Abstract
The L1 major capsid proteins of six human papillomavirus type 16 (HPV-16) strains were expressed in insect cells by using recombinant baculoviruses. Virus-like particles (VLPs) which appeared similar to empty virions were identified by electron microscopy for all HPV strains investigated. However, the yield of VLPs produced varied in a range from 1 to 79 depending on the HPV-16 strain. The L1 proteins of these strains differed by up to 15 amino acids from the L1 protein of the prototype HPV-16 strain. Mutations in the amino acid region from residues 83 to 97 seemed to affect the level of expression of the L1 protein. These results are important when considering the development of HPV vaccines and serological tests. They indicate that strains inducing high levels of VLP production must be selected for the development of vaccines. Moreover, the L1 proteins of all strains investigated were able to bind with DNA. We also investigated the seroreactivities of VLPs derived from three different HPV-16 strains from Algeria, Senegal, and the Philippines by testing sera from women from 11 countries in immunoglobulin G-specific enzyme-linked immunosorbent assays. We observed a strong correlation between the reactivities of the three different VLP variants, independent of the geographical origin of the sera investigated. These results indicate that the three strains investigated are serologically cross-reactive despite the fact that their L1 proteins differ in 14 amino acids and suggest that VLPs derived from only one HPV-16 strain could be sufficient for the development of an HPV-16 vaccine and anti-HPV-16 tests.
Infection by human papillomavirus type 16 (HPV-16) is causally associated with cervical cancer (14, 31), one of the most common cancers worldwide (23). Native virions of HPV are nonenveloped 50- to 60-nm-diameter icosahedral structures composed of 72 capsomers, and each capsomer is composed of five L1 molecules (1, 28). Immunization with virus-like particles (VLPs) obtained by self-assembly of the major capsid protein, L1, can induce protection against papillomavirus infection in animal models, and it appears that neutralizing antibodies recognize conformationally dependent epitopes. Immunization with HPV-11 virions elicits neutralizing antibodies in animals, and these were shown to inhibit the infectivity of the virus in a mouse xenograft experiment (3). These and other studies (8, 9) have also shown that neutralizing antibodies recognize conformational epitopes of the viral capsid protein and are type specific.
However, numerous nucleotide sequence variants of HPV-16 have been identified, and many of these variants have been isolated in distinct geographic locations (5, 6, 13). In contrast to the extensive genotype analysis, only one study using VLPs from two different HPV-16 strains obtained in Germany and Zaire has been performed (7), and this study found that serotypes for HPV-16 do not exist. On the other hand, Sasagawa et al. (25) reported that with some HPV-16 variants, the level of VLPs produced in fission yeast is 64 times higher than that produced with other variants. Few studies of this type have been performed because large amounts of HPV virions have not been available for use in serological assays until recently.
To investigate further the possibility that HPV variants are distinct serotypes, we compared the reactivities of 91 anti-HPV-16-positive and 122 anti-HPV-16-negative human sera from 11 countries by enzyme-linked immunosorbent assays (ELISAs) based on HPV VLPs composed of L1 proteins derived from three different strains isolated in Senegal, Algeria, and the Philippines. Levels of L1 proteins and VLPs produced in insect cells by recombinant baculoviruses which carried the L1 genes of six different HPV-16 strains were also investigated for the purpose of developing HPV vaccines and serological tests.
MATERIALS AND METHODS
Source of HPV-16 DNA and introduction of the HPV-16 L1 open reading frame into baculovirus.
HPV-16 DNA was extracted from infected cervical cells obtained by scrapings or from biopsies. The Sen32 strain was isolated from a woman with severe dysplasia living in Dakar, Senegal; the Alg1 strain was isolated from a woman with cancer of the cervix living in Algiers, Algeria. The Fra25 strain was obtained from a human immunodeficiency virus-seropositive man with anal warts, and Fra63 was obtained from a woman with cutaneous warts. These two patients lived in Besançon, France. Tha7 and Phi1 strains were isolated from women suffering from invasive cancer of the cervix living in Sonkla, Thailand, and in Manila, Philippines, respectively.
The L1 coding sequence was cloned after PCR amplification from purified genomic DNA, with primers designed to introduce BglII restriction enzyme sites (boldface letters) at the 5′ and 3′ ends of the PCR products. The forward and reverse primer sequences were 5′-CCAGATCTATGTCTCTTTGGCTGCCTAGTGAGGC-3′ and 5′-CCAGATCTTTACAGCTTACGTTTTTTGCGTTTAG-3′, respectively. Amplification was performed with a 0.7 mM concentration of each primer and 1.25 U of Taq DNA polymerase (Boehringer, Mannheim, Germany), and the PCR products were then cloned into the TAG vector (R & D Systems, Abingdon, United Kingdom). After digestion by BglII (Boehringer), the HPV-16 L1 gene was cloned into pBlueBacIII vector (Invitrogen, San Diego, Calif.). The resulting constructs were used to cotransfect Spodoptera frugiperda cells (Sf21) with linearized Autographa californica multiple nuclear polyhedrosis virus (AcMNPV) genomic DNA (linear AcMNPV transfection module; Invitrogen).
