Summary
Background
GBP510 vaccine contains self-assembling, recombinant nanoparticles displaying SARS-CoV-2 spike receptor-binding domains. We report interim phase 3 immunogenicity results for GBP510 adjuvanted with AS03 (GBP510/AS03) compared with ChAdOx1-S (Vaxzevria, AstraZeneca) in healthy adults aged ≥18 years, up to 6 months after the second dose.
Methods
This was a randomised, active-controlled, observer-blinded, parallel group, phase 3 study, conducted at 38 sites across six countries (South Korea, Philippines, Thailand, Vietnam, Ukraine and New Zealand). Cohort 1 (no history of SARS-CoV-2 infection/COVID-19 vaccination) was randomised 2:1 to receive two doses of GBP510/AS03 or ChAdOx1-S (immunogenicity and safety), while Cohort 2 (regardless of baseline serostatus) was randomised 5:1 (safety). Primary objectives were to demonstrate superiority in geometric mean titre (GMT) and non-inferiority in seroconversion rate (SCR; ≥4-fold rise from baseline) of GBP510/AS03 vs. ChAdOx1-S for neutralising antibodies against the ancestral strain by live-virus neutralisation assay. Secondary objectives included assessment of safety and reactogenicity (long-term 6 months cut-off date: 09 August 2022). This study was registered on ClinicalTrials.gov (NCT05007951).
Findings
Between 30 August 2021 and 11 January 2022, a total of 4913 participants were screened and 4036 participants (1956 in Cohort 1 and 2080 in Cohort 2) who met eligibility criteria were enrolled and randomised to receive 2 doses of GBP510/AS03 (n = 3039) or ChAdOx1-S (n = 997). Most participants were Southeast Asian (81.5%) and aged 18–64 years (94.7%). The primary objectives assessed in per-protocol set included 877 participants in GBP510/AS03 and 441 in ChAdOx1-S group: at 2 weeks after the second vaccination, the GMT ratio (GBP510/AS03/ChAdOx1-S) in per-protocol set was 2.93 (95% CI 2.63–3.27), demonstrating superiority (95% CI lower limit >1) of GBP510/AS03; the between-group SCR difference of 10.8% (95% CI 7.68–14.32) also satisfied the non-inferiority criterion (95% CI lower limit > −5%). Neutralizing antibody titres sustained higher for the GBP510/AS03 group compared to the ChAdOx1-S group through 6 months after the second vaccination. In Safety analysis (Cohort 1 & 2), the proportion of participants with adverse events (AEs) after any vaccination was higher with GBP510/AS03 vs. ChAdOx1-S for solicited local AEs (56.7% vs. 49.2%), but was similar for solicited systemic AEs (51.2% vs. 53.5%) and unsolicited AEs (13.3% vs. 14.6%) up to 28 days after the second vaccination. No safety concerns were identified during follow-up for 6 months after the second vaccination.
Interpretation
Our interim findings suggested that GBP510/AS03 met the superiority criterion for neutralising antibodies and non-inferiority criterion for SCR compared with ChAdOx1-S, and showed a clinically acceptable safety profile.
Funding
This work was supported, in whole or in part, by funding from CEPI and the Bill & Melinda Gates Foundation Investments INV-010680 and INV-006462. The Bill & Melinda Gates Foundation supported this project for the generation of IND-enabling data and CEPI supported this clinical study.
Keywords: SARS-CoV-2, COVID-19, Recombinant protein vaccine, Nanoparticle vaccine, Immunogenicity, Safety
Research in context.
Evidence before this study
Immunobridging has been proposed as an approach for assessing new COVID-19 vaccines by comparing the immunogenicity of candidate vaccines with an active comparator with demonstrated clinical efficacy. We searched PubMed up to 26 October 2022 for immunobridging clinical trials comparing a candidate vaccine with an approved vaccine, using the terms “immunobridging”, “SARS-CoV-2”, “COVID-19”, and “vaccine”. We identified immunobridging was used to assess immunogenicity of candidate vaccine in following trials. A post hoc analysis of phase 2 data found that MVC-COV1901 vaccine (a protein subunit vaccine developed by Medigen Vaccine Biologics Corporation, Taiwan) was non-inferior to ChAdOx1 with respect to neutralising antibody titres. A phase 3 study found that VLA2001 (an adjuvanted, inactivated whole-virus vaccine developed by Valneva, Austria) was superior to ChAdOx1 with respect to neutralising antibody titres and non-inferior with respect to seroconversion rates.
Added value of this study
This is the first study comparing the immunogenicity of recombinant SARS-CoV-2 protein nanoparticle vaccine GBP510 adjuvanted with AS03 vs. ChAdOx1-S. Interim analysis found that two-dose vaccination with GBP510/AS03 induced stronger neutralising antibody immune responses compared with ChAdOx1-S against the ancestral D614G strain at 2 weeks after the second dose. Although declined over time, neutralizing antibody immunity was still favourable for the GBP510/AS03 group compared to the ChAdOx1-S group through 6 months after the second vaccination. Also, GBP510/AS03 showed an acceptable safety profile during 6 months of follow-up.
Implications of all the available evidence
GBP510/AS03 induces strong neutralising antibody responses against ancestral SARS-CoV-2 strain and has an acceptable safety profile after a primary vaccination series. Additional studies on the long-term immunogenicity of GBP510/AS03 booster vaccination after homologous or heterologous priming are ongoing, and they would provide important information when considering once-a-year COVID-19 vaccination strategy.
Introduction
Multiple vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), based on different underlying technologies, have been approved in different countries.1
GBP510 is a recombinant protein vaccine consisting of self-assembling, two-component nanoparticles displaying SARS-CoV-2 spike receptor-binding domains (RBDs).2,3 It is adjuvanted with AS03, which contains α-tocopherol and squalene4 and enhances the immune response to the vaccine antigen.4 The vaccine can be stored at regular refrigerator temperatures (2–8 °C) which makes it suitable for rollout in parts of the world where the requirement for ultra-cold chain handling can be challenging. A phase 1/2 study showed that GBP510 adjuvanted with AS03 (hereafter GBP510/AS03) was highly immunogenic and well tolerated in healthy adults aged 19–85 years.5
Thresholds for immune correlates of protection based on antibody levels or functional activity have not yet been established.6 However, in 2021, the International Coalition of Medicines Regulatory Authorities determined that immunobridging studies were acceptable as part of the strategy for assessing new COVID-19 vaccines,7 a position which has since been adopted by regulatory bodies, including members of the Access Consortium (national regulatory authorities of Australia, Canada, Singapore, Switzerland and the UK).8
The aim of the current study was to assess the immunogenicity and safety of GBP510/AS03 (SKYCovione™, SK Bioscience Co., Ltd., Korea) for the prevention of COVID-19, based on the assumption that neutralising antibody titres would predict efficacy against the parental D614G strain. The primary objective was to demonstrate that the immune response induced by two doses of GBP510/AS03 administered at a 1-month interval in seronegative adults was superior (based on geometric mean titre [GMT] of neutralising antibodies) and non-inferior (based on seroconversion rate [SCR]) to the immune response induced by two doses of the ChAdOx1-S vaccine (Vaxzevria, AstraZeneca)9 against the ancestral strain using live virus neutralising assays. The secondary objective was to assess the safety profile of GBP510/AS03 regardless of baseline serostatus.
Follow-up of participants until 12 months after their second vaccination is ongoing. The interim analysis reported here encompasses immunogenicity data up to 2 weeks and safety data up to 4 weeks after the second dose (data cut-off date: 18 March 2022). In addition, the long-term follow-up data is included up to 6 months after the second vaccination (data cut-off date: 09 August 2022).
Methods
Study design and participants
This was a randomised, active-controlled, observer-blinded, parallel group, phase 3 study, conducted at 38 sites across six countries (South Korea, Philippines, Thailand, Vietnam, Ukraine and New Zealand).
Healthy or medically stable adults aged ≥18 years were enrolled into one of two cohorts (Figure S1). Cohort 1 (Immunogenicity cohort) to enrol 1950 individuals with no history of SARS-CoV-2 infection or COVID-19 vaccination (confirmed by a rapid antibody kit and medical interview). Cohort 2 (Safety cohort) to enrol 2040 individuals irrespective of their serostatus at screening (Figure S1).
Individuals were excluded from either cohort if they met any of the following criteria: clinically significant respiratory symptoms, febrile illness, or acute illness within 72 h; previous SARS or middle east respiratory syndrome (MERS) infection, or SARS/MERS vaccination confirmed by medical history check; receipt of medications/vaccinations aimed at preventing COVID-19; immunocompromised conditions; autoimmune diseases; bleeding disorders, thrombocytopenia, thrombosis or capillary leak syndrome; malignancy within 1 year; hypersensitivity to any vaccines; receipt of any other vaccine between 4 weeks (2 weeks for influenza vaccines) before first and 28 days after last study vaccination; receipt of immunoglobulins, blood products or systemic corticosteroids within 12 weeks; meeting any restriction for ChAdOx1-S; significant unstable chronic illness; pregnancy or breast feeding.
The study was designed by SK Bioscience with support from GlaxoSmithKline (GSK), the International Vaccine Institute and the Coalition for Epidemic Preparedness Innovations (CEPI). The study was performed in accordance with the Declaration of Helsinki and the International Council for Harmonization Good Clinical Practice guidelines. The protocol (Supplementary Material) was approved by the Institutional Review Board for each participating health facility, and written informed consent was obtained from all participants. The reporting of this study complies with the Consolidated Standards of Reporting Trials (CONSORT) 2010 Statement. This study is registered on ClinicalTrials.gov (NCT05007951).
Randomisation and masking
For Cohort 1 sites, participants were randomised in a 2:1 ratio to receive GBP510/AS03 or ChAdOx1-S. For Cohort 2 sites, participants were randomised in a 5:1 ratio to GBP510/AS03 or ChAdOx1-S. Centralised interactive response technology was used to randomly allocate participants to treatment according to pre-generated block randomisation schedules (fixed block size of 6 for Cohort 1; 12 for Cohort 2) stratified by age (18–64 or ≥65 years) and trial site. Except for pharmacy staff and vaccinators, all other study and laboratory personnel, and participants were blinded to treatment assignment.
