Abstract
A questionnaire relating to Clostridium difficile disease incidence and diagnostic practices was sent to 380 Canadian hospitals (all with >50 beds). The national questionnaire response rate was 63%. In-house testing was performed in 17.6, 61.5, and 74.2% of the hospitals with <300, 300 to 500, and >500 beds, respectively. The average test positivity rates were 17.2, 15.3, and 13.2% for hospitals with <300, 300 to 500, and >500 beds, respectively. The average disease incidences were 23.5, 30.8, and 40.3 cases per 100,000 patient days in the hospitals with <300, 300 to 500, and >500 beds, respectively. In the 81 hospitals where in-house testing was performed, cytotoxin testing utilizing tissue culture was most common (44.4%), followed by enzyme-linked immunosorbent assay (38.3%), culture for toxigenic C. difficile (32.1%), and latex agglutination (13.6%). The clinical criteria for C. difficile testing were variable, with 85% of hospitals indicating that a test was done automatically if ordered by a doctor. Our results show that C. difficile-associated diarrhea is a major problem in hospitals with ≥200 beds. Despite a lower disease incidence in smaller hospitals, there was a higher diagnostic test positivity rate. This may reflect the preference of smaller hospitals for culture and latex agglutination tests.
Clostridium difficile is an opportunistic, gram-positive anaerobe whose pathogenicity is associated with the production of two exotoxins: toxin A (enterotoxin) and toxin B (cytotoxin) (42). Toxin A is a potent enterotoxin with cytotoxic activity. When injected into rabbit ileal loops, toxin A is capable of inducing epithelial damage, hemorrhage, and fluid secretion (32, 42). Toxin B, although incapable of mucosal damage, is a 1,000-fold more potent cytotoxin than toxin A. Picogram quantities of toxin B are sufficient to cause cell rounding (10, 16). Toxigenic strains of C. difficile are known to cause various clinical symptoms, ranging from asymptomatic colonization to life-threatening pseudomembranous colitis (3). Asymptomatic colonization is found in ∼3 to 4% of healthy adults (25) but in as many as 15 to 75% of neonates (30, 39).
Nosocomial acquisition and transmission of C. difficile have been well documented (19, 25, 28, 33, 41). Outbreaks of C. difficile-associated diarrhea (CAD) have occurred on geriatric wards (6), orthopedic wards (34), medical wards (26), surgical wards (43), and long-term care facilities (5). The incidence rate of nosocomial CAD may vary with hospital populations and is influenced by the presence of predisposing factors, such as increased patient age, type and duration of antimicrobial therapy, severity of underlying illness(es), and length of hospital stay (1, 5, 8, 31). As the most frequently isolated nosocomial gastrointestinal pathogen, C. difficile is believed to be the leading cause of infectious nosocomial diarrhea, accounting for 20 to 45% of all cases (21, 24, 33). Despite the prevalence of the disease, the mechanism(s) of nosocomial acquisition or transmission is still not fully understood. The environment (28, 36), cross-infection between patients (11, 23), and carriage by hospital employees have all been cited as plausible sources of infection.
In the United States, nosocomial infections affect over 4 million patients annually (9, 22). Aside from the morbidity and mortality rates associated with nosocomial infections, there is also a significant financial drain on the health care system. One study modestly estimated the cost of treating a single CAD infection to be in excess of £4,000 (44). Although C. difficile is a significant nosocomial pathogen, little documentation is available to reflect disease prevalence or the diagnostic testing methods employed by Canadian hospitals.
The study described here was undertaken to assess current testing methods and testing criteria for C. difficile, as well as to survey CAD incidence rates across Canada. Data collected in the survey was intended to help hospitals compare their CAD incidence rates and diagnostic standards of practice with those of other, similar-size institutions.
Participants.
A questionnaire requesting information on hospital size (number of beds), hospital type, disease incidence, and diagnostic test methods was distributed in 1995 to 380 hospitals and provincial laboratories across Canada. Participants were instructed to complete the questionnaire by using their most recent 12 consecutive months of data. Statistical analysis was done by using Graphpad InStat (Graphpad Program Software, San Diego, Calif.). A follow-up telephone call was made 1.5 months after distribution of the questionnaire to optimize compliance and to validate the data. Those laboratories which participated in the survey were sent a summary report of collated data from all of the other Canadian hospitals surveyed. Private laboratories were not included in the survey.
