Abstract
To compare the sensitivity and specificity of RT-PCR with that of virus isolation in the detection of human rhinoviruses, we tested nasopharyngeal aspirates from 200 patients on the 1st and 7th days after the onset of the common cold. An assay utilizing a short amplicon in the conserved 5′ noncoding region was found highly sensitive. Of 192 positive samples altogether, 65 were found positive by RT-PCR only, 6 were positive by isolation exclusively, and 121 gave positive results in both tests.
Human rhinoviruses (HRVs), together with enteroviruses and hepatitis A virus, belong to the picornaviruses infecting humans. They are small icosahedral particles, consisting of a single-stranded RNA genome surrounded by viral capsid proteins. HRV infections are considered the most frequent cause of the common cold, and they can affect the whole respiratory tract (17). HRVs induce mucosal changes in the airways which may result in sinusitis (6, 15), otitis media (2), or exacerbation of asthma (13). Virus isolation followed by an acid lability test is the most widely used method for the detection of HRVs. This method is, however, laborious and time consuming. Immunological assays are complicated by the existence of more than 100 HRV serotypes. Therefore, recently developed reverse transcription-PCR (RT-PCR) assays, which are likely to detect virtually all HRVs in a single test, have a potential to replace isolation in the near future.
Most RT-PCR tests described for rhino- and enteroviruses take advantage of the conserved primer sequences in the 5′ noncoding region (5′NCR) of the genome (see, e.g., references 4, 5, 7, and 10), and specific probes are used to discriminate between the two picornavirus groups. Another approach utilizes the difference in length between the amplicons covering part of the 5′NCR and capsid protein-coding region, which can be used for identification of rhino- and enteroviruses after agarose gel electrophoresis (1, 11, 14). We have evaluated the usefulness of these assays in the detection of HRVs in nasopharyngeal aspirates from adult patients with the common cold by comparing the RT-PCR results with those obtained by virus isolation.
Nasopharyngeal aspirates were collected from 200 young adults with symptoms of the common cold (12). Patients with allergic rhinitis or chronic illness were excluded. The aspirates were collected with a disposable mucus extractor on the 1st and 7th days after the onset of the symptoms. Three sterile cotton swabs were dipped into mucus and then transferred into separate viral transport medium tubes containing 0.5% bovine serum albumin and antibiotics in 2 ml of tryptose phosphate broth. All the virus culture tubes were immediately frozen and stored at −70°C prior to virus isolation and RT-PCR.
HeLa Ohio-Salisbury and human foreskin fibroblast cells were used for virus isolation. HeLa cells were maintained in BME (Gibco 073-1300) containing 2% inactivated fetal calf serum, 5% tryptose phosphate, and 30 mM MgCl2 (pH 7.3). The maintenance medium for human foreskin fibroblast cells was Dulbecco’s modified Eagle medium-HEPES (pH 7.0) (Gibco 13016-035), with 2% inactivated fetal calf serum. Of each sample, 200 μl was inoculated per tube, and the tubes were incubated at 33°C in a roller drum. To minimize possible toxic effects of the sample, the maintenance medium was changed the following day, and the tubes were observed for 6 days for cytopathic effect (CPE). If the cultures were CPE negative, a blind passage was made after storage at −70°C, followed by strong agitation of the cells. The viruses in the samples exhibiting CPE were further typed either by HRV-specific RT-PCR (see below) or by an acid lability test. In the latter method, the cells of CPE-positive cultures were pelleted and the supernatants, diluted 1:10, were incubated at pH 3 and pH 7 for 60 min at 4°C. After neutralization of the pH, the supernatants in dilutions of 1:10 and 1:100 were inoculated into cell cultures. After 2 h of incubation at 33°C in a roller drum, the supernatants were replaced by fresh maintenance medium. The test was then completed as in routine virus isolation.
Two previously described RT-PCR assays were used for the detection of picornavirus RNA in the specimens. Prior to the tests, sample nucleic acids were isolated from the cotton swabs, containing nasopharyngeal aspirate, by proteinase K-sodium dodecyl sulfate treatment followed by phenol extraction and ethanol precipitation (9). First, the samples were tested with an assay utilizing primers derived from the conserved regions in the 5′NCR (4+) and the VP2 gene (5−) (1). These primers give rise to amplification products of approximately 530 and 650 bp for rhino- and enteroviruses, respectively. The conditions in the PCR were as follows: 1 min at 95°C, 1 min at 55°C, and 2 min at 72°C, for 40 cycles. In the other PCR test, an approximately 120-bp product from the 5′NCR was amplified with primers 4− and 3+ (7, 10). The PCR profile in this test was as follows: 1 min at 95°C, 1 min at 55°C, and 1 min at 72°C, for 40 cycles.
