Abstract
Increasing evidence shows that metabolic factors are involved in the pathological process of osteoarthritis (OA). Lactate has been shown to contribute to the onset and progression of diseases. While whether lactate is involved in the pathogenesis of OA through impaired chondrocyte function and its mechanism remains unclear. This study confirmed that serum lactate levels were elevated in OA patients compared to healthy controls and were positively correlated with synovial fluid lactate levels, which were also correlated with fasting blood glucose, high-density lipoprotein, triglyceride. Lactate treatment could up-regulate expressions of the lactate receptor hydroxy-carboxylic acid receptor 1 (HCAR1) and lactate transporters in human chondrocytes. We demonstrated the dual role of lactate, which as a metabolite increased NADPH levels by shunting glucose metabolism to the pentose phosphate pathway, and as a signaling molecule up-regulated NADPH oxidase 4 (NOX4) via activating PI3K/Akt signaling pathway through receptor HCAR1. Particularly, lactate could promote reactive oxygen species (ROS) generation and chondrocyte damage, which was attenuated by pre-treatment with the NOX4 inhibitor GLX351322. We also confirmed that lactate could increase expression of catabolic enzymes (MMP-3/13, ADAMTS-4), reduce the synthesis of type II collagen, promote expression of inflammatory cytokines (IL-6, CCL-3/4), and induce cellular hypertrophy and aging in chondrocytes. Subsequently, we showed that chondrocyte damage mediated by lactate could be reversed by pre-treatment with N-Acetyl-l-cysteine (NAC, ROS scavenger). Finally, we further verified in vivo that intra-articular injection of lactate in Sprague Dawley (SD) rat models could damage cartilage and exacerbate the progression of OA models that could be countered by the NOX4 inhibitor GLX351322. Our study highlights the involvement of lactate as a metabolic factor in the OA process, providing a theoretical basis for potential metabolic therapies of OA in the future.
Keywords: Lactate, Osteoarthritis, Chondrocytes, NADPH oxidase 4
Highlights
-
•
Elevated serum lactate in OA patients compared to healthy controls.
-
•
The dual role of lactate, as a metabolite increasing NADPH levels by shunting glucose metabolism to the pentose phosphate pathway, and as a signaling molecule up-regulating NADPH oxidase 4 (NOX4) via activating PI3K/Akt signaling pathway through receptor HCAR1 in human chondrocytes.
-
•
Lactate promoted ROS generation and chondrocyte damage, which was attenuated by NOX4 inhibitor GLX351322.
-
•
Lactate induced cartilage damage in animal models in vivo and aggravated the progression of OA models that can be countered by GLX351322.
Abbreviations
- OA
osteoarthritis
- MCT
monocarboxylate transporter
- MMP
matrix metalloproteinase
- ADAMTS
a disintegrin and metalloproteinase with thrombospondin motifs
- IL
interleukin
- CCL
C–C motif chemokine ligand
- SA-β-Gal
senescence-associated β-galactosidase
- ROS
reactive oxygen species
- NAD
nicotinamide adenine dinucleotide
- NADP
nicotinamide adenine dinucleotide phosphate
- NAC
N-Acetyl-l-cysteine
- NOX
NADPH oxidase
- 2-DG
2-deoxy-d-glucose
- HCAR1
hydroxy-carboxylic acid receptor 1
- NS
normal saline
- OARSI
Osteoarthritis Research Society International
1. Introduction
Osteoarthritis (OA) is a common disabling disease that occurs in middle-aged and elderly people, which seriously affects the quality of life of the affected population. Despite about 7% of the global population currently affected [1], the pathogenesis of OA remains poorly understood. New findings related to the pathology of OA have prompted its classification into distinct phenotypes, including Inflammatory, post-traumatic, aging, genetic, pain, and metabolic [2,3]. Although metabolic OA has recently received extensive attention as a new OA subtype [4,5], evidence for the involvement of metabolic factors in OA pathogenesis is still limited.
For a long time after its discovery in the early 20th century, lactate was thought to be a waste product of anaerobic metabolism. With the introduction of the lactate shuttle theory, the function of lactate as a major energy source, a major gluconeogenic precursor, and a signaling molecule has been gradually emphasized [6,7]. The discovery of lactate transporters (monocarboxylate transporter 1–4, MCT1-4, SLC5A8, and SLC5A12) and lactate receptor (hydroxy-carboxylic acid receptor 1, HCAR1) also shed more light on the role of lactate between producer cells and consumer cells [8]. In arthritic joints (e.g., rheumatoid arthritis, RA), the accumulation of lactate affects the function of immune cells by interfering with metabolic pathways, providing a pathological basis for the occurrence of RA [9]. In an animal study, nuclear magnetic resonance spectroscopy analysis revealed elevated lactate levels in OA synovial fluid compared to normal synovial fluid, although not as extreme as in RA [10]. To date, there is still a lack of evidence that abnormal lactate levels affect chondrocyte functions in the chronic low-grade inflammatory microenvironment of OA.
OA is a degenerative joint disease, mainly characterized by progressive cartilage degradation. Oxidative stress mediated by increased reactive oxygen species (ROS) levels can disrupt cartilage homeostasis and lead to cartilage damage in osteoarthritis [11]. Under normal conditions, ROS are generated at low concentrations in articular chondrocytes, mainly by NADPH oxidases (NOXs), participating in intracellular signaling mechanisms, regulating cartilage metabolism, and cytokine production [12]. The PI3K/Akt signaling pathway is crucial in maintaining the normal function of chondrocytes, but abnormal activation is also involved in the occurrence and development of OA [13]. The PI3K/Akt signaling pathway has been confirmed to regulate ROS levels. The studies of Chatterjee and Chen found that PI3K inhibitors or Akt knockout could specifically down-regulate NOX activity and reduce ROS generation [14,15]. Usatyuk et al. revealed that blocking the PI3K/Akt pathway could reduce ROS generation by inhibiting NOX subunit translocation [16].
This study aims to explore whether lactate affects the function of OA chondrocytes and its underlying molecular mechanisms, providing evidence of metabolic factors involved in OA and new ideas for the prevention and treatment of OA.
2. Materials and methods
2.1. Bioinformatics analysis
The genomic expression profiling data screened by the GEO database were imported into Gene set enrichment analysis software (GSEA, version 4.1.0, San Diego, USA), and the Hallmarks gene sets and Kyoto Encyclopedia of Genes and Genomes (KEGG) gene sets were selected for enrichment analysis. Differential expressed genes (DEGs, adjust p value < 0.05) were obtained via the GEO2R online analysis tool. The top 250 most statistically significant DEGs were screened for protein-protein interaction (PPI) analysis using the STRING database (https://string-db.org) and data visualization using Cytoscape software (version 3.8.2, San Diego, USA). Volcano charts and bubble charts were drawn by Hiplot (https://hiplot.com.cn).
2.2. Patients and healthy controls
Patients with end-stage knee osteoarthritis (KOA, n = 40) who underwent total knee arthroplasty at the department of orthopedic surgery in the first hospital of Jilin University were included in the OA group, and healthy people (n = 22) who received routine annual physical examinations at the health examination center of our hospital were included in the healthy control group. Human specimens were obtained from the department of biobank, division of clinical research, the first hospital of Jilin University. All participants in the study obtained the consent of themselves or their families. The study was approved by the ethics committee of the first hospital of Jilin University (IRB ID: 2015-284). Demographics are shown in Supplementary Table 1. Serum metabolic indicators of OA patients were recorded.
2.3. Isolation of chondrocytes and cell culture
The cartilage tissue sourced from KOA patients was cut into 1 mm3 pieces, digested with 0.25% trypsin for 0.5 h and 2 mg/ml type II collagenase for 6 h. The isolated cells were cultured in DMEM medium containing 10% fetal bovine serum and 1% penicillin-streptomycin. Chondrocytes were passaged when reaching approximately 90% confluence. Chondrocytes within three passages were utilized to avoid altered metabolism and dedifferentiation.
2.4. Treatment of chondrocytes with lactate
Cell Counting Kit-8 (CCK-8) was used to determine the viability of chondrocytes incubated with lactate (Sigma) in a concentration gradient (0, 10, 20, 40 mmol/L) and a time gradient (6, 12, 24, 48, 72 h) (Fig. S1A). Apoptosis of chondrocytes treated with lactate was detected by flow cytometry labeled 7-AAD/Annexin V (Fig. S1B). Optimal concentration of lactate was determined to be 20 mmol/L, and it was also verified that MMP-3 expression increased most obviously under this concentration (Fig. S1C).
2.5. Flow cytometry analyses for cell apoptosis and ROS detection
Annexin V (FITC, 2.5 μl/test, eBioscience) and 7-AAD (PerCP, 2.5 μl/test, BD) were used to mark apoptosis. DCFH-DA (FITC, 1:1000, AAT Bioquest) was used to detect ROS.
2.6. Immunofluorescence analysis
Chondrocytes were fixed with pre-cooled 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, and blocked with 1% BSA. Primary antibodies anti-Collgen II (eBioscience, 1:100) and anti-NOX4 (eBioscience, 1:200) were incubated at 4 °C overnight, fluorescent secondary antibody (Abbkine, 1:1000) was added the next day. DAPI (Beyotime) was incubated prior to photographing, and images were collected under a fluorescence microscope (Olympus). Quantification analysis of the density of Immunocytochemistry staining for Collgen II and NOX4 was performed by Image J.
