Abstract
In anisogamous species, sexual selection is expected to be stronger in males. Bateman's principles state that the variance in (i) reproductive and (ii) mating success is greater for males, and (iii) the relationship between reproductive success and mating success (the Bateman gradient) is also stronger for males than for females. Sexual selection, based on Bateman's principles, has been demonstrated in animals and some angiosperms, but never in a seaweed. Here we focus on the oogamous haploid-diploid rhodophyte Gracilaria gracilis in which previous studies have shown evidence for non-random mating, suggesting the existence of male–male competition and female choice. We estimated mating and reproductive success using paternity analyses in a natural population where up to 92% of fertilizations occurred between partners of that population. The results show that the variance in mating success is significantly greater in males than in females and that the Bateman gradient is positive only in males. Distance to female partners also explains a minor part of the variance in male mating success. Although there is no evidence for sexual dimorphism, our study supports the hypothesis that sexual selection occurs in G. gracilis, probably on male traits, even if we cannot observe, characterize or quantify them yet.
Keywords: Bateman gradient, paternity analyses, sessile marine organisms, mating and reproductive success, haploid diploid life cycle, dioicous species
1. Introduction
Sexual selection arises from competition among individuals for the access to mates and/or to their gametes [1]. Historically developed to explain the sexual dimorphism of secondary sexual traits in animal species [2], sexual selection theory postulates that anisogamy should lead to strong competition among males for the access to females and ovules [3]. Therefore, under this postulate, any trait that increases the successful access to female mating partners should greatly affect male reproductive success [4]. This theory thus constitutes a satisfying explanation for the frequently observed male-biased sexual dimorphism in several categories of traits, including ornaments and armaments (but see [5]). Sexual selection in both sexes has been quantified in a large number of animal species using metrics derived from Bateman's principles [6]: (i) the opportunity for selection I (i.e. the variance in relative reproductive success); (ii) the opportunity for sexual selection Is, i.e. the variance relative in mating success and (iii) the Bateman gradient (i.e. the slope of the regression of relative reproductive success as a function of relative mating success [7]). The sex associated with the highest value of these three metrics is considered as the one under stronger sexual selection. A recent meta-analysis focusing on animals indeed shows that both the Bateman gradient and the opportunity for sexual selection are more often higher in males [8]. Although this trend appears stronger in species with male-biased sexual dimorphism in terms of morphological or behavioural traits (i.e. males developing large body, bright colours or exuberant and acrobatic courtship displays), a number of studies have reported stronger sexual selection, or opportunities of sexual selection, in males within species in which no sexual dimorphism has been documented [8].
The use of these metrics of sexual selection has been largely biased towards animals, mainly vertebrates and non-sessile invertebrates [8]. Although it has been acknowledged that stronger sexual selection in males should occur in any anisogamous species [9], very few studies have attempted to directly verify this prediction in other anisogamous eukaryotes which typically lack copulation and for which cognitive traits (such as those involved in partner choice behaviours, sensu stricto) usually play no role in sexual selection. Therefore, these anisogamous eukaryotes, such as plants, provide stimulating study models for the empirical test of sexual selection. Sexual selection in angiosperms, although once fiercely debated [9,10], is now widely recognized as a possible evolutionary force, which may indeed be stronger through the male function of hermaphrodites or in male individuals in dioecious species, at least when pollen supply is not limiting [11–13]. Among the arguments in favour of the existence of sexual selection in plants, the occurrence of male-biased sexual dimorphism in dioecious plant species (e.g. males with larger or more numerous flowers than females) suggests that selection through the male function to attract pollinators or to invest in reproduction may be indeed stronger [14–16]. Nevertheless, Bateman's metrics have been estimated and compared between males and females in only three angiosperm species. In all three cases, sexual selection was found to be stronger in males, and results were at least partly consistent with the sexual dimorphism documented in these species [17–19]. Nonflowering plant species have been investigated even less on the topic of sexual selection. Only one study carried out on a non-sexually dimorphic moss, estimated Bateman gradients and found evidence for a steeper relationship between reproductive and mating success in male gametophytes [20]. These results suggest a potential for stronger sexual selection in males of this anisogamous species, but this does not appear to have driven the evolution of sexually dimorphic traits. Noteworthily, this particular study was performed in a population with an extreme male-biased sex ratio, a feature that has been found to be associated with higher opportunities for sexual selection and higher Bateman gradients in males [21].
One situation in which stronger opportunity for sexual selection in males would not be associated with sexual dimorphism is when variation in mating success is not determined by heritable traits. In plants, their inability to move forces pollen or male gametes to disperse in the environment via pollinators or abiotic factors, so that the exact spatial location of individuals is likely to be a strong determinant of mating success. Several studies have explored the impact of geographical location and of local sex ratio on male and/or female reproductive success [22–26], but without explicitly linking their results to sexual selection. Like plants and some animals, red algae are sessile and anisogamous [27]. Male gametes are released in the water and fertilizations occur on the females, which is referred as spermcast mating for sessile marine invertebrates [28]. In red algae, male gametes are non-flagellated and we recently demonstrated that isopod-mediated fertilization increases fertilization success [29]. In Florideophyceae, after fertilization, the diploid zygote divides mitotically in a diploid structure, protected and nourished by the parental female haploid gametophyte [30]. These characteristics of the reproductive life cycle suggest that fertilization success in this group may be driven by different selection pressures between males and females (i.e. competition between males for access to females or selective cystocarp abortion by the females) [31]. In the dioicous red alga Gracilaria gracilis, male fertilization success shows high variance [31,32], suggesting that there may be male–male competition or female choice, a sign of sexual selection. However, to date in this alga, the relationship between reproductive success and mating success has not been tested to determine if the Bateman gradient is steeper in males. Here, we compare potentials for sexual selection among male and female gametophytes in G. gracilis by estimating the three Bateman indexes in a natural population characterized by a balanced sex ratio. We also explore the role of individual location, relative to individuals of the other sex, on the variation of mating success in both males and females. The investigation of Bateman principles in species outside the clades classically studied for sexual selection should help to understand how and when anisogamy triggers differences in the magnitude of sexual selection between sexes, and when such differences can drive the evolution of sexual dimorphism.
2. Material and methods
(a) . Study species
Populations of the rhodophyte Gracilaria gracilis are patchily distributed in the intertidal zone on rocky shores. Individuals occupying rock pools remain immersed at ebb tide. Its life cycle is haploid-diploid, including male and female haploid gametophytes and diploid tetrasporophytes (figure 1). Tetrasporophytes produce haploid spores via meiosis that develop into dioicous male and female gametophytes. This species is characterized by a highly specialized type of oogamous reproduction [26]. Spermatia (non-flagellated male gametes) are released into the environment, but female gametes (carpogonium) are retained on the female gametophyte. After fertilization, the zygote remains on the female gametophyte and develops into diploid sporogenous tissue (gonimoblast) protected and nurtured by the parental female thallus, forming a complex, macroscopic post-fertilization structure called the cystocarp. The cystocarp is fully mature approximately one month after fertilization. One fertilization event gives rise to thousands of identical diploid spores that, after release, can germinate into new tetrasporophyte individuals [33]. The tetrasporophyte, female gametophyte and male gametophyte individuals are considered isomorphic, even though males are slightly smaller than females and tetrasporophytes [34]. Both haploid and diploid individuals are long-lived [35]. The thallus is formed of a few erect deciduous main axes, irregularly branched, reaching up to 30 cm long, arising from a small perennial discoid holdfast, fixing the individual to the bedrock. Previous studies have shown that the reproductive period occurs from March to September with cystocarp production peaking in August–September [33,36]. Individuals less than 8 cm in length are generally non-reproductive.
