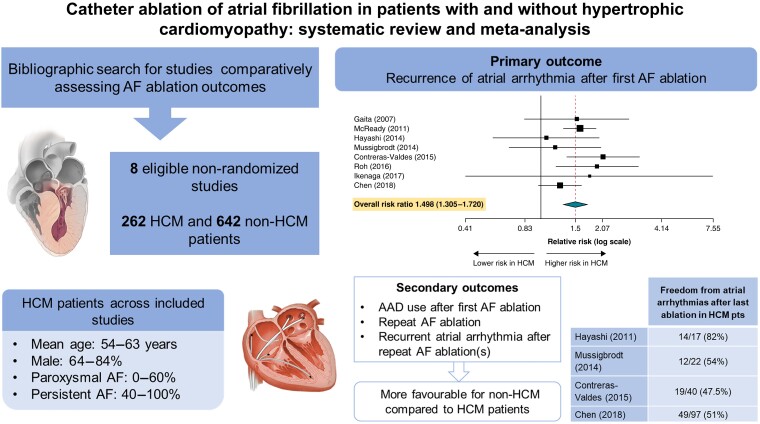
Abstract
Background
Atrial fibrillation (AF) is common in hypertrophic cardiomyopathy (HCM). There is limited data regarding the outcomes of AF catheter ablation in HCM patients. In this study, we aimed to synthesize all available evidence on the effectiveness of ablation of AF in patients with HCM compared to those without HCM.
Methods and results
We systematically reviewed bibliographic databases to identify studies published through February 2023. We included cohort studies with available quantitative information on rates of recurrent atrial arrhythmias, anti-arrhythmic drug (AAD) therapy, and repeat ablation procedures after initial AF ablation in patients with vs without HCM. Estimates were combined using random-effects meta-analysis models and reported as risk ratios (RR) and 95% confidence intervals (CI). Eight studies were included in quantitative synthesis (262 HCM and 642 non-HCM patients). During median follow-up 13–54 months across studies, AF recurrence rates ranged from 13.3% to 92.9% in HCM and 7.6% to 58.8% in non-HCM patients. The pooled RR for recurrent atrial arrhythmia after the first AF ablation in HCM patients compared to non-HCM controls was 1.498 (95% CI = 1.305–1.720; P < 0.001). During follow-up, HCM patients more often required AAD therapy (RR = 2.844; 95% CI = 1.713–4.856; P < 0.001) and repeat AF ablation (RR = 1.544; 95% CI = 1.070–2.228; P = 0.02). The pooled RR for recurrent atrial arrhythmias after the last AF ablation was higher in patients with HCM than those without HCM (RR = 1.607; 95% CI = 1.235–2.090; P < 0.001).
Conclusions
Compared to non-HCM patients, those with HCM had higher rates of recurrent atrial arrhythmias, AAD use, and need for repeat AF ablation after initial ablation of AF.
Keywords: Atrial fibrillation, Catheter ablation, Hypertrophic cardiomyopathy, Meta-analysis
Graphical Abstract
Graphical abstract.
What’s new?
This updated meta-analysis shows that rhythm control is feasible in patients with hypertrophic cardiomyopathy (HCM) and atrial fibrillation (AF).
Patients with HCM and AF appear to have higher rates of recurrent atrial arrhythmia after ablation, are more likely to need anti-arrhythmic drug (AAD) therapy, and more frequently require repeat ablations compared to patients without HCM.
Introduction
Atrial fibrillation (AF) is common in patients with hypertrophic cardiomyopathy (HCM), with a reported prevalence between 18% and 28%.1–3 Several factors contribute to left atrial (LA) dilation and remodelling in patients with HCM leading to an increased risk of AF, including elevated filling pressures due to diastolic dysfunction, atrial myopathy, left ventricular outflow tract obstruction, and mitral regurgitation.4–6 Due to underlying impaired left ventricular filling, AF is poorly tolerated by patients with HCM and is associated with an increased risk of heart failure–related morbidity and mortality, stroke, and functional disability.1
A prior single-arm study demonstrated increased AF recurrence rates in HCM patients undergoing pulmonary vein isolation (PVI), suggesting that more extensive ablation and elimination of possible non-pulmonary vein (PV) triggers may be required.7 There are additional uncertainties regarding the benefit of catheter ablation (CA) in patients with AF and HCM given the underlying myopathy and often extensive substrate abnormalities, which may affect the success of AF ablation in these patients, especially those with persistent AF. No randomized studies of AF ablation in HCM have been conducted. Therefore, we conducted a systematic review and meta-analysis of all observational cohort studies that assessed the long-term outcomes of AF ablation in patients with HCM compared to patients without HCM.
Methods
Data sources and searches
A comprehensive search of multiple databases was performed on 2 February 2023. No publication date or language restrictions were applied. Databases searched were Ovid MEDLINE(R), Ovid Embase, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, Web of Science Core Collection via Clarivate Analytics, and Scopus via Elsevier. Reference lists of eligible articles were also searched for further eligible studies. Controlled vocabulary supplemented with keywords was used to search for studies reporting on CA of AF in patients with HCM. The search strategy was designed by a librarian with input from the study investigators as detailed in Supplementary material online, Appendix S1. This systematic review and meta-analysis was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.8 The protocol of this study was registered at the International Prospective Register of Systematic Reviews (PROSPERO) database (CRD42023395391). This work was exempt from institutional review board approval due to its nature that involved the use of published data only.
