Abstract
Aims
Same-day discharge (SDD) is feasible after pulmonary vein isolation (PVI). We aim to compare prospectively cryoballoon (CRYO) vs. radiofrequency (RF) ablation in a systematic SDD programme.
Methods and results
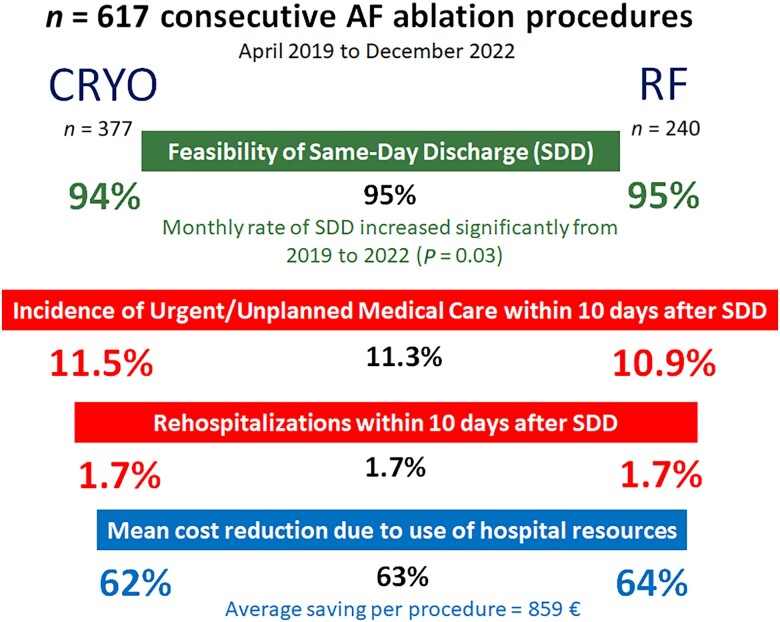
We prospectively analysed the 617 scheduled PVI performed consecutively at our institution (n = 377 CRYO, n = 240 RF) from 1 April 2019 to 31 December 2022 within a systematic programme of SDD. The feasibility of SDD, the 10-day incidence of urgent/unplanned medical care after discharge (UUC-10), and the cost per procedure due to hospital resource use were studied. The 100 procedures performed during the previous year, in which patients were systematically hospitalized, were used as a control group. Same-day discharge was achieved in 585/617 (95%) procedures, with a significant trend towards a higher monthly SDD rate from 2019 to 2022 (P = 0.03). The frequency of SDD was similar in CRYO (356/377; 94%) vs. RF (229/240; 95%). After SDD, the UUC-10 was 66/585 (11.3%), being similar for CRYO (41/356; 11.5%) and RF (25/229; 10.9%); P = 0.8 (log-rank test). Of these, 10 patients were re-hospitalized, with an identical rate in CRYO-treated (6/356; 1.7%) and RF-treated (4/229; 1.7%) patients and owing to similar causes (4 haematomas, 4 pericarditis, and 2 symptomatic sinus node dysfunction). Same-day discharge was associated with an average savings per procedure of 63% (P < 0.001), but no differences were found between the CRYO and RF (P = 0.8).
Conclusion
In a systematic SDD programme, feasibility (95%, increasing over time), safety (11% UUC-10, 1.7% re-hospitalizations), and savings (63% per procedure) were similar for CRYO and RF ablation procedures.
Keywords: Atrial fibrillation, Catheter ablation, Outcome, Cost, Same-day discharge
Graphical Abstract
Graphical Abstract.
Clinical and economic outcomes of a systematic same-day discharge strategy after atrial fibrillation ablation.
What’s new?
A systematic same-day discharge (SDD) programme after pulmonary vein isolation, including cryoballoon (CRYO) and radiofrequency (RF) in a similar ratio, allows discharge on the day of the procedure in 95% of patients (with no difference in CRYO vs. RF), a percentage that increases significantly over time.
After SDD, the cumulative incidences of urgent medical care and re-hospitalization at 10 days were 11 and 1.7%, respectively. The incidence, causes, and timing of unplanned medical care were similar for both CRYO and RF ablation procedures.
Only 1.5% of patients were admitted the day after discharge, and none were re-hospitalized.
Same-day discharge results in a 63% reduction in costs due to hospital resource use, the amount being similar for both CRYO and RF ablation procedures.
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia, affecting at least 4% of individuals over the age of 40 years.1 In addition, the incidence of AF is increasing due to the progressive ageing of the population and the high prevalence of risk factors such as hypertension and obesity.2
Pulmonary vein isolation (PVI) by catheter ablation is extremely effective in symptom control.3 This procedure also improves the prognosis of selected patients with heart failure and impaired systolic function.4 In both clinical settings, PVI by catheter ablation is superior to the use of antiarrhythmic drugs for maintaining sinus rhythm and for reducing the burden of AF.4,5
The excellent efficacy and safety profile of this technique, along with a progressive rise in the number of referred patients, have led to PVI becoming the most frequently performed procedure in developed countries, where it accounts for one of every three catheter ablation procedures.6 Consequently, the use of this procedure and its increasing frequency imply the need for strategies to optimize resources, minimizing costs and hospitalization.
Several research groups have reported their results on outpatient AF ablation, with the rate of feasibility ranging from 60 to 90%.7–11 However, there are little prospective data comparing the two mostly used PVI strategies [cryoballoon (CRYO) and radiofrequency (RF) catheter ablation] on cost analyses. Here, we present our findings on the feasibility, safety, and cost of hospital resource use when using CRYO vs. RF ablation in a systematic programme of same-day discharge (SDD) after PVI.
Methods
Study population
From April 2018, we prospectively collected all scheduled PVI procedures carried out at our Institution. A systematic SDD programme for all scheduled AF catheter ablation procedures (SDD being the default strategy for all patients) was established in our service on 1 April 2019. The outcomes of 617 consecutive catheter ablation procedures for AF performed until 31 December 2022, of which 377 corresponded to CRYO ablation and 240 to RF ablation, were prospectively analysed. Table 1 summarizes the demographic and epidemiological characteristics of the study population which were similar; however, in the RF group, there were more cases of redo and concomitant cavotricuspid isthmus ablation, and in the CRYO group, the procedure was shorter. As controls for the analysis of safety and costs, we examined the 100 procedures performed from April 2018 to March 2019 according to the conventional strategy that included 1 day of hospitalization after the intervention.