Classification of hospitals.
Survey participants were asked to classify their hospitals based on the following categories: tertiary care, hospital with an intensive care unit and affiliation with medical teaching; general medicine, hospital with an intensive care unit but no affiliation with medical teaching; community medicine, hospital with no intensive care unit and no affiliation with medical teaching; geriatric, hospital providing primary care for the elderly; other, any institution which does not fit into any of the other four categories.
In our tertiary care hospital (a teaching hospital with approximately 700 beds in 1994), the CAD incidence rate was 40.5/100,000 patient days in 1994. Review of the overall isolation statistics for 1996 showed that CAD was a significant cause, accounting for about 44% of the 126 patients who were subjected to some form of isolation precautions (Table 1). Patient morbidity and mortality and increased costs associated with controlling this infection further support the value of determining national data on CAD as a nosocomial disease.
TABLE 1.
Distribution of underlying reasons for patient isolationa
| Infective material or diagnosis | Total no. of patients isolated | % of total no. isolated |
|---|---|---|
| Drainage from lesions | ||
| Viruses (e.g., herpesvirus, varicella-zoster virus) | 7 | 5.56 |
| Streptococcus pyogenes | 4 | 3.17 |
| Scabies | 1 | 0.79 |
| Feces | ||
| Clostridium difficile | 55 | 43.65 |
| Viruses (e.g., rotavirus, hepatitis A virus) | 5 | 3.97 |
| Salmonellae | 6 | 4.76 |
| Other | 2 | 1.59 |
| Campylobacters | 1 | 0.79 |
| Respiratory secretions | ||
| Methicillin-resistant Staphylococcus aureus pneumonia | 1 | 0.79 |
| Tuberculosis (or suspected tuberculosis) | 10 | 7.94 |
| Salmonellae | 1 | 0.79 |
| Chicken pox contact | 1 | 0.79 |
| Bacterial meningitis | 3 | 2.38 |
| Human immunodeficiency virus in blood and body fluids | 2 | 1.59 |
| Other | ||
| Methicillin-resistant Staphylococcus aureus | 11 | 8.73 |
| Granulocytopenic patient | 3 | 2.38 |
| Chicken pox | 1 | 0.79 |
| Borderline oxacillin-resistant Staphylococcus aureus | 1 | 0.79 |
| Resistant gram-negative organisms | 11 | 8.73 |
The total number of patients placed in isolation at St. Boniface General Hospital in 1996 was 126. St. Boniface General Hospital is currently a 560-bed tertiary care hospital.
Completed questionnaires were received from 238 of the 380 institutions surveyed (63% response rate). Because there was a telephone follow-up, we feel that the survey was an accurate representation of C. difficile testing and disease incidence across Canada.
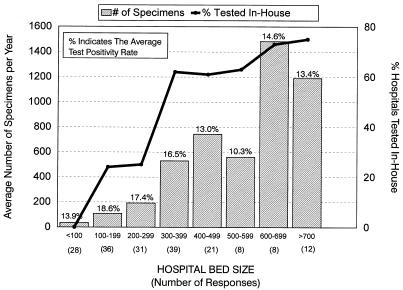
Stratification of data based on hospital size suggested that smaller hospitals (<300 beds) sent most of their stool specimens off site for diagnostic testing (Fig. 1). In-house testing was performed in 17.6, 61.5, and 74.2% of the hospitals with <300, 300 to 500, and >500 beds, respectively. There was a general trend for larger hospitals to submit more specimens for C. difficile testing (Fig. 1). Despite some diversity in the number of specimens submitted each year for testing, the diagnostic test positivity rate was greatest in smaller hospitals. The average test positivity rates were 17.2, 15.3, and 13.2% for hospitals with <300, 300 to 500, and >500 beds, respectively. The average test positivity rate was greatest for hospitals having 100 to 199 beds (18.6%) and least for hospitals having 500 to 599 beds (10.3%) (Fig. 1). Since many hospitals sent their specimens to provincial laboratories, two provincial laboratories were sent surveys to obtain information on diagnostic test methods. These centers processed an average of 2,879 specimens per year, with a test positivity rate of 20.4% (data not shown).
