Abstract
Mineral oil hydrocarbons (MOH) are composed of saturated hydrocarbons (MOSH) and aromatic hydrocarbons (MOAH). Due to the complexity of the MOH composition, their complete chemical characterisation is not possible. MOSH accumulation is observed in various tissues, with species‐specific differences. Formation of liver epithelioid lipogranulomas and inflammation, as well as increased liver and spleen weights, are observed in Fischer 344 (F344) rats, but not in Sprague–Dawley (SD) rats. These effects are related to specific accumulation of wax components in the liver of F344 rats, which is not observed in SD rats or humans. The CONTAM Panel concluded that F344 rats are not an appropriate model for effects of MOSH with wax components. A NOAEL of 236 mg/kg body weight (bw) per day, corresponding to the highest tested dose in F344 rats of a white mineral oil product virtually free of wax components, was selected as relevant reference point (RP). The highest dietary exposure to MOSH was estimated for the young population, with lower bound–upper bound (LB–UB) means and 95th percentiles of 0.085–0.126 and 0.157–0.212 mg/kg bw per day, respectively. Considering a margin of exposure approach, the Panel concluded that the present dietary exposure to MOSH does not raise concern for human health for all age classes. Genotoxicity and carcinogenicity are associated with MOAH with three or more aromatic rings. For this subfraction, a surrogate RP of 0.49 mg/kg bw per day, calculated from data on eight polycyclic aromatic hydrocarbons, was considered. The highest dietary exposure to MOAH was also in the young population, with LB–UB mean and 95th percentile estimations of 0.003–0.031 and 0.011–0.059 mg/kg bw per day, respectively. Based on two scenarios on three or more ring MOAH contents in the diet and lacking toxicological information on effects of 1 and 2 ring MOAH, a possible concern for human health was raised.
Keywords: Mineral oil hydrocarbons (MOH), MOSH, MOAH, alkanes, aromatic hydrocarbons, human dietary exposure, toxicity
Short abstract
This publication is linked to the following EFSA Journal article: https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2023.p210901
Summary
The mineral oil hydrocarbons (MOH) considered in this opinion contain about 10–50 carbon atoms and are separated into mineral oil saturated hydrocarbons (MOSH) and mineral oil aromatic hydrocarbons (MOAH). They can enter food in many ways: through environmental contamination, use of lubricants for machinery, release agents, processing aids, food or feed additives and migration from food contact materials. The European Commission asked the European Food Safety Authority (EFSA) to assess the toxicity studies on MOH that have become available since the EFSA scientific opinion on MOH in 2012, along with an exposure assessment allowing an updated risk characterisation.
The methods presently used to determine the concentration of the sum of the MOSH and MOAH or fractions by volatility ranges in food, feed or food contact materials are based mainly on on‐line coupled liquid chromatography‐gas chromatography with flame ionisation detection (LC‐GC‐FID). Comprehensive two‐dimensional GC (GC × GC) with FID or mass spectrometry allows for further characterisation of the MOSH and a differentiation between non‐alkylated and highly alkylated MOAH as well as by number of aromatic rings. It is acknowledged that due to the complexity of MOH mixtures, a complete chemical characterisation is not possible. Applying enrichment and elimination of interferences, analytical methods can generally reach detection limits below 0.5 mg total MOSH or MOAH/kg, but these auxiliary methods have not always been applied.
MOSH
Since the former EFSA opinion on MOH in 2012, studies on the retention of MOSH in liver, spleen, adipose tissue and the remaining carcass of female Fischer 344 (F344) rats, following exposure to various MOSH mixtures in the feed for up to 120 days, became available. The application of a MOSH mixture broad in molecular mass distribution indicated that accumulation in liver and spleen ranges from n‐C20 to about n‐C45, with a maximum at n‐C29; in the adipose tissue, it was from below n‐C16 to about n‐C35, with a maximum at around n‐C16. The concentrations in the tissues increased far less than proportionally to the dose. In the spleen and adipose tissue, there was no apparent tendency to reach a steady‐state level, while a plateau between 90 and 120 days appeared to be reached in the liver. At all doses, the MOSH concentration was an order of magnitude higher in the liver than in the spleen, adipose tissue or carcass. Following depuration for 30 days, there was still substantial retention of MOSH in all the studied tissues. The concentrations in liver, spleen and carcass were reduced (e.g. in liver by up to 53% after 30 days of depuration), while there was little change in the adipose tissue. The composition of MOSH was similar in liver and spleen and dominated by unresolved alkyl‐substituted naphthenes and highly branched iso‐alkanes. In adipose tissue, there were more n‐alkanes and hydrocarbons with less branched alkyl moieties.
As a follow‐up, MOSH mixtures with a narrower range of molecular masses were used, that is, S‐C25 (from n‐C16 to n‐C33, with a maximum at n‐C23 and containing a small amount of n‐alkanes), L‐C25 (from n‐C25 to n‐C50, with a maximum at n‐C34, deparaffinated, i.e. almost free of n‐alkanes) and a 1:1 mixture of L‐C‐25 with a wax (largely consisting of n‐alkanes from C23 to C45). n‐Alkanes up to n‐C25 were absent in liver and spleen, but strongly enriched above n‐C25. Enrichment of n‐alkyl‐monocyclic naphthenes above n‐C28 was also observed (together with n‐alkanes being typical wax components). It appears that n‐alkanes and probably other wax components are poorly biotransformed and eliminated in F344 rats. Following exposure to L‐C25, the retention in liver and spleen was dominated by substituted naphthenes and highly branched iso‐alkanes. The formation of granulomas was related to the presence of n‐alkanes. Experiments in female Sprague Dawley (SD) rats indicate that paraffinic and synthetic gas‐to‐liquid (GTL) wax components are poorly retained in the liver. In SD rats, the retention of naphthenic MOSH is stronger than that of iso‐alkanes of a similar range of carbon numbers, particularly in the liver.
New data on the presence and composition of MOSH in human tissues, taken at autopsy, were available. The concentrations varied greatly between individuals and between tissues; levels were higher in spleen, mesenteric lymph node (MLN), liver and adipose tissue than in heart and kidney and below the detection limit in the brain. The MOSH composition was similar in liver and spleen, with unresolved, highly isomerised iso‐alkanes and largely alkylated naphthenes centred on C‐27 and ranging from n‐C20 to n‐C46; hardly any n‐alkanes (including those naturally occurring in food) or terpenes were detected. The compositions in adipose tissue and MLN were similar, but clearly different from those in liver and spleen, ranging from about n‐C16 to n‐C36 and centred around n‐C23, including wax components, diterpenes (e.g. from dairy products) and n‐alkanes (from plants). Except for the n‐alkanes accumulated by the F344 rats, the molecular mass distribution and the compositional pattern observed by GC × GC analysis of the MOSH residues in human organs appeared to be similar to those in F344 rats and SD rats.
In line with the 2012 Opinion, the CONTAM Panel concluded that there was minimal acute toxicity associated with MOSH. Studies on MOSH in F344 rats confirmed the previous findings of granuloma formation in liver and MLN, associated signs of inflammation and of increased liver, spleen and MLN weights. The effects in liver and spleen were concluded to be F344‐specific, due to the higher propensity to retain n‐alkanes compared to SD rats and other test animals. Treatment of F344 rats with L‐C25, a deparaffinated MOSH product (treated to minimise the presence of wax components including n‐alkanes), did not induce organ weight changes or liver granulomas up to the highest tested dose of 236 mg/kg bw per day.
Limited data were available on MOSH and GTL oils (studies on GTL products were considered as supporting information in view of their similar composition as mineral oil‐derived products) in SD, Wistar and Long Evans rats and Beagle dogs. These data did not show the effects associated with n‐alkanes observed in F344 rats. In SD rats treated with a paraffinic oil in a subchronic study, minimal signs of liver inflammation were reported at 1,624 mg/kg bw per day. In a study with a GTL oil in SD rats, mild to moderate apoptosis and necrosis in the intestinal mucosa were observed at 1,267 mg/kg bw per day. In a subchronic study on paraffin wax, no adverse effects were observed in SD rats up to 9 g/kg bw per day.
There is no genotoxicity associated with MOSH.
A new chronic and carcinogenicity study in F344 rats was retrieved on ozokerite, a wax containing 81% saturated hydrocarbons in the range n‐C22 to n‐C38. In addition to the typical effects observed in F344 rats in liver, spleen and MLN, increased incidence in hepatic preneoplastic GST‐P positive foci and hepatocyte adenomas were observed. The CONTAM Panel concluded that these changes were secondary to the F344 rat‐specific chronic granulomatous inflammation caused by the liver granulomas.
New information on two GTL oils supports the conclusion in the previous Opinion of no potential for MOSH to induce developmental and reproductive toxicity. Based on new studies, the Panel concluded that there is no evidence that dietary exposure to MOSH induces autoimmunity.
In the absence of relevant new data on lipogranulomas induced by MOSH in humans, the conclusion of the previous EFSA Opinion that lipogranulomas observed together with MOSH in human liver, spleen, lymph nodes and other organs are not associated with adverse consequences is still valid. The Panel noted that the lipogranulomas observed in humans differ from the epithelioid granulomas observed in F344 rats.
In new studies, pharmaceutical grade mineral oil products used as placebo (1–4 g/day) in clinical trials of oil‐based agents might have caused adverse effects. While some large long‐term studies showed increases in atherogenic lipoproteins and inflammatory biomarkers, others that were small and of short duration did not. The CONTAM Panel noted the observational nature of this evidence and the large uncertainty related to potential hazard of mineral oil.
Regarding the mode of action of MOSH, the Panel concluded that epithelioid granulomas in the liver and increased liver weights of F344 rats and associated inflammatory response are related to hepatic accumulation of n‐alkanes > C25 and other wax components. Increased spleen weights were also observed, which were considered to be, at least in part, related to the inflammatory response in the liver. New evidence indicated that n‐alkanes are not accumulated in human liver. Therefore, the Panel concluded that the formation of hepatic epithelioid granuloma and associated effects in F344 rats exposed to MOSH are not critical endpoints relevant to humans. Macrophage aggregation and granuloma formation in MLN, and MLN increased weights were considered an adaptive response and not adverse.
In view of the lack of critical effects clearly identified for MOSH, a NOAEL of 236 mg/kg bw per day in F344 rats, corresponding to the highest tested dose of L‐C25, was selected as the relevant reference point (RP) for MOSH. The L‐C25 composition was considered to best represent what was found in human tissues with regard to mass range and low occurrence or absence of n‐alkanes. In addition, there were no adverse effects following exposure to MOSH at or below this value in other experimental animal models tested, albeit in limited studies. As the limitations of the data set precluded the setting of a health‐based guidance value (HBGV), a margin of exposure (MOE) approach was applied.
As regards occurrence data, 80,632 analytical results on MOSH and MOAH in food samples were extracted from the EFSA Data Warehouse (sampling years 2011–2021). After a first assessment and cleaning of the data, a total of 73,122 analytical results on MOSH and MOAH (7,840 samples) were available. Two types of data providers were identified: European countries (~56%) and food associations (~44%). The CONTAM Panel considered the two data sets suitable for exposure assessment and that they provided consistent and complementary information. Therefore, they were merged to perform the dietary exposure assessment.
Most samples were analysed for the MOSH and MOAH C‐fractions specified by the JRC Guidance on mineral oils, including ‘Total MOAH’ and ‘Total MOSH’. When the analytical results for ‘Total MOAH’ and/or ‘Total MOSH’ were not provided, the CONTAM Panel derived the values by summing the individual C‐fractions.
For MOSH, 7,675 food samples were available for the dietary exposure estimations. The highest mean concentrations were found in vegetable oils, the highest being for ‘Olive pomace oil’ (n = 51, lower bound (LB) = upper bound (UB) = 108.7 mg/kg). As compared to the 2012 EFSA opinion (EFSA CONTAM Panel, 2012), a decrease in MOSH levels was observed across the different food commodities, which, at least partially, could be explained by the different measures introduced by authorities and industry since 2012. Still, the comparison of the two occurrence data sets is difficult, considering the limitations of the data set used in 2012, e.g. the database was smaller, the data were mainly from one data provider and the conversion factors were used for some food groups.
The highest dietary exposure to MOSH was estimated for the young population, in particular ‘Infants’, with LB–UB means of 0.085–0.126 mg/kg bw per day and LB–UB 95th percentiles of 0.157–0.212 mg/kg bw per day. Across the different age classes, the food groups with the highest average contribution to the mean LB dietary exposure to MOSH were ‘Grains and grain‐based products’, ‘Milk and dairy products’ and ‘Animal and vegetable fats and oils and primary derivatives thereof’. For infants, ‘Food products for young population’ (18–76%, median = 60%) and ‘Milk and dairy products’ (6–51%, median = 24%) were the main contributors. For ‘Infants’, in a scenario considering sustained consumption of highly contaminated samples of ‘Infant formula’, mean exposures to MOSH (LB–UB) could be as high as 0.22–0.30 mg/kg bw day, while the P95 exposure (LB–UB) could be 0.27–0.37 mg/kg bw day. Using MOSH levels in human milk as reported in the literature and an average daily milk consumption of about 800 mL and a high consumption of 1,200 mL, the dietary exposure to MOSH of an infant of 6.1 kg exclusively fed with breast milk might be between 0.11 μg/kg bw per day (mean exposure) and 0.17 μg/kg bw per day (high exposure).
The consumption of certain foods, such as dairy products and other products of animal origin, may result in selective exposure to already accumulated MOSH components, which also have a higher propensity to accumulate in humans. Possible consequences for human health have not been investigated and are uncertain.
The Panel determined the MOE above which no concern for human health would arise, considering default assessment factors for interspecies differences (factor of 10), for intra‐species differences (factor of 10) and for extrapolation from short‐ to long‐term exposure (factor of 2). In addition, in conjunction with the uncertainty analysis, the CONTAM Panel selected an additional factor of 6 to cover for the deficiencies in the database for MOSH. As a result, the Panel considered that MOEs ≥ 1,200 are sufficient to conclude that there is no concern for human health risks. For all age classes, MOEs were at or above this value. Infants exclusively fed with infant formula with high MOSH content could have exposure with MOEs ranging approximately from 790 to 1,070 for mean exposure and from 640 to 870 for P95 exposure. Considering the short duration of exposure via infant formula, these MOEs were not considered to raise concerns. Taking into account the identified uncertainties, it is likely to very likely (with 66–95% certainty) that exposure levels do not raise health concern in either mean or high‐consuming toddlers. This conclusion can be extended to all age groups. Overall, the CONTAM Panel concluded that the current dietary exposure towards to MOSH for all age classes raises no concern for human health.
MOAH
For MOAH, no new studies on acute toxicity, repeated‐dose toxicity or carcinogenicity were retrieved. New studies confirm the conclusions of the previous EFSA Opinion that the genotoxicity of MOH is associated with the presence of some MOAH with three or more aromatic rings. As shown in new in vitro studies in human and rat microsomes, alkylation of MOAH could both shift ring oxidation to side chain oxidation and alter the position of ring oxidation, thereby either increasing or decreasing the ability to form reactive and genotoxic metabolites and carcinogenic potential.
As concluded in the previous EFSA opinion, there is evidence that MOAH include non‐genotoxic components acting as tumour promoters. No new evidence was available.
Fetotoxic and developmental effects were observed in dermal toxicity studies on petroleum products containing three or more ring MOAH. Studies on DMSO extracts from such products also showed a correlation between the developmental toxicity potency and both the presence of three or more ring MOAH and the extent of trans‐activation of the Ah receptor. No effects were observed in an oral screening reproductive and developmental toxicity study with a lubricating base oil treated to reduce the concentration of three or more ring MOAH.
Since the possible presence of genotoxic and carcinogenic components within MOAH prevents the setting of an HBGV, an MOE approach was applied. However, no studies were available to define a reference point (RP) for three or more ring MOAH. Under a conservative approach, using a surrogate RP for the risk characterisation of the exposure to three or more ring MOAH, the CONTAM Panel selected the BMDL10 of 0.49 mg/kg bw per day for increased incidence of total tumour‐bearing animals that was calculated from a carcinogenicity study of non‐alkylated polycyclic aromatic hydrocarbons (PAH), using the sum of eight PAH (PAH8). The lack of robust data on the oral toxicity of MOAH hampers the identification of potential critical effects and an RP related to the non‐genotoxic and non‐carcinogenic fraction of MOAH.
For MOAH, data of 6,472 out of 7,742 samples (~84%) were left censored. To minimise the impact of the left‐censored MOAH data on the LB–UB occurrence and exposure estimations, different LOQ cut‐offs based on the maximum LOQs described in the 2019 JRC Guidance on MOH were applied to the samples analysed in 2020 and 2021. After the exclusion of a total of 364 samples, the final data set for MOAH consisted of 7,378 samples. The highest mean levels of MOAH were reported for samples of ‘Olive pomace oil’ (n = 51, LB–UB = 13.54–13.56 mg/kg). MOAH levels were quantified in ‘Food products for young population’, although at low levels and with 90% of the samples being left‐censored. The highest concentrations were reported for ‘Ready‐to‐eat meal for infants and young children’ and ‘Simple cereals for infants or children, reconstituted’. MOAH were also quantified in some samples of ‘Infant formulae, liquid’ and ‘Follow‐on formulae, liquid’.
The highest dietary exposure was estimated for the young population, in particular ‘Infants’, with LB–UB mean estimations of 0.003–0.031 mg/kg bw per day and LB–UB 95th percentile estimations of 0.011–0.059 mg/kg bw per day. Across the different age classes, the food groups with the highest average contribution to the mean LB dietary exposure to MOAH were ‘Grains and grain‐based products’, ‘Animal and vegetable fats and oils and primary derivatives thereof’ and ‘Coffee, cocoa, tea and infusions’ (based exclusively on analytical data in cocoa beverages and tea and herbal infusions, and assuming a 100% transfer of MOAH during the brewing process). For ‘Infants’, ‘Food products for young population’ was the most important contributor (11–88%, median 64%).
Few data were available on the concentrations of three or more aromatic ring MOAH in food. Hence, an indirect approach was taken, based on the estimated proportion of the three or more aromatic ring MOAH in the total MOAH in the diet. The CONTAM Panel acknowledged that MOH products treated to reduce the content of the genotoxic MOAH are likely the main contributors to the presence of MOAH in food. However, certain sources with higher levels of three or more ring MOAH, such as batching oils, are also expected to contribute to the presence of MOAH in food. Environmental sources may also contribute to the background presence of three or more ring MOAH in food, but no specific data are available. As the contribution of different sources to the overall presence of MOAH will vary for different food categories as well as within food categories (e.g. same food contaminated from different sources), an estimation of the presence of three or more ring MOAH in the diet is uncertain. The CONTAM Panel considered two risk scenarios that it assumed to encompass reality. In Scenario 1, a conservative average of 10% of three or more ring MOAH within the MOAH fraction present across different foods in the dietary exposure was assumed, while it was 1% in Scenario 2. Using the selected BMDL10 of 0.49 mg/kg bw per day as a surrogate RP, under the Scenario 1, MOEs were consistently lower than 10,000 for most of the consumption surveys for mean consumers (ranging from 158 to 12,250 across age group and consumption surveys) and for all high consumers (83–4,900). Under the Scenario 2, MOEs were below 10,000 for UB estimates only, for most of the dietary surveys at the mean exposure, and for all dietary surveys at P95 exposure. However, MOEs higher than 10,000 were calculated for all the LB mean exposure levels and for most of the LB P95 exposure levels, except for some surveys in the younger age groups showing P95 LB MOEs in the range 4,000–8,000. Overall, the CONTAM Panel, considering the EFSA Opinion on assessment of genotoxic and carcinogenic substances (EFSA, 2005), concluded that Scenario 1 would raise a health concern related to the presence of three or more ring MOAH in food for all age groups, while Scenario 2 would raise a health concern for the high consumers in the younger age groups. It considers the conclusion as being conservative, as the risk characterisation was based on a surrogate RP related to PAH8. Taking into account the identified uncertainties in the expert elicitation exercise, it is extremely likely (with 99–100% certainty) that MOEs lower than 10,000, raising concern for human health, are present for mean and high‐consuming toddlers. For the other age groups, it turned out likely (certainty above 66%) that MOEs are below 10,000. The CONTAM Panel noted that a full risk characterisation would require additional data on toxicity and exposure to three or more ring MOAH.
Due to the lack of adequate oral toxicity studies, it was not possible to identify a reference point for the 1–2 ring MOAH. Therefore, a risk characterisation of this MOAH fraction could not be performed. The CONTAM Panel concluded that, in the absence of reliable toxicity data, the dietary exposure to 1–2 ring MOAH might raise a concern.
Generation of further data for the refinement of the risk assessment was recommended.
For MOSH, the following recommendations were issued:
Improvement of analytical methodology for better characterisation of MOSH and consistency in reporting are needed. MOSH concentrations in food should be determined according to the JRC guidance document (Bratinova and Hoekstra, 2023).
Better investigation of the sources of the hydrocarbons in food, which would enable better specification of the type of hydrocarbons present and their fate before ending in the food.
Data are needed on the formation, fate and toxicity of biotransformation products of MOSH, including their accumulation potential.
Investigation is recommended on the structural features of MOSH (particularly regarding alkylated naphthenes) that hinder metabolism and elimination and result in accumulation. This would also enable a better comparison of mineral oils with GTL oils.
Additional toxicity data are needed in relevant experimental models and design, in particular on bioaccumulating MOSH following their characterisation. Particular attention is needed in relation to effects in the liver, spleen as well as immune and nervous system.
Additional data generation is recommended on possible effects of MOSH on lipoproteins and on inflammation and inflammatory markers as seen in recent clinical trials.
More data on human MOSH tissue concentrations or development and use of biomarkers of exposure are needed, particularly from individuals born after 1995.
Contribution from environmental sources, compared to other sources, needs further investigation with regard to occurrence of MOSH and potential compositional modification and bioaccumulation.
The contribution from the environment needs further investigation. On the one hand, it is difficult to avoid this source of contamination. On the other hand, the risks may have been underestimated because of a much higher propensity of accumulation of the most persistent MOSH.
For MOAH:
More data on MOAH composition by aromatic ring number in food are needed, in particular with respect to the levels of three or more ring MOAH.
Sources of food contamination should be investigated when MOAH are detected. To this end, more selective and sensitive analytical method should be implemented.
More data are needed on the influence of ring alkylation on genotoxicity and carcinogenic potency of three or more ring MOAH.
Oral toxicity data are needed for MOAH, in particular with respect to 1–2 ring MOAH.
Technical specifications of white mineral oils and waxes used as food additives and food packaging materials should be updated, with detailed information about the MOAH content and composition.
MOAH concentrations in food should be determined according to the JRC guidance document (Bratinova and Hoekstra, 2023).
1. Introduction
1.1. Background and terms of reference as provided by the requestor
Background
Mineral oil hydrocarbons (MOH) are hydrocarbons containing 10 to about 50 carbon atoms which have been divided into two main types: mineral oil saturated hydrocarbons (MOSH) and mineral oil aromatic hydrocarbons (MOAH). MOH are chemical compounds derived mainly from crude oil, but also produced synthetically from coal, natural gas and biomass. MOH can be present in food in many ways, such as environmental contamination, lubricants for machinery used during harvesting and food production, processing aids like release agents or dust binders, food or feed additives and food contact materials. So‐called “food grade” MOH products are treated in a way that the MOAH content is minimised.
In 2012 the Scientific Panel on Contaminants in the Food Chain (CONTAM Panel) of the European Food Safety Authority (EFSA) concluded 1 that the potential human health impact of groups of substances among the MOH vary widely. MOAH may act as genotoxic carcinogens, in particular 3–7 ring MOAH, while some mineral oil saturated hydrocarbons (MOSH) can accumulate in human tissue and may cause adverse effects in the liver. Due to a lack of analytical data, a monitoring of MOH in food and in materials and articles intended to come into contact with food has been organised by the Commission. 2
The possible setting of maximum levels for MOH in certain foods is considered. It is therefore appropriate to request EFSA to assess recent occurrence data as well as new toxicity studies for an update of the scientific opinion.
Terms of Reference
In accordance with Art. 29 (1) of Regulation (EC) No 178/2002, the European Commission asks the European Food Safety Authority.
-
–
To assess the toxicity studies on mineral oil hydrocarbons which have become available since the EFSA scientific opinion on mineral oil hydrocarbons in 2012 and to update the scientific opinion if necessary as regards hazard characterisation.
-
–
To provide an updated exposure assessment taking into account recent occurrence data.
-
–
To update the section on risk characterisation, as necessary.
1.2. Interpretation of the terms of reference
As indicated in the mandate background, MOH can enter food from multiple sources, including environmental contamination, lubricants from machinery used during harvesting and food production, processing aids like release agents or dust binders, food or feed additives and food contact materials. The composition of the MOH varies correspondingly.
Basically, MOH are hydrocarbons of mineral origin, but the definition is somewhat arbitrary. Some hydrocarbons synthetically produced from coal, natural gas and biomass are subsumed under MOH. Hydrocarbons formed by plants do not belong to MOH, even though they may be the same as those in mineral oils, such as n‐alkanes, and are not included in the MOH data. Crude mineral oils are subjected to refining processes to obtain mineral oil products. Most products are obtained not only by fractionation (distillation), but also modified by chemical reactions like cracking (splitting larger molecules to smaller units), condensation (build‐up of larger molecules, ring formation) and addition or abstraction of hydrogen. Many products only remotely resemble the composition of crude mineral oil. A limit is drawn at which the hydrocarbons are no longer considered as MOH, even though they are petroleum‐derived, such as oligomers of plastics or poly alpha olefins (PAO), made of natural gas components or fractionated small molecular‐sized cracking products of petroleum.
In this opinion, the term MOH includes:
-
–
oils and waxes commonly termed mineral oil products even when they are strongly modified;
-
–
oils and waxes manufactured by Fischer–Tropsch synthesis using coal, methane or biomass as sources, also known as gas to liquid (GTL) oils or waxes, when the resulting products are similar to products made from mineral oil, i.e. in monodimensional GC seem similar or equal to MOH products.
-
–
MOAH include heterocyclic compounds, primarily thiophenes.
In principle (analytically not always possible), the following types of hydrocarbons are excluded:
-
○
hydrocarbons naturally present as food components, such as surface waxes of plants, predominantly consisting of n‐alkanes of odd‐numbered carbon atoms from C21 to C35, and hydrocarbons of terpenic origin;
-
○
degradation products of food constituents like sterenes from sterols or squalene isomerisation products generated, e.g. by the refining of edible oils;
-
○
polycyclic aromatic hydrocarbons (PAH) formed by pyrolysis processes from materials other than mineral oil; largely non‐alkylated aromatic hydrocarbons present in MOAH in very minor proportions (up to a few percent) among the respective alkylated MOAH (Grob et al., 1991a,b,c,d);
-
○
oligomeric hydrocarbons released from polyolefins, such as polyolefin oligomeric saturated hydrocarbons (POSH), largely consisting of branched or linear alkanes, unsaturated oligomers and oligomers including aromatic moieties like oligomers of polystyrene and its co‐polymers;
-
○
oils synthesised from crudely fractionated MOH cracking products, such as poly alpha olefins (PAO), used, e.g. as solvents or lubricants;
-
○
MOH of low molecular mass, such as gaseous hydrocarbons, hydrocarbons used for the extraction of foods, and the low and intermediate molecular mass part of gasoline and solvents, such as white spirits.
The CONTAM Panel recognises that by this definition, some hydrocarbons are excluded from MOH even though they also occur in mineral oils (e.g. n‐alkanes, if they are natural constituents of the food, and PAH from pyrolysis), while others are included even though not present in crude mineral oils (e.g. those resulting from chemical modifications during refining or Fischer–Tropsch products). The definition of ‘MOH’ does not consider toxicological properties; it does not rule out that hydrocarbons not falling under this definition of MOH have similar toxicological properties. For instance, synthetic hydrocarbons from plastics, adhesives and inks and degradation products of food components, or even hydrocarbons naturally present in food, may add to the effect of MOSH. The toxicity of the PAHs from pyrolysis processes (EFSA, 2008) is evaluated separately, despite some overlapping with MOAH.
Industry uses different terms, focusing on their products, whereas this opinion focuses on analytically observed hydrocarbons. According to CONCAWE, 3 mineral oils are composed of hydrocarbons with more than 20 carbon atoms. Products of lower number of carbon atoms are termed hydrocarbon solvents. Mineral oils are roughly divided in two groups; ‘Lubricant Base Oils (LBOs) and Highly Refined Base Oils (HRBOs). LBOs are petroleum‐derived mineral oils which have been dewaxed (normal paraffin significantly removed or transformed) to prevent crystallisation at low temperatures which would adversely impact their performance. HRBOs, also known as “white oils”, are colourless petroleum‐derived mineral oils from non‐carcinogenic LBOs, which are further highly refined to achieve low levels of aromatics to eliminate colour and improve stability’. ‘Pharmaceutical white oils are colourless oils derived from technical white oils, which are highly refined in a second hydrogenation or acid treatment to achieve extremely low levels of aromatics’.
In the literature, the use of the terms MOAH, PAH and PAC is inconsistent. MOAH include all aromatic hydrocarbons of MOH as defined above, including polyaromatic heterocyclic compounds containing sulfur and nitrogen atoms. PAH can be pyrolysis products or a subset of MOAH or PAC. PAC or three to seven PAC are used for polyaromatic aromatic hydrocarbons of three to seven condensed aromatic rings. In this opinion, the term MOAH, when possible with the specification of the number of aromatic rings, is used instead of PAC as long as the origin is of mineral oil according to the above definition. In the present opinion, the definition ‘three or more ring MOAH’ is preferred since the upper number of rings is unknown.
Analytical separation of hydrocarbons defined as MOH from those excluded, particularly when mixed, may be demanding and not complete, even by the best methods available.
1.3. Supporting information for the assessment
1.3.1. Chemistry
1.3.1.1. Composition of mineral oil hydrocarbons
Examples of hydrocarbons subsumed as MOH are shown in Figure 1.
Figure 1.
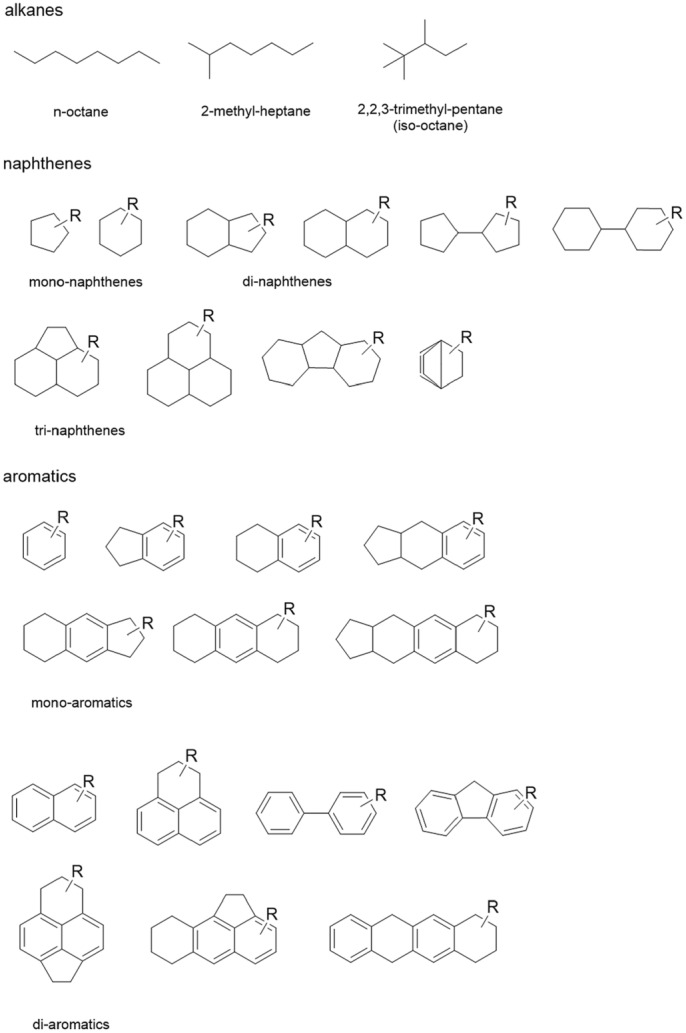
Examples of hydrocarbons for the different classes found in crude mineral oil, whereby the low molecular mass species are outside the definition of MOH used for this opinion. Figure adapted from the EFSA opinion 2012
Apart from hydrocarbons, crude mineral oils also contain hetero‐compounds with sulfur, oxygen and/or nitrogen. Thiophenes, mainly benzothiophenes, dibenzothiophenes and benzonaphthothiophenes are most abundant and were included in the MOAH definition in Section 1.2. However, the products derived from the crude oils are generally treated to reduce or eliminate these hetero‐compounds.
A schematic flow diagram of a typical integrated oil refinery and the description of the various treatments at the various steps is provided in the opinion from 2012 (EFSA CONTAM Panel, 2012).
Commercial products are mainly specified by their physical properties, such as viscosity, boiling point range, density, dielectric constant and octane or cetane number, rather than by their composition. The majority are the result of final blending of different intermediate products. Equivalent properties can be obtained from different hydrocarbon sources and by different processes.
The toxicological evaluation of mineral oil products is hampered by the intrinsic complexity of the MOH composition and the limited possibility of analytical separation and identification of the individual components. The composition of MOH products is determined by the crude mineral oil used as starting material, the physical treatment during refining (such as distillation, extraction) and chemical modifications (such as cracking, hydrogen addition or subtraction). Mineral waxes have a more constant composition than oils: They consist of a high proportion of n‐alkanes next to some mono‐methyl n‐alkanes, n‐alkyl cyclo‐hexanes and ‐pentanes and even less of other hydrocarbons.
1.3.1.2. Industry approach for the removal of the carcinogenic MOAH
Most oils on the market are treated with the intention of removing the carcinogenic MOAH, either with oleum followed by extraction of the sulfones, solvent extraction (e.g. by furfural) or/and by partial hydrogenation. The treatments preferentially remove MOAH with an elevated number of aromatic rings, considered to include the carcinogenic constituents (Mackerer et al., 2003), as these are more reactive to sulfonation and hydrogenation, and have a better affinity to polar solvents.
The IP 346 method 4 (recently reviewed by Carrillo et al., 2019) was developed to predict carcinogenicity of mineral oil products. In this test, oils are extracted with dimethyl sulfoxide (DMSO), which is back extracted into cyclohexane. The cyclohexane is evaporated and the residue gravimetrically compared to the extracted oil. Primarily based on correlation between IP346 and the mouse skin painting carcinogenicity assays, industry considers mineral oil products containing less than 3% w/w DMSO extract as not to represent a genotoxic or carcinogenic risk (see Section 3.1.2.4 on carcinogenicity). The method only applies to ‘unused, additive‐free lubricating base oils having an atmospheric boiling point of 300°C minimum at 5% recovered sample’. The industry specification regarding 3% DMSO extract has been endorsed by EU Regulation EC 1272/2008 on Classification, Labelling and Packaging (CLP), i.e. oils listed in Annex VI are not considered carcinogenic (category 1B), if it can be shown that they contain less than 3% w/w DMSO extract (CLP Note L).
Carrillo et al. (2019) reviewed the argumentation why DMSO is selective for the MOAH fraction that includes the carcinogenic constituents. The range of boiling point (BP) approximately from 300 to 600°C focuses on potentially carcinogenic MOAH species that have three to seven aromatic rings and excludes naphthalene (BP = 218°C). The method of characterisation was based on a mouse skin painting experiment by Agarwal et al. (1988). Fractions free of MOAH or only containing two‐ and three‐ring MOAH were not carcinogenic, whereas all mice treated with a MOAH fraction of more than three aromatic rings and subsequently treated with a promoting agent developed tumours. Application of the recombined fractions developed tumours in all animals without the need of a promoting agent. The authors concluded that the MOAH fraction of more than three aromatic rings needed the presence of constituents in the other fractions to be fully carcinogenic in this assay, but also that oils from which the > 3‐ring fraction has been eliminated may contain MOAH and are still not carcinogenic. Furthermore, the evaluation of carcinogenicity of mineral oils should be done by assessing the whole un‐fractioned oil, as only in this way the complex interactions of constituents can be fully evaluated.
The Panel noted that Agarwal et al. (1988) separated the two‐ and three‐ring MOAH from MOAH of more than three aromatic rings using a Sephadex LH‐20 column, which is gel permeation chromatography, i.e. at least predominantly by molecular size. However, since highly alkylated species overlap with compounds of more aromatic rings but lower degree of alkylation, this separation is incomplete. For instance, also highly alkylated benzenes are expected to be in the fraction of the benzopyrenes, as shown by comprehensive two‐dimensional chromatography (GC × GC) in Annex B. As a result, the content of MOAH with more than three rings was overestimated; there may be no such components at all.
The Panel agrees that the partitioning from oil into DMSO (the extraction efficiency) increase with the number of aromatic rings, i.e. provides selectivity for MOAH of an elevated number of aromatic rings. However, DMSO affinity also decreases with an increasing degree of alkylation, which means that highly alkylated MOAH with more than three aromatic rings may not be extracted into DMSO.
1.3.1.3. Composition of some mineral oil products
Fuels
Diesel oils are produced from fractional distillation of crude oil at between 200°C and 350°C and atmospheric pressure, resulting in mixtures of hydrocarbons typically containing between 9 and 25 carbon atoms. They contain around 15–25% MOAH. The common heating oils (extra light) have the same composition, kerosene/jet fuels are cuts reaching to lower molecular mass.
Solvents
The broad range of MOH solvents in use and of interest in this opinion are composed of hydrocarbons with about 8–20 carbon atoms, containing from almost 0 up to almost 100% MOAH. They are used, e.g. for inks, such as off‐set inks for paper and board, paints and cleaning agents.
White oils
White oils are made from vacuum‐distilled fractions that are treated to comply with the IP 346 specification. They are subsequently submitted to solvent or catalytic dewaxing (to remain clear liquids at low temperatures) and hydrogen finishing to further reduce the MOAH concentration (generally less than 5%) and are then often called ‘food grade’. The molecular mass distributions vary according to the required property. Applications include:
-
•
lubricating and hydraulic oils for food processing, bottling and canning;
-
•
release agents, e.g. for bakery ware and candies;
-
•
coatings for confectionaries, fruits, vegetables, cheese and sausages; eggshell sealants;
-
•
additives used in food contact materials (FCMs), such as water repellents on paper and board or plasticisers in polymers (e.g. polyolefins), inks and adhesives;
-
•
dust suppressants for grain or animal feed; carriers for adding fines to feeds, like minerals or vitamins;
-
•
carriers of pesticide formulations;
-
•
household cleaners and polishes;
-
•
ingredients of cosmetics and medicinal formulations.
White oils are classified by viscosity. For the molecular mass distribution, usually an approximation based on simulated distillation is used, i.e. gas chromatography (GC) with flame ionisation detection (FID) on a non‐polar stationary phase (dimethyl polysiloxane), assuming that elution simulates volatility in a distillation. For most products, a hump of incompletely resolved components is obtained that is integrated using cuts at n‐alkanes. For instance, the 5% distillation point comprises the fraction integrated up to the n‐alkane C25. Based on this method, the high viscosity oils (P100) are specified by an average mass of ≥ 500 Da and a 5% distillation point at a carbon number of ≥ 25, the medium and low viscosity class I (P70) oils by 480–500 Da and ≥ 25, respectively. The class II (N70) oils are specified by an average mass of 400–480 Da and a 5% distillation point at ≥ 22 and class III (P15, N15) oils by 300–400 Da and ≥ 17, respectively (FAO/WHO, 2002).
Waxes
Waxes derived from mineral oil or Fischer–Tropsch synthesis are classified by their molecular mass distribution: the low‐melting point waxes (LMPW), with characteristics similar to candle wax, are centred at around C30, the microcrystalline waxes above C40. By their crystallisation from oils, they are rich in n‐alkanes and contain little MOAH. They are usually treated to further reduce the MOAH.
Technical‐grade lubricant oils
Technical lubricating oils are made from base oils obtained by vacuum distillation, treatment to comply with the IP 346 specification and removal of the wax constituents to prevent crystallisation at low temperature. Their molecular mass distribution is usually centred on C26–C29. Technical lubricating oils used for, e.g. vehicles contain around 25% MOAH. They are of interest here because of their emission from diesel engines and contamination of foods and feeds exposed to the air (gasoline‐powered cars do not emit lubricating oil owing to the catalyst; Neukom et al., 2002).
Extender oils
Extender oils, process or softening oils mainly used for caoutchouc usually consist of treated distillate aromatic extracts (tDAE), i.e. solvent extracts of (poly)‐aromatic hydrocarbons e.g. from base oils to make lubricating oils, and are used in the production of tyres, handles for tools and other rubber goods. They may contain a high proportion of MOAH. There is a REACH restriction on the presence of PAHs derived from the use of extender oils in various plastic and rubber articles. 5
Bitumen
Bitumen is obtained from the residues of the vacuum distillation of crude mineral oils and has a molecular mass mainly above 500 Da. It consists of MOSH and MOAH with a high content of sulfur to obtain the properties needed for road paving.
1.3.2. Sources
MOH in food are from many sources: There is an environmental contribution from the air, through the soil or aquatic ecosystem. Machinery used during harvesting and processing adds MOH in several ways. Mineral oil products are used as processing aids and additives or migrate from FCMs. A list of known sources is reported in Annex A. Most sources have been identified before 2010 and were reported in the EFSA opinion from 2012.
The widespread and rather loose use of mineral oil products was noted in the late 1980s and early 1990s by the Kantonales Labor Zürich, an official food control authority, which then searched for sources and took measures to stop or reduce the contamination. Many uses were phased out, major ones being release agents, e.g. used for bakery products, spraying of rice to make it shiny, cleaning agents in edible oil refineries or additives in feeds. As a consequence, exposure of the consumers decreased, possibly by as much as two orders of magnitude, e.g. by phasing out the use of mineral oil products as release agent in bakery ware, particularly toast bread. This mainly occurred in the 1990s and the early 2000s. Further reduction came after 2008, when the contamination of Ukrainian sunflower oil and the migration from recycled paperboard became public issues (Biedermann and Grob, 2009).
MOH found in food and considered as contaminants often have similar or even the same composition as mineral oil products for authorised uses. In some cases, MOH as contaminants are considered as of potential concern at lower concentration than as authorised products. Differentiation between them is difficult and often unclear.
There is a high variation in the composition of the MOH in terms of molecular mass range, composition and proportion of MOAH. Some mineral oil products also contain additives and impurities, such as viscosity improvers in lubricating oils, e.g. emitted by diesel engines. By combustion, but also in the air, oxidation products are formed. Furthermore, microorganisms, plants or animals can biotransform oils and waxes. As a result, the MOSH composition in some foods will be enriched in hydrocarbons that are difficult or impossible to metabolise and, thus, primarily consist of those hydrocarbons that humans accumulate. Therefore, the source is of interest for the characterisation of the oil and the by‐products/impurities to be expected.
Intentional or unintentional addition of mineral oil products to food or migration by wetting contact with FCMs is not selective in terms of volatility, i.e. the MOH composition found in food is similar or the same as that in the original mineral oil product. However, other products are changed in composition as a result of, e.g. biodegradation or selective migration. MOH entering through the gas phase (e.g. from paperboard into dry foods) are restricted to hydrocarbons of sufficient volatility (mainly below n‐C24 at ambient temperature, Lorenzini et al., 2010), whereas contamination from ambient air is predominantly by particulate matter and, hence, restricted to constituents above about n‐C20 and those resisting degradation in the atmosphere.
Often MOH from several sources are combined. An example was provided by Barp et al. (2015) for pasta packed in a box of recycled paperboard. Migration occurred from this paperboard, but also from the adhesives used to close the boxes and from the transport boxes (about 30% and 25% of the total contamination, respectively). Usually, already the wheat flour to make the pasta contains MOH. Brühl (2016) reviewed the many potential sources in oilseeds and vegetable oils.
1.3.3. Analytical methods
The analytical methods are outlined below and described in more detail in Annex B.
Measurement of MOSH and MOAH concentrations
The presently used methods to determine the concentration of the sum of the MOSH and MOAH or fractions by volatility ranges in food, feed or FCMs are mostly based on on‐line coupled liquid chromatography (LC)‐GC‐FID. LC isolates the hydrocarbons from other sample components and separates the MOSH from the MOAH. FID is used because it provides virtually the same response for all hydrocarbons. The original methods are from the early 1990s (Grob et al., 1991a,b,c,d; Castle et al., 1991) and were limited to the MOSH. The LC‐GC‐FID method was updated to include the MOAH in 2009 (Biedermann et al., 2009). Auxiliary techniques were developed for the elimination of interfering hydrocarbons that do not belong to the MOH according to the definition in Section 1.2. These included the epoxidation of olefins, the reduction in the level of natural n‐alkanes or the enrichment for the samples with a high fat content, as summarised in Biedermann and Grob (2012a,b).
Those PAHs that are part of the MOAH fraction are also captured with the method (though incompletely if the sample is submitted to epoxidation, depending on the conditions applied and on the matrix). However, the LC‐GC‐FID is insensitive and non‐specific. It is not possible to use the method to differentiate between the largely non‐alkylated PAHs and the highly alkylated MOAH (e.g. alkyl benzenes). Statements on the number of aromatic rings cannot be made with the presently used online LC‐GC‐FID. Therefore, a differentiated exposure assessment of the MOAH is not possible with this method, e.g. the differentiation of the one‐ and two‐ring aromatics from the three‐ to seven‐ring aromatics. However, a differentiation is possible using GC × GC up to a size of four‐ring aromatics (Annex B). If the results of the GC × GC are compared with the results of the LC‐GC‐FID, when cutting fractions in the region corresponding to the retention time of the PAHs, as proposed by Roy et al. (1988), one usually finds that the LC‐GC‐FID underestimates the alkylated benzenes and overestimates the content of MOAH with higher ring numbers (see Annex B). An alternative method to separate one‐ and two‐ring MOAH from three or more ring MOAH using donor–acceptor complex LC in normal phase was proposed by Koch et al. (2020).
In the meantime, the LC‐GC method has been updated with regard to the LC separation between the MOSH and MOAH (Biedermann et al., 2017). For sample preparation, the method of epoxidation has been improved, although it is still imperfect because of loss of some MOAH and incomplete removal of certain interferences (Nestola and Schmidt, 2017; Biedermann et al., 2020). Saponification has been introduced for samples rich in lipids (Moret et al., 2016) and for the extraction of encapsulated MOH. For fats and oils, EN 16995:2017 and the standard method C‐VI 22 of the Deutsche Gesellschaft für Fettwissenschaft e. V. (DGF) have been introduced.
The European Union Reference Laboratory for food contact materials together with EU National Reference Laboratories for food contact materials, the Kantonales Labor Zürich and other experts of food analysis developed a ‘Guidance on sampling, analysis and data reporting for the monitoring of mineral oil hydrocarbons in food and food contact materials’ (Bratinova and Hoekstra, 2023).
The LOD is determined by the signal‐to‐noise ratio and the stability of the baseline tested with technical mineral oil products. To cover the entire range (C10–50), a low boiling point naphthenic oil and a high boiling point grease are used. For the LOQ, the maximum signal from the mineral oil hump in the chromatogram must be at least a factor of 10 above the baseline. For the LOD, the LOQ is divided by three (for more information, see Annex B).
Standardising the MOSH/MOAH analysis has proven to be a difficult task. Decisions have to be made on a case‐by‐case basis whether or not certain pretreatments and checks are needed. Some treatments may cause losses of MOSH and MOAH or contamination, and should only be applied when necessary (e.g. epoxidation or removal of n‐alkanes). The plausibility of the results should be checked and further analysis by GC × GC may be needed to check for interferences.
There have been, and still are, discussions about the reliability of the results, particularly for measurements at low concentrations. The LC‐GC‐FID method can be considered as standard and reliable, validated by collaborative tests. Nonetheless, sometimes there were large differences in the results from different laboratories. There were several reasons for this. (i) The interpretation of the chromatograms and the results is demanding; some laboratories provided data without the necessary experience, e.g. in recognising interferences. (ii) Steps to remove interferences may have been necessary. It is not always clarified whether they have been applied and results were corrected accordingly. (iii) The limit of quantitation has decreased since the previous CONTAM opinion, with the effect that analytical interferences have an increased impact. (iv) Contamination of the sample during transport or work‐up may be a problem due to the ubiquitous presence of MOH.
The use of mass spectrometry (MS) instead of FID was advocated (e.g. Spack et al., 2017). However, the lack of specific ions and the problems in calibration were the reasons to stay with FID (e.g. Biedermann et al., 2017).
With a few exceptions, analytical methods are available to reach detection limits below 0.5 mg total MOSH or MOAH/kg for all types of samples as well as to recognise and eliminate almost all interferences, either physically or by disregarding them from the integrated area. However, they are not always applied when needed because of costs or nor being available in the laboratory.
Compositional analysis of MOSH and MOAH
The toxicological evaluation requires compositional data. For the MOSH, it is mainly about the species that are strongly accumulated in tissues or in the environment (which is a small proportion of all MOSH). For the MOAH, the elucidation by number of aromatic rings (including partially hydrogenated polycyclic hydrocarbons) and the number and position of the alkyl substituents would be needed.
As mentioned above, the carbon number distribution of MOH is characterised by ‘simulated distillation’, i.e. assuming that GC retention times on a non‐polar stationary phase reflect boiling points. n‐Alkanes are used to delimit fractions. However, one needs to be aware that this does not directly reflect carbon numbers. iso‐Alkanes of the same molecular mass as the n‐alkanes can be eluted up to about two carbon numbers earlier, naphthenes later by up to several carbons. n‐Alkanes are also used for the specification of ranges of MOAH. However, MOAH tend to be eluted later than n‐alkanes of the same carbon number, whereby the shift varies substantially. The difference is small for alkylated benzenes: for instance, n‐octadecyl benzene with totally 24 carbon atoms is eluted close to the n‐C25 alkane, whereas benzopyrene (C20H12) is eluted in the region of the n‐C29 alkane, i.e. at the retention time of an n‐alkane containing nine more carbon atoms. Assuming an n‐alkyl on benzopyrene contributing to the retention time to the same extent as for the MOSH, at the retention time of the n‐alkane C40, only a C11‐alkylated benzopyrene (with totally 31 carbon atoms) would be eluted. A polyaromatic MOAH comprising 40 carbons could be eluted at or even beyond the retention time of the n‐alkane C50. Being aware of these shifts, nonetheless the simulated distillation is used to characterise MOSH and MOAH fractions in this opinion.
Conventional GC‐FID enables removal of some interferences from the part integrated, for example hydrocarbons of natural origin or from high‐density polyethylene as well as aromatic compounds (including elevated amounts of PAH) standing out of the hump of the MOAH. This is important for quantitative determinations but provides little information about the composition of the MOSH and MOAH.
Over the last 10 years, progress in compositional analysis has been achieved through comprehensive two‐dimensional GC (GC × GC) with FID and MS. The main features of GC × GC are not only significantly better separation and lower detection limits, but also placing structurally related compounds in an order, e.g. MOAH according to the number of aromatic rings. In this way, GC × GC may provide structural information if just a single compound, or even no compound of the series could be identified owing to lacking standards or reference mass spectra.
Since the commonly used GC × GC configuration does not fully separate MOSH and MOAH, GC × GC is usually applied after LC pre‐separation of MOSH and MOAH. Usually a polar first dimension column (typically 50% phenyl methyl polysiloxane) is combined with an apolar second dimension column (typically dimethyl polysiloxane; Biedermann et al., 2014).
For the MOSH, GC × GC plots have the following characteristics (see examples in Annex B):
-
○
hydrocarbons of basically the same structure, but differing in carbon number are at smoothly changing height in the plot (second dimension retention time) and separated by constant intervals (first dimension retention time);
-
○
usually the n‐alkanes are present in quantities forming an easily recognised row of spots, facilitating orientation in the plot;
-
○
multibranched hydrocarbons, including terpenes like pristane and phytane or the oligomers of polypropylene, are located above the n‐alkanes (at higher second dimension retention time);
-
○
little branched alkanes, such as the 2‐ and 3‐methyl n‐alkanes, are eluted at virtually the same height as the n‐alkanes, but between the n‐alkane of the same carbon number and that with one carbon less;
-
○
alkenes, e.g. n‐alkenes with terminal double bonds from polyethylene, form a row at a slightly lower position than n‐alkanes;
-
○
naphthenes are generally located below the n‐alkanes;
-
○
n‐alkyl cyclopentanes and n‐alkyl cyclohexanes are just about separated from each other and form a row of double signals clearly below the n‐alkanes;
-
○
cyclopentanes and cyclohexanes with branched alkyl groups of the same mass are strung with increasing branching in a line from the n‐alkyl compound extending upwards to the left (a behaviour also observed for other types of compounds);
-
○
the position of the naphthenes decreases with the number of rings;
-
○
steranes and hopanes are indicative of MOH products (e.g. Populin et al., 2004), though, in principle, also other sources, like microorganisms, are possible.
For the MOAH, GC × GC with the same column configuration provides the following features:
-
○
MOAH of the same number or aromatic rings form bands positioned the lower the higher is the number of aromatic rings;
-
○
the number of alkyl groups on the aromatic ring system can be determined by MS (2 mass units less for each alkyl group);
-
○
for each aromatic compound with a given carbon number in the alkyl group, a string is formed going upward to the left with increasing isomerisation (detectable in GC × GC‐MS);
-
○
polycyclic hydrocarbons with both aromatic and saturated rings (at least mainly formed by partial hydrogenation) are located between the fully aromatic MOAH of the same number of rings and one less, and partly overlapping with fully aromatic ring systems;
-
○
thiophenes are located clearly below the MOAH of the same ring number.
In general, using GC × GC with FID or MS can provide information beyond that available using single dimension GC:
-
○
MOSH in mineral oil products and residues in various matrices, including tissues, can be characterised in far more detail;
-
○
in many cases, POSH and MOSH can be distinguished;
-
○
usually interferences by natural hydrocarbons differing in their structure from MOH can be recognised;
-
○
MOAH can be separated by ring number;
-
○
the limit of quantitation is approximately 10 times lower owing to the sharp signals and the baseline being established for each second dimension chromatogram.
However, so far, the following potentially critical tasks for a toxicological assessment of MOSH and MOAH has not been accomplished:
-
○
the identification of the structural features that hinder metabolism and elimination of MOSH in living organisms; signals representing resistant MOSH (i.e. including such structural features) were detected in GC × GC, but the mass spectra could not be interpreted;
-
○
the determination of the position(s) of the alkyl group(s) on polyaromatic systems, which depends on finding the specific mass fragments in GC × GC‐MS.
1.3.4. Previous assessments
EFSA
The EFSA Panel on Contaminants in the Food Chain (CONTAM) assessed the risks related to the presence of MOH in food in 2012 (EFSA CONTAM Panel, 2012). It estimated a chronic dietary exposure ranging from 0.03 to 0.3 mg/kg bw per day for MOSH, although it was noted that specific production practices of bread and rice (release agents and spraying to obtain shiny surfaces, respectively) may have provided higher MOSH exposure levels. Insufficient data were available to calculate the exposure to MOAH, which was estimated to be about 20% of that of MOSH. For MOSH, the CONTAM Panel identified hepatic microgranulomas associated with inflammation in Fischer F344 rats (F344 rats) as the critical adverse effect and selected a NOAEL of 19 mg/kg bw per day from a sub‐chronic study on low‐melting point waxes (LMPW) as the Reference Point (RP) for calculating margins of exposure (MOEs) for background MOSH exposure. For MOSH, MOEs ranged from 59 to 680 across different age groups, indicating a potential concern. Based on hazard identification data on single substances and MOAH mixtures, the CONTAM Panel identified three‐ to seven‐ring MOAH with no‐ or a low degree of alkylation as the components of main concern in view of their genotoxic and carcinogenic nature. Three‐ to seven‐ring MOAH with a high degree of alkylation could act as tumour promoters, and some MOAH with less than three rings, like naphthalene, could act as carcinogens via non‐genotoxic modes of action. However, due to the lack of relevant dose–response data on carcinogenicity, it was not possible to draw conclusions on the risks related to the presence of MOAH in food and the CONTAM Panel concluded that in view of the possible presence of genotoxic and carcinogenic substances, the dietary exposure to MOAH was of potential concern.
In 2013, the Panel on Food Additives and Nutrient Sources added to Food (ANS) evaluated the safety of using medium viscosity white mineral oil (MVMO) as a food additive. Based on the results of a 2‐year feeding study in F344 rats, the ANS Panel established a group ADI of 12 mg/kg bw per day. The ANS Panel noted that for the proposed use as food additives, the potential dietary intake of MVMO and/or the previously evaluated high viscosity white mineral oil (HVMO) would be below the established group ADI. However, the ANS Panel also noted that additional exposure to MVMO and/or HVMO via other sources could represent a major source of exposure. Previous evaluations of various grades of white mineral oil and waxes for application as food additives were performed by the European Scientific Committee on Food between 1989 and 1995 and were summarised in the previous EFSA opinion (SCF, 1995; EFSA CONTAM Panel, 2012).
In 2019, an EFSA rapid risk assessment was published following the detection of MOAH in batches of infant and follow‐on formula from the EU market reported by a consumer rights organisation. The assessment was performed on the basis of limited occurrence data showing high variability in terms of number of samples with quantifiable MOAH. In the samples with quantifiable MOAH, concentrations ranged from 0.2 to 3 mg/kg. Exposure of infants, estimated from this limited data set, ranged from 0.8 to 44.6 and from 1.7 to 78.8 μg/kg bw per day for the average and the 95th percentile estimations, respectively. No information was available on the presence of three to seven MOAH. In line with to the conclusion of the 2012 CONTAM Panel opinion, the estimated exposure levels for infants and toddlers were considered of concern for human health due to the possible presence of genotoxic and carcinogenic compounds.
National risk assessment organisations
In the recommendation XXXVI on paper and board for food contact (BfR, 2021), the German Federal Institute for Risk Assessment (BfR) recommends a maximum migration of 12 mg/kg food (preliminary limit) for MOSH in the range of C10 to C16 (BfR, 2011) and of 4 mg/kg food (preliminary limit) for the range of C16 to C20 (BfR, 2012).
The French Agency for Food, Environmental and Occupational Health & Safety (ANSES) published an opinion on the migration of MOH from paper and paperboard packaging (ANSES, 2017). It endorsed the main conclusions reached by the 2012 EFSA opinion on the toxicity of MOSH and MOAH, noting that the new evidence published since 2012 did not allow the refinement of the assessment.
In 2018, the Dutch National Institute for Public Health and the Environment (RIVM) reviewed the available data on toxicity and national exposure to MOH (Van de Ven et al., 2018). It concluded that the limited number of studies on MOSH published since the EFSA CONTAM opinion 2012 would reduce the concerns expressed by EFSA for the MOSH fraction and that the focus should be more on MOAH in view of the carcinogenic properties of some MOAH. The exposure assessment, carried out by means of a probabilistic approach, provided results for MOSH in the same range as estimated by EFSA in 2012. MOAH exposure were estimated to be around 10–20% of those for MOSH.
The Food Standards Australia New Zealand (FSANZ, 2018) conducted a survey to quantify MOSH and MOAH levels in paper and paperboard packaging and in food products contained in these packaging materials but on the basis of the low incidence of detection in sampled foods a quantitative risk assessment was not performed. The results of MOSH and MOAH found in the food are summarised in Section 3.3 Previous occurrence data.
International risk assessment organisations
The Joint FAO/WHO Expert Committee on Food Additives (JECFA) evaluated the use of white mineral oils and waxes as food additives on several occasions. The evaluations performed in 1995 and 2002 (FAO/WHO, 1995, 2002) were summarised in the previous EFSA opinion (EFSA CONTAM Panel, 2012). Briefly, JECFA concluded that, with the exception of high MW microcrystalline waxes, white oils accumulate in the organism in a dose‐ and time‐dependent manner. For microcrystalline waxes and high‐viscosity oils (P100) an ADI of 20 mg/kg bw per day was established. For medium‐ and low‐viscosity oils class I (P70), the ADI was established at 10 mg/kg bw per day. For medium‐ and low‐viscosity oils class II (N70) and III (P15, N15), a temporary group ADI of 0.01 mg/kg bw per day was established, based on the increased incidence of histiocytosis in the mesenteric lymph nodes observed in female F344 rats and in view of the uncertainty about the long‐term significance of the observed inflammatory response (FAO/WHO, 2002; EFSA CONTAM Panel, 2012).
In 2012, JECFA re‐evaluated the safety of medium‐ and low‐viscosity class II and III white oils used as food additives (FAO/WHO, 2012), following the availability of new toxicokinetic data in F344 rats, Sprague Dawley rats and human volunteers. For these substances JECFA concluded that the newly submitted data did not adequately clarify the human relevance of the toxicity data in F344 rats. As data supporting the establishment of a full ADI had not been made available, the temporary group ADI (0.01 mg/kg bw per day) was withdrawn.
In 2012, the International Agency on Research on Cancer (IARC, 2012) classified untreated or mildly treated mineral oils as carcinogenic to humans (Group 1), based on the conclusion of sufficient evidence for carcinogenicity in humans. For experimental animals, it was concluded on sufficient evidence for carcinogenicity for various oil fractions, including untreated vacuum distillates, acid‐treated oils, extracts from solvent‐treated distillates, the high‐boiling fraction of catalytically cracked oils, mildly hydro‐treated oils and used gasoline‐engine oil. The evidence on the mechanism underlying the effects in humans, based on genotoxic (mutagenic) activity in bacteria and a single cytogenetic study of glassworkers exposed to aerosols of mineral oils, was considered as weak.
In 2020, JECFA evaluated class II and III white oils as acceptable for previous cargoes (FAO/WHO, 2020). It used a NOAEL of 22 mg/kg bw per day, based on the incidence of liver granulomas in F344 rats exposed to C14–C50 MOSH (including class II and III mineral oils) as a reference point. It estimated an overall exposure of 0.4 mg/kg bw per day (0.3 mg/kg bw per day from previous cargoes plus 0.1 mg/kg per day from other sources) and calculated an MOE of 55. JECFA considered it sufficient to address the uncertainties in the toxicological database. This took into account that the reference point was based on the most sensitive species, sex and strain and the conservative estimation of the exposure. In the absence of evidence that class II and III oils may induce allergic response or react with edible fats and oils, JECFA concluded that these oils meet the criteria for acceptability as previous cargoes. JECFA specified that the evaluation is based on the assumption that MOH products shipped as previous cargoes are highly refined food‐grade products. The Committee noted that ‘crude mineral oil is banned as previous cargo and MOAH, which contain mutagenic and carcinogenic substances, would be unacceptable as previous cargoes’.
1.3.5. Legislation
Below is short overview of legislation in the EU for mineral oils.
Food packaging materials
For FCMs, there are no specific measures regarding mineral oils, except for the provisions on their use as additives in plastic materials and articles, as laid down by Regulation (EU) 10/2011 with amendments. The following mineral oils are covered by the positive list of additives:
-
a)
FCM substance No 95 – White mineral oils, paraffinic, derived from petroleum‐based hydrocarbon feedstocks. No specific migration limit (SML) is defined (i.e. its use is restricted only by the overall migration limit of 60 mg/kg food or 10 mg/dm2 food contact surface).
-
b)
FCM substance No 94 – Waxes, refined, derived from petroleum‐based or synthetic hydrocarbon feedstocks. No SML is specified (i.e. their use is restricted only by the overall migration limit).
-
c)
FCM substance No 93 – Waxes, paraffinic, refined, derived from petroleum based or synthetic hydrocarbon feedstocks, low viscosity. An SML of 0.05 mg/kg food is specified. In addition, these products are not to be used for articles in contact with fatty foods.
In Europe, countries such as Germany, the Netherlands, Spain, France and Switzerland have recommendations or legislation for MOH in FCM.
