Abstract
Fish invasions threaten native freshwater ecosystems worldwide, yet methods to map biodiversity in data-deficient regions are scarce. Rainbow trout (Oncorhynchus mykiss) and brown trout (Salmo trutta fario) have been introduced to the Himalayan ecoregion where they are sympatric with vulnerable native snow trout Schizothorax plagiostomus and Schizothorax richardsonii. We aim to evaluate potential habitat overlap among snow trout and non-native trout in the Indus and Ganges River basins, Himalayan ecoregion. We transferred maximum entropy (MaxEnt) models developed with spatially continuous freshwater-specific environmental variables to map the distribution of potentially suitable habitats for rainbow and brown trout in the Himalayas. We adopted a similar procedure to map suitable habitats for snow trout species. There were substantial habitat overlaps (up to 96%) among snow trout and non-native trout. Yet, the physiography of receiving basins could play a role minimizing the impacts of each non-native trout on native snow trout. We generate high-resolution classified stream suitability maps as decision support tools to help managers in habitat allocation and policy formation to balance recreational fisheries with conservation of snow trout. Our workflow can be transferred to other basins and species for mapping freshwater biodiversity patterns in species-rich yet data-poor regions of the world.
Subject terms: Biodiversity, Biogeography, Conservation biology, Ecological modelling, Freshwater ecology, Invasive species
Introduction
Introduction of fishes has placed freshwater ecosystems among those most affected by biological invasions worldwide1. Non-native fishes can modify recipient ecosystems, thereby negatively impacting the diversity and distribution of native fishes2. Salmonids have been introduced globally for recreational and commercial purposes, with little regard to their effects on native species3. Globally, rainbow trout (Oncorhynchus mykiss, Walbaum, 1792), and brown trout (Salmo trutta fario, Linnaeus, 1758), are the two most problematic invasive salmonids4. The high adaptability of these species outside of their native ranges makes them top ranked in the IUCN’s (International Union for the Conservation of Nature) worst invasive species list5. Negative effects of introduced trout species have been documented in many regions including Japan6, New Zealand7, Chile8, Pakistan9, and India10. Many remote mountainous regions with pristine freshwaters are yet to be studied, including the Himalayas.
Systematic conservation planning in developing countries is difficult to achieve due to limited understanding of freshwater ecosystem functioning, paucity of baseline research, limited professional infrastructure, and inadequate investment in research and monitoring11. In the Himalayas, there is a lack of baseline knowledge about freshwater ecosystems including primary biodiversity whereas datasets about suitable habitats at regional scale have been overlooked. Under such data-poor settings, ecological niche models (ENMs) can play a crucial role in providing the best available information on potential distributions based on local and global geospatial information12. Though the entire invasion process is complex and multifaceted8, ENMs follow ecological theory which suggest that abiotic conditions in the native range of species can be used to predict potential distribution in their introduced range13. ENMs are routinely used as assessment tools to anticipate, and prevent the establishment and spread of non-native species14.
Unfortunately, datasets and tools needed to implement ENMs in freshwater systems are still limited, especially in understudied regions of the world such as South America, Africa, and Asia. These regions, despite having richest freshwater biodiversity worldwide15, are data-poor in terms of primary biodiversity information. High resolution instream and topographic variables are available only for some regions including North America, Europe, and New Zealand16. However, high resolution digital elevation models (DEM), which are freely available for most parts of the world, can be used to extract instream and topographic variables. This could be a computationally intensive process, depending on the resolution of DEM. Efficient GIS tools are therefore required to extract spatially continuous topographic variables that better represent stream conditions. These variables can describe the intrinsic potential of habitats17 that differentially support both native and non-native species18, 19, and be used as covariates to implement ENMs in data poor regions.
In the Himalayas, patterns of occurrence of native and introduced species across riverscapes have been understudied. This ecoregion support high diversity and endemism, with approximately 17% of all the freshwater fishes inhabiting cold waters20. Currently, the region faces challenges for the conservation of freshwaters due to pollution, overfishing, glacial retreat, flow regulation, climate change, and non-native species21. These factors affect native coldwater cyprinids of the genus Schizothorax, commonly known as ‘snow trout’. Although taxonomically misleading, the name ‘snow trout’ is likely attributed to their freshwater residence and similar ecological requirements than trout and other salmonids22. Two native snow trout S. plagiostomus (Heckel, 1838) and S. richardsonii (Gray, 1832) are listed as vulnerable on the IUCN red list9, 23 due to commercial and recreational fishing pressure. In addition, a considerable truncation and range shift has been observed for these snow trout species attributed to both climate change and non-native rainbow and brown trout24. Nonetheless, the geographic distribution of potential habitat overlap between native snow trout and non-native trout species warrants further scrutiny.
Native snow trout and non-native trout thrive in cold, clear waters in high-elevation lakes, streams, and rivers. Both taxonomic groups have produced species complexes independently via convergent evolution with similar ecological roles and requirements in their native ranges22, 25. In invaded rivers, naturalized trout populations occupy different habitats and gradients26, with rainbow trout using higher elevations27 whereas brown trout prefer lower portions of catchments28. Here, we use maximum entropy (MaxEnt) models29 with attributes of stream networks to evaluate whether introduced non-native trout species would establish differentially across river habitats in the Himalayas. This region has higher mountains and steep elevation gradients, compared to other mountain ranges in the world where these non-native trout have been introduced. We hypothesized (1) a substantial overlap in the distribution of potentially suitable rivers among native snow trout and non-native trout, and (2) a differential overlap pattern between each non-native trout and native snow trout (i.e., more overlap between native snow trout and brown trout at lower elevations, more overlap between native snow trout and rainbow trout at higher elevations).
Our study (1) provides baseline information on species-specific suitable river habitats for native and introduced cold-water species, (2) quantifies the degree of overlap in suitable river habitats among species, and (3) develops a complete workflow for implementing MaxEnt models with ecologically relevant and spatially continuous variables in stream networks. Our findings demonstrate how freely available climatic, landcover, and remotely-sensed topographic variables can be used to create ENMs creating a tool that adds biogeographic realism to the conservation of freshwaters in data-deficient regions of the world. Collectively, understanding the patterns of suitable habitats for native and non-native species and potential habitat overlaps are critical to anticipate and prevent invasions, and to balance the provision of recreational fisheries with the conservation of native species in the Himalayas and elsewhere.