Study selection and outcomes
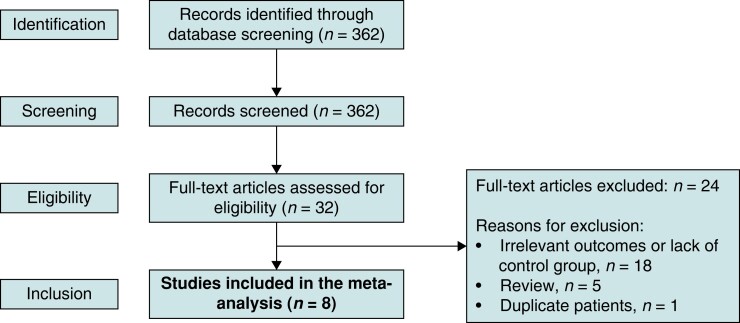
Two authors (F.M.E. and K.M.A.) independently assessed 362 potentially eligible articles identified by the search strategy above (Figure 1). Disagreements were resolved by consensus and further reviewed by a third author (K.C.S.). We included observational studies where AF ablation was performed in an HCM and a non-HCM control group with available quantitative relative risk information about post-ablation outcomes that could be included in the meta-analysis. Single-arm studies were not included.
Figure 1.
Flow diagram of study selection.
The prespecified primary outcome was recurrent atrial arrhythmias after the first AF ablation procedure, including AF, atrial flutter, or atrial tachycardia, according to the definitions of each included study. Secondary outcomes included the use of anti-arrhythmic drugs (AAD) during follow-up, the need for repeat AF ablation, and freedom from atrial arrhythmias after the last ablation (including repeat ablations if performed). Studies lacking information regarding follow-up duration and the number of subjects in each group were excluded.
Data extraction and study quality assessment
Data were extracted using a standardized tool, including study characteristics, patient demographics, and clinical outcomes. Any discrepancies were resolved by consensus. Since the studies identified and included in this meta-analysis were observational cohort studies, the Newcastle–Ottawa Scale for assessing the quality of nonrandomized studies in meta-analyses was used for quality assessment.9 Included studies were assessed independently by two authors (F.M.E. and K.M.A.) for risk of bias.
Data synthesis
For data synthesis, a random-effects model (DerSimonian and Laird) was used to calculate pooled risk ratios (RRs) and 95% confidence intervals (CIs) to account for different effect sizes and variations across studies for primary and secondary outcomes. Stepwise inclusion of one study at a time was used as a sensitivity analysis to determine the impact of individual studies on the primary outcome. We also performed a subgroup analysis assessing the association of paroxysmal vs non-paroxysmal AF with recurrent arrhythmias post-ablation within the HCM groups. Statistical significance was set at a two-tailed P-value of <0.05. A formal test of heterogeneity was performed using the I2 statistic. I2 values <25%, 25–50%, 50–75%, and >75% indicate absent, low, moderate, and high heterogeneity, respectively.10 The statistical analysis was conducted using the OpenMeta Analyst software.
Results
Characteristics of included studies
A total of eight studies met inclusion criteria.11–18 These studies included a total of 262 patients with HCM and 642 patients without HCM who underwent CA of AF. Of the eight studies included the control group was matched to the HCM group in 5 studies. Matching criteria are summarized in Table 1. Patients in the control group had no structural heart disease, except for dilated cardiomyopathy and valvular heart disease in the study by Gaita et al.11 and left ventricular hypertrophy secondary to hypertension in the study by Müssigbrodt et al.14 Most of the studies included in this meta-analysis had a low risk of bias (Table 2).
Table 1.
Baseline characteristics in the included studies
| Study | Country | Study design |
Matching parameters |
Study dates |
HCM group (n) |
Mean age in years (HCM) |
% Male (HCM) | AF type (HCM) |
Control group (n) |
Mean age in years (control) |
% Male (control) |
AF type (control) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gaita (2007)11 | Italy | Retrospective cohort | *Age *Sex *AF type |
2002–2005 | 26 | 58 ± 11 | 69% | Paroxysmal: 13 (50%) Persistent: 13 (50%) |
52 | 59 ± 11 (secondary) 58 ± 8 (idiopathic AF) |
67% | Paroxysmal: 26 (50%) Persistent: 26 (50%) |
| McCready (2011)12 | England | Retrospective cohort | 2003–2009 | 14 | Paroxysmal: 0 (0%) Persistent: 14 (100%) |
177 | Paroxysmal: 0 (0%) Persistent: 177 (100%) |
|||||
| Hayashi (2014)13 | Japan | Retrospective cohort | *Age *Sex *AF type *LA dimension |
2006–2012 | 17 | 63 ± 12 | 71% | Paroxysmal: 8 (47%) Persistent: 9 (53%) |
34 | 66 ± 9 | 71% | Paroxysmal: 16 (47%) Persistent: 18 (53%) |
| Müssigbrodt (2014)14 | Germany | Prospective cohort | *LV hypertrophy *Systolic LV function *AF type |
2009–2012 | 22 | 57 ± 8 | 68% | Paroxysmal: 10 (45.5%) Persistent: 12 (54.5%) |
22 | 63 ± 10 | 64% | Paroxysmal: 10 (45.5%) Persistent: 12 (54.5%) |
| Contreras-Valdes (2015)15 | United States | Retrospective cohort | 2006–2012 | 40 | 54 ± 7 | 70% | Paroxysmal: 13 (32.5%) Persistent: 27 (67.5%) |
64 | 56 ± 6 | 70% | Paroxysmal: 49 (29.7%) Persistent: 45 (70.3%) |
|
| Roh (2016)16 | Korea | Retrospective cohort | *Age *Sex *AF type |
2005–2014 | 31 | 57 ± 10 (apical HCM) 57 ± 8 (septal HCM) |
84% | Paroxysmal: 13 (41.9%) Persistent: 18 (58.1%) |
90 | 57 ± 10 | 89% | Paroxysmal: 35 (38.9%) Persistent: 55 (61.1%) |
| Ikenaga (2017)17 | Japan | Retrospective cohort | 2009–2013 | 15 | 62 ± 8 | 80% | Paroxysmal: 9 (60%) Persistent: 6 (40%) |
106 | 61 ± 9 | 77% | Paroxysmal: 68 (64.2%) Persistent: 38 (35.8%) |
|
| Chen (2018)18 | China | Prospective cohort | *Age *AF type *AF duration *LA diameter |
2007–2016 | 97 | 59 ± 11 (apical HCM) 56 ± 11 (non-apical HCM) |
71% | Paroxysmal: 56 (57.7%) Persistent: 41 (42.3%) |
97 | 57 ± 9 | 79% | Paroxysmal: 55 (56.7%) Persistent: 42 (43.3%) |
Empty cells indicate that the respective data were not reported in the specific study.
