Abstract
Response surface methodology was selected to explore the ultrasonic-assisted cellulase extraction conditions of Garcinia mangostana rind polysaccharides (GMRPs), and the optimum values of each condition were as follows: ratio of raw material to liquid of 1:50 g/mL, ultrasonic time of 40 min, enzyme concentration of 4 %, and ultrasonic power of 179 W. Based on the above conditions, the average extraction rate of GMRPs was 15.56 %. GMRPs were modified by carboxymethylation, and the relationship between the amount of chloroacetic acid and the substitution degree of carboxymethylated derivative was compared. Based on the results of single factor experiment, it was shown that the amount of chloroacetic acid significantly affected the degree of substitution of derivative products. The above research provides some valuable theoretical references for the preparation of GMRPs and its carboxymethylation products.
Keywords: Ultrasonic/cellulase-assisted extraction, Polysaccharide from Garcinia mangostana rinds, Carboxymethylated derivative
1. Introduction
Garcinia mangostana (mangosteen) is a tropical evergreen tree belonging to the genus Garcinia of the garcinaceae family, which has long been distributed in tropical and subtropical areas. Its flesh is sweet and sour and delicious, and is often called the “Queen of fruits”. At the same time, as early as hundreds of years ago, mangosteen's peel was used as an external medicine. In view of this, the edible and medicinal value of mangosteen has been widely studied, and its peel is particularly concerned because of its special pharmacological activities. Modern studies have clearly indicated that mangosteen rinds has anti-tumor, antibacterial, hypoglycemic and other biological activities [1]. In addition, the substances extracted from its peel are rich in polysaccharides, polyphenols and other active ingredients, and the active effects of polysaccharides are particularly obvious, mainly reflected in antioxidant, immunomodulatory and other aspects [2], [3], [4]. Hence, it is necessary to carry out more in-depth research on polysaccharides from Garcinia mangostana rinds (GMRPs).
The traditional extraction method of natural active substances such as polysaccharides is solvent extraction method, which often enhances the heat transfer effect by heating so that polysaccharides are released into solvent water. The advantages and disadvantages of this method are obvious. Namely, the equipment and operation are simple, the cost is low, but the yield is generally low and time/energy-consuming exists. In order to solve this problem, more new methods begin to emerge, such as microwave-assisted extraction, ultrasonic-assisted extraction, enzyme-assisted extraction and so on [5], [6], [7], [8], [9]. It is worth noting that many studies in recent years have begun to focus more and more on combining multiple extraction methods to effectively extract natural substances, and the common method is to combine enzymatic method with microwave method or ultrasonic method, so as to achieve the purpose of economy, environmental protection, green and high efficiency [10], [11]. The former mainly uses hydrolases such as cellulase and papain to degrade cell wall components, and then rapidly penetrates plant tissues through microwave non-ionizing irradiation to promote the release of natural substances [12]. The latter is to break the cell structure through the decomposition effect of enzymes, and then to further destroy the cell wall or enhance the enzyme activity through the thermal effect, mechanical effect and cavitation effect generated by ultrasound waves, thereby improving the yield [13], [14], [15]. However, the study on the extraction of GMRPs by enzymatic method combined with ultrasonic method or microwave method is rarely reported. In view of this, the response surface methodology (RSM) was adopted in this study to optimize the process conditions of ultrasonic-assisted cellulase extraction of GMRPs [16]. From the perspective of statistics, four important factors such as enzyme concentration, ultrasonic power, ultrasonic time and ratio of raw material to liquid were explored to determine the optimal extraction conditions.
The important factors that affect the activity of polysaccharides often include molecular weight, spatial structure, substituents and so on. It is worth noting that the change and quantity of substituents can most easily affect the activity of polysaccharides [17]. In view of this, many studies have begun to apply various chemical modifications to achieve the above purpose. Common modification methods include carboxymethylation, acetylation, sulfation, phosphorylation, etc. [18], [19]. In this study, carboxymethylation modification of GMRPs was mainly carried out, and the amount of chloroacetic acid was used as a single factor experiment to conduct relevant exploration, and carboxymethylation derivative with the best degree of substitution were obtained within a certain range. It is believed that this will provide some reference values for the study of the chemical modification of polysaccharides from Garcinia mangostana rinds.
2. Materials and methods
2.1. Materials and reagents
Fresh mangosteen was sourced and purchased from Thailand, their rinds were washed and dried in advance, and then crushed into powder for use. Cellulase (98% purity) was purchased from Shanghai Macklin Biochemical Technology Co., Ltd. (Shanghai, China). Other relevant reagents in this study were of analytical purity.
2.2. Experimental
2.2.1. Ultrasonic-assisted cellulase extraction of GMRPs
1.00 g of dried mangosteen rind power was accurately weighed, and then cellulase solution with a certain mass concentration (w/w) was mixed with 1.00 g raw material according to different ratio of raw material to liquid, and transferred to a round-bottom flask. Next, the flask was fixed in the ultrasonic machine with the power already set for extraction. After a period of extraction, the extracted solution was collected and deproteinized twice, that is, the extracted solution was fully mixed with Sevag reagent (Vchloroform: Vn-butanol = 4:1) at the volume ratio of 2:1, then transferred to the separation funnel for 15 min for shock, and then left for 5 min. After centrifugation, the supernatant was collected and put into 3500 Da dialysis bag for 1 day of tap water dialysis and 1 day of distilled water dialysis. Finally, the dialysate and anhydrous ethanol were mixed according to the volume ratio of 1:3 and stirred evenly. After placing for 1 h, the solid precipitates were collected by centrifugation, and the precipitates were washed with acetone and ether for three times in turn, and then freeze-dried to obtain GMRPs.
2.2.2. Glucose standard curve and extraction rate of GMRPs
Based on the phenol–sulfuric acid method, the absorbance of glucose standard solution with different concentrations at 490 nm was measured by ultraviolet spectrophotometer, so as to obtain the linear regression equation related to absorbance and concentration and draw the glucose standard curve [20]. The specific steps were as follows: firstly, the 25 mg of dried glucose power was fully dissolved with an appropriate amount of distilled water, and then transferred to a 250 mL volumetric bottle and added distilled water to obtain a concentration of 0.1000 mg/mL of glucose standard solution. Then, the standard solution of different volumes (0, 0.40, 0.80, 1.20, 1.60, 2.00 mL) was accurately transferred into the corresponding centrifuge tube (all are in 10 mL capacity) by using pipettor, and then different volumes of distilled water were added successively, so that the volume of solution in each centrifuge tube reached 2 mL. Finally, phenol (5%, 1 mL) and concentrated sulfuric acid (98%, 5 mL) were successively added to the above solution of different concentrations (0, 20, 40, 60, 80, 100 μg/mL). After shaking well, the absorbance of each solution at 490 nm was measured and the correlation regression equation (R2 = 0.9997) with a linear range of 0–100 μg/mL was obtained:
| (1) |
here, X is the concentration of the solution to be measured, μg/mL; Y is the absorbance of the solution to be measured.
According to the extraction method in 2.2.1, the polysaccharide extraction solution treated by ultrasonic-assisted cellulase method was collected, and the polysaccharide solution to be measured was prepared after dilution treatment. After the above solution was treated with phenol–sulfuric acid method, the absorbance at 490 nm was determined by ultraviolet spectrophotometer, and the value was substituted into the linear regression equation (1) to calculate the polysaccharide concentration of the solution to be measured. Finally, the extraction rate of polysaccharide was calculated by substituting the concentration of polysaccharide into the following formula:
| (2) |
here, C is the polysaccharide concentration of the solution to be measured, mg/mL; V is the volume of polysaccharide extraction solution, mL; N is the dilution; m is the mangosteen rinds of powder mass, g.