Procedures
The study vaccine GBP510, which contains self-assembling, two-component nanoparticles (RBD-16GS-I53-50) that display SARS-CoV-2 spike (S) protein RBDs, was developed by the Institute for Protein Design at the University of Washington and SK Bioscience. The AS03 adjuvant (an α-tocopherol-containing oil-in-water emulsion) was developed by GlaxoSmithKline.4 The control vaccine ChAdOx1-S was Chimpanzee Adenovirus encoding the SARS-CoV-2 spike glycoprotein (Vaxzevria, AstraZeneca).10
Study participants with no history of SARS-CoV-2 infection and COVID-19 vaccination confirmed by a SARS-CoV-2 rapid antibody kit at screening were enrolled in Cohort 1. Participants in Cohort 2 were enrolled regardless of their serostatus at screening.
Each participant received two intramuscular injections (0.5 mL volume) of vaccine into the deltoid muscle at a 28-day interval. Each dose of the GBP510/AS03 vaccine contained RBD 25 μg (0.25 mL) adjuvanted with AS03 (0.25 mL). Each dose of ChAdOx1-S contained no less than 2.5 × 108 infectious units/0.5 mL.
Safety evaluations were performed for all participants who received at least one dose of study intervention (Cohort 1 and 2). Immunogenicity assessments were performed in Cohort 1 only.
All participants were observed for at least 30 min after each vaccination for immediate adverse events. Participants used diary cards to record solicited local adverse events (AEs) (redness, swelling, and pain at injection site) and solicited systemic AEs (fever, nausea/vomiting, diarrhoea, fatigue, myalgia, arthralgia, chills) for 7 days after each vaccination, and unsolicited AEs within 28 days after each vaccination. Serious AEs (SAEs), medically-attended AEs (MAAEs), AEs leading to study withdrawal, and AEs of special interest (AESIs; Tables S1 and S2) were recorded throughout the entire study period. AE severity was based on the US Food and Drug Administration (FDA) toxicity grading scale.11 All solicited local and systemic AEs were considered related to the study intervention; the causal relationship for unsolicited AE was assessed by the investigators. The participants were instructed to report SARS-CoV-2 infection to investigator immediately with valid test results (i.e., polymerase chain reaction [PCR] test). Safety data were reviewed during the study by an independent safety monitoring board.
In vitro qualitative detection of antibodies to SARS-CoV-2 N-protein, which has higher accuracy than rapid antibody kit, was performed at 2 weeks after the second dose to ensure exclusion of seropositive participants for the primary immunogenicity analysis.
Blood samples for assessment of IgG antibody response and neutralisation assays were planned to be obtained from Cohort 1 participants at baseline, 4 weeks after the first vaccination, and at 2 weeks, 4 weeks, 6 months, and 12 months after the second vaccination. Blood samples for cell-mediated immunity assessments were collected at baseline and 2 weeks, 6 months, and 12 months after the second vaccination from a subset of approximately 10% of Cohort 1, selected pragmatically in advance to retain the randomisation ratio between study groups.
The neutralising antibody response to SARS-CoV-2 was measured using a focus reduction neutralisation test (FRNT) which assesses neutralising antibody titres in serum induced by viral infection/vaccination with live virus. The IgG antibody response to SARS-CoV-2 RBD was measured using enzyme-linked immunosorbent assay (ELISA). Cell-mediated immune responses were assessed using FluoroSpot assays to measure cytokine secretion in cells induced by external antigens such as viruses or vaccines, and intracellular cytokine staining (ICS), a flow-cytometry-based assay that detects specific T-cell types (e.g., CD4+ and CD8+) after cellular immunity is induced by external antigens. Cytokines included interferon (IFN)-γ, tumour necrosis factor (TNF)-α, interleukin (IL)-2, and IL-4.
Outcomes
The co-primary endpoints were neutralising antibody titres (GMT and SCR) to SARS-CoV-2 parental strain (D614G) measured by live virus neutralisation assay (FRNT) at 2 weeks after the second vaccination. Comparison between groups was measured as GMT ratio and SCR difference, defined as the percentage of participants with ≥4-fold rise in live virus neutralising antibody titre from baseline to 2 weeks after the second vaccination.
Secondary immunogenicity endpoints included titres of SARS-CoV-2 RBD-binding IgG antibody measured by ELISA and neutralising antibody to SARS-CoV-2 measured by live virus neutralisation assay, at each timepoint post-vaccination, measured as GMT, GMT ratio, geometric mean fold rise (GMFR) from baseline, SCR and SCR difference. Additionally, cell-mediated responses for cytokines, measured by FluoroSpot and, for CD4+ and CD8+ T-cells, measured by ICS, after the second vaccination were considered. Safety endpoints (secondary endpoints) included: immediate unsolicited systemic reactions (within 30 min post-vaccination); solicited local and systemic AEs within 7 days post-vaccination; unsolicited AEs within 28 days post-vaccination; SAEs, MAAEs and AESIs during the whole study period.
The interim analysis includes immunogenicity up to 2 weeks and safety data (including immediate unsolicited systemic AEs, solicited local/systemic AEs, unsolicited AEs, SAEs, MAAEs, AEs leading to withdrawal, and AESIs) up to 4 weeks after the second vaccination. Additionally, 6 months of long-term immunogenicity and safety follow-up key results after the last vaccination is included in this interim analysis report. The final results of 12 months (durability of response and safety) are not yet available, expected to be available by the end of 2023. In addition, extension study of homologous and heterologous booster vaccination is ongoing.
Statistical analysis
The sample size for Cohort 1 (Immunogenicity Cohort) was based on neutralisation assay results from the Phase 3 study of ChAdOx1-S8 and the Phase 1/2 study for GBP510/AS035 to demonstrate superiority of GMT ratio and non-inferiority of SCR difference in terms of neutralising antibody titres. The planned sample size for Cohort 1 was 1950 (1300 test group, 650 control group) and that for Cohort 2 was 2040 (1700 test group, 340 control group); overall, this would ensure safety data were available for at least 3000 recipients of the two-dose GBP510/AS03 regimen. This sample size was based on FDA guidance for vaccine industry,12 which may allow the observance of at least one adverse event occurring at a frequency of 1 in 1000. If the true AE rate was 0.1%, there would be a 95.03% probability of observing at least 1 AE in a test group comprising 3000 participants. As calculated sample size was sufficient to test immunogenicity hypothesis and the safety was evaluated in randomised/observer blind manner to avoid preferential bias, the study was approved by national regulatory authorities.
All participants who received at least one dose of study vaccine were included in the safety set (Cohort 1 & 2). The full analysis set comprised all participants who received at least 1 dose of the study vaccine and had valid pre- and at least one post-vaccination immunogenicity assessment results. The interim analysis of immunogenicity was primarily reported for the per-protocol set—who completed vaccination schedule without SARS-CoV-2 infection with no major protocol deviations. In addition, the baseline neutralizing antibody titer below LLOQ (Lower Limit of Quantification) in live neutralization assay and a negative result for the in vitro qualitative detection of antibodies to SARS-CoV-2 N-protein at 2 weeks after the second dose was required for per-protocol set (PPS) inclusion; presence of neutralizing antibody below the detectable range of assay is widely accepted as ‘no infection or exposure’ in the field. For the co-primary endpoints, adjusted post-vaccination GMT ratio estimate and 95% CIs were determined using analysis of covariance (ANCOVA) on log-transformed titres with treatment group and age (18–64 or ≥65 years) as factors, and baseline antibody level (titre) as covariate, while the 95% CIs for the difference in SCRs were calculated based on Chan and Zhang methodology (exact method for the difference of binomial proportion).13 Superiority of post-vaccination GMT was demonstrated if the lower limit of the two-sided 95% CI for the ratio of post-vaccination GMTs at 2 weeks after the second study vaccination (GBP510/AS03/ChAdOx1-S) was greater than 1. Non-inferiority of SCR was demonstrated if the lower limit of the two-sided 95% CI for the difference in the percentage of participants with a ≥4-fold rise from baseline in neutralisation antibody titre at 2 weeks after the second vaccination (GBP510/AS03—ChAdOx1-S) exceeded the non-inferiority margin of −5%. For secondary immunogenicity endpoints, point estimates and 95% CI, or summary statistics (n, mean, standard deviation [SD], median, min and max), were presented for each treatment group. For safety endpoints, number and percentages of participants with at least one AE were presented by treatment group.
Role of the funding source
The funders had no role in the study design, collection, analysis, and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication. All authors had full access to all the data in the study and responsibility for the decision to submit for publication.
