared to MC with very few small peptides (< 500 Da), the MALDI-TOF data of JFC showed 54 peptide precursor ions (1–3 kDa) that have been reported here for the first time. By applying an appropriate strategy for exploitation, jellyfish biomass could be used as sustainable resource for collagen extraction to meet its current demand in the global market. This will in turn create circular economy in the era of zero waste.
Acknowledgements
The authors thank the Director of CSIR-National Institute of Oceanography, Donapaula Goa, for constant support and encouragement. We thank CSIR funded project "BIOPROSmar" (MLP2019) for financial support. SJ is also grateful to UGC funding agency for the award of Senior Research Fellowship (SRF).
Author’s Contribution
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Saira James executed the experiments supervised by S. Tilvi and wrote the MS. M. Gautankar did amino acid analysis under the supervision of R. Khandeparkar. Sreepada and N. Thakur checked and edited the manuscript.
Funding
The funding was provided by Council of Scientific and Industrial- National Institute of Oceanography Research through project "BIOPROSmar" (MLP2019).
Availability of data and material
All data generated or analyzed during this study are included in this published article and also will be available from the corresponding author on reasonable request.
Code availability
NA.
Declarations
Conflicts of interest
The authors declare no conflict of interest.
Ethical approval
NA.
Consent to participate
NA.
Consent for publication
NA.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Addad S, Esposito JY, Faye C, Ricard-Blum S, Lethias C. Isolation, characterization and biological evaluation of jellyfish collagen for use in biomedical applications. Mar Drugs. 2011;9:967–983. doi: 10.3390/md9060967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliarsingh SK, Lotliker AA, Srichandan S, Samanta A, Kumar N, Nair TMB. A review of jellyfish aggregations, focusing on India’s coastal waters. Ecol Proc. 2020;9:58. doi: 10.1186/s13717-020-00268-z. [DOI] [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Carvalho AM, Marques AP, Silva TH, Reis RL. Evaluation of the potential of collagen from codfish skin as a biomaterial for biomedical applications. Mar Drugs. 2018;16:495. doi: 10.3390/md16120495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Shao Z, Li C, Yu L, Raja MA, Liu C. Isolation, characterization and evaluation of collagen from jellyfish Rhopilema esculentum Kishinouye for use in hemostatic applications. PLoS ONE. 2017;12:e0169731. doi: 10.1371/journal.pone.0169731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi C-F, Cao Z-H, Wang B, Hu F-Y, Li Z-R, Zhang B. Antioxidant and functional properties of collagen hydrolysates from spanish mackerel skin as influenced by average molecular weight. Molecules. 2014;19:11211–11230. doi: 10.3390/molecules190811211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Domenico S, De Rinaldi G, Paulmery M, Piraino S, Leone A. Barrel jellyfish (Rhizostoma pulmo) as source of antioxidant peptides. Mar Drugs. 2019;17:134. doi: 10.3390/md17020134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards CA, O'Brien WD., Jr Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin Chim Acta. 1980;104:161–167. doi: 10.1016/0009-8981(80)90192-8. [DOI] [PubMed] [Google Scholar]
- Eun S, So YK, Taehoon C, Hyun JB, Young ML. Collagen scaffolds derived from a marine source and their biocompatibility. Biomaterials. 2006;27:2951–2961. doi: 10.1016/j.biomaterials.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Felician FF, Yu R-H, Li M-Z, Li C-J, Chen H-Q, Jiang Y, Tang T, Qi W-Y, Xu H-M. The wound healing potential of collagen peptides derived from the jellyfish Rhopilema esculentum. Chin J Traumatol. 2019;22:12–20. doi: 10.1016/j.cjtee.2018.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.globenewswire.com/fr/news-release/2022/06/02/2455201/0/en/Collagen-Market-worth-5-3-billion-by-2026.html.
- Jenkins TA, Nguyen JCD, Polglaze KE, Bertrand PP. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients. 2016;8:56. doi: 10.3390/nu8010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F, Moran J. Dietary habits, nutrients and bone mass in Spanish premenopausal women: the contribution of fish to better bone health. Nutrients. 2013;5:10–22. doi: 10.3390/nu5010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khong NMH, Yusoff FM, Jamilah B, Basri M, Maznah I, Chan KW, Nishikawa J. Nutritional composition and total collagen content of three commercially important edible jellyfish. Food Chem. 2016;196:953–960. doi: 10.1016/j.foodchem.2015.09.094. [DOI] [PubMed] [Google Scholar]
- Leone A, Lecci R, Durante M, Meli F, Piraino S. The bright side of gelatinous blooms: Nutraceutical value and antioxidant properties of three mediterranean jellyfish (Scyphozoa) Mar Drugs. 2015;13:4654–4681. doi: 10.3390/md13084654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone A, Lecci RM, Milisenda G, Piraino S. Mediterranean jellyfish as novel food: effects of thermal processing on antioxidant, phenolic, and protein contents. Eur Food Res Technol. 2019;245:1611–1627. doi: 10.1007/s00217-019-03248-6. [DOI] [Google Scholar]
