Abstract
A wider application of naturally derived polysaccharides is of great interest as materials for food packaging industry. Biocompatibility and biodegradability of polysaccharide-based films and coatings ally with a shift from application of non-biodegradable petrochemical polymers to the more environmentally friendly ones. Due to a range of inherent features in chemical structure and bioactivity, the polysaccharide materials could bring additional functionality to food packaging. The chelating ability of the polysaccharides provides also their application as carriers of additional active components, such as nanoparticles, essential oils and polyphenols. The improved physicochemical, antibacterial and antioxidant properties of the filled films allows to consider the edible polysaccharide-based films as functional food products. This review is aimed at analysis of evolution of polysaccharide-based food packaging materials from inert one starting from cellophane to recent research works on development of multicomponent polysaccharide-based functional food films and coatings.
Keywords: Polysaccharides, Films, Coatings, Packaging, Functional food
Introduction
More than a century has passed since the beginning of industrial production of cellophane, a film material made of regenerated cellulose. In the middle of the twentieth century, polysaccharide-based packaging was almost completely replaced by materials based on petrochemical polymers, which are cheaper to produce and have new attractive properties. However, recent requirements for the transformation of packaging material’s production and utilization to a more ecologically friendly approach are forcing the manufacturers to return to biodegradable polymers, which certainly include polysaccharides (Dey et al. 2021). In the food industry, polysaccharide films are widely used to preserve various food products. A new option of polysaccharides application in this field is production of edible packaging films and coatings. Such materials could not only to protect the food, but also to serve as functional food itself. They supply the human needs for proteins, fats and carbohydrates, as well as enhance immunity, and improve the function of internal organs contributing to reducing body weight. In recent years, there has been an increase in the number of research works aimed at providing a new functional and physico-mechanical properties to polysaccharide films and coatings by filling them with essential oils, polyphenols and nanoparticles of various origins. A range of polysaccharides and their derivatives used in the food industry is also expanding. This work is aimed at analysis of the present state of the art of application of the polysaccharide-based films and coatings in food industry starting from packaging materials to functional food.
Polysaccharides: structure, properties and application in the food industry
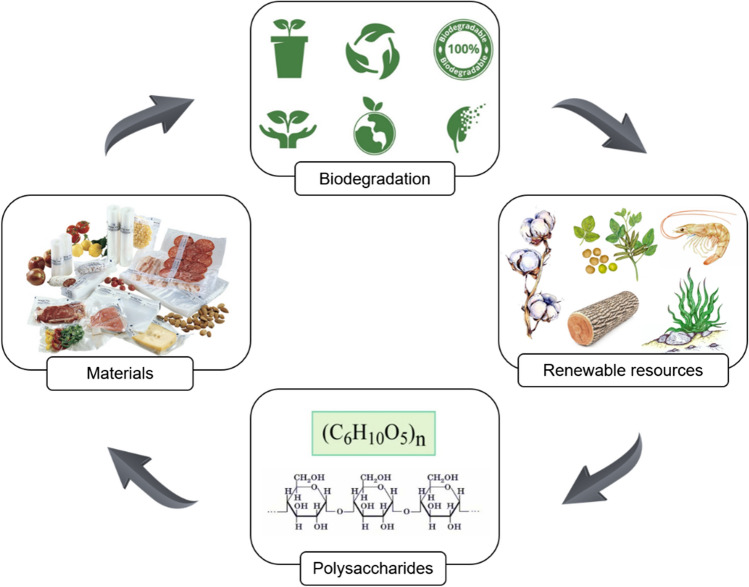
Polysaccharides are high-molecular weight carbohydrates composed of monosaccharide units linked by glycosidic bonds with a wide range of physicochemical and biological properties. They are derived from natural sources (plants, animals and microorganisms) and are edible and biocompatible (Zhou et al. 2022). The natural sources of the polysaccharides are easily renewable and environmentally friendly, so the polysaccharide-based materials can undergo a natural life cycle (Fig. 1), which is a positive factor for reduction of environmental pollution (Tajeddin and Arabkhedri 2020).
Fig. 1.
Life cycle of polysaccharide-based materials
A lot of works with detailed description of the processes of isolation of the polysaccharides from natural sources have been already published (Kaur and Dhillon 2014; Chopra and Manikanika 2021; Dehghani Soltani et al. 2021). We would like to emphasize only a fundamental difference in the methods of extraction. Some polysaccharides, such as guar and starch, are isolated from raw sources directly by mean of extraction (Dehghani Soltani et al. 2021). Other polysaccharides, such as chitin and cellulose, require a multistage treatment of the raw material, while their derivatives are obtained by chemical transformations with several steps of synthesis, extraction, and purification (Zhang et al. 2021; Akopova et al. 2021).
The most frequently used polysaccharides in food industry are (Cutter 2006; Lu et al. 2019):
starch – a mixture of semi-crystalline amylose (20–30%) and amorphous branched amylopectin (70–80%), consisting of D-glucopyranose units linked by α-1–4 glycosidic bonds; in amylopectin, side chains is also consisting of α-D-glucopyranose residues, which are attached to the main chain at regular intervals (1–6); obtained from cereals (mainly rice and corn) and root crops (potatoes and cassava);
cellulose − consisting of D-glucopyranose units linked by glycosidic bonds β-1–4, is obtained both from plant sources (cotton, wood and a number of herbaceous plants) and using microorganisms (bacteria Acetobacter, Sarcina ventriculi and Agrobacterium);
chitosan consists of D-glucosamine and N-acetyl-D-glucosamine units linked by β-(1–4) glycosidic bonds; product of deacetylation of chitin extracted from crustacean shells and some fungi and microorganisms;
alginates consist of β-D-mannuronic acid and α-L-hyaluronic acid units linked (1–4) by glycoside bonds; sodium and calcium salts of a natural polysaccharide, alginic acid, extracted from red, brown and some green algae of the genera Laminaria and Macrocystis;
guar (guar gum)—consists of β-(1–4) glycosidically linked mannose residues to which galactose residues are (1–6)-linked at every second mannose unit; obtained from the seeds of the legume Cyamopsis tetragonolobus.
These polysaccharides could effectively act as thickeners, emulsifiers, stabilizers, gel-formers, encapsulating and moisture-retaining agents even in small amounts of 1–3 wt.% (Venugopal 2016). They allow to preserve and improve the properties of various food products (Lu et al. 2019). The high molecular weight and the ability to organize complex hydrogen bond systems determine the good film-forming properties of polysaccharides. They are widely proposed to producing films and coatings for the packaging of various products, fruits or vegetables (Mangaraj et al. 2019). More detailed properties and possible applications of the above-mentioned polysaccharides in the food industry are summarized in Table 1.