Table 1.
Characteristics of the study population and PVI procedure
| CRYO n = 377 |
RF n = 240 |
Statistical analysis | |
|---|---|---|---|
| Age, years | 60 ± 10 | 61 ± 9 | 95% CI of the difference: (−7; 2); P = 0.3 |
| Female gender | 116 (31%) | 62 (26%) | OR = 1.2 (95% CI: 0.2; 1.6); P = 0.2 |
| AF type | |||
| Paroxysmal | 199 (53%) | 117 (49%) | OR = 1.1 (95% CI: 0.3; 2.2); P = 0.6 |
| Persistent | 178 (47%) | 63 (51%) | |
| CHA2DS2-Vasc | 1.7 ± 1.4 | 1.7 ± 1.2 | 95% CI of the difference: (−0.2; 0.2); P = 0.8 |
| Previous oral anticoagulation | 309 (82%) | 204 (85%) | OR = 0.9 (95% CI: 0.4; 3); P = 0.9 |
| Hypertension | 140 (37%) | 96 (40%) | OR = 0.9 (95% CI: 0.8; 2.4); P = 0.5 |
| Diabetes | 75 (20%) | 40 (17%) | OR = 1.2 (95% CI: 0.8; 1.8); P = 0.3 |
| Obesity (body mass index >30) | 109 (29%) | 74 (31%) | OR = 0.9 (95% CI: 0.3; 4); P = 0.7 |
| Previous heart failure | 83 (22%) | 48 (20%) | OR = 0.5 (95% CI: 0.5; 1.5); P = 0.6 |
| Left ventricular ejection fraction, % | 56 ± 11 | 57 ± 9 | 95% CI of the difference: (−4; 3); P = 0.1 |
| Left ventricular ejection fraction ≤45% | 69 (18%) | 41 (17%) | OR = 0.7 (95% CI: 0.4; 3); P = 0.7 |
| ICD, CRT-P, or CRT-D carrier | 42 (10%) | 25 (11%) | OR = 0.9 (95% CI: 0.5; 5); P = 0.8 |
| Left atrial size, mm2 | 27 ± 10 | 32 ± 9 | 95% CI of the difference: (−1.5; 3.2); P = 0.7 |
| Glomerular filtration rate, mL/min | 73 ± 15 | 71 ± 13 | 95% CI of the difference: (−4; 4); P = 0.7 |
| Previous AF ablation | 19 (5%) | 65 (27%) | OR = 0.5 (95% CI: 0.1; 0.7); P = 0.03 |
| Concomitant cavotricuspid isthmus ablation | 7 (2%) | 36 (15%) | OR = 0.6 (95% CI: 0.2–0.8); P = 0.02 |
| Ablation of substrates beyond pulmonary veins | 7 (2%) | 53 (22%) | OR = 0.5 (95% CI = 0.2–0.7); P = 0.01 |
| Duration of procedure, min | 91 ± 18 | 112 ± 38 | 95% CI of the difference: (6; 27); P = 0.04 |
| Isolation of all pulmonary veins | 98% | 98% | OR = 1.1 (95% CI: 0.8; 1.9); P = 0.9 |
| Immediate procedure-related complications | 10 (2.6%) | 5 (2.1%) | OR = 1.2 (95% CI: 0.4; 3.8); P = 0.6 |
| Mild groin haematoma | 4 (1.1%) | 2 (0.85%) | |
| Femoral pseudo-aneurysm | 1 (0.3%) | — | |
| Transient ST elevation | 4 (1.1%) | 2 (0.8%) | |
| Transient ischaemic attack | — | 1 (0.4%) | |
| Cardiac tamponade | 1 (0.3%) | — | |
| Procedure finish time: | |||
| <12:00 h | 101 (27%) | 74 (31%) | OR = 1.1 (95% CI: 0.3–2.1); P = 0.8 |
| 12:00–13:00 h | 101 (27%) | 55 (23%) | |
| 13:00–14:00 h | 83 (21%) | 53 (22%) | |
| 14:00–15:00 h | 84 (22%) | 51 (21%) | |
| >15:00 h | 8 (2%) | 7 (3%) | |
| Hospitalization per procedure | 0 (0–0) | 0 (0–0) | Non-parametric P = 1 |
| Distance from patient’s home to hospital >100 km | 147 (39%) | 96 (40%) | OR = 1 (95% CI: 0.7; 2.7); P = 0.6 |
AF, atrial fibrillation; CI, confidence interval; CRT-D, cardiac resynchronization defibrillator; CRYO, cryoballoon ablation; CRT-P, cardiac resynchronization pacemaker; ICD, implantable cardioverter-defibrillator; OR, odds ratio; PVI, pulmonary vein isolation; RF, point-to-point radiofrequency ablation.
Indication for PVI was carried out according to the current clinical practice guidelines set by the European Society of Cardiology.2,12 The protocol was approved by the Research Ethics Committee of our hospital (reference number: FI-19-12-32), and all patients were informed about the procedure and provided written consent to participate in the study.
Flow of patients in a systematic same-day discharge ablation strategy
Our protocol for outpatient AF ablation has been previously described.7 In brief, patients underwent a left atrial angio-computed tomography (CT) before their first PVI to rule out the presence of a thrombus in the left atrium and to determine the anatomy of the left atrium and pulmonary veins. Before redo PVI, the presence of thrombus in the left atrial appendage was excluded by transoesophageal echocardiography.