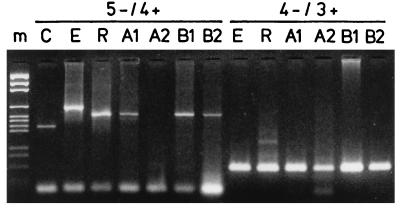
We originally used the RT-PCR assay specifically amplifying a fragment covering part of the 5′NCR and coding region of the HRV genome (1). An obvious advantage of this test is direct discrimination between HRVs and enteroviruses after agarose gel electrophoresis without a need for hybridization protocols. One hundred six of the 400 samples were positive by the assay. However, during the study, we observed that the sensitivity of this assay was somewhat lower (Fig. 1) than that of the RT-PCR test utilizing the shorter (approximately 120-bp) amplicon from the 5′NCR (16). Therefore, the samples that had remained negative in the original assay were also tested by RT-PCR using primers 4− and 3+. By this means, 79 new positive specimens were identified. In the samples from 17 individuals where identification could not be based on the size difference in the HRV-specific RT-PCR, the identities of the amplicons were confirmed by hybridization using oligonucleotide probes (7).
FIG. 1.
Detection of HRV RNA in nasopharyngeal specimens by the two RT-PCR assays. Nucleic acids were isolated from the aspirates, and the RT-PCR amplification products were analyzed by electrophoresis in agarose gels. m, molecular weight markers; C, truncated echovirus 11 control RNA (1); E, enterovirus (coxsackievirus A9) RNA; R, HRV type 1B RNA. A1, A2, B1, and B2 are the 1st and 2nd samples collected from patients A and B, respectively. The primer pairs (5− and 4+, 4− and 3+) used in the assays are indicated.
Of the 400 specimens (two samples from each of 200 patients) (12) tested, 192 (48%) were positive for HRV either by virus isolation or by RT-PCR. Sixty-five were diagnosed by RT-PCR only, 6 were detected exclusively by isolation, and 121 gave positive results in both tests. When the cell culture-grown HRV isolates from the six specimens originally negative in the RT-PCR tests were analyzed by RT-PCR, one of the isolates gave rise to an amplicon corresponding to HRVs while another amplicon was larger, resembling that obtained in enteroviruses. To investigate whether these nasopharyngeal aspirates contained inhibitors of RT-PCR, control picornavirus RNA (1) was added to the nucleic acid preparations prior to RT-PCR. The results indicated that the samples did not contain significant amounts of inhibitory factors.
The sensitivities of RT-PCR and isolation were 98 and 66%, respectively. Among the 200 common-cold patients, a positive HRV diagnosis was obtained by RT-PCR for 52% and by isolation for 40%. With virus isolation, the first sample was positive for 70 patients and the second specimen was positive for 56 patients, 10 of whom had originally tested negative. The corresponding figures for HRV RT-PCR were 96 and 90 patients (7 of whom had originally tested negative), respectively. The sensitivity of the RT-PCR assay was particularly critical in the study of samples collected 1 week after the onset of the symptoms: 64 of the 79 specimens (81%) which were positive only with the more sensitive primer pair 3+ and 4− were second samples.
The applicability of RT-PCR to rapid detection of HRVs in clinical samples has been documented in a number of reports. HRVs have been successfully detected by RT-PCR in nasal and throat swabs, nasal washings, and nasopharyngeal-aspirate specimens which have been positive for HRV by conventional culture methods (see, e.g., references 3, 7, and 11). Recently, Pitkäranta et al. (15) reported detection of HRV RNA in 10 maxillary aspirates or nasal swabs of 20 adult patients with sinusitis by RT-PCR, indicating an etiological role for these pathogens in a considerable proportion of other respiratory infections in addition to the common cold. Since HRVs have also been detected in a significant number of culture-negative specimens (7, 11), it has been suggested that RT-PCR could replace the laborious and time-consuming isolation methods in the diagnosis of HRV infections.
In the present study, we used two primer pairs to detect HRVs in nasopharyngeal-aspirate specimens from young adults with the common cold. The differences in sensitivity between the two assays can be explained by the differences in length between the amplicons (530 versus 120 bp), allowing more efficient amplification of the shorter RT-PCR product. It is a known fact that the primer site in the VP2 gene exhibits more variation than those in the 5′NCR (8), evidently leading to lower sensitivity or even to false-negative results. Further knowledge of the sequence differences in the human picornavirus genomes may enable development of more sensitive group-specific assays in the future.
In conclusion, our results show that RT-PCR can be applied for rapid diagnosis of HRV infections. The test can be completed during 1 working day instead of the 1 to 2 weeks needed for isolation and the acid lability test. The costs of RT-PCR and isolation are currently comparable, but the potential for automation of the PCR tests may allow for cost-effective detection of multiple respiratory pathogens in the same assay in the near future.
Acknowledgments
We thank Marja Aaltonen and Marita Maaronen for excellent technical assistance and Pekka Halonen and Juhana Santti for stimulating discussions.
This study was supported by grants from Glaxo Research and Development Limited, Uxbridge, Middlesex, United Kingdom, the Research Foundation of the Orion Corporation, Helsinki, Finland, the Academy of Finland, and the Sigrid Juselius Foundation.