Figure 1.
Life cycle of Gracilaria gracilis. The individuals are fixed to the rocky substrate via a perennial holdfast. The deciduous erect thalli decay after reproduction in late autumn and grow back from the holdfast in spring. Diploid tetrasporophytes produce haploid spores from meiosis that develop into dioicous male and female haploid gametophytes. Spermatia (non-flagellated male gametes) are released into the water column. After fertilization, the zygote is retained on the female and develops into a complex, macroscopic post-fertilization structure: the cystocarp. One fertilization gives rise to thousands of identical diploid carpospores that, after release and germination, grow into new tetrasporophyte individuals.
(b) . Study population and sampling
The study population was located on the coast of the North Sea at Cape Gris-Nez (in the Strait of Dover region, northern France, 50°53′N, 1°35′E) in a pool of approximately 5 m2 isolated from other populations at low tide [31,33]. In August 2020, every individual in the rock pool was sampled and spatially mapped relative to a pair of fixed points. Maximum thallus length and maximum diameter, measured using all the thalli emerging from the holdfast, were recorded for each individual to estimate biomass [35]. Several branches of each individual were sampled and examined under a dissecting microscope to establish the sexual phenotype (tetrasporophyte, female or male gametophyte) of each individual based on the observed reproductive structures. Of a total of 238 reproductive individuals sampled, 97 tetrasporophytes, 70 females and 71 males were identified (figure 2). There was a balanced sex ratio (1 : 1). For every female, cystocarps were counted on the 10 cm distal extremity of five branches. Thallus fragments were excised from each individual for DNA extraction. An average of 32 large cystocarps (ranging from 12 to 48 cystocarps) were randomly sampled and dissected from each female and stored at −20°C.
Figure 2.
Map of the Cape Gris-Nez intertidal rock pool showing the locations of the 71 male (triangles) and the 70 female (circles) gametophyte individuals sampled within the population (the tetrasporophyte individuals are not shown). Mating success (i.e. the numbers of sexual partners) is proportional to filled symbol size. Females with missing information that were not included in Bateman analyses are indicated with open circles. The rock pool edges are shown in grey. The polygons within the rock pool indicate the presence of rocks.
(c) . DNA extraction and microsatellite genotyping
For individuals, DNA extraction was performed on approximately 10 mg of dried thallus tissue using the NucleoSpin 96 Plant II kit (Macherey-Nagel, GmbH & Co. KG, Düren, Germany) following the manufacturer's instructions. For cystocarps, DNA extraction was performed using the Chelex method (Chelex TM 100; Biorad, Hercules, California), following the protocol described in Wattier et al. [37]. All male and female gametophyte individuals as well as the sampled cystocarps were genotyped using 13 microsatellite loci: Gv2CT [37], Gg121, Gg155, Gg173, Gg182, Gg216 [38], Ggrac_02, Ggrac_03, Ggrac_04, Ggrac_05, Ggrac_08, Ggrac_09, Ggrac_11 [39]. PCR was performed in a total volume of 10 µl containing 2 µl of DNA template diluted 1 : 50 (gametophytes) or not diluted (cystocarps), 1× GoTaq Flexi buffer (Promega Corporation), 2 mM MgCl2, 150 µM of each dNTP (Thermo Fisher Scientific Inc., USA), 30 pmol of forward fluorescent-labelled primer, 30 pmol of reverse primer (Eurofins MWG Operon, Germany), and 0.35 U GoTaq Flexi Polymerase (Promega Corporation). Amplifications were carried out under the following conditions: initial denaturation at 95°C for 5 min, 30 cycles of denaturation at 95°C for 30 s, annealing at 60°C for 30 s, and extension at 72°C for 30 s, followed by a final extension of 72°C for 10 min. Next, 2 µl of the diluted PCR product 1 : 10 was added to 10 µl of loading buffer made up of 0.5 µl of the SM594 size standard [40] and 9.5 µl of Hi-Di formamide, denatured at 95°C for 3 min, and run in an ABI 3130 XL capillary sequencer (Applied Biosystems, USA). Genotypes were scored manually in Genemapper version 4.0 (Applied Biosystems). The gametophytes being haploid, for each cystocarp, we were able to deduce the paternal genotype by subtracting the maternal genotype from the diploid cystocarp genotype across all loci using the Multilocus Matches option in GenAlEx ver. 6.5 [41,42].
(d) . Reproductive and mating success
Mating and reproductive success were estimated on the 50 females (out of the 70 initially present in the population; see figure 2) for which there was sufficient information (i.e. estimated biomass, cystocarp count per centimetre, and more than 24 cystocarps genotyped).
Mating success is the total number of mating partners per individual. In this study, we estimated the genetic mating success, i.e. the number of individuals of the other sex with which at least one zygote was produced, based on the results of the parentage assignment (electronic supplementary material, table S1). Slightly unequal numbers of cystocarps were genotyped across females, which may cause an artificial overestimation of mating success for females associated with a higher sampling effort. Mating success for both sexes was thus estimated using a bootstrapping procedure in which we resampled 24 genotyped offspring (cystocarps) 1000 times for each female. The genetic mating success for each individual was calculated from each resampling and, in further analyses, the mean ‘individual’ mating success value was used.
Female reproductive success is the total number of offspring a female produces. We estimated the female reproductive success by multiplying the mean number of cystocarps per cm3 counted on 5 branches by the total volume of the individual (electronic supplementary material, table S2) as a proxy for individual biomass [34].
Male reproductive success is the total number of offspring a male sires. Male reproductive success can thus be estimated as the proportion of each female's cystocarps sired by that male, as determined from the parental assignment (electronic supplementary material, table S1), multiplied by female reproductive success and summed over all surveyed females (electronic supplementary material, table S3). At each sampling step of the bootstrapping procedure performed for estimating mating success described above, we calculated the proportion of cystocarps sired by each male in all male-female pairs, to obtain an estimate of male reproductive success for each male individual. The mean value of male reproductive success resulting from the bootstrapping procedure was retained as an estimate of male reproductive success.
The relative reproductive and mating success of females and males were calculated by dividing the obtained values for each individual by the respective mean for each sex (electronic supplementary material, tables S2 and S3).
(e) . Distance to partners
To estimate the effect of distance on variation in male and female mating success, the distance of each individual to all potential mating partners (i.e. individuals of the other sex) was calculated using individual geographic coordinates within the population. The mean of these distances was used as a proxy for the potential access to mating partners (electronic supplementary material, tables S2 and S3).
(f) . Statistical analyses
All analyses were performed using R statistical software (v 4.0.3; R Core Team 2020) [43]. Function lm() was used for all the linear regression analysis. Function leveneTest() was used for Levene tests. The complete script of bootstrapping procedure and path analyses are available in Dryad (see data availability section).
