Abstract
Older adults with cardiovascular disease (CVD) contend with deficits across multiple domains of health due to age-related physiological changes and the impact of CVD. Multimorbidity, polypharmacy, cognitive changes, and diminished functional capacity, along with changes in the social environment, result in complexity that makes provision of CVD care to older adults challenging. In this review, we first describe the history of geriatric cardiology, an orientation that acknowledges the unique needs of older adults with CVD. Then, we introduce 5 essential principles for meeting the needs of older adults with CVD: 1) recognize and consider the potential impact of multicomplexity; 2) evaluate and integrate constructs of cognition into decision-making; 3) evaluate and integrate physical function into decision-making; 4) incorporate social environmental factors into management decisions; and 5) elicit patient priorities and health goals and align with care plan. Finally, we review future steps to maximize care provision to this growing population.
Key words: cognitive impairment, frailty, geriatric cardiology, multimorbidity, patient-centered care, polypharmacy
Central Illustration
Highlights
-
•
Cardiovascular disease in older adults occurs amidst multiple comorbid conditions and geriatric syndromes.
-
•
To meet the needs of older adults, clinicians should consider multicomplexity, cognition, physical function, and social factors.
-
•
Clinicians should formulate comprehensive geriatric cardiology care plans grounded in individualized health care goals and preferences.
Cardiovascular disease (CVD) is the leading cause of mortality and a major cause of morbidity worldwide, particularly among older adults.1 With an aging population, CVD is expected to impose an increasingly significant societal burden in terms of disability, functional decline, and health care costs.1,2 More than 23 million people in the United States are aged ≥75 years; of these, 6.5 million are above the age of 85 years. These numbers are expected to nearly triple by 2060.3 In fact, it is expected that the number of older adults (aged >65 years) will outnumber children (aged <18 years) for the first time around 2034. This demographic shift toward an older population will be accompanied by a dramatic increase in the prevalence of clinical and subclinical CVD1,4 due both to increased survival of those who developed disease at a younger age and incident disease that is mediated by pathophysiological risks associated with aging.
Common CVD conditions that mount with older age include hypertension, coronary heart disease, heart failure, cerebrovascular disease, valvular disease, arrhythmias, and peripheral artery disease.1 Importantly, few older adults with CVD have a single isolated condition; rather, older adults with CVD often have multiple conditions that include both cardiovascular and noncardiovascular disorders.5 This complexity is further enhanced by the fact that older adults are heterogeneous not only with respect to medical and cardiovascular health but also regarding their cognition and functional health, social and financial circumstances, and their health care goals and preferences. To date, there are no formal guidelines to assist with such complexity; rather, clinical practice guidelines have historically been disease centric, in some cases leading to conflicting recommendations across guidelines.6 Moreover, older adults have largely been excluded from major clinical trials in cardiovascular medicine, creating important evidence gaps with regard to real-world efficacy and safety.7 Taken together, older adults with CVD require comprehensive and integrative patient-centered approaches to organize complex care and optimize outcomes.
This state-of-the-art review highlights the increasing relevance of geriatric cardiology within the modern-day clinical environment and enumerates key principles essential for addressing the needs and vulnerabilities of older adults with CVD. Integration of fundamental cardiovascular care precepts within relevant geriatric principles is increasingly necessary to optimize current and future health care delivery to vulnerable older adults.
What is geriatric cardiology
Geriatric cardiology merges the management of diseases of the heart and blood vessels with a focus on the health care of older people and thus infuses cardiovascular medicine with the principles of geriatrics to provide individualized, holistic, patient-centered care to older individuals with or at risk for CVD.8,9 Geriatric cardiology is intrinsically collaborative and multidisciplinary, combining expertise of cardiologists, geriatricians, primary care clinicians, advanced practice providers, nurses, pharmacists, dieticians, therapists, and social workers to address the complex needs of older patients.
Evolution of geriatric cardiology
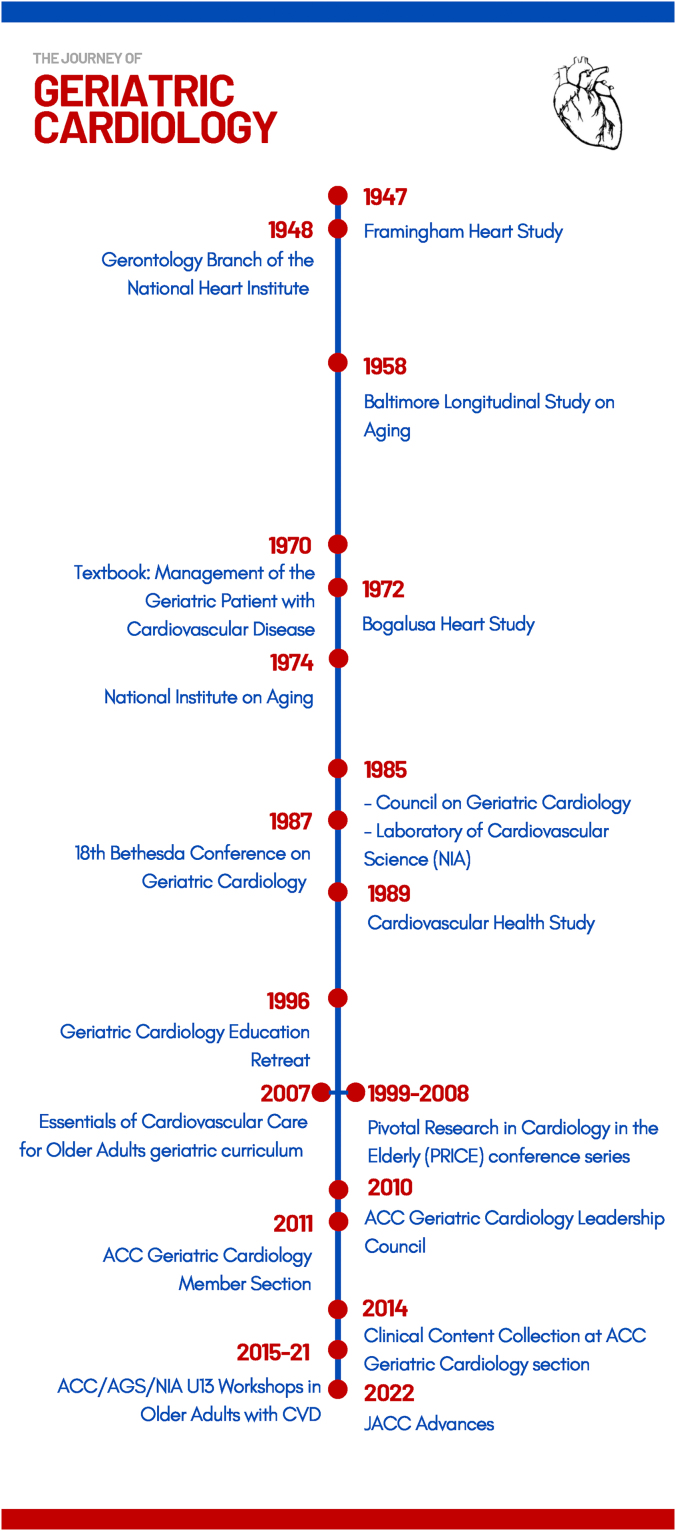
The concept of geriatric cardiology dates to the ancient Egyptians, who recognized an association between age and heart disease. In the mid-1600s, the British physician Thomas Sydenham introduced the concept of vascular aging when he stated “a man is as old as his arteries.” However, it was not until the mid-20th century when study of the aging cardiovascular system and of older adults with CVD began to accelerate (Figure 1). Although initially limited to adults aged 30 to 62 years, the landmark Framingham Heart Study, which began in 1948, set the stage for future epidemiologic studies, including the Baltimore Longitudinal Study of Aging (1958), the Bogalusa Heart Study (1972), and the Cardiovascular Health Study (1989), along with many others. These studies have provided a wealth of scientific information on normal aging physiology, risk factors for CVD in older adults, and the clinical course and prognosis of CVD in older adults. The year 1948 was also notable for establishing the Gerontology Branch in the newly formed National Heart Institute. In 1966, the Gerontology Branch became the Gerontology Research Center, an intramural laboratory of the National Institute of Child Health and Development (NICHD) that focused on the biology of aging. In 1974, the National Institute on Aging (NIA) was established, and in 1975, the Gerontology Research Center separated from NICHD to become one of the core components of NIA. In 1985 the Laboratory of Cardiovascular Science was commissioned as an additional intramural branch of the National Institute on Aging.
Figure 1.
Timeline of Geriatric Cardiology
Key advances in the development and emergence of geriatric cardiology are shown.
