Abstract
Most of the evidences for beneficial effects of beta-blockers in patients with acute myocardial infarction (AMI) were from the clinical studies published in the pre-reperfusion era when anti-platelet drugs, statins or inhibitors of renin-angiotensin-aldosterone system which are known to reduce cardiovascular mortality of patients with AMI were not introduced. In the reperfusion era, beta-blockers’ benefit has not been clearly shown except in patients with reduced ejection fraction (EF; ≤40%). In the era of the early reperfusion therapy for AMI, a number of patients with mildly reduced EF (>40%, <50%) or preserved EF (≥50%) become increasing. However, because no randomized clinical trials are available until now, the benefit and the optimal duration of oral treatment with beta-blockers in patients with mildly reduced or preserved EF are questionable. Registry data have not showed the association of oral beta-blocker therapy with decreased mortality in survivors without heart failure or left ventricular systolic dysfunction after AMI. In the Korea Acute Myocardial Infarction Registry-National Institute of Health of in-hospital survivors after AMI, the benefit of beta-blocker therapy at discharge was shown in patients with reduced or mildly reduced EF, but not in those with preserved EF, which provides new information about beta-blocker therapy in patients without reduced EF. However, clinical practice can be changed when the results of appropriate randomized clinical trials are available. Ongoing clinical trials may help to answer the unresolved issues of beta-blocker therapy in patients with AMI.
Keywords: adrenergic beta-antagonist, myocardial infarction, treatment outcome, ventricular ejection fraction
INTRODUCTION
Early coronary reperfusion therapy and evidence-based medications are recommended in patients with acute myocardial infarction (AMI) to reduce cardiac events. In hospitalized patients with ST-elevation myocardial infarction (STEMI) and non-ST-elevation myocardial infarction (NSTEMI), beta-blocker therapy prescribed at discharge is one of the measure sets in the clinical performance and quality measures [1], and it was used as one of the items of quality measures of AMI in the Korean National Health Insurance value incentive program to provide financial incentives [2]. However, most of the evidences for beneficial effects of beta-blockers were from the clinical studies reported in the early 1980s when anti-platelet drugs, statins or inhibitors of renin-angiotensin-aldosterone system which are known to reduce cardiovascular mortality in patients with AMI were not introduced. These early clinical studies of beta-blockers rarely required the estimation of left ventricular (LV) function by imaging modalities in their inclusion criteria. Although a meta-analysis of clinical trials before the reperfusion era showed the benefit of beta-blockers on cardiac mortality after AMI [3], in the reperfusion era, the effect of beta-blockers on the clinical outcomes has not been definitely shown [4,5] except in patients with left ventricular ejection fraction (LVEF) ≤40% [6]. In this review paper, the evidence gap of beta-blocker therapy in patients with AMI and future perspectives are discussed.
MECHANISMS OF ACTIONS OF BETA-BLOCKERS IN AMI
After AMI, a medical therapy to decrease the oxygen demand and increase the oxygen supply is an essential component in the initial and long-term treatment. In patients with AMI, sympathetic nervous system is usually activated in response to chest pain, anxiety, and impaired cardiac function. Activation of cardiac beta-1 adrenergic receptors increases the heart rate of the sinoatrial node, and the contractility of the myocardium, resulting in increased cardiac output and blood pressure. However, prolonged adrenergic activation exacerbates myocardial ischemia by increasing myocardial oxygen demand, and provokes ventricular arrhythmias. In this situation of adrenergic activation, beta-blockers decrease myocardial contractility, heart rate, and elevated blood pressure, which reduce myocardial oxygen demand. They also increase myocardial oxygen supply by improving coronary diastolic perfusion (Figure 1). Fatal and non-fatal ventricular arrhythmias are not an uncommon complication after AMI. As a class II anti-arrhythmic agent, beta-blockers reduce the cardiac automaticity in infarction-related, scarred myocardium and the risk of critical ventricular arrhythmias after AMI [7].
Figure 1.
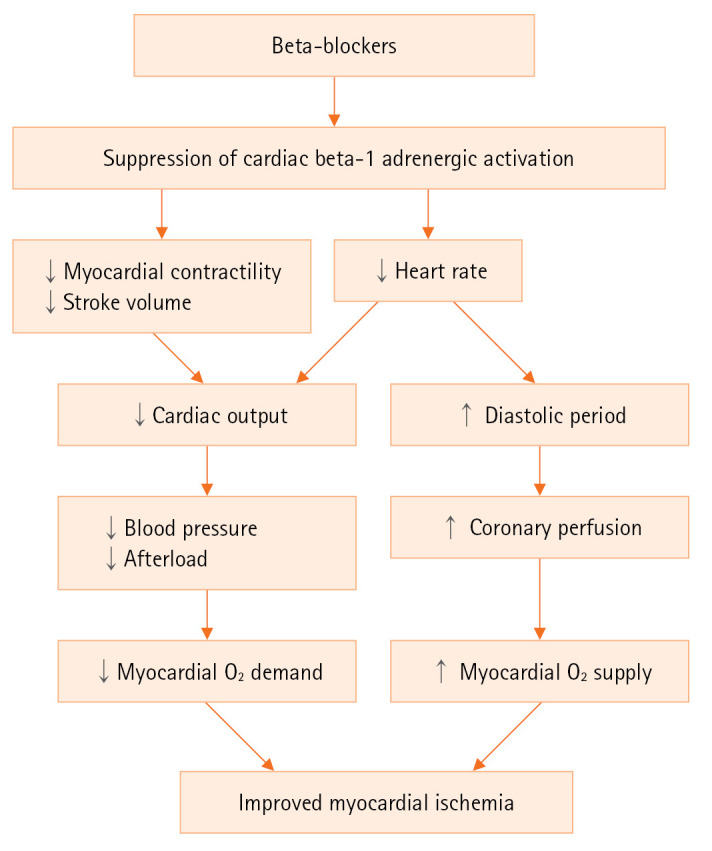
Mechanisms of beta-blocker therapy to improve myocardial ischemia in acute myocardial infarction. ↓: decrease;↑: increase.
