SUMMARY
Long noncoding RNA (lncRNA) are regulatory RNAs >200 nt. We previously showed that lncRNA GAS5 decreases significantly in serum of type 2 diabetes mellitus (T2DM) patients. Hence, we sought to decipher the molecular mechanisms underlying the role of GAS5 in T2DM in adipose tissue. Using CHIP-RIP, we demonstrate that GAS5 binds to promoter of insulin receptor to regulate its expression, and its depletion inhibits glucose uptake and insulin signaling. Toward stabilizing GAS5 levels in T2DM, we incorporated a strategy to limit the degradation of GAS5 by blocking the interaction of GAS5 and UPF1 with a small molecule identified using OBTC screening strategy. NP-C86 binds to GAS5 with high affinity, and increases GAS5 levels and glucose uptake in diabetic patient adipocytes. As a broader impact, NP-C86 may be used as a molecular probe to investigate the intricacies of GAS5 in relevant biological systems as it offers specificity, efficient cellular uptake and is non-cytotoxic.
In Brief
This study provides evidence that lncRNA GAS5 is a target for therapeutic intervention in type 2 diabetes management. The small molecule NP-C86 identified here increases GAS5 levels and glucose uptake in diabetic adipocytes and has high potential as a ncRNA targeted therapeutic.
Graphical Abstract
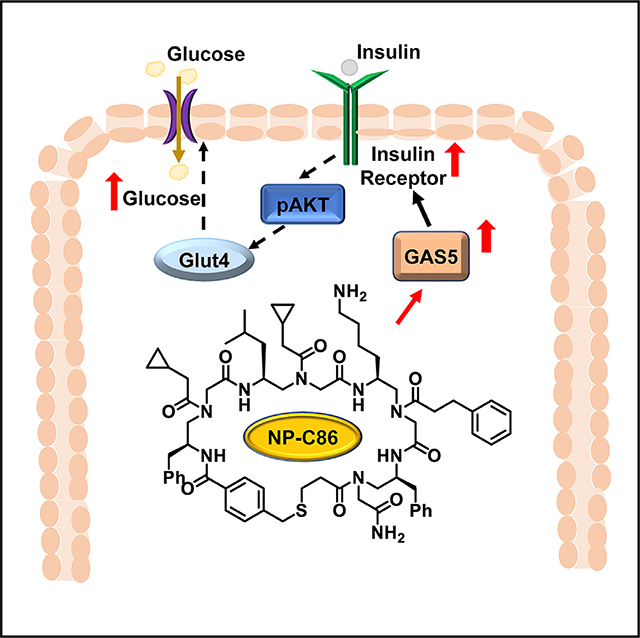
INTRODUCTION
Diabetes mellitus comprises of a group of metabolic diseases characterized by hyperglycemia, impaired insulin action and/or insulin secretion. Type 1 diabetes mellitus is juvenile onset diabetes in which the insulin-producing pancreatic β cells are destroyed (insulin dependent). Type 2 diabetes mellitus (T2DM) is polygenic in nature and is commonly diagnosed in middle-aged individuals. T2DM is frequently associated with insulin resistance and impaired insulin signal transduction with less than 15% of individuals being insulin sensitive. Further, insulin receptor double knockouts can cause T2DM (Bluher et al., 2002; Kitamura et al., 2003). While the underlying genetics of T2DM has been extensively studied, the emphasis has been on genes encoding transcripts that are translated to proteins. However, it is becoming increasingly clear that regulatory noncoding transcripts play an important role in disease manifestation.
Long noncoding RNAs (lncRNAs) (>200 nts) are orchestrators of essential biological networks exerting their effects although varied mechanisms including regulation of alternative splicing and signaling pathways, acting as molecular decoys, functioning as scaffolding for transcription and translation complexes and guiding ribonucleoprotein complexes. lncRNAs are essential for normal pathological and physiological processes, and multiple lines of evidence link aberrant expression of regulatory lncRNAs to human diseases (Ding et al., 2018; Qin et al., 2014; Schmidt et al., 2014; Zhao et al., 2014; Zhu et al., 2014). The lncRNA growth arrest-specific transcript 5 (GAS5) is a 5′ terminal oligopyrimidine class of gene shown to regulate cell growth, proliferation, and survival (Coccia et al., 1992; Smith and Steitz, 1998). The biogenesis of GAS5 is well established, and it is known that the GAS5 gene transcribes several small nuclear RNAs (snoRNAs) as well as four splice variants of GAS5 mRNA. However, due to presence of a STOP codon, none of the transcripts are translated into protein and they are degraded via the nonsense-mediated decay pathway when translation is initiated. The RNA levels of GAS5 are regulated by its degradation instead of regulation at its transcriptional level (Raho et al., 2000). GAS5 is encoded at 1q25, a locus displaying abnormalities in a number of cancers (Smedley et al., 2000) and associated with retinopathy and coronary heart disease (Qi et al., 2013). GAS5 inhibits actions of rapamycin, which is a mammalian target of rapamycin inhibitor and this affects both leukemic and untransformed human T lymphocytes (Williams et al., 2011). It is downregulated in breast cancer (Mourtada-Maarabouni et al., 2009). GAS5 acts as a riborepressor by repressing transcription of glucocorticoid receptor (Kino et al., 2010). However, the role of GAS5 in T2DM adipocytes and its underlying mechanisms are unknown.
Adipose tissue (AT) is an important endocrine regulator of energy homeostasis and glucose metabolism. AT is a primary organ for glucose uptake and storage of excess energy. AT also secretes metabolites such as leptin, adiponectin, free fatty acids, inflammatory cytokines, which affect glucose metabolism in the body. It is known that insulin increases the rate of glucose transport across the membrane and increases glycolysis. AT plays a central role in the development of insulin resistance and subsequently of T2DM. The importance of AT in T2DM is underscored by its impact through lipodystrophy and obesity in the development of insulin resistance.
Hence, we evaluated AT to understand the role of GAS5 in glucose metabolism. Our results indicate that GAS5 regulates insulin signaling in adipocytes, manifesting its potential as novel ncRNA target for T2DM. We also show that GAS5 levels could be manipulated by a small molecule targeted to GAS5 transcript such that it inhibited UPF1-mediated turnover via nonsensemediated RNA decay pathway. The small-molecule compound is highly effective in restoring GAS5 levels in T2DM adipocytes, thereby demonstrating its potential as a therapeutic agent manipulating noncoding RNA for the management of type 2 diabetes.
RESULTS
GAS5 Is Decreased in Adipocytes from Type 2 Diabetic Patients
Previously, we used a transcriptomics approach to screen lncRNA levels in serum of patients with or without T2DM. We observed a marked decrease in lncRNA GAS5 expression in T2DM samples compared with nondiabetic samples, while the other lncRNAs that were detected consistently in patient’s serum did not change significantly. We demonstrated that individuals with serum GAS5 < 10 ng/μL have almost 12 times higher odds of having T2DM (exact odds ratio [OR] = 11.79 [95% confidence interval: 3.97, 37.26], p < 0.001) (Carter et al., 2015). Hence, we focused on evaluating the role of GAS5 in T2DM adipocytes.
Since AT is an important regulator of glucose metabolism and development of insulin resistance, we determined the expression of GAS5 in the abdominal subcutaneous and omental adipose depots from nondiabetic and T2DM patients (IRB20295; all patients with BMI 24.1 ± 4; 6 nondiabetic patients HbA1c 5.4 ± 0.7; 6 T2DM patients HbA1c 8 ± 0.7; both groups are non-smokers, no cancers, other criteria matched). Omental and subcutaneous AT was digested with collagenase and purified to obtain adipocytes (free from other cells and macrophages). Adipocytes were maintained in culture for 48 hr (adipocyte media from ZenBio) and conditioned medium was collected to analyze its secretome. Using SYBR Green absolute qPCR for GAS5 we show that T2DM patients have significantly decreased expression of GAS5 in adipocytes and its secretome compared with adipocytes from nondiabetic patients (Figure 1A).
Figure 1. GAS5 Levels are Decreased in Diabetic Adipocytes.

(A) GAS5 levels are decreased in T2DM adipocytes and its secretome: adipocytes from subcutaneous (sc) or omental (on) depots were isolated from type 2 diabetic (DM) or nondiabetic (NDM) patients and maintained in culture for 48 hr. Total RNA was extracted from cells or conditioned media (CM). SYBR Green real-time qPCR GAS5 primers was performed for absolute quantification (AQ). GAPDH served as control. Experiments were repeated four times with similar results. The results were analyzed with two-tailed Student’s t test using PRISM4 statistical analysis software (GraphPad, San Diego, CA). A level of p < 0.05 was considered statistically significant. ***p < 0.0001 is highly significant between DM and NDM.
(B) High glucose does not affect levels of GAS5: adipose stem cells derived from NDM patients were differentiated in vitro to mature adipocytes and treated with 25 mM glucose. Total RNA was isolated on days 0, 3, and 5. SYBR Green real-time qPCR GAS5 primers was performed for relative quantification (RQ) using non-treated day 0 as reference. Experiments were repeated five times with similar results. No significant change was observed in the GAS5 levels between the samples.
To maintain reproducibility over a larger number of experiments, adipose stem cells (ASCs) derived from normal or type 2 diabetic patients were purchased from ZenBio (BMI 20–24; nondiabetic ASCs with HbA1c 5.9 [hereafter referred as NDM]; T2DM ASCs with HbA1c 8.5 [hereafter referred as DM]). The ASCs were differentiated in vitro to mature adipocytes (see STAR Methods) and used in experiments as described previously. To address whether GAS5 levels changed due to T2DM or GAS5 levels contributed to metabolic changes which ultimately result in T2DM, we incubated nondiabetic (NDM) adipocytes with 25 mM glucose for 0 to 5 days. Our real-time qPCR results (Figure 1B) indicated that hyperglycemia did not influence the levels of GAS5. This indicates that decrease in GAS5 levels is not a consequence of T2DM.
Depletion of GAS5 Decreases Glucose Uptake
AT is the primary target for insulin-mediated glucose uptake. Hence, we sought to evaluate the effect of GAS5 depletion on glucose uptake. GAS5 small interfering RNA (siRNA) (25 nM, Ambion n272331, validated; scrambled siRNA as control) was transfected in NDM pre-adipocytes for 48 hr and differentiated to mature NDM adipocytes for 8 days. We determined the effect of GAS5 depletion on glucose uptake by NDM adipocytes. Cells were serum starved, 1 μM of insulin was added to cells for 20 min, and 2-deoxyglucose uptake was measured. Results (Figure 2A) indicated a decrease in insulin-mediated glucose uptake in GAS5-depleted NDM adipocytes. These results indicate that GAS5 modulates uptake of glucose in adipocytes.
