Abstract
Background:
Total joint arthroplasties are common orthopedic surgeries that carry risk for developing chronic post-surgical pain. In addition to pre- and post-operative pain severity, psychological distress (e.g., anxiety, pain catastrophizing) is a risk factor for chronic postsurgical pain. Cognitive behavioral therapy (CBT) for chronic pain is an empirically supported approach to managing chronic pain, functional impairment, and related distress. While CBT has been used extensively in patients with established chronic pain, using it as a preventive intervention targeting the transition from acute to chronic postsurgical pain is a novel application.
Objectives:
The Perioperative Pain Self-Management (PePS) program is a pain self-management intervention based on the principles of CBT. This innovative intervention is brief, flexible, and is delivered remotely. The current study aims to determine the efficacy of PePS compared to standard care on reducing the incidence of significant surgical site pain at 6-months post-surgery. The current study also aims to evaluate the context for subsequent implementation.
Methods:
This study is a hybrid type I efficacy-preparing for implementation trial. It is a two-site, single-blind, two-arm, parallel, randomized control trial. Surgical patients will be randomized to either receive: 1) PePS plus standard care, or 2) Standard care. The primary end point will be surgical site pain severity at 6-months post-surgery.
Conclusion:
Results from this study are expected to result in support for a brief scalable intervention (PePS) that can prevent the development of chronic pain and prolonged post-surgical opioid use, as well as key details to inform subsequent implementation.
Keywords: Prevention, persistent postsurgical pain, randomized control trial protocol, pain self-management, veteran
Introduction
Chronic pain is prevalent among Veterans, with nearly two-thirds reporting pain in the previous three months [1]. Surgery may precipitate both chronic pain and long-term opioid use [2, 3]. Chronic pain and substance use disorders commonly co-occur in the general population and among Veterans [4]. As post-operative pain severity is a risk factor for developing chronic post-surgical pain [5], pharmacological pain management is central in the immediate post-surgical stage. Due to known risks associated with opioids, as well as long-term use of non-opioid analgesics, strategies are needed to reinforce perioperative pharmacotherapeutic management of pain with non-pharmacologic strategies, specifically for preventing long-term sequelae.
Total joint arthroplasties are common orthopedic surgeries that carry risk for chronic post-surgical pain, affecting up to 20% of patients who undergo these procedures [6–9]. In addition to pre- and post-operative pain severity, psychological distress (e.g., anxiety, pain catastrophizing) is a risk factor for persistent postsurgical pain [10]. Psychological interventions, such as Cognitive Behavioral Therapy (CBT), can effectively reduce distress and improve functioning among patients with chronic pain [11]. CBT for chronic pain is an approach that is widely implemented in the Veterans Health Administration (VHA) as part of pain care guidelines [12]. While CBT has been used extensively in patients with established chronic pain, applying it as preventive intervention in the perioperative period is novel.
The Perioperative Pain Self-Management (PePS) intervention is a CBT-based intervention that was designed for use with surgical patients. This 4-session telephone-based intervention was designed to be flexibly responsive to patient needs by providing them with a range of pain self-coping skills. The participants can select which coping strategies they will practice in during the pre-and post-operative period. The PePS intervention invites participants to actively manage their postsurgical pain with a deliberate, planful, approach. Further, pilot data provide preliminary support for a significant impact of PePS on reducing the incidence of moderate to severe persistent postsurgical pain at 3-months post-surgery [13]. A larger trial is needed to confirm these preliminary findings.
The current study uses a Hybrid Type 1 Randomized Controlled Trial design to: 1) Evaluate the efficacy of the PePS program versus Standard Care (SC) for reducing prolonged postsurgical site pain and opioid use and 2) evaluate the context of implementation by examining acceptability, feasibility, and appropriateness of the intervention.
Methods
Design.
This is a Hybrid Type I, two-site, single-blind, two-arm (PePS and SC), parallel, randomized controlled trial to test intervention efficacy under standardized conditions. To prepare for future widespread implementation, qualitative interviews will be completed with both participants and orthopedic surgery clinic staff (i.e., surgeons, nurses, and schedulers). The qualitative interviews will be used to determine the acceptability, feasibility, and appropriateness of contextual factors associated with the setting that might facilitate or create barriers for future implementation in a real-world setting [14]. Understanding contextual factors will facilitate future implementation within the VA healthcare system.
