Abstract
Six years ago, a 60‐year‐old man presented to our hospital with a cough and sputum. Upon suspicion of nontuberculous mycobacterial (NTM) infection, he was followed up at our hospital. Because the abnormal shadows in the bilateral lung fields deteriorated slightly over 6 years, bronchoscopy was performed. Exophiala dermatitidis and Mycobacterium intracellulare were detected in the bronchial lavage fluid. The patient underwent follow‐up examinations without drug administration. Currently, the patient's condition remains stable. E. dermatitidis is regulatory found in the lungs of patients with cystic fibrosis, but only rarely is it found in respiratory samples from patients without cystic fibrosis. However, NTM complications have been reported more frequently in recent years. Due to the increasing number of NTM patients, E. dermatitidis pulmonary infections may also increase. Additional research is required to develop strategies for treating this infection.
Keywords: bronchiectasis, Exophiala dermatitidis, nontuberculous mycobacteria
Here, we describe a case of Exophiala dermatitidis coinfection with nontuberculous mycobacteria (NTM) and review clinical reports describing the features of pulmonary infections caused by E. dermatitidis in non‐CF patients.
INTRODUCTION
Exophiala dermatitidis is a black fungus that is distributed worldwide in natural environments, such as soil and dead plant material. This fungus is regularly isolated from respiratory samples of cystic fibrosis (CF) patients as colonies and sometimes causes exacerbations. 1 , 2 , 3 Pulmonary infections caused by E. dermatitidis have been reported in non‐CF patients, albeit rarely compared to CF patients. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18
Here, we describe a case of E. dermatitidis coinfection with nontuberculous mycobacteria (NTM) and review clinical reports describing the features of pulmonary infections caused by E. dermatitidis in non‐CF patients.
CASE REPORT
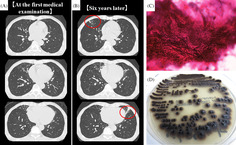
Six years ago, a 60‐year‐old man presented to our hospital with a cough and sputum. Chest computed tomography revealed nodules, infiltrative shadows, and bronchiectasis in the right middle and left upper lobes (Figure 1A). Upon suspicion of NTM infection, the patient was followed up at our hospital. He had no history of smoking or allergies. He had been diagnosed with prostatitis, but there were no past histories of respiratory diseases. Laboratory data showed a white blood cell count of 3860/μL and C‐reactive protein level of 0.05 mg/dL. Tests for β‐D‐glucan, anti‐aspergillus antigen, and anti‐cryptococcus antigen returned negative results but that for anti‐Mycobacterium avium complex antibody returned positive results. His symptoms improved by the administration of expectorant 3 months later of first visit. But after 6 years, the abnormal shadows in the bilateral lung fields deteriorated slightly (Figure 1B). Therefore, bronchoscopy was performed. Filamentous fungi (Figure 1C) and Mycobacterium intracellulare were detected in the bronchial lavage fluid. After 25 days of culturing the fungi at 35°C, olive‐black colonies grew on a potato‐dextrose agar (Figure 1D). Sequencing analysis of the internal transcribed spacer region in the ribosomal DNA revealed that the fungus was E. dermatitidis. The patient underwent a follow‐up examination without receiving drug treatment at his request. His condition is stable at present.
FIGURE 1.
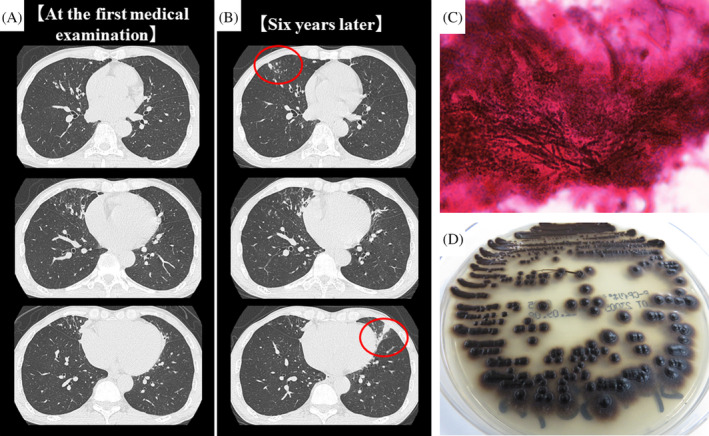
Chest computed tomography (CT) findings (A, B). (A) CT at the first medical examination showed nodules, infiltrative shadows, and bronchiectasis in the right middle and left upper lobes. (B) Six years later, these shadows were partially deteriorated (red circle). Microscopic and Macroscopic findings of the fungi from the bronchial lavage fluid (C, D). (C) Gram staining of bronchial lavage fluid showed filamentous fungi (×1000). (D) Olive‐black colour colonies were detected after culturing the fungi for 25 days in potato‐dextrose agar incubated at 35°C.