2.2.3. Single factor experiments and Box-Behnken design (BBD) experiment
Based on the control variable method, four factors were selected successively as single variables to carry out related exploration experiments, including ratio of raw material to liquid (1:30 g/mL, 1:40 g/mL, 1:50 g/mL, 1:60 g/mL, 1:70 g/mL), ultrasonic time (30 min, 40 min, 50 min, 60 min, 70 min), enzyme concentration (1 %, 2 %, 3 %, 4 %, 5 %)and ultrasonic power (144 W, 180 W, 216 W, 252 W, 288 W). That is, the optimal values of the above four variables were determined by four single factor experiments with the extraction rate of polysaccharide as the reference index.
The initial range of the four variable factors was reduced through the single factor experiments, and the four optimal values were preliminarily determined. On this basis, Box-Behnken Desigh (BBD) experiment in RSM was selected to further optimize the process conditions of ultrasonic-assisted cellulase extraction of GMRPs. The specific steps were as follows: the extraction rate of GMRPs was taken as the response value (Y), and the four factors were named X1 (ratio of raw material to liquid), X2 (ultrasonic time), X3 (enzyme concentration) and X4 (ultrasonic power), respectively, and the optimal value of each factor was designed as the central level to conduct the RSM experiment with four factors and three levels (Table 1).
Table 1.
Factors and levels.
| Factors | Levels |
||
|---|---|---|---|
| −1 | 0 | 1 | |
| X1 (ratio of raw material to liquid, g/mL) | 40 | 50 | 60 |
| X2 (ultrasonic time, min) | 30 | 40 | 50 |
| X3(enzyme concentration, %) | 3 | 4 | 5 |
| X4 (ultrasonic power, W) | 144 | 180 | 216 |
2.2.4. Preparation of carboxymethylation of GMRPs(CM - GMRPs)
On the basis of slight modification, CM - GMRPs were prepared according to the method proposed by Chen et al. [21]. The specific steps were as follows: 0.25 g of freeze-dried GMRPs were accurately weighed and added to a conical bottle containing 20 mL of NaOH solution (20%, w/w), and the GMRPs were fully dissolved with the help of a magnetic stirrer. After dissolution, the above solution was transferred to a 50 mL flask containing 7.5 mL chloroacetic acid solution (isopropyl alcohol as solvent, 4 mol/L) and reacted at 55 ℃ for 5 h. After the reaction, the reaction solution was cooled to room temperature, titrated with 1 mol/L HCI solution to the end point, and the Ph meter was used to detect whether the solution was neutral. Finally, the above solution was dialyzed for two days by using a dialysis bag of 3500 Da, and then the dialysate was concentrated at low pressure, followed by the addition of three times the volume of anhydrous ethanol. After standing for 12 h, the alcohol-precipitated solids were obtained by centrifugation, and the precipitates were freeze-dried to obtain CM - GMRPs. The yield of carboxymethylated polysaccharides was calculated by the following formula:
| (3) |
here, W1 is the mass of carboxymethylated polysaccharides (CM - GMRPs), g; W2 is the mass of raw material (GMRPs), g.
According to the above preparation method, the mass of fixed GMRPs was 0.25 g, and then the ratio of GMRPs and chloroacetic acid solution (w/v) was adjusted within a certain range to conduct relevant exploration experiments to obtain carboxymethylated derivatives with the best degree of substitution. That is, with the degree of substitution as a reference index, experiments with four different ratio of raw material to liquid of 1:20 g/mL, 1:30 g/mL, 1:40 g/mL and 1:50 g/mL were set for exploration, and the prepared products were named CM-20, CM-30, CM-40 and CM-50 respectively.
The degree of substitution of CM - GMRPs was determined based on acid-base titration [20]. That is, a certain amount of HCI solution (0.1 mol/L) was removed into a beakers containing 15 mg CM - GMRPs, and then heated at 45 ℃ by magnetic stirring for 0.5 h to fully dissolve it. After completion, the above solution was titrated with 0.1 mol/L NaOH solution, and the volume V1 (mL) and V2 (mL) of NaOH solution consumed at pH 2.1 and pH 4.3 of the mixed solution were recorded, respectively. Finally, the following formulas (4) and (5) were used to calculate the degree of substitution of carboxymethylated polysaccharides:
| (4) |
| (5) |
here, M is the mass of the carboxymethylated polysaccharides, g.
2.2.5. Characterization analysis methods
The characteristic functional groups of GMRPs and four carboxymethylated derivatives were determined by infrared spectrometer (the detection wavenumbers were set at 4000–400 cm−1). The specific steps were as follows: after the KBr powder was dried, with its thin slice as the background, 2 mg GMRPs, CM-20, CM-30, CM-40, CM-50 were mixed evenly with 100 mg KBr respectively, and then pressed them into thin slices with a tablet press to measure their transmittance.
GMRPs and its four carboxymethylated derivatives (all 30 mg) were dissolved in 0.6 mL deuterium water, respectively. The supernatant after centrifugation was determined by 13C NMR spectra.
3. Results and discussions
3.1. Single-factor experimental analysis
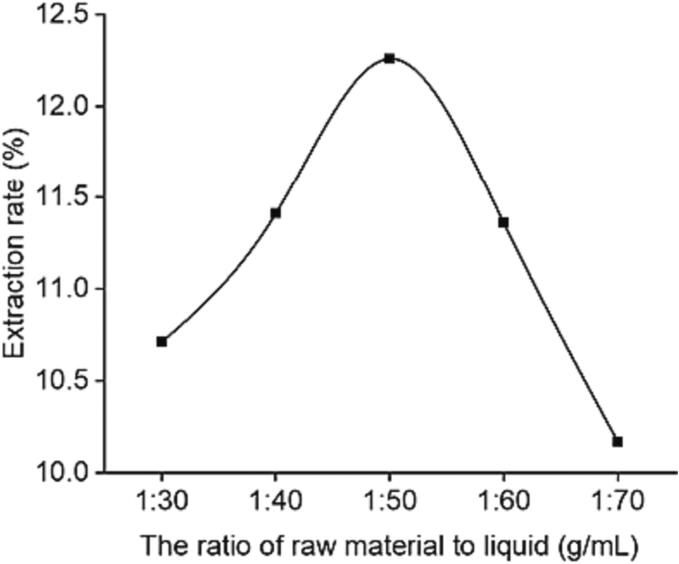
The relationship between polysaccharide extraction rate and ratio of raw material to liquid was shown in Fig. 1. In the lower range of 1:30 g/mL to 1:50 g/mL, the extraction rate of polysaccharides increased rapidly with the increase of solvent. The reasons for the above situation may be related to the increase of solid–liquid contact area to promote the release of natural active substances, and the decrease of extraction system viscosity to promote the ultrasonic cavitation effect [14], [22]. When the ratio of raw material to liquid was 1:50 g/mL, the release of polysaccharide reached the maximum. After that, the extraction rate of polysaccharides was significantly reduced with the increase of solvent content. The possible reasons for this were as follows: on the one hand, too much solvent inhibited the effect of penetration of ultrasound waves, thus reducing the thermal effect of the entire system [23]; on the other hand, the overflow of solvent content led to cellulase also promoted the production of non-target active products, thereby reducing the release of polysaccharides [24].
Fig. 1.
Effect of ratio of raw material to liquid on extraction rate of GMRPs.
