1. Introduction:
Pediatric acute myeloid leukemia (AML) is a devastating disease which carries a high relapse rate. Current risk stratification divides children into groups with higher and lower risk of relapse. However, within the group of children classified as being at lower risk of relapse, nearly 30 percent of patients still suffer a relapse.1 Because a second complete remission is often not achievable due to chemo-refractory disease, it is crucial to improve our ability to identify at-risk children and provide them with more intensive upfront therapy, or targeted therapies, to prevent relapses and improve long-term cure rates. Therefore, investigating additional clinical and biological characteristics to optimize risk stratification is critical for future progress in treating pediatric AML.
Historically risk stratification in pediatric AML was based primarily on cytogenetic factors and response to therapy. Through intensive work interrogating large groups of patients treated on Children’s Oncology Group (COG) protocols, new molecular and flow cytometry-based criteria were identified and have been incorporated into the current COG clinical trial risk stratification, refining early identification of high risk patients.2 However, components of the host environment have only recently begun to be extensively studied to determine their impact on clinical outcome in pediatric AML.
Previous studies have investigated the relationship between cytokines and clinical outcome in adults with AML, and several cytokines have been shown to be prognostic. For example, elevated concentrations of interleukin (IL)-6 in peripheral blood (PB) predicted poorer overall survival, whereas elevated IL-10 correlated with improved outcome in adult cohorts.3,4 Additionally, elevated concentrations of some cytokines in the bone marrow niche of adults with AML have been described, including IL-6.5 Recently, a study of a small pediatric AML cohort showed elevated IL-6 concentrations in peripheral blood compared with healthy age-matched controls.6 We previously reported that several interleukins in bone marrow plasma from pediatric patients at AML diagnosis are elevated when compared with bone marrow plasma from normal healthy children. Furthermore, we showed that elevated IL-6 was associated with inferior clinical outcome, particularly in “low risk” patients.7
Here, we expand our investigation of the potential use of cytokines for risk stratification to PB samples because the use of PB allows for the inclusion of all newly diagnosed patients. Some children at the time of diagnosis of AML are too ill to undergo a sedated procedure, and diagnostic studies are done with PB if the blast count is sufficiently high. If a potential new criterion for risk stratification requires bone marrow plasma sample collection, those very ill patients would not be able to benefit. On the other end of the spectrum, the diagnosis of AML is made from the initial bone marrow aspirate only. Collecting and processing bone marrow plasma from every child for whom a bone marrow aspirate is obtained to capture the small number who ultimately are diagnosed with AML has associated logistical hurdles. However, if PB plasma cytokine concentrations could be used to predict outcome, samples could be obtained from all patients, making a potential biomarker more widely applicable. To compare cytokine concentrations in PB of pediatric AML patients to those of normal healthy children and to probe the data for associations with clinical outcome, we used a unique repository of diagnostic PB plasma from pediatric AML patients.
2. Materials and Methods:
2.1. Patient samples
Peripheral blood plasma samples from pediatric AML patients enrolled on the multi-institutional COG protocol AAML1031 were studied.8 We selected diagnostic PB plasma for 80 patients initially classified as having lower risk of relapse (Supplemental Table 1). All samples were collected into Cellsave® tubes (https://www.cellsearchctc.com/product-systems-overview/cellsave-preservative-tubes), which contain a mild fixative to preserve tumor cells and cytokines during transport. Selection of samples was based on three criteria. First, we prioritized samples that arrived at our facility cold and in under three days. Second, our cohort was curated to ensure that 30% of included patients had relapsed to ensure sufficient statistical power. Third, in order to evaluate the effect of soluble factor concentrations on disease recurrence, patients who suffered treatment related mortality were excluded. As these patients were all classified as being at lower risk of relapse, upfront treatment did not include stem cell transplant. In order to allow for both description of normal pediatric PB soluble factor concentrations and for comparison with data from our cohort, we obtained normal PB (NPB) plasma from 11 healthy siblings undergoing blood draws for HLA typing at the Texas Children’s Cancer and Hematology Centers during 2015-2017. All patients or guardians provided written informed consent for the use of tissue for research purposes in accordance with the Declaration of Helsinki. These studies were approved by the Institutional Review Board of Baylor College of Medicine.