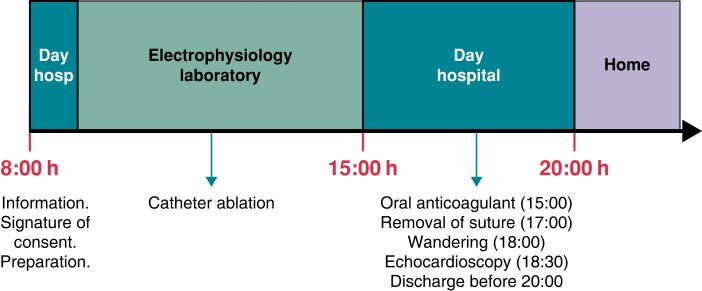
Patients discontinued taking antiarrhythmic drugs (except amiodarone) at least five half-lives before the day of the procedure. On the day of the intervention, only the morning dose of oral anticoagulant was not administered, and the patients arrived at the day hospital (i.e. a short-stay cardiology unit) between 08:00 and 8:30 h. Upon arrival, they received information, signed the consent form, and were prepared for treatment. The procedure started between 09:00 and 12:00 h, depending on when the CT scan was performed (either the day before or that morning) and when the electrophysiology study was scheduled. The intervention was usually completed before 15:00 h (Table 1). Patients were then returned to the day hospital for clinical observation. Administration of the oral anticoagulant was resumed between 15:00 and15:30 h, always using a direct-acting anticoagulant that was to be taken for at least 3 months. Likewise, in the afternoon, the antiarrhythmic drugs taken by the patient were re-introduced. Once the anticoagulant had been administered and after a minimum of 3 h of bed rest starting from the end of the procedure, the ‘figure-of-eight’ suture was removed. In addition, the patient is lifted out of bed to start walking, and after a clinical check-up, including echocardioscopy with Vscan ExtendTM (GE HealthCare Technologies, Chicago, IL, USA) pocket ultrasound, the patient was discharged before 20:00 h. The decision to discharge the patient was made by the physician in charge at the time. According to the established protocol, patients with complications detected intra- or post-procedurally were hospitalized. Patients were contacted by phone within 48 h and then 10 days after discharge to determine their clinical status and to provide any recommendations, if necessary (Figure 1).
Figure 1.
Flow of patients undergoing a scheduled pulmonary vein catheter ablation procedure. According to the protocol, all patients were referred to a same-day discharge strategy as ‘intention to treat’.
Description of the ablation procedure
The procedure was initiated via conscious sedation using dexmedetomidine in continuous perfusion (20–40 μg/h),13 midazolam (3 mg just before starting the femoral punctures), and fentanyl (75 μg boluses) as a rescue drug in case the patient perceived pain during the procedure. The management of sedoanalgesia was performed by the operating team, without the presence of an anaesthesiologist. Venous punctures were performed under vascular echo guidance. In the case of CRYO ablation, three punctures were performed in the right femoral vein for the diagnostic catheters placed in the paraseptal region with His electrogram recording (6 F introducer) and in the coronary sinus (5 F) and for the cryoablation system (12 F). For the RF ablation procedure, the same number of punctures were made for placing the ablation catheter (8F), the circular multi-polar pulmonary vein catheter (8F), and a tetrapolar coronary sinus catheter (5F). Trans-septal access was performed using electrical and anatomical references together with ∼5–10 cc of iodinated contrast (iodixanol, Visipaque®). Unfractionated sodium heparin was administered to reach an activated clotting time of >300 s. The CRYO ablation procedure was performed using the Arctic Front AdvanceTM system (Medtronic) until May 2019. After this date, the Arctic Front Advance ProTM (Medtronic) was employed using a 28 mm balloon in 98% of the cases. The RF ablation procedure employed the Ensite PrecisionTM (Abbott) non-fluoroscopic navigation system together with a 10-pole circular catheter for pulmonary vein mapping and an irrigated ablation catheter (FlexAbilityTM, Abbott, until 2019, and TactiCath Sensor Enable, Abbott, from 2020). In both cases, CRYO and RF, the procedure was performed according to the previously established strategies, to isolate all pulmonary veins exhibiting a bidirectional and maintained block (at least 30 min after the last application).2 At the physician’s discretion, in cases of repeated RF ablation, substrates other than the pulmonary veins were addressed. In addition, RF ablation of the cavotricuspid isthmus was performed in patients with previously documented common atrial flutter (Table 1). Haemostasis at the venous access site was performed according to the ‘figure-of-eight’ suture technique.14
Objectives
The primary objective of assessing feasibility was to determine the percentage of patients discharged on the same day as the procedure and with a length of hospital stay of <12 h. The primary objective regarding safety was to describe the cumulative incidence of urgent or unplanned medical care presumably related to the procedure (UUC-10) within 10 days after discharge. As a secondary objective, the analysis of the cost per procedure due to hospital resource use was carried out.
Economic analysis
The difference in the direct cost of hospital resource use was calculated as the mean difference in cost per procedure for stays at the day hospital, UUC-10, and hospitalization, including hospital resource use related to the index procedure and re-hospitalizations occurring within 10 days after discharge. Hospitalization was defined as an overnight stay plus one main meal (lunch or dinner).15 The cost was based on the most recent fees charged by the Spanish National Health System.16 This value was compared with that of the 100 procedures performed in the previous year (from April 2018 to March 2019) according to the conventional strategy including 1 day of hospitalization after the intervention. As previously reported, the average cost in this period was 1372€ [95% confidence interval (CI): 1303–1440] per procedure.7
Statistical analyses
The analysis was conducted using IBM SPSS (version 25.0) for Windows (SPSS Inc., Chicago, IL, USA). The continuous variables with normal distribution are described by the mean and standard deviation, while categorical variables are expressed as absolute numbers and percentages. The continuous variables without normal distribution (as determined by the Kolmogorov–Smirnov test) are described by the median and inter-quartile range (IQR). The comparison of categorical variables was performed by the χ2 test (or Fisher's exact test if n < 5). The comparison of two normally distributed continuous variables was carried out using Student’s t-test. The comparison of two non-normally distributed continuous variables was performed using the Mann–Whitney U test. The log-rank method was used to compare the cumulative incidence of UUC-10, expressed graphically using Kaplan–Meier curves. Multivariate analysis was performed using the logistic regression method. A value of P < 0.05 was considered statistically significant.
