Abstract
When prioritizing regions for conservation protection, decisions are often based on the principle that a single large reserve should support more species than several small reserves of the same total area (SLOSS). This principle remains a central paradigm in conservation planning despite conflicting empirical evidence and methodological concerns. In urban areas where small parks tend to dominate and policies to promote biodiversity are becoming increasingly popular, determining the most appropriate prioritization method is critical. Here, we document the role of SLOSS in defining the seasonal diversity of birds in 475 parks in 21 US cities. Collections of small parks were consistently associated with higher species richness, spatial turnover and rarity. Collections of both small and large parks were associated with higher phylogenetic and functional diversity whose patterns varied across seasons and cities. Thus, collections of small parks are a reliable source of species richness driven by higher spatial turnover and rarity, whereas collections of both small and large parks contain the potential to support higher phylogenetic and functional diversity. The presence of strong intra-annual and geographical variation emphasizes the need for regional prioritization strategies, where multiple diversity metrics are examined across parks and seasons.
Keywords: functional diversity, phylogenetic diversity, seasonal bird assemblages, single large or several small, species richness, urbanization
1. Introduction
When prioritizing regions for conservation protection, decisions are often made based on the principle that a single large (SL) reserve should contain more species than several small (SS) reserves of the same total area. This ‘SL > SS principle' originates from the general tenets of nature reserve design proposed by Diamond [1] and inspired by island biogeography theory [2]. As the ‘single large or several small' (SLOSS) debate developed, early tests found little support for SL > SS, with most studies providing evidence for the opposite outcome, SS > SL [3,4]. Subsequent studies confirmed the prevalence of SS > SL across taxa and geographical regions [5]. These findings, however, have been criticized as misleading due to methodological concerns regarding empirical SLOSS comparisons including the use of cross-scale species accumulation curves [6]. Nevertheless, the application of the SL > SS principle remains a dominant paradigm in conservation planning and management where large patches of natural habitat are often preserved at the expense of small patches [7]. The application also occurs in cities where the SL > SS principle has been used to justify the clearing of small patches of natural habitat for urban development, whose loss is then offset by the preservation of a large patch of natural habitat, typically on land having lower economic value [8] or on peri-urban land along the periphery of the urban matrix [9].
Current theory and observation suggest a single large reserve will outperform several small reserves when natural habitat patches are distributed within a low-quality matrix [10]. A primary example are urban parks within large cities where parks are distributed across a hazardous matrix of anthropogenic structures and activities. Studies that have tested the quality of the SL > SS principle among urban parks have examined a range of different taxa, including woodland [11] and grassland floras [8], and bird [12] and butterfly assemblages [13]. In all cases, these studies found that SS > SL was the dominant outcome for urban parks [5]. Natural habitat patches within urban areas are at a premium for conservation and the emerging practice of repurposing vacant or abandoned properties into parks [14,15] is providing unique opportunities to test reserve design theories and urban development strategies. The practice of ‘vacant lot greening' is especially prevalent in the post-industrial US cities where declining urban populations have resulted in large-scale land abandonment [16]. Thus, given the need for information to guide conservation choices in large urban areas, some of which are going through rapid landscape transformations, there is significant value in documenting the quality of the SL > SS principle among urban parks.
Birds represent one of the best-studied urban taxa [17] and provide a unique case study to test the quality of the SL > SS principle. In addition, the high mobility of birds increases the potential for inter-park movements, which should create conditions that support SS > SL over SL > SS [10]. We would therefore expect the species richness of birds in urban parks to display little support for SL > SS. Seasonal variation in the presence and absence of migratory species in urban parks, many of which only occur in urban areas during migration, has the potential to affect not only species richness but other facets of avian diversity including phylogenetic and functional diversity. The role of the SL > SS principle beyond its effects on species richness, however, has not been broadly explored. The primary exception is beta diversity or spatial turnover in species composition among habitat patches. Beta diversity has been shown to be a central factor promoting the prevalence of SS > SL where spatial turnover in species composition is higher among collections of small habitat patches compared with collections of large habitat patches of similar total area [10,18–20].
Here, we use bird occurrence information from the eBird citizen science programme [21] to identify well-surveyed urban parks (n = 475) in 21 large urban areas within the contiguous USA. We use a refined version of the methods proposed by Quinn & Harrison [4] to test the level of evidence for SL > SS versus SS > SL during four seasons of the year using seven measures of avian diversity. We include the traditional measure, species richness, in combination with six other measures that capture different facets of bird diversity [22,23]. This includes beta diversity, rarity, three phylogenetic metrics that capture species' evolutionary history, and one functional metric that estimates species' functional role at the community and ecosystem levels.
Based on the broad empirical support for SS > SL and the role of beta diversity as a factor driving this outcome [10], we predict SS > SL will be the dominant result across urban areas for species richness and beta diversity during all four seasons. Based on evidence that rare species tend to be confined to large habitat fragments [24] and islands [25] and specialist species tend to show stronger area dependence across habitat fragments [26], we predict SL > SS will be the dominant outcome when assessing rarity across parks. Based on the presence of unique habitat features that can only occur in large urban parks [27], we predict birds in larger parks will have higher functional diversity [28], which should result in SL > SS being the dominant outcome for functional diversity. Current evidence for birds indicates that functional diversity is a poor proxy for phylogenetic diversity [29] and phylogenetic diversity does not reliably capture functional diversity [30], suggesting these two metrics will have different outcomes. Lastly, we expect the presence and absence of migratory species from one season to the next to generate strong seasonal variation in phylogenetic and function diversity. In summary, we predict SS > SL will be the dominant outcome for species richness and beta diversity, SL > SS will be the dominant outcome for rarity and functional diversity, and the outcomes for functional and phylogenetic diversity will differ and both will display strong seasonal variation.
2. Material and methods
(a) . Urban areas and urban park polygons
We identified urban areas within the contiguous USA using the 2022 cartographic boundary file generated by the US census based on data from the 2010 census (tl_2022_us_uac10; scale 1:500 000; effective spatial resolution 250 m). From the 3601 designations in the cartographic boundary file, we considered 481 urban areas in our analysis that were classified as containing 50 000 or more people within the contiguous USA. We identified urban parks in the 481 urban areas using the 2022 ParkServe database maintained by the Trust for Public Lands (https://www.tpl.org/parkserve/about). The ParkServe database contains a total of 156 796 park polygons distributed across 110 916 parks. For the park polygons that occurred within the boundaries of the 481 urban areas, we estimated seven landcover characteristics: (1) per cent tree canopy cover, (2) per cent impervious surface, (3) per cent open water, (4) habitat heterogeneity, (5) isolation, (6) proximity, and (7) shape. We estimated per cent canopy cover using the 30-m spatial resolution National Land Cover Database (NLCD) 2016 US Forest Service Tree Canopy Cover product [31]. We estimated per cent impervious surface and per cent open water using the 30-m spatial resolution NLCD 2019 Land Cover product [32]. We estimated habitat heterogeneity using the standard deviation of peak vegetation greenness during the summer growing season [33]. Heterogeneity metrics based on vegetation greenness outperform metrics derived from topography or categorical landcover data in their ability to capture spatial variation in habitats and their ability to predict avian diversity [33,34]. Peak vegetation greenness was estimated at a 30-m spatial resolution using Landsat 8 Enhanced Vegetation Index (EVI) [35] images that were available between May and September from 2013 to 2017 [33]. The standard deviation was calculated using a 5 × 5 pixel moving window (2.25 ha), which is large enough to encompass the area of a typical breeding bird territory [33]. We defined park isolation as the shortest distance (30-m spatial resolution) from the edge of the park to the edge of the urban area. We defined park proximity as the average shortest distance (30-m spatial resolution) from the park boundary to all the other park boundaries in the urban area, including those not selected for analysis. We estimated park shape using the ratio between the perimeter and the square root of the area of the park [36]. The ratio is independent of park size and higher values indicate the shape of the park deviates from a perfect circle (ratio = 3.5449).