Hypotheses.
The primary hypothesis is that a lower proportion of participants randomized to PePS will report moderate to severe pain at the surgical site at 6-months post-surgery, compared to SC participants. Secondary hypotheses are that participants randomized to PePS will have a shorter duration of postsurgical opioid use, and better mood and pain-related functioning compared to SC participants at 6-months post-surgery. The null hypothesis for the primary and secondary hypotheses is that there will be no differences between groups. We also hypothesize that we will be able to identify contextual factors that will facilitate future implementation as well as potential barriers to implementation.
Study Population.
The recruitment target is 400 surgical patients from two sites: the Iowa City Veterans Affairs Healthcare System and the Minneapolis Veteran Affairs Health Care System. Patients indicated for surgery at the Iowa City or Minneapolis VA for unilateral primary total joint arthroplasty will be approached to participate. Inclusion criteria include: 1) at least 18 years old; 2) scheduled for total hip, knee, or shoulder joint arthroplasty through the VA. Exclusion criteria include: 1) inability to complete study forms/procedures because of a language/literacy barrier; 2) untreated bipolar or psychotic disorder; 3) history of brain injury with lasting effects; 4) dementia; 5) CBT therapy for chronic pain within the past year (which could confound study results); 6) lack of access to a telephone for PePS sessions; and 7) allergy to opioid medications.
Screening and Recruitment.
Orthopedic clinic schedules will be reviewed on a weekly basis to identify patients who are indicated or scheduled for eligible surgeries. Medical records will be reviewed to determine if patients have any exclusionary factors. Patients passing this initial chart screen will be approached in clinic or contacted via telephone if they are indicated for surgery by the surgical team, inviting them to participate. The study procedures, risks, and benefits will be explained, and questions answered. If willing to participate, patients will be further screened for inclusion and exclusion criteria (above) and those who are eligible will be consented.
Blinding and Randomization.
This is a single-blind study. The outcomes assessor will be blinded to group assignment. Participants will be randomized by study personnel who will not complete follow-up data collection. Using a sequence generated by the study statistician, randomization will occur in permuted blocks of 4 and 6 and will be stratified by surgery type, facility, and presence or absence of preoperative opioid use. The permuted blocks of 4 and 6 will prevent overly long strings of randomization to a single arm. Allocation to treatment arm will remain concealed until inclusion and exclusion criteria have been determined and as close in time to the pre-surgery assessment as possible. Randomization status will be concealed via waiting to enter the participant number into the excel randomization file which does not reveal randomization status of the next participant until a new participant number is entered. This approach minimizes the potential for investigator and participant bias by protecting the randomization sequence in a central location and maintaining concealment of treatment allocation until the last moment.
Study Procedures.
Following consent and randomization, all participants will be asked to complete a pre-surgery assessment survey either in person at the clinic or remotely via electronic survey or paper survey (per their preference). The survey will then be repeated at 3-months and 6-months post-surgery (Table 1).
Table 1.
Data Collection Schedule
| Enrollment | 6 weeks of phone calls post-surgery | 3-months post-surgery | 6-months post-surgery | |||||
|---|---|---|---|---|---|---|---|---|
| Demographics/Medical | X | PePS | Surgery | PePS | Qualitative Interview | |||
| Primary Outcome: | ||||||||
| Pain Intensity | X | X | X | |||||
| Secondary Outcomes: | ||||||||
| Opioid and Other Analgesics | X | X | X | X | ||||
| Depressive Symptoms | X | |||||||
| Anxious Symptoms | X | |||||||
| Pain-Related Function | X | X | X | |||||
| Potential Mediators: | ||||||||
| Pain Catastrophizing | X | X | X | |||||
| Pain Self-Efficacy | X | X | X | |||||
| Chart Review: | ||||||||
| Chart Review | X | X | X |
Postoperative medication use will be collected from all participants via weekly phone calls which have been informed by pre-phone call chart review of medications, for the first six weeks following surgery. Those randomized to receive the PePS intervention will receive the first session prior to surgery and the subsequent three sessions at 2-, 3-, and 4-weeks post-surgery, respectively (Figure 1). A subset of PePS participants will also be offered a qualitative interview 1-2 weeks following completion of the PePS intervention, until data saturation is reached. Postoperative medical chart review will be completed by research team members to record type of anesthesia used during surgery and opioid and other analgesic medications prescribed following surgery.
