Abstract
Autism spectrum disorders (ASD) are a complex sequelae of neurodevelopmental disorders which manifest in the form of communication and social deficits. Currently, only two agents, namely risperidone and aripiprazole have been approved for the treatment of ASD, and there is a dearth of more drugs for the disorder. The exact pathophysiology of autism is not understood clearly, but research has implicated multiple pathways at different points in the neuronal circuitry, suggesting their role in ASD. Among these, the role played by neuroinflammatory cascades like the NF-KB and Nrf2 pathways, and the excitotoxic glutamatergic system, are said to have a bearing on the development of ASD. Similarly, the GPR40 receptor, present in both the gut and the blood brain barrier, has also been said to be involved in the disorder. Consequently, molecules which can act by interacting with one or multiple of these targets might have a potential in the therapy of the disorder, and for this reason, this study was designed to assess the binding affinity of taurine, a naturally-occurring amino acid, with these target molecules. The same was scored against these targets using in-silico docking studies, with Risperidone and Aripiprazole being used as standard comparators. Encouraging docking scores were obtained for taurine across all the selected targets, indicating promising target interaction. But the affinity for targets actually varied in the order NRF-KEAP > NF-κB > NMDA > Calcium channel > GPR 40. Given the potential implication of these targets in the pathogenesis of ASD, the drug might show promising results in the therapy of the disorder if subjected to further evaluations.
Keywords: Autism spectrum disorder, Glutamate, Calcium-channels, NF-KB, Nrf2, Taurine
Highlights
-
•
Binding affinity of taurine evaluated against ASD-related targets in-silico.
-
•
Binding affinity followed the order NRF-KEAP > NF-κB > NMDA > Calcium channel > GPR40.
-
•
Taurine showed promising docking scores against NMDA receptors.
-
•
These scores were comparable to risperidone and higher than aripiprazole.
-
•
Taurine may as such be a promising candidate for binding to NMDA receptors in ASD.
-
•
Docking scores over other receptors also indicated its potential as an adjunct therapy.
1. Introduction
Autism spectrum disorders (ASD) are known to be a complex set of neurodevelopmental sequelae resulting from the interplay between numerous responsible factors, which together contribute to deficits in communication and social interaction, often accompanied by stereotypical behaviors and other defects in general neuronal functions. The symptoms of autism can run the gamut from mild and high-functioning forms (which require no to minor assistance) to severe or low functioning forms requiring significant and often lifelong assistance. Hence the name autism “spectrum" disorders (Diagnostic and Statistical Manual of Psychiatric Disorders, 2013). Additionally, many other behavioral anomalies, such as depression, anxiety and hyperactivity, have also been associated with autism (Hudson et al., 2019, Leitner et al., 2014, Vasa and Mazurek, 2015). Currently, the global incidence of the disorder stands at 1 in 100 children (World Health Organization, 2022), with 1 in every 36 children reporting suffering from ASD in the United States (Maenner et al., 2023). With the statistics already very high and growing at an alarming rate, the disorder now presents a problem that requires urgent attention.
While the understanding of the factors that play a role in the development of autistic phenotypes has been growing steadily over the last few years owing to an increase in the number of children being diagnosed with the disorder as well as enhanced interest in the same, the exact pathways that are involved in the pathogenesis are still not completely defined. Environmental and social factors have been said to play important roles in the generation of autistic symptoms, and a genetic predisposition has also been implicated. Dysfunction of pathways involved in normal neuronal signaling is believed to be an important contributor to the development of an autistic phenotypes, and a number of signaling cascades have been correlated to signs of autism.
1.1. Pathways suggested to be involved in ASD
Primarily, damage to neuronal cells mediated through overactivation of pathways like the ERK/MAPK (Tidyman and Rauen, 2009), PI3K/Akt (Sharma and Mehan, 2021), NRF2/NFKB (Canning et al., 2015; Young et al., 2011), and glutamatergic cascades (Brown et al., 2013), among others, has been found to have a direct relation to autism-related symptoms, and the mechanisms through which this is brought about can be far and varied, but are believed to occur mainly through processes like excitotoxicity, generation of reactive oxygen species, and neuroinflammation (Eissa et al., 2020) through an increase in the levels of cytokines and other mediators, among others.
The contribution of these factors to the generation of autistic symptoms can occur at different levels of molecular signaling, and interestingly, all of these pathways ultimately converge in damage to neuronal cells through apoptotic cascades. These cascades can in turn be activated by multiple mechanisms, the most commonly encountered of which are inflammatory pathways mediated through NFKB and NRF2 (Canning et al., 2015; Eissa et al., 2020; Yan et al., 2015; Young et al., 2011), as well as the calcium-induced (Nguyen et al., 2018) excitotoxic systems helmed mainly by NMDA-glutamate signaling and the attending calcium influx (Gargus and Schmunk, 2014; Nguyen et al., 2018; Rojas, 2014).