DNA sequencing was performed with an ABI PRISM 377 automated sequencing system (Perkin-Elmer/Applied Biosystems, Courtaboeuf, France). L1 gene sequences were obtained with M13 forward and reverse dye-labeled primers (Perkin-Elmer/Applied Biosystems) and four HPV-16 L1 gene internal primers (Table 1).
TABLE 1.
HPV-16 L1 gene internal sequencing primers
| Strand | Primer sequence (5′–3′) | Postion in HPV-16 genome |
|---|---|---|
| Plus | TTGAGGTAGGTCGTGGTC | 5951–5968 |
| Plus | GTTGTTGATACTACACGCAG | 6637–6653 |
| Minus | GTCCATAGCACCAAAGCC | 6247–6264 |
| Minus | CCCATGTCGTAGGTACTC | 6651–6738 |
Monolayer cultures of Sf21 cells (Invitrogen) were grown at 27°C in Grace’s medium (Life Technologies, Cergy-Pontoise, France) supplemented with 10% fetal calf serum. Recombinant baculoviruses encoding the L1 protein were selected in one round of plaque purification and visual examination of β-galactosidase-positive cells, except for the Sen32 strain, which was previously purified by four rounds of plaque purification (18).
Production and characterization of L1 proteins.
Sf21 cells, which were maintained in supplemented Grace’s insect medium with 10% fetal calf serum, were infected at a multiplicity of infection (MOI) of 20 with the recombinant baculoviruses. Cells were harvested 4 days postinfection and fractionated into cytoplasmic and nuclear fractions by Nonidet P-40 treatment (0.5%), followed by centrifugation (10,000 × g, 15 min). The nuclear pellet was resuspended in 8 M urea–1% β-mercaptoethanol (9). Nuclear fractions were separated by sodium dodecyl sulfate–12% polyacrylamide gel electrophoresis, electroblotted onto BA 83 nitrocellulose (Schleicher and Schuell, Dassel, Germany), and probed with CamVir-1 monoclonal antibody (Pharmingen, Newcastle, United Kingdom). Specifically bound antibodies were detected with an anti-mouse immunoglobulin G (IgG) alkaline phosphatase conjugate (Sigma, St. Quentin-Fallavier, France) used at a dilution of 1/1,000. Immunoreactive proteins were revealed with 4- nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate (BCIP) (Life Technologies).
Purification of HPV VLPs.
VLPs were purified essentially as previously described for HPV-16 VLPs (18). Infected insect cells were collected by centrifugation 4 days postinfection with the respective recombinant baculoviruses and resuspended in ice-cold phosphate-buffered saline (PBS) containing 0.5% Nonidet P-40. Cell lysates were centrifuged (10,000 × g, 15 min) after incubation on ice for 15 min. Nucleus pellets were resuspended in ice-cold PBS and sonicated by three 15-s bursts at 60% maximal power (Vibra-cell; Bioblock Scientific, Strasbourg, France). Nuclear lysates were loaded onto a 40% (wt/vol) sucrose cushion and centrifuged in a Beckman SW28 rotor (4°C, 150 min, 111,800 × g). Pellets were resuspended in PBS, loaded on CsCl, and centrifuged to equilibrium in the same rotor (20 h, 130,400 × g, 4°C). CsCl gradient fractions were harvested. Their densities were determined by refractometry, and their contents of VLPs were examined by electron microscopy (EM). Positive fractions were pooled, diluted in PBS, and ultracentrifuged in a Beckman SW28 rotor (4°C, 3 h, 141,000 × g). VLP pellets were resuspended in PBS after centrifugation, and protein content was evaluated with the MicroBCA kit (Pierce, Touzart et Matignon, France).
Detection of DNA binding to L1 protein by Southwestern assay.
The Southwestern assay, which allows the detection of DNA-protein interactions, was based on previously published procedures with some modifications (19, 20). Briefly, purified VLPs were boiled in the presence of 1% sodium dodecyl sulfate and the denatured L1 proteins were separated on a sodium dodecyl sulfate–10% polyacrylamide gel and transferred to a BA 83 nitrocellulose sheet by electroblotting (Hoefer Semiphor; Pharmacia). Nuclear extract of Sf21 insect cells and bovine serum albumin (BSA) were used as the negative controls for the DNA binding property. After a blocking step with 0.2% BSA for 30 min at 25°C, a renaturation step involving overnight incubation of the filters at 4°C in 50 mM Tris-HCl (pH 7.4)–1 mM EDTA–200 mM NaCl–0.1% Nonidet P-40–10% glycerol was carried out. The DNA binding assay was performed for 30 min at room temperature in a buffer containing 30 mM HEPES (pH 7.4), 5 mM MgCl2, and 50 mM NaCl by using digoxigenin-labeled plasmid DNA (Dig DNA labelling and detection kit; Boehringer). The membranes were then washed four times with binding buffer, and bound DNA was revealed by an anti-digoxigenin alkaline phosphatase-conjugated antibody (Boehringer), with 4-nitroblue tetrazolium and BCIP as the substrates.
Detection of anti-VLP antibodies by ELISA.