AF, atrial fibrillation; CTI, cavotricuspid isthmus; LA, left atrial; PV, pulmonary vein; PVI, pulmonary vein isolation; PWI, posterior wall isolation.
Table 2.
Risk bias assessment of the included studies using the Newcastle–Ottawa scale
| Study | Selection | Comparability | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Representation of exposed cohort | Selection of non-exposed cohort | Ascertainment of exposure | Change in outcome of interest | Comparability of cohorts | Assessment of outcome | Sufficient follow-up duration |
Adequate follow-up cohorts |
Risk of bias | |
| Gaita (2007)11 | * | * | * | * | * | * | * | * | Low |
| McCready (2011)12 | * | * | * | * | * | * | * | Moderate | |
| Hayashi (2014)13 | * | * | * | * | * | * | * | * | Low |
| Müssigbrodt (2014)14 | * | * | * | * | * | * | * | * | Low |
| Contreras-Valdes (2015)15 | * | * | * | * | * | * | Moderate | ||
| Roh (2016)16 | * | * | * | * | * | * | * | * | Low |
| Ikenaga (2017)17 | * | * | * | * | * | * | * | Moderate | |
| Chen (2018)18 | * | * | * | * | * | * | * | * | Low |
Asterisks indicate the star rating according to the Newcastle–Ottawa Scale. A maximum of one star can be given for each item within the Selection and Exposure categories. A maximum of two stars can be given for Comparability.
In patients with HCM, the mean age ranged from 54 to 63 years, and the percentage of male patients ranged between 68% and 84% across included studies. In patients without HCM, the mean age ranged from 56 to 66 years, and the percentage of male patients ranged between 64% and 89% across included studies. All studies included patients with paroxysmal and persistent AF except for the study by McCready et al.12, which only included patients with persistent AF. In the studies including patients with paroxysmal and persistent AF and unmatched controls,15,17 the proportions of patients with paroxysmal and persistent AF were similar between patients with and without HCM. In patients with HCM, the percentage of patients with paroxysmal AF (PAF) ranged between 32.5% and 60% across studies, and that of patients with persistent AF ranged between 40% and 100% across studies (Table 1).
Catheter ablation approach
In all studies, irrigated radiofrequency ablation was utilized. None of the studies utilized cryoballoon ablation. The specific ablation approach varied between the studies as shown in Table 3. Pulmonary vein isolation was performed in all of the studies with possible additional linear ablation in cases of persistent AF, except in the studies conducted by Gaita et al. and Hayashi et al.11,13 where a standardized ablation approach included PVI, LA roof line, and cavotricuspid isthmus ablation. Gaita et al. also added a linear lesion between the left inferior PV and mitral annulus.11 Hayashi et al. added posterior inferior linear lesions to isolate the posterior LA.13 Ablation of non-PV triggers was only performed in the study by Roh et al.16 In this study, triggers of sustained AF were evaluated and targeted after PVI. Atrial fibrillation was induced by burst pacing from the high right atrium with decremental pacing from 250 ms to the atrial refractory period. During the protocol, the mapping catheter, the ablation catheter, and a quadripolar catheter were positioned in the left and right pulmonary veins and superior vena cava, respectively. The beat initiating AF was considered a trigger and analysed for the site of origin. The same protocol was repeated at least three times.16
Table 3.