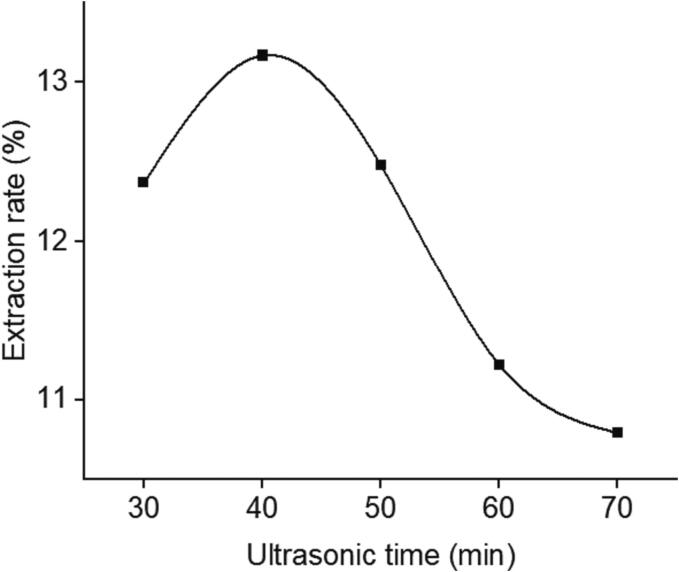
The relationship between extraction rate of polysaccharides and ultrasonic time was shown in Fig. 2. Ultrasonic time increased from 30 min to 40 min, during which the extraction rate of polysaccharide increased continuously and reached the maximum value, which may be related to the enhancement of the mass transfer effect of the extraction system promoted by ultrasound waves. However, with the increase of ultrasonic time, the extraction rate of polysaccharides began to decline significantly, which may be related to two factors. First, the ultrasonic shearing effect caused by too long ultrasonic time resulted in the continuous destruction of polysaccharide structure [11]. Second, the extension of ultrasonic time promoted the heat transfer effect of the whole system to a certain extent, and the change of temperature may affect the enzyme activity or lead to partial degradation of polysaccharides [25].
Fig. 2.
Effect of ratio of ultrasonic time on extraction rate of GMRPs.
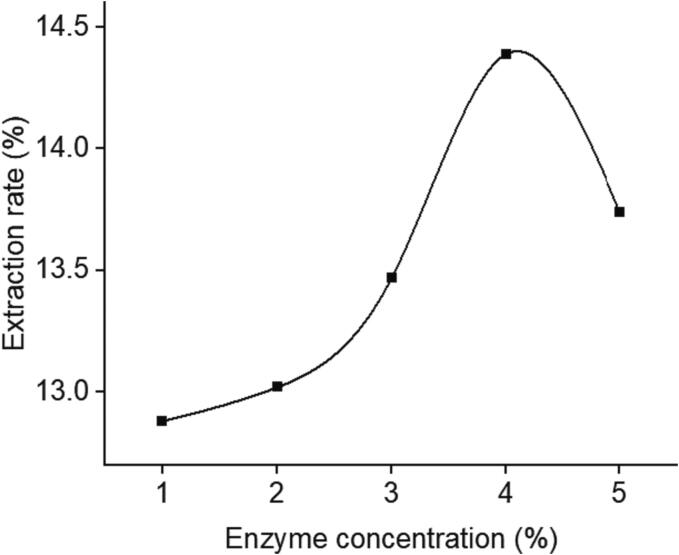
The influence of enzyme concentration on polysaccharide extraction rate was shown in Fig. 3. In the low concentration range, the effect of the enzyme was relatively small and the extraction rate increased relatively slowly. However, as the enzyme concentration changed from 2 % to 4 %, the release of polysaccharides increased significantly until it reached the maximum. After that, the increase of enzyme concentration caused the decrease of extraction rate of polysaccharides. The above situation occurred because, on the one hand, cellulase could promote the decomposition of cell wall to increase the release of natural active substances within the appropriate concentration range; on the other hand, the increase of enzyme content would increase the viscosity of enzyme solution, resulting in the decline of its decomposition ability, as so to affect the progress of enzymatic reaction [26].
Fig. 3.
Effect of ratio of enzyme concentration on extraction rate of GMRPs.
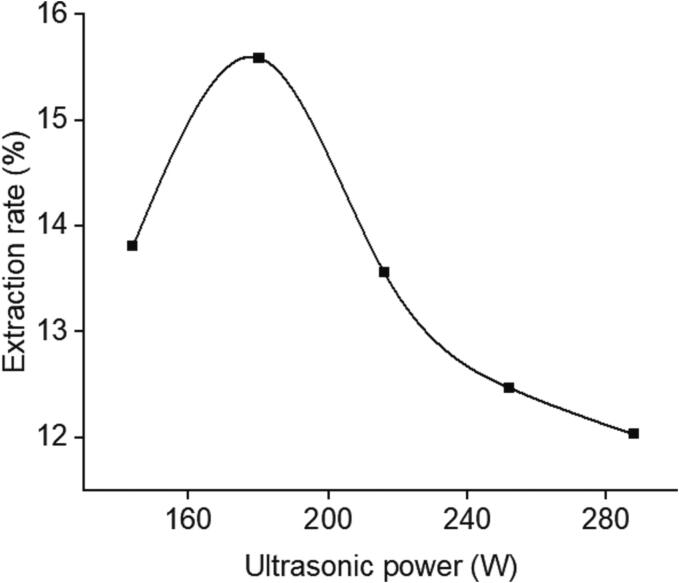
The influence of ultrasonic power on polysaccharide extraction rate was shown in Fig. 4. The relationship between the power change from 144 W to 180 W and the release of polysaccharides showed that the ultrasonic power had a greater promoting effect on the release of polysaccharides in the lower range. On the contrary, in the higher power range of 180 W to 288 W, the extraction rate of polysaccharides decreased continuously. The emergence of the above two situations might be related to two reasons. First, ultrasound waves with appropriate power agitated the extraction solvent and produced cavitation bubbles, which not only increased the pressure of the entire extraction system, but also promoted the contact between cellulase and raw materials, so that the combined action of the two made the cell wall more easily decomposed and broken [27], [28]. Second, the excessive increase of power also promoted the generation of more chemical decomposition related to polysaccharides, resulting in the decline of extraction rate [29].
Fig. 4.
Effect of ratio of ultrasonic power on extraction rate of GMRPs.
3.2. Results and analysis of BBD experiment for RSM
3.2.1. Model fitting and analysis of statistical results
BBD experiment in RSM was used to optimize the extraction conditions of GMRPs, and the rationality of statistical model was determined by variance analysis, fitting degree analysis and sufficiency analysis and so on. As shown in Table 2, 24 operations were carried out randomly according to four independent factors, and then the optimal values of the four factors of ratio of raw material to liquid (X1), ultrasonic time (X2), enzyme concentration (X3) and ultrasonic power (X4) were selected for 5 operations, so a total of 29 operations were performed. Multiple regression analysis was performed on the response value (Y) and each factor through the operational data in the table, and the relevant second-order polynomial regression equation was obtained as follows:
| (6) |
Table 2.
Box-Behnken experimental design and results for extraction rate of GMRPs.