(i) . Bateman's metrics
We quantified the strength of sexual selection in each sex using Bateman's metrics [44–46]. First, we estimated the standardized variance in reproductive success, I and in mating success Is. I and Is were calculated by dividing respectively the variance of reproductive and mating success by the square of their mean value. We tested for differences in reproductive and mating success variances between sexes using Levene's test. In addition, we estimated the slope of the linear regression of relative reproductive success as a function of relative mating success (Bateman gradient), on each sex separately. We then analysed the whole dataset with a linear model that tested for the effect of mating success and of the interaction between mating success and sex. Because uneven sampling between males and females may artificially increase the estimates of variance and the statistical power of the estimate of the Bateman gradient in males, a bootstrapping procedure was used to (i) compare both I and Is between males and females, and (ii) test for the Bateman gradient in males, based on a subsample of 50 males only.
(ii) . Distance to partners
A linear regression of relative mating success over the mean distance to potential partners was tested for males and females separately. A similar model was built, this time to test the effect of distance to partners on reproductive success, again testing males and females separately. Finally, we built a multiple linear regression of relative reproductive success over relative mating success and distance to potential partners (i.e. the equivalent of a partial Bateman gradient), to test whether any detected effect of the distance to partners on reproductive success was mediated through an impact of distance on mating success.
(iii) . Path analyses: indirect effects of distance and biomass on mating and reproductive success
In addition, we used a pathway analysis approach (structural equation modelling; see also [47]), in order to dissect direct from indirect effects of distance to potential mating partners on reproductive success. More precisely, we tested whether the distance to potential partners affected mating success and whether this had an indirect effect on reproductive success, while testing the relationship between mating and reproductive success. We also assessed the direct relationship between distance to partners and reproductive success. This was done simultaneously on males and females, using the piecewiseSEM package in R [48]. We then performed a multigroup analysis using local estimation of the parameters, by first providing statistics for sex × distance interaction using type III ANOVA. This allowed us to test whether the direct or indirect effects of distance to partners on reproductive success were significantly different between sexes. Testing the effect of sex × mating success interaction on reproductive success provided an additional test of a difference in the Bateman gradient between sexes. In a second step, the analysis provides correlation estimates and significant sex × variable interactions are free to vary between males and females. These results were compared to results of the linear models described above. Finally, we used the same path analysis approach by additionally testing for indirect and direct effects of individual biomass on reproductive success. Because biomass was used to estimate female reproductive success, this last analysis was performed only on males.
3. Results
Paternity analysis, performed by genotyping 1495 cystocarps from 50 females, revealed that 92% of the fertilization events (1371 cystocarps) occurred with males located in the population and only 8% (124 cystocarps) with males from outside. Of the 71 reproductive males in the population, we did not detect any sired cystocarps for five of them (electronic supplementary material, tables S1 and S2) and these five were thus assigned a null reproductive and mating success.
(a) . Bateman's metrics
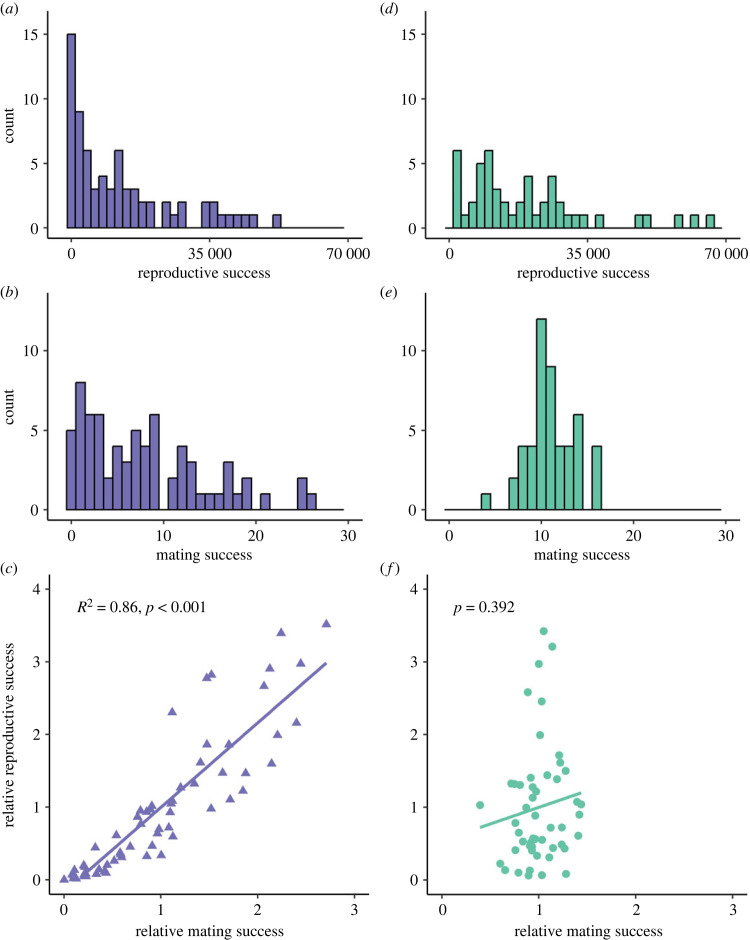
Reproductive success, i.e. the total number of offspring, ranged from 0 to 48 112 for males and from 1215 to 66 495 for females (figure 3a–d; electronic supplementary material, tables S1 and S3). Mating success (i.e. the number of sexual partners) ranged from 0 to 27 for males and from 4 to 18 for females (figure 3b–e; electronic supplementary material, tables S1 and S3). Both the opportunity for selection I and the opportunity for sexual selection Is were stronger for males than for females (I: 1.18 versus 0.66; Is: 0.74 versus 0.05), although this difference was significant only for mating success (Levene's test: I: F1,119 = 0.47, p = 0.49; Is: F1,119 = 28.75, p < 0.001). These results were not qualitatively affected when these analyses were performed on a subsample of 50 males to obtain equal sample sizes for males and females. Bateman gradients investigated for each sex separately provided different results in males and females: no significant relationship was found between relative mating and reproductive success in females (slope ± s.e.: 0.46 ± 0.53, p = 0.39), whereas it was significantly positive in males (slope ± s.e.: 1.17 ± 0.05, p < 0.001) (figure 3c–f). This result still held when the Bateman gradient was analysed on a subsample of 50 males to have the same sample size as in the female dataset. However, when relative reproductive success of males and females were analysed conjointly, mating success had no effect (slope ± s.e.: 0.46 ± 0.39, p = 0.25) and the interaction between mating success and sex was non-significant but with a p-value of 0.08 (slope ± s.e.: 0.71 ± 0.40, p = 0.08). When using the path analysis approach, we detected a significant effect of sex x mating success interaction on reproductive success, with mating success positively affecting reproductive success in males only (electronic supplementary material, figure S2).
Figure 3.
Relationship between reproductive and mating success and their distribution in males (dark blue) and females (green). Distribution of reproductive success (a,d), mating success (b,e) and the Bateman gradient (c,f) for males and females, respectively. The significance of the Bateman gradient, estimated by the slope of the linear regression, is indicated on the graph (c,f).
