Abstract
Gastrointestinal (GI) peptide hormones are chemical messengers that regulate secretory, mechanical, metabolic, and trophic functions of the gut. Restorative proctocolectomy (RPC) or resection of the colon and rectum with maintenance of intestinal continuity through the construction of an ileal pouch reservoir and preservation of the anal sphincters has become the standard of care for the surgical treatment of ulcerative colitis and familial adenomatous polyposis. The manipulation of the digestive system to create the ileal pouch involves altering gut-associated lymphoid tissue among other anatomic changes that lead to changes in GI peptides. In addition, the ileal pouch epithelium responds to a wide variety of stimuli by adjusting its cellularity and function. These adaptive mechanisms involve systemic factors, such as humoral and neural stimuli, as well as local factors, such as changes in intestinal peristalsis and intraluminal nutrients. There have been conflicting reports as to whether the alterations in GI hormones after RPC have actual clinical implications. What the studies on alterations of GI peptides’ response and behavior after RPC have contributed, however, is a window into the possible etiology of complications after pouch surgery, such as pouchitis and malabsorption. Given the possibility of pharmacologically modifying GI peptides or select components of adaptation as a therapeutic strategy for patients with ileal pouch dysfunction or pouchitis, a clear understanding of human pouch mucosal adaptation is of paramount importance. In this review, we summarize the evolution of the RPC and its effects on the GI hormones as well as their possible clinical implications.
Keywords: Restorative proctocolectomy, Evolution of functional outcome, Complications, Gut hormones, Epithelial integrity, Quality of life
Introduction
Gastrointestinal (GI) peptide hormones are chemical messengers that regulate secretory, mechanical, metabolic, and trophic functions of the gut. In addition, they can lead to disease states through hyper- or hyposecretion or through anomalies of sensitivity and/or metabolism. These GI hormones are useful as diagnostic tools and may have therapeutic applications for disease states. Restorative proctocolectomy (RPC) or ileal pouch anal anastomosis (IPAA) preserves anal sphincters and has become the standard of care for the surgical treatment of ulcerative colitis (UC) and familial adenomatous polyposis (FAP). Plasma and gastric levels of GI hormones after RPC have been reported [1–14], suggesting that postoperative GI motility may have a period of relative hypomotility that is associated with random, disorganized bursts of electrical activity [15] and that the gut hormones are involved in mediating the adaptive response of the intestine after RPC. The manipulation of the digestive system to create the pouch in this procedure involves altering gut-associated lymphoid tissue that leads to adaptive changes in GI peptides. There have been conflicting reports on whether these alterations in fasting and meal-stimulated plasma levels of different intestinal hormones after RPC have actual clinical implications. What the studies on alterations of gut peptides’ response and behavior after RPC have contributed, however, is a window into the possible etiology of complications after pouch surgery, such as pouchitis and malabsorption. In this review, we summarize the evolution of the RPC and its effects on the GI hormones and their possible clinical implications.
History
The surgical treatment for UC and FAP in the early 20th century required proctocolectomy and permanent end ileostomy despite poor stomal appliances and ileostomy techniques. Because of these stoma difficulties and the poor aesthetic and esteem issues associated with it, surgeons and patients wanted to attempt to maintain intestinal continuity. The evolution of the RPC, therefore, started with a direct anastomosis between the anus and the terminal ileum without a pouch being created. In 1933, Nissen [16] reported a successful result after an ileo–anal pull through procedure in a patient with familial adenomatous polyposis (FAP). In 1947, Ravitch and Sabiston [17] reported a proctocolectomy with rectal mucosal stripping followed by the distal ileum being passed through the remaining tube of rectal muscle and stitched to the anal skin as a straight ileo–anal anastomosis. In 1948, Ravitch followed the initial report with one of a proctocolectomy with mucosal proctectomy (excising the distal rectal wall as well) and straight ileo–anal anastomosis [18]. Later in 1951, Ravitch [19] claimed satisfactory functional and metabolic outcome with these procedures, but other researchers [20–22] reported frequent defecation, poor continence, and perineal pain (likely due to perineal irritation from frequent bowel function) with these methods. Moreover, mucosectomy was often technically difficult with a high rate of septic complications of about 70–80%, and therefore, the technique was essentially abandoned.
The return to intestinal continuity seemed even less necessary with the introduction by Brooke in 1952 [23] of an improved ileostomy technique through eversion and immediate mucocutaneous suturing (Fig. 1) and the development of better stoma appliances. Therefore, proctocolectomy and Brooke ileostomy became the first choice for the treatment of ulcerative colitis. During this period, several reports of animal studies [24–26] and human studies [17, 18] produced evidence that the incorporation of an ileal reservoir with ileoanal anastomosis improved functional results considerably as compared to a straight ileoanal (Fig. 2) and/or ileorectal (Fig. 3) technique. Despite initial concerns about preservation of normal bowel function, the operation was sporadically reported during the following decades until later studies showed that the presence of an ileal reservoir, physiologically, was consistent with normal intestinal function. In 1969, Kock [27] introduced the continent ileal reservoir through construction of an internal ileal pouch and creation of an ileostomy after complete proctocolectomy (Fig. 4).
Fig. 1.
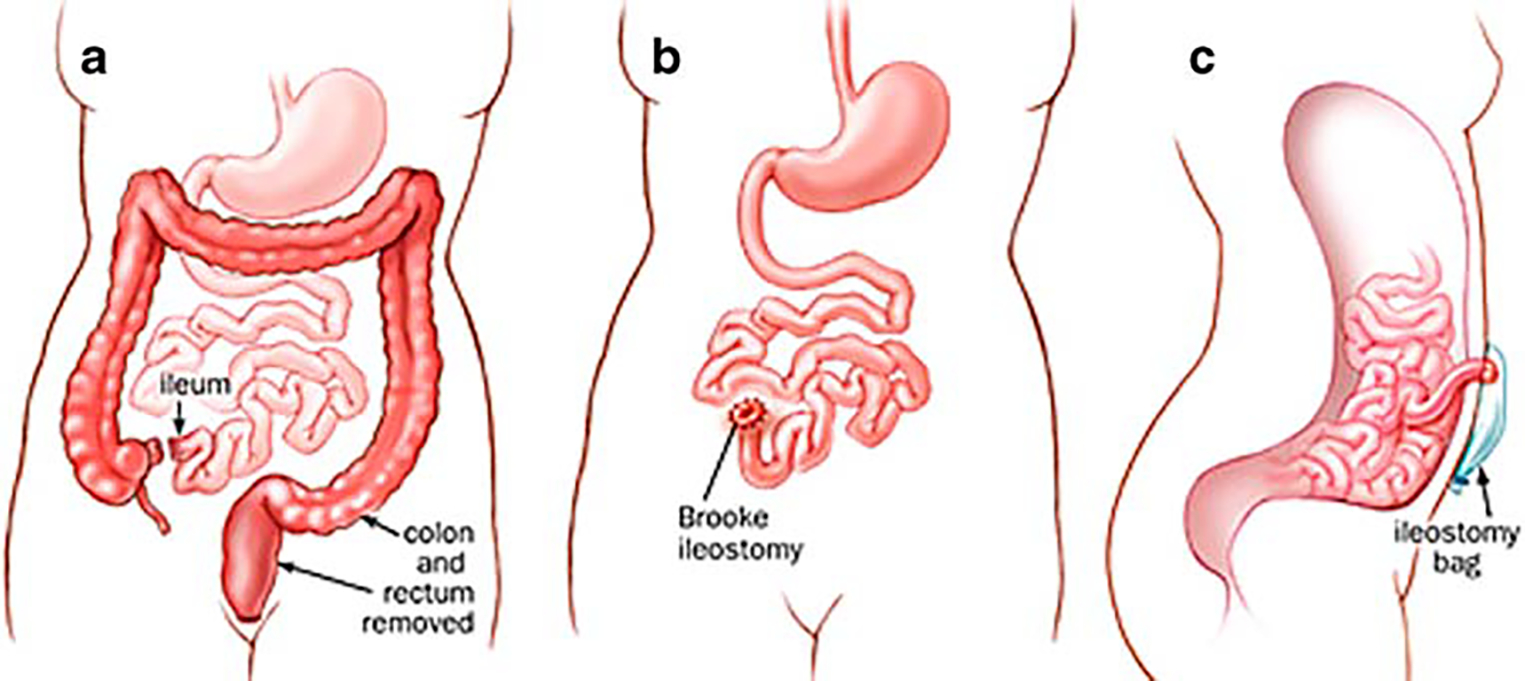
Total proctocolectomy with Brooke ileostomy. a Proctocolectomy; b Brooke ileostomy; c side view with ileostomy bag
Fig. 2.
Proctocolectomy with straight ileoanal anastomosis
Fig. 3.
Colectomy with straight ileorectal anastomosis (ileorectostomy)
Fig. 4.
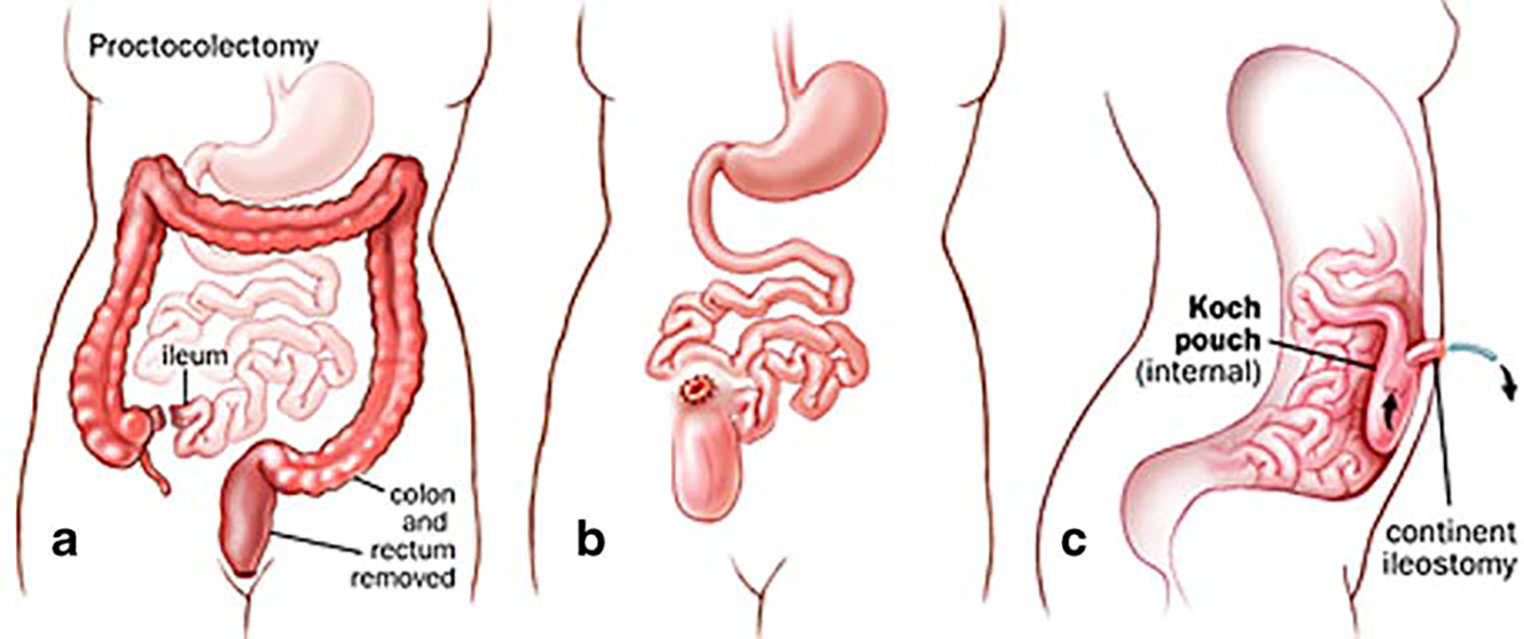
Total proctocolectomy with Koch pouch (continent ileostomy). a Proctocolectomy; b with internal Koch ileostomy; c side view
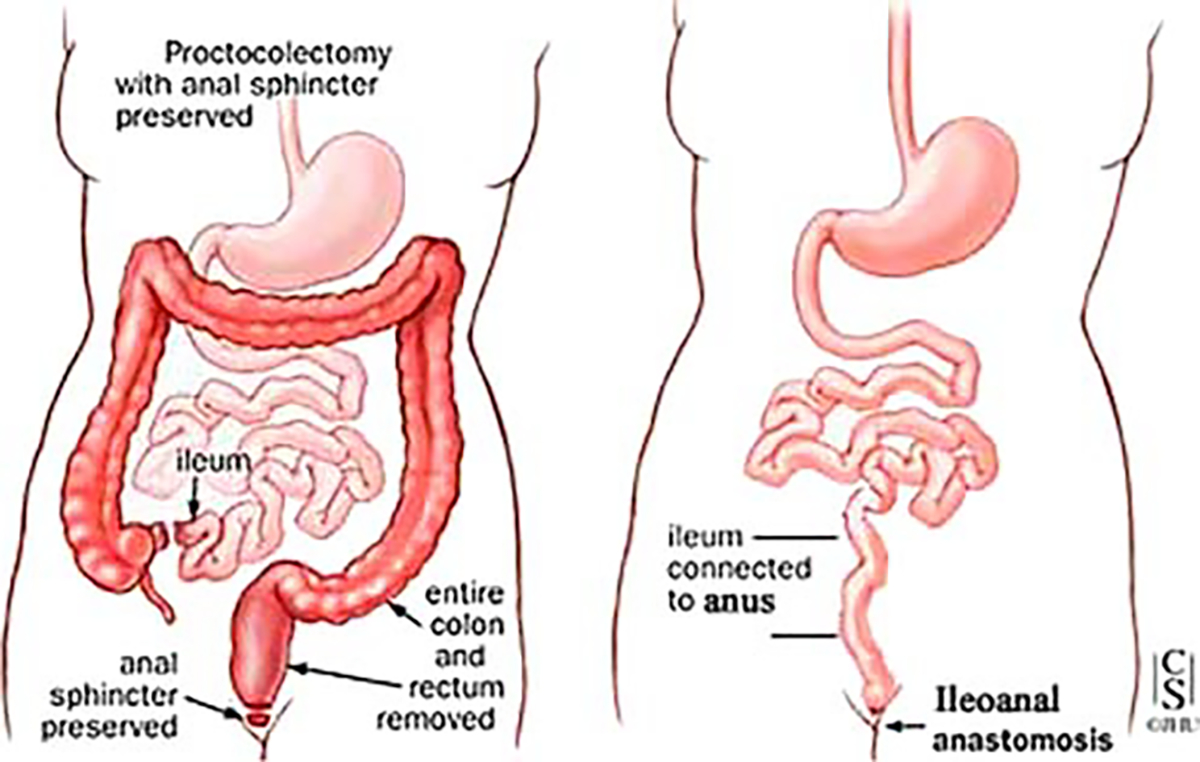
In 1978, Parks and Nicholls [28] presented the first successful use in humans of the pelvic ileal pouch (Fig. 5). The functional results, in terms of stool frequency and anal continence, were very encouraging compared to those reported after straight ileo–anal anastomoses. However, 50% of the patients (with S-shaped reservoir) were not able to defecate spontaneously and therefore emptied the reservoir with a catheter [28–30]. Moreover, there was a high incidence of major complications with a reported pelvic sepsis rate of about 20% [31–35]. Parks et al. [28] advocated preservation of a long rectal muscle cuff almost enclosing the ileal pouch. The procedure then involved mucosal dissection performed via abdominal and transanal approaches with a long outlet between the pouch and the anal canal. The reason for such an approach was, firstly, to preserve rectal sensation considered essential for continence [31, 32], and secondly, to reduce disturbances in sexual function commonly encountered after conventional proctocolectomy [32]. Mucosal dissection of the low muscular cuffs contributed to a high pelvic sepsis rate, and a long pouch outlet lead to functional imperfection [30]. Shortening of the efferent conduits and the muscle cuffs reduced these complications significantly [30], and postoperative continence was improved significantly after reduction of anal dilatation and preservation of the transitional zone.
Fig. 5.