Procedural data
| A. HCM group | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Number of patients | Ablation Procedure | PVI | CTI | Linear-roof | Linear-peri mitral isthmus | PWI | Complications | Notes |
| Gaita (2007)11 | 26 | All patients: PVI + CTI + roof line + linear lesions (left inferior PV to mitral annulus) |
26 (100%) | 26 (100%) | 26 (100%) | 26 (100%) | 5 (19.2%) | Mitral isthmus block in 9 patients and conduction slowing in 17 patients Pericardial effusion in 5 patients |
|
| McCready (2011)12 | 14 | All patients: PVI ± linear ablation and complex fractioned atrial electrogram ablation (per operator discretion) |
14 (100%) | ||||||
| Hayashi (2014)13 | 17 | All patients: PVI + CTI + PWI Persistent AF: all the above + mitral isthmus lesions |
17 (100%) | 17 (100%) | 17 (100%) | 17 (100%) | 0 (0%) | Successful PWI in 15 patients | |
| Müssigbrodt (2014)14 | 22 | All patients: PVI ± linear lesions (per operator discretion) |
22 (100%) | 1 (5%) | Additional lesions at the discretion of the operator in 7 patients Pulmonary vein stenosis in 1 patient |
||||
| Contreras-Valdes (2015)15 | 40 | ||||||||
| Roh (2016)16 | 31 | All patients: PVI + non-PV triggers Persistent AF: complex fractionated atrial electrogram ablation + CTI |
31 (100)% | 18 (58.1%) | 6 (19.4%) | 11 (35.5%) | 1 (3.2%) | Tamponade in 1 patient | |
| Ikenaga (2017)17 | 15 | All patients: PVI + CTI | 15 (100%) | 15 (100%) | 0 (0%) | ||||
| Chen (2018)18 | 97 | All patients: PVI ± CTI (if typical flutter induced) Persistent AF: CTI + roof line + mitral isthmus |
97 (100%) | 40 (41.2%) | 40 (41.2%) | ||||
| B. Control group | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Number of patients | Ablation Procedure | PVI | CTI | Linear-roof | Linear-peri mitral isthmus | PWI | Complications | Notes |
| Gaita (2007)11 | 52 | All patients: PVI + CTI + roof line + linear lesions (left inferior PV to mitral annulus) |
52 (100%) | 52 (100%) | 52 (100%) | 52 (100%) | |||
| McCready (2011)12 | 177 | All patients: PVI ± linear ablation and complex fractioned atrial electrogram ablation (per operator discretion) |
177 (100%) | ||||||
| Hayashi (2014)13 | 34 | All patients: PVI + CTI + PWI Persistent AF: all the above + mitral isthmus lesions |
34 (100%) | 34 (100%) | 34 (100%) | 34 (100%) | 0 (0%) | Successful PWI in 28 patients | |
| Müssigbrodt (2014)14 | 22 | All patients: PVI ± linear lesions (per operator discretion) |
22 (100%) | 0 (0%) | Additional lesions at the discretion of the operator in 5 patients | ||||
| Contreras-Valdes (2015)15 | 64 | ||||||||
| Roh (2016)16 | 90 | All patients: PVI + non-PV triggers Persistent AF: complex fractionated atrial electrogram ablation + CTI |
90 (100%) | 35 (38.9%) | 16 (17.8%) | 12 (13.3%) | 5 (5.6%) | Tamponade in 1 patient | |
| Ikenaga (2017)17 | 106 | All patients: PVI + CTI | 106 (100%) | 106 (100%) | 0 (0%) | ||||
| Chen (2018)18 | 97 | All patients: PVI ± CTI (if typical flutter induced) Persistent AF: CTI + roof line + mitral isthmus |
97 (100%) | 42 (43.3%) | 42 (43.3%) | 58 patients had linear ablation/fractionated potentials ablation | |||
Empty cells indicate that the respective data were not reported in the specific study.
AF, atrial fibrillation; CTI, cavotricuspid isthmus; HCM, hypertrophic cardiomyopathy; LA, left atrial; PV, pulmonary vein; PVI, pulmonary vein isolation; PWI, posterior wall isolation.
Outcomes
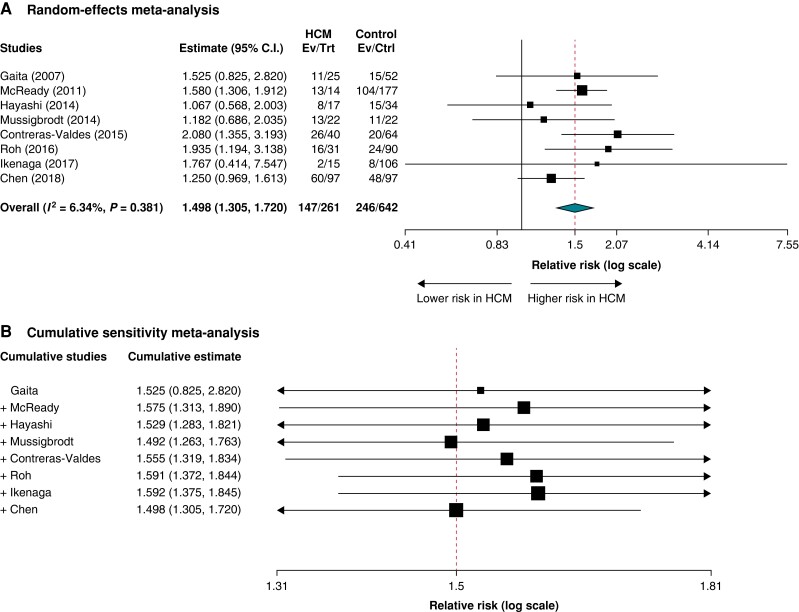
Median follow-up ranged from 13 to 54 months across studies. All studies reported single-procedure success rates (Table 4). The pooled RR for recurrent atrial arrhythmia after AF ablation in patients with HCM compared to patients without HCM was RR = 1.498 (95% CI = 1.305–1.720; P < 0.001; I2 = 6.34%) (Figure 2A). Sensitivity analysis sequentially adding each study demonstrated consistent results (Figure 2B).
Table 4.