The first textbook on geriatric cardiology was published in 1970 by Dr Raymond Harris, who in 1985 founded the first geriatric cardiology professional society, the Council on Geriatric Cardiology. The organization’s flagship publication, the American Journal of Geriatric Cardiology, was led by Editor-in-Chief, Dr Nanette Wenger. In the early 21st century, the geriatric cardiology community began to build relationships with the American College of Cardiology (ACC), culminating in the formation of the ACC Geriatric Cardiology Leadership Council in 2010 and the broader Geriatric Cardiology Section (GCS) in 2011.10 Over the years, the GCS has made numerous contributions to the field, including several timely publications7, 8, 9,11, 12, 13, 14, 15, 16, 17, 18, 19, 20 (Supplemental Table 1) and a series of workshops co-sponsored by the ACC, National Institute on Aging, and American Geriatrics Society. The GCS currently includes 5 productive Working Groups (Research, Advocacy, Palliative Care, Education and Training, and Fellows-in-Training and Early Career Professionals). In the educational realm, the Essentials of Cardiovascular Care for Older Adults geriatric cardiology curriculum was first published in 2007 and updated in 2017 to 2018; the online geriatric cardiology clinical content section featured on the GCS website was initiated in 2014; and GCS authors have contributed extensively to CardioSmart, ACC’s patient education portal.
Essential principles for caring for older adults with CVD
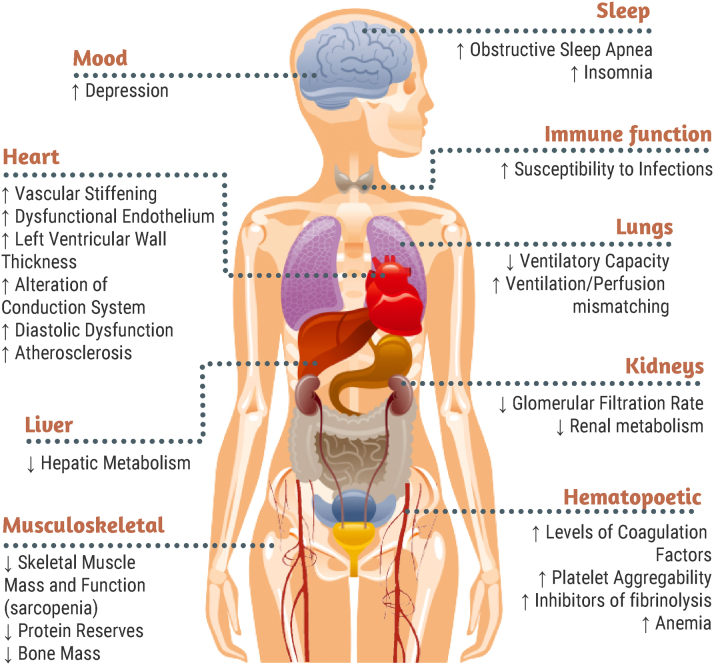
With aging, myriad physiological changes occur within multiple organ systems (Figure 2). Within the cardiovascular system, structural changes include increased vascular stiffness, endothelial dysfunction, increased left ventricular wall thickness resulting in diastolic dysfunction, and atherosclerosis; functional changes are characterized by diminished capacity to compensate for increased workload resulting in exercise intolerance, higher risk of heart failure, and conduction system alterations causing higher risk of arrhythmias and heart block.21, 22, 23 In addition, the brain undergoes shrinking in volume, white matter changes, and vascular changes, predisposing to cognitive impairment and dementia24; the musculoskeletal system undergoes significant losses of mass and functional capacity, contributing to frailty and increased risk for falls25; and kidneys sustain anatomical and functional changes including decreased number of glomeruli, reduced cortical volume, and diminished filtration reserve, predisposing to chronic kidney disease and higher risk for acute kidney injury.26 Age-related changes to these organ systems are further accelerated by the presence of clinical and subclinical CVD.27 Accordingly, physiological aging coupled with CVD leads to an increased risk of developing multimorbidity and also impacts other domains of health including cognition and physical function. The social environment can also change with age—increased caregiving needs for oneself and/or increased caregiving duties for others, loss of social support systems, and loneliness all become more common with age.28,29 Importantly, these changes do not operate individually; rather, they interact with one another, further adding to the complexities and challenges of providing care to older adults with CVD.
Figure 2.
Age-Related Changes to Organ Systems
Age-related changes within multiple organ systems are shown.
Multimorbidity and polypharmacy, cognitive impairment, functional abnormalities, and the social environment inform optimal care for older adults. First, the presence of these deficits could be a consequence of a known condition or could stem from subclinical disease that merits additional evaluation. The accumulation of deficits also increases the risk of harm from diagnostic and therapeutic interventions and may decrease life expectancy, altering risk-benefit calculations for treatment. Decision-making among older adults may be particularly complex, given variations in health outcome goals with age,30 and evidence gaps related to the use of therapeutic interventions that were primarily studied in younger healthier adults.7 Finally, it is important to recognize that abnormalities across these domains reflect physiological aging, which may be a more precise reflection of the consequences of aging compared with chronological age. Reliance on chronological age may, in some cases, lead to inappropriate implicit biases in care provision.31
Given the age-related biological changes and rising incidence of complex conditions across multiple domains among an aging population, geriatric cardiology is not just a preferred approach to optimizing cardiovascular care to the population—it is a necessary approach. We call for all cardiovascular clinicians to incorporate geriatric cardiology principles into the routine care of older adults with cardiovascular conditions. As a starting point, we describe 5 essential principles necessary to appropriately and effectively address the aforementioned vulnerabilities intrinsic to many older adults with CVD (Table 1).
Table 1.
Essential Principles for Caring for Older Adults With CVD
| 1. Recognize and consider the potential impact of multicomplexity |
| 2. Evaluate and integrate cognition into decision-making |
| 3. Evaluate and integrate constructs of physical function into decision-making |
| 4. Incorporate social environmental factors into management decisions |
| 5. Elicit patient priorities and health goals and align with care plan |
CVD = cardiovascular disease.
Recognize and consider the potential impact of multicomplexity
The prevalence of multimorbidity, defined as 2 or more chronic conditions, rises with advancing age due to age-related changes and the cumulative effects of CVD and other diseases. For example, aging and inflammatory processes, often jointly described as inflammaging,32 are compounded by lifestyle and aggregate effects of traditional CVD risk factors (eg, sedentariness, obesity, tobacco). The presence of multiple conditions can worsen prognosis and complicate diagnostic and therapeutic decision-making.33 In the setting of multimorbidity, it is important to consider life expectancy, competing risk of death from both CVD and non-CVD conditions,34 and overall treatment burden when formulating care plans for older adults with CVD. With advancing age, noncardiovascular death becomes increasingly common—it is therefore important to consider these competing risks and time to benefit when making decisions about interventions or medications. For example, although the implantable cardioverter-defibrillator has demonstrated robust evidence to prevent sudden cardiac death, the benefits are attenuated with advancing age, especially in patients with multiple chronic conditions, because of the competing risk of noncardiovascular death.35 Finally, as the number and severity of medical conditions increase, complex disease management plans can lead to increased burden for the patient to manage these conditions, which can impair quality of life.36
Polypharmacy is closely linked with multimorbidity. Often defined as the taking of at least 5 medications,37 polypharmacy is common in patients with CVD because of a proliferation of drugs that can alter the natural history of coronary artery disease, arrhythmias, and heart failure. In fact, polypharmacy is nearly universal in patients with heart failure; a recent study showed that more than 50% of older adults hospitalized for heart failure were taking 10 or more medications at discharge.38 On the one hand, this reflects major advances in science over the past 2 decades; however, this also increases the risk for adverse drug reactions.39 Given the prevalence of polypharmacy, multimorbidity, and age-related changes in body composition and hepatic and renal function that mediate pharmacokinetics and pharmacodynamics, older adults are at especially high risk for adverse drug reactions. Although cardiovascular medications have the potential to substantially reduce morbidity and mortality in some older adults, such agents are among the most common causes of adverse drug events requiring emergency room visits and/or hospitalization.40,41 Moreover, with an increasing number of chronic conditions and medications, therapeutic competition may occur, whereby an agent prescribed for one condition may exacerbate another.42
The key to managing polypharmacy is to ensure safe and effective medication prescribing (or deprescribing).17 A useful strategy to achieve this objective is to routinely assess for adverse events from CVD medications. If any adverse drug event is present, reduction in dose, class-switch, and/or discontinuation may be reasonable strategies, with subsequent re-evaluation of clinical status at a follow-up encounter. It is similarly important to confirm that the medications being prescribed are indicated, reflect a favorable risk-benefit calculation, and are consistent with the patient’s health outcome goals. For example, a recent study from an anticoagulation registry in Michigan showed that one-third of patients with an indication for anticoagulation but without an indication for aspirin were prescribed both; the group that took both agents experienced increased bleeding.43 Finally, it may be reasonable to review noncardiovascular medications to ensure that they are not causing harm. Some medications of concern are outlined in scientific statements from the American Heart Association44 and the American Geriatrics Society (known as Beers criteria).45 Tools that can potentially improve prescribing quality include the Screening Tool of Older Persons' Prescriptions and Screening Tool to Alert to Right Treatment (STOPP/START), although these are yet to be routinely used in practice.46
Evaluate and integrate cognition into decision-making
Older adults universally experience anatomical and functional changes in the brain. These changes can occur as a result of age, medical conditions, and/or therapeutic interventions such as medication or procedures. Neurons do not regenerate,47 and CVDs such as atrial fibrillation48 and heart failure49 have been implicated as important contributors to worsened cognition and subsequent dementia. Cellular senescence has also been implicated as an important mechanism driving histologic and physiological changes, with subsequent alterations in cognition.27 Finally, subclinical thromboembolic events that track with CVD50 result in reduced cerebral blood flow.51,52 Impaired cognition can be classified along a spectrum from mild cognitive impairment to dementia, although measurement can be confounded by superimposed delirium, depression, and/or hearing loss. Impairment can span any of several cognitive domains, including learning and memory, executive function, and attention.53 Each domain is important for self-care practices such as symptom monitoring and medication adherence, hallmarks of cardiovascular management. Cognitive impairment can compromise patient capacity to engage in these behaviors and lead to adverse clinical events.54,55 Moreover, limitations in self-care behavior engagement may be misconstrued as noncompliance, even when lack of engagement is unintentional rather than volitional. Depending on its severity, cognitive impairment may be associated with reduced life expectancy,56 highlighting its importance for consideration in risk/benefit calculations for procedures as well as medications.