Results
Characteristics of participants
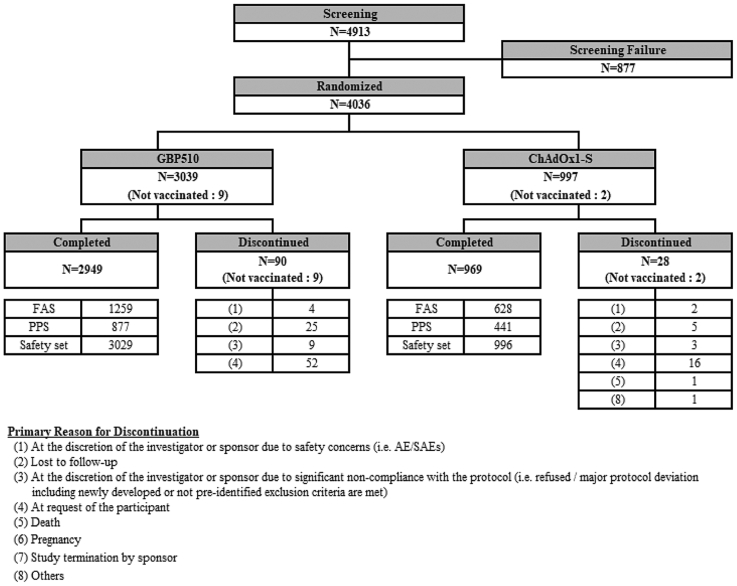
Between 30 August 2021 and 10 February 2022, a total of 4036 participants (intention-to-treat set [ITT set], 1956 in Cohort 1 and 2080 in Cohort 2) were randomised to receive GBP510/AS03 (n = 3039) or ChAdOx1-S (n = 997); Fig. 1. Total 11 participants were excluded after randomization and did not receive study vaccine (8 requested to be withdrawn; 2 excluded due to significant non-compliance with the protocol; 1 excluded due to safety concerns). Those who received at least 1 dose of study vaccination was included in the Safety Set, which were a total 4025 participants (3029 in the GBP510/AS03 group and 996 in the ChAdOx1-S group). Among Cohort 1 participants, 1887 (1259 in the GBP510/AS03 group and 628 in the ChAdOx1-S group) received at least 1 dose of the study vaccine and had valid pre- and at least one post-vaccination immunogenicity results (Full Analysis Set). The per-protocol set for the immunogenicity up to 2 weeks after the second vaccination included 1318 participants (877 in the GBP510/AS03 group and 441 in the ChAdOx1-S group) (Figure S2). The long-term follow-up of 6 months data includes total 3629 participants (2750 participants from GBP510/AS03 and 879 participants from ChAdOx1-S group). Total 370 participants discontinued the study, and 37 participants were enrolled to participate in extension stage of the booster vaccination (See Tables S3 and S5). Overall, the per-protocol set for the extension long-term immunogenicity stage (from 2 weeks to 6 months after the second vaccination) included 604 participants in GBP510/AS03 and 310 participants in ChAdOx1-S group.
Fig. 1.
Study diagram. After randomization, total 11 participants were not treated with study vaccine and not included in the safety set (8 requested to be withdrawn; 2 excluded due to significant non-compliance with the protocol–i.e., COVID-19 infection and receiving prohibited vaccination after randomization; 1 excluded due to safety concerns–i.e., mild degree of fever after randomization and within 72 h prior to 1st vaccination). FAS, full analysis set; PPS, per-protocol set.
The demographics and baseline characteristics of study participants (ITT set) are summarised in Table 1. Most participants were Southeast Asian (81.5%). There were more males (59.1%) than females (40.9%). The mean age of participants was 38.2 (SD, 13.8) years; the mean age of the GBP510/AS03 group was slightly lower than that of the ChAdOx1-S group (37.8 [SD, 13.8] vs. 39.3 [SD, 13.8]). However, the age strata distribution did not differ between the groups, with a total of 94.7% of participants aged 18–64 years. The mean body mass index was 23.7 (4.3) kg/m2. The overall trends of demographics and baseline characteristics between two groups in the ITT were also similar in the PPS (See Table S6). Participants with one or more comorbidities, defined by the Centres for Disease Control and Prevention as a risk factor for severe Covid-19, at baseline were reported by 17.7% of GBP510/AS03 group and 21.3% of ChAdOx1-S group (See Table S33).
Table 1.
Baseline characteristics (intention-to-treat set).
| GBP510/AS03 (N = 3039) | ChAdOx1-S (N = 997) | Total (N = 4036) | |
|---|---|---|---|
| Age (years), mean (SD) | 37.8 (13.8) | 39.3 (13.8) | 38.2 (13.8) |
| 18–24 years, n (%) | 596 (19.6) | 165 (16.6) | 761 (18.9) |
| 25–49 years, n (%) | 1864 (61.3) | 608 (61.0) | 2472 (61.3) |
| 50–64 years, n (%) | 418 (13.8) | 170 (17.1) | 588 (14.6) |
| 18–64 years, n (%) | 2878 (94.7) | 943 (94.6) | 3821 (94.7) |
| ≥65 years, n (%) | 161 (5.3) | 54 (5.4) | 215 (5.3) |
| Male/Female, n (%) | |||
| Male | 1814 (59.7) | 572 (57.4) | 2386 (59.1) |
| Female | 1225 (40.3) | 425 (42.6) | 1650 (40.9) |
| Race, n (%) | |||
| Asian | 2856 (94.0) | 931 (93.4) | 3787 (93.8) |
| Korean | 329 (10.8) | 168 (16.9) | 497 (12.3) |
| Southeast Asian | 2526 (83.1) | 762 (76.4) | 3288 (81.5) |
| Other Asian | 1 (0.03) | 1 (0.1) | 2 (0.05) |
| Caucasian | 177 (5.8) | 64 (6.4) | 241 (6.0) |
| Black | 0 | 0 | 0 |
| Hispanic | 0 | 0 | 0 |
| Other | 6 (0.2) | 2 (0.2) | 8 (0.2) |
| Body mass index (kg/m2), mean (SD) | 23.6 (4.4) | 23.9 (4.3) | 23.7 (4.3) |
| Concomitant illness, n (%) | 486 (16.0) | 204 (20.5) | 690 (17.1) |
| Concomitant medication, n (%) | 1232 (40.5) | 411 (41.2) | 1643 (40.7) |
SD = standard deviation, Min = minimum, Max = maximum, BMI = body mass index. Denominator of percentage is the number of participants in each group.
BMI (kg/m2) = weight (kg)/[height (cm) × 0.01]2.
Immunogenicity outcomes
Live virus neutralisation assays (FRNT)
The co-primary endpoints were based on measurement of the neutralising antibody response (ND50 presented as IU/mL using the WHO standard) to SARS-CoV-2 using live virus neutralisation assays (FRNT). Post-vaccination GMTs were adjusted for age group (18–64 or ≥65 years) and baseline antibody level.
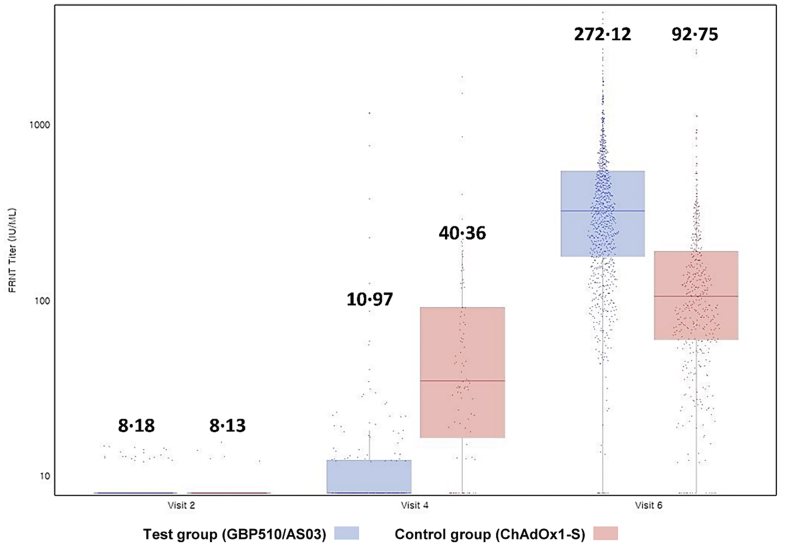
At baseline, there was no significant difference in GMT between the GBP510/AS03 group and the ChAdOx1-S group (8.18 vs. 8.13 IU/mL, respectively). At 4 weeks after the first vaccination, GMT, GMFR, and SCR were higher in the ChAdOx1-S group than in the GBP510/AS03 group, with between-group differences for the GMT ratio and SCR achieving statistical significance (Fig. 2 and Table 2).
Fig. 2.
Boxplots and individual data for the natural logarithmic titre by live virus neutralisation assay at baseline (visit 2), 4 weeks after first vaccination (visit 4), and 2 weeks after second vaccination (visit 6) (per-protocol set). A total of 877 participants in GBP510/AS03 and 441 participants in ChAdOx1-S were evaluated for Neutralization antibody by FRNT ND50 (Unit: IU/mL). Visit 4 (4 weeks after first vaccination) assessment was conducted only in the subset population, comprised of approximately 20% of PPS population (195 and 96 participants from GBP510/AS03 and ChAdOx1-S, respectively). The adjusted post vaccination GMTs was used in the superiority test. FRNT, focus reduction neutralisation test; PPS, per-protocol set; GMT, geometric mean titre.
Table 2.
Neutralising antibody response to SARS-CoV-2 by FRNT ND50 up to 2 weeks after the second vaccination (per-protocol set).
| GBP510/AS03 (N = 877) | ChAdOx1-S (N = 441) | |
|---|---|---|
| Baseline | ||
| n | 877 | 441 |
| GMT (SD) | 8.18 (1.08) | 8.13 (1.06) |
| 95% Confidence Interval | [8.14, 8.22] | [8.09, 8.17] |
| Ratio of GMTs (GBP510/ChAdOx1-S) (SD) | 1.01 (NA) | |
| 95% Confidence Interval | [1.00, 1.01] | |
| p-valuea | 0.094 | |
| Visit 4 (4 weeks after 1st vaccination) | ||
| n | 195 | 96 |
| GMT (SD) | 11.32 (2.25) | 41.43 (3.46) |
| 95% Confidence Interval | [10.10, 12.69] | [32.22, 53.28] |
| Ratio of GMTs (GBP510/ChAdOx1-S) (SD) | 0.27 (NA) | |
| 95% Confidence Interval | [0.21, 0.36] | |
| p-valuea | <0.0001 | |
| GMFR(SD) | 1.39 (2.25) | 5.10 (3.42) |
| 95% Confidence Interval | [1.24, 1.56] | [3.98, 6.55] |
| Adjusted GMT (SE) | 10.97 (1.14) | 40.36 (1.15) |
| 95% Confidence Interval | [8.43, 14.28] | [30.45, 53.51] |
| Ratio of GMTs (GBP510/ChAdOx1-S) (SE) | 0.27 (1.13) | |
| 95% Confidence Interval | [0.21, 0.34] | |
| p-valueb | <0.0001 | |
| Participants with ≥4-fold rise, n (%) | 11 (5.64) | 54 (56.25) |
| 95% Confidence Interval | [2.85, 9.87] | [45.75, 66.36] |
| Difference in Proportions of the Participant with ≥4-fold rise | −50.61 | |
| 95% Confidence Interval | [-60.89, −39.62] | |
| p-valuec | <0.0001 (c) | |
| Visit 6 (2 weeks after 2nd vaccination) | ||
| n | 877 | 441 |
| GMT (SD) | 300.66 (2.54) | 102.21 (2.76) |
| 95% Confidence Interval | [282.68, 319.78] | [92.95, 112.39] |
| Ratio of GMTs (GBP510/ChAdOx1-S) (SD) | 2.94 (NA) | |
| 95% Confidence Interval | [2.63, 3.29] | |
| p-valuea | <0.0001 | |
| GMFR (SD) | 36.76 (2.54) | 12.58 (2.76) |
| 95% Confidence Interval | [34.56, 39.11] | [11.44, 13.83] |
| Adjusted GMT (SE) | 272.12 (1.07) | 92.75 (1.07) |
| 95% Confidence Interval | [240.40, 308.02] | [80.79, 106.48] |
| Ratio of GMTsd (GBP510/ChAdOx1-S) (SE) | 2.93 (1.06) | |
| 95% Confidence Interval | [2.63, 3.27] | |
| p-valueb | <0.0001 | |
| Lower Limit >1 | Yes | |
| Participants with ≥4-fold rise, n (%) | 860 (98.06) | 385 (87.30) |
| 95% Confidence Interval | [96.91, 98.87] | [83.83, 90.26] |
| Difference in Proportions of the Participant with ≥4-fold rised | 10.76 | |
| 95% Confidence Interval | [7.68, 14.32] | |
| p-valuec | <0.0001 (c) | |
| Lower Limit >−5% | Yes | |
GMT = geometric mean titer, SD = standard deviation, SE = standard error, GMFR = geometric mean fold rise, ANCOVA = analysis of covariance, NA = not applicable.