Material and methods
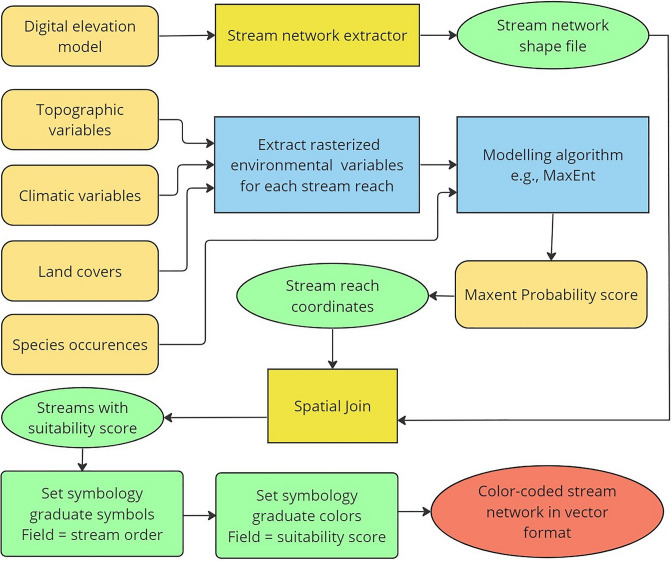
We designed a geoprocessing workflow (Fig. 1) in the form of a toolbox that can be used to extract stream networks with associated topographic attributes in data-deficient regions of the world. The topographic attributes of stream reaches can then be compiled to climatic and landcover variables to model the distribution of freshwater taxa. The integrated set of variables represents ecologically relevant predictors for freshwater species distribution which helps in developing more robust ENMs, using different algorithms. Here, we adopted a maximum entropy (MaxEnt) modelling approach29 using species occurrence data from both native and introduced ranges, and then the resulting algorithms were applied to the Himalayan basins for predicting habitat suitability for native and non-native trout. We used independently collected species occurrences and experts’ knowledge to evaluate the performance of selected algorithms of habitat suitability.
Figure 1.
Geoprocessing workflow to extract and display classified stream networks with suitability scores as attribute table in ArcGIS Pro. The brown colored round-edged rectangles represent spatial data as inputs for different stages of the workflow. The yellow rectangles and green ellipses represent geoprocessing tools and their outputs, respectively. Blue rectangles show part of the workflow outside ArcGIS Pro (in R, Python, and other standalone programs e.g., MaxEnt GUI). The green rectangles are different steps in setting symbology (color-coding) for displaying final output. The red rectangles represent outputs that are being used as inputs, whereas the red ellipse represents the classified streams.
Extracting spatially continuous topographic variables: the stream network extractor (SNE)
We developed a complete and customizable workflow as a geoprocessing tool. Stream Network Extractor (SNE) can be downloaded (please see data availability section) and used in ArcGIS Pro for extracting stream networks from DEMs (Fig. 1). Some of the advantages of SNE over existing alternatives are that it allows users to choose the length of stream reach (grain size), and minimum catchment area threshold for delimiting rivers. The order of geoprocessing tools compiled in SNE also helps to reduce computational timing significantly than running all geoprocessing routines separately. SNE can be used in data-deficient parts of the world to extract stream variables from freely available DEM. These reach-scale, spatially continuous stream segments are extracted in vector format, which preserves the hierarchical linear structure of stream networks. In addition to climatic requirements of species, topographic variables derived from DEM add information to the model about the intrinsic potential of streams17, 30 to support different fish species18, 19. SNE can be used to extract critical habitat features along the hierarchy of the stream network including reach gradient, total upstream gradient, and stream order, which are often associated with hydrological features, and are important for shaping the distribution of fishes31. Other instream variables that can be extracted via SNE such as stream density, total upstream catchment area, sinuosity, and density of confluences (stream nodes). These variables are good proxies for capturing habitat characteristics including stream complexity and heterogeneity, which are important drivers of fish distributions32, 33.
Delineation of stream networks
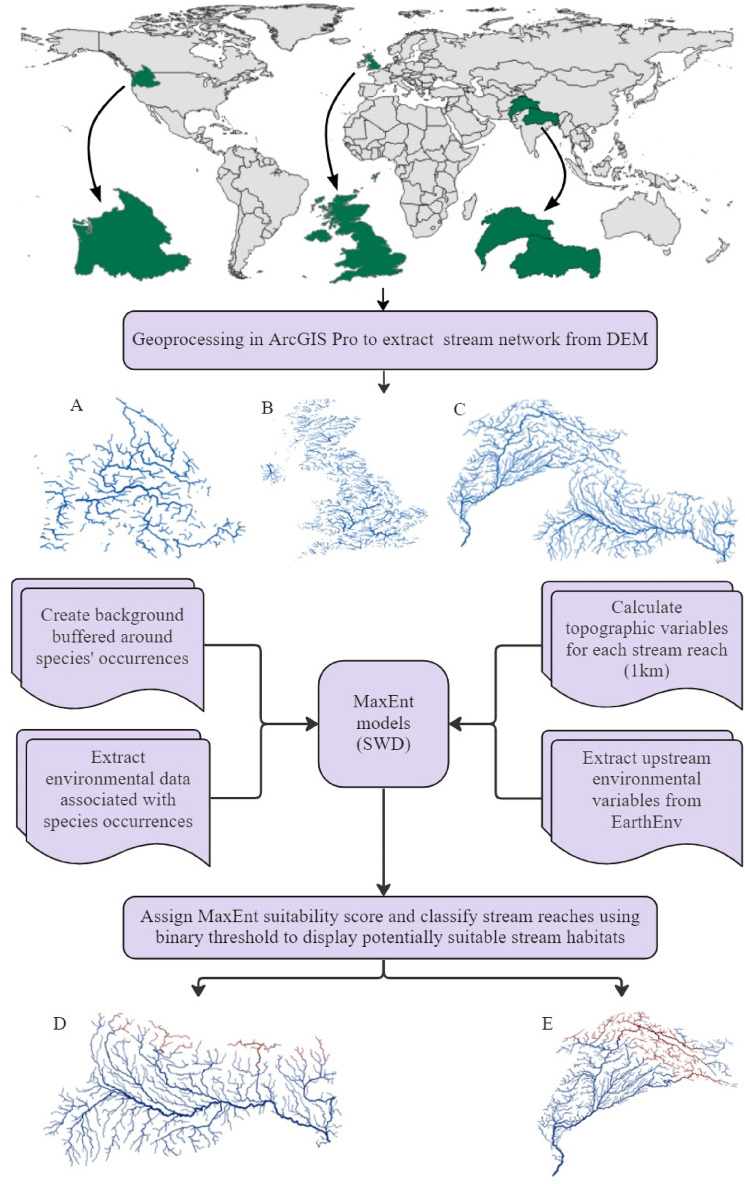
We used 12.5 m resolution L-Band DEMs (ALOS PALSAR) for stream network extraction; the best available space-borne topographic data for hydrological modeling34. Given that populations and communities of stream fishes generally carry out important aspects of their life histories at intermediate spatial scales35, we split the seamless stream network (Fig. 2) into 1.0 km stream reaches. We used 2.0 km2 as the starting threshold for delimiting headwater streams (i.e., a headwater stream should have, at least, a catchment area of 2 km2). We used SNE for extracting the stream network with associated topographic variables in our study area. We choose to extract our own stream network over already available datasets with environmental attributes e.g., HydroSHEDS36. HydroSHEDS has a coarser resolution of DEM (~ 450 m) from which the stream network is derived, and larger threshold to delineate headwater streams limits our ability to assess suitable habitats within smaller basins (< 10 km2).