DISCUSSION
E. dermatitidis is a melanized yeast‐like organism that belongs to the dematiaceous family of fungi 19 and is one of causative pathogen of phaeophyphomycosis. In humans, the fungus is rare as a cause of fungal infections. The most commonly infected organ is the skin, and the most frequent type of deep infection is pulmonary infection. 19 Although several studies on E. dermatitidis pulmonary infections have been reported in CF patients, only 16 cases have been reported in non‐CF patients. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18
A summary of non‐CF patients with E. dermatitidis pulmonary infections is presented in Table 1. Most patients were middle‐aged and older females and were from Asia. Patients frequently had cough and sputum, but some also had hemoptysis (4/17) and fever (3/17). The patient described in the present case is also a Japanese in his 60s but not a female patient. He had cough and sputum, but there were no other symptoms such as fevers, sweats and weight loss for follow‐up periods. It seems there are not specific symptoms for E. dermatitidis pulmonary infections, and it may be difficult to distinguish this infections from only patient's symptoms. Regarding the reported region, immunological differences in the host or differences in exposure to fungal propagules may affect disease progression. 11
TABLE 1.
Summary of non‐cystic fibrosis patients with pulmonary Exophiala dermatitidis infection.
| Author (references) | Age | Sex | Region | Underlying disease | Symptoms | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| Barenfanger et al. 4 | 79 | F | USA | Bronchiectasis | Hemoptysis, fever | AMPH‐B, 5‐FC | Survival |
| Kenney et al. 5 | 21 | F | USA | Chronic granulomatous disease | Fever, chill, shortness of breath | Op, AMPH‐B, 5‐FC, FLCZ | Survival |
| Mukaino et al. 6 | 54 | F | Japan | Bronchiectasis | Cough, sputum | MCZ, AMPH‐B | Survival |
| Taj‐Aldeen et al. 7 | 54 | F | Qatar | Diabetes, cervical cancer | Cough, sputum, hemoptysis | FLCZ, ITCZ, AMPH‐B | Death |
| Ozawa et al. 8 | 81 | F | Japan | None | Hemoptysis | ITCZ | Survival |
| Tanamachi et al. 9 | 53 | F | Japan | Bronchiectasis | Sputum, chest pain | MCZ, 5‐FC, ITCZ | Survival |
| Bulloch 10 | 86 | F | USA | Dementia | No symptoms | VRCZ | Survival |
| Suzuki et al. 11 | 65 | M | Japan | Multiple myeloma | No symptoms | VRCZ, Op | Survival |
| Mukai et al. 12 | 63 | F | Japan | None | Chest pain | ITCZ | Survival |
| Shintani et al. 13 | 56 | F | Japan | Bronchiectasis | Sputum, fever | ITCZ | Survival |
| Goto et al. 14 | 70 | F | Japan | NTM | Sputum, chest pain | VRCZ | Survival |
| Masuo et al. 15 | 58 | F | Japan | NTM | Cough | VRCZ | Survival |
| Sekiguchi et al. 16 | 65 | F | Japan | RA, bronchiectasis | Cough, sputum | VRCZ | Survival |
| Li et al. 17 | 52 | M | China | None | Cough, sputum, hemoptysis | VRCZ, AMPH‐B, PCZ | Survival |
| Watanabe et al. 18 | 65 | F | Japan | NTM, sinusitis | Cough, sputum | VRCZ | Survival |
| Watanabe et al. 18 | 47 | F | Japan | RA, NTM, sinusitis | Impaired sense of smell, nasal discharge | AMPH, VRCZ, ITCZ | Survival |
| Present case | 66 | M | Japan | NTM | Cough, sputum | None | Survival |
Abbreviations: AMPH‐B, amphotericin B; FLCZ, fluconazole; ITCZ, itraconazole; MCZ, miconazole; NTM, nontuberculous mycobacteria; Op, operation; PCZ, posaconazole; RA, rheumatoid arthritis; USA, United States of America; VRCZ, voriconazole; 5‐FC, 5‐fluorocytosine.
