Abstract
Patients with obstructive sleep apnea (OSA) reveal functional changes in brain sites involved in autonomic, cognitive, and mood regulations. However, it is unclear whether these brain changes reverse with short-term positive airway pressure (PAP) treatment. Our aim was to examine brain functional changes in response to 3-months of PAP treatment using regional homogeneity (ReHo) measures, where increased and decreased ReHo value indicates hyper- and hypo- local neural activities, respectively, and considered as functional deficits. We collected brain magnetic resonance imaging data as well as mood, cognitive, and sleep variables from 17 treatment-naïve OSA at baseline and after 3-months of PAP treatment and 25 age- and gender-matched healthy controls. Whole-brain ReHo maps were calculated and compared between OSA and controls and OSA subjects before and after PAP treatment. At baseline, treatment-naïve OSA subjects showed higher ReHo in the bilateral thalamus, putamen, postcentral gyrus, paracentral lobule, supplementary motor area, and right insula, and lower ReHo in the frontal and parietal cortices, compared to controls. After 3-months of PAP treatment, abnormal sleep and mood scores decreased significantly to normal levels. ReHo decreased in the autonomic and somatosensory control areas, including the thalamus, putamen, postcentral gyrus, and insula, and increased in the cognitive and affective regulatory parietal regions. The normalized ReHo was correlated with improved sleep quality and reduced anxiety symptoms. These findings suggest that 3-months of PAP use can improve sleep, mood issues, and partly recover brain activities, however, longer PAP treatment may be required to fully and permanently reverse brain functional deficits.
Keywords: Functional magnetic resonance imaging, Regional homogeneity, Sleep disordered breathing, Autonomic, Cognition
INTRODUCTION
Obstructive sleep apnea (OSA) is a sleep disorder characterized by recurrent episodes of complete or partial obstruction of the upper airway, with continued diaphragmatic efforts to breathe during sleep [1]. The pattern leads to episodic oxygen desaturations accompanied by dramatic blood pressure changes [1]. OSA is associated with an increased risk of cardiovascular morbidity and mortality [2]. Acting through sympathetic neural mechanisms, OSA may contribute to elevated blood pressure in a large segment of hypertensive patients. OSA is associated with significantly increased daytime sympathetic nerve activity, which is thought to result from the repetitive intermittent periods of hypoxemia during sleep [3]. In addition to cardiovascular symptoms, OSA patients also show cognitive deficits, depression, and anxiety symptoms [4]. If left untreated, OSA can result in altered cognition with reduced attention, memory, and executive functioning, and cardiovascular diseases [5].
Positive airway pressure (PAP) is the primary non-surgical approach for treating OSA and is effective at improving daytime sleepiness and reducing the risks of cardiovascular complications [6]. It has been demonstrated that after 6-months of PAP treatment, patients showed less daytime sleepiness, reduced sympathetic tones and nocturnal symptoms, and improved mental acuity [3, 6]. Structural brain imaging studies showed partial brain white matter integrity recovery after 3-months of PAP and moderate improvements in mood, attention, and executive-functioning, paralleled with white matter changes [7]. Longer treatments (more than 12 months) in OSA patients lead to changes in brain metabolite levels, gray and white matter integrity in multiple brain regions closer to the levels seen in good sleepers, indicating that both acute and mild brain damage can be reversed with effective non-surgical treatments [7, 8]. Using simultaneously-recorded electrophysiological and brain imaging data, multiple studies showed that elevated neural signal intensity in the striatum, insula, thalamus, and sensorimotor cortex covaried with increased muscle sympathetic nerve activity in treatment-naïve OSA patients [3, 9], which falls significantly after 6 months of PAP treatment. In addition, the reduction in resting sympathetic nerve activity was coupled with significant lower neural activities in the related brain areas [3, 9]. However, the effects of short-term PAP treatment (i.e., less than 6 months) on brain functions and related regional neural activities are still unclear.
Resting-state functional magnetic resonance imaging (fMRI) measures the blood-oxygen-level dependent (BOLD) signal changes induced by spontaneous neural activities [10]. The technique has been widely-applied in various clinical studies examining neural activities and brain functions in OSA, and the results suggest an intrinsic neural reorganization that may explain the neuropsychological deterioration in the condition [11–13]. Among the fMRI procedures, Regional homogeneity (ReHo) is a sensitive measure of local coherence of neuronal activities, and can be used to examine regional brain functional integrity of OSA [12, 13]. Increased and decreased ReHo measures over controls indicate hyper- and hypo- local neural activities, respectively, and considered as functional deficits.
Our aim was to examine brain functional changes in newly-diagnosed, treatment-naïve OSA after 3-month PAP treatment using ReHo procedures. We hypothesize that PAP use will help restore regional neural activities in areas that showed functional abnormalities in OSA over healthy controls, and that these restored brain activities will show associations with improved sleep quality and neuropsychological functions.
MATERIALS AND METHODS
Subjects
We recruited 17 newly-diagnosed, treatment-naïve OSA subjects from the Sleep Disorders Laboratory at the University of California at Los Angeles (UCLA) Medical Center and 25 age- and gender-comparable healthy controls from the UCLA Medical Center and the West Los Angeles area. Demographic, sleep, mood, cognitive, and physiologic data of OSA and control subjects are summarized in Table 1. OSA subjects were diagnosed based on overnight polysomnography, and all OSA subjects had a moderate-to-severe disease severity [apnea-hypopnea index (AHI) ≥ 15 events/hour]. OSA and control subjects had no diagnosed history of neurological illness or psychiatric disorders. We determined the potential for sleep disordered breathing in controls by interviewing subjects, and subjects suspected of showing such disturbed patterns were excluded. Also, any subject with a metallic implant, weight over 285 pounds, or condition contraindicated for the MRI scanner environment was excluded. All participants gave written informed consent before MRI scanning or other data collection, and the study protocol was approved by the UCLA Institutional Review Board.
Table 1.