Table 1.
Main properties of the polysaccharides and their application in the food industry
| Polysaccharide | Properties | Food processing applications | References |
|---|---|---|---|
| Starch | Non-ionic polymer capable of dissolving in hot water and dispersing in cold water; | Inert filler in bakery products; | Santana and Angela Meireles (2014), Tester et al. (2004) |
| A reversible decrease in viscosity and gel-forming ability with increasing temperature; | Thickener in soups, sauces and pie fillings; | ||
| Gel-forming ability is higher in starches with high amylose content (wheat, corn); | Jelly-forming agent in puddings, confectionery and cheese products; | ||
| Slowly absorbed by the body in the process of breaking it down to glucose; | Emulsifier for salad dressings and mayonnaise; | ||
| Ability to exhibit thermoplastic properties when plasticizers (glycerin, water, etc.) are added due to a sharp decrease in the glass transition temperature | Stabilizer in dairy products, ice cream; | ||
| Moisture-retaining, structure-forming component in sausage products; | |||
| Filler in packaging materials | |||
| Cellulose and its derivatives | In native form is unable to dissolve in water due to the complex structure of hydrogen bonds; | Structure-forming component in various products: milk powder, sausages, cookies; | Liu et al. (2021), Perumal et al. (2022) |
| Ability to regenerate from a number of water-soluble derivatives to form insoluble films and fibers with good mechanical characteristics; | Emulsifier in sauces, cheese products, dairy desserts; | ||
| The possibility of extraction of micro- and nanocrystalline forms capable of gelation in water; | Texturizing agent and stabilizer for frozen foods; | ||
| Hypolipidemic activity due to the ability to adsorb cholesterol; | A filler that prevents caking in loose mixes and clumping in bakery products; | ||
| Hypoglycemic activity due to its inhibitory effect on starch digestion and processes of glucose diffusion and absorption; | As dietary fiber; | ||
| Neither native cellulose nor its derivatives are absorbed in the body | Packaging materials | ||
| Chitosan | Good solubility in aqueous solutions of monoprotic mineral and organic acids; | Preservative and clarifier in fruit juices and wine; | Friedman and Juneja (2010), Venugopal (2016) |
| High chelating and adsorption capacity towards to both organic substances and radionuclides and salts of heavy metals; | To regulate the fat content of milk and as a stabilizer and immunomodulator in dairy products; | ||
| Antibacterial and antifungal activity; | As dietary fiber and rheological modifier in gluten-free products; | ||
| A wide range of molecular weights of commercial products, and hence the viscosity of aqueous salt solutions; | As structure-forming and stabilizing additives in food compositions containing oils and fats; | ||
| Film- and fiber-forming ability; | In fish and seafood to increase shelf life, stability during storage, protection against pathogens; | ||
| Ability to complex with fatty acids and therefore suppress the adsorption of steroids, bile acids and triglycerides in the body; | Packaging materials | ||
| Ability to lower cholesterol and glucose levels; | |||
| Is not digested in the body | |||
| Alginates | High water-holding properties (up to 25 ml of water per gram of its own weight); | Thickener in jams, marmalade, fruit sauces, desserts, baked goods; | (Menon (2011), Rehm (2009) |
| Water solubility; | Stabilizer for beer foam, mousse; | ||
| Good gel- and film-forming properties; | Emulsifier for mayonnaise and salad dressings; | ||
| Thermal stability; | As dietary fiber; | ||
| A wide range of solution viscosity; | For packaging products to ensure their preservation at high and low temperatures; | ||
| High sorption ability against radionuclides, salts of heavy metals, fatty acids; | |||
| Not digested in the body | |||
| Guar | High water-holding properties (up to 40 ml per gram of own weight); | As a rheological modifier in the baking industry (frozen dough, bread, cakes and cakes), in the production of gluten-free products (pasta and bread); | (Miyazawa and Funazukuri (2006), Mudgil et al. (2014) |
| Water solubility; | Stabilizer in the dairy industry (ice cream, milkshakes, yogurts); | ||
| High viscosity even at low solution concentrations (up to 1.5%); | Thickener in the production of beverages, sauces and ketchup; | ||
| Good gel- and film-forming abilities; | As dietary fiber; | ||
| Ability to lower cholesterol and blood glucose levels; | Packaging materials | ||
| Not absorbed in the body |
Thus, all the above-mentioned polysaccharides are promising and actively used in the food industry. They perform many functions, favorably influencing the consistency and useful properties of the food products. Application of polysaccharide-based materials as packaging materials are receiving a lot of attention in a recent works.
Polysaccharide-based packaging films and coatings
Packaging materials have certain requirements to ensure their successful use for preservation of packaged food products from deterioration during transportation, storage, or sale (Gokularaman et al. 2017). Significant differences in the shelf life and quality of food products are mainly affected by the absence of water vapor and oxygen, and, thus, the high vapor and gas barrier properties of the packaging material (Bourtoom 2008). In addition, these materials should have good mechanical properties, as they are often shaped and applied directly to the products themselves. The material should not deform, peel or decompose during the packaging process and further storage (Gokularaman et al. 2017). The material’s antimicrobial properties provide protection from various pathogenic microorganisms and their toxins, so they could significantly increase the product shelf life (Cha and Chinnan 2004). In recent years, the preference is given to materials that are environmentally friendly and capable of further safe biodegradation within a short period of time. Therefore, the most promising types of packaging materials in the food industry are recognized to be biocomposite thin-layer films and coatings (Tajeddin and Arabkhedri 2020).
Due to the complexly organized system of hydrogen bonds providing the high cohesion energy, the polysaccharides are incapable of melting without decomposition. Therefore, methods for casting of polysaccharide films and fibers are limited to solution-based technologies. Food packaging films and coating production preferably required the use of non-toxic solvents, such as water, ethyl alcohol, water-alcohol solutions, diluted aqueous solutions of acetic acid, etc. (Bourtoom 2008; Gokularaman et al. 2017). To shape the films in the food industry, two casting methods are mainly used—continuous and discontinuous. In the continuous method, the solution is applied through a nozzle with special holes for uniform distribution on a continuously moving belt, followed by drying. In the discontinuous method, the polymer solution is cast onto special precipitation substrates, followed by drawing and drying (Fryer and Versteeg 2008). Polymer solutions could be also used to form coatings directly to the products by applying them on vegetables, fruits or berries by spraying and electrospraying methods, as well as by dipping (Khan et al. 2012).