BETA-BLOCKER THERAPY IN THE PRE-REPERFUSION ERA
After the introduction of propranolol in 1960s, beta-blockers have been used to reduce myocardial oxygen demand in patients with ischemic heart diseases. The first study to evaluate the efficacy of a beta-blocker, practolol, in patients with AMI was terminated prematurely because of serious oculocutaneous and peritoneal side-effects, but a significant reduction in overall mortality, sudden deaths and all cardiac events was observed in patients treated with practolol [8]. Although practolol has been withdrawn from the market, this study suggested the favorable role of adrenergic inhibition with beta-blockers after AMI.
In 1981, two clinical studies showed the reduction of mortality by beta-blockers in patients with AMI. In the Norwegian Timolol study, the long-term treatment with timolol reduced mortality and the rate of reinfarction [9]. The mortality benefit at 90 days was also observed in patients with metoprolol in Swedish Gothenburg-study [10]. The Beta-Blocker Heart Attack Trial (BHAT) in 1982, the long-term treatment with propranolol reduced total mortality, arteriosclerotic heart disease mortality and sudden cardiac death [11]. The BHAT investigators recommended an oral treatment with propranolol for at least 3 years in patients with recent myocardial infarction (MI) unless contraindicated. In the first International Study of Infarct Survival, the immediate intravenous atenolol therapy within mean 5 hours after the onset of suspected AMI followed by oral medication for 7 days lowered the vascular mortality during the treatment period (0–7 days) [12].
In a landmark meta-analysis of clinical studies in 1970s and 1980s, the long-term beta-blocker therapy reduced all-cause mortality of 23% [3], and authors suggested beta-blockers to be continued indefinitely. Based on these early studies in the era of pre-reperfusion therapy, oral beta-blocker therapy was recommended in all patients with AMI for at least 3 years irrespective of LV systolic function if they have no contraindications to beta-blockers [13].
BETA-BLOCKER THERAPY IN THE REPERFUSION ERA
Since late 1980s, the immediate reperfusion therapy for occluded coronary arteries with either thrombolytic drugs or percutaneous coronary intervention (PCI) has become a main, emergent treatment of patients with STEMI. In addition, the evidence-based medical therapy other than beta-blockers such as anti-platelet drugs, statins or inhibitors of renin-angiotensin-aldosterone system was introduced to reduce cardiac mortality and morbidity of patients with AMI. The wide use of imaging modalities to evaluate LV systolic function was another advance in the management of patients with MI or heart failure (HF).
In the reperfusion era, the short-term clinical effect of metoprolol was studied in the Clopidogrel and Metoprolol in Myocardial Infarction Trial (COMMIT). The immediate intravenous metoprolol therapy within 24 hours after the onset of suspected AMI followed by oral medication for 28 days reduced the risks of MI and ventricular fibrillation. Because the immediate beta-blocker therapy increased the risk of cardiogenic shock, the COMMIT investigators recommended beta-blocker therapy to be started after hemodynamic stabilization after AMI to prevent reinfarction and sudden cardiac death [14]. In patients with AMI and LV systolic dysfunction (EF ≤40%), a long-term treatment with carvedilol lowered total mortality, cardiac mortality and non-fatal MI [6]. As a result, a life-long beta-blocker therapy has been recommended in patients with EF ≤40% [13].
However, other clinical studies in the reperfusion era have not clearly showed a long-term impact of beta-blocker therapy on cardiovascular events. Rather, one registry data showed the use of beta-blockers was not associated with a lower cardiovascular outcome in a propensity score-matched analysis [4]. In a meta-analysis of randomized clinical trials, a different effect of beta-blockers between pre- and post-reperfusion era was reported that beta-blockers reduced cardiovascular events in the pre-reperfusion era, but not in the reperfusion era [5].