Figure 1.
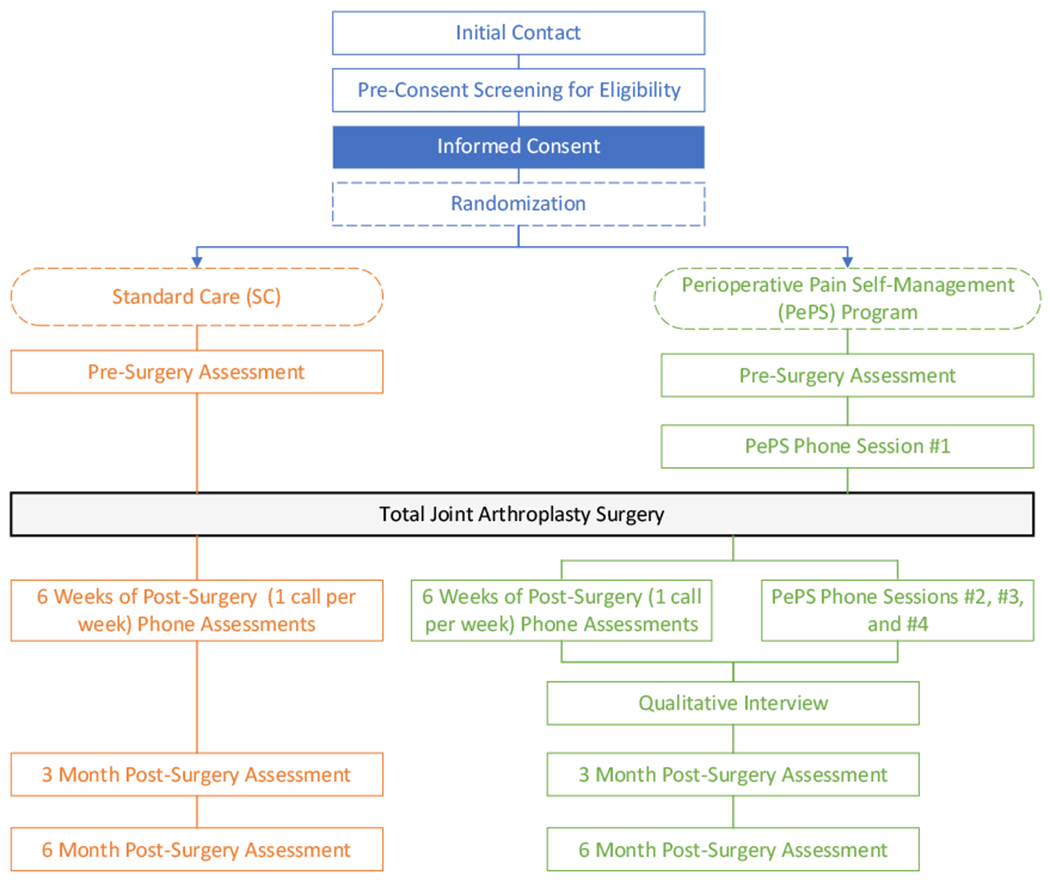
PePS Trial Procedures
To prepare for implementation, orthopedic clinic staff and physicians will be invited to participate in individual qualitative interviews. Staff and physician interviews will be completed in the last year of the trial, to maximize staff and provider familiarity with PePS processes. All surgeons, clerks, and nurses at both sites will be invited to participate and interviews will be conducted until data saturation is reached for each professional group. Interviews will be completed by qualitative experts. Qualitative data will be collected to determine the acceptability, appropriateness and feasibility of delivering the intervention in the context of clinic workflow. Further, to estimate additional workload required to implement the PePS program, labor will be tracked. The PePS interventionists will time each phone session and record this in the tracking database immediately following each call. Interventionists will also track preparation time and documentation time per session. All research team members will document the number of scheduling attempts and the time taken to schedule for each PePS session for each participant as well as the time it takes to mail a participant manual. Study personnel times required can then be used to estimate future staffing needs for PePS therapists and schedulers. These data will facilitate the ability to plan implementation workload requirements.
Primary Outcome Measure.