Due to their intermolecular structure the polysaccharide films have gas barrier properties against O2 and CO2 allowing them to prevent rapid product spoilage and providing extended shelf life for fruits, vegetables, sausages, meat and fish products (Bourtoom 2008; Coma 2013; Mangaraj et al. 2019). In addition, such packaging is able to retain moisture loss from the products and prevents them from drying out (Venugopal 2016; Hassan et al. 2018). Polysaccharide films and coatings are often edible, oil- and fat-resistant, transparent, low-calorie and odorless (Coma 2013; Hassan et al. 2018). They have a high sorption capacity, so that when ingested they adsorb and remove from the body metal ions, radionuclides and other harmful compounds. In addition, polysaccharide films and coatings successfully serve as carriers of components and additives of various nature: dyes, flavors, sweeteners, antimicrobial and antioxidant agents used to improve the properties and organoleptic characteristics of food products (Gómez-Estaca et al. 2014; Hassan et al. 2018). The disadvantages of polysaccharide films are their low antimicrobial properties (except for chitosan (Dutta et al. 2009)), low mechanical strength, weak vapor barrier properties, unstable against water, acids and alkalis due to their hydrophilic nature (Venugopal 2016; Mangaraj et al. 2019). In addition, most polysaccharide films have an increased stiffness, which is counteracted by the addition of safe plasticizers, such as glycerol or sorbitol (Lim et al. 2020). To prevent water solubility or loss of mechanical strength instead in high humidity, various methods of the macromolecule cross-linking are used (Azeredo and Waldron 2016). To overcome the above disadvantages and to optimize the properties of polysaccharide-based packaging materials, they are also used in combination with each other. Table 2 shows some combinations of polysaccharides and their effect on the properties of the food films and coatings.
Table 2.
Effect of polysaccharides combinations on the properties of the based on films and coatings as well as on the food products
| Polysaccharides combination | Effects | Referenes |
|---|---|---|
| Rice starch/chitosan | Improved vapor barrier capability; reduced water solubility; increased tensile strength and reduced film elasticity | Bourtoom and Chinnan (2008) |
| Cellulose/chitosan | Improved vapor barrier ability; antimicrobial activity against Escherichia coli and Staphylococcus aureus | Wu et al. (2004) |
| CMC/chitosan | Improved barrier properties; increased mechanical strength and resistance of the coating to water | Arnon et al. (2015) |
| Manioc starch/CMC | Reduced solubility in water; improved mechanical characteristics: increased tensile strength, reduced elongation at break | Tongdeesoontorn et al. (2011) |
| Sodium alginate/MC | Improvement of gas and vapor barrier properties of coatings | Maftoonazad et al. (2008) |
| Sodium alginate/cellulose | Improved vapor barrier ability; oil resistance; increased tensile strength | Sirviö et al. (2014) |
| Chitosan/guar | Good vapor barrier properties; decreased permeability to O2; increased tear and puncture strength; antimicrobial activity against Escherichia coli and Staphylococcus aureus | Rao et al. (2010) |
| Pea starch/guar | Improved vapor barrier capability; reduced water solubility; increased tear and puncture strength due to increased film density | Saberi et al. (2016, 2017) |
As can be seen from the data in Table 2, the combination of different polysaccharides allows improving a number of important food packaging properties, such as mechanical, barrier and antimicrobial (by adding chitosan) ones. However, this could be not enough for their further large-scale application. Various functional fillers into the polysaccharide matrix for targeted optimization of the material properties are proposed.
Functional film materials
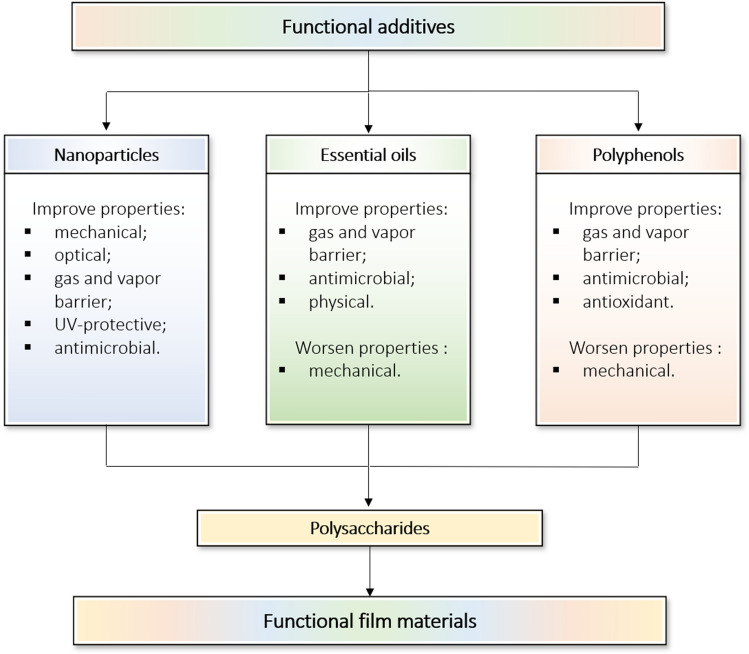
The high sorption and chelating ability of the polysaccharides determines their application as carriers of various active components, along with fabrication of nanocomposite film materials (Hassan et al. 2018). Recently, a lot of research works are focusing on functionalization of polysaccharide-based packaging materials by filling them with nanoparticles of various nature, essential oils, and polyphenols. Such fillers can both improve the physicochemical and mechanical properties of the materials and play the role of antimicrobial and antioxidant agents. It allows upgrading the packaging materials to supplement food products bearing the bioactive components to positively affect the human health and quality of life.
Filling with nanoparticles
The nanotechnology allows producing a large variety of nanoparticles with a size ≤ 100 nm, and provides a tremendous benefit on their use as fillers for various materials. The nanoparticles are classified into two groups based on their chemical nature: inorganic (metals, metal oxides, clay, etc.) and organic (natural or synthetic polymers) ones. The nanoparticles dispersed within a polysaccharide matrix could improve the mechanical, optical, barrier and antimicrobial properties of film packaging materials (Chaudhary et al. 2020).
For example, it was found that the complexation processes of starch hydroxyl groups with silver nanoparticles (Abreu et al. 2015) resulted in enhancement of mechanical properties and adding antimicrobial activity to the materials. The inclusion of titanium oxide nanoparticles reduced the hydrophilicity of the starch films, which led to an increase in their vapor barrier properties and a decrease in solubility (Goudarzi et al. 2017). The ability of the filled nanoparticles to shield the ultraviolet range of light could protect the food from UV radiation.