Demographic, sleep, mood, and cognitive variables of OSA and control subjects.
| Variables | OSA baseline (n = 17) | OSA 3-month (n = 17) | Controls (n = 25) | p values |
|---|---|---|---|---|
| Age range (years) | 31.4–64.0 | 31.7–64.3 | 32.8–62.2 | - |
| Mean age (years) | 48.9 ± 10.3 | 49.2 ± 10.3 | 46.4 ± 9.2 | 0.93/0.87/0.83 |
| Gender (Male:Female) | 8:9 | 8:9 | 12:13 | 1/1/0.9 |
| BMI (kg/m2) | 32.5 ± 4.8 | 32.0 ± 4.8 | 24.8 ± 3.5 | 0.79/0.0001/0.0001 |
| AHI (events/h) | 36.0 ± 23.3 | - | - | - |
| BAI | 7.2 ± 5.1 | 4.6 ± 4.6 | 3.8 ± 4.9 | 0.009/0.006/0.22 |
| BDI-II | 7.6 ± 4.3 | 3.8 ± 4.3 | 3.8 ± 4.9 | 0.005/0.004/0.68 |
| PSQI | 8.4 ± 3.6 | 6.1 ± 4.0 | 3.8 ± 2.5 | 0.015/0.0003/0.13 |
| ESS | 8.6 ± 4.0 | 6.1 ± 2.9 | 5.3 ± 3.6 | 0.007/0.008/0.40 |
| MoCA | 27.1 ± 2.3 | 28.2 ± 2.9 | 27.9 ± 2.6 | 0.18/0.23/0.12 |
p values, baseline vs. 3-month/baseline vs. controls/3-month vs. controls; OSA, Obstructive sleep apnea; BMI, Body mass index; AHI, Apnea-hypopnea index; BAI, Beck anxiety inventory; BDI-II, Beck depression inventory II; ESS, Epworth sleepiness scale; PSQI, Pittsburgh sleep quality index; MoCA, Montreal cognitive assessment.
Assessment of Sleep, Mood, and Cognition
We assessed sleep quality and daytime sleepiness in all OSA and control subjects using the Epworth Sleepiness Scale (ESS) and Pittsburgh Sleep Quality Index (PSQI) self-administered questionnaires, respectively. Higher scores on the ESS and PSQI indicate worse sleep. Depressive symptoms were evaluated by the Beck Depression Inventory II (BDI-II), and anxiety symptoms by the Beck Anxiety Inventory (BAI) in both groups. The BDI-II and BAI self-administered questionnaires include 21 questions (each question score ranges from 0–3), with total scores ranging from 0–63, based on depression or anxiety symptoms. Higher scores on the BDI-II and the BAI indicate worse depression/anxiety status. We used the Montreal Cognitive Assessment (MoCA) test for cognitive assessment (cognitive domains included attention, visuospatial/executive functions, memory, language, visuospatial skills, conceptual thinking, calculations, and orientation) with a maximum score of 30. Scores less than 26 on the MoCA indicate cognitive deficit.
Magnetic Resonance Imaging
All participants underwent brain structural and functional MRI in a 3.0-Tesla scanner (Siemens, Prisma Fit, Erlangen, Germany). We used foam pads on either side of the head to minimize head motion during scanning. High-resolution T1-weighted images were acquired using a magnetization prepared rapid acquisition gradient-echo (MPRAGE) pulse sequence [repetition time (TR) = 2200 ms; echo time (TE) =2.34, 2.41ms; flip angle (FA) = 9°; field of view (FOV) = 230×230 mm2; matrix size = 320×320; voxel size = 0.72×0.72×0.9 mm3]. BOLD-fMRI data were collected with an echo planar imaging (EPI)-based pulse sequence in the axial plane (TR = 2000 ms; TE = 30 ms; FA = 90°; FOV = 230×230 mm2; matrix size = 64×64; slice thickness = 4.2 mm; voxel size = 3.59×3.59×4.2 mm3; volumes = 150). During the fMRI scanning, all subjects were instructed to rest with their eyes open, without focusing on any specific thoughts.
Data Preprocessing
We first examined anatomical scans for any serious brain pathology using high-resolution T1-weighted images of all subjects. We also assessed fMRI data for imaging or head motion-related artifacts before data preprocessing. None of the subjects included here showed any serious brain injury, excessive head motion-related, or other imaging artifacts.
Prior to ReHo analysis, fMRI data were preprocessed using DPARSFA (http://rfmri.org/DPARSF) and SPM12 (https://www.fil.ion.ucl.ac.uk/spm) software: the initial 10 brain volumes were discarded to avoid signal saturation issues; the remaining 140 volumes were realigned to eliminate potential head-motion, and co-registered to T1-weighted images. The time series of each voxel was then band-pass filtered (0.01–0.08 Hz), and effects of linear drifting trend, six rigid-body motion parameters, their first and second derivatives, and global white matter, cerebrospinal fluid, and global brain signal changes were regressed. Images were then spatially-normalized to a canonical Montreal Neurological Institute space template using nonlinear transformation procedures, and spatially smoothed with a 4 mm full-width at half-maximum Gaussian kernel. Averaged cortical maps, derived from T1-weighted images of OSA and controls, and averaged whole-brain T1-weighted images, calculated from normalized T1-weighted images of all OSA and controls, were used for anatomical references.
ReHo Analysis
After finishing the preprocessing steps, ReHo maps were generated using the resting-state fMRI Data Analysis Toolkit [14]. ReHo value was determined for each voxel within the brain by calculating the Kendall’s coefficient of concordance (KCC) of the BOLD fMRI time course of the voxel with those of its nearest neighbors (26 voxels). Individual ReHo maps were converted into z-scored maps with Fisher’s z transformation to improve normality.
Network Analysis
In addition to ReHo analysis, which examines regional or local-level neural activities, we also examined network-level neural integrity/functional connectivity (FC) changes before and after CPAP treatment in OSA using independent component analysis (ICA). Different networks that are related to sleep and cognition, such as default mode network (DMN), executive control network, and frontoparietal network (FPN), were extracted using group ICA. Individual network component maps were converted into z-scored maps with Fisher’s z transformation to improve normality.
Statistical Analyses
Demographic, sleep, mood, cognitive, and physiologic data were examined by Chi-square (categorical values) and independent samples t-tests (numerical values) between OSA and control subjects. We compared the z-scored ReHo maps between OSA and controls at baseline, as well as between OSA after 3 months of PAP treatment and control subjects using analysis of covariance (ANCOVA; covariates, age and gender; Alphasim corrected, p < 0.05). We compared the z-scored ReHo and ICA network FC maps in OSA patients before and after 3 months PAP treatment using paired t-tests (Alphasim corrected, p < 0.05). Brain areas that showed significant changes in ReHo after treatment were selected as regions of interest (ROIs). The criteria to include these regions were: (a) voxel-wise p value < 0.005, and (b) the cluster size larger than 27 voxels. Regions surviving these criteria were: the bilateral ventral medial thalamus, bilateral putamen, bilateral postcentral gyrus, right anterior insula, left MPFC, left precuneus, left inferior parietal lobule, left angular gyrus, and SMA. We then examined correlations between the changes of regionally-averaged ReHo values in these ROIs, before and after the treatment, and the changes of sleep and neuropsychological variables using partial correlations (covariates: age and sex).