Anti-HPV-16 VLP antibodies were investigated by ELISA. Purified VLPs (100 ng/well) were diluted in PBS (pH 7.4) according to the EM results (see below): 100-fold for the Phi1 strain, 50-fold for the Sen32 strain, and 10-fold for the Alg1 strain. Microtiter plates (Maxisorp; Nunc, Life Technologies) were then incubated overnight at 4°C. After four washes of the plates with PBS–0.1% Tween 20, nonspecific binding sites were blocked by incubation for 30 min at 37°C with PBS–1% newborn bovine serum (NBS; Sigma). The blocking solution was replaced by 100 μl of human sera diluted 1/20 in 5× PBS containing 10% NBS and 2% Tween 20. Following incubation of the plates at 45°C for 90 min and four washes, bound antibodies were detected with mouse anti-human IgG antibodies covalently linked to horseradish peroxidase (Southern Biotechnology Associates, Birmingham, Ala.) and 100 μl of substrate solution containing o-phenylenediamine and H2O2 was added after incubation at 45°C for 90 min and four washes. The reaction was stopped after 30 min by the addition of 100 μl of 4 N H2SO4, and optical densities at 492 nm (OD492s) were read with an automated plate reader (Microplate reader EL 311; Biotek Instruments). Anti-VLP antibodies in 222 sera from 11 countries were investigated in order to ascertain the existence of serotypes within the HPV-16 genotype (Table 2). All these sera had previously been screened for anti-VLP antibodies by an ELISA using VLPs derived from the Sen32 strain of HPV-16 as the antigen. The cutoff value was set at an OD of 0.200.
TABLE 2.
Selected sera from 11 countries investigated for immune reactivity against VLPs derived from three different strains of HPV-16a
| Country of origin | No. of sera with indicated reslt against VLPsa
|
Total no. of sera | |
|---|---|---|---|
| + | − | ||
| France | 16 | 32 | 48 |
| Spain | 6 | 5 | 11 |
| Algeria | 1 | 14 | 15 |
| Tunisia | 7 | 14 | 21 |
| Senegal | 15 | 20 | 35 |
| Burundi | 10 | 17 | 27 |
| Gabon | 19 | 8 | 27 |
| Uganda | 1 | 1 | 2 |
| Tanzania | 4 | 1 | 5 |
| Colombia | 4 | 2 | 6 |
| Philippines | 10 | 17 | 27 |
| Total | 93 | 131 | 224 |
The anti-VLP immune reactivity was based on previous testing with VLPs derived from the Sen32 HPV-16 strain. +, positive; −, negative.
Detection and quantification of HPV-16 VLPs by EM.
VLP preparations were applied to carbon-coated grids, negatively stained with 1.5% uranyl acetate, and were observed at ×50,000 nominal magnification with a JEOL 1010 electron microscope. Each preparation was mixed with an equal volume of a standard suspension of 15-nm-diameter gold beads conjugated to anti-rabbit IgG (British BioCell International, Cardiff, United Kingdom) in order to compare the yields for the HPV-16 strains and then was observed by EM as previously described. Conjugation of gold beads to an Ig enhances the adhesion of the beads to the grid. The total numbers of HPV VLPs and gold beads in five randomly chosen fields were counted at the above magnification. Results were expressed as a ratio of the number of VLPs to the number of gold beads. The quantification of VLP production was performed two times with two preparations of the six different HPV-16 VLPs at an interval of 1 month by using the same MOI, and results are expressed as the means of the values obtained in these two experiments.
Quantification of L1 protein and L1 VLPs by ELISA.
The levels of expression of the six HPV-16 L1 proteins were determined by ELISA. Insect cells infected with recombinant baculoviruses at a MOI of 20 were sonicated and used as sources of antigen for the determination of L1 protein production. The lysates were boiled in the presence of 1% sodium dodecyl sulfate and 100 mM dithiothreitol for 5 min. Lysates were diluted twofold in 0.1 M carbonate buffer (pH 9.6). An ELISA was performed as described above, and common-epitope rabbit antiserum (Novocastra Laboratories, Buckinghamshire, United Kingdom) and peroxidase-conjugated anti-rabbit antibody (Sigma) were used at a dilution of 1/1,000. The results are expressed as L1 antigen titers, which are the reciprocals of the last positive sample dilutions. The cutoff was determined by using an insect cell lysate obtained from cells infected with an irrelevant recombinant baculovirus carrying the capsid gene of the hepatitis B virus (unpublished data).