Food additives
According to Regulation (EU) 1333/2008 on food additives other than colours and sweeteners, microcrystalline waxes (E 905) are approved for use in the surface treatment of confectionery (excluding chocolate), melons, papaya, mango, avocado, and chewing gums at quantum satis.
Pesticides
Paraffin oils with the CAS numbers 64742‐46‐7 (C11–C25), 72623‐86‐0 (C15–C30), 8042‐47‐5 (C17–C31) and 97862‐82‐3 (C11–C30) are approved for use in the EU according to Regulation 1107/2009 and 540/11 concerning the placing of plant protection products on the market, but only until 31 December 2023 (Regulation 2020/1511). They are included in Annex IV to Regulation 395/2005, which means that there are no toxicological reference values or maximum residue levels (MRLs).
According to Regulation 889/2008, laying down detailed rules on production and labelling of organic products, the following MOH are allowed to be used as pesticides:
-
–
paraffin oil (as insecticide and acaricide);
-
–
mineral oils (as insecticide and fungicide; only in fruit trees, vines, olive trees and tropical crops, e.g. bananas).
In addition, paraffin oils or other white mineral oils can be used as co‐formulants in plant protection products.
Veterinary medicine
According to Regulation (EEC) No 2377/90 (repealed by Regulation (EC) No 470/2009 on EU procedures for establishing limit of pharmacologically active substances in foodstuffs of animal origin), it is allowed to use ‘mineral hydrocarbons’ in veterinary medicine for all food‐producing species. The mineral hydrocarbons allowed are ‘of low to high viscosity including microcrystalline waxes, approximately C10‐C60 and both aliphatic, branched aliphatic and alicyclic compounds’. The mineral hydrocarbons excluded are ‘aromatics and unsaturated compounds’.
2. Data and methodologies
The current update of the EFSA risk assessments on MOH in food was developed applying a structured methodological approach, which implied developing a priori the protocol or strategy of the full risk assessments and performing each step of the risk assessment in line with the strategy and documentation of the process. The protocol in Annex C to this Opinion contains the method that was proposed for all the steps of the risk assessment process, including subsequent refinements/changes made.
The CONTAM Panel used its previous risk assessment on MOH in food (EFSA CONTAM Panel, 2012) as a starting point for drafting the current Opinion.
The draft scientific Opinion underwent a public consultation from 15 March until 30 April 2023. The comments received and how they were taken into account when finalising the scientific Opinion are available in Annex I.
2.1. Supporting information for the assessment
An extensive literature search was outsourced via an EFSA procurement (Licht et al., 2023). The search was aimed to retrieve and select published peer review publications on MOH pertaining to the following areas:
-
•
Area 1: Data on chemical identification and characterisation
-
•
Area 2: Data on sources and occurrence in food, including human milk
-
•
Area 3: Data on toxicokinetics (absorption, distribution, metabolism, excretion) in experimental animals and humans and from in vitro studies
-
•
Area 4: Data on toxicity in experimental animals
-
•
Area 5: Data on in vitro and in vivo genotoxicity and mode of action
-
•
Area 6: Data on observations in humans (including epidemiological studies, case reports, biomarkers of exposure)
The search was performed between 16 January and 5 February 2021 and targeted to studies published since 2010. The search was repeated on 10 October 2022 by the EFSA secretariat. Additional literature was retrieved by means of the ‘snowball method’.
Information on previous risk assessments by national and international bodies was retrieved by checking organisations' websites and performing specific searches. Information on legislation was retrieved from current EU and EU MS national legislations.
2.2. Hazard identification and characterisation
The selection of the scientific papers for inclusion or exclusion was based on consideration of the extent to which the study was relevant to the assessment and on general study quality considerations (e.g. sufficient details on the methodology, performance and outcome of the study, on dosing, substance studied and route of administration and on statistical description of the results), irrespective of the results. Limitations in the information used are documented in this Scientific Opinion.
2.3. Occurrence data submitted to EFSA
2.3.1. Data collection and validation
The data submission to EFSA followed the requirements of the EFSA Guidance on Standard Sample Description (SSD) for Food and Feed (EFSA, 2010a).
Data collection on MOH was boosted by the Commission Recommendation 2017/84 on the monitoring of MOH in food as well as materials and articles intended to come into contact with food. Analytical data were reported to EFSA as MOSH, MOAH as well as subfractions of these (so‐called C‐fractions). Most C‐fractions referred to those indicated in the JRC Technical Report ‘Guidance on sampling, analysis and data reporting for the monitoring of mineral oil hydrocarbons in food and food contact materials’ (Bratinova and Hoekstra, 2023), although analytical results codified as ‘Mineral oils’ and other C‐fractions were also submitted.
MOSH fractions were mainly determined and reported as follows:
-
–
> C9 – ≤ C16;
-
–
> C16 – ≤ C20;
-
–
> C20 – ≤ C25;
-
–
> C25 – ≤ C35;
-
–
> C35 – ≤ C40;
-
–
> C40 – ≤ C50.
MOAH fractions were mainly determined and reported as follows:
-
–
> C9 – ≤ C16;
-
–
> C16 – ≤ C25;
-
–
> C25 – ≤ C35;
-
–
> C35 – ≤ C50.
Taking into account that the previous EFSA scientific opinion on MOH included samples collected up to 2010, the current assessment focused on sampling years between 2011 and 2021.
2.3.2. Data analysis
To ensure the appropriate quality of the occurrence data used for the dietary exposure estimations, data cleaning and data validation steps were followed according to EFSA SOPs. 6 Together with identifying duplicate samples, attention was paid to the information provided on analytical methods and their sensitivity, sampling strategy, FoodEx2 classification, expression of the results, etc. Data providers were contacted when needed to confirm the information provided (e.g. reported data initially identified as potential outliers).
To identify questionable MOH data, e.g. those that might be affected by incomplete removal of interferences (e.g. natural olefins or olefins formed by heat treatment), the ratio for the MOAH/MOSH concentrations was checked across all samples, considering the ratios expected for the mineral oil that might have contaminated the food. Although no guidance on MOAH/MOSH ratios exists, the JRC Guidance on MOH proposes to check this ratio as first step to verify the reliability of the analysis (Bratinova and Hoekstra, 2023). Based on the available analytical data for MOAH and MOSH and considering measurement uncertainty, the CONTAM Panel applied two different cut‐offs: If the MOSH concentration exceeded 10 mg/kg, MOAH/MOSH ratios above 0.5 were considered questionable; if MOSH concentrations were below 10 mg/kg, the cut‐off was at 1, taking into account higher uncertainty. For samples above these cut‐offs, data providers were contacted to gather additional information (e.g. chromatograms or specifications of the sample preparation). The CONTAM Panel decided to exclude those samples when no information was provided or the information did not support the data in a plausible manner. The outcome of the data analysis is presented in Section 3.2.1.
The left‐censored data were treated by the substitution method using the lower bound (LB) and upper bound (UB) approach (WHO/IPCS, 2009; EFSA, 2010b). Applying the LB approach, results below the LOD/LOQ were replaced by zero; for the UB approach, the results below the LOD were replaced by the value reported as the LOD; results below the LOQ and above the LOD were replaced by the value reported as the LOQ. As explained later (see section 3.2.1), in certain samples, the sum of the reported C‐fractions was used to estimate the total MOAH/total MOSH content. In these samples, the left‐censoring limits of each of the C‐fractions were summed up to estimate the UB when the C‐fractions were reported as left‐censored data. The LB was estimated summing up all C‐fractions, using zero for those below LOD/LOQ and the reported value when quantified.
2.4. Food consumption data
The EFSA Comprehensive European Food Consumption Database (Comprehensive Database) provides a compilation of national information on food consumption at individual level and was first built in 2010. Details on how the Comprehensive Database is used are published in the Guidance of EFSA (EFSA, 2011a).
Food consumption data were retrieved from the Comprehensive Database in March 2022. That version of the Comprehensive Database, updated in July 2021, contained results from a total of 72 dietary surveys carried out in 24 European countries covering 137,165 individuals. Detailed information on the dietary surveys can be found on the dedicated page of the EFSA website. 7 The following age classes were considered:
-
•
Infants: < 12 months old;
-
•
Toddlers: ≥ 12 months to < 36 months old;
-
•
Other children: ≥ 36 months to < 10 years old;
-
•
Adolescents: ≥ 10 years to < 18 years old;
-
•
Adults: ≥ 18 years to < 65 years old;
-
•
Elderly: ≥ 65 years to < 75 years old;
-
•
Very elderly: ≥ 75 years old.
Nine additional surveys provided information on specific population groups: six on ‘Pregnant women’ (Austria: ≥ 19 years to ≤ 48 years old, Cyprus: ≥ 17 years to ≤ 43 years old; Latvia: ≥ 15 years to ≤ 45 years old, Romania: ≥ 19 years to ≤ 49 years old, Spain: ≥ 21 years to ≤ 46 years old, Portugal: 17 years old to 46 years old), two on ‘Lactating women’ (Greece: ≥ 28 years to ≤ 39 years old, Estonia: 18 years old to 45 years old) and one on ‘Vegetarians’ (Romania: ≥ 12 years to ≤ 74 years old).
When two dietary surveys were available for a country and age class, only the most recent one was used. Only dietary surveys with more than 1 day per subject were used to estimate chronic dietary exposure to MOAH and MOSH, following the recommendations from the EFSA Guidance on the use of the Comprehensive Database (EFSA, 2011a). This resulted in a total of 47 dietary surveys (86,117 subjects, 22 European countries) used for the chronic dietary exposure assessment (Annex D). Owing to the differences in the methods used for data collection, direct country‐to‐country comparisons can be misleading.
2.5. Food classification
Consumption and occurrence data were both codified according to the FoodEx2 classification system. FoodEx was developed by EFSA in 2009 with the objective of simplifying the linkage between occurrence and food consumption data when assessing the exposure to hazardous substances (EFSA, 2011b). Following its first publication, a testing phase was carried out in order to highlight strengths and weaknesses, and to identify possible issues and needs for refinement. Based on the outcome of the testing phase, EFSA published in 2015 the FoodEx2 revision 2 (EFSA, 2015).
The FoodEx2 catalogue hosts several hierarchies used for different data collections, e.g. ‘Reporting hierarchy’ for the collection of occurrence data and ‘Exposure hierarchy’ for the collection of food consumption data. It consists of a large number of individual food items aggregated into food groups and broader food categories in a hierarchical parent–child relationship. It contains 21 main food categories at the first level of the ‘Exposure hierarchy’, which are further divided into subcategories, resulting in seven levels with more than 4,000 items in total. In addition, FoodEx2 allows the further description of food items with facets. Facets are descriptors that provide additional information for a particular aspect of a food and are divided into implicit facets, which are integrated in the catalogue, and explicit facets, which are added by users while coding a food item.
2.6. Exposure assessment
Dietary chronic exposure to MOAH and MOSH was assessed at individual level by multiplying the average daily consumption for each food with the corresponding mean occurrence estimate for MOAH and MOSH (LB and UB), summing up the respective intakes throughout the diet and dividing the results by the individual's body weight. For each dietary survey, the mean and 95th percentile dietary exposure to MOAH and MOSH were estimated from the distribution of the individual exposure results. In accordance with the specifications of the EFSA Guidance on the use of the Comprehensive Database, 95th percentile estimates for dietary surveys/age classes with less than 59 observations were not calculated, since they may not be statistically robust (EFSA, 2011a). Minimum, median and maximum LB and UB exposure levels across national dietary surveys were reported for all population groups, both for mean and 95th percentile estimations.
The whole diet was taken into account, except for food not covered by occurrence data and/or for which an assumption on their contamination level was not possible. The food commodities were grouped under different food categories to better explain their contribution to the total dietary exposure to MOAH and MOSH in each age class.
Dilution factors were used to convert the occurrence and consumption data reported for solid food groups to their respective liquid forms. 8 The dilution factors were 75 for ‘Tea and herbs for infusions (Solid)’, 60 for ‘Cocoa powder‘, 10 for ‘Cocoa beverage‐preparation, powder’, five for ‘Porridge’ and eight for ‘Infant formula’, ‘Follow‐on formulae’ and ‘Milk powder, full fat’, among others. Due to insufficient specific data, no losses of MOAH and MOSH were assumed to happen during food processing when using the occurrence data reported in commodities that need to be processed at different extent before being consumed (e.g. brewing tea leaves, boiling pasta, etc.).
Together with the general chronic dietary exposure scenario for MOSH and MOAH, a few specific exposure scenarios were conducted. Some of them referred to food samples with high levels of MOAH and MOSH considered as not representative of the ‘background’ levels typically found in these foods; the high levels seem to have their origin in production practices, such as the use of specific releasing agents, anti‐dust agents, etc. These samples were excluded from the general exposure scenario but used in specific scenarios to cover consumers that might regularly consume these commodities. Examples are wheat groats and chocolate cakes, among others (see Sections 3.2 and 3.3).
To better characterise the contribution of infant formula to the dietary exposure to MOAH and MOSH of infants below 16 weeks of age, an exposure scenario was conducted using 170 mL/kg bw per day and 210 mL/kg bw per day for mean and P95 consumption, respectively, as recommended by the Guidance of the EFSA Scientific Committee (SC) on the risk assessment of substances present in food intended for infants below 16 weeks of age (EFSA Scientific Committee, 2017a).
To assess the dietary exposure to MOSH of breastfed infants below 6 months of age, a scenario considering a median age of 3 months was selected, equivalent to a body weight of about 6.1 kg, with an estimated average daily milk consumption of about 800 mL and a high consumption of 1,200 mL (IOM, 1991; EFSA, 2019b). The concentration of MOSH in human milk was derived from Concin et al. (2008), as no more recent data were available. From the data in the milk fat reported for 144 samples taken at day 20 after birth from individuals from the area of Innsbruck (Austria), a value of 0.87 mg/L was considered as representative of average MOSH concentration in human milk during the first months.
All analyses were run using the SAS Statistical Software (SAS enterprise guide 8.2).
2.7. Risk characterisation
The general principles of the risk characterisation for chemicals in food as described by the WHO/IPCS (2009 and subsequent updates) were applied as well as the different EFSA guidance documents relevant to this step of the risk assessment (see Annex A).
3. Assessment
3.1. Hazard identification and characterisation
The details of the in vivo studies summarised in the following sections are available in Appendix A.
3.1.1. Toxicokinetics
Summary from the previous CONTAM Panel Opinion
In the EFSA Opinion from 2012 (EFSA CONTAM Panel, 2012), information on the toxicokinetics of various MOH had been collected and reviewed. Data on the fate of MOH in animals and humans were scarce and mostly came from rodents.
MOSH
Absorption
It was concluded that the absorption and distribution of MOSH occurred by passive processes. Information was collected both for mixtures and single compounds. For aliphatic hydrocarbons, the absorption was inversely associated with carbon number. Generally, it appeared that both n‐alkanes and cycloalkanes were well absorbed at low exposure levels (< 100 mg/kg bw). Absorption was up to 90% for n‐C14 and n‐C18 and varied from 25% to 75% in the range of n‐C26 to n‐C29. The uptake of branched alkanes of equimolecular weight was less than that of the n‐alkanes in all tested animal species. The uptake via the lymphatic system appeared to play a major role and was strongly enhanced by co‐administration of lipids. Lower molecular weight hydrocarbons, e.g. hexadecane and below, may also be taken up and transported through the hepatic portal vein. In F344 rats, two peaks on the concentration curve were observed in blood, supporting the two independent routes of transport, a faster one via portal vein and a slower via the lymphatic system. However, only one peak was observed in SD rats.
Distribution and accumulation
Animal experiments provided evidence that MOSH are distributed to and accumulated in various tissues, such as the liver, spleen, mesenteric lymph nodes and fat. In a comparative study using one single dose of MOH, F344 rats appeared to be more prone to MOH accumulation in the liver, particularly for alkanes > n‐C25, than SD rats.
There was no information on transplacental transport of MOSH. Because of the lipophilic nature of the material, it is assumed that they will cross the placenta.
Metabolism
Metabolism may take place both in the small intestine and the liver. For alkanes, it involves omega oxidation to fatty alcohols and fatty acids that can be incorporated into lipids or undergo beta‐oxidation. Oxidation of branched alkanes ends up in tertiary alcohols, while cycloalkanes undergo oxidation of side chains and ring oxidation to cyclanols. For shorter alkanes (n‐C17), there were no differences in the rate of oxidation by liver microsomes from F344, SD and Wistar rats. Microsomes from human donors had a higher oxidation rate than those from F344 rats. The branched alkanes tested were not biotransformed by the rat and human liver microsomes.
Elimination
Studies in rats using radiolabelled individual compounds of different classes in the lower molecular weight group indicated excretion of > 60% of the administered radioactivity after 7 days. However, the amount excreted, and the main excretion pathway differed depending on the classes of MOSH. Though most studies address the retention of the administered radioactivity in the body, in studies with pristane and dodecylcyclohexane, respectively, the urine was shown not to contain the unmetabolised test substance. Elimination via the breast milk appears to be the only excretion pathway of unchanged MOSH from the body.
The current Panel noted that the main information available is on unmetabolised MOSH in tissues. One might expect water‐soluble oxidised metabolites to be excreted, but fatty alcohols and acids of higher molecular mass might be incorporated into lipids.
Kinetics in humans
Although MOSH were observed in human tissues at autopsy, no studies on the rate of absorption of mixtures or single compounds were identified. Based on data available at the time of the previous opinion on levels in abdominal fats and human milk, absorption in humans appeared to take place up to n‐C35. Saturated hydrocarbons have been analysed in abdominal adipose tissue and breast milk from women undergoing caesarean section. The gas chromatograms showed similar MOSH compositions with odd‐numbered n‐alkanes dominating over the even numbered. These were largely of plant origin from n‐C21 to n‐C33 on a hump of unresolved MOSH that was centred on n‐C23/n‐C24 and ranged from n‐C16 to about n‐C30. The concentration in the abdominal adipose tissue appeared to increase with age of the mother and with number of children; it decreased with body mass. Among the identified sources were cosmetic products, such as lipsticks, hand and sunscreen creams. The molecular mass distribution and composition in tissues differed from the mineral oil hydrocarbons to which humans are likely to have been exposed, indicating selective accumulation, with preference for branched alkanes and cycloalkanes (naphthenes).
MOAH
Limited studies on toxicokinetics in mammals suggested that MOAH were well absorbed and extensively biotransformed, particularly those with a low degree of substitution, which, as a result, do not accumulate. Biotransformation by CYP (P450) enzymes may lead to metabolic activation and formation of reactive and genotoxic metabolites from MOAH with three to seven aromatic rings and with no or a low degree of alkylation. The above‐mentioned bioactivation, described in detail for PAHs in EFSA (2008), includes in particular epoxide formation in specific positions of the polycyclic ring system, followed by formation of other reactive electrophilic metabolites capable of binding to cellular macromolecules, such as proteins and nucleic acids. In the case of MOAH, however, alkyl substitution may affect the bioactivation process by blocking or impairing the oxidation of the aromatic ring system.
The fate of MOAH was unknown, but it was assumed that degradation of long alkyl chain was similar to that of substituted cycloalkanes.
New studies
In vitro studies.
MOSH
No new in vitro studies on MOSH were identified.
MOAH
In a study by Wang et al. (2020), the in vitro metabolism of naphthalene and six alkyl‐substituted derivatives (1‐methyl‐naphthalene, 2‐methyl‐naphthalene, 1‐ethyl‐naphthalene, 2‐ethyl‐naphthalene, 1‐n‐hexyl‐naphthalene, 1‐n‐dodecyl‐naphthalene) were investigated using human and rat liver microsomes. Metabolites were determined using ultraperformance liquid chromatography (UPLC) with photodiode array detection and identified by GC–MS/MS. For naphthalene, the main metabolites were 1,2‐dihydro‐1,2‐naphthalenediol and 1‐naphthol, with the first one dominating upon incubation with human microsomes, while the latter dominated with rat microsomes. Alkyl substitution reduced aromatic ring oxidation. For all methyl‐ and ethyl‐substituted derivatives, there was a shift from ring to side chain oxidation, most markedly seen with rat microsomes. For ethyl‐substituted naphthalenes, oxidation took place in the 1‐position of the side chain. No side chain oxidation occurred for 1‐n‐hexyl‐ or 1‐n‐dodecyl‐naphthalene, but a minor amount of ring oxidation was observed for 1‐n‐hexyl‐naphthalene. No oxidation products were observed for 1‐n‐dodecyl‐naphthalene. Generally, human microsomes were less active than those from rats.
Wang et al. (2022a,b) investigated the impact of alkyl‐substitution on the oxidative biotransformation and mutagenicity of phenanthrene using human and rat liver microsomes. Nine alkyl‐substituted derivatives of phenanthrene were investigated and the details are given in the section on genotoxicity (Section 3.1.2.3) The impact of alkylation on bioactivation was investigated for the four mono‐methyl‐phenanthrenes (methylated in four different positions) by means of mutagenicity testing in S. Typhimurium, using S‐9 mix from livers of Aroclor‐1254‐treated SD rats. Metabolites were determined using UPLC with photodiode array detection and identified using GC–MS/MS.
Alkyl substitution shifted the aromatic oxidation to side chain oxidation for all alkylated derivatives except 1‐n‐dodecyl‐phenanthrene for which the biotransformation was negligible under the test conditions. The overall oxidation rate decreased upon increased chain length of the substituent.
For ethyl substituted derivatives, oxidation took place both in position 1 and 2 of the ethyl group. Liver microsomes from rats and two human donors oxidised the hexyl‐substituted derivate at four different non‐specified positions. In the mutagenicity tests with S‐9 mix, 1‐methyl‐phenanthrene was clearly positive in both test strains, whereas 9‐methyl‐phenanthrene was positive, but close to the background. Phenanthrene, 2‐ and 3‐methyl‐phenanthrene were all negative (see Section 3.1.2.3).
The authors also examined metabolites formed following incubation of phenanthrene and the four methylated derivatives with S9 mix. This yielded ring dihydrodiols, side‐chain alcohols, hydroxy‐phenanthrenes and a shift to side chain oxidation at the expense of aromatic oxidation as was also seen in the microsome experiments. In addition, a further metabolite was identified, hydroxymethyl‐hydroxy‐phenanthrene (hydroxymethyl‐phenanthrol). Formation of 1,2‐ and 7,8‐dihydrodiols was observed for all tested compounds. Variable amounts of 3,4‐ or 5,6‐dihydrodiols and 9,10‐dihydrodiols were formed from the methyl‐derivatives. Only 9,10‐dihydrodiols formed from 2‐ and 3‐methyl‐phenanthrene, and higher levels of 3,4‐ and 5,6‐dihydrodiols were formed from 1‐methyl‐ and 9‐methyl‐phenantrene. The authors hypothesised that for the mutagenic methyl derivatives, the methyl group substitution at or near the K‐region, i.e. 9,10‐position, of phenanthrene might create a bay region‐like structural motif and facilitate formation of a reactive bay region dihydrodiol‐epoxide.
Wang et al. (2022b) also examined the impact of alkyl‐substitution on the oxidative biotransformation of benzo(a)pyrene and the mutagenicity of benzo(a)pyrene with alkyl‐substituents in various positions. Oxidative biotransformation was investigated using liver microsomes from humans and SD‐rats, respectively. Also, in this case, metabolic oxidation shifted from aromatic ring oxidation to side chain oxidation. The overall rate of oxidative metabolism, including also aromatic oxidation, decreased with the length of the alkyl substituent. Alkylation shifted the position of aromatic ring oxidation. At a chain length of > 6 C atoms, oxidation was strongly reduced. There was a marked change in rates and pattern of metabolites in microsomes of benzo(a)pyrene methylated at different positions. There were also differences in rates and pattern of metabolites between human and rat microsomes. The authors noted that hydroxymethyl derivatives could be substrates for sulfotransferases leading to reactive sulfate esters. Details on the impact of alkylation of benzo(a)pyrene on mutagenicity are given in the section on genotoxicity (Section 3.1.2.3).
Studies in experimental animals
MOSH
In a study by McKee et al. (2012), female F344 rats were given white mineral oil P7H (carbon numbers ranging from n‐C14 to n‐C32; average n‐C20; containing 48% < C20) at 0.02%, 0.2% and 2% for 90 days in the diet (equal to average doses of about 17, 170 and 1,690 mg/kg bw per day according to the authors, respectively). Extracts from livers of treated rats were analysed by GC‐FID. MOSH were detected in liver tissues from the two highest dose levels only (2,280 ± 313 μg/g and 3,000 ± 329 μg/g, respectively). The average carbon number of the retained constituents was relatively high compared to the test oil given, with MOSH constituents from n‐C15 to n‐C20 being less abundant or largely absent. As these alkanes are usually absorbable, this suggests that aliphatic alkanes below n‐C20 are more easily eliminated, e.g. exhaled or biotransformed possibly followed by excretion. No MOH were detected in spleen, without a limit of detection being reported.
A summary of the results from the EFSA‐sponsored study; Cravedi et al., 2017, Barp et al., 2017a,b) in female F344 rats exposed to MOSH in the feed is reported below, with a more extensive description in Appendix B. Accumulation of MOSH was determined in liver, spleen and adipose tissue.
In the first experiment, the rats were exposed to a mixture of white oils of broad molecular mass range (n‐C14 to n‐C50), i.e. broader than the mass range found in human tissues (n‐C16 to about n‐C45), in order to determine the most strongly accumulated MOSH. The rats were fed with this MOSH mixture at nominal concentrations of 40, 400 and 4,000 mg/kg feed (equal to mean external doses of 2.6, 25 and 260 mg/kg bw per day, calculated on the basis of individual body weights and cage averaged feed intake) for up to 120 days. Separate groups received control feed for depuration during the last 30 days. Groups of rats were sacrificed at 30, 60, 90 and 120 days. Liver, spleen, adipose tissue and carcass were collected and analysed for MOSH.
In the feed, only small amounts of n‐alkanes were present above around n‐C25, as the oils of higher molecular mass were de‐waxed. As shown by GC × GC‐MS, the mixture contained little‐branched alkanes (i.e. with small and few branches, such as mono‐methyl n‐alkanes) and highly branched alkanes. Cyclic hydrocarbons consisted of alkyl‐cyclo‐pentanes and cyclohexanes as well as hydrocarbons with more rings (naphthenes). In GC × GC, these generally form a cloud of unresolved components owing to the presence of many isomers in terms of ring conformation and alkylation. The steranes and hopanes, widely used as markers for mineral oil, belong to these polycyclic saturated hydrocarbons (example plots shown in Appendix C).
At all doses, the MOSH concentrations were highest in the liver, followed by spleen and adipose tissue with roughly one order of magnitude lower concentrations. Concentrations in the remaining carcasses (the rest of the body after removal of liver, spleen, abdominal adipose tissue and gastro‐intestinal tract including digestive content) were low. After depuration for 30 days, the concentrations in liver, spleen and carcass were reduced (e.g. in liver about 53% reduction was observed at 2.6 and 25 mg/kg bw per day, and 34% reduction at 260 mg/kg bw per day), while there was little change in the adipose tissue. Up to day 30, there was a rapid increase in the retained amounts, then a slower increase between days 30 and 90. In spleen, the concentrations increased up to 120 days; there was no tendency to plateau; and no steady state was observed. For the other tissues, no consistent statistically significant increase of MOSH content was observed between day 90 and day 120, although an increasing trend was observed for adipose tissue and carcass. In liver, a plateau appeared to be reached between day 90 and day 120.
About 50% (49–61%) of the retained material ended up in the liver, with a mass range from n‐C16 to n‐C40, the major part between n‐C24 and n‐C34. The strongest accumulation was at n‐C29. In the spleen, the distribution was almost identical to that in the liver. Approximately 38–50% of the retained MOSH was found in the adipose tissue and carcass, with a range up to n‐C31, centred around n‐C19. The strongest retention was at n‐C15 – n‐C16, the low end of the applied mixture. It was noted that the n‐alkanes were enriched, approximately threefold, in the adipose tissue compared to the administered mixture.
Concentrations in the tissues increased far less than proportionally with the dose. Despite a very low background content of MOSH in the control feed (1.5 mg/kg feed), these MOSH were detected in the control animals. At day 30, the total retained amounts recovered in the animals excluding the gastrointestinal tract were 10.9, 6.9 and 2.0% of that ingested for the doses of 40, 400 and 4,000 mg/kg feed, respectively. At day 120, the retained amounts were 6.2, 3.5 and 1.5%, respectively. Depuration for 30 days resulted in a reduction of retention to 3.9, 2.0, 0.9%, respectively. Upon characterisation of the retained MOSH by GC × GC, it was found that in the liver and spleen, alkyl‐substituted naphthenes (forming a cloud) as well as highly branched iso‐alkanes dominated, whereas n‐alkanes and hydrocarbons with little branched alkyl moieties dominated in the adipose tissue.
In the second experiment (Barp et al., 2017b), three groups of female F344 rats were exposed to the following MOSH mixtures at nominal doses of 0, 400, 1,000 and 4,000 mg/kg feed during 120 days:
-
(i)
S‐C25, ranging from n‐C16 to n‐C34, with 73% ≤ n‐C25, containing a small amount of wax n‐alkanes (mean doses: 23, 61 and 226 mg/kg bw per day);
-
(ii)
L‐C25, ranging from n‐C25 to n‐C50, with 1.5% ≤ n‐C25, deparaffinated, i.e. with hardly any n‐alkanes (mean doses: 25, 62 and 236 mg/kg bw per day);
-
(iii)
a 1:1 (w:w) mixture of L‐C25 with a wax of a melting point around 65°C largely consisting of n‐alkanes from C23 to C45 (mean doses: 22, 53, 218 mg/kg bw per day).
For all three MOSH mixtures, a dose‐dependent tissue accumulation was observed, with the highest concentrations in the liver. The bioconcentration factor (BCF) at the low dose varied from about 12 for S‐C25 and L‐C25W to 3.5 for L‐C25 and decreased with increasing dose for all tissues. In the spleen, the BCFs were below 1 at all doses in all groups and decreased with increasing doses. The BCF in spleen of S‐C25 was about the double of that in L‐C25 and L‐C25W.
Compared with the total composition of the MOSH, n‐alkanes and n‐alkyl monocyclic naphthenes (typical wax components) were enriched in adipose tissue. In liver and spleen, n‐alkanes up to n‐C25 were absent, but enriched starting from n‐C25 and decreasing again beyond n‐C30. n‐Alkyl monocyclic naphthenes were enriched starting from n‐C28. For L‐C25, the centre of n‐C35 in the mixture applied shifted downwards to around n‐C30 in the liver, and the residues were dominated by naphthenes and iso‐alkanes. For the L‐C25W, the patterns in the tissues were strongly dominated by n‐alkanes in the range of n‐C25 to n‐C35. The authors suggested that the resistance to biotransformation and elimination of the n‐alkanes and possibly of other wax components might be associated with crystallisation.
Carrillo et al. (2021a,b) compared the tissue accumulation and retention of a white mineral oil product (N70H, CAS 8042‐47‐5) with a gas to liquid (GTL) synthetic oil product (CAS 1262661‐88‐0) of similar carbon range distribution. The N70H oil was a medicinal grade naphthenic oil with carbon numbers in the range of n‐C18 to n‐C48 and consisting of approximately 84% naphthenes, 3% linear alkanes, 7% mono/dimethyl alkanes and 4% more branched alkanes, whereas the GTL oil consisted of ∼ 99% iso‐alkanes, with 3% mono/dimethyl alkanes and the rest multibranched iso‐alkanes in the n‐C20 to n‐C42 range.
Groups of female SD rats received a diet spiked with 3,000 mg/kg (equal to ∼ 200 mg/kg bw per day according to the authors) of either of the two oils for 134 days or 90 days followed by 44 days on a control diet. A control group was fed untreated diet shown to contain 90 mg/kg MOSH throughout the 134 days.
The hydrocarbon content in blood and liver was determined by LC‐GC‐FID and GC × GC ‐TOF‐MS at days 1, 29, 57, 92 and 134 and also at days 106, 120 and 134 in the group only treated for 90 days followed by a 44‐day recovery period. Other tissues were analysed semi‐quantitatively at various time points to observe trends in MOSH retention.
The MOSH content was determined in most often three to four animals per time point and dose and often showed high variation. At day 92, roughly four to five times higher concentrations were found for the N70H oil (largely naphthenes) than for the GTL oil (largely iso‐alkanes) in liver (approximately 660 and 150 mg/kg, respectively). After 134 days in the continuous dose group, the MOSH contents differed by a factor of about 2 (∼ 650 and 300 mg/kg for N70H and GTL oil, respectively). The same trend was observed in MLN and visceral fat. This suggests that the accumulation of iso‐alkanes in the range n‐C20 to n‐C42 is less than that of naphthenes of a similar range of carbon numbers. After 92 days of exposure followed by 44 days of recovery, concentrations in liver were reduced by a factor of ∼ 3 (180 and 56 mg/kg for N70H and GTL oil, respectively), but no reduction was seen in the mesenteric lymph nodes.
In a new study submitted in the context of a Food Contact Material application, male and female SD rats were fed a paraffin wax (EFSA CEP Panel, 2023) in the diet. Nominal dose levels of 0, 0.2, 2 and 10 g/kg were administered for 13 weeks with a further 6‐week recovery period in an OECD TG 408 compliant study (ERBC, 2022). Group mean doses achieved in the animals were 0.19, 1.9 and 9 g/kg bw per day for males and 0.24, 2.0 and 10 g/kg bw per day for females. Tissue levels were analysed by means of LC‐GC‐FID in three to five animals/sex per time point from the high‐dose group and control animals, sacrificed at day 30, 60, 90 and 132 in liver, MLN, adipose tissue, spleen and lung. Tissue levels were in general three to five times higher in female than in male rats. The highest levels were measured in MLN at 60 days of treatment (average levels of 1,010 and 234 mg/kg in female and male rats, respectively), followed by adipose tissue, liver spleen and lung. Most of the increase in liver content of MOSH occurred during the first 30 days of exposure (average levels of 195 and 46 mg/kg in males and females, respectively) followed by a slight increase up to 90 days (242 and 72 mg/kg), and a substantial reduction in the recovery period (21 and 6 mg/kg), but it was still slightly above background (8–10 mg/kg in females and 4–5 mg/kg in males through the various time points). In the MLN, the reduction during the recovery period was much less than that seen in the liver. Further chemical characterisation of the wax administered and MOSH found in tissues from female animals was conducted by means of GC × GC ‐MS/FID. Of the wax administered, n‐alkanes constituted the majority, followed by iso‐alkanes and monocyclic alkanes (see typical alkane distribution in Table 1 of EFSA CEP Panel, 2023). In the liver, there was an enrichment in the fraction of substances in n‐C31‐35 and in iso‐alkanes (67% at 132 days), whereas the relative amounts of n‐alkanes (33% at 132 days) and monocyclics (< 1% at 132 days) were reduced. In MLN, adipose tissue, spleen and lung, the fraction of substances in n‐C16‐C25 was enriched. Besides an increase in n‐alkanes in adipose tissue, less changes were observed in chemical composition with regard to relative amounts of n‐alkanes, iso‐alkanes and monocyclics in MLN, spleen and lung. This would suggest that in contrast to F344 rats wax n‐alkanes are not resistant to biotransformation and elimination in SD rats.
Table 1.
Detailed information on the composition of the five mineral oils tested for genotoxicity in Tarnow et al. (2016). Information on individual and groups of substances derived via GC × GC ‐MS (personal communication)
| No (a) | Amount of MOAH in % | Number of carbon atoms (MOAH) | Composition (MOAH) | Result Comet assay |
|---|---|---|---|---|
| 1 | 27.3 | 14–24 |
|
Negative |
| 3 | – | – | – | Negative |
| 4 | 25.7 | 13–34 |
|
Negative |
| 9 | 20.5 | 13–21 |
|
Positive |
| 13 | 57.1 | 13–23 |
|
Positive |
Mineral oil number from Tarnow et al. (2016).
MOAH
No new data available.
Human observations
MOSH concentrations in various tissues were determined in a study on humans from Austria (n = 37) undergoing autopsy, using on‐line LC‐GC‐FID (Barp et al., 2014). The individuals had a mean age of 67 years, ranging from 25 to 91 years (an extended summary of the study is provided in Appendix C). MOH were determined in subcutaneous abdominal adipose tissue, MLN, spleen, liver and lung, and in some cases in kidney, heart and brain.
No MOAH were detected even for tissues with very high MOSH concentrations. Detection limits for MOAH ranged from 0.5 to 5 mg/kg across different tissues.
The MOSH concentrations varied greatly in all tissues investigated. The highest levels were found in the spleen (1,400 mg/kg) and the MLN (1,390 mg/kg), followed by the liver (900 mg/kg). Median values were 166, 71, 87, 28 and 7 mg/kg for MLN, liver, adipose tissue, spleen and lung, respectively. Those in heart and kidney were both 6 mg/kg; in the brain, they were below the detection limit of around 2 mg/kg.
Generally higher amounts of MOSH were found in females in comparison with males.
The composition of MOSH in the adipose tissue was similar to that reported in the previous opinion of the CONTAM Panel (EFSA CONTAM Panel, 2012). The bulk formed a hump of unresolved, highly isomerised hydrocarbons ranging from about n‐C16 to n‐C36 and centred at around n‐C23. There were also diterpenes and n‐alkanes, according to the study authors transiently occurring and originating from ongoing exposure to dairy products and foods of plant origin. The MOSH composition in the MLN was similar to that in adipose tissue, but different from that in liver and spleen.
The gas chromatograms obtained from liver and spleen showed similar composition, i.e. a hump of unresolved hydrocarbons centred on n‐C27 ranging from n‐C20 to n‐C46 with hardly any compounds forming peaks on top, such as terpenes or n‐alkanes. The MOSH in kidney, heart and lung had a pattern between those of adipose tissue, liver and spleen.
The accumulated MOSH were further characterised by GC × GC and compared with the composition of four mineral oil products to which humans are likely to be exposed (batching oil, hydraulic oil, motor oil and paraffin oil; Biedermann et al., 2015). The MOSH fraction in these four MOH mainly differed by the presence or absence of n‐alkanes and the cloud of unresolved components, presumably due to differences in refining processes during the manufacturing of mineral oil products (primarily naphthenes resulting from the hydrogenation of MOAH).
The analysis of extracts from the liver and spleen confirmed that most MOSH components forming distinct signals in the chromatograms of the oils were absent. Hence, many branched and cyclic MOSH, including steranes and hopanes, appear to be eliminated, whereas some highly isomerised iso‐alkanes and naphthenes resist elimination. There was similarity between the GC × GC plots obtained from human tissues with those obtained from the F344 rats exposed to the L‐C25 oil (Barp et al., 2017b).
3.1.1.1. Kinetic modelling
There is no new information identified on toxicokinetic modelling.
3.1.1.2. Human relevance of data on accumulation of MOSH in experimental animals
The new studies in experimental animals showed that the tissue concentrations relative to the external dose decreased with increasing doses. Furthermore, the composition of the MOSH found in the organs differed from that to which the rats were exposed, indicating differences in the elimination rates for different types of hydrocarbons. Finally, the composition of the retained MOSH material in liver and spleen was similar but differed from that in adipose tissue. A similar difference in retained material between organs was seen in human tissues.
In contrast to liver and spleen of F344 rats, n‐alkanes and other saturated hydrocarbons with a straight chain alkyl group (e.g. n‐alkyl cyclo‐hexanes and ‐pentanes) were virtually absent in human liver and spleen, though present in adipose tissue of both, humans and F344 rats. Assuming that differences between F344 rats and humans in the tissue distribution of the various types of MOSH are small, the findings suggest that, as the main difference, all n‐alkanes are efficiently eliminated from liver and spleen in humans, whereas only those up to n‐C25 were eliminated in F344 rats. In F344 rats, also n‐alkyl monocyclic naphthenes (other wax components) were only eliminated up to n‐C28. This phenomenon seemed to be confined to the F344 rat strain as confirmed by a recent study in SD rats exposed to paraffin wax. It has been hypothesised that above a critical mass the n‐alkanes (possibly the wax compounds) crystallise and are no longer accessible to biotransformation. The melting points of pure n‐C25 and n‐C30 are 54°C and 66°C, respectively; melting points of mixtures are lower. The retention in F344 rats, but not in other rat strains or humans would have to be explained either by impaired biotransformation capacity, building up higher wax concentrations that subsequently precipitate, by more rapid crystallisation or a combination of the two.
It appeared that at similar doses and duration of exposure, the retention of both iso‐ and cycloalkanes in the tissues was higher in the F344 than in SD rats. This is based on studies described above for SD rats given GTL oil (mainly isoalkanes) and N70H oil (mainly cycloalkanes) by Carrillo et al. (2021a,b) and F344 rats given L‐C25 (virtually free of wax components, containing iso‐and cycloalkanes above n‐C‐25) by Barp et al. (2017a,b). However, since the composition of the products administered may not be comparable, there is uncertainty in this comparison. In both rat strains, there seems to be a preferential retention of cycloalkanes (naphthenes) in comparison with iso‐alkanes.
In terms of molecular mass, the maximum retention of the MOSH in liver and spleen was at n‐C29, and the most prominent structures of the accumulated material were the highly branched paraffins and the alkylated naphthenes. This was in agreement for F344 rats, SD rats and humans (see Appendix C), as well as the trend of preferential accumulation of alkylated naphthenes compared to branched open chain alkanes mentioned above. Except for the n‐alkanes accumulated by the F344 rats, the molecular mass distribution and the compositional pattern observed by GC × GC of the MOSH residues in human organs appeared to be similar to those in F344 rats and SD rats.
The Panel noted that, based on the available data, the comparison of the lifetime MOSH accumulation in experimental animals and humans is associated with significant uncertainty. In order to estimate whether the differences in MOSH accumulation between rats and humans are within the range of common toxicokinetic differences (as covered by default interspecies toxicokinetic uncertainty factors), a human equivalent dose (HED) reaching similar tissue levels to those observed in F344 rats was calculated and compared with human exposure, as shown by Pirow et al. (2019).
HED were calculated for different tissues considering the following equation:
The details on the calculations are presented in Appendix E and discussed in Section 3.4.
For liver and spleen, the HEDs (based on median liver and spleen content as well as maximum liver content in humans) resulted in the range of the estimated dietary exposure levels. However, especially for adipose tissue, the HEDs are approximately one order of magnitude higher than the estimated dietary exposure. Hence, for the extrapolation of the dose/organ content relation from F344 rats to humans, the allometric scaling factor of 4, used as default value to account for toxicokinetic differences between rats and humans (EFSA Scientific Committee, 2012), appears to be sufficient for MOSH accumulation in liver and spleen. This conclusion is consistent with the assessment of Pirow et al. (2019). For adipose tissue, an additional scaling factor might be needed, acknowledging that the MOSH level in this tissue could reflect the long‐term accumulation in humans compared with the relatively shorter exposure period in the rat studies.
For other rat strains (e.g. SD rats), accumulation of MOSH in the organs is significantly lower than in F344 rats, likely due to the lower capacity of F344 rats to eliminate not only n‐alkanes above C25, but also certain other types of MOSH. Hence, for extrapolation from these strains to humans, an additional scaling factor might be needed for all organs.
3.1.2. Toxicity in experimental animals
3.1.2.1. Acute toxicity (single exposure)
The previous opinion concluded that the acute toxicity of MOSH and MOAH was low to moderate in laboratory animals (EFSA CONTAM Panel, 2012). One report of an acute oral toxicity study has been published (reviewed by Boogaard et al., 2017). SD rats (male and female) were treated with GTL products to cover the entire range of carbon chain lengths (C4‐C70). The study was carried out according to OECD guideline 420 (acute oral toxicity – fixed dose procedure). No acute toxicity was observed and an LD50 was determined as > 5,000 mg/kg bw. Two waxes, GTL light waxy raffinate and GTL wax, were also tested for acute oral toxicity (OECD guideline 401) and no toxic effects were observed up to the limit of 5,000 mg/kg bw.
3.1.2.2. Repeated dose toxicity studies
Summary from the previous CONTAM Panel opinion
The assessment of the subchronic and chronic toxicity of MOSH and MOAH was performed using a series of studies on mixtures and single substances.
For MOSH, subchronic oral dosing of light individual alkanes or petroleum products ranging from n‐C10 to n‐C14 and content of aromatic hydrocarbons < 0.5–2% showed the increased incidence of α2u‐globulin‐mediated nephrotoxicity in male rats at all tested doses, and increased liver weight and hepatocellular hypertrophy. The renal effects were considered as not relevant for humans and a lowest NOEL of 100 mg/kg bw per day was identified for the hepatocellular hypertrophy, that, in the absence of other pathological changes, was considered as an adaptive change of no or minimal adversity by the CONTAM Panel.
A series of subchronic oral toxicity studies of highly refined mineral oils and waxes in F344 rats showed accumulation in a dose‐related fashion in the liver and mesenteric lymph node (MLN). For some oils and waxes (in particular class II and III low‐ and medium‐viscosity mineral oils and LMPW, see also Section 1.3.4), the accumulation was associated with the formation of granulomas and microgranulomas in the liver and histiocytosis/microgranulomas in the MLN (see e.g. Baldwin et al., 1992; Firriolo et al., 1995; Smith et al., 1996). The microgranuloma and granuloma formation in the liver was associated with focal aggregations of macrophages surrounded by inflammatory cells and occasionally necrotic cells as well as fibrosis, whereas in the MLN, it consisted of macrophage accumulation without inflammatory changes or findings of necrotic and fibrotic tissues. The presence of microgranulomas/histiocytosis in MLN was considered as a non‐specific, adaptative change of low toxicological concern by the CONTAM Panel. These effects were specifically observed in F344 rats, with females showing a higher sensitivity than males (see e.g. Smith et al., 1995).
Sparse data are available in SD rats and Long Evans rats, generally indicating a lower toxicity than in F344 rats. For instance, in a comparative study in which F344 rats and SD rats were exposed to a low viscosity paraffinic oil, increased incidence of granulomas was observed in F344 rats at ≥ 161 mg/kg bw per day, minimal signs of liver inflammation were reported for SD rats at the highest tested dose of 1,624 mg/kg bw per day (Firriolo et al., 1995).
Although a chronic study carried out for two high viscosity oils did not show any progression of the inflammatory changes observed following subchronic exposure in female F344 rats, the CONTAM Panel concluded that the incidence of liver microgranulomas could be potentially relevant to humans and was selected as the critical effect for the risk assessment of MOSH (see Section 1.3.4 for further details).
For MOAH, 13‐week of exposure by gavage to a heavy paraffinic distillate aromatic extract (defined as composed by ‘77.7% total aromatics, of which 37.2% < 3 ring PAH, 23% 3–5 ring PAH, 12.8% sulfur containing‐PAC, 2.3 % nitrogen containing‐PAC, and 1.6 non‐basic’) in SD rats caused a series of adverse effects at both the tested doses (125 and 500 mg/kg bw per day), including haematological changes (decreased red blood cell count, haemoglobin concentration and haematocrit), and histopathological changes including atrophy in the thymus, prostate and seminal vesicles, fibrosis and decreased cellularity in the bone marrow and hepatocyte necrosis and vacuolation (API, 2003; ECHA, 2012b). In another study, 13 weeks of exposure of rats and mice to a jet fuel‐aromatic extract caused slight decreases in red blood cell count, haemoglobin concentration and haematocrit in rats, with a NOAEL of 20 mg/kg bw per day. No haematological changes were observed in mice (Smith et al., 1999). A series of oral toxicity studies on individual MOAH including non‐alkylated aromatic hydrocarbons (naphthalene, fluorene and pyrene), alkylated aromatic hydrocarbons (1‐ and 2‐methylnaphthalene), partially hydrogenated aromatic hydrocarbons (tetralin) and sulfur‐containing aromatic compounds (Benzo[b]thiophene) were also assessed by the CONTAM Panel. While these studies showed several different effects and potencies, none of the individual substances could be identified as representative of the complex MOAH mixture relevant for human exposure.
New studies
MOSH
McKee et al. (2012) examined the effect of treating female F344 rats with a white P7H mineral oil (carbon number range n‐C14 to n‐C32; average n‐C20; 48% < n‐C20) at 0.02%, 0.2% and 2% (equal to average doses of about 17, 170 and 1,690 mg/kg bw per day according to the authors, respectively) for 90 days in the diet (See study description in Section 3.1.1). There were no adverse effects observed at 17 mg/kg bw per day, considered as NOAEL by the authors. At the two highest doses, increases in liver and MLN weights were seen. At the highest dose, there was an increase in absolute numbers of neutrophils and limited evidence of microgranuloma formation in the liver. Serum liver enzymes and proteins were within the normal physiological range. The highest MOH levels were measured in the liver and MLN (3,000 +/− 329 mg/kg in the liver and 1,860 +/− 389 mg/kg in the MLN in the highest dose treatment). The analysis revealed that the fraction corresponding to n‐C15 to n‐C20 constituents was under‐represented in the liver in comparison with the test oil.
Nygaard et al. (2023) assessed the relationship between the toxicity of MOSH and the accumulation in rat liver as part of the EFSA‐sponsored study conducted in female F344 rats mentioned above (Barp et al., 2017a,b, see detailed study description in Section 3.1.1). In the first experiment with the broad MOSH mixture, rats were exposed to 2.6, 25 and 260 mg/kg bw per day for 30, 60, 90 or 120 days. An additional group was exposed for 90 days and kept for a 30‐day depuration period before sacrifice (Barp et al., 2017a; Nygaard et al., 2023). General toxicity (body weight gain and feed intake) was not affected by the treatments. At the highest dose, statistically significant increases in absolute and relative liver weights were observed at 30, 90 and 120 days. In the group fed for 90 days +30 days of depuration, these increases were no longer observed, indicating a reversible effect. MOSH concentrations in the liver at 120 days were 30, 220, 1,604 and 5,511 mg/kg liver for the control feed and the 40, 400 and 4,000 mg/kg dose, respectively. The liver granuloma density increased at 90 and 120 days in the highest dose group and was still observed at this dose after 30 days depuration. There was an apparent exponential relationship between accumulation of MOSH and granuloma density (number of granulomas/cm2). Lymphoid cell clusters were observed in the liver of rats from the middle‐ and high‐dose groups, but there was no dose–response relationship, and it was unclear whether these effects were treatment‐related.
In the second experiment (Barp et al., 2017b; Nygaard et al., 2023; see detailed study description in Section 3.1.1), female F344 rats were exposed to S‐C25 (23, 61 and 226 mg/kg bw per day), L‐C25 (25, 62 and 236 mg/kg bw per day) and L‐C25W (22, 53 and 218 mg/kg bw per day) for 120 days. No effects were observed after treatment with L‐C25, despite accumulation of MOSH in the liver. Groups exposed to S‐C25 showed a statistically significant increase in absolute liver weights at the top dose only as well as in absolute spleen weights and relative liver weights at all tested doses. The L‐C25W oil/wax mixture caused statistically significantly increased absolute liver and spleen weights as well as relative liver weight at all tested doses. In the S‐C25 group, granulomas were observed at the highest dose. No granulomas were seen in the L‐C25 groups. In the L‐C25W group, granulomas were seen in all treated groups. Lymphoid clusters in the portal tracts and parenchyma were seen at all doses of L‐C25W and in the highest dose of S‐C25. Liver cell vacuolisation was seen in the L‐C25W and the highest dose S‐C25 groups. Also needle‐shaped open clefts were observed that could represent precipitated n‐alkane crystalloid material. No such effects were observed in L‐C25‐treated animals.
Summarising the Nygaard et al. (2023) studies, exposure to MOSH increased the weight of the liver and spleen associated with the MOSH accumulation. In addition, granuloma formation and vacuolisation associated with lymphoid cell clusters occurred. These effects varied with the chemical composition of the administered MOSH and/or the accumulated MOSH. For S‐C25, no NOAEL could be identified for increased absolute spleen weight and relative liver weight (LOAEL of 23 mg/kg bw per day); for L‐C25, a NOAEL of 236 mg/kg bw per day was identified, the highest tested dose; for L‐C25W, no NOAEL could be identified for increased absolute spleen as well as absolute and relative liver weights, and incidence of liver granuloma (LOAEL of 22 mg/kg bw per day).
Male and female SD rats were fed a diet of nominal doses of 0, 0.2, 2 and 10 g/kg bw per day paraffin wax (EFSA CEP Panel, 2023) for 13 weeks with a further 6‐week recovery period in a study performed in accordance with OECD TG 408 (ERBC, 2022). Measured doses in the animals were 0.19, 1.9 and 9 g/kg bw per day for male rats, and 0.24, 2.0 and 10 g/kg bw per day for female rats. No changes were observed in any of the parameters measured, except for an increase in MLN weights achieving statistical significance in females from all treatment groups. At necropsy, aggregates of macrophages (granulomas) were found in MLNs, with a higher incidence and severity (expressed in terms of pathology scores) in females compared to males and with a dose–response relationship. After the 6‐week recovery period, the aggregates were still observed in females treated at 10 g/kg bw per day, but were reduced in severity. Changes in MLN weights and macrophage accumulation in MLN were considered not adverse by the study authors. In view of the low biological relevance for the changes in MLN (see Section 3.1.4), the CONTAM Panel identified a NOAEL of 9 g/kg bw per day.
A series of repeated toxicity studies on GTL oils and waxes were reported in a review paper by Boogaard et al. (2017) and are summarised here below.
The GTL base oil was tested at 0, 50, 200 and 1,000 mg/kg bw per day in SD rats by gavage in a 90‐day toxicity study. No toxicity was observed. There was some accumulation of alveolar macrophages with vacuolated cytoplasm in the lungs of rats exposed to the two highest doses. These changes did not regress with depuration. In the female rats, MLNs showed vacuolated histiocytes, which did not regress. Both effects were considered as not biologically relevant by the study authors. The effects in the lungs were attributed to the aspiration of the test material during the gavage procedure. The vacuolated histiocytes observed in the MLNs were interpreted as a response to poorly absorbed material. The CONTAM Panel agreed with the conclusions of the authors.
In a follow‐up, 28‐day repeated dose dietary toxicity study in SD rats, using a GTL base oil ranging from n‐C18 to n‐C30 at 0, 750, 3,750 and 15,000 mg/kg feed (equal to average dose levels of 0, 63, 308 and 1,267 mg/kg bw per day according to the authors), there were no effects on the lungs. Mild to moderate apoptosis and necrosis in the lamina propria, crypts of duodenum, jejunum and ileum were observed in male rats at the highest dose. Minimal lymphocytosis was seen in the spleen of males of the highest dose group as well as in MLN and Peyer's patches in males at the low and mid dose, which changed to moderate at the highest dose.
Wistar rats (male and female) were treated by gavage for 28 consecutive days with 0, 30, 300 and 1,000 mg/kg bw of the GTL residual oil (n‐C40 to n‐C70, branched, cyclic and linear alkanes) dissolved in polyethylene glycol 400. Two recovery groups were treated with the highest dose for 28 days, then for a further 14 days without treatment. Haematopoiesis in the spleen of female rats was observed at 300 and 1,000 mg/kg in the absence of haematological changes. This effect was reversible. No other toxicological, histological or behavioural effects were seen.
Three GTL waxes were fed to female F344 and SD rats to compare the effects of waxes on these rat strains (Boogaard et al., 2017). The Sarawaxes SX30 (C18‐C25), SX50 (C19‐C36) and SX701 (C25‐C48) were fed to F344 rats for 90 days at 0, 0.002%, 0.02%, 0.2% and 2% (equivalent to 1.8, 18, 180 and 1,800 mg/kg bw per day) and SX701 at 5% (4,500 mg/kg bw per day). An additional group of F344 rats was fed with the 2% dose to enable the measurement of the tissue concentrations of saturated hydrocarbons. SD rats were fed with all waxes only at 0% and 2% to compare the two strains. No general signs of toxicity (body weight, food intake, behaviour, condition) were observed in either strain. The absolute and relative liver weights were increased in F344 rats fed 2% SX50, 2 and 5% SX701. In SD rats, there was an increase in relative liver weight with 2% SX30 only. In F344 rats, the absolute and relative weights of MLNs and spleen increased after feeding 2% SX30, 0.2 and 2% SX50 and 0.2, 2 and 5% SX701. Absolute and relative weights of MLNs increased in SD rats fed 2% SX30 and SX50, while only the absolute MLN weight increased with SX701. There was no change in spleen weight in SD rats. Histological treatment‐related effects were observed in F344 rats with microgranulomas in the liver after treatment with SX50 and SX701 at greater than 0.2% and vacuolisation in the periportal region with SX701 at 2% and 5%. Foci of inflammatory cells were also noted after treatment with SX701 at low concentrations. Similar foci were observed in SD rats at 2% SX50 and SX701. No liver lesions were seen in F344 or SD rats with SX30. Histiocytosis, reactive nodes and adenitis were seen in F344 rats after feeding the highest doses of all three waxes and 5% SX701. Heart lesions, including increased basophilia of mitral valve, were noted after treatment with 2% SX50 and 2% and 5% SX701. Saturated hydrocarbons from SX50 and SX701 accumulated in the liver of F344 rats. Similar accumulation was found in MLNs with all three waxes.
In a separate 90‐day repeat dose study, the waxes SX 701, SX 702 (C25‐C48) and SX100 (C38‐C90) were tested. SX 701 was administered at 2% and 5%, SX 702 and SX 100 at 0.002, 0.02, 0.2 and 2% (equivalent to 1,800 and 4,500 mg/kg bw per day for SX701 and 1.8, 18, 180 and 1,800 mg/kg bw per day for SX702 and SX100) in feed to both F344 and SD rats. In addition, a 4‐, 8‐ and 13‐week study and a 13‐week study with 12‐week reversal was carried out with F344 rats at 5% SX701 and with SD rats at 2% SX701. No effects were observed except at 13 weeks with 5% SX701, where red staining on the face and around the genital area and unkempt fur was noted in F344 rats. This improved during the reversal period. Absolute and relative liver and spleen weights increased in all treated F344 rats. In SD rats, absolute and relative MLN weight increased. SX702 administered to F344 rats caused a relative increase in spleen weight at 0.02–2%. Liver and spleen in SD rats were not affected. Treatment‐related lesions in liver, MLNs and mitral valve of the heart were observed in F344 rats with SX 701. Lesions in MLNs were noted with SX 702, with the exception of the lowest dose. The severity of all lesions decreased with reversal. SX100 showed no evidence of lesions.
In a recent study, Carrillo et al. (2021a,b) compared the tissue accumulation and retention of a naphthenic white mineral oil product with a GTL oil product of similar carbon range distribution (for details, see Section 3.1.1). Groups of female SD rats received a diet spiked with 3,000 mg/kg (∼ 200 mg/kg bw per day) of either of the two oils for 134 or 90 days followed by 44 days on a control diet. Average tissue levels were highest for the 134 days mineral oil group (660 mg/kg in caudate liver lobe, 51 mg/kg in visceral fat tissue, 0.73 mg/kg in blood and 430 mg/kg in MLN; the spleen content not measured). At all‐time points and for all dose groups, no statistically significant change in body weight, absolute or relative organ weights (liver, MLN, spleen) were observed. In addition, no clinical alterations or gross pathological lesions were found.
The GTL naphtha studies were not considered, as the molecular mass range fell outside the scope of this Opinion.
MOAH
In the context of an application for the use of paraffin wax as Food contact material (ERBC, 2022), male and female SD rats were fed a diet of nominal concentrations of 0, 0.2, 2 and 10 g/kg bw per day paraffin wax (FCM 93 58) for 13 weeks with a further 6‐week recovery period in a study performed in accordance with OECD TG 408 (ERBC, 2022; see Section 3.1.2.2). The top daily dose achieved was 9 g/kg bw and 10 g/kg bw for male and female rats, respectively.
Seven batches of this wax were reported to contain different concentrations of MOAH ranging from the limit of detection up to a maximum of 1.14% (see EFSA CEP Panel, 2023 for additional information). The MOAH was comprised principally of alkylated‐benzenes and naphthalenes (1–2 ring MOAH). Although no signs of toxicity were reported at any dose level studied (other than non‐adverse changes in MLN, see Section 3.1.2.2), a NOAEL for the MOAH constituents could not be determined since the specific batch of wax employed in this toxicity study was not defined in terms of MOAH content.
Summary
Overall, the new studies on MOSH in F344 rats confirmed the previous findings of granuloma formation in liver and MLN, associated signs of inflammation, and of increased liver, spleen and MLN weights. These effects are F344‐specific and likely to be primarily related to a higher tendency for F344 rats to retain n‐alkanes compared to other test animals (e.g. SD rats). Increased organ weights and incidence of liver granulomas with local inflammatory signs were associated with paraffin‐containing MOSH. However, L‐C25, a deparaffinated MOSH product (treated to minimise the presence of wax components including n‐alkanes) in the range of n‐C25 to n‐C50 L‐C25, did not induce organ weight changes and formation of liver granulomas up to the highest tested dose of 236 mg/kg bw per day. This was further supported by studies with synthetic waxes.
Limited data were available on MOSH and synthetic GTL oils in test animals other than F344 rats, including SD, Wistar and Long Evans rats and Beagle dogs. The data showed a lower sensitivity compared to the effects observed in F344 rats. In SD rats, minimal signs of liver inflammation were reported at 1,624 mg/kg bw per day following subchronic exposure to a paraffinic oil. In a subacute dietary study with a synthetic GTL oil in SD rats, mild to moderate apoptosis and necrosis in the intestinal mucosa were observed at the highest tested dose of 1,267 mg/kg bw per day. Notably, a new subchronic study in SD rats with a paraffin wax (FCM 93 58) caused no adverse effects up to the highest tested dose of 9 g/kg bw per day. The tested paraffin wax is comparable in term of physicochemical properties to LMPW, which was the most potent MOSH product in F344 rats (see Section 1.3.4).
A general overview comparing the main findings recorded in repeated dose toxicity studies on white mineral oil products for F344 rats and other strains/species is available in Appendix F.
The Panel noted that studies of waxes, including synthetic GTL waxes, in F344 rats may have low relevance to humans due to their specific sensitivity to wax components. The possible mode of action and human relevance of the effects of MOSH in F344 rats is further discussed in Section 3.1.4.
Regarding MOAH, the new study on the effect of a paraffin wax in SD rats gave a NOAEL of 9 g/kg bw per day. Although batches of this wax were found to contain variable concentrations of 1–2 ring MOAH, the actual level of MOAH in the studied wax was not clear and a NOAEL specifically for the MOAH content could not be established.
3.1.2.3. Genotoxicity
Summary from previous CONTAM Panel opinion.
In the previous EFSA Opinion on mineral oils (EFSA CONTAM Panel, 2012), it was established that all unrefined mineral oils were mutagenic when tested in Salmonella Typhimurium (Ames test with metabolic activation). The MOAH, in particular including alkylated polycyclic aromatic hydrocarbons of three to seven fused rings and those containing sulfur (such as thiophenes), were concluded to be responsible for mutagenicity and for formation of DNA adducts in mouse skin. Non‐mutagenic refined mineral oils can also become mutagenic after use or when subjected to high temperatures. In contrast, various low molecular weight MOSH mixtures were negative in Ames tests. Some samples were also tested for chromosomal aberrations, sister chromatid exchange in vitro or production of micronuclei in vivo; all these tests were negative. Furthermore, mixtures of alkanes with a low content of aromatics were shown to be negative in the Ames test.
New studies
Since the previous EFSA Opinion, Tarnow et al. (2016) provided more information on genotoxicity of mineral oils. Two out of the five mineral oils tested weakly positive for the production of DNA strand breaks in the Comet assay in human epithelial keratinocytes. Information on composition of the MOAH fraction of the respective mineral oils is given in Table 1 (personal communication). Two oils (identified as number 13 and number 9 in Table 1) gave positive results in the Comet assay, showing similar levels of DNA breaks. Oil number 13 had the highest MOAH content (57.1%). However, the Oil number 9 contained less MOAH than the two other MOAH‐containing oils, which were negative in the Comet assay (Table 1). Although no association between composition of the MOAH containing mineral oils and result in the Comet assay is apparent from the study, the findings do not contradict the conclusions by EFSA CONTAM Panel (2012) that genotoxicity is associated with some 3 or more ring MOAH. Hochegger et al. (2022) used preparative LC to separate different MOSH or MOAH fractions of a mineral oil (MOLTOX reference oil no.1) and tested DMSO extracts of the fractions for mutagenicity in an Ames test employing S. Typhimurium strain TA98 in the presence of metabolic activation (S9). The total MOSH fraction was negative in this assay while the total MOAH fraction was positive. Using subfractions of MOAH, it was found that the fraction of 1‐ and 2‐ring MOAH were negative but the fraction of three or more aromatic ring constituents was tested positive for mutagenicity.