BETA-BLOCKER THERAPY IN THE GUIDELINES FOR THE MANAGEMENT OF AMI
Because clinical trials of beta-blockers in AMI patients without LV systolic dysfunction or HF have not been available yet, the guidelines for AMI provide inconsistent recommendations. In STEMI, the 2013 American guideline recommends oral beta-blockers to be initiated within the first day unless they have HF, low cardiac output, high risk for cardiogenic shock, or other contraindications (e.g. 2nd- or 3rd-degree heart block, PR interval ≥0.24 seconds, active asthma, or reactive airways disease), and to be continued during and after hospitalization for all patients as a class I recommendation [15]. The 2017 European guidelines also recommend that oral beta-blocker therapy is indicated in patients with HF and/or LVEF ≤40% unless contraindicated as a class I indication, but in other patients without HF or with LVEF >40%, oral beta-blocker therapy is recommended as a class IIa indication [16] (Table 1).
Table 1.
Oral beta-blocker therapy in the guidelines for the management of patients with acute myocardial infarction
| Guidelines | Class | LOE |
|---|---|---|
| Guidelines for the management of patients with STEMI | ||
| 1. 2013 ACC/AHA guideline [15] | ||
| Recommendations | ||
| · Oral beta-blockers should be initiated in the first 24 hours in patients with STEMI who do not have any of the following: signs of HF, evidence of a low output state, increased risk for cardiogenic shock, or other contraindications to use of oral beta blockers (PR interval more than 0.24 seconds, second- or third-degree heart block, active asthma, or reactive airways disease). | I | B |
| · Beta-blockers should be continued during and after hospitalization for all patients with STEMI and with no contraindications to their use. | I | B |
| 2. 2017 ESC guidelines [16] | ||
| Recommendations | ||
| · Oral treatment with beta-blockers is indicated in patients with HF and/or LVEF ≤40% unless contraindicated. | I | A |
| · Routine oral treatment with beta-blockers should be considered during hospital stay and continued thereafter in all patients without contraindications. | IIa | B |
| Guidelines for the management of patients with NSTEMI | ||
| 1. 2014 AHA/ACC guideline [17] | ||
| Recommendations | ||
| · Oral beta-blocker therapy should be initiated within the first 24 hours in patients who do not have any of the following: (1) signs of HF, (2) evidence of low-output state, (3) increased risk for cardiogenic shock, or (4) other contraindications to beta-blockade (e.g., PR interval >0.24 second, second- or third-degree heart block without a cardiac pacemaker, active asthma, or reactive airway disease). | I | A |
| · In patients with concomitant NSTE-ACS, stabilized HF, and reduced systolic function, it is recommended to continue beta-blocker therapy with 1 of the 3 drugs proven to reduce mortality in patients with HF: sustained-release metoprolol succinate, carvedilol, or bisoprolol. | I | C |
| · It is reasonable to continue beta-blocker therapy in patients with normal LV function with NSTE-ACS. | IIa | C |
| 2. 2020 ESC guidelines [18] | ||
| Recommendations | ||
| · Early initiation of beta-blocker treatment is recommended in patients with ongoing ischemic symptoms and without contraindications to the respective drug class. It is recommended to continue chronic beta-blocker therapy unless the patient is in Killip class III or higher. | I | C |
| · Long-term beta-blockers are recommended in patients with systolic LV dysfunction or HF with reduced LVEF (<40%). | I | A |
| · In patients with prior MI, long-term oral treatment with a beta-blocker should be considered in order to reduce all-cause and cardiovascular mortality and cardiovascular morbidity | IIa | B |
LOE: level of evidence; STEMI: ST-elevation myocardial infarction; ACC: American College of Cardiology; AHA: American Heart Association; HF: heart failure; ESC: European Society of Cardiology; LVEF: left ventricular ejection fraction; NSTEMI; non-ST-elevation myocardial infarction; NSTE-ACS: non-ST-elevation-acute coronary syndrome; LV: left ventricular; MI: myocardial infarction.
In case of NSTEMI, the 2014 American guideline recommends oral treatment with beta-blockers to be initiated within the first day unless patients with NSTEMI have the same contraindications as those with STEMI. It also recommends a long-term oral therapy with beta-blockers in patients with HF or reduced LV systolic function as a class I indication, but as a class IIa indication in patient with normal systolic function [17]. The 2020 European guidelines for patients with NSTEMI recommend beta-blocker treatment to be initiated early in patients with ongoing ischemic symptoms and to be continued chronically as a class I indication unless the patient is in over HF or Killip class ≥III. Although the benefit of a long-term oral therapy with beta-blockers over 1 year was questionable in observational studies or meta-analyses, the 2020 European guidelines recommend beta-blockers to be considered in all patients with prior MI as a class IIa indication to decrease all-cause mortality and cardiovascular morbidity [18] (Table 1).