(b) . Bird occurrence data
We compiled daily bird occurrence data from within the contiguous USA (between 125°–66° W longitude and 24°–50° N latitude) for the period 1 January 2008 to 31 December 2022 from eBird [21]. eBird contains bird observations in checklist format where species detected by sight or sound are recorded by one or more observers during a sampling event. Sampling effort in eBird is greatest within the vicinity of large cities [37] and observations tend to be concentrated within urban parks [38]. Thus, eBird is a primary source of information on the seasonal occurrence of birds in urban parks. We queried the database on 14 March 2023, and we retained checklists for analysis that used stationary, travelling or area sampling protocols, and we retained checklists where all the species observed by the eBird participant were recorded. We only considered observations that were identified as valid in the database, we combined observations in grouped checklists into single checklists, and we excluded all pelagic seabird species. We retained all checklists that used the stationary sampling protocol. We retained travelling protocol checklists where the length travelled was less than the diameter of the park if the park was a circle. We retained area protocol checklists where the survey area was less than the area of the park. The last two steps increased the probability that the observations originated from within each park polygon.
(c) . Survey completeness of bird occurrence
We used the methodology developed by Lobo et al. [39] to identify well-surveyed urban parks with reliable species composition information. We used the ‘exact' estimator from [40] to first estimate survey completeness of bird occurrence for all years combined during four seasons of the year: nonbreeding, spring migration, breeding and autumn migration. We defined the four seasons based on the dates that encompassed the region's peak migration periods: spring migration (15 March–10 June) and autumn migration (15 August–10 November) [41,42]. We estimated the relationship between the accumulated number of species and survey effort for each park using the eBird checklist as a surrogate of sampling effort. We defined survey completeness for each park as the percentage of observed species richness captured by the species richness estimates derived from the species accumulation curves [39]. We identified parks that were well surveyed for each season based on the following criteria: the ratio between the number of occurrence records and the number of observed species was greater than 15, the slope of the species accumulation curve was less than 0.02, and survey completeness was greater than 90 [39]. We retained parks for analysis that were well surveyed during all four seasons, and we retained urban areas for analysis that contained at least 10 parks that were well surveyed during all four seasons.
(d) . Measures of avian diversity
We examined seven measures of avian diversity in our analysis. The first is species richness, which we estimated by summing the number of unique species in the eBird checklists for each season. The second is beta diversity, which we estimated by calculating the dissimilarity in species composition among parks during each season using the Simpson multiple-site dissimilarity index [43,44]. The third is rarity, which we defined as the proportion of parks where the species did not occur during each season. We computed three phylogenetic diversity metrics to capture the evolutionary history contained in the seasonal bird assemblages. The first is Faith's phylogenetic diversity (PD), which is the sum of branch lengths of the phylogenetic tree linking all species in the assemblage [45]. The second is the mean pairwise distance (MPD) and the third is the mean nearest taxon distance (MNTD) between all species in the assemblage [46]. We calculated the three metrics using a consensus tree [47] generated from a total of 2000 phylogenies (1000 Hackett backbone and 1000 Ericson backbone) [48]. To account for differences in species richness, we calculated the standardized effect size (SES) for each of the phylogenetic metrics by comparing the observed metric to the pattern expected under a null model of phylogenetic randomization. The SES estimates to what extent assemblages are phylogenetically clustered (negative SES values) or dispersed (positive SES values) than expected by chance. Expected values were calculated based on 1000 randomizations of the tip labels of the phylogeny for all species in each urban area's annual species pool. We estimated functional diversity of the avian assemblages using a combination of 10 functional traits [49]. We included seven numerical measures of avian morphology: body mass, hand-wing index, beak length (tip of the beak to the base of the skull), beak width, beak depth, tail length and tarsus length. We included three nominal measures that describe species' habitat and trophic associations: habitat density, trophic niche and primary lifestyle. We estimated function diversity based on the Gower distance [50] among all pairs of species using an attribute-diversity framework [51]. This framework is a generalization of Hill numbers of order q [52] that includes a threshold of functional distinctiveness between any two species (tau) that refines and improves on the conventional species-equivalent approach [51]. Each species contributed equally to the estimates (q = 0), and we used the mean threshold level to identify functionally indistinct sets of species [53].
(e) . Statistical analysis
We assessed how the seven landcover characteristics varied among parks using the following procedure. We first divided the parks into three classes of equal sample size using the 0.333 and 0.666 quantiles of the distribution of log10 transformed park area. We then tested if the six landcover characteristics differed among the three park classes using heteroscedastic one-way ANOVA for trimmed means [54]. We selected this method to minimize the influence of extreme outliers. We used the percentile t bootstrap method with a 20% trim level and 10 000 bootstrap samples. We report the percentile t bootstrap test statistic as T, and we followed significant tests (p < 0.05) with the corresponding bootstrap post hoc tests.
For the parks that we selected for analysis, we examined how species richness, the three phylogenetic diversity metrics, and functional diversity varied among seasons using mixed-model ANOVA with urban area included as a random effect. When significant differences were detected, we followed the mixed-model ANOVA with pairwise t-tests. We examined how these metrics varied as a function of park area for each season using mixed-model ANCOVA with the intercept for urban area included as a random effect. We log10 transformed species richness and park area to improve their distributional properties for analysis. We included park area, season and the interaction between the two as predictors in the mixed-model ANCOVA. We generated parametric bootstrapped 95% confidence bands for the fitted lines using 1000 bootstrap iterations [55]. We summarized the results using a Type I ANOVA with Satterthwaite's method for denominator degrees-of-freedom and F statistic [56].
We used a derivation of the species accumulation curve approach first proposed by Quinn & Harrison [4] to test the level of evidence for SL > SS versus SS > SL for the seven diversity metrics for each season. The method consisted of measuring the accumulation of each diversity metric compiled from small-to-large parks and the accumulation of each diversity metric compiled from large-to-small parks in conjunction with the accumulation of park areas (see electronic supplementary material, appendices S1–S7, for examples). With species richness, this procedure involved combining the species lists across accumulated parks and counting the cumulative number of unique species. Here, we would expect both accumulation curves to be monotonically increasing to a common maximum corresponding to the combination of unique species across all parks [4]. With beta diversity, the procedure involved calculating the Simpson multiple-site dissimilarity index among parks using the presence/absence matrix compiled during each step of the process. Here, the process started with the two smallest parks (small-to-large) and the two largest parks (large-to-small). With rarity, the procedure involved combining species lists across accumulated parks and calculating the median proportion of parks where the species did not occur. With the three phylogenetic diversity metrics, the procedure involved merging the species lists across the accumulated parks and calculating the metric across all the unique species. With functional diversity, this procedure involved merging the species lists across the accumulated parks and calculating functional diversity across the unique species. Unlike species richness, we do not expect the accumulation curves for the remaining six diversity metrics to be monotonically increasing.
We assessed the relationship between the small-to-large accumulation curve and the large-to-small accumulation curve for each diversity metric, urban area and season using the following approach. We first identified where each point on the large-to-small curve occurred on the small-to-large curve, which we then connected with vertical lines (see electronic supplementary material, appendices S1–S7, for examples). We then summed the length of the vertical lines where the small-to-large curve occurred above the large-to-small curve, which we then divided by the sum of the lengths of all the vertical lines that occurred both above and below the small-to-large curve. This ratio estimates the proportion of area between the two curves where the small-to-large curve occurs above the large-to-small curve. Proportions close to 1.0 provide evidence in support of SS > SL, proportions close to 0.50 provide evidence in support of SL = SS, and proportions close to 0.0 provide evidence in support of SL > SS. This method represents an improvement over the integral ratio method proposed by Quinn & Harrison [4] in that it is not computed from the origin, which tends to bias the outcome towards SS > SL dominance [57]. Lastly, we implemented a jackknife sensitivity analysis to address how our choice of functional traits affected our results for functional diversity [58]. This procedure involved estimating functional diversity 10 times after systematically removing one of the ten traits with each iteration. We summarized the results of the jackknife procedure by calculating the mean and standard deviation of the 10 proportions for each urban area and season.