Filling of guar and cellulose derivative-based films with silver or copper nanoparticles (Arfat et al. 2017) as well as with nanocrystalline polysaccharides (Sotnikova et al. 2017; Mel’nikov et al. 2020) resulted in improvement of mechanical, antimicrobial and gas barrier properties. Filling the thermoplastic starch films with chitin nanoparticles in concentrations of 5 to 20% led to significant enhancement of the mechanical and thermal properties of the material, mostly pronounced when a fibrous filler is used (Salaberria et al. 2015). Such films had enhanced barrier properties against water vapor and oxygen and significant antifungal activity.
The addition of copper sulfide nanoparticles to sodium alginate films improved UV protection and mechanical strength, as well as increased the hydrophobicity and vapor barrier properties of the material (Roy and Rhim 2020). This work also revealed the antibacterial activity of the filled films against Gram-negative bacteria.
Chitosan-based films were filled with zinc oxide nanoparticles and hydrophobically modified with octadecylammonium clay (Rodrigues et al. 2020). Zinc oxide nanoparticles were homogeneously distributed over chitosan matrix in the presence of clay, which led to a significant improvement in the mechanical characteristics in comparison with films made of chitosan only. The addition of clay nanoparticles had no effect on the thermal degradation temperature of chitosan, whereas an improvement in thermal stability of the films was observed with the addition of zinc oxide nanoparticles. In the antimicrobial test, it was found that chitosan and zinc oxide nanoparticles had a synergistic effect against Escherichia coli and Staphylococcus aureus.
The synthesis of silver nanoparticles within a mixture of CMC and guar provide them the antimicrobial activity to pathogenic food bacteria and fungi as well as led to the improved mechanical properties (Kanikireddy et al. 2020). For example, the tensile strength of the filled films was 1.5-times higher while the modulus of elasticity was twice as low. The coating of strawberries by this nanocomposite mixture led to a decrease in weight loss and prolongation of the product's shelf life.
Thus, the analysis of literature sources showed that the addition of nanoparticles, both organic and inorganic nature, complexly affects the mechanical characteristics of the film materials, significantly increasing their tensile strength with some non-critical reduction in elasticity. Barrier properties, both in relation to water and gases, are also higher in all the above studies. Antimicrobial properties of the polysaccharide-based films can be improved by using chitosan both in pure form and in mixed polymer matrices, as well as by including silver nanoparticles and/or metal oxides and salts.
Encapsulation of essential oils
Essential oils − extracted from a variety of plants and spices are aromatic oily liquids that exhibit antimicrobial and immunomodulatory properties (Atarés and Chiralt 2016). Essential oils are highly effective against a wide range of Gram-positive and Gram-negative bacteria as well as some major foodborne pathogenic bacteria, i.e. Salmonella enterittidis, Escherichia coli, Campylobacter jejuni and Staphylococcus aureus (Burt 2004). More than three thousand species of essential oils are known, of which only about three hundred are of commercial interest for food applications (Atarés and Chiralt 2016). However, because of their hydrophobic nature, intense aroma and flavor, there are limitations and difficulties in incorporating oils directly into many food products. Encapsulating essential oils into polysaccharide films is a perspective way to solve these problems, since the polymer can inhibit the taste and aroma of the oils, level out the hydrophobicity, and allow controlling the rate of their release (Liu et al. 2019).
Encapsulation of orange peel essential oil into chitosan films led to extended the shelf life of the shrimps from 7 to 15 days, and also inhibited lipid oxidation and microbial growth (Alparslan and Baygar 2017). Orange essential oil was also added to corn starch via emulsion approach to increase antibacterial activity against Staphylococcus aureus and Listeria monocytogenes (do Evangelho et al. 2019). However, a decrease in the morphological homogeneity of the films resulted in the formation of micropores and a decrease in the tensile strength and elongation rate. The encapsulation of bergamot essential oil in chitosan-based films enhanced antimicrobial activity against Penicillium italicum as well as improved vapor barrier properties (Sánchez-González et al. 2010).
Emulsion films based on HPMC with tea tree essential oil were also fabricated and studied in (Sánchez-González et al. 2009). Increasing the concentration of essential oil led to an increase in the vapor barrier properties of the films and a decrease in the sorption capacity to water. Thus, when the oil concentration in the matrix was more than 2%, the vapor permeability of the films decreased by 30–40%, while the films became less transparent and lost their luster. As in the previous work, a decrease in mechanical characteristics due to violations of the film's continuity was also observed. The addition of the garlic essential oil in the alginate food film at a concentration of more than 0.2% resulted in a significant inhibitory effect against Staphylococcus aureus and Bacillus cereus (Pranoto et al. 2005). The authors also noted that they did not observe any decrease in the mechanical properties of the obtained emulsion films.
Other studies on the encapsulation of essential oils in polysaccharides have shown similar results (Ojagh et al. 2010; Bonilla et al. 2012). Generally, the encapsulation leads to a decrease in the mechanical characteristics and an increase in their vapor-barrier and antimicrobial properties of the films.
Polyphenols filling
Polyphenols are bioactive compounds found in various plants and include phenolic acids, flavonoids, polyphenolic amides, phytoestrogens (resveratrol and lignans). Polyphenols are shown to be promising as antioxidants, anti-allergic, anti-inflammatory, antitumor, anti-diabetic and antimicrobial agents helpful for the prevention of various diseases (Pinto et al. 2021). Due to this broad spectrum of properties, polyphenols and polyphenol containing plant extracts are added to film materials for food packaging.
For example, extracts of guava leaves were added to sodium alginate films resulting in a significant increase of antioxidant and antibacterial activity as well as the increased tensile strength of the filled films. The extract reduced also the film’s solubility in water, and improved vapor barrier capacity (Luo et al. 2019). The authors noted the denser structure of the films, which they attribute to the presence of intermolecular hydrogen bonds between the biologically active substances and the sodium alginate.
CMC-based films filled with Chinese onion root extract showed a decrease in the tensile strength and water solubility, while vapor barrier properties, antibacterial and antioxidant properties were improved (Riaz et al. 2020). The addition of green tea extracts to chitosan significantly increased the vapor barrier and antioxidant properties of the polysaccharide films, while their mechanical properties were decreased (Peng et al. 2013). The filling of starch films with mango peel powder improved the barrier properties of the films and promoted the homogenization of their structure (Rojas-Bravo et al. 2019). The composite films exhibited the high antioxidant properties depending on the filler concentration. The experiments showed the prospects of the developed composite films as edible coatings for peeled fruits. When starch films were filled with sunflower extract they showed the high antioxidant properties even at small amounts of the added extract (1–2 wt.%) (Menzel et al. 2019). The mechanical properties of the films decreased with increasing amounts of the extract. At the same time, all the composite samples had good gas and vapor barrier properties.