Figure 2.
Workflow diagram of data and processes used to develop MaxEnt models of suitable habitats for native and non-native fishes. Stream networks were extracted in ArcGIS Pro, MaxEnt models were built in R, and outputs were visualized back in ArcGIS Pro using appropriate binarization threshold. The geographic regions on the top shows regions of calibration (A-Columbia Basin for rainbow trout, B-United Kingdom for brown trout, C-Himalayan range for snow trout species), whereas stream network at the bottom represent receiving basins (D-Ganges River basin, E-Indus River basin). The figure was produced with ArcGIS Pro 3.0.0 with extensions provided by Oregon State University (https://www.esri.com/en-us/arcgis/products/arcgis-pro/overview).
Environmental variables
In stream networks, climate, geology, and topography at large scales set the context for geomorphic processes that create and maintain habitat at finer scales37. Freshwater ecosystems are also shaped by hydrological processes occurring upstream and therefore, we used hydroclimatic and landcover information summarized over the upstream catchment area38 (Table 1). In addition to rasterized hydroclimatic variables, which regulate fish distribution at large biogeographic scales39, topographic variables are associated with fluvial geomorphology of streams40, shaping fish distribution at local habitat scale. Landcover and soil organic carbon from EarthEvn38 serve proxies for stream productivity41, and are important in limiting fish distributions.
Table 1.
Hydroclimate, topography, landcover and soil predictors of suitable habitats for target species used in MaxEnt models.
| Class | Variable | Native snow trout | Non-native brown trout | Non-native rainbow trout |
|---|---|---|---|---|
| Hydroclimate | Annual mean temperature (°C)a | × | × | × |
| Temperature seasonality (°C)a | × | |||
| Annual upstream precipitation (mm)a | × | × | × | |
| Precipitation seasonality (mm)a | × | × | × | |
| Stream network, and topography | Density of stream confluences (Nodes/km2) | × | × | × |
| Strahler stream order (Unitless) | × | × | × | |
| Stream density (Km of stream/km2) | × | × | × | |
| Reach gradient (% slope) | × | × | × | |
| Sinuosity index (Unitless) | × | × | × | |
| Catchment area (km2)a | × | × | × | |
| Average slope (° * 100)a | × | × | × | |
| Land cover | Deciduous needleleaf trees (%)a | × | × | × |
| Evergreen broadleaf trees (%)a | × | × | × | |
| Mixed/other trees (%)a | × | × | × | |
| Shrubs (%)a | × | × | × | |
| Herbaceous vegetation (%)a | × | × | ||
| Soil | Average soil carbon (%)a | × |
aMeasured as the upstream catchment area.
We started with a variety of hydrologic, topographic, and climatic variables, some of which were highly correlated with each other (Supplementary Figures S8–13). Variable selection was guided by ecological relevance, correlation coefficient, data availability, and explanatory power based on iterative model runs. We selected different sets of variables for each species based on the correlation among variables in their native range for snow trout species, and pooled across native and introduced ranges for non-native trout. Only those variables having Pearson’s correlation coefficient < 0.7 were retained42 (Table 1). Among the correlated variables, we dropped those that had limited availability in the Himalayas (e.g., we selected upstream catchment area rather than discharge/flow). Detailed hydrological data is limited in most parts the world43. We applied the same criterion for climatic and topographic variables when they were correlated with hydrological variables.
Occurrence data for native and non-native trout
We used presence-background data for MaxEnt model development. Absence data is not informative in data-deficient settings where sampling sites are not thoroughly surveyed, and where sampling methods and intensities are inconsistent12. This is often the case in developing countries due to limited resources for research44. We contrasted occurrence data with pooled, random, background points (stream reaches), carefully selected inside a buffer around occurrences (see details in Supplementary Fig. S3).
In the native range of rainbow trout, we compiled occurrences (n = 1801) from the Oregon Department of Fish and Wildlife, and Pacific States Marine Fisheries Commission. In the native range of brown trout, we obtained occurrences (n = 2279) from National Biodiversity Network United Kingdom. In the Himalayas, we obtained occurrences of snow trout (S. plagiostomus; n = 255), and non-native trout (n = 98 for rainbow trout; n = 82 for brown trout) by conducted field sampling using cast and scoop nets between January 2017 and September 2019. We only recorded the coordinates of fish presence, i.e., the caught fishes were released back immediately to their natural habitat. Our involvement with fish was least invasive and according to the guidelines of the fisheries department of Khyber Pakhtunkhwa, Pakistan and local government authorities. Lastly, we extracted occurrence data for snow trout (S. richardsonii; n = 244) from published literature45, and the Global Biodiversity Information Facility. We filtered all occurrences for potential errors associated to unknown/assumed datum, duplicates, and fuzzy references. We discarded occurrences with geographic uncertainty > 100 m.
Model development and transfer
We used maximum entropy model (MaxEnt) version 3.4.446 for all four species. MaxEnt has been widely used for presence-only data producing consistently competitive and ecologically meaningful predictions47. We transferred MaxEnt models from a significant portion of the native ranges of rainbow trout (Columbia River Basin; 440,718 km of streams), and brown trout (United Kingdom; 234,867 km), to predict suitable habitats in the Indus (147,541 km), and Ganges (36,457 km) Basins from the Himalayan (Fig. 2). The portion of native ranges where the models were developed exhibited sufficient heterogeneity to capture the environmental limits of non-native trout, as evidenced by the generation of Gaussian response curves for the employed environmental variables. We adopted a similar procedure to map suitable habitats for native snow trout species in these two Himalayan basins. We used Kuenm R package48 to fit MaxEnt models. This R package allowed for comparisons in MaxEnt among candidate models under different regularization multipliers, and feature classes, balancing predictive power with appropriate complexity and statistical significance. Candidate models were evaluated for statistical significance (partial ROC), omission rate (E) and model complexity (AICc), to select the best model (see Supplementary Figs. S4–7). Given the good quality of occurrence data for S. plagiostomus and non-native trout, we used “minimum training presence” threshold to binarize MaxEnt probabilities. For S. richardsonii, since part of the data comes from GBIF, we used “10 percentile training presence” threshold, which leaves a 10% margin of error in the occurrence records and assumes that 10% of occurrence records in the least suitable habitat are not occurring in regions that are representative of the species overall habitat, and thus should be omitted.