Assay unit: IU/mL.
GMT = anti-logarithm [mean of natural logarithm (titer at visit n)], GMFR = anti-logarithm [mean of natural logarithm (titre at visit n/titre at visit 2)].
The test results are collected for randomly selected participants (about 20% of all participants) at Visit 4.
When two samples had unequal variance, the standard deviation for the ratio of GMT is presented ‘NA’.
The 95% confidence intervals for GMT and GMFR are calculated using Wald method with t-distribution.
The 95% CI of percentage of participants ≥ 4-fold rises is calculated based on Clopper-Pearson method.
The 95% CI of the difference between groups is calculated based on Chan and Zhang method.
If ANCOVA model has infinite likelihood, ANCOVA under the assumption of homoscedasticity is used.
Testing for difference between treatment groups (two sample t-test).
ANCOVA model with treatment group, age group (18∼64, ≥65) as factors, and baseline antibody level as covariate.
Testing for difference between treatment groups (chi-square test (c) or Fisher's exact test (f)).
Co-primary Endpoint (superiority based on the 2-sided 95% CI of the ratio of post-vaccination GMT (GBP510 over ChAdOx1-S); non-inferiority based on the 2-sided 95% CI of the difference in the percentages of participants with ≥ 4-fold rise from baseline (GBP510—ChAdOx1-S) in neutralization antibody titre).
At 2 weeks after the second vaccination (when the co-primary endpoints were evaluated), GMT and SCR were higher in the GBP510/AS03 group than in the ChAdOx1-S group, as follows. The GMTs adjusted for age group (18–64 years, ≥65 years) and baseline antibody level was 272.12 IU/mL (95% CI 240.4–308.0) for GBP510/AS03 and 92.75 IU/mL (95% CI 80.8–106.5) for ChAdOx1-S group. The adjusted GMT ratio (GBP510/AS03/ChAdOx1-S) was 2.93 (95% CI 2.63–3.27), satisfying the hypothesis of superiority (lower limit of 95% CI > 1); The SCR of two groups were 98.1% (860/877 participants) in GBP510/AS03 group (95% CI 96.9–98.9) and 87.3% (385/441 participants) in ChAdOx1-S group (95% CI 83.8–90.3). The SCR difference between groups was 10.76% (95% CI 7.68–14.32), satisfying the hypothesis of non-inferiority (lower limit of 95% CI > −5%). The test for independence between SCR and group was statistically significant (p < 0.0001). In addition, the GMFR (SD) was also higher in the GBP510/AS03 group, 36.76 (2.54), than in the ChAdOx1-S group, 12.58 (2.76), at 2 weeks after the second vaccination (Table 2).
The neutralising antibody response decreased gradually through 6 months after primary vaccination series. However, it was still favourable for the GBP510/AS03 group compared to the ChAdOx1-S group, with higher GMT and SCR. The adjusted GMT was 218.0 IU/mL (95% CI 170.7–278.4) in GBP510/AS03 group (n = 604) and 130.0 IU/mL (95% CI 99.0–170.7) in ChAdOx1-S group (n = 310), respectively (Table S7). The adjusted GMT ratio was 1.68 (95% CI 1.34–2.09), with statistical significance (the lower limit of the 95% CI > 1.0) (p < 0.0001). The SCR were 89.6% (541/604 participants) in GBP510/AS03 group (95% CI 86.9–91.9) and 66.1% (205/310 participants) in ChAdOx1-S group (95% CI 60.6–71.4).
In subgroup analyses, higher neutralising response with GBP510/AS03 were also seen at 2 weeks after the second vaccination in younger (18–64 years) and older (≥65 years) age groups, in male and female, across ethnicities (Korean, Southeast Asian and Caucasian), and in subgroup participants who had neutralising antibody above LLOQ at baseline, as seen in the PPS (i.e., seronegative population) (Tables S9–S12).
In addition, neutralising antibody GMTs against VOCs (Variants of Concerns) assessed by FRNT ND50 (not converted to IU/mL) at 2 weeks after the second vaccination were also higher with GBP510/AS03 than with ChAdOx1-S; against the Delta (B.1.617.2) variant (2644.2 vs. 96.96; GMT ratio 27.27 [95% CI 18.88–39.39], p < 0.0001), the Omicron BA.1 (B.1.1.529) variant (129.09 vs. 12.27; GMT ratio 10.52 [95% CI 8.18–13.53], p < 0.0001), and the Omicron BA.5 sub-lineage (61.61 vs. 13.56; GMT ratio 4.54 [95% CI 3.51–5.88], p < 0.0001) in a same subset of participants as observed against ancestral strain (1666.4 for GBP510/AS03 and 415.1 for ChAdOx1-S group, respectively) (Tables S13–S16).
Enzyme-linked immunosorbent assay (ELISA)
The GMTs of SARS-CoV-2 RBD-binding IgG antibody measured by ELISA at baseline were similar between the two groups with no significant difference (p = 0.74). At both 4 weeks after the first vaccination, and at 2 weeks after the second vaccination, GMT, GMFR, and the SCR were higher in the GBP510/AS03 group than in the ChAdOx1-S group, with differences in the GMT ratio and SCR achieving statistical significance at both timepoints (both p < 0.0001) (Table 3 and Figure S3). As seen in the neutralising antibody response, binding antibody response decreased gradually through 6 months of long-term follow-up for both test groups and it was still favourable for the GBP510/AS03 group than reported in the ChAdOx1-S group, in terms of both GMT and SCR (Table S8).
Table 3.
SARS-CoV-2 RBD-binding IgG antibody measured by enzyme-linked immunosorbent assay (ELISA) (per-protocol set).
| GBP510/AS03 (N = 877) | ChAdOx1-S (N = 441) | |
|---|---|---|
| Baseline | ||
| n | 877 | 441 |
| GMT (SD) | 10.91 (1.75) | 10.79 (1.70) |
| 95% Confidence Interval | [10.51, 11.32] | [10.27, 11.34] |
| Ratio of GMTs (GBP510/ChAdOx1-S) (SD) | 1.01 (1.74) | |
| 95% Confidence Interval | [0.95, 1.08] | |
| p-valuea | 0.74 | |
| Visit 4 (4 weeks after 1st vaccination) | ||
| n | 877 | 441 |
| GMT (SD) | 171.73 (2.28) | 119.46 (2.42) |
| 95% Confidence Interval | [162.58, 181.40] | [109.98, 129.75] |
| Ratio of GMTs (GBP510/ChAdOx1-S) (SD) | 1.44 (2.33) | |
| 95% Confidence Interval | [1.30, 1.58] | |
| p-valuea | <0.0001 | |
| GMFR(SD) | 15.74 (2.57) | 11.07 (2.72) |
| 95% Confidence Interval | [14.79, 16.76] | [10.08, 12.15] |
| Adjusted GMT (SE) | 131.24 (1.06) | 91.91 (1.06) |
| 95% Confidence Interval | [117.90, 146.09] | [81.55, 103.57] |
| Ratio of GMTs (GBP510/ChAdOx1-S) (SE) | 1.43 (1.05) | |
| 95% Confidence Interval | [1.30, 1.57] | |
| p-valueb | <0.0001 | |
| Participants with ≥4-fold rise, n (%) | 811 (92.47) | 374 (84.81) |
| 95% Confidence Interval | [90.52, 94.13] | [81.11, 88.03] |
| Difference in Proportions of the Participant with ≥4-fold rise | 7.67 | |
| 95% Confidence Interval | [3.89, 11.44] | |
| p-valuec | <0.0001 (c) | |
| Visit 6 (2 weeks after 2nd vaccination) | ||
| n | 877 | 441 |
| GMT(SD) | 3230.35 (2.10) | 248.45 (2.23) |
| 95% Confidence Interval | [3074.90, 3393.67] | [230.46, 267.84] |
| Ratio of GMTs (GBP510/ChAdOx1-S) (SD) | 13.00 (2.15) | |
| 95% Confidence Interval | [11.91, 14.19] | |
| p-valuea | <0.0001 | |
| GMFR(SD) | 296.10 (2.57) | 23.02 (2.48) |
| 95% Confidence Interval | [278.11, 315.24] | [21.14, 25.06] |
| Adjusted GMT(SE) | 2850.45 (1.05) | 215.67 (1.06) |
| 95% Confidence Interval | [2586.51, 3141.32] | [193.52, 240.36] |
| Ratio of GMTs (GBP510/ChAdOx1-S) (SE) | 13.22 (1.04) | |
| 95% Confidence Interval | [12.13, 14.40] | |
| p-valueb | <0.0001 | |
| Participants with ≥4-fold rise, n (%) | 873 (99.54) | 427 (96.83) |
| 95% Confidence Interval | [98.84, 99.88] | [94.73, 98.25] |
| Difference in Proportions of the Participant with ≥4-fold rise | 2.72 | |
| 95% Confidence Interval | [1.02, 4.41] | |
| p-valuec | <0.0001 (c) | |
GMT = geometric mean titer, SD = standard deviation, SE = standard error, GMFR = geometric mean fold rise, ANCOVA = analysis of covariance.