In general, studies of the polysaccharide-based films filled with various polyphenols also revealed high antioxidant properties of the modified films with a decrease in their mechanical properties, which were still enough for their usage as edible coatings (Liu et al. 2017; Wang et al. 2019).
Combinations of functional additives
Thus, the use of functional additives within the polysaccharide-based films leads to a significant improvement of their functional properties. However, the fillers could negatively affect the mechanical characteristics of the polymer matrices (Fig. 2). Therefore, it is advisable to combine different additives to achieve optimal material properties of functional packaging film for particular application. Table 3 summarizes the literature data on the properties and application of the composite films made using several functional additives within the polysaccharide matrix. The combination of nanoparticles with essential oils and/or polyphenols could balance the negative effect of the latter ones on the mechanical properties of the films. The combination of essential oils and polyphenols often leads to a significant increase in the antimicrobial properties of the films. Analysis of the literature data shows the effectiveness of this approach, and the large number of the studied functional additives could provide a variety of options for their combination. As a result, the range of polysaccharide-based functional films and coatings can be significantly expanded, and their properties can be optimized according to the criteria applied to packaging materials for the food industry.
Fig. 2.
Effect of various fillers on the properties of the functional polysaccharide-based film materials
Table 3.
Combination of functional additives and their effect on the packaging material properties and the application options
| Composition of the functional film | Properties | Application | References |
|---|---|---|---|
| Chitosan/guar/zinc oxide nanoparticles/rosella extract | The composite films showed improved antimicrobial, antioxidant, mechanical, and preservative properties compared to unfilled films | Protection of cheese from photo-oxidation, free radicals and microbes, extending its shelf life | El-Sayed et al. (2020) |
| Chitosan/clove essential oil/clay nanoparticles | The nanoparticles dispersed in the emulsion film matrix leveled the reduction in the mechanical properties of the films coming from the encapsulation of essential oil. An increase in vapor-barrier and antimicrobial properties of the materials was also noted | Extends the shelf life of sliced bread up to 3 months due to mold growth inhibition by films | Lee et al. (2018) |
| Chitosan/zinc oxide nanoparticles/sandalwood essential oil | Zinc oxide nanoparticles reduced the negative effect of the introduction of oil into the chitosan matrix on the mechanical properties of the films. Also, a decrease in light transmission, film roughness and an increase in antifungal activity against Peniccilium italicum were observed | Extend the shelf life of mandarins and protect them from fungal decay | Aditya et al. (2022) |
| Chitosan/starch/pomegranate peel extract/essential oil of Thymus kotschyanus | The composite films had optimal mechanical characteristics and a high antibacterial effect against L. Monocytogenes | Extending the shelf life of beef meat to 21 days at 4 °C and inhibiting lipid oxidation | Mehdizadeh et al. (2020) |
|
Cellulose/zinc oxide nanoparticles/extract grapefruit seeds |
The composite films had high transparency with improved mechanical, UV-protective and vapor barrier properties. The films also exhibited effective antimicrobial and antioxidant protection | Functional packaging for food products | Roy et al. (2021) |
| Sodium alginate/kiwi peel extract/silver nanoparticles | The composite films had high tensile strength, good vapor barrier, UV-protective and antioxidant properties, and antibacterial activity against Staphylococcus aureus and Escherichia coli | Extends the shelf life of cherries, inhibits berry moisture loss and protects against microbial spoilage | Sun et al. (2021) |
Conclusion
Polysaccharides are promising and actively used polymers in the food industry. They could serve as biodegradable packaging material with satisfactory mechanical characteristics. Moreover, combination of different polysaccharides allows improving a number of film properties, such as mechanical, barrier and antimicrobial ones. The high sorption and chelating ability of polysaccharides provides the possibility of incorporating various active components into the structure of polysaccharide-based films. Nanoparticles of different nature, essential oils and polyphenols are most commonly used for this purpose. Such fillers can both improve physical, chemical and mechanical properties of materials and play the role of functional antimicrobial and antioxidant agents. Combination of fillers of different nature in one matrix allows optimizing their consumer properties of the edible packaging films. This approach will allow to further supplement the food products with useful components having a favorable effect on the human health.
Abbreviations
- UV
Ultra violet
- CMC
Carboxymethylcellulose
- HPMC
Hydroxypropylmethylcellulose
- MC
Methylcellulose
Authors' contributions
Writing—original draft preparation, T.N.P.; visualization, T.N.P; writing—review and editing, T.S.D., T.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially financially supported by the Ministry of Science and Higher Education of the Russian Federation, theme number FFSM-2021-0006.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Declarations
Conflicts of interest
The authors declare that there is no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tatiana N. Popyrina, Email: popyrina@ispm.ru
Tatiana S. Demina, Email: demina@ispm.ru
Tatiana A. Akopova, Email: akopova@ispm.ru
References