Wang et al. (2022a,b) tested the genotoxicity of methylated phenanthrenes as representatives of alkyl‐substituted PAHs presumably present in MOAH. The following nine alkyl‐substituted derivatives of phenanthrene were investigated: 1‐methyl‐phenanthrene, 2‐methyl‐phenanthrene, 3‐methyl‐phenanthrene, 9‐methyl‐phenanthrene, 2‐ethyl‐phenanthrene, 9‐ethyl‐phenanthrene, 10‐methyl‐9‐ethyl‐phenanthrene, 1‐n‐hexyl‐phenanthrene, 1‐n‐dodecyl‐phenanthrene. The impact of alkylation on bioactivation was investigated for the four cited mono‐methyl‐phenanthrenes (methylated in four different positions) by means of mutagenicity testing in S. Typhimurium, using S‐9 mix from livers of Aroclor‐1,254‐treated SD rats. 1‐ and 9‐ methyl‐phenanthrene were positive in the S. Typhimurium mutagenicity assay using the strains TA100 and TA98, whereas phenanthrene and phenanthrene methylated in the two and three positions were negative. The authors hypothesised that for the mutagenic methyl derivatives, the methyl group substitution at or near the K‐region, i.e. 9,10‐position, of phenanthrene might create a bay region‐like structural motif and facilitate formation of a reactive bay region dihydrodiol‐epoxide. In spite of being positive for mutagenicity, the Panel noted that neither 1‐methyl‐ nor 9‐methyl‐phenanthrene were active tumour initiators in earlier skin‐painting tests (EFSA CONTAM Panel, 2012). The discrepancy may relate to a number of differences in the two systems (e.g. differences in induced liver vs. skin metabolism, and species differences).
Wang et al. (2022a) also report that with increasing chain length the intrinsic clearance (metabolism) decreased substantially for phenanthrenes with an alkyl side chain. With more than three carbon atoms, metabolism was limited or even not detectable (see Section 3.1.1). Wang et al. (2022b) showed that (as with phenanthrene) alkylation of benzo(a)pyrene shifted liver microsomal metabolic oxidation to the aliphatic side chain at the expense of aromatic ring oxidation and metabolism. In addition, aromatic ring oxidation was reduced with elongation of the alkyl side chain. In the Ames test with metabolic activation, methyl substitution of benzo(a)pyrene resulted in an increase or decrease of the mutagenic potency, depending on the substitution position. It can be predicted that reduced metabolism that results from increased side chain carbon number would reduce the chances of bioactivation to reactive metabolites of MOAH as would a shift to side chain oxidation. It should be noted that hydroxymethyl substituents may be subject to sulfation resulting in reactive sulfate esters.
McKee et al. (2013) noted from the literature that all of 43 different types of high boiling‐point petroleum products tested revealed bacterial mutagenicity in assays optimised for the detection of mutagenic PAC (MOAH). The optimisation included the use of DMSO to concentrate polycyclic aromatic compounds and the use of strain TA98 and hamster S‐9 mix. In contrast, a review of numerous cytogenetic tests showed that the results of the majority of studies on chromosomal effects in rodent bone marrow assays or in in vitro chromosomal aberration assays were negative, but details of these studies were not available.
The Panel noted that genotoxicity testing of oils as mixtures is less sensitive for assessing individual components, since their levels might be too low with respect to the sensitivity of the assays. To optimise sensitivity, EFSA recommends maximising the identification and testing of individual components where possible in addition to the testing of an uncharacterised fraction (EFSA Scientific Committee, 2019). In addition, the top concentration of a tested mixture may need to be higher than that recommended in the relevant OECD guideline for individual chemicals. However, for complex MOAH, such testing strategies are not feasible because of the low water solubility and limited possibilities to fractionate the mixture.
3.1.2.4. Chronic toxicity and carcinogenicity studies
Summary from previous CONTAM Panel Opinion
MOSH
A series of 2‐year oral rat toxicity studies on mineral oils and waxes used in food applications were reviewed in the previous assessment. The studies dated from 1962 to 2004, reflecting therefore possible differences in the degree of purity in respect to the residual aromatic fraction present in the products and the variability in the experimental design of long‐term bioassays. However, no evidence for treatment‐related increase in carcinogenicity was observed.
When tested in the mouse skin‐painting initiation/promotion model, mineral oil preparations with low or no detected PAH but containing longer chain aliphatic compounds were found to act as tumour promoters following initiation by a single treatment with a genotoxic compound.
MOAH
No oral toxicity studies were identified in the previous opinion for non‐food grade mineral oil products and as a result, carcinogenicity studies via other routes were used to provide some indication of the biological activity. In particular, the mouse skin‐painting studies constituted the main source of evidence to characterise the carcinogenic potential of MOAH. In general, studies on oils containing MOAH with more than three rings, as well as non‐alkylated and alkylated PAH with more than three rings were carcinogenic acting as initiating agents. The mutagenicity of MOH was concluded to be caused mainly by the three to seven ring MOAH, including (non‐alkylated) PAHs. Many MOAH with three or more aromatic rings can be activated by CYP450 to reactive genotoxic carcinogens. Some highly alkylated MOAH, including one‐ to two‐ring MOAH, could act as tumour promoters in the mouse skin model. Certain MOAH (e.g. naphthalene) were considered carcinogenic by a non‐genotoxic mode of action.
New studies
MOSH
Kuroda et al. (2013) examined the long‐term exposure of F344 rats (male and female) to Ozokerite (81% saturated hydrocarbons in the range of n‐C22 to n‐C38 and n‐C39 to n‐C58 saturated hydrocarbons as minor components; Ozokerite is a solid hydrocarbon wax used as a food additive and as an ingredient in cosmetic products) at 0, 0.05, 0.1 and 0.2% in the diet (equal to 0, 25.5, 50.3, 104.2 mg/kg bw per day for males and 0, 27.8, 54.9, 110.6 mg/kg bw per day for females) in a 52‐week study and at 0, 0.1 and 0.2% in the diet (0, 42.8, 86.7 mg/kg bw per day for males and 0, 47.8, 97.9 mg/kg bw per day for females) for a 104‐week carcinogenicity study.
In the 52‐week study, there were no deaths, and the general condition of animals showed no significant changes. There was a decrease in body weight gain from week 20 in males at the highest dose and changes in blood biochemistry parameters (increases in ALT and AST, as well as decreases in total protein, albumin and triglycerides) in males from 0.05% and in females from 0.1%. The absolute and relative weights of the lungs were increased in all treatment groups and in both sexes. Above 0.1%, the weight of spleen and liver increased in males and females, with increases in weights of kidney and testes in males at 0.2%. Histiocytosis was seen in liver and MLNs; granulomas and microgranulomas with crystalline material were present in liver and spleen together with hepatic vacuolisation in all treatment groups. There was also an increase in GST‐P positive foci in both sexes. In males, a dose‐related increase was observed, achieving statistical significance at all doses (mean number of GST‐P positive foci/cm2: 1.88, 4.53, 5.06 and 6.15 at 0, 0.05%, 0.1% and 0.2%, respectively). In females, a statistically significant increase was observed at 0.2% only, with a dose related increasing trend observed at lower doses (mean number of GST‐P positive foci/cm2: 0.43, 0.80, 1.23 and 1.77 at 0, 0.05%, 0.1% and 0.2%, respectively). Such foci are considered to be pre‐neoplastic (Sato et al., 1992). The authors considered the increase in GST‐P positive foci as secondary to the granulomatous chronic inflammation in liver of the F344 rats.
In the carcinogenicity study, increased absolute and relative weights of the lungs, liver and spleen were observed in males and females at both tested doses. Increased absolute and relative kidney weights as well as absolute testes weights were observed in males at 0.2%. The absolute and relative weight of the heart were increased in male rats at 0.2% and in all treated females. Increased incidence for the sum of hepatocellular adenomas, cholangiocarcinoma (1) and cholangiosarcomas (1) were observed in male rats at the two doses (Table 2). Considering the presence of pre‐neoplastic GST‐P positive foci observed in both sexes after 52‐weeks of exposure and the marginally significant increase in tumour incidence in the males only, the authors concluded that gender‐specific differences regarding the susceptibility to carcinogenicity induced by Ozokerite were unlikely. The CONTAM Panel noted that the increased incidence for the sum of tumours in male rats, reported by the authors, is actually driven by a statistically significant increase in hepatocellular adenomas without clear dose–response relationship, and only one case of cholangiocarcinoma and one case of cholangiosarcoma were observed at 0.1 and 0.2%, respectively. No information is reported on historical control data for the incidence of these malignant tumours in the testing laboratory. The authors reported that Ozokerite had tested negative for genotoxicity and concluded that a non‐genotoxic mode of action appears to be involved in the tumour formation. In the absence of evidence for genotoxicity of MOSH, as noted in the previous Opinion (EFSA CONTAM Panel, 2012), the Panel considered that a non‐genotoxic mechanism is likely (see Section 3.1.4).
Table 2.
Incidence of hepatic tumours in male and female F344 rats exposed to Ozokerite for two consecutive years (Kuroda et al., 2013)
| Males | Females | |||||
|---|---|---|---|---|---|---|
| Doses | 0 | 0.1% | 0.2% | 0 | 0.1% | 0.2% |
| Number of animals | 50 | 50 | 50 | 50 | 50 | 50 |
| Hepatocellular adenoma | 0 | 6 (a) | 5 (a) | 0 | 3 | 2 |
| Cholangiocarcinoma | 0 | 1 | 0 | 0 | 0 | 0 |
| Cholangiosarcoma | 0 | 0 | 1 | 0 | 0 | 0 |
| Total | 0 | 7 (a) | 6 (a) | 0 | 3 | 2 |
Statistically different from control (p < 0.05).
MOAH
No new information on chronic toxicity and carcinogenicity was retrieved for MOAH.
Summary
Overall, the long‐term oral studies on white oils summarised in the previous opinion did not indicate any concern of possible carcinogenic effects of MOSH. A new chronic and carcinogenicity study in F344 rats was retrieved on Ozokerite, a wax containing 81% saturated hydrocarbons in the range n‐C22 to n‐C38. In this study, 52‐week chronic exposure induced the typical effects observed in F344 rats in liver, spleen and MLN. In addition, treatment‐related increase in preneoplastic GST‐P‐positive foci was observed following 52 weeks of exposure and an increased incidence of hepatocyte adenomas, although with an unclear dose–response relationship in the only two tested doses, was observed in male F344 rats in the 104‐week carcinogenicity study. In addition to the hepatocellular adenomas, one case of cholangiocarcinoma and one case of cholangiosarcoma were reported in male rats treated at 0.1% and 0.2% Ozokerite, respectively. Noting the low incidence in one sex only and the lack of dose–response, the Panel concluded that the malignant tumours may not be treatment related. The authors concluded a non‐genotoxic mechanism appeared to be involved in the observed hepatocarcinogenesis and that the development of GST‐P positive foci and neoplastic changes were secondary to the chronic granulomatous inflammation caused by the liver granulomas and that this is a response specific for F344 rats. The CONTAM Panel agreed with this conclusion.
As no additional studies on MOAH were retrieved, the CONTAM Panel considered the conclusions from the previous opinion as still applicable.
3.1.2.5. Developmental and reproductive toxicity
Summary from previous CONTAM Panel Opinion
Only few oral studies on reproductive and developmental toxicity were retrieved and, therefore, information from studies performed via other routes of exposure were included in the assessment.
MOSH
Study summaries were retrieved from the ECHA website for two studies in SD rats, an oral developmental toxicity study (OECD 415 compliant) on a food‐grade white mineral oil product (CAS 8042‐47‐), and an oral screening reproductive and developmental toxicity study (OECD 421) on a lubricating base oil meeting the IP346 criteria (CAS 64742‐54‐7). No adverse effect on reproduction and development were reported from the summaries of these two studies up to the maximum tested doses of 5,000 and 1,000 mg/kg bw per day, respectively. Additional data from developmental toxicity tests in rats exposed by inhalation to various white spirits (mixtures with prevalent content of n‐C4 – n‐C13 saturated hydrocarbons and with aromatic hydrocarbons varying in the range 0.4–24% v/v), and from an oral reproductive study on n‐undecane in rats did not show any adverse effects on reproduction and development.
MOAH
Regarding MOAH, a study summary was retrieved for a developmental toxicity study in SD rats exposed dermally to a heavy distillate aromatic extract (DAE; CAS No 64742–04‐7, defined as a complex combination of hydrocarbons obtained consisting predominantly of aromatic hydrocarbons predominantly in the range of n‐C20 – n‐C50 and likely to contain ≥ 5% wt of four‐ to six‐membered condensed ring aromatic hydrocarbons.). The tested doses ranged from 8 to 1,000 mg/kg bw per day. Fetal toxicity was observed at > 30 mg/kg bw per day, with developmental anomalies observed at the top dose only. However, increased incidence of fetal late resorption was observed from 30 mg/kg bw per day, achieving statistical significance at higher doses. Maternal toxicity was observed at all tested doses (red vaginal discharge), with increased incidence of dams with no viable offspring, lower litter size, decreased maternal body weight gain and haematological changes observed at > 30 mg/kg bw per day.
In a gavage study according to OECD TG 414, the administration of tetralin up to of 135 mg/kg bw per day, as an example for partially hydrogenated aromatic hydrocarbons, did not cause developmental effects. No specific studies have been performed on the reproductive toxicity of tetralin. However, in order to determine whether tetralin may be a reproductive toxicant, vaginal cytology, sperm and reproductive organs were evaluated in subchronic inhalation studies with tetralin in rats and mice, respectively (OECD, 2004). Whilst there were no indications of adverse effects in rats, in mice uterus atrophy and atrophy of the ovary were found in the absence of other systemic toxicity.
New studies
MOSH – synthetic gas‐to‐liquid (GTL) oils
Unpublished studies on the developmental and reproductive toxicity of synthetic oils were extensively summarised by Boogaard et al. (2017). Two oils were tested, one being a GTL gas oil (C8–C26, branched and linear alkanes) and a GTL base oil (C18–C50, branched, cyclic and linear alkanes). Both products were tested for prenatal developmental toxicity and two‐generation reproductive toxicity, performed according to OECD TGD 414 and 416, respectively.
With the GTL gas oil, a 2‐generation study in line with OECD TGD no. 416 was performed in SD rats (25 rats/sex per group in the F0), dosed by gavage with 0, 50, 200 or 750 mg/kg bw per day. No effects were observed on reproductive performance, gestation and parturition of both the F0 and F1 generations. A statistically significant increase in the mean and adjusted age of attainment of balano‐preputial separation was observed in F1 males in the top dose group. The change fell within the historical control range and was considered as not adverse by the authors. No other treatment‐related effects were observed on F1 and F2 litter parameters. A statistically significant increase in the percentage of abnormal sperm of F1 males given 750 mg/kg bw per day was found, again falling within the historical control range and considered as not relevant by the authors. In addition, no effects were observed on the F1 reproductive performance parameters and no histological changes were reported in the testes. The control males from the F0 generation showed an unexplained low fertility with male mating indices. Histopathological findings revealed changes in the lungs of both males and females of the top dose group for the F0 and F1 generations. α2u‐globulin‐mediated nephropathy was observed in F1 males. The effects were consistent with those observed in subchronic studies of GTL naphtha and base oils (see Section 3.1.2.2). In addition, increased tubular mineralisation was observed in the kidney of F0 males dosed at 750 mg/kg bw per day and considered an equivocal effect by the authors. Overall, the Panel identified a NOAEL at the highest tested dose of 750 mg/kg bw per day.
For the GTL base oil, SD rats were dosed by gavage with 0, 50, 250 and 1,000 mg/kg bw following the same study design of the GTL gas oil study. Nephropathy associated with α2u‐globulin accumulation was observed in F0 males, consistently with the findings of subchronic toxicity studies in GTL oils (see Section 3.1.2.2). No adverse effects on reproductive performance were reported. However, a small but statistically significant decrease in the adjusted anogenital distance was observed in F1 females exposed to 250 and 1,000 mg/kg bw per day in comparison to controls, but not in F2 females. In the absence of other findings suggestive of adverse effects in the female reproductive performance, the authors concluded that the small decrease in the adjusted anogenital distance was not adverse and identified a study NOAEL at the highest tested dose of 1,000 mg/kg bw per day.
The prenatal developmental toxicity study for the GTL gas oil was conducted by administering 0, 50, 200 and 750 mg/kg bw per day by gavage to pregnant SD rats (24/group) between day 5 and 19 post‐coitum. No treatment‐related maternal or developmental effects were observed. The authors identified the highest tested dose of 750 mg/kg bw per day as the study NOAEL.
For the GTL base oil a prenatal developmental toxicity study was also conducted on pregnant RccHan:WIST rats administered 0, 50, 200 and 1,000 mg/kg bw per day by gavage to between day 6 and day 20 post‐coitum. No treatment‐related effects were reported with the exclusion of a slight increase in the body weight of live fetuses from the high‐dose group in comparison to controls, which was considered of no toxicological relevance. The authors identified the highest tested dose of 1,000 mg/kg bw per day as the study NOAEL.
MOAH
A summary for a new developmental toxicity study was retrieved in the ECHA dissemination website for light paraffinic distillate solvent, defined by the applicant to ‘consist predominantly of aromatic hydrocarbons in the range C15–C30 and likely to contain 5% or more of four‐ to six‐membered condensed ring aromatic hydrocarbons’ 9 (CAS number: 64742‐05‐8 10 ). In the study, performed according to OECD TGD 414, pregnant SD rats were exposed dermally to 0, 5, 25, 150, 450 mg/kg bw per day. Maternal toxicity, consisting of decreased food consumption and bw or bw gains and decreased absolute and relative thymus weights, was observed at ≥ 25 mg/kg bw per day. Developmental effects (increased post‐implantation loss with a corresponding decrease in the mean numbers and litter proportions of viable fetuses) were reported at 150 and 450 mg/kg bw per day groups. Lower mean male, female and combined fetal weights were reported at ≥ 25 mg/kg bw per day. Fetal developmental variations (unossified sternebrae, reduced ossification of the skull and vertebral arches) were noted at 150 and 450 mg/kg bw per day. A NOAEL of 5 mg/kg bw per day was reported for both maternal and fetal toxicity.
Studies relevant to prenatal developmental toxicity of various MOAH compositions were performed by Kamelia et al. (2017, 2019a,b). In a first study, Kamelia et al. (2017) applied the embryonic stem cell test (EST) on DMSO‐extracts from nine petroleum products and two MOAH‐free GTL oils. The nine petroleum products and the ‘PAH’ content of the related DMSO extracts are listed here below (as reported in Kamelia et al., 2019a):
one heavy fuel oil (HFO) sample – ‘PAH’ 48% w/w, mainly 3–5 ring PAHs;
three DAE samples – PAH ranging from 9% to 12% w/w, 4 and 5‐ring PAHs predominating in two samples, 5‐, 6‐ and ≥ 7‐ring ‘PAHs’ predominating in the third sample;
two gas oil (GO) samples – ‘PAH’ 4.2% and 5.5% w/w, 3‐ring PAHs predominating;
one vacuum tower overhead – ‘PAH’ 6.7% w/w, 2‐ and 3‐ring PAHs predominating; and
two residual aromatic extract (RAE) sample – ‘PAH’ 1.5% and 3.3% w/w, 5‐, 6‐ and ≥ 7‐ring PAHs predominating.
Mouse embryonic stem cell line D3 (ES‐D3) was used for the test and the inhibition of the cell differentiation into cardiomyocytes used as indication of embryotoxicity. ES‐D3 were exposed to the chemicals for 5 days (up to maximum concentrations of 500 μg/mL, with the exclusion of HFO sample, for which a maximum concentration of 50 μg/mL was attained. 5‐fluorouracil (0.065 μg/mL) was included as positive control. With the exclusion of GTL oil extracts, inhibited differentiation of ES‐D3 into cardiomyocytes was observed for all the tested samples. BMC50s calculated after day 1 and 5 of exposure showed a good correlation between the ‘PAH’ content and the embryotoxic potency of the product DMSO‐extracts. A good correlation was also observed with results of previous in vivo studies (decreased fetal body weight and increased fetal resorption). No inhibition of differentiation was observed for the two GTL oil extracts, confirming the absence of embryotoxicity described in the previous paragraphs.
The Panel noted that the ‘PAHs’ were determined by the method developed by Roy et al. (1988), i.e. by one‐dimensional GC. As explained in Annex B, these fractions included a high proportion of alkylated polyaromatic hydrocarbons and would be termed MOAH according to the definitions in Section 1.2. However, the fractions by ring number are neither complete nor well separated from fractions of lower ring number, i.e. the method underestimates the amount of the fractions of low ring number and overestimate those of higher ring number. Some of the tested fractions may not have contained the MOAH of higher ring number at all.
In a follow‐up study (Kamelia et al., 2019a), the same group of extracts was tested in vitro at non cytotoxic concentrations for the affinity to the Ah receptor and various hormone receptors, including the androgen receptor (AR), oestrogen receptor alpha (ERα) progesterone receptor (PR), thyroid receptor beta (TRβ), by means of the CALUX assay. Tested extracts containing relatively high levels of 4‐ to 7‐ring PAHs were more active (ant)agonists, showing anti‐androgen, anti‐oestrogen, anti‐progestogen and weak anti‐thyroid activities and strong AhR agonism. HFO was the most potent product, in line with its much higher content of ‘PAHs’. Conversely, no activity was observed for the GTL oils. The AhR activity of the different petroleum products well correlated with the in vitro results of the EST (Kamelia et al., 2017), suggesting a possible AhR‐mediated mode of action in the prenatal developmental effects of PAHs (see section 3.1.4).
Finally, Kamelia et al. (2019b) tested the same mineral oil product extracts in the zebrafish embryotoxicity test (ZET). All samples were tested at a range of concentrations up to 250 μg/mL, except for HFO, which was tested up to 15 μg/mL. Embryos were scored daily for mortality and developmental abnormalities for up to 96 h of exposure. With the exclusion of GTL oils, a dose‐dependent increase in fetal mortality, delayed development and malformations was observed for all the petroleum products. As for the EST, the HFO extract showed the highest potency and GTL oils did not induce prenatal developmental toxicity. A moderate correlation was observed between the results of the ZET, the results obtained with the EST and with the AhR‐mediated activity. Differences in potency ranking were observed comparing the EST and the ZET results. The authors attributed them to possible differences in solubility in the media used in the two assays, the possible activity of a different group of ‘PAH’ constituents, or the higher metabolic activity of the zebrafish embryos compared to the D3 cell line.
Summary
Overall, the evidence from studies assessed in the previous CONTAM Panel Opinion indicates no potential for MOSH to induce developmental and reproductive toxicity. New information on two synthetic GTL oils, showing no adverse effects on reproduction and development up to 750–1,000 mg/kg bw per day support the previous conclusion, although it is noted that GTL oils do not have the same composition as mineral oil‐derived products, particularly regarding the naphthenes.
Dermal studies on petroleum extracts containing three or more ring MOAH (heavy distillate aromatic extract and light paraffinic distillate solvent) showed fetotoxic effects with developmental NOAELs of 30 and 5 mg/kg bw per day, respectively. Conversely, an oral screening reproductive and developmental toxicity study with a lubricating base oil meeting the IP346 criteria (i.e. with a DMSO extract < 3% w/w) did not show any effect at the only oral dose tested of 1,000 mg/kg bw per day. A series of studies using alternative testing methods on various DMSO‐extracts from nine petroleum products showed a clear correlation between the developmental toxicity potency and the presence of three or more ring MOAH. However, the Panel also noted that the products tested may have contained less MOAH of the given ring number than assumed, as the applied analytical method was inadequate (see Section 1.3.1.2).
3.1.2.6. Immunotoxicity
Summary from previous CONTAM opinion
There were several reports on an autoimmune potential of high dose MOH exposures to animals, mainly by the injection route. In mice, a single injection of pristane (C19 isoalkane) induced auto‐antibodies and clinical signs relevant for human systemic lupus erythematosus (SLE). Similarly, a number of mineral oils acted as adjuvants by promoting elevated concentrations of autoantibodies. Another autoimmune endpoint, i.e. joint‐specific arthritis, has been reported to be induced in Dark Agouti (DA) rats by intradermal injection of squalene, pristane, medicinal white oils, as well as baby oils, cosmetic products containing MOH and incomplete Freund's adjuvant (the latter being an emulsion of water and mineral oil). Oral exposure to an intradermally potent white mineral oil did not give any arthritic reactions. Major limitations for these reports on autoimmunity induced by MOH in animal models are the application of high doses with a lack of dose–response studies, via injection and percutaneous routes. Overall, there is inadequate evidence in humans and in animal models for the promoting or exacerbating effect of relevant MOH exposure on autoimmune diseases, and no indication of altered immune function or autoimmunity after peroral exposures.
New studies
The EFSA‐sponsored project reported above (Cravedi et al., 2017; Nygaard et al., 2023) also investigated possible impact of a broad MOSH mixture and subfractions on the immune response in F344 rats. Keyhole limpet haemocyanin (KLH)‐specific IgM antibody levels in serum after KLH immunisation were determined. Overall, no significant effects on the KLH‐specific IgM concentrations in serum were observed after 120 days of exposure to the three doses of the broad MOSH mixture or to the three MOSH fractions, S‐C25, L‐C25 and L‐C25W. The KLH immunisation assay has been recommended as a useful marker of immunosuppressive or ‐stimulating effects in the OECD guideline 407 repeated dose 28‐day oral study in rodents. 11
In a second experiment, Andreassen et al. (2017) investigated the effect of dietary exposure to pristane or a MOSH mixture on the development of auto immune arthritis in arthritis‐prone Dark Agouti (DA) rats. The rats were given feed containing 4,000 mg/kg pristane (equal to 260 mg/kg bw per day for males and 244.5 mg/kg bw per day for females according to the authors) or the broad MOSH mixture described above in concentrations of 0, 40, 400 and 4,000 mg/kg feed (equal to 0, 2.9, 29.4 and 280.1 mg/kg bw per day for males and 0, 2.3, 25.7, 238.8 mg/kg bw per day for females according to the authors) for 90 days, or a single intradermal injection of 200 μL pristane as a positive control. Arthritis scores as well as serum and splenocyte markers were determined. There were no differences between controls and treated groups in body weight gain, no clinical arthritis symptoms and no increases in common arthritis‐associated biological markers in serum and spleen, i.e. splenocytes, toll‐like receptors 2 and 3, spontaneous cytokine release from splenocytes or cytokine release from splenocytes stimulated with lipopolysaccharide (LPS) or concanavalin A (ConA). However, all rats injected with pristane displayed arthritis symptoms and higher levels of associated serum markers, i.e. IgG‐RF, IL‐17. The Panel concluded that there is no evidence that dietary exposure to MOSH induces autoimmunity.
3.1.2.7. Cellular toxicity in vitro
In recognition of the difficulties in establishing the toxicity and modes of action of components of complex mixtures, House et al. (2021) assessed whether New Alternative Methodology (NAM)‐based biological activity fingerprints could distinguish between different categories of petroleum products. A total of 141 petroleum products were tested as DMSO extracts, using a dilution series in a range of cell types (liver, endothelial, cardio, neuronal and macrophage) in vitro. Bioactivity ‘signatures’ and points of departure were determined for a range of functional and toxicological end points (e.g. related to energy, oxidative stress, and altered physiology and cellular toxicity) to obtain a Toxicological Priority Index (ToxPi) for each category of substance.
Both overall and within groups, the 3–7 ring MOAH content (expressed as a proportion of DMSO‐extractable MOAH) of tested substances correlated strongly with bioactivity (ToxPi scores). Thus, the use of biological activity parameters across multiple cell types, combined with extant physico‐chemical properties, improves the ability to group and rank‐order petroleum products. The authors suggested that these approaches may contribute to develop read across in hazard identification and modes of action of different categories of petroleum mineral oils.
3.1.3. Observations in humans
Summary from the previous Opinion
In five human autopsy studies between 1950 and 1990, oil droplets, often together with macrophages, were observed in the liver and spleen. Also, lipogranulomas were reported in liver, spleen, lymph nodes and other organs. This was attributed to intestinal absorption and accumulation of MOSH in organs. In some studies, MOSH were extracted and analysed by thin‐layer chromatography or GC. In one study, correlations were found between MOSH contents and oil droplets observed in histopathological sections of the liver and spleen. There was little information on external exposure or tissue concentrations in relation to oil droplets observed.
Hepatic granulomas were investigated in two studies in liver biopsies in the periods between 1952–1953 and 1978–1980. There were significantly higher frequencies of lipogranulomas in the later period and were found in about 80% of the non‐fatty livers. Extracts from non‐fatty livers with lipogranulomas revealed presence of MOSH of a similar composition as commercial white oil products. The lipogranulomas have not been associated with inflammatory responses or clinical abnormalities, and the Panel considered their clinical significance as probably not of concern.
A case report describing a patient with a high dietary intake of natural long chain n‐alkanes from apples had granulomas with giant macrophagic cells containing crystalline inclusions of material in micronodules of the lung, possibly due to a defect in alkane degradation combined with a high intake of n‐alkanes.
In all four case reports describing single patients with disorders in liver (n = 3) and lung (n = 1) that reported in their medical history prior intake of MOH, no clear causal connection to MOH could be established.
Moreover, humans have been exposed to highly refined oils used as ingredients of pharmaceutical products in oral doses of up to 100 mL/day (approximately 1,500 mg/kg bw per day) over longer periods, without evidence of major adverse effects.
No human studies had been identified on the immunotoxic effects from oral exposure to mineral oils. However, two epidemiological studies reported associations between increased risk of autoimmune diseases and occupational exposure to high doses of mineral oils or exposure related to living near an oil field waste site, respectively.
Based on the above, the CONTAM Panel concluded in the previous Opinion that ‘lipogranulomas have been observed in humans in liver, spleen, lymph nodes and other organs, together with MOSH, but these changes are pathologically different from other granulomas and have not been associated with adverse consequences. There is no information on exposure levels at which these effects occur in humans.’
New studies
On the assumption of their biologically inert properties, food grade or pharmaceutical grade mineral oils have commonly been used as placebo in clinical trials assessing the efficacy of oil‐based agents, such as n‐3 polyunsaturated fatty acids. In a recent randomised double‐blind intervention study (REDUCE‐IT) (Bhatt et al., 2019) the impact of eicosapentaenoic acid ethyl ester (Icosapent ethyl) supplementation on plasma lipids and cardiovascular disease risk in patients with established cardiovascular disease or diabetes was investigated. The study population was in total 8,179 patients that were followed for 4.9 years. In comparison with the placebo group given 4 g of MOH (light liquid paraffin) per day, a 25% relative risk (hazard ratio) reduction in cardiovascular composite endpoint was observed in the actively treated group. As this effect could not be explained by the observed decrease in triglyceride (TG) levels, it was later discussed whether the beneficial effects noted were rather due to adverse effects related to the mineral oil placebo as there was an increase in the levels in LDL‐cholesterol and C‐reactive protein (CRP) (Kastelein and Stroes, 2019).
On the background of the observations made on mineral oil in the REDUCE‐IT study, Olshansky and co‐workers (2020) reviewed (outside a systematic review framework) this and other clinical trials that used MOH (pharmaceutical grade) as placebo. They identified 80 eligible studies in which the most common adverse events were considered unrelated to the study treatment including the MOH placebo; adverse effects related to MOH were abdominal pain, distention, and watery stools, all likely related to laxative properties. Mineral oil doses were mostly between 1 and 5 g per day. Only 28 studies reported changes in blood lipids or blood pressure from baseline to end of treatment; of these, 11 studies included participants with prior history of cardiovascular disease (CVD) or diabetes mellitus (DM), and 17 studies included healthy volunteers or participants with non‐cardiovascular conditions. Of the 11 studies on CVD and DM, 8 studies were relatively small (sample size range for the mineral oil study arm = 14–75) and of relative short duration (follow up duration range, 8–12 weeks). One study had 227 participants and lasted for 12 weeks (Ballantyne et al., 2012), while another study, REDUCE‐IT, had 4,090 participants receiving 4 g of MOH per day during a median period 4.9 years. While these latter two studies reported an increase in LDL‐cholesterol of 8.8% and 10.5% respectively, another six other studies showed lower increases and two studies showed a decrease or no change. HDL‐cholesterol increased in the two largest studies whereas there was no consistent change in TG level. In the 17 non‐CVD, non‐DM studies there were no consistent changes in blood lipids. In the REDUCE‐IT study, there was minimal association between changes in lipid levels and clinical outcomes. In 16 studies, high‐sensitivity C‐reactive protein (hsCRP), a marker of inflammation, was reported. There was no consistent change in this parameter in the groups receiving MOH. There were also no changes observed in seven studies measuring systolic blood pressure. Overall, there was an increase in LDL‐cholesterol of the two larger studies within the mineral oil placebo groups, while other changes in blood pressure, triglycerides, HDL‐cholesterol, high‐sensitivity C‐reactive protein, and other biomarkers were inconsistent.
In a newly published biomarker sub‐study of the REDUCE‐IT study discussed above (Ridker et al., 2022), atherosclerosis‐associated and inflammatory‐related biomarkers interleukin‐1β (IL‐1β), interleukin‐6 (IL‐6), high‐sensitive C‐reactive protein (hs‐CRP), oxidised low‐density lipoprotein cholesterol (OxLDL), homocysteine (HCys), lipoprotein(a) (Lp(a)) and lipoprotein‐associated phospholipase A2 (Lp‐PLA2) were investigated. At baseline, in both groups, the serum concentrations of the biomarkers were similar. After 12 and 24 months, in the mineral oil group, there were significant increases in all biomarkers: IL‐1β, 29%; hsCRP, 22%; Lp‐PLA2, 19%; IL‐6, 16%; OxLDL, 11%; Lp(a), 2%; and HCys, 1.5%. The authors noted that, in studies in similar populations receiving placebos that did not include mineral oil, no substantive biomarker changes were observed in the placebo groups (receiving various inert tablet placebos or corn oil) over long periods of time (Elkind et al., 2009; The STABILITY Investigators, 2014; O'Donoghue et al., 2014; Ridker et al., 2008, 2017, 2019; Nicholls et al., 2020; Nissen et al., 2021). The authors concludes that findings that icosapent ethyl had minimal effects on biomarkers associated with atherosclerotic disease and increases in these in the placebo group makes the interpretation of the study results unclear.
The CONTAM Panel noted that all the evidence described above, including data coming from the REDUCE‐IT megatrial and despite the fact that they are nested within randomised controlled trials are of an observational nature with considerable methodological limitations as they pertain to before‐and‐after (pre‐post) data without an appropriate control group even if similar changes were not seen similar populations from other studies using non‐MOH placebos. Hence, it is not possible unequivocally to determine whether mineral oil had an adverse impact on inflammation biomarkers important for cardiovascular diseases. Observations were inconsistent among other small studies of short duration using MOH as placebo.
3.1.4. Mode of action (MoA)
Summary from the previous opinion
In the previous EFSA Opinion (2012), it was concluded that granulomas, as observed in rat liver and mesenteric lymph nodes (MLN), were linked to accumulation of MOSH and appeared to be unique to F344 rats due to a high deposition of MOSH. MLN granulomas were considered an adaptive response and not adverse. The liver granulomas were associated with T and B lymphocyte influx and sometimes necrosis and fibrosis, unlike MLN in which no associated inflammation or necrosis was evident. Also in humans, lipogranulomas have been observed in liver, spleen, lymph nodes and other organs, attributed to intestinal absorption of MOH. This was in the absence of clinical abnormalities. It was not possible to exclude that the effects in F344 rat liver are relevant to human.
Although hydrocarbons can cause inflammation and act as adjuvants enhancing immune responsiveness, EFSA considered that autoimmune effects were not anticipated following oral exposure to mineral oil products, since no inflammation or formation of neo‐lymphatic tissue were evident.
Some MOAH containing three or more rings may be converted to metabolites that bind covalently to DNA and exert genotoxicity. In previous mice skin painting studies, the five tested monomethylated phenanthrenes did not induce tumours, while certain dimethyl phenanthrenes did (EFSA CONTAM Panel, 2012).
Highly alkylated MOAH and some long chain MOSH had tumour‐promoting activity (non‐genotoxic) in a two‐stage mouse skin carcinogenicity model. This was considered to be due to a cell proliferative response secondary to cell toxicity.
PAHs (non‐alkylated MOAH) can interact with the Ah receptor, which may also contribute to carcinogenicity. However, the concentrations of the PAH in MOAH are low.
Although MOH can cause nephropathy and kidney tumours specifically in male rats, the MoA is mediated by α2u‐globulin binding and not relevant to humans.
New studies
Carcinogenicity
Alkylation of the MOAH ring systems can block bioactivation into mutagenic metabolites, but also enhance bioactivation, depending on the position and degree of the alkylation (Wang et al., 2022a,b).
In the study of Kuroda et al. (2013), in which hepatic adenomas were observed in rats following exposure to MOSH, the evidence suggests that these effects were due to a MoA involving non‐genotoxic hepatic proliferation (see Section 3.1.2.4). The single cholangiocarcinoma and the single cholangiosarcoma also observed may not be treatment‐related.
Toxicity of MOAH
Regarding reproductive toxicity (see Section 3.1.2.5), Kamelia et al. (2017, 2019a,b) assessed the prenatal developmental toxicity of DMSO extracts of nine petroleum products containing different levels of ‘PAH’ (actually MOAH components) in comparison to two MOAH‐free GTL products, using three methods (mouse embryonic stem cell line D3 (ES‐D3) differentiation, affinity for AhR and hormonal receptors, and zebrafish embryotoxicity test). All extracts of petroleum products studied showed a concentration‐dependent potential for developmental toxicity, with the potency correlated with their three‐ to five‐ring MOAH content (though measured in a way considered inadequate by the Panel). In contrast, the GTL products did not show reproductive toxicity potential, which implied a role of MOAH in this effect.
The AhR responsiveness of MOAH was also demonstrated in the study by Tarnow et al. (2016), in which MCF‐7 cells treated with MOAH (54 μg/mL) showed an increase in transcripts of CYP 1A1, 1B1 and (to a lesser extent) 1A2, all of which are AhR responsive genes. Tsitou et al. (2015), in a review of the literature, considered that PAH have the potential to cause adverse birth outcomes and noted evidence from toxicogenomic studies that various PAHs have the ability to modify the expression of gene families (HOX, FOX, SHH and PAX) involved in development. The Panel noted that such effects could also be caused by non‐alkylated or alkylated polycyclic hydrocarbons within MOAH.
Toxicity of MOSH
The Panel noted further evidence of the role of α2u‐globulin in the causation of nephropathy in male rats in response to GTL products. Although proteins similar to α2u‐globulin have been detected in humans, lesions in the kidney characteristic of the nephropathy associated with α2u‐globulin in male rats have not been reported and it was concluded by Hard et al. (1993) that nephropathy caused by chemically induced α2u‐globulin accumulation in male rats is unlikely to occur in humans.
As part of the EFSA‐sponsored study, Nygaard et al. (2023) assessed in female F344 rats the effect of various MOSH fractions (0–4,000 mg/kg feed administered for up to 120 days; see Section 3.1.2.2). Vacuolisation and formation of granulomas with lymphoid cell clusters were observed in the liver. Liver and spleen weights were increased and were found to accumulate isoalkanes and alkylated naphthenes as well as n‐alkanes above C25. The liver granulomas appeared to relate to the accumulation of n‐alkanes starting from C25 and perhaps other wax‐type hydrocarbons.
Adenuga et al. (2017) addressed the question as to whether the granuloma formation observed in F344 rats following exposure to mineral oils is relevant to human and developed a ‘mode of action/human relevance framework’. They assessed components of a MoA using the modified Bradford‐Hill considerations. The key components were identified as intestinal absorption, preferential hepatic retention and subsequent formation of granulomas encased by infiltrating inflammatory lymphocytes. In light of differences in the key components between rat strains (F344 vs. SD rats) and between species, it was considered that the effects of MOH seen in the F344 rats were not relevant to humans. The authors concluded that this was consistent with a lack of evidence for formation of epithelioid granulomas in humans. This conclusion is supported by a proposed adverse outcome pathway (AOP) analysis (Carrillo et al., 2021a,b). In humans, liver lesions consisted of accumulation of saturated hydrocarbons and lipoid granulomas of no clinical relevance. These changes are markedly different from the epithelioid granulomas, with cytokine‐secreting giant cells, observed in F344 rats. The Panel noted that the immune response seen in F344 rats following MOSH exposure is likely to be related to the inflammatory response in the liver. Despite the frequent occurrence of lipogranulomas in humans, there is no evidence for accumulation of macrophages from the literature. Although this suggests that the immune system is not activated, detailed investigations, e.g. haematology or determination of spleen weight, are often missing.
In a review evaluating the current evidence of the risk of MOH in food to humans, Bevan et al. (2020) noted that there is uncertainty around the relevance for humans of the toxicity findings in F344 rats, since the data from F344 rats do not compare well with data from other rat strains or dogs.
Overall, the Panel concluded that the formation of epithelioid granuloma leading to liver inflammation in F344 rats exposed to MOSH is not a critical end‐point relevant to humans.
Changes in organ weights
The weight of liver, MLN and spleen was found to be increased following exposure to MOSH in F344 rats. Increased liver weight and centrilobular hepatic hypertrophy were observed in SD rats without the presence of granuloma (EFSA CONTAM Panel, 2012). As noted in the previous EFSA Opinion, the reason for hepatic hypertrophy is not known, as was not the increase in MLN weight. In F344 rats exposed to white oils and waxes, changes in liver and MLN weights are likely related to the formation of granuloma. Increased MLN weights associated with aggregates of macrophages (granuloma formation) were also observed in SD rats, although to a lower extent than in F344 rats. The CONTAM Panel confirmed the previous conclusion (EFSA CONTAM Panel, 2012) that these changes are considered as an adaptive response to poorly soluble, high MW materials and concluded that they are of low biological relevance for human health risk assessment.
In F344 rats, a dose‐dependent increase in spleen weights was seen following exposure to various MOSH mixtures (see summary table of relevant data in Appendix F). Animal studies from 1980 onwards were screened for evidence on possible MoA for reported spleen effects. Six studies were found for F‐344 rats only (Baldwin et al., 1992; Smith et al., 1996; Trimmer et al., 2004; McKee et al., 2012; Barp et al., 2017a,b; Cravedi et al., 2017; Nygaard et al., 2023), three studies comparing F‐344 and SD rats (Firriolo et al., 1995; Griffis et al., 2010a,b; Boogaard et al., 2017) and one study on SD rats only (Carrillo et al., 2021a,b), respectively.
The increase in spleen weight depended on the MOSH composition. In F344 rats, increased spleen weight was correlated with the presence of n‐alkanes in the administered MOSH. For L‐C25 (deparaffinated product, i.e. with minimised content of n‐alkanes) and L‐C25W (L‐C25 + wax 1:1, of similar molecular mass distribution), the correlation became most obvious: L‐C25W exposure resulted in a spleen weight increase already in the lowest dose, whereas L‐C25 produced no such increase in any dose (Barp et al., 2017b; Nygaard et al., 2023). However, the correlation was less clear when comparing S‐C25 with L‐C25W: S‐C25 contained a small proportion of n‐alkanes, but negligible amounts above C25, and the concentrations of the n‐alkanes in the liver and spleen were hardly detectable. Spleen weight increases in F344 rats were generally observed at the same dose level as that inducing the formation of granuloma in the liver. In the case of S‐C25, however, increased spleen weights were recorded at all tested doses, whereas an increased density of liver granulomas was only observed in the high‐dose group. For the mid‐dose tested, increased spleen weight was observed in combination with an increased density of lymphoid clusters in the liver parenchyma. n‐Alkanes were detectable in liver and spleen from all the treatment groups, but were below the limit of quantification. Comparing the highest doses tested, a similar spleen weight increase was recorded for L‐C25W and for S‐C25, despite a more than 30 times lower concentration of n‐alkanes in the spleen (see Appendix C). It is noted, however, that size of spleen weight increases was around 100% in all treatment group for L‐C25W without a clear dose–response relationship, possibly indicating that a maximum effect size was reached already at the lowest tested dose. In the case of S‐C25, conversely, a clear dose‐related increase in spleen weight, ranging approximately from 15% to 100%, was observed. It is notable that in the studies where immune response biomarkers were measured, a correlation between an increased immune response, increased spleen weight and MOSH‐induced liver granuloma in F344 rats was observed. In light of the above information, the Panel concluded that the spleen weight increase seen in MOSH‐exposed F344 rats is at least partly linked to a sustained increase in the activity of the immune system, apparent from higher numbers of granulocytes or other white blood cells. This in turn is associated with n‐alkane‐induced liver epithelioid granuloma. As discussed above, the latter liver effects are considered by the Panel not to be relevant to humans because of the lack of n‐alkane accumulation in human liver compared to that seen in F344 rats. It therefore follows that the increased spleen weight seen in F344 rats is also likely not to be relevant to humans.
Further support for this MoA comes from studies in SD and Long‐Evans rats, as well as in Beagle dogs, in which no significant increases were seen in either liver or spleen weights along with no evidence of haematological alterations or liver granuloma produced by various MOSH treatments (see general overview in Appendix D).
Although the suggested mechanism is supported by the data and is biologically plausible, there might still be other potential modes of action contributing to increased spleen weights, and this is reflected in the uncertainty analysis (Section 3.5.2).
No new information on organ weight changes from human studies was available. A summary of the information on the spleen effects observed in older human studies is reported in Annex G. Two studies reported on spleen weights in human subjects exposed to MOH. In a single case study (Nochomovitz et al., 1975), an increased spleen weight of 225 g was reported after chronic high ingestion of MOSH from white oils used as a laxative. However, the CONTAM Panel noted that the reported spleen weight was still within the physiological range. Liber and Rose (1967) investigated 13 spleens from autopsies, seven of which showed lipogranulomas and six did not. The authors found no clear relationship between the weight and MOH content of the spleens. However, spleens weighing > 400 g were excluded from the investigation. In addition, the number of subjects is low, and the determined MOH contents might be subject to uncertainty due to the analysis technology available at that time.
Summary
For MOSH, the MoA involving epithelioid granuloma formation in the liver and granulomas in spleen is only relevant for the particularly sensitive F344 rats. This is due to the retention of n‐alkanes above C25 and other wax components that may cause crystallisation. Such accumulation of n‐alkanes has not been reported in human liver (Section 3.1.1.2 and EFSA CONTAM Panel, 2012). The retention of n‐alkanes and other wax components, associated with the above‐mentioned n‐alkane‐induced epithelioid granuloma in the liver and subsequent enhanced immune system response, is considered, at least in part, to cause the increase in spleen weights observed in F344 rats. Increased MLN weights and formation of MLN granulomas were confirmed to be of low biological relevance for human health risk assessment.
The modes of toxic action of MOAH considered to be relevant to humans focus on genotoxicity and on potential developmental toxicity, both of which are associated with the three or more ring fraction. However, the Panel noted that this fraction was determined by a method not properly separating highly alkylated species of lower ring numbers from those of high ring number, which primarily means that the concentration of the three or more ring MOAH is uncertain and likely to be overestimated.
3.1.5. Hazard characterisation
3.1.5.1. Considerations of critical effects and dose–response analysis
MOSH
As concluded in the section on MoA (Section 3.1.4), the presence of liver epithelioid granuloma, activation of the immune system and an associated increase in liver and spleen weights have been associated with the hepatic accumulation of n‐alkanes in F‐344 rats, as seen in the new studies (McKee et al., 2012; Nygaard et al., 2023), as well as in older studies summarised in the previous CONTAM Panel Opinion (EFSA CONTAM Panel, 2012). Increased MLN weights associated with MLN macrophage aggregates and granuloma formation were observed in F344 and SD rats, and considered as not adverse changes as described in Section 3.1.4. Accumulation of n‐alkanes has not been found in human liver and spleen, and therefore, the effects seen in liver and spleen of F344 rats were not taken by the Panel as critical. Moreover, these effects have not been observed in limited studies in other strains of rats or in Beagle dogs.
Chronic exposure to hydrocarbon wax (Ozokerite) has been shown to cause liver adenomas and carcinomas in F344 rats (Kuroda et al., 2013). The Panel noted that the hydrocarbon wax studied was not characterised sufficiently, leaving 19% of unidentified material. In addition, at all doses, granulomas were observed in the liver, probably resulting from n‐alkanes contained in the test item. Hence, the effects are likely to be related to chronic inflammation in the recognised relatively highly sensitive F344 rats. These effects were considered to be due to a non‐genotoxic mechanism associated with chronic inflammation and cell proliferation, secondary to the n‐alkane accumulation specific to the F344 rat liver. Moreover, in the chronic study by Trimmer et al. (2004), in which F344 rats were exposed to white mineral oil products, no such effects were observed.
In SD rats, no adverse effects were observed up to 9 g/kg bw per day in a subchronic study with a paraffin wax (ERBC, 2022). Evidence of minimal multifocal chronic liver inflammation was observed after subchronic exposure (90 days) to a high dose of a paraffinic oil (Firriolo et al., 1995). Apoptosis and necrosis of the intestinal mucosa were observed after subacute exposure to a GTL oil (Boogaard et al., 2017). However, the Panel noted a relative paucity of information in strains other than F344 rats and had reservation on the use of synthetic GTL oil data to read‐across for the toxicity of MOSH. Overall, noting the uncertainties, a critical effect for MOSH relevant to humans could not be clearly identified from the available data.
MOAH
In 2012, the CONTAM Panel concluded that it was not possible to characterise the hazards associated with the MOAH fraction in view of the limited data available and the chemical complexity of the MOAH mixture. A potential concern was expressed about the presence of a genotoxic and carcinogenic fraction, constituted by substances with three to seven aromatic rings and various degrees of side chain alkyl moieties. While these components are likely to exert their genotoxic and carcinogenic effects via bioactivation of the aromatic ring system, well established for (unsubstituted) PAH, their potency is expected to be modulated by the number, size and position of the alkyl side chains.
New in vitro metabolism data on methylated phenanthrene and naphthalene showed a preferential metabolic oxidation of the alkyl‐side chains over the aromatic ring system, or an overall reduced metabolism in the presence of long side chains with higher steric hindrance (Wang et al., 2020, 2022a,b). The presence of methyl groups in specific positions of the ring system was shown to favour the bioactivation of phenanthrene (Wang et al., 2022a,b). The residence time of MOAH in the tissue might influence the occurrence of side chain and aromatic ring oxidation. The CONTAM Panel agreed with the conclusion of Pirow et al. (2019) that it is not predictable how alkylation affects the carcinogenic potential of MOAH compared to non‐alkylated PAHs, apart from the expectation that genotoxicity would be reduced with decreased metabolism associated with increased side chain carbon length.
In the present assessment, no additional studies were identified to solve the uncertainties identified in the previous opinion (EFSA CONTAM Panel, 2012). Overall, there is sufficient information to conclude that unrefined MOH products act as tumour inducers in the induction/promotion mouse skin painting model and cause adverse effects on development in dermal rat studies.
Little is known regarding the toxicity of MOAH other than genotoxicity of some three or more ring components. There is evidence that some components may act as tumour promoters following initiation in mouse skin‐painting studies, as reported in the previous opinion (EFSA CONTAM Panel, 2012). Certain aromatic hydrocarbons like naphthalene are also known non‐genotoxic carcinogens. However, no information is available on the impact of alkyl side chains in the toxicity of 1–2 ring MOAH. Overall, the lack of robust data on the oral toxicity of MOAH hampers the possibility to identify the critical effects related to the non‐genotoxic and carcinogenic fraction of MOAH.
In view of the lack of suitable data, no dose–response analysis could be performed for MOAH.
3.1.5.2. Establishment of reference point for a margin of exposure approach
MOSH
In view of the lack of critical effects clearly identified for MOSH (see Section 3.1.5.2), the Panel considered the available evidence in F344 rats for the establishment of the Reference Point (RP) for MOSH. The study of Nygaard et al. (2023) on the broad MOSH mixture and fractions (L‐C25, S‐C25 and L‐C25W) was selected, although it had limitations in the endpoints analysed. Out of this study, the L‐C25 composition was considered to best represent what was found in human tissues with regard to mass range and low occurrence or absence of n‐alkanes. Hence, the NOAEL of 236 mg/kg bw per day, corresponding to the highest tested dose of L‐C25, was selected as the relevant RP (see Section 3.1.1.2).
There were no adverse effects following exposure to MOSH at or below this value of 236 mg/kg bw per day in other experimental animal models tested (SD and Long‐Evans rats and beagle dogs), albeit in limited studies. In SD rats, minimal signs of liver inflammation were reported at 1,624 mg/kg bw per day following subchronic exposure to a paraffinic oil (Firriolo et al., 1995) and mild to moderate apoptosis and necrosis in the intestinal mucosa were observed at the highest tested dose of 1,267 mg/kg bw per day of a synthetic GTL oil (reviewed by Boogaard et al., 2017). No effects in these two studies were observed at 158 and 308 mg/kg bw per day, respectively. Because the data set in SD rats is limited, the Panel considered that the selection of the NOAEL of 236 mg/kg bw per day, taken from the top dose of L‐C25 administered to F344 rats, is appropriate. The n‐alkane‐mediated effects observed in F344 rats were deemed not relevant to humans; however, this strain was considered appropriate to detect other adverse effects.
The limitations of the data set prevented the setting of a health‐based guidance value (HBGV) for MOSH, and a MOE approach was warranted based on the NOAEL of 236 mg/kg bw per day, as established above. At the NOAEL, the corresponding tissue contents in F344 rats after 120 days were 3,805 mg/kg in liver, 419 mg/kg in spleen and 36 mg/kg in adipose tissue.
MOAH
The present uncertainties related to the characterisation of the carcinogenic potency of three or more ring MOAH discussed in Section 3.1.5 together with the general lack of robust oral studies on MOAH‐containing products hamper the derivation of specific reference points (RPs) for the hazard characterisation. The CONTAM Panel considered alternative strategies for the risk characterisation of MOAH in food. The possible presence of genotoxic and carcinogenic substances anyway prevents the setting of a HBGV for MOAH and a MOE approach was warranted in accordance with the Opinion of the EFSA Scientific Committee (EFSA, 2005).
In view of the structural similarity and the plausible common MoA for genotoxicity and carcinogenicity, the CONTAM Panel considered the possibility to make use of the RPs defined for PAH (EFSA, 2008) as surrogate RPs for the risk estimation related to the exposure to three or more ring MOAH. In the CONTAM Panel opinion on PAHs, a series of BMDL10 values for the incidence of total tumour‐bearing animals were derived from two 2‐year oral studies on coal tar mixtures, using benzo[a]pyrene alone or combinations of different PAHs as markers of the carcinogenic effects, as reported in Table 3.
Table 3.
Lowest BMDL10 for incidence of total tumours calculated in the EFSA opinion on polycyclic aromatic hydrocarbons (PAHs) (EFSA, 2008)
| Marker for genotoxic PAHs in food (a) | Lowest BMDL10 (mg/kg bw per day) |
|---|---|
| BaP | 0.07 |
| PAH2 | 0.17 |
| PAH4 | 0.34 |
| PAH8 | 0.49 |
BaP: benzo[a]pyrene; PAH2: BaP and chrysene; PAH4: PAH2, benzo[b]fluoranthene and benz[a]anthracene; PAH8; PAH4, benzo[k]fluoranthene, benzo[ghi]perylene, dibenzo[a,h]anthracene and indeno[1,2,3‐cd]pyrene.
The CONTAM Panel considered it most appropriate to select the BMDL10 of 0.49 mg/kg bw per day for the PAH8 mixture as representative and sufficiently conservative, considering the likely high variability in the potency of the components in the three or more ring‐MOAH fraction. Further supporting the conservative nature of the surrogate RP, it is noted that a substantial part of MOAH in food, quantified in mg/kg, consists of alkyl substituents.
Due to the lack of adequate oral toxicity studies, it was not possible to identify a Reference Point for the 1–2 ring MOAH.
The CONTAM Panel acknowledged a substantial degree of uncertainty related to the hazard characterisation approach for MOSH and MOAH, which is discussed in Section 3.5.
3.2. Occurrence data
3.2.1. Occurrence data on food submitted to EFSA
On 21 September 2021, a total of 92,851 analytical results on MOSH and MOAH from food and feed samples and food contact materials (FCMs) were extracted from the EFSA Data Warehouse (see Annex E for the raw data). After excluding the analytical data on feed (n = 142), on FCMs (n = 12,077) and selecting the sampling years from 2011 to 2021, an initial number of 80,632 analytical results were available.
A thorough analysis of the occurrence data set was carried out to prepare the data for the dietary exposure assessment; data providers were contacted as needed to clarify any doubt about the data submitted. Below is a brief description of the data excluded based on the feedback received, and the expert judgement of the data:
-
•
Analytical results codified as ‘Mineral oils’ without further information on whether they referred to MOSH or MOAH (n = 1,712).
-
•
Analytical results confirmed as duplicates by data providers (n = 1,676).
-
•
Analytical results for which reliability could not be confirmed by the data providers (n = 2,887).
-
•
Analytical results part of migration experiments where food samples (in some cases also the FCMs) were analysed before and after storage (two different analytical results provided for each sample); only the results after storage were retained in the final data set (n = 320).
-
•
Analytical results for ‘MOAH 3 to 7 rings’ that were analysed for ‘presence/absence’ (qualitative evaluation) (n = 2).
-
•
Analytical results from one sample analysed twice; the average of the two results was used (n = 1).
-
•
Analytical results of samples collected with sample strategy reported as ‘Suspect sampling’ 12 (n = 421).
-
•
Analytical results codified as MOSH C‐fraction C18‐42 with LOQs between 14.7 and 50 mg/kg (n = 11).
-
•
Analytical results of samples identified as suspicious based on their MOAH/MOSH ratio (see Section 2.3.2); a total of 480 analytical results (59 samples) were excluded.
After the exclusion of these results, a total of 73,122 analytical data (from 7,840 samples) on MOAH and MOSH were available. Figure 2 shows the total number of samples, those with information on both MOAH and MOSH levels, and those with only data on either MOAH or MOSH.
Figure 2.
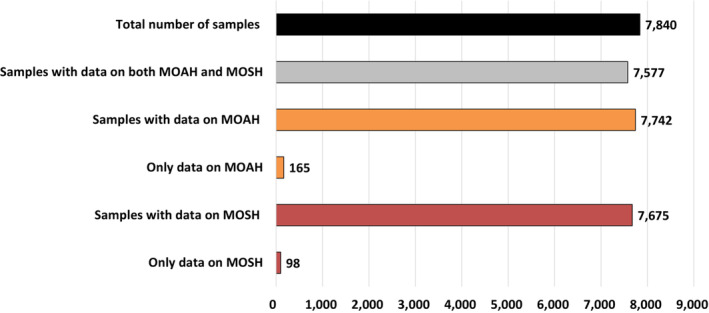
Number of samples with data on MOAH and/or MOSH
Two types of data providers were identified in the final data set of 7,840 samples: European countries and food associations. The data provided by food associations corresponded to 33,295 analytical results (3,413 samples, 43.5%) while data from European countries referred to 39,827 analytical results (4,427 samples, 56.5%).
The data from food associations were submitted by five organisations: the Federation of European Rice Millers (56 samples), the Organisation of Consumers and Users (105 samples), the European Cocoa Association (281 samples), the EU Vegetable Oil and Proteinmeal Industry Association (FEDIOL; 2,502 samples) and Specialised Nutrition Europe (469 samples). Among the analytical results excluded during data analysis (see above), the following corresponded to data submitted by food associations:
-
–
One analytical result reported as feed (one sample).
-
–
201 analytical results (21 samples) for which the MOAH/MOSH ratio was identified as suspicious (see Section 2.3.2).
-
–
2,593 analytical results (1,299 samples) for which reliability could not be confirmed by the data providers.
Out of the 3,413 samples reported by food associations, a total of 3,369 reported data on MOAH and 3,263 on MOSH. The data covered only few specific food categories, in particular ‘Vegetable fats and oils, edible’ (2,495 samples, ∼ 73%), ‘Food products for young population’ (458 samples, ∼ 13%) and ‘Chocolate and chocolate products’/‘Cocoa ingredients’ (225, ∼ 7%). Samples were collected between 2016 and 2021, with around 56% in 2020; for most of the samples, the sampling country was not specified but reported as ‘European Union’ (2,520 samples, 74%).
Before deciding to continue with a unique data set for the dietary exposure assessment, the data sets submitted by the European countries and the food association were compared. In general, for the same food matrices, the analytical methods used by the European countries were less sensitive than those used by the food associations. This could explain, at least to certain degree, the higher number of left‐censored data submitted by the European countries. Exceptions were identified, for instance for vegetable oils for which the food associations reported LOQs as high as 10 mg/kg. These LOQs are in line with the CEN – EN 16995. 13
Table 4 shows MOAH and MOSH levels in selected food categories. Direct comparisons are difficult as the analytical results are affected by different factors: sampling design, sampling year, sampling of suspect samples, the laboratory performance in preparing and analysing the samples, etc. In some food groups, higher mean levels seem to be present in the samples provided by the European countries. This is the case, for instance, in ‘Bitter chocolate’, ‘Cocoa powder’ or ‘Simple cereals which have to be reconstituted with milk or other appropriate nutritious liquids’. The different sampling years could explain, at least partially, such differences since different measures have been introduced in the last few years (e.g. limiting the use of recycled paperboard or placing functional barriers in the FCM) as the knowledge on the source of mineral oil contamination improved. Therefore, lower MOAH and MOSH levels could be expected in samples analysed more recently as those provided by food associations.
Table 4.