RECENTLY PUBLISHED DATA AND UNSOLVED ISSUES
Although short-term and long-term benefits of oral treatment with beta-blockers in AMI patients with reduced LV systolic function (EF ≤40%) or clinical HF have been well documented in clinical trials [6,19], its effects in those with EF >40% or without HF have not been definitely shown because of the lack of randomized clinical trials so far. In a recent guideline, HF has been classified as distinct phenotypes based on LVEF: HF with reduced EF (HFrEF; ≤40%), HF with mildly reduced EF (HFmrEF; >40%, <50%), and HF with preserved EF (HFpEF; ≥50%) [20]. In patients with HFpEF, the benefit of beta-blocker therapy was not demonstrated, and in patients with HFmrEF, only one meta-analysis of clinical trials showed reduced all-cause mortality of 41% in patients with overall median EF 40% (interquartile range, 40%–43%) [21].
In the era of the prompt primary PCI or thrombolytic therapy for STEMI or an early invasive strategy for NSTEMI, a number of patients with LVEF >40% after AMI becomes increasing. In the Korea Acute Myocardial Infarction Registry-National Institute of Health (KAMIR-HIH), 86% of patients had LVEF >40% and 62% had LVEF ≥50% [22]. However, because of the lack of randomized clinical trials, only registry data with inherent limitations are available until now. Therefore, the impact of beta-blocker therapy in patients with mildly reduced or preserved EF after AMI is controversial, especially when they undergo successful coronary reperfusion.
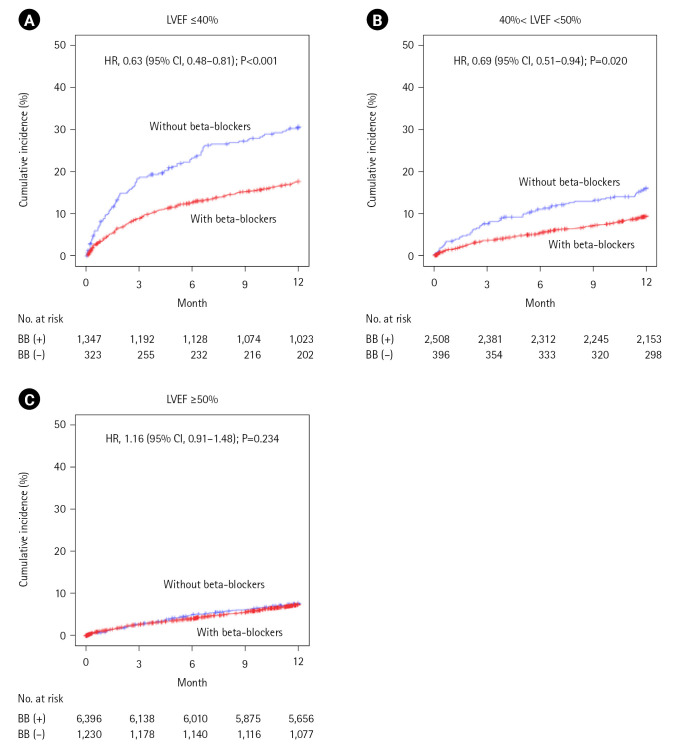
In one registry data of patients with STEMI and undergoing successful primary PCI, beta-blocker therapy was associated with reduced all-cause mortality [23]. However, other registry data have not showed the association of oral beta-blocker therapy with decreased mortality in survivors without HF or LV systolic dysfunction after AMI. In the nationwide French registry of patients with AMI, taking early beta-blockers was associated with 54% lower 30-day mortality in patients without LVEF ≤40% or HF, but 1-year mortality was not different between patients with and without beta-blockers [24]. The national English and Welsh registry data of patients with AMI also showed no significant difference of 1-year mortality in patients without LVEF <30% or HF whether oral beta-blockers were taken or not [25]. The KAMIR-NIH data of in-hospital survivors after AMI showed that patients with beta-blockers at hospital discharge had 16% lower 1-year composite cardiac events compared with those without beta-blockers. This impact was also observed in patients with successful early coronary reperfusion. However, this association had a significant interaction with LVEF. The benefit of beta-blocker therapy at discharge was demonstrated in patients with reduced or mildly reduced EF, but not in those with preserved EF (Figure 2) [22].
Figure 2.
Unadjusted Kaplan-Meier curves and adjusted hazard ratios (HR) for 1-year major adverse cardiac events in the entire cohort with vs. without beta-blockers (BB) according to left ventricular ejection fraction (LVEF). (A) LVFE ≤40% (n=1,670 patients). (B) 40%< LVEF <50% (n=2,904 patients). (C) LVEF ≥50% (n=7,626 patients). CI: confidence interval. Adapted from Joo et al. Eur Heart J Cardiovasc Pharmacother 2021;7:475-82 [22].
There are feasible explications why beta-blockers’ benefit was not proved in patients with AMI and preserved EF. Beta-blockers effectively reduce myocardial ischemia, recurrent MI or critical arrhythmia after AMI when a patient has large amount of infarction-related, scarred or non-viable myocardium. Lesser amount of infarction-related myocardium is expected in patients with preserved EF and clinical benefits of beta-blockers will consequently be diminished [5,22,25]. Another beneficial mechanism is heart rate slowing by beta-blockers and consequent reduction of myocardial oxygen consumption and improved diastolic coronary perfusion (Figure 1). The clinical benefit of heart rate slowing was demonstrated in patients with HFrEF using ivabradine [26]. However, in stable coronary artery disease, ivabradine did not improve clinical outcomes despite heart rate slowing [27], which suggested that in patients with preserved EF after AMI, heart rate reduction itself may not act as an effective mechanism in reducing cardiac events [28].