To identify changes in cognition, it may be helpful to ask patients and/or their family members about observed changes in memory or forgetfulness especially as it relates to taking their medications. The MiniCog and AD8 are validated ultrabrief screening tools57,58 for cognitive impairment and can be administered in just 3 minutes. Screening for depression via the validated Patient Health Questionnaire59 or the Geriatric Depression Scale60,61 is appropriate in the setting of potential cognitive impairment because depression is common with age and can itself impact cognition. In the acute setting, it may also be reasonable to screen for delirium, which is a transient state of altered consciousness, and is associated with mortality as well as an elevated risk of developing dementia.62 The Confusion Assessment Method is a validated tool for screening for delirium.63,64
Suspected cognitive impairment should lead to a referral for further neurocognitive function evaluation. An improved understanding of neurocognitive function can help identify specific impairments that require accommodation. In the presence of cognitive impairment, it may be reasonable to simplify medication regimens to minimize the risk for medication errors and subsequent adverse drug events. Involving family members and caretakers to assist with medication administration and other self-care behaviors may also be important. In this setting, family members and caretakers are critical for discussions about health priorities and subsequent medical decision-making about medications as well as procedures, especially because the presence of cognitive impairment itself impacts overall life expectancy. When depression is present, it is recommended to pursue behavioral (psychotherapy, cognitive behavioral therapy, and exercise) and pharmacologic interventions (selective serotonin reuptake inhibitors and serotonin norepinephrine reuptake inhibitors), which have shown efficacy and safety for the treatment of depression among older adults.65
Evaluate and integrate constructs of physical function into decision-making
Physical function is closely tied to mortality, preservation of quality of life, and independence—key priorities for older adults.66,67 Indeed, physical function declines more rapidly among older adults with CVD than in those without CVD.68,69 Physiological changes that impact physical function include age-related declines of cardiorespiratory fitness, decreased muscle mass, reduced strength, bone loss, and degenerative changes in connective tissue.70, 71, 72 CVD-related changes to physical function occur via cardiovascular instability or reduced perfusion of muscle due to atherosclerosis, reduced exercise tolerance due to heart failure, and consequent sedentary lifestyle aggravating physical deconditioning.66 These changes may be further accelerated by medications and/or hospitalizations. For example, a longitudinal study showed that 4% of leg lean mass is lost after 5 days of bedrest among older adults.73
To incorporate physical function into cardiovascular care, clinicians may consider the following domains: disability as assessed by activities of daily living (ADLs), physical frailty, and falls. Although they overlap, each represents unique constructs that merit consideration. On the most fundamental level, physical function can be characterized based on the ability to perform ADLs, as defined by Katz,74 to include bathing, dressing, toileting, transferring, continence, and feeding. Impairments in these basic skills have major implications on prognosis. For example, an impairment in at least 1 ADL is associated with almost halving of life expectancy.75 Impairment in ADLs is not only associated with higher mortality but also with reduced quality of life and loss of independence.75 When substantial impairments in quality of life and loss of independence have occurred, discussions about health priorities and subsequent modifications to treatment plans may be necessary.
Frailty is also an important construct of function and is defined76 as “a clinical syndrome of increased vulnerability resulting from age-associated declines in reserve and function across multiple physiologic systems such that the ability to cope with everyday acute stress is compromised.” It may be operationalized based on physical attributes alone, as delineated by Fried76—weight loss, weakness, exhaustion, slowness, and low physical activity level—or based on an accumulation of deficits across multiple domains, calculated as a frailty index.77,78 Regardless of definition, the prevalence of frailty is considerable among patients with CVD13—for example, it can affect up to half of adults with coronary artery disease, heart failure, and severe aortic stenosis. There is a dose-dependent bidirectional association between CVD and frailty, whereby CVD can lead to frailty, and frailty can lead to CVD. Indeed, a recent study showed that physical frailty was an independent risk factor for incident CVD.79,80 Shared pathophysiological mechanisms such as inflammation likely account for this bidirectional association and sometimes make it difficult to extricate frailty from CVD. Frailty has well-known associations with all-cause mortality, loss of independence, and disability.79,81,82 Moreover, frailty increases the risk of various interventions and impacts the risk-benefit ratio for medications.13
Falling is another important construct within the physical function domain, especially because the risk of falls is higher among those with CVD.83, 84, 85 This is the case for multiple reasons. First, older adults are already at risk for hypotension and/or falls due to age-related physiological changes, including reduced baroreceptor or autonomic reflex, impaired homeostasis of volume and electrolyte balance, neurologic disorders, decreased perception due to hearing or vision impairment, or cognitive impairment.86,87 Second, CVD itself can exacerbate many of these abnormalities through inadequate perfusion to muscle or the brain, which are necessary for central nervous system mediated balance control, and inadequate augmentation of heart rate from postural changes increasing the risk of syncope.87,88 Third, these risks are further exacerbated by medications commonly prescribed to treat CVD.89 For example, antihypertensives, diuretics, nitrates, and beta-blockers can cause hypotension and syncope and thus predispose older adults to falls.87
Assessment of ADLs, frailty, and falls can be done in a few ways. Clinicians can ask about ADLs during routine patient encounters as a part of history taking and/or through a questionnaire. The tools to specifically assess frailty include Fried’s Frailty Phenotype Criteria, the Frailty Index, Clinical Frailty Scale, FRAIL scale, grip strength, Timed Up and Go test, and the Short Physical Performance Battery test (Table 2).76,90, 91, 92, 93, 94, 95, 96 These tools vary in the required time and skills to conduct and should be matched to the resources available in each setting. For assessment of falls, a one-question inquiry about any falls occurring in the prior 6 months is a simple approach. Formal screening tools that quantify falls risks include the Johns Hopkins Fall Risk Assessment Tool, Henderich II Fall Risk Model, and Fall Risk Questionnaire, which can subsequently be incorporated into decision-making and a customized care plan.
Table 2.
Tools for Implementing the Essential Principles for Caring for Older Adults With CVD
| 1. Recognize and consider the potential impact of multicomplexity |
| Screening Tool of Older Persons' Prescriptions and Screening Tool to Alert to Right |
| Treatment (STOPP/START) |
| Beers criteria |
| 2. Evaluate and integrate cognition into decision-making |
| MiniCog test |
| AD8 |
| Patient Health Questionnaire |
| Geriatric Depression Scale |
| Confusion Assessment Methods |
| 3. Evaluate and integrate constructs of physical function into decision-making |
| Katz’s Activities of Daily Living |
| Fried’s phenotype of frailty |
| Frailty index |
| Clinical Frailty Scale |
| FRAIL scale |
| Grip strength test |
| Timed Up and Go test |
| Short Physical Performance Battery Test |
| Johns Hopkins Fall Risk Assessment Tool |
| Henderich II Fall Risk Model |
| Fall Risk Questionnaire |
| 4. Incorporate social environmental factors into management decisions |
| Heathy people 2030 |
| UCLA Loneliness Scale |
| 5. Elicit patient priorities and health goals, and align with care plan |
| Patient priorities care model |
CVD = cardiovascular disease.
Referral of patients with physical function impairment to exercise programs can be effective in improving physical function by increasing strength, balance, and aerobic endurance, leading to improvements in ADLs, mitigation of frailty, and a reduced risk for falls.66,97 Physical rehabilitation therapy has been shown to improve all 3 physical function constructs.98 In addition, given the bidirectional association between physical function and CVD, optimization of CVD conditions may also be important in improving function.
Integrating the assessment of physical function is critical in cardiovascular practice because these parameters have important implications on the decision-making process. If aggressive treatment of CVD can meaningfully improve function, it may be reasonable to pursue even high-risk procedures such as left ventricular assist devices. On the other hand, if impairment in function is severe and/or unrelated to CVD, such high-risk procedures may be less likely to alter the disease trajectory and therefore may not be warranted. This underscores the importance of quantifying physical function and incorporating it into decision-making.