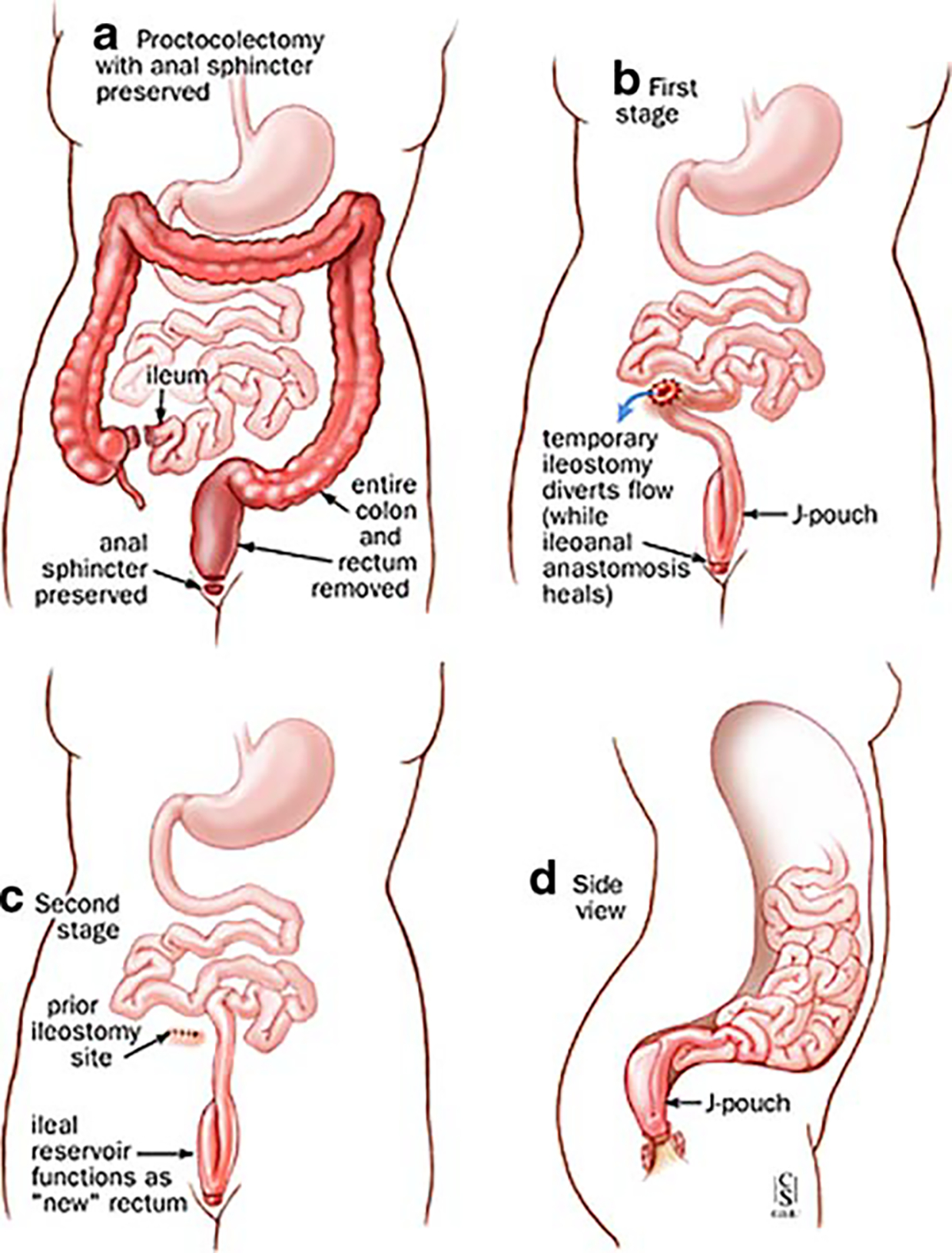
Restorative proctocolectomy (ileoanal pouch anastomosis, two stages procedure). a–d Two stages of restorative proctocolectomy. a Proctocolectomy with anal sphincter preserved. b First stage. c Second stage. d Side view
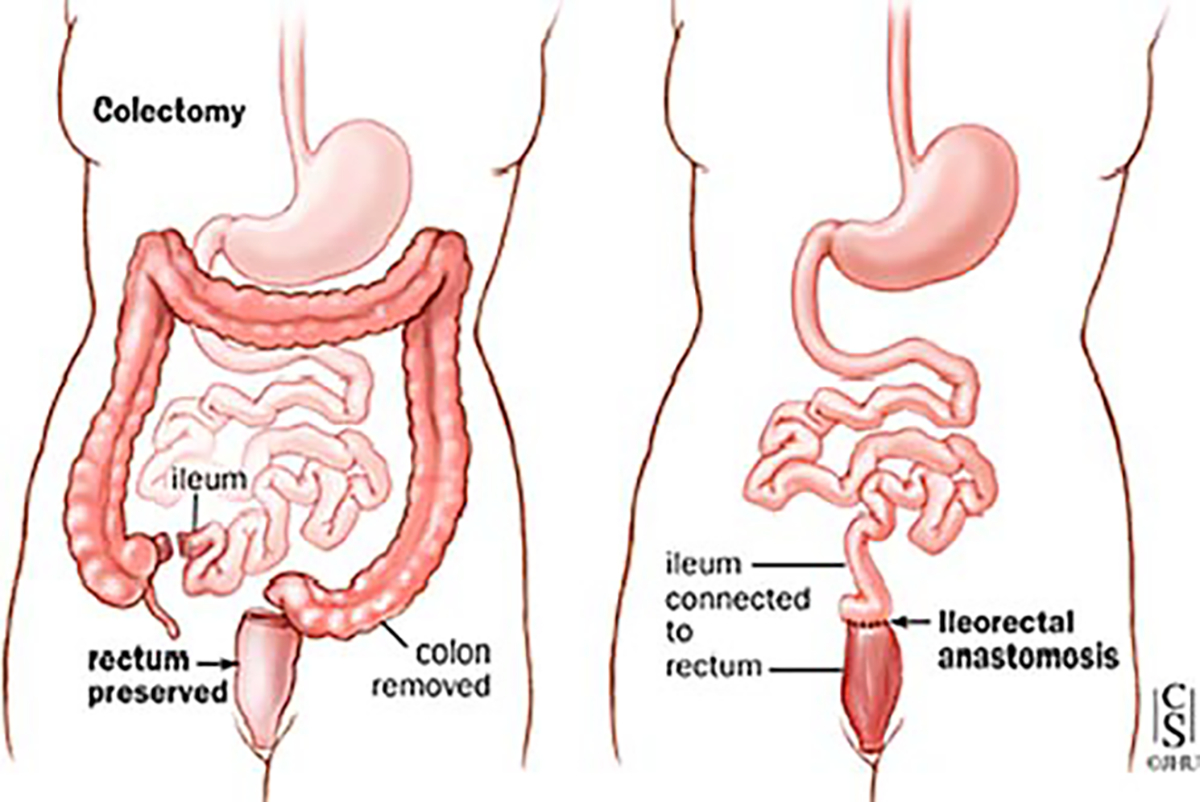
The relative importance of the sphincter mechanism and distal rectum as a contributory factor to overall continence is not fully understood, and therefore, alternate different techniques have been employed for the proctectomy as well. The transanal approach with performing mucosectomy from below has been thought to cause direct sphincter damage due to stretch, so a different technique has been employed to avoid the stretch by performing the dissection entirely from above—both technically demanding procedures depending on the pelvic anatomy. Transection of the rectum just above the pelvic floor, e.g., omitting the tedious mucosal dissection from above or below and leaving only a short rectal muscle cuff, and thus, a short outlet eliminated the need for catheterization [33–35] without affecting continence adversely [36, 37]. Avoidance of the mucosectomy with low division of the rectum and maintenance of the most distal 2–3 cm of rectal mucosa (and utilization of a double-stapled technique for the ileal-pouch anastomosis) has shortened operative times with good functional results but need for continued mucosal surveillance. Unfortunately, results of mucosectomy vs double-stapled technique are contradictory in many reports and head-to-head comparative studies are scarce [38–41].
The initial poor functional results of ileo–anal pouches led to a search for other techniques for the ileal pouch construction for which different pouch conformations were attempted (Fig. 6) [30, 42, 43]. In the original S-shaped reservoir design [28], the terminal ileum is folded into three loops 12–15 cm long, leaving a short segment of bowel projecting distally. The loop is sutured side-to-side and the lumina opened. The ileo–anal anastomosis is made end-to-end between the short projecting ileal segment and the anal canal. The J-shaped reservoir with a direct reservoir–anal anastomosis, introduced by Utsunomyia of Kyoto [29] (Fig. 7), soon became one of the most popular pouch configurations, partly due to its technical simplicity but also due to its favorable functional characteristics. It is constructed by folding the terminal ileum into two segments about 15–20 cm long which are sutured side-to-side, opened and joined together forming a pouch by a lateral anastomosis. The ileo–anal anastomosis is constructed side-to-end at the apex of the reservoir. Later, Nicholls [43] introduced the W-shaped or quadruple pouch, which is accomplished by adding another J to the first, which necessarily requires an additional length of ileum to be used in its construction. Attempts have been made to evaluate the functional merits of different pouch designs. Nicholls and Pezim [43] report superior results with the W-pouch compared to the S-pouch both with regard to defecation frequency and leakage. However, other studies fail to confirm this statement [44, 45]. There appear to be no major functional differences between the S- and J-shaped pouches [42, 45]. With its technically easier construction, especially with the advent of surgical stapling devices, the J-pouch has become the RPC pouch configuration of choice [29], and during the 1980s, the RPC became the first effectively functional continent alternative for the curative treatment of UC and FAP.
Fig. 6.
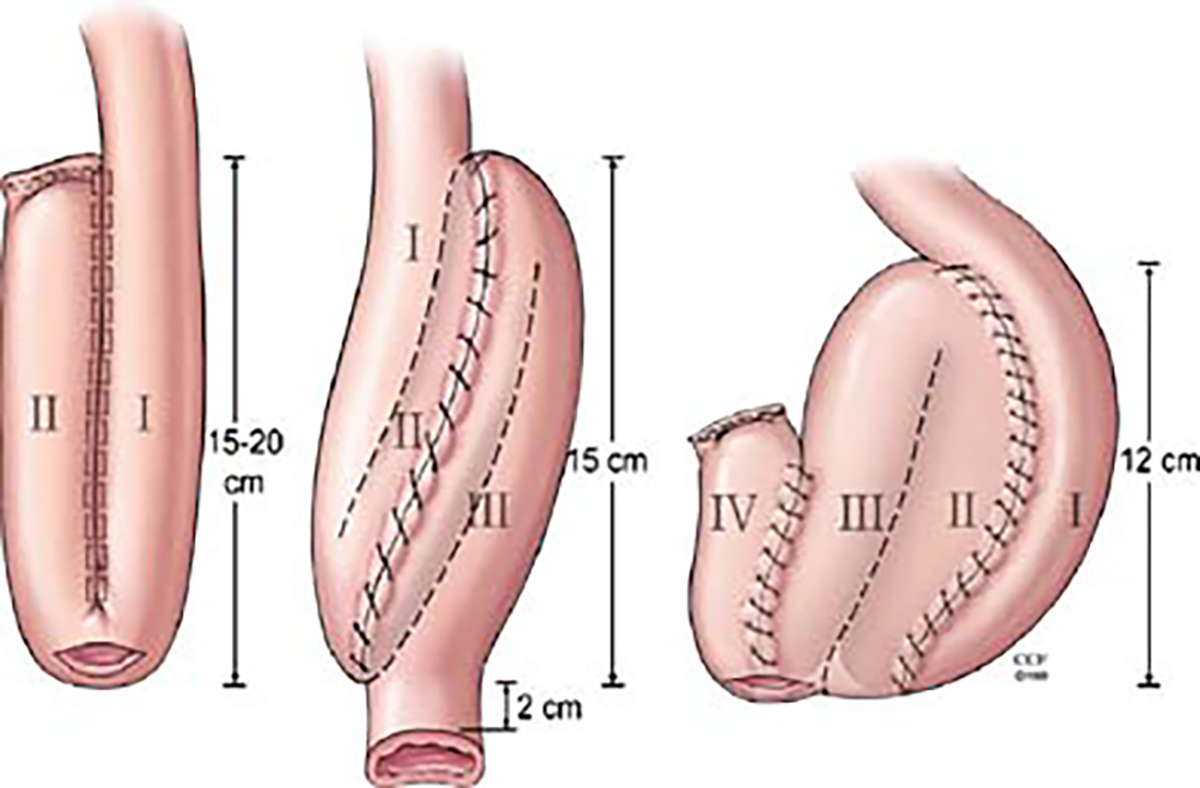
Different pouch conformations (J-, S- and W-shaped reservoirs)
Fig. 7.
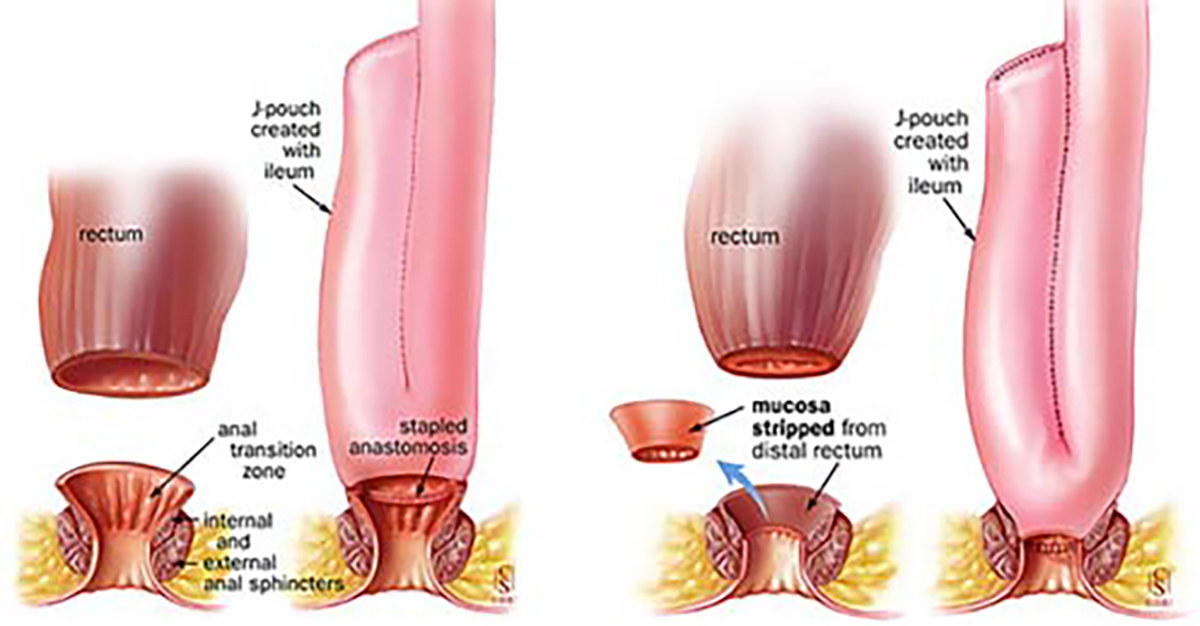
J-shaped reservoir (Utsunomyia). Ileal pouch anal anastomosis without stripping (double-stapled technique, left) and with rectal stripping (mucosectomy, right)
The functional outcome [1, 47–84] and patient quality of life [47, 50, 54, 63, 66–82] after RPC has been extensively reported. Although patient satisfaction is considered to be high, results are not perfect regardless of technique. There are consistent reports on changes of enteric bacteriology [85–87], mucosal histological morphology [86–91], and absorption [84–87, 92, 53] of the acceptably functioning reservoir, however, regardless of the operative technique for ileal pouch construction. Studies regarding short- and long-term postoperative morbidity have been widely reported (Tables 1 and 2) [2, 23, 28, 34, 93–99]. The enigmatic syndrome of pouchitis (incidence range: 20–60%) caused by an unspecific inflammation of the reservoir mucosa seems to be the main cause of late morbidity [2, 93–99].
Table 1.