Results
Immediate results of the procedure
Among the 617 procedures, PVI of all pulmonary veins was achieved in 605 (98%) cases.
There were 15 (2.4%) immediate complications: 6 inguinal haematomas, 1 femoral artery pseudo-aneurysm, 6 cases of transient ST elevation with normal coronary arteries and spontaneous resolution, 1 cardiac tamponade, and 1 transient ischaemic attack. As shown in Table 1, the immediate results of the procedure, in terms of efficacy and safety, were similar in both energies.
Same-day discharge feasibility
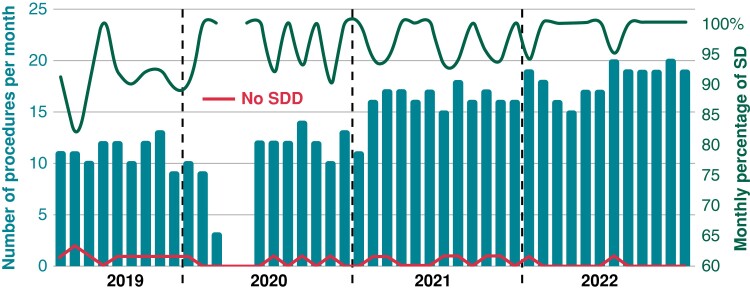
In 585 of 617 procedures (95%), the patient was discharged within 12 h after hospital arrival (range for length of hospital stay: 7–10 h). As shown in Figure 2, there was a significant trend towards a higher monthly SDD rate from 2019 to 2022 (91 vs. 95 vs. 97 vs. 99%, respectively; P = 0.03).
Figure 2.
Graph showing the time course of early discharges after the PVI procedure. The blue columns refer to the number of PVI procedures per month, the red line indicates the number of patients who were hospitalized after the procedure, and the green line represents the monthly percentage of SDD. PVI, pulmonary vein isolation; SDD, same-day discharge.
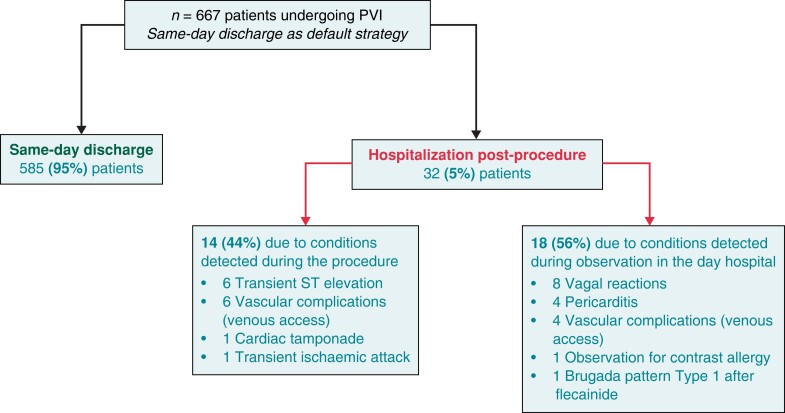
The frequency of SDD was similar for CRYO (356/377; 94%) and RF ablation procedures (229/240; 95%); odds ratio = 0.8 (95% CI: 0.4–1.7); P = 0.6. A total of 32 (5%) individuals were hospitalized after the procedure. Causes for hospitalization are displayed in Table 2. Figure 3 represents the timing of the diagnosis of the condition that prevented SDD. No differences were found between the values obtained for both the CRYO and RF groups. In the multivariate analysis (logistic regression) of predictors of SDD, no variable reached statistical significance (Table 3).
Table 2.
Causes of hospitalization after AF ablation procedure
| CRYO* n = 21/377 (5.5%) |
RF* n = 11/240 (4.6%) |
|
|---|---|---|
| Inguinal haematoma | 6 (1.6%) | 3 (1.25%) |
| Vagal reaction | 6 (1.6%) | 2 (0.85%) |
| Pericarditis | 1 (0.3%) | 3 (1.25%) |
| Transient ST elevation | 4 (1.1%) | 2 (0.85%) |
| Transient ischaemic attack | 0 | 1 (0.4%) |
| Pseudo-aneurysm of femoral artery | 1 (0.3%) | 0 |
| Observation for contrast allergy | 1 (0.3%) | 0 |
| Cardiac tamponade | 1 (0.3%) | 0 |
| Brugada pattern Type 1 after flecainide administration | 1 (0.3%) | 0 |
AF, atrial fibrillation; CRYO, cryoballoon ablation; RF, radiofrequency catheter ablation.
P > 0.1 for all comparisons (χ2 test).
Figure 3.
Flow chart showing the timing of detection of conditions that prevented same-day discharge following the ablation procedure. PVI, pulmonary vein isolation.
Table 3.
Multivariate analysis of the predictors of SDD
| Odds ratio | 95% confidence interval | P-value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Age, years | 1.05 | 0.95 | 1.15 | 0.3 |
| Female gender | 2.7 | 0.2 | 27 | 0.4 |
| Heart failure | 2.1 | 0.5 | 22 | 0.5 |
| Ischaemic heart disease | 2 | 0.4 | 3.7 | 1 |
| Previous oral anticoagulation | 1.7 | 0.3 | 2.6 | 0.5 |
| Left atrial size, mm2 | 1.1 | 0.9 | 1.2 | 0.8 |
| Left ventricular ejection fraction <45% | 1.09 | 0.2 | 4.5 | 0.6 |
| Paroxysmal AF | 1.2 | 0.2 | 6.1 | 0.8 |
| Cryoballoon ablation | 0.7 | 0.1 | 4.4 | 0.7 |
| Body mass index >30 | 0.5 | 0.1 | 2.7 | 0.4 |
| Procedure finish time >13:00 h | 0.7 | 0.4 | 2.7 | 0.7 |
| Distance from patient’s home to hospital >100 km | 0.8 | 0.2 | 4.2 | 0.5 |
AF, atrial fibrillation; SDD, same-day discharge.