| Run number | X1 The ratio of raw material to liquid (g/mL) | X2 Ultrasonic time (min) | X3 Enzyme concentration(%) | X4 Ultrasonic power (W) | Extraction rate of GMRPs (%) |
|
|---|---|---|---|---|---|---|
| Actual Value | Predicted Value | |||||
| 1 | 60 | 40 | 4 | 144 | 13.88 | 13.89 |
| 2 | 40 | 40 | 3 | 180 | 13.55 | 13.59 |
| 3 | 60, | 30 | 4 | 180 | 13.47 | 13.47 |
| 4 | 40 | 40 | 4 | 216 | 13.77 | 13.81 |
| 5 | 50 | 30 | 3 | 180 | 13.25 | 13.31 |
| 6 | 40 | 40 | 4 | 144 | 14.02 | 14.05 |
| 7 | 50 | 50 | 4 | 144 | 13.69 | 13.73 |
| 8 | 50 | 50 | 3 | 180 | 13.27 | 13.24 |
| 9 | 50 | 30 | 4 | 144 | 13.82 | 13.75 |
| 10 | 50 | 40 | 5 | 216 | 13.78 | 13.77 |
| 11 | 50 | 40 | 4 | 180 | 15.60 | 15.56 |
| 12 | 60 | 40 | 4 | 216 | 13.91 | 13.94 |
| 13 | 50 | 50 | 4 | 216 | 13.67 | 13.73 |
| 14 | 60 | 40 | 5 | 180 | 13.76 | 13.71 |
| 15 | 50 | 40 | 5 | 144 | 13.85 | 13.88 |
| 16 | 40 | 50 | 4 | 180 | 13.59 | 13.55 |
| 17 | 50 | 40 | 3 | 144 | 13.76 | 13.72 |
| 18 | 50 | 30 | 5 | 180 | 13.23 | 13.31 |
| 19 | 50 | 50 | 5 | 180 | 13.52 | 13.52 |
| 20 | 50 | 40 | 4 | 180 | 15.59 | 15.56 |
| 21 | 50 | 40 | 3 | 216 | 13.71 | 13.64 |
| 22 | 50 | 40 | 4 | 180 | 15.51 | 15.56 |
| 23 | 50 | 40 | 4 | 180 | 15.54 | 15.56 |
| 24 | 60 | 40 | 3 | 180 | 13.36 | 13.40 |
| 25 | 50 | 30 | 4 | 216 | 13.63 | 13.58 |
| 26 | 50 | 40 | 4 | 180 | 15.55 | 15.56 |
| 27 | 40 | 30 | 4 | 180 | 13.51 | 13.49 |
| 28 | 60 | 50 | 4 | 180 | 13.57 | 13.54 |
| 29 | 40 | 40 | 5 | 180 | 13.62 | 13.57 |
Analysis of variance was used to further verify the significance and suitability of the model. As shown in Table 3, the F-value of the regression model was 318.19, and its P-value was lower than 0.0001, indicating that the significance of the whole model was extremely good. R2 could be used to account for the proportion of variability in the model data, with a value of 0.9969, indicating that only 0.31% of the total variability could not be explained by the fitted model. The adjusted R2 value was 0.9937, which further confirmed the high significance of the model. In addition, for Lack of Fit and coefficient of variation (C.V.), the F-value of the former was 3.34 and the corresponding p-value was 0.1279, indicating that it was not significant for pure error. The value of the latter was relatively low, that is, 0.4335, indicating that the experimental data had high reliability. All the above showed that the statistical model had high accuracy and good effectiveness.
Table 3.
Analysis of variance for the regression model of extraction of GMRPs.
| Source | Sum of Square | Df | Mean Squares | F-value | P-value |
|---|---|---|---|---|---|
| Model | 16.32 | 14 | 1.17 | 318.19 | < 0.0001 |
| Residual | 0.0513 | 14 | 0.0037 | ||
| Lack of Fit | 0.0458 | 10 | 0.0046 | 3.34 | 0.1279 |
| Pure Error | 0.0055 | 4 | 0.0014 | ||
| Cor Total | 16.38 | 28 | |||
| R2 = 0.9969 | Adjusted R2 = 0.9937 | C.V. = 0.4335 | |||
The significance of each regression coefficient and the relationship between them were showed in Table 4. It could be seen that within the range of experimental variables simulated by the model, the P-values of the linear coefficients X3 and X4 and the cross-product coefficients X1X3, X1X4 and X2X3 were all less than 0.05, and the P-values of the quadratic coefficients X12, X22, X32 and X42 were all less than 0.0001, indicating that they all had significant or extremely significant effects on the extraction of GMRPs. At the same time, by comparing the significance of each linear coefficient and the significant differences of the cross-product coefficients formed after their interaction, it could be concluded that the order of influence of four factors on the response value was enzyme concentration (X3) > ultrasonic power (X4) > ultrasonic time (X2) > ratio of raw material to liquid (X1).