Short- and long-term postoperative classification of complications of the restorative proctocolectomy and ileal pouch–anal anastomosis
| Complication | Percentage (%) | Cited references |
|---|---|---|
|
| ||
| Pouchitis | 30–59 | Sandborn et al. 1994, Hurst et al.1996, Meagher et al.1998, Simchuk et al. 2000, Tiainen and Matikainen 2000, Heuschen et al. 2001 |
| Pelvic sepsis | 3–18.6 | Meagher et al. 1998, Johnson et al. 2001 |
| Anastomotic stricture/stenosis | 4–11 | Dayton et al. 2002, Prudhomme et al. 2003, Machelassi et al. 2003 |
| Fistulae | 1.6–9 | Lee et al. 1997, Belliveau et al. 1999, Dayton et al. 2002, Breen et al. 1998, Dayton et al. 2002, Zinicola et al. 2004, Gorfine et al. 2004, Tekkis et al. 2005 |
| Dysplasia/neoplasia/cancer | 0.5–15 | Baker et al. 1978, Grudfest et al. 1981, Johnson et al. 1983, Puthu et al. 1992, Hoenner et al. 1994, Ziv et al. 1994, von Herbay et al. 1996, Sequens et al. 1997, Sarigol et al. 1999, Vuilleumier et al. 2000, Thompson-Fawcett et al. 2001, Heuschen et al. 2001b, Gorfine et al. 2004, Borjesson et al. 2004, Rutter et al. 2004 |
| Pouch failure | 0.3–15 | Heppel et al. 1983, Pemberton et al. 1987, Keränen et al. 1997, Dayton et al. 2002, Fazio et al. 1995, Lepistö et al. 2002, Öresland et al. 2006 |
| Cholelithiasis | 2–5 | Harvey et al. 1991, Makino et al. 194, Mibu et al. 1995, Mibu et al. 2001 |
| Iron deficiency anemia | 6–20.8 | M’Koma et al. 1994a, M’Koma et al. 1994b, Tiainen et al. 2000, Kuisma et al. 2001, M’Koma et al. 2006 |
| Vitamin B12 deficiency anemia | 3–9 | Jagenburg et al. 1975, Nilsson et al. 1984, Bayat et al. 1994, M’Koma et al. 1994a, M’Koma et al. 1994b, M’Koma et al. 2006 |
| Crohn’s disease | 2.7–13 | Gemlo et al. 1992, Goldstein et al. 1997, Deutch et al. 1991, Neille et al. 1999, Yu et al. 2000, Keighley et al. 2000, Peyregne et al. 2000, Fazio et al. 2003, Shen et al. 2005, Fukushima et al. 2006 |
| Cuffitis | 2–7 | Schmitt et al. 1992, Shen et al. 2002, Shen et al. 2004, Mattioli et al. 2005, Abdelrazeq et al. 2005 |
| Osteoporosis | 2.3–5.0 | Sher et al. 1996, Abitbol et al. 1997, Teixeira et al. 1999, Valentine et al. 1999, Kuisma et al. 2002 |
| Sinuses | 2.1–12 | Beck et al. 2001, Prudholmme et al. 2003, Swain et al. 2004, Gorfine et al. 2004, Mehdi et al. 2004 |
| Leaks | 13–20 | Shen et al. 2005, M’Koma et al. 2006 |
| Abscess | 1.3 –6 | Belliveau et al. 1999, Bursics et al. 1999, Rossi et al. 2002, Heuschen 2002, Banerjee et al. 2004 |
| Adhesion | 5–23 | Van de Pavoordt et al. 1987, Edwards et al. 1998, Ikeuchi et al. 2004, Parc et al. 2004, Patel et al. 1995, Parker et al. 2005, Wong et al. 2005 |
| Prolapsy | 0.4–2 | Moskowitz et al. 1986, Sheperd et al. 1987, Nicholls et al. 1989, Blazeby 1994, Belliveau et al. 1999, Ehsan et al. 2002, Williams et al. 2003, Williams et al. 2004 |
| Bacterial overgrowth | – | Stryker 1985, Nasmyth et al. 1989, Natori, et al. 1992, Natori et al. 1992, Salemans et al. 1993, Taylor et al. 1998, Gionchetti et al. 1999, Whinerary et al. 2000, Kelly et al. 2004, Gionchetti et al. 2004, Gosselink et al. 2004 |
| CMV infection | 0.5–1 | Moskowitz et al. 1986, Mooka et al. 1998, Munoz-Juarez et al. 1999, Munoz-Juarez et al. 2000, Pfau et al. 2005 |
| Clostridium difficile infection | 1 –5 | LaMont et al. 1980, Bolton et al. 1986, Fekety et al. 1997, Cleary et al. 1998, Heuschen et al.2001, Mann et al. 2003, Shen et al. 2003 |
| Inflammatory polyps | 0.5–2 | Keighley et al. 1987, Thompson et al. 1998, Freeman et al. 2001, Saigusa et al. 2003, Polese et al. 2003, Watanabe et al. 2006 |
| Adenoma | 7–15 | Malassagne et al. 1995, Enriquez et al. 1998, van Duijvendijk et al. 1999, Parcy et al. 2001, Remzi et al. 2001, Tulchinsky et al. 2005 |
Includes: surgical/mechanical, inflammatory/infectious, dysplasia/neoplasia/cancer, and systemic/metabolic
Table 2.
Short- and long-term postoperative classification of functional outcome complications of the restorative proctocolectomy and ileal pouch–anal anastomosis
| Function | Percentage (%) | Cited references |
|---|---|---|
|
| ||
| Number of stools/24 h | 5–7 | Keränen et al. 1997, Meagher et al. 1998, Johnson et al. 2001 |
| Need for night evacuation | 40–80 | Romanos et al. 1997, Keränen et al. 1997 |
| Urgency | 6–10 | Romanos et al. 1997 |
| Incontinence | ||
| Occasional | 10–39 | Meagher et al. 1998, Tiainen and Matikainen 2000 |
| Frequent | 3–7 | Pemberton et al. 1987, Tuckson et al. 1991, Michelassi et al. 1993 |
| Use of retarding drugs | 29–75 | Keränen et al. 1997, Romanos et al. 1997, Johnson et al. 2001 |
| Protective pads | 17–30 | Meagher et al. 1998, Johnson et al. 2001 |
| Perianal soreness | 30–48 | Meagher et al. 1998, Johnson et al. 2001 |
| Irritable pouch syndrome | 43–46 | Camiller et al. 1999, Shen et al. 2002, Hahnloser et al. 2004, Shen et al. 2004 |
| Liver dysfunction | 7–19 | Nicholls et al. 1981, Max et al. 1987, Fiorentini et al. 1987, Jensen et al. 1992, M’Koma et al. 1994, 2006 and 2007 |
| Pelvic floor dysfunction | 8–40 | Öresland et al. 1989, Gemlo et al. 1992, Björk et al. 2001, Kraugz et al. 2005, Öresland et al. 2006, Arberg et al. 2006, Tilney et al. 2006, Prete et al. 2006, Baig et al. 2006, Strong et al. 2006, Kartheuser et al. 2006, Börjesson et al. 2006 |
| Poor pouch compliance | Groom et al. 1994, Goldberg et al. 2006 | |
| Pseudo-obstruction | 3–11.7 | Stabile et al. 1992, Stewart et al. 1994, Suilleahhain et al. 2001, Machelassi et al. 2003, Stabile et al. 2005 |
Gastrointestinal hormones
GI hormones are defined as peptides produced by endocrine cells located in the GI tract mucosa [100–111]. These hormones are released into the circulation under the influence of alimentary stimuli and are then involved in the regulation of secretion, motility, and growth of the digestive system [101–104]. A number of GI peptides are also produced by neurons in the central and peripheral nervous systems, particularly enteric neurons [111]. They are delivered locally (paracrine function) to their target cells, without entering the blood circulation, and/or exert biological effects outside the digestive system (endocrine function).
Cells producing GI hormones
Two types of cells synthesize and secrete peptides in the GI tract: endocrine cells [112, 113] found in the GI mucosa and pancreatic islets and neurons [114] located in all layers of the GI wall. Outside the digestive tract, only neurons produce GI peptides [114]. Together with the central and peripheral nervous systems, GI peptides influence (in a stimulatory or inhibitory manner) a number of GI functions, including other exocrine and endocrine secretions, motility, growth, and blood flow. Some peptides also affect secretion of other hormones and/or motility at extragastrointestinal sites, particularly in the circulatory, respiratory, and urogenital systems. They also control liver metabolism and exert various effects in the central nervous system, one of which is the regulation of appetite. Many of these affects result from a direct interaction of the peptides with epithelial, smooth muscle, or endocrine target cells, but some effects are hormonally and/or neurally mediated.
Exocrine secretions of the gastrointestinal tract
GI hormones regulate the secretion and/or absorption of water, electrolytes, enzymes, and mucus by epithelial cells of the GI, pancreatic, and biliary tracts. Thus, in glands of the gastric fundus, which consist of three morphologically and functionally distinct cell types, GI hormones affect the secretion of hydrogen ions by parietal (or oxyntic) cells [115], that of pepsinogen by chief cells, and the secretion of mucus by mucosal cells. Similarly, in the exocrine pancreas, which also shows cellular heterogeneity, GI hormones affect the secretion of water and bicarbonate ions by duct cells and that of enzymes by acinar cells. In intestinal epithelium, they regulate secretion in the crypts and absorption by the villi. Gastric acid secretion is stimulated primarily by gastrin and gastrin-releasing peptide (GRP)/bombesin. Gastric pepsin secretion is stimulated by gastrin, cholecystokinin (CCK), secretin, and vasoactive intestinal polypeptide (VIP). Intestinal secretion of water and electrolytes is also stimulated by VIP. Pancreatic secretion of enzymes is primarily stimulated by CCK but also by secretin, VIP, substance P (SP), and bombesin. Bile secretion is stimulated by most peptides of the secretin/glucagons and gastrin/CCK families. Virtually, all GI secretions in the basal and hormone-stimulated state are inhibited by SS.
Endocrine secretions of the gastrointestinal tract
Most peptides of the gastrin/CCK and glucagons/secretin families stimulate the release of pancreatic hormones (especially insulin and SS). Bombesin and SS affect the secretion of virtually all peptides; the former is stimulatory, and the later inhibitory. Many of these effects occur via direct interaction of the peptides with endocrine effector cells, although the inhibitory effects of glucagons, VIP, and secretin on gastrin release are mediated by an increase in release of SS [105, 116].
Gastrointestinal motility
The effects of GI peptides on motor function of the GI tract are based on mechanical and/or myoelectrical activity in humans [117]. In general, gastrin, CCK, motilin, bombesin, and neurotensin are primarily stimulatory, whereas glucagon, VIP, gastric inhibitory peptide (GIP), secretin, and SS have mostly inhibitory affects on motility. Some peptides regulate the migrating motor complex (MMC) or myoelectric complex, a cyclical pattern of interdigestive motor activity that propagates from the stomach to the distal intestine [118]. Motilin initiates a fasting pattern (MMC propagation), in contrast to gastrin, CCK, bombesin, and neurotensin, which induce a fed pattern. The rate of gastric emptying and intestinal transit time are traditionally parameters affected by GI hormones in vivo, which in part, reflect changes in motor activity. The effects of GI peptides on GI motility observed in vitro are generally consistent with those observed in vivo [118, 119], although the type of smooth muscle affected and the mechanism of the response (direct and/or neurally mediated) varies depending on the particular peptide and GI region.
Gastrointestinal growth
GI hormones have effects on the growth of epithelial cells in the GI tract and pancreas [119–121]. Some peptides, such as gastrin, CCK, and bombesin, are stimulatory, whereas other peptides, such as VIP and SS, are inhibitory. Effects of GI hormones on the growth of GI epithelial cells can be functionally dissociated from the effects on secretion. GI hormones, especially gastrin, play an important role in the structural and functional changes of the GI mucosa, which occur in response to feeding, in neonatal development, and postoperatively, all of which reduce the amount or alter the position of absorbing mucosa.
Gastrointestinal blood flow
GI peptides regulate GI blood flow in association with the autonomic nervous system, biogenic amines (catecholamines, histamine), non-GI peptides (bradykinin, vasopressin, angiotensin), and intrinsic mechanisms (metabolic and myogenic) [121]. Thus, gastrin, CCK, secretin, glucagons, and neurotensin increase gastric, intestinal and/or pancreatic blood flow, as do VIP and SP (which also affect other vascular beds in the body). It has been suggested that endogenous CCK, secretin, and GIP play a part in the phenomenon of postprandial intestinal hyperemia, and neurotensin has been implicated as a mediator of the increased intestinal capillary permeability seen with the absorption of fat.
Non-gastrointestinal effects of GI peptides
A number of GI peptides, especially those produced by neurons, exert effects outside the GI tract [122]. These include: contraction or relaxation of various types of smooth muscle, especially vascular smooth muscle (VIP, SP, neurotensin), inotropic and chronotropic effects on the heart (VIP), stimulation of water and electrolyte secretion in various exocrine glands (VIP), and stimulation or inhibition of pituitary hormone release (CCK, VIP, SS, SP).
Colon as an endocrine organ
While the colon mainly functions to control propulsion of the fecal stream and to absorb fluids (and some nutrients), it is also considered an endocrine organ [123–131] by producing regulatory peptides via mucosal endocrine cells [128, 131, 132]. With the availability of radioimmunoassay and immunofluorescence techniques, it has been shown that not only are endocrine cells present in the colonic mucosa, but peptides could be identified in mucosal extract and the venous effluent from the colon. Among inhibitory peptides found in the human colonic mucosa are enteroglucagon, VIP, and neurotensin [128, 133].
It was not until the early to mid-20th century that there was an appreciation of the endocrine function of the colon. This was first noted in studies on the colon’s effect on gastric function. In 1930, Kosaka and Lim [134] demonstrated that extracts from colonic mucosa that had been exposed to luminal fat were able to inhibit gastric acid secretion. Seal and Debas [123] later showed that colonic perfusion with several types of test substances was indeed a potent inhibitor of gastric acid secretion in animals. Soon-Shiong et al. [124] and Jian et al. [128] confirmed that such an inhibitory influence of the colon on gastric secretion was noted to occur similarly in humans.
Further observations on endocrine functions of the colon, has shown that the colon also influences pancreatic exocrine secretion [110, 111]. Hage et al. [125] noted that colonic perfusion with oleic acid markedly inhibited secretin-stimulated pancreatic secretion. In 1974, Harper et al. [126] demonstrated that colonic extracts given intravenously inhibited stimulated pancreatic secretion (see “Gastric Inhibitory Peptide” below).
Postoperative mucosal adaptation
With removal of the colon and rectum during RPC [28, 29, 44, 135], there is a resulting loss of endocrine cells and neurons in the enteric nervous system. The loss of the hormones produced by these cells, therefore, exerts an influence on the remaining bowel, including its proliferative adaptive abilities [136–138]. There are clear histological adaptive changes in the pouch epithelium both in animals [12] and in humans [87, 88] after RPC. The main features are chronic inflammatory infiltrate in the lamina propria, loss of villous length, and crypt hyperplasia making the pouch mucosa appear more like colorectal mucosa. Such changes were first reported by Senn et al. in 1888 [139] after surgical intestinal manipulations in patients with intestinal obstruction. Similar observations were noted by Flint et al. [140] confirming adaptive changes in patients with extensive resections of the small intestine. After these initial studies detailed the morphological and functional changes in the bowel, research is seeking to understand the basic science pathways underlying this adaptive process, thought to be linked to changes in luminal nutrition, endogenous secretions, and GI peptides.
Effects of Restorative Proctocolectomy on Gastrointestinal Hormones
The Gastrin family
The gastrin family hormones include gastrin, ghrelin, and CCK (Table 3).
Table 3.
Gut hormones: gastrin family: their mode of delivery, site of action, their physiologic effects, and the levels post-RPC surgery and cited references
| Hormone | Modes of delivery | Site of action | Effects | Levels in RPC | Cited references |
|---|---|---|---|---|---|
|
| |||||
| Gastrin | Endocrine | Stomach (parietal cells) | ↑ Gastric acid secretion | Identical | Inoue et al. 1982, Greenberg et al. 1989, M’Koma et al. 1999 |
| Ghrelin | Endocrine | Stomach (A-like cells) | ↑ Gastric emptying | No study in RPC | – |
| Paracrine | ↑ Gastric motility | ↑ in modulating immune response inflammatory colitis | Chang et al. 2003, Brzozowski et al. 2004, Dixit et al. 2004, Korbonits et al. 2004, Granado et al. 2205 | ||
| Cholecystokinin | Endocrine | Gall bladder | ↑ Contraction of gallbladder | ↑ in RPC | Salemans et al. 1995, Hallgren et al.1995, van Battum et al. 1999 |
| Small intestine | ↓ Gastric emptying | ||||
| ↑ Pancreatic enzyme secretion | |||||
| ↑ Memory enhancement Satiation | |||||
RPC Restorative proctocolectomy, IPAA ileal pouch anal anastomosis
Gastrin
The main sites of gastrin synthesis are in the upper small intestine and in open-type G cells in the gastric antrum [104]. It is the most important peptide in the physiologic regulation of gastric acid secretion [141–143] in response to a meal [115, 142–144]. Excessive secretion of gastrin in humans may lead to basal acid hypersecretion and hyperplasia of the acid-secreting mucosa [145], peptic ulcers, and diarrhea [3, 146, 147].
Gastrin has been studied in UC [3, 148–152], FAP [153], and in RPC-patients [3, 8, 129]. In UC RPC-patients without pouchitis, a Swedish study [3] demonstrated no significant changes in serum gastrin levels compared to the pre-RPC values. Despite this, in the same subjects, a significant increase in gastric acid secretion (based on direct measurements and pH monitoring) vs pre-RPC levels was found in fasting retention, basal, and after-pentagastrin stimulation. Interestingly, in UC and FAP RPC-patients with pouchitis, a Canadian study [2] found a low level of gastric acid secretion. This may suggest a link between gastric acid reduction and pouchitis that does not appear to be linked to gastrin levels. Gastric hypoacidity in patients with pouchitis after RPC might reduce the sterilizing barrier of the gastric acidity [2], which may promote gut microbial proliferation and potentiation of the pouchitis. The microbial etiology of pouchitis is supported by the fact that it is often relieved by antibiotics such as metronidazole [63, 65]. The possible link between pouchitis and gastric hypoacidity may warrant clinical trials looking at the use of agents to increase gastric acidity (e.g., gastrin analogs) or avoiding antacids (e.g., proton pump inhibitors or H2 receptor blockers) in RPC patients with pouchitis.
Ghrelin
Ghrelin is a novel gastrointestinal growth hormone-releasing peptide produced by P/D1 cells lining the fundus of the human stomach, with potential endogenous anti-inflammatory activities ameliorating some pathologic inflammatory conditions [154]. It is capable of down-regulating the production of proinflammatory cytokines (IL-1β, IL-6, and TNF-ά) and chemokines (IL-8) by activated monocytes and endothelial cells (Th1-mediated immune response). Newer data demonstrates novel therapeutic actions for ghrelin in a mouse model of colitis [154]. Ghrelin, also, stimulates gastric motility and emptying without affect on acid secretion and gastric endocrine cells [155] and acts as an appetite stimulant. Its role as a prokinetic is not well established in humans [154, 157].
