Abstract
The drug screen test on a 12-year-old male patient was positive for opiates by a kinetic interaction of microparticles in solution (KIMS) immunoassay method on the Roche Cobas C502. The positive opiates result was not confirmed by the liquid chromatography-tandem mass spectrometry (LC-MS/MS) method. A chart review revealed that the patient had tuberculosis and was on rifampin. We spiked rifampin into drug-free urine and tested opiates with the Cobas method. Once again, a positive result was obtained. This case showed that rifampin can still cause false positive opiate results measured with the KIMS method. We want to stress the importance of confirming positive screen results by more specific methods such as LC-MS/MS.
Keywords: Rifampin, Opiate, HPLC, LC-MS/MS, False-positive
Highlights
-
•
Rifampin can cause false positive results with the opiate immunoassays.
-
•
Drugs of abuse immunoassay screens should be confirmed by an alternative method such as LC-MS/MS.
1. Case description
A 12-year-old male with hypoplastic left heart syndrome presented to our institution for heart transplant evaluation. His blood pressure was 110/72 mmHg (<120/<80 mmHg), and B-natriuretic peptide (BNP) was 109 pg/mL (<100 pg/mL). The patient's medications included metoprolol, furosemide, and captopril. Additionally, this overseas patient tested positive for tuberculosis on the MTB-Quantiferon-Gold ELISA, for which rifampin (351 mg/day) was prescribed. As per pre-transplant procedures, he was tested for drugs of abuse in urine, which includes barbiturates, benzodiazepines, cannabinoids, cocaine, ethanol, fentanyl, methadone, methamphetamine/amphetamines, hydrocodone, opiates, and oxycodone. All drugs tested negative except for opiates, which were positive by the Opiates II (OPI2) assay on the Roche Cobas C502 analyzer using the kinetic interaction of microparticles in solution (KIMS) methodology. High-performance liquid chromatography (HPLC) was used to confirm the immunoassay results, which were negative for hydrocodone, hydromorphone, codeine, morphine, 6-acetyl morphine, noroxycodone, noroxymorphone, oxycodone, oxymorphone, and norhydrocodone. In addition, the patient's parents denied drug use by their child and assured social workers that there were no such drugs in the house. A follow-up urine sample collected two days later again tested positive for opiates by the same KIMS method on the Cobas. We then reviewed the patient's medication list and found that the patient was on rifampin. The sample's urine rifampin concentration was 52390 ng/mL by an HPLC method.
2. Discussion
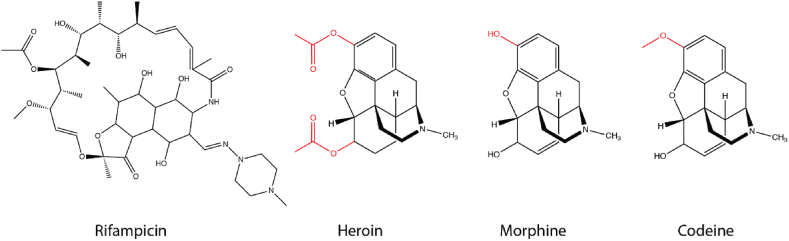
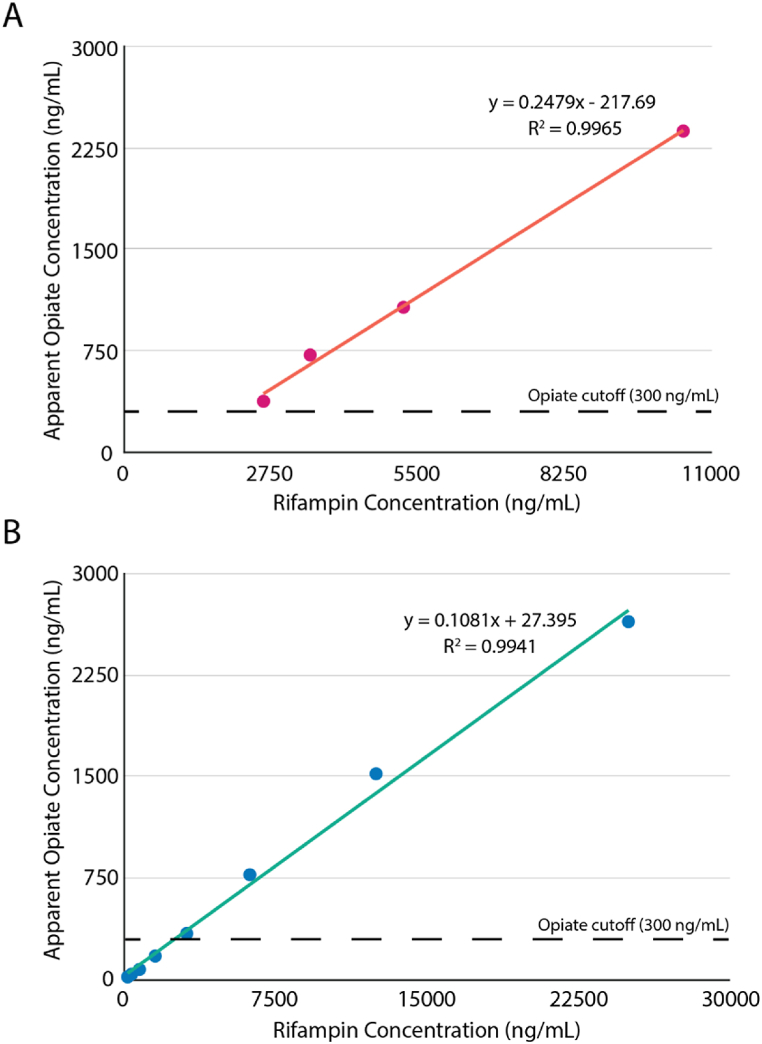