REFERENCES
- 1.Arola A, Santti J, Ruuskanen O, Halonen P, Hyypiä T. Identification of enteroviruses in clinical specimens by competitive PCR followed by genetic typing using sequence analysis. J Clin Microbiol. 1996;34:313–318. doi: 10.1128/jcm.34.2.313-318.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arola M, Ziegler T, Puhakka H, Lehtonen O-P, Ruuskanen O. Rhinovirus in otitis media with effusion. Ann Otol Rhinol Laryngol. 1990;99:451–453. doi: 10.1177/000348949009900607. [DOI] [PubMed] [Google Scholar]
- 3.Arruda E, Hayden F G. Detection of human rhinovirus RNA in nasal washings by PCR. Mol Cell Probes. 1993;7:373–379. doi: 10.1006/mcpr.1993.1055. [DOI] [PubMed] [Google Scholar]
- 4.Gama R E, Hughes P J, Bruce C B, Stanway G. Polymerase chain reaction amplification of rhinovirus nucleic acids from clinical material. Nucleic Acids Res. 1988;16:9346. doi: 10.1093/nar/16.19.9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gama R E, Horsnell P R, Hughes P J, North C, Bruce C B, Al-Nakib W, Stanway G. Amplification of rhinovirus specific nucleic acids from clinical samples using the polymerase chain reaction. J Med Virol. 1989;28:73–77. doi: 10.1002/jmv.1890280204. [DOI] [PubMed] [Google Scholar]
- 6.Gwaltney J M, Jr, Phillips C D, Miller R D, Riker K. Computed tomographic study of the common cold. N Engl J Med. 1994;330:25–30. doi: 10.1056/NEJM199401063300105. [DOI] [PubMed] [Google Scholar]
- 7.Halonen P, Rocha E, Hierholzer J, Holloway B, Hyypiä T, Hurskainen P, Pallansch M. Detection of enteroviruses and rhinoviruses in clinical specimens by PCR and liquid-phase hybridization. J Clin Microbiol. 1995;33:648–653. doi: 10.1128/jcm.33.3.648-653.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horsnell C, Gama R E, Hughes P J, Stanway G. Molecular relationships between 21 human rhinovirus serotypes. J Gen Virol. 1995;76:2549–2555. doi: 10.1099/0022-1317-76-10-2549. [DOI] [PubMed] [Google Scholar]
- 9.Hyypiä T, Stålhandske P, Vainionpää R, Pettersson U. Detection of enteroviruses by spot hybridization. J Clin Microbiol. 1984;19:436–438. doi: 10.1128/jcm.19.3.436-438.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hyypiä T, Auvinen P, Maaronen M. Polymerase chain reaction for human picornaviruses. J Gen Virol. 1989;70:3261–3268. doi: 10.1099/0022-1317-70-12-3261. [DOI] [PubMed] [Google Scholar]
- 11.Johnston S L, Sanderson G, Pattemore P K, Smith S, Bardin P G, Bruce C B, Lambden P R, Tyrrell D A J, Holgate S T. Use of polymerase chain reaction for diagnosis of picornavirus infection in subjects with and without respiratory symptoms. J Clin Microbiol. 1993;31:111–117. doi: 10.1128/jcm.31.1.111-117.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mäkelä M J, Puhakka T, Ruuskanen O, Leinonen M, Saikku P, Kimpimäki M, Blomqvist S, Hyypiä T, Arstila P. Viruses and bacteria in the etiology of the common cold. J Clin Microbiol. 1998;36:539–542. doi: 10.1128/jcm.36.2.539-542.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicholson K G, Kent J, Ireland D C. Respiratory viruses and exacerbations of asthma in adults. Br Med J. 1993;307:982–986. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olive D M, Al-Mufti S, Al-Mulla W, Khan M A, Pasca A, Stanway G, Al-Nakib W. Detection and differentiation of picornaviruses in clinical samples following genomic amplification. J Gen Virol. 1990;71:2141–2147. doi: 10.1099/0022-1317-71-9-2141. [DOI] [PubMed] [Google Scholar]
- 15.Pitkäranta A, Arruda E, Malmberg H, Hayden F G. Detection of rhinovirus in sinus brushings of patients with acute community-acquired sinusitis by reverse transcription-PCR. J Clin Microbiol. 1997;35:1791–1793. doi: 10.1128/jcm.35.7.1791-1793.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santti J, Hyypiä T, Halonen P. Comparison of PCR primer pairs in the detection of human rhinoviruses in nasopharyngeal aspirates. J Virol Methods. 1997;66:139–147. doi: 10.1016/s0166-0934(97)00049-9. [DOI] [PubMed] [Google Scholar]
- 17.Turner R B. Epidemiology, pathogenesis and treatment of the common cold. Ann Allergy Asthma Immunol. 1997;78:531–539. doi: 10.1016/S1081-1206(10)63213-9. [DOI] [PMC free article] [PubMed] [Google Scholar]