In terms of neuroinflammation, the NF-KB cascade is well-known. The NF-ΚB pathway is an inflammatory cascade which has a key role in neuroinflammatory processes (Yan et al., 2015; Young et al., 2011), and its presence has been detected in different regions of the brains of ASD subjects (Malik et al., 2011). While it is debatable whether this molecule is a primary contributor to the pathogenesis of autism or has more of a role in the later stages, it is now considered to be important in the etiology of the disorder. As such, therapeutic agents which can bind to and inhibit this pathway may have potential in the treatment of ASD.
Similarly, The Nrf2 pathway involves a transcription factor controlling the expression of genes playing a role in cytoprotection. This cascade is under negative regulation by different molecules, among which, the Kelch-like ECH-associated protein 1 (KEAP1) is the main regulator, mediating degradation of the factor through ubiquitination. This pathway has been found to be implicated in the synthesis and homeostasis of a number of antioxidants, including glutathione, catalase and NADPH, among others (Canning et al., 2015). Oxidative stress is believed to play a role in the disorder (Giulivi et al., 2010; Tang et al., 2013), and the same may be mediated through imbalance of this cascade. As such, binding of agents which can restore this balance may be a potential therapeutic strategy.
Importantly, the Nrf2 and NF-KB pathways are not isolated, but rather occur in tandem with each other, displaying a delicate balance between their functioning and activity. This is because on the one hand, Nrf2 has the capacity of downregulating the expression of NF-KB by causing an elevation in the levels of multiple antioxidant molecules, while on the other hand, NF-KB is also directly involved in inhibiting the expression of Nrf2. In the case of autism, this balance is tipped to one side, resulting eventually in an increased expression of the inflammatory factor while the levels of Nrf2 are seen to decline. So, therapeutics acting on even one of these pathways will likely show an effect on the functioning of both these cascades (Shah et al., 2023).
Glutamate signaling is one of the most important pathways that has been implicated in the pathophysiology of autism (Rojas, 2014), and has been said to be involved in causing damage to neuronal cells mainly through excitotoxic processes (Essa et al., 2013). While baseline functioning of glutamate receptors is essential for normal synaptic plasticity, an elevation in the same (Brown et al., 2013, Page, 2016) is suggested to be responsible for causing cell death through apoptotic pathways, with calcium overload through an increase in the activity of calcium channels (Gargus and Schmunk, 2014, Liao et al., 2020, Nguyen et al., 2018) being an important route for excitotoxicity (Essa et al., 2013). This calcium overload, in turn, can be mediated through the stimulation of intracellular calcium channels, or the voltage-gated channels present on the cell surface. These elevated levels of calcium are then known to interact with multiple downstream molecules such as calcium calmodulin-dependent kinase II (CamKII) and calcinurein, bringing about the activation of inflammatory and apoptotic cascades through the activity of certain pro-inflammatory mediators. Mitochondrial dysfunction and generation of reactive oxygen species (ROS) follow, ultimately resulting in neuronal cell (Creamer, 2020, Hudmon and Schulman, 2002, Palmieri et al., 2010). Given this context, it is reasonable to believe that agents which can inhibit and restore the normal functioning of glutamate receptors and/or calcium channels may be beneficial in ASD.
Finally, the role of GPR40 in autism is through its importance in the maintenance of intestinal integrity (Usuda et al., 2021) and neuronal function (Yang et al., 2018). Bacteria present in the gut microbiota are known to convert sugars and fibers present in the diet to short chain fatty acids (SCFAs) like acetate, propionate and butyrate, which are involved in physiological processes like gluconeogenesis, as well as certain inflammatory processes like the activation of NLRP inflammasomes which are important in maintaining gut integrity through the stimulation of interleukin receptors (particularly IL-8 activated by NLRP3). Improper function of these GPRs can result in increased intestinal permeability (Usuda et al., 2021), leading to the release of toxins into the bloodstream, which has been implicated in the etiology of autism through neuroinflammation and immunoexcitotoxic processes (Strunecka et al., 2018). Additionally, the digestive tract-associated problems in ASD include leaky gut syndrome, further consolidating the relation. Interestingly, it has also been suggested that GPR40 is expressed in the central nervous system, and affects the regulation of neuronal function, including in particular, the function of NMDA receptors, which again, might be connected to their role in ASD (Yang et al., 2018). As such, molecules showing affinity for GPR40 might also be promising candidates for treatment of ASD.
Keeping these findings in mind, we proposed assessing the docking scores and binding affinity of taurine against these target molecules, so as to suggest potential strategies for the therapy of ASD.
2. Material and methods
2.1. Modeling platform
The docking study was performed using the Protein Preparation Wizard,LigPrep,Receptor Grid Generation, Ligand Docking, Glide modules of Maestro 12.3 version of the Schrodinger suite (Schrödinger, LLC, New York, NY, USA).