FIG. 1.
Specimen processing and test positivity rates stratified according to hospital size (number of beds). A total of 81 hospitals performed in-house testing. Some hospitals referred specimens out for diagnostic testing. Data on the volume of specimens processed and test positivity rates was not provided by all hospitals.
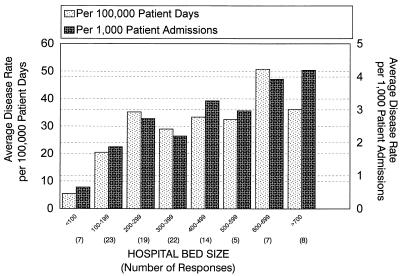
Analysis of data based on patient populations also showed evidence of diversity in test positivity rates. Hospitals classified under tertiary care, community, and general medicine had average test positivity rates of about 15%. Geriatric medicine and other types of centers had lower test positivity rates. Furthermore, the incidence of CAD per 100,000 patient days or per 1,000 patient admissions was highest in tertiary care hospitals, followed by general medicine hospitals, community hospitals, geriatric hospitals, and other centers. A similar correlation between hospital size and disease incidence was also seen when data was stratified based on hospital size (Fig. 2).
FIG. 2.
C. difficile-associated diarrhea rates. Data is stratified according to hospital size (number of beds). C. difficile-associated diarrhea rates are expressed as the number of cases per 100,000 patient days and per 1,000 patient admissions.
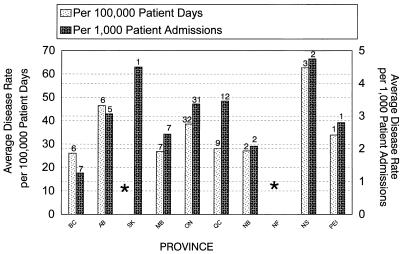
The provincial CAD incidence rates across Canada, for all hospitals with ≥200 beds, is shown in Fig. 3. With the exception of Nova Scotia, which had very few hospitals reporting incidence data, Ontario and Alberta seemed to have the highest CAD incidence rates.
FIG. 3.
National C. difficile disease incidence rates. Asterisks indicate that data on disease incidence from hospitals having fewer than 200 beds were excluded. Data on disease incidence was not available for Newfoundland. Data on disease incidence per 100,000 patient days was not available for Saskatchewan. The numbers above the bars are the numbers of respondents.
Of the 81 centers which performed in-house testing, the most commonly employed diagnostic test method for CAD was cytotoxin testing using tissue culture (44.4%), followed by enzyme-linked immunosorbent assay (ELISA) (38.3%). Culture of C. difficile was performed by 26 (32.1%) of the 81 hospitals doing in-house testing. Although culture was utilized by a large percentage of the hospitals, only eight (9.9%) centers used it as the sole method. Subsequent verification of toxin production was also performed by five of these eight centers employing culture. Latex agglutination was utilized by 11 (13.6%) of the 81 hospitals surveyed. Of the 11 hospitals utilizing this method, only 4 (4.9% of the 81 hospitals) used it as the sole method of testing.
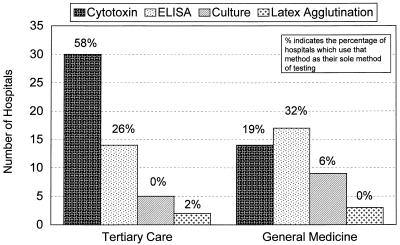
Stratification of testing methods based on hospital size showed that smaller hospitals (<300 beds) tended to use easier and more readily available methods, such as culture, ELISA, and latex agglutination (Table 2). Interestingly, 3 (7.5%) of 40 hospitals that had between 300 and 500 beds used latex agglutination as the sole method of diagnostic testing. When methods were stratified based on hospital type, it was apparent that general medicine hospitals (100- to 200-bed median) used ELISA the most, followed by the cytotoxin test (Fig. 4). In tertiary care hospitals (400- to 500-bed median), cytotoxin testing was the preferred method, followed by ELISA (Fig. 4).
TABLE 2.