Among the patients hospitalized, the mean of hospitalization was 1 (IQR = 1–2), with no differences in CRYO [1 (IQR = 1–2)] vs. RF [1 (IQR = 1–2)]; non-parametric P = 0.8. Most patients (60%) were discharged the following day. The longest period of hospitalization (7 days) corresponded to the patient with a femoral artery pseudo-aneurysm.
Primary safety endpoint
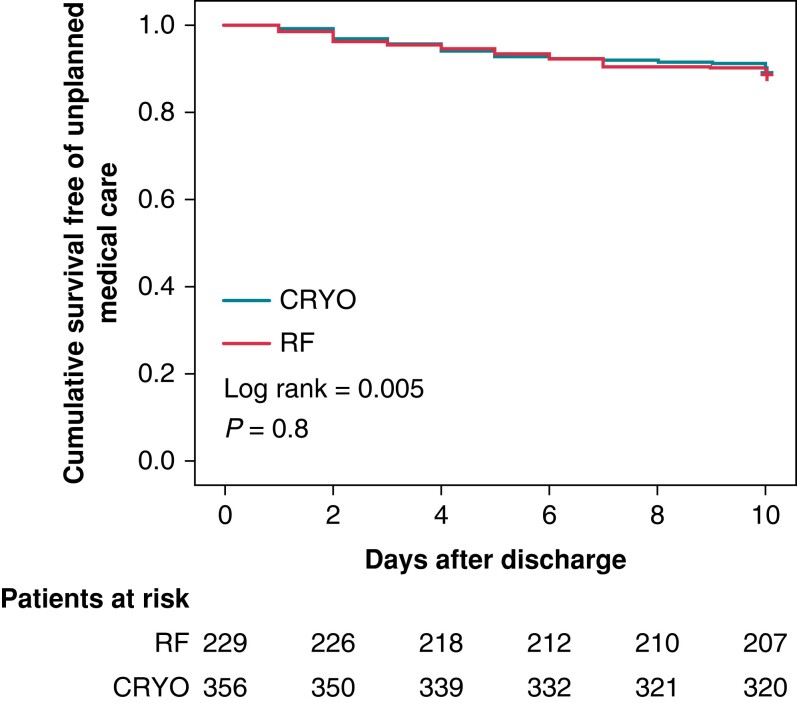
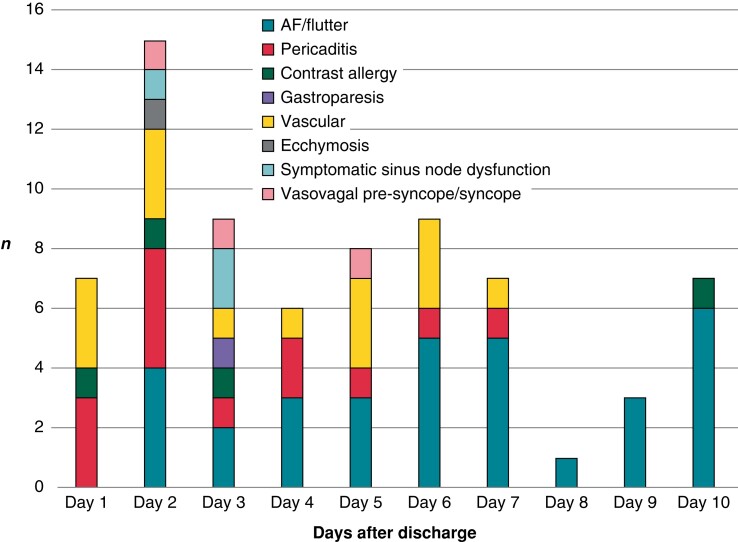
The 10-day cumulative incidence of UUC-10 was 69/617 (11.2%), being similar for patients who were hospitalized (3/32; 9.4%) vs. those who were discharged on the same day of the intervention (66/585; 11.3%); P = 0.7 (log-rank test). Among the 585 patients with SDD, the cumulative incidence of UUC-10 was similar for the CRYO (41/356; 11.5%) and RF (25/229; 10.9%) groups (Figure 4). The causes of UUC-10, which did not differ between CRYO and RF, are detailed in Table 4. Figure 5 shows the temporal distribution of causes of UUC-10. While recurrence of atrial arrhythmias occurred within 10 days following discharge, vascular complications and pericarditis were concentrated in the first 7 days.
Figure 4.
Kaplan–Meier plot depicting the timing of unplanned medical assistance after outpatient AF ablation, according to the energy used. CRYO, cryoballoon ablation; RF, point-to-point radiofrequency catheter ablation.
Table 4.
Causes of urgent/unplanned medical care within 10 days after discharge
| CRYO n = 42 |
RF n = 27 |
|
|---|---|---|
| AF/flutter recurrence | 16 | 14 |
| Vascular complications | 10 | 5 |
| Inguinal haematoma | 7 | 4 |
| Severe inguinal haematoma | 3a | 1a |
| Urticaria | 3 | 1 |
| Pericarditis | 8a | 5a |
| Gastroparesis | 1 | 0 |
| Symptomatic sinus node dysfunction | 1a | 1a |
| Vasovagal syncope/pre-syncope | 2 | 1 |
| Ecchymosis | 1 | 0 |
AF, atrial fibrillation; CRYO, cryoballoon ablation; RF, point-to-point radiofrequency catheter ablation.
Patients who were hospitalized due to severe haematoma (3 after CRYO and 1 after RF), symptomatic sinus dysfunction (CRYO and RF), and pericarditis (2 after CRYO and 2 after RF).
Figure 5.
Causes and timing of urgent or unplanned medical care within 10 days of discharge.
Most of the patients who received UUC-10 were discharged from the medical centre within <8 h. Only 10/69 (14%) patients were hospitalized due to the following causes: symptomatic sinus dysfunction (n = 2), pericarditis (n = 4), and vascular complications (n = 4) (Table 4). The frequency of re-hospitalization after outpatient ablation was identical for both the CRYO (6/356; 1.7%) and RF (4/229; 1.7%) groups. The mean length of re-admission was also identical, corresponding to 1 day of hospitalization (IQR = 1–1).