2.7. Seahorse real-time cell metabolic analysis
Chondrocytes (10,000 cells/well, confluence 80–90%) were seeded on Seahorse XF cell culture plates (Agilent), and probes were hydrated for 4 h before measurement. The detection solution was prepared according to the manufacturer’s instructions (Agilent): 1 ml glucose solution (1 mol/L), 1 ml pyruvate solution (100 mmol/L) and 1 ml glutamine solution (200 mmol/L) were added to 97 ml of Seahorse XF DMEM medium. Lactate and 2-deoxy-d-glucose (2-DG, Sigma) were diluted to 10-fold the working concentration using detection solution, and added them to the probe plate in sequence. Measurements were performed every 8 min with 3 cycles for each injection. After the calibration of the probe board, the Seahorse assay was performed, and the data was imported into GraphPad Prism 8 for mapping and analysis.
2.8. Measurements of NADH, NADPH and Acetyl-CoA
Chondrocytes were collected after lactate treatment, fully lysed by using specific lysate, and supernatants were collected after centrifugation. Levels of Acetyl-CoA, NAD+/NADH, and NADP+/NADPH were detected according to the kit instruction (Jiancheng, Beyotime).
2.9. Small interfering RNA (siRNA) mediated knockdown of HCAR1 in chondrocytes
siRNA sequence (sense: 5′-UCAUCAUGGUGGUGGCAAUTT-3′, antisense: 5′-AUUGCCACCACCAUGAUGATT-3′, General Biology) targeting HCAR1 was used. The siRNAs were transfected into chondrocytes using Lipofectamine 2000 (Invitrogen). Western blotting was performed to verify the transfection effect.
2.10. Quantitative gene expression and western blot analyses
Total cellular RNA was extracted using RNA extraction reagent (Accurate) according to the manufacturer’s instructions. cDNA synthesis (Abm) and quantitative real-time PCR (qRT-PCR, Accurate) were performed according to the manufacturer’s instructions. Gene data of MCT1-4, SLC5A8, SLC5A12, NOX1-5, DUOX1, and DUOX2 were expressed as 2−△ct, rest of the gene data was expressed as 2−△△ct. mRNA expression was calculated relative to the β-actin level. All primer sequences are shown in Supplementary Table 2. Western blot analyses were conducted with protein lysates from chondrocytes. Following primary antibodies were used: anti-PI3K (1:1000, CST), anti-p-PI3K (1:1000, CST), anti-Akt (1:1000, CST), anti-p-Akt (1:1000, CST), anti-HCAR1 (1:500, ABclonal), anti-NOX4 (1:500, ABclonal), and anti-β-actin (1:1000, CST). The intensity of each band was quantified using AlphaEaseFC software, the expression was calculated relative to the β-actin level.
2.11. Quantitation of catabolic enzymes and inflammatory cytokines
Supernatants of cultured chondrocytes were collected after lactate treatment for 24 h. The levels of IL-6, CCL-3, CCL-4, MMP-3, MMP-13, and ADAMTS-4 were detected according to enzyme-linked immunosorbent assay (ELISA) kit instructions (Jiancheng).
2.12. Senescence-associated β-galactosidase (SA-β-Gal) staining for cell senescence
SA-β-Gal staining was performed by using cell senescence β-galactosidase staining kit (Beyotime) according to the manufacturer’s protocol. The images were collected by a microscope (Nikon). Four fixed fields were selected, the SA-β-Gal positive cells and total cells were counted via image J. The SA-β-Gal positive cell rate (%) = the number of positive cells/total number of cells × 100%, and the average of the four fields was finally calculated.
2.13. Intraarticular injection of lactate in rats model
Eight-week-old male Sprague Dawley (SD) rats (n = 45) weighing between 240 g and 280 g were purchased from the Experimental Animal Center of Dalian Medical University. The rats were randomly assigned to different groups with 5 rats in each group. Lactate injection for 4 weeks or 12 weeks, the control group with injection of normal saline (NS). OA model and OA model with lactate injection for 4 weeks, the control group with sham operation and NS injection. OA model with injection of lactate and GLX351322 for 4 weeks, the control group with OA surgery and lactate injection. Eight-week-old male Rag2(−/−)/Il2rg(−/−) immunodeficient SD rats (n = 10) were purchased from Shanghai Model Organisms, Inc., the immunodeficient rats were housed under specific pathogen-free conditions, lactate injection for 12 weeks, the control group with injection of NS. The breeding environment complied with the animal management regulations of Dalian Medical University. This study was approved by the Laboratory animal ethics committee of Dalian Medical University (AEE19092).
Rats were anaesthetized with 1% sodium pentobarbital (0.4 ml/100 g) by intraperitoneal injection. Surgically induced OA by anterior cruciate ligment transection (ACLT) combined with destabilization of the medial meniscus (DMM) was performed at the right knee joint followed the procedure published previously [17]. 50 μl lactate (200 mmol/L) or NS was injected into the articular cavity using a microsyringe directly through the skin. Rats were sacrificed by intraperitoneal injection of 1% sodium pentobarbital (1.2 ml/100 g). Rat knee joints were collected 4 weeks or 12 weeks after injection for histologic analysis, Mankin’s scoring, and OARSI (Osteoarthritis Research Society International) scoring [18].
2.14. Statistical analysis
All data between the two groups were analyzed statistically and plotted using GraphPad Prism software (version 8.0, San Diego, USA). The normality of the data distribution was evaluated using the Kolmogorov–Smirnov test. Levene test was used to assess the homogeneity of variance. Independent samples t-tests were applied to analyze normally distributed values. Data with non-Gaussian distribution were analyzed by using the non-parametric Mann–Whitney U test. Categorical variables were assessed using the Pearson chi-square test. Correlation analysis was performed using Spearman’s method. Two-tailed values of p < 0.05 were considered statistically significant.
3. Results
3.1. Elevated lactate levels in OA patients
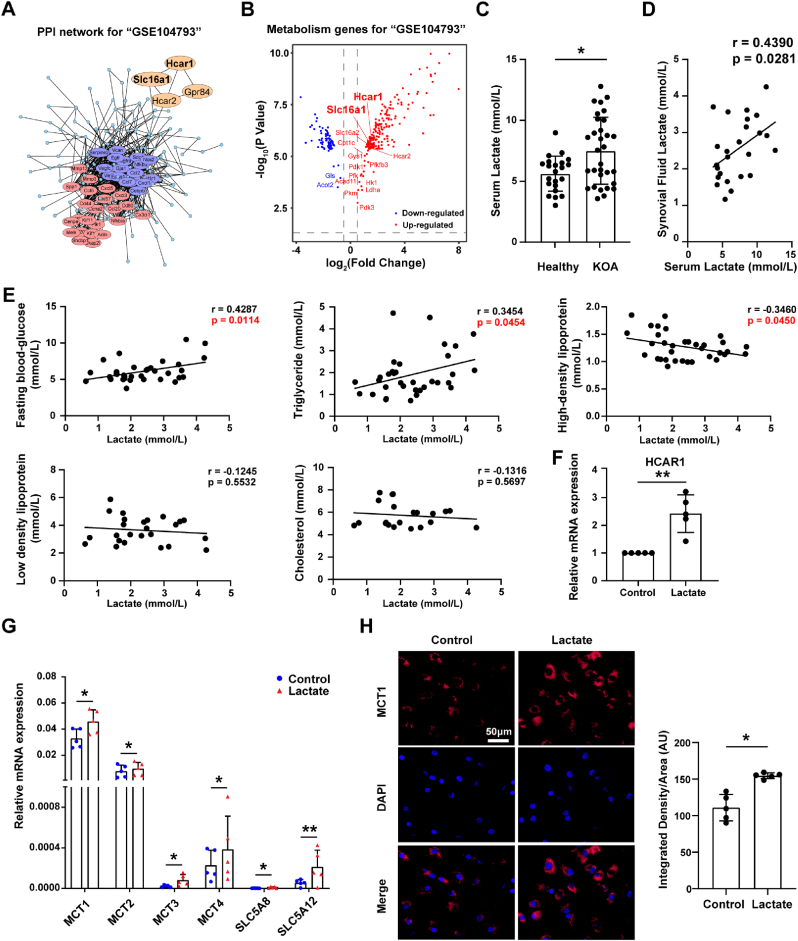
The gene set GSE104793 which contains mouse chondrocytes treated or untreated with IL-1β (OA chondrocyte model) was screened through the GEO database. PPI analysis identified a group of lactate-related genes such as HCAR1 and SLC16A1 (encoding lactate transporter MCT1) (Fig. 1A), which were up-regulated in OA (Fig. 1B). Among these DEGs, the glycolysis rate-limiting enzyme genes such as HK1, PFKFB3, PKM and LDHA were significantly up-regulated in OA chondrocytes (Fig. 1B), indicating an enhanced glycolysis pathway also by GSEA analysis (Fig. S2A). The above bioinformatic analysis suggests that lactate, known as an end-product of the glycolysis pathway, might be abnormal in OA. We confirmed that serum lactate levels of OA patients were higher than those of healthy controls (Fig. 1C), and had a positive correlation with lactate levels in the synovial fluid (Fig. 1D). Moreover, correlation analysis showed that synovial fluid lactate levels of OA patients were positively correlated with fasting blood glucose, triglyceride, and negatively correlated with high-density lipoprotein (Fig. 1E, Supplementary table 3). More interestingly, we still found that lactate treatment obviously up-regulated mRNA expressions of lactate receptor HCAR1 and all of lactate transporters in chondrocytes, among which MCT1 was the highest expressed isoform (Fig. 1F and G), and MCT1 protein level was also significantly increased by immunofluorescence staining (Fig. 1H). These results indicate that higher levels of lactate in OA serum and synovial fluid may be key metabolic products contributing to aberrant activities of chondrocyte via HCAR1 and lactate transporters.