Diagnostic testing methods of in-house testing centers stratified according to hospital sizea
| Hospital size (no. of beds) | No. of responses | Cytotoxin assay
|
ELISA
|
Culture
|
Latex agglutination test
|
||||
|---|---|---|---|---|---|---|---|---|---|
| Use method | Use as sole method | Use method | Use as sole method | Use method | Use as sole methodb | Use method | Use as sole method | ||
| 100–199 | 11 | 1 | 1 | 5 | 4 | 5 | 2 | 3 | 1 |
| 200–299 | 8 | 1 | 0 | 5 | 3 | 4 | 3 | 0 | 0 |
| 300–399 | 26 | 14 | 11 | 9 | 5 | 6 | 1 | 4 | 2 |
| 400–499 | 14 | 5 | 5 | 5 | 3 | 4 | 0 | 4 | 1 |
| 500–599 | 5 | 3 | 2 | 1 | 1 | 2 | 1 | 0 | 0 |
| 600–699 | 8 | 6 | 4 | 2 | 0 | 4 | 1 | 0 | 0 |
| >700 | 9 | 6 | 4 | 4 | 3 | 1 | 0 | 0 | 0 |
| Total (%) | 81 | 36 (44.4) | 27 (33.3) | 31 (38.3) | 19 (23.5) | 26 (32.1) | 8 (9.9) | 11 (13.6) | 4 (4.9) |
The table represents the use of diagnostic testing methods at various-size hospitals. Not all hospitals were able to provide responses. The provincial laboratories surveyed utilized ELISA and cytotoxin assays (data not shown).
Verification of toxin production was performed by five of the eight centers which utilized culture as the sole diagnostic tool for C. difficile.
FIG. 4.
C. difficile testing methods for tertiary care versus general medicine hospitals. Responses were obtained from 43 tertiary care hospitals and 31 general medicine hospitals.
The criteria for C. difficile testing were also assessed at the different institutions surveyed. A summary of the questions asked is provided in Table 3. Data from the survey showed a great diversity in the approaches taken by hospitals regarding the clinical criteria that had to be met before a C. difficile diagnostic test was performed. Of the institutions which responded, 85% said the assay was done automatically if ordered by a doctor.
TABLE 3.
C. difficile questionnaire summary
| Question | No. of respondents | No. (%) that answered:
|
|
|---|---|---|---|
| Yes | No | ||
| Do you do routine enteric cultures on patients who have been hospitalized for >3 days? | 194 | 57 (29) | 137 (71) |
| What factor must be present before a C. difficile assay is performed? | |||
| Automatically done if doctor orders test | 184 | 156 (85) | 28 (15) |
| Diarrhea | 199 | 90 (76) | 29 (24) |
| Current/recent antibiotic therapy | 107 | 66 (62) | 41 (38) |
| Hospitalized for >3 days | 91 | 30 (33) | 61 (67) |
| Do you have any stool samples submitted for C. difficile testing on patients <1 year old? | 182 | 54 (30) | 128 (70) |
Hospital-acquired infections are important concerns for many health care institutions and contribute significantly to patient morbidity and mortality, as well as to the costs associated with prolonged patient stays and care. Despite some geographical variation, diarrhea is considered to account for 1 to 14% of all nosocomial infections throughout the world (24, 45). Surveillance data from one Turkish hospital indicated that nosocomial diarrhea was ranked fifth and comprised about 7% of all nosocomial infections according to Centers for Disease Control 1988 criteria (17). Furthermore, in this hospital, C. difficile was responsible for 22.2% of the nosocomial diarrhea cases that occurred within a general medicine ward (40). There is good evidence that routine enteric cultures should not be done for patients hospitalized for 3 or more days (18). Our data indicated that 29% of the hospitals surveyed have not yet adopted this practice (Table 3).
Disease severity and patient suffering can be reduced if proper treatment and intervention strategies are made available. These factors are influenced by rapid, reliable, and accurate diagnosis of CAD. The currently available diagnostic tests include colonoscopy, tissue culture cytotoxin assay, stool culture for toxigenic C. difficile, latex agglutination, counterimmunoelectrophoresis, ELISA, and molecular diagnostics such PCR (35). Results from our survey indicate that for diagnostic testing, cytotoxin testing and ELISA predominate, followed by culture for toxigenic C. difficile and latex agglutination.