2.2. Protein preparation
The crystal structures of the target receptor binding sites of NF-KB (PDB ID-3GUT), NRF-Keap, (PDB ID-5WVF), NMDA (PDB ID-5EWJ), Calcium channel (PDB ID-6KZP), GPR 40 (PDB ID-4PHU) were retrieved from the RCSB Protein Data Bank (http://www.rcsb.org/pdb/home/home.do). The proteins were prepared by using the Protein Preparation Wizard. In general, the wizard offers two gears involving, preparation and refinement of the protein structure. The protein structures were refined for their bond orders, formal charges and missing hydrogen atoms, topologies. The water molecules beyond 5 Å from proteins were removed. The possible ionization states were generated in the protein structure and the most stable state was chosen. The hydrogen bonds were assigned and orientations of the retained water molecules were corrected. Finally, a restrained minimization of the protein structure was carried out using OPLS2005 force field with default Root Mean Square Deviation (RMSD) value of 0.30.
2.3. Active site prediction and receptor grid generation
The active site (binding pockets) and functional residues of different proteins were identified and characterized by Site-Map module from Schrodinger package. SiteMap calculation began with an initial search step that helped us in identification or characterization of one or more regions on the protein surface that are suitable for binding ligands to the receptor. The Site- Map module generates a number of active binding pockets and based on the parameters including site-score the best pocket for receptors were identified. Then, the shape and properties of these best site were computed by generating a grid box. A Gride box was produced to characterize the centroid of the dynamic site which is utilized for docking by utilizing the Glide grid generation wizard. The Glide grid box measurements (the centroid of a docked posture) of the protein were set to 20 × 20 × 20 Å around the pocket.
2.4. Ligand preparation
The two-dimensional (2D) structures of the ligands were drawn by 2D sketcher panel in the workspace of the Maestro 12.3. These 2D structures were converted into their corresponding three-dimensional (3D) structures. Hydrogens were added and their energy was reduced to obtain most stable conformation using Macromodel's Minimization module. The minimized ligand was then optimized by using the Optimized Potentials for Liquid Simulations-2005 (OPLS-2005) force field in a Schrodinger suit at 7.4 pH until an RMSD of 1.8 A° was achieved in the LigPrep module. The optimised ligands were selected for docking analysis with the receptor..
Fig. 1.
Diagrammatic hypothesis of the present study.
3. Theory
Despite the growing number of children across the globe being diagnosed with autism spectrum disorder, very limited drugs and therapeutic agents are currently available for the treatment of the disorder. Currently, drugs which can alleviate behavioral symptoms such as irritability are the first line of treatment for ASD, and these include agents like risperidone (3-[2-[4-(6-fluoro-1,2- benzisoxazol-3-yl)− 1-piperidinyl]ethyl]− 6,7,8,9-tetrahydro-2-methyl-4 H-pyrido[1,2- a]pyrimidin-4-one (Mano-Sousa et al., 2021) and aripiprazole (7-[4-[4-(2,3-dichlorophenyl)− 1- piperazinyl]butoxy]− 3,4-dihydrocarbostyril) (Blankenship et al., 2010). Nevertheless, research has been continuing towards developing therapeutic strategies for autism, and focus is being laid on a range of signaling pathways, with some receiving more attention than the others. Among them are included the NF-κB (Shih et al., 2015, Young et al., 2011) and NRF2-Keap-mediated (Canning et al., 2015) inflammatory cascade, the NMDA-glutamatergic (Rojas, 2014) and calcium-mediated excitation pathways (Liao et al., 2020), and the free fatty acid receptor 1 (FFAR1/GPR40) receptor-mediated cascades (Usuda et al., 2021; Yang et al., 2018). In the present research, we put forward the hypothesis that the inhibition of these pathways by taurine can alleviate the neurological deficits seen in ASD..
Fig. 2.
(a): Structure of risperidone, (b): Structure of aripiprazole, (c): Structure of taurine.
Taurine (2-aminoethanesulfonic acid) is a naturally-occurring sulfur-containing amino acid, which is the most abundantly present free amino acid in the human body. Responsible for multiple processes in the body, it is known to bring about inhibition of the above-mentioned pathways at least to some extent, and this forms the basis of our hypothesis that it may show potential as a therapeutic for autism. A conditionally essential amino acid, it has found use as a neuroprotectant, owing mainly to its activities as an anti-inflammatory agent (Marcinkiewicz and Kontny, 2014) and in the regulation of electrolytes, particularly the levels of calcium (Liu et al., 2006). It is believed to reduce calcium levels inside the cells, and block certain apoptotic pathways as well, thereby preventing neuronal damage (Liu et al., 2006; Wu and Prentice, 2010) through excitotoxic and inflammatory processes.
In particular, taurine has been found to exert significant effect on glutamatergic pathways (Bulley and Shen, 2010) and put a stop to glutamate-induced excitotoxicity through blockade of an intracellular calcium spike. And since this calcium spike is often responsible for the activation of apoptotic cascades, the agent can well be believed to have a positive effect on neurons, the damage to which is implicated in neurological disorders including autism. Similarly, apoptosis is also mediated by neuroinflammatory pathways like the NF-KB system, and since taurine has a major effect in preventing this apoptosis (Wu and Prentice, 2010), it may be worth assessing if it has any effect on this molecule. And with the Nrf2 pathway working in the opposite direction to NF-KB, the potential effect drugs for autism may have on this cascade is also worth studying. The GPR40 receptor has a role in autism through the indirect induction of neuroinflammation (Eissa et al., 2020), and so, blockade of this receptor too might exert a neuroprotective effect, giving it credibility as a potential pathway through which autism might be tackled.