2.2. Quantification of Cytokines
Concentrations of IL-6, other growth factors, and cytokines present in plasma from PB samples were determined using both the Milliplex MAP 21-plex Human High Sensitivity T Cell Cytokine/Chemokine Magnetic Bead Panel and the 15-plex Human Myokine Magnetic Bead Panel (EMD Millipore). The high sensitivity panel was used to assure that we were able to accurately quantify PB cytokine concentrations even at low pg/mL concentrations, as it accurately measures IL-6 down to a minimal detectable concentration of 0.18 pg/mL. The myokine panel was run concurrently to accurately quantify samples with IL-6 levels > 750 pg/mL and to quantify levels of oncostatin M, another gp130-family cytokine that was elevated in a subset of AML patients from our original cohort.7 The measured cytokines included: interferon-inducible T-cell α chemoattractant (ITAC), granulocyte-macrophage colony-stimulating factor, fractalkine, interferon (IFN)-γ, macrophage inflammatory protein (MIP)-3α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12 (p70), IL-13, IL-15, IL-17A, IL-21, IL-23, tumor necrosis factor (TNF)-α, macrophage inflammatory protein (MIP)-1α, MIP-1β, apelin, brain-derived neurotrophic factor, erythropoietin, osteonectin, leukemia inhibitory factor, myostatin, fatty acid binding protein 3, irisin, follistatin-like 1, oncostatin M, fibroblast growth factor 21, and osteocrin/musclin. Samples were run in triplicate as previously described.7 For NPB plasma controls, concentrations that fell below the limit of quantification were replaced with the lower limit of quantification which was required for one NPB IL-6 concentration.
2.3. Statistics
Differences between cytokine and growth factor concentrations for NPB and AML PB plasma samples were analyzed by the Mann-Whitney U test, and correlation of cytokine concentrations with clinical outcome was assessed by the Kaplan-Meier method.
For each cytokine, the mean concentration in pg/mL was reported. When cytokine level data passed the Shapiro Wilks normality test, healthy donor and AML patient samples were compared using a two-sample Student T test. In cases where data did not pass the normality test, a Wilcoxon non-parametric test was used. To account for multiple comparisons (n=34) within the multiplex analysis, the Bonferroni method produced an adjusted significance level of 0.0015 to identify significant differences between cytokines in patients vs. healthy controls.
Cytokine concentrations were analyzed using cut-point analysis to determine whether levels were associated with clinical outcome. Five cut-points for each of the three differentially expressed cytokines were tested with the selection interval including the inner 80% of the continuous covariate’s distribution. The five cut-points tested were the integer values closest to the 10th, 25th, 50th, 75th, and 90th percentiles. The optimal cut-point was determined by the minimum p-value approach, and clinical outcomes were compared between groups. Relapse-free survival (RFS) was defined as the time from study entry to the identification of relapse. Overall survival (OS) was defined as the time from study entry until death. Within this cohort there were no toxic deaths prior to identification of relapse so RFS is equivalent to event free survival. Estimates of RFS were reported with corresponding Greenwood standard errors. Groups were compared for significant differences using the log-rank test. Adjustment by the Bonferroni method due to the use of 5 cutpoints produced a significance threshold of 0.01. P values falling between 0.05 and 0.01 are notated within the text and figure legends. Clinical data were frozen on June 30, 2018. All analyses were performed using GraphPad Prism version 6.05 for Windows (GraphPad Software, La Jolla, California).
3. Results:
3.1. Patient Characteristics
Patient characteristics are reported in Table 1. When compared to the eligible remaining low risk patients on AAML1031, our cohort was enriched for older patients and those with a higher white blood count at diagnosis. Characteristics of healthy controls are reported in Supplemental Table 2
Table 1.