MOH levels (mg/kg) in key food categories divided according to the origin of data
| N (a) | ND (b) | Mean | 95th percentile (c) | ||||
|---|---|---|---|---|---|---|---|
| LB | UB | LB | UB | ||||
| Food associations | |||||||
| Rice grain | MOAH | 76 | 55 | 0.50 | 0.87 | 5.1 | 5.3 |
| MOSH | 76 | 18 | 2.84 | 3.24 | 13.3 | 13.3 | |
| European countries | |||||||
| MOAH | 215 | 145 | 0.71 | 1.50 | 2.17 | 5 | |
| MOSH | 220 | 88 | 2.94 | 3.83 | 14.3 | 14.35 | |
| Wheat semolina | Food associations | ||||||
| MOAH | 21 | 15 | 0.56 | 1.15 | – | – | |
| MOSH | 21 | 10 | 6.98 | 7.79 | – | – | |
| European countries | |||||||
| MOAH | 164 | 138 | 0.06 | 0.54 | 0.4 | 2 | |
| MOSH | 166 | 45 | 1.32 | 1.99 | 4.2 | 6 | |
| Bitter chocolate | Food associations | ||||||
| MOAH | 17 | 17 | 0.0 | 0.50 | – | – | |
| MOSH | 21 | 0 | 2.85 | 2.85 | – | – | |
| European countries | |||||||
| MOAH | 33 | 26 | 0.52 | 2.45 | – | – | |
| MOSH | 33 | 12 | 5.05 | 7.24 | – | – | |
| Milk chocolate | Food associations | ||||||
| MOAH | 74 | 67 | 0.13 | 0.55 | 0.7 | 0.82 | |
| MOSH | 78 | 0 | 3.88 | 3.95 | 7.8 | 7.92 | |
| European countries | |||||||
| MOAH | 208 | 184 | 0.09 | 0.94 | 0.6 | 2.4 | |
| MOSH | 208 | 61 | 2.97 | 3.80 | 8.5 | 8.6 | |
| Rape seed oil, edible | Food associations | ||||||
| MOAH | 433 | 426 | 0.04 | 4.37 | 0 | 10 | |
| MOSH | 433 | 247 | 3.59 | 7.44 | 12.5 | 12.5 | |
| European countries | |||||||
| MOAH | 62 | 59 | 0.17 | 1.47 | 0 | 5 | |
| MOSH | 62 | 22 | 3.35 | 4.45 | 15.8 | 15.8 | |
| Sunflower seed oil, edible | Food associations | ||||||
| MOAH | 561 | 546 | 0.04 | 3.62 | 0 | 10 | |
| MOSH | 567 | 242 | 2.71 | 6.02 | 8.6 | 10 | |
| European countries | |||||||
| MOAH | 98 | 71 | 0.58 | 1.81 | 2.7 | 5 | |
| MOSH | 99 | 12 | 10.01 | 10.61 | 63 | 63.1 | |
| Linseed oil | Food associations | ||||||
| MOAH | 22 | 11 | 0.67 | 0.95 | – | – | |
| MOSH | 22 | 3 | 6.01 | 6.28 | |||
| European countries | |||||||
| MOAH | 22 | 21 | 0.56 | 1.36 | – | – | |
| MOSH | 22 | 0 | 8.49 | 8.63 | – | – | |
| Coconut oil/fat | Food associations | ||||||
| MOAH | 207 | 76 | 3.73 | 5.39 | 11.5 | 12.7 | |
| MOSH | 210 | 4 | 27.48 | 28.58 | 57 | 57 | |
| European countries | |||||||
| MOAH | 43 | 25 | 3.42 | 4.27 | – | – | |
| MOSH | 43 | 1 | 18.23 | 18.40 | – | – | |
| Cocoa powder | Food associations | ||||||
| MOAH | 24 | 24 | 0.0 | 1.0 | – | – | |
| MOSH | 26 | 1 | 5.71 | 5.75 | – | – | |
| European countries | |||||||
| MOAH | 24 | 8 | 2.45 | 2.96 | – | – | |
| MOSH | 24 | 2 | 11.97 | 12.30 | – | – | |
| Follow‐on formula, milk‐based, powder | Food associations | ||||||
| MOAH | 160 | 137 | 0.11 | 1.82 | 0.99 | 2.09 | |
| MOSH | 95 | 15 | 2.02 | 4.02 | 5.6 | 6.8 | |
| European countries | |||||||
| MOAH | 30 | 28 | 0.08 | 1.32 | – | – | |
| MOSH | 30 | 8 | 2.49 | 3.65 | – | – | |
| Infant formula, milk‐based, powder | Food associations | ||||||
| MOAH | 152 | 137 | 0.08 | 1.94 | 0.7 | 2.26 | |
| MOSH | 109 | 10 | 3.10 | 5.06 | 7.5 | 9 | |
| European countries | |||||||
| MOAH | 40 | 38 | 0.03 | 1.53 | – | – | |
| MOSH | 40 | 2 | 2.93 | 4.39 | – | – | |
| Simple cereals which have to be reconstituted with milk or other appropriate nutritious liquids | Food associations | ||||||
| MOAH | 50 | 47 | 0.01 | 1.30 | – | – | |
| MOSH | 49 | 28 | 0.75 | 3.06 | – | – | |
| European countries | |||||||
| MOAH | 47 | 45 | 0.57 | 1.57 | – | – | |
| MOSH | 47 | 32 | 5.58 | 6.68 | – | – | |
N: number of samples.
ND: Non‐detected.
95th percentile estimates with less than 59 samples were not calculated since they may not be statistically robust (EFSA, 2011a).
Despite these differences, the CONTAM Panel considered that the two data sets were suitable for exposure assessment and that they provided consistent and complementary information. Therefore, the CONTAM Panel concluded that the data sets could be merged to perform the dietary exposure assessment.
Figure 3 shows the number of samples by sampling country in the merged data set. The maximum was from Germany (around 40%), and an important number was reported as collected in the European Union without specifying the country. Almost 90% of the samples were collected after 2017, probably boosted by the Commission Recommendation 2017/84 to monitor mineral oil hydrocarbons (Figure 4).
Figure 3.
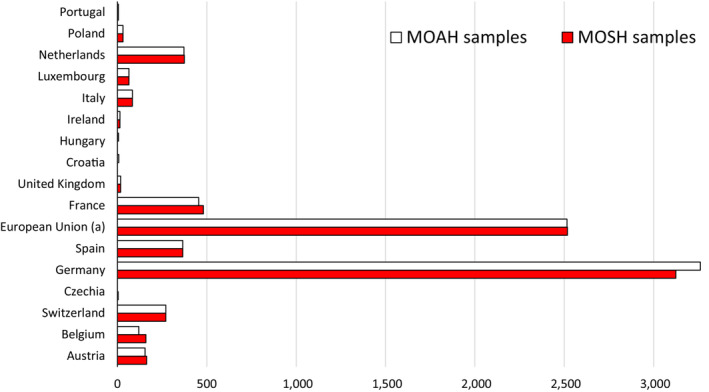
- (a): Samples reported by European food associations without specifying the sampling country.
Figure 4.
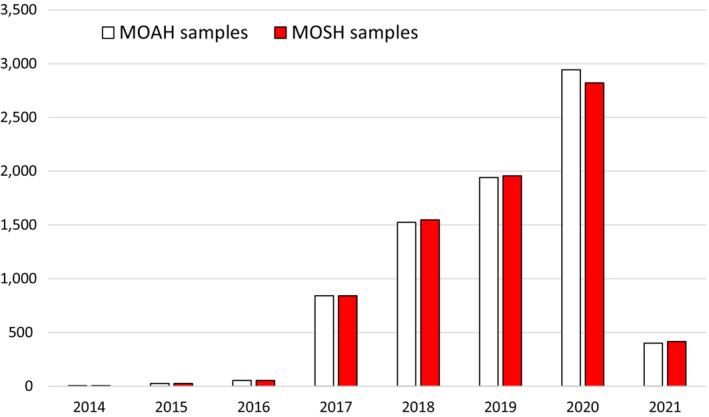
Number of samples by sampling year
Most samples were analysed for the MOSH and MOAH C‐fractions specified by the JRC Guidance on MOH, including ‘Total MOAH’ and ‘Total MOSH’ (Bratinova and Hoekstra, 2023). For MOAH, the C‐fractions ranged between the retention times of the n‐alkanes C9–C16, C16–C25, C25–C35 and C35–C50; for MOSH, the ranges were between C9–C16, C16–C20, C20–C25, C25–C35, C35–C40 and C40–C50. These C‐fractions represented 99.6% of the whole data set (a total of 72,841 out of the 73,122 analytical results available). The remaining results covered different MOAH and MOSH C‐fractions from C10 to C50 (see Table 5). For 21 samples, 14 the fraction ‘MOAH 3 to 7 ring’ was also analysed (all left‐censored data).
Table 5.
MOSH and MOAH data in the final data set (73,122 analytical results, 7,840 samples) by C‐fraction
| Number of results | % of the total | |
|---|---|---|
| Total MOSH | 4,615 | 6.3 |
| Total MOAH | 4,608 | 6.3 |
| MOSH C9–C16 | 6,698 | 9.2 |
| MOSH C16–C20 | 6,846 | 9.4 |
| MOSH C20–C25 | 6,828 | 9.3 |
| MOSH C25–C35 | 6,849 | 9.4 |
| MOSH C35–C40 | 5,747 | 7.9 |
| MOSH C40–C50 | 5,294 | 7.2 |
| MOAH C9–C16 | 5,376 | 7.4 |
| MOAH C16–C25 | 6,843 | 9.4 |
| MOAH C25–C35 | 6,903 | 9.4 |
| MOAH C35–C50 | 6,213 | 8.5 |
| MOAHs 3–7 ring | 21 | 0.03 |
| Other C‐fractions (a) | 281 | 0.4 |
MOAH fractions: one result on C10–C25, 42 on C10–C35, six on C16–C20, one on C16–C24, 55 on C16–C35, five on C20–C25, one on C24–C35. MOSH fractions: 40 results on C10–C35, one on C16–C24, 26 on C16–C25, 47 on C16–C35, four on C18–C42, 23 on C20–C25, one on C24–C35, 28 on C35–C50.
When the analytical result was provided for ‘Total MOAH’ and/or ‘Total MOSH’, the CONTAM Panel decided to use it. Otherwise, the total MOAH and total MOSH concentrations were derived by summing the individual C‐fractions reported. Uncertainties associated with this approach will be covered in Section 3.5. Out of the 7,675 samples submitted on MOSH, a total of 3,060 samples reported values only on the C‐fractions (40%), 761 samples only on ‘Total MOSH’ and 3,854 on both C‐fractions and ‘Total MOSH’. For the 7,742 samples available on MOAH, the numbers were 3,134 (40.5%) for samples reporting only C‐fractions, 731 for only ‘Total MOAH’ and 3,877 reporting both C‐fractions and ‘Total MOAH’.
Table 6 shows the number of MOAH and MOSH C‐fractions per sample in those samples for which C‐fractions were used to derive the total levels (3,134 for MOAH and 3,060 for MOSH). As can be seen, in almost 60% of the samples analysed, MOAH data were reported for four C‐fractions; the typical four C‐fractions reported were C9–C16, C16–C25, C25–C35 and C35–C50. For MOSH, typically the six fractions indicated in the JRC Guidance on MOH (Bratinova and Hoekstra, 2023) were reported (67% of the cases): C9–C16, C16–C20, C20–C25, C25–C35, C35–C40 and C40–C50. The total MOAH and MOSH levels were derived regardless the number of C‐fractions reported.
Table 6.
Number of MOSH or MOAH C‐fractions analysed per sample among those that did not report analytical results on ‘Total MOSH’ and ‘Total MOAH’
| MOSH | MOAH | ||||
|---|---|---|---|---|---|
| Number of C‐fractions reported | Number of MOSH samples | % | Number of C‐fractions reported | Number of MOAH samples | % |
| 1 | 34 | 1.1 | 1 | 67 | 2.1 |
| 2 | 2 | 0.07 | 2 | 263 | 8.4 |
| 3 | 1 | 0.03 | 3 | 930 | 29.7 |
| 4 | 666 | 21.8 | 4 | 1,849 | 59.0 |
| 5 | 309 | 10.1 | 5 | 23 | 0.7 |
| 6 | 2,048 | 66.9 | 6 | 1 | 0.03 |
| – | 7 | 1 | 0.03 | ||
Analytical methods
The main analytical method reported for the measurement of MOAH and MOSH was on‐line LC‐GC‐FID (86%), followed by GC‐FID (13%), and GC‐LRMS (0.09%). In few occasions (n = 32), no information was provided on the detection technique (‘Gas chromatography’, 1.1% 15 ).
The information provided in this section should be interpreted with care as EFSA confirmed with data providers that diverse approaches were used to derive and report the left‐censoring limits. As an example, in few samples, the LOD/LOQ estimated for one C‐fraction or for the total MOAH and/or total MOSH was assigned to all C‐fractions reported. In other samples, data providers quantified the MOAH and MOSH hump, divided the hump into C‐fractions and assigned arbitrary LODs and LOQs to the resulting C‐fractions, before submitting quantified values for total MOAH and/or total MOSH and for each of the C‐fractions. To provide the most realistic view on the sensitivity of the analytical methods, in this section, the range of LODs/LOQs refers exclusively to the samples for which only C‐fractions were reported (see above; n = 3,134 for MOAH and n = 3,060 for MOSH).
For MOAH C‐fractions, the reported LOQs ranged from 0.1 mg/kg up to 8 mg/kg and from 0.05 mg/kg to 2 mg/kg for the LOD. Similarly, for MOSH C‐fractions, varied between 0.1 and 5 mg/kg and between 0.03 and 1 mg/kg for LOQ and LOD, respectively. When ‘total MOAH’ was reported, the LOQs were between 0.05 mg/kg and 25 mg/kg, and from 0.05 mg/kg to 1 mg/kg for the LODs. Similarly, for ‘total MOSH’, the ranges were 0.03–10 mg/kg and 0.05–1 mg/kg for LOQ and LOD, respectively. LOQs and LODs mainly depended on the food type (e.g. fat content) and the pretreatment of the sample.
Figure 5 shows the percentage of non‐detected, non‐quantified and quantified results for the MOAH and MOSH C‐fractions specified in the JRC Guidance on MOH (Bratinova and Hoekstra, 2023). The left‐censored data accounted for 74.3% of all the data (54,395 analytical results). They were particularly high for MOAH, where for the four individual C‐fractions, approximately 93% of the results were reported either as below the LOD or below the LOQ.
Figure 5.
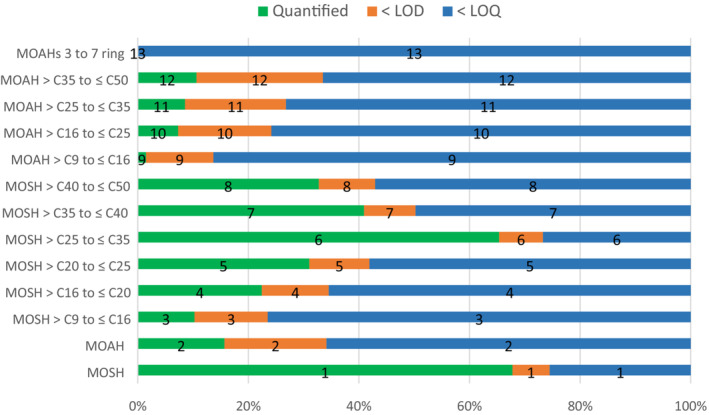
Percentage of quantified, non‐quantified and non‐detected analytical results across the different C‐fractions
At sample level, 6,472 of 7,742 samples with data on MOAH (84%) were left censored (all analytical data reported as below LOD or LOQ), while only 2,212 samples (29%) for MOSH. This proportion of left‐censored data, in particular for MOAH, will impact the occurrence levels and subsequently the dietary exposure estimations when applying the substitution method (LB/UB estimations). The exposure is likely to be underestimated with the LB approach and overestimated with the UB approach.
The EFSA guideline ‘Use of LOQ cut‐off values for dietary exposure to chemical contaminants’ (EFSA, 2018a) was used to identify possible ways to reduce the impact of the MOAH left‐censored data on the LB–UB estimations. As described in this guideline, method performance criteria on LODs/LOQs might be used to establish cut‐offs on the reported left‐censoring limits. The JRC Guidance on MOH specifies performance requirements for MOAH and MOSH analysis, among them maximum LOQs for each C‐fraction, depending on the type of food (Bratinova and Hoekstra, 2023):
-
–
Dry, low fat content (< 4% fat/oil), maximum LOQ = 0.5 mg/kg (e.g. bread and rolls, grains, pasta, etc.).
-
–
Intermediate fat/oil content (> 4% fat/oil), maximum LOQ = 1 mg/kg (e.g. fine bakery ware, chocolate, fish meat, fish products, etc.).
-
–
Fat/oils, maximum LOQ = 2 mg/kg (e.g. animal fat, vegetable oils, etc.).
Although the JRC Guidance on MOH refers to the C‐fractions, it was decided to also apply these cut‐offs to the analytical results reported as ‘total MOAH’. This is supported by the recent draft joint statement from EU Member States regarding the presence of MOAH in food, including food for infants and young children 16 that makes use of these LOQs to set limits for the total concentration of MOAH in different foods. Considering the publication date of the JRC Guidance on MOH, the LOQ cut‐offs were only applied to the MOAH samples analysed in 2020 and 2021. By applying these cut‐offs, 364 samples with data on MOAH were excluded. Most of the samples excluded were vegetable oils, in particular ‘Rape seed oil, edible’ (n = 155) and ‘Sunflower seed oil, edible’ (n = 140), mostly (n = 351) due to an LOQ of 10 mg/kg reported for ‘total MOAH’. Only six of the 364 samples excluded reported quantified levels of MOAH.
The final data set consisted of 7,839 samples, with 7,675 samples with data on MOSH and 7,378 samples with data on MOAH.
Food samples
The large majority of the 7,839 samples in the final data set were analysed for both MOAH and MOSH. At FoodEx2 Level 1, the food categories with the highest number of samples were ‘Animal and vegetable fats and oils and primary derivatives thereof’ (MOAH/MOSH, n = 2,988/3,310), ‘Grains and grain‐based products’ (MOAH/MOSH, n = 1,796/1,848), and ‘Food products for young population’ (MOAH/MOSH, n = 644/525; Table 7).
Table 7.
Number of samples (at FoodEx2 Level 1) with data on MOAH and MOSH
| Number of samples | ||
|---|---|---|
| MOAH | MOSH | |
| Grains and grain‐based products | 1,796 | 1,848 |
| Vegetables and vegetable products | 12 | 11 |
| Starchy roots or tubers and products thereof, sugar plants | 30 | 30 |
| Legumes, nuts, oilseeds and spices | 193 | 211 |
| Fruit and fruit products | 22 | 23 |
| Meat and meat products | 105 | 112 |
| Fish, seafood, amphibians, reptiles and invertebrates | 171 | 169 |
| Milk and dairy products | 266 | 263 |
| Eggs and egg products | 29 | 29 |
| Sugar and similar, confectionery and water‐based sweet desserts | 704 | 721 |
| Animal and vegetable fats and oils and primary derivatives thereof | 2,988 | 3,310 |
| Fruit and vegetable juices and nectars (including concentrates) | 1 | 1 |
| Coffee, cocoa, tea and infusions | 165 | 170 |
| Food products for young population | 644 | 525 |
| Products for non‐standard diets, food imitates and food supplements | 72 | 72 |
| Composite dishes | 52 | 52 |
| Seasoning, sauces and condiments | 82 | 82 |
| Major isolated ingredients, additives, flavours, baking and processing aids | 46 | 46 |
Before linking occurrence and consumption data and in order to obtain more accurate, representative and robust dietary exposure estimations, some samples were excluded from the general exposure scenario, as explained below. The occurrence data were grouped based on the number of samples and the levels reported.
Food categories with left‐censored data for all samples and for which the presence of MOAH or MOSH was not expected were excluded from the assessment to avoid artificially increasing the UB exposure estimations. In the case of MOSH samples, there were only few categories that were excluded, for instance samples of ‘Fruit/vegetable spreads and similar’ (n = 10) or ‘Salt’ (n = 5). For samples analysed for the presence of MOAH, this situation was observed for many food groups; as an example, food categories such as ‘Meat and meat products’ (n = 105), ‘Meat and dairy imitates’ (n = 45), ‘Fish and seafood non processed’ (n = 33), ‘Eggs and egg products’ (n = 29) among others, were excluded. The lists with all the food categories excluded are provided in Annexes F (for MOSH) and G (for MOAH). Samples analysed for MOAH (n = 659) and MOSH (n = 668) reported as ‘Vegetable oil’ without further information on the type of oil were also excluded. Likewise, food categories with less than five samples were excluded, except the three samples of ‘Margarines and similar’, as they are made of vegetable oils (mean LB–UB = 16.2–17.3 mg/kg 17 ), and two samples of ‘Imitation cheese’ (MOSH mean 2.9 mg/kg, LB = UB), a product that contains vegetable oil as one of their main ingredients.
Few samples with exceptionally high MOH levels were reported, in particular for MOSH. The high levels are probably explained by particular production practices, such as the use of release and anti‐dusting agents, practices that nowadays seem to have been largely phased out. These samples were kept in the data set, although not used in the general exposure scenario but in dedicated scenarios to cover consumption by consumers with a preference for a particular brand or limited access to a wide variety of food choices. Examples were one sample of ‘Cake marbled, with chocolate’ with 710–715 mg/kg MOSH (LB–UB) and MOAH levels of 24–29 mg/kg (LB–UB), and two samples codified as ‘Wheat groats’ with mean MOSH levels of 8,167 mg/kg (LB = UB) and mean MOAH levels of 35.4 mg/kg (LB = UB). The low proportion of MOAH as compared to MOSH suggests that the presence of MOH might have their origin in the use of release and/or anti‐dusting agents. Similarly, two samples of ‘Sticks, salty’ and one of ‘Cheese powder’ were not included in the general scenario after being informed by the data providers that the high MOH concentrations were due to the presence of grated cheese rind. For the two samples of ‘Sticks, salty’, the reported mean concentrations were 329.1 mg/kg for MOSH (LB = UB) and 14.3 mg/kg for MOAH (LB = UB), while for the sample of ‘Cheese powder’, MOAH was not quantified, but the MOSH concentration was 701.4 mg/kg (LB = UB). An ad hoc exposure scenario was conducted to cover the intake of the ‘Sticks, salty’, but not for ‘Cheese powder’, as only few eating occasions were available.
Particular attention was paid to few samples initially codified as ‘Food supplements and similar preparations’ (n = 12). Triggered by the relatively high mean MOSH content (LB = UB, 264.1 mg/kg), data providers were contacted to obtain further details. This revealed that these samples were either gelatine capsules or tablets of heterogenous composition (phytochemicals from plant extracts, beeswax, emulsifiers, different vegetable oils, etc.), which rendered an adequate linkage with the consumption data difficult. The potential exposure to MOSH and MOAH following the consumption of this type of food supplements is assessed in Section 3.4.1.
MOSH data (as used for the exposure assessment)
Table 8 shows the samples with analytical data on MOSH as they were used for the dietary exposure estimations. Data belong to 17 food categories at FoodEx2 Level 1 grouped under 103 food groups that were used for the linkage with the consumption data. Samples were grouped at FoodEx2 Levels 2–5 (see also Annex F for summary statistics). As compared to MOAH data, the number of left‐censored data was lower, resulting in lower differences between mean LB and mean UB estimations.
Table 8.
MOSH mean values in mg/kg, lower bound (LB) and upper bound (UB), for different food samples as used for the dietary exposure assessment
| Mean MOSH levels (mg/kg) | ||||||
|---|---|---|---|---|---|---|
| FoodEx2 level 1 | FoodEx2 level | Food group | Number of samples | % Left‐censored | LB | UB |
| Animal and vegetable fats and oils and primary derivatives thereof | 3 | Butter | 33 | 42 | 7.01 | 8.77 |
| 3 | Margarines and similar | 3 | 0 (a) | 16.17 | 17.17 | |
| 3 | Blended fat and oils | 97 | 1 | 12.59 | 12.59 | |
| 5 | Walnut oil | 18 | 0 (a) | 15.84 | 15.94 | |
| 5 | Palm kernel oil, edible | 56 | 18 | 5.40 | 6.59 | |
| 5 | Coconut oil/fat | 253 | 2 | 25.91 | 26.85 | |
| 5 | Sunflower seed oil, edible | 666 | 38 | 3.79 | 6.70 | |
| 5 | Maize oil, edible | 37 | 19 | 4.51 | 6.00 | |
| 5 | Soya bean oil, refined | 126 | 18 | 6.60 | 7.73 | |
| 5 | Pumpkin seed oil | 6 | 17 | 5.00 | 6.00 | |
| 5 | Cocoa butter | 80 | 13 | 4.19 | 4.34 | |
| 5 | Peanut oil, edible | 41 | 10 | 6.98 | 7.75 | |
| 5 | Olive oils (b) | 299 | 7 | 9.49 | 9.60 | |
| 5 | Olive pomace oil | 51 | 0 (a) | 108.67 | 108.71 | |
| 5 | Linseed oil | 44 | 7 | 7.25 | 7.45 | |
| 5 | Palm oil/fat | 283 | 18 | 11.49 | 12.94 | |
| 5 | Rape seed oil, edible | 495 | 54 | 3.56 | 7.06 | |
| Coffee, cocoa, tea and infusions | 3 | Tea beverages | 5 | 0 | 0.72 | 0.73 |
| 3 | Cocoa beverages | 72 | 17 | 0.14 | 0.19 | |
| 3 | Herbal and other non‐tea infusions | 12 | 0 (a) | 0.34 | 0.35 | |
| 5 | Cocoa mass | 76 | 5 | 4.28 | 4.33 | |
| Composite dishes | 2 | Fried or extruded cereal, seed or root‐based products | 14 | 0 (a) | 3.76 | 3.99 |
| 2 | Dishes, incl. Ready to eat meals (excluding soups and salads) | 21 | 76 | 0.83 | 2.55 | |
| 3 | Salads | 9 | 0 (a) | 2.27 | 3.44 | |
| 3 | Dishes excluding pasta or rice dishes, sandwiches and pizza | 6 | 67 | 0.66 | 2.83 | |
| Eggs and egg products | 3 | Whole eggs | 29 | 52 | 2.08 | 3.28 |
| Fish, seafood, amphibians, reptiles and invertebrates | 2 | Fish (meat) | 22 | 86 | 0.15 | 1.97 |
| 2 | Crustaceans | 5 | 80 | 0.23 | 1.63 | |
| 3 | Processed or preserved seafood | 5 | 0 (a) | 6.70 | 7.20 | |
| 4 | Canned/jarred fish | 72 | 28 | 8.42 | 9.97 | |
| 4 | Smoked fish | 36 | 61 | 1.25 | 3.14 | |
| Food products for young population | 2 | Special food for children's growth | 14 | 36 | 2.17 | 4.32 |
| 2 | Ready‐to‐eat meal for infants and young children | 14 | 64 | 1.73 | 2.05 | |
| 3 | Simple cereals for infants or children, reconstituted | 96 | 63 | 0.45 | 0.69 | |
| 3 | Cereals with an added high protein food reconstituted | 5 | 60 | 0.16 | 0.69 | |
| 3 | Biscuits, rusks and cookies for children | 18 | 61 | 1.28 | 2.87 | |
| 4 | Infant formulae, liquid (c) | 213 | 9 | 0.47 | 0.78 | |
| 4 | Follow‐on formulae, liquid (c) | 160 | 18 | 0.30 | 0.56 | |
| Fruit and fruit products | 3 | Dried fruit | 8 | 75 | 0.34 | 0.96 |
| 4 | Strawberries and similar | 5 | 80 | 0.27 | 1.31 | |
| Grains and grain‐based products | 3 | Cereal bran | 11 | 64 | 0.76 | 1.70 |
| 3 | Cereal and cereal‐like flours | 80 | 20 | 2.70 | 3.12 | |
| 3 | Cakes | 19 | 42 | 2.41 | 5.78 | |
| 3 | Breakfast cereals, plain | 57 | 26 | 2.54 | 3.08 | |
| 3 | Biscuits | 86 | 33 | 3.38 | 4.53 | |
| 3 | Muesli and similar mixed breakfast cereals | 130 | 45 | 2.31 | 3.80 | |
| 3 | Yeast leavened pastry | 7 | 0 | 4.86 | 4.86 | |
| 3 | Various pastry | 21 | 10 | 2.79 | 2.95 | |
| 3 | Semolina | 207 | 30 | 1.84 | 2.56 | |
| 3 | Rusk | 11 | 64 | 0.94 | 2.43 | |
| 3 | Processed and mixed breakfast cereals | 81 | 30 | 2.89 | 3.20 | |
| 3 | Crisp bread | 35 | 66 | 0.49 | 2.19 | |
| 3 | Leavened bread and similar | 114 | 75 | 0.39 | 1.94 | |
| 4 | Pasta, plain (not stuffed), uncooked | 321 | 40 | 3.33 | 3.71 | |
| 4 | Groats (not from wheat) | 25 | 60 | 0.86 | 3.49 | |
| 4 | Breadcrumbs | 66 | 27 | 1.34 | 1.93 | |
| 4 | All cereal grains except rice | 40 | 55 | 1.00 | 1.88 | |
| 4 | Wheat groats | 19 | 32 | 1.57 | 2.95 | |
| 4 | Rice and similar‐ | 404 | 35 | 2.63 | 3.28 | |
| 4 | Raw doughs and pre‐mixes | 48 | 60 | 1.17 | 3.13 | |
| 4 | Pasta‐like products | 27 | 48 | 2.40 | 3.35 | |
| 5 | Bread and similar products | 240 | 60 | 0.87 | 2.20 | |
| 5 | Fine bakery wares, unspecified | 6 | 0 (a) | 7.84 | 8.73 | |
| 5 | Pasta and similar products, unspecified | 7 | 29 | 14.69 | 14.96 | |
| 5 | Pita bread | 12 | 75 | 4.13 | 5.63 | |
| Legumes, nuts, oilseeds and spices | 3 | Tree nuts | 88 | 33 | 2.73 | 2.96 |
| 3 | Pulses (dried legume seeds) | 40 | 15 | 1.09 | 1.14 | |
| 3 | Oilseeds | 49 | 43 | 2.00 | 2.41 | |
| 3 | Legumes fresh seeds (beans, peas, etc.) | 7 | 43 | 0.76 | 1.03 | |
| 3 | Fruit spices | 18 | 17 | 2.25 | 3.00 | |
| 4 | Pulses flour | 8 | 0 (a) | 5.48 | 5.51 | |
| Major isolated ingredients, additives, flavours, baking and processing aids | 2 | Starches | 39 | 82 | 0.50 | 1.48 |
| Meat and meat products | 2 | Animal liver | 11 | 0 (a) | 4.93 | 5.21 |
| 2 | Animal kidney | 7 | 29 | 1.00 | 1.41 | |
| 3 | Fresh raw sausages | 7 | 57 | 1.21 | 1.93 | |
| 3 | Preserved or partly preserved sausages | 57 | 39 | 3.14 | 4.49 | |
| 3 | Cured pork fat | 6 | 0 | 6.10 | 6.10 | |
| Milk and dairy products | 2 | Dairy dessert and similar | 31 | 90 | 0.34 | 2.87 |
| 3 | Processed cheese and spreads | 40 | 10 | 11.24 | 12.42 | |
| 3 | Milk and dairy powders | 5 | 80 | 0.10 | 1.78 | |
| 3 | Milk | 21 | 5 | 0.35 | 0.46 | |
| 5 | Cheese (fresh, ripened, brined) | 164 | 26 | 3.69 | 5.57 | |
| Products for non‐standard diets, food imitates and food supplements | 3 | Meat imitates | 42 | 62 | 9.36 | 9.91 |
| 5 | Imitation cheese | 2 | 0 | 2.93 | 2.93 | |
| Seasoning, sauces and condiments | 3 | Gravy ingredients | 42 | 14 | 1.68 | 2.43 |
| 4 | Mayonnaise, hollandaise and related sauces | 17 | 94 | 0.06 | 0.54 | |
| 4 | Chutneys | 8 | 13 | 1.84 | 2.24 | |
| Starchy roots or tubers and products thereof, sugar plants | 5 | Potato flakes | 5 | 80 | 0.30 | 0.82 |
| 5 | Mashed potato powder | 9 | 22 | 0.89 | 1.46 | |
| 5 | Dried potato products | 12 | 67 | 0.93 | 1.18 | |
| Sugar and similar, confectionery and water‐based sweet desserts | 3 | Sweet bars and other formed sweet masses | 8 | 50 | 4.69 | 5.53 |
| 3 | Candies (soft and hard) | 39 | 23 | 5.27 | 5.48 | |
| 4 | Sucrose (common sugar) | 5 | 40 | 0.45 | 0.93 | |
| 5 | Pralines | 61 | 23 | 2.24 | 2.75 | |
| 5 | Milk chocolate | 286 | 21 | 3.22 | 3.84 | |
| 5 | Filled chocolate | 20 | 30 | 2.84 | 3.51 | |
| 5 | White chocolate | 11 | 0 (a) | 2.00 | 2.10 | |
| 5 | Couverture chocolate | 22 | 27 | 5.53 | 5.91 | |
| 5 | Chocolate spread | 5 | 20 | 2.17 | 3.57 | |
| 5 | Chocolate coated confectionery | 13 | 0 | 19.08 | 19.15 | |
| 5 | Chocolate and similar | 188 | 24 | 3.60 | 4.80 | |
| 5 | Bitter chocolate | 54 | 22 | 4.19 | 5.53 | |
| Vegetables and vegetable products | 5 | Spinaches | 7 | 71 | 0.71 | 1.21 |
Although all samples were quantified for this particular food group, the presence of quantified and non‐quantified C‐fractions within some of the quantified samples explains the differences between LB and UB mean estimations.
Olive oil refers to food samples reported as ‘Olive oil, virgin or extra‐virgin’ and ‘Olive oil, refined’.
Most of the samples of Infant formula/Follow‐on formula analysed for MOAH and MOSH were powder samples. The values shown in the table are obtained after applying a dilution factor of eight prior to the linkage to the consumption data (EFSA, 2018b).
The main food categories in number of samples were the same as described for MOAH, ‘Animal and vegetable fats and oils and primary derivatives thereof’ with 2,588 samples, followed by ‘Grains and grain‐based products’ with 2,074 samples.
The highest mean concentrations were found in vegetable oils, the highest for ‘Olive pomace oil’ (n = 51, LB–UB = 108.7–108.7 mg/kg). Pomace oils are obtained by extraction (centrifugation or solvent extraction) from residues after the extraction of extra virgin or pure olive oil, and refined before consumption. The C‐fraction from C25 to C35 was on average the highest contributor to the total MOSH (∼ 61%). Other edible oils with relatively high levels of MOSH were ‘Coconut oil/fat’ (n = 253, mean LB–UB = 25.91–26.85 mg/kg), ‘Walnut oil’ (n = 18, mean LB–UB = 15.84–15.94 mg/kg) and ‘Palm oil/fat’ (n = 283, mean LB–UB = 11.49–12.54 mg/kg).
MOSH were also found in a great variety of samples codified as ‘Grains and grain‐based products’, among them foods widely consumed (see Table 8). The highest mean levels were reported for the food group ‘Pasta and similar products, unspecified’ (n = 7, mean LB–UB = 14.67–14.96 mg/kg). In the raw primary and minimally processed commodities (e.g. rice and flours), the main average contributors to the total levels were the low C‐fractions C16–C20 and C20–C25, assumed to come partly or predominantly from recycled paper board. In more elaborated commodities (e.g. cakes, biscuits), the heavier C‐fractions, in particular C25–C35 and C35–C40, gained relevance.
The mean LB–UB concentrations reported for samples codified as ‘Canned/jarred fish’ (all but two samples were canned fish) were 8.42–9.97 mg/kg (n = 72). Although fish can contain MOSH from environmental contamination, it seems that the main source could be the lubrication oil used for can manufacturing (Grob et al., 1997; EFSA CONTAM Panel, 2012). In fact, the average levels reported for ‘Fish (meat)’ were almost one order of magnitude lower (LB–UB = 0.15–1.97 mg/kg, n = 22). The predominant average contributor in canned fish was the C‐fraction C25–C35.
The highest mean MOSH concentrations in chocolate and chocolate/cocoa‐based products were reported for ‘Chocolate coated confectionery’ (LB–UB = 19.08–19.15 mg/kg, n = 13) followed by ‘Couverture chocolate’ (LB–UB = 5.53–5.91 mg/kg, n = 22). Within the same FoodEx2 Level 1 (‘Sugar and similar, confectionery and water‐based sweet desserts’), relatively high values (LB–UB = 5.27–5.48 mg/kg, n = 39) were also reported for samples of ‘Candies’. Glazing agents and/or waxed paper have been described as possible source (EFSA CONTAM Panel, 2012). The C25–C35 fraction was the main contributor. MOSH concentrations in butter and cheese were similar when related to the fat content, also with the C‐fraction C25–C35 being the most abundant.
As compared to the 2012 EFSA SO (EFSA CONTAM Panel, 2012), it seems there is a decrease in MOSH levels across the different food commodities, which, at least partially, could be explained by the different measures introduced by industry since 2012. Still, a comparison is difficult considering the limitations in the data set used in 2012 e.g. the database was smaller, the data were mainly from one data provider, and the conversion factors used for some food groups.
MOAH data (as used for the exposure assessment)
Table 9 shows the samples with the analytical data on MOAH used for the dietary exposure estimations; data belong to 11 food categories at FoodEx2 Level 1 grouped under 72 food groups that were used for the linkage with the consumption data. Samples were grouped at different FoodEx2 levels, from FoodEx2 Level 2 to FoodEx2 Level 5 (see also Annex G for summary statistics).
Table 9.
MOAH mean values in mg/kg, lower bound (LB) and upper bound (UB), for different food samples as used for the dietary exposure assessment
| FoodEx2 level 1 | FoodEx2 level | Food group | Mean MOAH levels (mg/kg) | |||
|---|---|---|---|---|---|---|
| Number of samples | % Left‐censored | LB | UB | |||
| Animal and vegetable fats and oils and primary derivatives thereof | 3 | Blended fat and oils, margarine | 100 | 41 | 1.38 | 2.41 |
| 3 | Butter | 38 | 97 | 0.10 | 1.80 | |
| 5 | Coconut oil/fat | 248 | 40 | 3.69 | 5.17 | |
| 5 | Cocoa butter | 78 | 90 | 0.16 | 1.06 | |
| 5 | Olive oil (a) | 298 | 92 | 0.34 | 1.22 | |
| 5 | Olive pomace oil | 51 | 0 (b) | 13.54 | 13.56 | |
| 5 | Maize oil, edible | 37 | 97 | 0.03 | 1.91 | |
| 5 | Linseed oil | 44 | 73 | 0.62 | 1.16 | |
| 5 | Palm oil/fat | 276 | 66 | 1.02 | 2.75 | |
| 5 | Peanut oil, edible | 41 | 80 | 0.36 | 1.60 | |
| 5 | Soya bean oil, refined | 126 | 98 | 0.05 | 1.48 | |
| 5 | Sunflower seed oil, edible | 519 | 92 | 0.15 | 1.61 | |
| 5 | Palm kernel oil, edible | 56 | 84 | 0.26 | 1.80 | |
| 5 | Sesame seed oil, edible | 11 | 64 | 1.94 | 2.93 | |
| 5 | Rape seed oil, edible | 340 | 97 | 0.08 | 1.33 | |
| 5 | Walnut oil | 17 | 41 | 1.48 | 2.23 | |
| Coffee, cocoa, tea and infusions | 3 | Tea beverages | 5 | 20 | 0.11 | 0.12 |
| 3 | Herbal and other non‐tea infusions | 13 | 46 | 0.04 | 0.07 | |
| 3 | Cocoa beverages | 70 | 73 | 0.03 | 0.07 | |
| 5 | Cocoa mass | 73 | 73 | 0.21 | 0.59 | |
| Composite dishes | 2 | Fried or extruded cereal, seed or root‐based products | 14 | 79 | 0.22 | 0.49 |
| 3 | Pastas and rice (or other cereal)‐based dishes | 20 | 95 | 0.09 | 1.14 | |
| Fish, seafood, amphibians, reptiles and invertebrates | 4 | Smoked fish | 36 | 97 | 0.04 | 1.29 |
| 4 | Canned/jarred fish | 69 | 96 | 0.22 | 1.58 | |
| Food products for young population | 2 | Ready‐to‐eat meal for infants and young children | 14 | 93 | 0.05 | 0.49 |
| 3 | Simple cereals for infants or children, reconstituted | 97 | 95 | 0.04 | 0.20 | |
| 4 | Infant formulae, liquid (c) | 277 | 91 | 0.01 | 0.26 | |
| 4 | Follow‐on formulae, liquid (c) | 215 | 87 | 0.01 | 0.23 | |
| Grains and grain‐based products | 4 | Bulgur | 15 | 73 | 0.22 | 3.21 |
| 4 | Barley and similar‐ | 18 | 94 | 0.04 | 0.68 | |
| 4 | Rice and similar‐ | 399 | 69 | 0.51 | 1.07 | |
| 4 | Wheat and similar‐ | 8 | 88 | 0.24 | 1.13 | |
| 4 | Millet flour | 10 | 70 | 0.88 | 1.06 | |
| 4 | Wheat flour | 26 | 77 | 0.15 | 0.44 | |
| 4 | Rye flour | 20 | 70 | 0.14 | 0.78 | |
| 4 | Wheat semolina | 185 | 83 | 0.11 | 0.61 | |
| 4 | Maize semolina | 5 | 60 | 0.10 | 0.66 | |
| 4 | Spelt flour | 13 | 46 | 0.15 | 0.34 | |
| 4 | Rice flour | 5 | 60 | 2.60 | 2.91 | |
| 4 | Wheat groats | 19 | 84 | 0.11 | 1.39 | |
| 4 | Pasta, plain (not stuffed), uncooked | 319 | 82 | 0.18 | 0.58 | |
| 5 | Pasta and similar products, unspecified | 7 | 43 | 0.81 | 0.91 | |
| 4 | Pasta‐like products | 26 | 85 | 0.25 | 1.08 | |
| 5 | Cake pre‐mixes (dry) | 24 | 50 | 1.38 | 1.98 | |
| 5 | Fine bakery wares unspecified | 6 | 67 | 0.37 | 2.00 | |
| 3 | Biscuits | 85 | 82 | 0.20 | 1.66 | |
| 3 | Cakes | 19 | 79 | 0.24 | 3.69 | |
| 5 | Bread and similar products, unspecified | 217 | 92 | 0.07 | 1.08 | |
| 4 | Breadcrumbs | 64 | 83 | 0.09 | 0.54 | |
| 3 | Leavened bread and similar | 105 | 95 | 0.07 | 1.23 | |
| 3 | Rusk | 11 | 91 | 0.30 | 1.02 | |
| 2 | Breakfast cereals | 261 | 81 | 0.19 | 0.96 | |
| Legumes, nuts, oilseeds and spices | 2 | Pulses flour | 9 | 25 | 0.83 | 0.92 |
| 2 | Legumes | 39 | 92 | 0.05 | 0.31 | |
| 3 | Tree nuts | 80 | 74 | 0.26 | 0.57 | |
| 3 | Oilseeds | 47 | 94 | 0.22 | 0.72 | |
| Milk and dairy products | 4 | Processed cheese wedges and similar | 30 | 93 | 0.12 | 1.76 |
| 4 | Firm – ripened cheeses | 72 | 97 | 0.05 | 1.24 | |
| 5 | Cow milk | 20 | 95 | 0.01 | 0.17 | |
| Seasoning, sauces and condiments | 2 | Savoury extracts and sauce ingredients | 42 | 90 | 0.03 | 0.62 |
| 3 | Relishes | 8 | 88 | 0.11 | 0.34 | |
| Starchy roots or tubers and products thereof, sugar plants | 5 | Dried potato products | 26 | 88 | 0.05 | 0.48 |
| Sugar and similar, confectionery and water‐based sweet desserts | 3 | Sweet bars and other formed sweet masses | 8 | 63 | 0.89 | 1.51 |
| 3 | Candies (soft and hard) | 39 | 90 | 0.25 | 0.92 | |
| 5 | Chocolate and similar, unspecified | 188 | 91 | 0.08 | 1.17 | |
| 5 | White chocolate | 11 | 91 | 0.03 | 0.65 | |
| 5 | Milk chocolate | 282 | 89 | 0.10 | 0.84 | |
| 5 | Bitter chocolate | 50 | 86 | 0.34 | 1.78 | |
| 5 | Filled chocolate | 20 | 85 | 0.39 | 1.01 | |
| 5 | Chocolate coated confectionery | 9 | 22 | 2.23 | 2.59 | |
| 5 | Couverture chocolate | 22 | 68 | 0.45 | 1.04 | |
| 5 | Pralines | 60 | 93 | 0.04 | 0.51 | |
Olive oil refers to food samples reported as ‘Olive oil, virgin or extra‐virgin’ and ‘Olive oil, refined’.
Although all samples were quantified for this particular food group, the presence of quantified and non‐quantified C‐fractions within some of the quantified samples explains the differences between LB and UB mean estimations.
Most of the samples of Infant formula/Follow‐on formula analysed for MOAH and MOSH were powder samples. The values shown in the table are obtained after applying a dilution factor of eight prior to the linkage to the consumption data (EFSA, 2018b).
The highest MOAH mean levels were reported for samples of ‘Olive pomace oil’ (n = 51, LB–UB = 13.54–13.56 mg/kg); relatively high levels of MOAH have been described in the literature for olive pomace oil. As observed for MOSH, the most relevant C‐fraction in the samples of olive pomace oil seems to be C25–C35 that accounts on average for ~ 83% of the reported MOAH levels, probably mainly due to the reduction of the MOAH subfractions ≤ C24 during the deodourisation step of the refining process. Another vegetable oil with relatively high MOAH levels was ‘Coconut oil/fat’ (n = 248, mean LB–UB = 3.69–5.17 mg/kg), with the predominant fraction from C35 to C50 representing on average ~ 75% of the total. The lowest mean MOAH concentrations among edible vegetable oils were reported for ‘Maize oil, edible’ (n = 37, LB–UB = 0.03–1.91 mg/kg), ‘Soya bean oil, refined’ (n = 126, LB–UB = 0.05–1.48 mg/kg) and ‘Rape seed oil, edible’ (n = 340, LB–UB = 0.08–1.33 mg/kg) (see Table 9).
As also commented for MOSH, MOAH were found in a great variety of samples codified as ‘Grains and grain‐based products’, among them foods widely consumed such as ‘Breakfast cereals’ (n = 261, mean LB–UB = 0.19–0.96 mg/kg), ‘Pasta, plain (not stuffed), uncooked’ (n = 319, mean LB–UB = 0.18–0.58 mg/kg) and ‘Biscuits’ (n = 70, mean LB–UB = 0.20–1.66 mg/kg). The highest mean levels were reported for few samples of ‘Rice flour’ (n = 5, mean LB–UB = 2.60–2.91 mg/kg) and ‘Cake pre‐mixes (dry)’ (n = 24, LB–UB = 1.38–1.98 mg/kg). The C‐fraction from C16 to C25 was the main average contributor to the MOAH levels across ‘Grains and grain‐based products’, suggesting transfer by the gas phase from, e.g. recycled paper board.
MOAH levels were also quantified in ‘Food products for young population’, although at low levels and with 90% of the samples being left‐censored. The highest concentrations were reported for ‘Ready‐to‐eat meal for infants and young children’ and ‘Simple cereals for infants or children, reconstituted’ with mean LB–UB = 0.05–0.49 mg/kg and mean LB–UB = 0.04–0.20 mg/kg, respectively. MOAH was also quantified in few of the analysed samples of ‘Infant formulae, liquid’ and ‘Follow‐on formulae, liquid’, with mean LB–UB = 0.01–0.26 mg/kg for the first (n = 277) and mean LB–UB = 0.01–0.23 mg/kg (n = 215) for the latter.
In 2019, EFSA conducted a rapid risk assessment to assess possible risks due to the presence of MOAH in infant formula and follow‐on formula (EFSA, 2019). At that time, dietary exposure estimations were based on the minimum and maximum results among the quantified samples (37 of 719 samples, min–max LB = 0.2–3 mg/kg dry weight). In the current data set, quantitative data were reported for 24 samples of ‘Infant formulae, liquid’ out of the 277 samples analysed, and for 29 samples of the 215 of ‘Follow‐on formulae, liquid’. Mean levels (LB–UB) among the quantified samples were 0.13–0.19 mg/kg (1.1–2.0 mg/kg dry weight) and 0.11–0.20 mg/kg (0.9–1.6 mg/kg dry weight) for infant formula and follow‐on formula, respectively. The Panel noted that the reported MOAH levels are within the range reported in 2019. The main average contribution to the MOAH concentrations in infant/follow‐on formula came from the C‐fractions C16–C25 and C25–C35, in particular the latter one.
3.2.2. Previously reported occurrence data in the open literature
Since the last opinion, some studies have been published on occurrence of MOSH and MOAH in foods. In this paragraph, only studies on food directly are mentioned i.e. studies on formation are not included. Some of the studies are also mentioned in Appendix A.
A summary of the data is shown in Table 10 for MOSH and in Table 11 for MOAH.
Table 10.
Summary of occurrence data reported in literature for MOSH
| MOSH C‐fractions analysed | Food | Positive/analysed samples | Information on the concentration (mg/kg) of positive samples | Analytical method | Additional information | Reference |
|---|---|---|---|---|---|---|
| C10‐C40 | Different types of foods | 142/198 | Below LOQ in several food groups. Maximum concentration in positive samples from 0.74 (Pasta etc.) up to 84.82 mg/kg (Confectionery including chocolate) | LC‐GC‐FID LOQ = 0.5 mg/kg | Different clean‐up methods used samples purchased on the Belgium market | Van Heyst et al. (2018) |
| Different fractions | Olive fruits olive oils | 9/9 (fruit); 5/5 (oil) | 0.4–3.2 (fruit); 10.3–38.0 (oil) | LC‐GC‐FID LOQ ≈ 0.1 mg/kg | Results also reported for PAHs | Gharbi et al. (2017) |
| C16–C25 and C25–C35 fractions | Infant formula (dairy milk‐based and goat milk‐based) | C16–C25: 17/51 C25–C35:0/51 | 1.5–3.5 | GC‐FID/MS LOQ = 0.5 mg/kg | SPE to separate MOAH from MOSH. For the positive samples, all the MOSH and MOAH humps fell into the C16–C25 fraction. | Sui et al. (2020) |
| C12–C35 | Dry products (rice, pasta, couscous, breadcrumbs, chocolate powder) | 11/11 | 7–44 | LC‐GC‐FID LOQ = 0.1 mg/kg | MOH removal during boiling dry foods in water | Biedermann‐Brem and Grob (2011a,b) |
| Three different fractions between C10 and C35 | Cereal products (semolina, pasta, bread, biscuits, cakes) | 53/53 | Sum of all fractions from about 0.1 to about 26 mg/kg | LC‐GC‐FID LOQ = 0.1 mg/kg | Samples purchased in Italy. Results shown in stacked bars for the different fractions and therefore only the sum is given. | Moret et al. (2016) |
| Five different fractions between C10 and C50 | Fresh and packaged fish and fish products | 16/21 | Sum of all fractions from 0.5 to 9.5 mg/kg | LC‐GC‐FID LOQ ≈ 0.1 mg/kg | Samples purchased in Italy | Srbinovska et al. (2020) |
| ≤ C16; ≤ C24; ≤ 35; > C35 | Cocoa butter, sunflower oil, palm oil | ≤ C16: 4/175 ≤ C24: 50/175 ≤ 35: 89/175 > C35: 63/175 | < LOQ‐3.8 < LOQ‐45 < LOQ‐103 < LOQ‐44 | LC‐GC‐FID LOQ = 2.5 mg/kg | Minimum concentration in positive samples are not given in the paper Samples from German confectioners and raw material suppliers | Stauff et al. (2020) |
| C20‐C44 | Vegetable oils | 18/18 | 7.7–180.6 | LC‐GC‐FID LOQ = 2 mg/kg | Purchased in Italy | Tranchida et al. (2011) |
| C14–C31 | Raw cocoa butter | 40/8 | UCM: 14–47 n‐alkanes: 32–72 | LC‐GC‐FID LOQ = 2 mg/kg | UCM = branched and cyclic‐n‐alkanes from C14 to C22 centred on C17 Samples originated from a factory in Spain | Bonvehi and Coll (2014) |
| C16–C35 | Infant formula Adult milk powder | 38/24 12/9 | 0.09–0.65 0.07–2.55 | LC‐GC‐FID LOQ = 0.1 mg/kg | Samples purchased in China | Zhan et al. (2019) |
| Not stated | Dried unprepared foods | 1/56 | 17 |
Refer to BfR (2012) LOQ = 10 mg/kg |
FSANZ (2018) |
Table 11.
Summary of occurrence data reported in literature for MOAH
| MOAH C‐fractions analysed | Food | Positive/analysed samples | Information on the concentration (mg/kg) | Analytical method | Additional information | Reference |
|---|---|---|---|---|---|---|
| C10–C40 | Different types of foods | 23/198 | Below LOQ in most food groups. Maximum in positive samples between 0.64 (Cereals) up to 2.24 mg/kg (Confectionery including chocolate) | LC‐GC‐FIDLOQ = 0.5 mg/kg | Different clean‐up methods used Samples purchased on the Belgium market | Van Heyst et al. (2018) |
| Not described | Olive fruits, olive oils | 0/9 (fruit) 0/5 (oil) | LC‐GC‐FIDLOQ ≈ 0.1 mg/kg | Results also reported for PAHs | Gharbi et al. (2017) | |
| C16–C25 and C25–C35 fractions | Infant formula (dairy milk‐based and goat‐milk based) | C16–C25: 7/51C25–C35:0/51 | 0.5–1.7 | GC‐FID/MS LOQ = 0.5 mg/kg | SPE to separate MOAH from MOSH. For the positive samples, all the MOSH and MOAH humps fell into the C16–C25 fraction. | Sui et al. (2020) |
| C10–C35 | Cereal products (semolina, pasta, bread, biscuits, cakes) | 17/53 | From about LOQ up to 3.6 | LC‐GC‐FID LOQ = 0.1 mg/kg | Samples purchased in Italy. Results shown in stacked bars for the different fractions and therefore only the sum is given. | Moret et al. (2016) |
| Four different fractions between C10 and C40 | Fresh and packaged fish and fish products | 4/21 | Sum of all fractions from 0.7 to 1.4 | LC‐GC‐FID LOQ ≈ 0.1 mg/kg | Samples purchased in Italy | Srbinovska et al. (2020) |
| ≤ C24; ≤ 35; > C35 | Cocoa butter, sunflower oil, palm oil | ≤ C24: 19/175 ≤ 35: 24/175 > C35: 15/175 |
< LOQ – 36 < LOQ – 23 < LOQ – 18 |
LC‐GC‐FID LOQ = 2.5 mg/kg | Minimum concentration in positive samples are not given in the paper. Samples from German confectioners and raw material suppliers | Stauff et al. (2020) |
| C10–40 | Cereals products (primarily rice) and beans | 10/38 | 0.07–1.45 (in positive samples) | LC‐GC‐FID LOQ = 0.05 mg/kg | Samples purchased in Beijing. Fraction found from C13 to C24 centred about C18 or C19 | Xie et al. (2019) |
| Not stated | Infant formula Adult milk powder | 0/240/9 | LC‐GC‐FID LOQ = 0.1 mg/kg | Samples purchased in China | Zhan et al. (2019) | |
| Not stated | Dried unprepared foods | 4/56 | 15–150 |
Refer to BfR (2012) LOQ = 10 mg/kg |
FSANZ (2018) | |
| C16–35 | Dry foods in paperboard boxes | 102/119 | 0.2–101 (in positive samples) | LOQ below 0.2 mg/kg | Samples purchased on the German market in 2010 | Biedermann et al. (2013) |
Van Heyst et al. (2018) analysed in total 198 samples, purchased from the Belgium market, for the concentrations of MOSH and MOAH. The samples were sampled in food items suspected to contain a high amount of mineral oil and highly consumed both in quantity and frequency, e.g. coffee, chocolate, pasta, cereals, oils, fish, etc. The number of samples taken for each food item was between 1 and 7. For MOSH, 142 samples have concentrations above the LOQ of 0.5 mg/kg. The maximum in the positive samples were between 0.74 mg/kg and 84.82 mg/kg. For MOAH, 23 samples were positive with concentrations between 0.64 and 2.24 mg/kg.
FSANZ (2018) has analysed 56 samples of dried unprepared foods with a LOQ of 10 mg/kg for what was called total MOSH and total MOAH. In the report, there is no indication of which fractions that are analysed. One sample of dry chocolate cake mix was found to have a MOSH level of 71 mg/kg. For MOAH detections above the LOQ were found in couscous (85 mg/kg) and three samples of chocolate cake mix (17–150 mg/kg). The positive samples have very high concentrations of MOAH compared to what is normally found and the levels are higher than for MOSH which is also unusual and in contrast to normal findings. It should also be noted that the foods were taken among samples where a high concentration was expected.
Gharbi et al. (2017) have analysed nine sample of olive fruit and five samples extra virgin olive oil for MOSH and MOAH (see also Appendix A) and all samples were positive with concentrations between, respectively, 0.4–3.2 mg/kg (even though the authors stated that LOQ was about 1 mg/kg) in the fruit and 10.3–38.0 mg/kg in the olive oil. No MOAH was detected in any samples.
Stauff et al. (2020) reported findings of MOSH and MOAH in 175 samples of cocoa butter (see also Appendix A) as well as sunflower and palm oil. Different fractions were analysed. The number of positive samples was between 4 and 89 for MOSH dependent on fraction. The highest concentrations were between 3.8 and 103 mg/kg. For MOAH, between 15 and 24 samples were positive and the highest concentrations were between 18 and 36 mg/kg.
Results for MOSH and MOAH in infant formula from the Chinese market have been reported by Zhang et al. (2019) and Sui et al. (2020). For MOSH, 24 of 38 samples and 17 of 51 samples were positive, respectively. Concentrations in positive samples were between 0.09 and 3.5 mg/kg in the two studies. In Zhang et al. also what the authors called ‘adult milk powder’ was analysed. Here, nine of 12 samples were positive with concentrations between 0.07 and 2.55 mg/kg. For MOAH, none of the samples analysed in Zhang et al. (2019) were positive while in Sui et al. (2020), seven samples were positive with concentrations between 0.5 and 1.7 mg/kg. It should be noted that the samples show similar MOSH and MOAH concentrations which are in contrast with other data showing normally MOSH > MOAH.
Also, fresh and frozen fish as well as fish dishes have been analysed for MOSH and MOAH (Srbinovska et al., 2020) and for MOSH, 16 of 21 samples were positive with concentrations from 0.5 (LOQ) to 9.5 mg/kg. For MOAH, four samples have concentrations above LOQ that were between 0.7 and 1.4 mg/kg. This study is also included in Appendix A.
Concentrations in different cereal products from the Italian market were reported for both MOSH and MOAH in Moret et al. (2016). MOSH was found in all 53 samples in concentrations from about 0.1 mg/kg to about 26 mg/kg. For MOAH, 17 samples have concentration from about the LOQ of 0.1 and up to 3.6 mg/kg.
In 2010, 119 samples of dry food, such as semolina, rice, cereals and noodles, packed in paperboard boxes were sampled from the German market. The focus was on the migration from paperboard and printing inks, but other sources could not be excluded. The samples were analysed twice, first as collected (Vollmer et al., 2011, see EFSA opinion from 2012), another time when at or approaching the end of the shelf‐life (Biedermann et al. (2013). In the second round of analysis, the average MOSH concentration in the foods was 14.3 mg/kg, which was an increase by 60% from the first analysis, with a maximum of 101 mg/kg. The MOAH concentration exceeded 1 mg/kg in 58% of the samples, with a maximum at 13 mg/kg.
The removal of MOH migrated from paperboard into dry foods during cooking in boiling water was investigated by Biedermann‐Brem and Grob (2011). Rice, pasta, couscous, breadcrumbs and chocolate powder packed in recycled paperboard boxes were analysed for MOSH. All samples contained between 7 and 44 mg/kg MOSH. In rice under normal cooking conditions about 40% was removed. For couscous and pasta, no significant decreases were seen under normal cooking conditions.
Raw cocoa butter was analysed for MOSH in 40 samples (Bonvehi and Coll, 2014) and concentrations above the LOQ were found in eight samples in amounts from 14 to 72 mg/kg (see also Appendix A).
Different vegetables oils were analysed for MOSH (Tranchida et al., 2011). In all 19 samples, MOSH were found with concentrations between 7.7 and 180 mg/kg.
Cereals products (primary rice) and beans from the Beijing area were analysed for MOAH. Ten of 38 samples had concentrations between 0.07 and 1.45 mg/kg (Xie et al., 2019).
In general, the levels found in the literature for MOSH and MOAH are similar to the data submitted to EFSA. It seems that in the literature, higher maximum concentration is found in cereals and oils; contents in foods packed in recycled and printed paperboard seem to be higher.
3.3. Dietary exposure assessment for humans
3.3.1. Current dietary exposure assessment
Chronic dietary exposure to MOSH and MOAH was estimated following the methodology described in Section 2.6, using the occurrence data shown in Tables 8 and 9 in Section 3.2.1.
Mean and high dietary exposure to MOSH (general scenario)
Table 12 shows a summary of the chronic dietary exposure estimates to MOSH across 47 dietary surveys carried out in 22 European countries. The exposure estimates calculated for each dietary survey together with further details on the contribution of the food groups are presented in Annex F.
Table 12.
Summary statistics of the chronic dietary exposure assessment (mg/kg bw per day) to MOSH across European dietary surveys. n, number of dietary surveys
| Mean dietary exposure (mg/kg bw per day) | |||||||
|---|---|---|---|---|---|---|---|
| Lower bound (LB) | Upper bound (UB) | ||||||
| n | Min | Median | Max | Min | Median | Max | |
| Infants | 12 | 0.026 | 0.055 | 0.085 | 0.041 | 0.081 | 0.126 |
| Toddlers | 15 | 0.043 | 0.050 | 0.070 | 0.061 | 0.079 | 0.104 |
| Other children | 19 | 0.034 | 0.040 | 0.053 | 0.051 | 0.062 | 0.069 |
| Adolescents | 21 | 0.017 | 0.021 | 0.032 | 0.026 | 0.031 | 0.045 |
| Adults | 22 | 0.009 | 0.015 | 0.020 | 0.016 | 0.023 | 0.028 |
| Elderly | 19 | 0.009 | 0.013 | 0.017 | 0.014 | 0.021 | 0.025 |
| Very elderly | 14 | 0.009 | 0.016 | 0.017 | 0.015 | 0.024 | 0.027 |
| Pregnant women | 6 | 0.010 | 0.013 | 0.018 | 0.016 | 0.020 | 0.027 |
| Lactating women | 2 | 0.014 | – | 0.020 | 0.025 | – | 0.028 |
| Vegetarians | 1 | – | – | 0.012 | – | – | 0.018 |
| 95th percentile dietary exposure (mg/kg bw per day) | |||||||
| n | Lower bound (LB) | Upper bound (UB) | |||||
| Min | Median | Max | Min | Median | Max | ||
| Infants | 12 | 0.054 | 0.098 | 0.157 | 0.086 | 0.138 | 0.212 |
| Toddlers | 15 | 0.070 | 0.086 | 0.125 | 0.095 | 0.130 | 0.176 |
| Other children | 19 | 0.058 | 0.069 | 0.086 | 0.085 | 0.104 | 0.119 |
| Adolescents | 21 | 0.033 | 0.038 | 0.059 | 0.048 | 0.055 | 0.081 |
| Adults | 22 | 0.020 | 0.029 | 0.037 | 0.029 | 0.041 | 0.049 |
| Elderly | 19 | 0.016 | 0.025 | 0.034 | 0.025 | 0.035 | 0.048 |
| Very elderly | 14 | 0.016 | 0.028 | 0.036 | 0.027 | 0.041 | 0.050 |
| Pregnant women | 6 | 0.020 | 0.025 | 0.033 | 0.028 | 0.036 | 0.046 |
| Lactating women | 2 | 0.025 | – | 0.037 | 0.040 | – | 0.050 |
| Vegetarians | 1 | 0.027 | 0.038 | ||||
The highest dietary exposure was estimated for the young population, in particular ‘Infants’, with LB–UB means of 0.085–0.126 mg/kg bw per day and LB–UB 95th percentiles of 0.157–0.212 mg/kg bw per day. In the adult population, 18 dietary exposure was similar across the different population groups with maximum LB–UB estimates of 0.020–0.028 mg/kg bw per day and 0.037–0.050 mg/kg bw per day for mean and 95th percentile, respectively. The exposure estimates for ‘Pregnant women’ and ‘Lactating women’ were within those in the adult population. The same was observed for the only dietary survey on ‘Vegetarians’.
In general, the maximum exposure estimates to MOSH (mean and 95th percentile) across dietary surveys showed a 1.5‐ to 2.5‐fold decrease in comparison to the exposure assessment performed in 2012 (EFSA CONTAM Panel, 2012). However, comparisons with the previous exposure estimates are difficult as the consumption data are different, food coverage at that time was probably lower, part of the occurrence data was derived using conversion factors, foods were codified in FoodEx1 instead of FoodEx2, etc.
Contribution of food categories to the chronic dietary exposure to MOSH (LB estimations)
Across the different age classes, the food groups with the highest average contribution to the mean dietary exposure to MOSH were ‘Grains and grain‐based products’, ‘Milk and dairy products’ and ‘Animal and vegetable fats and oils and primary derivatives thereof’.