- Abreu AS, Oliveira M, De Sá A, Rodrigues RM, Cerqueira MA, Vicente AA, Machado AV. Antimicrobial nanostructured starch based films for packaging. Carbohyd Polym. 2015;129:127–134. doi: 10.1016/j.carbpol.2015.04.021. [DOI] [PubMed] [Google Scholar]
- Aditya A, Kingwascharapong P, Putri L. The antifungal effect against Penicillium italicum and characterization of fruit coating from chitosan/ZnO nanoparticle/Indonesian sandalwood essential oil composites. Food Packag Shelf Life. 2022;32:100849. doi: 10.1016/j.fpsl.2022.100849. [DOI] [Google Scholar]
- Akopova TA, Demina TS, Khavpachev MA, Popyrina TN, Grachev AV, Ivanov PL, Zelenetskii AN. Hydrophobic modification of chitosan via reactive solvent-free extrusion. Polymers. 2021;13(16):2807. doi: 10.3390/polym13162807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alparslan Y, Baygar T. Effect of chitosan ailm coating combined with orange peel essential oil on the shelf life of deepwater pink shrimp. Food Bioprocess Technol. 2017;10(5):842–853. doi: 10.1007/s11947-017-1862-y. [DOI] [Google Scholar]
- Arfat YA, Ejaz M, Jacob H, Ahmed J. Deciphering the potential of guar gum/Ag-Cu nanocomposite films as an active food packaging material. Carbohyd Polym. 2017;157:65–71. doi: 10.1016/j.carbpol.2016.09.069. [DOI] [PubMed] [Google Scholar]
- Arnon H, Granit R, Porat R, Poverenov E. Development of polysaccharides-based edible coatings for citrus fruits: a layer-by-layer approach. Food Chem. 2015;166:465–472. doi: 10.1016/j.foodchem.2014.06.061. [DOI] [PubMed] [Google Scholar]
- Atarés L, Chiralt A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci Technol. 2016;48:51–62. doi: 10.1016/j.tifs.2015.12.001. [DOI] [Google Scholar]
- Azeredo HMC, Waldron KW. Crosslinking in polysaccharide and protein films and coatings for food contact - a review. Trends Food Sci Technol. 2016;52:109–122. doi: 10.1016/j.tifs.2016.04.008. [DOI] [Google Scholar]
- Bonilla J, Atarés L, Vargas M, Chiralt A. Effect of essential oils and homogenization conditions on properties of chitosan-based films. Food Hydrocolloids. 2012;26(1):9–16. doi: 10.1016/j.foodhyd.2011.03.015. [DOI] [Google Scholar]
- Bourtoom T. Edible films and coatings : characteristics and properties. Int Food Res J. 2008;15(3):237–248. [Google Scholar]
- Bourtoom T, Chinnan MS. Preparation and properties of rice starch-chitosan blend biodegradable film. LWT Food Sci Technol. 2008;41(9):1633–1641. doi: 10.1016/j.lwt.2007.10.014. [DOI] [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods - a review. Int J Food Microbiol. 2004;94(3):223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Cha DS, Chinnan MS. Biopolymer-based antimicrobial packaging: a review. Crit Rev Food Sci Nutr. 2004;44(4):223–237. doi: 10.1080/10408690490464276. [DOI] [PubMed] [Google Scholar]
- Chaudhary P, Fatima F, Kumar A. Relevance of nanomaterials in food packaging and its advanced future prospects. J Inorg Organomet Polym Mater. 2020;30(12):5180–5192. doi: 10.1007/s10904-020-01674-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra L, Manikanika, Extraction of cellulosic fibers from the natural resources: a short review. Materials Today: Proceedings. 2021;48:1265–1270. doi: 10.1016/j.matpr.2021.08.267. [DOI] [Google Scholar]
- Coma V. Polysaccharide-based biomaterials with antimicrobial and antioxidant properties. Polimeros. 2013;23(3):287–297. doi: 10.4322/polimeros020ov002. [DOI] [Google Scholar]
- Cutter CN. Opportunities for bio-based packaging technologies to improve the quality and safety of fresh and further processed muscle foods. Meat Sci. 2006;74(1):131–142. doi: 10.1016/j.meatsci.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Dehghani Soltani M, Meftahizadeh H, Barani M, Rahdar A, Hosseinikhah SM, Hatami M, Ghorbanpour M. Guar (Cyamopsis tetragonoloba L.) plant gum: from biological applications to advanced nanomedicine. Int J Biol Macromol. 2021;193:1972–1985. doi: 10.1016/j.ijbiomac.2021.11.028. [DOI] [PubMed] [Google Scholar]
- Dey A, Dhumal CV, Sengupta P, Kumar A, Pramanik NK, Alam T. Challenges and possible solutions to mitigate the problems of single-use plastics used for packaging food items: a review. J Food Sci Technol. 2021;58(9):3251–3269. doi: 10.1007/s13197-020-04885-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Evangelho JA, da Silva Dannenberg G, Biduski B, Halal SLM, Kringel DH, Gularte MA, Fiorentini AM, da Rosa Zavareze E, Antibacterial activity, optical, mechanical, and barrier properties of corn starch films containing orange essential oil. Carbohyd Polym. 2019;222:114981. doi: 10.1016/j.carbpol.2019.114981. [DOI] [PubMed] [Google Scholar]
- Dutta PK, Tripathi S, Mehrotra GK, Dutta J. Perspectives for chitosan based antimicrobial films in food applications. Food Chem. 2009;114(4):1173–1182. doi: 10.1016/j.foodchem.2008.11.047. [DOI] [Google Scholar]
- El-Sayed SM, El-Sayed HS, Ibrahim OA, Youssef AM. Rational design of chitosan/guar gum/zinc oxide bionanocomposites based on Roselle calyx extract for Ras cheese coating. Carbohyd Polym. 2020;239:116234. doi: 10.1016/j.carbpol.2020.116234. [DOI] [PubMed] [Google Scholar]
- Friedman M, Juneja VK. Review of antimicrobial and antioxidative activities of chitosans in food. J Food Prot. 2010;73(9):1737–1761. doi: 10.4315/0362-028X-73.9.1737. [DOI] [PubMed] [Google Scholar]
- Fryer PJ, Versteeg C. Processing technology innovation in the food industry. Innov Manag Policy Pract. 2008;10(1):74–90. doi: 10.5172/impp.453.10.1.74. [DOI] [Google Scholar]
- Gokularaman S, Stalin Cruz A, Pragalyaashree MM, Nishadh A. Nanotechnology approach in food packaging - review. J Pharm Sci Res. 2017;9(10):1743–1749. [Google Scholar]
- Gómez-Estaca J, López-de-Dicastillo C, Hernández-Muñoz P, Catalá R, Gavara R. Advances in antioxidant active food packaging. Trends Food Sci Technol. 2014;35(1):42–51. doi: 10.1016/j.tifs.2013.10.008. [DOI] [Google Scholar]
- Goudarzi V, Shahabi-Ghahfarrokhi I, Babaei-Ghazvini A. Preparation of ecofriendly UV-protective food packaging material by starch/TiO2 bio-nanocomposite: characterization. Int J Biol Macromol. 2017;95:306–313. doi: 10.1016/j.ijbiomac.2016.11.065. [DOI] [PubMed] [Google Scholar]