Figure 2. GAS5 Regulates Glucose Uptake in Adipocytes.

(A) Depletion of GAS5 decreases glucose uptake: 25 nM GAS5 siRNA was used to deplete NDM adipocytes. Glucose uptake was performed after treatment with 1 μM insulin as described in the STAR Methods. Glucose up-take in pmol was calculated from the standard curve. The experiment was repeated five times with similar results. The results were analyzed with two-tailed Student’s t test using PRISM4 statistical analysis software (GraphPad). A level of p < 0.05 was considered statistically significant. ***p < 0.0001 is highly significant between NDM and GAS5 depleted NDM after insulin treatment.
(B) Depletion of GAS5 decreases Glut4 translocation: GAS5 siRNA (25 nM) was used to deplete NDM adipocytes. NDM adipocytes were serum starved for 3 hr and treated with 100 nM insulin for 30 min. The cytosolic and plasma membrane fractions were separated by ultracentrifugation. The proteins were separated and analyzed by western blot analysis using antibody against Glut4. The experiments were repeated five times with similar results.
(C) Depletion of GAS5 decreases insulin receptor expression: GAS5 siRNA (25 nM) was used to deplete NDM adipocytes. SYBR Green real-time qPCR using GAS5, IR-A, and IR-B primers was performed for RQ using non-treated day 0 as reference. Separately, PCR products were separated by PAGE and silver-stained for visualization of products (representative gel shown in inset). Experiments were repeated five times with similar results. Experiments were repeated four times with similar results. The results were analyzed with two-tailed Student’s t test using PRISM4 statistical analysis software (GraphPad). A level of p < 0.05 was considered statistically significant. ***p < 0.0001 is highly significant between NDMand GAS5 siRNA-treated NDM.
Following binding of insulin to the insulin receptor (IR) and activation of insulin signal transduction pathways, glucose transporters are recruited from the intracellular pool to the plasma membrane, thereby exposing functional glucose transporters to the extracellular medium containing glucose. In AT, the primary glucose transporter is GLUT4 (with GLUT1 to a lesser extent). Hence, subcellular fractionation was performed (Elmendorf, 2003; Kleiman et al., 2009) to determine translocation of Glut4 from cytosol to plasma membrane in NDM adipocytes depleted of GAS5 using siRNA (as above). Our results (Figure 2B) show that depletion of GAS5 inhibited insulin-mediated GLUT4 translocation.
Since our data showed that GAS5 depletion decreased uptake of glucose, we evaluated the genes involved in glucose metabolism that may be affected by depletion of GAS5. Customized human diabetes array (QIAGEN) was used to screen for expression of 84 genes related to the onset, development, and progression of diabetes. GAS5 depletion resulted in dramatic inhibition of insulin receptors (IR-A and IR-B), while transfection with scrambled siRNA had no effect on GAS5 levels. These results were also individually verified using quantitative SYBR Green qPCR (Figure 2C, NDM set as reference in calculating relative quantification in qPCR; inset shows silver-stained PCR products).
GAS5 Binds to the Promoter of IR
lncRNAs have structural and spatial features that allow it to bind to DNA, RNA, or protein partners, thereby regulating transcription of genes. Since our data showed that GAS5 depletion significantly inhibited both insulin receptors IR-A and IR-B, it suggested that GAS5 may regulate transcription of IR and not alternative splicing or other post-transcriptional mechanism.
We investigated whether GAS5 could bind to the IR promoter and interact with RNA polymerase II for transcription initiation. We performed a co-immunoprecipitation assay to determine the region on the IR promoter that interacts with GAS5 and simultaneously determine association of GAS5 with RNA polymerase II in NDM adipocytes. Following chromatin fixation, immunoprecipitations were performed with anti-RNA polymerase II (immunoglobulin G [IgG] immunoprecipitation [IP] serves as control). Immunoprecipitated DNA was analyzed by semi-qPCR using primers amplifying two regions on the IR promoter: −580 to −860 bp; −1,480 to −1,810 bp. These regions were assessed as putative GAS5 binding regions based on computational analysis for complementary sequence. Input DNA (1:10 of released chromatin) was included in control PCRs. The results (Figure 3A) indicated that GAS5 binds to the IR promoter between −580 and −860 bp. Further computational analysis showed that the sequence “aacgtttttat” on IR promoter region (at −826 bp) is complementary to a region of the GAS5 DNA binding domain. Subsequently, the crosslinking was reversed, RNA was isolated from the immunoprecipitated complex and GAS5 was detected using SYBR Green qPCR. We observed a 43-fold enrichment of GAS5 in RNA polymerase II immunoprecipitate over the control IgG immunoprecipitate. U1RNA levels in snRNP70 IP serve as positive control. Our results suggest that GAS5 acts as a riboactivator and promotes transcription of IR.
Figure 3. GAS5 Regulates Expression of Insulin Receptor.

(A) GAS5 binds to the promoter of the insulin receptor: following chromatin fixation of NDM cells, immunoprecipitations were performed using antibody against RNA polymerase II, SNRNP70 which served as positive control or IgG that served as negative control. Immunoprecipitated DNA was analyzed by semi-qPCR using primers at positions on insulin receptor promoter region as indicated on the schematic. Chromatin immunoprecipitation (ChIP) assay was performed as described in the STAR Methods. Cross linking was reversed and RNA was isolated from the immunoprecipitated complex. SYBR Green real-time qPCR was performed using primers for GAS5 and U1 RNA (binds to SNRNP70). The experiment was repeated five times with similar results.
(B) Binding of GAS5 to insulin receptor (IR) promoter increases transcription of IR: GAS5 siRNA 25 nM was transfected in NDM preadipocytes for 48 hr followed by transfection of human IR promoter deletion plasmid series with CAT reporter: pIRC1 (−1,823), pIRC 3 (−1,311), or pIRC5 (−746). The parental vector pBLCAT8+ served as control. Expression of the reporter gene chloramphenicol acetyltransferase (CAT) was measured using SYBR Green real-time qPCR. Experiments were repeated four times with similar results. The results were analyzed with two-tailed Student’s t test using PRISM4 statistical analysis software (GraphPad). A level of p < 0.05 was considered statistically significant. ***p < 0.0001 is highly significant between pIRC1 NDMand GAS5 siRNA-treated pIRC1 NDM; ***p < 0.0001 is highly significant between pIRC3 NDMand GAS5 siRNA-treated pIRC3 NDM.
(C) Insulin treatment increases GAS5 levels: 100 nM insulin was added to NDM adipocytes and maintained until day 4. RNA was isolated every 48 hr. SYBR Green real-time qPCR using GAS5 primers was performed for RQ using non-treated day 0 as reference. Experiments were repeated five times with similar results. The results were analyzed with two-tailed Student’s t test using PRISM4 statistical analysis software (GraphPad). A level of p < 0.05 was considered statistically significant. ***p < 0.0001 is highly significant between day 0 and day 2; ***p < 0.0001 is highly significant between day 0 and day 4.
(D) Depletion of GAS5 decreases phosphorylation of AKT: GAS5 siRNA 25 nM was transfected in NDM preadipocytes for 48 hr. Whole-cell lysates were collected and proteins were analyzed using automated western blot analysis (WES) using antibodies against pAKT (1:10) and AKT (1:100). The inset shows a gel view image and the graph represents five experiments performed individually with similar results. The results were analyzed with a two-tailed Student’s t test using PRISM4 statistical analysis software (GraphPad). A level of p < 0.05 was considered statistically significant. ***p < 0.0001 is highly significant for phospho-AKT between control and GAS5 siRNA cells.
Binding of GAS5 to Promoter of IR Activates Transcription
We sought to determine whether binding of GAS5 to IR promoter could increase transcription of IR. IR promoter deletion plasmid series with a chloramphenicol acetyltransferase (CAT) reporter gene (Webster et al., 1996) were a kind gift from Dr. Webster (UC, San Diego). We depleted GAS5 as described above in NDM preadipocytes for 48 hr followed by transfection of human IR promoter deletion plasmid series with CAT reporter: pIRC1 (−1,823), pIRC 3 (−1,311), or pIRC5 (−746). The parental vector pBLCAT8+ served as control. Expression of the reporter gene. CAT was measured using real-time qPCR. Results (Figure 3B) show that depletion of GAS5 decreases expression of the CAT reporter gene in pIRC1 and pIRC3, demonstrating that GAS5 binding to the IR promoter activates transcription. This effect is abolished in pIRC5, indicating that the GAS5 binding site on the IR promoter was deleted.
GAS5 Modulates the Insulin Signaling Pathway
Since our results demonstrated that GAS5 regulates the expression of IR, we sought to evaluate whether insulin levels affected GAS5 levels: 100 nM insulin (high insulin) was added to NDM adipocytes and evaluated every 48 hr (days 0, 2, and 4). Real-time qPCR analysis showed an increase in GAS5 levels (Figure 3C), indicating that hyperinsulinemia promoted GAS5 expression and suggesting a feedback loop of regulation.
Separately, we sought to evaluate whether depletion of GAS5 could affect the insulin signaling pathway. To do so, we evaluated phosphorylation of AKT, which is a downstream kinase of the insulin receptor-mediated phosphatidylinositol 3-kinase pathway. GAS5 was depleted in NDM adipocytes using GAS5 siRNA, as described above. Whole-cell lysates were analyzed using automated western blot analysis using antibodies against p-AKT and total AKT. Results demonstrated a decrease in phosphorylation of AKT with depletion of GAS5, while the total AKT levels remained constant (Figure 3D). These results indicated that depletion of GAS5 levels inhibited insulin signaling.
Small-Molecule Design to Stabilize GAS5 Levels
Our data show that depleted GAS5 levels affect glucose metabolism and insulin signaling, which are critical components attributed to metabolic syndrome and T2DM. The hallmark of T2DM is the development of insulin resistance. While in a normal state, improving insulin sensitivity would promote obesity, in a diabetic state, reducing insulin resistance would revert glucose homeostasis to its normal functioning. Current treatment regimens in T2DM aim at reducing insulin resistance and improving glucose metabolism. Hence, we sought to identify small molecular probes that could stabilize GAS5 levels in vivo. The goal of the drug design is not to boost insulin sensitivity in adipocytes to higher levels, but to bring back GAS5 levels to their pre-disease conditions, and thus to normal physiological functioning.