Table 4.
Regression relationship model between response variable and independent variables.
| Variables | Sum of Squares | Df | Mean Square | F-value | P-value |
|---|---|---|---|---|---|
| X1 | 0.001 | 1 | 0.001 | 0.2752 | 0.6081 |
| X2 | 0.0133 | 1 | 0.0133 | 3.64 | 0.0772 |
| X3 | 0.0616 | 1 | 0.0616 | 16.82 | 0.0011 |
| X4 | 0.0252 | 1 | 0.0252 | 6.88 | 0.0201 |
| X1X2 | 0.0001 | 1 | 0.0001 | 0.0273 | 0.8712 |
| X1X3 | 0.0272 | 1 | 0.0272 | 7.43 | 0.0164 |
| X1X4 | 0.0196 | 1 | 0.0196 | 5.35 | 0.0365 |
| X2X3 | 0.0182 | 1 | 0.0182 | 4.97 | 0.0426 |
| X2X4 | 0.0072 | 1 | 0.0072 | 1.97 | 0.1821 |
| X3X4 | 0.0001 | 1 | 0.0001 | 0.0273 | 0.8712 |
| X12 | 5.38 | 1 | 5.38 | 1467.9 | < 0.0001 |
| X22 | 8.35 | 1 | 8.35 | 2277.84 | < 0.0001 |
| X32 | 7.56 | 1 | 7.56 | 2062.32 | < 0.0001 |
| X42 | 3.42 | 1 | 3.42 | 932.08 | < 0.0001 |
3.2.2. Confirmation and analysis of optimal extraction conditions of RSM
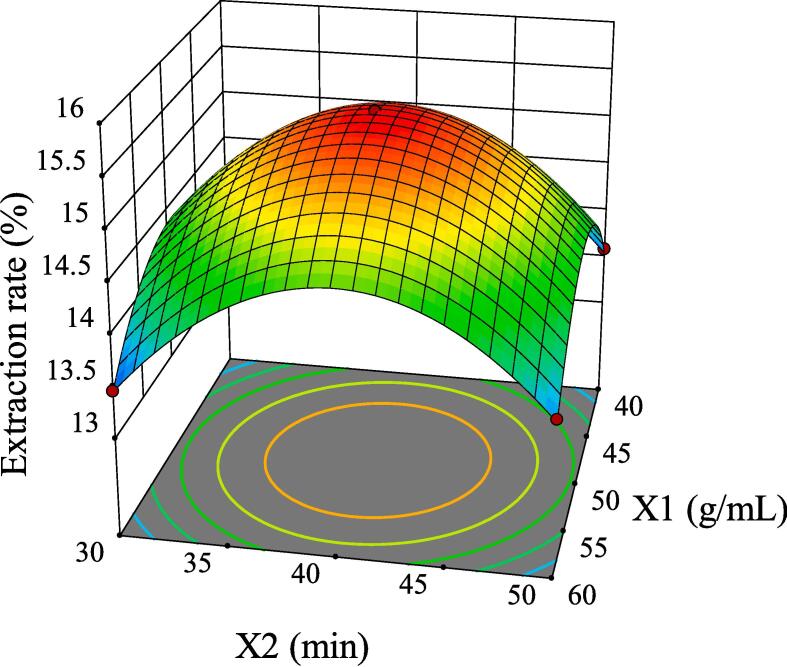
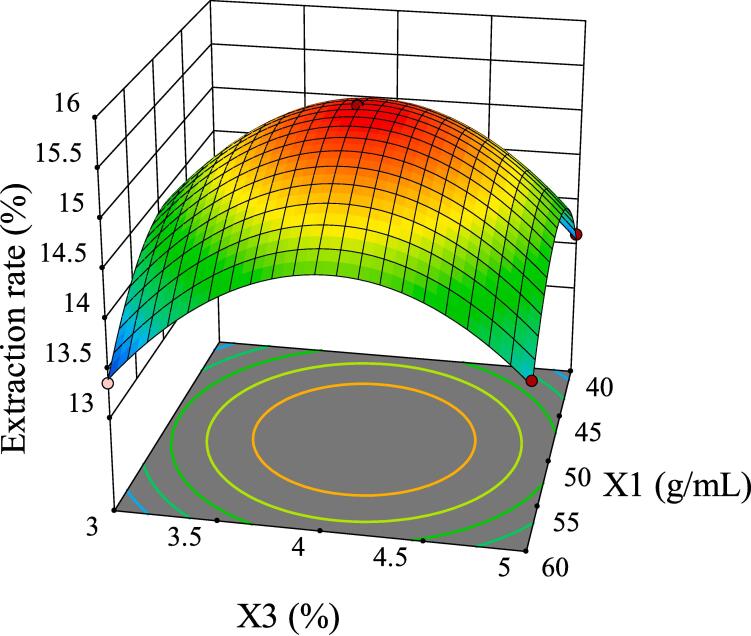
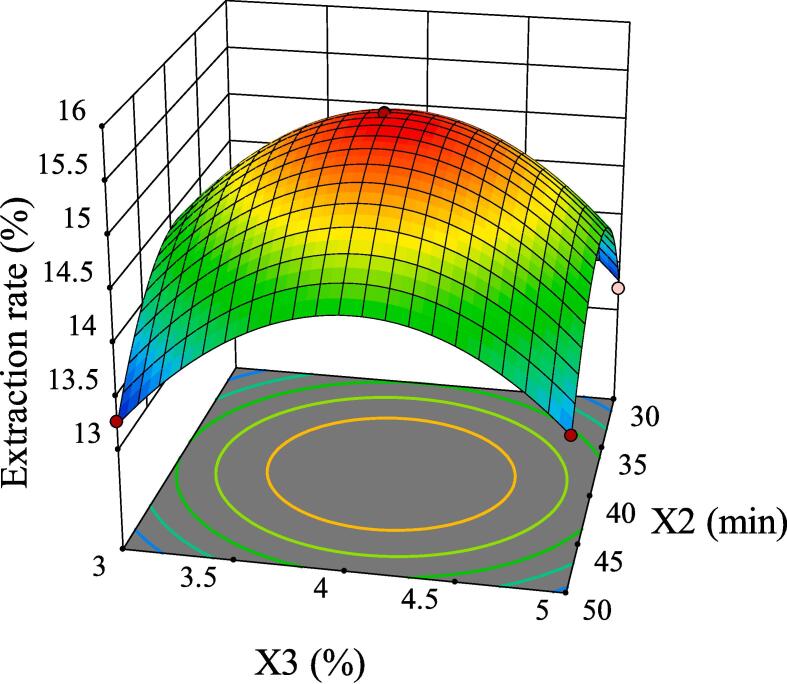
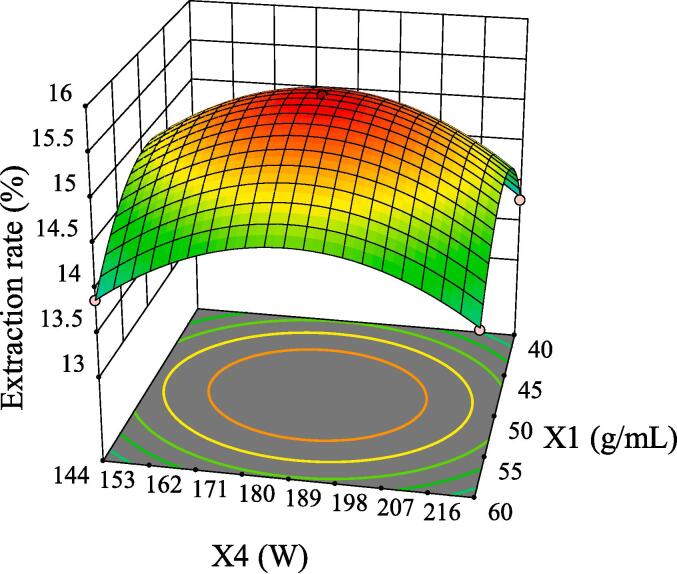
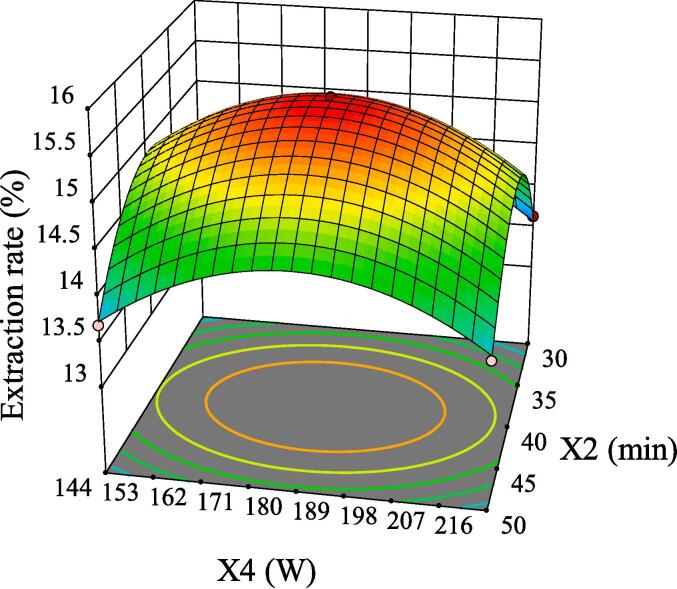
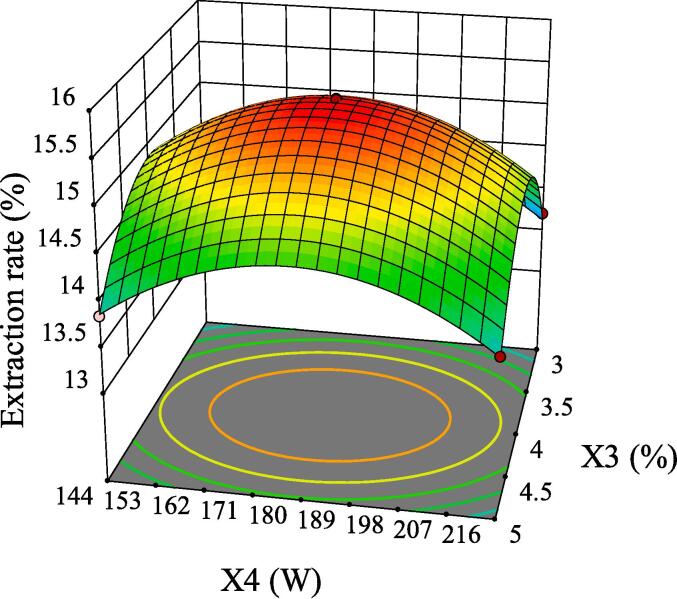
The intuitive representation of regression function can be represented by three-dimensional (3D) response surface and two-dimensional (2D) contour line. As shown in Fig. 5, although the 3D surface of X1X2 was comparatively steep, the color change of the contour line was slow and its shape was relatively circular, indicating that its interaction had no significant influence on the extraction rate of GMRPs. Fig. 6 and Fig. 8 showed the interaction effect of X1X3 and the interaction effect of X2X3, respectively. The 3D surfaces of X1X3 and X2X3 were relatively steep, and the contour line of the former changed color quickly and presented a relatively elliptical shape. Although the color change of the latter contour line was relatively slow, its shape was more elliptical than that of the former. All the above conditions indicated that both X1X3 interaction and X2X3 interaction had significant influences on the extraction rate of GMRPs, which was also corresponding to the statistical results in Table 4. The interaction effect of X1X4 was shown in Fig. 7, although the steepness of its 3D surface was relatively low, the shape of contour line was oval and the color changed quickly, so it also had a significant influence on the extraction rate to a certain extent. Fig. 9 and Fig. 10 showed the interaction effect of X2X4 and X3X4 respectively. The steepness of their 3D surfaces was low and the color change of their contour lines was slow, indicating that the extraction rate of GMRPs was little affected by X2X4 and X3X4. Through the above response surface analysis and combined with the Design results of Desigh-Expert software, it was obtained that the optimal conditions for ultrasonic-assisted cellulase extraction of GMRPs were as follows: the optimal value of ratio of raw material to liquid was 1: 49.947 g/mL, the optimal ultrasonic time was 40.1519 min, the optimal enzyme concentration was 4.0339%, and the optimal ultrasonic power was 178.862 W. Under the above conditions, the extraction rate of GMRPs was 15.56%.
Fig. 5.
Effect of interaction between X1 and X2 on extraction rate of GMRPs.
Fig. 6.
Effect of interaction between X1 and X3 on extraction rate of GMRPs.
Fig. 8.
Effect of interaction between X2 and X3 on extraction rate of GMRPs.
Fig. 7.
Effect of interaction between X1 and X4 on extraction rate of GMRPs.