Fig. 1.
Elevated lactate levels in osteoarthritis (OA) patients. The gene set GSE104793 that contains mouse chondrocytes treated (n = 3) or untreated (n = 3) with IL-1β (OA chondrocyte model) was screened through the GEO database. (A) Protein-protein interaction analysis of differentially expressed genes (DGEs) was performed by STRING (https://string-db.org), and cluster analysis was performed using Cytoscape software. (B) Volcano plots for metabolic DGEs of chondrocytes treated with IL-1β versus normal chondrocytes. Red represents up-regulation genes and blue represents down-regulation genes in chondrocytes treated with IL-1β. (C) Serum lactate levels in OA patients (n = 40) and healthy controls (n = 22). (D) Correlation analysis between serum lactate levels and synovial fluid lactate levels in OA patients (n = 25). (E) Correlation analysis between synovial fluid lactate levels and serum fasting blood glucose, triglycerides, high density lipoprotein, low density lipoprotein, and cholesterol in OA patients. Relative mRNA expression levels of (F) HCAR1, (G) MCT1, MCT2, MCT3, MCT4, SLC5A8, and SLC5A12 in chondrocytes treated with 20 mmol/L lactate for 6 h and controls were detected by quantitative real-time PCR (qRT-PCR) (n = 5 per group). The gene data was expressed as 2-△ct. (H) Immunofluorescence staining of MCT1 in chondrocytes treated with lactate for 24 h and controls (n = 5 per group). 50 μm scale bar. Quantification of MCT1 expression in chondrocytes via Image J. The data are mean ± SD, *p < 0.05, **p < 0.01. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.2. Lactate promotes NADPH production via shunting glucose metabolism to pentose phosphate pathway in chondrocytes
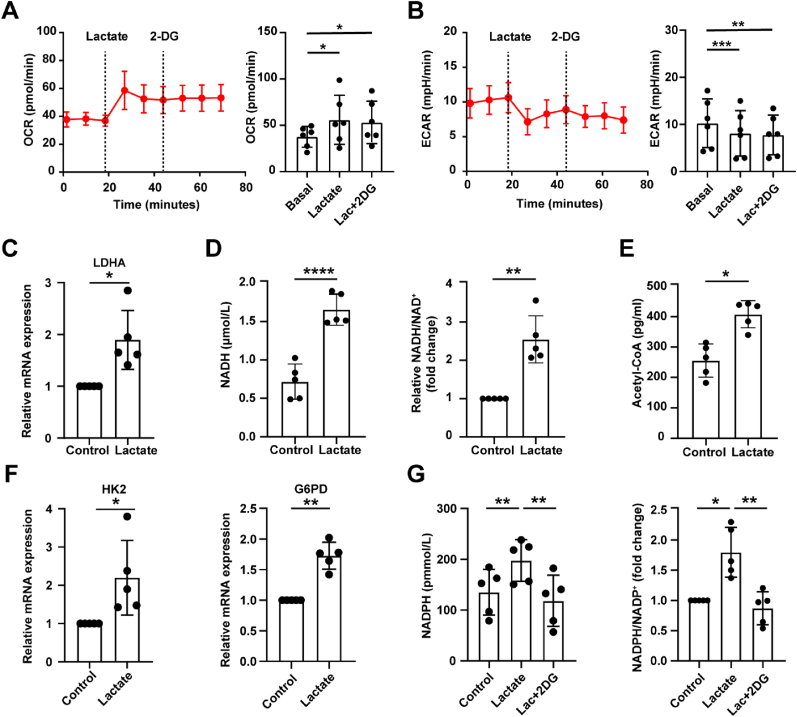
Large amounts of extracellular lactate entering the cell contributes to altered glucose metabolism [19]. We first showed that lactate increased the oxygen consumption rate (OCR) and decreased extracellular acidification rate (ECAR) of chondrocytes (Fig. 2A and B) with seahorse assay, and that the addition of 2-deoxy-d-glucose (2-DG), an inhibitor of glucose metabolism targeting hexokinase, did not affect OCR and ECAR, indicating that lactate could inhibit glycolysis pathway and enhance oxidative phosphorylation (OXPHOS).
Fig. 2.
Lactate promotes NADPH production via shunting glucose metabolism to pentose phosphate pathway in chondrocytes. (A) Oxygen consumption rate (OCR) and (B) extracellular acidification rate (ECAR) measurements of chondrocytes treated with 20 mmol/L lactate and 10 mmol/L 2-deoxy-d-glucose (2-DG) (Basal line: no treatment, lactate injection, 2-DG injection) were measured via Seahorse assay, n = 6. Measurements were performed every 8 min, with 3 cycles for each injection. (C) Relative mRNA expression levels of LDHA in chondrocytes treated with lactate for 6 h and controls were detected by qRT-PCR, n = 5. OA chondrocytes were treated or untreated with lactate for 24 h, and then the level of intracellular (D) NADH, NADH/NAD+ ratio, and (E) Acetyl-CoA level were measured by using kits (n = 5). (F) Relative mRNA expression levels of HK2 and G6PD in chondrocytes treated with lactate for 6 h and controls were detected by qRT-PCR, n = 5. (G) The NADPH levels and the relative NADPH/NADP+ ratio in chondrocytes treated with lactate for 24 h with or without 2-DG were measured by kit (n = 5). The data are mean ± SD, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Then, we found up-regulated lactate dehydrogenase A (LDHA) expression (Fig. 2C), increased NADH levels and the NADH/NAD+ ratio mediated by lactate (Fig. 2D), proving that lactate was oxidized to pyruvate after entering chondrocytes. Elevated acetyl-CoA levels (Fig. 2E) indicated that lactate-generated pyruvate entered the mitochondria to fuel OXPHOS, consistent with the seahorse results. Whether obstruction of the glycolytic pathway may enhance the branch pentose phosphate pathway (PPP)? Interestingly, the mRNA expression of G6PD, the rate-limiting enzyme of PPP, and hexokinase 2 (HK2) were increased with quantitative real-time PCR (qRT-PCR) (Fig. 2F). And then we found that NADPH levels and NADPH/NADP+ ratio was elevated in chondrocytes with lactate treatment, which was counteracted by 2-DG (Fig. 2G). These data suggest that lactate can promote the shunting of glucose metabolism to PPP in chondrocytes.
3.3. Lactate induces NADPH-dependent NOX4 expression via binding HCAR1 to activate the PI3K/Akt signaling pathway
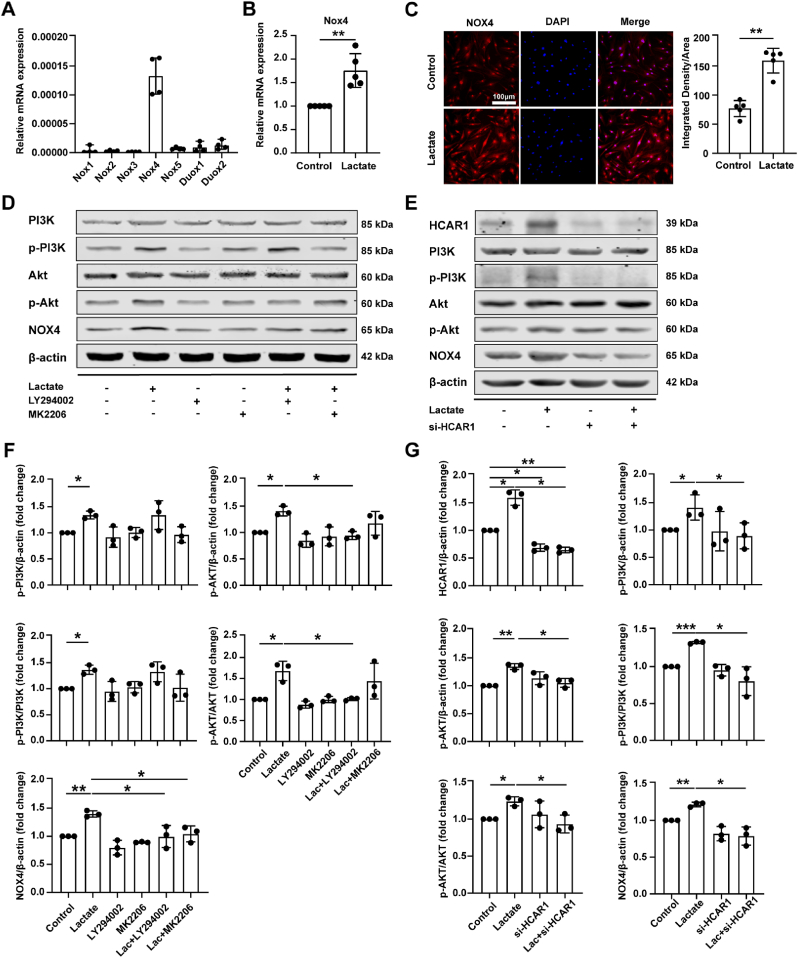
NADPH, a product of PPP, is regarded as the substrate catalyzed by NOXs [20]. We confirmed that NOX4 was the main isoform in chondrocytes (Fig. 3A), and lactate could boost NOX4 expression via qRT-PCR and immunofluorescence staining (Fig. 3B and C).