Pain intensity will be measured using a numeric rating scale (0-10 NRS) where 0 represents no pain and 10 represents “pain as bad as you can imagine”. Participants will be specifically asked to rate surgical site pain. A score ≥ 4 will be used to indicate moderate-severe pain. Numeric rating scale measurement of pain severity has been utilized in surgical patients and has been validated for use with surgical patients [13, 15, 16]. Pain assessment will also include a self-report measure of general (i.e., not surgical site specific) pain intensity and pain interference using the Brief Pain Inventory (BPI) Short Form. The BPI is a widely used 15-item measure that has been validated for use with postoperative pain [15, 16].
Secondary Outcomes.
Current opioid use will be assessed via self-report survey prior to surgery and at 3-months and 6-months post-surgery. Post-surgical opioid cessation will be assessed via daily opioid use collected via weekly phone calls informed by chart review. Opioid cessation will be defined as 7 consecutive days of taking no opioid medications. Weekly phone calls will be completed up to six weeks post-surgery or until the patient has discontinued all analgesic use. Information regarding the use of non-opioid analgesics and sedative-hypnotics will also be collected during weekly phone calls.
The Personal Health Questionnaire Depression Scale (PHQ-9) will be used to assess depressive symptoms. The PHQ-9 is based on diagnostic criteria for Major Depressive Disorder in the Diagnostic and Statistical Manual of Mental Disorders – Fourth Edition (DSM-IV). This measure has demonstrated validity and reliability for assessing depressive symptom severity [17]. The Generalized Anxiety Disorder Scale (GAD-7) will be used to assess for symptoms of anxiety. The GAD-7 is a 7-item self-report measure designed to assess Generalized Anxiety Disorder. Scores can range between 0–30. This measure has demonstrated reliability and validity as a measure of anxiety in the general population [18–20].
Pain-related functioning will be assessed via the Pain Disability Index (PDI). Participants will be instructed to complete this measure in relation to pre- and post-surgical pain at the site of surgery. The PDI is a 7-item measure assessing the impact of pain on functioning across multiple domains. This measure has demonstrated good validity and reliability [21, 22].
Potential Mediators of Treatment Effects.
Pain catastrophizing and pain self-efficacy will be assessed as potential mechanisms of treatment effects. The Pain Catastrophizing Scale (PCS) will assess for pain catastrophizing [23]. The PCS is a 13-item self-report measure that produces a total score and three subscale scores: Rumination, Magnification, and Helplessness. The PCS has good internal consistency (α = 0.87) and PCS scores predict pain intensity ratings in an acute pain paradigm [24]. The Pain Self-Efficacy Questionnaire (PSEQ) will assess for self-efficacy beliefs. The PSEQ is a 10-item scale with each item rated on 0 (not at all confident) to 10 (completely confident) for total scores ranging from 0-60. The PSEQ is a widely used measure among patients with chronic pain with established validity and reliability [25].
Implementation Outcomes.
Qualitative data will be collected to determine the acceptability, appropriateness, and feasibility of delivering the intervention in relation to surgery. Key stakeholders including patients, surgeons, clinic nurses, and clerical staff will be interviewed using guided semi-structured interviews. The interviews will elicit clinician and patient perspectives on the overall use of PePS as a method of pain management for surgical patients, as well as barriers and facilitators to uptake and implementation of PePS.
Description of Treatment Arms.
All participants will receive surgery and standard perioperative care as clinically indicated. Half of participants will also be randomized to receive the four telephone-based PePS program sessions.
Standard Care (SC).
All enrolled participants will receive SC. Current pre-surgery treatment includes instruction on pre-surgical preparation, and what to expect in the post-surgical period, including expectations for pain control and recovery. Patients may be taking analgesia (i.e., opioids and/or non-opioids) preoperatively and may be prescribed analgesics, sedatives and/or anxiolytics immediately prior to surgery. Intraoperatively, regional (i.e., spinal and femoral) or general anesthesia and analgesia is administered. In the immediate post-surgery period, patients receive opioids, non-opioids, anticonvulsants, and/or anxiolytics. Patients return home with analgesia (often a combination medication of an opioid and acetaminophen) for breakthrough pain.
Perioperative Pain Self-Management (PePS) intervention.