Model evaluation
Evaluation using independent data
We evaluated our final models using independent datasets. Testing on an independent dataset has often been considered the most robust type of evaluation49. The final MaxEnt models (average of 10 folds cross validation) for non-native trout had omission rates of 1–2% on validation data. Omission rates on independent data increased to 7–18%, which is to be expected for cross-continental model transfer12. Unfortunately, we could not conduct any sampling to collect independent data for S. richardsonii. Using Receiver Operating Characteristic (ROC) analysis for model evaluation has been criticized for giving equal weight to omission and commission errors50. Models for predicting suitable habitats for non-native/invasive species may have less tolerance for omission error than for commission error. Therefore, we used partial ROC (pROC) developed for ENM evaluation50. pROC uses AUC ratios (The partial AUC divided by random expectation), where a value of 1.0 represents model performance no better than random, whereas models with AUC ratios near or greater than 2.0 are considered good51. The p values of pROC indicate whether the ratios of model AUC to the random AUC is statistically significant. The details of evaluation metrics for our final MaxEnt models for each species are provided in the Supplementary Table S1.
Evaluation using experts’ opinions
Although our models performed well on independent data, we also included expert opinion in the evaluation process. We used the Delphi method52 and conducted Qualtrics surveys requesting respondents to evaluate maps of suitable habitats for our target species. We requested respondents to give a score (between 0 and 10) about the overall accuracy of the maps of Himalayan native fish. In the first round, mapped outputs of our final models were shared with 51 coldwater fisheries experts from Pakistan, India, Nepal, and Bhutan. A total of 16 out of 51 experts responded our survey. We adjusted model parameterization incorporating suggestions from experts before producing our final suitability maps. The final adjusted maps were made available to all the respondents. In the second round, the respondents agreed to the adjustments made and showed confidence in the final adjusted maps. Details of the gridded maps for each species, and questions asked to the experts, are provided in the Supplementary information. We reviewed the decision tree from the Office of Research Integrity and Institutional Review Board and confirm that all methods were carried out in accordance with relevant guidelines and regulations from Oregon State University. Institutional pre-screening indicates that our work is exempted from IACUC and IRB as no animals nor human subjects were used to conduct our research.
Results
Species-specific distribution of potentially suitable habitats
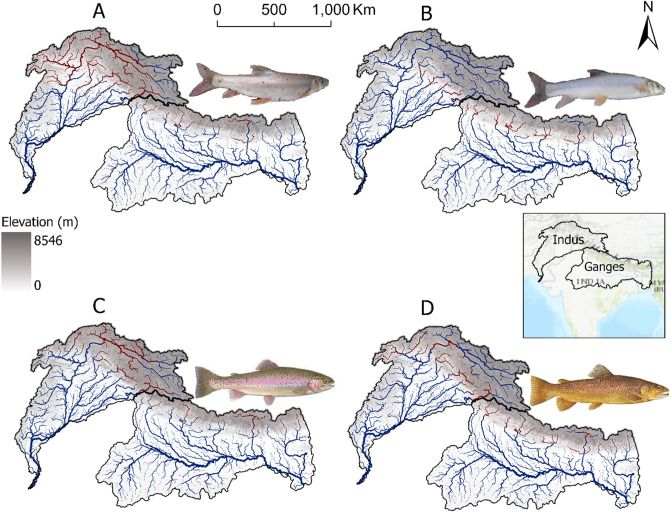
Species-specific distribution maps of potentially suitable habitats (Fig. 3) indicated that the snow trout S. plagiostomus had an extended distribution in the Indus (151,974 km) compared to the Ganges (36,457 km) River basin. This contrsated with snow trout S. richardsonii, that had more suitable habitats in the Ganges (101,898 km) compared to the Indus (29,177 km) River basin. Although non-native trout have potentially suitable habitats in both Himalayan basins, they were differentially distributed gradient-wise. Suitable habitats for rainbow trout dominated higher elevation areas, whereas brown trout habitats were more abundant at lower elevations. Assigning MaxEnt suitability score as an attribute to the stream network allowed us to quantify the total length of potentially suitable streams for each species. The total length of potentially suitable streams for rainbow trout in the Indus and Ganges Basins were 124,596 km and 13,861 km, respectively. Similarly, the total length of potentially suitable streams for brown trout in the Indus, and Ganges River basins, were 103,701 km, and 62,102 km, respectively. We evaluated the performance of our final maps with experts knowledge. We received a reasonable average score (8.2/10) for our final adjusted distribution maps.
Figure 3.
Maps of habitat suitability for native snow trout and non-native trout species in the Indus and Ganges River basins, Himalayas. Dark red represents potentially suitable streams whereas dark blue represents unsuitable streams. Only 4th and higher order streams are shown for better visualization. Map A and B represent potentially suitable habitats for native snow trout S. plagiostomus and S. richardsonii, respectively. Map C and D represent potentially suitable habitats for non-native rainbow and brown trout, respectively. The figure was produced with ArcGIS Pro 3.0.0 with extensions provided by Oregon State University.