Fig. 3.
Lactate induces NADPH-dependent NOX4 expression via binding HCAR1 to activate the PI3K/Akt signaling pathway. (A) Relative mRNA expression levels of 7 isoforms of NADPH oxidase (NOX) in chondrocytes were detected by qRT-PCR (n = 4). Gene data was expressed as 2-△ct. (B) Relative mRNA level of NADPH oxidase 4 (NOX4) in chondrocytes treated with lactate for 6 h was measured by qRT-PCR (n = 5). The data was expressed as 2−△△ct. (C) Protein levels of NOX4 in chondrocytes treated with lactate for 24 h were measured by immunofluorescence staining (n = 5). Scale bar 100 μm. Quantification of NOX4 expression in chondrocytes via Image J. (D) Western blot analysis of PI3K, p-PI3K, Akt, p-Akt, and NOX4 protein levels in chondrocytes treated with lactate in the presence or absence of PI3K inhibitor LY294002 or Akt inhibitor MK2206 (n = 3). Chondrocytes were pretreated with 25 μmol/L LY294002 or 5 μmol/L MK2206 for 2 h prior to 20 mmol/L lactate incubation for 24 h. (E) Chondrocytes were transfected with small interfering RNA targeting hydroxy-carboxylic acid receptor 1 (si-HCAR1) mediated by Lipo2000 for 48 h and then treated with 20 mmol/L lactate for 24 h. Western blot analysis of PI3K, p-PI3K, Akt, p-Akt, and NOX4 protein levels in chondrocytes treated with lactate in the presence or absence of si-HCAR1 (n = 3). (F, G) The protein gray values were measured by AlphaEaseFC, and the expression was calculated relative to the β-actin level. Data are mean ± SD, *p < 0.05, **p < 0.01, ***p < 0.001.
Lactate transporters, especially MCT1 and MCT4, control the import and export of lactate, and the knockout of MCT1 or MCT4 could result in abnormal intracellular lactate levels [21,22]. We respectively selected MCT1 knockout, MCT4 knockout, and OA datasets for enrichment analysis of DEGs. The results showed that the dysregulation pathways in the three datasets all contained a “PI3K-Akt signaling pathway” (Fig. S3A-C). Next, we found that lactate could increase phosphorylated PI3K (p-PI3K) and phosphorylated Akt (p-Akt) levels, as well as p-PI3K/PI3K and p-Akt/Akt ratios. The addition of the LY294002 (PI3K inhibitor) attenuated the elevated p-Akt level and p-Akt/Akt ratio caused by lactate. Importantly, adding LY294002 or MK2206 (Akt inhibitor) could attenuate the NOX4 level elevated by lactate (Fig. 3D and F). The above results suggest that lactate up-regulates the expression of NOX4 by activating the PI3K/Akt signaling pathway.
HCAR1 is currently the only known endogenous receptor for lactate under physiological conditions, and studies have reported that it is related to the activation of PI3K/Akt signaling [23]. We used a siRNA knockdown of HCAR1 to determine whether HCAR1 is involved in PI3K/Akt signaling. The results showed that the p-PI3K and p-Akt levels, p-PI3K/PI3K and p-Akt/Akt ratios, and NOX4 levels, which were elevated by lactate, could be significantly reduced with si-HCAR1 treatment (Fig. 3E and G). In conclusion, we confirm that lactate up-regulates NOX4 expression by inducing and binding HCAR1 to activate the PI3K/Akt signaling pathway.
3.4. Lactate promotes ROS generation and chondrocyte damage by up-regulating NOX4 expression
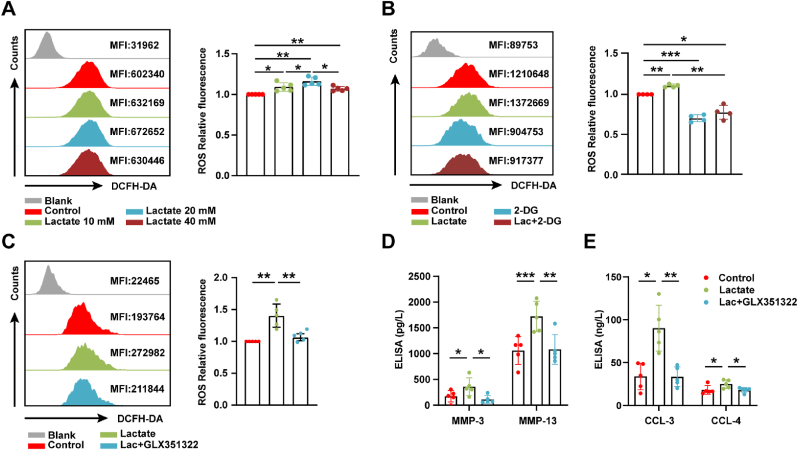
Studies have shown that the main source of ROS production in chondrocytes during the preliminary redox imbalance process is mediated by NOXs [24]. We found that lactate could increase ROS levels (Fig. 4A), which was offseted by 2-DG (Fig. 4B). In order to further verify whether lactate-induced ROS was catalyzed by NOX4, GLX351322, a specific inhibitor of NOX4, was utilized. The addition of GLX351322 could counteract increased ROS derived from lactate (Fig. 4C), and significantly reduce the secretion of MMP-3, MMP-13, CCL-3, and CCL-4 (Fig. 4D and E). The above results reveal that lactate could increase the ROS level by up-regulating NOX4 expression.
Fig. 4.
Lactate promote ROS generation and chondrocyte damage by up-regulating NOX4 expression. (A) Flow cytometry plots of intracellular reactive oxygen species (ROS) in chondrocytes incubated with lactate in a concentration gradient (0, 10, 20, 40 mmol/L) for 6 h (n = 5), (B) in chondrocytes pretreated with 10 mmol/L 2-DG for 2 h before 20 mmol/L lactate incubation for 6 h, (n = 4), and (C) in chondrocytes pretreated with 10 mmol/L GLX351322 for 1 h before 20 mmol/L lactate incubation for 6 h (n = 5). (D) Protein levels of MMP-3, MMP-13, (E) CCL-3, and CCL-4 in the culture supernatant of chondrocytes pretreated with GLX351322 for 1 h prior to lactate incubation for 24 h were measured by enzyme-linked immunosorbent assay (ELISA) (n = 5). Blank represents unstained control. Data are mean ± SD, *p < 0.05, **p < 0.01, ***p < 0.001.
3.5. Lactate mediates chondrocyte damage by increasing ROS production
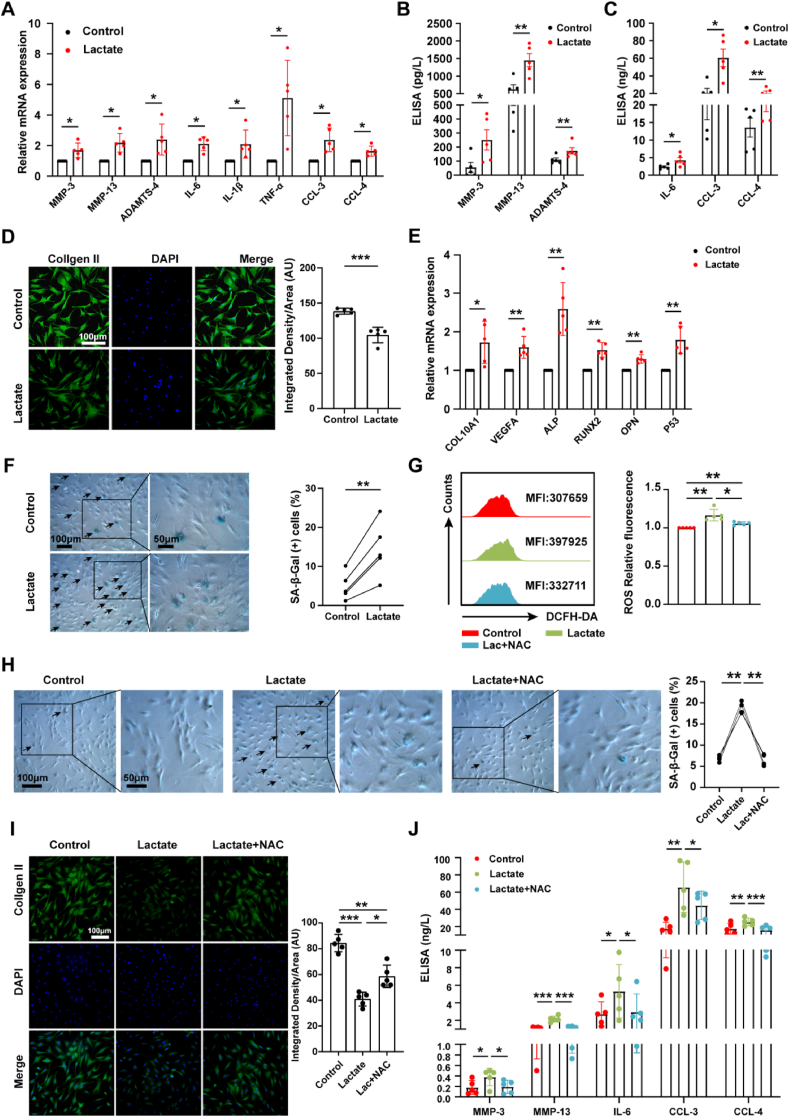
Previous studies reported that increased ROS levels contributed to cartilage destruction by preventing extracellular matrix (ECM) synthesis, degrading ECM components, and inducing chondrocyte death [11]. We found that catabolic genes, inflammatory genes, inflammatory response pathways and cellular senescence pathways were up-regulated in OA chondrocytes through bioinformatic analysis (Fig. S2A and B). Next, the effect of lactate on chondrocyte function was investigated. Our results showed increased expressions of MMP-3, MMP-13, and ADAMTS-4 by qRT-PCR and ELISA (Fig. 5A and B) and decreased expression of type II collagen by immunofluorescence staining (Fig. 5E) in chondrocytes treated with lactate, indicating the destructive effect of lactate on ECM. Elevated expressions of IL-6, CCL-3, and CCL-4 in chondrocytes (Fig. 5A and C) demonstrated the proinflammatory effect of lactate. Lactate increased the mRNA expressions of chondrocyte hypertrophic markers COL10aA1, ALP, RUNX2, VEGFA, and OPN (Fig. 5E), inducing a shift to the hypertrophic phenotype. Lactate also increased P53 mRNA expression (Fig. 5E) and the proportion of β-galactosidase-positive chondrocytes (Fig. 5F). These data demonstrate that lactate could induce OA chondrocyte damage, characterized by promoting expression of catabolic enzymes, reducing the synthesis of type II collagen, secreting inflammatory cytokines and chemokines, and causing hypertrophy and aging. Importantly, using N-Acetyl-l-cysteine (NAC), a ROS scavenger, reversed lactate-induced cellular senescence (Fig. 5G and H) and alleviated the inhibitory effect of lactate on type II collagen synthesis (Fig. 5I). Meanwhile, the secretion of MMP-3, MMP-13, IL-6, CCL-3, and CCL-4 was significantly decreased with NAC treatment (Fig. 5J). These results reveal that lactate could induce chondrocyte damage by promoting ROS generation.