We implemented our analysis using R, version 4.2.3 [59]. We used the R package KnowBR to implement the survey completeness analysis [39]. We used the function beta.multi in the betapart R package to calculate the Simpson multiple-site dissimilarity index [60]. We used the ses.pd, ses.mpd and ses.mntd functions in the picante R package [61] to calculate the standardized effect size of PD, MPD and MNTD, respectively. We used the alpha.fd.hill function in the mFD R package to calculate functional diversity [62]. We used the t1waybt and mcppb20 functions in the WRS2 R package to implement the heteroscedastic one-way ANOVA and corresponding post hoc tests, respectively [63]. We used the function lmer in the lme4 R package to implement the mixed-model ANOVA and mixed-model ANCOVA [64], and the lmerTest R package to generate the F-tests and pairwise t-tests [65]. Lastly, we used the visreg function in the visreg R package [66] in combination with the bootpredictlme4 R package [55] to generate the bootstrapped 95% confidence bands.
3. Results
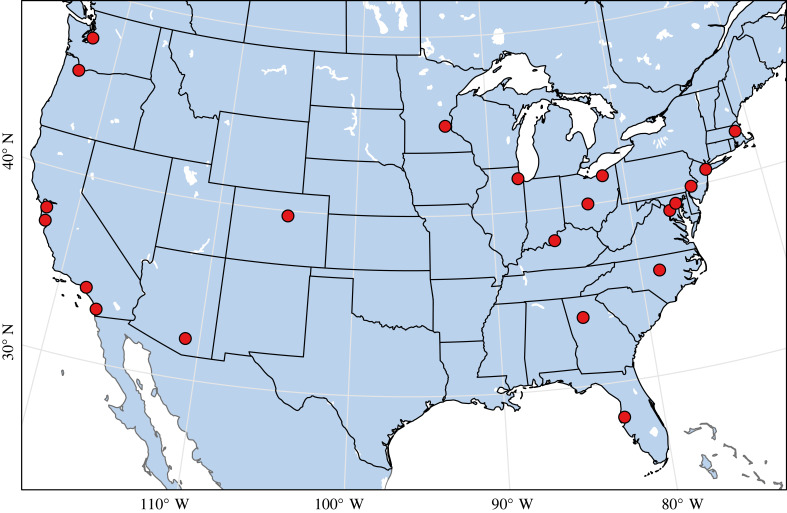
We retained 21 urban areas for analysis that contained a total of 475 park polygons that were well surveyed during all four seasons of the year (figure 1; electronic supplementary material, appendix S8: table S1). The parks contained a total of 580 unique bird species in 76 families (electronic supplementary material, appendix S9: table S2). More eBird checklists were submitted in large parks compared with small parks (F1,1892 = 122.3, p < 0.001; electronic supplementary material, appendix S10: figure S1). This relationship did not differ on average among seasons (F3,1892 = 1.36, p = 0.255) but fewer checklists were submitted on average during the breeding season (F3,1892 = 132.9, p < 0.001).
Figure 1.
The 21 urban areas within the contiguous USA considered in the analysis (electronic supplementary material, appendix S8: table S1).
(a) . Urban park landcover characteristics
We divided the 475 parks into three size classes of roughly equal sample size based on park area: small (0.003–0.315 km2), medium (0.316–1.033 km2) and large (1.042–25.098 km2). Per cent canopy cover did not differ on average among the three classes (T = 1.47, p = 0.102; electronic supplementary material, appendix S10: figure S2a). Per cent impervious surface differed on average among the three classes (T = 14.38, p < 0.001; electronic supplementary material, appendix S10: figure S2b). Large parks had less impervious surface on average compared with medium (p < 0.001) and small parks (p < 0.001), and medium parks had less impervious surface on average compared with small parks (p < 0.001). Per cent open water differed on average among the three classes (T = 9.37, p < 0.001; electronic supplementary material, appendix S10: figure S2c). Large parks had more open water on average compared to medium (p = 0.010) and small parks (p = 0.001). Habitat heterogeneity differed on average among the three classes (T = 6.23, p = 0.003; electronic supplementary material, appendix S10: figure S2d). Large parks had higher habitat heterogeneity on average compared with medium (p = 0.003) and small parks (p = 0.002). Park isolation from the boundary of the urban area differed on average among the three classes (T = 3.68, p = 0.027; electronic supplementary material, appendix S10: figure S2e). Large parks were closer on average to the urban boundaries compared with small parks (p = 0.009). Proximity to other parks within the urban areas differed on average among the three classes (T = 4.64, p = 0.012; electronic supplementary material, appendix S10: figure S2f). Large parks occurred in closer proximity to other parks on average compared with medium (p = 0.032) and small parks (p = 0.004). Park shape differed on average among the three classes (T = 3.78, p = 0.027; electronic supplementary material, appendix S10: figure S2g). The shape of large parks deviated more from a perfect circle compared with small parks (p = 0.010). Among the seven landcover characteristics, the strongest positive correlation occurred between habitat heterogeneity and per cent open water (r = 0.58, t = 15.5, d.f. = 473, p < 0.001) and strongest negative correlation occurred between per cent canopy cover and per cent impervious surface (r = −47, t = −11.43, d.f. = 473, p < 0.001; electronic supplementary material, appendix S10: figure S3).
(b) . Avian diversity: seasonal patterns
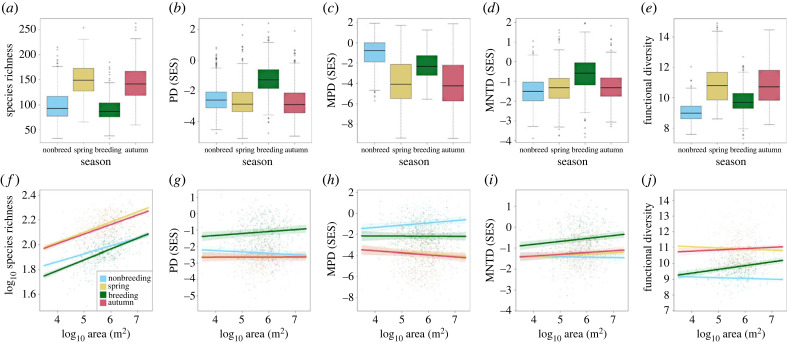
Species richness of birds within the 475 parks differed on average among seasons (F3,1876.6 = 508.91, p < 0.001; figure 2a). All the pairwise comparisons differed on average (p < 0.001) with spring migration having the highest species richness on average (149.4) followed by autumn migration (143.8), the nonbreeding season (99.7) and the breeding season (90.9; figure 2a). PD differed on average among seasons (F3,1875.5 = 349.28, p < 0.001; figure 2b). All the pairwise comparisons differed on average (p < 0.001) except between spring and autumn migration (p = 0.412; figure 2b). PD was highest on average during the breeding season and lowest on average during migration (figure 2b). MPD differed on average among seasons (F3,1876.2 = 350.46, p < 0.001; figure 2c). All the pairwise comparisons differed on average (p < 0.001) except between spring and autumn migration (p = 0.466; figure 2c). MPD was highest on average during the nonbreeding season and lowest on average during migration (figure 2c). MNTD differed on average among seasons (F3,1875.3 = 130.93, p < 0.001; figure 2d). All the pairwise comparisons differed on average (p < 0.001) except between spring and autumn migration (p = 0.179; figure 2d). MNTD was highest on average during the breeding season and lowest on average during the nonbreeding season (figure 2d). Functional diversity differed on average among seasons (F3,1875.7 = 432.94, p < 0.001; figure 2e). All the pairwise comparisons differed on average (p < 0.001) except between spring and autumn migration (p = 0.857; figure 2e). Functional diversity was highest on average during spring and autumn migration and lowest on average during the nonbreeding season (figure 2e). Among the five avian diversity metrics, the strongest correlation occurred between the three phylogenetic metrics, especially between PD and MNTD during the breeding (r = 0.834) and nonbreeding (r = 0.825) seasons (electronic supplementary material, appendix S10: figure S4; appendix S11: table S3).