In addition, we used general linear model to explore the relationships between baseline AHI and sleepiness and sleep quality scores (ESS and PSQI), as well as mood symptoms (BDI-II and BAI scores).
RESULTS
Demographics, sleep, mood, and cognitive variables
There were no significant differences in age or gender between OSA and control groups. OSA patients had higher BMI than control subjects. Both sleep quality and daytime sleepiness (PSQI and ESS) and mood scores (BDI-II and BAI) were significantly higher in OSA at baseline over control subjects (independent samples t-tests, p<0.05; Table 1). After 3 months of PAP treatment, OSA patients showed significant decline (i.e., improved sleep and depression symptoms) in ESS, PSQI, BDI-II, and BAI scores (paired t-tests, p<0.05; Table 1), and showed no significant differences between OSA after PAP over controls (independent samples t-tests, p>0.05; Table 1). No significant differences in MoCA scores emerged between OSA before and after treatment or compared to controls (Table 1). Also, no significant correlations were observed between baseline OSA severity, sleepiness and sleep quality, and mood symptoms (p>0.15).
ReHo differences between treatment-naïve OSA and controls
Treatment-naïve OSA patients showed significantly higher ReHo signals (indicating hyperactivity) in the bilateral ventral medial thalamus, bilateral putamen, bilateral postcentral gyrus, paracentral lobule, supplementary motor area (SMA), right anterior, and posterior insula (Figure 1) in comparison to controls. OSA patients showed significantly lower ReHo (hypo-activity) in the bilateral medial prefrontal cortex (MPFC), left superior frontal gyrus, right superior parietal lobule, left precuneus, left inferior parietal lobule, and left angular gyrus (Figure 1).
Figure 1:
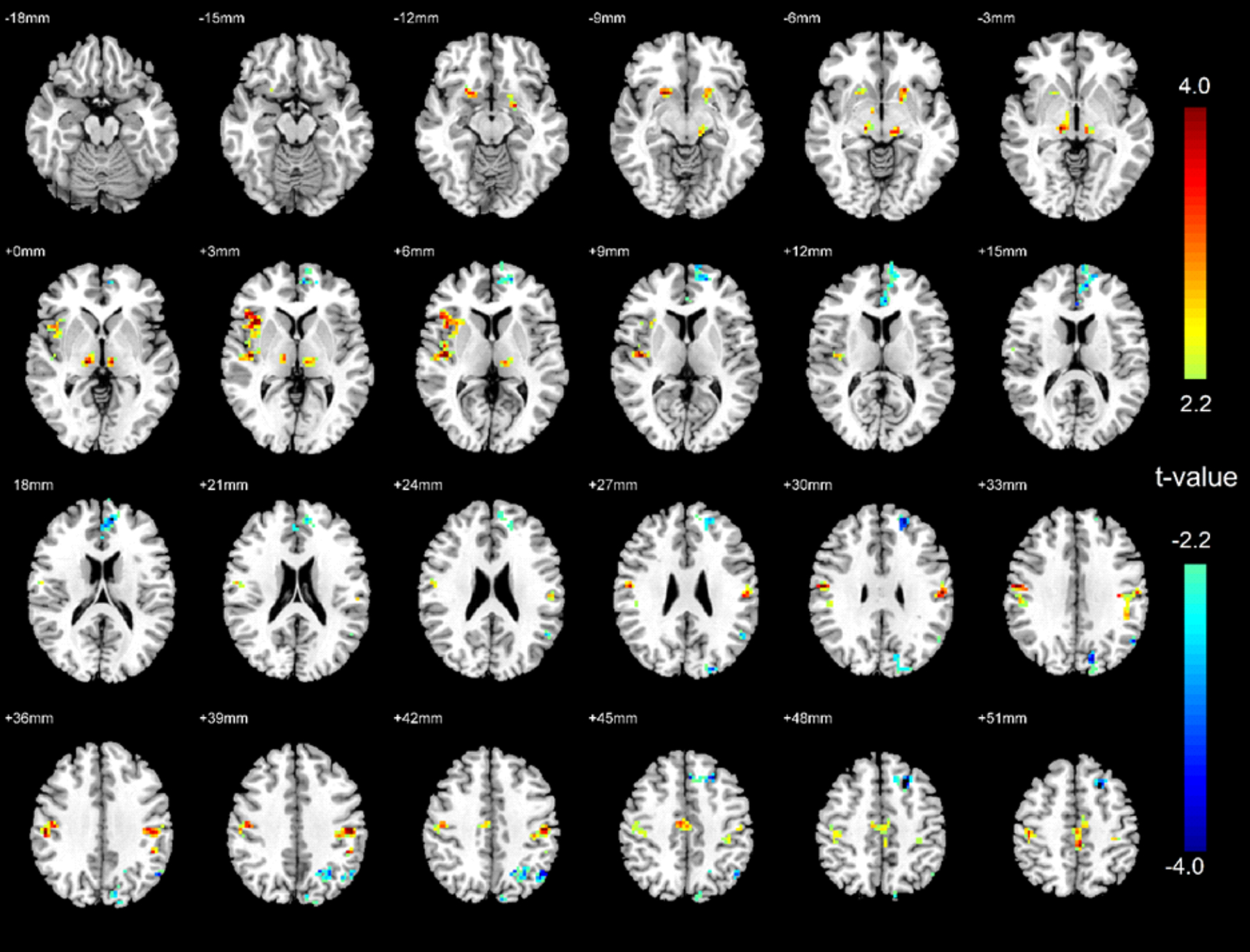
Brain regional homogeneity (ReHo) activity difference between treatment-naïve OSA patients and healthy controls. Areas with significantly higher or lower ReHo signals in OSA patients are shown in warm or cold color respectively.
ReHo differences between post-treatment OSA and controls
After 3 months of PAP use, OSA patients still showed higher ReHo (hyperactivity in comparison to controls) in the supplementary motor area and right posterior insula, and lower (hypo-activity) ReHo in the MPFC, left superior frontal gyrus, and right superior parietal lobule compared to healthy controls (Figure 2).