Fig. 9.
Effect of interaction between X2 and X4 on extraction rate of GMRPs.
Fig. 10.
Effect of interaction between X3 and X4 on extraction rate of GMRPs.
3.2.3. Verification of the extraction conditions given by the predictive model
In order to further verify the reliability of the optimal extraction conditions given by the response surface model, based on the existing conditions in this study, the optimal values of the four conditions given by the model were slightly adjusted, that is, the ratio of raw material to liquid was 1: 50 g/mL, ultrasonic time was 40 min, enzyme concentration was 4 % and ultrasonic power was 179 W. Three extraction experiments were carried out according to the above conditions, and the extraction rates of GMRPs were 15.52 %, 15.62 % and 15.55 % respectively. The average extraction rate of the three experiments was 15.56 %, which was consistent with the predicted value. Therefore, the statistical model is accurate and reliable, and takes into account the influence and interaction of the four variables more than the traditional method.
3.3. Results and analysis of substitution degree of CM-GMRPs
The principle of carboxymethylation of polysaccharides is mainly to alkalize polysaccharides through sodium hydroxide solution, and then use the electrophilicity of chloroacetic acid (carboxymethylation reagent) to promote the etherification of polysaccharides, and obtain related derivatives. It has been reported that the degree of carboxymethylation of polysaccharides was related to reaction temperature, reaction time and the amount of chloroacetic acid, among which the amount of chloroacetic acid had a particularly significant effect on the degree of substitution [30]. Based on this, this study investigated the relationship between the degree of substitution of CM - GMRPs and the amount of chloroacetic acid, and the specific results were shown in Table 5 below. With the increasing amount of chloroacetic acid, the substitution degree of CM-GMRPs showed a trend of first increasing and then decreasing, which may be due to the fact that too little chloroacetic acid produced fewer carboxymethyl groups, resulting in poor derivatization effect; too much chloroacetic acid would promote the occurrence of side reactions and change the alkaline environment of the solution, resulting in a decrease in the carboxymethylation reaction rate [31]. It could be seen that the amount of carboxymethylation reagent had an important effect on the derivatization of GMRPs. Meanwhile, it could be found from Table 5 that with the increase of chloroacetic acid content, the sugar content and yield of CM - GMRPs also began to decline. The possible reasons for this situation were as follows: under the medium temperature reaction condition for a long time, the acidity of the solution was relatively low when the amount of chloroacetic acid was small, and the polysaccharide was less hydrolyzed; as the amount of chloroacetic acid increased, the contact with the raw material also increased, resulting in more polysaccharides being hydrolyzed, which affected the yield and sugar content of the derivatives. Therefore, from all the above analyses, it can be seen that when the ratio of material to liquid was 1:30 g/mL, the carboxymethylation modification effect of GMRPs was relatively ideal, and the degree of substitution was 0.52.
Table 5.
Effect of different ratio of material to liquid on carboxymethylation of GMRPs.
| The ratio of material to liquid (g/mL) | substitution degree | Sugar content (%) | Yield (%) |
|---|---|---|---|
| 1:20(CM–20) | 0.25 | 61.01 | 74.32 |
| 1:30(CM–30) | 0.52 | 52.41 | 68.32 |
| 1:40(CM–40) | 0.36 | 37.97 | 57.60 |
| 1:50(CM–50) | 0.24 | 37.13 | 55.20 |
3.4. Analysis of infrared spectroscopy
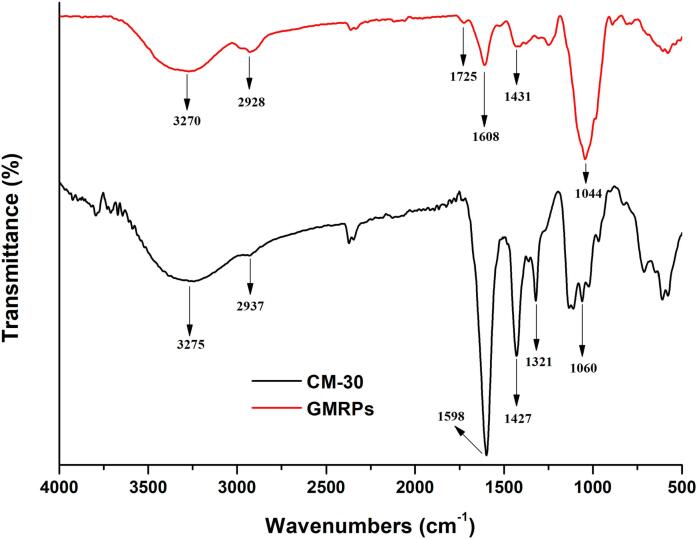
The functional groups and specific structures of GMRPs and four carboxymethylated derivatives were detected by infrared spectroscopy. The spectra of infrared detection results of GMRPs and CM-30 were shown in Fig. 11. In the picture, two wide peaks and two narrow peaks were formed near 3270 cm−1, 3275 cm−1, and 2928 cm−1, 2937 cm−1. The former was caused by the stretching vibration of the O-H bond of the hydroxy-group on the sugar ring of the above two polysaccharides. The latter was caused by the stretching vibration of the C-H bonds of the methyl and methylene groups of the two polysaccharides, and they showed a certain degree of peak shape change and chemical shift in the absorption peak region, which indicated that carboxymethylation of polysaccharides would affect their own hydroxyl groups and change their number. In addition, the weak small peak at 1725 cm−1 and the peaks at 1608 cm−1 and 1431 cm−1 could be attributed to the stretching vibration of carboxyl groups, which indicated that GMRPs might contain a very small amount of uronic acid [13]. At the same time, GMRPs and CM-30 had absorption peaks near 1044 cm−1 and 1060 cm−1, respectively, which were caused by asymmetric stretching vibration of C-O-C bond, and this further indicated that both were dominated by pyranose sugars [32]. Moreover, for the infrared spectroscopy of CM-30 in Fig. 11, it can be clearly seen that the characteristic absorption peaks of carboxymethyl group appeared near the three places of 1598 cm−1, 1427 cm−1 and 1321 cm−1, and the first two peaks were attributed to the stretching vibration of the C O bond and –COO bond, respectively, and the third peak was attributed to the variable angle vibration of the –CH bond. From the above analysis, it can be seen that the carboxymethylation of GMRPs was successful, and the main structure of polysaccharide did not change before and after chemical modification.
Fig. 11.
Infrared spectra of GMRPs and CM-30.
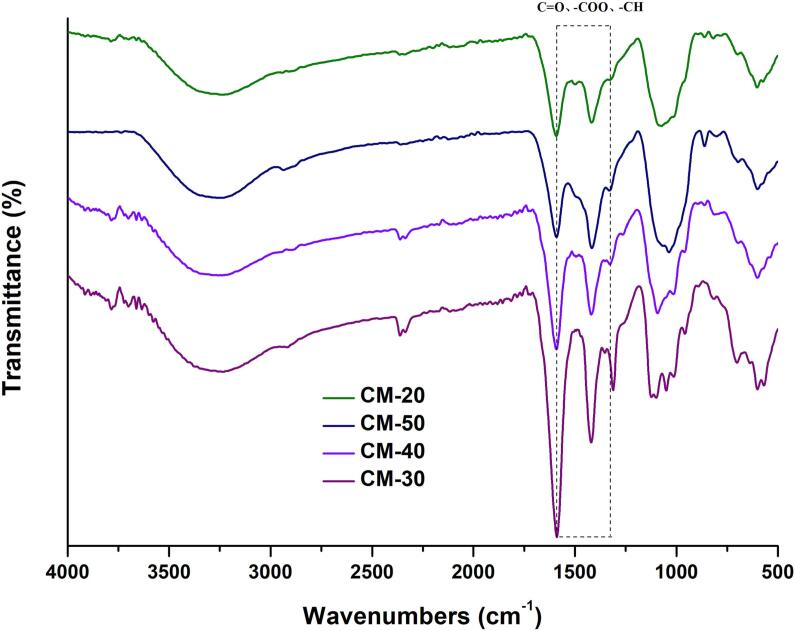
The infrared spectrum results of four carboxymethylated derivatives were shown in Fig. 12. It could be seen that three characteristic absorption peaks of carboxymethyl group appeared in all four polysaccharide derivatives, and the intensity changes of these peaks were related to the degree of substitution, which further indicated that the degree of carboxymethylation increased with the increase of the degree of substitution, and the main structure of polysaccharide did not change.