Characteristics of Low Risk Patients on Study
| Patients Stuudied (n=80) | Patients Not Included on Study (n=756) | ||||
|---|---|---|---|---|---|
| N | % | N | % | p-value* | |
| Sex | |||||
|
| |||||
| Male | 41 | 51% | 390 | 52% | 0.954 |
|
| |||||
| Female | 39 | 49% | 366 | 48% | |
|
| |||||
| Age, yr olds | |||||
|
| |||||
| Median (range) | 12.3 | (0.5 - 26.6) | 8.8 | (0 - 29.5) | 0.009 |
|
| |||||
| 0-2 y | 14 | 18% | 222 | 29% | 0.025 |
|
| |||||
| 3-10 y | 20 | 25% | 198 | 26% | 0.818 |
|
| |||||
| 11-29 y | 46 | 58% | 336 | 44% | 0.026 |
|
| |||||
| Race | |||||
|
| |||||
| White | 54 | 78% | 534 | 79% | 0.814 |
|
| |||||
| Black or African American | 11 | 16% | 85 | 13% | 0.438 |
|
| |||||
| Asian | 2 | 3% | 40 | 6% | 0.416 |
|
| |||||
| Other | 2 | 3% | 13 | 2% | 0.642 |
|
| |||||
| Unknown | 11 | 84 | |||
|
| |||||
| Ethnicity | |||||
|
| |||||
| Hispanic or Latino | 14 | 18% | 135 | 18% | 0.879 |
|
| |||||
| Not Hispanic or Latino | 65 | 82% | 598 | 82% | |
|
| |||||
| Unknown | 1 | 23 | |||
|
| |||||
| WBC x103 μL - median (range) | 25.8 | (4.8 - 308) | 17.4 | (0.6 - 2730) | 0.002 |
|
| |||||
| BM Blasts % | 71.5 | 0 - 100 | 63 | 0 - 99 | 0.058 |
|
| |||||
| Cytogenetics | |||||
|
| |||||
| Normal | 19 | 24% | 148 | 20% | 0.404 |
|
| |||||
| t(8;21) | 21 | 26% | 144 | 19% | 0.138 |
|
| |||||
| inv(16) | 15 | 19% | 95 | 13% | 0.131 |
|
| |||||
| t(9;11)/11q23 | 15 | 19% | 206 | 28% | 0.090 |
|
| |||||
| Other | 10 | 13% | 154 | 21% | 0.084 |
|
| |||||
| Unknown | 0 | 9 | |||
|
| |||||
| FLT3/ITD, high AR | |||||
|
| |||||
| ITD +, high AR | 0 | 0% | 0 | 0% | - |
|
| |||||
| ITD+, low AR | 2 | 3% | 47 | 6% | 0.218 |
|
| |||||
| ITD- | 78 | 98% | 708 | 94% | |
|
| |||||
| Unknown | 0 | 1 | |||
|
| |||||
| CEBPα mutation | |||||
|
| |||||
| Positive | 10 | 13% | 56 | 7% | 0.110 |
|
| |||||
| Negative | 70 | 88% | 698 | 93% | |
|
| |||||
| Unknown | 0 | 2 | |||
|
| |||||
| NPM mutation | |||||
|
| |||||
| Positive | 11 | 14% | 67 | 9% | 0.154 |
|
| |||||
| Negative | 69 | 86% | 688 | 91% | |
|
| |||||
| Unknown | 0 | 1 | |||
|
| |||||
| Treatment Arm | |||||
|
| |||||
| Arm A | 35 | 44% | 382 | 51% | 0.249 |
|
| |||||
| Arm B | 45 | 56% | 374 | 49% | |
|
| |||||
| Course 1 response | |||||
|
| |||||
| CR | 71 | 89% | 645 | 87% | 0.586 |
|
| |||||
| Not in CR | 9 | 11% | 100 | 13% | |
|
| |||||
| Not evaluable | 0 | 11 | |||
AML: acute myeloid leukemia. Age: given in years at the time of initial diagnosis. WBC: white blood count. BM: bone marrow. MRD: minimal residual disease by flow cytometry. CR: complete remission.
χ2 or Fisher exact test when data are sparse
3.2. Plasma cytokine profiles of pediatric patients with AML compared with those of healthy donors
We evaluated PB cytokine concentrations from AML patients prior to start of therapy (n=80) and compared them to healthy controls (n=11). In our cohort of samples, nine of the tested cytokines and growth factors were significantly higher in AML patients v. controls. These were IL-6, IL-10, IL-17A, TNFα, IL-2, IL-1β, IL-12(p70), MIP-3a, and fractalkine (Figure 1A–I). Interestingly, and discrepant from what has been described previously in adult and pediatric studies, IL-8 concentrations were not significantly different when compared to normal controls (Figure 1J).4,5,7 When corrected for multiple comparisons by the Bonferroni method, IL-6, TNFα, MIP-3a, and IL-1β remained significantly different. For IL-6, the median in NPB was 2.8 pg/mL (interquartile range [IQR] 1.43-6.3) vs 12.31 pg/mL for AML (IQR 7.29-21.09, p<.0001). For TNFα, the median in NPB was 12.1 pg/mL (IQR 6.5-18.2) vs. 21.7 pg/mL for AML (IQR 14.9-34.9, p<.0001). For IL-10, the median in NPB was 23.9 pg/mL (IQR 14.4-67.8) vs 64.0 pg/mL for AML (IQR 38.9-90.9, p=.007). Cytokine concentrations in healthy controls were not associated with the age of the donor in this cohort (data not shown).
Figure 1. Cytokine levels in peripheral blood (PB) plasma are elevated at diagnosis in a subset of patients when compared to PB plasma of healthy children.