Figure 2:
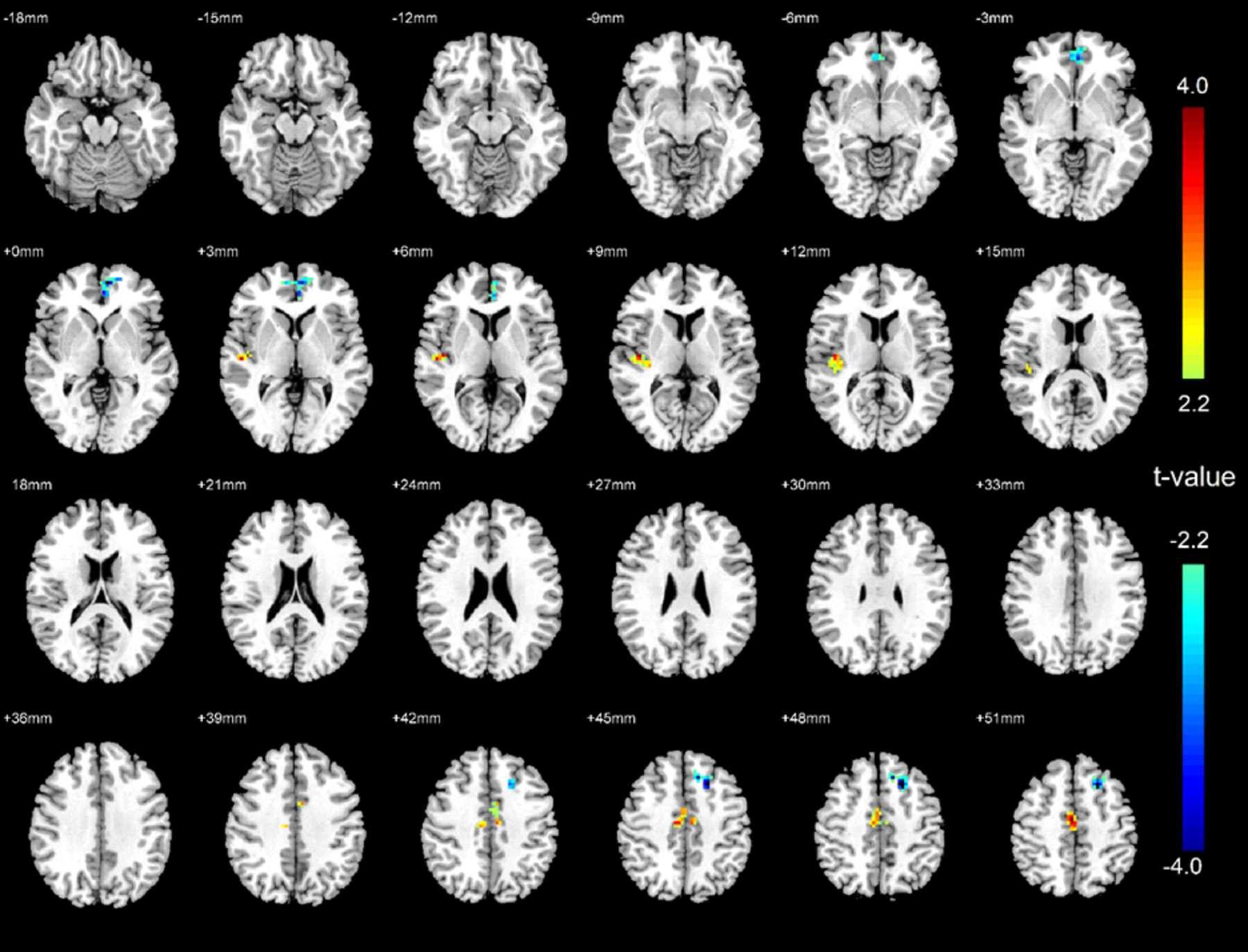
Brain regional homogeneity (ReHo) activity difference between OSA patients with 3-month PAP treatment and healthy controls. Areas with significantly higher or lower ReHo signals in OSA patients are shown in warm or cold color respectively.
ReHo changes after PAP treatment in OSA
Significantly decreased ReHo emerged in the bilateral ventral medial thalamus, bilateral putamen, bilateral postcentral gyrus, right anterior insula, and left MPFC after 3 months of PAP treatment in OSA subjects. In addition, OSA patients also showed increased ReHo in the left precuneus, left inferior parietal lobule, left angular gyrus, and SMA after 3 months PAP treatment (Figure 3). These findings show partial recovery of several brain sites (ReHo nearing control levels), including bilateral ventral medial thalamus, putamen, postcentral gyrus, right anterior insula, left precuneus, left inferior parietal lobule, and left angular gyrus.