Assay unit: BAU/mL.
GMT = anti-logarithm [mean of natural logarithm (titre at visit n)], GMFR = anti-logarithm [mean of natural logarithm (titre at visit n/titre at visit 2)].
95% confidence intervals for GMT and GMFR are calculated using Wald method with t-distribution.
The 95% CI of percentage of participants ≥ 4-fold rises is calculated based on Clopper-Pearson method.
The 95% CI of the difference in percentage of participants ≥ 4-fold rises between groups was calculated based on Wald method.
Testing for difference between treatment groups (two sample t-test).
ANCOVA model with treatment group, age group (18∼64, ≥65) as factors, and baseline antibody level as covariate.
Testing for difference between treatment groups (chi-square test (c) or Fisher's exact test (f)).
Cell-mediated immune response
The cell-mediated immune response was evaluated in a subset of Cohort 1 participants. At 2 weeks after the second vaccination, the median cell counts for IFN-γ, TNF-α, and IL-2 increased from baseline in both groups based on FluoroSpot assays (Table 4 and Figures S4–S6). In particular, noticeable increases in TNF-α response (from median 23 at baseline to 52 spot forming cell [SFC]/2.5 × 105 peripheral blood mononuclear cells [PBMCs] at 2 weeks after the second vaccination) and IL-2 response (from 24 at baseline to 57 SFC/2.5 × 105 PBMCs at 2 weeks after the second vaccination) were seen in the GBP510/AS03 group. No change in IL-4 was seen in either group (Table 4 and Figure S4–S7).
Table 4.
Cell-mediated immune response by FluoroSpot assay (quantification of cytokine-secreting cells) (per-protocol set).
| GBP510/AS03 (N = 877) | ChAdOx1-S (N = 441) | |
|---|---|---|
| [cytokines: IFN-γ] | ||
| Baseline | ||
| n | 76 | 40 |
| Mean (SD) | 16.3 (36.9) | 11.5 (9.6) |
| Median | 7 | 7 |
| IQR | 7–16 | 7–13 |
| Min, Max | 0, 304 | 0, 39 |
| p-valuea | 0.29 | |
| Visit 6 (2 weeks after 2nd vaccination) | ||
| n | 73 | 37 |
| Mean (SD) | 22.1 (17.9) | 16.7 (15.0) |
| Median | 20 | 15 |
| IQR | 7–27 | 7–20 |
| Min, Max | 0, 103 | 0, 86 |
| p-valuea | 0.12 | |
| [cytokines: IL-2] | ||
| Baseline | ||
| N | 76 | 40 |
| Mean (SD) | 34.6 (33.6) | 28.9 (26.9) |
| Median | 24 | 19.5 |
| IQR | 12.5–45.5 | 12.5–36.5 |
| Min, Max | 0, 159 | 6, 108 |
| p-valuea | 0.35 | |
| Visit 6 (2 weeks after 2nd vaccination) | ||
| n | 71 | 37 |
| Mean (SD) | 71.5 (57.2) | 42.5 (51.4) |
| Median | 57 | 27 |
| IQR | 34–95 | 18–44 |
| Min, Max | 0, 300 | 0, 284 |
| p-valuea | 0.011 | |
| [cytokines: IL-4] | ||
| Baseline | ||
| n | 75 | 40 |
| Mean (SD) | 17.8 (27.7) | 9.3 (9.1) |
| Median | 13 | 13 |
| IQR | 13–13 | 0–13 |
| Min, Max | 0, 166 | 0, 44 |
| p-valuea | 0.017 | |
| Visit 6 (2 weeks after 2nd vaccination) | ||
| n | 68 | 35 |
| Mean (SD) | 23.4 (23.1) | 13.5 (10.9) |
| Median | 13 | 13 |
| IQR | 13–30 | 13–13 |
| Min, Max | 0, 125 | 0, 51 |
| p-valuea | 0.0036 | |
| [cytokines: TNF-α] | ||
| Baseline | ||
| n | 73 | 38 |
| Mean (SD) | 21.8 (25.4) | 22.1 (23.3) |
| Median | 23 | 23 |
| IQR | 0–23 | 0–23 |
| Min, Max | 0, 112 | 0, 87 |
| p-valuea | 0.95 | |
| Visit 6 (2 weeks after 2nd vaccination) | ||
| N | 69 | 33 |
| Mean (SD) | 58.9 (48.6) | 56.9 (42.7) |
| Median | 52 | 47 |
| IQR | 23–84 | 23–76 |
| Min, Max | 0, 216 | 23, 178 |
| p-valuea | 0.84 | |
SD = standard deviation, IQR = Interquartile range, Min = minimum, Max = maximum, CMI = cell medicated immunity.
Assay unit: SFC/2.5 × 105 PBMCs.
Testing for difference between treatment groups (two sample t-test). The test result was collected for randomly selected participants (about 10% of all participants).
In ICS assessments of CD4+ T cells, some response was observed for TNF-α (median 0.23) and IL-2 (median 0.06) in the GBP510/AS03 group at 2 weeks after 2nd vaccination (Table 5 and Figures S8–S11). No response was observed in CD8+ T cells in either group (Table S19).
Table 5.
Cell-mediated immune response by intracellular cytokine staining (CD4+ T cells expressing cytokines) (per-protocol set).
| GBP510/AS03 (N = 877) | ChAdOx1-S (N = 441) | |
|---|---|---|
| [cytokines: IFN-γ] | ||
| Baseline | ||
| n | 70 | 36 |
| Mean (SD) | 0 (0.01) | 0 (0) |
| Median | 0 | 0 |
| IQR | 0–0.01 | 0–0 |
| Min, Max | 0, 0.05 | 0, 0.02 |
| p-valuea | 0.12 | |
| Visit 6 (2 weeks after 2nd vaccination) | ||
| n | 68 | 33 |
| Mean (SD) | 0.01 (0.02) | 0 (0.01) |
| Median | 0.01 | 0 |
| IQR | 0–0.01 | 0–0.01 |
| Min, Max | 0, 0.07 | 0, 0.02 |
| p-valuea | 0.018 | |
| [cytokines: IL-2] | ||
| Baseline | ||
| n | 70 | 36 |
| Mean (SD) | 0.02 (0.04) | 0.01 (0.02) |
| Median | 0.01 | 0.01 |
| IQR | 0.01–0.02 | 0–0.02 |
| Min, Max | 0, 0.20 | 0, 0.11 |
| p-valuea | 0.30 | |
| Visit 6 (2 weeks after 2nd vaccination) | ||
| n | 68 | 33 |
| Mean (SD) | 0.08 (0.08) | 0.03 (0.05) |
| Median | 0.06 | 0.02 |
| IQR | 0.04–0.11 | 0.01–0.04 |
| Min, Max | 0.01, 0.48 | 0, 0.24 |
| p-valuea | 0.0002 | |
| [cytokines: IL-4] | ||
| Baseline | ||
| n | 70 | 36 |
| Mean (SD) | 0 (0) | 0 (0) |
| Median | 0 | 0 |
| IQR | 0–0 | 0–0 |
| Min, Max | 0, 0.02 | 0, 0.01 |
| p-valuea | 0.51 | |
| Visit 6 (2 weeks after 2nd vaccination) | ||
| n | 68 | 33 |
| Mean (SD) | 0 (0) | 0 (0) |
| Median | 0 | 0 |
| IQR | 0–0 | 0–0 |
| Min, Max | 0, 0.02 | 0, 0.01 |
| p-valuea | 0.90 | |
| [cytokines: TNF-α] | ||
| Baseline | ||
| n | 70 | 36 |
| Mean (SD) | 0.24 (0.55) | 0.11 (0.22) |
| Median | 0.06 | 0.03 |
| IQR | 0.03–0.16 | 0.02–0.11 |
| Min, Max | 0, 3.20 | 0, 1.06 |
| p-valuea | 0.10 | |
| Visit 6 (2 weeks after 2nd vaccination) | ||
| n | 68 | 33 |
| Mean (SD) | 0.72 (1.11) | 0.39 (0.72) |
| Median | 0.23 | 0.09 |
| IQR | 0.11–1.00 | 0.07–0.24 |
| Min, Max | 0.06, 4.58 | 0, 3.12 |
| p-valuea | 0.073 | |
SD = standard deviation, IQR = Interquartile range, Min = minimum, Max = maximum, CMI = cell medicated immunity.
Assay unit: % of RBD-specific CD4+ T cells.
Testing for difference between treatment groups (two sample t-test). The test result was collected for randomly selected participants (about 10% of all participants).
Safety outcomes
Of 3029 participants in the GBP510/AS03 group and 996 participants in the ChAdOx1-S group analysed for the Safety Set, immediate systemic AEs (occurring within 30 min) after any vaccination were reported by six participants (0.2%) in the GBP510/AS03 group and in no participants in the ChAdOx1-S group (Table 6).
Table 6.