Habitat overlap between non-native trout and native snow trout
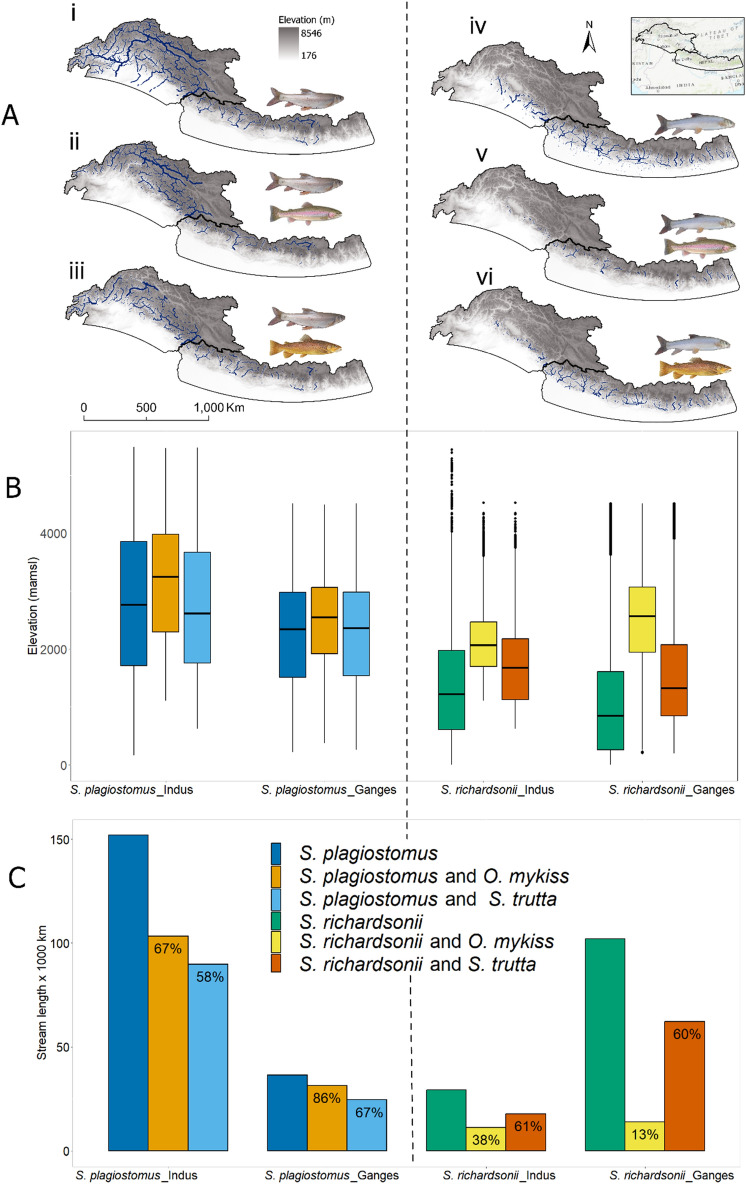
The highest overlap in suitable habitats between native and non-native trout species occurred for the snow trout S. plagiostomus (Fig. 4a). In the Indus River basin, suitable habitats for this snow trout species overlapped with suitable habitats for rainbow trout in 67% of stream reaches, and for brown trout in 58% of stream reaches. Overall, 78% of suitable stream reaches for this native snow trout overlapped with at least one non-native trout. In the Ganges River basin, the overlap of suitable stream reaches was higher, with 86% for rainbow trout and 67% for brown trout. In addition, 96% of suitable habitats for this native snow trout overlapped with at least one non-native trout in the Ganges River basin (Fig. 4c).
Figure 4.
Map of suitable habitats for native snow trout species and the overlap with non-native trout species in the Indus and Ganges Basins. Left-side panels correspond to the native snow trout S. plagiostomus, whereas right-side panels refer to the native snow trout S. richardsonii. (A) Maps i, ii, and iii show the distribution of S. plagiostomus and its overlap with rainbow and brown trout, respectively. Similarly, maps iv, v, and vi correspond to S. richardsonii. Only 4th and higher order streams are shown to improve visualization. (B) Violin plots show the gradient-wise distribution of individual and overlapped habitats. Figures B and C share the same legend. (C) Total length (km) of individual and overlapped habitats. The percentages on the bars represent habitat overlaps between paired native snow trout and non-native trout species. The figure was produced with ArcGIS Pro 3.0.0 with extensions provided by Oregon State University.
In the case of S. richardsonii, 38% and 61% of potentially suitable stream reaches overlapped with rainbow and brown trout in the Indus River basin, respectively. In the Ganges River basin, the overlap resulted in 13% and 60%, respectively. Overall, 63% and 62% of suitable stream reaches for this native snow trout overlapped with at least one non-native trout in the Indus and Gages River basins, respectively (Fig. 4c).
As mentioned above, suitable stream reaches for rainbow trout occurred at relatively higher elevations compared to brown trout, in both basins. The wide elevation range of suitable habitats for the native snow trout S. plagiostomus (mean elevation = 2784 m.a.m.s.l.) resulted in a higher overlap with both non-native trout species. In contrast, suitable stream reaches for the native snow trout S. richardsonii had more restricted elevations (mean elevation = 1078 m.a.m.s.l.), resulting in a higher overlap with brown trout compared with rainbow trout.
Despite the substantial overlap between the distribution of snow trout and non-native trout, the mean elevations at which potentially suitable streams are distributed were statistically significant (Kruskal–Wallis test p < 0.01) and different for each native-non-native pair (Fig. 4b). Potentially suitable stream reaches for O. mykiss were distributed at significantly (Wilcoxon test p < 0.01) higher elevations than suitable stream reaches for S. trutta fario. On the other hand, potentially suitable stream reaches for S. plagiostomus were distributed over a wide range of elevations. As a result, overlapped in potentially suitable stream reaches between S. plagiostomus and O. mykiss were at significantly (Wilcoxon test p < 0.01) higher elevations than overlapped streams between S. plagiostomus and S. trutta fario. Potentially suitable streams for S. richardsonii overlap more with S. trutta fario compared to O. mykiss, as both species inhabit relatively lower elevations (Fig. 3a). Elevation of potentially suitable stream reaches were also significantly different by basin. Overlapped streams between S. plagiostomus and non-native trout were at significantly higher elevations in the Indus (2942 m.a.m.s.l.) compared to the Ganges (2347 m.a.m.s.l.) River basin. Similarly, overlapped streams between S. richardsonii and non-native trout were at significantly (Wilcoxon test p < 0.01) higher elevations in the Indus (1847 m.a.m.s.l.) compared to the Ganges (1690 m.a.m.s.l.) River basins.
Discussion
Our findings lay out baseline information on reach-level (1 km) potential suitability of streams to support species-specific habitats for native snow trout that can be used to reduce the risk of new introductions, and conserve sensitive habitats in the Himalayas. Our geoprocessing workflow characterizes stream networks to be used in ENMs and is transferable for modelling freshwater species distribution at fine scales (< 1 km). The last is especially relevant in data-deficient, but species-rich parts of the world. MaxEnt models outputs provide native snow trout potential suitability maps on a continuous probabilistic scale and help the identification of conservation areas most suited for this species (Supplementary Fig. S1). Specifically, highly suitable streams for snow trout in the Indus River basin mainly occur in Chitral, Swat, Dir upper, North-eastern Gilgit, with some fragmented segments of the Jhelum River, and the upper parts of the Chenab, Ravi, and Bias Rivers in the Indian territory. Whereas in the Ganges Basin, highly suitable streams for snow trout lie mostly in north-eastern India (Uttarakhand and Himanchal Pradesh), Nepal, and Bhutan (Supplementary Fig. S1). In addition, we provide habitat suitability maps that can be used to inform stocking practices of non-native trout in streams where they could potentially establish naturalized populations.