The development of this intervention was informed by CBT interventions for chronic pain [11, 26–32]. CBT for chronic pain is a self-management approach that emphasizes a collaborative relationship between the patient and therapist, then enacted to 1) modify maladaptive cognitive and behavioral coping strategies and 2) introduce alternate strategies to cope with chronic pain (i.e., graded activity, cognitive restructuring, task persistence, positive self-statements, distraction, relaxation, etc.) [33]. Similar to CBT for chronic pain interventions [34], the PePS program will target reducing fearful and catastrophic responses to pain, thereby increasing physical activity levels and engagement in valued domains of living. Participants randomized to PePS will receive a treatment workbook to serve as an instructional aid during the telephone sessions and will be completed both during and between sessions. Each week participants will be asked to complete written assignments in their workbook.
There are four PePS sessions. Session one (preoperative) is an introduction to relaxation and the importance of interpretation in the pain experience. Session two (postoperative) is a review of relaxation and the connection between thoughts/interpretation and pain, and introduction to thought records. Session three includes a review of thought records and relaxation, and introduction to goal-setting and cognitive restructuring. Session four includes a review of goal setting, relaxation, and cognitive restructuring. The final session will also involve creating a plan for continued use of the skills learned in the intervention (Table 2). The full protocol will be offered to patients with encouragement for them to try the various strategies and to continue practicing the strategies which work well for their pain management needs.
Table 2.
PePS Intervention
| Session | Content | Time to Surgery |
|---|---|---|
| 1 | Introduction to relaxation and the relevance of interpretation in the pain experience. | 1-2 weeks pre-surgery |
|
| ||
| Surgery | ||
|
| ||
| 2 | Review of relaxation, interpretation and pain, and introduction to thought records. | 2-weeks post-surgery |
| 3 | Review of thought records and relaxation. Introduction to goal-setting and cognitive restructuring. | 3-weeks post-surgery |
| 4 | Review of goal setting, relaxation, and cognitive restructuring. | 4-weeks post-surgery |
Sample Size Determination.
The study was powered to detect a significant difference in pain outcomes between the PePS and SC arms. It assumes the rate of persistent post-surgical pain post-arthroplasty is 20% following usual care [6–9]. Pilot data found an odds ratio of 0.34; to be conservative we used a moderately smaller odds ratio (0.4) for the sample size calculation, putting expected rates of persistent pain in the treatment arm at 9%. With 80% power and a 5% Type I error rate for a test of difference in proportions between arms, 160 participants would be needed per arm. To account for 20% attrition, a total of 400 participants will be needed. This sample size will allow for >80% power to detect differences between arms on secondary outcomes of opioid cessation, anxiety, depression, and pain-related functioning. We anticipate being able to recruit this sample across a three-year recruitment timeframe, based on the projected available surgical patients across the two sites and the recruitment rates from the pilot study.
For the qualitative interviews with patients, we anticipate completing 40 interviews (20 from each site) to reach data saturation. For the qualitative interviews with staff and providers at the two sites, we plan to interview about 20 participants (10 from each site), including surgeons (2-3 per site), clerical staff (2 per site), and clinic nurses (LPNs and RNs: at least 4 per site). We will oversample clinic nurses because they play a significant role in coordinating pre- and post-surgical care, via collecting clinical data to clear patients for surgery, coordinating outpatient visits, and providing patient education. We expect clinic nurses will be critical in future PePS program implementation. If we do not reach data saturation in a specific sub-group (i.e., surgeons, clerks, or nurses), we will continue to recruit from that sub-group until we reach data saturation or have attempted to recruit every member of the team.
Data Analysis Plan
The data analysis plan was approved by the Data Safety and Monitoring Board (DSMB) who convene annually to monitor the trial. Descriptive statistics (means, medians, percentages, standard deviations, and inter-quartile ranges) for all variables will be computed for each arm (PePS and SC). The distributions of continuous variables will be evaluated for normality. If data are non-normal, statistical analyses appropriate for non-normal data will be utilized (see below). Pre-surgical (i.e. baseline) assessment of variables will be compared across intervention groups using a t-test or Wilcoxon-rank sum test for continuous variables and Pearson Chi-square test for categorical variables. Variables that are found to significantly differ (p ≤ 0.10) between the groups (including pre-operative opioid use for comorbid conditions) will be used as covariates in the comparison of outcome measures between the treatment groups.