At the same time, despite the potential implications of these cascades in ASD, a majority of them have not been explored as targets for drugs aimed at the therapy of autism. Moreover, with the growing incidence of the disorder across the globe, there is an increasing need for drugs to tackle the symptoms of ASD, but the same have not yet been developed since the pathways involved continue to remain elusive. And most drugs that are available only focus on symptomatic relief (risperidone and aripirazole). Since taurine is known to have an efficacy in maintaining neuronal health and countering the imbalances in at least a few of these pathways, and owing to its relatively favorable safety profile, we chose this agent for the present study. As such, in silico docking studies with these receptors were conducted in comparison to risperidone and aripiprazole as standards to establish data regarding target binding.
3.1. Evaluation of hypothesis idea
The Evaluation of the hypothesis idea was carried out through structure-based computer-aided drug design (CADD) and molecular docking. CADD makes use of computational methods to search, synthesize, and validate pharmaceutic agents. These methods are chiefly divided into three broad categories, with the first being the discovery stage where potential drug targets and active compounds (“hits") are identified. The second stage encompasses preclinical studies and drug development. Finally, the registration of medications for clinical trials also makes use of CADD on some levels, and hence can be included as the third major category (Lavecchia and Cerchia, 2016). Drug discovery is increasingly making use of CADD at various stages, from target recognition and validation, molecular synthesis, as well as target interaction, and has greatly improved industry economics in terms of cost and time (Kapetanovic, 2008). Molecular docking, in particular, is a method through which tiny ligands are docked into macromolecular proteins, and their complimentary values are scored against binding sites (Saikia and Bordoloi, 2018).
4. Results and discussion
In order to investigate the binding capacity of taurine in comparison to available reference drugs on selected proteins related to autism in humans, we docked them using Glide module of Schrodinger suite of Maestro 12.3. So, the docking results collected in the form of docking scores, types of interactions involved, and interacting residues, are shown in the Table 1, Table 2, supported with Fig. 3, Fig. 4, Fig. 5 illustrating 2D and 3D ligand-protein interaction diagrams..
Fig. 6.
(a): 2D representation showing the interaction of Taurine with active amino residues of NMDA receptor (- - - - - - hydrogen bonds). (b): 3D representation of predicted binding modes of the Taurine.
Fig. 7.
(a): 2D representation showing the interaction of Aripiperazole with active amino residues of NMDA receptor (- - - - - - hydrogen bonds,- - - - - - - aromatic). (b): 3D representation of predicted binding modes of the Aripiperazole.
Fig. 8.
(a): 2D representation showing the interaction of Risperidone with active amino residues of NMDA receptor (- - - - - - hydrogen bonds, - - - - - - - aromatic). (b): 3D representation of predicted binding modes of the Risperidone.
Table 1.
Site score of different proteins predicted by Sitemap tool.
| Protein Site | Rank | Site score |
||||
|---|---|---|---|---|---|---|
| NF-κB | NRF-Keap | NMDA | Calcium channel | GPR-40 | ||
| 1 | 1 | 1.040 | 1.107 | 1.063 | 1.142 | 1.022 |
| 2 | 2 | 1.035 | 1.094 | 1.019 | 1.043 | 0.968 |
| 3 | 3 | 1.029 | 0.993 | 1.018 | 1.032 | 0.637 |
| 4 | 4 | 1.009 | 0.979 | 0.99 | 1.030 | - |
| 5 | 5 | 0.992 | 0.871 | 0.962 | 1.005 | - |
Table 2.
Docking results of ligands with different receptors.
| Compounds | Docking score |
||||
|---|---|---|---|---|---|
| NF-κB (PDB:3GUT) |
NRF-Keap (PDB:5WFV) |
NMDA (PDB:5EWJ) |
Calcium channel (PDB: 6KZP) |
GPR-40 (PDB: 4PHU) |
|
|
Taurine (Test) |
-5.485 | -6.115 | -5.227 | -4.559 | -4.385 |
| Risperidone (Reference) | -7.732 | -9.184 | -4.259 | -6.487 | -4.005 |
|
Aripiperazole (Reference) |
-5.081 | -6.761 | -2.973 | -6.446 | -4.796 |
Fig. 3.
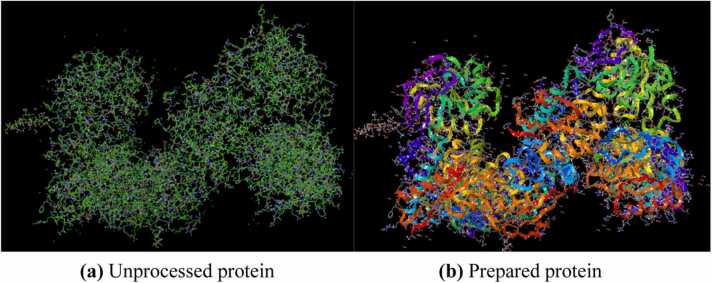
(a) Unprocessed protein (b) Prepared protein.