Despite the technical nature of the “gold standard” (35) tissue culture cytotoxin assay, this was the most prevalent method used for the diagnosis of CAD, with 44.4% of in-house testing facilities employing the assay. In larger institutions, such as tertiary care hospitals, cytotoxin testing use was at 58%. Test rapidity (2.5 to 3.5 h) and ease of performance due to automation likely account for the ELISA being the second most common (38.3%) in-house diagnostic test. Several investigators (2, 7, 12, 13, 15, 35) have found ELISA sensitivity to approach or equal that of cytotoxin assays (87 to 98%). Although culturing is considered to be the most sensitive assay for C. difficile (35), a subsequent cytotoxin assay must be performed to determine if the isolate is toxigenic, making the procedure very time consuming and labor intensive. The advantage of the culturing method is that the organism is available for epidemiological typing, which may be beneficial when outbreaks occur. In our survey, culturing was prevalent, with 32.1% of the hospitals that did in-house testing employing the technique; however, only eight (9.9%) centers employed it as a stand-alone test. Five of these eight centers also performed subsequent cytotoxin assays to confirm that the organisms were toxigenic. The latex agglutination test, although rapid and easy to perform, is not recommended as a sole diagnostic test, since it is not specific for toxigenic C. difficile and frequently results in false-positive reactions (27, 29). Our survey indicated that most hospitals are aware of this, as only four (4.9%) centers used it as the sole method of testing. When we performed a telephone follow-up, we found that subsequent to receiving our survey summary report (1 year later), all of the centers using latex agglutination as the sole test method had switched over to ELISA technology. Similarly, the centers previously employing culture as the stand-alone test now employ cytotoxin assays and ELISA.
Asymptomatic carriage of toxigenic strains of C. difficile makes it critical that diagnostic testing be done only for symptomatic patients. Data from Table 3 indicates that most centers do not use stringent criteria when accepting stools submitted for CAD testing. Testing of stool from patients without clinical indications of CAD is not only an unnecessary cost but may also complicate patient care if unnecessary antibiotic treatment is given. Testing for CAD should be based not on length of hospitalization but, rather, on the presence of clinically significant diarrhea (29) (e.g., four or more bowel movements per day for 3 or more days) with a history of antibiotic therapy or the presence of acute abdominal syndrome with little or no diarrhea (20). Communication of these criteria to clinicians who order tests for CAD is important. In our study, 54 (30%) of 182 respondents said they received stool samples for cytotoxin testing on patients less than 1 year old. Although there are rare instances in which toxin B in children less than 1 year old is significant (38), up to 50% of neonates may be colonized with toxigenic strains without clinical symptoms (4, 14, 18). It is important that laboratory workers performing tests on children less that 1 year old be sure that the clinicians ordering this test are aware of the high rate of asymptomatic carriage within the neonatal population. It has been reported that 35% of patients with cystic fibrosis can also be asymptomatic carriers of toxigenic C. difficile (37).
The results of our survey indicated higher test positivity rates in smaller hospitals. Since smaller hospitals were more likely to use easier and more readily available methods, such as latex agglutination, culturing, and ELISA, higher test positivity rates may have been reflective of false-positive reactions, particularly for the latex agglutination test. Variations in test positivity rates may also have been influenced by the number of repeat specimens taken from an individual patient or by stool samples taken from patients without clinically significant diarrhea. Data from the survey showed a higher disease incidence in larger hospitals. Since severe underlying illnesses are predisposing factors for CAD, larger centers with larger critically ill patient populations should be expected to have higher disease incidence rates.
In summary, C. difficile is an important nosocomial pathogen in Canada, with a national average CAD incidence of 36.18/100,000 patient days or 3.06/1,000 admissions in hospitals with ≥200 beds. Diversity in test positivity rates may have been influenced by testing criteria or choice of testing method. The use of latex agglutination as a sole diagnostic test is not recommended. Implementation of standardized clinical criteria for C. difficile testing should be considered, as it would increase the reliability and accuracy of diagnosis.
Acknowledgments
The assistance of Nancy Olson in data collection and the translation of the questionnaire into French by Pat DeGagne are acknowledged.