Intent-to-treat (ITT) analyses will be conducted to assess treatment efficacy on post-operative pain and opioid use on all participants that have been randomized and had surgery performed. Only those who receive surgery are included in these analyses to test the efficacy of PePS because our underlying model for treatment efficacy assumes that PePS will prevent the development of chronic post-surgical pain by helping patients cope with postoperative pain. Therefore, receipt of surgery is necessary.
Primary Outcome: Chronic Post-Surgical Pain.
Multivariable logistic regression adjusting for facility will be employed to determine the relationship between treatment arm and the dichotomous outcome: moderate to severe pain at 6-months post-surgery. To determine if any demographic or clinical measures modify the relationship between treatment arm and the outcomes, multivariable models will be fitted. For the primary outcome analyses, it is expected that randomization will lessen the need for covariate or moderator adjusted analyses. However, if any demographic, baseline (including pre-operative opioid use for comorbid conditions), or perioperative variables (i.e., perioperative anesthesia/analgesic) are found to differ between intervention groups, the model will be expanded to include these variables as covariates or effect moderators to estimate and test intervention group differences, or odds ratio, adjusting for these variables. Secondary analyses will examine changes in pain catastrophizing and pain self-efficacy (between baseline and 3-months) on pain severity at 6-months to determine their impact on this outcome variable as mediators of treatment effect.
Secondary Outcome: Opioid Cessation.
Efficacy of PePS compared to SC for post-operative opioid cessation will be examined using survival analysis methods. Time from date of surgery to cessation will be used as the endpoint. Those without the endpoint by six-weeks post-surgery will be considered censored observations at the time of last known follow-up. Kaplan-Meier curves will be constructed showing the product-limit estimate of the cumulative probability of survival (non-cessation) at the follow-up times for the observed endpoints. Median time to cessation will also be computed for each endpoint. PePS treatment effect size relative to SC, expressed as hazard ratio for these endpoints, will be estimated by fitting a Cox proportional hazard regression model that includes treatment group, treatment facility, and covariates (such as differences in pre-operative opioid use for comorbid conditions) to account for baseline group differences. The hazard ratio will compare the hazard rate in PePS versus SC groups, with the hazard rate defined as the probability that at a given time, an individual who remained event-free up to that time would experience the event. For this study, the hazard ratio will be the relative likelihood of cessation in the PePS versus SC subjects at any given point in time.
Secondary Outcome: Other Analgesic Cessation.
Similar to analyses for opioid cessation, survival analyses will also be utilized to determined time to cessation of all other post-operative analgesics (and sedative medications) comparing the PePS to SC arms. The same analyses described above will be used. In addition, descriptive analyses will characterize patterns of non-opioid analgesic and sedative prescriptions and rates of transition from opioid to other analgesic medications (e.g., discontinuing opioids but initiating gabapentin or duloxetine for pain) in both arms.
Secondary Outcomes: Depression, Anxiety, and Pain-Related Functioning.
Differences between the PePS and SC arms on these three measures will each be analyzed using independent samples t-tests comparing change scores between pre-surgery and 6-months post-surgery on each measure between arms. Alternately, Mann Whitney-U tests will be used if indicated by non-normal data distributions on these continuous measures.
Additional secondary analyses will include both follow-up timepoints: 3-months and 6-months post-surgery. All primary and secondary outcomes as well as opioid and other analgesic use at 3- and 6-months will be examined using repeated measures analyses.
Handling Missing Data.
In the case of missingness, reasons for missing will be recorded and compared between arms (PePS and SC). Participant characteristics at baseline for those that drop out post-surgery will be compared to those that complete the study. In the presence of a disparity of the distribution of the characteristics between missing and complete groups, under the assumption of missing at random (MAR), an inverse probability weighting procedure will be applied by building the propensity score link between missingness and participant characteristics at baseline. It will remove potential bias of estimates and lead to valid statistical inference. However, since the data under analysis cannot distinguish if data is MAR or it is missing not at random (MNAR), sensitivity analysis will also be performed using pattern mixture models. Multiple imputation will be used for sensitivity analysis by imputing from a non-random pattern mixture model.
Implementation Outcomes: Qualitative Analyses.