Species-specific maps provide valuable guidance for prioritizing habitats where native snow trout populations could be protected or restored, but additional knowledge about the life history traits of native species can be included to minimize competition and spatial overlap with non-native fishes53. We show that both rainbow and brown trout follow global parallelism resulting in a significant habitat overlap with native snow trout, although with different distributions following elevation as shown in invaded systems elsewhere (e.g. in Japan6, New Zealand7, and Chile8). Rainbow trout tends to inhabit slow-moving, deep-water streams with a 1:1 pool-riffle ratio54. Thus, this species might be unable to establish self-sustaining populations at high elevation at northern Himalayan streams where the likelihood of spawning and rearing would be low due to high water velocities. At high elevations, snow trout might have a competitive advantage over rainbow trout due to their specialized morphological traits (e.g., modified lower lip for adhesion), and to preference for torrential streams. Yet, non-native trout invasion would affect S. plagiostomus as most suitable for the last are located at lower elevations compared to S. richardsonii. Our findings can contribute to the conservation of both snow trout species as the IUCN9 recommends the reduction of stocking of non-native trout via hatcheries, and their restriction to stream segments that would minimize any likelihood of naturalization.
Recent research has documented range truncation for native snow trout due to non-native trout introductions9 and naturalized populations in some parts of the Ganges10 and Indus River basins55. However, naturalization to a new stream and climate is a necessary, but not sufficient condition for invasion success56. Nonetheless, the growing concerns regarding potential invasiveness57 are valid as non-native trout may become invasive, as observed in other basins10 under continuous and high propagule pressure. Previous research demonstrated that even sub-optimal climatic conditions allow colonization of invasive species if propagule pressure is sufficiently high58. Yet, the lack of consolidated data on propagule pressure may underestimate the risk of non-native trout establishing themselves and ultimately invading the Indus and Ganges River basins in places our models identified as suitable stream reaches. Further research to document the frequency and magnitude of trout stocking areas warrants further attention in this region.
Our results highlight streams vulnerable to non-native trout invasions at the basin scale in the Indus and Ganges River basins. Although at the basin scale climatic conditions are likely the main drivers of fish distribution59, non-native trout would experience a series of local scale filters in the form of physiological thermal constraints, biotic interactions, and access to ideal stream habitat with intrinsic potential17 before they can establish naturalized populations in a particular stream or watershed. Although we are unable to incorporate local-scale biotic interactions due to a lack of data, our results can identify both potential locations to further study the ecological interactions among native and non-native fishes10 and areas where stocking trout might be less detrimental for native species. For instance, rainbow trout stocking at the upper reaches of River Swat may not as ecologically detrimental as in River Kumrat as it has more chances of naturalization in Kumrat watershed55. Similarly, Rivers in Chitral watersheds where non-native trout outcompete native snow trout21, reduced stocking and management by modifying impacts of non-native trout, as outlined by Dunham et al.53 would help in the conservation of native snow trout.
Our approach has some limitations that need to be acknowledged to implement best practices and proper use of ENMs in the management of biological invasions. First, the regulatory role of climatic and abiotic variables in freshwater fish distributions is typically observed at broader biogeographic scales. However, the direct use of air temperature and precipitation as surrogates for actual instream hydrological conditions is tenuous60. Stream temperature and hydrology are fundamental determinants of fish distributions61, and are highly correlated to air temperature and precipitation. This association is also affected by other factors such as riparian vegetation62 total catchment area, hyporheic exchange, slope, and watershed elevation40. In addition, the correlation between air and water temperature also becomes weaker over time60 and at higher elevation potentially affecting habitat modelling for cold-water fish species63. We minimize these potential limitations by testing our final models with independent data from the receiving basins and with expert judgement. The final predicted habitat suitability for all species in this study closely approximated the expert’s knowledge of fish distributions, giving us confidence in the utility of our models for decision support to managers across Himalayan countries.
Additional limitations of our modelling approach include the data deficiency in developing countries as well as the overall assessment of impacts of invasive species in freshwaters. Here, we provide a geoprocessing tool SNE that can be used to extract important topographic stream variables from freely available DEM that can be combined with climatic data and species occurrences to model species distributions. Fortunately, even in situations where occurrence data is absent, experts knowledge about species ecology can be used to model the intrinsic potential of habitat to support fish populations18, 19. The SNE tool can extract instream variables (discharge, gradient, and valley confinement) for modelling the intrinsic potential of streams to support species-specific habitat based on ecological knowledge of species requirements. We recommend using DEMs with the finest available resolution to extract more reliable positioning of stream network. With SNE users can run analyses at multiple scales by choosing desired grain size (reach length) and can customize drainage threshold for delimiting headwater streams.
Globally, the Himalayas is one of the most vulnerable regions to climate change. This region is warming at twice the rate of the global average, and glacial retreat is happening six times faster than in other regions of the world64. The geographic distribution of native snow trout is likely shrinking, and shifting, under climate change24. Many developing countries have extensive freshwater systems with high species diversity and endemism, which demands approaches that maximize the utility of freely available information (e.g., climatic, landcover, and other geospatial data). This study demonstrates that freely available georeferenced species collections from different inventories, DEM, and climatic and environmental data can be used to develop ENMs that provide baseline information for policy making. Therefore, despite the limitations of this study, which are germane to many species distribution studies, it is reasonable and urgent to balance the need for clear baseline research with the reality of limited resources and data deficiency in developing countries.
Supplementary Information
Author contributions
A.J. conceived and led the study design. A.J. ran the analyses and drafted the original manuscript. I.A. and G.G. assisted with the design of the study and provided substantial edits to the original manuscript. All authors helped with reviewing and editing the final version of the manuscript.
Data availability
All programming code, model outputs, raw data used, and SNE tool were made available for this peer-review process through the open data repository Dryad (https://datadryad.org/stash/share/3U5qi2Xo52W7uPlPA7sRRk9Hgagi1fEvvGFNSdaapr8). Any additional information related to study can be obtained from corresponding author upon reasonable request. Rainbow trout data: https://nrimp.dfw.state.or.us/FHD_FPB_Viewer/index.html. https://maps.psmfc.org/server/rest/services. Brown trout data: https://nbnatlas.org/. S. richardsonii data from GBIF: 10.15468/dl.m3xxse.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-41778-y.
References
- 1.Bernery C, et al. Freshwater fish invasions: A comprehensive review. Annu. Rev. Ecol. Evol. Syst. 2022;53:427–456. [Google Scholar]
- 2.Leprieur F, Beauchard O, Blanchet S, Oberdorff T, Brosse S. Fish invasions in the world’s river systems: When natural processes are blurred by human activities. PLoS Biol. 2008;6:0404–0410. doi: 10.1371/journal.pbio.0060028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shepard, B.et al. Global perspectives on the management of trout and char. In Trout and Char of the World. American Fisheries Society, Bethesda, Maryland 605–644 (2019).