RESULTS
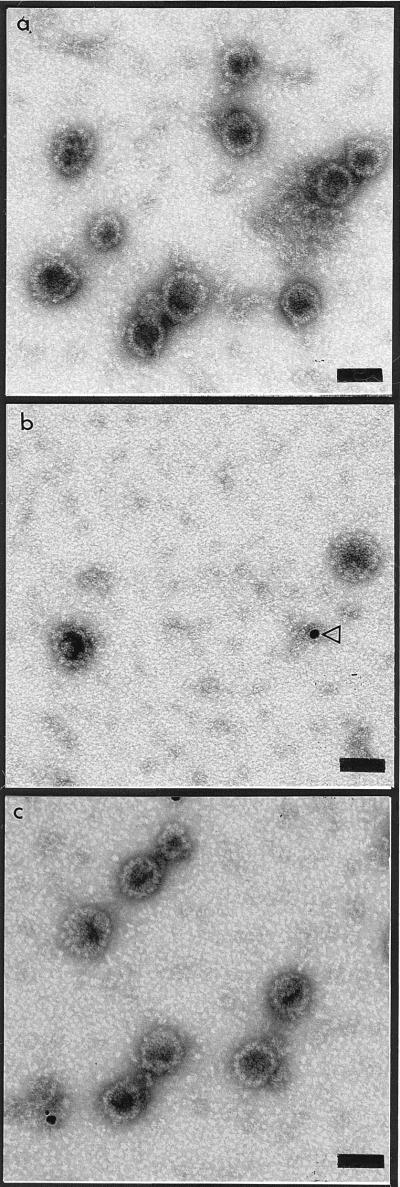
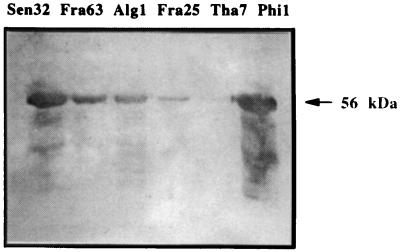
Electron micrographs showed VLPs with diameters of approximately 50 nm, consistent with the 55-nm diameter of other papillomavirus virions (Fig. 1), for all the six HPV-16 strains expressed in insect cells. Some tubular structures and some capsomer aggregates could also be identified. The VLPs were observed in CsCl gradient fractions with densities ranging from 1.27 to 1.30 g/cm3. The anti-HPV-16 L1 monoclonal antibody CamVir-1 recognized denatured recombinant L1 proteins as 56-kDa proteins from the six strains investigated by Western blot assay (Fig. 2). The intensity of the 56-kDa band varies according to the amount of protein expressed.
FIG. 1.
Electron micrographs of VLPs obtained by expression of the L1 genes of three different variants of HPV-16. (a) Phi1 VLPs; (b) Alg1 VLPs (the arrowhead indicates a gold bead used for the quantification of VLPs); (c) Sen32 VLPs. Bar, 50 nm.
FIG. 2.
Detection of L1 proteins from six different strains of HPV-16 by Western blotting using the CamVir-1 monoclonal antibody. L1 proteins were obtained by disruption of VLPs.
The levels of expression of L1 protein and VLPs were investigated by ELISA and by EM. The level of VLP expression ranged from 1 to 79, and the level of L1 protein expression ranged from 1 to 32, with perfect agreement between these two end points (Table 3). High levels of both L1 protein and VLPs were observed with strains Phi1, Sen32, and Fra63. In contrast, very low levels of L1 and VLPs were observed with strains Tha7 and Fra25. An intermediate level of expression of L1 and VLPs was observed for the Alg1 strain.
TABLE 3.
Comparison of the amounts of VLPs produced to the levels of L1 protein expressed for six HPV-16 strains
| Strain | Origin | L1 protein level
|
VLP amounta
|
||
|---|---|---|---|---|---|
| ELISA titer | Ratio | No. of VLPs/100 beads ± SD | Ratio | ||
| Phi1 | Philippines | 64,000 | 32 | 316 ± 8.5 | 79 |
| Tha7 | Thailand | 4,000 | 2 | 12 ± 1.4 | 3 |
| Alg1 | Algeria | 16,000 | 8 | 95 ± 14.1 | 24 |
| Sen32 | Senegal | 32,000 | 16 | 160 ± 5.6 | 40 |
| Fra25 | France | 2,000 | 1 | 4 ± 1.4 | 1 |
| Fra63 | France | 32,000 | 16 | 168 ± 8.5 | 42 |
As determined by EM.
Sequencing of the entire L1 genes of the six strains revealed that the L1 amino acid sequences of the strains differed by up to 15 amino acids from that of the HPV-16 prototype strain (Table 4). All had a mutation of His to Asp at position 202, in agreement with the results of Kirnbauer et al. (17), who had suggested that this mutation is a prerequisite for self-assembly of the L1 protein. The Phi1 strain was identical to the 114K strain (17). Three of the strains (Sen32, Fra25, and Fra63) were closely related and had eight identical mutations. It must be noted that the Sen32 and Fra63 strains were identical at the amino acid level. However, these two strains differed at the nucleotide level by 1 bp. Tha7 and Alg1 strains each revealed one specific mutation: the Tha7 strain had Arg in place of Pro at position 78, and the Alg1 strain had Trp in place of Arg in position 97.
TABLE 4.