Immunoassays remain the preferred method for screening drugs of abuse in most clinical laboratories due to their availability, simplicity, and fast turnaround time. However, the sensitivity (300 ng/mL cut-off for opiates by Cobas) and specificity of immunoassays for drug testing are not as good as liquid chromatography-tandem mass spectrometry (LC-MS/MS) (20 ng/mL cut-off) or gas chromatography-tandem mass spectrometry (GC-MS/MS). One drawback of immunoassays is that they are prone to false positive or false negative results due to antibody cross-reactivity with the metabolites of certain medications or with structurally related compounds, autoantibody or other interference substances present in the sample, complex matrixes effect, or the limitation of the sensitivity of the assay [1]. The Roche opiates immunoassay uses the KIMS method for opiates test, in which the opiates present in a sample can inhibit microparticle aggregation formed due to the interactions between the reagent antibody and the morphine conjugate derivatives. Although the skeleton structure of rifampin does not appear to resemble that of morphine or other opiates (Fig. 1), it is possible that some configurations of rifampin in solution partially resemble the structure of the morphine conjugate, resulting in antibody cross-reactivity. This hypothesis is consistent with the apparent opiate concentration containing rifampin, which yielded a linear response curve between rifampin concentration and the apparent opiate concentration (Fig. 2A). When the sample was diluted 5, 10, 15, and 20 times, the apparent opiate concentration obtained with KIMS OPI2 on the cobas exhibited a linear relationship with a slope of 0.25 (Fig. 2A). Similarly, we spiked drug-free urine with rifampin (Fisher Scientific) and ran them on the Roche opiate immunoassay; this resulted in an apparent opiate concentration linearly related to rifampin concentration with a slope of 0.11 (Fig. 2B). Together, this suggests that rifampin can cause false positivity due to cross-reactivity with the OPI2 assay at around 10%, similar to previous reports [2,3].
Figure 1.
Structure comparison of various opiates and rifampin with structural differences highlighted in red.
Fig. 2.
Rifampin dilution and spiking studies. Opiate concentrations were measured with Cobas OPI2 assay. (A) The apparent opiates results in diluted urine samples of the patient. The concentration of rifampin in each of the diluted urine samples was calculated using the neat rifampin concentration (52390 ng/mL) and the associated dilution factors. B) The apparent opiates results in urine specimens containing various spiked rifampin. Additionally, to achieve a positive result using the OPI2 from Roche required 2521 ng/mL of Rifampin.