Finally, among the patients undergoing outpatient ablation, only seven (1.2%) received UUC-10 the day after the procedure, and none of these patients were hospitalized.
Economic analysis
Compared with the 100 procedures performed in the previous year, the SDD programme was associated with an average reduction of 1 day of hospitalization per procedure [1 (1–1) vs. 0 (0–0); non-parametric P < 0.001], with no increase in either UUC-10 or re-hospitalizations. The average cost per procedure in hospital resource use (not including personnel, ablation equipment, or drugs) in the SDD period was 513€ (95% CI: 466–560). This value was similar for both the CRYO [523€ (457–589)] and RF groups [497€ (433–560)]; P = 0.6. Therefore, the SDD programme was associated with a relative saving of 63% in hospital resource use, corresponding to 859€ (95% CI: 817–923), P < 0.001, in absolute terms (Table 5).
Table 5.
Description of the use of hospital resources and associated costsa
| Controls | SDD-CRYO | SDD-RF | Statistical analysis | |
|---|---|---|---|---|
| Overall time in hospital in relation to the index procedure, hoursb | 28 (24–32) | 7 (7–7) | 7 (7–7) | Non-parametric P < 0.001 (SDD vs. controls) |
| Stays in the day hospital in relation to the index procedureb | 1 (1–1) | 1 (1–1) | 1 (1–1) | Non-parametric P = 1 (SDD vs. controls) |
| Hospital stays in relation to the index procedureb | 1 (1–1) | 0 (0–0) | 0 (0–0) | Non-parametric P = 1 (SDD vs. controls) |
| UUC-10 | 12% | 11.5% | 10.9% | P = 0.9 (SDD vs. controls) |
| Re-hospitalizations after discharge | 0% | 1.7% | 1.7% | P = 0.8 (SDD vs. controls) |
| Hospital stays due to re-hospitalizations after UCC-10b | 0 (0–0) | 0 (0–0) | 0 (0–0) | Non-parametric P = 1 |
| Cost due to use of hospital resourcesc | 1372€ (1303–1440) | 523€ (457–589) |
497€ (433–560) |
P < 0.001 (SDD vs. controls) |
CRYO, cryoballoon ablation; RF, point-to-point radiofrequency ablation; SDD, same-day discharge; UUC-10 urgent or unplanned medical care presumably related to the procedure within 10 days after discharge.
Fees applied (reference 16): day hospital stay: 306€. Hospital stay: 986€. Hospital emergency room stay without subsequent hospitalization: 174€.
Data expressed as median (inter-quartile range).
Data expressed as mean (95% confidence interval).
Discussion
We present the broadened results of the first systematic outpatient AF ablation programme, performed in the Spanish Public Health System.7 According to our findings, the SDD strategy allows the vast majority of patients to be discharged within <12 h of hospitalization, without increasing the incidence of unplanned or urgent medical attention 10 days after discharge. Both the CRYO and RF ablation procedures present virtually identical feasibility and safety profiles. Finally, the reduction in hospitalization stays results in significant economic savings.
Same-day discharge programme after atrial fibrillation ablation
In experienced centres, AF ablation is a safe technique, with a complication rate of around 5%, of which <1% of cases are severe.17 The keys to adequate results in terms of efficacy and safety are patient selection, protocolization of the procedure, and the level of experience at the centre and the physicians carrying out the procedure.18,19 The possibility of performing outpatient procedures requires continuous multidisciplinary care in which the central component is the day hospital that allows rapid and efficient preparation and continuous clinical observation (especially after sedation) once the procedure has been completed.20 Conscious sedoanalgesia, which generates patient comfort and procedural safety, avoiding general anaesthesia, facilitates early discharge even in procedures that take longer. This sedation strategy is not associated with a lower efficacy of the procedure, as shown by the meta-analysis done by Li et al.,21 which includes more than nine studies on patients undergoing AF ablation with RF. In addition, most of the acute complications that appear in AF ablation procedures are related to vascular access and haemostasis.17 In our experience, the ‘figure-of-eight’ suture technique14 is safe, allowing rapid and effective haemostasis despite a vigorous concomitant anticoagulation regimen.
In addition, several series have been published that show the feasibility of an outpatient approach to AF ablation. In selected patients, the frequency of daytime discharge ranges from 20 to 90%.8–10,22,23 However, much of the available scientific evidence is of low quality, based on retrospective studies and/or unsystematic SDD programmes.24 The most recent study on a Canadian series of patients reports an SDD rate of 90%.11 According to our data, in a systematic SDD programme after AF ablation, which was implemented for >3 years, the feasibility of this strategy was slightly higher (95%) and similar to that found for the CRYO and RF groups. Moreover, there are two relevant aspects worth noting. First, this approach is not suitable for a priori patient profile. This is relevant considering the characteristics of our non-selected population (30% obese, 40% living >100 km from the hospital). Secondly, the monthly percentage of SDD has increased significantly over time, which indicates that the experience and confidence of the team performing the procedure has allowed us to improve the efficacy of the strategy and to avoid unnecessary hospitalization.
Incidence, causes, and chronology of urgent medical care after same-day discharge
Our previously published experience based on a prospective comparison of two AF ablation strategies (with homogeneous protocols in which the only difference was hospitalization after the procedure) showed that SDD is safe because it does not increase the incidence of UUC-10.7 However, there are little data comparing CRYO vs. RF in this context. The results from the Canadian series, comprised mostly of patients treated with RF (80%) and some (n = 82) with CRYO, present a similar level of safety for both energies.11 In our work, which included a more equal ratio of patients receiving either CRYO or RF (3:2), a detailed analysis of the UUC-10 within 10 days following discharge was performed, showing a similar safety profile between the two energies. Interestingly, Deyell et al.11 reported that the incidence of urgent care at 1 month was significantly higher for patients who had been previously hospitalized. On the contrary, in our series, the UUC-10 was similar for patients hospitalized after the index procedure and for those who were discharged on the same day. It is likely that the different composition of the population and the duration of follow-up may account for this discrepancy.