Fig. 4.
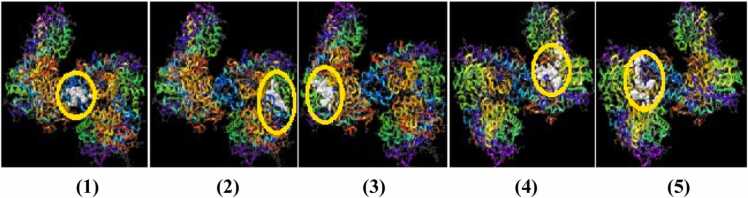
Five Potential binding pockets obtained by Sitemap analysis.
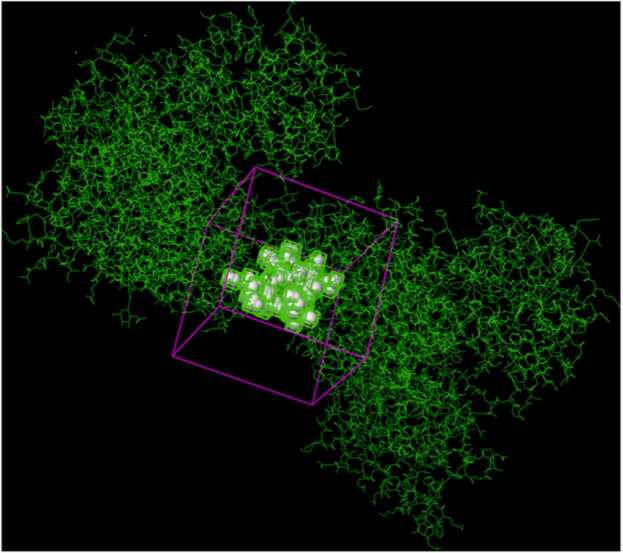
Fig. 5.
Receptor grid generation around the active site.
The x-ray crystallographic structure of selected targets were downloaded from the Protein Data Bank (PDB) and prepared using Protein Preparation Wizard in Schrodinger suite. Figs. 3a and 3b showed the unprocessed protein structure and the processod protein structure of NMDA respectively. Active site of the proteins was obtained using SiteMap tool, which provides a fast and effective means of identifying potential binding pockets of proteins. Five binding sites with different site score were identified as shown in Fig. 4 and Table 1. Site 1 with highest site score was used for further docking analysis and selected for Receptor grid generation. A grid was generated around the active site for effective binding as shown in Fig. 5. Glide docking tool of Schrodinger was used to dock the ligands to all the different proteins. In docking study binding affinity is being represented by scoring function known as docking score, that provides information about the extent of binding between the ligand and protein. The negative docking score corresponds to a strong binding and a less negative or even positive docking score corresponds to a weak or non-existing binding.
Encouraging outcomes of Taurine with negative docking score ranging from − 6.115 to − 4.385 (kcal/mol) indicating its binding potential for all the selected proteins (Table 2). But affinity for the selected protein varies and has been observed that Taurine showed binding potential with all the different receptors in the order of NRF-KEAP > NF-κB > NMDA > Calcium channel > GPR 40. But compared to all the receptors, taurine showed the highest docking score and good binding affinity with NRF-keap receptors. The high score of Taurine could be due to the presence of high numbers of amino acids residues involved in hydrogen bonding as compared to other receptors as shown in Table 3. Fig. 5 is indicating the established hydrogen bonding between the amine group and oxygen of sulphonyl group in Taurine with active amino acid residues VAL 512, VAL 465 and residues ILE 559, VAL 606, GLY 367 respectively. These interactions makes the ligands fit better within the pocket of the receptor NRF-Keap.
Table 3.
Types of interactions with active amino acid residues.
| Compounds | Receptors | No. of Residues | Hydrogen bonding | Aromatic bonding | Salt bridges |
|---|---|---|---|---|---|
| Taurine | NF-κB | 05 | ARG 354 | - | ARG 354 |
| Risperidone | 17 | ARG 354 | ASN 547 | - | |
| Aripiperazole | 22 | ARG 354 | - | - | |
| Taurine | NRF-Keap | 11 | VAL 512, VAL 465, ILE 559, VAL 606, GLY 367 | - | - |
| Risperidone | 32 | THR 560 | LEU 365 | - | |
| Aripiperazole | 33 | ARG 415 | VAL 561, LEU 365 |
- | |
| Taurine | NMDA | 08 | SER 319, LYS 327, GLU 316 |
- | LYS 327, GLU 316 |
| Risperidone | 15 | - | GLU 316 | GLU 316 | |
| Aripiperazole | 18 | - | PRO 317, GLU 316 |
- | |
| Taurine | Calcium channel | 08 | ASN 952, LYS 1462 | - | LYS 1462 |
| Risperidone | 18 | LYS 1462 | LEU 499 | - | |
| Aripiperazole | 17 | LYS 1462 | - | - | |
| Taurine | GPR-40 | 11 | GLU 65 | TYR 12 | LYS 62, GLU 65 |
| Risperidone | 21 | - | - | - | |
| Aripiperazole | 23 | SER 8 | LEU 2253 |
Further, when compared the results of taurine with reference drugs i.e Aripiperazole and Risperidone, it was found that taurine showed low docking score as compared to Risperidone except for NMDA receptor. Similarly for aripiperzole, taurine showed a comparable results with all the target receptors except for NMDA for which it showed high docking score. Comparable docking score of taurine with aripiperazole could be due to the involvement of more number and similar amino acid residues SER 319, LYS 318, PRO 317, LYS 327, VAL 465,ALA 466, ARG 354, GLU 316,LYS 1462, GLU 65, VAL 512, ILE 559. Taurine (selected ligand), was found to have good binding potentials with NMDA receptor by affording high docking score (−5.227) to reference drug risperidone (−4.259), aripiperazole (−2.973). This may be possible due to the absence of hydrogen bonding interactions with active site of amino acids in both the reference drugs. On the other hand, hydrogen bond interaction is present in Taurine with the amino acid of the active site namely SER 319, LYS 327, GLU 316 of the target protein NMDA. Further, these interactions make the taurine fit within the pocket of the receptor NMDA and indicated its potential for binding.