Figure 3:
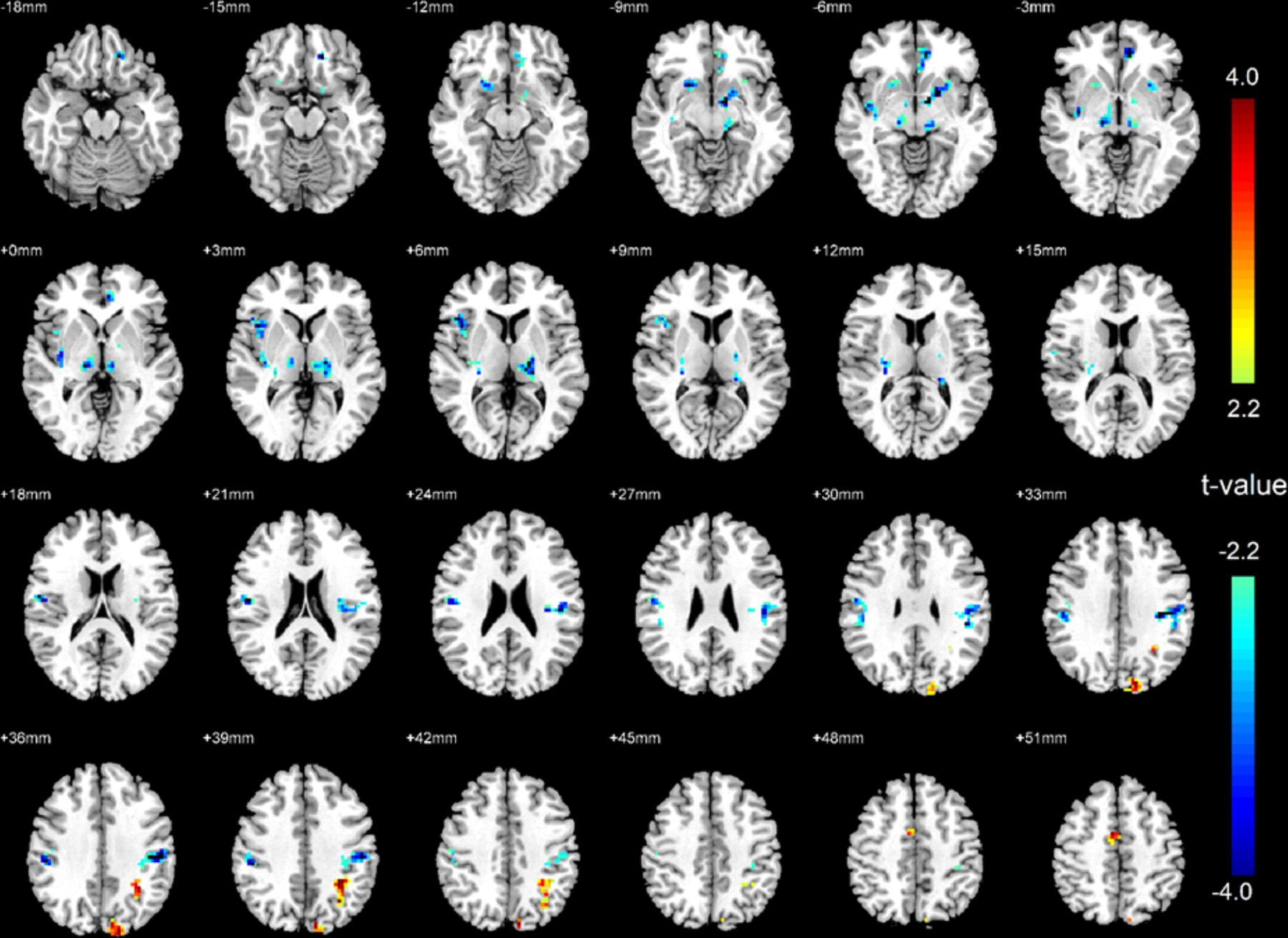
Brain regional homogeneity (ReHo) activity changes in OSA patients after 3-month PAP treatment compared to baseline. Areas with significantly higher or lower ReHo signals in after PAP treatment are shown in warm or cold color respectively.
Correlations between behavioral and ReHo changes in OSA
Decreased BAI scores (i.e., less anxiety) were correlated with ReHo signals decreasing in comparison to baseline OSA values in the right anterior insula and bilateral putamen after 3 months PAP treatment in OSA. In addition, the decreased PSQI scores (less abnormal sleep symptoms) were correlated with decreased ReHo in the bilateral postcentral gyrus (Table 2 and Figure 4). These results suggest association between improved behavioral symptoms and restored regional neural activities. The changes of ReHo in other brain areas were not significantly correlated with the behavioral variables.
Table 2.
Correlation between behavioral variable changes and ReHo changes.
| Variables | Brain areas | MNI coordinate | Cluster size (voxels) | R values | p values |
|---|---|---|---|---|---|
| BAI | Right Anterior Insula | 36 21 −9 | 44 | 0.54 | <0.001 |
| BAI | Left Putamen | −18 18 −6 | 35 | 0.51 | <0.001 |
| BAI | Right Putamen | 19 14 −3 | 30 | 0.47 | <0.001 |
| PSQI | Left Postcentral Gyrus | 54 −18 39 | 54 | 0.64 | <0.001 |
| PSQI | Right Postcentral Gyrus | 43 −27 39 | 65 | 0.86 | <0.001 |
BAI, Beck anxiety inventory; PSQI, Pittsburgh sleep quality index.
Figure 4:
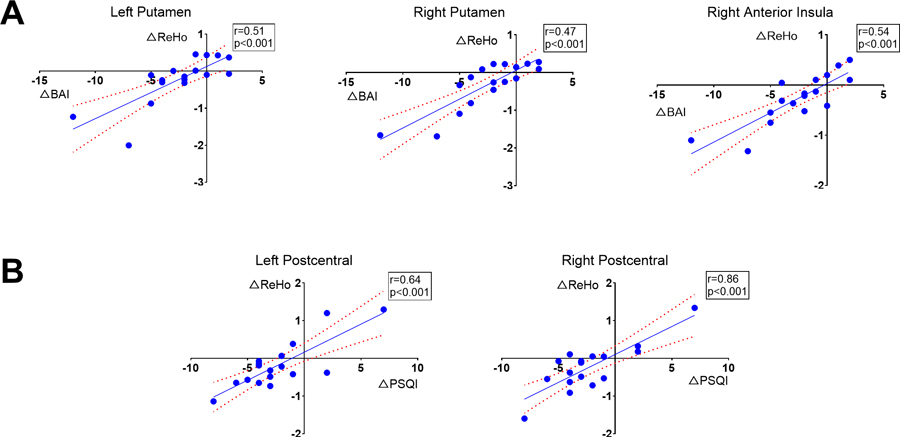
Correlations between the BAI (A), PSQI (B), and ReHo changes in OSA patients before and after 3-month CPAP treatment. (A) Changes in BAI are strongly correlated with the changes of ReHo in left and right putamen, as well as in right anterior insula. (B) The PSQI changes are strongly correlated with the changes of ReHo in left and right postcentral cortices.
Network changes after CPAP treatment in OSA
As a supplementary analysis, we evaluated FC changes before and after CPAP treatment in OSA using ICA. Significantly increased FC between the left and right angular gyrus in the FPN, and significantly increased FC in the precuneus cortex within the DMN, were observed in OSA after CPAP treatment over baseline (see Supplementary Figure 1). However, none of these FC changes were significantly correlated with BAI, BDI-II, ESS, or PSQI changes. Also, no significant changes of FC were observed in any other networks.