REFERENCES
- 1.Aronsson B, Mollby R, Nord C E. Antimicrobial agents and Clostridium difficile in acute enteric disease: epidemiological data from Sweden, 1980–1982. J Infect Dis. 1985;151:476–481. doi: 10.1093/infdis/151.3.476. [DOI] [PubMed] [Google Scholar]
- 2.Barbut F, Kajzer C, Planas N, Petit J-C. Comparison of three enzyme immunoassays, a cytotoxicity assay, and a toxigenic culture for diagnosis of Clostridium difficile-associated diarrhea. J Clin Microbiol. 1993;31:963–967. doi: 10.1128/jcm.31.4.963-967.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartlett J. Clostridium difficile: clinical considerations. Rev Infect Dis. 1990;12:S243–S251. doi: 10.1093/clinids/12.supplement_2.s243. [DOI] [PubMed] [Google Scholar]
- 4.Bartlett J G. Antibiotic-associated diarrhea. Clin Infect Dis. 1992;15:573–581. doi: 10.1093/clind/15.4.573. [DOI] [PubMed] [Google Scholar]
- 5.Bender B S, Bennett R, Laughon B E, Greenough W B, Gaydos C, Sears S D, Forman M S, Bartlett J G. Is Clostridium difficile endemic in chronic-care facilities? Lancet. 1986;ii:11–13. doi: 10.1016/s0140-6736(86)92559-6. [DOI] [PubMed] [Google Scholar]
- 6.Bennett G C J, Allen E, Millard P H. Clostridium difficile diarrhoea: a highly infectious organism. Age Ageing. 1984;13:363–366. doi: 10.1093/ageing/13.6.363. [DOI] [PubMed] [Google Scholar]
- 7.Borriello S P, Vale T, Brazier J S, Hyde S, Chippeck E. Evaluation of a commercial enzyme immunoassay kit for the detection of Clostridium difficile toxin A. Eur J Clin Microbiol Infect Dis. 1992;11:360–363. doi: 10.1007/BF01962079. [DOI] [PubMed] [Google Scholar]
- 8.Brown E, Talbot G M, Axelrod P, Provencher M, Hoegg C. Risk factors for Clostridium difficile toxin-associated diarrhea. Infect Control Hosp Epidemiol. 1990;11:283–290. doi: 10.1086/646173. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control. Surveillance, prevention, and control of nosocomial infections. Morbid Mortal Weekly Rep. 1992;41:783–787. [PubMed] [Google Scholar]
- 10.Chang T W, Lauermann M, Bartlett J G. Cytotoxicity assay in antibiotic-associated colitis. J Infect Dis. 1979;140:765–770. doi: 10.1093/infdis/140.5.765. [DOI] [PubMed] [Google Scholar]
- 11.Cumming A D, Thomson B J, Sharp J, Poxton I R, Fraser A G. Diarrhoea due to Clostridium difficile associated with antibiotic treatment in patients receiving dialysis: the role of cross infection. Br Med J. 1986;292:238–239. doi: 10.1136/bmj.292.6515.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeGirolami P C, Hanff P A, Eichelberger K, Longhi L, Teresa H, Pratt J, Cheng A, Letourneau J M, Thorne G M. Multicenter evaluation of a new enzyme immunoassay for detection of Clostridium difficile enterotoxin A. J Clin Microbiol. 1992;30:1085–1088. doi: 10.1128/jcm.30.5.1085-1088.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delmee M, Mackey T, Hamitou A. Evaluation of a new commercial Clostridium difficile toxin A enzyme immunoassay using diarrheal stools. Eur J Clin Microbiol Infect Dis. 1992;11:246–249. doi: 10.1007/BF02098089. [DOI] [PubMed] [Google Scholar]
- 14.DiPersio J R, Varga F J, Conwell D L, Kraft J A, Kozak K J, Willis D H. Development of rapid enzyme immunoassay for Clostridium difficile toxin A and its use in the diagnosis of C. difficile-associated disease. J Clin Microbiol. 1991;29:2724–2730. doi: 10.1128/jcm.29.12.2724-2730.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doern G V, Coughlin R T, Wu L. Laboratory diagnosis of Clostridium difficile-associated gastrointestinal disease: comparison of a monoclonal antibody enzyme immunoassay for toxins A and B with a monoclonal antibody enzyme immunoassay for toxin A only and two cytotoxicity assays. J Clin Invest. 1992;90:822–829. doi: 10.1128/jcm.30.8.2042-2046.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donta S T, Sullivan N, Wilkins T D. Differential effects of Clostridium difficile toxins on tissue-cultured cells. J Clin Microbiol. 1982;15:1157–1158. doi: 10.1128/jcm.15.6.1157-1158.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eskiturk A, Soyletir G. A dilemma: infections in intensive care unit. J Turk Microbiol Soc. 1994;25:110–115. [Google Scholar]