Fig. 12.
Infrared spectra of four carboxymethylated derivatives.
3.5. Analysis of NMR detection results
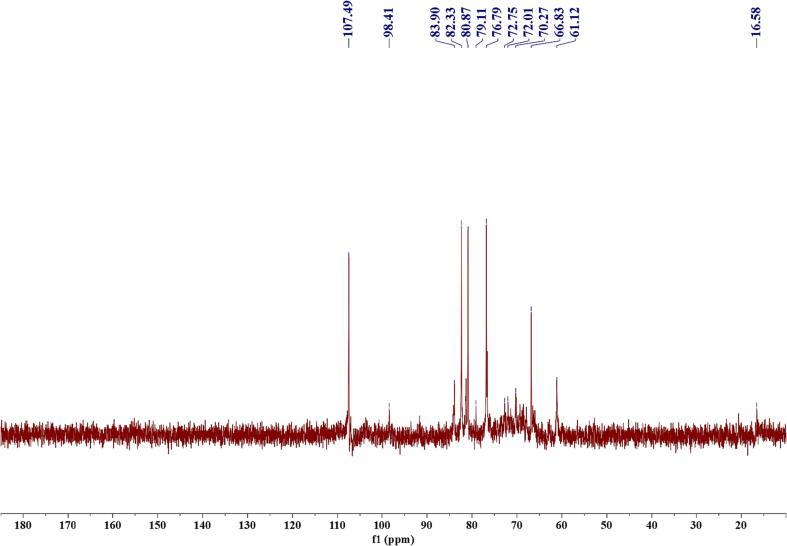
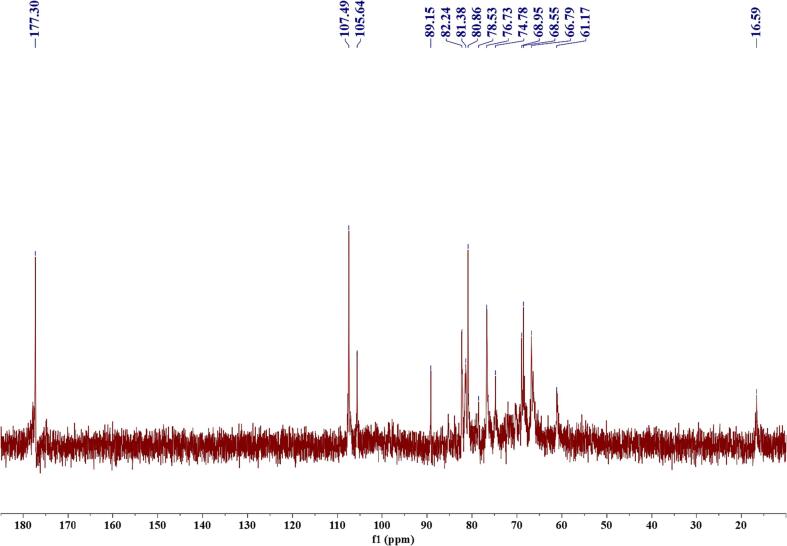
The NMR results of GMRPs and CM-30 are shown in Fig. 13, Fig. 14. The NMR spectrum of GMRPs was mainly divided into two regions, that is, 90–110 ppm is the anomeric carbon signal range of polysaccharide, and within this range, GMRPs showed two signal peaks, which indicated that it may be made up of two types of glycosidic residues. And multiple signal peaks in the range of 65–85 ppm correspond to the resonance signals of C2–C5 on the polysaccharide sugar ring. In the two signal peaks of δ61.12 and δ16.58, the former was the signal peak of C6 and indicated that GMRPs were hexose, while the weak signal peak of the latter indicated that it may contain a small amount of rhamnose [32]. In addition, there was no characteristic peak of carboxyl group in the signal range of 170–180 ppm in the low field region, indicating that the amount of uronic acid contained in GMRPs was too small, which was also consistent with the results of infrared spectroscopy. For the 13C NMR of CM-30, some changes can be clearly seen in Fig. 14. A strong carbonyl signal peak was added at δ177.30 in the low field region, which indicated that carboxymethyl group had been successfully introduced by GMRPs. At the same time, the 13C NMR of CM-30 also produced two new signal peaks at δ105.43 and δ89.15, which were most likely caused by methylene in the carboxymethyl group. Moreover, compared with GMRPs, it can also be seen that most of the signal peaks of CM-30 had a certain degree of chemical shift in the signal range of 60–85 ppm, which indicated that the chemical modification occurred at some sites in this range. Therefore, the results of NMR analysis further proved that the carboxymethylation of GMRPs was successful.
Fig. 13.
13C NMR spectrum of GMRPs.
Fig. 14.
13C NMR spectrum of CM-30.
4. Conclusion
RSM was selected to optimize and determine the optimal conditions for the extraction of GMRPs by ultrasonic-assisted cellulase method, that is, three extraction experiments were carried out under the conditions of ratio of raw material to liquid of 1:50 g/mL, ultrasonic time of 40 min, enzyme concentration of 4 %, and ultrasonic power of 179 W. The average extraction rate was 15.56 %. The carboxymethylation of GMRPs was performed and the relationship between different ratio of raw material to liquid and the degree of substitution of carboxymethylated derivatives was explored. Through single-factor experiment, it was found that when the optimal ratio of raw material to liquid was 1:30 g/mL, the degree of substitution of carboxymethylation of GMRPs was relatively ideal, which was 0.52. Finally, the success of carboxymethylation of GMRPs was confirmed by infrared spectroscopy and NMR analysis.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Gangliang Huang, Email: huangdoctor226@163.com.
Hualiang Huang, Email: hlhuang@wit.edu.cn.