The levels of GAS5 are regulated by its degradation instead of regulation at its transcriptional level (Raho et al., 2000). GAS5 transcript has a premature termination codon which renders greater susceptibility for nonsense-mediated RNA decay. It was demonstrated that GAS5 levels were increased when UPF1, an essential component of nonsense-mediated RNA decay, was depleted (Tani et al., 2013). This demonstrated an inverse relationship of UPF1 and GAS5. We measured GAS5 and UPF1 levels in NDM and DM adipocytes. Our data indicated that GAS5 levels were low in DM adipocytes concurrent with increased expression of UPF1 compared with NDM adipocytes (Figure 4A).
Figure 4. Small Molecule NP-C86 Binds to GAS5.

(A) UPF1 levels are increased in DM adipocytes: total RNA was isolated from DM or NDM adipocytes, and SYBR Green real-time qPCR was performed using primers for GAS5 and UPF1 for RQ using NDM as reference. Experiments were repeated five times with similar results. The results were analyzed with a two-tailed Student’s t test using PRISM4 statistical analysis software (GraphPad). A level of p < 0.05 was considered statistically significant. ***p < 0.0001 is highly significant between NDM and DM for GAS5 and UPF1.
(B) Determination of binding affinity (Kd) of FITC NP-C86 for GAS5 by fluorescence polarization (FP). The FP experiment was performed by incubating 50 nM FITC-labeled AApeptide with the 111-bp GAS5 RNA (0.03125–0.5 μM) in 1× PBS. Dissociation constants (Kd) were determined by plotting the fluorescence anisotropy values as a function of RNA concentration, and the plots were fitted to the equation (STAR Methods). The Lst is the concentration of the AApeptide and the x stands for the concentration of the protein. The experiments were conducted in triplicates and repeated three times.
(C) NP-C86 binds to GAS5: RNA electrophoretic mobility shift assay was performed with biotin-labeled GAS5 transcript and 20 nM NP-C86, and detected using a Biotin Chromogenic detection kit. The gel shows shift in mobility and is representative of experiments repeated five times with similar results.
(D) NP-C86 treatment disrupts binding of UPF1 to GAS5: DM adipocytes were treated with 20 nM NP-C86 for 24 hr. RIP assay was performed with UPF1 immunoprecipitation and immunoprecipitated RNA was analyzed by qPCR for GAS5. The graph is plotted as fold enrichment of GAS5 using qPCR in the RIP assay with UPF1 IP; fold enrichment of U1 RNA using SNRP70 IP (positive control). Inset using automated WES show immunoblot of UPF1 IP and IgG IP (negative control) using antibody against UPF1. Experiments were repeated four times with similar results.
However, it is not advisable to inhibit UPF1, since it is integral to nonsense-mediated decay, which is a crucial surveillance mechanism to reduce errors in gene expression. In addition, in humans, UPF1 is required for S phase progression and genome stability (Azzalin, 2012; Azzalin and Lingner, 2006). Hence, we used a screening strategy to discover novel molecules that could disrupt the binding of UPF1 to GAS5 thereby inhibiting GAS5 turnover. GAS5 has premature stop codons UAA upstream of the poly(A) tail. UPF1 binds to this region and tags it for nonsense-mediated decay (Tani et al., 2013). As such, molecules that bind to the same region would competitively inhibit UPF1 binding and thereby disrupt the interaction between UPF1 and GAS5. Recently, we developed a new class of peptidomimetics, γ-AApeptide, with diverse side chains for chemical diversity and resistance to proteolytic cleavage (Shi et al., 2016). The γ-AApeptides are based on a one-bead-one-compound combinatorial library (Wu et al., 2014; Teng et al., 2014) and a one-bead-two-compound (OBTC) combinatorial library (Shi et al., 2017). We hypothesized that, with cyclic constraints and analysis of binding affinity to targets, this OBTC cyclic γ-AApeptide combinatorial library could identify molecular hits that bound to GAS5. To this end, we synthesized a new cyclic library with a diversity of 214,375 (Schemes 1 and S1) and used it to identify molecules that target GAS5.
Scheme 1. Design and Preparation of S-Bridged Cyclic γ-AApeptide Library.

(a) Soak in water; (b) (Boc)2O, DCM/ether; (c) Fmoc-Met-OH/HOBt/DIC (2:4:4 equiv.) in DMF; (d) 20% piperidine in DMF; (e) split in to five portions; (f) Dde-protected amino acids/PyBOP/NEM (2:6:6) in DMF; (g) TFA/triisopropylsilane/H2O/thioanisole (v/v/v/v, 94:2:2:2); (h) Fmoc-protected γ-AApeptide/HOBt/DIC (2:4:4) in DMF; (i) Pd(PPh3)4 and Me2NH•BH3 in DCM; (j) Dmt-protected mercaptopropionic acid/HOBt/DIC (2:4:4 equiv.) in DMF; (k) deprotect Dde by NH2OH•HCl and imidazole in NMP/DCM; (l) split-and-pool synthesis, repeated the previous steps; (m) 20% piperidine in DMF; (n) 4-(bromomethyl)benzoyl chloride/DIPEA (2:4 equiv.) in DCM; (o) TFA/triisopropylsilane/DCM (2:2:96); (p) (NH4)2CO3 (10 equiv.) in 1:1 (v/v) DMF/H2O. Additional information in Scheme S1.
To conduct the library screening, we synthesized a fluorescein-tagged oligonucleotide (Eurofins Scientific), which spanned 30 nucleotides on either side of the UAA sequences on GAS5. This is at the 3′ end of the transcript (near poly(A) tail) and does not interfere with the 5′ DNA binding domain of GAS5 through which it interacts with the IR. The length of the oligonucleotide was required to maintain the stem-loop structure of GAS5, which allows specific binding of ligands. Further, we modeled the oligonucleotide (RNAfold software) to evaluate its folding and verified the absence of any unwanted secondary structures. A fluorescein tag was attached to the 5′ end to aid in screening. The oligonucleotide was used to screen against 214,375 molecules in the combinatorial library in the presence of ~1,000-fold of tRNA as competitor (as tRNA has similar stem-loop secondary structures) to eliminate non-specific binding (Supplemental Information, Library Screening). The beads emitting green fluorescence were picked up as the putative positive hits (Figure S1). Followed by the on-bead cleavage and the sequence analysis, the structures of the putative hits were identified. The putative hits were resynthesized (both unmodified and fluorescein isothiocyanate-labeled derivatives; Supplemental Information), and validated in vitro for their ability to bind to GAS5. These series of experiments led to the identification of the lead compound NP-C86 and its FITC derivative, which exhibited a binding affinity (Kd) of 153 nM toward GAS5 by fluorescence polarization (Figures 4B and S5). Compound 94, which was part of the library, served as negative control as the Kd data showed that 94-NC has no binding with GAS5 (Figure S2).
GAS5-Stabilizing Compound NP-C86 Binds to lncRNA GAS5
As fluorescence polarization assay showed that NP-C86 bound to GAS5, we performed an in vitro RNA-electrophoretic mobility shift assay (REMSA) to validate the binding of NP-C86 to the GAS5 transcript. In brief, GAS5 was cloned in the TOPO vector, which has the T7 promoter. In vitro transcription assay was performed and the biotin-labeled GAS5 transcript was incubated with 20 nM NP-C86 in the presence of 10 U yeast tRNA and 10 units of RNase inhibitor in a final volume of 10 μL of RNA shift buffer for 20 min at room temperature, and detected using a Biotin Chromogenic Detection kit (Thermo Fisher Scientific). Our results with REMSA demonstrate that NP-C86 bound to the GAS5 transcript (Figure 4C).
To demonstrate that the association of lncRNA GAS5 and UPF1 was disrupted by NP-C86, we performed the RNA immunoprecipitation (RIP) assay. DM adipocytes (obtained by in vitro differentiation as described above) were treated with NP-C86 (20 nM) for 24 hr. UPF1 was immunoprecipitated from adipocytes and pull down of the associated lncRNA GAS5 was evaluated using real-time qPCR. Our results demonstrate that UPF1 associated with GAS5 robustly in DM adipocytes, while treatment with NP-C86 dramatically reduced the association as shown as decrease in fold enrichment (Figure 4D).
Compound NP-C86 Increases GAS5 Levels in DM Adipocytes
Next, we evaluated whether NP-C86 could stabilize GAS5 levels in DM adipocytes. The goal of dose optimization is to restore GAS5 levels to normal physiological levels and not promote overexpression, which is detrimental to the cells. DM adipocytes (obtained by in vitro differentiation as described above) were treated with NP-C86 (10–200 nM) for 24 hr. GAS5, IR-A and IR-B, and Glut4 levels were measured using SYBR Green qPCR. Our results indicate that NP-C86 at a concentration of 20 nM is sufficient to increase GAS5 levels by 4-fold (Figure 5A), with concurrent increases in IR-A and IR-B, while Glut4 levels did not change significantly with the treatment. Next, DM adipocytes were treated with 20 nM NP-C86 or a structural analog from the library (94-NC as control, the Kd data showed that the 94-NC has no binding with GAS5, Figure S2) for 24 hr. Our results show that NP-C86 substantially increased GAS5 levels in DM adipocytes to the levels observed in NDM without affecting UPF1 levels (Figure 5B). Further, NP-C86 was efficiently taken up by DM adipocytes and increased GAS5 levels without causing cellular toxicity (Figures 5C and 5D). NP-C86 did not affect levels of MALAT1 or NEAT1, the other lncRNAs present in the adipocytes (Figure 5E), which demonstrates that compound NP-C86 has high specificity for lncRNA GAS5. Since our results show that NP-C86 could stabilize GAS5 levels in DM adipocytes, we sought to evaluate whether treatment with NP-C86 could improve insulin-mediated glucose utilization. DM adipocytes were treated with NP-C86 (20 nM) for 24 hr, stimulated with insulin for 20 min, followed by measurement of glucose uptake. Our results (Figure 5F) demonstrate that NP-C86 restored insulinmediated glucose uptake in DM adipocytes.
Figure 5. NP-C86 Increases Glucose Uptake in Diabetic Adipocytes.

(A) NP-C86 increases GAS5 levels in DM adipocytes: total RNA was isolated from DM or NP-C86 treated (10–200 nM) DM adipocytes. SYBR Green real-time qPCR was performed using primers for GAS5, IR-A, IR-B, and Glut4 for RQ using untreated DM as reference. Experiments were repeated five times with similar results.