Alignment of L1 protein amino acid sequences corresponding to the different strains used to produce recombinant VLPs with the sequence of the HPV-16 L1 prototype (27)a
| HPV-16 strain | L1 amino acid at positionb:
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 76 | 78 | 83 | 97 | 176 | 181 | 194 | 202 | 266 | 282 | 319 | 346 | 353 | 422 | 474 | |
| Prototype | His | Pro | Phe | Arg | Thr | As | Val | His | Thr | Ser | His | Ser | Ala | Thr | Leu |
| 114K | - | - | - | - | - | - | - | Asp | - | - | - | - | - | - | - |
| Phi1 | - | - | - | - | - | - | - | Asp | - | - | - | - | - | - | - |
| 114B | - | - | - | - | - | - | Ile | Asp | Ala | - | - | - | - | - | - |
| Tha7 | - | Arg | - | - | Asn | - | - | Asp | - | - | - | - | Pro | - | - |
| Alg1 | - | - | - | Trp | - | - | - | Asp | - | - | Arg | - | - | - | Phe |
| Z-1194 | Tyr | - | - | - | Asn | Thr | - | Asp | Ala | Pro | - | - | Pro | - | Phe |
| Sen32 | Tyr | - | - | - | Asn | - | - | Asp | Ala | - | Arg | Val | - | Ala | Phe |
| Fra25 | Tyr | - | Ser | - | Asn | - | - | Asp | Ala | - | Arg | Val | - | Ala | Phe |
| Fra63 | Tyr | - | - | - | Asn | - | - | Asp | Ala | - | Arg | Val | - | Ala | Phe |
The reactivities of human sera to the different VLPs in previously selected anti-HPV-positive and -negative samples, including the sera from 93 anti-HPV-16-positive women and 131 anti-HPV-16-negative women from 11 different countries (France, Spain, Algeria, Tunisia, Senegal, Uganda, Burundi, Gabon, Tanzania, Colombia, and Philippines), were investigated (Table 2). Sera were investigated for anti-VLP antibodies with VLPs derived from HPV-16 variants Sen32, Phi1, and Alg1. Due to the low level of production of VLPs by strains Fra25 and Tha7, these antigens were not used in this comparison. Moreover, VLPs from the Fra63 strain were not used because its amino acid sequence is identical to that of the Sen32 strain.
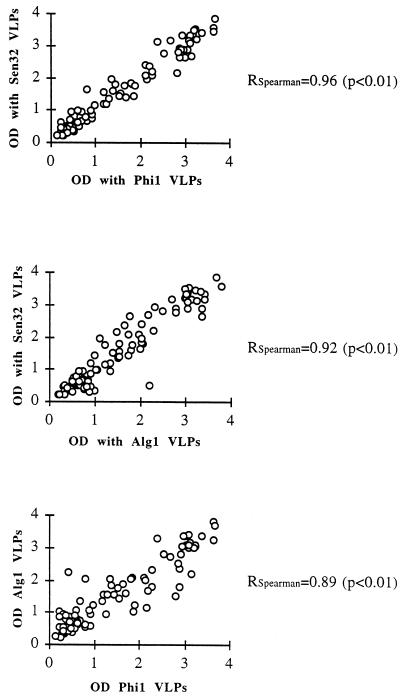
All sera which reacted with the Sen32 variant also reacted with the two other strains, except for one weakly positive serum which gave negative results with the other two VLP variants, with, however, OD values close to the cutoff (Fig. 3). Accordingly, all samples found to be seronegative when tested with the Sen32 VLPs were also found to be negative when tested with the two other HPV-16 strains, except for one sample which was positive, with an OD value close to the cutoff, with the Alg1 VLPs. This sample gave negative results with the other two strains but had OD values close to the cutoff.
FIG. 3.
Reactivities of 93 sera from women of 11 countries to HPV-16 VLPs obtained from Alg1, Phi1, and Sen32 strains (the cutoff is set at OD = 0.200). Correlation coefficients (RSpearman) were determined by Spearman’s method.
The HPV-16 ELISA OD values obtained with the three HPV strains are shown in Fig. 3. Agreement of the results for the different strains of HPV-16 used as antigens was very good. The correlation coefficients were 0.96 (Sen32 versus Phi1; P < 0.01), 0.92 (Sen32 versus Alg1; P < 0.01), and 0.89 (Alg1 versus Phi1; P < 0.01). The anti-VLP seroreactivities of the three HPV-16 strains were also analyzed with respect to the geographical origins of the sera investigated by using the data from groups with at least five anti-Sen32 VLP-positive patients (Table 5). The analysis indicates that comparisons of the seroreactivities of origin-grouped sera with the three different HPV-16 VLPs exhibit Spearman’s correlation coefficients ranging from 0.78 to 1 (P < 0.01). However, reactivities of sera from Tunisia with Sen32 and Alg1 strains had a correlation coefficient of 0.82 (P < 0.05) and the reactivities of the same sera with Alg1 and Phi1 strains had a correlation coefficient of 0.60 (not significant).
TABLE 5.
Comparison of anti-VLP seroreactivities of sera from seven countries to Phi1, Alg1, and Sen32 VLPs
| Origin of sera (n) | Spearman’s correlation coefficient (P value) for:
|
||
|---|---|---|---|
| Phi1 vs Sen32 | Sen32 vs Alg1 | Phi1 vs Alg1 | |
| France (16) | 0.93 (<0.01) | 0.94 (<0.01) | 0.94 (<0.01) |
| Spain (6) | 0.94 (<0.01) | 0.94 (<0.01) | 1.00 (<0.01) |
| Tunisia (7) | 0.89 (<0.01) | 0.82 (<0.05) | 0.60 (NSa) |
| Senegal (15) | 0.97 (<0.01) | 0.99 (<0.01) | 0.97 (<0.01) |
| Burundi (10) | 0.94 (<0.01) | 0.96 (<0.01) | 0.97 (<0.01) |
| Gabon (19) | 0.93 (<0.01) | 0.90 (<0.01) | 0.88 (<0.01) |
| Philippines (10) | 0.98 (<0.01) | 0.78 (<0.01) | 0.72 (<0.05) |
NS, not significant.