Figure 2.
Seasonal (a) species richness, (b) phylogenetic diversity (PD), (c) mean pairwise distance (MPD), (d) mean nearest taxon distance (MNTD), and (e) functional diversity of bird assemblages in urban parks (n = 475) located in 21 urban areas within the contiguous USA (figure 1; electronic supplementary material, appendix S8: table S1). The relationships between park area and seasonal (f) species richness, (g) PD, (h) MPD, (i) MNTD, and (j) functional diversity. The four seasons include nonbreeding, spring migration, breeding, and autumn migration. The fitted lines and bootstrapped 95% confidence bands are from mixed-model ANCOVA with the intercept for urban area included as a random effect. The three phylogenetic metrics (PD, MPD and MNTD) were converted to standardized effect size (SES). The SES estimates to what extent assemblages are phylogenetically clustered (negative SES values) or dispersed (positive SES values) than expected by chance.
(c) . Avian diversity: park area relationships
Species richness had a significant positive relationship on average with park area (F1,1890.5 = 402.59, p < 0.001; figure 2f). The intercepts for species richness differed on average among seasons (F3,1872.5 = 619.79, p < 0.001) but the slopes did not (F3,1872.5 = 1.88, p = 0.132; figure 2f). PD did not have a significant relationship on average with park area (F1,1876.4 = 0.13, p = 0.714) but the intercepts differed on average among seasons (F3,1871.7 = 349.52, p < 0.001; figure 2g). MPD did not have a significant relationship on average with park area (F1,1880.9 = 0.28, p = 0.598) but the intercepts differed on average among seasons (F3,1872.2 = 351.00, p < 0.001; figure 2h). MNTD had a significant positive relationship on average with park area (F1,1879.4 = 5.60, p = 0.018; figure 2i). The intercepts for MNTD differed on average among seasons (F3,1871.3 = 131.35, p < 0.001) but the slopes did not (F3,1871.3 = 1.46, p = 0.224; figure 2i). Functional diversity did not have a significant relationship on average with park area (F1,1882.5 = 1.82, p = 0.178) but the intercepts (F3,1871.7 = 435.21, p < 0.001) and slopes (F3,1871.7 = 4.03, p = 0.007) differed on average among seasons with the strongest positive slope occurring during the breeding season (figure 2j).
(d) . SLOSS assessment
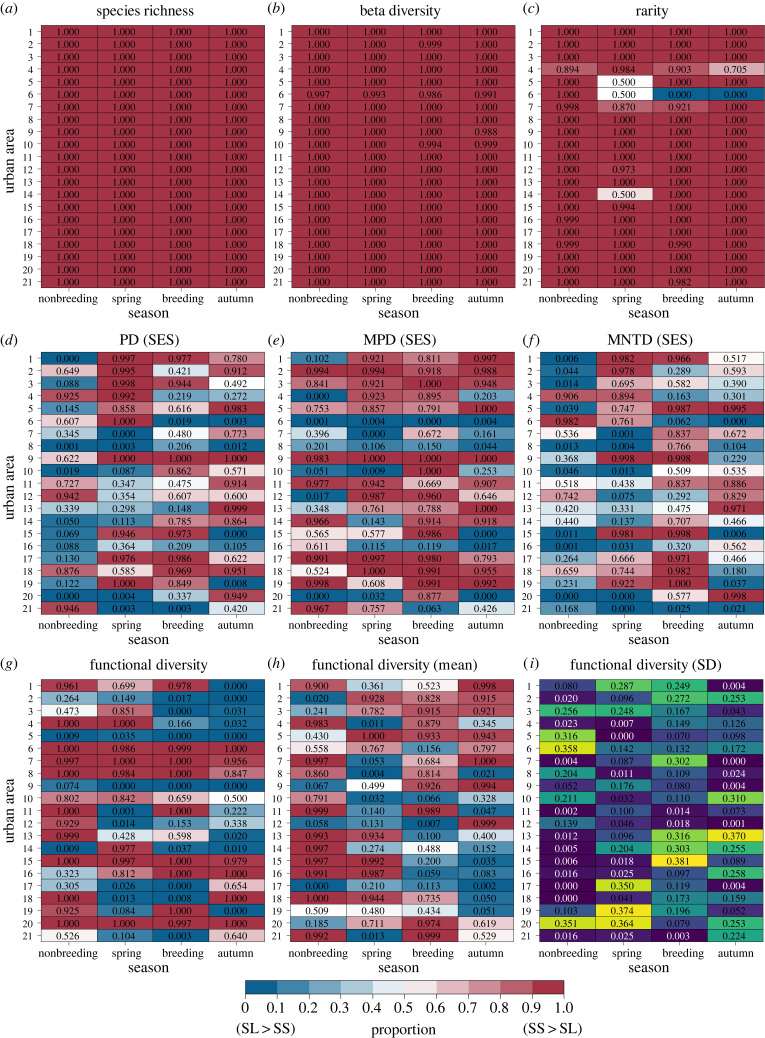
The seven measures of avian diversity presented different levels of support for SL > SS versus SS > SL (figure 3 and table 1). With species richness (figure 3a; electronic supplementary material, appendix S1) and beta diversity (figure 3b; electronic supplementary material, appendix S2), all 21 urban areas and all four seasons presented consistent support for SS > SL (table 1). With rarity, support for SS > SL was the dominant outcome in almost all cases with Columbus, OH being the primary exception where SL > SS was the dominant outcome during the breeding season and autumn migration (figure 3c and table 1; electronic supplementary material, appendix S3). With PD, more urban areas presented evidence for SL > SS during the nonbreeding season, more evidence for SS > SL during autumn migration, and nearly equal evidence during spring migration and the breeding season (figure 3d; table 1; electronic supplementary material, appendix S4). With MPD, more urban areas presented evidence for SS > SL during all four seasons, especially during the breeding season (figure 3e; table 1; electronic supplementary material, appendix S5). With MNTD, more urban areas presented evidence for SL > SS during the nonbreeding season, more evidence for SS > SL during the breeding season, and nearly equal evidence during spring and autumn migration (figure 3f and table 1; electronic supplementary material, appendix S6). With functional diversity, more urban areas presented evidence for SL > SS during autumn migration, more urban areas presented evidence for SS > SL during the nonbreeding season, and nearly equal evidence during spring migration and the breeding season (figure 3g and table 1; electronic supplementary material, appendix S7). These results differed based on the jackknife sensitivity analysis, with more urban areas presenting evidence for SL > SS during spring migration and more evidence for SS > SL during the breeding season (figure 3h–i and table 1).
Figure 3.