One potential weakness of our approach is that we set a maximum value of female mating success (i.e. the number of genotyped offspring per female), which could artificially decrease the estimated female mating success and flatten the Bateman gradient in females. This is an unavoidable limit of such an approach, when exhaustive genotyping is not an option, as already underlined in similar studies [19,49]. However, in our case, the observed shared paternity among offspring of the same female (see electronic supplementary material, table S1) suggests that estimated female mating success would not necessarily strongly increase if we had genotyped a higher number of offspring for each female.
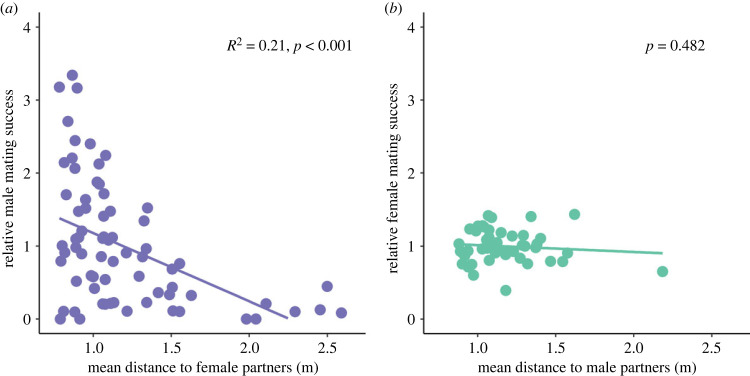
(b) . Distance to partners and biomass explained part of the variance in mating success in males
The mean distance to potential mating partners ranged from 0.88 m to 2.18 m for females, and from 0.78 m to 2.59 m for males (electronic supplementary material, tables S1 and S3). In males, mating success significantly decreased when their distance to potential female partners increased (slope ± s.e.: −0.93 ± 0.21, p < 0.001) (figure 4a). The same result was observed for reproductive success (slope ± s.e.: −1.01 ± 0.28, p < 0.001) (electronic supplementary material, figure S1). However, when the distance to potential partners and mating success were simultaneously included in a linear regression of reproductive success, only mating success was found to have a significant effect (slope ± s.e.: 1.21 ± 0.07, p < 0.001) and the distance effect was no longer significant (p = 0.40). This result suggests that the effect of distance to potential partners on male reproductive success was mediated through an effect on male mating success. In contrast, distance to potential male partners had no effect on either female mating success (p = 0.48) (figure 4b) or reproductive success (p = 0.23) (electronic supplementary material, figure S1). The path analysis approach provided very consistent results, with an indirect effect of the distance to potential partners on reproductive success, mediated through the interaction between mating and reproductive success, in males but not in females (electronic supplementary material, figure S2).
Figure 4.
Relationship between mating success and mean distance to partners (a) in males and (b) in females. The significance of the relationship, estimated by the slope of the linear regression, is shown on each graph.
Finally, when focusing on males only, we found that individual biomass positively affected mating success, thus having an indirect positive effect on reproductive success. Adding the biomass in the model did not qualitatively modify the effects of distance to potential female partners described above (electronic supplementary material, figure S3).
4. Discussion
In this first-ever test of Bateman's principles in an oogamous red alga, we found evidence in support of two of Bateman's three principles, suggesting a greater potential for sexual selection in males than in females in Gracilaria gracilis. We also detected an effect of individual location relative to potential mating partners on male mating success, which indirectly affects their reproductive success. Below, we discuss these different results in the light of red algal reproduction biology and their possible implications for processes of sexual selection in this species.
The first result of our study was that the variance in mating success was significantly higher in males than in females, thereby validating Bateman's second principle. This result is consistent with the Darwinian sex-role hypothesis linked to anisogamy, which predicts that sexual selection is often strongest in the sex that produces the smallest and most abundant gametes (i.e. males by definition) [5]. This expected consequence of anisogamy [3,50], even if not observed in all studies (see [8]), has been detected in a large number of animal species [1,8], but also in some angiosperms [12,13,51], mosses [20], fungi [52,53] and a red alga [31,32].
Second, our results also validate Bateman's third principle because the Bateman gradient showed a significant positive relationship between reproductive and mating success in males, but not in females. The stronger dependence of reproductive success on mating success in males compared with females has been widely documented in animals (see for review [8]), but has only recently been reported in three species of angiosperms [17–19] and one aquatic species of peat moss [20], while it had never been tested in seaweeds before. Bateman's third principle has generally been interpreted as the existence of strong competition between males for access to females, whereas the reproductive success of females is not strongly tied to their ability to access mates. The transferability of methods used for investigating the Bateman gradient and how to interpret it in animals to angiosperms was recently discussed in Tonnabel et al. [17] and Barbot et al. [19]. In particular, as frequently reported in angiosperms (see for review [54]) but rare in motile animals, it may bias the estimation of sexual selection. Multiple mating partners should result in high values of mating success, but the fact that genetic mating success is typically estimated on a subsample of offspring may lead to an underestimation of the mating success at least in some individuals, and ultimately to an underestimation of the variance in mating success. Such bias has been discussed in a few papers based on meta-analyses of studies focused in animals and simulated data sets, suggesting that the Bateman gradient may be overestimated in such a case [49,55]. In red algae, gametophytes are haploid and produce gametes by mitosis, and—given that male gametophytes thus produce genetically identical gametes—fertilization of multiple females by individual males can be readily detected. To date, the few paternity analyses published in red algae (i.e. G. gracilis [31] and Chondrus crispus [56]) have confirmed high levels of polyandry in natural populations, which suggests that in our study the Bateman gradient, as in other plants, may be overestimated in males.
Nevertheless, in the handful of investigated plant species, the positive Bateman gradient found in males, but not in females, has been interpreted as the direct result of female reproductive success not being limited by pollen supply, at least in the experimental conditions in which these studies were conducted [17,19]. Pollen limitation is another common feature in angiosperms [11,57] that should, when it occurs, decrease the difference in magnitude of sexual selection between females and males because in pollen-limiting situations, female reproductive success becomes limited by access to male gametes. Although this relationship has not been directly tested yet, in situations of low plant and/or pollinator densities, despite the much higher production of male gametes compared with female gametes at the population level, female Bateman gradients are expected to become significant [3,19]. In sessile marine organisms, female fertility has been assumed to be limited due to short sperm lifespans and their dilution in turbulent flow [58,59], but several mechanisms such as synchronous release of motile gametes and chemotaxis have evolved in a range of invertebrates and brown seaweed, to ensure fertilization rates of up to 80–100% (for reviews, see [60,61]). In red algae, it has long been hypothesized that fertilization success should be particularly low due to the characteristics of their life cycle [62,63]. Male gametes are not flagellated, have a short lifespan and fertilization takes place on a remote female organ. The absence of any documented mechanism of synchronous gamete release or chemotaxis has raised questions on the efficiency of gamete encounters in rhodophytes [63]. Searles [62] even hypothesized that the evolution of cystocarps in which the female nurtures, amplifies and protects the zygote is probably a response to low rates of fertilization. Rare gamete encounters should lead to a positive Bateman gradient in female G. gracilis, a result that was not found in the current study. Therefore, in the studied population, dispersal appears to be efficient enough to ensure the sufficient access of female gametophytes to reproduction and thus the production of cystocarps. This argument is further supported by our evidence that all measured females had multiple fertilization partners. Although data on natural fertilization success are still sparse in red algae, Engel et al. [31] and Maggs et al. [64] reported that male gametes did not appear to be limiting because the majority of fertilization takes place during low tide when the spermatium concentration is high [65]. In addition, isopod-mediated transport of male gametes, a phenomenon similar to pollination, recently demonstrated in a red alga [29] may compensate for the lack of flagella, increasing fertilization rates and thus explaining any absence of gamete limitation. Further work is needed to investigate whether the Bateman gradient can become significant for females under spermatium-limiting conditions (i.e. without ‘pollinators’ or in a more open environment).