For ‘Infants’, the contribution of two food groups stands out: ‘Food products for young population’ (18–76%, median = 60%) and ‘Milk and dairy products’ (6–51%, median = 24%). In the first, ‘Infant formulae, liquid’, ‘Follow‐on formulae, liquid’ and ‘Ready‐to‐eat meal for infants and young children’ were the most important foods, with different relevance depending on the dietary survey (Table 13). Within ‘Milk and dairy products’, the main contribution came from the consumption of milk in almost all dietary surveys.
Table 13.
Average contribution of selected food groups to the mean dietary exposure to MOSH (at the LB estimations) across the different population groups. Number of dietary surveys by population group: Infants (n = 12), toddlers (n = 15), other children (n = 19), adolescents (n = 21), adults (n = 22), elderly (n = 19), very elderly (n = 14), pregnant women (n = 6), lactating women (n = 2), vegetarians (n = 1)
| Animal and vegetable fats and oils and primary derivatives thereof | Coffee, cocoa, tea and infusions | Composite dishes | Food products for young population | Grains and grain‐based products | Meat and meat products | Milk and dairy products | Sugar and similar, confectionery and water‐based sweet desserts | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | P50 | Max | Min | P50 | Max | Min | P50 | Max | Min | P50 | Max | Min | P50 | Max | Min | P50 | Max | Min | P50 | Max | Min | P50 | Max | |
| Infants | 2 | 6 | 20 | 0 | 1 | 4 | 0 | 0.1 | 1 | 18 | 60 | 76 | 2 | 8 | 21 | 0 | 0.2 | 2 | 56 | 24 | 51 | 0 | 0.1 | 1 |
| Toddlers | 6 | 15 | 30 | 0.1 | 1 | 9 | 0.3 | 1 | 8 | 1 | 11 | 32 | 16 | 29 | 40 | 0.4 | 4 | 11 | 18 | 27 | 37 | 1 | 3 | 9 |
| Other children | 9 | 16 | 34 | 1 | 3 | 14 | 0.2 | 2 | 28 | 0 | 0.4 | 4 | 20 | 36 | 42 | 0.4 | 7 | 12 | 15 | 22 | 32 | 2 | 6 | 14 |
| Adolescents | 6 | 16 | 32 | 1 | 3 | 15 | 0 | 2 | 8 | 0 | 0 | 1 | 23 | 38 | 55 | 1 | 6 | 10 | 13 | 21 | 29 | 2 | 8 | 15 |
| Adults | 8 | 18 | 36 | 1 | 7 | 24 | 0.1 | 1 | 21 | 0 | 0 | 0.2 | 17 | 30 | 42 | 1 | 5 | 16 | 13 | 20 | 27 | 1 | 5 | 10 |
| Elderly | 9 | 25 | 47 | 3 | 11 | 33 | 0 | 0.5 | 17 | 0 | 0 | 0.1 | 15 | 25 | 38 | 1 | 5 | 15 | 11 | 20 | 29 | 1 | 2 | 6 |
| Very elderly | 12 | 27 | 49 | 3 | 12 | 35 | 0.1 | 2 | 18 | 0 | 0 | 0.4 | 15 | 26 | 39 | 1 | 5 | 13 | 10 | 17 | 25 | 1 | 3 | 6 |
| Pregnant women | 8 | 18 | 25 | 1 | 5 | 19 | 1 | 1 | 3 | 0 | 0 | 0.4 | 33 | 38 | 42 | 1 | 3 | 5 | 19 | 21 | 25 | 2 | 3 | 7 |
| Lactating women | 12 | – | 16 | 1 | – | 18 | 0 | – | 19 | 0.2 | – | 0.3 | 26 | – | 26 | 1 | – | 5 | 26 | – | 30 | 3 | – | 5 |
| Vegetarians | 15 | 12 | 1 | 0 | 34 | 0 | 12 | 4 | ||||||||||||||||
For ‘Toddlers’, the main contributors were ‘grains and grain‐based products’ (16–40%, median = 29%) and ‘Milk and dairy products’ (18–37%, median = 27%). In the survey with the highest estimate, the latter was more relevant, in particular due to the consumption of cheese. Overall, cheese became the main source of MOSH within ‘Milk and dairy products’. Among ‘grains and grain‐based products’, many food groups contributed, with ‘Biscuits’ and ‘Pasta, plain (not stuffed), uncooked’ being the main sources. ‘Food products for young population’ were also among the main contributors (1–32%, median = 11%). Additionally, ‘animal and vegetable fats and oils and primary derivatives thereof’ became a relevant source (6–30%, median = 15%).
The same food groups dominated for ‘other children’, with the exception of ‘food products for young population’ that almost disappeared. As the diet becomes more varied in this age class, other food groups appeared, such as ‘meat and meat products’ (0.4–12%, median = 7%), in particular due to the reported consumption of ‘preserved or partly preserved sausages’, and ‘sugar and similar, confectionery and water‐based sweet desserts’ (2–14%, median = 6%), with ‘chocolate coated confectionery’ as recurrent main contributor.
In the adult population, there were three main contributors to the exposure to MOSH: ‘grains and grain‐based products’, ‘animal and vegetable fats and oils and primary derivatives thereof’, and ‘milk and dairy products’. Their relevance varied among dietary surveys (see Table 13). In the case of ‘grains and grain‐based products’, a wide variety of foods added to the exposure, among which ‘pasta, plain (not stuffed), uncooked’ and ‘yeast leavened pastry’. Depending on the country, the main sources of exposure to ‘animal and vegetable fats and oils and primary derivatives thereof’ were ‘margarines and similar’, ‘olive oils’ and, to a lesser extent, ‘butter’. The consumption of cheese was the main driver in the relatively high average contribution of ‘milk and dairy products’. To mention that for ‘Vegetarians’, the above food groups identified in the adult population were less relevant (with the exception of ‘grains and grain‐based products’), and at the same time, new groups appeared: ‘legumes, nuts, oilseeds and spices’ (9%) and ‘products for non‐standard diets, food imitates and food supplements’ (12%), the latter mainly due to the consumption of meat imitates.
Mean and high dietary exposure to MOAH (general scenario)
Table 14 shows a summary of the chronic dietary exposure estimates to MOAH across 47 dietary surveys carried out in 22 European countries. The estimates, calculated for each dietary survey together with further details on the contribution of the food groups, are presented in Annex G.
Table 14.
Summary statistics of the chronic dietary exposure assessment (mg/kg bw per day) to MOAH across European dietary surveys. n, number of dietary surveys
| Mean dietary exposure (mg/kg bw per day) | |||||||
|---|---|---|---|---|---|---|---|
| Lower bound (LB) | Upper bound (UB) | ||||||
| n | Min | Median | Max | Min | Median | Max | |
| Infants | 12 | 0.001 | 0.002 | 0.003 | 0.011 | 0.018 | 0.031 |
| Toddlers | 15 | 0.002 | 0.002 | 0.003 | 0.013 | 0.017 | 0.021 |
| Other children | 19 | 0.001 | 0.002 | 0.003 | 0.011 | 0.013 | 0.015 |
| Adolescents | 21 | 0.001 | 0.001 | 0.002 | 0.005 | 0.007 | 0.010 |
| Adults | 22 | 0.0004 | 0.001 | 0.001 | 0.003 | 0.005 | 0.006 |
| Elderly | 19 | 0.0004 | 0.001 | 0.002 | 0.003 | 0.004 | 0.006 |
| Very elderly | 14 | 0.001 | 0.001 | 0.002 | 0.004 | 0.005 | 0.006 |
| Pregnant women | 6 | 0.001 | 0.001 | 0.001 | 0.004 | 0.005 | 0.006 |
| Lactating women | 2 | 0.001 | – | 0.001 | 0.005 | – | 0.006 |
| Vegetarians | 1 | 0.001 | 0.004 | ||||
| 95th percentile dietary exposure (mg/kg bw per day) | |||||||
| n | Lower bound (LB) | Upper bound (UB) | |||||
| Min | Median | Max | Min | Median | Max | ||
| Infants | 12 | 0.003 | 0.004 | 0.011 | 0.029 | 0.036 | 0.059 |
| Toddlers | 15 | 0.003 | 0.004 | 0.009 | 0.024 | 0.029 | 0.040 |
| Other children | 19 | 0.003 | 0.004 | 0.006 | 0.018 | 0.024 | 0.029 |
| Adolescents | 21 | 0.001 | 0.002 | 0.004 | 0.010 | 0.014 | 0.017 |
| Adults | 22 | 0.001 | 0.002 | 0.003 | 0.007 | 0.009 | 0.012 |
| Elderly | 19 | 0.001 | 0.002 | 0.003 | 0.006 | 0.008 | 0.013 |
| Very elderly | 14 | 0.001 | 0.002 | 0.004 | 0.008 | 0.009 | 0.013 |
| Pregnant women | 6 | 0.001 | 0.001 | 0.003 | 0.007 | 0.010 | 0.010 |
| Lactating women | 2 | 0.001 | – | 0.003 | 0.009 | – | 0.009 |
| Vegetarians | 1 | 0.002 | 0.010 | ||||
The highest dietary exposure was estimated in the young population, in particular in ‘Infants’, with LB–UB mean estimations of 0.003–0.031 mg/kg bw per day and LB–UB 95th percentile estimations of 0.011–0.059 mg/kg bw per day. As also observed for the exposure to MOSH, in the adult population, 17 dietary exposure was rather similar across the different population groups with maximum LB–UB estimates of 0.002–0.006 mg/kg bw per day and 0.004–0.013 mg/kg bw per day for mean and 95th percentile, respectively. The exposure estimates for ‘Pregnant women’ and ‘Lactating women’ were within those in the adult population; the same was observed for the only dietary survey on ‘Vegetarians’.
Contribution of food categories to the chronic dietary exposure to MOAH (LB estimations)
Overall, the food groups with the highest average contribution to the mean dietary exposure to MOAH in the general population were ‘grains and grain‐based products’, ‘animal and vegetable fats and oils and primary derivatives thereof’, and ‘coffee, cocoa, tea and infusions’.
Two aspects are noted in relation to the food group ‘coffee, cocoa, tea and infusions’ (FoodEx2 Level 1). First, no samples of coffee were analysed for the presence of MOAH (Table 9); therefore, there is no contribution of this specific commodity to the overall exposure to MOAH in any of the population groups. Second, for the different types of tea and infusions and in the absence of specific data, a 100% transfer of the MOAH quantified in the dry product was considered during the brewing process (Section 2.6). A dilution factor of 75 was applied to assign the final MOAH concentration in ‘Tea beverages’ and ‘Herbal and other non‐tea infusions’ (Table 9).
For ‘Infants’, the most important contributor was ‘Food products for young population’ with an average contribution between 11% and 88% (median 64%) across dietary surveys (Table 15). The main drivers were ‘Follow‐on formulae, liquid’ and ‘Infant formulae, liquid’, although ‘Ready‐to‐eat meal for infants and young children’ was also an important source in several dietary surveys. A second food group in importance was ‘grains and grain‐based products’ (11–69%, median = 23%). This is a group that encompasses many food commodities; in those countries with high contribution of this group and with relatively high levels of exposure, the main contributors were ‘rice flour’ and ‘breakfast cereals’.
Table 15.
Average contribution of selected food groups to the mean dietary exposure to MOAH (at the LB estimations) across the different population groups. Number of dietary surveys by population group: Infants (n = 12), toddlers (n = 15), other children (n = 19), adolescents (n = 21), adults (n = 22), elderly (n = 19), very elderly (n = 14), pregnant women (n = 6), lactating women (n = 2), vegetarians (n = 1)
| Animal and vegetable fats and oils and primary derivatives thereof | Coffee, cocoa, tea and infusions | Composite dishes | Food products for young population | Grains and grain‐based products | Legumes, nuts, oilseeds and spices | Milk and dairy products | Sugar and similar, confectionery and water‐based sweet desserts | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | P50 | Max | Min | P50 | Max | Min | P50 | Max | Min | P50 | Max | Min | P50 | Max | Min | P50 | Max | Min | P50 | Max | Min | P50 | Max | |
| Infants | 0.2 | 5 | 33 | 0 | 2 | 18 | 0 | 0.1 | 1 | 11 | 64 | 88 | 11 | 23 | 69 | 0.04 | 0.4 | 2 | 0.3 | 1 | 4 | 0 | 0.03 | 1 |
| Toddlers | 3 | 12 | 44 | 0.1 | 5 | 24 | 0 | 1 | 3 | 1 | 7 | 41 | 32 | 53 | 70 | 0.3 | 1 | 2 | 3 | 5 | 7 | 0.2 | 4 | 13 |
| Other children | 4 | 14 | 49 | 2 | 10 | 35 | 0.3 | 2 | 23 | 0 | 0.1 | 2 | 29 | 52 | 72 | 0.2 | 1 | 2 | 2 | 4 | 7 | 1 | 8 | 24 |
| Adolescents | 2 | 14 | 47 | 2 | 12 | 36 | 0 | 2 | 5 | 0 | 0 | 2 | 26 | 50 | 74 | 0.3 | 1 | 2 | 2 | 4 | 9 | 1 | 10 | 23 |
| Adults | 4 | 18 | 40 | 4 | 21 | 45 | 0.1 | 1 | 4 | 0 | 0. | 0.2 | 22 | 43 | 65 | 0.6 | 2 | 5 | 2 | 3 | 5 | 1 | 7 | 15 |
| Elderly | 5 | 19 | 57 | 8 | 24 | 55 | 0 | 0.3 | 4 | 0 | 0 | 0.1 | 20 | 38 | 64 | 0.8 | 2 | 5 | 1 | 3 | 5 | 0.3 | 3 | 11 |
| Very elderly | 6 | 24 | 59 | 10 | 32 | 55 | 0 | 0.3 | 5 | 0 | 0 | 0.4 | 18 | 35 | 65 | 0.5 | 2 | 3 | 1 | 2 | 4 | 0.4 | 3 | 8 |
| Pregnant women | 4 | 9 | 21 | 4 | 16 | 42 | 1 | 1 | 2 | 0 | 0 | 0.5 | 46 | 55 | 64 | 2 | 3 | 5 | 2 | 3 | 6 | 3 | 4 | 11 |
| Lactating women | 10 | – | 16 | 4 | – | 45 | 0.02 | – | 19 | 0.1 | – | 0.3 | 34 | – | 47 | 2 | – | 2 | 2 | – | 5 | 6 | – | 6 |
| Vegetarians | 10 | 30 | 1 | – | 37 | 13 | 1 | 7 | ||||||||||||||||
As seen in Table 15, the group ‘grains and grain‐based products’ was the most important contributor for ‘toddlers’ (32–70%, median = 53%), with several types of foods as main sources: ‘breakfast cereals’, ‘pasta, plain (not stuffed), uncooked’, ‘rice and similar’, ‘biscuits’ and ‘leavened bread and similar’. It is important to mention ‘animal and vegetable fats and oils and primary derivatives thereof’ (3–44%, median = 12%), in particular the combined contribution of ‘blended fat and oils’ and ‘margarine’ that accounted for most of the contribution of this food group. Although less relevant than for ‘infants’, the group ‘food products for young population’ was still an important source of exposure to MOAH (1–41%, median = 7%).
The relevance of ‘food products for young population’ for ‘other children’ was rather low. For them, again ‘grains and grain‐based products’ (29–72%, median = 52%) were the main source, with the same foods as mentioned for ‘toddlers’ and the addition of ‘cakes’. A source shared with ‘toddlers’ was ‘animal and vegetable fats and oils and primary derivatives thereof’, with the highest contribution due to the consumption of ‘blended fat and oils’ and ‘margarine’. The reported consumption of ‘tea beverages’ in some dietary surveys made ‘coffee, cocoa, tea and infusions’ relatively important (2–35%, median = 10%). Similarly, the consumption of ‘chocolate coated confectionery’ increased the average contributions of the group ‘sugar and similar, confectionery and water‐based sweet desserts’ up to 24%.
In the adult population, the most relevant contributors to the exposure to MOAH were similar to those in other age classes. The food group contributing the most was again ‘grains and grain‐based products’. The groups ‘animal and vegetable fats and oils and primary derivatives thereof’ and ‘coffee, cocoa, tea and infusions' were also relevant sources (see Table 15), in particular due to the high consumption of ‘tea beverages' (e.g. in the ‘very elderly’ population). As mentioned above, coffee did not contribute to the dietary exposure, as no occurrence data were available. The contribution of cocoa beverages was negligible as compared to that from ‘Herbal and other non‐tea infusions' and in particular ‘Tea beverages'.
The main sources of exposure for ‘pregnant women’ and ‘lactating women’ were similar to those in the adult population. For ‘vegetarians’, the contribution of ‘grains and grain‐based products’ and ‘animal and vegetable fats and oils and primary derivatives thereof’ was lower as compared to other population groups (38% and 10%, respectively), while ‘legumes, nuts, oilseeds and spices’ appeared as a relevant contributor (13%; only single survey).
Ad hoc dietary exposure scenarios for MOAH and MOSH
As mentioned in Section 2.6, different ad hoc scenarios were carried out to complement the general exposure scenario.
Infant formula scenario
Although a relatively high number of samples of ‘Infant formula’ with analytical data on MOAH and MOSH was available, the accuracy on the contribution of this commodity to the total exposure in infants is affected by the limited number of infants with 0–3 months present in the comprehensive database. To have a better understanding of the relevance of ‘Infant formula’ in the dietary exposure to MOAH and MOSH for infants, the CONTAM Panel decided to use two default consumption values: 170 and 210 mL/kg bw per day for mean and P95 consumption, respectively. These are the consumption values recommended by the Guidance of the EFSA Scientific Committee (SC) on the risk assessment of substances present in food intended for infants below 16 weeks of age (EFSA Scientific Committee, 2017a,b), and also used in the previous EFSA risk assessment on the presence of MOAH in infant formula (EFSA, 2019a,b).
Two possible scenarios were considered: one for average consumers (scenario 1) using the mean occurrence values that are considered as the most representative for chronic exposure estimates, and a second scenario (scenario 2) where infants were supposed to have a continuously consumption of a highly contaminated product. In scenario 2, the P95 occurrence levels for MOAH and MOSH were used. The different exposure estimates are shown in Table 16. For scenario 2, mean exposures (LB–UB) could be as high as 0.22–0.30 mg/kg bw day (MOSH) and 0.019–0.051 mg/kg bw day (MOAH), while the P95 exposure (LB–UB) could be 0.27–0.37 mg/kg bw day (MOSH) and 0.023–0.063 mg/kg bw day (MOAH).
Table 16.
Dietary exposure estimates for MOSH and MOAH via the consumption data of ‘Infant formula’ using default consumption data as recommended by the EFSA Scientific Committee (a) and the occurrence data reported to EFSA (Section 3.2.1)
| Dietary exposure to MOSH (mg/kg bw day) | ||||
|---|---|---|---|---|
| Mean exposure | P95 exposure | |||
| LB | UB | LB | UB | |
| Scenario 1 (b) | 0.08 | 0.13 | 0.10 | 0.16 |
| Scenario 2 (c) | 0.22 | 0.30 | 0.27 | 0.37 |
| Dietary exposure to MOAH (mg/kg bw day) | ||||
| Mean exposure | P95 exposure | |||
| LB | UB | LB | UB | |
| Scenario 1 (d) | 0.002 | 0.045 | 0.003 | 0.056 |
| Scenario 2 (e) | 0.019 | 0.051 | 0.023 | 0.063 |
For the exposure estimations, default consumption values of 170 mL/kg bw per day (mean) and 210 mL/kg bw per day (P95) were used (EFSA Scientific Committee, 2017).
Reported mean levels for MOSH in ‘Infant formula’ = 0.47–0.78 mg/kg (LB–UB).
Reported P95 levels for MOSH in ‘Infant formula’ = 1.28–1.78 mg/kg (LB–UB).
Reported mean levels for MOAH in ‘Infant formula’ = 0.012–0.266 mg/kg (LB–UB).
Reported P95 levels for MOAH in ‘Infant formula’ 0.109–0.300 mg/kg (LB–UB).
Dietary exposure to MOSH in breastfed infants (0–6 months)
The dietary exposure to MOSH of breastfed infants below 6 months of age was estimated assuming an average daily milk consumption of about 800 mL and a high consumption of 1,200 mL, representative for a breastfed infant of 3 months and a body weight of 6.1 kg. The MOSH concentration used in this scenario was 0.87 mg/L (see Section 2.6 for details). Under these assumptions, the dietary exposure to MOSH of an infant of 6.1 kg exclusively fed with breast milk might have been between 0.11 μg/kg bw per day (mean exposure) and 0.17 μg/kg bw per day (high exposure). Since the data on MOSH levels in human milk are from 2008, the Panel noted that the exposure to MOSH of the mothers in the period before 2008 might have been higher than the recent one by a factor of around two and, therefore, the current dietary exposure to MOSH of breastfed infants could be correspondingly lower.
Other scenarios
Additional exposure scenarios were conducted for ‘Cake marbled, with chocolate’, ‘Wheat groats’ and ‘Sticks, salty’, making use of reported values for MOAH and MOSH considered as not representative of the ‘background’ levels typically found in these foods. This refers to one sample of ‘Cake marbled, with chocolate’ (710–715 mg/kg for MOSH and 24–29 mg/kg for MOAH, LB–UB), 19 two samples of ‘Wheat groats’ (8,167 mg/kg for MOSH and 35.4 mg/kg for MOAH, mean LB = UB) and two samples of ‘Sticks, salty’ (329.1 mg/kg for MOSH and 14.3 mg/kg for MOAH, mean LB = UB).
The relatively high values in these samples seem to have their origin in different food‐grade mineral oil products used for instance as release agents and anti‐dust agents, a practice that nowadays is largely phased out. For the particular case of the two samples of ‘Sticks, salty’, the data provider indicated the presence of grated cheese rind in the final product as communicated by the manufacturer. A similar situation was reported with a sample of ‘Cheese powder’ (MOSH = 701.4 mg/kg). Even higher levels of MOSH and MOAH in similar products (cheese biscuits and cheese powder, above 1,000 mg/kg for MOSH) were recently reported to the Netherlands Food and Consumer Product Safety Authority (NVWA). 20
Table 17 shows the exposure estimations for the three selected food commodities; within each age class, only dietary surveys with at least 50 eating occasions for each commodity were considered.
Table 17.
Dietary exposure estimates to MOAH and MOSH in ‘consumers only’ of ‘Cake marbled, with chocolate’, ‘Wheat groats’ and ‘Sticks, salty’ across different age classes, using consumption data from the comprehensive database and occurrence data reported to EFSA (ad hoc scenarios using exceptionally high MOH levels) (a)
| Dietary exposure to MOSH (mg/kg bw day) (b) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Wheat groats | Cake marbled, with chocolate | Sticks, salty | ||||||
| Mean | P95 | N | Mean | P95 | N | Mean | P95 | ||
| min–max | min–max | min–max | |||||||
| Toddlers | 3 | 1.6–5.8 | 5 | 7 | 1.0–1.4 | 2.3–3.4 | 2 | 0.1–0.4 | 0.3–0.9 |
| Other children | 3 | 1.9–4.2 | 5.9–8.6 | 14 | 0.7–1.5 | 1.8–3.9 | 3 | 0.1–0.4 | 0.2–1.1 |
| Adolescents | 3 | 0.1–3.4 | 6.3–10.4 | 16 | 0.5–1.1 | 1.2–2.5 | – | – | – |
| Adults | 3 | 0.1–2.2 | 0.5 | 18 | 0.3–0.8 | 0.8–2.0 | 4 | 0.1–0.2 | 0.3 |
| Elderly | – | – | – | 11 | 0.3–0.7 | 0.6–1.7 | 1 | 0.1 | |
| Very elderly | – | – | – | 5 | 0.3–0.7 | 0.6–1.8 | – | – | – |
| Pregnant women | 1 | 2.4 | 5.4 | 3 | 0.6–0.7 | 1.4–1.6 | – | – | – |
| Dietary exposure to MOAH (mg/kg bw day) (b) | |||||||||
| N | Wheat groats | Cake marbled, with chocolate | Sticks, salty | ||||||
| Mean | P95 | N | Mean | P95 | N | Mean | P95 | ||
| min–max | min–max | min–max | |||||||
| Toddlers | 3 | 0.007–0.025 | 0.022 | 7 | 0.039–0.058 | 0.092–0137 | 2 | 0.04–0.018 | 0.013–0.040 |
| Other children | 3 | 0.008–0.018 | 0.026 | 14 | 0.030–0.067 | 0.085–0.156 | 3 | 0.04–0.018 | 0.010–0.048 |
| Adolescents | 3 | 0.0004–0.015 | 0.027–0.045 | 16 | 0.022–0.043 | 0.054–0.103 | – | – | – |
| Adults | 3 | 0.0004–0.010 | 0.002 | 18 | 0.012–0.030 | 0.032–0.083 | 4 | 0.005–0.007 | 0.013–0.015 |
| Elderly | – | – | – | 11 | 0.011–0.029 | 0.025–0.068 | 1 | 0.004 | – |
| Very elderly | – | – | – | 5 | 0.012–0.029 | 0.023–0.072 | – | – | – |
| Pregnant women | 1 | 0.01 | 0.023 | 3 | 0.023–0.026 | 0.056–0.064 | – | – | – |
Occurrence values reported to EFSA: ‘Cake marbled, with chocolate’ (710–715 mg/kg for MOSH and 24–29 mg/kg for MOAH, LB–UB, n = 1), ‘Wheat groats’ (8,167 mg/kg for MOSH and 35.4 mg/kg, mean LB = UB, n = 2), ‘Sticks, salty’ (329.1 mg/kg for MOSH and 14.3 mg/kg for MOAH, mean LB = UB, n = 2). For the sample ‘Cake marbled, with chocolate’, the UB levels were used for the exposure estimations. The reported consumption of ‘Cakes’ was used to estimate the dietary exposure from ‘Cake marbled, with chocolate’ and the consumption of ‘Groats’ was used to estimate the exposure from ‘Wheat groats’.
Dietary exposure estimates are shown only when the number of eating occasions was at least 50 within each dietary surveys. The P95 estimates in the dietary surveys are only shown when the eating occasions were ≥ 59.
For MOSH, the highest mean exposure estimate would be reached by the consumption of ‘Wheat groats’ by ‘Toddlers’ (5.8 mg/kg bw day, LB = UB), while the highest P95 exposure estimate would be 10.4 mg/kg bw day (LB = UB) in ‘Adolescents’, also via the consumption of ‘Wheat groats’. For MOAH, the highest mean and P95 exposure would be reached by the consumption of ‘Cake marbled, with chocolate’ by ‘Other children’, with estimates of 0.067 mg/kg bw day (UB) and 0.156 mg/kg bw day (UB), respectively. This exposure would add to the background dietary exposure from the rest of the diet.
As described in Section 3.2.1, few samples of food supplements (capsules/tablets) containing various plant extracts, beeswax, emulsifiers, different vegetable oils, etc. were reported to EFSA with relatively high levels of MOSH (LB = UB, 264.1 mg/kg). Assuming an average intake of one capsule per day (1 g) by an adult of 70 kg, the exposure to MOSH would be around 0.004 mg/kg bw.
3.3.2. Previously reported dietary exposure
Besides the dietary exposures referred to in the previous assessments (Section 1.3.4), only one more recent paper was found on the dietary exposure of the Belgium population (van Heyst et al., 2020), using the occurrence data from van Heyst et al. (2018). The exposure was calculated for the individuals in the consumer groups of children (3–6 years of age), adolescents (10–17 years of age) and adults (18–64 years of age). Two scenarios were considered, namely for the mean and the highest concentration in food. The 95th percentile exposure was used to represent the ‘high‐level consumers’. The consumption data were from the 2014 to 2015 Belgian food consumption survey (Van Heyst et al., 2018).
For MOSH, children had the highest exposure. It varied from 6.6 μg/kg bw per day using mean concentrations and 30 μg/kg bw per day for P95 and the highest concentrations. For adolescents and adults, the exposure was similar and in the range of 4–20 μg/kg bw per day. Cereal products were the highest contributors to MOSH for all age groups.
To MOAH, the adults had the highest exposure, namely ranging between 0.23 and 2.1 μg/kg bw per day, while for children, the exposure was in the range of 0.15–0.98 and for adolescent 0.2–1.1 μg/kg bw per day. Coffee was the highest contributor to the exposure to MOAH in the adult population. It should be noted that the concentration of MOAH and MOSH in coffee was determined in coffee powder and that for the exposure estimation, a dilution factor was included, but a transfer rate of 100% from the powder to the beverage was applied (most probably an overestimation of the concentration in the brewed coffee). Fats and oils were the main contributors to the exposure for children.
It is not completely possible to compare the exposures calculated in this opinion, including the exposure from the Belgian population with the exposures calculated by van Heyst et al. (2020) due to differences in age classes. In general, the exposures are similar.
3.3.3. Non‐dietary sources of exposure
White mineral oils are used as medicine or ingredients of pharmaceutical products. People have been exposed to oral doses of up to 100 mL/day over many days without evidence of major adverse effects.
White mineral oils are also used in cosmetic preparations and personal care products. They are excellent moisturisers and emollients as well as a lipophilic base to deliver active ingredients. Formulas for baby oils, breast salves, creams and lotions, bath oils, lipsticks and lip gloss, sunscreens, hair products and make‐up removers often contain MOH (Heimbach et al., 2002; Noti et al., 2003).
Babies may be exposed to mineral paraffins by direct licking off salves (often consisting of petroleum jelly) from the breast. As a worst case, daily intake from breast care products was estimated to reach 40 mg/kg bw per day (Noti et al., 2003).
Many cosmetic products containing MOH or consisting of MOH are applied to the skin. Mineral oils also get into contact with the skin when working on metals, repairing machinery, etc. However, they would add to the burden of MOH in the human body only if they passed the skin.
In a review of the data on dermal penetration of MOSH, Petry et al. (2017) concluded that there was no evidence that MOH could become systemically available. Thirteen studies were assessed, including four human volunteer studies. Although MOAH were not part of the assessment, the authors concluded that also MOAH would not be systemically available via dermal application. However, extraction of skin with solvent after thorough washing with soap showed that MOH penetrate the skin to an extent that they can no longer be removed by washing (unpublished data, Kantonales Labor Zurich). As they remain in the skin for long periods, slow uptake, longer than assessed by available tests, could nonetheless contribute to systemic exposure.
Most lip care products contained MOSH, Ozokerite and polyolefins (mainly polybutene, which are difficult to separate from MOSH, owing their similarity in composition). They largely end up in oral uptake and can be an important contribution to exposure: For instance, it was shown that with frequent use of lipsticks, the highest exposure to MOSH/POSH from dietary sources, as estimated by EFSA CONTAM Panel (2012), is exceeded when the MOSH/POSH content in the lip care product exceeds 32%. This was the case for 31% of the 175 products tested (Niederer et al., 2016).
3.4. Risk characterisation
3.4.1. MOSH
The limitations of the data set prevented the Panel from setting a HBGV for MOSH, and a MOE approach was warranted. Due to data gaps concerning toxicokinetic differences between F344 rats and humans as well as the quantitative correlation between a lifetime exposure in humans and the resulting organ contents, the Panel decided to base risk characterisation on the MOEs to the NOAEL of 236 mg/kg bw per day. As an alternative approach, MOEs were based on internal exposure, i.e. on ratios of MOSH contents in human and animal tissues.
Calculation of MOEs based on external exposure
Tables 18 and 19 show the MOEs calculated from the selected RP of 236 mg/kg bw per day (L‐C25, Barp et al., 2017b; Section 3.1.5.2) and human exposure estimations in different population groups. For adults, MOEs are in the range of 8,429–26,222 (medians: 8,906–19,667) for mean consumption and in the range of 4,720–14,750 (medians: 5,244–9,440) for 95th percentile consumption, respectively. No significant difference in MOEs is seen for particularly sensitive adult groups (pregnant or lactating women) or vegetarians compared to other adult groups. For children and adolescents, MOEs are significantly lower than for adults, being in the range of 1,873–13,882 (medians: 2,914–11,238) for mean consumption and 1,113–7,152 (medians: 1,710–6,211) for high consumption, respectively. The lowest MOE resulted for infants. The upper bound and lower bound approach for exposure calculation resulted in a difference between MOEs of a factor of approximately 1.5 for all age classes. The difference in MOEs for mean and 95th percentile consumption is approximately a factor of 2 for all age classes.
Table 18.
Calculated MOEs for different age classes and European surveys based on the selected Reference Point of 236 mg/kg bw per day and exposure derived from mean consumption; LB = lower bound, UB = upper bound
| Population groups | N surveys | LB median (max–min) | UB median (max–min) |
|---|---|---|---|
| Infants | 12 | 4,291 (2,776–9,077) | 2,914 (1,873–5,756) |
| Toddlers | 15 | 4,720 (3,371–5,488) | 2,987 (2,269–3,869) |
| Other children | 19 | 5,900 (4,453–6,941) | 3,806 (3,420–4,627) |
| Adolescents | 21 | 11,238 (7,375–13,882) | 7,613 (5,244–9,077) |
| Adults | 22 | 15,733 (11,800–26,222) | 10,261 (8,429–14,750) |
| Elderly | 19 | 18,154 (13,882–26,222) | 11,238 (9,440–16,857) |
| Very elderly | 14 | 14,750 (13,882–26,222) | 9,833 (8,741–15,733) |
| Pregnant women | 6 | 18,154 (13,111–23,600) | 11,800 (8,741–14,750) |
| Lactating women | 2 | 11,800–16,857 | 8,429–9,440 |
| Vegetarians | 1 | 19,667 | 13,111 |
Table 19.
Calculated MOEs for different age classes and European surveys based on the selected Reference Point of 236 mg/kg bw per day and exposure derived from 95th percentile of consumption; LB = lower bound, UB = upper bound
| Population groups | N surveys | LB median (min–max) | UB median (min–max) |
|---|---|---|---|
| Infants | 12 | 2,408 (1,503–4,370) | 1,710 (1,113–2,744) |
| Toddlers | 15 | 2,744 (1,888–3,371) | 1,815 (1,341–2,484) |
| Other children | 19 | 3,420 (2,744–4,069) | 2,269 (1,983–2,776) |
| Adolescents | 21 | 6,211 (4,000–7,152) | 4,291 (2,914–4,917) |
| Adults | 22 | 8,138 (6,378–11,800) | 5,756 (4,816–8,138) |
| Elderly | 19 | 9,440 (6,941–14,750) | 6,743 (4,917–9,440) |
| Very elderly | 14 | 8,429 (6,556–14,750) | 5,756 (4,720–8,741) |
| Pregnant women | 6 | 9,440 (7,152–11,800) | 6,556 (5,130–8,429) |
| Lactating women | 2 | 6,378–9,440 | 4,720–5,900 |
| Vegetarians | 1 | 8,741 | 6,211 |
Evaluation of the MOEs
In the discussion of the MOE above which no concern for human health would arise, the Panel started by taking into consideration the default assessment factors (EFSA Scientific Committee, 2012) for interspecies differences with respect to toxicokinetics and ‐dynamics between humans and rats (factor of 10), for the intraspecies differences in humans (factor of 10) and for the shorter duration of the key study (120 days) compared to a lifetime exposure (factor of 2). The analysis of uncertainties related to the hazard identification and characterisation of MOSH indicated that an additional factor was warranted (see uncertainties described in Section 3.5.2).
According to the EFSA Scientific Committee Guidance on selected default values (EFSA Scientific Committee, 2012), an additional factor can be considered in case of deficiencies in the database on a case‐by‐case basis. A default value has not been proposed, as it will be directly dependent on the data set available. The WHO/IPCS (1994, 1999) has recommended a factor of 3 or 5 if there are minor deficiencies in the database and a factor of 10 if there are major deficiencies in the database. In conjunction with the uncertainty analysis, the CONTAM Panel considered an additional factor of 6 to be appropriate.
As a result, the Panel considered that MOEs ≥ 1,200 are sufficient to conclude that there is no concern for human health risks related to the current dietary exposure to MOSH.
With the exception of the minimum MOE for infants resulting from the 95th percentile of consumption and UB calculation, showing an MOE slightly lower than 1,200, all MOEs reported in Tables 18 and 19 are above 1,200. Especially for adolescents and adults, which are the most important age classes with respect to lifetime accumulation, MOEs are approximately one order of magnitude above the value of 1,200.
Calculations from comparison of MOSH tissue concentrations and body burdens in humans and animals
Compared to the approach by external exposure, the assessment of tissue concentrations and estimated body burdens is based on the same animal data (L‐C25) and on the human data from 2014 (Barp et al., 2014). Advantages of using internal exposure data are that calculations are independent of:
-
•
long‐term accumulation of some MOSH components;
-
•
the non‐linearity of the uptake;
-
•
possible dependence of the uptake on the food matrix;
-
•
discrepancy between the MOSH compositions in L‐C25 compared to the composition to which humans are exposed.
However, the Panel also noted the uncertainties in the data: The human data used for comparison are based on only 37 individuals from Austria (Barp et al., 2014). Their exposure is not accurately known. The long‐term mean dietary exposure of the individuals in Barp et al. (2014) was probably higher than the current dietary exposure. In addition, the MOSH contents were measured in autopsy tissue material, and it is unknown, whether incidences shortly before the death of the individuals (e.g. illness, weight loss, medical treatment) had a significant influence on these tissue levels – especially important with regard to the individuals with high MOSH tissue levels. Finally, it is unknown to what extent non‐dietary sources contributed to the measured tissue concentrations.
Comparison of tissue levels
Table 20 shows the minimum, average, median, 90th percentile and maximum MOSH contents, respectively, measured in human tissues by Barp et al. (2014). For comparison, the respective content of MOSH in organ tissues in F344 rats used are from the same experiment as used for MOE calculations on external exposure: the highest dose of L‐C25 (236 mg/kg bw per day) administered for 120 days (Barp et al., 2017b). They amount to 3,805 mg/kg in liver, 419 mg/kg in spleen and 36 mg/kg in adipose tissue. The resulting ratios between the tissue contents in humans and F344 rats are shown in Table 20.
Table 20.
Calculated ratios of MOSH contents in tissues of F344 rats (Barp et al., 2017b) and humans (Barp et al., 2014). The following tissue levels in F344 rats at the reference point of 236 mg/kg bw per day L‐C25 were used to calculate the F344/human ratios: 3,805 mg/kg liver, 419 mg/kg spleen and 36 mg/kg adipose tissue
| Human organ content | MOSH concentrations (mg/kg) | Ratios F344/human | ||||
|---|---|---|---|---|---|---|
| Liver | Spleen | Adipose tissue | Liver | Spleen | Adipose tissue | |
| Minimum | 14 | 6 | 17 | 272 | 76 | 2.1 |
| Average | 131 | 93 | 130 | 29 | 4.5 | 0.28 |
| Median | 71 | 28 | 87 | 54 | 15 | 0.41 |
| 90th percentile | 357 | 185 | 233 | 11 | 2.3 | 0.15 |
| Maximum | 901 | 1,397 | 493 | 4.2 | 0.3 | 0.07 |
For liver, ratios are in the range of 4.2–272. Hence, all measured MOSH contents in human liver are significantly lower than in the animal tissue. For spleen, ratios are in the range of 0.3–76, i.e. again most of the MOSH contents measured in human spleen are lower than in the spleen of the F344 rats. For adipose tissue, ratios are in the range of 0.07–2.1, i.e. the measured MOSH contents in human fat tissue were usually higher than the content in the fat tissue of the F344 rats. However, it should be noted, that n‐alkanes, which highly contribute to the fat tissue burden of humans, are not present in L‐C25. Hence, a better test item to compare organ contents with would be the broad mixture from Barp et al. (2017a) or the S‐C25 or L‐C25W from Barp et al. (2017b), respectively. The corresponding ratios would be about an order of magnitude higher and thus well above 1 for mean, median and 90th percentile adipose tissue MOSH content in humans from Barp et al. (2014). In addition, for fat tissue also the longer exposure time of the humans from Barp et al. (2014) is likely a reason for the comparably higher MOSH contents in human fat tissue (for details, see paragraph below and Appendix E).
Comparison of organ and body burdens
Another feasible approach to account for differences in the internal exposure of humans and animals in studies used for risk assessment is the body or organ burden approach. In addition to the comparison of MOSH contents in the organs of humans and animals, the body burden gives information on the overall exposure of the individuals as well as on the contribution of the levels in the individual organs to the total exposure.
Selected data on organ and body burden are presented in Table 21. For comparison between F344 rats and humans, the overall body burden is also presented in relation to body weight. Further data, information and calculation are shown in Appendix E.
Table 21.
Body burden of MOSH in relation to body weight and organ contributions in humans from Barp et al. (2014) in comparison to F344 rats from selected studies, doses and test items
| Study | Species | Percentile | Overall body burden in mg MOSH/kg bw | Contribution of liver burden in % | Contribution of spleen burden in % | Contribution of fat tissue burden in % | |
|---|---|---|---|---|---|---|---|
| Barp et al. (2014) | Humans | Median | 28 | 5.3 | 0.2 | 93 | |
| P5 | 6 | 1.5 | 0.0 | 81 | |||
| P95 | 140 | 17 | 0.8 | 98 | |||
| Study | Species | Test item | Dose in mg MOSH/kg bw per day | Overall body burden in mg MOSH/kg bw | Contribution of liver burden in % | Contribution of spleen burden in % | Contribution of fat tissue + carcass burden in % |
| Cravedi et al. (2017), narrow mixtures | F344 rat | L‐C25 | 25 | 55 | 83 | 1.1 | 16 |
| L‐C25 | 236 | 143 | 89 | 0.9 | 10 | ||
| S‐C25 | 23 | 212 | 79 | 0.4 | 21 | ||
| S‐C25 | 226 | 697 | 84 | 0.4 | 16 | ||
| L‐C25W | 22 | 230 | 83 | 0.3 | 16 | ||
| L‐C25W | 218 | 405 | 82 | 0.4 | 17 | ||
| Cravedi et al. (2017), broad mixture | F344 rat | Broad, day 120 | 2.6 | 13 | 53 | 0.7 | 47 |
| Broad, day 120 | 260 | 307 | 65 | 0.4 | 35 | ||
| Study | Species | Test item | Dose in mg MOSH/kg bw per day | OVERALL body burden in mg MOSH/kg bw | Contribution of liver burden in % | Contribution of spleen burden in % | Contribution of fat tissue burden in % |
|
Smith et al. (1996) |
F344 rat | N15H | 1,951 | 224 | 78 | n.d. | 9.3 |
| P15H | 1,951 | 169 | 69 | n.d. | 9.9 | ||
| P70H | 1,951 | 34 | 97 | n.d. | n.m. | ||
| P100H | 1,951 | 37 | 53 | n.d. | 3.4 | ||
| LMPW 2 | 1,951 | 718 | 100 | n.d. | n.m. | ||
| HMPW | 1,951 | 51 | 11 | n.d. | 9.0 |
n.m.: not measured; n.d.: not detected.
The median overall body burden in relation to body weight is in the same order of magnitude for the humans from Barp et al. (2014) as for F344 rats receiving a broad MOSH mixture at 2.6 mg/kg bw per day over 120 days (Cravedi et al., 2017) – 28 mg/kg bw as compared to 13 mg/kg bw. When compared to the RP for the MOE calculation (ingestion of L‐C25 at 236 mg/kg bw per day for 120 days), the resulting body burden in relation to body weight is ca. 5 times higher in rats than in humans (median value) – 143 compared to 28 mg/kg bw. The 95th percentile of the human body burdens in relation to body weights from Barp et al. (2014) is 140 mg/kg bw, and therefore equals the cited value for F344 rats at the RP.
However, it should be noted, that the main contribution to the body burden in humans results from the adipose tissue (> 90%), whereas liver burden is the main contribution in F344 rats (ca. 80%). This might be due to the longer exposure of the humans from Barp et al. (2014) or point to toxicokinetic differences between the species.
In F344 rats and in humans, a steady state for the liver burden seems to be reached after a relatively short time compared to fat tissue burden, where neither in humans nor F344 rats a steady state is observed.
Evaluation of the results on tissue level and body burden comparison
As expected with regard to the interspecies differences between rats and humans, the ratios of tissue concentrations in F344 rats and humans are generally substantially smaller than the MOE calculated above. In some human individuals, the spleen content was higher than the mean content in F344 rats receiving L‐C25 at 236 mg/kg bw per day for 120 days. The adipose tissue content was higher in almost all human individuals from Barp et al. (2014) than in the cited group of F344 rats.
The median body burden in relation to body weight was also substantially lower in humans than in F344 rats receiving L‐C25 at 236 mg/kg bw per day for 120 days, although the margin was only a factor of 5. However, in addition to the uncertainties in the approach discussed above, it has to be noted that the main contribution to body burden in humans results from adipose tissue burden. In the adipose tissue, no adverse effects have been described in any study in any species at any dose. Hence, the Panel considers these findings unlikely to be of concern. Still, it should be noted that fat tissue seems to form a depot for certain types of MOSH. In case of a significant reduction of adipose tissue in short time, high amounts of MOSH might be released into the body, possibly leading to high MOSH content in other organs.
Influence of the source of the ingested MOSH
The occurrence data provided do not distinguish MOSH of different characteristics, particularly with regard to their propensity to accumulate. Waxes contain less constituents prone to be accumulated than oils, hydrogenated oils more than non‐hydrogenated and hydrocarbons below n‐C20 are hardly accumulated in human livers and spleens. The most important difference, however, is between oils and waxes ingested as such and those biotransformed by various organisms and enriched in bioaccumulating hydrocarbons before entering food, as mentioned in Section 1.3.2. The effect is that the concentration data of the MOSH in certain foods may not be comparable to the MOSH added to feed in the animal tests.
However, there are uncertainties in establishing the degree and implications of such differences. Whereas meat products are likely to contain higher levels of accumulated MOSH, the enrichment cannot be precisely quantified, but according to data from F344 rats may exceed a factor of 10. Moreover, it cannot be assumed that milk and dairy products only contain concentrated accumulated MOSH components. It is not possible to analytically determine differences in the profile of MOSH components between different food sources. Moreover, the impact of exposure to enriched bioaccumulating components cannot be ascertained in terms of toxicity, since the relative toxicity of different MOSH components is not known. Therefore, in the uncertainty analysis, the Panel considered the potential impact of enriched bioaccumulating components in certain animal products entering the human diet.
Conclusion on risk characterisation of MOSH
For the general population, the CONTAM Panel noted that MOEs for all age classes are at or above a value of 1,200. With the exception of fat tissue, median tissue levels and body burdens of humans are generally lower than in the group of F344 rats selected as RP. Infants exclusively fed with infant formula with high MOSH content could have exposure with MOEs ranging approximately from 790 to 1,070 for mean exposure and from 640 to 870 for P95 exposure. Considering the short duration of exposure via infant formula, these MOEs do not raise concerns. Hence, overall the CONTAM Panel concluded that the current dietary exposure towards MOSH for all age classes raises no concern for human health.
The consumption of certain foods, such as dairy products, may result in the exposure to MOSH enriched in strongly accumulating components. The consequences of long‐term accumulation of MOSH for human health have not been investigated and are uncertain.
3.4.2. MOAH
As discussed in Section 3.1.5, the hazard characterisation of the MOAH fraction poses difficulties due to the lack of oral toxicity data and the poor chemical characterisation of the composition. The CONTAM Panel confirmed the conclusions of the previous Opinion (EFSA CONTAM Panel, 2012) that the main concern for human health is related to the possible presence of MOAH with three or more aromatic rings and various side chain alkyl moieties.
The highest concentrations of MOAH with ≥ 3 aromatic rings are in crude mineral oils. Regarding contamination of food, little‐refined mineral oil products are used as batching oils for jute and sisal bags (Grob et al., 1991a,b,c,d) and can be considered as a worst case. A sample of batching oil was analysed in 1991, using an on‐line HPLC pre‐separation on an amino‐modified silica gel column. It contained 23% MOAH of two and more aromatic rings. The amount of three or more ring MOAH in the batching oil was 6.4%, predominantly alkylated dibenzothiophenes, anthracenes and phenanthrenes (Grob et al., 1991a,b,c,d). The amount of all MOAH in the batching oil could not be determined, since at that time, the analytical separation between MOSH and alkylated benzenes was not possible.
There are few data on the occurrence of three or more ring MOAH in food. Rice analysed in 1996, most likely contaminated from jute bags, contained 130 mg/kg MOSH and 30 mg/kg MOAH of two and more aromatic rings (21% of these MOH). Of these MOAH, 58% were naphthalenes, 17% fluorenes, 18% dibenzothiophenes, 6% anthracenes and phenanthrenes and < 2% pyrenes and fluoranthenes (on‐line HPLC‐HPLC‐GC/FID using an amino‐modified silica gel column for separation by ring number; Moret et al., 1997). A sample of non‐refined linseed oil contained about 300 mg/kg MOH (Moret et al., 1996). The alkylated naphthalenes amounted to 10.1% of the MOH, fluorene to 1.0%, the dibenzothiophenes to 0.9% and the sum of the anthracenes and phenanthrenes to 1.3%. The MOAH with more than three aromatic rings were not detectable (below about 0.2%). The presence of the dibenzothiophenes indicates a little‐refined mineral oil product and can, therefore, again be considered as a worst case. Other samples (chocolate, fish, edible oils) contained less MOAH and less MOAH of ≥ 3 aromatic rings.
In a recent study using on‐line HPLC‐GC × GC (Biedermann et al., 2022), in 10 samples of rice contaminated with 1.0–8.7 mg/kg MOAH, the MOAH with ≥ 3 aromatic rings made up 2–24% of the MOAH. Little‐refined mineral batching oils are still occasionally used in jute and sisal bags for packing food, such as rice, cocoa beans and copra, primarily for storage at the farmer and transport to the deals in their country, but according to Biedermann et al. (2022), it was unknown for which rice sample, jute or sisal bags were the source.
It should be noted that the occurrence of such batching oils in food accounts for only a small proportion of dietary exposure to MOAH.
Environmental sources may also contribute to the background presence of three or more ring MOAH in food, but no specific data are available.
As recently described by Carrillo et al. (2022), several MOAH‐containing mineral oil products (e.g. lubricating base oils) are treated to lower the content of three or more ring MOAH in order to pass the IP346 test (see Section 1.3 for further details). These products are likely the main contributors to the presence of MOAH in food. However, in certain products (e.g. fuels or bitumen), higher levels of three or more ring MOAH are still present.
EFSA received hardly any data on three or more ring MOAH concentrations in food. As the sources of MOAH, hence MOAH compositions, vary between food categories as well as within food categories (the same foods may be contaminated from various sources), the presence of three or more ring MOAH in the diet could not be estimated. The actual proportion of three or more ring MOAH in the total MOAH is likely to vary between about 25% (e.g. transfer from jute bags) and virtually 0% (e.g. a white mineral oil product) in and between food groups. For instance, rice may be contaminated from jute bags and contain MOAH with 25% of the three or more ring species. The concentration of the total MOAH (as measured) has to be considered, the proportion of such rice in other rice without or with negligible three or more ring MOAH and finally the contribution of rice to the total diet. Hence, a high content of three or more ring MOAH is ‘diluted’ by the other foods in the diet. Since there were not enough data for such a complex estimate, the CONTAM Panel decided to use two scenarios that are assumed to encompass reality. As a conservative one (scenario 1), an average of 10% of three or more ring MOAH within the MOAH fraction present across the different foods was assumed. Even for a high consumer of food contaminated with a high level of MOAH containing the highest proportion of three or more ring MOAH (e.g. a high consumer of a kind of rice that is usually contaminated from jute bags), the overall proportion would probably be below 10%. Without consumption of foods contaminated by little refined mineral oil products, the overall percentage could be as low as 1% (scenario 2). The two scenarios are, therefore, based on upper and lower limits of exposure to three or more ring MOAH estimated from the available data.
In considering the carcinogenicity risks of the MOAH subfraction with three or more aromatic rings, using the two scenarios, the CONTAM Panel selected the BMDL10 of 0.49 mg/kg bw per day as derived from the assessment of PAHs, as a surrogate RP in a MOE approach (see Section 3.1.5.2).
An overview of the MOE ranges calculated based on the above assumptions is reported in Table 22 for mean and P95 exposure levels.
Table 22.
Margin of exposure (MOE) ranges calculated for mean and high (P95) exposure levels for young (‘infants’, ‘toddlers’ and ‘other children’) and adult (‘adolescents’, ‘adults’, ‘elderly’ and ‘very elderly’) groups of the population. For each age group, the ranges include MOE calculated considering the minimum lower bound and maximum upper bound exposure (see Table 14). Two scenarios were considered, with average contents of 10% or 1% of three or more ring MOAH within the MOAH fraction present across different foods in the dietary exposure (defined as Scenario 1 and 2, respectively)
| Margins of exposure (Maximum UB–Minimum LB) | |||||
|---|---|---|---|---|---|
| Assumed fraction of three or more MOAH | 10% (Scenario 1) | 1% (Scenario 2) | |||
| Mean exposure | Infants and children | 158 | 4,900 | 1,581 | 49,000 |
| Adults | 490 | 12,250 | 4,900 | 122,500 | |
| P95 exposure | Infants and children | 83 | 1,633 | 830 | 16,333 |
| Adults | 288 | 4,900 | 2,882 | 49,000 | |
The MOEs calculated under the Scenario 1 are consistently lower than 10,000 for most of consumption surveys for mean consumers and for all high consumers. Scenario 1 would raise a health concern according to the EFSA Opinion on assessment of genotoxic and carcinogenic substances (EFSA, 2005). Under the Scenario 2, MOE below 10,000 were calculated for UB estimates only, for most of the dietary surveys at the mean exposure and for all dietary surveys at P95 exposure. However, MOE higher than 10,000 were calculated for all the LB mean exposure levels. MOE higher than 10,000 were calculated also for most of the LB P95 exposure levels, with the exception of some surveys in the younger age groups showing P95 LB MOEs in the range 4,000–8,000 (data not shown in Table 20). Scenario 2 would raise a health concern, in particular for the high consumers in the younger age groups.
The CONTAM Panel noted that a full risk characterisation would require additional data on toxicity and exposure to three or more ring MOAH.
Due to the lack of adequate oral toxicity studies, it was not possible to identify a reference point for the 1–2 ring MOAH. Therefore, a risk characterisation of this MOAH fraction could not be performed. The CONTAM Panel concluded that, in the absence of reliable toxicity data, the dietary exposure to 1–2 ring MOAH might raise a concern.
3.5. Uncertainty analysis
The purpose of the uncertainty analysis is to identify and quantify the specific uncertainties of the risk assessment and combine them to assess the overall certainty of the final conclusion, as recommended in EFSA's guidance on uncertainty analysis (EFSA Scientific Committee, 2018a).
In a first step, sources of uncertainties related to hazard identification and characterisation and exposure to MOSH and MOAH were listed and discussed (Annex H). The uncertainty analysis focusses on uncertainties, which are specific to the current assessment. Standard uncertainties as covered by extrapolation factors or the use of contaminations data reported to EFSA and food consumption data from national surveys were excluded. Their impact is part of every chemical risk assessment and discussed elsewhere.
It was considered which of those sources of uncertainty would have most impact on the outcome of the hazard identification and characterisation and the exposure estimations.
Each of these main sources of uncertainty related to hazard identification and characterisation were discussed individually. Additional evidence was reviewed to quantify its possible impact on the reference point used in the current assessment. Semi‐formal structured methods of Expert Knowledge Elicitation (semi‐formal EKE, Annex B.8 of EFSA Scientific Committee (2018b)) were applied to determine a credibility range for the ratio between the reference point of an assessment without this uncertainty or limitation and the current assessment.
Standard uncertainties, which are covered by the extrapolation factors used to determine the acceptable MOE, were assumed for both: the assessment without limitations and the current assessment. Their effect is therefore not included in the credibility range.
Finally, the combined impact of all main uncertainties, as well as the identified minor uncertainties, was judged by the experts on the hazard assessment giving the distribution of the overall uncertainty of the ratio between the reference value of an assessment without specific uncertainties or limitations and the current assessment, called the uncertainty factor for the hazard.
The potential impact of the main uncertainties affecting the exposure assessment was explored by sensitivity analysis using a simplified exposure model for the complete diet of the average European toddler. This age class was used, because the ratio of intake and body weight is most unfavourable, resulting in high risk for this age. The results from the sensitivity analysis were used to inform judgements of the experts on exposure assessment on the combined impact of all uncertainties affecting the exposure assessment for high consuming (P95) toddlers. Again the ratio between the exposure assessment without uncertainties or limitations and the current assessment was quantified as uncertainty distribution called the uncertainty factor for the exposure.
Both uncertainty assessments were combined by Monte‐Carlo simulation calculating the uncertainty distribution of the MOE for the high consuming and mean toddler:
The replications of the simulation can be interpreted as possible MOE values, when the chemical risk assessment would have been done under perfect conditions (assuming only standard uncertainties), without any specific uncertainties. Therefore, the likelihood of the MOE falling below the acceptable MOE can be estimated, expressing the certainty of the statement ‘MOE above the acceptable MOE (no concern)’ or ‘MOE below the acceptable MOE (concern)’.
During the uncertainty assessment, the acceptable MOE was set to 200 for MOSH and 10,000 for MOAH covering the standard uncertainties.
In the final step, the appropriateness of this calculation for toddlers and all other age groups were discussed. For the latter, the exposures were adjusted to the estimates of the specific age groups, while the uncertainty factors were taken from the uncertainty analysis of toddlers.
This exercise resulted in the following certainty bands for the final conclusions:
Regarding MOSH, it is likely to very likely (with 66–95% certainty) that neither mean nor high consuming toddlers give reason for concern. The certainty level of ‘no concern’ was calculated to be 87% for high‐consuming toddlers, 95% for toddlers with mean intake. Besides high‐consuming infants (85%), the calculated certainty level for all other age groups exceeds the values for toddlers.
Regarding MOAH, it is extremely likely (with 99–100% certainty) that mean and high‐consuming toddlers give reason for concern. The certainty level of ‘concern’ was calculated to be 100% for high‐consuming toddlers, 99% for toddlers with mean intake. For all other age groups, it is likely (above 66%) to give reason for concern. The minimal certainty level was calculated for elderly persons with mean intake (64%).
To note that after the identification of the specific uncertainties for MOSH an additional factor of 6 was selected, resulting in a reference MOE of 1,200 raising no concern for human health. This does not change the results of the uncertainty assessment or the overall conclusions of the risk assessment.
3.5.1. Assessment objectives
Assessments must indicate what sources of uncertainty have been identified and characterise their overall impact on the assessment conclusion. It is recommended to quantify the overall uncertainty of conclusions using probability to avoid the ambiguity of qualitative approaches. However, the conclusions may subsequently be reported without probabilities if legislation or risk managers require that, providing that the associated probabilities are somewhere defined (EFSA Scientific Committee, 2018a). The present uncertainty analysis was conducted with the objective to address the risk assessment question on the risks for human health related to the presence of MOSH and MOAH in food.
Both for MOSH and MOAH, a MOE approach was applied for the risk characterisation. The MOE is calculated as the ratio of the reference point and the P95 exposure estimate for the EU population in each age group. The uncertainties pertaining to each of these two components were identified and quantified separately and combined for the overall uncertainty assessment. Risk managers have indicated that the P95 exposure is more important for decision‐making, in order to protect the majority of the population. The uncertainty analysis, therefore, focussed on the MOE for the P95 exposure.
For MOAH, the MOE approach was applied to scenarios considering the possible presence of genotoxic and carcinogenic components. The risk for human health for substances that are genotoxic and carcinogenic is addressed by assessing whether the MOE exceeds the value of 10,000, using the BMDL10 for increased tumour incidence from experimental animal studies as RP (EFSA, 2005).
For MOSH, the MOE approach was applied in view of the limitations of the data set preventing the setting of a HBGV. For the definition of a MOE indicating no concern for human health, a default uncertainty factor of 200 was applied in the performance of the EKE. However, in the risk characterisation of MOSH, it was considered appropriate to account for the main uncertainties identified and an extra uncertainty factor of 6 was included for this purpose (see Section 3.4.1 for further details).
Specifically, both for the MOE approaches applied for MOSH and MOAH, the uncertainty analysis assessed the probability that the MOE for the 95th percentile of exposure in the EU average toddler would be below 200/10,000 for MOSH/MOAH, if all identified uncertainties affecting the exposure assessment, hazard assessment and risk characterisation were resolved.
The uncertainty analysis was conducted following the guidance of the EFSA Scientific Committee, 2018a, on uncertainty analysis in scientific assessments. The analysis follows the guidance for case‐specific assessments (Section 4 of EFSA Scientific Committee, 2018a) although it does contain some standardised elements (e.g. using a threshold MOE of 10,000 to identify the level of concern). The combined impact of uncertainties on the principal conclusions in each part of the assessment was quantified using % probabilities. These are reported below as % certainty for the more probable outcome for each conclusion, following EFSA's guidance on communication of uncertainty (EFSA, 2019b).
3.5.2. Hazard identification and characterisation
Possible limitations of the hazard assessment were systematically screened to identify those uncertainties, which may have a high impact on the reference point. The following uncertainties were considered of higher priority regarding the estimation of the reference point for MOSH. The magnitude and direction of the impact of these uncertainties on the reference point was evaluated by a structured expert elicitation (see Annex H for a more detailed description):
Uncertainty regarding differences in the composition of MOSH products used in toxicological studies vs. the MOSH profiles found in food.
Little knowledge of the absorption, distribution, metabolism and excretion of single MOSH compounds and components tested in subchronic assays, particularly potential long‐term bioaccumulation of MOSH including its metabolites.
Possible differences in accumulation of certain MOSH in human tissues compared to F344 rats, considering the known interspecies differences in ADME and the longer duration of human exposure.
Presence of adverse effects not identified in F344 rats (due to lack of long‐term studies) and in other strains/species (only few studies available not covering all MOSH components).
Uncertainty in the relevance of the adverse effects observed in experimental animals for human health.
Uncertainty related to human observations (unknown effects at levels observed in human tissues and possible adverse effects identified in clinical studies).
Uncertainty numbers 1–5 were carried forward for the EKE analysis, while it was agreed to describe narratively uncertainty number 6 as it has not a direct impact on the identification of the reference point for MOSH (based on F344 rat data). The analysis of uncertainties 1–5 led to an overall uncertainty factor of 0.39 (with 90% certainty range from 0.06 to 1.48). It is likely (85%) that a hazard assessment without uncertainties or limitations would lead to a lower reference point as indicated in the key study used in the assessment. The CONTAM Panel noted that an overall uncertainty factor of 0.17 corresponds to the 25th percentile of the range mentioned above. The reciprocal of this corresponds to the additional factor of 6 selected for the MOE approach for MOSH (see Section 3.4.1), thus covering 75% of the certainty range (see Annex H – Section 2.8).
With respect to uncertainty number 6, data on human observations are sparse. Overall, it is very likely that human lipogranulomas observed in liver and other organs in association with exposure to MOSH are not adverse changes; however, other possible effects related to long‐term accumulation of MOSH components on human health have not been investigated and are uncertain. Recent papers associated the use of pharmaceutical grade mineral oil products as placebo in clinical trials with an increase in biomarkers related to atherogenic lipoproteins and inflammation. As discussed in Section 4.1.4, the CONTAM Panel considered these findings as highly uncertain and concluded that they could not be used for hazard characterisation of MOSH.
For MOAH, the following uncertainties were identified with higher priority:
Uncertainty about the composition of MOAH/MOH products used in toxicological studies vs. the MOAH profiles found in food.
Uncertainty related to the incomplete data set on oral toxicity of MOAH.
Uncertainty about the use of a surrogate reference point from PAH8 for the hazard characterisation of three or more ring MOAH.
The analysis led to an overall uncertainty factor of 0.05 (with 90% certainty range from 0.006 to 0.26). Therefore, a hazard assessment, without uncertainties or limitations, is extremely likely (> 99%) to lead to a reference point higher than the one selected.