The level of evidence for SL > SL versus SS > SL by season for the seven measures of avian diversity calculated across urban parks (n = 475) located in 21 urban areas within the contiguous USA (figure 1; electronic supplementary material, appendix S8: table S1). The values in each cell display the proportion of the vertical area where the small-to-large accumulation curve occurred above the large-to-small accumulation curve during four seasons for (a) species richness, (b) beta diversity, (c) rarity, (d) phylogenetic diversity (PD), (e) mean pairwise distance (MPD), (f) mean nearest taxon distance (MNTD), (g) functional diversity, (h) the mean and (i) standard deviation (blue = low, yellow = high) of the 10 jackknife functional diversity iterations. The three phylogenetic metrics (PD, MPD and MNTD) were converted to standardized effect size (SES). Proportions close to 1.0 provide evidence in support of SS > SL, proportions close to 0.50 provide evidence in support of SL = SS, and proportions close to 0.0 provide evidence in support of SL > SS.
Table 1.
The sum of the proportions of total area (range = 0–21) during four seasons across 21 urban areas within the contiguous USA (figure 1; electronic supplementary material, appendix S8: table S1) where the small-to-large curve occurred above the large-to-small curve for seven measures of avian diversity: species richness, beta diversity, rarity, phylogenetic diversity (PD), mean pairwise distance (MPD), mean nearest taxon distance (MNTD), functional diversity (FD) and the mean FD from the jackknife sensitivity analysis (figure 3). The three phylogenetic metrics (PD, MPD, and MNTD) were converted to standardized effect size (SES). The per cent of the 21 urban areas where SS > SL received the greatest support (proportion greater than 0.50) are shown in parentheses.
| diversity measure | nonbreeding season | spring migration | breeding season | autumn migration |
|---|---|---|---|---|
| species richness | 21 (100) | 21 (100) | 21 (100) | 21 (100) |
| beta diversity | 21 (100) | 21 (100) | 21 (100) | 21 (100) |
| rarity | 21 (100) | 19.9 (95) | 19.8 (95) | 19.7 (95) |
| PD (SES) | 7.7 (38) | 11.9 (52) | 12.1 (52) | 12.2 (62) |
| MPD (SES) | 11.3 (57) | 12.7 (67) | 15.6 (81) | 12.3 (57) |
| MNTD (SES) | 6.4 (29) | 10.4 (52) | 13.3 (67) | 9.8 (48) |
| FD | 14.6 (67) | 11.0 (52) | 10.6 (52) | 9.2 (43) |
| FD (mean) | 13.6 (67) | 10.3 (43) | 11.8 (57) | 10.2 (48) |
4. Discussion
Although the SLOSS paradigm that a single large reserve is more effective for conservation than several small reserves of the same total area has been used to validate conservation priorities, our work shows that this does not generalize for all aspects of avian diversity in urban parks. Based on our approach for testing the relevance of SL > SS versus SS > SL, we found different levels of support for the SL > SS principle across the seven avian diversity metrics. Following our predictions and agreeing with previous assessments [5], SS > SL was the dominant outcome for species richness and beta diversity. Our prediction that SL > SS would be the dominant outcome for species rarity was not met, suggesting rare species accumulate more rapidly across collections of small parks, even when the smallest parks are excluded from the assessment. This finding agrees with evidence that specialist species tend to accumulate more rapidly across collections of small habitat fragments [5,24]. Our predictions that SL > SS would be the dominant outcome for functional diversity was partly supported, primarily during the nonbreeding season. We also found that the outcome for functional diversity was sensitive to the choice of functional traits. As we predicted, phylogenetic diversity displayed outcomes that differed from functional diversity with support broadly divided between SL > SS versus SS > SL. In summary, collections of small parks consistently promoted higher species richness, higher beta diversity and greater numbers of rare species. Phylogenetic and functional diversity displayed strong variation among seasons and urban areas, while the outcome for phylogenetic diversity was sensitive to the choice of diversity metric and the outcome for functional diversity was sensitive to the choice of functional traits.
Our findings for species richness and beta diversity show that higher species turnover among collections of small parks drives SS > SL across seasons. Thus, for each urban area, turnover in species composition across collections of small parks resulted in more unique species than turnover in species composition across a collection of larger parks of similar total size. The consistency of these results among urban areas and seasons suggests that collections of small parks play an important role in supporting higher numbers of resident and migratory species. Our findings for phylogenetic and functional diversity were considerably less consistent across urban areas and seasons. Our results for the three phylogenetic diversity metrics indicate that the influx of transitory species during spring and autumn migration does not result in higher phylogenetic diversity, suggesting that transient species do not expand the evolutionary history of bird assemblages in urban parks. Our findings for functional diversity, however, indicate that the presence of transients in the spring and autumn enhances the functional diversity of bird assemblages in urban parks.
Our findings indicate that large parks do not have more canopy cover, as we expected, but they do have less impervious surface, more water and greater habitat heterogeneity. Large parks also tended to be less isolated from the urban boundary, in closer proximity to other parks and less circular in shape. In general, we would expect these differences to support higher species richness and greater phylogenetic and functional diversity. This expectation was met with species richness, which displayed positive relationships with park area across seasons, replicating the findings from previous studies [67]. However, this expectation was not always met with phylogenetic and functional diversity, which did not present consistent relationships with park area. A possible exception occurred during the breeding season with functional diversity, suggesting that larger parks have the potential to enhance the functional diversity of breeding bird assemblages. Therefore, the effect of park area is largely constrained to species richness and the influx of migratory bird species in the spring and autumn results in higher species richness and functional diversity but not phylogenetic diversity.
Even though seasonal species richness had strong positive relationships with park area, our findings emphasize the importance of collections of small parks as sources of taxonomically diverse collections of both common and rare bird species across seasons. In addition, even though phylogenetic and functional diversity did not present strong relationships with park area, collections of small parks and collections of large parks stood out as key sources for phylogenetic and functional diversity within different urban areas during different seasons. Therefore, collections of large parks do not necessarily support more species or higher beta diversity, but collections of large parks do provide opportunities for the occurrence of species with unique evolutionary histories or functional traits, enhancing the overall diversity of urban bird assemblages. Urbanization tends to reduce and constrict the functional diversity of regional bird assemblages [68,69], and this outcome has the potential to reduce the breadth and quality of the ecosystem services that birds provide, e.g. as predators, pollinators, scavengers, seed dispersers, seed predators or ecosystem engineers [70]. If collections of large parks create opportunities for the presence of bird species with unique functional traits, the ecosystems in urban parks are likely to be enhanced through greater productivity, resilience and stability [71,72], creating opportunities for higher biodiversity [73] and reduced invasibility [74].
Our findings contain several caveats that can be used to inform and guide future research efforts. First, by examining species' occurrence within parks, we gave each species equal weight in our analysis. Within urban environments, however, non-native species often occur in higher abundance compared with native species [75]. The addition of abundance information would likely clarify how our findings are affected by the presence of native versus non-native species. Second, how long species occurred within parks during each season (duration of occurrence) was not considered in our analysis. Determining the duration of occurrence would clarify the role parks play in species' annual life cycles, e.g. as short-term stopover or dispersal sites or as long-term breeding or nonbreeding sites. Third, parks could function as population sources or sinks, a dynamic that has not been well studied in urban environments [76,77]. Determining how source/sink dynamics are defined based on park area would clarify the broader implications of our findings. For example, if large parks tend to be population sources, the ecological value of collections of large parks would likely increase.
A methodological consideration for future investigations is our use of species accumulation curves to test the relevance of SL > SS versus SS > SL. Species accumulation curves estimate how species richness changes with increasing patch area based on independent draws from the regional species pool [78] and do not estimate how species accumulate as patches are combined [79]. Thus, the use of species accumulation curves does not fully account for the scale dependence of species richness. As a consequence, through the effects of the species–area relationship, the probability of encountering new species is maximized when going from small-to-large parks and minimized when going from large-to-small parks [6], which results in an almost vertical slope of the initial small-to-large species accumulation curves (see electronic supplementary material, appendix S1). Our approach removed the steepest portion of the small-to-large species accumulation curve. Nevertheless, we still found overwhelming support for SS > SL when examining species richness, beta diversity and rarity. These findings were not replicated with phylogenetic and functional diversity. The accumulation of species in either direction did not generate monotonic relationships with phylogenetic and functional diversity, and support for SL > SS versus SS > SL was highly variable among urban areas and seasons (see electronic supplementary material, appendices S4–S7). These findings suggest that, unlike species richness, beta diversity and rarity, the use of species accumulation curves to test SL > SS versus SS > SL is considerably more informative when applied to phylogenetic and functional diversity and may provide a valuable resource for future research.