Here, the variance in mating success was greater in males and strongly correlated with their reproductive success. This relationship seems to suggest that variance in reproductive success is higher in males than in females. However, even if the opportunity for selection (I) tended to be stronger in males, the difference between sexes was not significant, and therefore did not confirm Bateman's first principle. These results differ from what has been found in other animal and plant species [7,8]. One possible explanation for this discrepancy is that female reproductive success depends on other factors not related to their access to reproduction but, for example, to their ability to mobilize resources. Interestingly, a trade-off between sexual reproduction and growth has been demonstrated in females for two species of red algae (G. chilensis [66] and G. domingensis [67]).
Our results thus suggest that sexual selection may be stronger in males in G. gracilis. Consequently, any heritable trait increasing individual access to mating partners may be under sexual selection in males, leading to some sort of sexual dimorphism. In animals, numerous studies have focused on dimorphic traits that allow males to increase the number of partners, such as ornaments or armaments [4]. In angiosperms, some sexually dimorphic traits such as different metrics of flower size and phenology have been shown to be under stronger selection [68,69] or sexual selection [19] in males. In the current study, some constraints in our data collection prevented us from investigating the impact of biomass on female reproductive success. When focusing on males, we found that biomass increased mating success, suggesting that this trait may thus be under sexual selection, although nothing is currently known about its heritability in G. gracilis.
If the variance in male mating success largely depends on the geographical location of individuals, a typically non-heritable trait, a distance effect may explain why sexual selection seems stronger in males in a species in which no obvious sexual dimorphism seems to occur. A distance effect on reproductive success has been commonly reported in plants [22–26] and also in G. gracilis [30]. In the current study, our tests indeed revealed that only male (and not female) reproductive success depends on the distance to potential mates. This effect arises from the dependence of mating success on distance. However, distance explains only a small part of the variance in male mating success, and specific male traits, not yet identified, may nevertheless be involved in this variable access to mates.
Given that red algae are cryptogams (i.e. without visible sexual organs) and have a simple morphology (i.e. thallus), the identification of dimorphic sexual characters remains a real challenge. In G. gracilis there is no marked sexual dimorphism [33,34]. Nevertheless, some male traits may help increase access to females by improving gamete quality and/or efficiency, such as the phenology of gamete production, or the level of production of mucilage strands in spermatia that may aid dispersal and animal transport [29,70]. These traits may be subject to sexual selection in males, and offer exciting avenues for future research.
By investigating the Bateman gradient, the current study mainly focused on the equivalent of pre-copulation sexual selection mechanisms [8], i.e. those involved in the transport of gametes from male to female reproductive structures. However, other processes can occur after mating such as the selection of some particular male gametes by female tissues, a mechanism often referred to as cryptic female choice in animals and which may also occur in plants [55]. It is thus possible that our estimate of the Bateman gradient in G. gracilis also captured the consequences of such cryptic choice. In red algae, female choice could occur within a female structure called the trichogyne [71]. Several spermatia may adhere to a trichogyne [72], but only one male nucleus goes on to fuse with the female gamete nucleus [73]. Due to the long and costly development of cystocarps, female choice may play an important role after fertilization in G. gracilis. Through this process, females can improve offspring quality by selectively aborting zygotes, on the basis of their paternity. This phenomenon has been widely described in plants where abortions are frequent [74–77] and also in fungi [78]. Post-mating abortions have also been described after controlled crosses in Gracilariaceae [79–81], including individuals of G. gracilis sampled from the same population as that studied here [32].
In conclusion, our study suggests that sexual selection occurs with a higher intensity in males than in females in G. gracilis, a species that shows no clear sexual dimorphism. Our results pave the way for future research to examine particular traits and their susceptibility to sex-specific sexual selection. Finally, our investigation on a non-obvious candidate species shed light on sexual selection being a possible evolutionary driver in a hitherto little-studied phylum of the tree of life.
Acknowledgements
We want to thank the Genomer platform of the Station Biologique de Roscoff in particular Gwenn Tanguy, Biogenouest genomics and EMBRC France partner core facility for their technical support. The authors are deeply grateful to Colin Destombe, Antoine Faure and Stéphane Loisel for sampling and Carolyn Engel for comments on the last version of the manuscript. We thank the associated editor Maurine Neiman and two anonymous reviewers for comments that have improved this article.
Contributor Information
M. Valero, Email: myriam.valero@sb-roscoff.fr.
M. Dufay, Email: mathilde.dufay@cefe.cnrs.fr.
Ethics
This work did not require ethical approval from a human subject or animal welfare committee.
Data accessibility
Genotypic data, complete script of bootstrapping procedure and path analyses were deposited and are available in Dryad [82]. All other data used in the analyses are available in the paper or the electronic supplementary material [83].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors' contributions
E.L.: conceptualization, formal analysis, investigation, methodology, resources, visualization, writing—original draft, writing—review and editing; M.V.: conceptualization, funding acquisition, investigation, project administration, supervision, writing—original draft, writing—review and editing; S.M.: data curation, methodology, resources, writing—review and editing; M.L.G.: funding acquisition, writing—review and editing; C.D.: conceptualization, investigation, writing—original draft, writing—review and editing; M.D.: formal analysis, investigation, methodology, validation, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
The authors declare that they have no competing interests.
Funding
This work was supported by the Centre National de la Recherche Scientifique (CNRS) PhD fellowship to E.L.; core funding was provided by the IRL 3614, Evolutionary Biology and Ecology of Algae, CNRS and Sorbonne Université and by the International Research Network (IRN), Diversity and Biotechnology of Marine Algae, CNRS, to M.V., S.M., C.D. and M.L.G. This work was also funded by ANID–Millenium Nucleus Marine Agronomy of Seaweed Holobionts (NCN2021_033) to M.L.G.