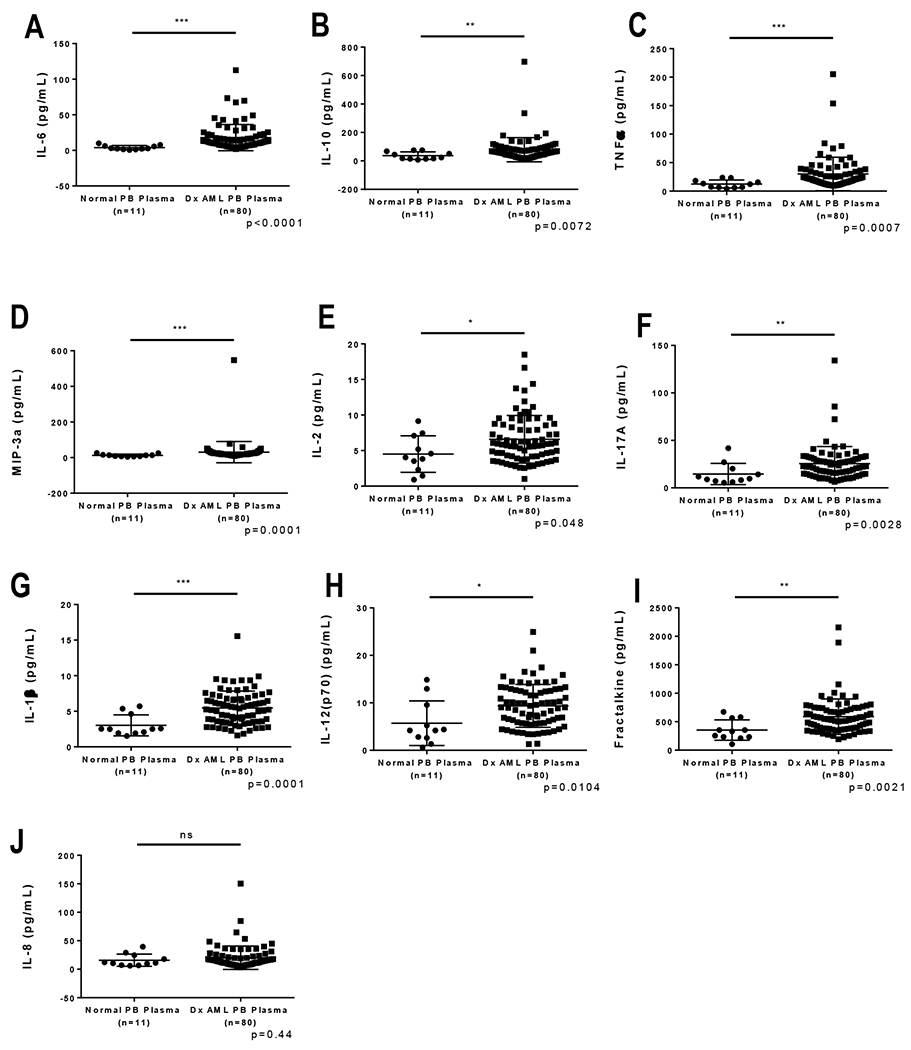
In this cohort of samples we found the following cytokines and growth factors to be significantly higher in AML patients v. controls: (A) IL-6, (B) IL-10, (C) TNFα, (D) MIP-3a, (E) IL-2, (F) IL-17A, (G) IL-1β, (H) IL-12(p70), and (I) Fractalkine. (J) IL-8 levels were not significantly different when compared to normal controls in this cohort. When corrected for multiple comparisons by the Bonferroni method, IL-6, TNFα, and IL-1β remained significantly different with p values <0.0012. For IL-6, the median in NPB was 2.8 pg/mL (25-75 Percentile 1.43-6.3) vs 12.31 pg/mL for AML (25-75 Percentile 7.29-21.09). For TNFα, the median in NPB was 12.1 pg/mL (25-75 Percentile 6.5-18.2) vs 21.7 pg/mL for AML (25-75 Percentile 14.9-34.9). For IL-10, the median in NPB was 23.9 pg/mL (25-75 Percentile 14.4-67.8) vs 64.0 pg/mL for AML (25-75 Percentile 38.9-90.9).