- 4.Hansen, M. J., Guy, C. S., Budy, P. & McMahon, T. E. Trout as native and nonnative species: A management paradox. In Trout and Char of the World’. (eds Kershner, J. L., Williams, J. E., Gresswell, R. E. & Lobon-Cervia, J.) 645–684 (2019).
- 5.Lowe S, Browne M, Boudjelas S, De Poorter M. 100 of the World’s Worst Invasive Alien Species: A Selection from the Global Invasive Species Database. Invasive Species Specialist Group Auckland; 2000. [Google Scholar]
- 6.Morita K, Tsuboi J-I, Matsuda H. The impact of exotic trout on native charr in a Japanese stream. J. Appl. Ecol. 2004;41:962–972. [Google Scholar]
- 7.Townsend CR. Invasion biology and ecological impacts of brown trout Salmo trutta in New Zealand. Biol. Conserv. 1996;78:13–22. [Google Scholar]
- 8.Arismendi I, et al. Differential invasion success of salmonids in southern Chile: Patterns and hypotheses. Rev. Fish Biol. Fish. 2014;24:919–941. [Google Scholar]
- 9.Jan, A. & Daniels, A. 2022. Schizothorax plagiostomus. The IUCN Red List of Threatened Species 2022: e.T128725859A139131270. https://dx.doi.org/10.2305/IUCN.UK.2022-1.RLTS.T128725859A139131270.en. Accessed on 03 September 2023.
- 10.Johal MS, et al. Invasive brown trout Salmo trutta induce differential growth strategies in the native snow trout Schizothorax richardsonii of Himalaya: Are natives in unaltered rivers better at picking the gauntlet of invasion? J. Appl. Ichthyol. 2021;37:723–734. [Google Scholar]
- 11.Esselman PC, Allan JD. Application of species distribution models and conservation planning software to the design of a reserve network for the riverine fishes of northeastern Mesoamerica. Freshw. Biol. 2011;56:71–88. [Google Scholar]
- 12.Peterson AT, et al. Ecological Niches and Geographic Distributions (MPB-49) Princeton University Press; 2011. [Google Scholar]
- 13.Peterson AT. Predicting the geography of species’ invasions via ecological niche modeling. Q. Rev. Biol. 2003;78:419–433. doi: 10.1086/378926. [DOI] [PubMed] [Google Scholar]
- 14.Jiménez-Valverde A, et al. Use of niche models in invasive species risk assessments. Biol. Invasions. 2011;13:2785–2797. [Google Scholar]
- 15.Abell R, et al. Freshwater ecoregions of the world: A new map of biogeographic units for freshwater biodiversity conservation. Bioscience. 2008;58:403–414. [Google Scholar]
- 16.McGarvey DJ, et al. On the use of climate covariates in aquatic species distribution models: Are we at risk of throwing out the baby with the bath water? Ecography. 2018;41:695–712. [Google Scholar]
- 17.Burnett KM, et al. Distribution of salmon-habitat potential relative to landscape characteristics and implications for conservation. Ecol. Appl. 2007;17:66–80. doi: 10.1890/1051-0761(2007)017[0066:dosprt]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 18.Manning MA, Arismendi I, Olivos JA, Giannico G. Assessing hybridization risk between ESA-listed native bull trout (Salvelinus confluentus) and introduced brook trout (S. fontinalis) using habitat modeling. Front. Environ. Sci. 2022;10:126. [Google Scholar]
- 19.Soto D, et al. Environmental risk assessment of non-native salmonid escapes from net pens in the Chilean Patagonia. Rev. Aquac. 2023;15:198–219. [Google Scholar]
- 20.Ghosh A. Himalayan fauna with special reference to endangered and endemic species. In: Dhar U, editor. Himalayan Biodiversity: Action Plan. GB Pant Institute of Himalayan Environment & Development; 1997. pp. 53–59. [Google Scholar]
- 21.Jan A, et al. Current scenario and threats to ichthyo-diversity in the foothills of Hindu Kush: Addition to the checklist of coldwater fishes of Pakistan. Pak. J. Zool. 2016;48:285–288. [Google Scholar]
- 22.Regmi B, et al. The Himalayan uplift and the evolution of aquatic biodiversity across Asia: Snowtrout (Cyprininae: Schizothorax) as a test case. bioRxiv. 2020 doi: 10.1101/2020.10.12.336149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vishwanath, W. 2010. Schizothorax richardsonii (errata version published in 2020). The IUCN Red List of Threatened Species 2010: e.T166525A174786567. https://dx.doi.org/10.2305/IUCN.UK.2010-4.RLTS.T166525A174786567.en. Accessed on 03 September 2023.
- 24.Sharma A, Dubey VK, Johnson JA, Rawal YK, Sivakumar K. Dendritic prioritization through spatial stream network modeling informs targeted management of Himalayan riverscapes under brown trout invasion. J. Appl. Ecol. 2021;58:2415–2426. [Google Scholar]
- 25.Keeley ER. Origins, Species Diversity, and Ecological Diversification in Trout and Char. American Fisheries Society; 2019. [Google Scholar]
- 26.Soto D, et al. Sur de Chile, país de truchas y salmones: patrones de invasión y amenazas para las especies nativas. Rev. Chil. Hist. Nat. 2006;79:97–117. [Google Scholar]
- 27.Crawford SS, Muir AM, Andrew AE, Muir M. Global introductions of salmon and trout in the genus Oncorhynchus: 1870–2007. Rev. Fish Biol. Fish. 2008;18:313–344. [Google Scholar]
- 28.Finstad AG, et al. Competitive exclusion along climate gradients: Energy efficiency influences the distribution of two salmonid fishes. Glob. Change Biol. 2011;17:1703–1711. [Google Scholar]
- 29.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006;190:231–259. [Google Scholar]
- 30.Olivos JA, et al. An environmental resistance model to inform the biogeography of aquatic invasions in complex stream networks. J. Biogeogr. 2023 doi: 10.1111/jbi.14621. [DOI] [Google Scholar]
- 31.Penaluna BE, et al. UPRLIMET: UPstream Regional LiDAR Model for Extent of Trout in stream networks. Sci. Rep. 2022;12:20266. doi: 10.1038/s41598-022-23754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benda L, et al. The network dynamics hypothesis: How channel networks structure riverine habitats. Bioscience. 2004;54:413. [Google Scholar]
- 33.Flitcroft RL, Arismendi I, Santelmann MV. A review of habitat connectivity research for Pacific salmon in marine, estuary, and freshwater environments. J. Am. Water Resour. Assoc. 2019;55:430–441. [Google Scholar]
- 34.Shawky M, Moussa A, Hassan QK, El-Sheimy N. Pixel-based geometric assessment of channel networks/orders derived from global spaceborne digital elevation models. Remote Sens. 2019;11:235. [Google Scholar]