Incorporate social environmental factors into management decisions
Social isolation defined as the physical lack of social support and loneliness defined as the emotional perception associated with the absence of intimate connections are highly prevalent at older age.28,29 Death of a spouse is more common among women due to the sex-related differences in life expectancy.99 Spousal support and living arrangements have direct implications on emotional and physical support as widowed older adults are more likely to live alone or in group homes.99 Social displacement with resulting social isolation and loss of independence is also more common among those with fewer children.100 The deaths of loved ones and friends with associated declines in social interactions and displacement to group homes result in lower life satisfaction, self-esteem, and sensations of belonging.101 These changes can be especially challenging for older adults when other capacities such as cognition and function are diminished.
In addition, household income often decreases substantially after retirement. This affects a large proportion of older adults, as the average retirement age in the United States is currently 66 years. With this shift, there is often a reliance on government programs such as Medicare for medical insurance, which may entail significant cost-sharing and high out-of-pocket expenditures. Although policies vary, many insurance carriers do not cover services frequently required by older adults, including medication costs; vision, hearing, and dental needs; and long-term care either at home or in group settings.102 The resulting financial challenges may be compounded by costs related to transportation and the need for additional assistance in the home.99,103 These factors can force many older adults to transition to smaller publicly owned or subsidized housing and preclude patients from accessing the medical care they need. Beyond the social context, economic stability, and health care access outlined here, neighborhood-related factors (such as pollution and violence) as well as health literacy levels and language proficiency are likely to impact health outcomes.104
Clinicians caring for older adults with CVD should be aware of these social vulnerabilities, especially in the presence of physiological deficits, when developing care plans to ensure that they are feasible. For example, simply inquiring about social support is a critical first step; then screening for loneliness through tools such as the UCLA Loneliness Scale105 could be considered. In addition, remaining sensitive and vigilant about financial wherewithal and the presence of other social determinants of health can provide invaluable information about patient context that drives behavior and health priorities. Finally, developing novel approaches to assist with some of these limitations, such as through remote monitoring and other forms of gerotechnology, could be valuable but will require further development and validation before broad implementation.18
Elicit patient priorities and health goals and align with care plan
In the face of complexity and uncertainty, cardiovascular care should start with what matters to the patient. This is critical to aligning care with individual health outcome goals and care preferences, which are heterogeneous and vary with advancing age.106,107 This is especially relevant in settings where older adults receive care from multiple clinicians, which is common for older adults with CVD, given the prevalence of multimorbidity. Without clarity on a patient’s health outcome goals, receiving care from multiple clinicians can lead to treatment strategies that compete with one another, whereby a treatment strategy that improves one condition may conflict with the patient’s goals for another condition. Understanding health outcome goals can also help facilitate decisions about tradeoffs.108 Although some therapeutic interventions can concurrently improve the quality of life and prolong life, other interventions can only achieve one at the expense of the other. There are also interventions that provide short-term benefits at the expense of long-term harms, and vice versa. For example, treatment of osteoarthritis with nonsteroidal anti-inflammatory drugs may reduce joint pain but increases the risk for future cardiovascular events. Complying with prescribed health plans also comes with tradeoffs—for example, attending medical appointments may come with added stress and anxiety related to the logistical challenges of getting to and from appointments or may come at the sacrifice of spending time with family; similarly, paying for medicines may come at the cost of paying for other things, which may even include basic necessities such as food and clothing. Clinical practice guidelines may not be helpful to reconcile these challenges because most guidelines are disease specific. Although health priorities of older adults often include longevity, health outcomes such as quality of life, function, and independence are often as important or more important than longevity.66 Accordingly, “What Matters” is critical for clinicians to effectively address the inherent complexities of managing CVD in older adults, serving as an anchor to maximize the likelihood of helping the patient to achieve their care goals with shared decision-making. It is important to recognize that the patient's health outcome goals may change over time and may be highly influenced by their responsibilities and position within their social structures.109,110 Goals may also evolve as medical conditions advance, disability progresses, and overall life expectancy decreases. Understanding overall prognosis and patient priorities can also help determine the appropriateness of palliative care, an underutilized resource in cardiovascular medicine.111
When initiating a conversation about “What Matters,” it may be helpful to explain the underlying reason for such a conversation (eg, “I would like to know what specific goals you would like to achieve through the care that I provide so that I can make sure that my care plan aligns with those goals.”) Follow-up questions may include “What do you want to focus on during your time with me?” or “What activities do you want to get back to doing?” The recently developed Patient Priorities Care toolkit is an excellent resource that can assist clinicians in addressing “What Matters”.110 These insights can then drive discussions about care preferences and tradeoffs, facilitate personalized care, and prepare clinicians to share a sentiment like the following: “There are several things we could do, but knowing what matters most to you, I suggest we…” This shift from a disease-centric encounter to a holistic patient-centered encounter is necessary to address the rising complexity of older adults with CVD.112
Geriatric cardiology frameworks and implementation
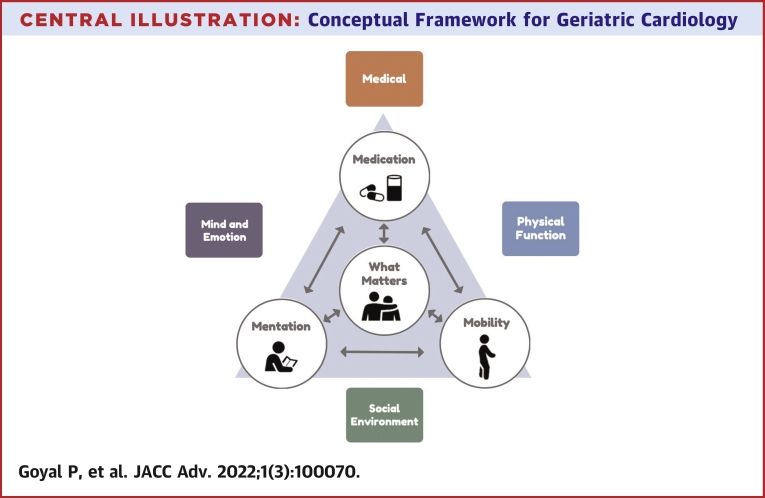
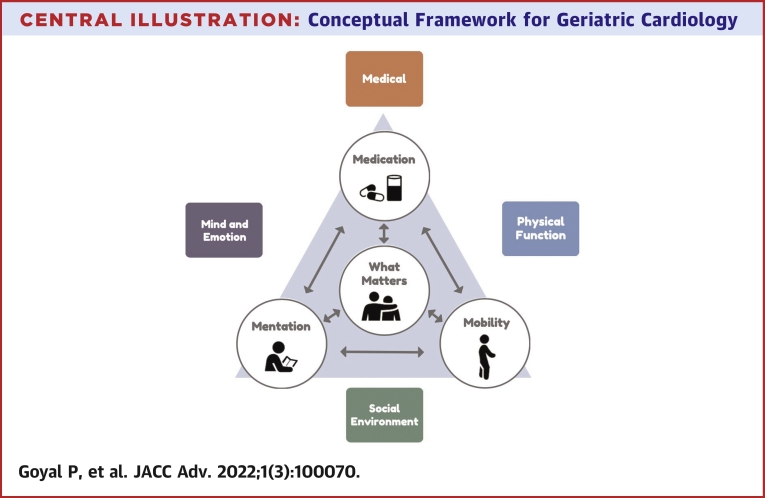
Conceptual frameworks for comprehensive integrative care of older adults have previously been outlined. In a 2018 State of the Art paper published in the Journal of American College of Cardiology, the ACC GCS outlined a multiple domain approach to the management of older adults with heart failure.113 This approach promulgates 4 key domains relevant to patient care—the medical domain, the mind and emotion domain, the functional domain, and the social domain. Although this approach was devised for the care of older adults with heart failure, the precepts can be broadly applied to any older adult with CVD. On the other hand, the Age-Friendly Health System 4 Ms framework, from the Institute for Healthcare Improvement and the John A. Hartford Foundation,114 seeks to address the needs pertinent to the older adults with complex medical conditions by integrating: 1) What Matters; 2) Medications; 3) Mentation; and 4) Mobility. In Central Illustration, we have merged these models to generate an overarching framework that combines key elements necessary to provide optimal care to older adults with CVD.
Central Illustration.
Conceptual Framework for Geriatric Cardiology
A framework that combines essential principles of care to older adults with CVD is shown.