Outcomes of catheter ablation of atrial fibrillation in the HCM and control groups
| Study | HCM group | Control group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Follow-up duration (months) |
Atrial arrhythmia recurrence (n, %) |
Absence of atrial arrhythmia at 1 year (n, %) |
Anti-arrhythmic drug (n, %) |
Redo ablation (n, %) |
Follow-up duration (months) |
Arial arrhythmia recurrence (n, %) |
Absence of atrial arrhythmia at 1 year (n, %) |
Anti-arrhythmic drug (n, %) |
Redo ablation (n, %) |
|
| Gaita (2007)11 | 19 ± 10 | 11/25 (44%) |
5/25 (20%) |
16 ± 8 (secondary) 20 ± 4 (idiopathic AF) |
15/52 (28.8%) |
12/52 (23%) |
||||
| McCready (2011)12 | 13 ± 8.9a | 13/14 (92.9%) |
13 ± 8.9a | 104/177 (58.8%) |
||||||
| Hayashi (2011)13 | 29 ± 17 | 8/17 (47%) |
10/17 (58.8%) |
8/17 (47%) |
8/17 (47%) |
24 ± 12 | 15/34 (44%) |
19/34 (55.9%) |
4/34 (12%) |
12/34 (35%) |
| Müssigbrodt (2014)14 | 13/22 (59%) |
6/22 (27.3%) |
5/22 (23%) |
11/22 (50%) |
0 (0%) | 3/22 (14%) |
||||
| Contreras-Valdes (2015)15 | 22 to 67 | 26/40 (65%) |
17/40 (42.5%) |
18/40 (45%) |
13/40 (32.5%) |
35 to 67 | 20/64 (31.3%) |
45/64 (70.3%) |
12/64 (18.8%) |
12/64 (18.8%) |
| Roh (2016)16 | 45 ± 31 (apical HCM) 58 ± 39 (septal HCM) |
16/31 (51.6%) |
8/31 (25.8%) |
54 ± 34 | 24/90 (26.7%) |
9/90 (10%) |
||||
| Ikenaga (2017)17 | 18.8 ± 8.4a | 2/15 (13.3%) |
18.8 ± 8.4a | 8/106 (7.6%) |
||||||
| Chen (2018)18 | 44.3 ± 29.6a | 60/97 (61.9%) |
69/97 (71%) |
44.3 ± 29.6a | 48/97 (49.5%) |
63/97 (64.9%) |
||||
Empty cells indicate that the respective data were not reported in the specific study.
HCM, hypertrophic cardiomyopathy.
Follow-up durations pertain to the overall population in these studies.
Figure 2.
Primary outcome of recurrence of atrial arrhythmia after the first ablation of atrial fibrillation in patients with hypertrophic cardiomyopathy versus controls: A) Random effects meta-analysis, and B) Cumulative sensitivity meta-analysis.
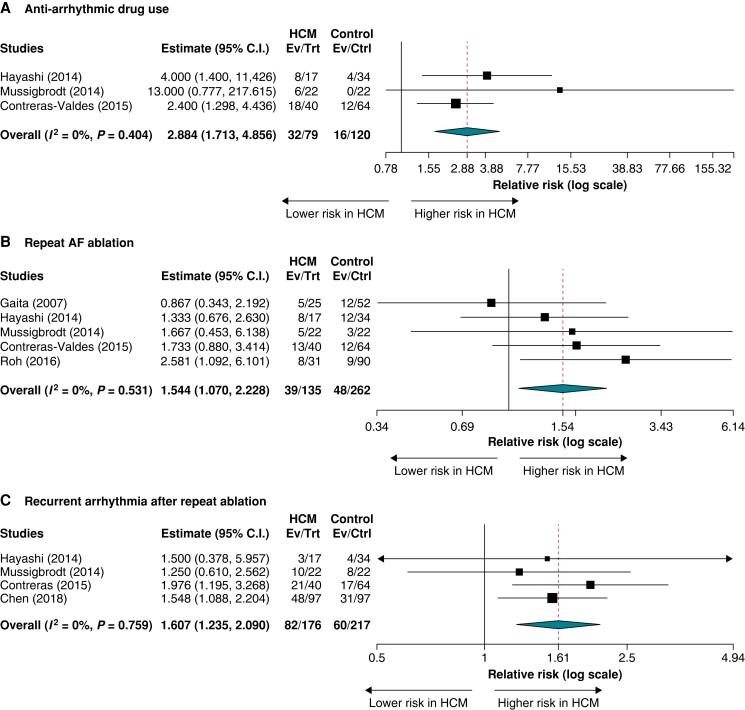
In three studies reporting AAD utilization post-ablation, the need for AADs during follow-up ranged from 27% to 47% in the HCM group and from 0% to 19% in the control group. The rates of AAD use after AF ablation were significantly higher in patients with HCM compared to patients without HCM (RR = 2.884; 95% CI = 1.713–4.856; P < 0.001; I2 = 0%) (Figure 3A).
Figure 3.
Secondary outcomes after atrial fibrillation ablation in patients with hypertrophic cardiomyopathy versus controls: (A) anti-arrhythmic drug use, (B) repeat atrial fibrillation ablation, and (C) recurrent atrial arrhythmias after repeat ablation(s).
In five studies reporting repeat AF ablation, the pooled RR for redo AF ablation in patients with HCM compared to patients without HCM was RR = 1.544 (95% CI = 1.070–2.228; P = 0.020; I2 = 0%) (Figure 3B). In four studies reporting recurrent atrial arrhythmias after the patient’s last ablation (end of follow-up), the rates of recurrent atrial arrhythmias ranged from 18% to 52.5% in the HCM group and from 12% to 36% in the control non-HCM group. In pooled analyses, HCM status was associated with a higher rate of recurrent atrial arrhythmias (RR = 1.607; 95% CI = 1.235–2.090; P < 0.001; I2 = 0%) (Figure 3C; Table 5).