3.3. Cytokine levels at diagnosis and clinical outcome
To determine whether elevated cytokine concentrations at diagnosis were associated with an increased risk of relapse, we performed cut-point analysis and evaluated outcomes for all patients. Among the cytokines that were elevated in diagnostic samples, TNFα and IL-10 were associated with clinical outcome. Patients with high TNFα (n=40) had a three-year relapse free survival (RFS) of 49.6±8% compared with 76.6±7% for those with low TNFα (n=40, cut at the median 21.7 pg/mL, p=.0053, log-rank test, Figure 2A). Patients with high TNFα (n=40) had a three-year overall survival (OS) of 74.27% compared with 91.6±5% for those with low TNFα (n=40, cut at the median 21.7 pg/mL, p=.033, log-rank test, Figure 2B). Patients with high IL-10 (n=11) had a three-year RFS of 36.4±15% compared with 67.3±6% for those with low IL-10 (n=69, cut-point of 112 pg/mL, p=.005, log-rank test, Figure 2C). Patients with high IL-10 (n=11) had a three-year OS of 54.5±15% compared with 87.4±4% for those with low IL-10 (n=69, cut-point of 112 pg/mL, p=.0175, log-rank test, Figure 2D). All differences in outcomes remained significant after Bonferroni correction. Of note, our previous study of bone marrow plasma showed that elevated IL-6 concentrations at diagnosis are associated with inferior RFS; however, in this PB study, IL-6 levels were not associated with a difference in RFS (Figure 2E,2F).7 Subgroup analysis confirmed inferior outcomes in patients classified as being at lower risk of relapse solely based on negative MRD at the end of induction (Supplemental Figure 1). In this subgroup analysis, we noted that elevations in TNFα and IL-10 were primarily seen in patients classified as LR based on MRD alone. This suggests that IL-10 and TNFa driven chronic immunosuppression, and possibly suppression of T cell infiltrate, may be more important in contributing to poor clinical outcomes for children who are classified as low risk solely due to MRD negative status and the absence of high risk features.
Figure 2. Relationship between cytokine levels and clinical outcomes.
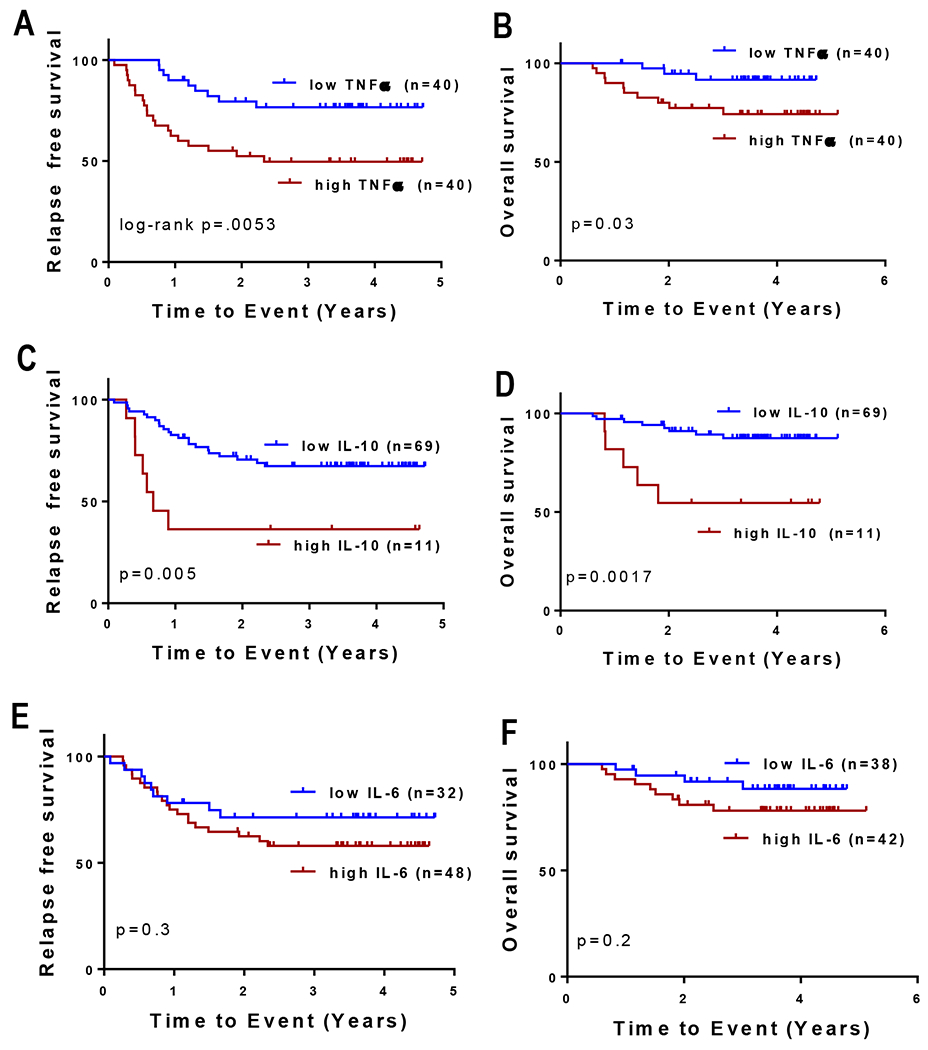
Among the cytokines that were found to be elevated in diagnostic samples, TNFα and IL-10 were associated with clinical outcome. Patients with high TNFα (n=40, A, B) had 3 year relapse free survival (RFS) of 49.6% compared to 76.6% for those with low TNFα (n=40, cut at the median 21.7 pg/mL. Patients with high IL-10 (n=11) had 3 year RFS of 36.4% compared to 67.3% for those with low IL-10 (n=69, cut-point of 112 pg/mL C, D). These differences in outcomes remained significant after Bonferroni correction. PB plasma IL-6 levels did not predict outcome at any cutpoint examined. The cutpoint that came closest to statistical significance is shown. (E, F).