Partnerships between the cardiologist and other clinicians involved in the care of older adults are critical to ensure that there is alignment of care plans. Communication across multiple clinicians is especially relevant, given the rise in the number of specialties seen by older adults in a given year—recent data indicate that almost one-third of Medicare beneficiaries see at least 5 different physicians per year.115 If multiple geriatric conditions are present, it may be reasonable for a cardiovascular clinician to partner with a geriatrician in the ambulatory setting and potentially pursue a geriatric co-management model in the inpatient setting. Geriatric co-management is a model of care for complex older adults whereby a designated geriatrician or geriatrics-trained nurse provides concurrent care alongside the cardiovascular clinician. Prior work in other countries has shown that geriatric co-management can prevent episodes of delirium and functional decline among older adults undergoing cardiovascular surgery116 and among older adults hospitalized to a cardiovascular service117; and a single-center study done in the United States showed that geriatric co-management reduced cost, days spent in the intensive care unit, and in-hospital mortality among older adults although this was not specific to those with CVD.118
Putting these principles into action will take time and effort; the current reimbursement structure for health care delivery in the United States (ie, favoring resource-intensive procedures) does not yet facilitate routine incorporation of these principles.119 In this setting, activation of a multidisciplinary team with allocation of efforts based on expertise could be helpful for implementing geriatric cardiology principles. For example, pharmacists can assist in characterizing and managing polypharmacy as part of a system-based approach to addressing complexity. Similarly, occupational therapists and physical therapists have unique expertise that could assist in the evaluation and management of the cognitive and functional domains. The perspectives and skillsets of nurses and social workers could likewise be utilized to develop comprehensive, holistic care plans that integrate multiple domains of health. Along these lines, a recent study of older adults with cancer showed that leveraging a multidisciplinary team to implement a systematized approach to assessing and managing geriatric conditions can reduce chemotherapy-related toxicity.120 In this study, the intervention group received lower doses of chemotherapy but experienced similar survival compared with the usual care group. Although some might argue that similar evidence is needed for older adults with CVD,121 increasingly frequent integration of geriatric principles into the care of older adults with cancer122,123 demonstrates that this approach to care is feasible even within the constraints of the current U.S. health care system. With advances in technology and mobile health,18 implementing mobile applications such as the Essential Frailty Toolset124 or GeriKit,125 which are freely available and easily accessible to clinicians, represent yet another specific strategy that can be used to implement geriatric principles into the routine care of older adults with CVD.
The Future
Even with substantive advances in geriatric cardiology over the last several decades, much work remains in the research, clinical care, education, and health policy realms (Table 3). As previously outlined, adults aged ≥75 years have been underrepresented in most cardiovascular clinical trials, and this deficiency is most pronounced among women, racial and ethnic minority groups, nursing home residents, and older adults with multimorbidity, cognitive impairment, and frailty. Moreover, few studies have examined outcomes valued by older adults, including quality of life and maintenance of function and independence. The National Institutes of Health Inclusion Across the Lifespan Policy,126 released in December 2017, was designed to ensure that clinical studies supported by the National Institutes of Health do not exclude older patients without compelling justification. Future efforts such as this will be necessary to ensure researchers actively recruit a broad spectrum of older adults’ representative of clinical practice, including complex patients with geriatric syndromes, and to integrate relevant outcomes into study design. In addition, continued basic and translational studies in geroscience will be necessary to increase understanding of mechanisms underlying cardiovascular aging and intersections with geriatrics, cancer, and other age-associated conditions—investigations into linkages between CVD and cellular mechanisms such as autophagy and senescence have begun to provide unique insights that will hopefully generate novel therapeutic strategies in the future.127,128
Table 3.
Gaps and Challenges in Geriatric Cardiology
| I. Research |
| A. Increased enrollment of older adults in clinical studies, including women, racial and ethnic minorities, nursing home residents, and complex elders with multimorbidity, frailty, functional and cognitive limitations |
| B. Incorporation of outcomes relevant to older adults into clinical study design, including quality of life, maintenance of independence, physical and cognitive function |
| C. Expanded studies in geroscience to clarify mechanisms of cardiovascular aging and intersections with geriatrics, oncology, and other specialties as related with aging |
| II. Clinical care |
| A. Integration of geriatrics constructs into the care of older adults with cardiovascular disease in the inpatient and ambulatory settings |
| B. Continued alignment of clinical practice guidelines, appropriate use documents, and consensus statements with current knowledge and evidence pertinent to older adults, acknowledging where data are insufficient to make recommendations |
| C. Use of gerotechnology for improved quality and efficiency of health care delivery |
| D. Activation and integration of multiple disciplines to provide care to older adults (pharmacists, physical and occupational therapists, social workers, etc) |
| III. Education |
| A. Increased education of clinicians caring for older adults, including physicians, advanced practice providers, and pharmacists, about geriatric principles of care, starting with professional school training and continuing throughout professional life |
| B. Integration of geriatric cardiology knowledge and competencies into the COCATS guidelines for training of fellows in cardiovascular disease |
| IV. Public policy |
| A. Revision of CMS reimbursement models to better reflect time required to provide optimal multidisciplinary patient-centered care to complex older adults and better support integrated interdisciplinary models of care |
| B. Increased requirements by FDA to ensure that drugs and devices intended for use in older adults have been adequately tested in this population and to ensure adequate post-approval surveillance to identify unanticipated adverse events |
CMS = Centers for Medicare & Medicaid Services; COCATS = Core Cardiology Training Symposium; FDA = Food and Drug Administration.
Development of strategies to seamlessly incorporate geriatric cardiology principles into the care of older adults is another major unmet need. This aligns closely with the need to revise current payment structures to encourage the use of comprehensive and integrated care for older adults. In addition, incorporation of geriatric cardiology precepts into the standards of cardiology fellowship training (COCATS: Core Cardiology Training Symposium)129 is warranted to enhance the skillset of future cardiologists who will inevitably need to contend with the many complexities of caring for older patients. The optimal strategy to ensure that there are sufficient number of clinicians sensitive to fundamental geriatric cardiology principles to provide high-quality care of this complex and rapidly growing subpopulation remains unclear. Further work is also necessary to develop strategies that can leverage technology and/or more broadly integrate multiple disciplines (pharmacists, physical and occupational therapists, social workers, etc) into the care of older adults with CVD.
Finally, at the health policy level, there is need to revise the compensation model for services provided to complex older patients, acknowledging the time and expertise required to deliver optimal multidisciplinary patient-centered care. There is also need for increased Food and Drug Administration requirements to ensure that pharmaceuticals and devices intended for use in older patients are adequately tested in this population before approval and that postmarketing surveillance is sufficient to identify unanticipated adverse events.
Conclusions
Aging is the most powerful risk factor for CVD, and the number of older adults with CVD is growing rapidly as the population ages. Older adults with CVD often have several non-CVD medical challenges, including multimorbidity, polypharmacy, cognitive impairment, and diminished physical function that add to the complexity of care. Moreover, older patients are often beset with an array of social, environmental, and financial challenges that may impact care preferences and health care delivery. To address these issues and to ensure high-quality, patient-centered care for older adults with CVD, care should involve 5 essential geriatric cardiology principles: 1) recognize and consider the potential impact of multicomplexity; 2) evaluate and integrate cognition into decision-making; 3) evaluate and integrate constructs of physical function into decision-making; 4) incorporate social environmental factors into management decisions; and 5) elicit patient priorities and health goals and closely align them with the care plan. By applying these 5 principles, cardiovascular clinicians have the opportunity to provide comprehensive geriatric cardiology care to their older patients.
Funding support and author disclosures
Dr Goyal is supported by American Heart Association grant 20CDA35310455, National Institute on Aging grant K76AG064428, and Loan Repayment Program award L30AG060521; and has received personal fees for medicolegal consulting related to heart failure and has received honoraria from Akcea Therapeutics Inc. Dr Kwak has received research funding from the U.S. Deprescribing Research Network (National Institute on Aging R24AG064025) and consult free from the Endocrine & Diabetes Plus Clinic of Houston and the Institute for Healthcare Improvement. Dr Damluji has received research funding from the Pepper Scholars Program of the Johns Hopkins University Claude D. Pepper Older Americans Independence Center funded by the National Institute on Aging P30-AG021334 and a mentored patient-oriented research career development award from the National Heart, Lung, and Blood Institute K23-HL153771-01. Dr Denfeld is currently funded by the National Institutes of Health/National Institute of Nursing Research (R01NR019054) and the Medical Research Foundation. Dr Forman has received funds from the National Institute on Aging through grants R01AG060499, R01AG058883, and P30AG024827. Dr Rich has received funds from the National Institute on Aging through grants R01AG060499; and from the National Heart, Lung, and Blood Institute through grants R01HL147862 and R01HL151431. The contents of this article are solely the responsibility of the authors and do not necessarily represent official views of the National Heart, Lung, and Blood Institute, National Institutes of Health, or the United States Department of Health and Human Services. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors would like to thank Hannah Curtis for her assistance in creating and organizing figures.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For the supplemental table, please see the online version of this paper.