DISCUSSION
In this study, we investigated whether mood, cognitive, sleep quality, and daytime sleepiness symptoms improvements can be achieved after 3 months of PAP treatment, and whether corresponding brain functions can be restored as early as 3 months with PAP treatment in treatment-naïve OSA patients. The findings show that after 3 months of PAP treatment, PSQI, ESS, BAI, and BDI-II scores decreased significantly over baseline, reaching to the normal levels as in controls. Brain ReHo data show that after treatment, ReHo decreased toward normal levels in the bilateral ventral medial thalamus, bilateral putamen, bilateral postcentral gyrus, and right anterior insula, and ReHo increased toward normal control levels in the left precuneus, left inferior parietal lobule, and left angular gyrus in OSA patients. Reduced mood symptoms and improved sleep quality was associated with these normalized brain activities.
Abnormal ReHo in treatment-naïve OSA
Previous functional MRI studies in treatment-naïve OSA patients using ReHo procedures demonstrated similar results as shown here, and reported increased local neural activity coherence in the thalamus, striatum, insula, and SMA, and decreased local coherence in the MPFC, precuneus, and superior and inferior parietal lobe [12, 13]. The increased ReHo in the striatal-limbic areas might be associated with altered autonomic functions and mood dysregulation, while the decreased ReHo in the cortical areas may lead to impairments in attention, cognition, and executive functions [12].
The increased ReHo in the thalamus, putamen, insula, and motor areas in treatment-naïve OSA found here might contribute to increased sympathetic tone in the condition. Growing evidence suggests that OSA may contribute to the initiation and progression of hypertension, heart failure, cardiac arrythmia, and stroke [15]. Chronic sympathetic activation appears to be a key mechanism underlying the cardiovascular morbidity in OSA [15]. A number of studies have consistently shown that patients with OSA have high levels of sympathetic nerve traffic [16]. During sleep, repetitive episodes of hypoxia, hypercapnia, and obstructive apnea act through chemoreceptor reflexes and other mechanisms to increase sympathetic drive [16]. Remarkably, the high sympathetic drive is present even during daytime wakefulness in OSA subjects with normal breathing and no evidence of hypoxia or chemoreflex activation is apparent [15]. Previous functional MRI studies showed increased neural activity and functional connectivity in the thalamus, putamen, insula, and postcentral gyrus during autonomic challenges that elicit a sequence of sympathetic activities in healthy subjects, and the increased neural activities in the thalamo-striatal-limbic system were associated with increased blood pressure and heart rate [17, 18]. Other studies reported a positive correlation between muscle sympathetic nerve activity and signal intensity in the left and right insula, and thalamus in both OSA and control subjects [9]. Moreover, in OSA subjects, the striatum showed augmented functional MRI signal changes compared with controls [9]. Several neural and humoral mechanisms may contribute to maintenance of higher sympathetic activity and blood pressure, and may include chemoreflex and baroreflex dysfunction, altered cardiovascular variability, vasoconstrictor effects of nocturnal endothelin release, and endothelial dysfunction [15, 16]. The increased resting-state local neural synchronization in the thalamo-striatal-limbic circuit might be associated with autonomic dysfunction in OSA subjects.
Reduced ReHo signals were observed in the MPFC, superior frontal gyrus, superior and inferior parietal lobule, and angular gyrus in OSA patients over controls. These areas are important nodes of the DMN that is thought to be involved in high-order cognition, consciousness, sleep regulations, self-referential processing, visuospatial attention, and executive control [19]. Previous studies showed decreased resting-state functional connectivity and gray matter volume in the MPFC of the anterior DMN in patients with OSA, indicating both structural and functional deficits in the MPFC, which may be related to impaired cognitive function in the condition [13]. Decreased resting-state the DMN activities and increased resting-state thalamo-cortical network activities are also associated with slower reaction to stimuli and impaired daytime vigilance [19]. Moreover, intermittent hypoxia has a negative correlation with cerebral activation in the bilateral frontal and left parietal regions, and may be a major factor in cognitive and memory dysfunction in OSA patients [20]. The precuneus, angular gyrus, and superior and inferior parietal lobules play important roles in an array of advanced cognition functions, such as visuospatial imagery, top-down attention, and episodic memory retrieval [11]. Several MRI studies showed impaired functional connectivity between these cortical areas with subcortical structures, such as the hippocampus and caudate nuclei, sites that are involved in mood disorder, as well as cognitive and attention deficits found in OSA [11]. Positron emission tomography measurements of glucose utilization also indicated that cerebral metabolism was decreased in the precuneus and parietal cortex in OSA [21]. Other studies found that decreased ReHo in the precuneus showed a significant correlation with sleep time in OSA, suggesting decreased sleep time might be an important factor for dysfunction in the DMN in OSA [13].
Short-term PAP treatment and brain activities in OSA
After 3 months of PAP treatment, ReHo signals decreased in the bilateral ventral medial thalamus, putamen, postcentral gyrus, and right anterior insula in OSA subjects, and these areas were no longer significantly different between controls and OSA patients, indicating restored autonomic regulation and decreased sympathetic tone. Brain fMRI studies concurrently with muscle sympathetic nerve activity recording from percutaneously common peroneal nerve in OSA patients demonstrated positive correlations between sympathetic never activities and functional MRI signals from several brain sites, including the insula, thalamus, sensorimotor cortex, and mid-cingulate cortex [9, 22]. After 6 months of PAP treatment, muscle sympathetic nerve activity significantly reduced in OSA subjects, and coupled BOLD signal changes within the autonomic regulatory areas also returned to control levels [22]. Both abnormal systolic and diastolic blood pressure in OSA patients can be reversed as early as 3 months after CPAP therapy, with progressive improvement in cardiovascular remodeling over one year, as assessed by transthoracic echocardiography and cardiac MRI studies [2]. Our data also highlight the effectiveness of PAP treatment for reducing elevated sympathetic activities, one of the most significant health issues associated with cardiovascular symptoms and elevated morbidity in OSA condition.
Previous studies reported increased ReHo in the bilateral thalamus, postcentral gyrus, and SMA in treatment-naïve OSA patients over controls [12, 13]. In this study, we showed similar findings that short-term PAP treatment significantly reduced ReHo signals in the bilateral thalamic nuclei and postcentral gyrus. The thalamic sites are sensitive to hypoxia and can be alleviated by using PAP. Chronic intermittent hypoxia decreases NAA/Cr ratios in animal studies, a reliable measure of neuronal integrity/functionality, in the thalamus [23]. Human studies showed significant negative correlations between AHI and cerebrovascular reactivity in the thalamus in OSA patients; however, thalamic cerebrovascular reactivity increased with therapeutic PAP treatment [24]. Considering the fundamental role of the thalamus in the arousal mechanism and sleep-wake transition process along with autonomic and motor controls [25], an alteration of spontaneous diurnal behavior could represent the effect of an aberrant overstimulation during the night, while choke sensation and increased breathing effort components of apneic episodes induce brief and partial cortical arousal. The incomplete electrical cortical reactivations, repeated several times during the night could also have generated an aberrant thalamic hyperactivation during the rest condition [12].