4. Discussion:
Our data demonstrate that in pediatric AML patients, IL-6, IL-10, and TNFα concentrations in diagnostic PB plasma were elevated in a subset of patients. Further, higher concentrations of TNFα and IL-10 were associated with inferior clinical outcomes. Previously we reported that elevated IL-6 concentrations in the bone marrow correlated with poorer outcomes in low-risk pediatric AML patients, while in the current study, we did not find IL-6 concentrations in the PB to be prognostic.7 One possible explanation for this discrepancy is that the prior study evaluated bone marrow samples from both high and low risk AML patients while the current study evaluated PB samples from the low-risk subset only. There may also be other selection biases in the patient cohorts including differences in ethnicity, as the Texas Children’s Hospital patient population that provided the bone marrow samples is enriched for patients of Hispanic ethnicity. Another potential source of selection bias is that our PB patient cohort was enriched for patients with older age and higher white count at diagnosis. This was likely due to the requirements for collecting the optional blood samples on the clinical trial, which included an absolute peripheral blast count of >1,000 cells/μL. Lastly, the bone marrow samples were obtained from our single institution and processed immediately, while the PB samples were shipped from other institutions nationwide. Though use of Cellsave tubes has been shown to be provide superior cytokine stability, shipping theoretically allows for possible alterations of soluble factor levels depending on shipping quality and delays.9 Additionally, no data set currently exists to validate the correlation of PB and BM soluble factor concentrations. With recent increased recognition of the importance of the microenvironment and increased banking of plasma this data gap may be addressed in the near future.
TNFα and IL-6 are known pro-inflammatory cytokines and therefore our findings support the idea that an abnormal chronic pro-inflammatory state may contribute to poor outcomes in pediatric AML patients. A heightened inflammatory state has been well established as correlating with inferior outcome in a wide variety of solid tumors frequently seen in adults, and in Hodgkin lymphoma in children and young adults.10–13 Specifically in AML, increased ferritin, an inflammatory marker, and inflammatory gene signatures have been shown to correlate with inferior outcomes.14,15 Historically, it was believed that the presence of an immune infiltrate in the tumor micro-environment (a “pro-inflammatory state”) served to control tumor growth similar to the role inflammation plays in combating pathogens and other foreign entities. However, more recent research has shown that cancer-related chronic inflammation leads to derangement of the immune response, disruption of tissue homeostasis, and promotion of malignant cellular propagation, leading to the unexpected effect of promoting tumorigenesis and progression through evasion of immune-surveillance..11 We hypothesize that this chronic pro-inflammatory cytokine milieu at diagnosis allows stem cell survival and resistance to therapy throughout the treatment course but the mechanism behind this process remains an area of active investigation.
One potential link is that chronic inflammatory states may suppress the host immune response to the malignant cells. Recently, deeper characterization of subgroups of lymphocytes and myeloid cells has led to the identification of specific populations of immune cells frequently found in association with solid malignancies. Importantly, the presence or absence of immune cells at diagnosis of malignancy can be associated with clinical outcome. For instance, quantification of CD3+CD8+ T cells infiltrating within the core of a malignancy is now an established factor indicating more favorable prognosis in colorectal cancer and is growing in use for hepatocellular carcinoma.16,17 Furthermore there is growing evidence suggesting that the immune cell microenvironment may impact osteosarcoma prognosis.18,19 In acute leukemias, the interaction between a pro-inflammatory state and possible dysregulation of host immune surveillance is less well understood. An intriguing theory currently under investigation is that soluble factors and stroma within the microenvironment interact with the malignant cells to enable the malignant cells to evade host immune surveillance.20,21 In pediatric precursor B cell acute lymphoblastic leukemia, it has been shown that a low absolute lymphocyte count at the end of the first cycle of induction correlates with poor clinical outcome.22 This was hypothesized to be due to a reduction in immune surveillance against the leukemic cells and may be impacted by an immunosuppressive state in the host. Specific to myeloid leukemia, AML blasts have been shown in vitro to secrete immunosuppressive factors that inhibit T cell function.23 Additional immune suppressive myeloid subsets, monocyte- and polymorphonuclear-myeloid derived suppressor cells (MDSCs), are elevated in the blood of adults with AML.24 However, the complexities of how these microenvironment-immune-AML interactions occur have yet to be fully characterized.