Supplementary data
References
- 1.Tsao C.W., Aday A.W., Almarzooq Z.I., et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. 2022;145:e153–639. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 2.Murphy S.L., Kochanek K.D., Xu J.Q., Arias E. National Center for Health Statistics; Hyattsville, MD: 2021. Mortality in the United States, 2020. NCHS Data Brief. no. 427. [PubMed] [Google Scholar]
- 3.Vespa J., Medina L., Armstrong D.M. U.S. Census Bureau; 2020. Demographic Turning Points for the United States: “Population Projections for 2020 to 2060,” Current Population Reports; pp. 25–1144. [Google Scholar]
- 4.Bergström G., Persson M., Adiels M., et al. Prevalence of subclinical coronary artery atherosclerosis in the general population. Circulation. 2021;144:916–929. doi: 10.1161/CIRCULATIONAHA.121.055340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Medicare & Medicaid Services. Chronic conditions charts: 2018. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Chronic-Conditions/Chartbook_Charts
- 6.Boyd C., Smith C.D., Masoudi F.A., et al. Decision making for older adults with multiple chronic conditions: executive summary for the American Geriatrics Society guiding principles on the care of older adults with multimorbidity. J Am Geriatr Soc. 2019;67:665–673. doi: 10.1111/jgs.15809. [DOI] [PubMed] [Google Scholar]
- 7.Rich M.W., Chyun D.A., Skolnick A.H., et al. Knowledge gaps in cardiovascular care of older adults: a scientific statement from the American Heart Association, American College of Cardiology, and American Geriatrics Society: executive summary. J Am Geriatr Soc. 2016;64:2185–2192. doi: 10.1111/jgs.14576. [DOI] [PubMed] [Google Scholar]
- 8.Bell S.P., Orr N.M., Dodson J.A., et al. What to expect from the evolving field of geriatric cardiology. J Am Coll Cardiol. 2015;66:1286–1299. doi: 10.1016/j.jacc.2015.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forman D.E., Rich M.W., Alexander K.P., et al. Cardiac care for older adults. Time for a new paradigm. J Am Coll Cardiol. 2011;57:1801–1810. doi: 10.1016/j.jacc.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American College of Cardiology Geriatric Member Section, American College of Cardiology. https://www.acc.org/membership/sections-and-councils/geriatric-cardiology-section
- 11.Afilalo J., Alexander K.P., Mack M.J., et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. 2014;63:747–762. doi: 10.1016/j.jacc.2013.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindman B.R., Alexander K.P., O'Gara P.T., Afilalo J. Futility, benefit, and transcatheter aortic valve replacement. J Am Coll Cardiol Intv. 2014;7:707–716. doi: 10.1016/j.jcin.2014.01.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ijaz N., Buta B., Xue Q.L., et al. Interventions for frailty among older adults with cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79:482–503. doi: 10.1016/j.jacc.2021.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curtis A.B., Karki R., Hattoum A., Sharma U.C. Arrhythmias in patients ≥80 years of age: Pathophysiology, management, and outcomes. J Am Coll Cardiol. 2018;71:2041–2057. doi: 10.1016/j.jacc.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kodali S.K., Velagapudi P., Hahn R.T., Abbott D., Leon M.B. Valvular heart disease in patients ≥80 years of age. J Am Coll Cardiol. 2018;71:2058–2072. doi: 10.1016/j.jacc.2018.03.459. [DOI] [PubMed] [Google Scholar]
- 16.Madhavan M.V., Gersh B.J., Alexander K.P., Granger C.B., Stone G.W. Coronary artery disease in patients ≥80 years of age. J Am Coll Cardiol. 2018;71:2015–2040. doi: 10.1016/j.jacc.2017.12.068. [DOI] [PubMed] [Google Scholar]
- 17.Krishnaswami A., Steinman M.A., Goyal P., et al. Deprescribing in older adults with cardiovascular disease. J Am Coll Cardiol. 2019;73:2584–2595. doi: 10.1016/j.jacc.2019.03.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnaswami A., Beavers C., Dorsch M.P., et al. Gerotechnology for older adults with cardiovascular diseases: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76:2650–2670. doi: 10.1016/j.jacc.2020.09.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Damluji A.A., Forman D.E., van Diepen S., et al. Older adults in the cardiac intensive care unit: factoring geriatric syndromes in the management, prognosis, and process of care: a scientific statement from the American Heart Association. Circulation. 2020;141:e6–e32. doi: 10.1161/CIR.0000000000000741. [DOI] [PubMed] [Google Scholar]
- 20.Volgman A.S., Nair G., Lyubarova R., et al. Management of atrial fibrillation in patients 75 years and older: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79:166–179. doi: 10.1016/j.jacc.2021.10.037. [DOI] [PubMed] [Google Scholar]
- 21.Fleg J.L., Strait J. Age-associated changes in cardiovascular structure and function: a fertile milieu for future disease. Heart Fail Rev. 2012;17:545–554. doi: 10.1007/s10741-011-9270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strait J.B., Lakatta E.G. Aging-associated cardiovascular changes and their relationship to heart failure. Heart Fail Clin. 2012;8:143–164. doi: 10.1016/j.hfc.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.North B.J., Sinclair D.A. The intersection between aging and cardiovascular disease. Circ Res. 2012;110:1097–1108. doi: 10.1161/CIRCRESAHA.111.246876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peters R. Ageing and the brain. Postgrad Med J. 2006;82:84–88. doi: 10.1136/pgmj.2005.036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frontera W.R. Physiologic changes of the musculoskeletal system with aging: a brief review. Phys Med Rehabil Clin N Am. 2017;28:705–711. doi: 10.1016/j.pmr.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Denic A., Glassock R.J., Rule A.D. Structural and functional changes with the aging kidney. Adv Chronic Kidney Dis. 2016;23:19–28. doi: 10.1053/j.ackd.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ungvari Z., Tarantini S., Sorond F., Merkely B., Csiszar A. Mechanisms of vascular aging, a geroscience perspective: JACC focus seminar. J Am Coll Cardiol. 2020;75:931–941. doi: 10.1016/j.jacc.2019.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yanguas J., Pinazo-Henandis S., Tarazona-Santabalbina F.J. The complexity of loneliness. Acta Biomed. 2018;89:302–314. doi: 10.23750/abm.v89i2.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Rourke H.M., Collins L., Sidani S. Interventions to address social connectedness and loneliness for older adults: a scoping review. BMC Geriatr. 2018;18:214. doi: 10.1186/s12877-018-0897-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fried T.R., McGraw S., Agostini J.V., Tinetti M.E. Views of older persons with multiple morbidities on competing outcomes and clinical decision-making. J Am Geriatr Soc. 2008;56:1839–1844. doi: 10.1111/j.1532-5415.2008.01923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamczyk M.R., Nevado R.M., Barettino A., Fuster V., Andrés V. Biological versus chronological aging: JACC focus seminar. J Am Coll Cardiol. 2020;75:919–930. doi: 10.1016/j.jacc.2019.11.062. [DOI] [PubMed] [Google Scholar]
- 32.Franceschi C., Garagnani P., Parini P., Giuliani C., Santoro A. Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14:576–590. doi: 10.1038/s41574-018-0059-4. [DOI] [PubMed] [Google Scholar]
- 33.Forman D.E., Maurer M.S., Boyd C., et al. Multimorbidity in older adults with cardiovascular disease. J Am Coll Cardiol. 2018;71:2149–2161. doi: 10.1016/j.jacc.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berry S.D., Ngo L., Samelson E.J., Kiel D.P. Competing risk of death: an important consideration in studies of older adults. J Am Geriatr Soc. 2010;58:783–787. doi: 10.1111/j.1532-5415.2010.02767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee D.S., Tu J.V., Austin P.C., et al. Effect of cardiac and noncardiac conditions on survival after defibrillator implantation. J Am Coll Cardiol. 2007;49:2408–2415. doi: 10.1016/j.jacc.2007.02.058. [DOI] [PubMed] [Google Scholar]
- 36.Boyd C.M., Darer J., Boult C., Fried L.P., Boult L., Wu A.W. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294:716–724. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 37.Masnoon N., Shakib S., Kalisch-Ellett L., Caughey G.E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(230) doi: 10.1186/s12877-017-0621-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Unlu O., Levitan E.B., Reshetnyak E., et al. Polypharmacy in older adults hospitalized for heart failure. Circ Heart Fail. 2020;13 doi: 10.1161/CIRCHEARTFAILURE.120.006977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hilmer S.N., Gnjidic D. The effects of polypharmacy in older adults. Clin Pharmacol Ther. 2009;85:86–88. doi: 10.1038/clpt.2008.224. [DOI] [PubMed] [Google Scholar]
- 40.Gurwitz J.H., Field T.S., Harrold L.R., et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA. 2003;289:1107–1116. doi: 10.1001/jama.289.9.1107. [DOI] [PubMed] [Google Scholar]