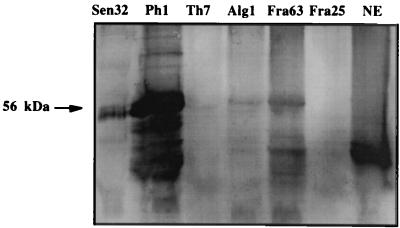
L1 proteins from the six HPV-16 strains were used to investigate DNA binding to L1 protein by Southwestern blotting using a digoxigenin-labeled DNA probe (Fig. 4). DNA binding to L1 protein (56 kDa) was detected for five of the strains investigated, with strong signals observed with Phi1, Sen32, and Fra63; the signal strength was related to the L1 protein content observed by ELISA and EM. Only a weak DNA-binding signal was observed with the Tha7 strain sample in which the L1 protein content was 8- to 16-fold less than those for the other strains, and DNA binding could not be observed with the Fra25 sample, in which the protein content was 16- to 32-fold lower than those for the Ph1, Sen32, and Fra63 strains. No DNA binding was observed with BSA (data not shown), and a strong signal was observed with the insect cell nuclear extract, but the signal corresponded to proteins of lower molecular weight.
FIG. 4.
Detection of DNA binding to HPV-16 L1 proteins in the six strains by Southwestern blotting using a digoxigenin-labelled probe. NE, insect cell nuclear extract.
DISCUSSION
Expression of the L1 capsid protein by six different HPV-16 strains from five different countries in a baculovirus system resulted in the formation of VLPs for all strains investigated. However, the yield of VLPs obtained varied from 1 to 79 depending on the HPV-16 strain and L1 gene sequence. These relative levels of expression of the L1 protein were obtained 4 days after infection of the insect cells by the recombinant baculovirus. This time period was chosen from previous experiments with the Sen32 strain. However, it is possible that the relative levels of recombinant protein would be different if they were investigated at another period after infection. Mutations in the amino acid region from residues 83 to 97 seem to affect the level of expression of L1 proteins but not their ability to self-assemble into VLPs. For example the only difference between the L1 proteins of strains Fra63 and Fra25 that has been detected is the replacement of Phe at position 83 by Ser. No other difference is encoded by the L1 genes of these two strains. However, Fra63 strains yielded 16 times more L1 protein and 42 times more VLPs than the Fra25 strain. It could thus be speculated that this mutation at position 83 in the Fra25 strain was related to the decrease in L1 protein production. Mutating strain Fra25 at position 83 to convert the Ser to Phe by site-directed mutagenesis would validate this hypothesis. It is clear from the present results that only the amount of L1 produced is variable depending on the strain of HPV-16, indicating that a point mutation in the L1 gene could be related to the amount of L1 protein produced, thus affecting the VLP yield. It could be speculated that with some strains the decreased expression of L1 is the result of truncated L1 RNA transcripts, as reported by Neeper et al. (21) for HPV-11 L1 expression in Saccharomyces cerevisiae. These results are important for the development of vaccines and serological tests. They suggest that strains inducing the production of high levels of VLP must be selected for the development of vaccines and anti-HPV-16 antibody detection tests.
In addition, we have shown by Southwestern blotting that the L1 protein of HPV-16 has the ability to bind to DNA. This result confirms those of two recent studies concerning HPV-11 and HPV-33, indicating that not only L2 but also L1 has the ability to interact with DNA (19, 29).
One of the goals of the study was to investigate further the serologic reactivity between HPV-16 strains in order to determine whether more than one serotype exists for this virus genotype, as has been observed for HPV-5 (11). The serologic reactivities of strains from Senegal, Algeria, and the Philippines with 93 anti-HPV-16-positive and 131 anti-HPV-16-negative sera from women from 11 different countries were investigated. Random assay variability was believed to be responsible for the divergent results observed with two of these sera, which gave OD values close to the cutoff. Moreover, we have found that sera from different countries react similarly to the three HPV-16 VLPs derived from virus strains isolated in Senegal, Algeria, and the Philippines. We conclude that strains Sen32, Phi1, and Alg1 are serologically cross-reactive despite a relatively large number of differences in the amino acids of the L1 proteins from these three strains. These data confirm those of Cheng et al. (7), indicating that HPV-16 strains belong to the same serotype.
These results also suggest that an ELISA based on a single variant could be used to evaluate anti-HPV-16 L1 antibodies in populations from different countries.