Most of clinical trials of MI or HF defined reduced LVEF as <35%–40%. Patients with HFmrEF (>40%, <50%) have been reported to show unique clinical feature compared with those with HFrEF (≤40%) or HFpEF (≥50%) [20]. The impact of beta-blockers in patients with mildly reduced EF after AMI has been only investigated in the registry data. The Portuguese Registry of Acute Coronary Syndromes showed a positive impact of in-hospital beta-blocker therapy on in-hospital mortality in patients with mildly reduced EF [29]. In the KAMIR-NIH, 24% of patients had mildly reduced EF, and in these patients, beta-blocker therapy showed about 30% reduction of 1-year and 2-year composite cardiac events. This benefit of beta-blockers was mainly caused by lower recurrent MI [22,30]. All of these findings suggest the benefit of oral treatment with beta-blockers in AMI patients with mildly reduced EF without racial difference.
The optimal duration of beta-blocker therapy after AMI is another unresolved issue. The American guideline recommend oral beta-blockers with evidence to reduce mortality (e.g., carvedilol, metoprolol succinate, or bisoprolol) to be used in all patients with prior MI and LVEF ≤40% as a class I indication, and had recommended beta-blocker therapy to be continued at least for 3 years in all patients even they had normal LV function [13]. Recently this guideline has been revised that it may be reasonable to reassess the necessity for a long-term (>1 year) beta-blocker therapy in order to reduce cardiac events in patients with prior MI and without LVEF ≤50% (Table 2) [31]. The European guidelines recommend a long-term (>1 year) treatment with beta-blockers in patients with prior MI and without HF or LV systolic dysfunction as a class IIa indication even though prior studies have questioned the benefit of this regimen [32]. In a 1-year landmark analysis of Korean nationwide medical insurance data, beta-blocker therapy over 1 year after the initial attack of AMI was associated with 19% reduction of all-cause death in patients without HF, but this association was lost beyond 3 years [33]. However, 1-year landmark analysis of the Danish registry data of AMI patients without HF showed no association of long-term beta-blocker therapy with cardiovascular mortality or recurrent MI following the patients from 3 months to 3 years after index admission [34]. One-year landmark analysis of the KAMIR-NIH data of stable patients without major cardiac events until 1 year after AMI also showed no association of beta-blocker therapy with cardiac mortality irrespective of initial EF [35].
Table 2.
Long-term beta-blocker therapy after acute myocardial infarction in the guidelines
| Guidelines | Class | LOE |
|---|---|---|
| 2012 ACC/AHA guideline for diagnosis and management for patients with stable IHD [13] | ||
| Recommendations | ||
| · Beta-blocker therapy should be started and continued for 3 years in all patients with normal LV function after MI or ACS. | I | B |
| · Beta-blocker therapy should be used in all patients with LV systolic dysfunction (EF ≤40%) with HF or prior MI, unless contraindicated (Use should be limited to carvedilol, metoprolol succinate, or bisoprolol, which have been shown to reduce risk of death.). | I | A |
| 2023 AHA/ACC guideline for the management for patients with CCD [31] | ||
| Recommendations | ||
| · In patients with CCD and LVEF ≤40% with or without previous MI, the use of beta-blocker therapy is recommended to reduce the risk of future MACE, including cardiovascular death. | I | A |
| · In patients with CCD and LVEF <50%, the use of sustained release metoprolol succinate, carvedilol, or bisoprolol with titration to target doses is recommended in preference to other beta blockers. | I | A |
| · In patients with CCD who were initiated on beta-blocker therapy for previous MI without a history of or current LVEF ≤50%, angina, arrhythmias, or uncontrolled hypertension, it may be reasonable to reassess the indication for long-term (>1 year) use of beta-blocker therapy for reducing MACE. | IIb | B |
| 2019 ESC guidelines for the diagnosis and management for patients with CCS [32] | ||
| Recommendations | ||
| · Beta-blockers are recommended in patients with LV dysfunction or systolic HF. | I | A |
| · In patients with a previous STEMI, long-term oral treatment with a beta-blocker should be considered. | IIa | B |
LOE: level of evidence; ACC: American College of Cardiology; AHA: American Heart Association; IHD: ischemic heart disease; LV: left ventricular; MI: myocardial infarction; ACS: acute coronary syndrome; EF: ejection fraction; HF: heart failure; CCD: chronic coronary disease; LVEF: left ventricular ejection fraction; MACE: major adverse cardiovascular event; ESC: European Society of Cardiology; CCS: chronic coronary syndrome; STEMI: ST-elevation myocardial infarction.