References
- 1.Hosken DJ, House CM. 2011. Sexual selection. Curr. Biol. 21, R62-R65. ( 10.1016/j.cub.2010.11.053) [DOI] [PubMed] [Google Scholar]
- 2.Darwin C. 1872. The descent of man, and selection in relation to sex. New York, NY: Appleton & Co. [Google Scholar]
- 3.Lehtonen J. 2022. Bateman gradients from first principles. Nat. Commun. 13, 3591. ( 10.1038/s41467-022-30534-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dewsbury DA. 2005. The Darwin-Bateman paradigm in historical context. Integr. Comp. Biol. 45, 831-837. ( 10.1093/icb/45.5.831) [DOI] [PubMed] [Google Scholar]
- 5.Kokko H, Jennions MD. 2008. Parental investment, sexual selection and sex ratios. J. Evol. Biol. 21, 919-948. ( 10.1111/j.1420-9101.2008.01540.x) [DOI] [PubMed] [Google Scholar]
- 6.Bateman AJ. 1948. Intra-sexual selection in Drosophila. Heredity 2, 349-368. ( 10.1038/hdy.1948.21) [DOI] [PubMed] [Google Scholar]
- 7.Jones AG. 2009. On the Opportunity for sexual selection, the Bateman gradient and the maximum Intensity of sexual selection. Evolution 63, 1673-1684. ( 10.1111/j.1558-5646.2009.00664.x) [DOI] [PubMed] [Google Scholar]
- 8.Janicke T, Häderer IK, Lajeunesse MJ, Anthes N. 2016. Darwinian sex roles confirmed across the animal kingdom. Sci. Adv. 2, e1500983. ( 10.1126/sciadv.1500983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnold SJ. 1994. Is there a unifying concept of sexual selection that applies to both plants and animals? Am. Nat. 144, S1-S12. ( 10.1086/285650) [DOI] [Google Scholar]
- 10.Grant V. 1995. Sexual selection in plants: Pros and Cons. Proc. Natl Acad. Sci. USA 92, 1247-1250. ( 10.1073/pnas.92.5.1247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burd M. 1994. Bateman's principle and plant reproduction: The role of pollen limitation in fruit and seed set. Bot. Rev. 60, 83-139. ( 10.1007/BF02856594) [DOI] [Google Scholar]
- 12.Delph LF, Ashman T-L. 2006. Trait selection in flowering plants: How does sexual selection contribute? Integr. Comp. Biol. 46, 465-472. ( 10.1093/icb/icj038) [DOI] [PubMed] [Google Scholar]
- 13.Moore JC, Pannell JR. 2011. Sexual selection in plants. Curr. Biol. 21, R176-R182. ( 10.1016/j.cub.2010.12.035) [DOI] [PubMed] [Google Scholar]
- 14.Hodgins KA, Barrett SCH. 2008. Natural selection on floral traits through male and female function in wild populations of the heterostylous daffodil Narcissus triandrus. Evolution 62, 1751-1763. ( 10.1111/j.1558-5646.2008.00404.x) [DOI] [PubMed] [Google Scholar]
- 15.Briscoe Runquist RD, Geber MA, Pickett-Leonard M, Moeller DA. 2017. Mating system evolution under strong pollen limitation: Evidence of disruptive selection through male and female fitness in Clarkia xantiana. Am. Nat. 189, 549-563. ( 10.1086/691192) [DOI] [PubMed] [Google Scholar]
- 16.Paterno GB, Silveira CL, Kollmann J, Westoby M, Fonseca CR. 2020. The maleness of larger angiosperm flowers. Proc. Natl. Acad. Sci. 117, 10 921-10 926. ( 10.1073/pnas.1910631117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tonnabel J, David P, Pannell JR. 2019. Do metrics of sexual selection conform to Bateman's principles in a wind-pollinated plant? Proc. R. Soc. B 286, 20190532. ( 10.1098/rspb.2019.0532) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwok A, Dorken ME. 2022. Sexual selection on male but not female function in monoecious and dioecious populations of broadleaf arrowhead (Sagittaria latifolia). Proc. R. Soc. B 289, 20220919. ( 10.1098/rspb.2022.0919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barbot E, Dufaÿ M, De Cauwer I. 2023. Sex specific selection patterns in a dioecious insect-pollinated plant. Evolution 77, 1578-1590. ( 10.1093/evolut/qpad069) [DOI] [PubMed] [Google Scholar]
- 20.Johnson MG, Shaw AJ. 2016. The effects of quantitative fecundity in the haploid stage on reproductive success and diploid fitness in the aquatic peat moss Sphagnum macrophyllum. Heredity 116, 523-530. ( 10.1038/hdy.2016.13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janicke T, Morrow EH. 2018. Operational sex ratio predicts the opportunity and direction of sexual selection across animals. Ecol. Lett. 21, 384-391. ( 10.1111/ele.12907) [DOI] [PubMed] [Google Scholar]
- 22.Graff A. 1999. Population Sex structure and reproductive fitness in gynodioecious Sidalcea malviflora (malvaceae). Evolution 53, 1714-1722. ( 10.1111/j.1558-5646.1999.tb04556.x) [DOI] [PubMed] [Google Scholar]
- 23.Oddou-Muratorio S, Klein EK, Demesure-Musch B, Austerlitz F. 2006. Real-time patterns of pollen flow in the wild-service tree, Sorbus torminalis (Rosaceae). III. Mating patterns and the ecological maternal neighborhood. Am. J. Bot. 93, 1650-1659. ( 10.3732/ajb.93.11.1650) [DOI] [PubMed] [Google Scholar]
- 24.Isagi Y, Saito D, Kawaguchi H, Tateno R, Watanabe S. 2007. Effective pollen dispersal is enhanced by the genetic structure of an Aesculus turbinata population. J. Ecol. 95, 983-990. ( 10.1111/j.1365-2745.2007.01272.x) [DOI] [Google Scholar]
- 25.De Cauwer I, Dufay M, Cuguen J, Arnaud JF. 2010. Effects of fine-scale genetic structure on male mating success in gynodioecious Beta vulgaris ssp. maritima. Mol. Ecol. 19, 1540-1558. ( 10.1111/j.1365-294X.2010.04586.x) [DOI] [PubMed] [Google Scholar]
- 26.De Cauwer I, Arnaud J-F, Schmitt E, Dufaÿ M. 2010. Pollen limitation of female reproductive success at fine spatial scale in a gynodioecious and wind-pollinated species, Beta vulgaris ssp. maritima. J. Evol. Biol. 23, 2636. ( 10.1111/j.1420-9101.2010.02119.x) [DOI] [PubMed] [Google Scholar]
- 27.Dixon PS. 1973. Biology of the Rhodophyta. University reviews in botany. Edinburgh: Oliver & Boyd. [Google Scholar]
- 28.Bishop JDD, Pemberton AJ. 2006. The third way: spermcast mating in sessile marine invertebrates. Integr. Comp. Biol. 46, 398-406. ( 10.1093/icb/icj037) [DOI] [PubMed] [Google Scholar]
- 29.Lavaut E, Guillemin M-L, Colin S, Faure A, Coudret J, Destombe C, Valero M. 2022. Pollinators of the sea: A discovery of animal-mediated fertilization in seaweed. Science 377, 528-530. ( 10.1126/science.abo6661) [DOI] [PubMed] [Google Scholar]