- 41.Budnitz D.S., Lovegrove M.C., Shehab N., Richards C.L. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365:2002–2012. doi: 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- 42.Lorgunpai S.J., Grammas M., Lee D.S., McAvay G., Charpentier P., Tinetti M.E. Potential therapeutic competition in community-living older adults in the US: use of medications that may adversely affect a coexisting condition. PLoS One. 2014;9 doi: 10.1371/journal.pone.0089447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schaefer J.K., Errickson J., Li Y., et al. Adverse events associated with the addition of aspirin to direct oral anticoagulant therapy without a clear indication. JAMA Intern Med. 2021;181:817–824. doi: 10.1001/jamainternmed.2021.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Page R.L., 2nd, O'Bryant C.L., Cheng D., et al. Drugs that may cause or exacerbate heart failure: a scientific statement from the American Heart Association. Circulation. 2016;134:e32–69. doi: 10.1161/CIR.0000000000000426. [DOI] [PubMed] [Google Scholar]
- 45.American Geriatrics Society 2019 updated AGS beers criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67:674–694. doi: 10.1111/jgs.15767. [DOI] [PubMed] [Google Scholar]
- 46.O'Mahony D., O'Sullivan D., Byrne S., O'Connor M.N., Ryan C., Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44:213–218. doi: 10.1093/ageing/afu145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steward M.M., Sridhar A., Meyer J.S. Neural regeneration. Curr Top Microbiol Immunol. 2013;367:163–191. doi: 10.1007/82_2012_302. [DOI] [PubMed] [Google Scholar]
- 48.Kalantarian S., Stern T.A., Mansour M., Ruskin J.N. Cognitive impairment associated with atrial fibrillation: a meta-analysis. Ann Intern Med. 2013;158:338–346. doi: 10.7326/0003-4819-158-5-201303050-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang M., Sun D., Wang Y., Yan M., Zheng J., Ren J. Cognitive impairment in heart failure: landscape, challenges, and future directions. Front Cardiovasc Med. 2022;8 doi: 10.3389/fcvm.2021.831734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen L.Y., Lopez F.L., Gottesman R.F., et al. Atrial fibrillation and cognitive decline-the role of subclinical cerebral infarcts: the atherosclerosis risk in communities study. Stroke. 2014;45:2568–2574. doi: 10.1161/STROKEAHA.114.005243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leeuwis A.E., Smith L.A., Melbourne A., et al. Cerebral blood flow and cognitive functioning in a community-based, multi-ethnic cohort: the SABRE study. Front Aging Neurosci. 2018;10:279. doi: 10.3389/fnagi.2018.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schoenenberger A.W., Zuber C., Moser A., et al. Evolution of cognitive function after transcatheter aortic valve implantation. Circ Cardiovasc Interv. 2016;9 doi: 10.1161/CIRCINTERVENTIONS.116.003590. [DOI] [PubMed] [Google Scholar]
- 53.Gale S.A., Acar D., Daffner K.R. Dementia. Am J Med. 2018;131:1161–1169. doi: 10.1016/j.amjmed.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 54.Toot S., Devine M., Akporobaro A., Orrell M. Causes of hospital admission for people with dementia: a systematic review and meta-analysis. J Am Med Dir Assoc. 2013;14:463–470. doi: 10.1016/j.jamda.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 55.Lee C.S., Gelow J.M., Bidwell J.T., et al. Blunted responses to heart failure symptoms in adults with mild cognitive dysfunction. J Cardiovasc Nurs. 2013;28:534–540. doi: 10.1097/JCN.0b013e31826620fa. [DOI] [PubMed] [Google Scholar]
- 56.Rizzuto D., Bellocco R., Kivipelto M., Clerici F., Wimo A., Fratiglioni L. Dementia after age 75: survival in different severity stages and years of life lost. Curr Alzheimer Res. 2012;9:795–800. doi: 10.2174/156720512802455421. [DOI] [PubMed] [Google Scholar]
- 57.Patel A., Parikh R., Howell E.H., Hsich E., Landers S.H., Gorodeski E.Z. Mini-cog performance: novel marker of post discharge risk among patients hospitalized for heart failure. Circ Heart Fail. 2015;8:8–16. doi: 10.1161/CIRCHEARTFAILURE.114.001438. [DOI] [PubMed] [Google Scholar]
- 58.Galvin J.E., Roe C.M., Powlishta K.K., et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65:559–564. doi: 10.1212/01.wnl.0000172958.95282.2a. [DOI] [PubMed] [Google Scholar]
- 59.Kroenke K., Spitzer R.L., Williams J.B. The patient health questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41:1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 60.Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aikman G.G., Oehlert M.E. Geriatric depression scale. Clin Gerontol. 2001;22:63–70. [Google Scholar]
- 62.Inouye S.K., Westendorp R.G., Saczynski J.S. Delirium in elderly people. Lancet. 2014;383:911–922. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Inouye S.K., van Dyck C.H., Alessi C.A., Balkin S., Siegal A.P., Horwitz R.I. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 64.Ely E.W., Inouye S.K., Bernard G.R., et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 65.Kok R.M., Reynolds C.F., 3rd Management of depression in older adults: a review. JAMA. 2017;317:2114–2122. doi: 10.1001/jama.2017.5706. [DOI] [PubMed] [Google Scholar]
- 66.Forman D.E., Arena R., Boxer R., et al. Prioritizing functional capacity as a principal end point for therapies oriented to older adults with cardiovascular disease: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2017;135:e894–e918. doi: 10.1161/CIR.0000000000000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fried T.R., Bradley E.H., Towle V.R., Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346:1061–1066. doi: 10.1056/NEJMsa012528. [DOI] [PubMed] [Google Scholar]
- 68.Wang L., van Belle G., Kukull W.B., Larson E.B. Predictors of functional change: a longitudinal study of nondemented people aged 65 and older. J Am Geriatr Soc. 2002;50:1525–1534. doi: 10.1046/j.1532-5415.2002.50408.x. [DOI] [PubMed] [Google Scholar]
- 69.Levine D.A., Davydow D.S., Hough C.L., Langa K.M., Rogers M.A.M., Iwashyna T.J. Functional disability and cognitive impairment after hospitalization for myocardial infarction and stroke. Circ Cardiovasc Qual Outcomes. 2014;7:863–871. doi: 10.1161/HCQ.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lavie C.J., Arena R., Swift D.L., et al. Exercise and the cardiovascular system: clinical science and cardiovascular outcomes. Circ Res. 2015;117:207–219. doi: 10.1161/CIRCRESAHA.117.305205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Deschenes M.R. Effects of aging on muscle fibre type and size. Sports Med. 2004;34:809–824. doi: 10.2165/00007256-200434120-00002. [DOI] [PubMed] [Google Scholar]
- 72.Kallman D.A., Plato C.C., Tobin J.D. The role of muscle loss in the age-related decline of grip strength: cross-sectional and longitudinal perspectives. J Gerontol. 1990;45:M82–M88. doi: 10.1093/geronj/45.3.m82. [DOI] [PubMed] [Google Scholar]
- 73.Tanner R.E., Brunker L.B., Agergaard J., et al. Age-related differences in lean mass, protein synthesis and skeletal muscle markers of proteolysis after bed rest and exercise rehabilitation. J Physiol (Lond) 2015;593:4259–4273. doi: 10.1113/JP270699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31:721–727. doi: 10.1111/j.1532-5415.1983.tb03391.x. [DOI] [PubMed] [Google Scholar]
- 75.Stineman M.G., Xie D., Pan Q., et al. All-cause 1-, 5-, and 10-year mortality in elderly people according to activities of daily living stage. J Am Geriatr Soc. 2012;60:485–492. doi: 10.1111/j.1532-5415.2011.03867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fried L.P., Tangen C.M., Walston J., et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M157. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 77.Mitnitski A.B., Mogilner A.J., Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323–336. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rockwood K., Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 79.Damluji A.A., Chung S., Xue Q., et al. Frailty and cardiovascular outcomes in the National Health and Aging trends study. Eur Heart J. 2021;42:3856–3865. doi: 10.1093/eurheartj/ehab468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Segar M.W., Singh S., Goyal P., et al. Prefrailty, impairment in physical function, and risk of incident heart failure among older adults. J Am Geriatr Soc. 2021;69:2486–2497. doi: 10.1111/jgs.17218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Damluji A.A., Huang J., Bandeen-Roche K., et al. Frailty among older adults with acute myocardial infarction and outcomes from percutaneous coronary interventions. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.013686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Patel A., Goodman S.G., Yan A.T., et al. Frailty and outcomes after myocardial infarction: insights from the CONCORDANCE registry. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.009859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Manemann S.M., Chamberlain A.M., Boyd C.M., et al. Fall risk and outcomes among patients hospitalized with cardiovascular disease in the community. Circ Cardiovasc Qual Outcomes. 2018;11 doi: 10.1161/CIRCOUTCOMES.117.004199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee P.G., Cigolle C., Blaum C. The co-occurrence of chronic diseases and geriatric syndromes: the health and retirement study. J Am Geriatr Soc. 2009;57:511–516. doi: 10.1111/j.1532-5415.2008.02150.x. [DOI] [PubMed] [Google Scholar]
- 85.Tinetti M.E., Williams C.S. Falls, injuries due to falls, and the risk of admission to a nursing home. N Engl J Med. 1997;337:1279–1284. doi: 10.1056/NEJM199710303371806. [DOI] [PubMed] [Google Scholar]
- 86.Kruschke C., Butcher H.K. Evidence-based practice guideline: fall prevention for older adults. J Gerontol Nurs. 2017;43:15–21. doi: 10.3928/00989134-20171016-01. [DOI] [PubMed] [Google Scholar]
- 87.Forman D.E., Lipsitz L.A. Syncope in the elderly. Cardiol Clin. 1997;15:295–311. doi: 10.1016/s0733-8651(05)70337-4. [DOI] [PubMed] [Google Scholar]