- Hassan B, Chatha SAS, Hussain AI, Zia KM, Akhtar N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: a review. Int J Biol Macromol. 2018;109:1095–1107. doi: 10.1016/j.ijbiomac.2017.11.097. [DOI] [PubMed] [Google Scholar]
- Kanikireddy V, Varaprasad K, Rani MS, Venkataswamy P, Mohan Reddy BJ, Vithal M. Biosynthesis of CMC-Guar gum-Ag0 nanocomposites for inactivation of food pathogenic microbes and its effect on the shelf life of strawberries. Carbohyd Polym. 2020;236:116053. doi: 10.1016/j.carbpol.2020.116053. [DOI] [PubMed] [Google Scholar]
- Kaur S, Dhillon GS. The versatile biopolymer chitosan: potential sources, evaluation of extraction methods and applications. Crit Rev Microbiol. 2014;40(2):155–175. doi: 10.3109/1040841X.2013.770385. [DOI] [PubMed] [Google Scholar]
- Khan MKI, Schutyser MAI, Schroën K, Boom R. The potential of electrospraying for hydrophobic film coating on foods. J Food Eng. 2012;108(3):410–416. doi: 10.1016/j.jfoodeng.2011.09.005. [DOI] [Google Scholar]
- Santana Á, Meireles AA, M, New starches are the trend for industry applications: a review. Food Public Health. 2014;4(5):229–241. doi: 10.5923/j.fph.20140405.04. [DOI] [Google Scholar]
- Lee MH, Kim SY, Park HJ. Effect of halloysite nanoclay on the physical, mechanical, and antioxidant properties of chitosan films incorporated with clove essential oil. Food Hydrocolloids. 2018;84:58–67. doi: 10.1016/j.foodhyd.2018.05.048. [DOI] [Google Scholar]
- Lim WS, Ock SY, Park GD, Lee IW, Lee MH, Park HJ. Heat-sealing property of cassava starch film plasticized with glycerol and sorbitol. Food Packag Shelf Life. 2020;26:100556. doi: 10.1016/j.fpsl.2020.100556. [DOI] [Google Scholar]
- Liu J, Meng C, Liu S, Kan J, Jin C. Preparation and characterization of protocatechuic acid grafted chitosan films with antioxidant activity. Food Hydrocolloids. 2017;63:457–466. doi: 10.1016/j.foodhyd.2016.09.035. [DOI] [Google Scholar]
- Liu Q, Huang H, Chen H, Lin J, Wang Q. Food-grade nanoemulsions: preparation, stability and application in encapsulation of bioactive compounds. Molecules. 2019;24(23):1–37. doi: 10.3390/molecules24234242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Ahmed S, Sameen DE, Wang Y, Lu R, Dai J, Li S, Qin W. A review of cellulose and its derivatives in biopolymer-based for food packaging application. Trends Food Sci Technol. 2021;112:532–546. doi: 10.1016/j.tifs.2021.04.016. [DOI] [Google Scholar]
- Lu X, Chen J, Guo Z, Zheng Y, Rea MC, Su H, Zheng X, Zheng B, Miao S. Using polysaccharides for the enhancement of functionality of foods: a review. Trends Food Sci Technol. 2019;86:311–327. doi: 10.1016/j.tifs.2019.02.024. [DOI] [Google Scholar]
- Luo Y, Liu H, Yang S, Zeng J, Wu Z. Sodium alginate-based green packaging films functionalized by guava leaf extracts and their bioactivities. Materials. 2019 doi: 10.3390/ma12182923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maftoonazad N, Ramaswamy HS, Marcotte M. Shelf-life extension of peaches through sodium alginate and methyl cellulose edible coatings. Int J Food Sci Technol. 2008;43(6):951–957. doi: 10.1111/j.1365-2621.2006.01444.x. [DOI] [Google Scholar]
- Mangaraj S, Yadav A, Bal LM, Dash SK, Mahanti NK. Application of biodegradable polymers in food packaging industry: a comprehensive review. J Packaging Technol Res. 2019;3(1):77–96. doi: 10.1007/s41783-018-0049-y. [DOI] [Google Scholar]
- Mehdizadeh T, Tajik H, Langroodi AM, Molaei R, Mahmoudian A. Chitosan-starch film containing pomegranate peel extract and Thymus kotschyanus essential oil can prolong the shelf life of beef. Meat Sci. 2020;163:108073. doi: 10.1016/j.meatsci.2020.108073. [DOI] [PubMed] [Google Scholar]
- Mel’nikov IS, Sotnikova YS, Demina TS, Goncharuk GP, Svidchenko EA, Veselov VI, Akopova TA, Babaevskii PG, Deformation-strength properties of films derived from hydroxyethylcellulose filled with micro- and nanocrystalline cellulose. Fibre Chem. 2020;51(5):340–345. doi: 10.1007/s10692-020-10108-7. [DOI] [Google Scholar]
- Menon VV. Seaweed polysaccharides - food applications. Handb Marine Macroalgae Biotechnol Appl Phycol. 2011;2011:541–555. doi: 10.1002/9781119977087.ch36. [DOI] [Google Scholar]
- Menzel C, González-Martínez C, Chiralt A, Vilaplana F. Antioxidant starch films containing sunflower hull extracts. Carbohyd Polym. 2019;214:142–151. doi: 10.1016/j.carbpol.2019.03.022. [DOI] [PubMed] [Google Scholar]
- Miyazawa T, Funazukuri T. Noncatalytic hydrolysis of guar gum under hydrothermal conditions. Carbohyd Res. 2006;341(7):870–877. doi: 10.1016/j.carres.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Mudgil D, Barak S, Khatkar BS. Guar gum: Processing, properties and food applications - a review. J Food Sci Technol. 2014;51(3):409–418. doi: 10.1007/s13197-011-0522-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojagh SM, Rezaei M, Razavi SH, Hosseini SMH. Development and evaluation of a novel biodegradable film made from chitosan and cinnamon essential oil with low affinity toward water. Food Chem. 2010;122(1):161–166. doi: 10.1016/j.foodchem.2010.02.033. [DOI] [Google Scholar]
- Peng Y, Wu Y, Li Y. Development of tea extracts and chitosan composite films for active packaging materials. Int J Biol Macromol. 2013;59:282–289. doi: 10.1016/j.ijbiomac.2013.04.019. [DOI] [PubMed] [Google Scholar]
- Perumal AB, Nambiar RB, Moses JA, Anandharamakrishnan C. Nanocellulose: recent trends and applications in the food industry. Food Hydrocolloids. 2022;127:107484. doi: 10.1016/j.foodhyd.2022.107484. [DOI] [Google Scholar]
- Pinto T, Aires A, Cosme F, Bacelar E, Morais MC, Oliveira I, Ferreira-Cardoso J, Anjos R, Vilela A, Gonçalves B. Bioactive polyphenols, volatile compounds from vegetables, medicinal and aromatic plants. Foods. 2021;10(1):106. doi: 10.3390/foods10010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranoto Y, Salokhe VM, Rakshit SK. Physical and antibacterial properties of alginate-based edible film incorporated with garlic oil. Food Res Int. 2005;38(3):267–272. doi: 10.1016/j.foodres.2004.04.009. [DOI] [Google Scholar]