Fig. 5.
Lactate mediates chondrocyte damage by increasing ROS production. (A) Relative mRNA expression levels of MMP-3, MMP-13, ADAMTS-4, IL-6, IL-1β, TNF-α, CCL-3, and CCL-4 in chondrocytes treated with 20 mmol/L lactate for 6 h and controls were detected by qRT-PCR (n = 5). The data was expressed as 2-△△ct. Protein levels of (B) MMP-3, MMP-13, ADAMTS-4, (C) IL-6, CCL-3, and CCL-4 in the culture supernatant of chondrocytes treated with lactate for 24 h were measured by ELISA (n = 5). (D) Immunofluorescence staining of type II collagen in chondrocytes treated with lactate for 24 h and controls (n = 5). Scale bar 100 μm. Quantification of type II collagen expression in chondrocytes via Image J. (E) Relative mRNA expression levels of COL10A1, ALP, RUNX2, VEGFA, and OPN were detected by qRT-PCR (n = 5). (F) Senescence-associated β-galactosidase (SA-β-Gal) staining of chondrocytes treated or untreated with lactate for 24 h. Scale bar 50 μm or 100 μm. The arrows represent β-Galactosidase positive cells. Quantification of β-galactosidase positive chondrocytes was calculated via Image J. (G) Flow cytometry plots of ROS in chondrocytes treated for 2 h with 5 mmol/L N-Acetyl-l-cysteine (NAC) prior to lactate incubation (n = 5). (H) SA-β-Gal staining (n = 4) and (I) immunofluorescence staining of type II collagen (n = 5) in chondrocytes pretreated with NAC for 2 h prior to lactate incubation for 24 h. Scale bar 50 μm or 100 μm. The arrows represent β-Galactosidase positive cells. Quantification of β-Galactosidase positive cells and type II collagen expression in chondrocytes were calculated via Image J. (J) Detection of MMP3, MMP13, IL-6, CCL3, CCL4 levels in the culture supernatant of chondrocytes with lactate treatment for 24 h with or without NAC treatment by ELISA (n = 5). The data are mean ± SD, *p < 0.05, **p < 0.01, ***p < 0.001.
3.6. NOX4 inhibitor attenuated cartilage damage of OA rat model caused by lactate
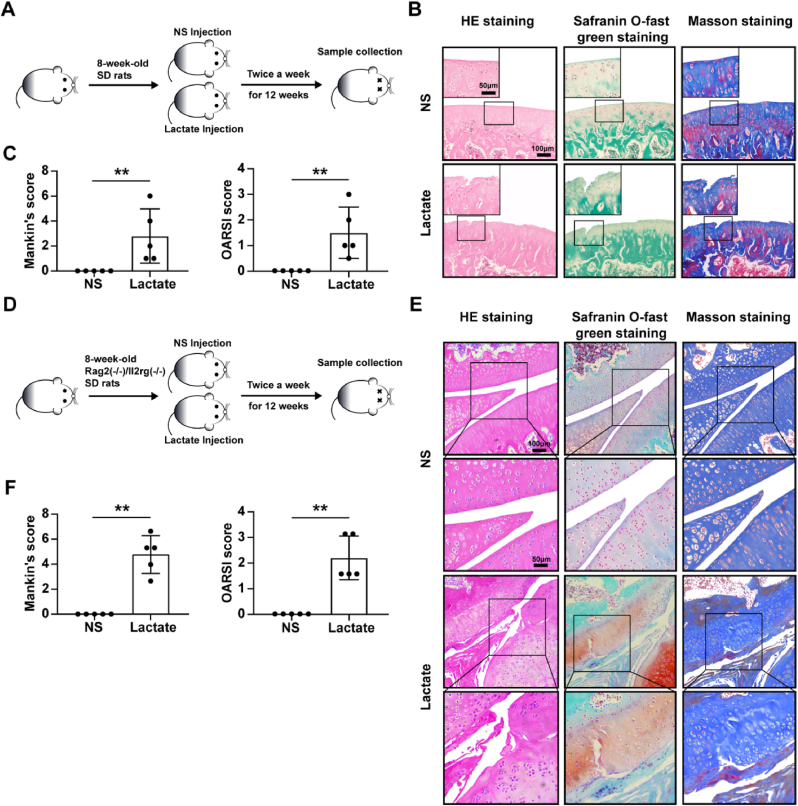
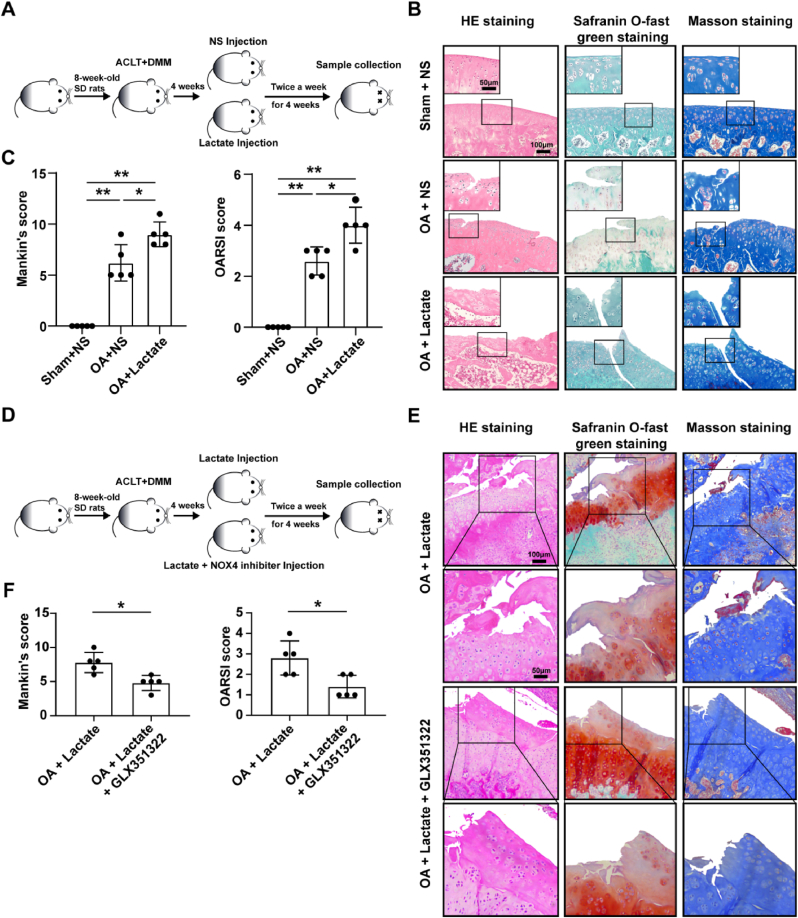
To investigate the role of lactate in the cartilage in vivo, continuous intra-articular injection of high-concentration lactate (200 mmol/L) was conducted in SD rats for 12 weeks, and cartilage showed OA-like changes (Fig. 6B), as well as increased Mankin’s score and OARSI score compared with NS treatment (Fig. 6C). To rule out lactate damaging chondrocytes by affecting immune cell function, we also administered lactate injection for 12 weeks in immunodeficient rats and found similar results (Fig. 6E and F). There were no changes between lactate treatment for 4 weeks and the control (Fig. S4B). More severe cartilage damage and higher Mankin’s score and OARSI score were observed in the ACLT + DMM model combined with lactate injection than in the OA models (Fig. 7B and C). Additional injection of GLX351322 attenuated cartilage damage induced by lactate in OA models (Fig. 7E and F). The above results indicate that a long-term high concentration of lactate in the articular cavity can induce cartilage damage, and aggravate the progression of OA by up-regulating NOX4.