It has been reported that formalin-inactivated bovine papillomaviruses (BPV) are effective as a vaccine for the prevention of experimental BPV infection in calves (22). In a canine model, immunization with formalin-inactivated canine oral papillomavirus (COPV) or baculovirus-expressed recombinant COPV VLPs protects dogs against COPV challenge and natural infection (2, 27). Moreover, immunization of rabbits with VLPs composed of the cottontail rabbit papillomavirus (CRPV) L1 major capsid protein, expressed in the baculovirus expression system or in S. cerevisiae, has recently been shown to protect rabbits against CRPV challenge (4, 10, 15). These results indicate that the L1 protein self-assembled into VLPs (12, 16, 17, 30) has the potential for the development of a subunit vaccine effective against naturally transmitted papillomavirus infection and related malignant lesions. Our results indicate that VLPs from a single HPV-16 variant might be effective worldwide for the prevention of HPV-16 infections. However, the test used did not measure neutralizing antibodies, and substitutions within the L1 gene that affect the amino acid sequence may be important in virus escape from neutralizing antibodies induced by vaccination. The possibility of using VLPs from only one strain in an HPV-16 vaccine would be definitively confirmed by testing neutralizing antibodies against different HPV-16 pseudotypes which have recently been developed for HPV-16 and -33 (25, 29).
ACKNOWLEDGMENTS
This work was supported by grants from INSERM/MGEN and the Association pour la Recherche sur le Cancer (No. 5064). A. Touzé was supported by a grant from the Association pour la Recherche sur le Cancer.
REFERENCES
- 1.Baker T S, Newcomb W W, Olson N H, Cowsert L M, Olson C, Brown J C. Structures of bovine and human papillomavirus: analysis by cryoelectron microscopy and three-dimensional image reconstruction. Biophys J. 1991;60:1445–1456. doi: 10.1016/S0006-3495(91)82181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell J A, Sundberg J P, Ghim S J, Newsome J, Jenson A B, Schlegel R. A formalin-inactivated vaccine protects against mucosal papillomavirus infection: a canine model. Pathobiology. 1994;62:194–198. doi: 10.1159/000163910. [DOI] [PubMed] [Google Scholar]
- 3.Bonnez W, Rose R C, DaRin C, Borkhuis C, De Mesy Jensen K L, Reichman R C. Propagation of human papillomavirus type 11 in human xenografts using the severe combined immunodeficiency (SCID) mouse to the nude mouse model. Virology. 1993;197:455–458. doi: 10.1006/viro.1993.1611. [DOI] [PubMed] [Google Scholar]
- 4.Breitburd F, Kirnbauer R, Hubbert N L, Nonnenmacher B, Trin-Dinh-Demarquet C, Orth G, Schiller J T, Lowy D R. Immunization with virus-like particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J Virol. 1995;69:3959–3963. doi: 10.1128/jvi.69.6.3959-3963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan S Y, Ho L, Ong C K, Chow V, Drescher B, Dürst M, ter Meulen J, Villa L, Luande J, Mgaya H N, Bernard H U. Molecular variants of human papillomavirus 16 from four continents suggest pandemic spread of the virus and its coevolution with humankind. J Virol. 1992;66:2057–2066. doi: 10.1128/jvi.66.4.2057-2066.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan S Y, Bernard H U, Ong C K, Chan S C, Hofmann B, Delius H. Phylogenetic analysis of 48 papillomavirus types and 28 subtypes and variants: a showcase of the molecular evolution of DNA viruses. J Virol. 1992;66:5714–5725. doi: 10.1128/jvi.66.10.5714-5725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng G, Icenogle J P, Kirnbauer R, Hubbert N L, Louis M E, Han C, Svare E I, Krüger Kjaer S, Lowy D R, Schiller J T. Divergent human papillomavirus type 16 variants are serologically cross-reactive. J Infect Dis. 1995;172:1584–1587. doi: 10.1093/infdis/172.6.1584. [DOI] [PubMed] [Google Scholar]
- 8.Christensen N D, Kreider J W, Cladel N M, Patrick S D, Welsh P A. Monoclonal antibody-mediated neutralization of infectious human papillomavirus type 11. J Virol. 1990;64:5678–5681. doi: 10.1128/jvi.64.11.5678-5681.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christensen N D, Kreider J W. Antibody-mediated neutralization in vivo of infectious papillomavirus. J Virol. 1990;64:3151–3156. doi: 10.1128/jvi.64.7.3151-3156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christensen N D, Reed C A, Cladel N M, Han R, Kreider J W. Immunization with virus-like particles induces long-term protection of rabbits against challenge with cottontail rabbit papillomavirus. J Virol. 1996;70:960–965. doi: 10.1128/jvi.70.2.960-965.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Favre M, Ramoz N, Orth G. Human papillomavirus: general features. Clin Dermatol. 1997;15:181–198. doi: 10.1016/s0738-081x(97)00008-4. [DOI] [PubMed] [Google Scholar]