A separate codebook will be developed for the Veteran and provider interviews based on deductive and inductive thematic analysis [35, 36]. The study team will meet and review the first three interviews for thematic content. Deductive, a priori codes will be based on previous literature and the interview guide, while inductive codes will be based on themes that emerge from the data. The codebook will be hierarchical in that broad themes (e.g., feasibility of PePS) will comprise successively narrower, more specific themes. After the team adjusts the codebook as necessary and according to consensus, the team will code the remaining transcripts independently, meeting at intervals to code a transcript in tandem. They will discuss and adjust for conceptual drift from initial code definitions and make adjustments accordingly. Finally, coded material will be analyzed for cross-cutting themes shared across sites and roles, as and site-specific themes, such as environmental barriers.
Conclusion
The proposed study offers a novel approach to preventing chronic post-surgical pain and prolonged opioid use. This study will both test efficacy and readiness for implementation. Should PePS prove efficacious in preventing chronic post-surgical pain, PePS has the potential to expand and improve perioperative pain care. Including the psychological components of the biopsychosocial model of pain may optimize long-term pain and opioid use outcomes. In addition, we anticipate gathering key details to directly inform subsequent implementation of the PePS program. The knowledge gained will inform the design of an implementation project to incorporate this non-opioid adjunct to surgical pain self-management within the VA health care delivery system.
Supplementary Material
Acknowledgments
This work was supported by the U.S. Department of Veterans Affairs Department of Health Services Research & Development (IIR 20-115). The work reported here was also supported by the US Department of Veterans Affairs Health Services Research and Development (HSR&D) Service through the Center for Access & Delivery Research and Evaluation (CADRE) Center (CIN 13-412). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the U.S. Department of Veterans Affairs or the United States Government.
Footnotes
Disclosures and Conflicts of Interest: Dr. Yoon is a paid consultant for Paragon 28, a medical device company, to consult on a medical device used to repair fifth metatarsal fractures of the foot. All other authors report that they have no disclosures. All authors report that they have no conflicts of interest
Registration with clinicaltrials.gov: The Perioperative Pain Self-Management Trial: NCT04979429.
ClinicalTrials.gov Identifier: NCT04979429
References
- 1.Nahin RL, Severe Pain in Veterans: The Effect of Age and Sex, and Comparisons With the General Population. J Pain, 2017. 18(3): p. 247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kehlet H, Jensen TS, and Woolf CJ, Persistent postsurgical pain: risk factors and prevention. Lancet, 2006. 367(9522): p. 1618–25. [DOI] [PubMed] [Google Scholar]
- 3.Clarke H, et al. , Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ, 2014. 348: p. g1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morasco BJ, et al. , Systematic review of prevalence, correlates, and treatment outcomes for chronic non-cancer pain in patients with comorbid substance use disorder. Pain, 2011. 152(3): p. 488–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schug SA and Bruce J, Risk stratification for the development of chronic postsurgical pain. Pain Rep, 2017. 2(6): p. e627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macrae WA, Chronic post-surgical pain: 10 years on. Br J Anaesth, 2008. 101(1): p. 77–86. [DOI] [PubMed] [Google Scholar]
- 7.Bjornholdt KT, et al. , Persistent pain is common 1-2 years after shoulder replacement. Acta Orthop, 2015. 86(1): p. 71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robertsson O, et al. , Patient satisfaction after knee arthroplasty: a report on 27,372 knees operated on between 1981 and 1995 in Sweden. Acta Orthop Scand, 2000. 71(3): p. 262–7. [DOI] [PubMed] [Google Scholar]
- 9.Bourne RB, et al. , Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res, 2010. 468(1): p. 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruce J and Quinlan J, Chronic Post Surgical Pain. Rev Pain, 2011. 5(3): p. 23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehde DM, Dillworth TM, and Turner JA, Cognitive-behavioral therapy for individuals with chronic pain: efficacy, innovations, and directions for research. Am Psychol, 2014. 69(2): p. 153–66. [DOI] [PubMed] [Google Scholar]