Fig. 6.
A high concentration of lactate induces cartilage damage in animal models. 50 μl lactate (200 mmol/L) or normal saline (NS) was injected in (A) SD rats and (D) Rag2(−/−)/Il2rg(−/−) immunodeficient SD rats twice a week for 12 consecutive weeks. (B and E) The representative images of HE staining, safranin O-fast green staining, and masson staining for joints from rats injected with lactate or NS (n = 5, per group). Scale bar, 50 μm, 100 μm. (C and F) The Mankin’s score of cartilage was evaluated according to HE staining and the Osteoarthritis Research Society International (OARSI) score of cartilage was evaluated according to safranin O-fast green staining. Data are presented as mean ± SD, *p < 0.05, **p < 0.01. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 7.
NOX4 inhibitor attenuates cartilage damage of OA rat model caused by lactate. (A) Lactate or NS injection was conducted twice a week for 4 weeks, and one week after anterior cruciate ligment transection (ACLT) combined with destabilization of the medial meniscus (DMM) surgery. The sham operation with the control rats was performed with a similar incision at the joint without the meniscus and ligaments section. (B) The representative images of HE staining, safranin O-fast green, and masson staining for joints from OA models injected with lactate or NS, and rats with sham and NS injection (n = 5, per group). Scale bar, 50 μm, 100 μm. (D) Lactate or lactate plus GLX351322 injection was conducted twice a week for 4 weeks, one week after OA surgery. (E) The representative images of HE staining, safranin O-fast green, and masson staining for joints from OA models injected with lactate or lactate and GLX351322 (n = 5, per group). Scale bar, 50 μm, 100 μm. (C and F) The Mankin’s score of cartilage was evaluated according to HE staining and the OARSI score of cartilage was evaluated according to safranin O-fast green staining. Data are presented as mean ± SD, *p < 0.05, **p < 0.01. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Metabolomic analysis of serum and synovial fluid has identified potential diagnostic biomarkers for OA [25]. Nonetheless, there are only a few reports that metabolites (e.g., long-chain polyunsaturated fatty acids and cholesterol) are involved in the pathogenesis of OA [26,27]. Most recently, the role of lactate has been redefined, with a large number of studies focusing on immune cells and tumor cells [9,28]. However, the effect of lactate on chondrocytes remains unclear. In our study, we combined bioinformatic analyses, and clinical correlation analyses, human OA chondrocyte experiments in vitro, and animal experiments in vivo, confirming that lactate can induce the impairment of human OA chondrocytes and damage of cartilage in animal models. Our findings provide a molecular mechanism by which lactate induces oxidative stress in chondrocytes by altering glucose metabolism and activation of PI3K/akt signaling pathway to up-regulate NOX4 expression.
In the chronic low-grade inflammatory microenvironment of OA, cartilage metabolism is characterized by the glycolytic pathway as the main metabolism [5]. Arra et al. demonstrated that IL-β up-regulated LDHA in mouse chondrocytes, which further promoted ROS generation by binding to NADH [29]. Although lactate, the catalytic product of LDHA, was mentioned in the authors' study, the effect of lactate on chondrocytes was not explored in detail. In our study, first, We verified enhanced glycolysis in OA chondrocyte models by GSEA, consistent with the results of Arra et al. Meanwhile, we found a set of up-regulated lactate-related genes (e.g., HCAR1 and SLC16A1) by protein-protein interaction analysis, guiding us to focus on lactate. Lacitignola et al. reported that the lactate level in the synovial fluid of the equine OA model was approximately 3-fold higher than that of normal synovial fluid [30]. In our study, we found elevated lactate levels in the serum and synovial fluid in OA patients. The recent view suggests that metabolic dysfunction is strongly associated with OA [25], so we attempted to discover the link between lactate and clinical metabolic indicators through correlation analysis. It is remarkable that lactate levels in the synovial fluid of OA patients were positively correlated with fasting blood glucose, triglyceride, and negatively correlated with high-density lipoprotein, indicating that local lactate levels in joints may be affected by systemic metabolic disorders. Previous studies have shown that MCT1 was widely expressed in most tissues and responsible for maintaining lactate homeostasis, and MCT4 was relatively highly expressed in chondrocytes [31], which was also confirmed by our results. Miranda-Gonçalves et al. demonstrated a role for MCT1 as a mediator of lactate signaling between glioma cells and brain endothelial cells, showing that MCT1 can mediate endothelial cells metabolic reprogramming, proliferation, and vessel sprouting in response to tumor signaling [32]. Therefore, MCT1 may play a key role in the entry of lactate into chondrocytes.
Next, we explored the flow of lactate into chondrocytes by detecting intermediate metabolites and rate-limiting enzymes. We first found that lactate could elevate NADH/NAD+ ratios and LDHA expression, as well as increased acetyl-CoA levels. As mentioned in the literature review, lactate accumulation could lead to a sharp increase in the ratio of lactate to pyruvate, and a large amount of monocarboxylic acid enters the mitochondria, causing increased acetyl-CoA [33]. Interestingly, seahorse assay showed decreased ECAR in chondrocytes with lactate treatment. These findings are consistent with that of Quinn et al. who reported that increased NADH/NAD+ ratio caused by high lactate concentration led to the inhibition of GAPDH, subsequently the inhibition of glycolysis [34]. Meanwhile, we found that the expressions of HK2 and G6PD, were significantly up-regulated, as well as elevated NADPH levels, the product of PPP, illustrating an enhanced PPP. Similarly, Pucino et al. observed that lactate could increase the ratio of NADPH/NADP+ in CD4+T cells and promote the shunting of glucose to the PPP by inhibiting pyruvate kinase [9]. Therefore, when the glycolytic pathway is blocked by lactate, glucose metabolism can be shunted to the branch PPP.
Several studies about elevated NADPH have focused on increased fatty acid synthesis or amino acid synthesis affecting cell function [9,35]. Notably, NADPH has another important role as the substrate of NADPH oxidases for catalyzing ROS production [20]. Although Latsenko et al. found that Lactobacillus plantarum-derived lactate triggered the activation of the NOXs in the fly gut [36], the exact mechanism is not unclear. First, we identified NOX4 as the main isoform in chondrocytes and subsequently confirmed that lactate could boost NOX4 expression. Many signaling pathways have been reported to be involved in the regulation of NOX4 expression. Through bioinformatic analysis, we found dysregulation of the PI3K/Akt signaling pathway in cells with high lactate levels and OA chondrocytes. Koundouros et al. reported that abnormal PI3K/Akt signaling can directly regulate NOXs to generate ROS [37]. Our results proved that lactate could activate the PI3K/Akt signaling pathway, and further confirmed that the PI3K/Akt signaling pathway inhibitors LY294002 or MK2206 could attenuate lactate-induced NOX4 elevation. Morland et al. have noted the importance of lactate receptor HCAR1 involved in the activation of the PI3K/Akt signaling pathway [38]. In our study, siRNA mediated HCAR1 knockdown was able to suppress the phosphorylation of the PI3K/Akt signaling pathway activated by lactate and the expression of NOX4. Thus, we demonstrated the dual role of lactate, which not only promoted the rise of NADPH levels through metabolic reprogramming, but also up-regulated NOX4 expression through HCAR1/PI3K/Akt signaling pathway.
A systematic review proposed that ROS (mainly H2O2) catalyzed by NOXs is another major source, accounting for about 40% [39]. Here, we found that lactate could increase levels of intracellular ROS. Particularly, it was further confirmed that using a specific inhibitor of NOX4 (GLX351322) could attenuate the elevated ROS and catabolic factors mediated by lactate. Moreover, when we used 2-DG to inhibit the progression of glucose metabolism, lactate-elevated ROS levels were reduced. 2-DG, a glucose analog, can competitively bind to hexokinase and lead to a decreased production of glucose-6-phosphate, a substrate of the PPP, which further reduced the production of NADPH and lowered ROS levels catalyzed by NADPH-dependent NOX4.
It has been reported that ROS overproduction in OA modified intracellular signaling, chondrocyte life cycle, metabolism of cartilage matrix, and contributed to synovial inflammation [12]. Our results showed that lactate could increase the levels of MMP-3, MMP-13, ADAMTS-4, and reduce the synthesis of type II collagen to degrade the ECM. We also found evidence of lactate-induced inflammatory responses, which can impair chondrocyte function and also recruit immune cells to promote the inflammatory responses, creating a vicious cycle. Finally, we observed that lactate induced a hypertrophic phenotype and a senescent phenotype in chondrocytes. Hypertrophy and senescence are natural processes in chondrocytes, but their abnormal activation accelerates the progression of OA [40]. Chondrocyte hypertrophy is characterized by high expression of COL10aA1, ALP, RUNX2, VEGFA, MMP-13 and OPN [41,42]. According to research, OA chondrocytes exhibit characteristics similar to aging cells, such as increased β-galactosidase activity and expressions of p16 and p53, which leads to cell cycle arrest [43].