References
- 1.Ovalle-Magallanes B., Eugenio-Pérez D., Pedraza-Chaverri J. Medicinal properties of mangosteen (Garcinia mangostana L.): A comprehensive update. Food Chem. Toxicol. 2017;109(Pt 1):102–122. doi: 10.1016/j.fct.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 2.Wathoni N., Yuan Shan C., Yi Shan W., Rostinawati T., Indradi R.B., Pratiwi R., Muchtaridi M. Characterization and antioxidant activity of pectin from Indonesian mangosteen (Garcinia mangostana L.) rind. Heliyon. 2019;5(8):e02299. doi: 10.1016/j.heliyon.2019.e02299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang S., Li Z., Wang X., An L., Bao J., Zhang J., Cui J., Li Y., Jin D.Q., Tuerhong M., Abudukeremu M., Ohizumi Y., Xu J., Guo Y. Isolation, structural elucidation, and immunoregulation properties of an arabinofuranan from the rinds of Garcinia mangostana. Carbohydr. Polym. 2020;246 doi: 10.1016/j.carbpol.2020.116567. [DOI] [PubMed] [Google Scholar]
- 4.Zhang S., An L., Li Z., Wang H., Shi L., Zhang J., Li Y., Jin D.Q., Tuerhong M., Ohizumi Y., Shuai L., Xu J., Guo Y. An active heteropolysaccharide from the rinds of Garcinia mangostana Linn.: Structural characterization and immunomodulation activity evaluation. Carbohydr. Polym. 2020;235(115929) doi: 10.1016/j.carbpol.2020.115929. [DOI] [PubMed] [Google Scholar]
- 5.Zhang W., Huang G. Preparation, structural characteristics, and application of taro polysaccharides in food. J. Sci. Food Agric. 2022;102(14):6193–6201. doi: 10.1002/jsfa.12058. [DOI] [PubMed] [Google Scholar]
- 6.Tang Z., Huang G. Extraction, structure, and activity of polysaccharide from Radix astragali. Biomed. Pharmacother. 2022;150 doi: 10.1016/j.biopha.2022.113015. [DOI] [PubMed] [Google Scholar]
- 7.Zhang W., Duan W., Huang G., Huang H. Ultrasonic-assisted extraction, analysis and properties of mung bean peel polysaccharide. Ultrason. Sonochem. 2023;98 doi: 10.1016/j.ultsonch.2023.106487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang Z., Wang Y., Huang G., Huang H. Ultrasound-assisted extraction, analysis and antioxidant activity of polysaccharide from the rinds of Garcinia mangostana L. Ultrason. Sonochem. 2023;97 doi: 10.1016/j.ultsonch.2023.106474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiong X., Yang W., Huang G., Huang H. Ultrasonic-assisted extraction, characteristics and activity of Ipomoea batatas polysaccharide. Ultrason. Sonochem. 2023;96 doi: 10.1016/j.ultsonch.2023.106420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng Z., Song H., Yang Y., Liu Y., Liu Z., Hu H., Zhang Y. Optimization of microwave-assisted enzymatic extraction of polysaccharides from the fruit of Schisandra chinensis Baill. Int. J. Biol. Macromol. 2015;76:161–168. doi: 10.1016/j.ijbiomac.2015.01.048. [DOI] [PubMed] [Google Scholar]
- 11.Chen W., Jia Z., Zhu J., Zou Y., Huang G., Hong Y. Optimization of ultrasonic-assisted enzymatic extraction of polysaccharides from thick-shell mussel (Mytilus coruscus) and their antioxidant activities. Int. J. Biol. Macromol. 2019;140:1116–1125. doi: 10.1016/j.ijbiomac.2019.08.136. [DOI] [PubMed] [Google Scholar]
- 12.Hashemifesharaki R., Xanthakis E., Altintas Z., Guo Y., Gharibzahedi S.M.T. Microwave-assisted extraction of polysaccharides from the marshmallow roots: Optimization, purification, structure, and bioactivity. Carbohydr. Polym. 2020;240 doi: 10.1016/j.carbpol.2020.116301. [DOI] [PubMed] [Google Scholar]
- 13.Li J., Chen Z., Shi H., Yu J., Huang G., Huang H. Ultrasound-assisted extraction and properties of polysaccharide from Ginkgo biloba leaves. Ultrason. Sonochem. 2023;93 doi: 10.1016/j.ultsonch.2023.106295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang W., Huang G. Preparation and analysis of polysaccharide from Solanum tuberdsm. Ultrason. Sonochem. 2023;98 doi: 10.1016/j.ultsonch.2023.106520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin B., Wang S., Zhou A., Hu Q., Huang G. Ultrasound-assisted enzyme extraction and properties of Shatian pomelo peel polysaccharide. Ultrason. Sonochem. 2023;98 doi: 10.1016/j.ultsonch.2023.106507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan Y., Huang G. Preparation and analysis of Pueraria lobata polysaccharides. ACS Biomater Sci. Eng. 2023;9(5):2329–2334. doi: 10.1021/acsbiomaterials.2c01479. [DOI] [PubMed] [Google Scholar]
- 17.Song Y., Yang Y., Zhang Y., Duan L., Zhou C., Ni Y., Liao X., Li Q., Hu X. Effect of acetylation on antioxidant and cytoprotective activity of polysaccharides isolated from pumpkin (Cucurbita pepo, lady godiva) Carbohydr. Polym. 2013;98(1):686–691. doi: 10.1016/j.carbpol.2013.06.049. [DOI] [PubMed] [Google Scholar]
- 18.Lin B., Huang G. Extraction, isolation, purification, derivatization, bioactivity, structure-activity relationship, and application of polysaccharides from White jellyfungus. Biotechnol. Bioeng. 2022;119(6):1359–1379. doi: 10.1002/bit.28064. [DOI] [PubMed] [Google Scholar]
- 19.Zhou S., Huang G. Extraction, purification and antioxidant activity of Juglans regia shell polysaccharide. Chem. Biol. Technol. Agric. 2023;10:75. [Google Scholar]
- 20.Huang S., Huang G. Extraction, structural analysis, and activities of rice bran polysaccharide. Chem. Biol. Drug Des. 2021;98(4):631–638. doi: 10.1111/cbdd.13916. [DOI] [PubMed] [Google Scholar]
- 21.Chen S., Huang H., Huang G. Extraction, derivatization and antioxidant activity of cucumber polysaccharide. Int. J. Biol. Macromol. 2019;140:1047–1053. doi: 10.1016/j.ijbiomac.2019.08.203. [DOI] [PubMed] [Google Scholar]
- 22.Chen C., You L.J., Abbasi A.M., Fu X., Liu R.H. Optimization for ultrasound extraction of polysaccharides from mulberry fruits with antioxidant and hyperglycemic activity in vitro. Carbohydr. Polym. 2015;130:122–132. doi: 10.1016/j.carbpol.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Zheng Q., Ren D., Yang N., Yang X. Optimization for ultrasound-assisted extraction of polysaccharides with chemical composition and antioxidant activity from the Artemisia sphaerocephala Krasch seeds. Int. J. Biol. Macromol. 2016;91:856–866. doi: 10.1016/j.ijbiomac.2016.06.042. [DOI] [PubMed] [Google Scholar]
- 24.Wu D., Gao T., Yang H., Du Y., Li C., Wei L., Zhou T., Lu J., Bi H. Simultaneous microwave/ultrasonic-assisted enzymatic extraction of antioxidant ingredients from Nitraria tangutorun Bobr. juice by-products. Ind. Crop. Prod. 2015;66:229–238. [Google Scholar]
- 25.Wang Y., Liu Y., Hu Y. Optimization of polysaccharides extraction from Trametes robiniophila and its antioxidant activities. Carbohydr. Polym. 2014;111:324–332. doi: 10.1016/j.carbpol.2014.03.083. [DOI] [PubMed] [Google Scholar]
- 26.Huang D., Zhou X., Si J., Gong X., Wang S. Studies on cellulase-ultrasonic assisted extraction technology for flavonoids from Illicium verum residues. Chem. Cent. J. 2016;10(1):56. doi: 10.1186/s13065-016-0202-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Contamine R.F., Wilhelm A.M., Berlan J., Delmas H. Power measurement in sonochemistry. Ultrason. Sonochem. 1995;2:S43–S47. [Google Scholar]
- 28.Chen F., Sun Y., Zhao G., Liao X., Hu X., Wu J., Wang Z. Optimization of ultrasound-assisted extraction of anthocyanins in red raspberries and identification of anthocyanins in extract using high-performance liquid chromatography-mass spectrometry. Ultrason. Sonochem. 2007;14(6):767–778. doi: 10.1016/j.ultsonch.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 29.Zhu C.P., Zhai X.C., Li L.Q., Wu X.X., Li B. Response surface optimization of ultrasound-assisted polysaccharides extraction from pomegranate peel. Food Chem. 2015;177:139–146. doi: 10.1016/j.foodchem.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 30.Shi M.J., Wei X., Xu J., Chen B.J., Zhao D.Y., Cui S., Zhou T. Carboxymethylated degraded polysaccharides from Enteromorpha prolifera: Preparation and in vitro antioxidant activity. Food Chem. 2017;215:76–83. doi: 10.1016/j.foodchem.2016.07.151. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X., Liu J., Wang X., Hu H., Zhang Y., Liu T., Zhao H. Structure characterization and antioxidant activity of carboxymethylated polysaccharide from Pholiota nameko. J. Food Biochem. 2022;46(7):e14121. doi: 10.1111/jfbc.14121. [DOI] [PubMed] [Google Scholar]
- 32.Li J., Shi H., Yu J., Lei Y., Huang G., Huang H. Extraction and properties of Ginkgo biloba leaf polysaccharide and its phosphorylated derivative. Ind. Crop. Prod. 2022;189 [Google Scholar]