Table 5.
Freedom from atrial arrhythmias after the last ablation (end of follow-up)
| Study | Freedom from atrial arrhythmias |
|---|---|
| Hayashi (2011)13 | 14/17 (82%) in the HCM group vs 30/34 (88%) in the non-HCM group (log-rank P = 0.35) |
| Müssigbrodt (2014)14 | 12/22 (54%) in the HCM group vs 14/22 (64%) in the non-HCM group (log-rank P = 0.121) |
| Contreras-Valdes (2015)15 | 19/40 (47.5%) in the HCM group vs 47/64 (73.4%) in the non-HCM group (log-rank P = 0.005) |
| Chen (2018)18 | 49/97 (51%) in the HCM group vs 66/97 (68%) in the non-HCM group (log-rank P = 0.008) |
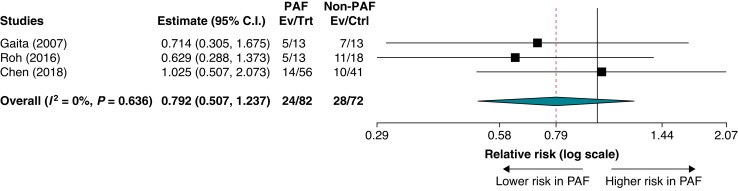
In three studies reporting the rhythm control outcome in patients with HCM stratified by the type of AF, the rates of recurrent atrial arrhythmias after the first ablation of AF ranged from 25% to 38% in the PAF group and from 24% to 61% in the non-PAF group. In meta-analysis, the recurrence rate of atrial arrhythmias was not significantly different between the PAF and non-PAF groups (RR = 0.792; 95% CI = 0.507–1.237; P = 0.305 ; I2 = 0%) (Figure 4).
Figure 4.
Subgroup analysis of recurrent atrial arrhythmias after the first ablation in patients with hypertrophic cardiomyopathy and paroxysmal atrial fibrillation versus non-paroxysmal atrial fibrillation.
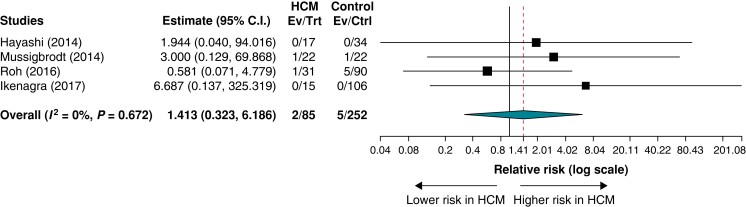
In four studies reporting procedural complications in both the HCM and control patient groups, the rates of procedural complications ranged from 0% to 4.5% in the HCM group and from 0% to 5.6% in the control group. There was no statistically significant difference in the rates of procedural complications for HCM patients versus controls (RR = 1.413; 95% CI = 0.323–6.186; P = 0.646; I2 = 0%) (Figure 5).
Figure 5.
Procedural complications in patients with hypertrophic cardiomyopathy versus controls.
Discussion
Major findings
The main findings of this meta-analysis are the following:
Patients with AF and HCM had higher rates of recurrent atrial arrhythmia after ablation, were more likely to need AAD therapy, and more frequently required repeat ablations compared to patients without HCM.
The percentage of HCM patients free of atrial arrhythmia recurrence surpassed 80% at long-term follow-up (after single or multiple procedures) in some of the analysed studies, suggesting that rhythm control is still feasible in this group of patients.
Comparison with other studies and interpretation of major findings
A previous meta-analysis assessing the efficacy of AF CA in patients with HCM compared to patients without HCM19 included five studies, and results were similar to our findings with a lower rate of freedom from atrial arrhythmias, higher AAD use, and more frequent repeat procedures after AF ablation in patients with HCM.19 Similarly, increased rates of recurrence have been shown in non-controlled observational studies. A recent large multicentre single-arm observational study found low rates of rhythm control maintenance at long-term follow-up after CA of AF in HCM patients.20 This reduction in ablation success was particularly pronounced in HCM patients with persistent atrial fibrillation, where the risk of recurrence was almost double that of HCM patients with PAF.20 Late recurrences, defined as those occurring after the initial 12-month period, were common among HCM patients with both paroxysmal and persistent AF.20 The higher use of AADs and frequent redo ablation post-AF ablation in patients with HCM is not unexpected, considering the complex arrhythmogenic substrate and ongoing remodelling predisposing to recurrent atrial arrhythmias. In another single-arm multicentre study of AF ablation in HCM, PVI alone resulted in high recurrence rates in this population. However, long-term success was obtained in most patients following repeat procedures, including non-PVI targets, particularly trigger mapping and ablation.7 This suggests that the complexity of arrhythmogenic substrate in HCM may warrant more extensive ablation beyond the standard PVI. The optimal ablation targets are likely to differ among HCM patients. Our meta-analysis did not have sufficient study-level data to inform on the relative effects of specific ablation strategies.