3.5.3. Dietary exposure assessment
The EKE analysis was conducted in toddlers as they were, together with infants, the age classes with the highest exposure estimations for MOAH and MOSH, and they were exposed to a more diverse diet than infants. The identified uncertainty in toddlers is expected to have a similar impact on the dietary exposure estimated in the other age classes.
For MOSH and MOAH, the discussions on uncertainty concluded that the main uncertainties identified during the EKE analysis refer to (1) the lack of representativity of some of the occurrence data for particular European countries where MOH levels could be higher as the different measures introduced by authorities and industry since 2012 to reduce levels could not be fully implemented, (2) different approaches used in the chromatographic analysis resulted in the estimation of the total levels using the individual C‐fractions, possibly leading to UB overestimations and LB underestimations, (3) lack of harmonisation among laboratories to report LOQs for the individual C‐fractions. Additionally, in the case of MOAH analysis, relevant uncertainties were also identified related to the high amount of left‐censored data reported, and to the analytical measurements at low concentrations.
For MOSH, the EKE analysis led to an overall uncertainty factor of 1.10 (with 90% certainty range from 0.962 to 1.19). It is likely (> 80%) that an exposure assessment for high‐consuming toddlers without uncertainties or limitations would lead to a higher exposure as indicated in the current assessment.
For MOAH, the EKE analysis led to an overall uncertainty factor of 1.30 (with 90% certainty range from 0.794 to 1.76). It is likely (> 75%) that an exposure assessment for high‐consuming toddlers without uncertainties or limitations would lead to a higher exposure as indicated in the current assessment.
Together with the most important uncertainties identified and categorised for the occurrence data by the informal EKE, the Panel also noted the uncertainties and limitations related to the use of the EFSA Comprehensive Food Consumption Database (see Annex H). The main uncertainties have been described by EFSA (2011a) and generally relate to the use of different dietary survey methodologies, standard portion sizes, representativeness of samples included in surveys, or to the inclusion of consumption surveys covering only few days to estimate high percentiles of chronic exposure. The Panel noted these uncertainties are common to dietary chronic exposure assessments performed using the Comprehensive Database, and have the potential to cause either an over‐ or underestimation of the exposure. The uncertainties affecting the food consumption data were not specific for the MOAH and MOSH exposure assessment. They are ‘standard uncertainties’ across all opinions and were considered to have low priority. It is generally accepted that the estimates from the Comprehensive Database are generally considered to be fit for purpose, provided there are no non‐standard uncertainties, as it is the case for MOAH and MOSH.
For MOSH, it is also important to mention that the occurrence data reported did not distinguish MOSH of different characteristics, particularly regarding MOSH components with propensity to be bioaccumulated in foods of animal origin. For MOAH, data are largely missing to distinguish MOAH by ring number.
Concerning the dietary exposure to MOAH, particularly in the adult population, there is probably an overestimation of the exposure via the consumption of ‘Tea beverages’ and ‘Herbal and other non‐tea infusions’ (under ‘Coffee, cocoa, tea and infusions’, one of the main contributors), since in the absence of specific data, a 100% transfer of the MOAH quantified in the dry product was considered during the brewing process.
Few samples with exceptionally high MOH levels were reported, in particular for MOSH (e.g. cakes). The high levels are probably explained by specific production practices, such as the use of release and anti‐dusting agents, practices that nowadays seem to have been largely phased out. However, subjects that might regularly consume this type of products for different reasons would be expose to much higher levels of MOH as compared to the general population.
4. Conclusions
Mineral oil hydrocarbons (MOH) are hydrocarbons mainly derived from crude mineral oil that can enter food from multiple intended and unintended sources. Due to their complexity and variable composition of MOH, their complete chemical characterisation is not possible.
The aliphatic and aromatic hydrocarbons present in food from MOH are separated into mineral oil saturated hydrocarbon (MOSH) and mineral oil aromatic hydrocarbons (MOAH). In the present opinion, the term MOH includes:
-
–
mineral oils and waxes obtained from crude oils by fractionation and chemical refining processes (e.g. cracking or hydrogenation);
-
–
oils and waxes manufactured by Fischer–Tropsch synthesis using coal, methane or biomass as sources, also known as gas‐to‐liquid (GTL) oils;
-
–
heterocyclic compounds, primarily thiophenes, may be part of the MOAH fraction.
Hydrocarbons naturally present as food components, as well as other hydrocarbons from different sources, such as plastics, are not included in the MOH definition, but their analytical separation may be demanding and not complete.
The toxicological data set used in the present opinion relies mainly on studies performed on MOH products derived from crude mineral oils. Data from toxicological studies on GTL products were also considered as supporting information, although there are limitations, as GTL oils do not have the same composition as mineral oil‐derived products.
The present Scientific Opinion is an update of the Scientific Opinion of the CONTAM Panel published in 2012 (EFSA CONTAM Panel, 2012), and in several instances in this section reference to the conclusions of the previous Opinion is made.
4.1. Hazard identification and characterisation
4.1.1. Toxicokinetics
MOSH
Experimental animals
- New studies in female F344 rats exposed to various MOSH mixtures in the feed for up to 120 days on retention of MOSH in liver, spleen, adipose tissue and remaining carcass (excluding gastro‐intestinal tract) became available.
-
○Application of a MOSH mixture broad in molecular mass distribution indicated that accumulation in liver and spleen ranges from n‐C20 to about n‐C45, with a maximum at about n‐C29; in the adipose tissue, it was from below n‐C16 to about n‐C35.
-
○The concentrations in the tissues increased far less than proportional with the dose.
-
○In the spleen and adipose tissue, there was no apparent tendency to plateau or achieve a steady‐state level as seen for MOSH content between 90 and 120 days, while a plateau between 90 and 120 days appeared to be reached in the liver.
-
○After 120 days, about 50% of the retained MOSH from the broad mixture ended up in the liver and 38–50% in adipose tissue and the carcass.
-
○At all doses, the MOSH concentration was at least one order of magnitude higher in the liver than in the spleen, adipose tissue or carcass. Following depuration for 30 days, organ contents were significantly lower. However, substantial amounts of MOSH still remained in liver, spleen and carcass.
-
○The MOSH composition was similar in liver and spleen and dominated by unresolved alkyl‐substituted naphthenes and highly branched iso‐alkanes. There were more n‐alkanes and hydrocarbons with little branched alkyl moieties in adipose tissue.
-
○As a follow up, MOSH mixtures with a narrower range of molecular masses were used. Up to n‐C25, n‐alkanes were absent in liver and spleen, but enriched above n‐C25. Also enrichment of n‐alkyl‐monocyclic naphthenes above n‐C28 was observed. It appears that in F344 rats, these wax components resist biotransformation and elimination.
-
○Following exposure to a dewaxed MOSH mixture ranging from n‐C25 to n‐C50 (L‐C25), the retention in liver and spleen was dominated by alkylated naphthenes and highly branched iso‐alkanes.
-
○
- New experiments in female SD rats indicate that:
-
○paraffinic and GTL wax components are hardly retained;
-
○the retention of naphthenic MOSH is stronger than that of iso‐alkanes of a similar range of carbon numbers, particularly in the liver.
-
○
No information is available on the pathways and rates of excretion for MOSH.
Humans
No new information was retrieved on absorption, distribution and elimination of MOSH mixtures or single compounds in humans.
- New data on the presence and composition of MOSH mixtures in human tissues from autopsies were reported.
-
○There was a large inter‐individual variation in the concentration of MOSH in tissues, which were higher in females than males.
-
○Among tissues, the concentrations varied greatly: Those in spleen, mesenteric lymph nodes (MLN), liver and adipose tissue were highest, lower in heart and kidney and below detection limit in the brain.
-
○The MOSH compositions were similar in liver and spleen, with highly isomerised branched and alkylated cyclic hydrocarbons centred on n‐C27, ranging from n‐C20 to n‐C46, and hardly any terpenes or n‐alkanes. In the adipose tissue and MLN, the compositions were similar, but clearly different from those in liver and spleen, ranging from about n‐C16 to n‐C36 and centred around n‐C23, including wax components, diterpenes (e.g. from dairy products) and n‐alkanes (from plants).
-
○Except for the n‐alkanes accumulated by the F344 rats, the molecular mass distribution and the compositional pattern observed by comprehensive two‐dimensional gas chromatography (GC × GC) analysis of the MOSH residues in human organs appeared to be similar to those in F344 rats and SD rats.
-
○
MOAH
New in vitro studies using human and rat microsomes on oxidative biotransformation of alkyl substituted MOAH showed that alkyl substitution shifted ring oxidation to side chain oxidation. The oxidation rate decreased with increased chain length. Methyl substitution could also shift the position of ring oxidation and impact ability to produce reactive metabolites.
No MOAH were detected in human tissues, with a detection limit of around 0.5% related to the MOSH.
4.1.2. Toxicity in experimental animals
MOSH
In line with the previous opinion, the CONTAM Panel concluded that there was minimal acute toxicity associated with MOSH.
The new studies on MOSH in F344 rats confirmed the previous findings of granuloma formation in liver and MLN, associated signs of inflammation and of increased liver, spleen and MLN weights. The effects in liver and spleen were concluded to be F344‐specific, due to their higher tendency to retain n‐alkanes compared to SD rats and other test animals.
Treatment of F344 rats with L‐C25, a MOSH product in the range of n‐C25 to n‐C50 treated to minimise the presence of wax components, including n‐alkanes, did not induce organ weight changes and formation of liver granulomas up to the highest tested dose of 236 mg/kg bw per day.
Limited data were available on MOSH and GTL oils in SD, Wistar and Long Evans rats and Beagle dogs. These data did not show the effects associated with n‐alkanes observed in F344 rats.
In SD rats treated with a paraffinic oil in a subchronic study, minimal signs of liver inflammation were reported at 1,624 mg/kg bw. In a study with a synthetic GTL oil in SD rats, mild to moderate apoptosis and necrosis in the intestinal mucosa were observed at 1,267 mg/kg bw per day.
In a subchronic study on paraffin wax, no adverse effects were observed in SD rats up to 9 g/kg bw per day. The only treatment‐related changes were observed in MLN and considered as non‐adverse.
There is no evidence of genotoxicity associated with MOSH.
A new chronic and carcinogenicity study in F344 rats was retrieved on Ozokerite, a wax containing 81% saturated hydrocarbons in the range n‐C22 to n‐C38. In addition to the typical effects observed in F344 rats in liver, spleen and MLN, increased incidence in hepatic preneoplastic GST‐P‐positive foci and hepatocyte adenomas were observed. The CONTAM Panel concluded that these changes were secondary to the F344 rat‐specific chronic granulomatous inflammation caused by the liver granulomas.
New information on two GTL oils supports the previous EFSA CONTAM Panel (2012) Opinion on the absence developmental and reproductive toxicity.
Based on new studies, the Panel concluded that there is no evidence that dietary exposure to MOSH induces autoimmunity.
MOAH
No new studies on acute toxicity, repeated‐dose toxicity or carcinogenicity were retrieved for MOAH.
New studies confirm the conclusions of the EFSA CONTAM Panel (2012) Opinion that the genotoxicity of MOH is associated with the presence of some three or more ring MOAH.
The Panel noted that genotoxicity testing of oils as mixtures is insensitive for assessing individual components.
Fetotoxic and developmental effects were observed in dermal toxicity studies on DMSO‐petroleum extracts enriched in three or more ring MOAH. No effects were observed in an oral screening reproductive and developmental toxicity study with a lubricating base oil treated to reduce three or more ring MOAH.
As concluded in the EFSA CONTAM Panel (2012) opinion, there is evidence that some MOH components act as tumour promoters following initiation in mouse skin‐painting studies and that certain aromatic hydrocarbons like naphthalene are known non‐genotoxic carcinogens.
4.1.3. Observations of toxicity in humans
MOSH
In the absence of relevant new data, the earlier conclusion that the lipogranulomas observed together with MOSH in human liver, spleen, lymph nodes and other organs are not associated with adverse consequences, is still valid. These lipogranulomas differ from the epithelioid granulomas observed in F344 rats.
In new studies, pharmaceutical grade mineral oil products used as placebo in clinical trials of oil‐based agents might have caused adverse effects. While some long‐term, large studies showed increases in atherogenic lipoproteins and inflammatory biomarkers, other studies that were small and of short duration did not. The CONTAM Panel noted the observational nature of this evidence and the large uncertainty related to potential hazard of mineral oil.
MOAH
No new data were retrieved on toxicity of MOAH in humans.
4.1.4. Mode of action
MOSH
Epithelioid granuloma in liver of F344 rats and associated inflammatory response are related to hepatic accumulation of n‐alkanes > C25 and other wax components. Increased spleen weights were observed, which were considered to be, at least in part, associated with enhanced immune system response associated with the effects in the liver.
In the absence of relevant new data on effects of MOSH in humans, the conclusion of the previous EFSA Opinion that lipogranulomas observed together with MOSH in human liver, spleen, lymph nodes and other organs are not associated with adverse consequences is still valid.
New evidence indicates that n‐alkanes do not accumulate in human liver. Therefore, the Panel concluded that the formation of epithelioid granuloma and associated effects in F344 rats exposed to MOSH are not critical endpoints relevant to humans.
Macrophage aggregation and granuloma formation in MLN, and MLN increased weights were considered an adaptive response and not adverse.
MOAH
Genotoxicity is due to some three or more ring MOAH, depending on alkylation, albeit with no clear relationship to position, size and structure of alkyl side chains.
Studies on DMSO‐extracts from petroleum products investigated showed a correlation between the developmental toxicity potency and the presence of three or more ring MOAH. Evidence indicates that developmental toxicity is correlated with the extent of trans‐activation of the Ah receptor.
4.1.5. Hazard characterisation
MOSH
The effects observed in liver and spleen of F344 rats following exposure to MOSH have been associated with the specific hepatic accumulation of n‐alkanes in this rat strain.
Since such accumulation of n‐alkanes has not been found in humans, these effects were not considered as critical. Moreover, these effects have not been observed in other strains of rat or in Beagle dogs.
In view of the lack of critical effects clearly identified for MOSH, a NOAEL of 236 mg/kg bw per day in F344 rats, corresponding to the highest tested dose of L‐C25, was selected as the relevant reference point for MOSH.
The L‐C25 composition best represents what was found in human liver and spleen with regard to mass range and low occurrence or absence of n‐alkanes. In addition, there were no adverse effects following exposure to MOSH at or below this value in other experimental animal models tested, albeit in limited studies.
The limitations of the data set precluded the setting of a health‐based guidance value (HBGV) for MOSH, and a margin of exposure (MOE) approach was applied.
MOAH
The CONTAM Panel confirmed the conclusion of the previous opinion that there is a potential concern regarding the presence of a genotoxic and carcinogenic fraction in MOAH, constituted by substances with three or more aromatic rings with various side chain alkyl moieties.
Since the possible presence of genotoxic and carcinogenic components within MOAH prevents the setting of an HBGV, an MOE approach was applied in accordance with the Opinion of the EFSA Scientific Committee (EFSA, 2005). However, in view of the lack of suitable data, no dose–response analysis could be performed for carcinogenicity, and no reference point could be defined for three or more ring MOAH.
In view of the structural similarity and the plausible common MoA for genotoxicity and carcinogenicity between MOAH and PAHs, the CONTAM Panel considered appropriate to make use of the RPs defined for carcinogenic PAHs.
Under a conservative approach, as a surrogate RP for the risk estimation related to the exposure to three or more ring MOAH, the CONTAM Panel selected the BMDL10 of 0.49 mg/kg bw per day for increased incidence of total tumour‐bearing animals, calculated from a carcinogenicity study using the sum of eight PAHs (PAH8) as found in coal tar.
Little is known regarding the toxicity of 1–2 ring MOAH. Overall, the lack of robust data on the oral toxicity of MOAH hampers the possibility to identify the critical effects and a RP related to the non‐genotoxic and non‐carcinogenic fraction of MOAH.
4.2. Occurrence and exposure
4.2.1. Occurrence in food
A total of 80,632 analytical results on MOSH and MOAH in food samples were initially extracted from the EFSA Database (sampling years 2011–2021). After a first assessment and cleaning of the data, 71,222 analytical results (7,840 samples) were available.
Data were provided by European countries (4,427 samples, 56.5%) and food associations (3,413 samples, 43.5%); the CONTAM Panel considered both data sets suitable for exposure assessment, providing consistent and complementary information.
The main analytical methods reported for the measurement of MOAH and MOSH were on‐line LC‐GC‐FID (86%), GC‐FID (13%) or GC‐LRMS (0.09%).
Most samples were analysed for the MOSH and MOAH C‐fractions specified by the JRC Guidance on MOH. Whenever possible, the results provided for ‘Total MOAH’ and ‘Total MOSH’ were used; otherwise, the total MOAH and total MOSH concentrations were derived by summing the individual C‐fractions reported.
MOSH
There were 7,675 samples (43,047 analytical results). Of these, 3,060 samples reported values only on C‐fractions (40%), 761 samples only on ‘Total MOSH’ and 3,854 on both C‐fractions and ‘Total MOSH’.
A total of 2,212 samples (29%) were left censored, i.e. all fractions or the total were below LOD or LOQ.
The highest mean concentrations were found in vegetable oils, the highest being for ‘Olive pomace oil’ (n = 51, LB = UB = 108.7 mg/kg).
MOAH
There were 7,742 samples (30,075 analytical results). Of these, 3,134 samples reported values only on C‐fractions (40.5%), 731 samples only on ‘Total MOAH’ and 3,877 on both C‐fractions and ‘Total MOAH’.
A total of 6,472 samples (84%) were left censored, i.e. all fractions or the total were below LOD or LOQ.
To minimise the impact of the left‐censored data, the samples reporting C‐fractions or total MOAH with LOQs above those specified by the JRC Guidance were excluded. The final data set used for the exposure estimations consisted of 7,378 samples.
The highest mean levels were reported for samples of ‘Olive pomace oil’ (n = 51, LB–UB = 13.54–13.56 mg/kg)
The Panel noted that the reported levels quantified in some samples of ‘Infant formulae, liquid’ and ‘Follow‐on formulae, liquid’ were within the range reported in the 2019 EFSA Rapid risk assessment (EFSA, 2019).
The current data do not allow conclusions on the levels of three or more ring MOAH in food.
4.2.2. Exposure assessment
MOSH general scenario
The highest dietary exposure was estimated for the young population, in particular ‘Infants’, with LB–UB means of 0.085–0.126 mg/kg bw per day and LB–UB 95th percentiles of 0.157–0.212 mg/kg bw per day.
Across the different age classes, the food groups with the highest average contribution to the mean LB dietary exposure to MOSH were ‘Grains and grain‐based products’, ‘Milk and dairy products’ and ‘Animal and vegetable fats and oils and primary derivatives thereof’.
For ‘Infants’, ‘Food products for young population’ (18–76%, median = 60%) and ‘Milk and dairy products’ (6–51%, median = 24%) were the main contributors.
Overall, exposure estimates to MOSH (mean and 95th percentile) across dietary surveys showed a 1.5–2.5 fold decrease in comparison with the exposure assessment performed in 2012 with data from 1999 to 2010 (EFSA CONTAM Panel, 2012).
MOAH general scenario
The highest dietary exposure was estimated in the young population, in particular ‘Infants’, with LB–UB mean estimations of 0.003–0.031 mg/kg bw per day and LB–UB 95th percentile estimations of 0.011–0.059 mg/kg bw per day.
Across the different age classes, the food groups with the highest average contribution to the mean LB dietary exposure to MOAH were ‘Grains and grain‐based products’, ‘Animal and vegetable fats and oils and primary derivatives thereof’, as well as ‘Tea beverages’ and ‘Herbal and other non‐tea infusions’.
For ‘Infants’, ‘Food products for young population’ was the most important contributor (11–88%, median 64%). The main drivers were ‘Follow‐on formulae, liquid’ and ‘Infant formulae, liquid’, although ‘Ready‐to‐eat meal for infants and young children’ was also an important source in several dietary surveys.
Ad hoc exposure scenarios
For ‘Infants’, in a scenario considering default consumption values (EFSA Scientific Committee, 2017a,b) and sustained consumption of highly contaminated samples of ‘Infant formula’, mean exposures (LB–UB) could be as high as 0.22–0.30 mg/kg bw day (MOSH) and 0.019–0.051 mg/kg bw day (MOAH), while the P95 exposure (LB–UB) could be 0.27–0.37 mg/kg bw day (MOSH) and 0.023–0.063 mg/kg bw day (MOAH).
Using MOSH levels in human milk as reported in the literature (2008) and an average daily milk consumption of about 800 mL and a high consumption of 1,200 mL, the dietary exposure to MOSH of an infant of 6.1 kg exclusively fed with breast milk might be between 0.11 μg/kg bw per day (mean exposure) and 0.17 μg/kg bw per day (high exposure).
Different scenarios were conducted with food samples that were considered as not representative of the levels typically found in those foods, e.g. wheat groat and chocolate cakes reporting relatively high MOSH and MOAH values. Mean exposure estimates as high as 5.8 mg MOSH/kg bw day (LB = UB) and 0.067 MOAH/kg bw day (UB) could be reached via the consumption of highly contaminated wheat groats and chocolate cakes, respectively.
4.3. Risk characterisation
MOSH
Considering the existing uncertainties in the toxicological data set of MOSH, the CONTAM Panel concluded that MOEs ≥ 1,200 are sufficient to indicate that there is a low concern for human health risks related to the current dietary exposure to MOSH.
For the general population, the CONTAM Panel noted that MOEs for all age classes are at or above a value of 1,200.
With exception of fat tissue, median tissue levels measured in a study on 37 human subjects and the related estimated body burdens were generally lower than tissue levels and body burdens in F344 rats exposed at the RP.
Infants exclusively fed with infant formula with high MOSH content could have exposure with MOEs ranging approximately from 790 to 1,070 for mean exposure and from 640 to 870 for P95 exposure. Considering the short duration of exposure via infant formula, these MOEs do not raise concerns.
The consumption of certain foods, such as dairy products and other products of animal origin, may result in the exposure to MOSH enriched in strongly accumulating components. The consequences of this increased exposure to accumulating components for human health have not been investigated and are uncertain.
Taking into account the identified uncertainties, it is likely to very likely (with 66–95% certainty) that exposure levels do not health raise concern in either mean or high‐consuming toddlers. This conclusion can be extended to all age groups.
Overall, the CONTAM Panel concluded that the current dietary exposure to MOSH for all age classes raises no concern for human health.
MOAH
Few data were available on the concentrations of three or more ring MOAH in food.
As the sources of MOAH, hence MOAH compositions, vary between food categories as well as within food categories, the presence of three or more ring MOAH in the diet could not be estimated.
The actual proportion of three or more ring MOAH in total MOAH is likely to vary between about 25% (e.g. transfer from jute bags) and virtually 0% (e.g. a white mineral oil product) in and among food groups.
- The CONTAM Panel decided to use two scenarios that are assumed to encompass realistic proportions of three or more ring MOAH in the MOAH fractions present in food.
-
○As a conservative one (scenario 1), an average of 10% of three or more ring MOAH within the MOAH fraction present across the different foods in the exposure was assumed.
-
○Without consumption of foods contaminated by little refined mineral oil products, the overall percentage could be as low as 1% (scenario 2).
-
○
In considering the carcinogenicity risks of the MOAH subfraction with three or more aromatic rings, using the two scenarios, the CONTAM Panel selected the BMDL10 of 0.49 mg/kg bw per day as derived from the assessment of PAHs, as a surrogate RP in an MOE approach.
In view of the possible presence of genotoxic and carcinogenic substances, MOEs ≥ 10,000 were considered of low concern for human health, in accordance with the Opinion of the EFSA Scientific Committee (EFSA, 2005).
Under the Scenario 1, MOEs were consistently lower than 10,000 for most of the consumption surveys for mean consumers and all high consumers.
Under the Scenario 2, MOEs were below 10,000 for UB estimates only, for most of the dietary surveys at the mean exposure and for all at P95 exposure. However, MOEs were higher than 10,000 for all the LB mean exposure levels and for most of the LB P95 exposure levels, with the exception of some surveys in the younger age groups showing P95 LB MOEs in the range 4,000–8,000.
The CONTAM Panel concluded that Scenario 1 would raise a health concern related to the presence of three or more ring MOAH in food for all the age groups. Scenario 2 would raise a health concern, in particular for the high consumers in the younger age groups.
Taking into account the identified uncertainties, an expert knowledge elicitation exercise resulted in an extreme likelihood (99–100% certainty) that MOEs are lower than 10,000 for mean and high‐consuming toddlers. For the other age groups, it is likely (certainty above 66%) that MOEs are below 10,000.
The CONTAM Panel noted that a full risk characterisation would require additional data on toxicity and exposure to three or more ring MOAH.
Due to the lack of adequate oral toxicity studies, it was not possible to identify a reference point for the 1–2 ring MOAH. Therefore, a risk characterisation of this MOAH fraction could not be performed. The CONTAM Panel concluded that, in the absence of reliable toxicity data, the dietary exposure to 1–2 ring MOAH might raise a concern.
5. Recommendations
MOSH
Improvement of analytical methodology for better characterisation of MOSH and consistency in reporting are needed. MOSH concentrations in food should be determined according to the JRC guidance document (Bratinova and Hoekstra, 2023).
Better investigation of the sources of the hydrocarbons in food, which would enable better specification of the type of hydrocarbons present and their fate before ending in the food.
Data are needed on the formation, fate and toxicity of biotransformation products of MOSH, including their accumulation potential.
Investigation is recommended on the structural features of MOSH (particularly regarding alkylated naphthenes) that hinder metabolism and elimination and result in accumulation. This would also enable a better comparison of mineral oils with GTL oils.
Additional toxicity data are needed in relevant experimental models and design, in particular on bioaccumulating MOSH following their characterisation. Particular attention is needed in relation to effects in the liver, spleen as well as immune and nervous system.
Additional data generation is recommended on possible effects of MOSH on lipoproteins and on inflammation and inflammatory markers as seen in recent clinical trials.
More data on human MOSH tissue concentrations or development and use of biomarkers of exposure are needed, particularly from individuals born after 1995.
Contribution from environmental sources, compared to other sources, needs further investigation with regard to occurrence of MOSH and potential compositional modification and bioaccumulation.
The contribution from the environment needs further investigation. On the one hand, it is difficult to avoid this source of contamination. On the other hand, the risks may have been underestimated because of a much higher propensity of accumulation of the most persistent MOSH.
MOAH
More data on MOAH composition by aromatic ring number in food are needed, in particular with respect to the levels of three or more ring MOAH.
Sources of food contamination should be investigated when MOAH are detected. To this end, more selective and sensitive analytical method should be implemented.
More data are needed on the influence of ring alkylation on genotoxicity and carcinogenic potency of three or more ring MOAH.
Oral toxicity data are needed for MOAH, in particular with respect to 1–2 ring MOAH.
Technical specifications of white mineral oils and waxes used as food additives and food packaging materials should be updated, with detailed information about the MOAH content and composition.
MOAH concentrations in food should be determined according to the JRC guidance document (Bratinova and Hoekstra, 2023).
Documentation as provided to EFSA
ERBC (European Research Biology), 2022. EWF FCM 93 58. 13‐Week oral toxicity study in rats followed by a 6‐week recovery period including toxicokinetics. ERBC Study No. A4471. Unpublished study report submitted by the European Wax Federation.
Abbreviations
- ADME
Absorption, Distribution, Metabolism, Elimination
- ADI
acceptable daily intake
- ALT
Alanine transaminase
- ANS
Panel on Food Additives and Nutrient Sources added to Food
- ANSES
The French Agency for Food, Environmental and Occupational Health & Safety
- AR
androgen receptor
- AST
aspartate transaminase
- BCF
bioconcentration factor
- BfR
German Federal Institute for Risk Assessment
- BMD
Benchmark dose modelling
- BMDL10
benchmark dose lower bound at 10% BMR
- BMDU10
benchmark dose upper bound at 10% BMR
- BMI
body mass index
- BMR
benchmark response value
- BP
boiling point
- CLP
Classification, Labelling and Packaging
- ConA
concanavalin A
- CONTAM
The EFSA Panel on Contaminants and in the Food Chain
- CRP
C‐reactive protein
- CVD
cardiovascular disease
- CYP450
Cytochrome P450
- DM
diabetes mellitus
- DMSO
dimethyl sulfoxide
- ECHA
European Chemicals Agency
- EMA
European Medicines Agency
- EKE
expert knowledge elicitation
- Erα
oestrogen receptor alpha
- ESI
electrospray ionisation mode
- EST
embryonic stem cell test
- EU
European Union
- FAO
Food and agriculture organisation
- FCM
food contact material
- FID
flame ionisation detection
- FoodEx
comprehensive food classification and description system
- FSANZ
The Food Standards Australia New Zealand
- GC
gas chromatography
- GC × GC
two‐dimensional GC
- GO
gas oil
- GST
glutathione‐S‐transferases
- GTL
gas to liquid
- HBGV
Health Based Guidance Value
- HCys
homocysteine
- HED
human equivalent dose
- HFO
heavy fuel oil
- HRBOs
Highly Refined Base Oils
- hsCRP
high‐sensitivity C‐reactive protein
- HVMO
high viscosity white mineral oil
- IL
interleukin
- IPCS
International Programme on Chemical Safety
- JEFCA
The Joint FAO/WHO Expert Committee on Food Additives
- KLH
Keyhole limpet haemocyanin
- LBOs
Lubricant Base Oils
- LB
lower bound
- LC
liquid chromatography
- LMPW
low‐melting‐point‐waxes
- LOAEL
lowest observed adverse effect level
- LOD
limit of detection
- LOQ
limit of quantification
- LP‐PLA2
lipoprotein‐associated phospholipase A2
- LPS
lipopolysaccharide
- MLN
mesenteric lymph nodes
- MOAH
mineral oil aromatic hydrocarbons
- MOE
margin of exposure
- MOH
Mineral oil hydrocarbons
- MOSH
mineral oil saturated hydrocarbons
- MRLs
Maximum Residue Levels
- MS
mass spectroscopy
- MVMO
medium viscosity white mineral oil
- MW
microcrystalline waxes
- NAM
New Alternative Methodology
- NOAEL
no‐observed adverse effect level
- NVWA
Netherlands Food and Consumer Product Safety Authority
- OECD
Organisation for Economic Co‐operation and Development
- OxLDL
oxidised Low‐Density Lipoprotein cholesterol
- PAC
polycyclic aromatic compounds
- PAH
polycyclic aromatic hydrocarbons
- PAO
poly alpha olefins
- PC
public consultation
- POSH
polyolefin oligomeric saturated hydrocarbons
- PR
progesterone receptor
- RAE
residual aromatic extract
- REACH
Registration, Evaluation, Authorisation and Restriction of Chemicals
- RIVM
the Dutch National Institute for Public Health and the Environment
- RP
reference point
- SC
scientific committee
- SLE
systemic lupus erythematosus
- SML
specific migration limit
- SOP
standard operating procedure
- SSD
Standard Sample Description
- tDAE
treated distillate aromatic extracts
- TG
triglyceride
- ToxPi
Toxicological Priority Index
- TRβ
thyroid receptor beta
- UB
upper bound
- UK
United Kingdom
- USA
United States of America
- WHO
World Health Organisation
- ZET
zebrafish embryotoxicity test
Appendix A – Summary tables of toxicological studies published since the previous opinion
1.
Table A.1.
Toxicokinetic studies
| Test substance | Exposure conditions | Result | Reference |
|---|---|---|---|
|
White P7H MO Paraffinic, low viscosityn‐C14 – n‐C32 (average C20) 48% was </= C20 |
F344 rats (f), age: 4 weeks, 10 per group Dose/route: 0, 0.02, 0.2 or 2% in feed, corresponding to 0, 17, 170 or 1,690 mg/kg bw per day (reported in the publication) Duration: 90 days |
MOSH constituents with molecular weight < C20 underrepresented in liver in comparison to distribution in test oil MOSH constituents with molecular weight > C20 overrepresented in liver in comparison to distribution in test oil |
McKee et al. (2012) |
|
Experiment broad MOSH mixture n‐C14 – n‐C50 |
F344 rats (f), age: 4 weeks, 25 per group Dose/route: 0, 400, 1,000 or 4,000 mg/kg in feed, corresponding to 0, 2.6, 25 or 260 mg/kg bw per day (calculated on the basis of individual body weights and cage averaged feed intake) Background contamination of control feed: 1.6 mg/kg feed Duration: 30, 60, 90, 120 days or 120 + 30 days of control feed for depuration |
All doses: MOSH concentration highest in liver, low in carcasses 0–30 days: rapid increase of retained amount 30–90 days: slower increase 90–120: still increasing Spleen, adipose tissue: no plateau, no steady state After 30 days depuration: concentration in liver, spleen, carcass reduced, little change in adipose tissue. Liver: 49–61% of retained material, range n‐C16 to n‐C40, major part n‐C24 to n‐C34, max n‐C29 Spleen: nearly identical mass distribution Adipose tissue and carcass: ~ 38–50% of retained MOSH, range to n‐C31, centre n‐C19, max n‐C15/16 Mass distribution in liver concentrated around n‐C23 to n‐C31 with n‐C16 to n‐C22 absent and n‐C25 to n‐C35 retained |
Cravedi et al. (2017), Barp et al. (2017a,b) |
|
Experiment with 3 narrow MOSH mixtures
|
F344 rats (f), age: 4 weeks, 8 per group Dose/route: 0, 400, 1,000 or 4,000 mg/kg in feed, corresponding to S‐C25: 0, 23, 61 or 226 mg/kg bw per day L‐C25: 0, 25, 62 or 236 mg/kg bw per day L‐C25W: 0, 22, 53 or 218 mg/kg bw per day (calculated on the basis of individual body weights and cage averaged feed intake) Background contamination of control feed: 1.5 mg/kg feed Duration: 120 days |
All mixtures: dose‐dependent tissue accumulation S‐C25: material recovered from liver and spleen mainly > n‐C20, in adipose tissue and carcass enrichment of < n‐C20, half as much retained as L‐C25W L‐C25: material recovered from liver and spleen n‐C23 – n‐C40, maximum at n‐C30, in adipose tissue up to n‐C34, maximum at n‐C28, carcass similar to adipose tissue. L‐C25W: molecular mass distribution similar to L‐C25, most strongly retained mixture |
|
|
Naphthenic MO N70H ∼ 84% naphthenes, 3% linear alkanes, 7% mono/dimethyl alkanes, 4% branched alkanes n‐C18 to nC48 Synthetic oil 99% iso‐alkanes (3% mono/dimethyl alkanes, rest multibranched iso‐alkanes) n‐C20 to n‐C42 |
SD rats (f), age: 8–10 w, 5 per group Dose/route: 3,000 mg/kg in feed, corresponding to 200 mg/kg bw per day (reported in the publication) Duration: 134 days, 90 + 44 days of control feed for depuration |
NOAEL: 200 mg/kg bw High intraindividual variability in hydrocarbon concentrations in the liver. Day 92: 4–5 times higher concentration for N70H than GTL oil in blood and liver (660 mg/kg and 150 mg/kg liver) Day 134: MOSH concentration differs by approx. factor 2 in liver (650 and 300 mg/kg liver) Same trend observed in mesenteric lymph nodes and visceral fat After 44 days depuration: concentrations in liver reduced by approx. factor 3 (56 and 180 mg/kg) No reduction seen in mesenteric lymph nodes in the group treated with N70H |
Carrillo (2021) |
| Paraffin wax (FCM 93 58) |
SD rats (m + f), age 53–55 days old at the start of administration, 10 animals/sex per group. Doses/route Oral via the diet Four treatment groups: 0, 0.2, 2 or 10 g/kg bw per day (nominal doses) exposed for 90 consecutive days, satellites groups (10 animals/sex/group) exposed to 0 or 10 g/kg bw per day for 30 or 60 days, or to 90 days + 6 weeks of recovery, for an overall period of 132 days. Measured doses: m: 0.19, 1.9 and 9 g/kg bw per day; f: 0.24, 2.0 and 10 g/kg bw per day. |
NOAEL: 9 g/kg bw per day Higher levels found in female than in male livers. After 6 weeks of recovery, an almost complete elimination was observed in liver. The fraction C31–C35 was more efficiently retained in liver, whereas spleen, adipose tissue, MLN and lungs retained more efficiently C21–C25, followed by C16–C20. In terms of chemical composition, n‐alkanes were more effectively retained in MLN, lungs, spleen and adipose tissue than in the liver, whereas the liver was relatively enriched with isoalkanes and monocyclic alkanes. |
ERBC Study A4471, 2022 |
Table A.2.
Acute toxicity studies
| Test substance | Exposure conditions | Result | Reference |
|---|---|---|---|
| GTL products (n‐C4 to n‐C70) |
OECD guideline 420 SD rats (m + f), age: unknown, number per group unknown Route: gavage Duration: single exposure |
LD50 > 5,000 mg/kg bw Effects: No gross signs of any toxicity |
Boogaard et al. (2017) |
|
Sarawax SX30 (GTL linear paraffin n‐C18 to n‐C25) Sarawax SX100 (GTL linear paraffin n‐C38 to n‐C90) |
OECD guideline 401 SD rats (m + f), age: unknown, number per group unknown Route: gavage Duration: single exposure |
LD50 > 5,000 mg/kg bw Effects: No treatment‐related changes in behaviour or condition, no findings in necropsy |
Table A.3.
Repeated dose toxicity studies
| Test substance | Exposure conditions | Result | Reference |
|---|---|---|---|
|
White P7H MO Paraffinic, low viscosityn‐C14 to n‐C32 (average C20) 48% was </= C20 |
F344 rats (f), age: 4 weeks, 10 per group Dose/route: 0, 0.02, 0.2 or 2% in feed, corresponding to 0, 17, 170 or 1,690 mg/kg bw per day (reported in the publication) Duration: 90 days |
NOAEL 17 mg/kg bw per day Tissue levels at 0, 17, 170, 1,690 mg/kg bw per day: Liver: ND, ND, 2,280 and 3,000 mg/kg, MLN: ND, ND, 2,910 and 1,860 mg/kg, Spleen: ND at all doses, Effects: Liver: abs weight ↑ (a) (mid and top dose), incidence of granulomas ↑ (top dose) Immune system: abs number of neutrophiles ↑ (top dose) MLN: weight ↑ and inflammation (mid and top dose), incidence ↑ and severity of granulomas (mid and top dose) Spleen: weight ↔, histopathology unchanged |
McKee et al. (2012) |
|
Experiment broad MOSH mixture: Broad white MO mixture n‐C14 – n‐C50 |
F344 rats (f), age: 4 weeks, 25 per group (5 rats/time point) Dose/route: 0, 400, 1,000 or 4,000 mg/kg in feed, corresponding to 0, 2.6, 25 or 260 mg/kg bw per day (calculated on the basis of individual body weights and cage averaged feed intake) Background contamination of control feed: 1.6 mg/kg feed Duration: 30, 60, 90, 120 days or 120 + 30 days of control feed for depuration |
NOAEL 25 mg/kg bw per day Tissue levels at exposure to 0, 2.6., 25, 260 mg/kg bw per day after 90 days: Liver: 30, 238, 1,411 and 5,492 mg/kg MLN: not analysed Spleen: 8, 31, 128 and 274 mg/kg Effects: Liver: abs weight ↑ (top dose), density of granulomas ↑ (top dose) Immune system: lymphoid cells in liver parenchyma ↑ (top dose) MLN: not analysed Spleen: weight ↔ Clinical chemistry and haematology analyses not performed |
Nygaard et al. (2023), |
Experiment 3 narrow MOSH mixtures
|
F344 rats (f), age: 4 weeks, 8 per group Dose/route: 0, 400, 1,000 or 4,000 mg/kg in feed, corresponding to S‐C25: 0, 23, 61 or 226 mg/kg bw per day L‐C25: 0, 25, 62 or 236 mg/kg bw per day L‐C25W: 0, 22, 53 or 218 mg/kg bw per day (calculated on the basis of individual body weights and cage averaged feed intake) Background contamination of control feed: 1.5 mg/kg feed Duration: 120 days |
S‐C25, LOAEL 23 mg/kg bw per day L‐C25, NOAEL 236 mg/kg bw per day LC25W, LOAEL 22 mg/kg bw per day Tissue levels after 90d exposure: Liver: S‐C25: 16.5, 4,784, 9,223 and 14,639 mg/kg at 0, 20, 52 and 222 mg/kg bw per day, respectively L‐C25: 16.5, 1,393, 2,590 and 3,805 mg/kg at 0, 22, 54 and 207 mg/kg bw per day, respectively L‐C25W: 16.5, 4,827, 6,851 and 7,709 mg/kg at 0, 19, 46 and 187 mg/kg bw per day, respectively MLN: Not analysed Spleen: S‐C25: 1.3, 287, 538 and 499 mg/kg at 0, 20, 52 and 222 mg/kg bw per day, respectively L‐C25: 1.3, 192, 263 and 419 mg/kg at 0, 22, 54 and 207 mg/kg bw per day, respectively L‐C25W: 1.3, 134, 147 and 243 mg/kg at 0, 19, 46 and 187 mg/kg bw per day, respectively Effects: Liver: S‐C25: relative weight ↑ (all doses), absolute weight ↑ (top dose), density of granulomas ↑ (top dose) L‐C25: No findings L‐C25W: absolute weight ↑ (all doses), density of granulomas ↑ (all doses) MLN: Not analysed Immune system: S‐C25: Lymphoid cells in liver parenchyma ↑ (top dose) L‐C25: No findings L‐C25W: ↑ (all doses) Spleen: S‐C25: absolute weight ↑ (all doses) L‐C25: No findings L‐C25W: absolute weight ↑ (all doses) Clinical chemistry and haematology analyses not performed |
|
| GTL base oil experiment: GTL base oil (n‐C18 to n‐C50, branched and linear alkanes) dissolved in arachis oil |
OECD testing guideline 408 SD rats (m + f), age: unknown, 20 per group Dose/route: 0, 50, 200 or 1,000 mg/kg bw per day by gavage Duration: 90 days, 90 at highest dose+28 days of control feed for depuration |
Effects: No toxicity observed, some accumulation of alveolar macrophages with vacuolated cytoplasm in lungs (two top doses) Female rats: MLN showed vacuolated histiocytes (both effects not considered biologically relevant by the study authors) |
Boogaard et al. (2017) |
| Follow up 28‐day study: GTL base oil n‐C18 to n‐C30 |
Rats (gender unknown), age: unknown, number per group: unknown Dose/route: 0, 750, 3,750, 15,000 mg/kg feed, corresponding to 0, 63, 308 and 1,267 mg/kg bw per day (reported in the publication) Duration: 28 days |
Effects: Lungs: No effects mild to moderate apoptosis and necrosis in lamina propria, crypts of duodenum, jejunum and ileum in male rats (top dose) Spleen: lymphocytosis in males (minimal in top dose) MLN and Peyer's patches: lymphocytosis in males (minimal in low and mid dose, moderate at top dose) |
|
|
GTL residual oil experiment: GTL residual oil (n‐C40 to nC70, branched, cyclic and linear alkanes) dissolved in polyethylene glycol 400 |
OECD testing Guideline 407 Wistar rats (m + f), age: unknown, 10 per group Dose/route: 0, 30, 300, 1,000 mg/kg bw per day by gavage Duration: 28 days, 28 at highest dose +14 days of control feed for depuration |
NOAEL 1,000 mg/kg bw Effects: Haematopoiesis in spleen of female rats (two top doses), effect reversible. Considered adaptive, not adverse. |
|
|
90‐d dietary repeated‐dose toxicity study: GTL waxes (n‐C15 to n‐C50, branched and linear alkanes) Sarawaxes
|
F344 rats (f), age: unknown, 5–10 per group Doses/route: SX30, SX50, SX701: 0, 0.002%, 0.02%, 0.2%, 2% in feed, corresponding to 0, 1.8, 18, 180, 1,800 mg/kg bw per day SX701: 5% in feed, corresponding to 4,500 mg/kg bw per day (calculated by EFSA default values) Duration: 90 days SD rats (unknown), age: unknown, 5 per group Dose/route: SX30, SX50, SX701: 0%, 2% in feed, corresponding to 0, 1,800 mg/kg bw per day (calculated by EFSA default values) Duration: 90 days |
Effects: Liver: absolute and relative liver weight ↑ (F344 at SX50 2% and at SX701 2% and 5%), relative liver weight ↑ (SD at SX30 2%) MLN: absolute and relative weight ↑ (F344 at SX30 2%, SX50 0.2 and 2%, SX701 0.02, 0.2, 2 and 5%, SD at SX30 and SX50 doses unknown), absolute weight ↑ (SD at SX701) Spleen: ↑ (F344 at SX30 2%, SX50 0.2% and 2%, SX701 0.2%, 2%, 5%), absolute and relative weight ↔ (SD rats) Microgranulomata in liver: present (F344 at SX50 and SX701 > 0.2%) Periportal vacuolisation: present (F344 at SX701 2% and 5%) Foci of inflammatory cells: present (F344 at SX701 0.002% and 0.02%, SD at SX50 and SX701 2%) Liver lesions: None (F344 and SD rats at SX30) MLN lesions, reactive nodes, histiocytosis, adenitis: present (F344 at SX30, SX50, SX701 at 2%, SX701 at 5%), histiocytosis (F344 at SX50, SX701 lower doses) Heart lesions, thickening, chronic inflammation, ↑ basophilia of mitral valve: (F344 SX50 2%, SX701 2% and 5%) Accumulation of non‐polar hydrocarbon material in liver: (F344 rats at SX50 and SX701), in MLN: (F344 at SX30, SX50, SX701) |
|
|
90‐d repeated‐dose study: GTL waxes (n‐C15 to n‐C50, branched and linear alkanes) Sarawaxes
|
SD and F334 rats (f), age: unknown, 5–10 per group Doses/route: SX701: 0, 2, 5% in feed, corresponding to 0, 1,800, 4,500 mg/kg bw per day SX702, SX100: 0, 0.002, 0.02, 0.2, 2% in feed, corresponding to 0, 1.8, 18, 180, 1,800 mg/kg bw per day (calculated by EFSA default values) Duration: 90 days F344 (f), age: unknown, 5 per group Doses/route: SX701: 0, 5% in feed, corresponding to 0, 4,500 mg/kg bw per day (calculated by EFSA default values) Duration: 4, 8, 13 week, 13 week + 6–12 weeks reversal SD rats (f), age: unknown, 5 per group Doses/route: SX701: 0, 2% in feed, corresponding to 0, 1,800 mg/kg bw per day (calculated by EFSA default values) Duration: 13 week |
Effects: Appearance: Red staining in face, genital area, unkempt fur (F344 at SX701 5%, 13 w, improved in reversal period) Liver and spleen: Absolute and relative weight ↑ (F344), ↔ (SD) MLN: absolute and relative weight ↑ (F344, SD) Spleen: Absolute and relative weight ↑ (F344), ↔ (SD) Lesions in liver, MLN, mitral valve of heart: (F344 at SX701) Lesions in MLN: (F344 at SX702 0.02, 0.2, 2%) Severity all lesions: ↓ in reversal period SX100 no evidence of lesions |
|
|
90‐day repeated dose study: Paraffin wax (FCM 93 58) |
SD rats (m + f), age 53–55 days old at the start of administration, 10 animals/sex per group. Doses/route Oral via the diet Four treatment groups: 0, 0.2, 2 or 10 g/kg bw per day (nominal doses), plus two groups (0 and 10 g/kg bw per day) allowed for 6‐week recovery after the 13‐week administration period. Measured doses: m: 0.19, 1.9, and 9 g/kg bw per day; f: 0.24, 2.0 and 10 g/kg bw per day. Duration: 13 weeks +6 weeks of recovery (only control and top dose groups) Study parameters compliant with OECD 408 (2018) |
NOAEL: 9 g/kg bw per day Effects: No adverse effects observed up to the highest tested dose. Increased MLN weights observed in both sexes (achieving statistical significance in female rats at all tested doses). Macrophage aggregates were observed in MLN of females rats with incidence and severity increasing with dosage. |
ERBC Study A4471 (2022) |
↑ indicates an increase, ↓ a decrease, ↔ indicates no changes.
Table A.4.
Chronic toxicity and carcinogenicity studies
| Test substance | Exposure conditions | Result | Reference |
|---|---|---|---|
|
Ozokerite (OZK), 81% C22–C38 Solid hydrocarbon wax used in lipstick and skincare products |
Chronic study: F344 rats (m + f), age: 4 weeks, 20 per group Dose/route: 0, 0.05, 0.1, 0.2% in feed, corresponding to 0, 25.5., 50.3, 104.2 mg/kg bw per day for males and 0, 27.8, 54.9, 110.6 mg/kg bw per day for females (reported in the publication) Duration: 52 week |
LOAEL 25 mg/kg bw per day Effects: Lungs: Absolute and relative weight ↑ (all doses, both sexes) Spleen and liver: absolute and relative weight ↑ (above 0.1%, both sexes) Kidney: absolute and relative weight ↑ (at 0.2%, males) Testes: absolute weight ↑ (at 0.2%, males) Blood chemistry: ↑ ALT, AST, ↓ total protein, albumin and triglycerides (males from 0.05%, females from 0.1%) Histiocytes in liver and MLN, granulomas and microgranulomas in liver and spleen, hepatic vacuolisation (all doses, both sexes) GST‐P positive foci: ↑ (from 0.1%, both sexes) |
Kuroda et al. (2013) |
|
Carcinogenicity study: F344 rats (m + f), age: 4 week, 100 per group Dose/route: 0, 0.1, 0.2% in feed, corresponding to 0, 42.8, 86.7 mg/kg bw per day for males and 0, 47.8, 97.9 mg/kg bw per day for females (reported in the publication) Duration: 104 week |
LOAEL 50 mg/kg bw per day Effects: Lungs, liver, spleen, kidney: absolute and relative weight ↑ (all doses, both sexes) Heart: absolute and relative weight ↑ i(males from 0.2%, females at all doses) GST‐P positive foci: ↑ (from 0.1%, both sexes) Hepatocellular adenomas and carcinomas: ↑ (males at 0.1% and 0.2%) |
Table A.5.
Developmental and reproductive toxicity studies
| Test substance | Exposure conditions | Result | Reference |
|---|---|---|---|
| GTL gas oil C8‐C26, branched and linear alkanes |
Two‐generation reproductive toxicity study OECD TG 416 Duration: F0: dosed 70 consecutive days before mating F1: dosed from PND22 F0 and F1 m: throughout mating phase until day prior of euthanasia F0 and F1 f: throughout mating, gestation, lactation until day prior of euthanasia Non selected F1 and F2 necropsied on PND21 All F2 necropsied on PND21 |
NOAEL: 750 mg/kg bw per day Effects: Mean and adjusted age of attainment of balano‐preputial separation: ↑ F1 m in top dose falling within the historical control range, not considered adverse percentage of abnormal sperm ↑ F1 m at 750 mg/g bw per day, falling within historical control range, not considered adverse unexplained low fertility with male mating indices: control F0 m Changes in lungs: F0 and f1, m + f at top dose group α2u‐globulin‐mediated nephropathy: F1 m Increased tubular mineralisation: F0 m at 750 mg/kg bw per day, considered equivocal effect |
Boogaard et al. (2017) |
|
SD rats (m + f), age: 42–46 days, F0: 25 per sex per group, F1: 28 per sex per group Dose/route: 0, 50, 200, 750 mg/kg bw per day by gavage | |||
| GTL base oil, C18–C50, branched, cyclic and linear alkanes |
SD rats (m + f), age: 42–46 days, F0: 25–26 per sex per group, F1: 25 per sex per group Dose/route: 0, 50, 250, 1,000 mg/kg bw per day by gavage |
NOAEL: 1,000 mg/kg bw per day Effects: α2u‐globulin‐mediated nephropathy: F0 m adjusted anogenital distance: small ↓ in F1 f (not F2) at 250 and 1,000 mg/kg bw per day |
|
| GTL gas oil C8–C26, branched and linear alkanes |
Prenatal developmental toxicity study OECD TGD 414 |
NOAEL: 750 mg/kg bw per day Effects: No maternal or developmental effects |
|
|
SD Crl:CD rats (f, pregnant), age: unknown, 24 per group Dose/route: 0, 50, 200, 750 mg/kg bw per day by gavage Duration: d5 to d19 post‐coitum, euthanised d20 | |||
| GTL base oil, C18–C50, branched, cyclic and linear alkanes |
RccHan:WIST rats (f, pregnant), age: unknown, 22 per group Dose/route: 0, 50, 200, 1,000 mg/kg bw per day by gavage Duration: d6–d20 post‐coitum, euthanised on d21 |
NOAEL: 1,000 mg/kg bw per day Effects: No relevant maternal or developmental effects |
|
| Light paraffinic distillate solvent, ‘consisting predominantly of aromatic hydrocarbons in the range C15–C30, likely contains 5% or more of 4–6 membered condensed ring aromatic hydrocarbons’ according to applicant |
OECD TG 414 SD rats (f, pregnant), age: 13 w, 25 per group Dose/route: 0, 5, 25, 150, 450 mg/kg bw per day by dermal exposure (reported in the study) Duration: 20 days |
NOAEL: 5 mg/kg bw per day for maternal and fetal toxicity Maternal effects (food consumption ↓, bw ↓ or ↑, absolute and relative thymus weight ↓) at ≥ 25 mg/kg bw per day |
Study summary (a) |
| Developmental effects (↑ post‐implantation loss, corresponding ↓ mean numbers and litter proportions of viable fetuses) at 150 and 450 mg/kg bw per day, mean m + f and combined fetal weights ↓ at ≥ 25 mg/kg bw per day, fetal developmental variations (unossified sternebrae, reduced ossification of skull and vertebral arches) at 150 and 450 mg/kg bw per day |
Table A.6.
Immunotoxicity studies
| Test substance | Exposure conditions | Result | Reference |
|---|---|---|---|
| Broad MOSH mixture and three MOSH fractions see Table E.1, Column Cravedi et al. (2017), Barp et al. (2017a,b) |
OECD test guideline 443 see Table E.1, Column Cravedi et al. (2017), Barp et al. (2017a,b) Duration: 120 days |
Effects: No significant effects on Keyhole limpet haemocyanin (KLH)‐specific IgM concentrations in serum | Cravedi et al. (2017), Nygaard et al. (2023) |
|
Broad MOSH mixture, n‐C14 to n‐C50 Pristane |
DA/OlaHsd rats (m + f), age: 7–8 weeks, 10 per group Dose/route: 4,000 mg/kg pristane in feed, corresponding to 260 mg/kg bw per day for males and 244.5 mg/kg bw per day for females 0, 40, 400 and 4,000 mg/kg broad MOSH mixture in feed, corresponding to doses of 0, 2.9, 29.4 and 280.1 mg/kg bw per day for males and 0, 2.3, 25.7, 238.8 mg/kg bw per day for females (reported in the publication) Positive control: Single intradermal injection of 200 μL pristane Duration: 90 days |
Effects: No effects (body weight ↑, clinical arthritis symptoms, ↑ in common arthritis‐associated biological markers in serum and spleen (splenocytes TLR2 and 3, spontaneous cytokine release from splenocytes or cytokine release from splenocytes stimulated with LPS or Con A) at all doses Positive control: Arthritis symptoms and higher levels of serum markers |
Andreassen et al. (2017) |
Appendix B – Toxicokinetic data from study sponsored by EFSA
1.
In the first part of the study sponsored by EFSA (GP/EFSA/CONTAM/2013/01; Cravedi et al., 2017; Barp et al., 2017a), female F344 rats were exposed to MOSH of a molecular mass range from n‐C14 to n‐C50 and aimed at determining the mass range accumulated and types of hydrocarbons accumulated in different tissues.
-
•
In the adipose tissue, the maximum retention was at the most volatile end of the applied mixture, i.e. n‐C15/16, and reached up to about n‐C31 (Figure B.1).
-
•
In the liver and spleen, the maximum was at n‐C29, the range covering n‐C16 to n‐C40. This distribution falls into that of the medium and low viscosity class I (P70) oils defined by FAO/WHO, 2002) for which an ADI of 10 mg/kg bw has been specified.
Figure B.1.
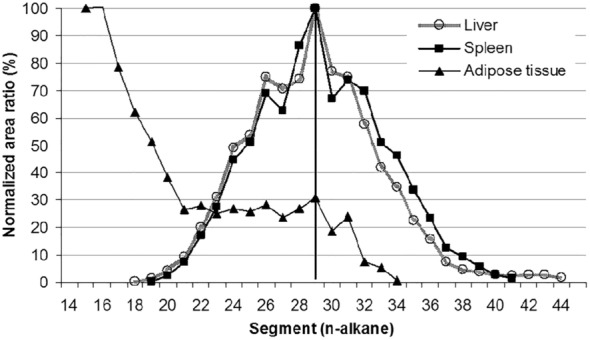
Relative retention of MOSH in liver, spleen and adipose tissue (40 mg/kg dose; 90 + 30 days exposure). Adapted from Cravedi et al. (2017) © INRA, KLZ, NIPH. Barp et al. (2017a)
GC × GC for the characterisation of the composition of the retained MOSH combined a polar first dimension (OV‐17) with a non‐polar (dimethylpolysiloxane) second dimension separation, further presented in Annex B. The upper left plot in Figure B.2 was obtained from the administered MOSH, the other three from extracts of the adipose tissue, liver and spleen exposed to the mixture for 120 days. Into each plot, a line was drawn at the level the n‐alkanes that were eluted at about the same second‐dimensional retention time (height in the GC × GC plot) as the little branched open chain alkanes. Above the n‐alkanes are the multibranched alkanes, mainly below them the naphthenes.
Figure B.2.
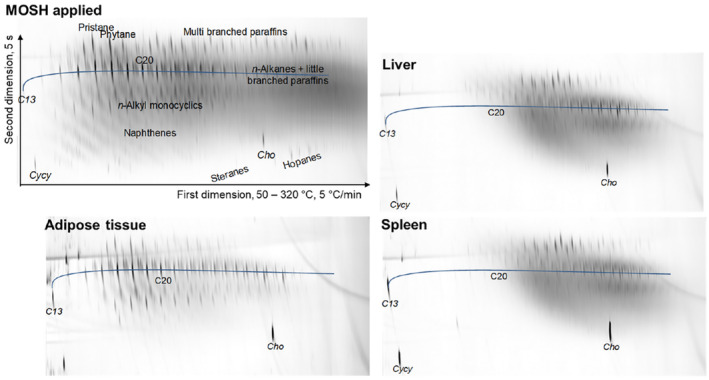
GC × GC /FID of the MOSH added to the feed (upper left) and in the extracts of adipose tissue, liver and spleen from F344 rats exposed to the 400 mg/kg dose during 120 days. Cycy and Cho, internal standards. Attenuation adjusted to result in similar intensity. Adapted from Cravedi et al. (2017) © INRA, KLZ, NIPH. Barp et al. (2017a)
The comparison of the tissue extracts with the MOSH in the feed shows that in the adipose tissue the low molecular mass components were enriched; the cloud of the unresolved material of high mass shown at the right in the plot of the administered mixture is almost missing. In the earlier eluted part, the clear signals stand out, particularly the n‐alkanes and the n‐alkyl monocyclics.
The plots obtained from the liver and spleen are similar among themselves, but strongly different from that of the adipose tissue. The early eluted part (at the left in the plot) is largely empty and also the latest part is missing (as observed by HPLC‐GC). The clear signals stand back. The strongest ones on the line of the n‐alkanes are little branched paraffins (the n‐alkanes are almost absent). There remained multibranched paraffins and mainly the cloud of unresolved hydrocarbons, at least mainly of naphthenes.
Less than 10% of the administered MOSH were retained in the tissues, which means that the hydrocarbons detected in the tissues are those for which metabolic elimination is difficult. GC × GC /MS of the liver and spleen extracts confirmed for the cyclohexanes and the cyclopentanes that the accumulation increased with the branching of the alkyl groups, while the opposite applied for adipose tissue. However, still most branched alkanes and naphthenes are eliminated. The search for the additional structural features that determine the persistent substances in liver and spleen failed.
The second part of the study sponsored by EFSA (GP/EFSA/CONTAM/2013/01; Cravedi et al., 2017; Barp et al., 2017b) was on oils largely below and above n‐C25, termed S‐C25 and L‐C25, respectively, and a 1:1 mixture of L‐C25 with a low melting point wax ranging from n‐C23 to n‐C45 (L‐C25W; Figure B.3). S‐C25 contained some n‐alkanes up to about C22, L‐C25 virtually none (Figure B.3). Doses of 400, 1,000 and 4,000 mg/kg feed were administered for 120 days. Tissues concentrations are reported in Table B.1.
Figure B.3.
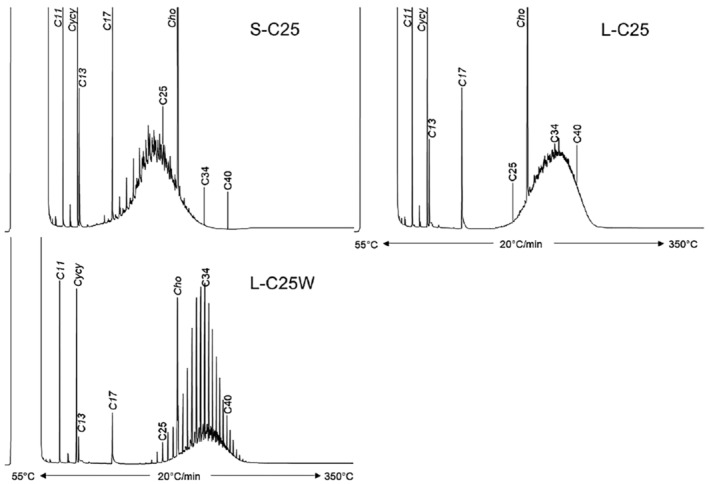
HPLC‐GC‐FID chromatograms of the three MOSH mixtures tested in the second part of the EFSA‐sponsored study. Internal standards in Italics: n‐alkanes C11, C13 and C17, cyclohexyl cyclohexane (Cycy) and cholestane (Cho). Adapted from Cravedi et al. (2017) © INRA, KLZ, NIPH. Barp et al. (2017b)
Table B.1.
MOSH concentrations (mg/kg) in liver, spleen, adipose tissue and carcass following 120 days of exposure to nominal concentrations of 0 (control containing about 1.6 mg/kg MOSH), 400, 1,000 and 4,000 mg/kg feed of the three MOSH mixtures. Adapted from Barp et al. (2017b)
| MOSH | Dose | Liver | Spleen | Adipose tissue | Carcass |
|---|---|---|---|---|---|
| Control | 0 | 16.5 | 1.3 | 1.1 | < LOQ |
| S‐C25 | 400 | 4,784 | 287 | 88.1 | 48.3 |
| 1,000 | 9,223 | 538 | 154 | 83 | |
| 4,000 | 14,639 | 499 | 347 | 119 | |
| L‐C25 | 400 | 1,393 | 192 | 12.8 | 9.8 |
| 1,000 | 2,590 | 263 | 17.4 | 20.2 | |
| 4,000 | 3,805 | 419 | 36 | 16.1 | |
| L‐C25W | 400 | 4,827 | 134 | 96.4 | 40.1 |
| 1,000 | 6,851 | 147 | 102 | 51.1 | |
| 4,000 | 7,709 | 243 | 200 | 76.5 |
As observed in the first experiments (Barp et al., 2017a), the concentrations in the tissues increased less than proportionally with the dose: for the 10fold difference in the dose (from 400 to 4,000 mg/kg feed), the increase merely amounted to factors of 1.7–3.9 for S‐C25, 1.7–2.8 for L‐C25 and 1.6–2.1 for L‐C25W. This means that human tissues may contain more MOSH residues than would be predicted from extrapolation from the dose applied in the rat experiments.
Bioconcentration factors (BCFs) for MOSH in liver and abdominal adipose tissue were calculated as the ratio of the MOSH concentration in the tissue to that in the feed (Table B.2). They showed the absence of bioconcentration in adipose tissue, but BCFs for the liver exceeding 1, irrespective of the mixture tested. In the liver, for the 400 mg/kg group, values of approximately 13, 4 and 15 were observed for S‐C25, L‐C25 and L‐C25W, respectively, again increasing with decreasing dose. They were in the same order as those calculated from other studies in rats (Trimmer et al., 2004; Griffis et al., 2010).