Taurine showed high binding with receptors NMDA to Aripiperazole and Risperidone, preferabledrug used for the treatment of autism. Taurine antagonizing NMDA pathway may show improvement in the patient by inhibiting this pathway and may help in improve neurological complications thereafter and may improve the patient’s quality of life.
5. Conclusions
Autism spectrum disorders (ASD) is a set of complex neurodevelopmental deficits manifesting in the form of issues with communication and social behavior, paired with repetitive actions. The exact mechanism at play is not well-understood, but an interaction between environmental and genetic factors has been implicated, potentially acting through neuroinflammatory and excitotoxic pathways. Till date, the number of therapeutic agents available for the therapy of ASD is very limited, with only two drugs being approved by the FDA for management of irritability in autistic patients. Literature suggests the potential role of a number of signaling pathways in the pathogenesis of the disorder, with particular emphasis being laid on the importance of the glutamatergic cascades and inflammatory processes. Impaired glutamate signaling has been implicated in excitotoxicity to neurons, through activation of calcium channels. Similarly, inflammation of neuronal cells is brought about through pathways such as the NF-KB cascade, and the same is indirectly antagonized by pathways such as the Nrf2 cascade. This inflammation may be triggered by agents like short-chain fatty acids, which leak into the brain owing to dysfunction of GPR40 receptors, which is noted in leaky gut, a hallmark of ASD. In this paper, we hypothesized the potential of Taurine in acting on these pathways and correcting their dysfunction as a therapeutic agent in autism, and put forward the idea of repurposing it for the same. In silico molecular docking studies, where tiny ligands are docked into macromolecular targets to score their complimentary values at binding sites, were used to back our hypothesis, with comparison against reference drugs risperidone and aripiprazole. During the same, we obtained encouraging results regarding the docking scores of taurine across all the target molecules involved in the study, hinting at a potentially promising interaction of the drug with these targets in the therapy of ASD. At the same time, the binding affinity was seen to go in the order of NRF-KEAP > NF-κB > NMDA > Calcium channel > GPR 40, and the best docking score and binding affinity was shown against Nrf-Keap. However, docking scores shown by taurine were less than those shown by risperidone and comparable to those shown by aripiprazole across all targets except against the NMDA receptor, where they were comparable and superior, respectively. As such, the interaction of taurine with NMDA receptors might be the key step in its potential application in ASD therapy.
With the current scenario in the pharmaceutical market being directed towards drug repurposing and polypharmacy to as opposed to developing new drugs due to the time crunch arising from the growing need for therapeutics for various disorders, including ASD, the present study was focused on providing a basis for potentially repurposing taurine for treatment of the disorder. Moreover, since this molecule has shown acceptable and promising binding scores to most of the potential targets used for the present study, the same can potentially be used as a starting point for planning future research, and help streamline procedures to save time and effort, as well as preventing the unnecessary death of animals during protcols.
CRediT authorship contribution statement
Dr. Ranjana Bhandari: Conceptualization, Methodology, Writing – review & editing, Funding acquistion, Resources. Dr. Neelima Dhingra: Conceptualization, Methodology, Software, Formal analysis, Resources. Dr. Anurag Kuhad: Supervision. Manasi Varma: Writing – original draft. Priyanka Rana: Investigation, Writing – original draft.
Declaration of Competing Interest
None.
Acknowledgements
The authors acknowledge the IBRO Early Career Grant 2020 awarded to Dr. Ranjana Bhandari.
Contributor Information
Ranjana Bhandari, Email: akb10in@yahoo.co.uk, rbhandari@pu.ac.in.
Neelima Dhingra, Email: neelimad08@pu.ac.in.
Anurag Kuhad, Email: anurag.kuhad@pu.ac.in, anurag_pu@yahoo.com.