There are no study reports on ghrelin in FAP or after RPC, but its levels have been noted to be elevated in the serum of UC patients [156]. A correlation between the severity of the inflammation and ghrelin levels has been suggested, although no association was found with clinical disease activity. Patients with ileal Crohn’s disease had higher ghrelin levels compared to colonic Crohn’s disease [154–157]. Further research may lead to advances in the treatment of conditions such as inflammatory bowel disease, pouchitis, and postoperative ileus.
Cholecystokinin (CCK)
CCK is secreted by open-type cells (I cells), which are most densely located in the small intestine and gradually decrease in number toward the large intestine [158–160]. CCK has a wide range of effects on the GI tract. Three physiological effects on gut motility have been identified: contraction of the gallbladder [158], relaxation of the sphincter of Oddi [159], and inhibition of gastric emptying [158]. CCK also increases pancreatic enzyme secretion [158]. Its role on colonic motility is controversial. While Behar et al. [158] were able to demonstrate increased colonic contractions after CCK administration, Niederau et al. [160], Chijiiwa et al. [161], and Makino et al. [162] could find no effect of CCK on colonic motility. Several studies in different species have reported that after colectomy, there are higher plasma concentrations of CCK, both basal and postprandial, compared to healthy controls [163–165]. It has been suggested that this is due to depletion of an inhibitory factor of CCK secretion which is released from the colon. It is not known if the elevated CCK levels observed in colectomy patients [163–165] have any impact on GI motility or health.
RPC is known to predispose patients to the formation of gallstones [165, 166]. Gallstones formed in proctocolectomy patients are mainly composed of cholesterol [162]. Harvey et al. [163] reported that 3 of 20 of his colectomy patients had gallstones classified as cholesterol stones. On the other hand, Galatola et al. [164] suggested that RPC did not predispose patients to the formation of cholesterol gallstones. Noshiro et al. [165] found pigment gallstones in seven of ten colectomized animals. The stones were composed mainly of sodium bilirubinate and proteins and differed from the black pigment stones formed in humans. These studies suggest that pigment gallstones may not be formed in colectomy patients. However, one case of pigment gallstone formation in animals after RPC has been reported [166]. The interrelationship between the colon, ileal pouch, CCK, and gallstone formation has yet to be clarified.
The Secretin family
The hormones in the secretin family include secretin, vasoactive intestinal polypeptide (VIP), peptide histidine methionine (PHM), enteroglucagon (GLP), and gastric inhibitory peptide (GIP) (Table 4).
Table 4.
Gut hormones: secretin family: their mode of delivery, site of action, their physiologic effects, and the levels post-RPC surgery and cited references
| Hormone | Modes of delivery | Site of action | Effects | Levels in RPC | Cited references |
|---|---|---|---|---|---|
|
| |||||
| Secretin | Endocrine | Pancreatic acini | ↑ HCO3-rich secretion | No study in RPC | |
| Liver | ↑ Bile secretion | ||||
| Vasoactive intestinal peptide (VIP) | Neurocrine | Stomach Liver Pancreas |
↓ Gastric acid secretion ↑ Glucose release ↑ Insulin release ↑ HCO3-rich secretion ↓ Absorption of water and sodium (inhibitor) |
↑ in RPC with pouchitis | Keränen et al. 1996a, Keränen et al. 1996b |
| Peptide histidine methionine (PHM) | Neurocrine | Ileum Colon Trachea Stomach Gallbladder Blood vessels |
↑ Intestinal secretion of water and electrolytes (stimulatory) ↑ Pancreatic secretion of water and bicarbonate in vivo and of amylase in isolated acini (stimulatory). Relaxation of tracheal, gastric and gallbladder smooth muscle and vasodilation ↑ Insulin, glucagon and prolactin secretion (stimulatory). |
No study in RPC | |
| Glucagon like peptide | Endocrine | Lower Ileum Colon |
↑ Gastric emptying ↑ Small bowel absorption ↑ Growth of small bowel mucosa ↓ Small bowel transit ↓ Gastric acid secretion ↓ Intestinal motility |
↑ in RPC | Pietroletti et al. 1990, Amstrong et al. 1991, Pironi et al. 1993, Palnaes Hansen et al. 1997 |
| Gastric inhibitor peptide (GIP) | Endocrine | Duodenum Jejunum Stomach |
↓ Gastric acid secretion ↑ Insulin release ↓ Water and electrolyte absorption in ileum |
↑ in RPC | Palnaes Hansen et al. 1997 |
| Ileum | |||||
Secretin
Secretin was the first hormone ever discovered. It is produced in the S cells of the duodenum in the crypts of Lieberkühn. Its primary effect is to regulate the pH of the duodenal contents via the control of gastric acid secretion through buffering with water and bicarbonate [167, 168]. There are no studies of secretin after colectomy and/or RPC.
Vasoactive Intestinal Polypeptide (VIP)
Initially, VIP was only considered to be a gut hormone, but it was later shown to be widely distributed throughout the central and peripheral nervous systems and to be associated exclusively with neurons [169]. The major effect of VIP is to inhibit absorption and stimulate the secretion of water and electrolytes (especially chloride) and to inhibit enzyme secretion in the small and large intestine [170, 171]. VIP also has a role in the relaxation of gut smooth muscle sphincters in response to certain stimulants [171].
VIP has been studied in RPC patients. The number and density of VIP-immunoreactive nerve fibers in the lamina propria were markedly increased in the pouch of RPC-patients with pouchitis when compared to the number of fibers in a normal RPC pouch, in the ileum of UC patients, and in the normal ileum [172]. This suggests that VIP may play a role in pouchitis perhaps by mediating inflammatory processes that may contribute to the regulation of intestinal motility [172, 173]. Hypothetically, increased VIP production could also lead to increased alkalinization of the intestinal effluent (neutralizing the gastric acid bacterial barrier), thus, permitting further bacterial overgrowth in the ileal pouch and worsening pouchitis. This has not been scientifically confirmed.
Peptide Histidine Methionine
Peptide histidine methionine (PHM) is structurally related to VIP [169, 174] and shares many of its biological activities, just less potent. It was first identified as part of the sequence of the VIP precursor, and it was later isolated from the colon [175, 176]. The mucosal–submucosal concentrations of PHM in UC show a significant decrease when compared to normal colon [177]. These results indicated that PHM, like VIP, is localized within the same neural structures that have been altered in idiopathic inflammatory bowel diseases. There are no studies regarding the effects of RPC on PHM.
Enteroglucagon
Enteroglucagon is produced by endocrine cells of the colonic mucosa and from L-cells in the distal small intestine [178–180]. It is immunochemically related to pancreatic glucagon but heterogeneous as to molecular size, charge, and nature of the glucagon-like peptides (GLP) [181–183]. Enteroglucagon stimulates liver glycogenolysis [184] and insulin release from the pancreas [185], albeit with lesser potency than pancreatic glucagons. Under both normal and pathologic conditions, enteroglucagon is an important regulator of intestinal epithelial proliferation [123, 124] by inhibiting gastric acid secretion and intestinal motility. There are at least two subtypes of enteroglucagon [186–188]: GLP-1 which stimulates the release of insulin in response to glucose and inhibits the release of pancreatic glucagon; and GLP-2 which shows a direct proliferative action on human intestinal epithelial cells suggesting that it can be a regulator of proliferation, depending on the target cell type. Most of the stimuli for the secretion of enteroglucagon are luminal [183, 185, 189] and include glucose, triglycerides, or the ingestion of a mixed meal. Two regulatory peptides known to influence the rate of enteroglucagon release are SS, which inhibits it, and bombesin, which stimulates its release.
Enteroglucagon has been noted to be increased in RPC-patients [4, 5, 13]. In contrast, Palnaes Hansen et al. [122] found a lower concentration of GLP-1 in patients after RPC than in controls or patients after proctocolectomy and end ileostomy. Therefore, increasing the circulating and tissue levels of enteroglucagon could be important in patients with intractable diarrhea by decreasing intestinal secretion, slowing intestinal transit and increasing small bowel absorption [4, 5]. Hypothetically, when the colon is resected, the production and release of enteroglucagon would increase in the remaining small bowel, suggesting that L-type endocrine cells may participate in adaptive responses that improve intestinal function after colonic surgery.
Gastric Inhibitory Peptide
In the human GI tract, gastric inhibitory peptide (GIP) is released from endocrine K-cells, which are present at highest concentration in the duodenum and jejunum [190]. GIP is a GI hormone whose major effects are to inhibit gastric acid secretion through gastric motor activity and stimulation of intestinal secretion of water and electrolytes. GIP also enhances insulin secretion in the presence of elevated glucose concentrations [122, 191]. Glucose metabolism by the β-cell is a prerequisite for GIP-stimulated insulin secretion to occur. At supraphysiological concentrations, GIP inhibits gastric acid secretion in humans.
Studies on GIP have shown how normal colon is capable of influencing pancreatic and gastric function [3, 122, 127]. Palnaes Hansen et al. [122] concluded from patients undergoing RPC for UC that the affects of the proctocolectomy on the “enteroinsular axis” may be due to adaptive changes in the small intestine and the absence of endocrine factors from the colon. This study on the enteroinsular axis in patients with UC after colectomy included patients with a conventional ileostomy, patients with an ileo–anal reservoir, and normal controls. The concentrations of GIP were measured in plasma during an oral glucose test with peak levels of GIP being significantly higher in RPC patients vs end ileostomy patients and controls [122]. The peak level was four times higher in RPC patients compared to controls... Patients with an ileo–anal reservoir in this study showed a slower GLP-1 response after intake of glucose. These results suggest that the colon, with its physiologic role as an endocrine organ, affects the enteroinsular axis through GIP release, perhaps leading to hyperinsulinemia and an impaired glucose tolerance. The clinical relevance of this is yet unclear.
The Pancreatic Polypeptide Family
The three hormones in the pancreatic polypeptide family include pancreatic polypeptide (PP), peptide tyrosine–tyrosine (PYY) and neuropeptide Y (NPY) (Table 5).
Table 5.
Gut hormones: pancreatic polypeptide family
| Hormone | Modes of delivery | Site of action | Effects | Levels in RPC | Cited references |
|---|---|---|---|---|---|
|
| |||||
| Pancreatic polypeptide (PP) | Endocrine | Pancreas | ↑ Gastric acid secretion ↓ Stimulated pancreatic & pentagastrin secretion ↑ Stomach and intestine motility |
↑ in colonic resection | Besterman et al. 1982, Flaten et al. 1982, Yamamura et al. 1985, Greenberg et al. 1989, Sharkey et al. 1990, Yanagi et al. 1991 |
| Peptide tyrosine–tyrosine (PYY) | Endocrine | Lower ileum | ↓ Gastric acid secretion (inhibitory) | ↑ in RPC | Adrian et al. 1987, Pietroletti et al. 1990, Amstrong et al. 1991, Pironi et al.1993, Tsukamoto et al. 1994, Imamura et al. 1999, Teixeira et al. 2001 |
| Colon | ↓ Pepsin secretion (inhibitory) | ||||
| ↓ Pancreatic exocrine secretion (inhibitory) ↓ GI motility (inhibitory) ↑ Potent intestinal vasoconstriction |
↓ in RPC | Greenberg et al. 1989, Falk et al. 1998, Ternent et al. 1998, van Battum et al. 1999 | |||
| Neuropeptide Y (NPY) | Neurocrine | GI tract | Acts on various smooth |
No study in RPC | |
| CNS | muscles | ||||
| PNS | ↓ Local blood flow ↑ Adrenergic vasoconstriction |
||||
Their mode of delivery, site of action, their physiologic effects, and the levels post-RPC surgery and cited references
GI Gastrointestinal, CNS central nervous system, PNS peripheral nervous system, IPAA ileal pouch anal anastomosis, RPC restorative proctocolectomy
Pancreatic Polypeptide
PP is a CCK antagonist polypeptide secreted by PP cells in the endocrine pancreas, predominantly in the head of the pancreas. It suppresses pancreatic secretion and stimulates gastric secretion, GI motility, and pancreatic growth [192]. The release of PP into the blood is stimulated by luminal nutrients, especially lipids and proteins. Studies after RPC and/or colonic resection showed an elevation in the levels of PP [8, 143, 193–196]. After intestinal resection, PP was the most elevated hormone observed [197]. This may also be due to the presence of inflammatory bowel disease as this condition alone is also associated with elevated levels of PP [196, 197]
Peptide tyrosine–tyrosine
Highest tissue concentrations of PYY are seen in the rectum, sigmoid colon, ascending colon, and ileum [198–201]. PYY is regulated by luminal stimulants, paracrine factors, and vagal influence. The peptide is released in response to the ingestion of a meal, particularly fat and proteins [198, 202]. PYY mediates the inhibitory effect of oleic acid on gastric and pancreatic secretion, and thus, corresponds to the so-called “enterogastrone”, “pancreatone”, and “anti-CCK hormone”. In the small intestine, PYY reduces jejunal contractility and increases small intestinal transit times. Furthermore, PYY is a potent inhibitor of water and chloride ion secretion from jejunal crypt cells and a trophic hormone that may be important in both the development of the intestinal mucosa and dietary adaptation.
There are conflicting reports on the effects of RPC on PYY [1, 4–14]. Some authors noted decreased PYY levels [1, 6–8], while others noted increased PYY levels [4, 5, 10–14] after RPC. Armstrong et al. [4] and Pietroletti et al. [5] observed significant increases in basal and postprandial plasma PYY concentrations after RPC for both UC and FAP patients compared to healthy controls. These increases in the release of PYY seems to be involved in the “ileal brake” [13], a physiologic phenomenon that mediates the adaptive response of the GI tract and is related to a compensatory increase in L-cell density in the mucosa of the ileal pouch, perhaps affecting functional results [1, 4, 5, 10, 11, 14, 118, 119, 149, 202–204]. These results include pouch volume, evacuation, and compliance along with sphincter function and volume of the stool reaching the pouch. PYY also inhibits gastric acid, pancreatic exocrine, and pepsin secretions, along with retention of gallbladder contents [122, 204]. It also acts as a potent intestinal vasoconstrictor and slows gastric and intestinal transit [198, 199, 204]. Thus, increased basal and meal-stimulated release of PYY after RPC might lead to improved pouch function by decreasing the volume of fluid reaching the pouch and its evacuation. Basal plasma PYY concentration and PYY response in RPC patients with pouchitis did not differ from those in patients without a history of pouchitis [4–7, 10]
Neuropeptide Y
NPY is a neurotransmitter peptide found in the brain and autonomic nervous system. It is a powerful vasoconstrictor and exerts numerous inhibitory effects on the GI tract, particularly on gut motility. In patients with slow transit chronic constipation (e.g., Arbuthnot Lane’s syndrome) that underwent surgery with subtotal colectomy and ileo–rectal anastomosis, NPY immunoreactivity was increased in the myenteric plexus [205] demonstrating that NPY acts as an autocrine neurotrophic factor for enteric neurons [206]. The NPY has also been associated with a number of physiologic processes in the brain, including the regulation of energy balance, memory, and learning [201, 204]. It also forms part of the “lipostat” system along with leptin and corticotrophin releasing hormone. NPY has not been studied in connection with RPC.
Miscellaneous
The miscellaneous group of hormones includes motilin, neurotensin, SS, gastric releasing peptide, bombesin, SP, galanin, thyrotropin-releasing hormone, and calcitonin gene-related hormone (CGRP) (Table 6). Of these, only motilin, neurotensin, SS, and SP has been studied in connection with RPC.
Table 6.