(B) NP-C86 increases GAS5 levels but does not affect UPF1 levels in DM adipocytes: total RNA was isolated from DM or NP-C86-treated (20 nM) DM adipocytes, or control compound 94 (20 nM, from the same library but with no binding to GAS5). SYBR Green real-time qPCR was performed using primers for GAS5 and UPF1 for RQ using untreated DM as reference. Experiments were repeated five times with similar results. The results were analyzed with two-tailed Student’s t test using PRISM4 statistical analysis software (GraphPad). A level of p < 0.05 was considered statistically significant. ***p < 0.0001 is highly significant between DM and DM + NP-C86 for GAS5. ns, not significant between DM and DM + NP-C86 for GAS5.
(C) NP-C86 is not toxic to adipocytes: WST-1 (Roche Molecular Biochemicals) was added to DM adipocytes (in triplicate) that were treated without or with NP-C86 to a final concentration of 10% (v/v). The graph is representative of five experiments performed with similar results.
(D) NP-C86 is efficiently taken up by adipocytes: FITC NP-C86 (10 nM) was added to adipocytes for 24 hr. The cells were labeled with DAPI and fixed. Images were captured on Nikon’s A1R + confocal microscope.
(E) NP-C86 increases GAS5 levels but does not affect lncRNA NEAT1 or MALAT1 levels in DM adipocytes: total RNA was isolated from DM or NP-C86-treated (20 nM) DM adipocytes. SYBR Green real-time qPCR was performed using primers for GAS5, NEAT1, and MALAT1 for RQ using untreated DM as reference. Experiments were repeated five times with similar results. The results were analyzed with two-tailed Student’s t test using PRISM4 statistical analysis software (GraphPad). A level of p < 0.05 was considered statistically significant. ***p < 0.0001 is highly significant between DM and DM + NP-C86 for GAS5.
(F) NP-C86 increases glucose uptake in DM adipocytes: 20 nM NP-C86 was added to DM adipocytes. NDM adipocytes were used as positive control for the assay for 24 hr. Glucose uptake was performed after treatment with 1 μM insulin as described in the STAR Methods. Glucose uptake in pmol was calculated from the standard curve. The experiment was repeated five times with similar results. The results were analyzed with two-tailed Student’s t test using PRISM4 statistical analysis software (GraphPad). A level of p < 0.05 was considered statistically significant. ***p < 0.0001 is highly significant between DM and NP-C86-treated DM in response to insulin; ns, not significant between DM and DM in response to insulin.
GAS5 Compound NP-C86 Increases GAS5 Levels in DM Adipose Explants
To evaluate the effect of NP-C86 on adipocytes in their physiological environment, ex vivo subcutaneous and omental AT explants were obtained from nondiabetic and type 2 diabetic patients (IRB20295; n = 3; BMI 24.1 ± 5; T2DM patients HbA1c 8 ± 0.7; nonsmokers, no cancers, other criteria matched as described above) and incubated in adipocyte medium (ZenBio); treated with or without NP-C86 (20 nM) for 48 hr. The tissue was homogenized and total RNA was extracted. GAS5 levels were measured using SYBR Green qPCR for absolute quantification. Our results show NP-C86 substantially increases GAS5 levels in ex vivo diabetic AT explants from subcutaneous and omental depots (Figure 6).
Figure 6. NP-C86 Increases GAS5 Levels in Adipose Explants.

Adipose tissue from subcutaneous (SubQ) and omental (Om) depots was treated with 20 nM NP-C86 for 48 hr. Total RNA was isolated and SYBR Green real-time qPCR GAS5 primers was performed for absolute quantification (AQ). GAPDH served as control. Experiments were repeated five times with similar results. The results were analyzed with two-tailed Student’s t test using PRISM4 statistical analysis software (GraphPad). A level of p < 0.05 was considered statistically significant. ***p < 0.0001 is highly significant between DM and NP-C86-treated DM.
DISCUSSION
The lncRNA GAS5 is encoded by the GAS5 host gene, which also encodes snoRNAs U44, U75, and U81. GAS5 was so named since it was observed that its expression increased upon serum starvation (Coccia et al., 1992). GAS5 was shown to promote apoptosis in breast and prostate cancer cells, where survival of tumors is the underlying function attributed to lncRNA GAS5 (Mourtada-Maarabouni et al., 2009; Pickard et al., 2013; Pickard and Williams, 2014; Yacqub-Usman et al., 2015). As stated above, the samples and cells in this study were from patients with no history of cancer. We previously showed that circulating GAS5 levels measured in serum of diabetic patients were lower compared with the nondiabetic patients (Carter et al., 2015). Here, we sought to determine the underlying mechanism for mode of action of lncRNA GAS5 in T2DM. We determined that the levels of GAS5 in AT (an important endocrine organ) and its secretome were lower in AT from T2DM compared with nondiabetic patients. We performed an unbiased transcriptomics screen to identify genes affected by depletion of GAS5 levels in adipocytes and identified IR as the target gene whose levels are significantly affected by GAS5. Our data demonstrate that GAS5 binds to the IR promoter, thereby regulating its expression in adipocytes at the transcriptional level. It will be interesting to evaluate if and how GAS5 may interact with HMG1-Y, C/EBPβ, and SP1 on the IR promoter; this is being investigated as a separate project.
A recent study by Jin et al. (2017) showed the role of GAS5 in pancreatic β cells and demonstrated that GAS5 was central to maintain β cell identity and function by affecting insulin synthesis and secretion from β cells. ANRIL and H19 lncRNAs in the pancreas are also shown to be involved in diabetes (Ding et al., 2012; Pasmant et al., 2011), while TUG1 was shown to regulate apoptosis and insulin secretion in pancreatic β cells (Yin et al., 2015). These studies add to our knowledge that several regulatory lncRNAs are involved in glucose metabolism, and often the function of an lncRNA is specific to the tissue and cell type.
Previously it was shown that GAS5 bound to the glucocorticoid receptor and served to repress its activity. In this scenario, GAS5 functions as a riborepressor as it bound to the DNA binding domain of the glucocorticoid receptor and acts as a decoy for glucocorticoid response elements (Kino et al., 2010). Distinct, unique features of GAS5 enable it to bind to DNA as well as RNA partners. GAS5 is also shown to bind to microRNAs (miRNAs). In gliomas, the role of GAS5 lncRNA is attributed to its ability to bind and repress the availability of miR-222 (Zhao et al., 2015), while in melanomas GAS5 binds to miR-137 to regulate tumorigenesis (Bian et al., 2017). The transcription of GAS5 itself is regulated by miR-21 and, interestingly, GAS5 regulates miR-21 levels in this feedback loop (Zhang et al., 2013). The study by Zhang et al. (2018) in a liver model showed that GAS5 acts as ceRNA to interact with miR-222 to regulate levels of p27.
RNA-based therapeutics are gaining utmost importance in their ability to treat several chronic diseases. However, antisense oligonucleotides and siRNAs that interact with RNA are vulnerable to degradation and require modifications for stability and efficient uptake. Previous studies based on interaction of lncRNA GAS5 with DNA, RNA, and protein (discussed above) showed evidence that GAS5 participates in multi-component complexes. This provides targetable regions to modulate the interactions of GAS5 with other biomolecules. Earlier reports had shown that GAS5 turnover is via the nonsense-mediated pathway and our data presented here showed that UPF1 levels were inversely related to GAS5 levels. This suggested that the GAS5 transcript is targeted for faster turnover by increased UPF1 levels via nonsense-mediated RNA decay in T2DM. Among known UPF1 targets are genes associated with diabetes (COIL, ITPR3, and TRIM32), which were upregulated when UPF1 was depleted (Tani et al., 2012). We thus hypothesized that molecules disrupting UPF1/GAS5 interaction could inhibit its turnover and stabilize GAS5 levels. Future work in the field is needed to address the mechanisms for increased UPF1 expression in diabetes and possible miRNA regulation of GAS5 levels in diabetes.
Since UPF1 is also known for critical roles in other biological processes, we sought to identify small molecules that could disrupt its binding to GAS5 rather than the approach of depleting UPF1 levels. To achieve this, we developed a cyclic γ-AApeptide combinatorial library. γ-AApeptides are oligomers of γ-substituted-N-acylated-N-aminoethyl amino acids, and were designed based on the chiral PNA backbone. As a new class of peptidomimetics, they have enhanced chemodiversity and cell permeability and are resistant to proteolytic degradation (Shi et al., 2016). In each building unit of the γ-AApeptides, half side chains are chiral and derived from the natural amino acids, thus they may retain functions of the conventional peptides. The other half side chains are introduced from the coupling reaction of the secondary amine on the backbone with the carboxylic acids or acyl chlorides. As a result, γ-AApeptides are versatile molecules with enormous diversity, which provides a rich source of unnatural ligands for combinatorial library screening (Teng et al., 2016; Wu et al., 2014). In the past few years, with the rapid development of chemical biology and biomedical sciences, ligands that can bind to the targets with high affinity and specificity are urgently needed. As an efficient strategy to reduce the conformational flexibility of the peptide backbone thus leading to a higher affinity, macrocyclization has been successfully applied into peptide libraries for target screening (Chen et al., 2013; Heinis et al., 2009; Kaniraj and Maayan, 2015; Kawamura et al., 2017; Lee and Lim, 2014; Qian et al., 2015; Upadhyaya et al., 2015). Our unpublished results and other studies (Li et al., 2018) show that overexpression of GAS5 may alter adipogenesis or may promote cancer as discussed above. Hence, NP-C86 could also be of importance to understand the molecular mechanisms of GAS5 in other diseases.
SIGNIFICANCE
This study demonstrates that lncRNA GAS5 binds to the insulin receptor and regulates its expression, and results show that GAS5 is a potential target to modulate insulin signaling and glucose uptake in type 2 diabetes (T2DM). While the underlying genetics of T2DM has been extensively studied, the emphasis has been on genes encoding transcribed and translated messages. However, it is becoming increasingly clear that noncoding transcripts play an important role in diseases and our results demonstrate the impact of lncRNA GAS5, a regulatory RNA, on metabolic pathways in T2DM in human adipose tissue. Although lncRNAs are proposed to play a role in T2DM, it has not been investigated mechanistically nor shown to regulate insulin receptor expression. This adds to our knowledge that lncRNA are robust disease markers and can be targeted for developing therapeutic agents in diseases. Of particular importance is identification of NP-C86 that stabilizes GAS5 levels to normal physiological concentrations demonstrating high therapeutic potential. Results show that NP-C86 is noncytotoxic, is efficiently taken up by cells and binds to GAS5 with high affinity and specificity, and, significantly, our results demonstrate that NP-C86 restored insulin-mediated glucose uptake in diabetic adipocytes. In designing the strategy to stabilize GAS5 in T2DM, the approach was refined such that UPF1, which destabilizes GAS5 was not knocked down or inhibited as it is an important cell-surveillance mechanism. The screening strategy comprised of disrupting the interaction of GAS5 with UPF1 and this furthermore presents NP-C86 as a molecular probe to investigate the structure-function relationship of GAS5 in relevant biological systems and its implications in disease manifestation.