References
- Blankenship, K., Erickson, C.A., Stigler, K.A., Posey, D.J., Mcdougle, C.J., 2010. Aripiprazole for irritability associated with autistic disorder in children and adolescents aged 6–17 years. [DOI] [PMC free article] [PubMed]
- Brown M.S., Singel D., Hepburn S., Rojas D.C. Increased glutamate concentration in the auditory cortex of persons with autism and first-degree relatives: a 1H-MRS study. Autism Res. 2013;6(1):1–10. doi: 10.1002/AUR.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulley S., Shen W. Reciprocal regulation between taurine and glutamate response via Ca 2+- dependent pathways in retinal third-order neurons. J. Biomed. Sci. 2010;17(1):1–15. doi: 10.1186/1423-0127-17-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning P., Sorrell F.J., Bullock A.N. Structural basis of Keap1 interactions with Nrf2. Free Radic. Biol. Med. 2015;88(B):101–107. doi: 10.1016/J.FREERADBIOMED.2015.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creamer T.P. Calcineurin. Cell Commun. Signal. 2020;18:137. doi: 10.1186/s12964-020-00636-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissa N., Sadeq A., Sasse A., Sadek B. Role of neuroinflammation in autism spectrum disorder and the emergence of brain histaminergic system. Lessons also for BPSD? Front. Pharmacol. 2020;11:886. doi: 10.3389/FPHAR.2020.00886/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essa M.M., Braidy N., Vijayan K.R., Subash S., Guillemin G.J. Excitotoxicity in the pathogenesis of autism. Neurotox. Res. 2013;23(4):393–400. doi: 10.1007/S12640-012-9354-3. [DOI] [PubMed] [Google Scholar]
- Gargus J.J., Schmunk G. Dysregulation of neurogenic calcium signaling and autism. Compr. Guide Autism. 2014:1285–1312. doi: 10.1007/978-1-4614-4788-7_35. [DOI] [Google Scholar]
- Giulivi, C., Zhang, Y.-F., Omanska-Klusek, A., Ross-Inta, C., Wong, S., Hertz-Picciotto, I., Tassone, F., Pessah, I.N., 2010. Mitochondrial Dysfunction in Autism. 〈http://www.jama.com〉. [DOI] [PMC free article] [PubMed]
- Hudmon A., Schulman H. Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem. J. 2002;364(3):593–611. doi: 10.1042/BJ20020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson C.C., Hall L., Harkness K.L. Prevalence of depressive disorders in individuals with autism spectrum disorder: a meta-analysis. J. Abnorm. Child Psychol. 2019;47(1):165–175. doi: 10.1007/S10802-018-0402-1. [DOI] [PubMed] [Google Scholar]
- Kapetanovic I.M. Computer-aided drug discovery and development (CADDD): in silico-chemico-biological approach. Chem. -Biol. Interact. 2008;171(2):165–176. doi: 10.1016/J.CBI.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavecchia A., Cerchia C. In silico methods to address polypharmacology: current status, applications and future perspectives. Drug Discov. Today. 2016;21(2):288–298. doi: 10.1016/j.drudis.2015.12.007. [DOI] [PubMed] [Google Scholar]
- Leitner, Y., Berger, I., -Hebrew, H., Martino, A.Di, & York, N. (2014). HUMAN NEUROSCIENCE The co-occurrence of autism and attention deficit hyperactivity disorder in children-what do we know? 10.3389/fnhum.2014.00268. [DOI] [PMC free article] [PubMed]
- Liao X., Liao X., Li Y. Genetic associations between voltage-gated calcium channels and autism spectrum disorder: a systematic review. Mol. Brain. 2020;13(1):1–10. doi: 10.1186/s13041-020-00634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H. ying, Gao W. yuan, Wen W., Zhang Y.Min. Taurine modulates calcium influx through L-type voltage-gated calcium channels in isolated cochlear outer hair cells in guinea pigs. Neurosci. Lett. 2006;399(1–2):23–26. doi: 10.1016/j.neulet.2006.01.070. [DOI] [PubMed] [Google Scholar]
- Maenner M.J., Warren Z., Williams A.R., et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years — autism and developmental disabilities monitoring network, 11 sites, United States, 2020. MMWR Surveill. Summ. 2023;72(No. SS-2):1–14. doi: 10.15585/mmwr.ss7202a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik, M., Tauqeer, Z., Sheikh, A.M., Wen, G., Nagori, A., Yang, K., Brown, W.T., Li, X., 2011. 10.1155/2011/785265. [DOI]
- Mano-Sousa B.J., Pedrosa A.M., Alves B.C., Galduróz J.C.F., Belo V.S., Chaves V.E., Duarte-Almeida J.M. Effects of risperidone in autistic children and young adults: a systematic review and meta-Analysis. Curr. Neuropharmacol. 2021;19(4):538. doi: 10.2174/1570159×18666200529151741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkiewicz J., Kontny E. Taurine and inflammatory diseases. Amino Acids. 2014;46(1):7–20. doi: 10.1007/s00726-012-1361-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen R.L., Medvedeva Y.V., Ayyagari T.E., Schmunk G., Gargus J.J. Intracellular calcium dysregulation in autism spectrum disorder: an analysis of converging organelle signaling pathways. Biochim. Et Biophys. Acta - Mol. Cell Res. 2018;1865(11):1718–1732. doi: 10.1016/J.BBAMCR.2018.08.003. [DOI] [PubMed] [Google Scholar]
- Page L. In vivo 1H-magnetic resonance spectroscopy study of amygdala-hippocampal and parietal regions in autism. Am. J. Psychiatry. 2016;163(12):2189. doi: 10.1176/APPI.AJP.163.12.2189. [DOI] [PubMed] [Google Scholar]
- Palmieri L., Papaleo V., Porcelli V., Scarcia P., Gaita L., Sacco R., Hager J., Rousseau F., Curatolo P., Manzi B., Militerni R., Bravaccio C., Trillo S., Schneider C., Melmed R., Elia M., Lenti C., Saccani M., Pascucci T., Persico A.M. Altered calcium homeostasis in autism-spectrum disorders: evidence from biochemical and genetic studies of the mitochondrial aspartate/glutamate carrier AGC1. Mol. Psychiatry. 2010;15(1):38–52. doi: 10.1038/mp.2008.63. [DOI] [PubMed] [Google Scholar]
- Psychiatry.org – DSM., 2013. Classification of ASD based on DSM-V. 〈https://psychiatry.org/psychiatrists/practice/dsm〉 accessed 25 August, 2022.