- 12.Hagensee M E, Yaegashi N, Galloway D A. Self-assembly of human papillomavirus type 1 capsids by expression of the L1 protein alone or by coexpression of the L1 and L2 capsid proteins. J Virol. 1993;67:315–322. doi: 10.1128/jvi.67.1.315-322.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho L, Chan S Y, Burk R D, Das B C, Fujinaga K, Icenogle J P, Kahn T, Kiviat N, Lancaster W, Mavromara-Nazos P, Labropoulou V, Mitrani-Rosenbaum S, Norrild B, Radhakrishna-Pillai M, Stoerker J, Syrjaenen K, Syrjaenen S, Tay S K, Villa L L, Wheeler C M, Williamson A L, Bernard H U. The genetic drift of human papillomavirus type 16 is a means of reconstructing prehistoric viral spread and the movement of ancient human populations. J Virol. 1993;67:6413–6423. doi: 10.1128/jvi.67.11.6413-6423.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International Agency for Research on Cancer. Monographs on the evaluation of the carcinogenic risk of chemicals to humans. 64. Human papillomavirus. Lyon, France: International Agency for Research on Cancer; 1995. [Google Scholar]
- 15.Jansen K U, Rosolowsky M, Schultz L D, Markus H Z, Cook J C, Donnelly J J, Martinez D, Ellis R W, Shaw A R. Vaccination with yeast-expressed cottontail rabbit papillomavirus (CRPV) virus-like particles protects rabbits from CRPV-induced papilloma formation. Vaccine. 1996;13:1509–1514. doi: 10.1016/0264-410x(95)00103-8. [DOI] [PubMed] [Google Scholar]
- 16.Kirnbauer R, Booy F, Cheng N, Lowy D R, Schiller J T. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are immunogenic. Proc Natl Acad Sci USA. 1992;89:12180–12184. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirnbauer R, Taub J, Greenstone H, Roden R, Dürst M, Gissmann L, Lowy D R, Schiller J T. Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. J Virol. 1993;67:6929–6936. doi: 10.1128/jvi.67.12.6929-6936.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Cann P, Coursaget P, Iochman S, Touzé A. Self-assembly of human papillomavirus type 16 capsids by expression of the L1 protein in insect cells. FEMS Microbiol Lett. 1994;117:269–274. doi: 10.1111/j.1574-6968.1994.tb06778.x. [DOI] [PubMed] [Google Scholar]
- 19.Li M, Cripe T P, Estes P A, Lyon M K, Rose R C, Garcea R L. Expression of the human papillomavirus type 11 L1 capsid protein in Escherichia coli: characterization of protein domains involved in DNA binding and capsid assembly. J Virol. 1997;71:2988–2995. doi: 10.1128/jvi.71.4.2988-2995.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreland R B, Montross L, Garcea R L. Characterization of the DNA-binding properties of the polyomavirus capsid protein VP1. J Virol. 1991;65:1168–1176. doi: 10.1128/jvi.65.3.1168-1176.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neeper M P, Hofmann K J, Jansen K U. Expression of the major capsid protein of human papillomavirus type 11 in Saccharomyces cerevisae. Gene. 1996;180:1–6. doi: 10.1016/s0378-1119(96)00388-5. [DOI] [PubMed] [Google Scholar]
- 22.Olson C, Segre D, Skidmore L V. Further observations on immunity to bovine cutaneous papillomatosis. Am J Vet Res. 1960;21:233–238. [PubMed] [Google Scholar]
- 23.Pisani P, Parkin D M, Munoz N, Ferlay J. Cancer and infection: estimates of the attributable fraction in 1990. Cancer Epidemiol Biomark Prev. 1997;6:387–400. [PubMed] [Google Scholar]
- 24.Roden R B S, Greenstone H L, Kirnbauer R, Booy F P, Jessie J, Lowy D R, Schiller J T. In vitro generation and type-specific neutralization of a human papillomavirus type 16 virion pseudotype. J Virol. 1996;70:5875–5883. doi: 10.1128/jvi.70.9.5875-5883.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sasagawa T, Pushko P, Steers G, Gschmeissner S E, Hajibagheri M A N, Finch J, Crawford L, Tommasino M. Synthesis and assembly of virus-like particles of human papillomaviruses type 6 and type 16 in fission yeast Schizosaccharomyces pombe. Virology. 1995;206:126–135. doi: 10.1016/s0042-6822(95)80027-1. [DOI] [PubMed] [Google Scholar]
- 26.Seedorf K, Krammer G, Dürst M, Suhai S, Rowenkamp W. Human papillomavirus type 16 DNA sequence. Virology. 1985;145:181–185. doi: 10.1016/0042-6822(85)90214-4. [DOI] [PubMed] [Google Scholar]
- 27.Suzich J A, Ghim S J, Palmer-Hill F, White W I, Tamura J K, Bell J A, Newsome J A, Jenson A B, Schlegel R. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci USA. 1995;92:11553–11557. doi: 10.1073/pnas.92.25.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trus B L, Roden R B S, Greenstone H L, Schiller J T. Novel structural features of bovine papillomavirus capsid revealed by a three-dimensional reconstruction to 9 Å resolution. Nat Struct Biol. 1997;4:413–420. doi: 10.1038/nsb0597-413. [DOI] [PubMed] [Google Scholar]
- 29.Unckell F, Streeck R E, Sapp M. Generation and neutralization of pseudovirions of human papillomavirus type 33. J Virol. 1997;71:2934–2939. doi: 10.1128/jvi.71.4.2934-2939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou J, Sun X Y, Stenzel D J, Frazer I H. Expression of recombinant HPV-16 L1 and L2 ORF proteins in epithelial cells is sufficient for assembly of HPV virion-like particles. Virology. 1991;185:251–257. doi: 10.1016/0042-6822(91)90772-4. [DOI] [PubMed] [Google Scholar]
- 31.zur Hausen H. Papillomaviruses in anogenital cancer as a model to understand the role of viruses in human cancers. Cancer Res. 1989;49:4677–4681. [PubMed] [Google Scholar]