STAR★METHOD
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Niketa A. Patel (npatel@health.usf.edu) and the co-corresponding author, Jianfeng Cai (jianfengcai@usf.edu).
EXPERIMENT MODEL AND SUBJECT DETAILS
Adipose Samples
White adipose tissue was obtained as discarded tissue from surgeries performed at Tampa General Hospital by Dr. Murr. Donors consented to their waste tissue to be used in research. The subcutaneous and omental depots were collected from the same subject. All patients (equal M/F ratio) with BMI 24.1±4; non-diabetic patients HbA1c 5.4±0.7; T2DM patients HbA1c 8±0.7; both groups are nonsmokers, no cancers, other criteria matched. The de-identified samples were obtained under an Institutional Review Board approved protocol (University of South Florida IRB #20295) with a ‘‘not human research activities determination’’ and was transported to the laboratory and processed within 24h of collection. Additional tissue with the same criteria was also obtained from ZenBio™.
Adipose-Derived Stem Cells
ASC were isolated as previously described by our lab (Watson et al., 2014). Briefly, adipose tissue (as described above) was cut up into small pieces and digested with 0.075% collagenase Type 1 (Worthington) in modified PBS for 2 h at 37°C. The digestion was stopped by adding α-MEM + 20% heat-inactivated FBS.
In Vitro Differentiation of ASC to Adipocytes
The adipose stem cells derived from normal or type 2 diabetic patients (Caucasian, equal M/F) were purchased from Zenbio™. The cells were cultured as follows. ASC were passaged with preadipocyte medium (PM-1; DMEM/Ham’s F-12 medium, HEPES, FBS, penicillin, streptomycin, amphotericin B; ZenBio™) and then plated at 50,000 cells/cm2 with PM-1. Cells were fed every other day with PM-1 until confluent.
METHOD DETAILS
Adipose Samples
White adipose tissue was obtained as discarded tissue from surgeries performed at Tampa General Hospital by Dr. Murr. Donors consented to their waste tissue to be used in research. The subcutaneous and omental depots were collected from the same subject. All patients with BMI 24.1±4; non-diabetic patients HbA1c 5.4±0.7; T2DM patients HbA1c 8±0.7; both groups are nonsmokers, no cancers, other criteria matched. The de-identified samples were obtained under an Institutional Review Board approved protocol (University of South Florida IRB #20295) with a “not human research activities determination” and was transported to the laboratory and processed within 24h of collection. Additional tissue with the same criteria was also obtained from ZenBio™.
Adipose-Derived Stem Cells
ASC were isolated as previously described by our lab (Watson et al., 2014). Briefly, adipose tissue was cut up into small pieces and digested with 0.075% collagenase Type 1 (Worthington) in modified PBS for 2 h at 37°C. The digestion was stopped by adding α-MEM + 20% heat-inactivated FBS. The suspension was filtered and centrifuged at 400g at room temperature. The pellet contains the stromal vascular fraction (SVF). The pellet was resuspended in 1mL of the erythrocyte lysis buffer (Stem Cell Technologies) for 10 min and washed in 20mL of PBS with 2% P/S/A before centrifugation, 300–500 g, 5 min. The supernatant was aspirated and the cell pellet resuspended in a 3mL stromal medium (α-MEM; Mediatech) with 20% FBS, 1% l-glutamine (Mediatech),1% P/S/A. Following three rinses in the stromal medium, SVF cells were plated for initial cell culture at 37°C with 5% CO2 in ASC medium from ZenBio™ (Cat# PM-1). Subconfluent cells were passaged by trypsinization. Experiments were conducted within passages 2–3.
In Vitro Differentiation of ASC to Adipocytes
The adipose stem cells derived from normal or type 2 diabetic patients were purchased from Zenbio™. These are tested in culture to differentiate into mature adipocytes and show accumulation of lipid and secrete adiponectin and leptin. At the start of all experiments, cells were grown to confluency such that all cells are synchronized and then differentiated. The cells were cultured as follows. ASC were passaged with preadipocyte medium (PM-1; DMEM/Ham’s F-12 medium, HEPES, FBS, penicillin, streptomycin, amphotericin B; ZenBio™) and then plated at 50,000 cells/cm2 with PM-1. Cells were fed every other day with PM-1 until confluent. To induce differentiation, PM-1 medium was replaced with differentiation medium (DM2; ZenBio™) which included biotin, pantothenate, human insulin, dexamethasone, isobutylmethylxanthine and a PPARγ agonist (Days 0–7). After 7 days, DM-2 medium was replaced with Adipocyte Medium (AM1; ZenBio™; Days 7–14), which included PM-1, biotin, pantothenate, human insulin and dexamethasone. By Day 14, cells contained large lipid droplets and were considered mature adipocytes. Cells were maintained at 37°C in a humidified 5% CO2 atmosphere.
Quantitative Real-Time qPCR
Total RNA was isolated from cells using RNA-Bee™ (Tel Test, Inc) as per manufacturer’s instructions. 2mg of RNA was used to synthesize first strand cDNA using random hexamer primers and Omniscript™ kit (Qiagen). 1μL of cDNA was amplified by real-time quantitative PCR using Maxima SYBR Green/Rox qPCR master mix (Thermo Scientific) in an ABI ViiA7 sequence detection system (PE Applied Biosystems) to quantify the relative levels of the transcripts in the samples. The primers are: GAS5 sense primer 5′- CTTCTGGGCTCAAGTGATCCT -3′ and anti-sense 5’ TTGTGCCATGAGACTCCATCAG -3′; IR-A sense primer 5′- GTTTTCGTCCCCAGGCCATC -3′ and anti-sense 5′ CCAACATCGCCAAGGGACCT -3′; IR-B sense primer 5′- CACTGGTGCCGAGGACCCTA -3′ and anti-sense 5′ GACCTGCGTTTCCGAGATGG -3′; IR promoter set 1 sense primer 5′- AGATCTCTGGCCATTGCACTCCAG -3′ and anti-sense 5′ TTCAATAAACAGTTTGCTAGGAGC -3′; IR promoter set 2 sense primer 5′- TCGAGTCACCAAAATAAACAT -3′ and anti-sense 5′ TGCAGGGGAGGGAGGTGCCGC -3′; GAPDH sense primer 5′- TGACGTGCCGCCTGGAGAAAC -3′ and anti-sense 5′- CCGGCATCGAAGGTGGAAGAG -3′; CAT sense: 5’ GCCGCTGGCGATTCAG 3’ and antisense 5′ TTCATTAAGCATTCTGCCGACAT 3’; UPF1 sense: 5’ AGATCACGGCACAGCAGAT 3’ and antisense 5′ TGGCAGAAGGGTTTTCCTT 3’; U1snRNA sense: 5’ TCCCAGGGCGAGGCTTATCCATT 3’ and antisense 5′ GAACGCAGTCCCCCACTACCACAAAT 3’. Amplification was performed on the ViiaA 7 (Applied Biosystems). Real-time PCR was then performed in triplicate on samples and standards. The plate setup included a standard series, no template control, no RNA control, no reverse transcriptase control, and no amplification control. After primer concentrations were optimized to give the desired standard curve and a single melt curve, relative quotient (RQ) was determined using the ΔΔCT method with U6snRNA or GAPDH as the endogenous control and ASC control samples as the calibrator sample. Experiments were repeated four times.
For absolute quantification, a standard curve was generated for each gene in every assay. To do so, 100 ng to 0.4 ng of RNA were reverse-transcribed as described above. The resulting cDNA was used to obtain a standard curve correlating the amounts with the threshold cycle number (Ct values). A linear relationship (r2 > 0.96) was obtained for each gene. Real time PCR was then performed on samples and standards in triplicates. The plate setup also included a standard series, no template control, no RNA control, no reverse transcriptase control and no amplification control. The dissociation curve was analyzed for each sample. Absolute quantification GAS5 expression levels was calculated by normalizing the values to GAPDH.
Western Blot Analysis
Automated western blot analysis using Simple WES system (ProteinSimple, Santa Clara, CA, USA) was used. The amount of lysate to antibody ratio was optimized as per manufacturer’s instructions. A concentration of 0.4mg/mL was found optimal to be used on all antibodies. The samples were separated on 12–230 kDa Wes Separation Module capillary cartridges of Simple Protein Wes system. Rabbit monoclonal antibody specific for phospho-AKT was used at a dilution of 1:10 while antibody specific for AKT was used at a dilution of 1:100 in the runs. β-actin was used as a loading control (1:250 dilution of antibody). Anti-rabbit detection module kits were specific for Wes (ProteinSimple) and include Luminol-S, Peroxide, Streptavidin-HRP and anti-rabbit secondary antibody. The proteins are separated by capillary technology and analyzed based on the chemiluminescence signal peaks generated, shown as digital images representing bands as observed in traditional western blot analysis. Using Compass software (ProteinSimple), the peak areas of Akt, pAkt, were estimated and normalized against β-actin.
Transient Transfection of Plasmid DNA
ASC were trypsinized and cell pellets were collected in 100 μL Nucleofector® solution (Lonza) and combined with pMAX GFP (2 μg. The cell/DNA solution was transferred to a cuvette and the program initiated (0.34kV, 960 microfarads). Medium (500 μL) was added immediately and cells were gently transferred to 60mm plates and allowed to grow for 24 hours or more according to the experiment.
siRNA Transfection
Three siRNAs (Ambion; ThermoFisher) that target separate areas were used to knockdown expression of GAS5 and GAS5 siRNA (ID: n272331. validated) with optimal knockdown was used in all subsequent experiments along with its scrambled control. Ambion’s PARIS kit (catalog 1921) was used to simultaneously isolate proteins and RNA to verify knockdown by siRNA transfection and evaluate expression of other genes affected by GAS5 depletion.