There is also a controversy about optimal dose of beta-blockers after AMI. In “real-world” registries, the lower than maximal tolerable doses of beta-blockers that are recommended in the guidelines are a usual prescription pattern. In one registry of the United States and Canada, 60% of patients were prescribed in less than 25% of target dose at discharge, and the lowest 2-year mortality was observed in patients with >12.5%–25% of target dose [36]. One-year landmark analysis of the same registry also showed that AMI patients treated with >12.5%–25% of the maximum tolerable dose had reduced all-cause death compared with no beta-blocker or other doses [37]. In the KAMIR-NIH, less than 25% of target dose of beta-blockers was prescribed at discharge, but this dose was associated with better clinical outcomes [22]. In the Swedish national registries, cardiovascular outcomes after AMI were not different between patients with <50% and with ≥50% of the target dose of beta-blockers [38]. Only a randomized clinical trial may answer the optimal dose of beta-blockers after AMI although such a clinical trial would not be easy to performed.
FUTURE PERSPECTIVES
Unresolved beta-blocker therapy issues in patients with AMI are the effect of beta-blocker therapy in patients with mildly reduced or preserved EF, the optimal duration of beta-blocker therapy, and the optimal dose of beta-blockers.
There are six on-going, randomized clinical trials enrolled in ClinicalTrial.gov to investigate the effect of oral treatment with beta-blockers in patients with AMI and without reduced EF or HF (Table 3). Four clinical trials are investigating the non-inferiority of immediate discontinuation (1–8 days after initial attack) of beta-blockers in patients with EF ≥40% (EF ≥50% in one trial). Other two clinical trials are evaluating the non-inferiority of discontinuation after at least 6 month to 1 year use of oral beta-blockers in patients with EF ≥40%. In the study designs of these clinical trials, only one (DANBLOCK: Danish trial of beta blocker treatment after myocardial infarction without reduced ejection fraction) recommends to use the highest dose of beta-blockers deemed tolerable for the patient at the time of randomization, but other trials permit dose at the discretion of attending investigators. Except for the optimal dose question, the results of these on-going clinical trials may answer the unresolved questions of beta-blocker therapy in patients with AMI.
Table 3.
Ongoing clinical trials evaluating beta-blocker therapy in patients with acute myocardial infarction and without reduced ejection fraction
| Trial | Country | No. of patients | Inclusion criteria | Primary end-point | Point of patients enrollment | Follow-up period | Expected completion date | Study Ida) |
|---|---|---|---|---|---|---|---|---|
| REDUCE | Sweden | 5,000 | EF ≥50% | All-cause death or nonfatal MI | 1–7 Days after MI | 3 yr | Dec 1, 2025 | NCT03278509 |
| REBOOT | Italy, Spain | 8,468 | EF >40% | All-cause death, nonfatal MI, or HF | At discharge | 3 yr | Nov 1, 2024 | NCT03596385 |
| BETAMI | Norway | 10,000 | EF ≥40% | All-cause death or nonfatal MI | 1–8 Days after PCI or thrombolysis | 2 yr | Oct 1, 2023 | NCT03646357 |
| DANBLOCK | Denmark | 3,570 | EF ≥40% | All-cause death, nonfatal MI, revascularization, stroke, ventricular arrhythmia, cardiac arrest with successful resuscitation or HF | 14 Days after MI | 6 mo–6 yr | Jun 1, 2024 | NCT03778554 |
| AβYSS | France | 3,700 | EF ≥40% | All-cause death, stroke, MI, or hospitalization for other CV reason | ≥6 Months after MI | 4 yr | Aug 1, 2023 | NCT03498066 |
| SMART-DECISION | Korea | 2,540 | EF ≥40% | All-cause death, MI, or hospitalization for HF | After at least 1 year of β-blocker therapy | 2.5 yr | Mar 1, 2026 | NCT04769362 |
REDUCE: randomized evaluation of decreased usage of betablocckers after myocardial infarction; REBOOT: treatment with beta-blockers after myocardial infarction without reduced ejection fraction; BETAMI: betablocker treatment after acute myocardial infarction in patients without reduced left ventricular systolic function; DANBLOCK: Danish trial of beta blocker treatment after myocardial infarction without reduced ejection fraction; AβYSS: assessment of βeta blocker interruption after uncomplicated myocardial infarction on safety and symptomatic cardiac events requiring hospitalization; SMART-DECISION: discontinuation of β-blocker therapy in stabilized patients after acute myocardial infarction; EF: ejection fraction; MI: myocardial infarction; HF: heart failure; PCI: percutaneous coronary intervention; CV: cardiovascular.
Study identification number enrolled in ClinicalTrials.gov.
CONCLUSIONS
In patients with AMI, oral treatment with beta-blockers is recommended when they have reduced EF or HF unless contraindicated, but in patients without reduced EF or HF, the benefit and the optimal duration of beta-blocker therapy is questionable. Ongoing clinical trials may help to answer the unresolved issues of beta-blocker therapy in patients with AMI.
HIGHLIGHTS
▪ In patients with acute myocardial infarction, oral treatment with beta-blockers is recommended when they have reduced ejection fraction (≤40%) or heart failure unless contraindicated.