Based on our data, the causes and chronology of UUC-10 after SDD are similar for the CRYO and RF patients. As expected, AF/flutter recurrence is the most frequent cause of the need for short-term medical care after AF ablation,11,25 with a homogeneous timing over the first 10 days after discharge. In contrast, vascular complications and pericarditis (both are the main causes of re-admission within 10 days after discharge) are concentrated in the first 7 days after the procedure. The incidence and duration of re-admissions at 10 days after SDD (1.7% and 1 hospital stay, respectively) were identical for CRYO and RF. Finally, only seven patients required medical attention the day after outpatient ablation, and none were readmitted. These are the only cases in which unplanned urgent care could have been avoided if the patient had been hospitalized for 1 day after the procedure.
Economic implications
There are little data on the cost-effectiveness of SDD after PVI. Retrospective analyses of hospitals with same-day discretionary discharge indicate that outpatient ablation is associated with significant cost savings.26,27 In our experience, the implementation of an outpatient AF ablation programme significantly reduces the need for hospitalization, even when compared with a previously optimized protocol where the conventional strategy only generated an average of 1 day of hospitalization per procedure.7 Since SDD does not increase short-term morbidity, this translates into a significant reduction of 63% in hospital resource costs, irrespective of the energy used in the index procedure. Furthermore, the outpatient nature of the procedures improves the efficiency of the system and the use of electrophysiology rooms.28
Limitations
The main limitation of our study is that the comparison between strategies was not randomized. Nevertheless, the exhaustive and prospective nature of recruitment allows us to provide an accurate perspective on clinical practice. In addition, our hospital is relatively high-volume institution, and most procedures are performed by highly experienced physicians. Therefore, our results may not be generalizable and extrapolated to low-volume settings.
Furthermore, the economic analysis did not include the costs derived from the consumables used during the procedure. However, since all procedures were performed according to the same protocol and did not differ in the clinical or anatomical substrate, no significant differences between the two strategies analysed should have been observed.
Conclusions
The majority of patients (95%) undergoing scheduled AF ablation can be discharged after <10 h of hospitalization. The frequency of SDD is similar for CRYO and RF ablation procedures and can be extrapolated to all patients. The SDD strategy is safe since the proportion of individuals requiring unplanned or urgent care in the 10 days following discharge is similar to those managed according to a conventional strategy. The outpatient ablation strategy is associated with an average reduction of 63% in the cost of hospital resource use.
Contributor Information
Javier Jimenez-Candil, Servicio de Cardiología, IBSAL-Hospital Universitario, Paseo de San Vicente, 58-182, 37007 Salamanca, Spain; CIBER-CV; Universidad de Salamanca, Salamanca, Spain.
Jesus Hernandez Hernandez, Servicio de Cardiología, IBSAL-Hospital Universitario, Paseo de San Vicente, 58-182, 37007 Salamanca, Spain.
Alba Cruz Galban, Servicio de Cardiología, IBSAL-Hospital Universitario, Paseo de San Vicente, 58-182, 37007 Salamanca, Spain.
Fabian Blanco, Servicio de Cardiología, IBSAL-Hospital Universitario, Paseo de San Vicente, 58-182, 37007 Salamanca, Spain.
Jose Luis Moriñigo, Servicio de Cardiología, IBSAL-Hospital Universitario, Paseo de San Vicente, 58-182, 37007 Salamanca, Spain; Universidad de Salamanca, Salamanca, Spain.
Manuel Sanchez García, Servicio de Cardiología, IBSAL-Hospital Universitario, Paseo de San Vicente, 58-182, 37007 Salamanca, Spain.
Armando Oterino, Servicio de Cardiología, IBSAL-Hospital Universitario, Paseo de San Vicente, 58-182, 37007 Salamanca, Spain.
Pedro L Sanchez, Servicio de Cardiología, IBSAL-Hospital Universitario, Paseo de San Vicente, 58-182, 37007 Salamanca, Spain; CIBER-CV; Universidad de Salamanca, Salamanca, Spain.
Funding
This work was supported in part by the Biomedical Research Institute of Salamanca (IBSAL)-University Hospital of Salamanca (A.C.G.).
Data availability
The data from this study are available from the corresponding author, upon appropriate and reasonable request.
References
- 1. Gomez-Doblas JJ, Muniz J, Martin JJ, Rodriguez-Roca G, Lobos JM, Awamleh Pet al. . Prevalence of atrial fibrillation in Spain. OFRECE study results. Rev Esp Cardiol (Engl Ed) 2014;67:259–69. [DOI] [PubMed] [Google Scholar]
- 2. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei Bet al. . 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC endorsed by the European Stroke Organisation (ESO). Europace 2016;18:1609–1678.27567465 [Google Scholar]
- 3. Packer DL, Mark DB, Lee KL. Catheter ablation compared with drug therapy for atrial fibrillation-reply. JAMA 2019;322:1106. [DOI] [PubMed] [Google Scholar]
- 4. Marrouche NF, Kheirkhahan M, Brachmann J. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018;378:417–27. [DOI] [PubMed] [Google Scholar]
- 5. Andrade JG, Wells GA, Deyell MW, Bennett M, Essebag V, Champagne Jet al. . Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med 2021;384:305–15. [DOI] [PubMed] [Google Scholar]
- 6. Cozar Leon R, Anguera Camos I, Cano Perez O; en representacion de los colaboradores del Registro Espanol de Ablacion con C . Spanish Catheter Ablation Registry. 20th official report of the Heart Rhythm Association of the Spanish Society of Cardiology (2020). Rev Esp Cardiol 2021;74:1073–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jimenez-Candil J, Perez J, Hernandez J, Morinigo JL, Sanchez Garcia M, Sanchez PL. Outpatient ablation for atrial fibrillation. Rev Esp Cardiol (Engl Ed) 2021;74:466–8. [DOI] [PubMed] [Google Scholar]