Overall adverse events after any vaccination up to four weeks after the second vaccination (safety set).
| (%) [no. of AEs] | GBP510/AS03 (N = 3029) | ChAdOx1-S (N = 996) |
|---|---|---|
| Immediate Unsolicited Systemic AEs (within 30 min) | 6 (0.20) [7] | 0 |
| Exact 95% CI | (0.07–0.43) | (0.00–0.37) |
| Immediate Unsolicited Systemic ADRs (within 30 min) | 5 (0.17) [6] | 0 |
| Exact 95% CI | (0.05–0.38) | (0.00–0.37) |
| Solicited Local AEs within 7 days | 1717 (56.69) [2946] | 490 (49.20) [677] |
| Exact 95% CI | (54.90–58.46) | (46.05–52.35) |
| Solicited Systemic AEs within 7 days | 1551 (51.21) [6133] | 533 (53.51) [2067] |
| Exact 95% CI | (49.41–53.00) | (50.36–56.65) |
| Unsolicited AEs within 28 days | 402 (13.27) [640] | 145 (14.56) [213] |
| Exact 95% CI | (12.08–14.53) | (12.43–16.90) |
| Unsolicited ADRs within 28 days | 106 (3.50) [164] | 41 (4.12) [59] |
| Exact 95% CI | (2.87–4.22) | (2.97–5.54) |
| SAEs within 28 days | 15 (0.50) [15] | 7 (0.70) [9] |
| Exact 95% CI | (0.28–0.82) | (0.28–1.44) |
| AESIs within 28 days | 2 (0.07) [2] | 1 (0.10) [1] |
| Exact 95% CI | (0.01–0.24) | (0.00–0.56) |
| MAAEs within 28 days | 147 (4.85) [172] | 50 (5.02) [69] |
| Exact 95% CI | (4.12–5.68) | (3.75–6.57) |
| MAADRs within 28 days | 17 (0.56) [25] | 10 (1.00) [18] |
| Exact 95% CI | (0.33–0.90) | (0.48–1.84) |
| AEs leading to study withdrawalawithin 28 days | 3 (0.10) [8] | 2 (0.20) [3] |
| Exact 95% CI | (0.02–0.29) | (0.02–0.72) |
| Participants with AEs leading to deathbwithin 28 days | 0 | 1 (0.10) [1] |
| Exact 95% CI | (0.00–0.12) | (0.00–0.56) |
AE = adverse event, ADR = adverse drug reaction, CI = confidence interval, SAE = serious adverse event, AESI = adverse event of special interest, MAAE = medically attended adverse event, MAADR = medically attended adverse drug reaction.
Data are presented as ‘number of participants (% participants) [number of events]’. Denominator of % is group N. 95% confidence interval was calculated by Clopper-Pearson Methods. SAEs, AESIs, MAAEs, MAADRs and death were reported for whole study period.
The case checked on ‘at the discretion of the investigator or sponsor due to safety concerns' as a primary reason for discontinuation and checked on ‘Stop Vaccination (only if prior to second vaccination)' as changes to IP (investigational product) vaccination.
The case checked on ‘Death’ as a primary reason for discontinuation and checked on ‘Fatal’ as outcome.
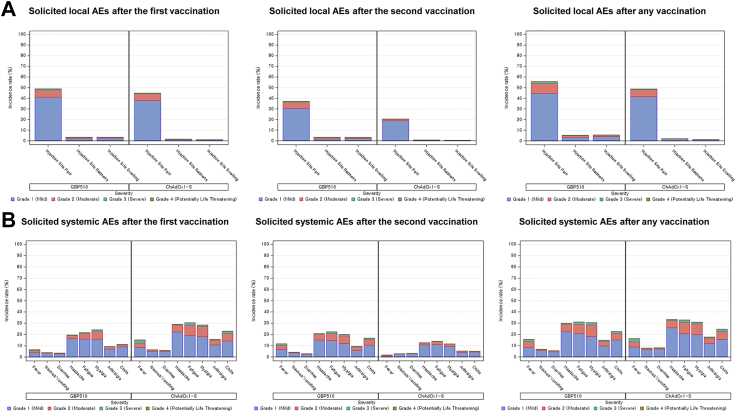
Solicited local AEs after any vaccination had a higher incidence in the GBP510/AS03 group (56.7% of participants) than in the ChAdOx1-S group (49.2% of participants; p < 0.0001). Injection site pain was the most common solicited local AE in both groups, reported by 55.7% of participants in the GBP510/AS03 group and 48.8% of participants in the ChAdOx1-S group; injection site redness was reported by 5.3% and 2.0% of participants, and injection site swelling by 5.6% and 1.2% of participants, respectively (Fig. 3).
Fig. 3.
Incidence rates for solicited local (A) and systemic (B) adverse events within 7 days after each-dose vaccination. Denominator of percentage is the number of participants in each group. Adverse events are displayed as ‘number of participants (percentage of participants)’. If one participant experienced the same adverse event more than once, the adverse event is counted only once with the most severe category.
Solicited systemic AEs after any vaccination were reported by 51.2% of participants in the GBP510/AS03 group and 53.5% of participants in the ChAdOx1-S group (p = 0.21). Headache (29.9% vs. 33.2%), fatigue (31.1% vs. 32.8%), and myalgia (30.5% vs. 30.8%) were the most commonly reported solicited systemic AEs in both groups after any vaccination. In the GBP510/AS03 group, the frequency of solicited systemic AEs was similar after the first (40.4%) and second doses (37.6%), while in the ChAdOx1-S group the frequency was higher after the first dose (49.5%) than after the second dose (25.0%) (Fig. 3).
Most solicited local and systemic AEs were grade 1 (mild) or 2 (moderate) in severity and lasted a mean of 1.3–3.1 days for both groups (Fig. 3, Tables S21 and S22). The proportion of participants who reported grade 3 (severe) solicited local AE after any vaccination was 1.9% in the GBP510/AS03 group and 0.6% in the ChAdOx1-S group. Grade 3 solicited systemic AEs were reported by 5.3% of participants in GBP510/AS03 group and 6.12% in ChAdOx1-S group. No grade 4 (potentially life threatening) solicited AE was reported in either group.
The proportions of participants aged 18–64 years with solicited local/systemic AEs were 56.7%/51.4% in the GBP510/AS03 group and 49.4%/53.7% in the ChAdOx1-S group, and in participants aged ≥65 years were 56.3%/47.5% and 45.5%/50.9%, respectively (Table S24). The proportion of Korean participants reporting solicited local/systemic AEs (96.9%/93.6% in the GBP510/AS03 group and 85.1%/92.9% in the ChAdOx1-S group) was higher than seen among Southeast Asian participants (49.9%/44.0% and 39.7%/43.8%, respectively) (Tables S34–S36; See Table S20 for Overall AEs presented by each Cohort).
Unsolicited AEs occurring within 28 days after any vaccination were reported by 13.3% of participants in the GBP510/AS03 group and 14.6% of participants in the ChAdOx1-S group (p = 0.3040), including 3.5% and 4.1%, respectively, of unsolicited adverse drug reactions (ADRs) (p = 0.37) (Table S23). The most commonly reported unsolicited AEs by System Organ Class (SOC) were ‘Infections and infestations’ in both the GBP510/AS03 (5.9% of participants) and the ChAdOx1-S (5.9% of participants) groups, which were predominantly due to COVID-19 infections. Most unsolicited AEs after any vaccination were grade 1 (mild) or 2 (moderate) in severity. Unsolicited AEs of grade ≥3 severity occurred in 0.7% of participants in the GBP510/AS03 group and 1.2% in the ChAdOx1-S group (p = 0.09) (Tables S37 and S38).
MAAEs were reported by 4.85% (147/3029 participants) of GBP510/AS03 group and 5.02% (50/996 participants) of ChAdOx1-S group, up to 4 weeks after the second vaccination, which includes 0.6% (17/3029 participants) and 1.0% (10/996 participants) of MAADRs, respectively. During 6-month follow-up, additional MAAEs were reported by 6.9% (210/3029 participants) of GBP510/AS03 group and 7.0% (70/996 participants) of ChAdOx1-S group (Table 7). The most frequently reported MAAEs by SOC in unsolicited AEs until 4 weeks after the second vaccination were ‘Infections and infestation’ (2.8% of GBP510/AS03 and 2.1% of ChAdOx1-S group), predominantly due to ‘COVID-19’ and ‘Suspected COVID-19’, followed by ‘Musculoskeletal and connective tissue disorders’ (0.5% of GBP510/AS03 and 0.7% of ChAdOx1-S group). This trend was also consistent that ‘Infections and infestation’ (5.5% of GBP510/AS03 and 5.4% of ChAdOx1-S group) and ‘Musculoskeletal and connective tissue disorders’ (0.3% of GBP510/AS03 and 0.4% of ChAdOx1-S group) were the most frequently reported unsolicited MAAEs during the 6-months of follow-up (See Tables S23, S26 and S27).
Table 7.
Overall adverse events after any vaccination up to six months after the second vaccination (safety set).
| (%) [no. of AEs] | GBP510/AS03 (N = 3029) | ChAdOx1-S (N = 996) |
|---|---|---|
| During 6-month follow-up period from 28 days after vaccination | ||
| Participants with SAEs | 16 (0.53) [17] | 5 (0.50) [7] |
| Exact 95% CI | [0.30, 0.86] | [0.16, 1.17] |
| Participants with AESIs | 1 (0.03) [1] | 2 (0.20) [2] |
| Exact 95% CI | [0.00, 0.18] | [0.02, 0.72] |
| Participants with MAAEs | 210 (6.93) [239] | 70 (7.03) [87] |
| Exact 95% CI | [6.05, 7.90] | [5.52, 8.80] |
| Participants with MAADRs | 5 (0.17) [6] | 0 |
| Exact 95% CI | [0.05, 0.38] | [0.00, 0.37] |
| Participants with AEs leading to study withdrawala | 0 | 0 |
| Exact 95% CI | [0.00, 0.12] | [0.00, 0.37] |
| Participants with AEs leading to deathb | 1 (0.03) [1] | 1 (0.10) [2] |
| Exact 95% CI | [0.00, 0.18] | [0.00, 0.56] |
AEs = adverse events, SAEs = serious adverse events, AESIs = adverse events of special interest, MAAEs = medically attended adverse events, MAADRs = medically attended adverse drug reactions.
Data are presented as ‘number of participants (% participants) [number of events]’. Denominator of percentage is the number of participants in each group. 95% confidence interval was calculated by Clopper-Pearson Methods.