▪ In patients without reduced ejection fraction or heart failure, the benefit and the optimal duration of beta-blocker therapy is questionable.
Acknowledgments
None.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
This work was supported by the 2023 education, research and student guidance grant funded by Jeju National University.
REFERENCES
- 1.Jneid H, Addison D, Bhatt DL, Fonarow GC, Gokak S, Grady KL, et al. 2017 AHA/ACC clinical performance and quality measures for adults with ST-elevation and non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol. 2017;70:2048–90. doi: 10.1016/j.jacc.2017.06.032. [DOI] [PubMed] [Google Scholar]
- 2.Kim SM, Jang WM, Ahn HA, Park HJ, Ahn HS. Korean National Health Insurance value incentive program: achievements and future directions. J Prev Med Public Health. 2012;45:148–55. doi: 10.3961/jpmph.2012.45.3.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freemantle N, Cleland J, Young P, Mason J, Harrison J. β Blockade after myocardial infarction: systematic review and meta regression analysis. BMJ. 1999;318:1730–7. doi: 10.1136/bmj.318.7200.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bangalore S, Steg G, Deedwania P, Crowley K, Eagle KA, Goto S, et al. β-Blocker use and clinical outcomes in stable outpatients with and without coronary artery disease. JAMA. 2012;308:1340–9. doi: 10.1001/jama.2012.12559. [DOI] [PubMed] [Google Scholar]
- 5.Bangalore S, Makani H, Radford M, Thakur K, Toklu B, Katz SD, et al. Clinical outcomes with β-blockers for myocardial infarction: a meta-analysis of randomized trials. Am J Med. 2014;127:939–53. doi: 10.1016/j.amjmed.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 6.Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet. 2001;357:1385–90. doi: 10.1016/s0140-6736(00)04560-8. [DOI] [PubMed] [Google Scholar]
- 7. Antman EM, Loscalzo J. ST-segment elevation myocardial infarction. In: Loscalzo J, Fauci A, Kasper DL, Hauser SL, Longo DL, Jameson JL, editors. Harrison’s principles of internal medicine. 21st ed. McGraw Hill; 2022. p. 2053-66. [Google Scholar]
- 8.Improvement in prognosis of myocardial infarction by long-term beta-adrenoreceptor blockade using practolol: a multicentre international study. Br Med J. 1975;3:735–40. doi: 10.1136/bmj.3.5986.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norwegian Multicenter Study Group Timolol-induced reduction in mortality and reinfarction in patients surviving acute myocardial infarction. N Engl J Med. 1981;304:801–7. doi: 10.1056/NEJM198104023041401. [DOI] [PubMed] [Google Scholar]
- 10.Hjalmarson A, Elmfeldt D, Herlitz J, Holmberg S, Málek I, Nyberg G, et al. Effect on mortality of metoprolol in acute myocardial infarction: a double-blind randomised trial. Lancet. 1981;2:823–7. doi: 10.1016/s0140-6736(81)91101-6. [DOI] [PubMed] [Google Scholar]
- 11.A randomized trial of propranolol in patients with acute myocardial infarction. I. Mortality results. JAMA. 1982;247:1707–14. doi: 10.1001/jama.1982.03320370021023. [DOI] [PubMed] [Google Scholar]
- 12.First International Study of Infarct Survival Collaborative Group Randomised trial of intravenous atenolol among 16 027 cases of suspected acute myocardial infarction: ISIS-1. Lancet. 1986;2:57–66. [PubMed] [Google Scholar]
- 13.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44–164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Chen ZM, Pan HC, Chen YP, Peto R, Collins R, Jiang LX, et al. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet. 2005;366:1622–32. doi: 10.1016/S0140-6736(05)67661-1. [DOI] [PubMed] [Google Scholar]
- 15.O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:529–55. doi: 10.1161/CIR.0b013e3182742c84. [DOI] [PubMed] [Google Scholar]
- 16.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39:119–77. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 17.Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Jr, Ganiats TG, Holmes DR, Jr, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2354–94. doi: 10.1161/CIR.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 18.Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289–367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 19.McMurray J, Køber L, Robertson M, Dargie H, Colucci W, Lopez-Sendon J, et al. Antiarrhythmic effect of carvedilol after acute myocardial infarction: results of the Carvedilol Post-Infarct Survival Control in Left Ventricular Dysfunction (CAPRICORN) trial. J Am Coll Cardiol. 2005;45:525–30. doi: 10.1016/j.jacc.2004.09.076. [DOI] [PubMed] [Google Scholar]