Gut hormones: miscellaneous: their mode of delivery, site of action, their physiologic effects, and the levels post-RPC surgery and cited references
| Hormone | Modes of delivery | Site of action | Effects | Levels in RPC | Cited references |
|---|---|---|---|---|---|
|
| |||||
| Motilin | Endocrine | Stomach Duodenum Jejunum Esophagus |
↑ Smooth-muscle contraction | ↑ in RPC and in end ileotomy than control | Greenberg et al. 1989 |
| Neurotensin | Endocrine Neurocrine |
Stomach Jejunum Ileum |
↓ Gastric secretion and/or emptying ↓ Pepsin secretion ↓ Pancreatic exocrine secretion ↓ Smooth muscle motility ↑ Pancreatic secretion ↑ Intestinal fluid secretion |
Identical | Hallgren et al. 1995 |
| Somatostatin | Endocrine Paracrine |
Stomach Ileum Pancreas and neurocrine normal pancreas |
↓ Gastric acid secretion ↓ Mucosal growth of the GI tract |
Used in clinical trials for difficult pouch dysfunction | Kusuhara et al. 1992, Sagar et al. 1994, Rabau et al. 1995, Spiliotis et al. 2003 |
| Gastric releasing peptide (GRP) | Neurocrine | Stomach Ileum |
↑ Release of gastrin and Somatostatin ↑ Gastric acid secretion ↑ Pancreatic enzyme secretion |
No study in RPC | |
| Bombesin | Neurocrine Endocrine |
Stomach Ileum CNS |
↑ Release of gastrin and Somatostatin ↑ Gastric acid secretion ↑ Pancreatic enzyme secretion |
Used to study CCK levels and gallbladder volume in RPC; CCK was increased in RPC patients and gallbladder volume decreased | Salemans et al. 1995 |
| Substance P | Endocrine Paracrine |
CNS Intestine |
↑ Nitric oxide synthesis ↑ GI motility ↑ Potent vasodilatation ↑ Salivary flow (stimulatory) |
↑ in RPC with pouchitis | Keränen et al. 1996a, Keränen et al. 1996b |
| Galanin | Neurocrine | CNS Stomach Small intestine |
↓ Gastric motility ↑ Gastric emptying Induce hyperphagia ↓ Gut-related NST and DMNV neurons ↓ Gut-related DVC neurons |
No study in RPC | |
| Thyrotropin-releasing hormone |
Endocrine | Pancreas Stomach Enteric nerves | ↓ Gastric secretion ↓ Amylase and lipase (inhibitory) Stimulates intestinal smooth muscle Inhibits cholesterol synthesis in intestinal mucosa |
No study in RPC | |
| Calcitonin gene-related hormone | Endocrine | Sensory nerves | Vasodilation Enteric nerves |
No study in RPC | |
DMNV Dorsal motor nucleus of the vagus nerve, DVC dorsal vagal complex, NST nucleus of the solitary tract, RCP restorative proctocolectomy, CNS central nervous system
Motilin
Motilin is found in open-type M cells of the epithelium throughout the small intestine, most dense in the duodenum and proximal jejunum [207]. Motilin regulates the interdigestive motility of the stomach and small intestine [208–220]. The release of motilin into the blood is stimulated by, among other things, luminal acid and pancreatico-biliary secretions and by circulatory bombesin [210, 215, 216]. Acidification of the duodenum produces a prompt increase in plasma motilin, whereas alkalization produces a decrease [215, 216].
Basal plasma motilin levels were noted to be elevated after RPC when compared to patients with a continent ileostomy, end ileostomy, active ulcerative colitis, and healthy controls. Similar changes also occurred in ulcerative colitis patients and those with conventional ileostomy [8]. These changes in motilin levels are difficult to interpret, but may be due to alterations in luminal acidification associated with UC or after RPC, but this has not been fully elucidated.
Neurotensin
Neurotensin causes vasodilatation and hypotension, but affects several GI functions as well [221, 222]. In the GI tract, neurotensin predominates in the epithelium of the distal jejunum and ileum where it is synthesized and released by endocrine N-cells. It inhibits basal and stimulated acid and pepsin secretion, pancreatic exocrine secretion, and GI motility. Hallgren et al. [223] reported effects of RPC on neurotensin in both RPC-patients with a diverting loop ileostomy and in those without. The neurotensin levels were noted to be identical. It seems therefore that maintenance of bowel continuity has no effect on the secretion physiology of neurotensin.
Somatostatin
In the human GI tract, SS reaches highest concentrations in the stomach and duodenum [116, 224] and is associated predominantly with the mucosa and submucosa. SS is released from GI and pancreatic endocrine D-cells. In humans, all major classes of nutrients and a number of GI peptides stimulate SS release. Inhibitors of its secretion include endorphins and SS itself. SS is the predominant inhibitory hormone of the GI tract and has also been shown to inhibit the growth of the GI mucosa and normal pancreas [225]. The mechanism by which it inhibits acid secretion may involve release of prostaglandins [226], but this has been disputed [227, 228].
SS’s inhibitory effects have been beneficially used in clinical trials [229–231], including reducing complications after RPC [230, 232–234]. Because of its anti-secretory effect (or its octapeptide analogue, octreotide), SS has been used in the treatment of pouch dysfunction [230, 232] and in the reduction of the effluent volume associated with high ileostomy output or post-RPC diarrhea [233]. Long-acting SS has been shown to be effective for prolonged diarrhea in patients who have undergone RPC and have not responded to conventional medications [232–234]. Gullichsen [235] observed that approximately 72% of post-RPC-patients with diarrhea, who were treated with long-acting octreotide, experienced a marked reduction in bowel movement frequency. The active peptide analogue of SS has also been effectively used as an agent to ameliorate symptoms after RPC from bowel and anastomotic strictures, pouchitis, or cuffitis [234–236].
Bombesin and Gastric Releasing Peptide
Bombesin/GRP has been identified in the GI tract associated with neurons and is found predominantly in the mucosa of the gastric fundus and the muscular layer of the antrum and intestine [237]. Bombesin, like GRP, stimulates the release of gastric acid secretion, gastrin, and a variety of other GI peptides [237]. Bombesin has been used in studies to stimulate plasma CCK levels and decrease gallbladder volume. In patients, after RPC was compared to normal controls, bombesin had a significantly greater effect on CCK levels [156]. These findings suggest that the colon may contain a factor that inhibits the release of CCK and that colectomy may have an impact on the formation of the reported cholelithiasis after RPC [158–163].
Substance P
In the GI tract, SP is found predominantly in the muscular layer of the intestine and is also present in a subpopulation of endocrine enterochromaffin cells [174, 238]. The biological action of SP is as a potent stimulator of GI motility, and it affects numerous other GI functions. In the circulatory system, SP causes potent vasodilatation.
The SP distribution in RPC patients has been documented by Keranen et al. [172, 173]. The number and intensity of SP immunoreactive nerve fibers were markedly increased in pouches of patients with pouchitis as compared to normal pouches, the ileum of UC patients, and to normal ileum. The results suggest that SP may play a role in mediating inflammatory processes in patients with pouchitis.
Galanin, thyroid releasing hormone, and calcitonin gene-related hormone
There are no studies on the effects of RPC on galanin, thyroid releasing hormone (TRH), and CGRP.
Effects of galanin on GI function have been widely reported [238–243]. Investigations show that galanin suppresses gastric emptying [239], increases gastric motility [240], and induces hyperphagia [228, 242–244]. Galanin is known to be involved in the regulation of feeding and gastric motility [239–242], and therefore, colectomy and RPC procedures alone may not negatively affect the physiological function of galanin.
TRH was the first hypothalamic-releasing factor to be isolated [244]. TRH is widely distributed throughout the GI tract with concentrations in the pancreas, stomach, and colon [245–248]. Secretion of TRH, and hence, thyroid-stimulating hormone (TSH), is inhibited by high blood levels of thyroid hormones in a classical negative feedback loop. Loss of TRH secretion cells from the colon, after colectomy, is compensated for by other sources of TRH secreting cells in the GI tract.
CGRP is a mediator of gastric mucosa and produces splanchnic and peripheral vasodilatation [249]. As CGRP release is stimulated by glucose, a role for CGRP in producing postprandial hypotension has been suggested. It regulates food intake at both peripheral sites and within the central nervous system. CGRP release is also stimulated by acid in the gastric mucosa. The CGRP released from primary afferent sensory nerves stimulates the release of SS, thus, decreasing gastric acid secretion.
Conclusions
Restorative proctocolectomy has become the standard of care for the surgical treatment of most UC and FAP patients. The RPC results in a series of adaptive responses by the GI tract, including morphologic changes and proliferation of enteroendocrine cells in the ileal pouch. Basal and postprandial plasma gastrin, enteroglucagon, neurotensin, VIP, CCK, GIP, PYY, motilin, SS, SP, and PP levels in RPC-patients have been studied. Observations on digestive function and circulating and morphologic gut endocrine responses after RPC are often contradicting and with unclear clinical implications. After an ileal reservoir, sufficient reserve apparently remains for the majority of GI hormones to maintain adequate release into the circulation, suggesting compensation for the presence of an ileal reservoir and the absence of a colon and rectum. Further studies of the ileal pouch after RPC for FAP vs UC should clarify whether the underlying disease process is responsible for some of the hormone changes noted after RPC. Reservoirs may show neuromorphologic and histologic alterations that appear to be related to mucosal inflammation but may be due to adaptive changes alone. Therapeutic strategies to improve intestinal function and enhance repair and regeneration of the intestinal epithelial integrity have been inadequate and remain the goal of future research into the GI peptide changes after RPC.
Acknowledgement
We thank Mayo Clinic and Foundation for Medical Education and Research and NIH-Institutional Research and Career Development Award (IRACDA) (5K12GM068543 to A.E.M). Copyright of published figures: Artwork is reproduced, with permission, from the Johns Hopkins Gastroenterology and Hepatology Resource Center (Figs. 1–5 and 7) and Courtesy of Cleveland Clinic Foundation (Table 6).
Contributor Information
Amosy E. M’Koma, Inflammatory Bowel Disease Center, Vanderbilt University School of Medicine, Nashville, TN 37232-2765, USA
Paul E. Wise, Department of General Surgery, Vanderbilt University School of Medicine, Nashville, TN 37232-2765, USA Inflammatory Bowel Disease Center, Vanderbilt University School of Medicine, Nashville, TN 37232-2765, USA.
Roberta L. Muldoon, Department of General Surgery, Vanderbilt University School of Medicine, Nashville, TN 37232-2765, USA Inflammatory Bowel Disease Center, Vanderbilt University School of Medicine, Nashville, TN 37232-2765, USA.
David A. Schwartz, Department of Medicine, Division of Gastroenterology, Vanderbilt University School of Medicine, Nashville, TN 37232-2765, USA Inflammatory Bowel Disease Center, Vanderbilt University School of Medicine, Nashville, TN 37232-2765, USA.
Mary K. Washington, Department of Pathology, Vanderbilt University School of Medicine, Nashville, TN 37232-2765, USA Inflammatory Bowel Disease Center, Vanderbilt University School of Medicine, Nashville, TN 37232-2765, USA.
Alan J. Herline, Department of General Surgery, Vanderbilt University School of Medicine, Nashville, TN 37232-2765, USA Inflammatory Bowel Disease Center, Vanderbilt University School of Medicine, Nashville, TN 37232-2765, USA.
References
- 1.Falk PM, Sentovich SM, Adrian TE (1998) Ileoanal pouch function and release of peptide YY. Dis Colon Rectum 41:868–874 [DOI] [PubMed] [Google Scholar]
- 2.Dubé S, Heyen F (1990) Pouchitis and gastric hyposecretion: cause or effect? Int J Colorectal Dis 4:142–143 [DOI] [PubMed] [Google Scholar]
- 3.M’Koma AE, Lindquist K, Liljeqvist L (1999) Effect of restorative proctocolectomy on gastric acid secretion and serum gastrin levels: a prospective study. Dis Colon Rectum 42:398–402 [DOI] [PubMed] [Google Scholar]
- 4.Armstrong DN, Ballantyne GH, Andrian TE, Bilchik AJ, MacMillen MA, Modlin IM (1991) Adaptive increase in peptide YY and enteroglucagon after proctocolectomy and pelvic ileal reservoir construction. Dis Colon Rectum 34:119–125 [DOI] [PubMed] [Google Scholar]
- 5.Pietroletti R, Slors FJM, Mariani P et al. (1990) Enteroglucagon and peptide YY response after construction of a pelvic reservoir in humans. Dis Colon Rectum 33:966–970 [DOI] [PubMed] [Google Scholar]
- 6.Ternent CA, Staab P, Thorson AG, Blatchford GJ, Christensen MA, Thompson JS, Lanspa SJ, Meade PG, Cali RA, Falk PM, Sentovich SM, Adrian TE (1998) Ileoanal pouch function and release of peptide YY. Dis Colon Rectum 41:868–874 [DOI] [PubMed] [Google Scholar]
- 7.van Battum PL, Hopman WP, Salemans JM, Kuijpers JH, Nagengast FM, Jansen JB (1999) Impaired release of peptide YY in patients with proctocolectomy and ileal pouch-anal anastomosis. Scand J Gastroenterol 34:404–408 [DOI] [PubMed] [Google Scholar]
- 8.Greenberg GR, Buchan AM, McLeod RS, Preston P, Cohen Z (1989) Gut hormone responses after reconstructive surgery for ulcerative colitis. Gut 30:1721–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adrian T, Savage A, Sagor G et al. (1985) Effect of peptide YY on gastric, pancreatic, and biliary function in humans. Gastroenterology 89:494–449 [DOI] [PubMed] [Google Scholar]
- 10.Teixeira FV, Pera M, Kelly KA (2001) Enhancing release of peptide YY after near-total proctocolectomy: jejunal pouch vs. ileal pouchdistal rectal anastomosis. J Gastrointest Surg 5:108–112 [DOI] [PubMed] [Google Scholar]
- 11.Imamura M, Nakajima H, Mikami Y, Yamauchi H (1999) Morphological and immunohistochemical changes in intestinal mucosa and PYY release following total colectomy with ileal pouch–anal anastomosis in dogs. Dig Dis Sci 44:1000–1007 [DOI] [PubMed] [Google Scholar]
- 12.Tsukamoto Y, Koh K (1994) Adaptation and effects of ileal reservoir on ileo-proctostomy following total colectomy in dogs. J Smooth Muscle Res 30:35–50 [DOI] [PubMed] [Google Scholar]
- 13.Pironi L, Stanghellini V, Miglioli M, Corinaldesi R, De Giorgio R et al. (1993) Fat-induced ileal brake in humans: a dose-dependent phenomenon correlated to the plasma levels of peptide YY. Gastroenterology 105:733–739 [DOI] [PubMed] [Google Scholar]
- 14.Adrian TE, Savage AP, Fuessl HS, Wolfe K, Besterman HS, Bloom SR (1987) Release of peptide YY (PYY) after resection of small bowel, colon, or pancreas in man. Surgery 101:715–719 [PubMed] [Google Scholar]
- 15.Wilson JP (1975) Postoperative motility of the large intestine in man. Gut 16:689–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nissen R (1933) Sitzungsberichte aus chirurgischen Geselishaften. Zentralbi Chir 15:888 [Google Scholar]
- 17.Ravitch MM, Sabiston DC (1947) Anal ileostomy with preservation of the sphincter. Surg Gyn Obstet 84:1095–1099 [PubMed] [Google Scholar]
- 18.Ravitch MM (1948) Anal ileostomy with sphincter preservation in patients requiring total colectomy for benign conditions. Surg 24:170–181 [PubMed] [Google Scholar]
- 19.Ratvich MM, Handelsman JC (1951) One stage resection of entire colon and rectum for ulcerative colitis and polypoid adenomatosis. Bull Johns Hopkins Hosp 88:59–82 [PubMed] [Google Scholar]