- Rao MS, Kanatt SR, Chawla SP, Sharma A. Chitosan and guar gum composite films: preparation, physical, mechanical and antimicrobial properties. Carbohyd Polym. 2010;82(4):1243–1247. doi: 10.1016/j.carbpol.2010.06.058. [DOI] [Google Scholar]
- Rehm BHA. Alginates: biology and applications. Germany: Münster; 2009. [Google Scholar]
- Riaz A, Lagnika C, Luo H, Nie M, Dai Z, Liu C, Abdin M, Hashim MM, Li D, Song J. Effect of Chinese chives (Allium tuberosum) addition to carboxymethyl cellulose based food packaging films. Carbohyd Polym. 2020;235:115944. doi: 10.1016/j.carbpol.2020.115944. [DOI] [PubMed] [Google Scholar]
- Rodrigues C, de Mello JMM, Dalcanton F, Macuvele DLP, Padoin N, Fiori MA, Soares C, Riella HG. Mechanical, thermal and antimicrobial properties of chitosan-based-nanocomposite with potential applications for food packaging. J Polym Environ. 2020;28(4):1216–1236. doi: 10.1007/s10924-020-01678-y. [DOI] [Google Scholar]
- Rojas-Bravo M, Rojas-Zenteno EG, Hernández-Carranza P, Ávila-Sosa R, Aguilar-Sánchez R, Ruiz-López II, Ochoa-Velasco CE. A potential application of mango (Mangifera indica L. cv Manila) peel powder to increase the total phenolic compounds and antioxidant capacity of edible films and coatings. Food Bioprocess Technol. 2019;12(9):1584–1592. doi: 10.1007/s11947-019-02317-8. [DOI] [Google Scholar]
- Roy S, Kim HC, Panicker PS, Rhim JW, Kim J. Cellulose nanofiber-based nanocomposite films reinforced with zinc oxide nanorods and grapefruit seed extract. Nanomaterials. 2021 doi: 10.3390/nano11040877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Rhim JW. Effect of CuS reinforcement on the mechanical, water vapor barrier, UV-light barrier, and antibacterial properties of alginate-based composite films. Int J Biol Macromol. 2020;164:37–44. doi: 10.1016/j.ijbiomac.2020.07.092. [DOI] [PubMed] [Google Scholar]
- Saberi B, Thakur R, Bhuyan DJ, Vuong QV, Chockchaisawasdee S, Golding JB, Scarlett CJ, Stathopoulos CE. Development of edible blend films with good mechanical and barrier properties from pea starch and guar gum. Starch/staerke. 2017;69(1–2):1–33. doi: 10.1002/star.201600227. [DOI] [Google Scholar]
- Saberi B, Thakur R, Vuong QV, Chockchaisawasdee S, Golding JB, Scarlett CJ, Stathopoulos CE. Optimization of physical and optical properties of biodegradable edible films based on pea starch and guar gum. Ind Crops Prod. 2016;86:342–352. doi: 10.1016/j.indcrop.2016.04.015. [DOI] [Google Scholar]
- Salaberria AM, Diaz RH, Labidi J, Fernandes SCM. Role of chitin nanocrystals and nanofibers on physical, mechanical and functional properties in thermoplastic starch films. Food Hydrocolloids. 2015;46:93–102. doi: 10.1016/j.foodhyd.2014.12.016. [DOI] [Google Scholar]
- Sánchez-González L, Cháfer M, Chiralt A, González-Martínez C. Physical properties of edible chitosan films containing bergamot essential oil and their inhibitory action on Penicillium italicum. Carbohyd Polym. 2010;82(2):277–283. doi: 10.1016/j.carbpol.2010.04.047. [DOI] [Google Scholar]
- Sánchez-González L, Vargas M, González-Martínez C, Chiralt A, Cháfer M. Characterization of edible films based on hydroxypropylmethylcellulose and tea tree essential oil. Food Hydrocolloids. 2009;23(8):2102–2109. doi: 10.1016/j.foodhyd.2009.05.006. [DOI] [Google Scholar]
- Sirviö JA, Kolehmainen A, Liimatainen H, Niinimäki J, Hormi OEO. Biocomposite cellulose-alginate films: promising packaging materials. Food Chem. 2014;151:343–351. doi: 10.1016/j.foodchem.2013.11.037. [DOI] [PubMed] [Google Scholar]
- Sotnikova YS, Demina TS, Istomin AV, Svidchenko EA, Subcheva EN, Surin NM, Akopova TA, Zelenetskii AN. Materials based on guar and hydroxypropylguar filled with nanocrystalline polysaccharides. Fibre Chem. 2017;49(3):188–194. doi: 10.1007/s10692-017-9867-x. [DOI] [Google Scholar]
- Sun X, Zhang H, Wang J, Dong M, Jia P, Bu T, Wang Q, Wang L. Sodium alginate-based nanocomposite films with strong antioxidant and antibacterial properties enhanced by polyphenol-rich kiwi peel extracts bio-reduced silver nanoparticles. Food Packag Shelf Life. 2021;29:100741. doi: 10.1016/j.fpsl.2021.100741. [DOI] [Google Scholar]
- Tajeddin B, Arabkhedri M. Polymers and food packaging. In Polym Sci Innov Appl. 2020 doi: 10.1016/b978-0-12-816808-0.00016-0. [DOI] [Google Scholar]
- Tester RF, Karkalas J, Qi X. Starch - Composition, fine structure and architecture. J Cereal Sci. 2004;39(2):151–165. doi: 10.1016/j.jcs.2003.12.001. [DOI] [Google Scholar]
- Tongdeesoontorn W, Mauer LJ, Wongruong S, Sriburi P, Rachtanapun P. Effect of carboxymethyl cellulose concentration on physical properties of biodegradable cassava starch-based films. Chem Central J. 2011 doi: 10.1186/1752-153X-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal V. Marine polysaccharides: food applications. New York, USA. 2016 doi: 10.1201/b10516. [DOI] [Google Scholar]
- Wang X, Yong H, Gao L, Li L, Jin M, Liu J. Preparation and characterization of antioxidant and pH-sensitive films based on chitosan and black soybean seed coat extract. Food Hydrocolloids. 2019;89:56–66. doi: 10.1016/j.foodhyd.2018.10.019. [DOI] [Google Scholar]
- Wu YB, Yu SH, Mi FL, Wu CW, Shyu SS, Peng CK, Chao AC. Preparation and characterization on mechanical and antibacterial properties of chitsoan/cellulose blends. Carbohyd Polym. 2004;57(4):435–440. doi: 10.1016/j.carbpol.2004.05.013. [DOI] [Google Scholar]
- Zhang Z, Zhong Z, Zhao Z. Preparation, characterization and antimicrobial activities of cyclic substituted chitosan derivatives. Int J Biol Macromol. 2021;193:474–480. doi: 10.1016/j.ijbiomac.2021.10.101. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Chen X, Chen T, Chen X. A review of the antibacterial activity and mechanisms of plant polysaccharides. Trends Food Sci Technol. 2022;123:264–280. doi: 10.1016/j.tifs.2022.03.020. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.