Finally, to verify the effect of lactate on articular cartilage in vivo, we constructed animal models. We observed that high concentrations of lactate injection in the articular cavity could induce mild OA-like changes, but it may take at least 12 weeks. Lactate has been reported to activate immune cell function [9], which may affect immune cells in the synovium of joints. We observed that lactate can also damage cartilage in immunodeficient rat models, indicating that lactate had a direct damaging effect on cartilage. The cartilage damage induced by lactate was more obvious on the basis of OA. The NOX4 inhibitor GLX351322 showed therapeutic effect against OA models using lactate. Lactate concentrations in the knee joint of OA patients were far from such a high level, but they often had a disease course of more than 10–20 years. Long-term accumulation of lactate in OA joints may accelerate the progression and shorten the course, and NOX4 inhibitors may hold promise as potential therapeutic agents.
There are some limitations to this study as well. The lack of synovial fluid samples from healthy controls in our study should be examined in the future. The mechanism of lactate-mediated chondrocyte damage shown in vivo was only linked to the involvement of NOX4. The evidence that lactate-mediated injury works via HCAR1 or other pathways in vivo is still lacking. However, more animal models (e.g., HCAR1 knockout) should be established to further explore the effects and mechanism of lactate on chondrocyte in future study.
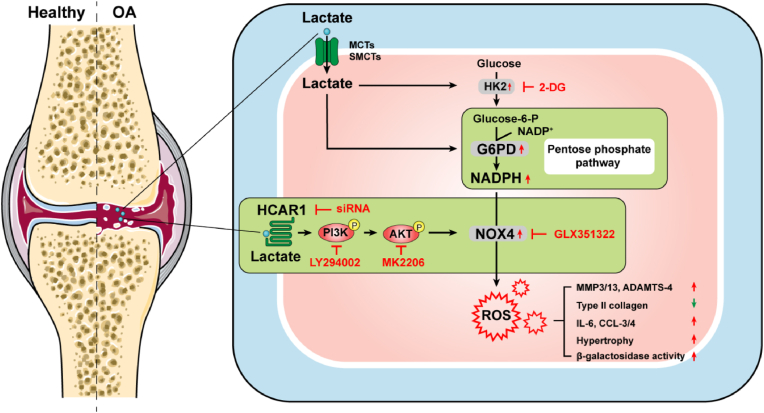
In summary, this study reported that lactate induces chondrocyte damage in vitro and in vivo through the involvement of NOX4. We further confirm that lactate as a metabolite increases NADPH levels by facilitating the shunting of glucose metabolism to PPP, and as a signaling molecule up-regulates NOX4 via activation of the PI3K/Akt signaling pathway through the receptor HCAR1, both of which ultimately increase ROS and mediate chondrocyte dysfunction in vitro (Fig. 8). We also confirmed that intra-articular injection of lactate can damage cartilage in vivo and exacerbate the progression of OA models that can be countered by the NOX4 inhibitor. Our study provides a new perspective for lactate as a metabolic factor involved in the OA procession, and laying the theoretical foundation for potential metabolic therapies for OA in the future.
Fig. 8.
Schematic representation of lactate-induced oxidative stress. Lactate induces the expression of MCTs and SMCTs in chondrocytes, and enters chondrocytes through them. Lactate increased NADPH levels by promoting shunting of glucose to the pentose phosphate pathway and up-regulates NOX4 expression via binding receptor HCAR1 to activate the PI3K/Akt signaling pathway, leading to elevated ROS and further induced impaired chondrocytes characterized by promoting expression of catabolic enzymes, reducing the synthesis of type II collagen, secreting inflammatory cytokines and chemokines, and causing hypertrophy and aging. OA, osteoarthritis; MCT, monocarboxylate transporter; SMCT, sodium-coupled monocarboxylate transporter; HCAR1, hydroxy-carboxylic acid receptor 1; NADP, nicotinamide adenine dinucleotide phosphate; NOX, NADPH oxidase; HK, hexokinase; Glucose-6-P, glucose-6-phosphate; G6PD, glucose-6-phosphate dehydrogenase; 2-DG, 2-deoxy-d-glucose; ROS, reactive oxygen species.
Author contributions
Huang YF, Wang G, Li X, Qi X for the conception and design of the study, Huang YF, Ding L, Leng Y, Tian JW, Zhang JZ, Bai ZR, Li YQ, Ahmad, Qin YH for acquisition of data, Huang YF, Wang G, Li X for analysis and interpretation of data, Huang YF, Wang G, and Li X for drafting the article and revising it critically for important intellectual content, Li X and Qi X for final approval of the version to be submitted.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China [82071834, 82101896, 82271839], Science and Technology Innovation of Dalian [2021JJ13SN48], Dalian key laboratory of human microorganism homeostasis and immunological mechanism research of diseases, Natural Science Funding of Jilin province [20200201548JC, 20210101324JC], Medicine & Health Funding of Jilin province [20200404145YY], and International Collaboration Funding of Jilin province [20200801045GH]. The authors would like to acknowledge all lab members for insightful discussions. We thank the Department of Biobank, Division of Clinical Research, the First Hospital of Jilin University for the providing of human tissues.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2023.102867.
Contributor Information
Xia Li, Email: lixia0416@dmu.edu.cn.
Xin Qi, Email: dr.qixin@jlu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Hunter D.J., March L., Chew M. Osteoarthritis in 2020 and beyond: a Lancet commission. Lancet. 2020;396:1711–1712. doi: 10.1016/S0140-6736(20)32230-3. [DOI] [PubMed] [Google Scholar]
- 2.Hunter D.J., Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393:1745–1759. doi: 10.1016/S0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 3.Cao Y., Tang S., Ding C. Inflammatory phenotype of osteoarthritis and its potential therapies. Rheumatol. Autoimmun. 2021;1:92–100. doi: 10.1002/rai2.12018. [DOI] [Google Scholar]
- 4.Zheng L., Zhang Z., Sheng P., Mobasheri A. The role of metabolism in chondrocyte dysfunction and the progression of osteoarthritis. Ageing Res. Rev. 2021;66 doi: 10.1016/j.arr.2020.101249. [DOI] [PubMed] [Google Scholar]
- 5.Mobasheri A., Rayman M.P., Gualillo O., Sellam J., van der Kraan P., Fearon U. The role of metabolism in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2017;13:302–311. doi: 10.1038/nrrheum.2017.50. [DOI] [PubMed] [Google Scholar]
- 6.Brooks G.A. The science and translation of lactate shuttle theory. Cell Metabol. 2018;27:757–785. doi: 10.1016/j.cmet.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Brooks G.A. Lactate as a fulcrum of metabolism. Redox Biol. 2020;35 doi: 10.1016/j.redox.2020.101454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun S., Li H., Chen J., Qian Q. Lactic acid: No longer an inert and end-product of glycolysis. Physiology. 2017;32:453–463. doi: 10.1152/physiol.00016.2017. [DOI] [PubMed] [Google Scholar]
- 9.Pucino V., Certo M., Bulusu V., Cucchi D., Goldmann K., Pontarini E., Haas R., Smith J., Headland S.E., Blighe K., Ruscica M., Humby F., Lewis M.J., Kamphorst J.J., Bombardieri M., Pitzalis C., Mauro C. Lactate buildup at the site of chronic inflammation promotes disease by inducing CD4+ T cell metabolic rewiring. Cell Metabol. 2019;30:1055–1074.e8. doi: 10.1016/j.cmet.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaggard M.K.J., Boulangé C.L., Akhbari P., Vaghela U., Bhattacharya R., Williams H.R.T., Lindon J.C., Gupte C.M. A systematic review of the small molecule studies of osteoarthritis using nuclear magnetic resonance and mass spectroscopy. Osteoarthritis Cartilage. 2019;27:560–570. doi: 10.1016/j.joca.2018.08.024. [DOI] [PubMed] [Google Scholar]
- 11.Bolduc J.A., Collins J.A., Loeser R.F. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic. Biol. Med. 2019;132:73–82. doi: 10.1016/j.freeradbiomed.2018.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lepetsos P., Papavassiliou K.A., Papavassiliou A.G. Redox and NF-κB signaling in osteoarthritis. Free Radic. Biol. Med. 2019;132:90–100. doi: 10.1016/j.freeradbiomed.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 13.Sun K., Luo J., Guo J., Yao X., Jing X., Guo F. The PI3K/AKT/mTOR signaling pathway in osteoarthritis: a narrative review. Osteoarthritis Cartilage. 2020;28:400–409. doi: 10.1016/j.joca.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 14.Chatterjee S., Browning E.A., Hong N., DeBolt K., Sorokina E.M., Liu W., Birnbaum M.J., Fisher A.B. Membrane depolarization is the trigger for PI3K/Akt activation and leads to the generation of ROS. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H105–H114. doi: 10.1152/ajpheart.00298.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Q., Powell D.W., Rane M.J., Singh S., Butt W., Klein J.B., McLeish K.R. Akt phosphorylates p47phox and mediates respiratory burst activity in human neutrophils. J. Immunol. 2003;170:5302–5308. doi: 10.4049/jimmunol.170.10.5302. [DOI] [PubMed] [Google Scholar]