- 30.Kamiya M, Kawai H. 2002. Dependence of the carposporophyte on the maternal gametophyte in three ceramiacean algae (Rhodophyta), with respect to carposporophyte development, spore production and germination success. Phycologia 41, 107-115. ( 10.2216/i0031-8884-41-2-107.1) [DOI] [Google Scholar]
- 31.Engel CR, Wattier R, Destombe C, Valero M. 1999. Performance of non–motile male gametes in the sea: Analysis of paternity and fertilization success in a natural population of a red seaweed, Gracilaria gracilis. Proc. R. Soc. Lond. B 266, 1879-1886. ( 10.1098/rspb.1999.0861) [DOI] [Google Scholar]
- 32.Engel CR, Valero M, Lagadeuc Y, Destombe C. 2002. Non-random mating in controlled multiple-donor crosses in Gracilaria gracilis (Gracilariaceae, Rhodophyta). Eur. J. Phycol. 37, 179-190. ( 10.1017/S096702620200358X) [DOI] [Google Scholar]
- 33.Destombe C, Valero M, Vernet P, Couvet D. 1989. What controls haploid—diploid ratio in the red alga, Gracilaria verrucosa ? J. Evol. Biol. 2, 317-338. ( 10.1046/j.1420-9101.1989.2050317.x) [DOI] [Google Scholar]
- 34.Kain(Jones) JM, Destombe C. 1995. A review of the life history, reproduction and phenology of Gracilaria. J. Appl. Phycol. 7, 269-281. ( 10.1007/BF00004001) [DOI] [Google Scholar]
- 35.Engel CR, Åberg P, Gaggiotti OE, Destombe C, Valero M. 2001. Population dynamics and stage structure in a haploid-diploid red seaweed, Gracilaria gracilis. J. Ecol. 89, 436-450. ( 10.1046/j.1365-2745.2001.00567.x) [DOI] [Google Scholar]
- 36.Destombe C, Godin J, Bodard M. 1988. The decay phase in the life history of Gracilaria verrucosa: the consequences in intensive cultivation. In Algal biotechnology (ed. Stadler T, et al.), pp. 287-303. Oxford, UK: Elsevier. [Google Scholar]
- 37.Wattier R, Dallas JF, Destombe C, Saumitou-Laprade P, Valero M. 1997. Single locus microsatellites in Gracilariales (Rhodophyta): high level of genetic variability within Gracilaria gracilis and conservation in related species. J. Phycol. 33, 868-880. ( 10.1111/j.0022-3646.1997.00868.x) [DOI] [Google Scholar]
- 38.Luo H, Möorchen M, Engel CR, Destombe C, Epplen JT, Epplen C, Saumitou-Laprade P, Valero M. 1999. Characterization of microsatellite markers in the red alga Gracilaria gracilis. Mol. Ecol. 8, 700-702. ( 10.1046/j.1365-294x.1999.00879.x) [DOI] [PubMed] [Google Scholar]
- 39.Mauger S, Baud A, Le Corguillé G, Tanguy G, Legeay E, Creis E, Valero M, Destombe C, Potin P. In press. Genetic resources of macroalgae: development of an efficient method using microsatellite markers in non-model organisms. Algal Res. ( 10.1016/j.algal.2023.103251) [DOI] [Google Scholar]
- 40.Mauger S, Couceiro L, Valero M. 2012. A simple and cost-effective method to synthesize an internal size standard amenable to use with a 5-dye system. Prime Res. Biotechnol. 2, 2315-5299. [Google Scholar]
- 41.Peakall R, Smouse PE. 2006. Genalex 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 6, 288-295. ( 10.1111/j.1471-8286.2005.01155.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peakall R, Smouse PE. 2012. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—an update. Bioinformatics 28, 2537-2539. ( 10.1093/bioinformatics/bts460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.R Core Team. 2020. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 44.Wade MJ. 1979. Sexual selection and variance in reproductive success. Am. Nat. 114, 742-747. ( 10.1086/283520) [DOI] [PubMed] [Google Scholar]
- 45.Wade MJ, Arnold SJ. 1980. The intensity of sexual selection in relation to male sexual behaviour, female choice, and sperm precedence. Anim. Behav. 28, 446-461. ( 10.1016/S0003-3472(80)80052-2) [DOI] [Google Scholar]
- 46.Arnold SJ, Wade MJ. 1984. On the measurement of natural and sexual selection: theory. Evolution 38, 709-719. ( 10.2307/2408383) [DOI] [PubMed] [Google Scholar]
- 47.Henshaw JM, Jennions MD, Kruuk LEB. 2018. How to quantify (the response to) sexual selection on traits. Evolution 72, 1904-1917. ( 10.1111/evo.13554) [DOI] [PubMed] [Google Scholar]
- 48.Lefcheck JS. 2016. PiecewiseSEM: piecewise structural equation modelling in R for ecology,evolution and systematics. Methods Ecol. Evol. 7, 573-579. ( 10.1111/2041-210X.12512) [DOI] [Google Scholar]
- 49.Anthes N, Häderer IK, Michiels NK, Janicke T. 2017. Measuring and interpreting sexual selection metrics: evaluation and guidelines. Methods Ecol. Evol. 8, 918-931. ( 10.1111/2041-210X.12707) [DOI] [Google Scholar]
- 50.Schärer L, Rowe L, Arnqvist G. 2012. Anisogamy, chance and the evolution of sex roles. Trends Ecol. Evol. 27, 260-264. ( 10.1016/j.tree.2011.12.006) [DOI] [PubMed] [Google Scholar]
- 51.Murphy CG. 1998. Interaction-Independent sexual selection and the mechanisms of sexual selection. Evolution 52, 8-18. ( 10.1111/j.1558-5646.1998.tb05133.x) [DOI] [PubMed] [Google Scholar]
- 52.Nieuwenhuis BPS, Aanen DK. 2012. Sexual selection in fungi. J. Evol. Biol. 25, 2397-2411. ( 10.1111/jeb.12017) [DOI] [PubMed] [Google Scholar]
- 53.Beekman M, Nieuwenhuis B, Ortiz-Barrientos D, Evans JP. 2016. Sexual selection in hermaphrodites, sperm and broadcast spawners, plants and fungi. Phil. Trans. R. Soc. B 371, 20150541. ( 10.1098/rstb.2015.0541) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pannell JR, Labouche A-M. 2013. The incidence and selection of multiple mating in plants. Phil. Trans. R. Soc. B 368, 20120051. ( 10.1098/rstb.2012.0051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tonnabel J, David P, Janicke T, Lehner A, Mollet J-C, Pannell JR, Dufay M. 2021. The scope for postmating sexual selection in plants. Trends Ecol. Evol. 36, 556-567. ( 10.1016/j.tree.2021.02.013) [DOI] [PubMed] [Google Scholar]
- 56.Krueger-Hadfield SA, Roze D, Correa JA, Destombe C, Valero M. 2015. O father where art thou? Paternity analyses in a natural population of the haploid–diploid seaweed Chondrus crispus. Heredity 114, 185-194. ( 10.1038/hdy.2014.82) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Knight TM, et al. 2005. Pollen limitation of plant reproduction: pattern and process. Annu. Rev. Ecol. Evol. Syst. 36, 467-497. ( 10.1146/annurev.ecolsys.36.102403.115320) [DOI] [Google Scholar]