Mechanisms for atrial fibrillation in hypertrophic cardiomyopathy
The pathogenesis of atrial arrhythmias in HCM is multifactorial. Underlying myocyte hypertrophy, myocyte disarray, and interstitial fibrosis associated with HCM ultimately result in left ventricular diastolic dysfunction, which increases left ventricular end-diastolic pressure drive and LA afterload.21 Increased LA pressure and distension drive pathological remodelling of the left atrium, creating a substrate for initiating and maintaining AF. Additionally, some evidence suggests primary atrial myopathy and fibrosis among HCM patients, which may be independent of a secondary haemodynamic mechanism.19,22 Potential mechanisms of AF in HCM patients also include dysregulation of intracellular ion concentration. Patients with specific familial or hereditary forms of HCM have been shown to have dysregulation of calcium cycling/handling, resulting in increased intracellular calcium levels.23 As such, triggered activity from early afterdepolarizations has been implicated as a trigger for atrial fibrillation in the setting of proarrhythmic atrial substrate and remodelling.19,23
Clinical implications
Atrial fibrillation is often poorly tolerated in HCM patients due to restrictive ventricular filling, loss of atrial contractility, and the potential for worsening outflow tract obstruction and decreased cardiac output with rapid ventricular rates. Therefore, most HCM patients benefit from intensive rhythm control. It should be emphasized that our study was not designed to address whether an ablation-based strategy is superior to AADs in patients with HCM. However, our meta-analysis suggests that a multifaceted approach may lead to favourable long-term rhythm control in HCM patients since freedom from atrial arrhythmias at the last follow-up ranged from 47.5% to 82% in the HCM groups across studies. As part of this multifaceted approach, CA might be more effective when implemented early in the disease course before extensive adverse remodelling has taken place.24 In general, HCM patients and clinicians should be primed for the requirement of concomitant AADs and repeat ablations. Eliminating arrhythmia with single procedures without adjunctive AADs or additional procedures may be unrealistic for many HCM patients. As such, it is important to appropriately frame the definition of success of AF ablation in HCM patients.
Patients with HCM and AF frequently present with non-PV triggers,7 both at the time of index AF diagnosis and with recurrent episodes following initial PVI. More extensive ablation beyond PVI with targeting of non-pulmonary vein triggers may be warranted in such patients. Patients at high risk for AF recurrence, such as those with HCM, might be best served with a more extensive ablation strategy upfront to optimize long-term rhythm control. Hybrid surgical and CA approaches may also be considered in patients with HCM for PVI and posterior wall isolation, with or without LA appendage ligation, particularly for those with persistent AF.25,26 However, further investigation of such management strategies is needed to determine potential efficacy and benefit. It is also noteworthy that conventional ablation techniques may be less effective in patients with HCM due to inadequate lesion formation related to myocyte hypertrophy and inability to form transmural lesions.
Limitations
Certain limitations of our study merit consideration. First, the ablation approach was not uniform among the individual studies. We did not have sufficient study-level data to determine the relative outcomes of the various ablation strategies. Second, control group selection was variable among studies. In three studies,12,15,17 controls were not selected based on matching. Controls had structural heart disease in two studies.11,14 Third, there was a variable prevalence of patients with PAF and non-PAF across the included studies. Only three of the studies reported outcomes stratified by AF type not allowing us to draw strong conclusions regarding the impact of AF type on ablation outcomes in HCM patients. Lastly, compared to the outcomes of AF ablation in the included studies, ablation outcomes may be superior in more contemporary HCM cohorts due to the widespread use of the latest AF mapping and ablation innovations, including ultra-high-density mapping, cryoballoon ablation, contact-force sensing, and index-guided ablation, among others.20 Despite these limitations, heterogeneity among the studies included in this meta-analysis was low, increasing the certainty of the evidence for each primary and secondary outcome.
Conclusions
In this meta-analysis of observational studies of AF ablation, patients with HCM had a higher risk of recurrent atrial arrhythmia, use of AADs, and need for redo AF ablation during follow-up compared to patients without HCM. While these results alone cannot support explicit management recommendations, they provide guidance for nuanced shared decision-making discussions and frame the role of CA in the context of the multifaceted AF management in this challenging patient population.
Supplementary Material
Contributor Information
Fatima M Ezzeddine, Department of Cardiovascular Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 59905, USA.
Kolade M Agboola, Department of Cardiovascular Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 59905, USA; Division of Cardiovascular Medicine, Brigham and Women’s Hospital, Boston, MA, USA.
Leslie C Hassett, Plummer Library, Mayo Clinic, Rochester, MN, USA.
Ammar M Killu, Department of Cardiovascular Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 59905, USA.
Freddy Del-Carpio Munoz, Department of Cardiovascular Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 59905, USA.
Christopher V DeSimone, Department of Cardiovascular Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 59905, USA.
Gurukripa N Kowlgi, Department of Cardiovascular Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 59905, USA.
Abhishek J Deshmukh, Department of Cardiovascular Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 59905, USA.
Konstantinos C Siontis, Department of Cardiovascular Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 59905, USA.
Supplementary material
Supplementary material is available at Europace online.
Data availability
The data supporting this paper’s findings are available from the corresponding author, K.C.S., upon reasonable request.