- 88.Juraschek S.P., Daya N., Appel L.J., et al. Subclinical cardiovascular disease and fall risk in older adults: results from the atherosclerosis risk in communities study. J Am Geriatr Soc. 2019;67:1795–1802. doi: 10.1111/jgs.16041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jaber D., Vargas F., Nguyen L., et al. Prescriptions for potentially inappropriate medications from the beers criteria among older adults hospitalized for heart failure. J Card Fail. 2022;28(6):906–915. doi: 10.1016/j.cardfail.2021.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rockwood K., Theou O. Using the clinical frailty scale in allocating scarce health care resources. Can Geriatr J. 2020;23:210–215. doi: 10.5770/cgj.23.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ekerstad N., Javadzadeh D., Alexander K.P., et al. Clinical frailty scale classes are independently associated with 6-month mortality for patients after acute myocardial infarction. Eur Heart J Acute Cardiovasc Care. 2022;11:89–98. doi: 10.1093/ehjacc/zuab114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chung K.J.N.C., Wilkinson C., Veerasamy M., Kunadian V. Frailty scores and their utility in older patients with cardiovascular disease. Interv Cardiol. 2021;16:e05. doi: 10.15420/icr.2020.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morley J.E., Malmstrom T.K., Miller D.K. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging. 2012;16:601–608. doi: 10.1007/s12603-012-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Calvo E., Teruel L., Rosenfeld L., et al. Frailty in elderly patients undergoing primary percutaneous coronary intervention. Eur J Cardiovasc Nurs. 2019;18:132–139. doi: 10.1177/1474515118796836. [DOI] [PubMed] [Google Scholar]
- 95.Podsiadlo D., Richardson S. The timed “up & go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 96.Guralnik J.M., Simonsick E.M., Ferrucci L., et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 97.Fiatarone M.A., O'Neill E.F., Ryan N.D., et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 98.Kitzman D.W., Whellan D.J., Duncan P., et al. Physical rehabilitation for older patients hospitalized for heart failure. N Engl J Med. 2021;385:203–216. doi: 10.1056/NEJMoa2026141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.National Research Council (US) Panel on Statistics for an Aging Population . The National Academies Press; 1988. The Aging Population in the Twenty-First Century: Statistics for Health Policy. [Social, Economic, and Demographic Changes among the Elderly. [PubMed] [Google Scholar]
- 100.Wu D., Gao X., Xie Z., Xu Z. Understanding the unmet needs among community-dwelling disabled older people from a linkage perspective. Int J Environ Res Public Health. 2021;18:389. doi: 10.3390/ijerph18020389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Victor C.R. Loneliness in care homes: a neglected area of research? Aging Health. 2012;8:637–646. [Google Scholar]
- 102.Jacobson G., Cicchiello A., Shah A., et al. When costs are a barrier to getting health care: reports from older adults in the United States and other high-income countries. Commonwealth Fund. 2021. https://www.commonwealthfund.org/publications/surveys/2021/oct/when-costs-are-barrier-getting-health-care-older-adults-survey
- 103.Boyer S., Trimouillas J., Cardinaud N., et al. Frailty and functional dependence in older population: lessons from the FREEDOM limousin - nouvelle aquitaine cohort study. BMC Geriatr. 2022;22:128. doi: 10.1186/s12877-022-02834-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.U.S. Department of Health and Human Services. Healthy people 2030. https://health.gov/healthypeople [DOI] [PubMed]
- 105.Russell D., Peplau L.A., Cutrona C.E. The revised UCLA loneliness scale: concurrent and discriminant validity evidence. J Pers Soc Psychol. 1980;39:472–480. doi: 10.1037//0022-3514.39.3.472. [DOI] [PubMed] [Google Scholar]
- 106.Brunner-La Rocca H.P., Rickenbacher P., Muzzarelli S., et al. End-of-life preferences of elderly patients with chronic heart failure. Eur Heart J. 2012;33:752–759. doi: 10.1093/eurheartj/ehr404. [DOI] [PubMed] [Google Scholar]
- 107.Hamel M.B., Lynn J., Teno J.M., et al. Age-related differences in care preferences, treatment decisions, and clinical outcomes of seriously ill hospitalized adults: lessons from SUPPORT. J Am Geriatr Soc. 2000;48:S176–S182. doi: 10.1111/j.1532-5415.2000.tb03129.x. [DOI] [PubMed] [Google Scholar]
- 108.Tinetti M.E., Naik A.D., Dodson J.A. Moving from disease-centered to patient goals-directed care for patients with multiple chronic conditions: patient value-based care. JAMA Cardiol. 2016;1:9–10. doi: 10.1001/jamacardio.2015.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lyons K.S., Lee C.S. The theory of dyadic illness management. J Fam Nurs. 2018;24:8–28. doi: 10.1177/1074840717745669. [DOI] [PubMed] [Google Scholar]
- 110.Patient priorities care. https://patientprioritiescare.org/
- 111.Braun L.T., Grady K.L., Kutner J.S., et al. Palliative care and cardiovascular disease and stroke: a policy statement from the American Heart Association/American Stroke Association. Circulation. 2016;134:e198–e225. doi: 10.1161/CIR.0000000000000438. [DOI] [PubMed] [Google Scholar]
- 112.Tinetti M.E., Esterson J., Ferris R., Posner P., Blaum C.S. Patient priority-directed decision making and care for older adults with multiple chronic conditions. Clin Geriatr Med. 2016;32:261–275. doi: 10.1016/j.cger.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 113.Gorodeski E.Z., Goyal P., Hummel S.L., et al. Domain management approach to heart failure in the geriatric patient: present and future. J Am Coll Cardiol. 2018;71:1921–1936. doi: 10.1016/j.jacc.2018.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Institute for Healthcare Improvement Age friendly health systems. http://www.ihi.org/Engage/Initiatives/Age-Friendly-Health-Systems/Pages/default.aspx
- 115.Barnett M.L., Bitton A., Souza J., Landon B.E. Trends in outpatient care for medicare beneficiaries and implications for primary care, 2000 to 2019. Ann Intern Med. 2021;174:1658–1665. doi: 10.7326/M21-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Thillainadesan J., Aitken S.J., Monaro S.R., et al. Geriatric comanagement of older vascular surgery inpatients reduces hospital-acquired geriatric syndromes. J Am Med Dir Assoc. 2022;23:589–595.e6. doi: 10.1016/j.jamda.2021.09.037. [DOI] [PubMed] [Google Scholar]
- 117.Van Grootven B., Jeuris A., Jonckers M., et al. Geriatric co-management for cardiology patients in the hospital: a quasi-experimental study. J Am Geriatr Soc. 2021;69:1377–1387. doi: 10.1111/jgs.17093. [DOI] [PubMed] [Google Scholar]
- 118.Bernstein J.M., Graven P., Drago K., Dobbertin K., Eckstrom E. Higher quality, lower cost with an innovative geriatrics consultation service. J Am Geriatr Soc. 2018;66:1790–1795. doi: 10.1111/jgs.15473. [DOI] [PubMed] [Google Scholar]
- 119.Goyal P., Gorodeski E.Z., Flint K.M., et al. Perspectives on implementing a multidomain approach to caring for older adults with heart failure. J Am Geriatr Soc. 2019;67:2593–2599. doi: 10.1111/jgs.16183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mohile S.G., Mohamed M.R., Xu H., et al. Evaluation of geriatric assessment and management on the toxic effects of cancer treatment (GAP70+): a cluster-randomised study. Lancet. 2021;398:1894–1904. doi: 10.1016/S0140-6736(21)01789-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Goyal P., Maurer M.S. A proposal to accelerate widespread implementation of geriatric cardiology. Trends Cardiovasc Med. Published online December 3, 2021 doi: 10.1016/j.tcm.2021.11.008. [DOI] [PubMed] [Google Scholar]
- 122.Wildiers H., Heeren P., Puts M., et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32:2595–2603. doi: 10.1200/JCO.2013.54.8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hamaker M.E., Te Molder M., Thielen N., van Munster B.C., Schiphorst A.H., van Huis L.H. The effect of a geriatric evaluation on treatment decisions and outcome for older cancer patients - a systematic review. J Geriatr Oncol. 2018;9:430–440. doi: 10.1016/j.jgo.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 124.Solomon J., Moss E., Morin J.F., et al. The essential frailty toolset in older adults undergoing coronary artery bypass surgery. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.020219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Viswanathan A.V., Dodson J.A., Blachman N.L. GeriKit: a novel app for comprehensive geriatric assessment. Gerontol Geriatr Educ. 2022:1–8. doi: 10.1080/02701960.2022.2048298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.National Institutes of Health The National Institutes of Health inclusion across the lifespan policy. https://grants.nih.gov/grants/guide/notice-files/NOT-OD-18-116.html
- 127.Xu M., Pirtskhalava T., Farr J.N., et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24:1246–1256. doi: 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kulkarni A.S., Gubbi S., Barzilai N. Benefits of metformin in attenuating the hallmarks of aging. Cell Metab. 2020;32:15–30. doi: 10.1016/j.cmet.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Halperin J.L., Williams E.S., Fuster V. COCATS 4 introduction. J Am Coll Cardiol. 2015;65:1724–1733. doi: 10.1016/j.jacc.2015.03.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.