- 20.Wangensteen OH, Toon RW (1948) Primary resection of the colon and rectum with particular reference to cancer and ulcerative colitis. Am J Surg 75:384–404 [DOI] [PubMed] [Google Scholar]
- 21.Goligher JC, Hughes ESR (1951) Sensibility of the rectum and colon, its role in the mechanism of anal continence. The Lancet 1260:513–517 [DOI] [PubMed] [Google Scholar]
- 22.Best RR (1952) Evaluation of ileoproctostomy to avoid ileostomy in various colon lesions. JAMA 150:637–642 [DOI] [PubMed] [Google Scholar]
- 23.Brooke BN (1952) The management of an ileostomy. Including its complications. Lacent 102–104 [DOI] [PubMed] [Google Scholar]
- 24.Valiente MA, Bacon HE (1955) Construction of a pouch using “pantaloon” technic for pull-through of ileum following total colectomy. Report of experimental work and results. Am J Surg 90:742–750 [DOI] [PubMed] [Google Scholar]
- 25.Karlan M, McPherson RC, Watman RN (1959) An experimental evaluation of fecal continence sphincter and reservoir—in the dog. Surg Gyn Obstat 108:469–475 [PubMed] [Google Scholar]
- 26.Peck DA, Hallenbeck GA (1964) Fecal continence in the dog after replacement of rectal mucosa with ileal mucosa. Surg Gyn Obstet 119:1312–1320 [PubMed] [Google Scholar]
- 27.Kock NG (1969) Intra-abdominal reservoir in patients with permanent ileostomy. Arch Surg 99:223–231 [DOI] [PubMed] [Google Scholar]
- 28.Parks AG, Nicholls RJ, Belliveau P (1980) Proctocolectomy with ileal reservoir and anal anastomosis. Br J Surg 67:533–538 [DOI] [PubMed] [Google Scholar]
- 29.Utsunomiya J, Iwama T, Imajo M, Matsud S, Sawal S, Yoegashi K, Hirayama R (1980) Total colectomy, mucosal proctectomy and ileo-anal anastomosis. Dis Colon Rectum 23:459–466 [DOI] [PubMed] [Google Scholar]
- 30.Liljeqvist L, Lindquist K, Ljungdahl I (1988) Alterations in ileoanal pouch technique, 1980 to 1987. Complications and functional outcome. Dis Colon Rectum 31:929–938 [DOI] [PubMed] [Google Scholar]
- 31.Johnston D, Williams NS, Neal DE, Axon AT (1981) The value of preserving the anal sphincter in operations for ulcerative colitis and polyposis: a review of 22 mucosal proctectomies. Br J Surg 68:874–878 [DOI] [PubMed] [Google Scholar]
- 32.Percy JP, Parks AG (1981) The nerve supply of the pelvic floor. Scheitz Rundschau Med 70:640–642 [PubMed] [Google Scholar]
- 33.Fonkalsrud EW (1980) Total colectomy and endorectal ileal pull-through with internal ileal reservoir for ulcerative colitis. Surg Gyn Obstet 150:1–8 [PubMed] [Google Scholar]
- 34.Lindquist K, Nilsell K, Liljeqvist (1987) Cuff abscesses and ileoanal anastomotic separations in pelvic pouch surgery: an analysis of possible etiologic factors. Dis colon Rectum 30:355–359 [DOI] [PubMed] [Google Scholar]
- 35.Kukt-Hansen L, Rosenkilde-Olsen P, Simonsen L (1985) Total colectomy, mucosal proctectomy and an ileal reservoir to an anal anastomosis. A comparison of short and long efferent legs. Scand J Gastroenterol 20:1091–1096 [DOI] [PubMed] [Google Scholar]
- 36.Grant D, Cohen Z, McHugh S, McLeod R, Sten H (1986) Restorative proctocolectomy. Clinical results and manometric findings with long and short rectal cuffs. Dis Colon Rectum 29:27–32 [DOI] [PubMed] [Google Scholar]
- 37.Peck DA (1980) Rectal mucosal replacement. Ann Surg 191:294–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lovegrove RE, Constantinides VA, Heriot AG, Athanasiou T, Darzi A, Remzi FH, Nicholls RJ, Fazio VW, Tekkis PP (2006) A Comparison of Hand-Sewn Versus Stapled Ileal Pouch Anal Anastomosis (IPAA) Following Proctocolectomy. Ann Surg 244:18–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reilly WT, Pemberton JH, Wolff BG, Nivatvongs S, Devine RM, Litchy WJ, McIntyre PB (1997) Randomized prospective trial comparing ileal pouch–anal anastomosis performed by excising the anal mucosa to ileal pouch–anal anastomosis performed by preserving the anal mucosa. Ann Surg 225:666–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Landi E, Fianchini A, Landa L, Marmorale C, Corradini G, De Luca S, Piloni V (1990) Proctocolectomy and stapled ileo–anal anastomosis without mucosal proctectomy. Int J colorec Dis 5:151–154 [DOI] [PubMed] [Google Scholar]
- 41.van Duijvendijk P, Slors JFM, Taat CW, Oosterveld P, Vasen HFA (1999) Functional outcome after colectomy and ileorectal anastomosis compared with proctocolectomy and ileal pouch–anal anastomosis in familial adenomatous polyposis. Ann Surg 5 (230):648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Engemann R, Hamelmann W, Wagner R, Thiede A (1994) Pelvic pouches. Zentralbl Cir 119:862–866 [PubMed] [Google Scholar]
- 43.Nicholls RJ, Pezim ME (1985) Restorative proctocolectomy with ileal reservoir for ulcerative colitis and familial adenomatous polyposis: a comparison of three reservoir designs. Br J Surg 72:470–474 [DOI] [PubMed] [Google Scholar]
- 44.Nasmyth DG, Williams NS, Johnston D (1986a) Comparison of the function of triplicated and duplicated pelvic ileal reservoir after mucosal proctectomy and ileo–anal anastomosis for ulcerative colitis and adenomatous polyposis. Br J Surg 73:361–366 [DOI] [PubMed] [Google Scholar]
- 45.Keighley MR, Yoshioka K, Kmiot W (1988) Prospective randomized trial to compare the stapled double lumen pouch and the sutured quadruple pouch for restorative proctocolectomy. Br J Surg 75:1008–1011 [DOI] [PubMed] [Google Scholar]
- 46.Lindquist K, Liljeqvist L, Sellberg B (1984) The topography of ileoanal reservoir in relation to evacuation patterns and clinical functions. Acta Chir Scand 150:573–579 [PubMed] [Google Scholar]
- 47.Delaney CP, Fazio VW, Remzi FH, Hammel J, Church JM, Hull TL, Senagore AJ, Strong SA, Lavery IC (2003) Prospective, age-related analysis of surgical results, functional outcome, and quality of life after ileal pouch–anal anastomosis. Ann Surg 238:221–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carmon E, Keidar A, Ravid A, Goldman G, Rabau M (2003) The correlation between quality of life and functional outcome in ulcerative colitis patients after proctocolectomy ileal pouch anal anastomosis. Colorectal Dis 5:228–232 [DOI] [PubMed] [Google Scholar]
- 49.Kayaalp C, Nessar G, Akoglu M, Atalay F (2003) Elimination of mucosectomy during restorative proctocolectomy in patients with ulcerative colitis may provide better results in low-volume centers. Am J Surg 185:268–272 [DOI] [PubMed] [Google Scholar]
- 50.Delaney CP, Dadvand B, Remzi FH, Church JM, Fazio VW (2002) Functional outcome, quality of life, and complications after ileal pouch–anal anastomosis in selected septuagenarians. Dis Colon Rectum 45:890–894 [DOI] [PubMed] [Google Scholar]
- 51.Dayton MT, Larsen KR, Christiansen DD (2002) Similar functional results and complications after ileal pouch–anal anastomosis in patients with indeterminate vs ulcerative colitis. Arch Surg 137:690–694 [DOI] [PubMed] [Google Scholar]
- 52.Halverson AL, Hull TL, Remzi F, Hammel JP, Schroeder T, Fazio VW (2000) Perioperative resting pressure predicts long-term postoperative function after ileal pouch–anal anastomosis. J Gastrointest Surg 6:316–320 [DOI] [PubMed] [Google Scholar]
- 53.Barton JG, Paden MA, Lane M, Postier RG (2001) Comparison of postoperative outcomes in ulcerative colitis and familial polyposis patients after ileoanal pouch operations. Am J Surg 182:616–620 [DOI] [PubMed] [Google Scholar]
- 54.Dunker MS, Bemelman WA, Slors JF, van Duijvendijk P, Gouma DJ (2001) Functional outcome, quality of life, body image, and cosmesis in patients after laparoscopic-assisted and conventional restorative proctocolectomy: a comparative study. Dis Colon Rectum 44:1800–1807 [DOI] [PubMed] [Google Scholar]
- 55.Harris GJ, Lavery IC, Fazio VW (2001) Function of a colonic J pouch continues to improve with time. Br J Surg 88:1623–1627 [DOI] [PubMed] [Google Scholar]
- 56.Ikeuchi H, Shoji Y, Kusunoki M, Yanagi H, Noda M, Yamamura T (2004) Clinical results after restorative proctocolectomy without diverting ileostomy for ulcerative colitis. Int J Colorectal Dis 19:234–238 [DOI] [PubMed] [Google Scholar]
- 57.Parc Y (2001) Factors influencing the functional outcome of restorative proctocolectomy in ulcerative colitis. Ann Chir 126:271–272 [DOI] [PubMed] [Google Scholar]
- 58.Fichera A, Michelassi F (2001) Long-term prospective assessment of functional results after proctectomy with coloanal anastomosis. J Gastrointest Surg 5:153–157 [DOI] [PubMed] [Google Scholar]
- 59.Andriesse GI, Gooszen HG, Schipper ME, Akkermans LM, van Vroonhoven TJ, van Laarhoven CJ (2001) Functional results and visceral perception after ileo neo-rectal anastomosis in patients: a pilot study. Gut 48:683–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gee SH, Seow-Choen F (2001) Functional outcome after restorative panproctocolectomy for ulcerative colitis decreases an otherwise enhance quality of life. Br J Surg 88:472. [DOI] [PubMed] [Google Scholar]
- 61.Barrier A, Martel P, Dugue L, Gallot D, Malafosse M (2001) Direct and reservoir colonic–anal anastomoses. Short and long term results. Ann Chir 126:18–25 [DOI] [PubMed] [Google Scholar]
- 62.Johnson E, Carlsen E, Nazir M, Nygaard K (2001) Functional outcome after reservoir surgery in ulcerative colitis. Tidsskr Nor Laegeforen 121:292–294 [PubMed] [Google Scholar]
- 63.Church JM (2000) Functional outcome and quality of life in an elderly patient with an ileal pouch–anal anastomosis: a 10-year follow-up. Aust N Z J Surg 70:906–907 [DOI] [PubMed] [Google Scholar]
- 64.Choi JS, Potenti F, Wexner SD, Nam YS, Hwang YH, Nogueras JJ, Weiss EG, Pikarsky AJ (2000) Functional outcomes in patients with mucosal ulcerative colitis after ileal pouch–anal anastomosis by the double stapling technique: is there a relation to tissue type? Dis Colon Rectum 43:1398–1404 [DOI] [PubMed] [Google Scholar]
- 65.Karlbom U, Raab Y, Ejerblad S, Graf W, Thorn M, Pahlman L (2000) Factors influencing the functional outcome of restorative proctocolectomy in ulcerative colitis. Br J Surg 87:1401–1408 [DOI] [PubMed] [Google Scholar]
- 66.Scarpa M, Angriman I, Ruffolo C, Ferronato A, Polese L, Barollo M, Martin A, Sturniolo GC, D’Amico DF (2004) Health-related quality of life after restorative proctocolectomy for ulcerative colitis: long-term results. World J Surg 28:124–129 [DOI] [PubMed] [Google Scholar]
- 67.Kalbassi MR, Winter DC, Deasy JM (2003) Quality-of-life assessment of patients after ileal pouch–anal anastomosis for slow-transit constipation with rectal inertia. Dis Colon Rectum 46:1508–1512 [DOI] [PubMed] [Google Scholar]
- 68.Holubar S, Hyman N (2003) Continence alterations after ileal pouch–anal anastomosis do not diminish quality of life. Dis Colon Rectum 46:1489–1491 [DOI] [PubMed] [Google Scholar]
- 69.Camilleri-Brennan J, Munro A, Steele RJ (2003) Does an ileoanal pouch offer a better quality of life than a permanent ileostomy for patients with ulcerative colitis? J Gastrointest Surg 7:814–819 [DOI] [PubMed] [Google Scholar]
- 70.Richards DM, Hughes SA, Irving MH, Scott NA (2001) Patient quality of life after successful restorative proctocolectomy is normal. Colorectal Dis 3:223–226 [DOI] [PubMed] [Google Scholar]
- 71.Gunther K, Braunrieder G, Bittorf BR, Hohenberger W, Matzel KE (2003) Patients with familial adenomatous polyposis experience better bowel function and quality of life after ileorectal anastomosis than after ileoanal pouch. Colorectal Dis 5:38–44 [DOI] [PubMed] [Google Scholar]
- 72.Berndtsson I, Oresland T (2003) Quality of life before and after proctocolectomy and IPAA in patients with ulcerative proctocolitis—a prospective study. Colorectal Dis 5:173–179 [DOI] [PubMed] [Google Scholar]
- 73.Parc Y, Piquard A, Dozois RR, Parc R, Tiret E (2004) Long-term outcome of familial adenomatous polyposis patients after restorative coloproctectomy. Ann Surg 239:378–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Efficace F, Bottomley A, Blazeby JM (2003) Randomized clinical trial comparing quality of life after straight and pouch coloanal reconstruction. Br J Surg 90:594–595 [DOI] [PubMed] [Google Scholar]
- 75.Weinryb RM, Liljeqvist L, Poppen B, Gustavsson JP (2003) A longitudinal study of long-term quality of life after ileal pouch–anal anastomosis. Am J Surg 185:333–338 [DOI] [PubMed] [Google Scholar]
- 76.Takahashi T, Ponce de Leon S, Cardenas S, Remes JM, Garcia-Osogobio S, Camilo Barreto J, Zarate Diaz X (2002) Quality of life after ileo–anal anastomosis. Rev Invest Clin 54:397–402 [PubMed] [Google Scholar]
- 77.Sailer M, Fuchs KH, Fein M, Thiede A (2002) Randomized clinical trial comparing quality of life after straight and pouch coloanal reconstruction. Br J Surg 89:1108–1117 [DOI] [PubMed] [Google Scholar]
- 78.Delaney CP, Remzi FH, Gramlich T, Dadvand B, Fazio VW (2002) Equivalent function, quality of life and pouch survival rates after ileal pouch–anal anastomosis for indeterminate and ulcerative colitis. Ann Surg 236:43–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Robb B, Pritts T, Gang G, Warner B, Seeskin C, Stoops M, James L, Rafferty J, Azizkhan R, Martin L, Nussbaum M (2002) Quality of life in patients undergoing ileal pouch–anal anastomosis at the University of Cincinnati. Am J Surg 183:353–360 [DOI] [PubMed] [Google Scholar]
- 80.Robb BW, Pritts TA, Warner BW (2002) Health-related quality of life after ileal pouch anal anastomosis for ulcerative colitis: right answer–wrong question. Gastroenterology 122:1180–1181 [DOI] [PubMed] [Google Scholar]
- 81.Coffey JC, Winter DC, Neary P, Murphy A, Redmond HP, Kirwan WO (2002) Quality of life after ileal pouch–anal anastomosis: an evaluation of diet and other factors using the Cleveland Global Quality of Life instrument. Dis Colon Rectum 45:30–38 [PubMed] [Google Scholar]
- 82.Giebel GD, Mennigen R, Karanjia ND (1994) Histochemical and metabolic changes in functioning ileal pouches after proctocolectomy for familial adenomatous polyposis and ulcerative colitis. JR Coll Surg Edin 39:228–231 [PubMed] [Google Scholar]
- 83.Hulten L, Kewenter J, Persson E, Åhren C (1970) Vitamin B12 absorption in ileostomy patients after operation for ulcerative colitis. Scand J Gastroenterol 5:113–116 [PubMed] [Google Scholar]
- 84.Kelly DG, Phillips SF, Kelly KA et al. (1983) Dysfunction of the continent ileostomy: clinical futures and bacteriology. Gut 24:193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.O’Connell PR, Rankin DR, Weiland LH, Kelly KA (1986) Enteric bacteriology, absorption, morphology and emptying after ileal pouch–anal anastomosis. Br J Surg 73:909–914 [DOI] [PubMed] [Google Scholar]
- 86.Philipson B, Brandberg A, Jagenburg R, Kock NG, Lager I, Ahren C (1975) Mucosal morphology, bacteriology, and absorption in intra-abdominal ileostomy reservoir. Scand J Gastroenterol 10:145–153 [PubMed] [Google Scholar]
- 87.Veress B, Reinholt FP, Lindquist K, Liljeqvist L (1990) Different types of mucosal adaptation in the ileal reservoir after restorative proctocolectomy. A two-year follow-up study. APMIS 98:786–796 [DOI] [PubMed] [Google Scholar]
- 88.Veress B, Reinholt FP, Lindquist K, Lofberg R, Liljeqvist L (1995) Long-term histomorphological surveillance of the pelvic ileal pouch: dysplasia develops in a subgroup of patients. Gastroenterology 109:1090–1097 [DOI] [PubMed] [Google Scholar]
- 89.Nilsson LO, Kock NG, Lindgren I, Myrvold HE, Philipson BM, Ahren C (1980) Morphological and histochemical changes in the mucosa of the continent ileostomy reservoir 6–10 years after its construction. Scand J Gastroenterol 15:737–747 [DOI] [PubMed] [Google Scholar]
- 90.Tiainen J, Matikainen M, Aitola P, Hiltunen KM, Mattila J (2001) Histological and macroscopic changes in the pelvic pouch: long-term follow up after restorative proctocolectomy for ulcerative colitis (UC). Colorectal Dis 3:28–32 [DOI] [PubMed] [Google Scholar]