- 20.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 21.Cleland JG, Bunting KV, Flather MD, Altman DG, Holmes J, Coats AJ, et al. Beta-blockers for heart failure with reduced, mid-range, and preserved ejection fraction: an individual patient-level analysis of double-blind randomized trials. Eur Heart J. 2018;39:26–35. doi: 10.1093/eurheartj/ehx564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joo SJ, Kim SY, Choi JH, Park HK, Beom JW, Lee JG, et al. Effect of beta-blocker therapy in patients with or without left ventricular systolic dysfunction after acute myocardial infarction. Eur Heart J Cardiovasc Pharmacother. 2021;7:475–82. doi: 10.1093/ehjcvp/pvaa029. [DOI] [PubMed] [Google Scholar]
- 23.Yang JH, Hahn JY, Song YB, Choi SH, Choi JH, Lee SH, et al. Association of beta-blocker therapy at discharge with clinical outcomes in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. JACC Cardiovasc Interv. 2014;7:592–601. doi: 10.1016/j.jcin.2013.12.206. [DOI] [PubMed] [Google Scholar]
- 24.Puymirat E, Riant E, Aissaoui N, Soria A, Ducrocq G, Coste P, et al. β blockers and mortality after myocardial infarction in patients without heart failure: multicentre prospective cohort study. BMJ. 2016;354:i4801. doi: 10.1136/bmj.i4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dondo TB, Hall M, West RM, Jernberg T, Lindahl B, Bueno H, et al. β-blockers and mortality after acute myocardial infarction in patients without heart failure or ventricular dysfunction. J Am Coll Cardiol. 2017;69:2710–20. doi: 10.1016/j.jacc.2017.03.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost-Brama A, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–85. doi: 10.1016/S0140-6736(10)61198-1. [DOI] [PubMed] [Google Scholar]
- 27.Fox K, Ford I, Steg PG, Tardif JC, Tendera M, Ferrari R, et al. Ivabradine in stable coronary artery disease without clinical heart failure. N Engl J Med. 2014;371:1091–9. doi: 10.1056/NEJMoa1406430. [DOI] [PubMed] [Google Scholar]
- 28.Nambiar L, Meyer M. β-Blockers in myocardial infarction and coronary artery disease with a preserved ejection fraction: recommendations, mechanisms, and concerns. Coron Artery Dis. 2018;29:262–70. doi: 10.1097/MCA.0000000000000610. [DOI] [PubMed] [Google Scholar]
- 29.Montenegro Sá F, Carvalho R, Ruivo C, Santos LG, Antunes A, Soares F, et al. Beta-blockers for post-acute coronary syndrome mid-range ejection fraction: a nationwide retrospective study. Eur Heart J Acute Cardiovasc Care. 2019;8:599–605. doi: 10.1177/2048872619827476. [DOI] [PubMed] [Google Scholar]
- 30.Joo SJ, Kim SY, Lee JG, Beom JW, Choi JH, Park HK, et al. Association of the medical therapy with beta-blockers or inhibitors of renin-angiotensin system with clinical outcomes in patients with mildly reduced left ventricular ejection fraction after acute myocardial infarction. Medicine (Baltimore) 2022;101:e30846. doi: 10.1097/MD.0000000000030846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Writing Committee Members, Virani SS, Newby LK, Arnold SV, Bittner V, Brewer LC, et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA guideline for the management of patients with chronic coronary disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2023;82:833–955. doi: 10.1016/j.jacc.2023.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–77. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 33.Kim J, Kang D, Park H, Kang M, Park TK, Lee JM, et al. Long-term β-blocker therapy and clinical outcomes after acute myocardial infarction in patients without heart failure: nationwide cohort study. Eur Heart J. 2020;41:3521–9. doi: 10.1093/eurheartj/ehaa376. [DOI] [PubMed] [Google Scholar]
- 34.Holt A, Blanche P, Zareini B, Rajan D, El-Sheikh M, Schjerning AM, et al. Effect of long-term beta-blocker treatment following myocardial infarction among stable, optimally treated patients without heart failure in the reperfusion era: a Danish, nationwide cohort study. Eur Heart J. 2021;42:907–14. doi: 10.1093/eurheartj/ehaa1058. [DOI] [PubMed] [Google Scholar]
- 35.Joo SJ, Boo KY, Beom JW, Lee JG, Choi JH, Kim SY, et al. Optimal medical therapy for patients with acute myocardial infarction beyond one year after the initial attack. J Am Coll Cardiol. 2022;79(9_Supplement):952. [Google Scholar]
- 36.Goldberger JJ, Bonow RO, Cuffe M, Liu L, Rosenberg Y, Shah PK, et al. Effect of beta-blocker dose on survival after acute myocardial infarction. J Am Coll Cardiol. 2015;66:1431–41. doi: 10.1016/j.jacc.2015.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldberger JJ, Subačius H, Marroquin OC, Beau SL, Simonson J, OBTAIN (Outcomes of Beta‐Blocker Therapy After Myocardial Infarction) Investigators One-year landmark analysis of the effect of beta-blocker dose on survival after acute myocardial infarction. J Am Heart Assoc. 2021;10:e019017. doi: 10.1161/JAHA.120.019017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mars K, Wallert J, Held C, Humphries S, Pingel R, Jernberg T, et al. Association between β-blocker dose and cardiovascular outcomes after myocardial infarction: insights from the SWEDEHEART registry. Eur Heart J Acute Cardiovasc Care. 2021;10:372–9. doi: 10.1093/ehjacc/zuaa002. [DOI] [PubMed] [Google Scholar]