Several cases of rifampin's interference with KIMS opiates assay have been reported outside the United States (US) [2,4]. However, there is a dearth of reports in the US. One possibility may be that we rarely encounter urine specimens containing rifampin due to the low prevalence of tuberculosis in the US compared with other countries, with 2.2 cases per 100,000 persons in the US but 24% of cases within California alone [5].
Illicit substances are implicated with organ damage; as such, current or former abusers often do not get accepted into transplant recipient lists [6,7]. The International Society of Heart and Lung Transplantation recommends that patients with current illicit substance abuse (including opiates) be unsuitable for heart transplantation due to the risk of poor outcomes [7,8]. Thus, differentiating between a true or a false positive result for opiate immunoassay, as presented in this case, can have consequential outcomes. In this case study, we confirm that the cross-reactivity of rifampin with the opiate immunoassay on Roche Cobas C502 KIMS assay persists. Due to many limitations of immunoassays mentioned elsewhere [9], it is essential that positive immunoassay results for transplantation and suspected child abuse be confirmed by a more sensitive and specific method such as LC-MS/MS or GC-MS/MS.
3. Conclusion
Our data demonstrate the interference of rifampin with the OPI2 assay using the KIMS method on the Cobas C502 analyzer. This study highlights the importance of confirming a positive opiate immunoassay results with a GC-MS/MS or LC-MS/MS method, especially in patients taking rifampin.
Patient consent
This study has complied with the University of California research policy & compliance review board's IRB procedures and protocols for relevant ethics guidelines.
Declaration of competing interest
The authors declare no competing interests.
Data availability
No data was used for the research described in the article.
References
- 1.Lam K.H.B., Sobolevsky T., Ahrens B., Song L., Metushi I.G., Description C. A suspected case of carbon monoxide poisoning consistent with fentanyl toxicity. J Appl Lab Med. 2023;8:413–417. doi: 10.1093/JALM/JFAC140. [DOI] [PubMed] [Google Scholar]
- 2.De Paula M., Saiz L.C., González-Revaldería J., Pascual T., Alberola C., Miravalles E. Rifampicin causes false-positive immunoassay results for urine opiates. Clin. Chem. Lab. Med. 1998;36:241–243. doi: 10.1515/CCLM.1998.041. [DOI] [PubMed] [Google Scholar]
- 3.Saitman A., Park H.D., Fitzgerald R.L. False-positive interferences of common urine drug screen immunoassays: a review. J. Anal. Toxicol. 2014;38:387–396. doi: 10.1093/JAT/BKU075. [DOI] [PubMed] [Google Scholar]
- 4.Daher R., Haidar J.H., Al-Amin H. Rifampin interference with opiate immunoassays. Clin. Chem. 2002;48:203–204. [PubMed] [Google Scholar]
- 5.Deutsch-Feldman M., Pratt R.H., Price S.F., Tsang C.A., Self J.L. vol. 70. 2021. p. 409. (Tuberculosis — United States, 2020, Morbidity and Mortality Weekly Report). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majumder P., Sarkar S. A review of the prevalence of illicit substance use in solid-organ transplant candidates and the effects of illicit substance use on solid-organ transplant treatment outcomes. Cureus. 2020;12 doi: 10.7759/CUREUS.8986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen L.A., Ambardekar A.V. vol. 9. Circ Heart Fail; 2016. (Hashing it Out over Cannabis : Moving toward a Standard Guideline on Substance Use for Cardiac Transplantation Eligibility that Includes Marijuana). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehra M.R., Canter C.E., Hannan M.M., Semigran M.J., Uber P.A., Baran D.A., Danziger-Isakov L., Kirklin J.K., Kirk R., Kushwaha S.S., Lund L.H., Potena L., Ross H.J., Taylor D.O., Verschuuren E.A.M., Zuckermann A. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10-year update. J. Heart Lung Transplant. 2016;35:1–23. doi: 10.1016/J.HEALUN.2015.10.023/ATTACHMENT/248C18F7-626A-4C72-9B82-9E0483119738/MMC1.PDF. [DOI] [PubMed] [Google Scholar]
- 9.Hoofnagle A.N., Wener M.H. The fundamental flaws of immunoassays and potential solutions using tandem mass spectrometry. J. Immunol. Methods. 2009;347:3–11. doi: 10.1016/J.JIM.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.