- 58.Levitan DR, Sewell MA. 1998. Fertilization success in free-spawning marine invertebrates: review of the evidence and fisheries implications. Can. Spec. Publ. Fish. Aquat. Sci. 125, 159-164. [Google Scholar]
- 59.Yund PO. 2000. How severe is sperm limitation in natural populations of marine free-spawners? Trends Ecol. Evol. 15, 10-13. ( 10.1016/S0169-5347(99)01744-9) [DOI] [PubMed] [Google Scholar]
- 60.Berndt M-L, Callow JA, Brawley SH. 2002. Gamete concentrations and timing and success of fertilization in a rocky shore seaweed. Mar. Ecol. Prog. Ser. 226, 273-285. ( 10.3354/meps226273) [DOI] [Google Scholar]
- 61.Serrão EA, Havenhand J. 2009. Fertilization strategies. In Marine hard bottom communities, pp. 149-164. [Google Scholar]
- 62.Searles RB. 1980. The strategy of the red algal life history. Am. Nat. 115, 113-120. ( 10.1086/283548) [DOI] [Google Scholar]
- 63.Santelices B. 2002. Recent advances in fertilization ecology of macroalgae. J. Phycol. 38, 4-10. ( 10.1046/j.1529-8817.2002.00193.x) [DOI] [Google Scholar]
- 64.Maggs CA, Fletcher HL, Fewer D, Loade L, Mineur F, Johnson MP. 2011. Speciation in red algae: members of the Ceramiales as model organisms. Integr. Comp. Biol. 51, 492-504. ( 10.1093/icb/icr075) [DOI] [PubMed] [Google Scholar]
- 65.Engel CR, Destombe C. 2002. Reproductive ecology of an intertidal red seaweed, Gracilaria gracilis: influence of high and low tides on fertilization success. J. Mar. Biol. Assoc. U. K. 82, 189-192. ( 10.1017/S0025315402005349) [DOI] [Google Scholar]
- 66.Guillemin ML, Valenzuela P, Gaitán-Espitia JD, Destombe C. 2014. Evidence of reproductive cost in the triphasic life history of the red alga Gracilaria chilensis (Gracilariales, Rhodophyta). J. Appl. Phycol. 26, 569-575. ( 10.1007/s10811-013-0072-x) [DOI] [Google Scholar]
- 67.Guimarães M, Plastino EM, Oliveira EC. 1999. Life history, reproduction and growth of Gracilaria domingensis (Gracilariales, Rhodophyta) from Brazil. Botanica Marina 42, 481-486. ( 10.1515/BOT.1999.055) [DOI] [Google Scholar]
- 68.Campbell DR. 1989. Measurements of selection in a hermaphroditic plant: variation in male and female pollination success. Evolution 43, 318-334. ( 10.1111/j.1558-5646.1989.tb04230.x) [DOI] [PubMed] [Google Scholar]
- 69.O'Connell LM, Johnston MO. 1998. Male and female pollination success in a deceptive orchid, a selection study. Ecology 79, 1246. ( 10.2307/176740) [DOI] [Google Scholar]
- 70.Brawley SH, Johnson LE. 1992. Gametogenesis, gametes and zygotes: An ecological perspective on sexual reproduction in the algae. Br. Phycol. J. 27, 233-252. ( 10.1080/00071619200650241) [DOI] [Google Scholar]
- 71.Pickett-Heaps JD, West J. 1998. Time-lapse video observations on sexual plasmogamy in the red alga Bostrychia. Eur. J. Phycol. 33, 43-56. ( 10.1080/09670269810001736523) [DOI] [Google Scholar]
- 72.Hommersand MH, Fredericq S. 1990. Sexual reproduction and cystocarp development. Cambridge: Cambridge University Press. [Google Scholar]
- 73.Fredericq S, Hommersand MH. 1989. Proposal of the Gracilariales ord. Nov. (Rhodophyta) based on an analysis of the reproductive development of Gracilaria verrucosa. J. Phycol. 25, 213-227. ( 10.1111/j.1529-8817.1989.tb00116.x) [DOI] [Google Scholar]
- 74.Hossaert M, Valero M. 1988. Effect of ovule position in the pod on patterns of seed formation in two species of Lathyrus (Leguminosae: papilionoideae). Am. J. Bot. 75, 1714-1731. ( 10.1002/j.1537-2197.1988.tb11248.x) [DOI] [Google Scholar]
- 75.Willson MF, Burley N. 1983. Mate choice in plants: tactics, mechanisms, and consequences. Princeton: Princeton University Press. [Google Scholar]
- 76.Rigney LP. 1995. Postfertilization causes of differential success of pollen donors in Erythronium grandiflorum (Liliaceae): nonrandom ovule abortion. Am. J. Bot. 82, 578-584. ( 10.1002/j.1537-2197.1995.tb11502.x) [DOI] [Google Scholar]
- 77.Havens K, Delph LF. 1996. Differential seed maturation uncouples fertilization and siring success in Oenothera organensis (Onagraceae). Heredity 76, 623-632. ( 10.1038/hdy.1996.89) [DOI] [Google Scholar]
- 78.Bruggeman J, Debets AJM, Hoekstra RF. 2004. Selection arena in Aspergillus nidulans. Fungal Genet. Biol. 41, 181-188. ( 10.1016/j.fgb.2003.10.007) [DOI] [PubMed] [Google Scholar]
- 79.van der Meer JP. 1986. Genetics of Gracilaria tikvahiae (Rhodophyceae). Xi. Further characterization of a bisexual mutant. J. Phycol. 22, 151-158. ( 10.1111/j.1529-8817.1986.tb04158.x) [DOI] [Google Scholar]
- 80.Richerd S, Destombe C, Cuguen J, Valero M. 1993. Variation of reproductive success in a haplo-diploid Red Alga, Gracilaria verrucosa : Effects of parental identities and crossing distance. Am. J. Bot. 80, 1379-1391. ( 10.1002/j.1537-2197.1993.tb15382.x) [DOI] [Google Scholar]
- 81.Gurgel CFD, Fredericq S, Norris JN, Yoneshigue-Valentin Y. 2008. Two new flat species of Gracilaria (Gracilariales, Rhodophyta) from Brazil. Phycologia 47, 249-264. ( 10.2216/PH06-59.1) [DOI] [Google Scholar]
- 82.Lavaut E, Valero M, Mauger S, Guillemin ML, Destombe C, Dufay M. 2023. Data from: Sexual selection in seaweed? Testing Bateman's principles in the red alga Gracilaria gracilis. Dryad Digital Repository. ( 10.5061/dryad.t4b8gtj6c) [DOI] [PMC free article] [PubMed]
- 83.Lavaut E, Valero M, Mauger S, Guillemin ML, Destombe C, Dufay M. 2023. Sexual selection in seaweed? Testing Bateman's principles in the red alga gracilaria gracilis. Figshare. ( 10.6084/m9.figshare.c.6824001) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Lavaut E, Valero M, Mauger S, Guillemin ML, Destombe C, Dufay M. 2023. Data from: Sexual selection in seaweed? Testing Bateman's principles in the red alga Gracilaria gracilis. Dryad Digital Repository. ( 10.5061/dryad.t4b8gtj6c) [DOI] [PMC free article] [PubMed]
- Lavaut E, Valero M, Mauger S, Guillemin ML, Destombe C, Dufay M. 2023. Sexual selection in seaweed? Testing Bateman's principles in the red alga gracilaria gracilis. Figshare. ( 10.6084/m9.figshare.c.6824001) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Genotypic data, complete script of bootstrapping procedure and path analyses were deposited and are available in Dryad [82]. All other data used in the analyses are available in the paper or the electronic supplementary material [83].