PAP treatment can prevent microarousals and repetitive incomplete nocturnal transition from sleep to wakefulness induced by apnea or hypopnea and thus can improve sleep quality. As in previous studies, our results show a pattern of increased regional ReHo activities in the bilateral postcentral gyrus and SMA in OSA patients before treatment. Short-term PAP treatment reduced ReHo signals in the bilateral postcentral gyrus, which was further correlated with improved sleep quality. Altered pattern of brain activity in motor and somatosensory circuits at baseline could be linked to an increased mechanical effort that may occur during pathological arousal in sleep, with an immediate restoration of breathing muscle tone to waking levels [12, 13]. By improving sleep quality, there is a gradual reduction in local coherence rearrangement in the thalamus and postcentral gyrus.
We show that OSA patients demonstrate higher levels of depression and anxiety symptoms. The ReHo reduced significantly in the right anterior insula and bilateral putamen after 3 months PAP treatment, and the decreased ReHo signals in these areas were correlated with reduced anxiety symptoms. Insular cortices and putamen sites are not only involved in motor and somatosensory input integration with interoceptive autonomic action [26], but also in affect and mood regulation, e.g., blood pressure and breath changes accompanying emotional reactions [27]. Aberrant functional interactions between insular cortices, striatum, and other cortical regions have been described to be associated with anxiety and depressive symptoms in OSA [11, 28, 29]. The putamen also showed higher functional connectivity with insula in patients with comorbid movement and anxiety disorders than healthy controls and patients without anxiety [30]. Structural MRI studies also showed that tissue damage occurs in the insular cortices in anxious OSA subjects vs non-anxious OSA, as indicated by prolonged T2-relaxation values [27]. Timely PAP treatment may prevent brain injury and oxidative and neuro-inflammatory effects on emotional regulatory brain sites in OSA [5, 31, 32], and restore tissue integrity in the internal and external capsule surrounding the putamen and insula [33].
ReHo signals decreased significantly in the right anterior insula, but the right posterior insula still showed hyperactivities in OSA patients after 3 months of PAP treatment. Multiple studies support the notion that the right insular cortex is associated with sympathetic activity, and left insular cortex with parasympathetic control [34]. They also found differed responses in both the right and left insular cortices during autonomic challenge between the gyri in an anterior-posterior order, with greater responses in the anterior gyri [17, 18, 35]. These studies suggest that the right anterior insula activation is associated with exaggerated sympathetic activities. Short-term PAP treatment can partly reduce the hyperactivities in the insula and associated sympathetic outflows in OSA patients, and longer treatment may further reduce activities in the posterior part of insula.
We observed decreased ReHo signals in multiple frontal-parietal regions in OSA subjects before treatment. However, the ReHo signals in the precuneus, left inferior parietal lobule, and angular gyrus increased to normal levels after 3 months of PAP treatment. A number of structural MRI studies have shown that treatment-naïve OSA subjects are accompanied by a range of tissue changes, including altered white matter integrity, free water content, and regional gray matter volume changes, especially in the frontal and parietal regions that are responsible for cognition, attention, memory, and mood regulations [36–38]. Among OSA patients and sleep-deprived healthy volunteers, reduced attention and impaired working memory, accompanied by lowered parietal-occipital activation, may underlie performance decrements seen in other higher cognitive domains [39, 40], and PAP treatment can improve these functions. Task-based fMRI studies indicated that the beneficial PAP treatment effects on attention and working memory are associated with greater deactivation in the right posterior insula and increased activation in the right inferior parietal lobule [40]. Consistently, our results showed short-term PAP usage can restore the resting-state neural activities in attention, memory, and cognition regulatory areas. Other studies suggested that even shorter treatment with PAP (one month) could lead to adaptive alterations in the neurocognitive architecture that underlies the reduced sleepiness, and improved attention and memory functions in OSA patients, and these improvement are correlated with partial neural recovery occurred during short periods of PAP treatment [41].
ReHo signals in the parietal regions increased significantly after short-term treatment, but the ReHo in the MPFC and superior frontal gyrus remained low, suggesting that longer PAP usage might be required to fully recover the functions in these sites. MRI volumetric measures showed no differences in gray matter or white matter volume between PAP and sham PAP usage after 2-month treatment in OSA patients [42]. OSA patients have brain metabolite changes in the frontal lobe detected by MRS, suggestive of decreased frontal lobe neuronal viability and integrity [43]. Significant hypometabolism was also observed in the cuneus, precentral gyri, and anterior cingulate in OSA patients before PAP treatment, and after 3 months of PAP treatment, significant increases in cortical glucose metabolism were only observed in the precentral gyri and anterior cingulate cortex [44]. However, such improvements in hypometabolism in both areas were insufficient to reach control levels, and hypometabolism in the bilateral prefrontal regions persisted after 3 months PAP treatment [44]. Even after 6 months of PAP treatment, slight changes in the prefrontal metabolism were seen [43], but cognitive impairments were not fully reversed in some OSA patients [6], indicating that long-term administration of PAP treatment might be necessary.
In addition to regional neural activity changes, network integrity in the DMN and FPN was also improved after CPAP treatment in OSA. The DMN and FPN play important roles in sleep and mood, attention, and cognition regulatory functions [19]. The increased FC in these networks after treatment suggested that improved local neural activities or normalized ReHo may lead to overall restoration of normal brain network functions.
Several mechanisms may underlie the beneficial effects of PAP treatment on the brain, including the prevention of tissue injury induced by intermittent hypoxia or ischemia, and dysfunctional brain networks caused by sleep disturbances and fragmented sleep [5, 31, 45]. Consistent PAP usage can efficiently reduce the effects of intermittent hypoxia and hypoxemia [8], and protect the neuronal cells, axons, and glial structures [46]. PAP treatment may also stop hypoxia from triggering endothelial cell dysfunction that can compromise the blood brain barrier, as shown in OSA [47]; a long-term breakdown in the blood brain barrier can promote tissue injury [46]. Our study also showed improved sleep quality with PAP treatment in OSA patients. Sleep deficits and fragmented rapid eye movement sleep can alter molecular signaling pathways that regulate synaptic strength, plasticity-related gene expression, and protein translation in mood and cognition regulatory circuitries [48]. By preventing frequent brief awakenings, and ensuing fragmented sleep, PAP treatment can help restore cognitive and emotional functions [5].