In sum, our findings suggest urban planners need to carefully consider the broader implications of park size for avian diversity. Collections of small parks will consistently support more bird species and higher species turnover among parks and more rare species. Thus, preserving or restoring natural habitats in collections of small parks or ‘greening' small vacant lots will benefit important aspects of urban bird diversity. This outcome, however, could be counterproductive if the bird species in small parks occur in low abundance for short durations and small parks function as population sinks. Determining the role of park size for avian survival and fitness would help clarify the value of small parks for conserving urban bird populations. Conversely, preserving or ‘greening' a similarly sized collection of large parks will likely not result in higher species numbers, but it will provide opportunities to enhance phylogenetic and functional diversity. Preserving or restoring natural habitats in large parks is therefore likely to increase the overall diversity of urban bird assemblages, and if native species occur in high abundance over longer durations and large parks are weaker population sinks, these changes are likely to provide broader and more persistent ecological benefits. Our findings suggest that this would take on greater relevance during the breeding season, the only season where multiple diversity metrics displayed positive relationships with park area. In total, our findings highlight the value of both small and large urban parks for seasonal bird diversity and the need for regional prioritization strategies where multiple diversity metrics are examined across seasons.
Acknowledgements
This research would not be possible without the contributions of the eBird participants, expert reviewers, and the many other individuals whose work and engagement make the eBird project successful. We thank D. Deane for valuable feedback on an earlier draft and K. Horton for assistance with the figures.
Ethics
This work did not require ethical approval from a human subject or animal welfare committee.
Data accessibility
The ParkServe data are available from https://www.tpl.org/parkserve/downloads and the bird occurrence data are available from https://science.ebird.org/en/use-ebird-data. Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.h44j0zprr [80].
Supplementary material is available online [81].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors' contributions
F.A.L.: conceptualization, formal analysis, methodology, visualization, writing—original draft; J.A.G.C.: data curation, methodology, writing—review and editing; C.A.L.: conceptualization, writing—review and editing; M.F.J.A.: conceptualization, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
The authors declare no competing interests.
Funding
This work was supported by the Wolf Creek Charitable Foundation and the National Science Foundation (ABI sustaining: DBI-1939187).
References
- 1.Diamond JM. 1975. The island dilemma: lessons of modern biogeographic studies for the design of natural reserves. Biol. Conserv. 7, 129-146. ( 10.1016/0006-3207(75)90052-X) [DOI] [Google Scholar]
- 2.MacArthur RH, Wilson EO. 1967. The theory of island biogeography. Princeton, NJ: Princeton University Press. [Google Scholar]
- 3.Simberloff D, Abele LG. 1982. Refuge design and island biogeographic theory: effects of fragmentation. Am. Nat. 120, 41-50. ( 10.1086/283968) [DOI] [Google Scholar]
- 4.Quinn JF, Harrison SP. 1988. Effects of habitat fragmentation and isolation on species richness: evidence from biogeographic patterns. Oecologia 75, 132-140. ( 10.1007/BF00378826) [DOI] [PubMed] [Google Scholar]
- 5.Fahrig L. 2020. Why do several small patches hold more species than few large patches? Glob. Ecol. Biogeogr. 29, 615-628. ( 10.1111/geb.13059) [DOI] [Google Scholar]
- 6.Deane DC. 2022. Species accumulation in small–large vs large–small order: more species but not all species? Oecologia 200, 273-284. ( 10.1007/s00442-022-05261-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armsworth PR, et al. 2018. Is conservation right to go big? Protected area size and conservation return-on-investment. Biol. Conserv. 225, 229-236. ( 10.1016/j.biocon.2018.07.005) [DOI] [Google Scholar]
- 8.Kendal D, Zeeman BJ, Ikin K, Lunt ID, McDonnell MJ, Farrar A, Pearce LM, Morgan JW. 2017. The importance of small urban reserves for plant conservation. Biol. Conserv. 213, 146-153. ( 10.1016/j.biocon.2017.07.007) [DOI] [Google Scholar]
- 9.McGregor D, Simon D. 2005. The peri-urban interface: approaches to sustainable natural and human resource use, p. 272. London, UK: Routledge. [Google Scholar]
- 10.Fahrig L, et al. 2022. Resolving the SLOSS dilemma for biodiversity conservation: a research agenda. Biol. Rev. 97, 99-114. ( 10.1111/brv.12792) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Godefroid S, Koedam N. 2003. How important are large vs. small forest remnants for the conservation of the woodland flora in an urban context? Glob. Ecol. Biogeogr. 12, 287-298. ( 10.1046/j.1466-822X.2003.00035.x) [DOI] [Google Scholar]
- 12.Kim J, Chae J, Koo T-H. 2007. Variation in bird diversity in relation to habitat size in the urban landscape of Seoul, South Korea. Acta Ornithol. 42, 39-44. ( 10.3161/068.042.0111) [DOI] [Google Scholar]
- 13.Lizee M-H, Tatoni T, Deschamps-Cottin M. 2016. Nested patterns in urban butterfly species assemblages: respective roles of plot management, park layout and landscape features. Urban Ecosyst. 19, 205-224. ( 10.1007/s11252-015-0501-5) [DOI] [Google Scholar]
- 14.Beauregard R. 2012. Strategic thinking for distressed neighborhoods. In The city after abandonment (eds Dewar M., Manning J.), pp. 227-243. Philadelphia, PA: University of Pennsylvania Press. [Google Scholar]
- 15.Schilling J, Logan J. 2008. Greening the Rust Belt: a green infrastructure model for right sizing America's shrinking cities. J. Am. Plann. Assoc. 74, 451-466. ( 10.1080/01944360802354956) [DOI] [Google Scholar]
- 16.Accordino J, Johnson GT. 2000. Addressing the vacant and abandoned property problem. J. Urban Aff. 22, 301-315. ( 10.1111/0735-2166.00058) [DOI] [Google Scholar]
- 17.Lepczyk CA, Warren PS. 2012. Urban bird ecology and conservation. Oakland, CA: University of California Press. [Google Scholar]
- 18.Deane DC, Nozohourmehrabad P, Boyce SSD, He F. 2020. Quantifying factors for understanding why several small patches host more species than a single large patch. Biol. Conserv. 249, 108711. ( 10.1016/j.biocon.2020.108711) [DOI] [Google Scholar]
- 19.Chase JM, Kraft NJB, Smith KG, Vellend M, Inouye BD. 2011. Using null models to disentangle variation in community dissimilarity from variation in α-diversity. Ecosphere 2, art24. ( 10.1890/ES10-00117.1) [DOI] [Google Scholar]
- 20.Riva F, Fahrig L. 2023. Landscape-scale habitat fragmentation is positively related to biodiversity, despite patch-scale ecosystem decay. Ecol. Lett. 26, 268-277. ( 10.1111/ele.14145) [DOI] [PubMed] [Google Scholar]
- 21.Sullivan BL, et al. 2014. The eBird enterprise: an integrated approach to development and application of citizen science. Biol. Conserv. 169, 31-40. ( 10.1016/j.biocon.2013.11.003) [DOI] [Google Scholar]