- 16.Usatyuk P.V., Fu P., Mohan V., Epshtein Y., Jacobson J.R., Gomez-Cambronero J., Wary K.K., Bindokas V., Dudek S.M., Salgia R., Garcia J.G.N., Natarajan V. Role of c-Met/phosphatidylinositol 3-kinase (PI3k)/Akt signaling in hepatocyte growth factor (HGF)-mediated lamellipodia formation, reactive oxygen species (ROS) generation, and motility of lung endothelial cells. J. Biol. Chem. 2014;289:13476–13491. doi: 10.1074/jbc.M113.527556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glasson S.S., Blanchet T.J., Morris E.A. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15:1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Gerwin N., Bendele A.M., Glasson S., Carlson C.S. The OARSI histopathology initiative – recommendations for histological assessments of osteoarthritis in the rat. Osteoarthritis Cartilage. 2010;18:S24–S34. doi: 10.1016/j.joca.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 19.Li X., Yang Y., Zhang B., Lin X., Fu X., An Y., Zou Y., Wang J.-X., Wang Z., Yu T. Lactate metabolism in human health and disease. Signal Transduct. Targeted Ther. 2022;7:305. doi: 10.1038/s41392-022-01151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor J.P., Tse H.M. The role of NADPH oxidases in infectious and inflammatory diseases. Redox Biol. 2021;48 doi: 10.1016/j.redox.2021.102159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Todenhöfer T., Seiler R., Stewart C., Moskalev I., Gao J., Ladhar S., Kamjabi A., Al Nakouzi N., Hayashi T., Choi S., Wang Y., Frees S., Daugaard M., Oo H.Z., Fisel P., Schwab M., Schaeffeler E., Douglas J., Hennenlotter J., Bedke J., Gibb E.A., Fazli L., Stenzl A., Black P.C. Selective inhibition of the lactate transporter MCT4 reduces growth of invasive bladder cancer. Mol. Cancer Therapeut. 2018;17:2746–2755. doi: 10.1158/1535-7163.MCT-18-0107. [DOI] [PubMed] [Google Scholar]
- 22.Hu K.Y., Wang D.G., Liu P.F., Cao Y.W., Wang Y.H., Yang X.C., Hu C.X., Sun L.J., Niu H.T. Targeting of MCT1 and PFKFB3 influences cell proliferation and apoptosis in bladder cancer by altering the tumor microenvironment. Oncol. Rep. 2016;36:945–951. doi: 10.3892/or.2016.4884. [DOI] [PubMed] [Google Scholar]
- 23.Hu J., Cai M., Liu Y., Liu B., Xue X., Ji R., Bian X., Lou S. The roles of GRP81 as a metabolic sensor and inflammatory mediator. J. Cell. Physiol. 2020;235:8938–8950. doi: 10.1002/jcp.29739. [DOI] [PubMed] [Google Scholar]
- 24.Drevet S., Gavazzi G., Grange L., Dupuy C., Lardy B. Reactive oxygen species and NADPH oxidase 4 involvement in osteoarthritis. Exp. Gerontol. 2018;111:107–117. doi: 10.1016/j.exger.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Batushansky A., Zhu S., Komaravolu R.K., South S., Mehta-D’souza P., Griffin T.M. Fundamentals of OA. An initiative of osteoarthritis and cartilage. Obesity and metabolic factors in OA. Osteoarthritis Cartilage. 2022;30:501–515. doi: 10.1016/j.joca.2021.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abshirini M., Ilesanmi-Oyelere B.L., Kruger M.C. Potential modulatory mechanisms of action by long-chain polyunsaturated fatty acids on bone cell and chondrocyte metabolism. Prog. Lipid Res. 2021;83 doi: 10.1016/j.plipres.2021.101113. [DOI] [PubMed] [Google Scholar]
- 27.Choi W.-S., Lee G., Song W.-H., Koh J.-T., Yang J., Kwak J.-S., Kim H.-E., Kim S.K., Son Y.-O., Nam H., Jin I., Park Z.-Y., Kim J., Park I.Y., Hong J.-I., Kim H.A., Chun C.-H., Ryu J.-H., Chun J.-S. The CH25H–CYP7B1–RORα axis of cholesterol metabolism regulates osteoarthritis. Nature. 2019;566:254–258. doi: 10.1038/s41586-019-0920-1. [DOI] [PubMed] [Google Scholar]
- 28.Brown T.P., Ganapathy V. Lactate/GPR81 signaling and proton motive force in cancer: role in angiogenesis, immune escape, nutrition, and Warburg phenomenon. Pharmacol. Therapeut. 2020;206 doi: 10.1016/j.pharmthera.2019.107451. [DOI] [PubMed] [Google Scholar]
- 29.Arra M., Swarnkar G., Ke K., Otero J.E., Ying J., Duan X., Maruyama T., Rai M.F., O’Keefe R.J., Mbalaviele G., Shen J., Abu-Amer Y. LDHA-mediated ROS generation in chondrocytes is a potential therapeutic target for osteoarthritis. Nat. Commun. 2020;11:3427. doi: 10.1038/s41467-020-17242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lacitignola L., Fanizzi F.P., Francioso E., Crovace A. 1H NMR investigation of normal and osteoarthritic synovial fluid in the horse. Vet. Comp. Orthop. Traumatol. 2008;21:85–88. doi: 10.3415/VCOT-06-12-0101. [DOI] [PubMed] [Google Scholar]
- 31.Halestrap A.P. The SLC16 gene family – structure, role and regulation in health and disease. Mol. Aspect. Med. 2013;34:337–349. doi: 10.1016/j.mam.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Miranda-Gonçalves V., Bezerra F., Costa-Almeida R., Freitas-Cunha M., Soares R., Martinho O., Reis R.M., Pinheiro C., Baltazar F. Monocarboxylate transporter 1 is a key player in glioma-endothelial cell crosstalk. Mol. Carcinog. 2017;56:2630–2642. doi: 10.1002/mc.22707. [DOI] [PubMed] [Google Scholar]
- 33.Passarella S., de Bari L., Valenti D., Pizzuto R., Paventi G., Atlante A. Mitochondria and L-lactate metabolism. FEBS Lett. 2008;582:3569–3576. doi: 10.1016/j.febslet.2008.09.042. [DOI] [PubMed] [Google Scholar]
- 34.Quinn W.J., Jiao J., TeSlaa T., Stadanlick J., Wang Z., Wang L., Akimova T., Angelin A., Schäfer P.M., Cully M.D., Perry C., Kopinski P.K., Guo L., Blair I.A., Ghanem L.R., Leibowitz M.S., Hancock W.W., Moon E.K., Levine M.H., Eruslanov E.B., Wallace D.C., Baur J.A., Beier U.H. Lactate Limits T cell proliferation via the NAD(H) Redox State. Cell Rep. 2020;33 doi: 10.1016/j.celrep.2020.108500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu J., Schwörer S., Berisa M., Kyung Y.J., Ryu K.W., Yi J., Jiang X., Cross J.R., Thompson C.B. Mitochondrial NADP(H) generation is essential for proline biosynthesis. Science. 2021;372 doi: 10.1126/science.abd5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iatsenko I., Boquete J.-P., Lemaitre B. Microbiota-derived lactate activates production of reactive oxygen species by the intestinal NADPH oxidase Nox and shortens Drosophila Lifespan. Immunity. 2018;49:929–942.e5. doi: 10.1016/j.immuni.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 37.Koundouros N., Poulogiannis G. Phosphoinositide 3-Kinase/Akt signaling and redox metabolism in cancer. Front. Oncol. 2018;8:160. doi: 10.3389/fonc.2018.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morland C., Andersson K.A., Haugen Ø.P., Hadzic A., Kleppa L., Gille A., Rinholm J.E., Palibrk V., Diget E.H., Kennedy L.H., Stølen T., Hennestad E., Moldestad O., Cai Y., Puchades M., Offermanns S., Vervaeke K., Bjørås M., Wisløff U., Storm-Mathisen J., Bergersen L.H. Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nat. Commun. 2017;8 doi: 10.1038/ncomms15557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sies H., Jones D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020;21:363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 40.Rim Y.A., Nam Y., Ju J.H. The role of chondrocyte hypertrophy and senescence in osteoarthritis initiation and progression. IJMS. 2020;21:2358. doi: 10.3390/ijms21072358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caron M.M.J., Emans P.J., Coolsen M.M.E., Voss L., Surtel D.a.M., Cremers A., van Rhijn L.W., Welting T.J.M. Redifferentiation of dedifferentiated human articular chondrocytes: comparison of 2D and 3D cultures. Osteoarthritis Cartilage. 2012;20:1170–1178. doi: 10.1016/j.joca.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 42.van Donkelaar C.C., Wilson W. Mechanics of chondrocyte hypertrophy. Biomech. Model. Mechanobiol. 2012;11:655–664. doi: 10.1007/s10237-011-0340-0. [DOI] [PubMed] [Google Scholar]
- 43.Coryell P.R., Diekman B.O., Loeser R.F. Mechanisms and therapeutic implications of cellular senescence in osteoarthritis. Nat. Rev. Rheumatol. 2021;17:47–57. doi: 10.1038/s41584-020-00533-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.