- Marjan M-R, Mehrangiz E, Aliasgar E. Copper, magnesium, zinc and calcium status inosteopenic and osteoporotic post-menopausal women. Clinic Cases Miner Bone Metab. 2015;18:8–21. doi: 10.11138/ccmbm/2015.12.1.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S, Kimura S. Jellyfish Mesogloea Collagen: characterization of molecules as α1α2α3 heterotrimers. J Bio Chem. 1985;260:15352–15356. doi: 10.1016/S0021-9258(18)95743-1. [DOI] [PubMed] [Google Scholar]
- Mokrejs P, Langmaier F, Mladek M, Janacova D, Kolomaznik K, Vasek V. Extraction of collagen and gelatine from meat industry by-products for food and non food uses. Waste Manag Res. 2009;27:31–37. doi: 10.1177/0734242X07081483. [DOI] [PubMed] [Google Scholar]
- Nagai T, Worawattanamateekul W, Suzuki N, Nakamura T, Ito T, Fujiki K, NakaoM YT. Isolation and characterization of collagen from rhizostomous jellyfish (Rhopilema asamushi) Food Chem. 2000;70:205–208. doi: 10.1016/S0308-8146(00)00081-9. [DOI] [Google Scholar]
- Nudelman R, Alhmoud H, Delalat B, Fleicher S, Fine E, Guliakhmedova T, Elnathan R, Nyska A, Voelcker NH, Gozin M, Richter S. Jellyfish-based smart wound dressing devices containing in situ synthesized antibacterial nanoparticles. Adv Funct Mater. 2019;29:1902783. doi: 10.1002/adfm.201902783. [DOI] [Google Scholar]
- Nurilmala M, Hizbullah HH, Karnia E, Kusumaningtyas E, Ochiai Y. Characterization and antioxidant activity of collagen, gelatin, and the derived peptides from yellowfin Tuna (Thunnus albacares) Skin. Mar Drugs. 2020;18:98. doi: 10.3390/md18020098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul C, Leser S, Oesser S. Significant amounts of functional collagen peptides can be incorporated in the diet while maintaining indispensable amino acid balance. Nutrients. 2019;11:1079. doi: 10.3390/nu11051079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzolini M, Scarfì S, Gallus L, Castellano M, Vicini S, Cortese K, Gagliani MC, Bertolino M, Costa G, Giovine M. Production, characterization and biocompatibility evaluation of collagen membranes derived from marine sponge Chondrosia reniformis Nardo, 1847. Mar Drugs. 2018;16:111. doi: 10.3390/md16040111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pustlauk W, Paul B, Brueggemeier S, Gelinsky M, Bernhardt A. Modulation of chondrogenic differentiation of human mesenchymal stem cells in jellyfish collagen scaffolds by cell density and culture medium. J Tissue Eng Regen Med. 2015;11:1710–1722. doi: 10.1002/term.2065. [DOI] [PubMed] [Google Scholar]
- Rastian Z, Pütz S, Wang Y, Kumar S, Fleissner F, Weidner T, Parekh SH. Type I collagen from jellyfish Catostylus mosaicus for biomaterial applications. ACS Biomater Sci Eng. 2018;4:2115–2125. doi: 10.1021/acsbiomaterials.7b00979. [DOI] [PubMed] [Google Scholar]
- Šimat V, Elabed N, Kulawik P, Ceylan Z, Jamroz E, Yazgan H, Čagalj M, Regenstein JM, Özogul F. Recent advances in marine-based nutraceuticals and their health benefits. Mar Drugs. 2020;18:627. doi: 10.3390/md18120627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumiyoshi H, Okamura Y, Kawaguchi AT, Kubota T, Endo H, Yanagawa T, Yasuda J, Matsuki Y, Nakao S, Inagaki Y. External administration of moon jellyfish collagen solution accelerates physiological wound healing and improves delayed wound closure in diabetic model mice. Regen Therapy. 2021;18:223–230. doi: 10.1016/j.reth.2021.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylingo R, Mania S, Panek A, Piątek R, Pawłowicz R. Isolation and characterization of acid soluble collagen from the skin of African catfish (Clarias gariepinus), Salmon (Salmo salar) and Baltic Cod (Gadus morhua) J Biotech Biomater. 2016;6:1000234. doi: 10.4172/2155-952X.1000234. [DOI] [Google Scholar]
- Weber K, Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969;244:4406–4412. doi: 10.1016/S0021-9258(18)94333-4. [DOI] [PubMed] [Google Scholar]
- Widdowson JP, Picton AJ, Vince V, Wright CJ, Spragg AM. In vivo comparison of jellyfish and bovine collagen sponges as prototype medical devices. J Biomed Mater Res Part B Appl Biomater. 2018;106(4):1524–1533. doi: 10.1002/jbm.b.33959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HC, Chen HM, Shiau CY. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus) Food Res Inter. 2003;36:949–957. doi: 10.1016/S0963-9969(03)00104-2. [DOI] [Google Scholar]
- Xu N, Peng X, Li H-R, Liu J-X, Cheng J-S-Y, Qi X-Y, Ye S-J, Gong H-L, Zhao X-H, Yu J, Xu G, Wei D-X. Marine-derived collagen as biomaterials for human health. Front Nutr. 2021;8:702108. doi: 10.3389/fnut.2021.702108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Yamamoto K, Sato Y, Inoue S, Morinaga T, Hirano E. Combination of aspartic acid and glutamic acid inhibits tumor cell proliferation. Biomed Res. 2016;37:153–159. doi: 10.2220/biomedres.37.153. [DOI] [PubMed] [Google Scholar]
- Zhang J, Duan R, Huang L, Song Y, Regenstein JM. Characterization of acid soluble and pepsin-solubilised collagen from jellyfish (Cyanea nozakii Kishinouye) Food Chem. 2014;150:22–26. doi: 10.1016/j.foodchem.2013.10.116. [DOI] [PubMed] [Google Scholar]
- Zhuang Y, Sun L, Zhao X, Wang J, Hou H, Li B. Antioxidant and melanogenesis-inhibitory activities of collagen peptide from jellyfish -(Rhopilema esculentum) J Sci Food Agric. 2009;89:1722–1727. doi: 10.1002/jsfa.3645. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and also will be available from the corresponding author on reasonable request.
NA.