IL-10, has been classified as anti-inflammatory, but its role in the pathogenesis of cancer remains controversial.25,26 Some studies suggest that as an immunosuppressive cytokine, IL-10 functions to promote immune escape of malignant cells, which has been supported by a correlation found between elevated IL-10 concentrations and inferior outcomes in certain solid tumors.27,28 Paradoxically, there is also ample evidence suggesting that IL-10 elicits potent anti-tumor effects. For example, studies have shown that tumor cells engineered to express IL-10 are rejected by murine models and established tumors have reduced growth rates when exposed to IL-10.29,30 As is seen with IL-6 and other soluble factors, IL-10 can come from either the microenvironment or from tumor cells. IL-10 has been shown to be expressed by tumor cells themselves, in this case possibly suppressing anti-tumor immune responses. Further studies are needed to determine the source of elevated concentrations of these cytokines.3,31 Our results support the former notion that IL-10 does promote cancer refractoriness.
We recognize that a weakness of the study is the relatively small sample size precluding an ideal study design with separate training and validation sets. Additional studies are needed to determine the mechanisms by which these cytokines may be reducing survival, and to further evaluate their use as prognostic biomarkers in pediatric AML patients. Improved quantification of measurable cytokines and growth factors and collection of RNA sequencing data at diagnosis may help to both risk stratify these challenging patients and may also lead to identification of new therapeutic targets and approaches to disrupting the protective environment within the bone marrow niche.21
Supplementary Material
Acknowledgements:
The authors would like to thank Carl Allen for the use of the Luminex 200 and Millliplex Analyst 5.1 software. Collection and storage of human specimens by the Children’s Oncology Group (COG) is supported through funding from the NIH (U24CA114766). This work was supported by grants from the Children’s Oncology Group Hematopoietic Malignancy Translational Pilot Study Program (AMS), a gift of funding from the Turn it Gold Foundation (AMS), and National Cancer Institute Grants R01CA17026 (MSR), R01CA164024 (TMH), and K12CA090433 (AMS).
References:
- 1.Aplenc R, Meshinchi S, Sung L, et al. Bortezomib with standard chemotherapy for children with acute myeloid leukemia does not improve treatment outcomes: a report from the Children’s Oncology Group. Haematologica. 2020;105(7):1879–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolouri H, Farrar JE, Triche T Jr., et al. The molecular landscape of pediatric acute myeloid leukemia reveals recurrent structural alterations and age-specific mutational interactions. Nature medicine. 2018;24(1):103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binder S, Luciano M, Horejs-Hoeck J. The cytokine network in acute myeloid leukemia (AML): A focus on pro- and anti-inflammatory mediators. Cytokine Growth Factor Rev. 2018;43:8–15. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez-Correa B, Bergua JM, Campos C, et al. Cytokine profiles in acute myeloid leukemia patients at diagnosis: survival is inversely correlated with IL-6 and directly correlated with IL-10 levels. Cytokine. 2013;61(3):885–891. [DOI] [PubMed] [Google Scholar]
- 5.Han Y, Ye A, Bi L, Wu J, Yu K, Zhang S. Th17 cells and interleukin-17 increase with poor prognosis in patients with acute myeloid leukemia. Cancer science. 2014;105(8):933–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanches FL, Nitsch TM, Vilela MM, Sgarbieri VC. Comparison of biochemical and immunological profile of pediatric patients with acute myeloid leukemia in relation to healthy individuals. Jornal de pediatria. 2015;91(5):478–484. [DOI] [PubMed] [Google Scholar]
- 7.Stevens AM, Miller JM, Munoz JO, Gaikwad AS, Redell MS. Interluekin-6 levels predict event-free survival in pediatric AML and suggest a mechanism of chemotherapy resistance. Blood Advances. 2017;1(18):1387–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aplenc R, Meshinchi S, Sung L, et al. The Addition of Bortezomib to Standard Chemotherapy for Pediatric Acute Myeloid Leukemia Has Increased Toxicity without Therapeutic Benefit: A Report from the Children’s Oncology Group. Blood. 2016;128(22):899–899. [Google Scholar]