- 35.Fausch KD, Torgersen CE, Baxter CV, Li HW. Landscapes to riverscapes: Bridging the gap between research and conservation of stream fishes. Bioscience. 2002;52:483–498. [Google Scholar]
- 36.Lehner B, Verdin K, Jarvis A. New global hydrography derived from spaceborne elevation data. EOS Trans. Am. Geophys. Union. 2008;89:93–94. [Google Scholar]
- 37.Montgomery DR. Process domains and the river continuum 1. JAWRA J. Am. Water Resour. Assoc. 1999;35:397–410. [Google Scholar]
- 38.Domisch S, Amatulli G, Jetz W. Near-global freshwater-specific environmental variables for biodiversity analyses in 1 km resolution. Sci. Data. 2015;2:1–13. doi: 10.1038/sdata.2015.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fournier A, Barbet-Massin M, Rome Q, Courchamp F. Predicting species distribution combining multi-scale drivers. Glob. Ecol. Conserv. 2017;12:215–226. [Google Scholar]
- 40.Allan JD, Castillo MM. Stream Ecology: Structure and Function of Running Waters. Springer; 2009. [Google Scholar]
- 41.Dudgeon D, et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. Camb. Philos. Soc. 2006;81:163–182. doi: 10.1017/S1464793105006950. [DOI] [PubMed] [Google Scholar]
- 42.Dormann CF, et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography. 2013;36:27–46. [Google Scholar]
- 43.McManamay RA, Griffiths NA, DeRolph CR, Pracheil BM. A synopsis of global mapping of freshwater habitats and biodiversity: Implications for conservation. Pure Appl. Biogeogr. 2018 doi: 10.5772/INTECHOPEN.70296. [DOI] [Google Scholar]
- 44.Graham CH, Ferrier S, Huettman F, Moritz C, Peterson AT. New developments in museum-based informatics and applications in biodiversity analysis. Trends Ecol. Evol. 2004;19:497–503. doi: 10.1016/j.tree.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 45.Sharma A, Dubey VK, Johnson JA, Rawal YK, Sivakumar K. Is there always space at the top? Ensemble modeling reveals climate-driven high-altitude squeeze for the vulnerable snow trout Schizothorax richardsonii in Himalaya. Ecol. Ind. 2021;120:106900. [Google Scholar]
- 46.Phillips SJ, Anderson RP, Dudík M, Schapire RE, Blair ME. Opening the black box: An open-source release of Maxent. Ecography. 2017;40:887–893. [Google Scholar]
- 47.Elith J, et al. A statistical explanation of MaxEnt for ecologists: Statistical explanation of MaxEnt. Divers. Distrib. 2011;17:43–57. [Google Scholar]
- 48.Cobos ME, Peterson AT, Barve N, Osorio-Olvera L. kuenm: An R package for detailed development of ecological niche models using Maxent. PeerJ. 2019;7:e6281. doi: 10.7717/peerj.6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Araújo MB, Guisan A. Five (or so) challenges for species distribution modelling. J. Biogeogr. 2006;33:1677–1688. [Google Scholar]
- 50.Peterson AT, Papeş M, Soberón J. Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecol. Model. 2008;213:63–72. [Google Scholar]
- 51.Escobar LE, Qiao H, Cabello J, Peterson AT. Ecological niche modeling re-examined: A case study with the Darwin’s fox. Ecol. Evol. 2018;8:4757–4770. doi: 10.1002/ece3.4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eycott AE, Marzano M, Watts K. Filling evidence gaps with expert opinion: The use of Delphi analysis in least-cost modelling of functional connectivity. Landsc. Urban Plan. 2011;103:400–409. [Google Scholar]
- 53.Dunham JB, et al. What to do when invaders are out of control? Wiley Interdiscip. Rev. Water. 2020;7:1–13. [Google Scholar]
- 54.Raleigh RF. Habitat Suitability Information: Rainbow Trout. Western Energy and Land Use Team, Division of Biological Services, Research; 1984. [Google Scholar]
- 55.Jan, A. Studies on the culture prospects of Snow Trout (Schizothorax plagiostomus) in Dir Upper, Khyber Pakhtunkwa (KP) to reduce the poverty and food security risk in the area: A pilot project (AS-147) (2019).
- 56.Simberloff D, Rejmánek M. Encyclopedia of Biological Invasions. University of California Press; 2011. [Google Scholar]
- 57.Rafique M, Khan NUH. Distribution and status of significant freshwater fishes of Pakistan. Rec. Zool. Surv. Pakistan. 2012;21:90–95. [Google Scholar]
- 58.Gallardo B, Vila L. Human influence, key to understand the biogeography of invasive species in the Anthropocene. Cuad. Investig. Geogr. 2019;45:61–86. [Google Scholar]
- 59.Bellard C, et al. Will climate change promote future invasions? Glob. Change Biol. 2013;19:3740–3748. doi: 10.1111/gcb.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arismendi I, Safeeq M, Dunham JB, Johnson SL. Can air temperature be used to project influences of climate change on stream temperature? Environ. Res. Lett. 2014;9:084015. [Google Scholar]
- 61.Lytle DA, Poff NL. Adaptation to natural flow regimes. Trends Ecol. Evol. 2004;19:94–100. doi: 10.1016/j.tree.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 62.Arismendi I, Johnson SL, Dunham JB, Haggerty R, Hockman-Wert D. The paradox of cooling streams in a warming world: Regional climate trends do not parallel variable local trends in stream temperature in the Pacific continental United States. Geophys. Res. Lett. 2012 doi: 10.1029/2012GL051448. [DOI] [Google Scholar]
- 63.Isaak DJ, et al. Slow climate velocities of mountain streams portend their role as refugia for cold-water biodiversity. Proc. Natl. Acad. Sci. 2016;113:4374–4379. doi: 10.1073/pnas.1522429113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dimri AP, Kumar D, Choudhary A, Maharana P. Future changes over the Himalayas: Mean temperature. Glob. Planet. Change. 2018;162:235–251. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All programming code, model outputs, raw data used, and SNE tool were made available for this peer-review process through the open data repository Dryad (https://datadryad.org/stash/share/3U5qi2Xo52W7uPlPA7sRRk9Hgagi1fEvvGFNSdaapr8). Any additional information related to study can be obtained from corresponding author upon reasonable request. Rainbow trout data: https://nrimp.dfw.state.or.us/FHD_FPB_Viewer/index.html. https://maps.psmfc.org/server/rest/services. Brown trout data: https://nbnatlas.org/. S. richardsonii data from GBIF: 10.15468/dl.m3xxse.