Glucose Uptake Assay
NDM adipocytes were serum starved for 3 hours, followed by glucose starvation in KRPH buffer supplemented with 2% BSA for 40 mins. Then cells were treated with 1μM insulin for 20 minutes, followed by 10 mM 2-deoxy glucose 6 phosphate (2DG6P). Glucose uptake was performed using Glucose Uptake Fluorometric assay kit (Sigma # MAK084). A standard curve with 2DG6P was generated on the same plate. Fluorescence was measured at Ex 535 nm and Em 587 nm and 2-deoxy glucose uptake of samples was calculated from the standard curve.
Chromatin-Immunoprecipitation (ChIP) Assay
The ChIP kit was purchased from Sigma and protocol followed as per manufacturer’s instruction. RNA polymerase II antibody and SNRNP70 antibody were purchased from Novus, and IgG antibody was included in kit (Sigma). Following cross-linking, cell lysate (10%) was removed for input sample. Immunoprecipitation was performed with 2μg RNA polymerase II antibody, snRNP70 antibody (positive control) or IgG antibody (as negative control). SYBR Green Real-time qPCR was performed as described above using GAS5 primers and primers for U1 RNA, the binding partner for the positive control SNRNP70. The binding events and specificity (fold enrichment) was calculated using Excel™ template for ChIP from manufacturer.
RNA-Immunoprecipitation (RIP) Assay
The RIP kit was purchased from Sigma and protocol followed as per manufacturer’s instruction. UPF1 antibody and SNRNP70 antibody were purchased from Novus, and IgG antibody was included in kit (Sigma). Cell lysate (10%) was removed for input sample. Immunoprecipitation was performed with 2μg UPF1 antibody, snRNP70 antibody (positive control) or IgG antibody (as negative control). RNA was purified and treated with DNAse to remove genomic DNA. SYBR Green Real-time qPCR was performed as described above using GAS5 primers and primers for U1 RNA, the binding partner for the positive control SNRNP70. The yield (% input) and specificity (fold enrichment) was calculated using Excel™ template for RIP from Sigma.
RNA Electrophoresis Mobility Assay
For RNA-EMSA, GAS5 was cloned in TOPO vector which has the T7 promoter. In vitro transcription assay was performed with biotinlabel using RiboScribe™ kit using T7 RNA polymerase at 37°C for 2 h in the presence of nucleotides, RNase inhibitor, and 5X transcription buffer. 0.1nM of transcribed, biotin-labeled GAS5 was incubated with 10nM NP-C86 in presence of 10U yeast tRNA and 10 units of RNase inhibitor in a final volume of 10μl of RNA shift buffer for 20 min at room temperature and detected using Biotin Chromogenic Detection kit (ThermoFisher).
Immunochemistry
Adipocytes were plated in 8 well chamber plates and were treated with fluorescein labeled NP-C86. After 24h, medium was removed, cells washed three times with PBS and fixed with 4% paraformaldehyde for 30 min. To visualize nucleus, cells were stained with DAPI for 15 minutes at room temperature. Images were obtained using Nikon’s A1R confocal microscope.
Preparation of One-Bead Two-Compound Library
The One-bead Two-compound cyclic γ-AApeptide library was prepared on TentaGel NH2 resin (4.2g, 0.97 mmol, 643125 beads) using split and pool method at room temperature following our previously reported protocol(Shi et al., 2017) (Scheme S1). Each bead was modified to display two layers, with the inner layer containing a coding peptide (Dde protected amino acids) and the outer layer composed of the cyclic γ-AApeptide ligand (γ-AApeptide). This was achieved by soaking the beads in water overnight, and then the resin was quickly washed with 1:1 (v/v) ether/DCM solution, which theoretically established an aqueous inner core and an organic-solvent-occupied outer shell on the beads. (Lam et al., 1991) A solution of (Boc)2O (0.5 equiv.) in 1:1 (v/v) DCM/diethyl ether was added to react with the free NH2 group of the outer layer. After shaking for 3 hours, the solvent was drained and the resin was washed with DCM (3×), DMF (3×). Next, Fmoc-Met-OH (0.5 equiv.) was then added together with HOBt (2 equiv) and DIC (2 equiv) to react with the inner layer of the resin. The Fmoc group was removed with 20 % piperidine in DMF (2 × 10 min). Subsequently, the beads were split into 5 portions, and each aliquot reacted with one of the 5 different Dde protected amino acids, Dde-Ala-OH, Dde-Phe-OH, Dde-Leu-OH, Dde-Val-OH, and Dde-Glu(OBn)-OH in 5 peptide synthesis vessels in the presence of PyBop (6 equiv) and NEM (6 equiv) in DMF for 3 hours, respectively. Subsequently, the Boc protecting group on the outer layer was removed with TFA/TIS (triisopropylsilane)/H2O/Thioanisole (94:2:2:2) for 1 hour, and the exposed amine was coupled with the corresponding γ-AApeptide building blocks/HOBt/DIC (2:4:4 equiv). Following that, the Alloc protecting group on the γ-AApeptide building blocks was removed by Pd(PPh3)4 (8 mg, 0.007 mmol) and Me2NH•BH3 (25 mg, 0.42 mmol) in 3 mL DCM (10 min × 2). After that the Dmt protected mercaptopropionic acid/HOBt/DIC (2:4:4 equiv) was added to react with the secondary amine. After all the beads were pooled and mixed thoroughly, the resin was then splited into 5 portions again. Next, the inner layer Dde group of was removed, 5 Dde protected amino acids were added subsequently to react as the previous step (Scheme S1). The Dde deprotection solution was prepared by suspending 1.25 g (0.180 mmol) of NH2OH•HCl and 0.918 g (0.135 mmol) of imidazole in 5 mL NMP, and the mixture was sonicated until complete dissolution. The solution was diluted with CH2Cl2 before use (5:1, v:v)(Díaz-Mochón et al., 2004). Then the Fmoc group of the outer layer was removed and was coupled with desired 5 different γ-AApeptide building blocks. The beads were pooled and split again, and the synthetic cycle was repeated three more times. At last, the terminal Dde protecting group was remained on the sequence, while the Fmoc group of the outer layer was removed and the N-terminal were capped with the 4-(bromomethyl)benzoyl chloride/DIPEA (2:4 equiv). Following that, the Dmt group was removed by CH2Cl2/TFA/triisopropylsilane (96:2:2) for 2 min (×5) until the deprotecting solution turned to colorless. The cyclization of γ-AApeptide was achieved by adding the solution of (NH4)2CO3 (10 equiv) in 1:1 (v/v) DMF/H2O to the resin and shaking the mixture for 8 h. The resin was washed with DMF (3×) and DCM (3×). Finally, protecting groups on the sidechains were removed with TFA/TIS (triisopropylsilane)/H2O/Thioanisole (94:2:2:2) for 1 h.
Unless otherwise noted, the Fmoc protecting group was removed by 20% (v/v) piperidine in DMF (10 min × 2). The Alloc protecting group was removed by Pd(PPh3)4 (8 mg, 0.007 mmol) and Me2NH•BH3 (25 mg, 0.42 mmol) in 3 mL DCM (10 min × 2). All the Alloc protected γ-AApeptide building blocks (2 equiv) and carboxylic acids (2 equiv) were coupled to desired amino groups on the solid phase using HOBt/DIC (4:4 equiv.) as the coupling reagents in DMF for 6 h twice. All the acyl chlorides (2 equiv.) were coupled to desired amino groups in DCM containing DIPEA (4 equiv.) for 30 min twice. The Dde protected amino acids (2 equiv.) were coupled to the desired amino groups in DMF for 3 h in the presence of PyBop (6 equiv.) and NEM (6 equiv) (Díaz-Mochón et al., 2004).
Library Screening
The screening of the cyclic γ-AApeptide library against GAS5 long non-coding RNA was performed by adapting previously reported protocols(Lian et al., 2014; Liu et al., 2002). The TentaGel beads (4.2g, 643,000 beads, 214,375 compounds) were swelled in DMF for 1 h, followed wash with Tris buffer for five times, then the beads were equilibrated in Tris buffer overnight at room temperature. After incubating the beads with the blocking buffer (1% BSA in Tris buffer) for 1 h, the resin was washed 5 times with Tris buffer. Next, the beads were incubated with the last 111 bps of GAS5 with FITC labeling at the 5’ (Flu- CUCCC AGUGG UCUUU GUAGA CUGCC UGAUG GAGUC UCAUG GCACA AGAAG AUUAA AACAG UGUCU CCAAU UUUAA UAAAU UUUUG CAAUC CAAAA AAAAA AAAAA AAAAA A) at a concentration of 20 nM for 4 h at room temperature in the presence of 25 μL of 10 mg/mL t-RNA (~1000 fold). The beads were washed and then transferred into the 6-well plate with Tris buffer, and observed under the Zeiss inverted fluorescence microscope. The beads emitting green fluorescence were picked up as putative hits for further validation (Figure S1). These hits were separately transferred to the Eppendorf microtubes, and each bead was stripped of bound Gas5 RNA by incubation in 100 μL 8M guanidine$HCl for 1h at room temperature, respectively. The beads were rinsed with Tris buffer 3 × 10 min, water 3 × 10 min, DMF 3 × 10 min, and ACN 3 × 10 min. At last the resin was placed in ACN overnight in each microtube and then ACN was evaporated. The inner layer decoding sequence was cleaved after soaking the bead overnight in the 50 mg/mL cyanogen bromide (CNBr) cocktail (ACN/ glacial acetic acid/H2O 5:4:1, v:v:v) at room temperature. The cleavage solution was allowed to evaporate, and the cleaved peptide was dissolved in 10 μL ACN/H2O (4:1, v:v) and subject to MALDI TOF-TOF analysis.
Synthesis of the FITC-Labeled Hits
The synthesis of the FITC-labeled hits was conducted on the Rink amide resin. After the Fmoc protecting group of the Rink amide was removed, the Fmoc-Lys(Dde)-OH was first attached the solid support, followed by the coupling of the β-Ala linker. Next, the desired building blocks which were determined by the analysis of MALDI MS/MS were subsequently coupled to the resin under the standard peptide coupling conditions. After the cyclization was achieved, the Dde group of the Fmoc-Lys(Dde)-OH was removed, then the exposed amine was coupled with 5,6-carboxyfluorescein (2 equiv.) and DIPEA (6 equiv.) in DMF by shaking the reaction for 12 h at room temperature. The resin was treated with 1:1 (v/v) DCM/TFA containing 2% triisopropylsilane to cleave off FITC labeled cyclic γ-AApeptide. The crude product was purified by the Waters HPLC system with flow rate of 1 mL/min for analytic module and 16 mL/min for preparative module with a linear gradient from 5% to 100% (CH3CN in water) in 40 min.
Fluorescence Polarization (FP)
The binding affinity (Kd) of the hits identified from library screening was obtained by fluorescence polarization (FP). The FP experiment was performed by incubating 50 nM FITC labeled AApeptide with the 111-bps GAS5 RNA (CUCCCAGUGGUCUUUGUAGACUGCCUGAUGGAGUCUCAUGGCACAAGAAGAUUAAAACAGUGUCUCCAAUUUUAAUAAAUUUUUGCAAUCCAAAAAAAAAAAAAAAAAAAA) (0.03125 to 0.5 μM) in 1×PBS. Dissociation constants (Kd) were determined by plotting the fluorescence anisotropy values as a function of protein concentration, and the plots were fitted to the following equation. The Lst is the concentration of the AApeptide and the x stands for the concentration of the protein. The experiments were conducted in triplicates and repeated for three times (Figures 4B and S5).
Synthesis, Purification and Analysis
Solid phase synthesis was conducted in peptide synthesis vessels on a Burrell Wrist-Action shaker. γ-AApeptides were analyzed and purified on a Waters Breeze 2 HPLC system, and then lyophilized on a Labcono lyophilizer. The purity of the compounds was determined to be >95% by analytical HPLC (Figure S3). Masses of γ-AApeptides and the MS/MS analysis were obtained on an Applied Biosystems 4700 Proteomics Analyzer (Figure S4).
QUANTIFICATION AND STATISTICAL ANALYSIS
All experiments were repeated 3–5 times to ensure reproducibility of results. Analyses were performed using PRISM™ software and analyzed using two-tailed Student’s t-test. * p < 0.05 was significant; ** p < 0.001 was highly significant; *** p < 0.001 was extremely significant. Analysis was performed either within group or between groups as determined by the experiment. The Kd data was fitted by the OriginPro and the equation below.
Supplementary Material
KEY RESOURCES TABLE.
| REAGENTor RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| RNA polymerase II antibody | Novus | Cat# NBP1-70155; RRID: AB_11014485 |
| SNRNP70 antibody | Novus/Millipore | Cat# 03-103; RRID: AB_11210069 |
| IgG antibody | Sigma-Aldrich | Cat# A9169; RRID: AB_258434 |
| UPF1 antibody | Novus | Cat# NB100-368; RRID: AB_2213199 |
| Biological Samples | ||
| White adipose tissue | Tampa General Hospital, Dr. Murr. Donors | N/A |
| Additional tissue | ZenBio™ | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Fmoc-protected amino acids | Chem-impex | N/A |
| TentaGel resin | RAPP Polymere | MB250002 |
| Rink Amide-MBHA resin | GL Biochem | N/A |
| 1-Hydroxybenzotriazole (HOBt) | Oakwood Chemical | Cat #24755 |
| 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) | Oakwood Chemical | Cat #024810 |
| N-ethylmorpholine (NEM) | Oakwood Chemical | A0358553 |
| 5,5-Dimethyl-1,3-cyclohexanedione | Oakwood Chemical | Cat #011151 |
| 4,4’-Dimethoxytrityl Chloride | Oakwood Chemical | Cat #00118 |
| 4-(Bromomethyl)benzoic acid | AK-Scientific | Cat # 6232-88-8 |
| 3-Mercaptopropionic Acid | Oakwood Chemical | Cat # 094485 |
| Benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate (PyBOP) | Oakwood Chemical | 009017 |
| Fluorescein isothiocyanate | Chemodex | Cat # 00860 |
| Erythrocyte lysis buffer | Stem Cell Technologies | N/A |
| ASC medium | ZenBio™ | Cat# PM-1 |
| Adipocyte Medium, AM1 | ZenBio™ | N/A |
| differentiation medium, DM2 | ZenBio™ | N/A |
| RNA-Bee™ | Tel Test, Inc | N/A |
| Nucleofector® solution | Lonza | N/A |
| Critical Commercial Assays | ||
| Omniscript™ kit | Qiagen | N/A |
| Glucose Uptake Fluorometric assay kit | Sigma | MAK084 |
| Biotin Chromogenic Detection kit | ThermoFisher | N/A |
| Experimental Models: Cell Lines | ||
| adipose stem cells (ASC) | ZenBio™ | N/A |
| Oligonucleotides | ||
| GAS5 sense primer 5’- CTTCTGGGCTCAAGTGATCCT -3’ anti-sense 5’ TTGTGCCATGAGACTCCATCAG -3’ | This paper | N/A |
| IR-A sense primer 5’- GTTTTCGTCCCCAGGCCATC -3’ and anti-sense 5’ CCAACATCGCCAAGGGACCT -3’ | This paper | N/A |
| IR-B sense primer 5’- CACTGGTGCCGAGGACCCTA -3’ and anti-sense 5’ GACCTGCGTTTCCGAGATGG -3’ | This paper | N/A |
| IR promoter set 1 sense primer 5’- AGATCTCTGGCCATTGC ACTCCAG -3’ and anti-sense 5’ TTCAATAAACAGTTTGCTA GGAGC -3’ | This paper | N/A |
| IR promoter set 2 sense primer 5’- TCGAGTCACCAAAATAAACAT -3’ and anti-sense 5’ TGCAGGGGAGGGAGGTGCCGC -3’ | This paper | N/A |
| GAPDH sense primer 5’- TGACGTGCCGCCTGGAGAAAC -3’ and anti-sense 5’- CCGGCATCGAAGGTGGAAGAG -3’; | This paper | N/A |
| CAT sense: 5’ GCCGCTGGCGATTCAG 3’ and antisense 5’ TTCATTAAGCATTCTGCCGACAT 3’ | This paper | N/A |
| UPF1 sense: 5’ AGATCACGGCACAGCAGAT 3’ and antisense 5’ TGGCAGAAGGGTTTTCCTT 3’ | This paper | N/A |
| U1snRNA sense: 5’ TCCCAGGGCGAGGCTTATCCATT 3’ and antisense 5’ GAACGCAGTCCCCCACTACCACAAAT 3’ | This paper | N/A |
| Software and Algorithms | ||
| Compass software | ProteinSimple | https://www.proteinsimple.com/compass/downloads/ |
| Excel™ | Microsoft Office | https://office.microso1tcom/excel/ |
| PRISM™ | Graphpad Software | https://www.graphpad.com/scientific-software/prism/ |
| OriginPro | OriginLab | https://www.originlab.com/ |
Highlights.
ncRNA GAS5 regulates expression of insulin receptor in adipocytes
NP-C86 increases GAS5 levels and glucose uptake in diabetic adipocytes
NP-C86 is specific, non-toxic with high potential as a ncRNA targeted therapeutic
NP-C86 may be used as a molecular probe for GAS5 in relevant biological systems
ACKNOWLEDGMENT
This work was supported by NSF CAREER 1351265 (to J.C.) and NIH 1R01GM112652 (to J.C.), and VAMR I01BX003836 (to N.A.P.). This work does not reflect the view or opinion of the James A. Haley VA Hospital nor the US Government.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing financial interest.
SUPPLEMENTAL INFORMATION
Supplemental Information includes five figures and one scheme and can be found with this article online at https://doi.org/10.1016/j.chembiol.2018.11.012.
REFERENCES
- Azzalin CM (2012). UPF1: a leader at the end of chromosomes. Nucleus 3, 16–21. [DOI] [PubMed] [Google Scholar]
- Azzalin CM, and Lingner J (2006). The human RNA surveillance factor UPF1 is required for S phase progression and genome stability. Curr. Biol. 16, 433–439. [DOI] [PubMed] [Google Scholar]
- Bian D, Shi W, Shao Y, Li P, and Song G (2017). Long non-coding RNA GAS5 inhibits tumorigenesis via miR-137 in melanoma. Am. J. Transl. Res. 9, 1509–1520. [PMC free article] [PubMed] [Google Scholar]
- Bluher M, Michael MD, Peroni OD, Ueki K, Carter N, Kahn BB, and Kahn CR (2002). Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev. Cell 3, 25–38. [DOI] [PubMed] [Google Scholar]
- Carter G, Miladinovic B, Patel AA, Deland L, Mastorides S, and Patel NA (2015). Circulating long noncoding RNA GAS5 levels are correlated to prevalence of type 2 diabetes mellitus. BBA Clin. 4, 102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Rentero Rebollo I, Buth SA, Morales-Sanfrutos J, Touati J, Leiman PG, and Heinis C (2013). Bicyclic peptide ligands pulled out of cysteine-rich peptide libraries. J. Am. Chem. Soc. 135, 6562–6569. [DOI] [PubMed] [Google Scholar]
- Coccia EM, Cicala C, Charlesworth A, Ciccarelli C, Rossi GB, Philipson L, and Sorrentino V (1992). Regulation and expression of a growth arrest-specific gene (gas5) during growth, differentiation, and development. Mol. Cell. Biochem. 12, 3514–3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Mochón JJ, Bialy L, and Bradley M (2004). Full orthogonality between Dde and Fmoc: the direct synthesis of PNA-peptide conjugates. Org. Lett. 6, 1127–1129. [DOI] [PubMed] [Google Scholar]
- Ding GL, Wang FF, Shu J, Tian S, Jiang Y, Zhang D, Wang N, Luo Q, Zhang Y, Jin F, et al. (2012). Transgenerational glucose intolerance with Igf2/H19 epigenetic alterations in mouse islet induced by intrauterine hyperglycemia. Diabetes 61, 1133–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Wang M, Sun D, and Li A (2018). TPGLDA: novel prediction of associations between lncRNAs and diseases via lncRNA-disease-gene tripartite graph. Sci. Rep. 8, 1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmendorf JS (2003). Fractionation analysis of the subcellular distribution of GLUT-4 in 3T3-L1 adipocytes. Methods Mol. Med. 83, 105–111. [DOI] [PubMed] [Google Scholar]
- Heinis C, Rutherford T, Freund S, and Winter G (2009). Phage-encoded combinatorial chemical libraries based on bicyclic peptides. Nat. Chem. Biol. 5, 502–507. [DOI] [PubMed] [Google Scholar]
- Jin F, Wang N, Zhu Y, You L, Wang L, De W, and Tang W (2017). Downregulation of long noncoding RNA Gas5 affects cell cycle and insulin secretion in mouse pancreatic beta cells. Cell. Physiol. Biochem. 43, 2062–2073. [DOI] [PubMed] [Google Scholar]
- Kaniraj PJ, and Maayan G (2015). A facile strategy for the construction of cyclic peptoids under microwave irradiation through a simple substitution reaction. Org. Lett. 17, 2110–2113. [DOI] [PubMed] [Google Scholar]
- Kawamura A, Munzel M, Kojima T, Yapp C, Bhushan B, Goto Y, Tumber A, Katoh T, King ON, Passioura T, et al. (2017). Highly selective inhibition of histone demethylases by de novo macrocyclic peptides. Nat. Commun. 8, 14773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kino T, Hurt DE, Ichijo T, Nader N, and Chrousos GP (2010). Noncoding RNA gas5 is a growth arrestand starvation-associated repressor of the glucocorticoid receptor. Sci. Signal. 3, ra8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Kahn CR, and Accili D (2003). Insulin receptor knockout mice. Annu. Rev. Physiol. 65, 313–332. [DOI] [PubMed] [Google Scholar]
- Kleiman E, Carter G, Ghansah T, Patel NA, and Cooper DR (2009). Developmentally spliced PKCbetaII provides a possible link between mTORC2 and Akt kinase to regulate 3T3-L1 adipocyte insulin-stimulated glucose transport. Biochem. Biophys. Res. Commun. 388, 554–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam KS, Salmon SE, Hersh EM, Hruby VJ, Kazmierski WM, and Knapp RJ (1991). A new type of synthetic peptide library for identifying ligand-binding activity. Nature 354, 82–84. [DOI] [PubMed] [Google Scholar]
- Lee KJ, and Lim H-S (2014). Facile method to sequence cyclic peptides/peptoids via one-pot ring-opening/cleavage reaction. Org. Lett. 16, 5710–5713. [DOI] [PubMed] [Google Scholar]
- Li M, Xie Z, Wang P, Li J, Liu W, Tang S, Liu Z, Wu X, Wu Y, and Shen H (2018). The long noncoding RNA GAS5 negatively regulates the adipogenic differentiation of MSCs by modulating the miR-18a/CTGF axis as a ceRNA. Cell Death Dis. 9, 554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian W, Jiang B, Qian Z, and Pei D (2014). Cell-permeable bicyclic peptide inhibitors against intracellular proteins. J. Am. Chem. Soc. 136, 9830–9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Marik J, and Lam KS (2002). A novel peptide-based encoding system for “one-bead one-compound” peptidomimetic and small molecule combinatorial libraries. J. Am. Chem. Soc. 124, 7678–7680. [DOI] [PubMed] [Google Scholar]
- Mourtada-Maarabouni M, Pickard MR, Hedge VL, Farzaneh F, and Williams GT (2009). GAS5, a non-protein-coding RNA, controls apoptosis and is downregulated in breast cancer. Oncogene 28, 195–208. [DOI] [PubMed] [Google Scholar]
- Pasmant E, Sabbagh A, Vidaud M, and Bieche I (2011). ANRIL, a long, noncoding RNA, is an unexpected major hotspot in GWAS. FASEB J. 25, 444–448. [DOI] [PubMed] [Google Scholar]
- Pickard MR, Mourtada-Maarabouni M, and Williams GT (2013). Long non-coding RNA GAS5 regulates apoptosis in prostate cancer cell lines. Biochim. Biophys. Acta 1832, 1613–1623. [DOI] [PubMed] [Google Scholar]
- Pickard MR, and Williams GT (2014). Regulation of apoptosis by long noncoding RNA GAS5 in breast cancer cells: implications for chemotherapy. Breast Cancer Res. Treat. 145, 359–370. [DOI] [PubMed] [Google Scholar]
- Qi L, Qi Q, Prudente S, Mendonca C, Andreozzi F, di Pietro N, Sturma M, Novelli V, Mannino GC, Formoso G, et al. (2013). Association between a genetic variant related to glutamic acid metabolism and coronary heart disease in individuals with type 2 diabetes. JAMA 310, 821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Z, Xu X, Amacher JF, Madden DR, Cormet-Boyaka E, and Pei D (2015). Intracellular delivery of peptidyl ligands by reversible cyclization: discovery of a PDZ domain inhibitor that rescues CFTR activity. Angew. Chem. Int. Ed. 54, 5874–5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Yao J, Geng P, Fu X, Xue J, and Zhang Z (2014). LncRNA TSLC1-AS1 is a novel tumor suppressor in glioma. Int. J. Clin. Exp. Pathol. 7, 3065–3072. [PMC free article] [PubMed] [Google Scholar]
- Raho G, Barone V, Rossi D, Philipson L, and Sorrentino V (2000). The gas 5 gene shows four alternative splicing patterns without coding for a protein. Gene 256, 13–17. [DOI] [PubMed] [Google Scholar]
- Schmidt LH, Gorlich D, Spieker T, Rohde C, Schuler M, Mohr M, Humberg J, Sauer T, Thoenissen NH, Huge A, et al. (2014). Prognostic impact of Bcl-2 depends on tumor histology and expression of MALAT-1 lncRNA in non-small-cell lung cancer. J. Thorac. Oncol. 9, 1294–1304. [DOI] [PubMed] [Google Scholar]
- Shi Y, Challa S, Sang P, She F, Li C, Gray GM, Nimmagadda A, Teng P, Odom T, Wang Y, et al. (2017). One-bead-two-compound thioether bridged macrocyclic gamma-AApeptide screening library against EphA2. J. Med. Chem. 60, 9290–9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Teng P, Sang P, She F, Wei L, and Cai J (2016). γ-AApeptides: design, structure, and applications. Acc. Chem. Res. 49, 428–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedley D, Sidhar S, Birdsall S, Bennett D, Herlyn M, Cooper C, and Shipley J (2000). Characterization of chromosome 1 abnormalities in malignant melanomas. Genes Chromosomes Cancer 28, 121–125. [DOI] [PubMed] [Google Scholar]
- Smith CM, and Steitz JA (1998). Classification of gas5 as a multi-small-nucleolar-RNA (snoRNA) host gene and a member of the 5’-terminal oligopyrimidine gene family reveals common features of snoRNA host genes. Mol. Cell. Biochem. 18, 6897–6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani H, Imamachi N, Salam KA, Mizutani R, Ijiri K, Irie T, Yada T, Suzuki Y, and Akimitsu N (2012). Identification of hundreds of novel UPF1 target transcripts by direct determination of whole transcriptome stability. RNA Biol. 9, 1370–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani H, Torimura M, and Akimitsu N (2013). The RNA degradation pathway regulates the function of GAS5 a non-coding RNA in mammalian cells. PLoS One 8, e55684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng P, Shi Y, Sang P, and Cai J (2016). gamma-AApeptides as a new class of peptidomimetics. Chemistry 22, 5458–5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng P, Zhang X, Wu H, Qiao Q, Sebti SM, and Cai J (2014). Identification of novel inhibitors that disrupt STAT3-DNA interaction from a gamma-AApeptide OBOC combinatorial library. Chem. Commun. (Camb.) 50, 8739–8742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyaya P, Qian Z, Selner NG, Clippinger SR, Wu Z, Briesewitz R, and Pei D (2015). Inhibition of Ras signaling by blocking Ras-effector interactions with cyclic peptides. Angew. Chem. Int. Ed. 54, 7602–7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JE, Patel NA, Carter G, Moor A, Patel R, Ghansah T, Mathur A, Murr MM, Bickford P, Gould LJ, et al. (2014). Comparison of markers and functional attributes of human adipose-derived stem cells and dedifferentiated adipocyte cells from subcutaneous fat of an obese diabetic donor. Adv. Wound Care (New Rochelle) 3, 219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster NJ, Resnik JL, Reichart DB, Strauss B, Haas M, and Seely BL (1996). Repression of the insulin receptor promoter by the tumor suppressor gene product p53: a possible mechanism for receptor overexpression in breast cancer. Cancer Res. 56, 2781–2788. [PubMed] [Google Scholar]
- Williams GT, Mourtada-Maarabouni M, and Farzaneh F (2011). A critical role for non-coding RNA GAS5 in growth arrest and rapamycin inhibition in human T-lymphocytes. Biochem. Soc. Trans. 39, 482–486. [DOI] [PubMed] [Google Scholar]
- Wu H, Li Y, Bai G, Niu Y, Qiao Q, Tipton JD, Cao C, and Cai J (2014). gamma-AApeptide-based small-molecule ligands that inhibit Abeta aggregation. Chem. Commun. (Camb.) 50, 5206–5208. [DOI] [PubMed] [Google Scholar]
- Yacqub-Usman K, Pickard MR, and Williams GT (2015). Reciprocal regulation of GAS5 lncRNA levels and mTOR inhibitor action in prostate cancer cells. Prostate 75, 693–705. [DOI] [PubMed] [Google Scholar]
- Yin DD, Zhang EB, You LH, Wang N, Wang LT, Jin FY, Zhu YN, Cao LH, Yuan QX, De W, et al. (2015). Downregulation of lncRNA TUG1 affects apoptosis and insulin secretion in mouse pancreatic beta cells. Cell. Physiol. Biochem. 35, 1892–1904. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Ye Y, and Zhao SJ (2018). LncRNA Gas5 acts as a ceRNA to regulate PTEN expression by sponging miR-222–3p in papillary thyroid carcinoma. Oncotarget 9, 3519–3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhu Z, Watabe K, Zhang X, Bai C, Xu M, Wu F, and Mo YY (2013). Negative regulation of lncRNA GAS5 by miR-21. Cell Death Differ. 20, 1558–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Li T, Qi J, Liu J, and Qin C (2014). The miR-545/374a cluster encoded in the Ftx lncRNA is overexpressed in HBV-related hepatocellular carcinoma and promotes tumorigenesis and tumor progression. PLoS One 9, e109782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Wang P, Liu J, Zheng J, Liu Y, Chen J, and Xue Y (2015). Gas5 exerts tumor-suppressive functions in human glioma cells by targeting miR-222. Mol. Ther. 23, 1899–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Chen Q, Liu X, Sun Q, Zhao X, Deng R, Wang Y, Huang J, Xu M, Yan J, et al. (2014). lncRNA H19/miR-675 axis represses prostate cancer metastasis by targeting TGFBI. FEBS J. 281, 3766–3775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