- 22.Naeem S, et al. 2016. Biodiversity as a multidimensional construct: a review, framework and case study of herbivory's impact on plant biodiversity. Proc. R. Soc. B 283, 20153005. ( 10.1098/rspb.2015.3005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyashevska O, Farnsworth KD. 2012. How many dimensions of biodiversity do we need? Ecol. Indic. 18, 485-492. ( 10.1016/j.ecolind.2011.12.016) [DOI] [Google Scholar]
- 24.Rösch V, Tscharntke T, Scherber C, Batáry P. 2015. Biodiversity conservation across taxa and landscapes requires many small as well as single large habitat fragments. Oecologia 179, 209-222. ( 10.1007/s00442-015-3315-5) [DOI] [PubMed] [Google Scholar]
- 25.Mac Nally R, Lake PS. 1999. On the generation of diversity in archipelagos: a re-evaluation of the Quinn-Harrison ‘saturation index’. J. Biogeogr. 26, 285-295. ( 10.1046/j.1365-2699.1999.00268.x) [DOI] [Google Scholar]
- 26.Matthews TJ, Cottee-Jones HE, Whittaker RJ. 2014. Habitat fragmentation and the species–area relationship: a focus on total species richness obscures the impact of habitat loss on habitat specialists. Divers. Distrib. 20, 1136-1146. ( 10.1111/ddi.12227) [DOI] [Google Scholar]
- 27.Chang H-Y, Lee Y-F. 2016. Effects of area size, heterogeneity, isolation, and disturbances on urban park avifauna in a highly populated tropical city. Urban Ecosyst. 19, 257-274. ( 10.1007/s11252-015-0481-5) [DOI] [Google Scholar]
- 28.Schütz C, Schulze CH. 2015. Functional diversity of urban bird communities: effects of landscape composition, green space area and vegetation cover. Ecol. Evol. 5, 5230-5239. ( 10.1002/ece3.1778) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calba S, Maris V, Devictor V. 2014. Measuring and explaining large-scale distribution of functional and phylogenetic diversity in birds: separating ecological drivers from methodological choices. Glob. Ecol. Biogeogr. 23, 669-678. ( 10.1111/geb.12148) [DOI] [Google Scholar]
- 30.Mazel F, et al. 2018. Prioritizing phylogenetic diversity captures functional diversity unreliably. Nat. Commun. 9, 2888. ( 10.1038/s41467-018-05126-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coulston JW, Moisen GG, Wilson BT, Finco MV, Cohen WB, Brewer CK. 2012. Modeling percent tree canopy cover: a pilot study. Photogramm. Eng. Remote Sens. 78, 715-727. ( 10.14358/PERS.78.7.715) [DOI] [Google Scholar]
- 32.Dewitz J. 2021. US Geological Survey National Land Cover Database (NLCD) 2019 Products (ver. 2.0, June 2021). US Geological Survey data release. ( 10.5066/P9KZCM54) [DOI]
- 33.Farwell LS, Elsen PR, Razenkova E, Pidgeon AM, Radeloff VC. 2020. Habitat heterogeneity captured by 30-m resolution satellite image texture predicts bird richness across the United States. Ecol. Appl. 30, e02157. ( 10.1002/eap.2157) [DOI] [PubMed] [Google Scholar]
- 34.Tuanmu M-N, Jetz W. 2015. A global, remote sensing-based characterization of terrestrial habitat heterogeneity for biodiversity and ecosystem modelling. Glob. Ecol. Biogeogr. 24, 1329-1339. ( 10.1111/geb.12365) [DOI] [Google Scholar]
- 35.Huete A, Didan K, Miura T, Rodriguez EP, Gao X, Ferreira LG. 2002. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 83, 195-213. ( 10.1016/S0034-4257(02)00096-2) [DOI] [Google Scholar]
- 36.Feder J. 1988. The perimeter-area relation. In Fractals (ed. Feder J), pp. 200-211. Boston, MA: Springer US. [Google Scholar]
- 37.Zhang G. 2020. Spatial and temporal patterns in volunteer data contribution activities: a case study of eBird. ISPRS Int. J. Geo-Inf. 9, 597. ( 10.3390/ijgi9100597) [DOI] [Google Scholar]
- 38.Newsome D, Simpson G. 2020. Green cities as bird watching destinations. In Routledge handbook of tourism cities (eds Morrison AM, Coca-Stefaniak A), pp. 262-275. London, UK: Routledge. [Google Scholar]
- 39.Lobo JM, et al. 2018. KnowBR: an application to map the geographical variation of survey effort and identify well-surveyed areas from biodiversity databases. Ecol. Indic. 91, 241-248. ( 10.1016/j.ecolind.2018.03.077) [DOI] [Google Scholar]
- 40.Ugland KI, Gray JS, Ellingsen KE. 2003. The species-accumulation curve and estimation of species richness. J. Anim. Ecol. 72, 888-897. ( 10.1046/j.1365-2656.2003.00748.x) [DOI] [Google Scholar]
- 41.Horton KG, Van Doren BM, Albers HJ, Farnsworth A, Sheldon D. 2021. Near-term ecological forecasting for dynamic aeroconservation of migratory birds. Conserv. Biol. 35, 1777-1786. ( 10.1111/cobi.13740) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horton KG, Nilsson C, Van Doren BM, La Sorte FA, Dokter AM, Farnsworth A. 2019. Bright lights in the big cities: migratory birds' exposure to artificial light. Front. Ecol. Environ. 17, 209-214. ( 10.1002/fee.2029) [DOI] [Google Scholar]
- 43.Baselga A. 2010. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 19, 134-143. ( 10.1111/j.1466-8238.2009.00490.x) [DOI] [Google Scholar]
- 44.Baselga A. 2012. The relationship between species replacement, dissimilarity derived from nestedness, and nestedness. Glob. Ecol. Biogeogr. 21, 1223-1232. ( 10.1111/j.1466-8238.2011.00756.x) [DOI] [Google Scholar]
- 45.Faith DP. 1992. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 61, 1-10. ( 10.1016/0006-3207(92)91201-3) [DOI] [Google Scholar]
- 46.Webb CO, Ackerly DD, McPeek MA, Donoghue MJ. 2002. Phylogenies and community ecology. Annu. Rev. Ecol. Syst. 33, 475-505. ( 10.1146/annurev.ecolsys.33.010802.150448) [DOI] [Google Scholar]
- 47.Sukumaran J, Holder MT. 2010. DendroPy: a Python library for phylogenetic computing. Bioinformatics 26, 1569-1571. ( 10.1093/bioinformatics/btq228) [DOI] [PubMed] [Google Scholar]
- 48.Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO. 2012. The global diversity of birds in space and time. Nature 491, 444-448. ( 10.1038/nature11631) [DOI] [PubMed] [Google Scholar]
- 49.Tobias JA, et al. 2022. AVONET: morphological, ecological and geographical data for all birds. Ecol. Lett. 25, 581-597. ( 10.1111/ele.13898) [DOI] [PubMed] [Google Scholar]
- 50.Gower JC. 1971. A general coefficient of similarity and some of its properties. Biometrics 27, 857-871. ( 10.2307/2528823) [DOI] [Google Scholar]
- 51.Chao A, Chiu C-H, Villéger S, Sun IF, Thorn S, Lin Y-C, Chiang J-M, Sherwin WB. 2019. An attribute-diversity approach to functional diversity, functional beta diversity, and related (dis)similarity measures. Ecol. Monogr. 89, e01343. ( 10.1002/ecm.1343) [DOI] [Google Scholar]
- 52.Chao A, Chiu C-H, Jost L. 2014. Unifying species diversity, phylogenetic diversity, functional diversity, and related similarity and differentiation measures through Hill numbers. Annu. Rev. Ecol. Evol. Syst. 45, 297-324. ( 10.1146/annurev-ecolsys-120213-091540) [DOI] [Google Scholar]
- 53.Magneville C, et al. 2022. mFD: an R package to compute and illustrate the multiple facets of functional diversity. Ecography 2022, e05904. ( 10.1111/ecog.05904) [DOI] [Google Scholar]
- 54.Wilcox R. 2021. Introduction to robust estimation and hypothesis testing, 5th edn. London, UK: Elsevier. [Google Scholar]
- 55.Duursma R. 2022. Bootpredictlme4: predict method for lme4 with bootstrap. R package version 0.1.