Many patients had bronchiectasis as an underlying disease (Table 1). In recent years, NTM complications have been reported more frequently. 14 , 15 , 18 Interestingly, previous cases did not report any immunocompromised patients, although immune status has proven to affect disease progression. 19 The present case was immunocompetent and complicated by NTM. A previous report showed that although prompt mucociliary and phagocytic clearance occurs in healthy lungs, anatomically abnormal and immunocompromised airways, such as those in bronchiectasis, are at a higher risk of fungal acquisition, colonization, and potential disease. 20 Additionally, the incidence and prevalence of NTM have increased worldwide. 21 Fujita et al. reported that approximately 45% of patients with Mycobacterium avium‐intracellulare complex had chronic coinfection with pathogenic microorganisms, such as methicillin‐sensitive Staphylococcus aureus, Pseudomonas aeruginosa, and Aspergillus spp., among whom 85.1% had bronchiectasis. 22 It is unclear whether NTM infection is associated with E. dermatitidis infection in non‐CF patients. On the other hand, Kondori et al. reported that serum levels of IgG antibodies to E. dermatitidis were significantly higher in the E. dermatitidis culture‐positive CF patients compared to culture‐negative CF patients. 23 CF patients with higher level of E. dermatitidis IgG antibodies were more often colonized with NTM. 23 It was also reported that CF patients with E. dermatitidis isolation had higher rates of NTM isolation compared to CF patients without E. dermatitidis isolation. 24 Currently, few reports on E. dermatitidis pulmonary infections in non‐CF patients have been published. However, it has the potential to increase in the future with the increase in the number of patients with NTM infection.
Appropriate treatments for E. dermatitidis pulmonary infections have not yet been established. Previous cases were treated with monotherapy or combination therapy with amphotericin B (AMPH‐B), 5‐fluorocytosine, itraconazole (ITCZ), voriconazole (VRCZ), and other antifungals. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 Several studies have demonstrated the susceptibility of various organisms to antifungal agents (Table 2). The minimum inhibitory concentrations of AMPH‐B, ITCZ and VRCZ are low according to these studies, while those of micafungin, caspofungin, and fluconazole are relatively high. Our patient did not receive medical treatment for E. dermatitidis pulmonary infection. Antifungal susceptibility testing in this study also revealed that the minimum inhibitory concentration of ITCZ was low. Therefore, we intend to administer ITCZ if our patient's condition deteriorates. Further studies are needed to establish the treatment strategy for E. dermatitidis pulmonary infection.
TABLE 2.
Summary of the susceptibility of various antifungal agents for Exophiala dermatitidis.
| Author (references) | MCFG | CPFG | AMPH‐B | 5‐FC | FLCZ | ITCZ | VRCZ | MCZ |
|---|---|---|---|---|---|---|---|---|
| Barenfanger et al. 4 | NA | NA | NA | NA | NA | NA | NA | NA |
| Kenney et al. 5 | NA | NA | NA | NA | NA | NA | NA | NA |
| Mukaino et al. 6 | NA | NA | NA | NA | NA | NA | NA | NA |
| Taj‐Aldeen et al. 7 | NA | NA | 0.25 | 32 | 192 | 0.094 | NA | NA |
| Ozawa et al. 8 | NA | NA | NA | NA | NA | NA | NA | NA |
| Tanamachi et al. 9 | 16 | NA | 0.25 | 4 | 8 | 0.25 | NA | 0.125 |
| Bulloch 10 | NA | NA | NA | NA | NA | NA | NA | NA |
| Suzuki et al. 11 | 16 | NA | 2 | 4 | 64 | 2 | 1 | 1 |
| Mukai et al. 12 | 16 | NA | 0.5 | 1 | 8 | 0.25 | 0.12 | 0.12 |
| Shintani et al. 13 | NA | NA | NA | NA | NA | NA | NA | NA |
| Goto et al. 14 | 32 | 8 | 0.25 | 128 | 8 | 0.12 | 0.06 | 0.5 |
| Masuo et al. 15 | 16 | 16 | 0.5 | 2 | 16 | 0.5 | 0.125 | 0.25 |
| Sekiguchi et al. 16 | 16 | NA | 1 | NA | NA | 0.25 | 0.12 | NA |
| Li et al. 17 | NA | NA | NA | NA | NA | NA | NA | NA |
| Watanabe et al. 18 | 8 | 16 | 0.25 | 2 | 8 | 0.25 | 0.12 | 0.5 |
| Watanabe et al. 18 | 16 | 16 | 0.25 | 4 | 8 | 0.25 | 0.12 | 5 |
| Present case | 16 | 8 | 8 | 8 | 32 | 0.5 | 8 | NA |
Abbreviations: AMPH‐B, amphotericin B; CPFG, caspofungin; FLCZ, fluconazole; ITCZ, itraconazole; MCFG, micafungin; MCZ, miconazole; NA, not available; VRCZ, voriconazole; 5‐FC, 5‐fluorocytosine.