- 12.Murphy JL, et al. , Cognitive behavioral therapy for chronic pain among Veterans: Therapist Manual, Washington DC: US Department of Veterans Affairs. [Google Scholar]
- 13.Hadlandsmyth K, et al. , Enhancing the Biopsychosocial Approach to Perioperative Care: A Pilot Randomized Trial of the Perioperative Pain Self-management (PePS) Intervention. Ann Surg, 2022. 275(1): p. e8–e14. [DOI] [PubMed] [Google Scholar]
- 14.Proctor E, et al. , Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health, 2011. 38(2): p. 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendoza TR, et al. , The utility and validity of the modified brief pain inventory in a multiple-dose postoperative analgesic trial. Clin J Pain, 2004. 20(5): p. 357–62. [DOI] [PubMed] [Google Scholar]
- 16.Zalon ML, Correlates of recovery among older adults after major abdominal surgery. Nurs Res, 2004. 53(2): p. 99–106. [DOI] [PubMed] [Google Scholar]
- 17.Kroenke K, Spitzer RL, and Williams JB, The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med, 2001. 16(9): p. 606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kroenke K, et al. , Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med, 2007. 146(5): p. 317–25. [DOI] [PubMed] [Google Scholar]
- 19.Spitzer RL, et al. , A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med, 2006. 166(10): p. 1092–7. [DOI] [PubMed] [Google Scholar]
- 20.Lowe B, et al. , Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population. Med Care, 2008. 46(3): p. 266–74. [DOI] [PubMed] [Google Scholar]
- 21.Chibnall JT and Tait RC, The Pain Disability Index: factor structure and normative data. Arch Phys Med Rehabil, 1994. 75(10): p. 1082–6. [DOI] [PubMed] [Google Scholar]
- 22.Tait RC, Chibnall JT, and Krause S, The Pain Disability Index: psychometric properties. Pain, 1990. 40(2): p. 171–82. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan MJL, Bishop S, and Pivik J, The pain catastrophizing scale: development and validation. Psychological Assessment, 1995. 7: p. 524–32. [Google Scholar]
- 24.Sullivan MJ and Bishop SR, The Pain Catastrophizing Scale: Development and Validation. Psychological Assessment, 1995. 4: p. 524–532. [Google Scholar]
- 25.Nicholas MK, The pain self-efficacy questionnaire: Taking pain into account. Eur J Pain, 2007. 11(2): p. 153–63. [DOI] [PubMed] [Google Scholar]
- 26.Williams AC, Eccleston C, and Morley S, Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev, 2012. 11: p. CD007407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aggarwal VR, et al. , Psychosocial interventions for the management of chronic orofacial pain. Cochrane Database Syst Rev, 2011(11): p. CD008456. [DOI] [PubMed] [Google Scholar]
- 28.Astin JA, et al. , Psychological interventions for rheumatoid arthritis: a meta-analysis of randomized controlled trials. Arthritis Rheum, 2002. 47(3): p. 291–302. [DOI] [PubMed] [Google Scholar]
- 29.Glombiewski JA, et al. , Psychological treatments for fibromyalgia: a meta-analysis. Pain, 2010. 151(2): p. 280–95. [DOI] [PubMed] [Google Scholar]
- 30.Henschke N, et al. , Behavioural treatment for chronic low-back pain. Cochrane Database Syst Rev, 2010(7): p. CD002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffman BM, et al. , Meta-analysis of psychological interventions for chronic low back pain. Health Psychol, 2007. 26(1): p. 1–9. [DOI] [PubMed] [Google Scholar]
- 32.Knittle K, Maes S, and de Gucht V, Psychological interventions for rheumatoid arthritis: examining the role of self-regulation with a systematic review and meta-analysis of randomized controlled trials. Arthritis Care Res (Hoboken), 2010. 62(10): p. 1460–72. [DOI] [PubMed] [Google Scholar]
- 33.Jensen MP, Nielson WR, and Kerns RD, Toward the development of a motivational model of pain self-management. J Pain, 2003. 4(9): p. 477–92. [DOI] [PubMed] [Google Scholar]
- 34.Otis JD, Managing Chronic Pain: A Cognitive Behavioral Therapy Approach. Treatments That Work, ed. Barlow DH. 2007, Oxford, U.K.: Oxford University Press. [Google Scholar]
- 35.Ryan G and Bernard H, Data management and analysis methods., in Handbook of Qualitative Research (2nd edn), ed. Lincoln N.K.D.a.Y.S.. 2000, London: Sage Publications. 769–802. [Google Scholar]
- 36.Ryan GW and Bernard HR, Techniques to Identify Themes. Field Methods 2003. 15: p. 85–109. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