References
- 1. Olivotto I, Cecchi F, Casey SA, Dolara A, Traverse JH, Maron BJ. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation 2001;104:2517–24. [DOI] [PubMed] [Google Scholar]
- 2. Maron BJ, Olivotto I, Bellone P, Conte MR, Cecchi F, Flygenring BPet al. Clinical profile of stroke in 900 patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 2002;39:301–7. [DOI] [PubMed] [Google Scholar]
- 3. Siontis KC, Geske JB, Ong K, Nishimura RA, Ommen SR, Gersh BJ. Atrial fibrillation in hypertrophic cardiomyopathy: prevalence, clinical correlations, and mortality in a large high-risk population. J Am Heart Assoc 2014;3:e001002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ho CY. Genetics and clinical destiny: improving care in hypertrophic cardiomyopathy. Circulation 2010;122:2430–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang H, Woo A, Monakier D, Jamorski M, Fedwick K, Wigle EDet al. Enlarged left atrial volume in hypertrophic cardiomyopathy: a marker for disease severity. J Am Soc Echocardiogr 2005;18:1074–82. [DOI] [PubMed] [Google Scholar]
- 6. Tuluce K, Tuluce SY. Predictors of atrial fibrillation risk in hypertrophic cardiomyopathy. J Atr Fibrillation 2015;7:1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Santangeli P, Di Biase L, Themistoclakis S, Raviele A, Schweikert RA, Lakkireddy Det al. Catheter ablation of atrial fibrillation in hypertrophic cardiomyopathy: long-term outcomes and mechanisms of arrhythmia recurrence. Circ Arrhythm Electrophysiol 2013;6:1089–94. [DOI] [PubMed] [Google Scholar]
- 8. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CDet al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos Met al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Oxford; 2000. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- 10. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gaita F, Di Donna P, Olivotto I, Scaglione M, Ferrero I, Montefusco Aet al. Usefulness and safety of transcatheter ablation of atrial fibrillation in patients with hypertrophic cardiomyopathy. Am J Cardiol 2007;99:1575–81. [DOI] [PubMed] [Google Scholar]
- 12. McCready JW, Smedley T, Lambiase PD, Ahsan SY, Segal OR, Rowland Eet al. Predictors of recurrence following radiofrequency ablation for persistent atrial fibrillation. Europace 2011;13:355–61. [DOI] [PubMed] [Google Scholar]
- 13. Hayashi H, Hayashi M, Miyauchi Y, Takahashi K, Uetake S, Tsuboi Iet al. Left atrial wall thickness and outcomes of catheter ablation for atrial fibrillation in patients with hypertrophic cardiomyopathy. J Interv Card Electrophysiol 2014;40:153–60. [DOI] [PubMed] [Google Scholar]
- 14. Müssigbrodt A, Kosiuk J, Koutalas E, Pastromas S, Dagres N, Darma Aet al. Results of catheter ablation of atrial fibrillation in hypertrophied hearts—comparison between primary and secondary hypertrophy. J Cardiol 2015;65:474–8. [DOI] [PubMed] [Google Scholar]
- 15. Contreras-Valdes FM, Buxton AE, Josephson ME, Anter E. Atrial fibrillation ablation in patients with hypertrophic cardiomyopathy: long-term outcomes and clinical predictors. J Am Coll Cardiol 2015;65:1485–7. [DOI] [PubMed] [Google Scholar]
- 16. Roh SY, Kim DH, Ahn J, Lee KN, Lee DI, Shim Jet al. Long-term outcome of catheter ablation for atrial fibrillation in patients with apical hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol 2016;27:788–95. [DOI] [PubMed] [Google Scholar]
- 17. Ikenaga H, Nakano Y, Oda N, Suenari K, Sairaku A, Tokuyama Tet al. Radiofrequency catheter ablation is effective for atrial fibrillation patients with hypertrophic cardiomyopathy by decreasing left atrial pressure. J Arrhythm 2017;33:256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen X, Dong JZ, Du X, Wu JH, Yu RH, Long DYet al. Long-term outcome of catheter ablation for atrial fibrillation in patients with apical hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol 2018;29:951–7. [DOI] [PubMed] [Google Scholar]
- 19. Providencia R, Elliott P, Patel K, McCready J, Babu G, Srinivasan Net al. Catheter ablation for atrial fibrillation in hypertrophic cardiomyopathy: a systematic review and meta-analysis. Heart 2016;102:1533–43. [DOI] [PubMed] [Google Scholar]
- 20. Creta A, Elliott P, Earley MJ, Dhinoja M, Finlay M, Sporton Set al. Catheter ablation of atrial fibrillation in patients with hypertrophic cardiomyopathy: a European observational multicentre study. Europace 2021;23:1409–17. [DOI] [PubMed] [Google Scholar]
- 21. Cui H, Schaff HV, Lentz Carvalho J, Nishimura RA, Geske JB, Dearani JAet al. Myocardial histopathology in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2021;77:2159–70. [DOI] [PubMed] [Google Scholar]
- 22. Ohtani K, Yutani C, Nagata S, Koretsune Y, Hori M, Kamada T. High prevalence of atrial fibrosis in patients with dilated cardiomyopathy. J Am Coll Cardiol 1995;25:1162–9. [DOI] [PubMed] [Google Scholar]
- 23. Lan F, Lee AS, Liang P, Sanchez-Freire V, Nguyen PK, Wang Let al. Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell 2013;12:101–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan Aet al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–16. [DOI] [PubMed] [Google Scholar]
- 25. van der Heijden CAJ, Vroomen M, Luermans JG, Vos R, Crijns H, Gelsomino Set al. Hybrid versus catheter ablation in patients with persistent and longstanding persistent atrial fibrillation: a systematic review and meta-analysis†. Eur J Cardiothorac Surg 2019;56:433–43. [DOI] [PubMed] [Google Scholar]
- 26. Khawaja T, Majmundar M, Zuzek Z, Arora S, Attizzani GF, Filby SJet al. Surgical and transcatheter left atrial appendage closure in patients with atrial fibrillation and hypertrophic cardiomyopathy. Europace 2023;25:euad101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this paper’s findings are available from the corresponding author, K.C.S., upon reasonable request.