The prognosis of E. dermatitidis pulmonary infection in patients without CF was good (Table 1), considering that only one death was reported. 7 The cause of death was cervical cancer progression and a complicated infection of Candida krusei fungemia, not E. dermatitidis pulmonary infection. Our patient's condition is stable at present. However, diseases caused by E. dermatitidis range from benign cutaneous infections to systemic infections, with a 40% fatality rate. 19 In addition, it was reported that a non‐CF patient with E. dermatitidis pulmonary infection relapsed after 11 months of antifungal therapy. 16 Therefore, careful follow‐up of this disease is necessary.
In conclusion, we described a rare case of pulmonary E. dermatitidis coinfection with NTM. The incidence of this infection may possibly increase with increased incidence of NTM infections. Additional research is needed because the treatment strategy for this infection has not been sufficiently clarified yet.
AUTHOR CONTRIBUTIONS
Writing‐original draft: Seigo Miyoshi. Writing review and editing: Seigo Miyoshi, Miyuki Tanabe, Mayuko Semba, Chika Sato, Sanae Aoyama, Akira Watanabe, Ryoji Ito, Kumi Hamada, Akira Watanabe, Masahiro Abe. All authors have read and agreed to the published version of the manuscript.
CONFLICT OF INTEREST STATEMENT
None declared.
ETHICS STATEMENT
The authors declare that appropriate written informed consent was obtained for the publication of this manuscript and accompanying images.
ACKNOWLEDGMENTS
We would like to thank Editage (www.editage.com) for English language editing.
Miyoshi S, Tanabe M, Semba M, Sato C, Aoyama S, Watanabe A, et al. Exophiala dermatitidis coinfection with nontuberculous mycobacteria: A case report and literature review. Respirology Case Reports. 2023;11:e01221. 10.1002/rcr2.1221
Associate Editor: Michael Maze
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- 1. Diemert D, Kunimoto D, Sand C, Rennie R. Sputum isolation of Wangiella dermatitidis in patients with cystic fibrosis. Scand J Infect Dis. 2001;33:777–779. [DOI] [PubMed] [Google Scholar]
- 2. Haase G, Skopnik H, Groten T, Kusenbach G, Posselt HG. Long‐term fungal cultures from sputum of patients with cystic fibrosis. Mycoses. 1991;34:373–376. [DOI] [PubMed] [Google Scholar]
- 3. Horré R, Schaal KP, Siekmeier R, Sterzik B, de Hoog GS, Schnitzler N. Isolation of fungi, especially Exophiala dermatitidis, in patients suffering from cystic fibrosis. A prospective study. Respiration. 2004;71:360–366. [DOI] [PubMed] [Google Scholar]
- 4. Barenfanger J, Ramirez F, Tewari RP, Eagleton L. Pulmonary phaeohyphomycosis in a patient with hemoptysis. Chest. 1989;95:1158–1160. [DOI] [PubMed] [Google Scholar]
- 5. Kenney RT, Kwon‐Chung KJ, Waytes AT, Melnick DA, Pass HI, Merino MJ, et al. Successful treatment of systemic Exophiala dermatitidis infection in a patient with chronic granulomatous disease. Clin Infect Dis. 1992;14:235–242. [DOI] [PubMed] [Google Scholar]
- 6. Mukaino T, Koga T, Oshita Y, Narita Y, Obata S, Aizawa H. Exophiala dermatitidis infection in non‐cystic fibrosis bronchiectasis. Respir Med. 2006;100:2069–2071. [DOI] [PubMed] [Google Scholar]
- 7. Taj‐Aldeen SJ, El Shafie S, Alsoub H, Eldeeb Y, de Hoog GS. Isolation of Exophiala dermatitidis from endotracheal aspirate of a cancer patient. Mycoses. 2006;49:504–509. [DOI] [PubMed] [Google Scholar]
- 8. Ozawa Y, Suda T, Kaida Y, Kato M, Hasegaw H, Fujii M, et al. A case of bronchial infection of Wangiella dermatitidis . Nihon Kokyuki Gakkai Zasshi. 2007;45:907–911. [PubMed] [Google Scholar]