- Rojas, D.C., 2014. The role of glutamate and its receptors in autism and the use of glutamate receptor antagonists in treatment. 10.1007/s00702-014-1216-0. [DOI] [PMC free article] [PubMed]
- Saikia S., Bordoloi M. Molecular docking: challenges, advances and its use in drug discovery perspective. Curr. Drug Targets. 2018;20(5):501–521. doi: 10.2174/1389450119666181022153016. [DOI] [PubMed] [Google Scholar]
- Shah, A., Varma, M., & Bhandari, R. 2023. Exploring sulforaphane as neurotherapeutic: targeting Nrf2-Keap & Nf-Kb pathway crosstalk in ASD. Metabolic brain disease. Advance online publication. 10.1007/s11011-023-01224-4. [DOI] [PubMed]
- Sharma A., Mehan S. Targeting PI3K-AKT/mTOR signaling in the prevention of autism. Neurochem. Int. 2021;147(February) doi: 10.1016/j.neuint.2021.105067. [DOI] [PubMed] [Google Scholar]
- Shih R.H., Wang C.Y., Yang C.M. NF-kappaB signaling pathways in neurological inflammation: a mini review. Front. Mol. Neurosci. 2015;8(DEC):77. doi: 10.3389/FNMOL.2015.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunecka A., Blaylock R.L., Patocka J., Strunecky O. Immunoexcitotoxicity as the central mechanism of etiopathology and treatment of autism spectrum disorders: a possible role of fluoride and aluminum. Surg. Neurol. Int. 2018;9(1) doi: 10.4103/SNI.SNI_407_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G., Gutierrez Rios P., Kuo S.H., Akman H.O., Rosoklija G., Tanji K., Dwork A., Schon E.A., DiMauro S., Goldman J., Sulzer D. Mitochondrial abnormalities in temporal lobe of autistic brain. Neurobiol. Dis. 2013;54:349–361. doi: 10.1016/J.NBD.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidyman W.E., Rauen K.A. The RASopathies: developmental syndromes of Ras/MAPK pathway dysregulation. Curr. Opin. Genet. Dev. 2009;19(3):230–236. doi: 10.1016/j.gde.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usuda H., Okamoto T., Wada K. Leaky gut: effect of dietary fiber and fats on microbiome and intestinal barrier. Int. J. Mol. Sci. 2021;22(14) doi: 10.3390/IJMS22147613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasa, R.A., & Mazurek, M.O. 2015. An update on anxiety in youth with autism spectrum disorders. https://doi.org/10.1097/YCO.0000000000000133. [DOI] [PMC free article] [PubMed]
- World Health Organization (2022). Autism. Retrieved from 〈https://www.who.int/news-room/fact-sheets/detail/autism-spectrum-disorders〉.
- Wu J.Y., Prentice H. Role of taurine in the central nervous system. J. Biomed. Sci. 2010;17(1):1–6. doi: 10.1186/1423-0127-17-S1-S1/FIGURES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Tian X., Xu D., Zheng F., Lu X., Zhang Y., Ma Y., Li Y., Xu X., Zhu B., Wang X. GPR40 modulates epileptic seizure and NMDA receptor function. Sci. Adv. 2018;4(10):1–13. doi: 10.1126/sciadv.aau2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., Lukiw W.J., Yang C.-M., Shih R.-H., Wang C.-Y. NF-kappaB signaling pathways in neurological inflammation: a mini review. Front. Mol. Neurosci. 2015;8:77. doi: 10.3389/fnmol.2015.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A.M.H., Campbell E., Lynch S., Suckling J., Powis S.J. Aberrant NF-KappaB expression in autism spectrum condition: a mechanism for neuroinflammation. Front. Psychiatry. 2011;2(MAY) doi: 10.3389/FPSYT.2011.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]