- 91.Andersson H, Dotevall G, Gillberg G, Jagenburg R, Kock NG (1971) Absorption studies in patients with Crohn’s disease and in patients with ulcerative colitis. Acta Med Scand 190:407–410 [DOI] [PubMed] [Google Scholar]
- 92.Nilsson LO, Andersson H, Hulten L, Jagenburg R, Kock NG, Myrvold HE, Philipson B (1979) Absorption studies in patients six to 10 years after construction of ileostomy reservoirs. Gut 20:499–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Heuschen UA, Hinz U, Allemeyer EH, Autschbach F, Stern J, Lucas M, Herfarth C, Heuschen G (2002) Risk factors for ileoanal J pouch-related septic complications in ulcerative colitis and familial adenomatous polyposis. Ann Surg 235:207–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Giebel GD, Sabiers H (1996) Ileal pouch–anal anastomosis for ulcerative colitis and polyposis coli: is the risk of carcinoma formation conclusively averted? Eur J Surg Oncol 22:372–376 [DOI] [PubMed] [Google Scholar]
- 95.Thompson-Fawcett MW, Marcus V, Redston M, Cohen Z, McLeod RS (2001) Risk of dysplasia in long-term ileal pouches and pouches with chronic pouchitis. Gastroenterology 12:275–281 [DOI] [PubMed] [Google Scholar]
- 96.Coull DB, Lee FD, Henderson AP, Anderson JH, McKee RF, Finlay IG (2003) Risk of dysplasia in the columnar cuff after stapled restorative proctocolectomy. Br J Surg 90:72–75 [DOI] [PubMed] [Google Scholar]
- 97.Remzi FH, Church JM, Bast J, Lavery IC, Strong SA, Hull TL, Harris GJ, Delaney CP, O’Riordain MG, McGannon EA, Fazio VW (2001) Mucosectomy vs. stapled ileal pouch–anal anastomosis in patients with familial adenomatous polyposis: functional outcome and neoplasia control. Dis Colon Rectum 44:1590–1596 [DOI] [PubMed] [Google Scholar]
- 98.Colwell JC, Gray M (2001) What functional outcomes and complications should be taught to the patient with ulcerative colitis or familial adenomatous polyposis who undergoes ileal pouch anal anastomosis? J Wound Ostomy Continence Nurs 28:184–189 Review [DOI] [PubMed] [Google Scholar]
- 99.Das P, Johnson MW, Tekkis PP, Nicholls RJ (2007) Risk of dysplasia and adenocarcinoma following restorative proctocolectomy for ulcerative colitis. Colorect Dis 9:15–27 [DOI] [PubMed] [Google Scholar]
- 100.Baylis WM, Starling EH (1902) The mechanism of pancreatic secretion. J Physiol 28:325–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Edkins JS (1905) The chemical mechanism of gastric secretion. Proc R Soc 69:352–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ivy AC, Oldberg E (1928) A hormonal mechanism of gallbladder contraction and evacuation. Am J Physiol 86:599–613 [Google Scholar]
- 103.Harper AA, Raper HS (1943) Pancreozymin, a stimulant of the secretion of pancreatic enzymes in the small intestine. J Physiol 102:115–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jorpes JE, Mutt V, Magnusson S, Steele BB (1962) Amino acid composition and N-terminal amino acid sequence of porcine secretin. Biochem Biophys Res Commun 9:275–279 [DOI] [PubMed] [Google Scholar]
- 105.Gregory RA, Tracy HJ (1964) The constitution and properties of two gastrins extract from hog antral mucosa. Gut 5:103–114 [PMC free article] [PubMed] [Google Scholar]
- 106.Jorpes E, Mutt V, Toczko K(1964) Further purification of cholecystokinin and pancreozymin. Acta Chem Scand 18:2408–2410 [Google Scholar]
- 107.McGuigan JE (1968) Immunochemical studies with synthetic human gastrin. Gastroenterology 54:1005–1011 [PubMed] [Google Scholar]
- 108.Noyes BE, Mevarech M, Stein R, Agarwal KL (1979) Detection and partial sequence analysis of gastrin mRNA by using an oligodeoxynucleotide probe. Proc Natl Acad Sci 76:1770–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Angood PB, Gingalewski CA, Andersen DK (2001) Surgical complication. In: Townsend CM Jr, Beauchamp RD, Evers BM, Mattox KL (eds) Sabiston textbook of surgery the biology basis of modern surgical practice. 16th ed. Saunders, Philadelphia, pp 198–225 [Google Scholar]
- 110.Oberg K (2001) Chemotherapy and biotherapy in the treatment of neuroendocrine tumours. Ann Oncol 12:111–114 [DOI] [PubMed] [Google Scholar]
- 111.Grube D, Forssmann WG (1979) Morphology and function of the entero-endocrine cells. Hormone Metab Res 11:589–606 [DOI] [PubMed] [Google Scholar]
- 112.Sjölund K, Sanden C, Hakanson R, Sundler F (1983) Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology 85:1120–1130 [PubMed] [Google Scholar]
- 113.Furness JB, Costa M (1980) Types of nerves in the enteric nervous system. Neuroscience 5:1–20 [DOI] [PubMed] [Google Scholar]
- 114.Soll AH, Walsh JH (1979) Regulation of gastric acid secretion. Ann Rev Physiol 41:35–53 [DOI] [PubMed] [Google Scholar]
- 115.Pern W, Wass JA, Butler MD et al. (1983) Distribution and characterization of immunoreactive somatostatin in human gastrointestinal tract. Regul Peptides 7:53–65 [DOI] [PubMed] [Google Scholar]
- 116.Wienbeck M, Erckenbrecht J (1982) The control of gastrointestinal motility by GI hormones. Clin Gastroenterol 11:523–543 [PubMed] [Google Scholar]
- 117.Itoh Z, Aizawa I, Sekiguchi T (1982) The interdigestive migrating complex and its significance in man. Clin Gastroenterol 497–521 [PubMed] [Google Scholar]
- 118.Johnson LR (1981) Regulation of gastrointestinal growth. In: Johnson LR (ed) Physiology of the gastrointestinal tract. Raven, New York, pp 169–196 [Google Scholar]
- 119.Grossman MI. (1974) Gastrointestinal hormones: spectrum of actions of structure–activity relations. In: Chey W, Books F. (eds) Endocrinology of the gut. CB Slack, Thorofare, NJ, pp 65–75 [Google Scholar]
- 120.Granger DN, Richardson PD, Kvietys PR, Mortillaro NA (1980) Intestinal blood flow. Gastroenterology 78:847–863 [PubMed] [Google Scholar]
- 121.Morley JE, Levine AS (1983) The central control of appetite. Lacent 1:394–401 [DOI] [PubMed] [Google Scholar]
- 122.Palnaes Hansen C, Andreasen JJ, Holst JJ (1997) The release of gastric inhibitory peptide, glucagon-like peptide-I, and insulin after oral glucose test in colectomized subjects. Scand J Gastroenterol 32:473–477 [DOI] [PubMed] [Google Scholar]
- 123.Seal AM, Debas HT (1980) Colonic inhibition of gastric acid secretion in dog. Gastroenterology 79:823–827 [PubMed] [Google Scholar]
- 124.Soon-Shiong P, Debas HT, Seal AM et al. (1980) Colonic inhibition of gastric acid secretion in man. Surg Forum 31:152–154 [Google Scholar]
- 125.Hage G, Tiscornia O, Palasciano G et al. (1974) Inhibition of pancreatic exocrine secretion by intra-colonic oleic acid infusion in the dog. Biomedicine 21:263–267 [PubMed] [Google Scholar]
- 126.Harper AA, Hood AJC, Mushens J et al. (1974) Inhabition of external pancreatic secretion by extracts of ileal and colonic mucosa. Gut 15:825–1974 [PubMed] [Google Scholar]
- 127.Gomez de Segura IA, Marijuan, Trillo P, Picornell M, Codoceo R, Diaz J, De Miguel E (1995) Changes in plasma levels of intestinal regulatory peptides following colonic resection in the rat. Rev Esp Enferm Dig 87:20–24 [PubMed] [Google Scholar]
- 128.Jian R, Besterman HS, Sarson DL, Aymes C, Hostein J, Bloom SR, Rambaud JC (1981) Colonic inhibition of gastric secretion in man. Dig Dis Sci 26:195–201 [DOI] [PubMed] [Google Scholar]
- 129.Inoue K, Wiener I, Fried GM, Lilja P, Watson LC, Thompson JC (1982) Effect of colectomy on cholecystokinin and gastrin release. Ann Surg 196:691–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Thompson JC, Townsend CM Jr. Endocrine pancreas. In: Townsend CM Jr, Beauchamp RS, Evers BM, Mattox KL (eds) Sabiston textbook of surgery: the biological basis of modern surgical practice. 16th ed. Philadelphia: Saunders, pp 646–661 [Google Scholar]
- 131.Moxey PC (1978) Is the human colon an endocrine organ? Gastroenterology 75:147–149 [PubMed] [Google Scholar]
- 132.Hedner P, Persson H, Rorsman G (1970) Effects of cholecystokinin on small intestine. Acta Physiol Scand 70:250–254 [DOI] [PubMed] [Google Scholar]
- 133.Hesselfeldt P, Christiansen J Rehfeldt JF et al. (1979) Meal-stimulated gastric acid and gastric secretion before and after jejuno-ileal shunt operation in obese patients. Scand J Gastroent 14:13–16 [DOI] [PubMed] [Google Scholar]
- 134.Kosaka T, Lim RKS (1930) Demonstration of the humoral agent in fat inhibition of gastric secretion. Pro Soc Exper Biol Med 27:890–891 [Google Scholar]
- 135.Heppell J, Kelly KA, Phillips SF (1982) Physiologic aspect of continence after colectomy, mucosal proctectomy and endorectal ileo-anal anastomosis. Ann Surg 195:435–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Londar JH, Alcancia EY, Fulkerson CC (1967) Effects of colectomy on gastric secretion in dogs. Am J Surg 113:32–36 [DOI] [PubMed] [Google Scholar]
- 137.Wettergren A, Maina P, Boesby S, Holst JJ (1997) Glucagon-like peptide-1 7–36 amide and peptide YY have additive inhibitory effect on gastric acid secretion in man. Scand J Gastroenterol 32:552–555 [DOI] [PubMed] [Google Scholar]
- 138.Hofmann S (1975) Surgical methods for adaptation of impaired absorption in cases of extensive resection of the small intestine in childhood. Padiatr Padol Suppl 3:47–53 [PubMed] [Google Scholar]
- 139.Senn N (1888) An experimental contribution to intestinal surgery with special reference to the treatment of intestinal obstruction. II Enterectomy. Ann Surg 7:99–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Flint JM (1912) The effect of extensive resections of the small intestine. Bull Johns Hopkins Hosp 23:127–144 [Google Scholar]
- 141.Mulholland MW, Debas HT (1988) Physiology and pathophysiology of gastrin: a review. Surgery 103:135–147 [PubMed] [Google Scholar]
- 142.Debas HT (1987) Gastrin. Clin Invest Med 10:222–225 [PubMed] [Google Scholar]
- 143.Besterman HS, Adrian TE, Mallinson CN, Christofides ND, Sarson DL, Pera A, Lombardo L, Modigliani R, Bloom SR (1982) Gut hormone release after intestinal resection. Gut 23:854–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dembiski A, Johnson L (1982) Role of gastrin in gastrointestinal adaptation after small bowel resection. Am J Physiol 243: G16–G20 [DOI] [PubMed] [Google Scholar]
- 145.Walsh J, Grossman M (1975) Medical progress: gastrin. N Engl J Med 292:1324–1332 [DOI] [PubMed] [Google Scholar]
- 146.Walsh J, Isenberg J, Ansfield J, Maxwell V (1976) Clearance and acid-stimulating action of human big and little gastrins in duodenal ulcer subjects. J Clin Invest 57:1125–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Walsh J, Lam SK (1980) Physiology and pathology of gastrin. Clinics in Gastroenterology 9:567–591 [PubMed] [Google Scholar]
- 148.Rumiantsev VG, Kabanov AV, Bulgakov SA (1988) Basal gastrin secretion in patients with ulcerative colitis and functional disorders of the large intestine. Klin Med (Mosk) 66:104–106 [PubMed] [Google Scholar]
- 149.Besterman HS, Mallinson CN, Modigliani R, Christofides ND, Pera A, Ponti V, Sarson DL, Bloom SR (1983) Gut hormones in inflammatory bowel disease. Scand J Gastroenterol 18:845–852 [DOI] [PubMed] [Google Scholar]
- 150.Spence RA, Buchanan KD (1982) Serum gastrin in inflammatory bowel disease. N Engl J Med 307:1530. [DOI] [PubMed] [Google Scholar]
- 151.Essop AR, Segal I, Ming R (1982) High serum gastrin in ulcerative colitis. N Engl J Med 15(307):192. [DOI] [PubMed] [Google Scholar]
- 152.Payer J, Huorka M, Duris I, Mikulecky M, Kratochvil’ova H, Ondrejka P (1993) Circadian rhythmicity and cross correlation of plasma gastrin, cortisol and somatostatin levels in ulcerative colitis patients and healthy subjects. Hepatogastroenterology 40:272–275 [PubMed] [Google Scholar]
- 153.Svendsen LB, Bisgard ML, Gustafsen J, Bulow S, Stadil F (1970) Serum gastrin values in patients with familial adenomatous polyposis. Dis Colon Rectum 37:22–25 [DOI] [PubMed] [Google Scholar]
- 154.Gonzalez-Rey E, Chorny A, Delgado M (2006) Therapeutic action of Ghrelin in a mouse model colitis. Gastroenterology 130:1707–1720 [DOI] [PubMed] [Google Scholar]
- 155.Stubbs RS, Stabile BE (1995) Role of cholecystokinin in pancreatic exocrine response to intraluminal amino acids and fat. Am J Physiol 248:347–352 [DOI] [PubMed] [Google Scholar]
- 156.Salemans JM, Thimister PW, Hopman WP, Kuijpers HC, Rosenbusch G, Nagengast FM, Jansen JB (1995) Plasma cholecystokinin levels and gallbladder volumes after proctocolectomy with ileal pouch–anal anastomosis. Surgery 117:705–711 [DOI] [PubMed] [Google Scholar]
- 157.Murray CDR, Kamm MA, Bloom ST, Emmanuel AV (2003) Ghrelin for the gastroenterologist: history and potential. Gastroenterology 125:1492–1502 [DOI] [PubMed] [Google Scholar]
- 158.Cantor P, Mortensen PE, Myhre J, Gjorup I, Worning H, Stahl E, Survill TT (1992) The effect of the cholecystokinin receptor antagonist MK-329 on meal-stimulated pancreaticobiliary output in humans. Gastroenterology 102:1742–1751 [DOI] [PubMed] [Google Scholar]
- 159.Behar J, Biancani P (1987) Pharmacologic characterization of excitatory and inhibitory cholecystokinin receptors of the cat gallbladder and sphincter of Oddi. Gastroenterology 92:764–770 [DOI] [PubMed] [Google Scholar]
- 160.Niederau C, Faber S, Karaus M (1992) Cholecystokinin’s role in regulation of colonic motility in health and in irritable bowel syndrome. Gastroenterology 102:1889–1898 [DOI] [PubMed] [Google Scholar]
- 161.Chijiiwa K, Makino I, Kozaki N, Tanaka M (1994) Differences in gallbladder bile lithogenicity in patients after gastrectomy and colectomy. Eur Surg Res 28:1–7 [DOI] [PubMed] [Google Scholar]
- 162.Makino I, Chijiiwa K, Higashijima H et al. (1994) Rapid cholesterol nucleation time and cholesterol gall stone formation after subtotal and total colectomy in humans. Gut 35:1760–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Harvey PRC, McLeod RS, Cohen Z, Strasberg SM (1991) Effect of colectomy on bile composition, cholesterol crystal formation, and gallstones in patients with ulcerative colitis. Ann Surg 214:396–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Galatola G, Fracchia M, Jazrawi RP (1995) Effect of colectomy with ileo–anal anastomosis on the biliary lipids. Eur J Clin Invest 25:534–538 [DOI] [PubMed] [Google Scholar]
- 165.Noshiro H, Hotokezaka M, Higashijima H, Iwamoto T, Nakahara S, Mibu R, Soloway RD, Chijiiwa K (1996) Gallstone formation and gallbladder bile composition after colectomy in dogs. Dig Dis Sci 41:2423–2432 [DOI] [PubMed] [Google Scholar]
- 166.Mibu R, Noshiro H, Hotokezaka M, Chijiiwa K, Tanaka M (2001) Pigment gallstone formation following proctocolectomy. Dig Dis Sci 46:2783–2785 [DOI] [PubMed] [Google Scholar]
- 167.Chey WY, Lee YM, Hendricks JG, Rhodes RA, Tai HH (1978) Plasma secretin in fasting and postprandial state in man. Digest Dis Sci 23:981–988 [DOI] [PubMed] [Google Scholar]
- 168.Schaffalitzky de Muchadell OB, Fahrenkrug J, Watt-Boolsen S, Worming GH (1987) Pancreatic response and plasma secretin concentration during infusion of low dose secretion in man. Scand J Gastroenterol 15:305–311 [DOI] [PubMed] [Google Scholar]
- 169.Itoh N, Obata KI, Yanaihara N, Okamoto H (1983) Human preprovasoactive intestinal polypeptide contains a moved PHI-27-like peptide, PHM-27. Nature 304:547–549 [DOI] [PubMed] [Google Scholar]
- 170.GJ Fordtran JS, Bloom SR et al. (1980) Effects of VIP infusion on water and ion transport in the human jejunum. Gastroenterology 78:722–727 [PubMed] [Google Scholar]
- 171.Makhlouf GM, Said SI (1975) The effect of vasoactive intestinal polypeptide (VIP) on digestive and hormonal function. In: Thompson JC (ed) Gastrointestinal hormones. University of Texas Press, Austin, pp 599–610 [Google Scholar]
- 172.Keranen U, Jarvinen H, Karkkainen P, Kiviluoto T, Kivilaakso E, Soinila S (1996) Substance P-an underlying factor for pouchitis? Prospective study of substance P- and vasoactive intestinal polypeptide-immunoreactive innervation and mast cells. Dig Dis Sci 41:1665–1671 [DOI] [PubMed] [Google Scholar]