- 9. Tanamachi C, Hashimoto K, Nakata K, Sagawa K. A case of pulmonary chromomycosis caused by Exophiala dermatitidis . J Jap Soc Clin Microbiol. 2008;18:25–30. [Google Scholar]
- 10. Bulloch MN. The treatment of pulmonary Wangiella dermatitidis infection with oral voriconazole. J Clin Pharm Ther. 2011;36:433–436. [DOI] [PubMed] [Google Scholar]
- 11. Suzuki K, Nakamura A, Fujieda A, Nakase K, Katayama N. Pulmonary infection caused by Exophiala dermatitidis in a patient with multiple myeloma: a case report and a review of the literature. Med Mycol Case Rep. 2012;1:95–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mukai Y, Nureki S, Hata M, Shigenaga T, Tokimatsu I, Miyazaki E, et al. Exophiala dermatitidis pneumonia successfully treated with long‐term itraconazole therapy. J Infect Chemother. 2014;20:446–449. [DOI] [PubMed] [Google Scholar]
- 13. Shintani R, Hagiwara E, Yamakawa H, Ikeda S, Kitamura H, Baba T, et al. Pulmonary phaeohyphomycosis caused by Exophiala dermatitidis . JJA Inf. 2017;91:785–789. [Google Scholar]
- 14. Goto Y, Murakami N, Yamasaki Y. Sequelae of pulmonary chromoblastomycosis caused by the viscous species Exophiala dermatitidis in a patient with nontuberculosis mycobacterial disease. Igakukensa. 2020;69:451–456. [Google Scholar]
- 15. Masuo M, Hanazawa S, Nukui Y. Pulmonary chromomycosis caused by Exophiala dermatitidis in a patient with pulmonary non‐tuberculous mycobacteriosis. J Jap Soc Respir Endos. 2021;43:619–623. [Google Scholar]
- 16. Sekiguchi R, Urabe N, Sakamoto S, Sasaki M, Homma S, Kishi K. Exophiala dermatitidis pneumonia with bronchiectasis required prolonged voriconazole treatment. Respirol Case Rep. 2021;9:e00783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li Z, Tang J, Zhu J, Xie M, Huang S, Li S, et al. The convoluted process of diagnosing pulmonary mycosis caused by Exophiala dermatitidis: a case report. BMC Infect Dis. 2022;22:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Watanabe Y, Sano H, Konno S, Kamioka Y, Hariu M, Takano K, et al. Sinobronchial syndrome patients with suspected non‐tuberculous mycobacterium infection exacerbated by Exophiala dermatitidis infection. Infect Drug Resist. 2022;15:1135–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Usuda D, Higashikawa T, Hotchi Y, Usami K, Shimozawa S, Tokunaga S, et al. Exophiala dermatitidis. World J Clin Cases. 2021;9:7963–7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chotirmall SH, Martin‐Gomez MT. Aspergillus species in bronchiectasis: challenges in the cystic fibrosis and non‐cystic fibrosis airways. Mycopathologia. 2018;183:45–59. [DOI] [PubMed] [Google Scholar]
- 21. Prevots DR, Marras TK. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clin Chest Med. 2015;36:13–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fujita K, Ito Y, Hirai T, Kubo T, Togashi K, Ichiyama S, et al. Prevalence and risk factors for chronic co‐infection in pulmonary Mycobacterium avium complex disease. BMJ Open Respir Res. 2014;1:e000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kondori N, Lindblad A, Welinder‐Olsson C, Wennerås C, Gilljam M. Development of IgG antibodies to Exophiala dermatitidis is associated with inflammatory responses in patients with cystic fibrosis. J Cyst Fibros. 2014;13:391–399. [DOI] [PubMed] [Google Scholar]
- 24. Tewkesbury DH, Looi E, Barry PJ, Edwards G, Green H, Smith M, et al. Isolation of Exophiala dermatitidis is not associated with worse clinical outcomes during acute pulmonary exacerbations in cystic fibrosis. J Med Microbiol. 2022;71:001431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors.