- 18.Fan K, Morris A J, Reller L B. Application of rejection criteria for stool cultures for bacterial enteric pathogens. J Clin Microbiol. 1993;31:2233–2235. doi: 10.1128/jcm.31.8.2233-2235.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fekety R, Kim K H, Brown D, Batts D H, Cudmore M, Silva J., Jr Epidemiology of antibiotic-associated colitis: isolation of Clostridium difficile from the hospital environment. Am J Med. 1981;70:906–908. doi: 10.1016/0002-9343(81)90553-2. [DOI] [PubMed] [Google Scholar]
- 20.Fekety R. Antibiotic-associated colitis. In: Mandell G L, Douglas R G, Bennett J E, editors. Principles and practice of infectious disease. 4th ed. New York, N.Y: Churchill Livingstone; 1995. p. 978. [Google Scholar]
- 21.Gerding D N, Olson M M, Peterson L R, Teasley D G, Gebhard R L, Schwartz M L, Lee J T. Clostridium difficile-associated diarrhoea and colitis in adults. Arch Intern Med. 1986;146:95–100. [PubMed] [Google Scholar]
- 22.Haley R W, Culver D H, White J W, Morgan W M, Emori T G. The nationwide nosocomial infection rate. Am J Epidemiol. 1985;121:159–167. doi: 10.1093/oxfordjournals.aje.a113988. [DOI] [PubMed] [Google Scholar]
- 23.Heard S R, O’Farrell S, Holland D, Crook S, Barnett M J, Tabaqchali S. The epidemiology of Clostridium difficile with use of a typing scheme: nosocomial acquisition and cross-infection among immunocompromised patients. J Infect Dis. 1986;153:159–162. doi: 10.1093/infdis/153.1.159. [DOI] [PubMed] [Google Scholar]
- 24.Hughes J M, Jarvis W R. Nosocomial gastrointestinal infections. In: Wenzel R P, editor. Prevention and control of nosocomial infections. Baltimore, Md: The Williams & Wilkins Co.; 1987. pp. 405–439. [Google Scholar]
- 25.Johnson S, Clabots C R, Linn F V, Olson M M, Peterson L R, Gerding D N. Nosocomial Clostridium difficile colonisation and disease. Lancet. 1990;336:97–100. doi: 10.1016/0140-6736(90)91605-a. [DOI] [PubMed] [Google Scholar]
- 26.Kaatz G W, Gitlin S D, Schaberg D R, Wilson K H, Kauffman C A, Seo S M, Fekety R. Acquisition of Clostridium difficile from the hospital environment. J Epidemiol. 1988;127:1289–1294. doi: 10.1093/oxfordjournals.aje.a114921. [DOI] [PubMed] [Google Scholar]
- 27.Kelly M T, Champagne S G, Sherlock C H, Noble M A, Freeman H J, Smith J A. Commercial latex agglutination test for detection of Clostridium difficile-associated disease. J Clin Microbiol. 1987;25:1244–1247. doi: 10.1128/jcm.25.7.1244-1247.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim K H, Fekety R, Batts D H, Brown D, Silva J, Waters D. Isolation of Clostridium difficile from the environment and contacts of patients with antibiotic-associated colitis. J Infect Dis. 1981;143:42–50. doi: 10.1093/infdis/143.1.42. [DOI] [PubMed] [Google Scholar]
- 29.Knoop F C, Owens M, Crocker I C. Clostridium difficile: clinical disease and diagnosis. Clin Microbiol Rev. 1993;6:251–265. doi: 10.1128/cmr.6.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larson H E, Barclay F E, Honour P, Hill I D. Epidemiology of Clostridium difficile in infants. J Infect Dis. 1982;146:727–733. doi: 10.1093/infdis/146.6.727. [DOI] [PubMed] [Google Scholar]