- 8. Bartoletti S, Mann M, Gupta A, Khan AM, Sahni A, El-Kadri Met al. . Same-day discharge in selected patients undergoing atrial fibrillation ablation. Pacing Clin Electrophysiol 2019;42:1448–55. [DOI] [PubMed] [Google Scholar]
- 9. Akula D, Wassef M, Luthra P, Friedrich E, Katz D, Levi Set al. . Safety of same day discharge after atrial fibrillation ablation. J Atr Fibrillation 2020;12:2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Deyell MW, Leather RA, Macle L, Forman J, Khairy P, Zhang Ret al. . Efficacy and safety of same-day discharge for atrial fibrillation ablation. JACC Clin Electrophysiol 2020;6:609–19. [DOI] [PubMed] [Google Scholar]
- 11. Deyell MW, Hoskin K, Forman J, Laksman ZW, Hawkins NM, Bennett MTet al. . Same-day discharge for atrial fibrillation ablation: outcomes and impact of ablation modality. Europace 2023;25:400–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist Cet al. . 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 13. Ichihara N, Miyazaki S, Taniguchi H, Usui E, Takagi T, Iwasawa Jet al. . Simple minimal sedation for catheter ablation of atrial fibrillation. Circ J 2015;79:346–50. [DOI] [PubMed] [Google Scholar]
- 14. Aytemir K, Canpolat U, Yorgun H, Evranos B, Kaya EB, Sahiner MLet al. . Usefulness of ‘figure-of-eight’ suture to achieve haemostasis after removal of 15-French calibre femoral venous sheath in patients undergoing cryoablation. Europace 2016;18:1545–50. [DOI] [PubMed] [Google Scholar]
- 15. GRS-SACYL . Precios públicos por actos asistenciales y servicios sanitarios prestados por la Gerencia Regional de Salud. BOCYL 2013:83725. [Google Scholar]
- 16. Osakidetza . Libro de tarifas para facturación de servicios sanitarios y docentes de Osakidetza para el año 2020. https://nam12.safelinks.protection.outlook.com/?url=https%3A%2F%2Fwww.osakidetza.euskadi.eus%2Fcontenidos%2Finformacion%2Fosk_servic_para_empresas%2Fes_def%2Fadjuntos%2FLIBRO-DE-TARIFAS_2020_osakidetza.pdf&data=05%7C01%7Ceuropace.oup%40novatechset.com%7C46a01f026c4848ec565e08dbb1e7a68e%7Ca03a7f6cfbc84b5fb16bf634dbe1a862%7C0%7C0%7C638299381318979921%7CUnknown%7CTWFpbGZsb3d8eyJWIjoiMC4wLjAwMDAiLCJQIjoiV2luMzIiLCJBTiI6Ik1haWwiLCJXVCI6Mn0%3D%7C3000%7C%7C%7C&sdata=9eeGQIq9jqyhl%2BOOcRyHo91s6iVKH8MyLDBlgORVJKo%3D&reserved=0
- 17. Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman Jet al. . Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010;3:32–8. [DOI] [PubMed] [Google Scholar]
- 18. Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SAet al. . 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace 2012;14:528–606. [DOI] [PubMed] [Google Scholar]
- 19. Kirchhof P, Breithardt G, Aliot E, Al Khatib S, Apostolakis S, Auricchio Aet al. . Personalized management of atrial fibrillation: proceedings from the fourth atrial fibrillation competence NETwork/European Heart Rhythm Association consensus conference. Europace 2013;15:1540–56. [DOI] [PubMed] [Google Scholar]
- 20. Gallego-Delgado M, Villacorta E, Valenzuela-Vicente MC, Walias-Sanchez A, Avila C, Velasco-Canedo MJet al. . Start-up of a cardiology day hospital: activity, quality care and cost-effectiveness analysis of the first year of operation. Rev Esp Cardiol (Engl Ed) 2019;72:130–7. [DOI] [PubMed] [Google Scholar]
- 21. Li KHC, Sang T, Chan C, Gong M, Liu Y, Jesuthasan Aet al. . Anaesthesia use in catheter ablation for atrial fibrillation: a systematic review and meta-analysis of observational studies. Heart Asia 2019;11:e011155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Opel A, Mansell J, Butler A, Schwartz R, Fannon M, Finlay Met al. . Comparison of a high throughput day case atrial fibrillation ablation service in a local hospital with standard regional tertiary cardiac centre care. Europace 2019;21:440–4. [DOI] [PubMed] [Google Scholar]
- 23. Sahashi Y, Kuno T, Tanaka Y, Passman R, Briasoulis A, Malik AH. The 30-day readmission rate of same-day discharge protocol following catheter ablation for atrial fibrillation: a propensity score-matched analysis from National Readmission Database. Europace 2022;24:755–61. [DOI] [PubMed] [Google Scholar]
- 24. Tang PT, Davies M, Bashir Y, Betts TR, Pedersen M, Rajappan Ket al. . Efficacy and safety of same-day discharge after atrial fibrillation ablation compared with post-procedural overnight stay: a systematic review and meta-analysis. Europace 2022;24:1569–84. [DOI] [PubMed] [Google Scholar]
- 25. Lopez F C, Ruiz G E, Verdu P P, Sanchez J M, Munoz JJ S, Esparza C Met al. . Emergency department attendance and reasons for consultation after cryoballoon ablation for pulmonary vein isolation of atrial fibrillation. Rev Esp Cardiol (Engl Ed) 2020;74:233–7. [DOI] [PubMed] [Google Scholar]
- 26. He H, Datla S, Weight N, Raza S, Lachlan T, Aldhoon Bet al. . Safety and cost-effectiveness of same-day complex left atrial ablation. Int J Cardiol 2021;322:170–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Creta A, Ventrella N, Providencia R, Earley MJ, Sporton S, Dhillon Get al. . Same-day discharge following catheter ablation of atrial fibrillation: a safe and cost-effective approach. J Cardiovasc Electrophysiol 2020;31:3097–103. [DOI] [PubMed] [Google Scholar]
- 28. Monnickendam G, De Asmundis C. Why the distribution matters: using discrete event simulation to demonstrate the impact of the distribution of procedure times on hospital operating room utilisation and average procedure cost. Oper Res Health Care 2018;16:20–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data from this study are available from the corresponding author, upon appropriate and reasonable request.