- 9.Horton TM, Hoff FW, van Dijk A, et al. The effects of sample handling on proteomics assessed by reverse phase protein arrays (RPPA): Functional proteomic profiling in leukemia. J Proteomics. 2021;233:104046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galdiero MR, Marone G, Mantovani A. Cancer Inflammation and Cytokines. Cold Spring Harb Perspect Biol. 2018;10(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer treatment reviews. 2013;39(5):534–540. [DOI] [PubMed] [Google Scholar]
- 13.Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nature reviews Cancer. 2008;8(11):887–899. [DOI] [PubMed] [Google Scholar]
- 14.Ihlow J, Gross S, Sick A, et al. AML: high serum ferritin at initial diagnosis has a negative impact on long-term survival. Leukemia & lymphoma. 2018:1–9. [DOI] [PubMed] [Google Scholar]
- 15.Narayanan P, Man TK, Gerbing RB, et al. Aberrantly low STAT3 and STAT5 responses are associated with poor outcome and an inflammatory gene expression signature in pediatric acute myeloid leukemia. Clin Transl Oncol. 2021;23(10):2141–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. [DOI] [PubMed] [Google Scholar]
- 17.Galon J, Pages F, Marincola FM, et al. Cancer classification using the Immunoscore: a worldwide task force. Journal of translational medicine. 2012;10:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchais A, Marques da Costa ME, Job B, et al. Immune Infiltrate and Tumor Microenvironment Transcriptional Programs Stratify Pediatric Osteosarcoma into Prognostic Groups at Diagnosis. Cancer research. 2022;82(6):974–985. [DOI] [PubMed] [Google Scholar]
- 19.Wu CC, Beird HC, Andrew Livingston J, et al. Immuno-genomic landscape of osteosarcoma. Nature communications. 2020;11(1):1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu H, Borsotti C, Schickel J-N, et al. A novel humanized mouse model with significant improvement of class-switched, antigen-specific antibody production. Blood. 2017;129(8):959–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan F, Shen N, Pang JX, et al. Fatty acid-binding protein FABP4 mechanistically links obesity with aggressive AML by enhancing aberrant DNA methylation in AML cells. Leukemia. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabin KR, Gramatges MM, Borowitz MJ, et al. Absolute lymphocyte counts refine minimal residual disease-based risk stratification in childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2012;59(3):468–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orleans-Lindsay JK, Barber LD, Prentice HG, Lowdell MW. Acute myeloid leukaemia cells secrete a soluble factor that inhibits T and NK cell proliferation but not cytolytic function--implications for the adoptive immunotherapy of leukaemia. Clinical and experimental immunology. 2001;126(3):403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pyzer AR, Stroopinsky D, Rajabi H, et al. MUC1-mediated induction of myeloid-derived suppressor cells in patients with acute myeloid leukemia. Blood. 2017;129(13):1791–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mannino MH, Zhu Z, Xiao H, Bai Q, Wakefield MR, Fang Y. The paradoxical role of IL-10 in immunity and cancer. Cancer Lett. 2015;367(2):103–107. [DOI] [PubMed] [Google Scholar]
- 26.Ouyang W, O’Garra A. IL-10 Family Cytokines IL-10 and IL-22: from Basic Science to Clinical Translation. Immunity. 2019;50(4):871–891. [DOI] [PubMed] [Google Scholar]
- 27.Boyano MD, Garcia-Vázquez MD, López-Michelena T, et al. Soluble interleukin-2 receptor, intercellular adhesion molecule-1 and interleukin-10 serum levels in patients with melanoma. Br J Cancer. 2000;83(7):847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li C, Li H, Jiang K, Li J, Gai X. TLR4 signaling pathway in mouse Lewis lung cancer cells promotes the expression of TGF-β1 and IL-10 and tumor cells migration. Biomed Mater Eng. 2014;24(1):869–875. [DOI] [PubMed] [Google Scholar]
- 29.Huang S, Xie K, Bucana CD, Ullrich SE, Bar-Eli M. Interleukin 10 suppresses tumor growth and metastasis of human melanoma cells: potential inhibition of angiogenesis. Clin Cancer Res. 1996;2(12):1969–1979. [PubMed] [Google Scholar]
- 30.Kundu N, Beaty TL, Jackson MJ, Fulton AM. Antimetastatic and antitumor activities of interleukin 10 in a murine model of breast cancer. Journal of the National Cancer Institute. 1996;88(8):536–541. [DOI] [PubMed] [Google Scholar]
- 31.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