The case checked on ‘at the discretion of the investigator or sponsor due to safety concerns' as a primary reason for discontinuation and checked on ‘Stop Vaccination (only if prior to 2nd vaccination)' as changes to IP (investigational product) vaccination.
The case checked on ‘Death’ as a primary reason for discontinuation and checked on ‘Fatal’ as outcome.
Cumulatively, a total of 32 SAEs from 31 participants of GBP510/AS03 group and 16 SAEs from 12 participants of ChAdOx1-S administered group were reported, up to 6 months of follow-up after the second vaccination (Tables S30 and S31). No clinically significant differences were found between groups up to 28 days and up to 6 months after the second vaccination (p = 0.44 and 0.92, respectively). As for the AESIs, a total of 6 cases were reported; 3 in the GBP510/AS03 group (acute kidney injury, rapidly progressive glomerulonephritis, and Cutaneous vasculitis) and 3 in the ChAdOx1-S group (acute pancreatitis, anaphylactic reaction, and psoriasis) (Tables 6 and 7). Of note, rapidly progressive glomerulonephritis in the GBP510/AS03 group was assessed as a suspected unexpected serious adverse reaction (SUSAR) and AESI (Table 6). As a conservative judgement, the investigator reported it as a vaccine-related event due to the temporal relationship with GBP510/AS03 administration, and because a causal relationship with GBP510/AS03 could not be fully excluded. However, the clinical presentation and available laboratory findings do not provide evidence to establish a possible autoimmune aetiology for this event. There was no thromboembolism-related event reported until data cut-off (see Tables S1, S2, S28, and S29 for the reported AESIs and the full lists of AESIs; See Supplementary Appendix for ‘Narratives of SAE Cases’). Cumulatively, one death occurred in the GBP510/AS03 group (brain neoplasm), and two deaths occurred in ChAdOx1-S group (cardiorespiratory failure from one participant; tubulointerstitial nephritis and acute cholecystitis from one participant), with neither considered to be related to the study vaccines (See Supplementary Appendix for ‘Narratives of Death Cases’).
Cumulatively, five pregnancies were reported in the GBP510/AS03 group and two in the ChAdOx1-S group; all individuals gave birth without any abnormal outcomes or SAEs. Three participants in the GBP510/AS03 group and two in the ChAdOx1-S withdrew due to AEs, with one participant in the GBP510/AS03 group reported to have vaccine-related AEs (urticaria rash, injection site pain, headache, fatigue, myalgia, and arthralgia), all of which recovered/were resolved (See Supplementary Appendix for ‘Narratives of Study Withdrawal Due to AEs’).
There were total 456 virologically-confirmed (by PCR) or suspected (diagnosed with rapid antigen test) COVID-19 cases from 454 participants (2 participants were re-infected) up to 6 months of follow-up. The incidence rates of COVID-19 were not statistically different between two groups, which were 11.2% (338/3029 participants) in GBP510/AS03 group and 11.7% in ChAdOx1-S group (p = 0.67) (Table S32). All COVID-19 cases were either non-severe or asymptomatic, as per WHO criteria, thus, they were not suspected as vaccine-associated enhanced disease/vaccine-associated enhanced respiratory disease.
Discussion
This interim analysis found that the immune response induced by two doses of GBP510/AS03 in seronegative adults was superior compared to ChAdOx1-S with respect to GMTs, and non-inferior with respect to SCRs, in terms of neutralising antibody response against the ancestral D614G strain of SARS-CoV-2 at 2 weeks after the second vaccination (primary endpoints). ELISA assessments of SARS-CoV-2 RBD-binding IgG antibody also indicated a higher immune response with GBP510/AS03 than with ChAdOx1-S at 2 weeks after the second vaccination.
Higher neutralising antibody responses with GBP510/AS03 against the ancestral strain were seen in participants regardless of age, ethnicity, or sex. In addition, a trend towards higher neutralising antibody responses against the Delta and Omicron variants was observed with GBP510/AS03 in comparison with ChAdOx-1S in a subset of participants.
Furthermore, when an additional analysis was performed by FRNT in the subset to observe the trend in neutralising antibody response after interim analysis, the GMT ratio of the two groups at 4 weeks after the second vaccination showed a similar trend to that seen at 2 weeks after the second vaccination (GMT ratio: 3.01 [95% CI 2.30–3.94]), whereas SCR in the ChAdOx1-S group showed a noticeable decrease compared to the GBP510/AS03 group (SCR: 94.7% [180/190 participants; GBP510/AS03] and 68.2% [60/88 participants; ChAdOx1-S]). This result confirmed that both test groups were equally evaluated at its peak of the second vaccination (i.e., 2 weeks after the second vaccination) for the primary endpoint.
The comparator vaccine was selected based on the availability of WHO emergency use listing (EUL) issued COVID-19 vaccine procurement at the time of study conduct, and after the extensive discussion with regulatory bodies through scientific advice meetings. Although there was a concern of adenoviral vector vaccines at the early stage of development, of which pre-existing immunity to human adenovirus serotype 5 (Ad5) might mitigate its immune response to target antigen, this was not the case for comparator vaccine used in this study. ChAdOx1-S COVID-19 vaccine was developed using their ChAd (Chimpanzee Ad) technology to circumvents pre-existing immunity to Ad5 and obtained its full approval in numerous countries and WHO EUL by demonstrating acceptable safety and efficacy up to the third dose.14, 15, 16, 17 Of note, 4-week interval dosing schedules for ChAdOx1-S vaccination was selected as the manufacturer claimed that differences in the primary dosing interval did not appear to have a significant impact on the immunogenicity of the ChAdOx1-S vaccine. Furthermore, allowing a different dosing interval for the study vaccine could be a confounding factor when comparing the immune responses.18
GBP510/AS03 had previously been found to be highly immunogenic in a phase 1/2 study.3 In that study, two-dose vaccination with GBP510 (25 μg RBD per dose)/AS03 induced high neutralising antibody titres measured by wild-type virus assay (PRNT; plaque-reduction neutralisation test), with an increase in GMT from 4.33 at baseline to 861 IU/mL at 2 weeks after the second dose. Direct comparison is not possible because a different assay method (FRNT) was used in current study; however, a high response was also seen in this phase 3 study (GMT increased from 8.18 to 272.12 IU/mL). In addition, a substantial increase in geometric mean concentration (GMC) of IgG binding antibody, by ELISA, was seen at 2 weeks after the second dose (2599 BAU/mL) of GBP510/AS03 in phase 1/2 study,3 which is also consistent with the level observed at that timepoint in our study (2850.45 BAU/mL).
A noticeable increase in antibody responses after the second dose was observed compared to the first dose in both the phase 1/2 and this phase 3 study, which may be due to some CD4+ T-cell response that was observed by ICS assessments after GBP510/AS03 vaccination.3 With CD4+ T-cell help, activated B cells initiate a germinal centre reaction, resulting in the generation of high-affinity memory B-cells and plasma cells, which are differentiated to antibody-secreting cells and provide overlapping layers of protection.19,20 A superior antigen-specific CD4+ T-cell response was also reported with AS03-adjuvantation in a randomised influenza vaccine trial.21 The cellular immune response data obtained in the current study showed insignificant amount of CD8+ T cell responses in both groups. While CD8+ T cells play an important role in controlling viral infections, the vaccine's effectiveness can be determined through rigorous clinical trials that evaluate its ability to prevent infection and reduce disease severity in vaccinated individuals. This is because the immune response to vaccines is complex and involves multiple components, including antibodies, CD4+ T cells, and CD8+ T cells.22
In the phase 2 clinical study of BNT162b2 (Pfizer-BioNTech), reported fold increase after 2 doses of 30 μg BNT162b2 was approximately 31 folds against ancestral strain at its peak from the baseline,23 which is similar to the fold increase (36-folds) observed in our study after 2 doses of GBP/AS03. Furthermore, fold reduction trend against Omicron variants BA.1 and BA.5 after primary dose of mRNA vaccines reported in other studies, were comparable to those observed in our study (11.8 and 24.8 folds in GBP510/AS03 group compared to ancestral strain). In this study, the main immunogenicity results were for the ancestral D614G strain after vaccination with GBP510/AS03. In addition, a subset analysis was conducted to evaluate the neutralising antibody responses against Omicron BA.1 and BA.5, which were the dominant circulating variants in regions at the time of study. Cross-neutralisation tests against currently circulating variants, such as XBB.1 lineage viruses, are planned for the ongoing subsequent studies after the homologous and heterologous booster vaccination with GBP510/AS03.
For the safety assessment, GBP510/AS03 showed a clinically acceptable safety profile, and no safety concerns were noted during the study up to 6 months after the second vaccination. The incidence rates of unsolicited AEs and SAEs were similar between the study groups. Reactogenicity after any vaccination was higher with GBP510/AS03 than with ChAdOx1-S in terms of local solicited AEs, especially after the second vaccination, but was similar to ChAdOx1-S in terms of systemic solicited AEs (being lower after the first vaccination with GBP510/AS03, but higher after the second one, compared with ChAdOx1-S). Both local and systemic solicited AEs were mostly mild or moderate in intensity in both groups. In the current study, GBP510/AS03 showed lower reactogenicity than that observed in the phase 1/2 study,5 which could be explained by a difference in study populations. Higher rates of solicited AEs were observed in Korean participants compared with Southeast Asians (who constituted the majority of study participants). The rates seen in Korean participants were consistent with those seen in the phase 1/2 study, which included only Korean participants.5
One of the main limitations of the data generated in this study is that the absence of vaccine efficacy data, and the lack of established correlates of protection for COVID-19 vaccines, make it difficult to extrapolate the results of the study. Although thresholds for immune correlates of protection based on antibody levels or functional activity have not yet been established, COVID-19 vaccine studies that have reported vaccine efficacy have shown robust correlations between antibody responses (neutralising antibody or binding antibody titres) and vaccine efficacy, despite the use of different assays, endpoints, and study populations.6,24, 25, 26 Efficacy was not evaluated as a part of the pivotal phase 3 clinical trial. At the time of study conducted, appropriately designed immunobridging studies were considered as an acceptable approach for COVID-19 vaccine authorisation. Immunobridging studies were designed to compare the immunogenicity and safety of study vaccine with an authorised vaccine, which demonstrated high efficacy.8 Another limitation is the small number of elderly participants due to high vaccination coverage in the elderly at the time of enrolment; however, the available results suggest that the immunogenicity of GBP510/AS03 against the parental strain was consistent across all age strata. The study included mostly Asian participants, and did not include individuals with severe or unstable comorbid conditions, those who were immunocompromised, or pregnant or lactating women, and post-authorisation studies are needed to collect safety data in these populations. Finally, safety data for this interim analysis were only available up to 6 months after the second vaccination; however, additional data up to 12 months of follow-up in current study, in addition to the ongoing extension study of homologous and heterologous booster vaccination (NCT05501522) will add more value on the GBP510/AS03 vaccine.