Table B.2.
MOSH bioconcentration factors in liver and adipose tissue (120 days). Adapted from Barp et al. (2017b)
| Mixture | Dose | Liver | Adipose tissue |
|---|---|---|---|
| Control | 1.6 | 10.2 | 0.7 |
| S‐C25 | 400 | 12.6 | 0.23 |
| 1,000 | 9.5 | 0.16 | |
| 4,000 | 4.4 | 0.10 | |
| L‐C25 | 400 | 3.6 | 0.03 |
| 1,000 | 2.6 | 0.02 | |
| 4,000 | 1.1 | 0.01 | |
| L‐C25W | 400 | 14.9 | 0.27 |
| 1,000 | 8.0 | 0.13 | |
| 4,000 | 2.3 | 0.06 |
The MOSH retention in the animals was calculated from the amount of feed consumed during the 120 days multiplied by the MOSH concentration in the feed and the concentrations in the tissues multiplied by the concentration of these when the animals were sacrificed. The digestive tract was not included in the calculations. Table B.3 shows the percentages for the three MOSH fractions. The difference between L‐C25 and L‐C25W reflects the strong retention of the n‐alkanes from the wax. At increased dose, the relative accumulation was again strongly reduced.
Table B.3.
Percentage of MOSH recovered from the body of the rats compared to the amount ingested (%). Adapted from Barp et al. (2017b)
| Dose (mg/kg) | Retention (%) | ||
|---|---|---|---|
| S‐C25 | L‐C25 | L‐C25W | |
| 400 | 9.3 | 2.5 | 10.1 |
| 1,000 | 2.9 | 1.9 | 5.8 |
| 4,000 | 3.2 | 0.7 | 1.7 |
Figure B.4 provides an overview of the HPLC‐GC/FID chromatograms, qualitatively comparing the MOSH administered (black) with those found in the tissue (red) in terms of molecular mass distribution and signals on top of the hump. It shows the strong accumulation of the n‐alkanes starting from n‐C25 in all tissues after administration on L‐C25W, which was associated with a strong formation of granulomas (Nygaard et al., 2023) and is considered specific for F344 rats.
Figure B.4.
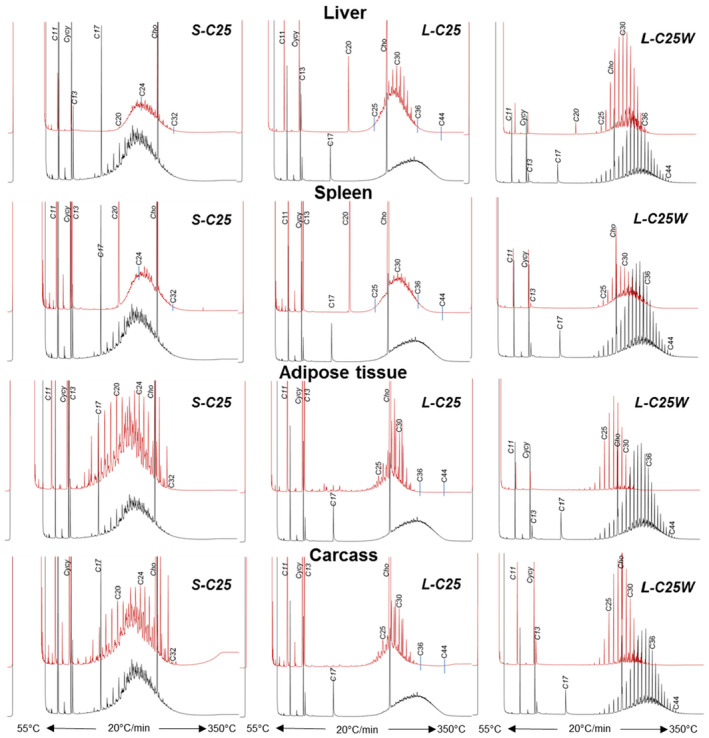
Comparison of HPLC‐GC‐FID chromatograms of extracts from the tissues (upper chromatograms in red) with the corresponding MOSH mixtures applied (lower chromatograms in black). 400 mg/kg doses; 90 days. Adapted from Cravedi et al. (2017) © INRA, KLZ, NIPH. Barp et al. (2017b).
The presence of n‐alkanes (and other wax compounds) was of interest in the context of the increased liver and spleen weight discussed in Section 3.1.4 and the conclusions that can be drawn for humans. The S‐C25 oil contained a minor amount of n‐alkanes up to about C22. As the n‐alkanes were enriched in the adipose tissue, they are better seen in this tissue (Figure B.4). The series of signals ends in the region of n‐C22 (green arrows). The sharp dominant signals starting from C23 have a slightly longer retention time and are from the same branched, unidentified hydrocarbons as observed for L‐C25 in the liver and mentioned above for the broad MOSH mixture. Since n‐alkanes are enriched in adipose tissue, this is evidence that the S‐C25 oil contained hardly any n‐alkanes above about C23, i.e. those enriched by the F344 rats and considered to trigger granuloma formation (Figures B.5 and B.6).
Figure B.5.
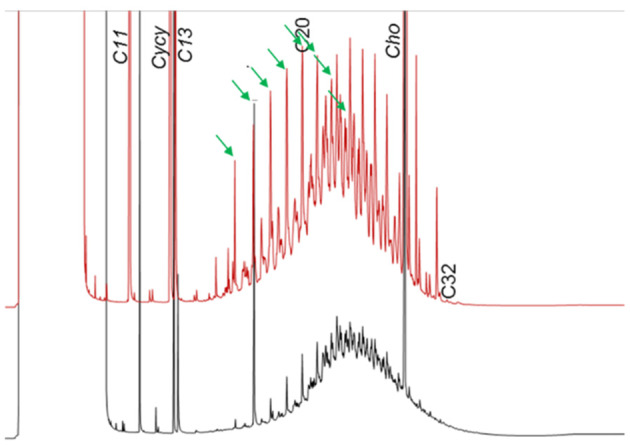
Extract from Figure B.4 focusing on the S‐C25 oil and the adipose tissue, showing that the n‐alkanes ranging up to about C22 (green arrows), then disappeared in other signals, particularly the little branched alkanes
Figure B.6.
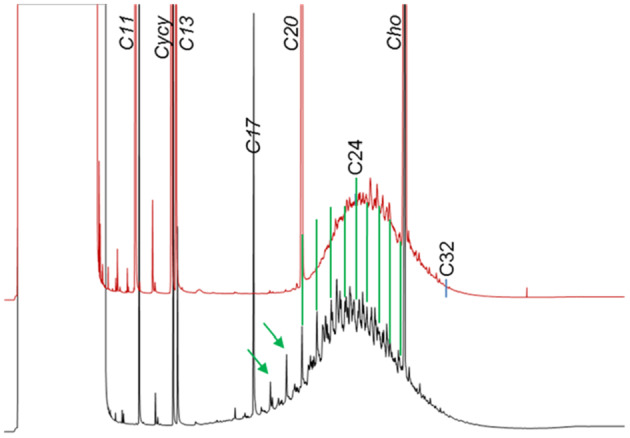
Extract from Figure B.4 focusing on the S‐C25 oil and the MOSH in the spleen, showing the almost complete absence of n‐alkanes in the spleen
Figure B.6 again shows the n‐alkanes in the administered S‐C25 and relates this to the MOSH in the spleen (red). In the early eluted part of the chromatogram from the spleen, the n‐alkanes are virtually absent, presumably owing to the metabolic elimination. In the later part, they are virtually absent owing to the low concentration in the S‐C25 oil. The green lines show the position of the n‐alkanes in the two chromatograms.
Figure B.7 does the same for the liver, again showing the almost complete absence of n‐alkanes.
Figure B.7.
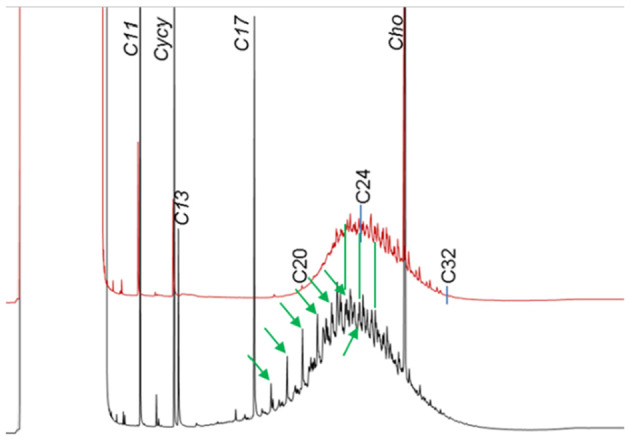
Extract from Figure B.4 focusing on the S‐C25 oil and the MOSH in the liver, showing the almost complete absence of peaks related to n‐alkanes
With respect to the presence of n‐alkanes, the chromatograms from all three doses applied are qualitatively similar (Kantonales Labor Zürich).
Figure B.8 shows the analogous extracts from Figure B.4 for L‐C25. The two chromatograms at the left show that the feed containing L‐C25 contained little n‐alkanes, but they were not completely absent: again, the n‐alkanes are better seen in the adipose tissue, where those of C25, 29, 30 and 31 are marked. n‐C29 and n‐C31 are from the feed (odd‐numbered species dominate). They are followed by the iso‐alkanes mentioned above. As for S‐C25, it was not possible to quantify the n‐alkanes in the spleen, but they were lower after administration of S‐C25 (those from the feed were also in the spleen of the rats fed with S‐C25).
Figure B.8.
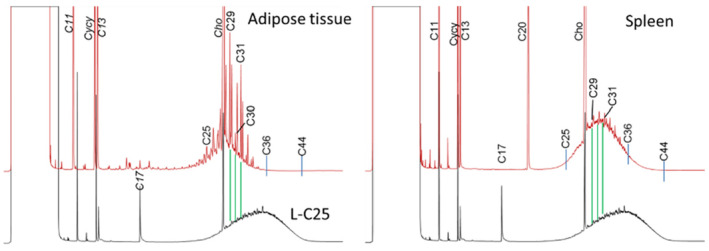
Extract from Figure B.4 focusing on the L‐C25 oil and the MOSH in the adipose tissue and the spleen, analogous to Figures B.5 and B.6. The concentration of the n‐alkanes was lower than after administration of S‐C25, but they were still present
In the GC × GC/FID plots (same configuration as above), the multibranched open chain hydrocarbons in the top region of the plots are encircled, the centres of the signals of the n‐alkanes and little branched open chain alkanes as well as of the n‐alkyl monocyclic naphthenes interconnected by lines. Attenuation was adjusted to provide similar overall intensities.
Figure B.9 compares the composition of the S‐C25 oil with the residues in the tissues. In the plot from the oil, distinct signals for the n‐alkanes, the branched open chain hydrocarbons and (scarcely separated) n‐alkyl cyclopentanes and cyclohexanes are well visible. There are two clouds of unresolved, highly isomerised hydrocarbons in the background (mainly representing the hump in the HPLC‐GC chromatograms), the upper one at the height (second dimension retention time) of the n‐alkanes, the weaker one in the upper region of the naphthenes. The n‐alkanes were dominant up to C21 and visible up to C23, but then a series of stronger signals at slightly higher position took over; the unidentified iso‐alkanes mentioned above.
Figure B.9.
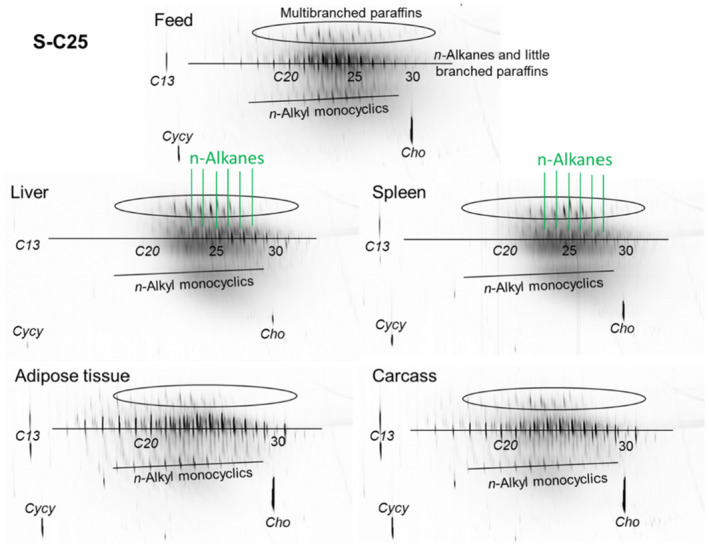
Sections of the GC × GC‐FID plots of the S‐C25 oil (top) and its residues in the tissues (400 mg/kg dose). Green lines point out the n‐alkanes that are barely visible. Axes as in Figure B.2. Adapted from Cravedi et al. (2017) © INRA, KLZ, NIPH. Barp et al. (2017b)
In the liver (400 mg/kg dose), some of the multibranched open chain hydrocarbons gained intensity relative to the total residue. The signals of the n‐alkanes largely disappeared up to C23, but are visible as weak signals above C24 eluted shortly before the dominant iso‐alkanes. The n‐alkyl monocyclics and the row of signals above them virtually disappeared. The two clouds gained in relative intensity. The plot obtained from the spleen is similar, though with the n‐alkanes hardly visible. In the adipose tissue and carcass, opposite changes are observed. Apart from the gain in the low molecular constituents, the proportion of the distinct signals belonging to the n‐ and iso‐alkanes as well as the n‐alkyl monocyclics was enhanced, whereas the cloud in the background was reduced. The MOSH residues in the carcass had virtually the same composition as in the adipose tissue.
The L‐C25 oil showed less distinct signals than S‐C25 (Figure B.10), presumably because of dewaxing, and was dominated by the cloud mainly of naphthenes with one to four rings (in general, in the plot, naphthenes are located further down the higher is the number of rings). In the liver (400 mg/kg dose), the series of iso‐alkanes between n‐C26 and n‐C35 stood out. The relative intensity of the distinct signals of the multibranched open chain compounds was reduced. The n‐alkanes from C26 to C35 are visible, increased by the natural n‐alkanes C29 and C31 from the feed. Several series of distinct signals below the n‐alkanes were enhanced. The cloud of the naphthenes gained in intensity, but there must have also been selectivity in their accumulation, since the hopanes disappeared. The same trend was observed in the spleen, where also some n‐alkanes are visible. In the adipose tissue and carcass, the strong gain in the distinct signals was on cost of the cloud in the background. The multibranched compounds were almost absent.
Figure B.10.
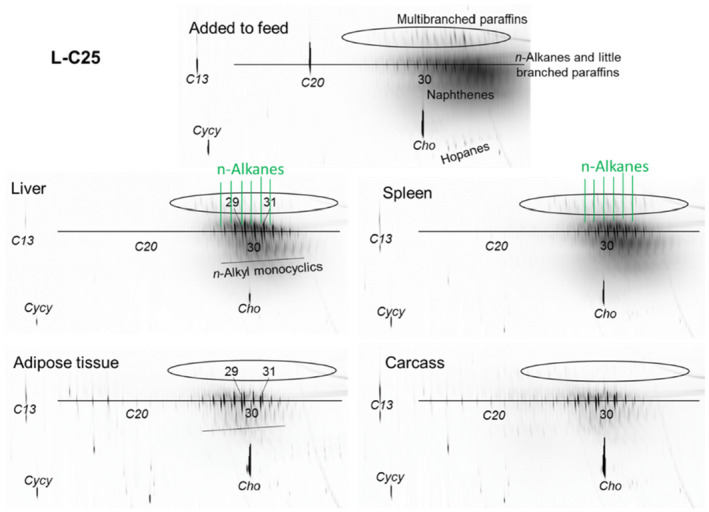
Sections of the GC × GC‐FID plots of L‐C25 and its residues in the tissues (400 mg/kg dose). Green lines point out the n‐alkanes, which produced small, but visible signals. Axes as in Figure B.2. Adapted from Cravedi et al. (2017) © INRA, KLZ, NIPH. Barp et al. (2017b)
Figure B.11 compares the composition of L‐C25W with the related residues in the tissues of the animals exposed to the 400 mg/kg dose. In all tissues, the wax components predominate even more strongly than in L‐C25W applied
Figure B.11.
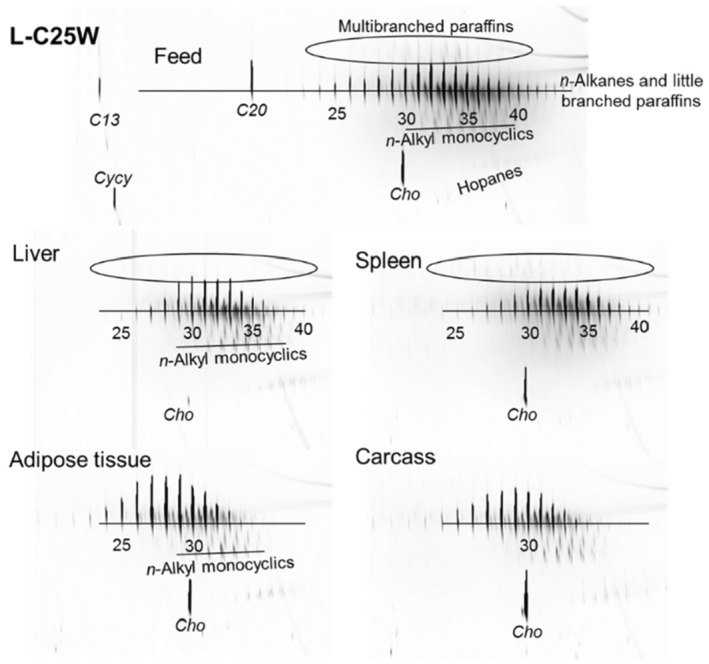
Sections of the GC × GC‐FID plots of L‐C25W and its residues in the tissues (400 mg/kg dose). Axes as in Figure B.2. Adapted from Cravedi et al. (2017) © INRA, KLZ, NIPH. Barp et al. (2017b)
The structure of the accumulated iso‐alkanes forming clear signals would have been of interest to explain their resistance to metabolic elimination. According to the second dimension GC retention (approximately at the height of the 2‐ and 3‐methyl n‐alkanes), it is little branched. In the first dimension GC, these iso‐alkanes are eluted earlier than the n‐alkane of the same carbon number by an equivalent of approximately 0.8 carbon atoms, which is more than the mono‐methyl branched ones in positions 2 and 3, suggesting a smaller molecular volume. However, their mass spectrum (GC × GC‐MS/EI) did not enable to attribute a structure (Barp et al., 2017b).
Appendix C – Summary of the new studies on MOH in human tissues.
1.
MOSH
Accumulated MOSH in various tissues were investigated in a study on humans from Austria (n = 37) undergoing autopsy (Barp et al., 2014). The individuals had a mean age of 67 years, ranging from 25 to 91 years (Figure C.1). The samples were from 2014, which means that the high exposure to MOH (at least in Switzerland) reduced between 1995 and 2000 was 14–19 years back.
Figure C.1.
Subjects sorted by age, with females in grey and males in black (modified from Barp et al., 2014. © Elsevier Ltd)
MOH were determined in subcutaneous abdominal adipose tissue, mesenteric lymph nodes (MLN), spleen, liver and lung, and in some cases also in kidney, heart and brain. The MOSH concentrations varied greatly in all tissues investigated. The highest levels were found in the spleen (1,400 mg/kg) and the mesenteric lymph nodes (MLN; 1,390 mg/kg), followed by the liver (900 mg/kg; Table C.1, Figure C.2). Higher concentrations of MOSH were found in females, possibly due to exposure from cosmetics.
Table C.1.
MOSH concentrations in human tissues (adapted from Barp et al., 2014)
| Tissue | Concentrations (mg/kg) | ||
|---|---|---|---|
| n = 37 | |||
| min–max | Average | Median | |
| MLN | 21–1,390 | 223 | 166 |
| Liver | 14–901 | 131 | 71 |
| Fat tissue | 17–493 | 130 | 87 |
| Spleen | 6–1,400 | 93 | 28 |
| Lung | < 2–91 | 12 | 7 |
| n = 14 | |||
| min–max | Average | Median | |
| Kidney | < 2–12 | 6 | 6 |
| Heart | < 2–41 | 9 | 6 |
| Brain | < 2 | ||
Figure C.2.
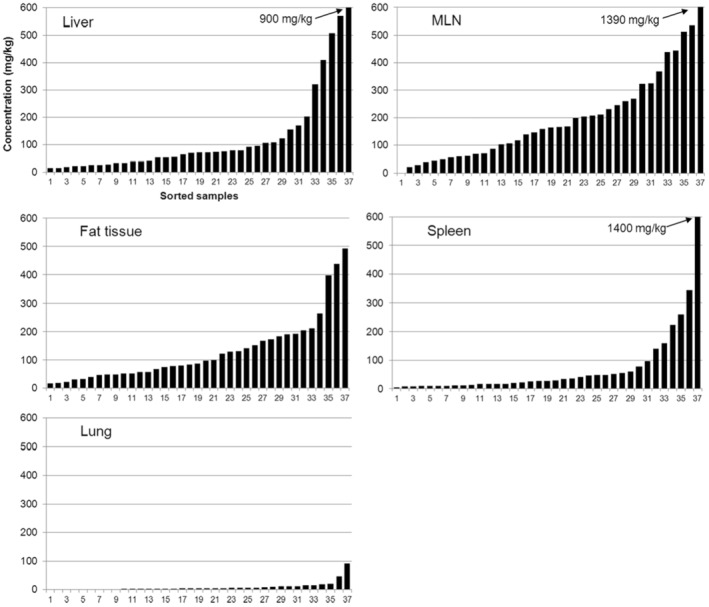
MOSH concentrations in five human tissues sorted by increasing values (from Barp et al., 2014). © Elsevier Ltd
In adipose tissue, the MOSH concentration was slightly higher, whereas the composition was similar to that in adipose tissue obtained from 144 volunteers with Caesarean sections reported by Concin et al. (2008) and referenced in the previous opinion of the CONTAM Panel (EFSA CONTAM Panel, 2012). The higher concentration may have been due to higher age.
As shown in Figure C.3, the bulk of the MOSH consisted of highly branched iso‐alkanes and alkylated naphthenes, ranging from about n‐C16 to n‐C36 and centred close at around n‐C23. There were also diterpenes and n‐alkanes, presumably transiently occurring from exposure to milk and dairy products as well as plants. The MOSH composition in MLN was similar to that in adipose tissue.
Figure C.3.
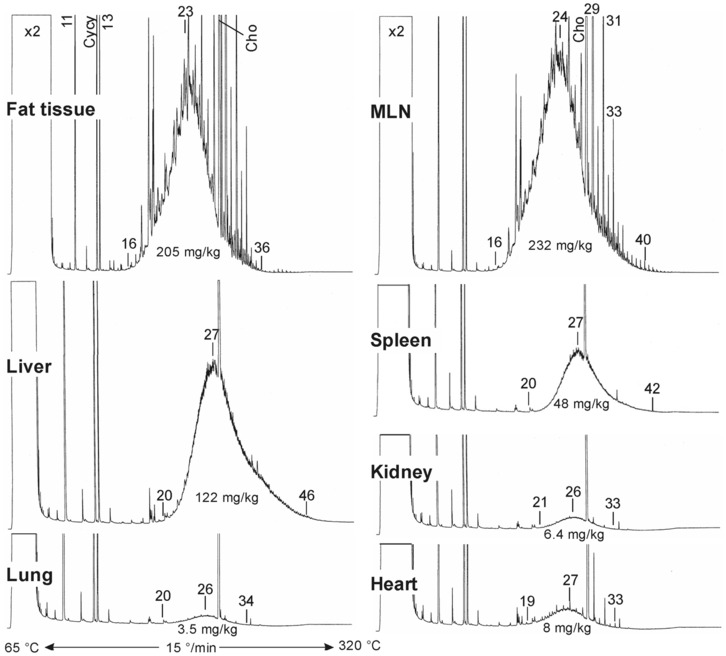
HPLC‐GC‐FID chromatograms from the various investigated tissues from the same subject. From Barp et al. (2014). © Elsevier Ltd
As observed in the rats, the MOSH compositions in liver and spleen were similar, but different from that in adipose tissue and MLN (Figure B.3), showing a hump of unresolved hydrocarbons centred on n‐C27, ranging from n‐C20 to n‐C46, without hardly any peaks on it. Also, the naturally occurring diterpenes and n‐alkanes, were virtually absent, confirming efficient elimination when taking into account that exposure to those is far higher than to MOSH. Kidney, heart and lung had a pattern between those of adipose tissue and liver/spleen.
The total amount of MOSH in the human body was calculated from the weight of a tissue multiplied by the MOSH concentration, assuming that all relevant tissues have been analysed and that the concentrations in all fat tissues are equal to that measured in the subcutaneous abdominal fat tissue (Figure C.4).
Figure C.4.
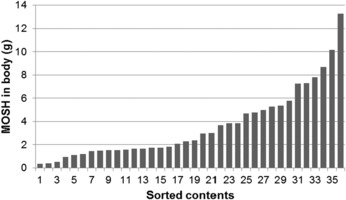
MOSH in human body (g) sorted by increasing values. © Elsevier Ltd
In the second study, the accumulated hydrocarbons were further characterised by GC × GC (Biedermann et al., 2015). As shown in Figure C.5, in liver, virtually all components forming distinct signals are absent. Hence, not only n‐alkanes and n‐alkyl monocyclics, but also many branched and cyclic MOSH, including steranes and hopanes, are efficiently eliminated. Highly isomerised hydrocarbons and alkylated naphthenes constitute a dominant cloud of unresolved MOSH that resisted elimination. The cloud extended to low second dimension retention times (low in the plot) more than in the rat tissues, indicative of an elevated number of rings. However, no further identifications of components were achieved.
Figure C.5.
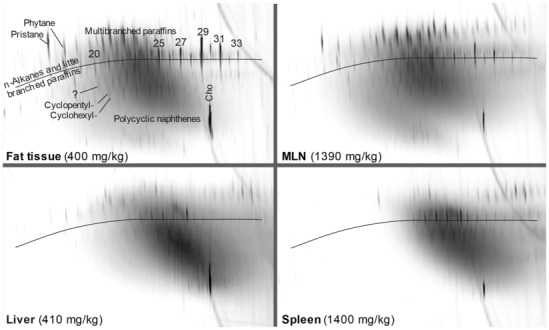
GC × GC‐FID plots of HPLC fractions of the saturated hydrocarbons from four tissues of the same male subject. Concentrations refer to total MOSH determined by HPLC‐GC. Horizonal axes: first dimension chromatography from polar column; vertical axes: second dimension chromatography on non‐polar stationary phase. From Biedermann et al. (2015). © 2014 Elsevier B.V.
A key question for the extrapolation of animal data to humans is the duration of accumulation, i.e. how much time it takes to reach a steady state. As accumulation differs for different hydrocarbons, this also involves a change in composition towards those most difficult to eliminate.
Some information can be obtained by the total amount found in a given human tissue and the time needed to build up this amount. The mean MOSH content in the liver of the 37 Austrians (Barp, et al., 2014) was 210 mg, the median 110 mg. The mean exposure of adults, assessed in this opinion was 0.023 mg/kg bw per day or 1.6 mg/day for a 70 kg bw individual. For the percentage of the MOSH distributed to the liver, the data from the broad mix administered during 30 days to the F344 rats at 40 mg/kg feed (Barp et al., 2017a) resulted in MOSH content of 125 mg/kg and 3.9 mg/kg in liver and adipose tissue, respectively. Assuming the mass of the adipose tissue in the rat to be around 20 times that of the liver, roughly 60% of the MOSH distributed to the liver. The analogous calculation for L‐C25 administered at 400 mg/kg feed during 120 days liver (1,393 mg/kg and 12.8 mg/kg in the liver and the adipose tissue, respectively) results in 84% distribution to the liver.
Assuming a liver distribution of 70% of the ingested MOSH in humans, 1.1 mg/days out of the estimated individual exposure of 1.6 mg/days, distribute to the liver. Of the 400 mg L‐C25 in the feed administered during 120 days, 2.5% remained in the rat body (Barp et al., 2017b), of which perhaps 70% in the liver, i.e. 1.75%. Using these residual 1.75% also for humans is a high value, since much of the MOSH found in the rat liver was from the last few days and would have reduced, whereas the contribution of the last days for humans is predicted to be small. Nonetheless, 1.75% of the 1.1 mg/d going to the liver would amount to 0.019 mg/day. For the mean MOSH content (210 mg), it would take 30 years to be reached, for the median (110 mg), 15.9 years.
Hence, potentially a far higher concentration builds up in humans per exposure than during the rat experiments.
MOAH
In the study of Barp et al. (2014), the tissues of a subject with high MOSH concentrations were analysed for MOAH. None were found at detection limits of 0.5 mg/kg (lung), 1 mg/kg (liver, adipose tissue) and 5 mg/kg (spleen and MLN). If set into relation to the MOSH found in the same samples, these detection limits (i.e. possible residue MOAH concentrations) correspond to 0.55% of the MOSH (lung) or less (other organs). Also for tissues of other subjects, no MOAH were detected. Since many of the mineral oils humans are exposed to contain MOAH at 10–30%, this means that MOAH are not accumulated. MOAH metabolites were not investigated.
Appendix D – Overview of main findings from repeat dose studies on various MOH products
1.
Table D.1.
is not intended to provide a comprehensive overview of the available data, but rather to illustrate the main findings in F344 rats exposed to different white mineral oil products. The endpoints listed include absolute and relative liver weight and histopathology, markers for immune system response, mesenteric lymph node weight and histopathology, and spleen weight. For each endpoint the lowest dose at which a relevant change is observed is reported in bracket (expressed in mg/kg bw per day), or the highest tested dose in case of no observed effects. The table includes studies reviewed in the previous opinion of the CONTAM Panel (EFSA CONTAM Panel, 2012), as well as studies reviewed in the present opinion
| Tested item | Species/strain | Liver | Immune system | Mesenteric lymph node | Spleen | Reference |
|---|---|---|---|---|---|---|
| P7H(paraffinic C14‐32) | F344 rats |
↑ weight (170) ↑ granulomas (1,690) |
↑ neutrophils (1,690) |
↑ weight and granulomas (170) |
No findings | McKee et al. (2012) |
| N10A (naphthenic C15‐30) | F344 rats |
↑ weight (17) ↑ granulomas (1,815) ↑ AST and GGT (1,815) |
↑ WBC (1,815) | ↑ weight and granulomas (173) | ↑ weight (1815) | Smith et al. (1996) |
| P15H (paraffinic C14‐32) | F344 rats |
↑ weight (17) ↑ granulomas (1815) ↑ GGT (1815) |
↑ WBC (1815) |
↑ weight (1815) ↑ granulomas (17) |
↑ weight (173) | |
| N15H (naphthenic C17‐30) | F344 rats |
↑ weight (17) ↑ granulomas (1815) ↑ AST and GGT (1815) |
↑ WBC (1815) |
↑ weight (1815) ↑ granulomas (17) |
↑ weight (1815) | |
| N70A (naphthenic C21‐35) | F344 rats |
↑ weight (17) ↑ granulomas (1815) |
↑ monocytes (1815) | ↑ weight (1815) | ↑ weight (1815) | |
| N70H (naphthenic C22‐37) | F344 rats | ↑ weight and granulomas (1815) | ↑ monocytes and neutrophils (1815) | ↑ weight (1815) | ↑ weight (1815) | |
| P70H (paraffinic C27‐43) | F344 rats |
↑ weight (1815) ↑ ALT (1815) |
No findings | No findings | ↑ weight (1815, recovery only) | |
| P100H (paraffinic C28‐45) | F344 rats | ↑ AST (1815) | ↑ WBC (1815) | No findings | No findings | |
| ‘Broad mixture’ | F344 rats |
↑ weight and granulomas (260) No clinical chemistry performed |
↑ lymphoid cells in liver parenchyma (260) No haematology performed |
Not analysed | No findings | Nygaard et al. (2023) |
|
S‐C25 (C16‐34, incl. paraffins) |
F344 rats |
↑ granulomas (226) ↑ weight (23) No clinical chemistry performed |
↑ lymphoid cells in liver parenchyma (226) No haematology performed |
Not analysed | ↑ weight (23) | |
| L‐C25 (C25‐50, deparaffinated) | F344 rats |
No findings No clinical chemistry performed |
No changes in liver parenchyma No haematology performed |
Not analysed | No findings | |
| L‐C25W (C25‐50, incl. paraffins) | F344 rats | ↑ weight and granulomas (22) |
↑ lymphoid cells in liver parenchyma (22) No haematology performed |
Not analysed | ↑ weight (22) | |
| P15H | SD rats | ↑ (minimal) multifocal inflammation (1,624) | No findings | No findings | No findings | Firriolo et al. (1995) |
| N70H | SD rats |
No findings (max dose: 200) Histopathology not performed |
No findings |
No findings Histopathology not performed |
No findings | Carrillo et al. (2022) |
| Marcol72 (paraffinic C14‐38)/LE rats | LE rats | No findings (max dose: 125) | No findings | No findings | Not analysed | Smith et al. (1995) |
| EZL550 (paraffinic C23‐44)/LE rats | LE rats | No findings | ↑ Leukocytes (125) | No findings | Not analysed | |
| Marcol72 (paraffinic C14‐38)/Beagle dogs | Beagle dogs | No findings (max dose: 52) | No findings | No findings | Not analysed | |
| EZL550 (paraffinic C23‐44)/Beagle dogs | Beagle dogs | No findings (max dose: 52) | No findings | No findings | Not analysed |
Appendix E – Estimation of Human equivalent doses (HEDs) and organ/body burden for MOSH in humans and F344 rats
E.1. Calculation of HEDs
Since risk characterisation for MOSH is based on animal data, toxicokinetic differences between animals and humans have to be taken into consideration. However, there are not sufficient data available for humans and F344 rats to be used to actually quantify these interspecies differences. In order to estimate whether or not the factor typically used as default value might be sufficient, HEDs are calculated by applying this default value and compared to the actual exposure of humans. Due to the noted gaps in the database, certain assumptions are necessary, which leads rather to a range of than to distinct HED and exposure values:
-
–
Organ contents in humans after a whole lifetime are available from the Barp et al. study (2014). It is assumed that the organ contents of the 37 persons investigated therein are representative for the whole population. This assumption is uncertain especially for the very few individuals with very high organ contents. Hence, HEDs calculated from median organ contents should be considered as more relevant in the assessment. In an older study, abdominal fat tissue from 144 caesarean sections was investigated (Concin et al., 2008), and the resulting MOSH contents were somewhat lower compared to Barp et al. (2014). However, the study population in Concin et al. (2008) was significantly younger, which might indicate lower exposure or less than a lifetime increase in adipose tissue content.
-
–
The actual MOSH exposure of the 37 humans from Barp et al. (2014) study is not precisely known. Dietary intake data from 1999–2010 (EFSA CONTAM Panel, 2012) was assumed. However, individual exposure varies and exposure in earlier times was higher, particularly before 2000, relevant for potential long‐term accumulation. Hence, by assuming recent exposure, the lifetime exposure of the humans investigated in Barp et al. (2014) might be underestimated.
-
–
Organ contents in animals (F344 rats) are available from the Cravedi et al. (2017) study. The broad MOSH mixture is well suited for comparison with human organ contents, because the lowest administered dose is closest to the human dietary exposure. The test items S‐C25 and L‐C25 also show similarity to human exposure (for more details please refer to critical effect section). Hence, the respective organ contents were used for comparison to humans. However, it has to be noted that accumulation strongly depends on the MOSH composition ingested and on the animal strain used in the study.
-
–
Cravedi et al. (2017) treated the animals for up to 120 days. For MOSH content in liver, data on the highest dose suggested a steady state being reached, but not for the lower doses. A statistically significant increase of MOSH content occurred for spleen and adipose tissue between day 90 and day 120. Hence, at least for spleen and adipose tissue, MOSH contents might be significantly higher after lifetime exposure compared to subchronic exposure in Cravedi et al. (2017), which would lead to lower HEDs. However, since based on the available data, extrapolation to lifetime exposure was not possible, the organ contents from Cravedi et al. (2017) were used for HEDs calculation.
-
–
The fraction of the administered dose retained in the organs significantly decreases with increasing doses. Hence, for a reliable comparison, a dose close to human exposure should be used. However, such a dose was only applied in the study part with the broad MOSH mixture (Cravedi et al., 2017). Using the data for the L‐C25 and S‐C25 may overestimate the HED.
Taking the data from Cravedi et al. (2017) and Barp et al. (2014) as shown in Table 1 and taking the standard allometric scaling factor of 4 21 , 22 for toxicokinetic extrapolation from rats to humans, HEDs were calculated according to the following formula:
In view of the uncertainties related to human exposure levels, HEDs were calculated using all animal doses and test items shown in Table E.1 in comparison to the maximum and median content found in the respective human organs. The median calculated HED are reported for all organs together with the respective minimum and maximum calculated HED (Table E.1).
Table E.1.
Human equivalent doses (HED) in mg/kg bw per day calculated from doses applied and organ contents determined in Cravedi et al. (2017) in comparison to the human organ contents determined in Barp et al. (2014)
| Tissue concentrations (mg/kg): F344 rats (Cravedi et al., 2017) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dose group ca. (mg/kg bw per day) | 230 | 25 | 2.6 | 230 | 25 | 2.6 | 230 | 25 | 2.6 |
| Liver | Spleen | Adipose tissue | |||||||
| S‐C25 | 14,639 | 4,784 | – | 499 | 287 | – | 347 | 88.1 | – |
| L‐C25 | 3,805 | 1,393 | – | 419 | 192 | – | 36 | 12.8 | – |
| Broad mixture | 5,511 | 1,604 | 220 | 383 | 202 | 32 | 274 | 95 | 13 |
| Tissue concentrations (mg/kg): Humans (Barp et al., 2014 ) | |||||||||
| Liver (mg/kg) | Spleen (mg/kg) | Adipose tissue (mg/kg) | |||||||
| Max content | 901 | 1,400 | 493 | ||||||
| Min content | 14 | 6 | 17 | ||||||
| Median content | 71 | 28 | 87 | ||||||
| Human equivalent doses (mg/kg bw per day) | |||||||||
| Liver, median (min–max) | Spleen, median (min–max) | Adipose tissue, median (min–max) | |||||||
| Max content in human organ | 3.5 (1.2–14) | 46 (28–210) | 82 (25–787) | ||||||
| Median content in human organ | 0.28 (0.093–1.1) | 0.91 (0.57–4.2) | 14 (4.4–139) | ||||||
Since the individual exposure of the humans investigated in Barp et al. (2014)) is not known, typical human exposure is assumed for comparison with the HEDs. EFSA CONTAM Panel (2012) estimated the typical human dietary exposure in the range of 0.03–0.3 mg/kg bw per day (adults 0.031–0.12 mg/kg bw per day). However, it noted that due to the use of release agents consisting of white mineral oil products, specific (adult) consumers of bread and a range of bakery products (particularly pre‐sliced toast bread) may have been exposed up to 0.69–2.5 mg/kg bw per day. Spraying of rice may have increased exposure to 0.08–1.3 mg/kg bw/day. Both practices were gradually abandoned in the 1990s and largely disappeared in the early 2000s.
In the second part of the 20th century, the mineral oil dietary exposure of humans probably was higher than today. Boitnott and Margolis (1966b) estimated the average yearly MOH dietary exposure at that time to 47.5 g (1.9 mg/kg bw per day for a person of 70 kg bw). Significant additional exposure might be caused by cosmetic products. In a worst case estimation, Pirow et al. (2019) estimated MOSH ingestion from lip care products to be in the range of 0.08–0.7 mg/kg bw per day. Hence, combined human exposure from the cited sources could have been in the range of 0.03–4.8 mg/kg bw per day. When comparing these data to the HEDs derived from the median liver and spleen content in humans (0.093–1.1 mg/kg bw per day and 0.57–4.2 mg/kg bw per day, respectively), the HEDs seem to be well in the range of the exposure data. This is also true for the maximum liver content in humans (especially regarding the median of 3.5 mg/kg bw per day). Especially for adipose tissue, the HEDs are approximately one order of magnitude higher than the estimated exposure.
E.2. Estimation of whole body burden and contribution of different organ burdens in humans
In several publications (e.g. Holländer, 2021; Holländer, 2020; Grandmaison, 2001; Molina and DiMaio, 2012, 2015), mean organ weights and organ weight distributions in humans were described. Due to differences in the study population (e.g. age, body weight, body mass index (BMI)), in organ sampling and due to possible other unknown influences, the results differ significantly – especially for liver weight (see Table E.2). Relative organ weights were not provided, but it is apparent from the data, that especially the weights of heart, liver and kidney are highly dependent on body weight (or BMI), whereas other organ weights (e.g. lungs, brain or spleen) do not differ much with body weight or BMI.
Table E.2.
Mean human organ weights as described in the literature
| Organ | Weight in g ± standard deviation (or range) | |||||
|---|---|---|---|---|---|---|
| Molina et al. (2012, 2015) | Holländer et al. (2020, 2021) | Grandmaison et al. (2001) | ||||
| Male | Female | Male | Female | Male | Female | |
| Left lung | 395 ± 147 | 299 ± 117 | 526 ± 157 | 425 ± 146 | 583 ± 216 | 467 ± 174 |
| Right lung | 445 ± 159 | 340 ± 123 | 624 ± 185 | 499 ± 161 | 663 ± 239 | 546 ± 207 |
| Liver | 1,561 ± 317 | 1,288 ± 330 | 1,770 ± 402 | 1,410 ± 357 | 1,677 ± 396 | 1,475 ± 362 |
| Liver (BMI < 18.5) | 1,236 (945–1,689) | 1,021 (824–1,513) | – | – | – | – |
| Liver (BMI 18.5–24.9) | 1,414 (838–2,013) | 1,185 (775–1,946) | 9,300–2,520 | 751–1,861 | – | – |
| Liver (BMI 25–29.9) | 1,633 (1,115–2,584) | 1,357 (1,066–2,037) | 1,048–2,929 | 1,040–2,850 | – | – |
| Liver (BMI ≥30) | 1,874 (1,238–2,530) | 1,622 (988–2,395) | 1,300–2,630 | 1,210–1,890 | – | – |
| Liver (BMI < 21/20*) | – | – | – | – | 1,581 ± 372 | 1,346 ± 328 |
| Liver (BMI 21/20* – ≤ 24) | – | – | – | – | 1,730 ± 405 | 1,521 ± 331 |
| Liver (BMI > 24) | – | – | – | – | 1,769 ± 390 | 1,609 ± 419 |
| Spleen | 139 ± 58 | 115 ± 51 | 167 ± 76 | 132 ± 68 | 156 ± 87 | 140 ± 78 |
| Left Kidney | 137 ± 28 | 116 ± 32 | 171 ± 39 | 135 ± 36 | 160 ± 41 | 136 ± 37 |
| Right Kidney | 129 ± 26 | 108 ± 27 | 165 ± 43 | 128 ± 33 | 162 ± 39 | 135 ± 39 |
| Brain | 1,407 ± 124 | 1,233 ± 115 | 1,445 ± 141 | 1,279 ± 123 | – | – |
| Heart | – | – | 379 ± 61 | 323 ± 68 | 365 ± 71 | 312 ± 78 |
Males/females respectively.
From the values shown in Table E.2, mean values for the organ weights and relative standard deviations were calculated (Table E.3). For liver, mean weights according to the BMI of the persons from Barp et al. (2014) were used. For kidney and heart, the Panel concluded that that the impact on the overall body burden is low due to low organ contents. Hence, no BMI‐dependent mean organ weights were used. For lung and kidney, the mean organ weight was calculated as sum of the mean weights of the left and right kidney or lung, respectively. Since no marked difference was seen in the standard deviations of the organ weights between men and women, these were calculated as mean for both sexes.
Table E.3.
Mean organ weights used by the Panel for body burden calculation
| Organ | Mean weight in g | Mean rel. standard deviation in % | |
|---|---|---|---|
| Male | Female | Male and female | |
| Left lung | 501 | 397 | 36 |
| Right lung | 577 | 462 | 35 |
| Lungs (overall) | 1,079 | 859 | 35 |
| Liver | 1,669 | 1,391 | 24 |
| Liver (BMI < 18.5) | 1,409 | 1,184 | 24 |
| Liver (BMI 18.5–24.9) | 1,572 | 1,353 | 24 |
| Liver (BMI 25–29.9) | 1,701 | 1,483 | 24 |
| Liver (BMI ≥30) | 1,822 | 1,616 | 24 |
| Spleen | 154 | 129 | 49 |
| Left Kidney | 156 | 129 | 25 |
| Right Kidney | 152 | 124 | 25 |
| Kidneys (overall) | 308 | 253 | 25 |
| Brain | 1,426 | 1,256 | 9 |
| Heart | 372 | 318 | 20 |
The amount of fat tissue of the body is highly dependent on the body weight of the person and its BMI. For adults, a good formula to estimate the amount of body fat from the BMI of a person was developed by Jessica Bruso, based on a study from Jackson et al. (2002):
The standard error of the estimate (measured vs. estimated body fat percentage) was 4.4% body fat (women) and 4.6% body fat (men) for the 3‐compartment model. Hence, 4.5% were assumed as standard error of the estimated amount of fat tissue in the persons from Barp et al. (2014). Statistics for the calculated amount of body fat are presented in Table E.4.
Table E.4.
Amount of body fat calculated for the persons from Barp et al. (2014) based on body mass index and the above‐cited formula
| Body fat in % (n = 36) | Body fat in kg (n = 36) | Standard deviation in kg (n = 36) | |
|---|---|---|---|
| Min | 15 | 7.8 | 0.35 |
| Max | 55 | 65 | 2.9 |
| Mean | 32 | 26 | 1.2 |
| Median | 31 | 24 | 1.1 |
| P5 | 19 | 13 | 0.58 |
| P25 | 25 | 17 | 0.78 |
| P75 | 38 | 32 | 1.4 |
| P95 | 47 | 48 | 2.2 |
For the MLN, 150 MLNs with a mean diameter of 10 mm and a density of 1 g/cm3 was assumed as a reasonable worst case (Fritsch and Kuhnel, 2014). Hence, the overall lymph node weight was estimated as 79 g.
From the estimated organ weights and the MOSH content in the organs, overall body burdens were calculated for the individuals from Barp et al. (2014), multiplying the mean organ weight with the individual organ content for MOSH. Statistics of the result are presented in Tables E.5 and E.6.
Table E.5.
Organ burden and overall body burden (sum) in mg MOSH ± standard deviation (SD) estimated for the individuals from Barp et al. (2014). Overall body burden related to body weight in mg/kg (ppm). Due to the low impact, no SD was calculated for brain. For lymph nodes, no SD was available from the estimation of mean lymph node weights; bw = body weight
| Liver | Fat tissue | Spleen | Lungs | Lymph nodes | Kidney | Heart | Brain | Sum | Sum in ppm bw. | |
|---|---|---|---|---|---|---|---|---|---|---|
| N | 36 | 36 | 36 | 36 | 35 | 13 | 13 | 12 | 36 | 36 |
| Min | 25 ± 6 | 273 ± 12 | 1 ± 0 | 0 ± 0 | 2 | 0 ± 0 | 0 ± 0 | 0 | 301 | 4 |
| Max | 1,219 ± 288 | 10,886 ± 490 | 215 ± 106 | 78 ± 28 | 109 | 3 ± 1 | 13 ± 3 | 0 | 11,988 | 178 |
| Mean | 210 ± 50 | 3,282 ± 148 | 14 ± 7 | 8 ± 3 | 18 | 1 ± 0 | 3 ± 1 | 0 | 3,532 | 46 |
| Median | 110 ± 26 | 2,056 ± 93 | 4 ± 2 | 4 ± 1 | 13 | 1 ± 0 | 2 ± 0 | 0 | 2,194 | 28 |
| P5 | 28 ± 7 | 311 ± 14 | 1 ± 1 | 0 ± 0 | 3 | 0 ± 0 | 0 ± 0 | 0 | 398 | 6 |
| P25 | 55 ± 13 | 1,333 ± 60 | 2 ± 1 | 2 ± 1 | 6 | 0 ± 0 | 1 ± 0 | 0 | 1,435 | 18 |
| P75 | 185 ± 44 | 4,535 ± 204 | 8 ± 4 | 7 ± 3 | 21 | 2 ± 0 | 3 ± 1 | 0 | 4,880 | 61 |
| P95 | 937 ± 221 | 8,711 ± 392 | 37 ± 18 | 23 ± 8 | 41 | 3 ± 1 | 8 ± 2 | 0 | 9,012 | 140 |
Table E.6.
Contribution of organ burdens to overall body burden in %
| Liver | Fat | Spleen | Lungs | Lymph nodes | Kidney | Heart | Brain | Sum excl. fat tissue | |
|---|---|---|---|---|---|---|---|---|---|
| N | 36 | 36 | 36 | 36 | 35 | 13 | 13 | 12 | 36 |
| Min | 1.3 | 75 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 1.6 |
| Max | 21 | 98 | 1.8 | 3.3 | 3.1 | 0.1 | 0.2 | 0.0 | 25 |
| Mean | 6.4 | 92 | 0.3 | 0.4 | 0.6 | 0.0 | 0.1 | 0.0 | 7.7 |
| Median | 5.3 | 93 | 0.2 | 0.1 | 0.5 | 0.0 | 0.1 | 0.0 | 6.7 |
| P5 | 1.5 | 81 | 0.0 | 0.0 | 0.2 | 0.0 | 0.0 | 0.0 | 2.0 |
| P25 | 2.4 | 91 | 0.1 | 0.0 | 0.3 | 0.0 | 0.0 | 0.0 | 3.1 |
| P75 | 8.4 | 97 | 0.5 | 0.3 | 0.7 | 0.0 | 0.1 | 0.0 | 9.3 |
| P95 | 17 | 98 | 0.8 | 1.5 | 1.3 | 0.1 | 0.2 | 0.0 | 19 |
The overall body burden varied in a range between 0.301 and 12 g/person with a median of 2.2 g/person (Table E.4). This is fair agreement with the estimates by Barp et al. (2014).
The MOSH content in the adipose tissue accounted for at least 75% of the overall body burden (median 93%; Table E.6). There was only a very weak correlation between overall MOSH body burden and BMI (R2 = 0.0522; linear approach) or body weight (R2 = 0.0046; linear approach). The same is true for the liver burden (R2 = 0.0026 and 0.0038, respectively), when using a linear approach. There was also no correlation between liver burden and age (R2 = 0). However, there was a weak correlation between the age and the overall body burden (R2 = 0.19) or adipose tissue burden (R2 = 0.21) or MOSH content in adipose tissue (R2 = 0.18), respectively. Although on a very small data basis, this might be an indication, that human fat tissue is acting as a depot with lifelong increasing MOSH content, whereas in liver and spleen, a steady‐state content is reached in significantly shorter time. At least for liver, this is also seen in F344 rats. When applying data for MOSH retention in liver after 120 days of feeding with the broad mixture in Cravedi et al. (2017), as well as median exposure data from EFSA CONTAM Panel (2012) and median liver contents and body weights from Barp et al. (2014), a plausible range of time needed to reach a steady state in humans can be estimated. The retention in liver of the broad MOSH mixture amount ingested varied between 1% and 3.3% after 120 days. The median exposure for mean and P95 consumption in EFSA CONTAM Panel (2012) ranged between 0.038 and 0.082 mg/kg bw per day. The median human liver burden and body weight in Barp et al. (2014) were 110 mg and 82 kg, respectively. Using these data, the time to reach this liver burden would be in the range of 1.4–10 years with a mean of roughly 5 years.
As was already discussed in Barp et al. (2014), there is also a correlation between MOSH content in liver and fat tissue (R2 = 0.32) and MOSH content in spleen and fat tissue (R2 = 0.31).
E.3. Estimation of whole body burden and contributions of different organ burdens in F344 rats
Several studies were identified providing information on MOH tissue contents. However, often only some organs were investigated, making calculation of overall body burden impossible – especially when the content in the fat tissue was missing (Baldwin, Trimmer, Firriolo, McKee). Hence, the studies by Cravedi et al. (2017) and Smith et al. (1996) were chosen for estimation of MOSH body burden of rats. They cover a broad range of MOSH compositions, e.g. white oils (Smith et al., 1996; Cravedi et al., 2017) and several types of waxes (Smith et al., 1996). However, due to missing information in the studies, several assumptions had to be made, e.g. on organ weights or body fat.
For all studies, liver, fat tissue (and carcass), spleen and MLN were assumed to be the relevant contributing organs.
In Smith et al. (1996), treatment‐related changes in liver weights are only given as percentage of the control group. In order to get absolute liver weights for the calculation, a typical liver weight of 6.49 g was assumed for the control group according to Marino (2012). In addition, MLN weights, amount of body fat and body weight were not reported in Smith et al. (1996). Hence, a typical value of 0.07% of the body weight was assumed for the lymph node weight (mean of data reported in Trimmer et al. (2004)), a typical body weight of 198 g was assumed according to Marino (2012), and a typical amount of body fat of 11.4% was assumed as mean of the values for young and old F344 rats (Schoeffner, 1999). The body fat values reported in Schoeffner (1999) were for male F344 rats, but in taking a mean for old and young animals, the possible incorrectness of the assumption is regarded to be small. The overall body burden was calculated as sum of the organ burdens from liver, MLN and fat tissue. Since neither daily intake nor the weekly body weight development were reported by Smith et al. (1996), a daily feed consumption of 10 g (as in Cravedi et al., 2017) was assumed to calculate the overall retention of MOSH in the animals. Only results for female F344 rats are shown, since MOSH content in organs of female rats was significantly higher than in males (Smith et al., 1996).
For all test items from Cravedi et al. (2017), MOSH content in MLN was not measured. However, from Smith et al. (1996), it can be concluded that the contribution of the MOSH content in the MLN to the overall body burden is small (see Table E.7). For the broad MOSH mixture from Cravedi et al. (2017), the amount of body fat and carcass weight were not reported. Hence, again a typical amount of body fat of 11.4% was assumed. The overall body burden was calculated from the cage‐individual consumption and the time‐ and dose‐dependent mean retention in the animals. The MOSH burden in carcass and fat tissue was consequently calculated by subtracting liver and spleen burden from the overall body burden. For the test items L‐C25, S‐C25 and L‐C25W from Cravedi et al. (2017), the overall retention of MOSH in the animals was not provided, but fat tissue and carcass weights were reported. Hence, the overall body burden was calculated as sum of the organ burdens from liver, spleen, fat tissue and carcass. From this, the total retention of MOSH was calculated in relation to the cage‐individual consumption data. The results are presented in Tables E.7 and E.8.
Table E.7.
Organ burden and overall body burden (in mg) in F344 rats after subacute to subchronic exposure towards MOSH with different compositions. Overall retention of MOSH in the animals as percentage of overall body burden to the ingested MOSH during the study
| Study | Test item | Dose in mg/kg feed | Dose in mg/kg bw per day | Liver burden in mg | Spleen burden in mg | MLN burden in mg | Fat tissue burden in mg | Carcass burden in mg | Fat tissue + carcass burden in mg | Overall body burden in mg | Overall retention in % ingested MOSH |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cravedi et al. (2017), narrow mixtures | L‐C25 | 394 | 25 | 9.5 | 0.1 | n.m. | 0.1 | 1.7 | 1.8 | 11 | 2.5 |
| L‐C25 | 985 | 62 | 17.9 | 0.2 | n.m. | 0.1 | 3.6 | 3.7 | 22 | 1.9 | |
| L‐C25 | 3,731 | 236 | 26.0 | 0.3 | n.m. | 0.2 | 2.7 | 3.0 | 29 | 0.7 | |
| S‐C25 | 368 | 23 | 35.6 | 0.2 | n.m. | 0.9 | 8.5 | 9.4 | 45 | 10.4 | |
| S‐C25 | 939 | 61 | 72.5 | 0.5 | n.m. | 1.2 | 14.3 | 15.5 | 89 | 7.7 | |
| S‐C25 | 3,630 | 226 | 122.6 | 0.6 | n.m. | 2.8 | 20.7 | 23.5 | 147 | 3.4 | |
| L‐C25W | 350 | 22 | 39.0 | 0.1 | n.m. | 0.7 | 6.8 | 7.5 | 47 | 11.3 | |
| L‐C25W | 836 | 53 | 61.8 | 0.2 | n.m. | 0.7 | 8.8 | 9.6 | 72 | 7.3 | |
| L‐C25W | 3,372 | 218 | 70.0 | 0.3 | n.m. | 1.3 | 13.3 | 14.6 | 85 | 2.1 | |
| Cravedi et al. (2017), broad mixture | Broad, day 30 | 37 | 2.6 | 0.7 | 0.0 | n.m. | 0.1 | – | 0.5 | 1.2 | 10.9 |
| Broad, day 30 | 361 | 25 | 5.6 | 0.0 | n.m. | 0.5 | – | 1.8 | 7.5 | 6.9 | |
| Broad, day 30 | 3,641 | 260 | 15 | 0.1 | n.m. | 2.4 | – | 7.0 | 22 | 2.0 | |
| Broad, day 60 | 37 | 2.6 | 1.1 | 0.0 | n.m. | 0.1 | – | 0.5 | 1.6 | 7.5 | |
| Broad, day 60 | 361 | 25 | 7.2 | 0.1 | n.m. | 0.8 | – | 2.5 | 9.8 | 4.6 | |
| Broad, day 60 | 3,641 | 260 | 22 | 0.1 | n.m. | 3.3 | – | 11 | 33 | 1.5 | |
| Broad, day 90 | 37 | 2.6 | 1.6 | 0.0 | n.m. | 0.2 | – | 0.7 | 2.3 | 7.1 | |
| Broad, day 90 | 361 | 25 | 9.6 | 0.1 | n.m. | 1.0 | – | 3.2 | 13 | 4.1 | |
| Broad, day 90 | 3,641 | 260 | 38 | 0.2 | n.m. | 4.2 | – | 10 | 48 | 1.5 | |
| Broad, day 120 | 37 | 2.6 | 1.4 | 0.0 | n.m. | 0.3 | – | 1.2 | 2.7 | 6.2 | |
| Broad, day 120 | 361 | 25 | 10 | 0.1 | n.m. | 2.3 | – | 4.0 | 14 | 3.5 | |
| Broad, day 120 | 3,641 | 260 | 41 | 0.3 | n.m. | 6.0 | – | 22 | 63 | 1.5 | |
|
Smith et al. (1996) |
Control 1 | 0 | 0 | 2.3 | n.d. | 0.1 | 2.7 | n.m. | – | 5.0 | – |
| Control 2 | 0 | 0 | 1.0 | n.d. | 0.0 | n.m. | n.m. | – | 1.0 | – | |
| Control 3 | 0 | 0 | 1.8 | n.d. | 0.0 | n.m. | n.m. | – | 1.8 | – | |
| N10A | 20,000 | 1,951 | 12.6 | n.d. | 0.4 | 9.5 | n.m. | – | 22 | 0.1 | |
| N15H | 20,000 | 1,951 | 34.6 | n.d. | 0.4 | 9.3 | n.m. | – | 44 | 0.2 | |
| P15H | 20,000 | 1,951 | 23.0 | n.d. | 0.5 | 9.9 | n.m. | – | 33 | 0.2 | |
| N70A | 20,000 | 1,951 | 28.2 | n.d. | 0.4 | 3.8 | n.m. | – | 32 | 0.2 | |
| N70H | 20,000 | 1,951 | 23.2 | n.d. | 0.2 | 4.7 | n.m. | – | 28 | 0.2 | |
| P70H | 20,000 | 1,951 | 6.6 | n.d. | 0.2 | n.m. | n.m. | – | 6.8 | 0.04 | |
| P100H | 20,000 | 1,951 | 3.9 | n.d. | 0.1 | 3.4 | n.m. | – | 7.4 | 0.04 | |
| LMPW 1 | 20,000 | 1,951 | 62.5 | n.d. | 0.4 | 11.5 | n.m. | – | 74 | 0.4 | |
| LMPW 2 | 20,000 | 1,951 | 141.5 | n.d. | 0.6 | n.m. | n.m. | – | 142 | 0.8 | |
| MP | 20,000 | 1,951 | 73.1 | n.d. | 0.3 | n.m. | n.m. | – | 73 | 0.4 | |
| IMPW | 20,000 | 1,951 | 76.8 | n.d. | 0.3 | n.m. | n.m. | – | 77 | 0.4 | |
| HSW | 20,000 | 1,951 | 3.5 | n.d. | 0.0 | 1.6 | n.m. | – | 5.2 | 0.03 | |
| HMPW | 20,000 | 1,951 | 1.1 | n.d. | 0.0 | 9.0 | n.m. | – | 10 | 0.06 |
n.m.: not measured; n.d.: not detected; −: not applicable.
Table E.8.
Contribution of respective organ burdens to overall body burden in F344 rats after subacute to subchronic exposure towards different MOSH products. Overall body burden of MOSH in the animals in relation to bodyweight (in ppm)
| Study | Test item | Dose in mg/kg feed | Dose in mg/kg bw per day | Contribution of liver burden in % | Contribution of spleen burden in % | Contribution of MLN burden in % | Contribution of fat tissue burden in % | Contribution of carcass burden in % | Contribution of fat tissue + carcass burden in % | Overall body burden in ppm bw |
|---|---|---|---|---|---|---|---|---|---|---|
| Cravedi et al. (2017), narrow mixtures | L‐C25 | 394 | 25 | 83 | 1.1 | n.m. | 0.8 | 15 | 16 | 55 |
| L‐C25 | 985 | 62 | 82 | 0.8 | n.m. | 0.6 | 16 | 17 | 103 | |
| L‐C25 | 3731 | 236 | 89 | 0.9 | n.m. | 0.8 | 9.4 | 10 | 143 | |
| S‐C25 | 368 | 23 | 79 | 0.4 | n.m. | 1.9 | 19 | 21 | 212 | |
| S‐C25 | 939 | 61 | 82 | 0.5 | n.m. | 1.4 | 16 | 18 | 424 | |
| S‐C25 | 3630 | 226 | 84 | 0.4 | n.m. | 1.9 | 14 | 16 | 697 | |
| L‐C25W | 350 | 22 | 83 | 0.3 | n.m. | 1.5 | 15 | 16 | 230 | |
| L‐C25W | 836 | 53 | 86 | 0.2 | n.m. | 1.1 | 13 | 14 | 345 | |
| L‐C25W | 3,372 | 218 | 82 | 0.4 | n.m. | 1.6 | 16 | 17 | 405 | |
|
Cravedi et al. (2017), broad mixture |
Broad, day 30 | 36.5 | 2.6 | 60 | 0.4 | n.m. | 5.1 | – | 40 | 9 |
| Broad, day 30 | 360.5 | 25 | 75 | 0.4 | n.m. | 7.3 | – | 24 | 53 | |
| Broad, day 30 | 3640.5 | 260 | 68 | 0.3 | n.m. | 10.9 | – | 32 | 155 | |
| Broad, day 60 | 36.5 | 2.6 | 67 | 0.6 | n.m. | 7.6 | – | 32 | 9 | |
| Broad, day 60 | 360.5 | 25 | 74 | 0.6 | n.m. | 8.3 | – | 26 | 57 | |
| Broad, day 60 | 3640.5 | 260 | 67 | 0.3 | n.m. | 10 | – | 32 | 190 | |
| Broad, day 90 | 36.5 | 2.6 | 67 | 0.8 | n.m. | 10 | – | 32 | 12 | |
| Broad, day 90 | 360.5 | 25 | 74 | 0.6 | n.m. | 8.0 | – | 25 | 67 | |
| Broad, day 90 | 3640.5 | 260 | 78 | 0.4 | n.m. | 8.8 | – | 22 | 256 | |
| Broad, day 120 | 36.5 | 2.6 | 53 | 0.7 | n.m. | 11 | – | 47 | 13 | |
| Broad, day 120 | 360.5 | 25 | 72 | 0.9 | n.m. | 16 | – | 28 | 73 | |
| Broad, day 120 | 3640.5 | 260 | 65 | 0.4 | n.m. | 10 | – | 35 | 307 | |
|
Smith et al. (1996) |
control 1 | 0 | 0 | 45 | n.d. | 1.2 | 54 | n.m. | – | 25 |
| control 2 | 0 | 0 | 96 | n.d. | 4.4 | n.m. | n.m. | – | 5 | |
| control 3 | 0 | 0 | 99 | n.d. | 0.5 | n.m. | n.m. | – | 9 | |
| N10A | 20,000 | 1,951 | 56 | n.d. | 1.8 | 42 | n.m. | – | 114 | |
| N15H | 20,000 | 1,951 | 78 | n.d. | 0.9 | 21 | n.m. | – | 224 | |
| P15H | 20,000 | 1,951 | 69 | n.d. | 1.4 | 30 | n.m. | – | 169 | |
| N70A | 20,000 | 1,951 | 87 | n.d. | 1.2 | 12 | n.m. | – | 164 | |
| N70H | 20,000 | 1,951 | 82 | n.d. | 0.8 | 17 | n.m. | – | 142 | |
| P70H | 20,000 | 1,951 | 97 | n.d. | 3.3 | n.m. | n.m. | – | 34 | |
| P100H | 20,000 | 1,951 | 53 | n.d. | 1.1 | 46 | n.m. | – | 37 | |
| LMPW 1 | 20,000 | 1,951 | 84 | n.d. | 0.6 | 15 | n.m. | – | 376 | |
| LMPW 2 | 20,000 | 1,951 | 100 | n.d. | 0.4 | n.m. | n.m. | – | 718 | |
| MP | 20,000 | 1,951 | 100 | n.d. | 0.4 | n.m. | n.m. | – | 371 | |
| IMPW | 20,000 | 1,951 | 100 | n.d. | 0.4 | n.m. | n.m. | – | 389 | |
| HSW | 20,000 | 1,951 | 68 | n.d. | 0.9 | 31 | n.m. | – | 26 | |
| HMPW | 20,000 | 1,951 | 11 | n.d. | 0.2 | 89 | n.m. | – | 51 |
n.m.: not measured; n.d.: not detected; −: not applicable.