- 31.Lyerly D M, Krivan H C, Wilkins T D. Clostridium difficile: its disease and toxins. Clin Microbiol Rev. 1988;1:1–18. doi: 10.1128/cmr.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyerly D M, Lockwood D E, Richardson S H, Wilkins T D. Biological activities of toxins A and B of Clostridium difficile. Infect Immun. 1982;35:1147–1150. doi: 10.1128/iai.35.3.1147-1150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McFarland L V, Mulligan M E, Kwok R Y Y, Stamm W E. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989;320:204–210. doi: 10.1056/NEJM198901263200402. [DOI] [PubMed] [Google Scholar]
- 34.McKay I, Coia J E, Poxton I R. Typing of Clostridium difficile causing diarrhoea in an orthopaedic ward. J Clin Pathol. 1989;42:511–515. doi: 10.1136/jcp.42.5.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merz C S, Kramer C, Forman M, Gluck L, Mills K, Senft K, Steiman I, Wallace N, Charache P. Comparison of four commercially available rapid enzyme immunoassays with cytotoxin assay for detection of Clostridium difficile toxin(s) from stool specimens. J Clin Microbiol. 1994;32:1142–1147. doi: 10.1128/jcm.32.5.1142-1147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mulligan M E, Rolfe R D, Finegold S M, George W L. Contamination of a hospital environment by Clostridium difficile. Curr Microbiol. 1979;3:173–175. [Google Scholar]
- 37.Peach S, Borriello S P, Gaya H, Barclay F E, Welch A R. Asymptomatic carriage of Clostridium difficile in patients with cystic fibrosis. J Clin Pathol. 1986;39:1013–1018. doi: 10.1136/jcp.39.9.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qualman S J, Petric M, Karmali M A, Smith C R, Hamilton S R. Clostridium difficile invasion and toxin circulation in fatal pediatric pseudomembranous colitis. Am J Clin Pathol. 1990;94:410–416. doi: 10.1093/ajcp/94.4.410. [DOI] [PubMed] [Google Scholar]
- 39.Richardson S A, Alcock P A, Gray J. Clostridium difficile and its toxin in healthy neonates. Br Med J. 1983;287:878. doi: 10.1136/bmj.287.6396.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soyletir G, Eskiturk A, Kilic G, Korten V, Tozun N. Clostridium difficile acquisition rate and its role in nosocomial diarrhoea at a university hospital in Turkey. Eur J Epidemiol. 1996;12:391–394. doi: 10.1007/BF00145303. [DOI] [PubMed] [Google Scholar]
- 41.Struelens M J, Maas A, Nonhoff C, Deplano A, Rost F, Serruys E, Delmee M. Control of nosocomial transmission of Clostridium difficile on sporadic case surveillance. Am J Med. 1991;91:138S–144S. doi: 10.1016/0002-9343(91)90359-6. [DOI] [PubMed] [Google Scholar]
- 42.Taylor N S, Thorne G M, Bartlett J G. Comparison of two toxins produced by Clostridium difficile. Infect Immun. 1981;34:1036–1043. doi: 10.1128/iai.34.3.1036-1043.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Testore G P, Pantosti A, Cerquetti M, Babudieri S, Panichi G, Mastrantonio Gianfrilli P. Evidence for cross-infection in an outbreak of Clostridium difficile-associated diarrhoea in a surgical unit. J Med Microbiol. 1988;26:125–128. doi: 10.1099/00222615-26-2-125. [DOI] [PubMed] [Google Scholar]
- 44.Wilcox M H, Cunniffe J G, Trundle C, Redpath C. Financial burden of hospital-acquired Clostridium difficile infection. J Hosp Infect. 1996;34:23–30. doi: 10.1016/s0195-6701(96)90122-x. [DOI] [PubMed] [Google Scholar]
- 45.Zaidi M, Ponce de Leon S, Ortiz R M, Ponce de Leon S, Calva J J, Ruiz-Palacios G, Camorlinga M, Cervantes L E, Ojeda F. Hospital acquired diarrhea in adults: a prospective case controlled study in Mexico. Infect Control Hosp Epidemiol. 1991;12:349–355. doi: 10.1086/646355. [DOI] [PubMed] [Google Scholar]