- 56.Satterthwaite FE. 1946. An approximate distribution of estimates of variance components. Biometrics Bull. 2, 110-114. ( 10.2307/3002019) [DOI] [PubMed] [Google Scholar]
- 57.Ramsey FL. 1989. Comments on a ‘Saturation Index’. Oecologia 81, 569-570. ( 10.1007/BF00378971) [DOI] [PubMed] [Google Scholar]
- 58.Legras G, Loiseau N, Gaertner JC, Poggiale JC, Gaertner-Mazouni N. 2020. Assessing functional diversity: the influence of the number of the functional traits. Theor. Ecol. 13, 117-126. ( 10.1007/s12080-019-00433-x) [DOI] [Google Scholar]
- 59.R Development Core Team. 2023. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. (https://www.R-project.org/). [Google Scholar]
- 60.Baselga A, Orme D, Villeger S, De Bortoli J, Leprieur F, Logez M. 2022. Betapart: partitioning beta diversity into turnover and nestedness components. R package version 1.5.6. See https://CRAN.R-project.org/package=betapart.
- 61.Kembel SW, Cowan PD, Helmus MR, Cornwell WK, Morlon H, Ackerly DD, Blomberg SP, Webb CO. 2010. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26, 1463-1464. ( 10.1093/bioinformatics/btq166) [DOI] [PubMed] [Google Scholar]
- 62.Magneville C, et al. 2021. mFD: A computation of functional spaces and functional indices. R package version 1.0.0. See https://github.com/CmlMagneville/mFD.
- 63.Mair P, Wilcox R. 2020. Robust statistical methods in R using the WRS2 package. Beh. Res. Meth. 52, 464-488. ( 10.3758/s13428-019-01246-w) [DOI] [PubMed] [Google Scholar]
- 64.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1-48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 65.Kuznetsova A, Brockhoff PB, Christensen RHB. 2017. lmerTest package: tests in linear mixed effects models. J. Stat. Softw. 82, 1-26. ( 10.18637/jss.v082.i13) [DOI] [Google Scholar]
- 66.Breheny P, Burchett W. 2017. Visualization of regression models using visreg. The R Journal 9, 56-71. ( 10.32614/RJ-2017-046) [DOI] [Google Scholar]
- 67.La Sorte FA, Aronson MFJ, Lepczyk CA, Horton KG. 2020. Area is the primary correlate of annual and seasonal patterns of avian species richness in urban green spaces. Landsc. Urban Plann. 203, 103892. ( 10.1016/j.landurbplan.2020.103892) [DOI] [Google Scholar]
- 68.La Sorte FA, et al. 2018. The phylogenetic and functional diversity of regional breeding bird assemblages is reduced and constricted through urbanization. Divers. Distrib. 24, 928-938. ( 10.1111/ddi.12738) [DOI] [Google Scholar]
- 69.Sol D, et al. 2020. The worldwide impact of urbanisation on avian functional diversity. Ecol. Lett. 23, 962-972. ( 10.1111/ele.13495) [DOI] [PubMed] [Google Scholar]
- 70.Whelan CJ, Wenny DG, Marquis RJ. 2008. Ecosystem services provided by birds. Ann. N. Y. Acad. Sci. 1134, 25-60. ( 10.1196/annals.1439.003) [DOI] [PubMed] [Google Scholar]
- 71.Hallett LM, Stein C, Suding KN. 2017. Functional diversity increases ecological stability in a grazed grassland. Oecologia 183, 831-840. ( 10.1007/s00442-016-3802-3) [DOI] [PubMed] [Google Scholar]
- 72.Vallina SM, Cermeno P, Dutkiewicz S, Loreau M, Montoya JM. 2017. Phytoplankton functional diversity increases ecosystem productivity and stability. Ecol. Model. 361, 184-196. ( 10.1016/j.ecolmodel.2017.06.020) [DOI] [Google Scholar]
- 73.Tilman D, Isbell F, Cowles JM. 2014. Biodiversity and ecosystem functioning. Annu. Rev. Ecol. Evol. Syst. 45, 471-493. ( 10.1146/annurev-ecolsys-120213-091917) [DOI] [Google Scholar]
- 74.Kennedy TA, Naeem S, Howe KM, Knops JMH, Tilman D, Reich P. 2002. Biodiversity as a barrier to ecological invasion. Nature 417, 636-638. ( 10.1038/nature00776) [DOI] [PubMed] [Google Scholar]
- 75.Lepczyk CA, La Sorte FA, Aronson MFJ, Goddard MA, MacGregor-Fors I, Nilon CH, Warren PS. 2017. Global patterns and drivers of urban bird diversity. In Ecology and conservation of birds in urban environments (eds Murgui E, Hedblom M), pp. 13-33. Cham, Switzerland: Springer International Publishing. [Google Scholar]
- 76.Pulliam HR. 1988. Sources, sinks, and population regulation. Am. Nat. 132, 652-661. ( 10.1086/284880) [DOI] [Google Scholar]
- 77.Lepczyk CA, Aronson MFJ, Evans KL, Goddard MA, Lerman SB, MacIvor JS. 2017. Biodiversity in the city: fundamental questions for understanding the ecology of urban green spaces for biodiversity conservation. Bioscience 67, 799-807. ( 10.1093/biosci/bix079) [DOI] [Google Scholar]
- 78.Scheiner SM, Chiarucci A, Fox GA, Helmus MR, McGlinn DJ, Willig MR. 2011. The underpinnings of the relationship of species richness with space and time. Ecol. Monogr. 81, 195-213. ( 10.1890/10-1426.1) [DOI] [Google Scholar]
- 79.Matthews TJ, Triantis KA, Rigal F, Borregaard MK, Guilhaumon F, Whittaker RJ. 2016. Island species–area relationships and species accumulation curves are not equivalent: an analysis of habitat island datasets. Glob. Ecol. Biogeogr. 25, 607-618. ( 10.1111/geb.12439) [DOI] [Google Scholar]
- 80.La Sorte FA, Clark JAG, Lepczyk CA, Aronson MFJ. 2023. Data from: Collections of small urban parks consistently support higher species richness but not higher phylogenetic or functional diversity. Dryad Digital Repository. ( 10.5061/dryad.h44j0zprr) [DOI] [PMC free article] [PubMed]
- 81.La Sorte FA, Clark JAG, Lepczyk CA, Aronson MFJ. 2023. Collections of small urban parks consistently support higher species richness but not higher phylogenetic or functional diversity. Figshare. ( 10.6084/m9.figshare.c.6806472) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- La Sorte FA, Clark JAG, Lepczyk CA, Aronson MFJ. 2023. Data from: Collections of small urban parks consistently support higher species richness but not higher phylogenetic or functional diversity. Dryad Digital Repository. ( 10.5061/dryad.h44j0zprr) [DOI] [PMC free article] [PubMed]
- La Sorte FA, Clark JAG, Lepczyk CA, Aronson MFJ. 2023. Collections of small urban parks consistently support higher species richness but not higher phylogenetic or functional diversity. Figshare. ( 10.6084/m9.figshare.c.6806472) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The ParkServe data are available from https://www.tpl.org/parkserve/downloads and the bird occurrence data are available from https://science.ebird.org/en/use-ebird-data. Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.h44j0zprr [80].
Supplementary material is available online [81].