In conclusion, this interim analysis found that two-dose vaccination with GBP510/AS03 induced stronger neutralising antibody immune responses compared with ChAdOx1-S against the ancestral D614G strain at 2 weeks after the second vaccination and sustained favourable trend to GBP510/AS03 up to 6 months and had a clinically acceptable safety profile up to 6 months after second vaccination.
Contributors
HJC, HK, JHR, SJL, YWP, FR, TB, PW and LC contributed to the conception and design of this study. SS contributed to the project administration. WSC, JYH, JSL, DSJ, SWK, KHP, JSE, SJJ, JL, KTK, HJC, JWS, YKK, BWY, IJJ, HJC, and MZC were the principal investigators of the study site. JYS, WSC, JYH, JSL, DSJ, SWK, KHP, JSE, SJJ, JL, KTK, HJC, JWS, YKK, BWY, IJJ, HJC, and MZC contributed to the acquisition of the clinical and laboratory data. SS contributed to the resources of the study. SS and MS contributed to the supervision of the study. JYS, SJJ, YKK, BWY, IJJ, PW, FR, TB, and HJC contributed to the interpretation of the data. JYS and HJC had full access to and verify all of the data in the study and take responsibility for the integrity of the data. SS and ND contributed to the review and edit of the manuscript. JYS and HJC prepared the manuscript and had responsibility for the decision to submit the manuscript. All authors reviewed the manuscript for intellectual content and approved the final draft for submission. All authors have full access to all the data in the study and accept responsibility to submit for publication. All authors critically reviewed and approved the final version.
Data sharing statement
Individual participant data will be made available when the trial is complete upon requests directed to the corresponding author. Proposals will be reviewed and approved by the sponsor, investigators, and collaborators on the basis of scientific merit. After approval of a proposal, data can be shared through a secure online platform.
Declaration of interests
FR, TB, and PW are employees of the GSK group of companies. FR and TB hold restricted shares in the GSK group of companies. HK, JHR, SJL, and YWP are full-time employees of SK Bioscience. HK, YWP and JHR own SK Bioscience stock as employees. SS, MS and ND are full-time employees of IVI. All other authors declare no competing interests.
Acknowledgements
This study was supported, in whole or in part, by the Bill & Melinda Gates Foundation (BMGF) and the Coalition for Epidemic Preparedness Innovations (CEPI). The authors thank David P. Figgitt PhD, ISMPP CMPP™, and Katherine F. Croom, Content Ed Net, for providing editorial support during the preparation of the manuscript, with funding from SK Bioscience.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102140.
Contributor Information
Hee Jin Cheong, Email: heejinmd@korea.ac.kr, heejinmd2@gmail.com.
GBP510/AS03 study group:
Agathe Philippot, Francesca Solmi, Maria Angeles Ceregido, Byoung-Shik Shim, Sang Hwan Seo, Simone D'Souza, Patchara Thaisrivichai, Josefina Carlos, Edison Alberto, Sorachai Nitayaphan, Winai Ratanasuwan, Piroon Mootsikapun, Romanee Chaiwarith, Luong Chan Quang, Olena Karpenko, Tatiana Yurkiv, Vitalii Kutovyi, Angela Bartko, Olga Gyrina, Olga Barna, Mykhailo Pugach, Claire Thurlow, Simon Carson, Susan Smith, Mike Williams, Tiwini Hemi Senior, Tim Humphrey, Davitt Sheahan, Hokeun Park, Yoon Yeong Lee, and Seung Gu Kang
Appendix A. Supplementary data
References
- 1.Fiolet T., Kherabi Y., MacDonald C.J., Ghosn J., Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect. 2022;28:202–221. doi: 10.1016/j.cmi.2021.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walls A.C., Fiala B., Schäfer A., et al. Elicitation of potent neutralizing antibody responses by designed protein nanoparticle vaccines for SARS-COV-2. Cell. 2020;183(5):1367. doi: 10.1016/j.cell.2020.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walls A.C., Miranda M.C., Schäfer A., et al. Elicitation of broadly protective sarbecovirus immunity by receptor-binding domain nanoparticle vaccines. Cell. 2021;184(21):5432. doi: 10.1016/j.cell.2021.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morel S., Didierlaurent A., Bourguignon P., et al. Adjuvant system AS03 containing alpha-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine. 2011;29:2461–2473. doi: 10.1016/j.vaccine.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Song J.Y., Choi W.S., Heo J.Y., et al. Safety and immunogenicity of a SARS-CoV-2 recombinant protein nanoparticle vaccine (GBP510) adjuvanted with AS03: a randomised, placebo-controlled, observer-blinded phase 1/2 trial. eClinicalMedicine. 2022;51 doi: 10.1016/j.eclinm.2022.101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilbert P.B., Donis R.O., Koup R.A., Fong Y., Plotkin S.A., Follmann D. A covid-19 milestone attained — a correlate of protection for vaccines. N Engl J Med. 2022;387:2203–2206. doi: 10.1056/NEJMp2211314. [DOI] [PubMed] [Google Scholar]
- 7.International Coalition of Medicines Regulatory Authorities . 2021. ICMRA COVID-19 vaccine development: future steps workshop.http://www.icmra.info/drupal/en/covid-19/24june2021 Available online: [Google Scholar]
- 8.Medicines and Healthcare Products Regulatory Agency, UK . 2021. Access consortium: alignment with ICMRA consensus on immunobridging for authorising new COVID-19 vaccines.https://www.gov.uk/government/publications/access-consortium-alignment-with-icmra-consensus-on-immunobridging-for-authorising-new-covid-19-vaccines/access-consortium-alignment-with-icmra-consensus-on-immunobridging-for-authorising-new-covid-19-vaccines Available online: [Google Scholar]
- 9.Falsey A.R., Sobieszczyk M.E., Hirsch I., et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) covid-19 vaccine. N Engl J Med. 2021;385:2348–2360. doi: 10.1056/NEJMoa2105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.European Medicines Agency . 2021. Vaxzevria (previously COVID-19 vaccine AstraZeneca): EPAR–product information.https://www.ema.europa.eu/documents/product-information/vaxzevria-previously-covid-19-vaccine-astrazeneca-epar-product-information_en.pdf [Google Scholar]
- 11.US Food and Drug Administration . 2007. Guidance for Industry: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials.http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm091977.pdf [DOI] [PubMed] [Google Scholar]
- 12.US Food and Drug Administration . 2020. Guidance for industry: development and licensure of vaccines to prevent COVID-19.https://www.fda.gov/regulatory-information/search-fda-guidance-documents/development-and-licensure-vaccines-prevent-covid-19 [Google Scholar]
- 13.Chan I.S., Zhang Z. Test-based exact confidence intervals for the difference of two binomial proportions. Biometrics. 1999;55:1202–1209. doi: 10.1111/j.0006-341x.1999.01202.x. [DOI] [PubMed] [Google Scholar]
- 14.Mendonça S.A., Lorincz R., Boucher P., Curiel D.T. Adenoviral vector vaccine platforms in the SARS-CoV-2 pandemic. NPJ Vaccines. 2021;6 doi: 10.1038/s41541-021-00356-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folegatti P.M., Ewer K.J., Aley P.K., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramasamy M.N., Minassian A.M., Ewer K.J., et al. Safety and immunogenicity of Chadox1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020;396(10267):1979–1993. doi: 10.1016/s0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrett J.R., Belij-Rammerstorfer S., Dold C., et al. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat Med. 2021;27:279–288. doi: 10.1038/s41591-020-01179-4. [DOI] [PubMed] [Google Scholar]
- 18.AstraZeneca . 2022. Type II variation r Assessment report (ChAdOx1 S [recombinant])https://www.ema.europa.eu/en/documents/variation-report/vaxzevria-previously-covid-19-vaccine-astrazeneca-h-c-5675-ii-0052-epar-assessment-report-variation_en.pdf [cited 2023 Jun 23] Available from: [Google Scholar]
- 19.Crotty S. A brief history of T cell help to B cells. Nat Rev Immunol. 2015;15:185–189. doi: 10.1038/nri3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laidlaw B.J., Ellebedy A.H. The germinal centre B cell response to SARS-CoV-2. Nat Rev Immunol. 2022;22:7–18. doi: 10.1038/s41577-021-00657-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Couch R.B., Bayas J.M., Caso C., et al. Superior antigen-specific CD4+ T-cell response with AS03-adjuvantation of a trivalent influenza vaccine in a randomised trial of adults aged 65 and older. BMC Infect Dis. 2014;14:425. doi: 10.1186/1471-2334-14-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barros-Martins J., Hammerschmidt S.I., Cossmann A., et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat Med. 2021;27:1525–1529. doi: 10.1038/s41591-021-01449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.European Medicines Agency . 2021. Cormirnaty: EPAR–public assessment report.https://www.ema.europa.eu/en/documents/assessment-report/comirnaty-epar-public-assessment-report_en.pdf [Google Scholar]
- 24.Khoury D.S., Cromer D., Reynaldi A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 25.Earle K.A., Ambrosino D.M., Fiore-Gartland A., et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39:4423–4428. doi: 10.1016/j.vaccine.2021.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng S., Phillips D.J., White T., et al. Oxford COVID Vaccine Trial Group. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27:2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.