Consistently through all studies and test items, the main MOSH contribution to the overall body burden resulted from the liver, and a minor one from the fat tissue/carcass. The MOSH contribution from all other organs was marginal.
The body burden increased with the ingested dose, though not linearly. For all test items in Cravedi et al. (2017), the increase in body burden from low to middle dose was higher than the increase from middle to high dose. However, this was more pronounced in the narrow MOSH mixtures than in the broad MOSH mixture from Cravedi et al. (2017).
The body burden was also highly dependent on the test item ingested. From L‐C25 and the broad mixture, the body burden was significantly lower than from S‐C25 and L‐C25W in comparable doses. For instance, the body burden of the 25 mg L‐C25/kg bw per day group rats was ca. four times lower than that of the L‐C25W or the S‐C25 group at the same dose. In Smith et al. (1996), body burden varied in almost two orders of magnitude, depending on the test item. This is likely to be due to the particular behaviour of the F344 rats in accumulating wax‐type MOSH constituents.
Finally, the body burden depended on exposure time. For overall body burden, MLN and fat tissue burden, an almost linear increase was observed from day 30 to day 120 for the broad mixture from Cravedi et al. (2017). However, for liver burden, a plateau was reached at somewhere between day 90 and 120 for the highest and lowest dose, but not for the middle dose. These findings are in line with other observations (Trimmer et al., 2004). However, the time needed to reach the plateau may be longer (e.g. several months for high viscosity white oils from Trimmer et al. (2004)).
Comparison of body burdens in humans and F344 rats
The median overall body burden in relation to body weight is in the same order of magnitude for the humans from Barp et al. (2014) as for F344 rats receiving a broad MOSH mixture at 2.6 mg/kg bw per day over 120 days (Cravedi et al., 2017) – 28 ppm as compared to 13 ppm. When compared to the PoD for the MOE calculation (ingestion of L‐C25 at 236 mg/kg bw per d for 120 days), the resulting body burden in relation to body weight is ca. 5 times higher in rats than in humans (median value) – 143 ppm compared to 28 ppm.
It is noticeable, that the main contribution to the human body burden results from the adipose tissue (> 90%), whereas in rats, the liver burden is the main contribution (ca. 80%). This might be due to the longer exposure of the humans or point to toxicokinetic differences between the species.
In F344 rats and in humans, a steady state for the liver burden seems to be reached after a relatively short time compared to fat tissue burden, where neither in humans nor F344 rats a steady state is observed.
Appendix F – Spleen weight increase or immune system activation, evidence from human studies
1.
Increased spleen weights in F344 rats were observed in several studies on MOSH. If measured, these were accompanied by alterations in haematology linked to inflammation and immune response – in particular increased number of neutrophilic granulocytes or white blood cells in general (Smith et al., 1996; Firriolo et al., 1995; Griffis et al. 2010; Boogaard et al., 2017). In contrast, if spleen weights were not increased, no significant change in haematology was observed (Smith et al., 1996; Trimmer et al., 2004; McKee et al., 2012).
For SD rats, no increase in spleen weight or alteration in haematology was seen in the studies available (Boogaard et al., 2017; Griffiset al., 2010; Firrioloet al., 1995).
As discussed earlier in more detail, these results point to the conclusion, that increased spleen weight is linked to a chronic increased activity of the immune system, apparent in higher numbers of granulocytes or other white blood cells. Hence, relevance of this effect observed in F‐344 rats for humans is linked to the question, whether mineral oil ingestion in humans is accompanied with increased immune system activity. Therefore, the available data for humans were investigated (Table F.1). Eight studies on spleen content of MOH and related effects were identified (Nochomovitz et al. 1975; Cruickshank et al. 1984; Wanless et al. 1985; Boitnott et al. 1966, 1970; Kelsall et al. 1969; Liber et al. 1967; Barp et al., 2014). In many studies, quite high incidences for follicular lipidosis (FL) – as formation of droplets of mineral oil in the organs was called in the older publications – were reported (up to 76% in Cruickshank et al. (1984)), but in very few cases increased amount of macrophages or even fibrosis of the spleen tissue (Cruickshank et al. 1984) were seen.
Table F.1.
Investigations in humans for MOSH in spleen
| Study | Study details | Results | New | Comment |
|---|---|---|---|---|
| Boitnott (1966) | 61 patients autopsy | Frequent finding of MOH in the spleen; no detailed information given | No | Spleen weight not measured, no information on inflammation |
| Liber (1967) | 13 patients autopsy | No clear correlation between spleen weight and MOH content or lipidosisno follicular lipidosis at content < 0.2 mg/g | No | Already C‐fraction accumulated in the spleen very well identified and difference to typical mineral oil noticed spleens with weight > 400 g not evaluated |
| Kelsall (1969) | 300 patients autopsy | Lipophages seen in 24% of the cases, sometimes with macrophages | No | Spleen weight not measured, in some cases, macrophages seen (incorporating lipid vacuoles) |
| Boitnott (1970) | 4,000 spleen samples hydrocarbon content measured from spleens of 34 patients | Content in spleen between 0.14 and 4.5 mg/g no histological oil droplets in spleen, when MOH content < 0.2 mg/g | No | Spleen weight not measured, in some cases, macrophages seen (incorporating lipid vacuoles) |
| Nochomovitz et al. (1975) | Human, case study | Autopsy of a human with prolonged MOH ingestion (for laxative purposes) liver (1,273 g) and spleen (225 g) not significantly enlarged many lipid containing vacuoles, no agglomeration of immune cells | No | No tendency of spleen enlargement in that highly exposed human |
| Cruickshank (1984) | Meta study of several older studies; 500 patients autopsies | Follicular lipidosis (76%), in very few patients accompanied with fibrosis or sarcoid type granulomas Samples without follicular lipidosis had lower MOH content (0.1–0.3 mg/g) than samples with follicular lipidosis (3.4–4.2 mg/g) | No | Spleen weight not measured, immune system response only in very few patients |
| Wanless (1985) | 465 patients autopsy | 46% with lipogranulomas in spleen47.5 g MOH assumed as per capita yearly exposure (Boitnott, 1966) | No | Spleen weight not measured, no information on inflammation |
| Barp et al. (2014) | 37 patients autopsy | MOSH content in spleen between 6 and 1.4 mg/g (median 0.028 mg/g) | Yes | Spleen weight not measured no haematology reported |
Noticeably, in older studies, much higher concentrations in human spleen were reported: mean for 13 samples of 912 mg/kg, maximum of 2,660 mg/kg (Liber and Rose 1967; determined by a combination of analytical methods available at that time), compared to 93 and 1,397 mg/kg, respectively (Barp et al., 2014). This was probably associated with a higher exposure and that the tissues were from American subjects for which a higher incidence of granulomas was found than for continental Europeans like Austrians (Barp et al., 2014). Possible higher exposure levels of the American population may be related to different dietary habits. For instance, EFSA CONTAM Panel (2012) noted that ‘Bread and rolls and Grains for human consumption may occasionally show high levels of MOSH from the use of white oils as release agents, e.g. for bread and bakery products, or spraying agents on rice. This particularly referred to toast bread and the use of release agents in the baking pan and to slice the bread that was and is more popular in America than in most European countries.
Only two studies reported spleen weights. In a single case study (Nochomovitz et al. 1975), enlarged spleen weight of 225 g is reported (which is still in the physiological range) after chronic ingestion of high levels of MOH for laxative purposes. Liber et al. (1967) investigated 13 spleens from autopsies with (seven) and without (six) FL. No clear correlation between MOH contents in spleen and the respective organ weights was observed. However, it has to be noted, that spleens weighing > 400 g were excluded from the investigation.
From the available evidence, the Panel considers it unlikely that MOSH accumulation in human spleen leads to chronic immune system activation or spleen weight increase. However, the Panel notes that the database is limited.
Annexes
1.
Annex A – Known sources of mineral oil hydrocarbons present in food. The annex is provided as a separate pdf file containing a list of known sources for the presence of mineral oil hydrocarbons in food as summarised in Section 1.3.2 of the opinion, and is available on the EFSA Knowledge Junction community on Zenodo at: https://doi.org/10.5281/zenodo.8198819
Annex B – Analytical methods for the quantification and characterisation of mineral oil hydrocarbons in food. The annex is provided as a separate pdf file containing a detailed description of the analytical methods for the quantification and characterisation of mineral oil hydrocarbons as summarised in Section 1.3.3 of the opinion, and is available on the EFSA Knowledge Junction community on Zenodo at: https://doi.org/10.5281/zenodo.8198908
Annex C – Protocol for the human health risk assessments related to the presence of mineral oil hydrocarbons in food. The annex is provided as a separate pdf file containing the protocol selected by the CONTAM Panel to update the previous risk assessments of mineral oil hydrocarbons in food and is available on the EFSA Knowledge Junction community on Zenodo at: https://doi.org/10.5281/zenodo.8198913
Annex D – Dietary surveys for chronic exposure assessment. The annex is provided as a separate Excel file containing the information on the food consumption data used for the exposure assessment of mineral oil hydrocarbons in food, and is available on the EFSA Knowledge Junction community on Zenodo at: https://doi.org/10.5281/zenodo.8198927
Annex E – Raw occurrence data set. The annex is provided as a separate Excel file containing the raw occurrence data set for mineral oil hydrocarbons in food, and is available on the EFSA Knowledge Junction community on Zenodo at: https://doi.org/10.5281/zenodo.8198939
Annex F – Exposure estimations – mineral oil saturated hydrocarbons. The annex is provided as a separate Excel file containing the chronic dietary exposure estimations for mineral oil saturated hydrocarbons, and is available on the EFSA Knowledge Junction community on Zenodo at: https://doi.org/10.5281/zenodo.8198947
Annex G – Exposure estimations – mineral oil aromatic hydrocarbons. The annex is provided as a separate Excel file containing the chronic dietary exposure estimations for mineral oil aromatic hydrocarbons, and is available on the EFSA Knowledge Junction community on Zenodo at: https://doi.org/10.5281/zenodo.8198960
Annex H – Expert knowledge elicitation protocol. The annex is provided as a separate pdf file containing the protocol and results of the expert knowledge elicitation performed for the uncertainty analysis of the assessment, and is available on the EFSA Knowledge Junction community on Zenodo at: https://doi.org/10.5281/zenodo.8198970
Annex I – Public Consultation Report. The annex is provided as a separate pdf file containing the comments received during the public consultation and the responses provided by the CONTAM Panel, and is available on the EFSA Knowledge Junction community on Zenodo at: https://doi.org/10.5281/zenodo.8198980
Suggested citation: EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) , Schrenk, D ., Bignami, M ., Bodin, L ., del Mazo, J ., Grasl‐Kraupp, B ., Hogstrand, C ., Hoogenboom, L ., Leblanc, J.‐C ., Nebbia, C. S ., Nielsen, E ., Ntzani, E ., Petersen, A ., Sand, S ., Schwerdtle, T ., Vleminckx, C ., Wallace, H ., Alexander, J ., Goldbeck, C ., … Chipman, J. K . 2023. Update of the risk assessment of mineral oil hydrocarbons in food. EFSA Journal 2023;21(9), 1–143. 10.2903/j.efsa.2023.8215
Requestor: European Commission
Question number: EFSA‐Q‐2020‐00664
Panel members: Margherita Bignami, Laurent Bodin, James Kevin Chipman, Jesús del Mazo, Bettina Grasl‐Kraupp, Christer Hogstrand, Laurentius (Ron) Hoogenboom, Jean‐Charles Leblanc, Carlo Stefano Nebbia, Elsa Nielsen, Evangelia Ntzani, Annette Petersen, Salomon Sand, Dieter Schrenk, Tanja Schwerdtle, Christiane Vleminckx and Heather Wallace.
Declaration of interest: If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
Acknowledgements: The Panel wishes to acknowledge the EFSA staff members Katia Schirmer, Federico Cruciani and Thomas Tietz* for the support provided to this scientific output. The Panel wishes to acknowledge all European competent institutions, Member State bodies and other stakeholders that provided consumption and occurrence data for this scientific output.
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
* GP/EFSA/BIOCONTAM/2021/01.
Adopted: 12 July 2023
This publication is linked to the following EFSA Journal article: https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2023.p210901
Note: A plain language summary of this scientific opinion is available at https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2023.p210901
Notes
Scientific Opinion on Mineral Oil Hydrocarbons in Food. EFSA Journal 2012;10(6):2704, 185 pp. https://doi.org/10.2903/j.efsa.2012.2704
Commission Recommendation (EU) 2017/84 of 16 January 2017 on the monitoring of mineral oil hydrocarbons in food and in materials and articles intended to come into contact with food. OJ L 12, 17.1.2017, p. 95.
CONCAWE, The use of the dimethyl sulfoxide (DMSO) extract by the IP 346 method as an indicator of the carcinogenicity of lubricant base oils and distillate aromatic extracts. 1994. 94/51.
The CONTAM Panel noted that 6‐ring MOAH would not fall within the given molecular mass range (C15‐C30). For the 5‐ring MOAH, only the non‐alkylated MOAH would be included in the range (n‐C29). The method of analysis was not specified.
Samples analysed to confirm or reject a suspicion of non‐conformity, i.e. not random sampling.
Method for the determination of mineral oil saturated hydrocarbons (MOSH) and mineral oil aromatic hydrocarbons (MOAH) with on‐line HPLC‐GC‐FID analysis.
Together with two samples analysed for presence/absence of this fraction (‘MOAH 3 to 7 ring’) that were not included in the final data set.
Assumed to be GC‐FID.
The three samples were quantified, but at least one C‐fraction was below LOQ.
In this scientific opinion, adult population refers to the population groups ‘Adults’, ‘Elderly’ and ‘Very elderly’.
The presence of quantified and non‐quantified C‐fractions in the sample explains the differences between LB and UB estimations.
Netherlands Food and Consumer Product Safety Authority. Front Office Assessment mineral oils in cheese biscuits. Report NVWA‐English.https://english.nvwa.nl/documents/consumers/food/safety/documents/front-office-assessment-mineral-oils-in-cheese-biscuits.
References
- Adenuga D, Goyak K and Lewis R, 2017. Evaluating the MoA/human relevance framework for F‐344 rat liver epithelioid granulomas with mineral oil hydrocarbons. Critical Reviews in Toxicology, 47, 754–770. [DOI] [PubMed] [Google Scholar]
- Agarwal R, Shukla Y, Kumar S and Mehrotra NK, 1988. Evaluation of carcinogenic effect of jute batching oil (JBO‐P) fractions following topical application to mouse skin. Archives of Toxicology, 62, 406–410. [DOI] [PubMed] [Google Scholar]
- Andreassen M, Hjertholm H, Cravedi JP, Grob K, Alexander J and Nygaard UC, 2017. Effect of dietary pristane and other saturated mineral oils (MOSH) on autoimmune arthritis in rats. Toxicology Reports, 4, 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANSES (Agence nationale de sécurité sanitaire, de l'alimentation, de l'environnement et du travail) , 2017. Avis de l'Anses relatif à la migration des composés d'huiles minérales dans les denrées alimentaires à partir des emballages en papiers et cartons recycles. Available online: https://www.anses.fr/fr/system/files/ESPA2015SA0070.pdf
- API (American Petroleum Institute) , 2003. Robust Summary Information on aromatic extracts. Available online: https://www.petroleumhpv.org/-/media/PetroleumHPV/Documents/2012_may21_Aromatic_Extracts_robustsummary_2012_May_21_FINAL.pdf?la=en&hash=1140D4B3E6FCC952545AF0310251DA2D0C52696F
- Baldwin MK, Berry PH, Esdaile DJ, Linnett SL, Martin JG, Peristianis GC, Priston RA, Simpson BJ and Smith JD, 1992. Feeding studies in rats with mineral hydrocarbon food grade white oils. Toxicologic Pathology, 20, 426–435. [DOI] [PubMed] [Google Scholar]
- Ballantyne CM, Bays HE, Kastelein JJ, Stein E, Isaacsohn JL, Braeckman RA and Soni PN, 2012. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin‐treated patients with persistent high triglycerides (from the ANCHOR study). American Journal of Cardiology, 110, 984–992. 10.1016/j.amjcard.2012.05.031 [DOI] [PubMed] [Google Scholar]
- Barp L, Kornauth C, Wuerger T, Rudas M, Biedermann M, Reiner A, Concin N and Grob K, 2014. Mineral oil in human tissues Part I: concentrations and molecular mass distributions. Food and Chemical Toxicology, 72, 312–321. [DOI] [PubMed] [Google Scholar]
- Barp L, Suman M, Lambertini F and Moret S, 2015. Migration of selected hydrocarbon contaminants into dry pasta packaged in direct contact with recycled paperboard. Food Additives and Contaminants, Part A, 32, 271–283. [DOI] [PubMed] [Google Scholar]
- Barp L, Biedermann M, Grob K, Blas‐Y‐Estrada F, Nygaard UC, Alexander J and Cravedi JP, 2017a. Accumulation of mineral oil saturated hydrocarbons (MOSH) in female Fischer 344 rats: comparison with human data and consequences for risk assessment. Science of Total Environment, 575, 1263–1278. [DOI] [PubMed] [Google Scholar]
- Barp L, Biedermann M, Grob K, Blas‐Y‐Estrada F, Nygaard UC, Alexander J and Cravedi JP, 2017b. Mineral oil saturated hydrocarbons (MOSH) in female Fischer 344 rats; accumulation of wax components; implications for risk assessment. Science of Total Environment, 583, 319–333. [DOI] [PubMed] [Google Scholar]
- Bevan R, Harrison PTC, Jeffrey B and Mitchell D, 2020. Evaluating the risk to humans from mineral oils in foods: current state of the evidence. Food and Chemical Toxicology, 136, 110966. [DOI] [PubMed] [Google Scholar]
- BfR (Bundesinstitut für Risikobewertung) , 2021. Recommendation XXXVI. Papiere, Kartons und Pappen für den Lebensmittelkontakt. Available online: https://bfr.ble.de/kse/faces/resources/pdf/360-english.pdf
- Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr, Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CM and Investigators REDUCE‐IT, 2019. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. New England Journal of Medicine, 380, 11–22. 10.1056/NEJMoa1812792 [DOI] [PubMed] [Google Scholar]
- Biedermann M and Grob K, 2009. How "white" was the mineral oil in the contaminated Ukrainian sunflower oils? European Journal of Lipid Science and Technology, 111, 313–319. [Google Scholar]
- Biedermann M and Grob K, 2012a. On‐line coupled high performance liquid chromatography – gas chromatography (HPLC‐GC) for the analysis of mineral oil. Part 1: method of analysis in foods, environmental samples and other matrices. A review. Journal of Chromatography A, 1255, 56–75. [DOI] [PubMed] [Google Scholar]
- Biedermann M and Grob K, 2012b. On‐line coupled high performance liquid chromatography – gas chromatography (HPLC‐GC) for the analysis of mineral oil; Part 1: Part 2: migrated from paperboard into dry foods: interpretation of chromatograms. A review. Journal of Chromatography A, 1255, 76–99. [DOI] [PubMed] [Google Scholar]
- Biedermann M, Fiselier K and Grob K, 2009. Aromatic hydrocarbons of mineral oil origin in foods: method for determining the total concentration and first results. Journal of Agricultural and Food Chemistry, 57, 8711–8721. [DOI] [PubMed] [Google Scholar]
- Biedermann M, Ingenhoff JE, Dima G, Zurfluh M, Biedermann‐Brem S, Richter L, Simat T, Harling A and Grob K, 2013. Migration of mineral oil from printed paperboard into dry foods: survey of the German market. Part II: advancement of migration during storage. European Food Research and Technology, 236, 459–472. [Google Scholar]
- Biedermann M, Castillo R, Riquet AM and Grob K, 2014. Comprehensive two‐dimensional gas chromatography for determining the effect of electron beam treatment of polypropylene used for food packaging. Polymer Degradation and Stability, 99, 262–273. [Google Scholar]
- Biedermann M, Barp L, Kornauth C, Wuerger T, Rudas M, Reiner A, Concin N and Grob K, 2015. Mineral oil in human tissues, Part II: characterization of the accumulated hydrocarbons by comprehensive two‐dimensional gas chromatography. Science of the Total Environment, 506–507, 644–655. [DOI] [PubMed] [Google Scholar]
- Biedermann M, Munoz C and Grob K, 2017. Update of on‐line coupled liquid chromatography – gas chromatography for the analysis of mineral oil hydrocarbons in foods and cosmetics. Journal of Chromatography A, 1521, 140–149. [DOI] [PubMed] [Google Scholar]
- Biedermann M, Munoz C and Grob K, 2020. Epoxidation for the analysis of the mineral oil aromatic hydrocarbons in food. An update. Journal of Chromatography A Volume, 1624, 461236. 10.1016/j.chroma.2020.461236 [DOI] [PubMed] [Google Scholar]
- Biedermann‐Brem S and Grob K, 2011. Removal of mineral oil migrated from paperboard packing during cooking of foods in boiling water. European Food Research and Technology, 232, 1035–1041. [Google Scholar]
- Biedermann M, Eicher A, Altherr T and McCombie G, 2022. Quantification of mineral oil aromatic hydrocarbons by number of aromatic rings via comprehensive two‐dimensional gas chromatography: first results in food. Journal of Chromatography Open, 2, 100072. 10.1016/j.jcoa.2022.100072 [DOI] [Google Scholar]
- Boitnott JK and Margolis S, 1966a. Mineral oil in human tissues I. Detection of saturated hydrocarbons using thin‐layer chromatography. Bulletin of The Johns Hopkins Hospital, 118, 402–413. [Google Scholar]
- Boitnott JK and Margolis S, 1966b. Mineral oil in human tissues II. Oil droplets in lymph nodes of the Porta Hepatis. Bulletin of The Johns Hopkins Hospital, 118, 414–421. [Google Scholar]
- Boitnott JK and Margolis S, 1970. Saturated hydrocarbons in human tissues. 3. Oil droplets in the liver and spleen. Johns Hopkins Medical Journal, 127, 65–78. [PubMed] [Google Scholar]
- Bonvehi J and Coll F, 2014. Mineral oil paraffins in jute bags and cocoa butter. Acta Alimentaria, 43, 40–52. [Google Scholar]
- Boogaard PJ, Carrillo JC, Roberts L and Whale G, 2017. Toxicological and ecotoxicological properties of gas‐to‐liquid (GTL) products. 1. Mammalian toxicology. Critical Reviews in Toxicology, 47, 121–144. [DOI] [PubMed] [Google Scholar]
- Bratinova S and Hoekstra E, 2023. Guidance on sampling, analysis and data reporting for the monitoring of mineral oil hydrocarbons in food and food contact materials. Publications Office of the European Union. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC115694 [Google Scholar]
- Brühl L, 2016. Occurrence, determination, and assessment of mineral oils in oilseeds and vegetable oils. European Journal of Lipid Science and Technology, 118, 361–372. [Google Scholar]
- Carrillo JC, van der Wiel A, Danneels D, Kral O and Boogaard PJ, 2019. The selective determination of potentially carcinogenic polycyclic aromatic compounds in lubricant base oils by the DMSO extraction method IP346 and its correlation to mouse skin painting carcinogenicity assays. Regulatory Toxicology and Pharmacology, 106, 316–333. [DOI] [PubMed] [Google Scholar]
- Carrillo JC, Danneels D and Woldhuis J, 2021a. Relevance of animal studies in the toxicological assessment of oil and wax hydrocarbons. Solving the puzzle for a new outlook in risk assessment. Critical Reviews in Toxicology., 51, 418–455. [DOI] [PubMed] [Google Scholar]
- Carrillo JC, Shen H, Momin F, Kral O, Schnieder H and Kühn S, 2021b. GTL synthetic paraffin oil shows low liver and tissue retention compared to mineral oil. Food and Chemical Toxicology, 159, 112701. 10.1016/j.fct.2021.112701 [DOI] [PubMed] [Google Scholar]
- Carrillo JC, Kamelia L, Roamnuka J, Kral O, Isola A, Niemela H and Steneholm A, 2022. Comparison of PAC and MOAH for understanding the carcinogenic and developmental toxicity potential of mineral oils. Regulatory Toxicology and Pharmacology, 132, 105193. 10.1016/j.yrtph.2022.105193 [DOI] [PubMed] [Google Scholar]
- Castle L, Kelly M and Gilbert J, 1991. Migration of mineral hydrocarbons into foods. 1. Polystyrene containers for hot and cold beverages. Food Additives and Contaminants, 8, 693–699. [DOI] [PubMed] [Google Scholar]
- Concin N, Hofstetter G, Plattner B, Tomovski C, Fiselier K, Gerritzen K, Fessler S, Windbichler G, Zeimet A, Ulmer H, Siegl H, Rieger K, Concin H and Grob K, 2008. Mineral oil paraffins in human body fat and milk. Food and Chemical Toxicology, 46, 544–552. [DOI] [PubMed] [Google Scholar]
- Cravedi JP, Grob K, Nygaard UC and Alexander J, 2017. Bioaccumulation and toxicity of mineral oil hydrocarbons in rats ‐ specificity of different subclasses of a broad mixture relevant for human dietary exposures. EFSA supporting publication 2017;EN‐1090, 98 pp. [Google Scholar]
- Cruickshank B, 1984. Follicular (mineral oil) lipidosis: I. Epidemiologic studies of involvement of the spleen. Human Pathology, 15, 724–730. [DOI] [PubMed] [Google Scholar]
- ECHA (European Chemicals Agency) , 2012b. Published information on the REACH Registration Dossier on Distillates (petroleum), solvent‐refined heavy paraffinic (CAS Number 64741–88‐4). Available online: https://echa.europa.eu/web/guest/information-on-chemicals/registered-substances
- EFSA (European Food Safety Authority) , 2005. Opinion of the Scientific Committee on a request from EFSA related to A Harmonised Approach for Risk Assessment of Substances Which are both Genotoxic and Carcinogenic. EFSA Journal 2005;3(10):282, 31 pp. 10.2903/j.efsa.2005.282 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2008. Polycyclic Aromatic Hydrocarbons in Food ‐ Scientific Opinion of the Panel on Contaminants in the Food Chain. EFSA Journal 2008;6(8):724, 114 pp. 10.2903/j.efsa.2008.724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2010a. Standard sample description for food and feed. EFSA Journal 2010;8(1):1457, 54 pp. 10.2903/j.efsa.2010.1457 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2010b. Management of left‐censored data in dietary exposure assessment of chemical substances. EFSA Journal 2010;8(3):1557, 96 pp. 10.2903/j.efsa.2010.1557 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2011a. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2011b. Report on the development of a Food Classification and Description System for exposure assessment and guidance on its implementation and use. EFSA Journal 2011;9(12):2489, 84 pp. 10.2903/j.efsa.2011.2489 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2015. The food classification and description system FoodEx2 (revision 2). EFSA supporting publication 2015;EN‐804, 90pp. 10.2903/j.efsa.2015.804 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2019. Guidance on Communication of Uncertainty in Scientific Assessments. EFSA Journal 2019;17(1):5520, 73 pp. 10.2903/j.efsa.2019.5520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Arcella D and Gómez Ruiz JA, 2018a. Technical report on use of cut‐off values on the limits of quantification reported in datasets used to estimate dietary exposure to chemical contaminants. EFSA supporting publication 2018;EN‐1452, 11 pp. 10.2903/sp.efsa.2018.EN-1452 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Arcella D, Ioannidou S and Sousa R, 2018b. Internal report on the harmonisation of dilution factors to be used in the assessment of dietary exposure. EFSA Journal 2018. 10.5281/zenodo.1256085 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Arcella D, Baert K and Binaglia M, 2019b. Rapid risk assessment on the possible risk for public health due to the contamination of infant formula and follow‐on formula by mineral oil aromatic hydrocarbons (MOAH). EFSA Supporting Publication 2019;EN‐1741, 18 pp. 10.2903/j.efsa.2019.1741 [DOI] [Google Scholar]
- EFSA CEP Panel (EFSA Panel on Food Contact Materials, Enzymes and Processing Aids) , 2023. Scientific Opinion on the safety assessment of ‘waxes, paraffinic, refined, derived from petroleum‐based or synthetic hydrocarbon feedstock, low viscosity’ for use in food contact materials. EFSA Journal 2023;21(2):7761, 19 pp. 10.2903/j.efsa.2023.7761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CONTAM Panel (European Food Safety Authority Panel on Contaminants) , 2012. Scientific Opinion on Mineral Oil Hydrocarbons in Food. EFSA Journal 2012;10(6):2704, 185 pp. 10.2903/j.efsa.2012.2704 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012. Guidance on selected default values to be used by the EFSA Scientific Committee, Scientific Panels and Units in the absence of actual measured data. EFSA Journal 2012;10(3):2579, 32 pp. 10.2903/j.efsa.2012.2579 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2017a. Guidance on the risk assessment of substances present in food intended for infants below 16 weeks of age. EFSA Journal 2017;15(5):4849, 58 pp. 10.2903/j.efsa.2017.4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2017b. Update: use of the benchmark dose approach in risk assessment. EFSA Journal 2017;15(1):4658, 41 pp. 10.2903/j.efsa.2023.4658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2018a. Guidance on Uncertainty Analysis in Scientific Assessments. EFSA Journal 2018;16(1):5123, 39 pp. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2018b. Scientific Opinion on the principles and methods behind EFSA's Guidance on Uncertainty Analysis in Scientific Assessment. EFSA Journal 2018;16(1):5122, 235 pp. 10.2903/j.efsa.2018.5122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2019. Statement on the genotoxicity assessment of chemical mixtures. EFSA Journal 2019;17(1):5519, 11 pp. 10.2903/j.efsa.2019.5519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkind MS, Leon V, Moon YP, Paik MC and Sacco RL, 2009. High‐sensitivity C‐reactive protein and lipoprotein‐associated phospholipase A2 stability before and after stroke and myocardial infarction. Stroke, 40, 3233–3237. 10.1161/STROKEAHA.109.552802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO (Food and Agriculture Organization/World Health Organization) , 1995. Summary of Evaluations Performed by the Joint FAO/WHO Expert Committee on Food Additives. Mineral oil. Available online: https://www.inchem.org/documents/jecfa/jeceval/jec_1655.htm
- FAO/WHO (Food and Agriculture Organization/World Health Organization) , 2002. Evaluation of certain food additives: fifty‐ninth report of the Joint FAO/WHO Expert Committee on food Additives. WHO Technical Report Series 913. Available online: https://whqlibdoc.who.int/trs/WHO_TRS_913.pdf [PubMed]
- FAO/WHO (Food and Agriculture Organization/World Health Organization) , 2012. Evaluation of certain food additives: seventy‐sixth report of the Joint FAO/WHO Expert Committee on food Additives. WHO Technical Report Series 974. Available online: https://www.inchem.org/documents/jecfa/jecmono/v50je04.htm
- FAO/WHO (Food and Agriculture Organization/World Health Organization) , 2020. Evaluation of certain contaminants: ninetieth report of the Joint FAO/WHO Expert Committee on food Additives. WHO Technical Report Series 1032. Available online: https://apps.who.int/iris/bitstream/handle/10665/363392/9789240036925-eng.pdf?sequence=1&isAllowed=y
- Firriolo JM, Morris CF, Trimmer GW, Twitty LD, Smith JH and Freeman JJ, 1995. Comparative 90‐day feeding study with low‐viscosity white mineral oil in Fischer‐344 and Sprague‐Dawley‐derived CRL:CD rats. Toxicologic Pathology, 23, 26–33. [DOI] [PubMed] [Google Scholar]
- Fritsch H and Kühnel W, 2014. Color Atlas of Human Anatomy. 2: Internal Organs. Thieme. [Google Scholar]
- Gharbi I, Moret S, Chaari O, Issaoui M, Conte L, Lucci P and Hammami M, 2017. Evaluation of hydrocarbon contaminants in olives and virgin olive oils from Tunisia. Food Control, 75, 160–166. [Google Scholar]
- Grandmaison GL, Clairand I and Durigon M, 2001. Organ weight in 684 adult autopsies: new tables for a Caucasoid population. Forensic Science International, 119, 149–154. [DOI] [PubMed] [Google Scholar]
- Griffis LC, Twerdok LE, Francke‐Carroll S, Biles RW, Schroeder RE, Bolte H, Faust H, Hall WC and Rojko J, 2010. Comparative 90‐day dietary study of paraffin wax in Fischer‐344 and Sprague‐Dawley rats. Food and Chemical Toxicology, 48, 363–372. [DOI] [PubMed] [Google Scholar]
- Grob K, Biedermann M, Caramaschi A and Pacciarelli B, 1991a. LC‐GC analysis of the aromatics in a mineral oil fraction: batching oil for jute bags. Journal of High Resolution Chromatography, 14, 33–39. 10.1002/jhrc.1240140109 [DOI] [Google Scholar]
- Grob K, Artho A, Biedermann M and Egli J, 1991b. Food contamination by hydrocarbons from lubricating oils and release agents ‐ determination by coupled LC‐GC. Food Additives and Contaminants, 8, 437–446. [DOI] [PubMed] [Google Scholar]
- Grob K, Biedermann M, Artho A and Egli J, 1991c. Food contamination by hydrocarbons from packaging materials determined by coupled LC‐GC. Zeitschrift für Lebensmitteluntersuchung und Forschung, 193, 213–219. [DOI] [PubMed] [Google Scholar]
- Grob K, Lanfranchi M, Egli J and Artho A, 1991d. Determination of food contamination by mineral oil from jute sacks using coupled LC‐GC. Journal of the Association of Official Analytical Chemists, 74, 506–512. [PubMed] [Google Scholar]
- Grob K, Huber M, Boderius U and Bronz M, 1997. Mineral oil material in canned foods. Food Additives and Contaminants, 14, 83–88. [DOI] [PubMed] [Google Scholar]
- Hard GC, Rodgers IS, Baetcke KP, Richards WL, McGaughy RE and Valcovic LR, 1993. Hazard evaluation of chemicals that cause accumulation of alpha 2u‐globulin, hyaline droplet nephropathy and tubule neoplasia in the kidneys of male rats. Environmental Health Perspectives, 99, 313–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimbach JT, Bodor AR, Douglass JS, Barraj LM, Cohen SC, Biles RW and Faust HR, 2002. Dietary exposures to mineral hydrocarbons from food‐use applications in the United States. Food and Chemical Toxicology, 40, 555–571. [DOI] [PubMed] [Google Scholar]
- Hochegger A, Wagenhofer R, Savić S, Mayrhofer E, Washüttl M and Leitner E, 2022. Combination of multidimensional instrumental analysis and the Ames test for the toxicological evaluation of mineral oil aromatic hydrocarbons. Journal of Agricultural Food Chemistry, 70, 16401–16409. 10.1021/acs.jafc.2c05970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holländer C, Ackermann H and Parzeller M, 2021. Current reference values of organ weights and indices for forensic practice, part 2: liver, lungs, spleen and kidneys. Rechtsmedizin, 31, 117–130. [Google Scholar]
- Holländer C, Ackermann H and Parzeller M, 2020. Current norm values of organ weights and indices for forensic practice, Part 1. Forensic Medicine, 30, 79–87. [Google Scholar]
- House JS, Grimm FA, Klaren WD, Dalzell A, Kuchi S, Zhang S‐D, Lenz K, Boogaard PJ, Ketelslegers HB, Gant TW, Wright FA and Rusyn I, 2021. Grouping of UVCB substances with new approach methodologies (NAMs) data. Alternative to Animal Experimentation, 38, 123–137. 10.14573/altex.2006262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- BfR (Bundesinstitut für Risikobewertung) , 2012. Bestimmung von Kohlenwasserstoffen aus Mineralöl (MOSH und MOAH) oder Kunststoffen (POSH, PAO) in Verpackungsmaterialien und trockenen Lebensmitteln mittels Festphasenextraktion und GC‐FID. Available online: https://www.bfr.bund.de/cm/343/10-sitzung-der-bfr-kommission-fuerbedarfsgegenstaende.pdf
- BfR (Bundesinstitut für Risikobewertung) , 2011. 7. Sitzung der BfR‐Kommission für Bedarfsgegenstände. Available online: https://www.bfr.bund.de/cm/343/7_sitzung_der_bfr_kommission_fuer_bedarfsgegenstaende.pdf [Google Scholar]
- FSANZ (Food Standards Australia New Zealand) , 2018. Mineral oil hydrocarbons in food and food packaging. Available online: https://www.foodstandards.gov.au/publications/Documents/Mineral%20oil%20hydrocarbons.pdf
- IOM (Institute of Medicine (US) Committee on Nutritional Status During Pregnancy and Lactation) , 1991. Nutrition During Lactation. Washington (DC): National Academies Press (US); 1991.PMID: 25144080 Bookshelf ID: NBK235593. 10.17226/1577 [DOI] [PubMed]
- IARC (International Agency for Research on Cancer) , 2012. Chemical agents and related occupations. Volume 100 F, A Review of Human Carcinogens. Available online: https://publications.iarc.fr/_publications/media/download/3076/73443059d4ec0adde733204bab30939c7470dd2b.pdf
- Jackson AS, Stanforth PR, Gagnon J, Rankinen T, Leon AS, Rao DC, Skinner JS, Bouchard C and Wilmore JH, 2002. The effect of sex, age and race on estimating percentage body fat from body mass index: The Heritage Family Study. International Journal of Obesity, 26, 789–796. [DOI] [PubMed] [Google Scholar]
- Kamelia L, de Haana L, Rietjens IMCM and Boogaard PJ, 2017. Prenatal developmental toxicity testing of petroleum substances: application of the mouse embryonic stem cell test (EST) to compare in vitro potencies with potencies observed in vivo. Toxicology in Vitro, 44, 303–312. [DOI] [PubMed] [Google Scholar]
- Kamelia L, de Haan L, Ketelslegers HB, Rietjens IMCM and Boogaard PJ, 2019a. In vitro prenatal developmental toxicity induced by some petroleum substances is mediated by their 3‐ to 7‐ring PAH constituent with a potential role for the aryl hydrocarbon receptor (AhR). Toxicology Letters, 315, 64–76. [DOI] [PubMed] [Google Scholar]
- Kamelia L, Brugman S, de Haan S, Ketelslegers HB, Rietjens IMCM and Boogaard PJ, 2019b. Prenatal developmental toxicity testing of petroleum substances using the zebrafish embryotoxicity test. ALTEX ‐ Alternatives to Animal Experimentation, 36, 245–260. 10.14573/altex.1808121 [DOI] [PubMed] [Google Scholar]
- Kastelein JJP and Stores ESG, 2019. Fishing for the miracle of Eicosapentaenoic acid. New England Journal of Medicine, 380, 89–90. 10.1056/NEJMe1814004 [DOI] [PubMed] [Google Scholar]
- Kelsall GR and Blackwell JB, 1969. The occurrence and significance of lipophage clusters in lymph nodes and spleen. Pathology, 1, 211–220. [DOI] [PubMed] [Google Scholar]
- Koch M, Becker E, Päch M, Kühn S and Kirchhoff E, 2020. Separation of the mineral oil aromatic hydrocarbons of three and more aromatic rings from those of one or two aromatic rings. Journal of Separation Science, 43, 1089–1099. 10.1002/jssc.201900833 [DOI] [PubMed] [Google Scholar]
- Kuroda K, Kijma A, Jin M, Ishii Y, Takasu S, Matsushita K, Nishikawa A and Umemura T, 2013. The effects of long‐term exposure to ozokerite mainly consisting of an aliphatic series of hydrocarbons using F344 rats. Food and Chemical Toxicology, 55, 476–483. [DOI] [PubMed] [Google Scholar]
- Liber AF and Rose HG, 1967. Rose Saturated hydrocarbons in follicular lipodosis of the spleen. Archives of Pathology, 83, 116–122. [PubMed] [Google Scholar]
- Licht O, Breuer F, Blümlein K, Schwonbeck S, Pallapies D, Kellner R, Wiedemeier P and Bitsch A, 2023. Extensive literature search on mineral oil hydrocarbons. EFSA supporting publication 2023;20(2):EN‐7893, 1543 pp. 10.2903/sp.efsa.2023.EN-7893 [DOI] [Google Scholar]
- Lorenzini R, Fiselier K, Biedermann M, Barbanera M, Braschi I and Grob K, 2010. Saturated and aromatic mineral oil hydrocarbons from paperboard food packaging: prediction of long‐term migration from contents in the paperboard; data from the market. Food Additives and Contaminants, Part A, 27, 1765–1774. [DOI] [PubMed] [Google Scholar]
- Mackerer CR, Griffis LC, Grabowski JS Jr and Reitman FA, 2003. Petroleum mineral oil refining and evaluation of cancer hazard. Applied Occupational and Environmental Hygiene, 18, 890–901. [DOI] [PubMed] [Google Scholar]
- Marino DJ, 2012. Age‐specific absolute and relative organ weight distributions for Fischer 344 rats. Journal of Toxicology and Environmental Health, Part A, 75, 1484–1516. [DOI] [PubMed] [Google Scholar]
- McKee R, Drummond J, Freeman J, Letinski D and Miller M, 2012. Light white oils exhibit low tissue accumulation potential and minimal toxicity in F344 rats. International Journal of Toxicology, 31, 175–183. [DOI] [PubMed] [Google Scholar]
- McKee R, Schreiner C, Nicolich M and Gray T, 2013. Genetic toxicity of high‐boiling petroleum substances. Regulatory Toxicology and Pharmacology, 67, S75–S85. [DOI] [PubMed] [Google Scholar]
- Molina DK and DiMaio VJ, 2012. Normal organ weights in men: part II—the brain, lungs, liver, spleen, and kidneys. The American Journal of Forensic Medicine and Pathology, 33, 368–372. [DOI] [PubMed] [Google Scholar]
- Molina DK and DiMaio VJ, 2015. Normal organ weights in women: part II—the brain, lungs, liver, spleen, and kidneys. The American Journal of Forensic Medicine and Pathology, 36, 182–187. [DOI] [PubMed] [Google Scholar]
- Moret S, Grob K and Conte LS, 1996. On‐line high‐performance liquid chromatography‐solvent evaporation‐high‐performance liquid chromatography‐capillary gas chromatography‐flame ionisation detection for the analysis of mineral oil polyaromatic hydrocarbons in fatty foods. Journal of Chromatography A, 750, 361–368. [DOI] [PubMed] [Google Scholar]
- Moret S, Grob K and Conte LS, 1997. Mineral oil polyaromatic hydrocarbons in foods, eg from jute bags, by on‐line LC‐solvent evaporation (SE)‐LC‐GC‐FID. Zeitschrift für Lebensmitteluntersuchung und ‐forschung a‐Food Research and Technology, 204, 241–246. [Google Scholar]
- Moret S, Scolaro M, Barp L, Purcaro G and Conte LS, 2016. Microwave assisted saponification (MAS) followed by on‐line liquid chromatography (LC)–gas chromatography (GC) for high‐throughput and high‐sensitivity determination of mineral oil in different cereal‐based foodstuffs. Food Chemistry, 196, 50–57. [DOI] [PubMed] [Google Scholar]
- Nestola M and Schmidt TC, 2017. Determination of mineral oil aromatic hydrocarbons in edible oils and fats by online liquid chromatography‐gas chromatography‐flame ionization detection ‐ evaluation of automated removal strategies for biogenic olefins. Journal of Chromatography A, 1505, 69–76. 10.1016/j.chroma.2017.05.035 [DOI] [PubMed] [Google Scholar]
- Neukom HP, Grob K, Biedermann M and Noti A, 2002. Food contamination by C‐20‐C‐50 mineral paraffins from the atmosphere. Atmospheric Environment, 36, 4839–4847. [Google Scholar]
- Nicholls SJ, Lincoff AM, Garcia M, Bash D, Ballantyne CM, Barter PJ, Davidson MH, Kastelein JJP, Koenig W, McGuire DK, Mozaffarian D, Ridker PM, Ray KK, Katona BG, Himmelmann A, Loss LE, Rensfeldt M, Lundström T, Agrawal R, Menon V, Wolski K and Nissen SE, 2020. Effect of high‐dose omega‐3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA, 324, 2268–2280. 10.1001/jama.2020.22258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederer M, Stebler T and Grob K, 2016. Mineral oil and synthetic hydrocarbons in cosmetic lip products. International Journal of Cosmetic Science, 38, 194–200. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Lincoff AM, Wolski K, Ballantyne CM, Kastelein JJP, Ridker PM, Ray KK, McGuire DK, Mozaffarian D, Koenig W, Davidson MH, Garcia M, Katona BG, Himmelmann A, Loss LE, Poole M, Menon V and Nicholls SJ, 2021. Association between achieved ω‐3 fatty acid levels and major adverse cardiovascular outcomes in patients with high cardiovascular risk: a secondary analysis of the STRENGTH trial. JAMA Cardiology, 6, 1–8. 10.1001/jamacardio.2021.1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nochomovitz LE, Uys CJ and Epstein S, 1975. Massive deposition of mineral oil after prolonged ingestion. South African Medical Journal, 49, 2187–2190. [PubMed] [Google Scholar]
- Noti A, Grob K, Biedermann M, Deiss U and Bruschweiler BJ, 2003. Exposure of babies to C‐15‐C‐45 mineral paraffins from human milk and breast salves. Regulatory Toxicology and Pharmacology, 38, 317–325. [DOI] [PubMed] [Google Scholar]
- Nygaard UC, Vege A, Rognum T, Grob K, Cartier C, Cravedi JP and Alexander J, 2023. Toxic effects of mineral oil saturated hydrocarbons (MOSH) and relation to accumulation in rat liver. Food and Chemical Toxicology, 177, 113847. 10.1016/j.fct.2023.113847 [DOI] [PubMed] [Google Scholar]
- O'Donoghue ML, Braunwald E, White HD, Lukas MA, Tarka E, Steg PG, Hochman JS, Bode C, Maggioni AP, Im K, Shannon JB, Davies RY, Murphy SA, Crugnale SE, Wiviott SD, Bonaca MP, Watson DF, Weaver WD, Serruys PW, Cannon CP, SOLID‐TIMI 52 Investigators and Steen DL, 2014. Effect of darapladib on major coronary events after an acute coronary syndrome: the SOLID‐TIMI 52 randomized clinical trial. JAMA, 312(10), 1006–1015. 10.1001/jama.2014.11061 Erratum in: JAMA. 2014 Oct 8;312(14):1473. Dylan P. Steen[corrected to Dylan L. Steen] [DOI] [PubMed] [Google Scholar]
- OECD (Organisation for Economic Co‐Operation and Development) , 1983. Guideline for the testing of chemicals. “One‐Generation Reproduction Toxicity Study”, 415. Available online: https://www.oecd.org/chemicalsafety/risk-assessment/1948458.pdf
- OECD (Organisation for Economic Co‐Operation and Development) , 2001a. OECD Guidelines for the testing of chemicals. Test No. 414: prenatal developmental toxicity study. Available online: https://www.oecd-ilibrary.org/environment/test-no-414-prenatal-development-toxicity-study_9789264070820-en, https://www.oecd.org/dataoecd/18/15/1948482.pdf
- OECD (Organisation for Economic Co‐Operation and Development) , 2001b. OECD Guidelines for the testing of chemicals. Test No. 416: two generation toxicity study. Available online: https://www.oecd-ilibrary.org/environment/test-no-416-two-generation-reproduction-toxicity_9789264070868-en
- OECD (Organisation for Economic Co‐Operation and Development) , 2004. High Production Volume Screening Information Dataset (OECD SIDS) on 1,2,3,4‐tetrahydronaphthalene. Available online: https://www.chem.unep.ch/irptc/sids/oecdsids/119642.pdf
- OECD (Organisation for Economic Co‐Operation and Development) , 2017. Guidelines for the testing of chemicals, section 4: health effects, test no. 421: reproduction/developmental toxicity screening test. Available online: https://read.oecd-ilibrary.org/environment/test-no-421-reproduction-developmental-toxicity-screening-test_9789264264380-en#page1
- OECD (Organisation for Economic Co‐Operation and Development) , 2018. Guideline for the testing of chemicals. Repeated Dose 90‐day Oral Toxicity Study in Rodents. 408. Available online: https://www.oecd.org/env/ehs/testing/Revision%20TG%20408%202018.pdf
- OECD (Organisation for Economic Co‐Operation and Development) , 2022. Guidelines for the testing of chemicals, section 4: health effects test no. 443: extended one‐generation reproductive toxicity study. Available online: https://read.oecd-ilibrary.org/environment/test-no-443-extended-one-generation-reproductive-toxicity-study_9789264185371-en#page1
- Olshansky B, Chung MK, Budoff MJ, Philip S, Jiao L, Doyle RT Jr., Copland C, Giaquinto A, Juliano RA, Bhatt DL, 2020. Mineral oil: safety and use as placebo in REDUCE‐IT and other clinical studies, European Heart Journal Supplements, Volume 22, Issue Supplement_J, October 2020. pp. J34–J48. 10.1093/eurheartj/suaa117 [DOI] [PMC free article] [PubMed]
- Petry T, Bury D, Fautz R, Hauser M, Huber B, Markowetz A, Mishra S, Rettinger K, Schuh W and Teichert T, 2017. Review of data on the dermal penetration of mineral oils and waxes used in cosmetic applications. Toxicology Letters, 280, 70–78. [DOI] [PubMed] [Google Scholar]
- Pirow R, Blume A, Hellwig N, Herzler M, Bm H, Hutzler C, Pfaff K, Thierse H‐J, Tralau T, Vieth B and Luch A, 2019. Mineral oil in food, cosmetic products, and in products regulated by other legislations. Critical Reviews in Toxicology, 49, 742–789. 10.1080/10408444.2019.1694862 [DOI] [PubMed] [Google Scholar]
- Populin T, Biedermann M, Grob K, Moret S and Conte L, 2004. Relative hopane content confirming the mineral origin of hydrocarbons contaminating foods and human milk. Food Additives and Contaminants, 21, 893–904. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ and JUPITER Study Group , 2008. Rosuvastatin to prevent vascular events in men and women with elevated C‐reactive protein. New England Journal of Medicine, 359, 2195–2207. 10.1056/NEJMoa0807646 [DOI] [PubMed] [Google Scholar]
- Ridker PM, Revkin J, Amarenco P, Brunell R, Curto M, Civeira F, Flather M, Glynn RJ, Gregoire J, Jukema JW, Karpov Y, Kastelein JJP, Koenig W, Lorenzatti A, Manga P, Masiukiewicz U, Miller M, Mosterd A, Murin J, Nicolau JC, Nissen S, Ponikowski P, Santos RD, Schwartz PF, Soran H, White H, Wright RS, Vrablik M, Yunis C, Shear CL and Tardif JC, 2017. SPIRE cardiovascular outcome investigators. cardiovascular efficacy and safety of bococizumab in high‐risk patients. New England of Journal of Medicine, 376, 1527–1539. 10.1056/NEJMoa1701488 [DOI] [PubMed] [Google Scholar]
- Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, Mam V, Hasan A, Rosenberg Y, Iturriaga E, Gupta M, Tsigoulis M, Verma S, Clearfield M, Libby P, Goldhaber SZ, Seagle R, Ofori C, Saklayen M, Butman S, Singh N, Le May M, Bertrand O, Johnston J, Paynter NP, Glynn RJ and CIRT Investigators , 2019. Low‐dose methotrexate for the prevention of atherosclerotic events. New England Journal of Medicine, 380, 752–762. 10.1056/NEJMoa1809798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, MacFadyen J, Glynn RJ, Jiao L, Steg PG, Miller M, Brinton EA, Jacobson TA, Tardif JC, Ballantyne CM, Mason RP and Bhatt DL, 2022. Effects of randomized treatment with icosapent ethyl and a mineral oil comparator on interleukin‐1β, interleukin‐6, C‐reactive protein, oxidized low‐density lipoprotein cholesterol, homocysteine, lipoprotein(a), and lipoprotein‐associated phospholipase A2: a REDUCE‐IT biomarker substudy. Circulation, 146, 372–379. 10.1161/CIRCULATIONAHA.122.059410 [DOI] [PubMed] [Google Scholar]
- Rose HG and Liber AF, 1966. Accumulation of saturated hydrocarbons in human spleens. Journal of Laboratory Clinical Medicine, 68, 475–483. [PubMed] [Google Scholar]
- Roy TA, Johnson SW, Blackburn GR and Mackerer CR, 1988. Correlation of mutagenic and dermal carcinogenic activities of mineral oils with polycyclic aromatic compound content. Fundamental and Applied Toxicology, 10, 446–476. [DOI] [PubMed] [Google Scholar]
- Sato K, Satoh K, Tsuchida S, Hatayama I, Shen H, Yokoyama Y, Yamada Y and Tamai K, 1992. Specific expression of glutathione S‐transferase Pi forms in (pre)neoplastic tissues: their properties and functions. Tohoku Journal of Experimental Medicine, 168, 97–103. 10.1620/tjem.168.97 [DOI] [PubMed] [Google Scholar]
- Schoeffner DJ, 1999. Organ weights and fat volume in rats as a function of strain and age. Journal of Toxicology and Environmental Health Part A, 56, 449–462. [DOI] [PubMed] [Google Scholar]
- SCF (Scientific Committee for Food) , 1995. Opinion on mineral and synthetic hydrocarbons, SCF reports: 37th series. 25 September 1995. Available online: https://ec.europa.eu/food/system/files/2020-12/sci-com_scf_reports_37.pdf [Google Scholar]
- Smith JH, Bird MG, Lewis SC, Freeman JJ, Hogan JK and Scala RA, 1995. Subchronic feeding study of four white mineral oils in dogs and rats. Drug and Chemical Toxicology, 18, 83–103. 10.3109/01480549509017859 [DOI] [PubMed] [Google Scholar]
- Smith JH, Mallett AK, Priston RA, Brantom PG, Worrell NR, Sexsmith C and Simpson BJ, 1996. Ninety‐day feeding study in Fischer‐344 rats of highly refined petroleum‐derived food‐grade white oils and waxes. Toxicologic Pathology, 24, 214–230. [DOI] [PubMed] [Google Scholar]
- Smith PB, Veley KE, Yarrington JT and Slauter RW, 1999. 90‐day oral gavage toxicity study of C9‐C16 aromatic fraction JET‐A in female Sprague‐Dawley CD rats and male C57BL/6 mice. United States Air Force Research Laboratory, Report Number AFRL‐HE‐WP‐TR‐1999‐0229.
- Spack LW, Leszczyk G, Varela J, Simian H, Gude T and Stadler RH, 2017. Understanding the contamination of food with mineral oil: the need for a confirmatory analytical and procedural approach. Food Additives and Contaminants: Part A, 34, 1052–1071. [DOI] [PubMed] [Google Scholar]
- Srbinovska A, Conchione C, Lucci P and Moret S, 2020. On‐line HP(LC)‐GC‐FID determination of hydrocarbon contaminants in fresh and packaged fish and fish products. Journal of AOAC International, 2020, 1–7. [DOI] [PubMed] [Google Scholar]
- STABILITY Investigators , White HD, Held C, Stewart R, Tarka E, Brown R, Davies RY, Budaj A, Harrington RA, Steg PG, Ardissino D, Armstrong PW, Avezum A, Aylward PE, Bryce A, Chen H, Chen MF, Corbalan R, Dalby AJ, Danchin N, De Winter RJ, Denchev S, Diaz R, Elisaf M, Flather MD, Goudev AR, Granger CB, Grinfeld L, Hochman JS, Husted S, Kim HS, Koenig W, Linhart A, Lonn E, López‐Sendón J, Manolis AJ, Mohler ER 3rd, Nicolau JC, Pais P, Parkhomenko A, Pedersen TR, Pella D, Ramos‐Corrales MA, Ruda M, Sereg M, Siddique S, Sinnaeve P, Smith P, Sritara P, Swart HP, Sy RG, Teramoto T, Tse HF, Watson D, Weaver WD, Weiss R, Viigimaa M, Vinereanu D, Zhu J, Cannon CP and Wallentin L, 2014. Darapladib for preventing ischemic events in stable coronary heart disease. New England Journal of Medicine, 370, 1702–1711. 10.1056/NEJMoa1315878 [DOI] [PubMed] [Google Scholar]
- Stauff A, Schnapka J, Heckel F and Matissek R, 2020. Mineral oil hydrocarbons (MOSH/MOAH) in edible oils and possible minimization by deodorization through the example of cocoa butter. European Journal of Lipid Science and Technology, 122, 1900383. [Google Scholar]
- Sui H, Gao H, Chen Y, Ke R, Zhong H, Zhong Q, Liu Z and Song Y, 2020. Survey of mineral oil hydrocarbons in infant formula from the Chinese market. Food Additives and Contaminants: Part A, 37, 1040–1048. [DOI] [PubMed] [Google Scholar]
- Tarnow P, Hutzler C, Grabiger S, Schön K, Tralau T and Luch A, 2016. Estrogenic activity of mineral oil aromatic hydrocarbons used in printing inks. PLoS One, 11, e0147239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranchida PQ, Zoccali M, Purcaro G, Moret S, Conte L, Beccaria M, Dugo P and Mondello L, 2011. A rapid multidimensional liquid‐gas chromatography method for the analysis of mineral oil saturated hydrocarbons in vegetable oils. Journal of Chromatography A, 1218, 7476–7480. 10.1016/j.chroma.2011.06.089 [DOI] [PubMed] [Google Scholar]
- Trimmer GW, Freeman JJ, Priston RA and Urbanus J, 2004. Results of chronic dietary toxicity studies of high viscosity (P70H and P100H) white mineral oils in Fischer 344 rats. Toxicologic Pathology, 32, 439–447. [DOI] [PubMed] [Google Scholar]
- Tsitou P, Heneweer M and Boogaard PJ, 2015. Toxicogenomics in vitro as an alternative tool for safety evaluation of petroleum substances and PAHs with regard to prenatal developmental toxicity. Toxicolicology In Vitro, 29, 299–307. [DOI] [PubMed] [Google Scholar]
- Van de Ven BM, Fragki S, te Biesebeek JD, Rietveld AG and Boon PE, 2018. Mineral oils in food; a review of toxicological data and an assessment of the dietary exposure in The Netherlands. RIVM Letter report 0182. Available online: https://www.rivm.nl/bibliotheek/rapporten/2017-0182.pdf
- Van Heyst A, Vanlacker M, Vercammen J, Van den Houwe K, Mertens B, Elskens M and Van Hoeck E, 2018. Analysis of mineral oil in food: results of a Belgian market survey. Food Additives and Contaminants: Part A, 35, 2062–2075. [DOI] [PubMed] [Google Scholar]
- Van Heyst A, Goscinny S, Bel S, Vandevijvere S, Mertens B, Elskens M and Van Hoeck E, 2020. Dietary exposure of the Belgian population to mineral oil. Food Additives and Contaminants: Part A, 37, 267–279. [DOI] [PubMed] [Google Scholar]
- Vollmer A, Biedermann M, Grundböck F, Ingenhoff J‐E, Biedermann‐Brem S, Altkofer W and Grob K, 2011. Migration of mineral oil from printed paperboard into dry foods: survey of the German market. European Food Research and Technology, 232, 175–182. [Google Scholar]
- Wang D, Bruyneel B, Kamelia L, Wesseling S, Rietjens IMCM and Boogaard PJ, 2020. In vitro metabolism of naphthalene and its alkylated congeners by human and rat liver microsomes via alkyl side chain or aromatic oxidation. Chemical‐Biological Interactions, 315, 108905. 10.1016/j.cbi.2019.108905 [DOI] [PubMed] [Google Scholar]
- Wang D, Schramm V, Pool J, Pardali E, Brandenburg A, Rietjens IMCM and Boogaard PJ, 2022a. The effect of alkyl substitution on the oxidative metabolism and mutagenicity of phenanthrene. Archives of Toxicology, 96, 1109–1131. 10.1007/s00204-022-03239-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Groot A, Seidel A, Wang L, Kiachaki E, Boogaard PJ and Rietjens IMCM, 2022b. The influence of alkyl substitution on the in vitro metabolism and mutagenicity of benzo[a]pyrene. Chemical‐Biological Interactions, 363, 110007. 10.1016/j.cbi.2022.110007 [DOI] [PubMed] [Google Scholar]
- Wanless IR and Geddie WR, 1985. Mineral oil lipogranulomata in liver and spleen. A study of 465 autopsies. Archives of Pathology and Laboratory Medicine, 109, 283–286. [PubMed] [Google Scholar]
- WHO/IPCS (World Health Organization/International Programme on Chemical Safety) , 1994. Assessing humanhealth risks of chemicals: Derivation of guidance values for health‐based exposure limits. Environmental HealthCriteria 170. WHO, Geneva. [Google Scholar]
- WHO/IPCS ((World Health Organization/International Programme on Chemical Safety) , 1999. Principles for theassessment of risks to human health from exposure to chemicals. Environmental Health Criteria 210. WHO, Geneva. [Google Scholar]
- WHO/IPCS (World Health Organization/International Programme on Chemical Safety) , 2009. Principles and Methods for the Risk Assessment of Chemicals in Food. International Programme on Chemical Safety (IPCS), Environmental Health Criteria 240. Available online: https://www.who.int/publications/i/item/9789241572408
- Xie Y, Li B, Liu L, Ouyang J and Wu Y, 2019. Rapid screening of mineral oil aromatic hydrocarbons (MOAH) in grains by fluorescence spectroscopy. Food Chemistry, 294, 458–467. 10.1016/j.foodchem.2019.05.057 [DOI] [PubMed] [Google Scholar]
- Zhang S, Liu L, Li B, Xie Y, Ouyang J and Wu Y, 2019. Concentrations of migrated mineral oil/polyolefin oligomeric saturated hydrocarbons (MOSH/POSH) in Chinese commercial milk powder products. Food Additives and Contaminats: Part A: Chemistry, Analysis, Control, Exposure and Risk Assessment, 36, 1261–1272. [DOI] [PubMed] [Google Scholar]