- 173.Keranen U, Jarvinen H, Kiviluoto T, Kivilaakso E, Soinila S (1996) Substance P- and vasoactive intestinal polypeptide-immunoreactive innervation in normal and inflamed pouches after restorative proctocolectomy for ulcerative colitis. Dig Dis Sci 41:1658–1664 [DOI] [PubMed] [Google Scholar]
- 174.Chang MM, Leeman SE (1970) Isolation of sialogogic peptide from bovine hypothalamic tissue and its characterization as substance P. J Biol Chem 245:4784–4790 [PubMed] [Google Scholar]
- 175.Tatemoto K, Mutt V (1981) Isolation of the intestinal peptide porcine PHI, a new member of the glucagons-secretion family. Proc Natl Acad Sci 78:6603–6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tatemoto J, Jörnvall H, McDonald TJ et al. (1984) Isolation and primary structure of human PHI (peptide HI). FEBS Lett 174:258–261 [DOI] [PubMed] [Google Scholar]
- 177.Kock TR, Michener SR, Carney JA, Go VL (1988) Decreased colonic peptide histidine–methionine in idiopathic inflammatory bowel (1988) diseases. Dig Dis Sci 33:423–428 [DOI] [PubMed] [Google Scholar]
- 178.Yusta B, Huang L, Munroe D, Wolff G, Fantaske R, Sharma S, Demchyshyn L, Asa SL, Drucker DJ (2000) Enteroendocrine localization of GPL-2 receptor expression in humans and in rodents. Gastroenterology 119:744–755 [DOI] [PubMed] [Google Scholar]
- 179.Munck A, Kervran A, Marie JC et al. (1984) Glucagon-37 (oxyntomodulin) and glucagons-29 (pancreatic glucagons) in human bowel: analysis by HPLC and radioreceptor assay. Peptides 5:553–561 [DOI] [PubMed] [Google Scholar]
- 180.Bataille DK, Tatemoto C, Gespach H et al. (1982) Isolation of glucagons-37 (bioactive enteroglucagon/oxyntomodulin) from porcine jejuno-ileum. Characterization of the peptide. FEBS Lett 146:79–86 [DOI] [PubMed] [Google Scholar]
- 181.Thim L, Moody AJ (1981) The primary structure of porcine glicentin (proglucagon). Peptides 2:139–151 [DOI] [PubMed] [Google Scholar]
- 182.Brazzeau P, Vale W, Burgus R et al. (1973) Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science 179:77–79 [DOI] [PubMed] [Google Scholar]
- 183.Unger RH, Orci L (1976) Physiology and pathophysiology of glucagon. Physiol Rev 56:78–826 Review [DOI] [PubMed] [Google Scholar]
- 184.Jaffer SS, Makhlouf GM, Schorr BA, Zfass AM (1974) Nature and kinetics of inhibition of lower esophageal sphincter pressure by glucagon. Gastroenterology 67:42–46 [PubMed] [Google Scholar]
- 185.Whalen GE (1974) Glucagon and the small gut. Gastroenterology 67:1284–1286 [PubMed] [Google Scholar]
- 186.Jasleen J, Ashley SW, Shimoda N, Zinner MJ, Whang EE (2002) Glocagon-like peptide 2 stimulates intestinal epithelial proliferation in vitro. Dig Dis Sci 47:1135–1140 [DOI] [PubMed] [Google Scholar]
- 187.Bromer WL, Sinn A, Sand O et al. (1957) The amino acid sequence of glucagons. Diabetes 6:45–49 [DOI] [PubMed] [Google Scholar]
- 188.Sasaki HB, Rubalcava D, Baetens E et al. (1975) Identification of glucagons in the gastrointestinal tract. J Clin Invest 56:135–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Dyck WP, Janowitz HD (1971) Effect of glucagon on hepatic bile secretion in man. Gastroenterology 60:400–404 [PubMed] [Google Scholar]
- 190.Brown JC, Mutt V, Pederson RA (1970) Further purification of a polypeptide demonstrating enterogastrone activity. J Physiol 209:57–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Andersen DK, Elahi D, Brown JC et al. (1978) Oral glucagon augmentation of insulin secretion. Interactions of gastric inhibitory polypeptide with ambient glucose and insulin levels. J Clin Invest 62:152–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kimmel JR, Hyden LJ, Pollock HG (1975) Isolation and characterization of a new pancreatic polypeptide hormone. J Biol Chem 24:9369–9376 [PubMed] [Google Scholar]
- 193.Sharkey MF, Kadden ML, Stabile BE (1990) Severe posthemicolectomy diarrhea: evaluation and treatment with SMS. Gastroenterology 99:1144–1148 [DOI] [PubMed] [Google Scholar]
- 194.Yamamura T (1985) Gut hormone profiles after various types of gastrointestinal surgery. Nippon Geka Gakkai Zasshi 86:1180–1183 [PubMed] [Google Scholar]
- 195.Flaten O, Hanssen LE, Karesen R, Aune S, Myren J (1982) Intestinal phase release of pancreatic polypeptide (PP) after oral glucose in patients with gastric resection. Horn Metab Res 14:612–614 [DOI] [PubMed] [Google Scholar]
- 196.Yanagi H, Kusunoki M, Sakanoue Y, Shoji Y, Yamamura T, Murai M, Kikkawa N, Utsunomiya J (1991) Vipoma of the pancreas complicating ulcerative colitis. Am J Gastroenterol 86:1066–1069 [PubMed] [Google Scholar]
- 197.Adrian TE, Besterman HS, Bloom SR (1979) The importance of cholinergic tone in the release of pancreatic polypeptide by gut hormones in man. Life Sci 24:1989–1993 [DOI] [PubMed] [Google Scholar]
- 198.Adrian T, Ferri G, Bacarese-Hamilton A et al. (1985) Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology 89:1070–1077 [DOI] [PubMed] [Google Scholar]
- 199.Bottcher G, Sjolund K, Ekbland E et al. (1984) Coexistence of peptide YY and glicentin immunoreactivity in endocrine cells of the gut. Reg Peptides 8:261–266 [DOI] [PubMed] [Google Scholar]
- 200.Tatemoto K (1982) Isolation and characterization of peptide YY (PYY), a candidate gut hormone that inhibits pancreatic exocrine secretion. Proc Natl Acad Sci USA 79:2514–2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tetamoto K, Mutt V (1980) Isolation of two novel candidate hormones using a chemical method for finding naturally occurring polypeptides. Nature 285:417–418 [DOI] [PubMed] [Google Scholar]
- 202.Pappas T, Debas H, Goto Y, Taylor (1985) Peptide YY inhibits meal-stimulated pancreatic and gastric secretion. Gastroent Liver Physiol 248:118–123 [DOI] [PubMed] [Google Scholar]
- 203.Niebergall-Roth E, Teyssen S, Rippel K, Singer MV (1997) Effects of peptide YY on functions of the gastrointestinal tract. Dtsch Tierarztl Wochenschr 104:108–113 [PubMed] [Google Scholar]
- 204.Lundberg J, Tatemoto K (1982) Vascular effects of the peptide PYY and PHI: comparison with APP and VIP. Eur J Pharmacol 83:143–146 [DOI] [PubMed] [Google Scholar]
- 205.Dolk A, Broden G, Holmstrom B, Johansson C, Schultzberg M (1990) Slow transit chronic constipation (Arbuthnot Lane’s disease). An immunohistochemical study of neuropeptide-containing nerves in resected specimens from the large bowel. Int J Colorectal Dis 5:181–187 [DOI] [PubMed] [Google Scholar]
- 206.Anitha M, Chandrasekharan B, Salgado JR, Grouzmann E, Mwangi S, Sitaraman SV, Srinivasan S (2006) Glial-derived neurotrophic factor modulates enteric neuronal survival and proliferation through neuropeptide Y. Gastroenterology 131:1164–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Brown CJ, Cook MA, Dryburgh JR (1973) Motilin, a gastric motor activity stimulating polypeptide: the complete amino acid sequence. Can J Biochem 51:533–537 [DOI] [PubMed] [Google Scholar]
- 208.Brown J, Mutt V, Dryburgh J (1971) The further purification of motilin, a gastric motor activity stimulating polypeptide from the mucosa of the small intestine of hogs. Can J Physiol Pharmacol 49:399–405 [DOI] [PubMed] [Google Scholar]
- 209.Vantrappen C, Janssens J, Peeters TL et al. (1979) Motilin and the interdigestive migrating motor complex in man. Digest Dis Sci 24:497–500 [DOI] [PubMed] [Google Scholar]
- 210.Wingate D, Ruppin H, Thompson H et al. (1975) 13-Norleucine motilin versus pentagastrin: contrasting and competitive effects on gastrointestinal myoelectrical activity in the conscious dog. Acta Hepatogastroenterol 22:409–410 [PubMed] [Google Scholar]
- 211.Itoh Z, Takeuchi s, Aizawa I et al. (1978) Recent advances in motilin research: its physiological and clinical significance. In: V Speraza N et al (eds) Gastrointestinal hormones and pathology of the digestive system. Plenum, New York: pp 241–58 [DOI] [PubMed] [Google Scholar]
- 212.Lee K, Chey W, Tai H, Yajima H (1978) Radioimmunoassay of motilin: validation and studies on the relationship between plasma motilin and interdigestive myoelectric activity of the duodenum of dog. Dig Dis 23:789–795 [DOI] [PubMed] [Google Scholar]
- 213.Peetrs T, Vantrappen G, Janssens J (1980) Fasting plasma motilin levels are related to the interdigestive motility complex. Gastroenterology 79:716–719 [PubMed] [Google Scholar]
- 214.Poitras P, Steinback J, Van Deventer G et al. (1980) Motilin independent ectopic fronts of the interdigestive myoelectric complex in dogs. Am J Physiol 239:215–220 [DOI] [PubMed] [Google Scholar]
- 215.You C, Chey W, Lee K (1980) Studies on plasma motilin concentration and interdigestive motility of the duodenum in humans. Gastroenterology 79:62–66 [PubMed] [Google Scholar]
- 216.Itoh Z, Honda R, Hiwatashi K et al. (1976) Motilin-induced mechanical activity in the canine alimentary tract. Scand J Gastroenterol 39:93–110 [PubMed] [Google Scholar]
- 217.Owyang C, Funakoshi A, Vinik A (1983) Evidence for modulation of motilin secretion by pancreatico-biliary juice in health and in chronic pancreatitis. J Clin Endocrin Metab 57:1015–1020 [DOI] [PubMed] [Google Scholar]
- 218.Christofides N, Bloom S, Besterman H et al. (1979) Release of motilin by oral and intravenous nutrients in man. Gut 20:102–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Mitznegg P, Bloom S, Domschke W et al. (1977) Effects of secretin on plasma motilin in man. Gut 18:468–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Varner AA, Modlin IM, Walsh JH (1981) High potency of bombesin for stimulation of human gastrin release and gastric acid secretion. Regul Pept 1:289–296 [DOI] [PubMed] [Google Scholar]
- 221.Carraway R, Leeman S (1975) The amino acid sequence of a hypothalamic peptide, neurotensin. J Biol Chem 250:1907–1911 [PubMed] [Google Scholar]
- 222.Carraway R, Kitabgi P, Leeman SE (1978) The amino acid sequence of radioimmunoassayable neurotensin from bovine intestine: identity to neurotensin from hypothalamus. J Biol Chem 253:7996–7998 [PubMed] [Google Scholar]
- 223.Hallgren T, Oresland T, Cantor P, Fasth S, Hulten L (1995) Intestinal intraluminal continuity is a prerequisite for the distal bowel motility response to feeding. Scand J Gastroenterol 30:554–561 [DOI] [PubMed] [Google Scholar]
- 224.Rosenthal LE, Yamashiro DJ, Rivier J et al. (1983) Structure–activity relationships of somatostatin analogs in the rabbit ileum and rat colon. J Clin Invest 71:840–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Tulassay Z (1998) Somatostatin in the gastrointestinal tract. Scand J Gastroenterol 228:115–121 [DOI] [PubMed] [Google Scholar]
- 226.Albinus M, Gomez-Pan A, Hirst B, Shaw B (1985) Evidence against prostaglandin-mediation of somatostatin-inhibition of gastric secretion. Reg Peptides 10:259–266 [DOI] [PubMed] [Google Scholar]
- 227.Mogard M, Kauffman G Jr, Pehlevanian M, Golanska E (1985) Prostaglandins may not mediate inhibition of gastric acid secretion by somatostatin in the rat. Reg Peptides 10:231–236 [DOI] [PubMed] [Google Scholar]
- 228.Reyl F, Lewin M (1982) Intracellular receptor for somatostatin in gastric mucosal cells: decomposition and reconstruction of somatostatin-stimulated phosphoprotein phosphatases. Proc Natl Acad Sci USA 79:978–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Edwards C, Cann PA, Read NW, Holdsworth CD (1986) Effect of two new antisecretory drugs on fluid and electrolyte transport in a patient with secretory diarrhoea. Gut 27:581–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Sagar PM, Finigan P, Taylor BA (1994) Lack of response of pouch dysfunction to the somatostatin analogue octreotide. Br J Surg 81:1064. [DOI] [PubMed] [Google Scholar]
- 231.Konturek SJ, Tasler J, Krol R, Dembinski A, Coy DH, Schally AV (1977) Effect of somatostatin analogs on gastrin and pancreatic secretion. Pro Soc Exp Biol Med 155:519–522 [DOI] [PubMed] [Google Scholar]
- 232.Rabau M, Scapa E, Vinograd I (1995) Use of somatostatin to reduce the incidence of complications after ileal pouch anal anastomosis. Eur J Surg 161:293. [PubMed] [Google Scholar]
- 233.Kusuhara K, Kusunoki M, Okamoto T, Sakanoue Y, Utsunomiya J (1992) Reduction of the effluent volume in high-output ileostomy patients by a somatostatin analogue, SMS 201–995. Int J Colorectal Dis 7:202–205 [DOI] [PubMed] [Google Scholar]
- 234.Spiliotis J, Tambasis E, Christopoulou A, Rogdakis A, Siambaliotis A, Zografos K, Datsis A (2003) Sandostatin as a “hormonal” temporary protective ileostomy in patients with total or subtotal colectomy. Hepatogastroenterology 50:1367–9239 [PubMed] [Google Scholar]
- 235.Gullichsen R (2006) Long-acting octreotide in the treatment of diarrhea after pelvic pouch surgery. Tech Coloproctol 10: 346–349 [DOI] [PubMed] [Google Scholar]
- 236.Bajetta E, Carnaghi C, Ferrari L, Spagnoli I, Mazzaferro V, Buzzoni R (1996) The role of somatostatin analogues in the treatment of gastro-enteropancreatic endocrine tumours. Digestion 57:72–76 [DOI] [PubMed] [Google Scholar]
- 237.Nagase HN (2002) Regulation of feeding behaviour, gastric emptying, and sympathetic nerve activity to interscapular brown adipose tissue by galanin and enterostatin: the involvement of vagal–central nervous system interaction. J Gastroenterol 37:118–127 [DOI] [PubMed] [Google Scholar]
- 238.Nawa H, Hirose T, Takashima et al. (1983) Nucleotide sequences of cloned cDNAs for two types of bovine brain substance P precursors. Nature 306:32–36 [DOI] [PubMed] [Google Scholar]
- 239.Davison JS, Mah CL (1997) Regulation of gastric function by intrahypothalamic galanin. Proc West Pharmacol Soc 40:105–106 [PubMed] [Google Scholar]
- 240.Akabayashi A, Koenig JI, Watanabe Y, Alexander JT, Leibowitz SF (1994) Galanin-containing neurons in the paraventricular nucleus: a neurochemical marker for fat ingestion and body weight gain. Proc Natl Acad Sci USA 91:10375–10379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Gundlach AL (2002) Galanin/GALP and galanin receptors: role in central control of feeding, body weight/obesity and reproduction? Eur J Pharmacol 440:255–268 Review [DOI] [PubMed] [Google Scholar]
- 242.Koegler FH, Ritter S(1998) Galanin injection into the nucleus of the solitary tract stimulates feeding in rats with lesions of the paraventricular nucleus of the hypothalamus. Physiol Behav 63:521–527 [DOI] [PubMed] [Google Scholar]
- 243.Tan Z, Fogel R, Jiang C, Zhang X (2004) Galanin inhibits gut-related vagal neurons in rats. J Neurophysiol 91:2330–2343 [DOI] [PubMed] [Google Scholar]
- 244.Burgus R, Ward DN, Sakiz E, Guillemin R (1996) Action des enzymes proteolytiques sur des preparations purifiees de l’hormone hypothalamique TSH-hypophysiotrope, TRF. CR Acad Sci Par 262:2643–2645 [PubMed] [Google Scholar]
- 245.Raber W, Nowotny P, Vytiska-Binstorfer E, Vierhapper H (2003) Thyroxine treatment modified in infertile women according to thyroxine-releasing hormone testing: 5 year follow-up of 283 women referred after exclusion of absolute causes of infertility. Hum Reprod 18:707–714 [DOI] [PubMed] [Google Scholar]
- 246.Heppell J, Taylor BM, Beart RW Jr, Dozois RR, Kelly KA (1983) Predicting outcome after endorectal ileoanal anastomosis. Can J Surg 26:132–134 [PubMed] [Google Scholar]
- 247.Prokai L (2002) Central nervous system effects of thyrotropin-releasing hormone and its analogues: opportunities and perspectives for drug discovery and development. Prog Drug Res 59:133–169 [DOI] [PubMed] [Google Scholar]
- 248.Geelissen SM, Beck IM, Darras VM, Kuhn ER, Van der Geyten S (2003) Distribution and regulation of chicken growth hormone secretagogue receptor isoforms. Gen Comp Endocrinol 134:167–174 [DOI] [PubMed] [Google Scholar]
- 249.Holzer-Petsche U, Seitz H, Lembeck F (1989) Effect of capsaicin on gastric corpus smooth muscle of the rat in vitro. Eur J Pharmacol 14(162):29–36 [DOI] [PubMed] [Google Scholar]