CONCLUSIONS
PAP treatment, as short as 3-months, can significantly improve sleep quality and reduce daytime sleepiness, as well as reduce mood symptoms in OSA patients. Decreased ReHo in the thalamo-striatal-cortical circuit and increased ReHo in the parietal regions of the DMN may underlie these behavioral changes. However, hyperactivities in the supplementary motor areas and deficits in the frontal and parietal areas persist after short-term PAP treatment, indicating longer and consistent treatment may be required.
Supplementary Material
STATEMENT OF SIGNIFICANCE.
Brain sites regulating autonomic, cognitive, and mood functions in patients with obstructive sleep apnea (OSA) show functional deficits. Positive airway pressure (PAP), a primary treatment for OSA, is effective in improving OSA symptoms, but it is unknown whether 3-month of PAP treatment can restore regional neural dysfunctions. Regional homogeneity (ReHo), a sensitive measure of local coherence of neuronal activities, is being reported in OSA subjects for the first time to examine the effect of 3-months PAP treatment. The findings from this study show partial recovery of functional dysfunctions in vital brain sites and associated improvement in sleep quality and reduced anxiety symptoms and suggest longer PAP treatment for complete reversal of brain functional deficits.
ACKOWLEDGEMENTS
This research work was supported by the National Institutes of Health R01 HL-113251 and R01 NR-015038. In addition, Dr. Vacas was supported by NIH K23GM132795 and NIH R21 AG070269, and Dr. Kumar was also supported by NIH R21 AG070269.
Footnotes
Conflicts of interest:
Financial Disclosure: All authors have no financial conflicts of interest to declare.
Non-financial Disclosure: All authors have no non-financial conflicts of interest to declare.
Data Availability Statement:
The data underlying this article will be shared on reasonable request to the corresponding author.
REFERENCES
- [1].Lee W, Nagubadi S, Kryger MH, Mokhlesi B. Epidemiology of Obstructive Sleep Apnea: a Population-based Perspective. Expert review of respiratory medicine 2008;2:349–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Colish J, Walker JR, Elmayergi N, Almutairi S, Alharbi F, Lytwyn M, et al. Obstructive sleep apnea: effects of continuous positive airway pressure on cardiac remodeling as assessed by cardiac biomarkers, echocardiography, and cardiac MRI. Chest 2012;141:674–81. [DOI] [PubMed] [Google Scholar]
- [3].Fatouleh RH, Lundblad LC, Macey PM, McKenzie DK, Henderson LA, Macefield VG. Reversal of functional changes in the brain associated with obstructive sleep apnoea following 6 months of CPAP. Neuroimage Clin 2015;7:799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sforza E, de Saint Hilaire Z, Pelissolo A, Rochat T, Ibanez V. Personality, anxiety and mood traits in patients with sleep-related breathing disorders: effect of reduced daytime alertness. Sleep medicine 2002;3:139–45. [DOI] [PubMed] [Google Scholar]
- [5].Rosenzweig I, Glasser M, Polsek D, Leschziner GD, Williams SC, Morrell MJ. Sleep apnoea and the brain: a complex relationship. Lancet Respir Med 2015;3:404–14. [DOI] [PubMed] [Google Scholar]
- [6].Gay P, Weaver T, Loube D, Iber C. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep 2006;29:381–401. [DOI] [PubMed] [Google Scholar]
- [7].Castronovo V, Scifo P, Castellano A, Aloia MS, Iadanza A, Marelli S, et al. White matter integrity in obstructive sleep apnea before and after treatment. Sleep 2014;37:1465–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kim H, Joo E, Suh S, Kim JH, Kim ST, Hong SB. Effects of long-term treatment on brain volume in patients with obstructive sleep apnea syndrome. Hum Brain Mapp 2016;37:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fatouleh RH, Hammam E, Lundblad LC, Macey PM, McKenzie DK, Henderson LA, et al. Functional and structural changes in the brain associated with the increase in muscle sympathetic nerve activity in obstructive sleep apnoea. Neuroimage Clin 2014;6:275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature 2001;412:150–7. [DOI] [PubMed] [Google Scholar]
- [11].Song X, Roy B, Kang DW, Aysola RS, Macey PM, Woo MA, et al. Altered resting-state hippocampal and caudate functional networks in patients with obstructive sleep apnea 2018:e00994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Santarnecchi E, Sicilia I, Richiardi J, Vatti G, Polizzotto NR, Marino D, et al. Altered cortical and subcortical local coherence in obstructive sleep apnea: a functional magnetic resonance imaging study. Journal of sleep research 2013;22:337–47. [DOI] [PubMed] [Google Scholar]
- [13].Peng DC, Dai XJ, Gong HH, Li HJ, Nie X, Zhang W. Altered intrinsic regional brain activity in male patients with severe obstructive sleep apnea: a resting-state functional magnetic resonance imaging study. Neuropsychiatr Dis Treat 2014;10:1819–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yan CG, Wang XD, Zuo XN, Zang YF. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics 2016;14:339–51. [DOI] [PubMed] [Google Scholar]
- [15].Narkiewicz K, Somers VK. Sympathetic nerve activity in obstructive sleep apnoea. Acta physiologica Scandinavica 2003;177:385–90. [DOI] [PubMed] [Google Scholar]
- [16].Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. The Journal of clinical investigation 1995;96:1897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Song X, Roy B, Fonarow GC, Woo MA, Kumar R. Brain structural changes associated with aberrant functional responses to the Valsalva maneuver in heart failure 2018;96:1610–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Song X, Roy B, Lai M, Sahib A, Fonarow GC, Woo MA, et al. Aberrant Brain Functional Connectivity Dynamic Responses to the Valsalva Maneuver in Heart Failure. Journal of Cardiac Failure 2019;25:757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Song X, Qian S, Liu K, Zhou S, Zhu H, Zou Q, et al. Resting-state BOLD oscillation frequency predicts vigilance task performance at both normal and high environmental temperatures. Brain Struct Funct 2017. [DOI] [PubMed] [Google Scholar]
- [20].Prilipko O, Huynh N, Schwartz S, Tantrakul V, Kim JH, