Highlights
-
•
COL1A1::PDGFB fusion uterine sarcomas are rare tumors.
-
•
These sarcomas have nonspecific clinical presentation and histologic appearance.
-
•
Accurate diagnosis relies on identification of the characteristic fusion.
-
•
This tumor may potentially respond to targeted therapy with imatinib.
Keywords: COL1A1::PDGFB fusion, Imatinib, Uterine sarcoma
Abstract
Uterine sarcomas are rare neoplasms of the uterus, some of which are associated with distinctive gene fusions. COL1A1::PDGFB fusion uterine sarcoma is a recently described entity that shares the same genetic alteration as dermatofibrosarcoma protuberans. These uterine sarcomas have a nonspecific spindle cell sarcoma appearance and are CD34 positive by immunohistochemistry. Accurate diagnosis relies on identification of the characteristic fusion by molecular genetic methods. The importance of diagnosing this entity lies in its potential response to targeted therapy with imatinib, a tyrosine kinase inhibitor successfully used in dermatofibrosarcoma protuberans, but only one prior case of COL1A1::PDGFB fusion uterine sarcoma treated with imatinib has been reported. Here, we describe a case of COL1A1::PDGFB fusion uterine sarcoma with response to imatinib after recurrence, with a brief review of this rare tumor.
1. Introduction
Uterine sarcomas are rare but aggressive neoplasms of the female genital tract that comprise less than 10% of uterine malignancies (Giannini, et al., 2023). The most common uterine sarcomas are leiomyosarcomas, followed by endometrial stromal sarcomas, and undifferentiated sarcomas (Giannini, et al., 2023). The current molecular era in diagnostics has defined novel uterine sarcomas that bear characteristic gene fusions, which may have previously been categorized as undifferentiated sarcomas (a diagnosis of exclusion). Many of these tumors have fibrosarcoma-like morphology and overlapping immunophenotypes, including NTRK-rearranged sarcomas, RET fusion sarcomas, FGFR1::TACC1 fusion sarcomas, and COL1A1::PDGFB fusion sarcomas (Croce, 2019, Croce, 2021, Devereaux, 2021).
COL1A1::PDGFB uterine sarcoma was first described in 2019. It shares the same gene fusion with dermatofibrosarcoma protuberans (DFSP), and as such may be amenable to treatment with the tyrosine kinase inhibitor imatinib. To date, seven cases of COL1A1::PDGFB uterine sarcomas have been reported (Croce, 2019, Hogeboom, 2023, Grindstaff, 2019, Lu, 2023, Panwar, 2023). Here, we present the eighth case of COL1A1::PDGFB fusion uterine sarcoma, and the second report to include treatment results with imatinib. This case highlights the importance of molecular characterization of uterine sarcomas in instances where morphology and immunophenotype fail to provide a definitive diagnosis.
2. Case presentation
A 43-year-old Black woman presented to the gynecology clinic with a one year history of increasing dysmenorrhea and menorrhagia. The patient had a supposed uterine fibroid diagnosed on ultrasound at an outside hospital three years prior to this presentation. Her pelvic exam was significant for an enlarged uterus (18–20-week size) with a mass extending into the introitus, displacing the bladder superiorly.
A pelvic ultrasound and computed tomography (CT) scan showed a large, heterogeneously-enhancing 10.6 cm mass located around the lower uterine segment, abutting the rectum and urinary bladder without invasion (Fig. 1A). An attempt at an endometrial/uterine biopsy was unsuccessful as the cervix was not visualized. The patient subsequently underwent a total abdominal hysterectomy with bilateral salpingo-oophorectomy. Intraoperatively, a 12 × 12 cm friable pelvic mass was seen anterior to the cervix, involving the upper vagina and the right and left pelvic sidewalls. The urinary bladder appeared attenuated over the mass with possible erosion into the bladder serosa. The bilateral fallopian tubes and ovaries appeared normal, and were removed along with the uterus and cervix, separate from the main pelvic mass. The mass was dissected off of the pelvic sidewalls,and an upper vaginectomy was performed, with no gross residual tumor seen at the end of surgery.
Fig. 1.
CT imaging findings. A) Preoperative CT scan demonstrating the tumor in the pelvis, associated with the lower uterus (yellow arrow). An intrauterine device is present in the uterine fundus. B) CT scan two months post-surgery, with thickening of the vaginal cuff (yellow arrow). C) CT scan showing decreased vaginal cuff fullness and near complete resolution of peritoneal nodularity post-imatinib treatment. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
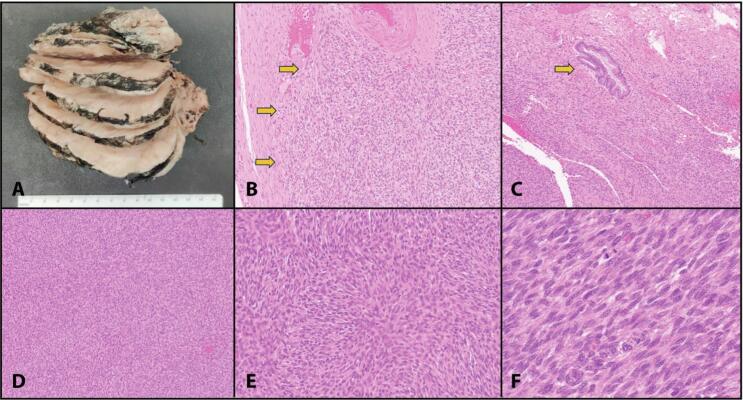
On gross pathologic examination, there were multiple fragments of tumor tissue, including a 12.0 × 9.5 × 5.5 cm mass with a tan-pink homogeneous cut surface (Fig. 2A). Necrosis and hemorrhage were absent. The separate uterus had a cauterized and roughened distal surface from where the mass was resected. No distinct cervix was identifiable grossly.
Fig. 2.
Gross and microscopic examination of COL1A1::PDGFB fusion-associated uterine sarcoma. A) Gross image of the main pelvic mass specimen. Tumor shows a tan-pink homogeneous cut surface without necrosis or hemorrhage. B) Infiltrative, poorly defined border (yellow arrows); H&E image at 100X original magnification. C) Tumor in relation to endocervical glands (yellow arow); H&E image at 100X. D) Fascicular architecture of tumor; H&E image at 40X magnification. E) Area with storiform architecture, H&E image at 100X magnification. F) Spindle cells with ovoid nuclei and coarse chromatin, H&E image at 200X magnification. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Microscopic examination revealed a spindle cell neoplasm with infiltrating borders invading into endocervical stroma (Fig. 2B-2C). The tumor was arranged predominantly in fascicles, with different areas demonstrating storiform and herringbone patterns (Fig. 2D-2E). Individual tumor cells showed mild to moderate atypia and had scant to moderate amounts of clear to eosinophilic cytoplasm with indistinct cytoplasmic borders (Fig. 2F). The nuclei were ovoid and spindle-shaped with coarse chromatin and indistinct nucleoli. Mitotic counts ranged from 12 to 22/10 high power fields. There was no lymphocytic infiltration. Lymphovascular invasion was not identified.
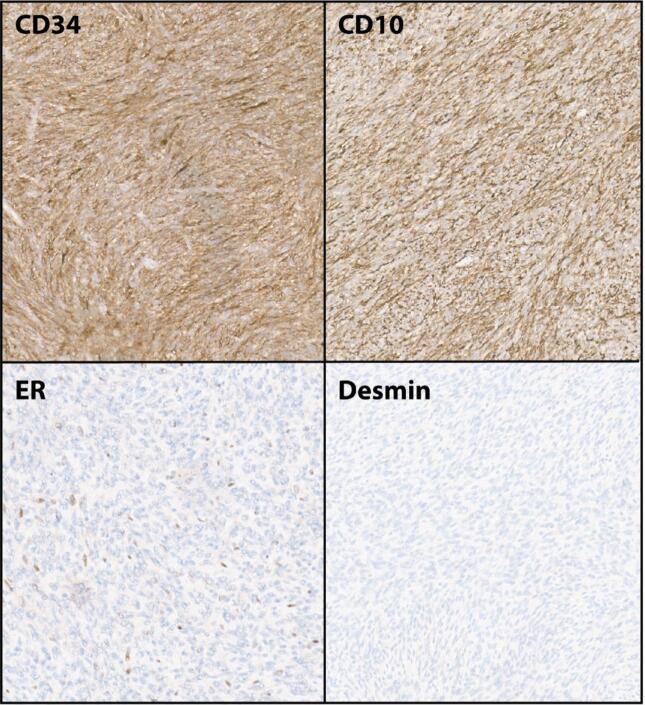
A panel of immunostains was performed to further characterize this tumor. Pax-8 was negative. The neoplasm was diffusely positive for CD10 and CD34 while negative for smooth muscle markers (smooth muscle actin, caldesmon, myogenin, and desmin) suggesting a non-myomatous origin (Fig. 3). Immunostains for estrogen and progesterone receptors showed strong nuclear reactivity in 10% of tumor cells. Cyclin D1 was trace positive. Uterine and pelvic tumors with diffuse CD34 positivity include solitary fibrous tumor and extra-intestinal gastrointestinal stromal tumor (GIST), which we ruled out with STAT, BCL2, DOG1, and CD117 immunostains, which were negative. S100 and HMB45 were performed for consideration of PEComa, but were also negative.
Fig. 3.
Representative immunohistochemical staining in COL1A1::PDGFB uterine sarcoma. Images show diffusely positive (brown) staining for CD34 and CD10, with focal nuclear staining for ER. Desmin is negative. Images at 100X original magnification.
Since the tumor showed positive CD10 IHC, with focal ER, PR, and cyclin D1, we considered endometrial stromal sarcoma. Fluorescence in situ hybridization (FISH) for rearrangement of JAZF1, PHF1, and/or YWHAE was negative. Finally, comprehensive genomic profiling by next generation sequencing (NGS) was ordered (Tempus xT assay, Tempus, Chicago, IL). RNA NGS showed a COL1A1::PDGFB gene fusion, supporting a diagnosis of COL1A1::PDGFB fusion sarcoma. Notably, transcriptome analysis also showed overexpression of PDGFRB.
The patient’s immediate postoperative course was uneventful. Two months following surgery, an MRI scan showed a 2.1 cm mass involving the right vaginal cuff representing recurrent/progressive disease (Fig. 1B). The patient was started on doxorubicin, and showed initial response. Nonetheless, two months after initiating chemotherapy, further tumor progression was noted on imaging with thickening of the vaginal cuff and peritoneal nodules. At this point, based on the molecular profile of the tumor showing COL1A1::PDGFB fusion, the patient was started on 400 mg imatinib dosed twice daily. Three months following imatinib therapy, imaging showed decreased fullness of vaginal cuff and near-complete resolution of peritoneal nodularities (Fig. 1C).
The patient tolerated imatinib treatment well with minimal side effects. At the time of this report, she is eight months post-surgery and is in the fourth month of continuing imatinib therapy. Her most recent follow-up imaging (eight months post-surgery) showed no evidence of disease progression.
3. Discussion
With the advent of sequencing technology, Croce et al initially determined uterine sarcomas bearing COL1A1::PDGFB fusion to be a distinct entity (Croce, 2019, Croce, 2021). The case described here, like four of the other seven previously reported cases, arose from the lower uterine segment/cervix in a premenopausal female. Limited experience to date with this neoplasm shows that it can progress even after surgery and chemotherapy, resulting in pelvic, peritoneal and pulmonary nodules and vaginal recurrences (Rutkowski, 2011, Mills, 2011). Our case showed initial progression while on treatment with doxorubicin, prior to imatinib therapy.
Shared similarities between cases published to date include spindle cell morphology in a storiform or fascicular pattern with infiltrative growth, with up to moderate nuclear atypia and diffuse CD34 positivity. The patchy/focal ER/PR staining and patchy cyclin D1 positivity reported here have also been described previously. In contrast to some prior reports in which CD10 was only focally positive or positive only in areas of atypia, our case additionally showed diffuse CD10 positivity (Grindstaff, 2019, Panwar, 2023). The current tumor lacked SMA positivity, but SMA was positive in two other reported cases (Hogeboom, 2023, Grindstaff, 2019). The morphology and immunohistochemical pattern of these tumors is not specific, increasing the diagnostic difficulty.
Definitive diagnosis requires identification of the COL1A1::PDGFB fusion. This fusion results in increased production of platelet derived growth factor B (PDGFB), the ligand for platelet derived growth factor receptor B (PDGFRB) which belongs to the family of receptor tyrosine kinases. This results in autocrine stimulation and cellular proliferation (Tomonari, et al., 2007). Imatinib is one of the tyrosine kinase inhibitors that is the gold standard in the treatment of inoperable, recurrent or metastatic DFSP. Owing to the identical characteristic gene fusion, and the success of imatinib in other malignancies with receptor tyrosine kinase activation, study of therapeutic role of imatinib in COL1A1::PDGFB uterine sarcomas is worthwhile.
One prior report showed that administration of imatinib resulted in only temporary benefit Grindstaff, 2019, Grindstaff, 2020; however, in that case the patient presented with stage IVA disease and was initially diagnosed with leiomyosarcoma before discovery of the COL1A1::PDGFB fusion. In the current case, the patient had stage IB disease, and imatinib therapy was started four months after surgical resection, at the time of initial recurrence. There was resolution of peritoneal nodules and decrease in size of vaginal involvement, with no progression four months afterbeginning imatinib therapy. While these findings are promising, it will be important to continue follow-up in this patient.
4. Conclusion
Our experience with this case and review of cases reported to date support the evidence that CD34 should be routinely included in the panel of immunostains used in evaluation of uterine sarcomas. In the presence of CD34 positivity and appropriate morphology, COL1A1::PDGFB fusion sarcoma should be considered in the differential diagnosis and molecular characterization performed where accessible. This is an important entity to diagnose since there are available kinase inhibitors that could prove beneficial in treatment. Long term follow-up of these treated patients is required to determine the effectiveness of this targeted therapy.
Consent.
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
CRediT authorship contribution statement
Udita Chapagain: Data curation, Visualization, Writing – original draft. Hannah R. Krigman: Investigation, Writing – review & editing. Ian S. Hagemann: Conceptualization, Writing – review & editing. Mia C. Weiss: Investigation, Writing – review & editing. Lulu Sun: Conceptualization, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Croce S., et al. Uterine and vaginal sarcomas resembling fibrosarcoma: a clinicopathological and molecular analysis of 13 cases showing common NTRK-rearrangements and the description of a COL1A1-PDGFB fusion novel to uterine neoplasms. Mod. Pathol. 2019;32(7):1008–1022. doi: 10.1038/s41379-018-0184-6. PMID: 30877273. [DOI] [PubMed] [Google Scholar]
- Croce S., et al. NTRK and other recently described kinase fusion positive uterine sarcomas: A review of a group of rare neoplasms. Genes Chromosom. Cancer. 2021;60(3):147–159. doi: 10.1002/gcc.22910. PMID: 33099837. [DOI] [PubMed] [Google Scholar]
- Devereaux K.A., et al. Neurofibrosarcoma Revisited: An Institutional Case Series of Uterine Sarcomas Harboring Kinase-related Fusions with Report of a Novel FGFR1-TACC1 Fusion. Am. J. Surg. Pathol. 2021;45(5):638–652. doi: 10.1097/PAS.0000000000001644. PMID: 33481389. [DOI] [PubMed] [Google Scholar]
- Giannini, A., et al., 2023. Uterine sarcomas: A critical review of the literature. European Journal of Obstetrics & Gynecology and Reproductive Biology, Volume 287,166-170, ISSN 0301-2115. [DOI] [PubMed]
- Grindstaff S.L., et al. COL1A1-PDGFB fusion uterine fibrosarcoma: A case report with treatment implication. Gynecol Oncol Rep. 2019;31 doi: 10.1016/j.gore.2019.100523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindstaff S.L., et al. COL1A1-PDGFB fusion uterine sarcoma and its response to Imatinib therapy. Gynecol Oncol Rep. 2020;34 doi: 10.1016/j.gore.2020.100653. PMID: 33364286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogeboom A., et al. A Case of COL1A1-PDGFB Fusion Uterine Sarcoma. Int. J. Gynecol. Pathol. 2023;42(2):147–150. doi: 10.1097/PGP.0000000000000875. PMID: 35551153. [DOI] [PubMed] [Google Scholar]
- Lu L., et al. Case Report: A case of COL1A1-PDGFB fusion uterine sarcoma at cervix and insights into the clinical management of rare uterine sarcoma. Front. Oncol. 2023;13:1108586. doi: 10.3389/fonc.2023.1108586. PMID: 36994196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills A.M., et al. Endocervical fibroblastic malignant peripheral nerve sheath tumor (neurofibrosarcoma): report of a novel entity possibly related to endocervical CD34 fibrocytes. Am. J. Surg. Pathol. 2011;35(3):404–412. doi: 10.1097/PAS.0b013e318208f72e. PMID: 21317712. [DOI] [PubMed] [Google Scholar]
- Panwar V., et al. COL1A1-PDGFB Fusion Associated Fibrosarcoma of the Uterine Corpus: A Case Report and Literature Review. Int. J. Gynecol. Pathol. 2023;42(2):143–146. doi: 10.1097/PGP.0000000000000850. PMID: 36729934. [DOI] [PubMed] [Google Scholar]
- Rutkowski P., et al. Advances in molecular characterization and targeted therapy in dermatofibrosarcoma protuberans. Sarcoma. 2011;2011 doi: 10.1155/2011/959132. PMID: 21559214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomonari, T., et al., 2007. Detection of COL1A1-PDGFB fusion transcripts and PDGFB/PDGFRB mRNA expression in dermatofibrosarcoma protuberans. Mod. Pathol. Volume 20, Issue 6, 668-675, ISSN 0893-3952, https://doi.org/10.1038/modpathol.3800783. [DOI] [PubMed]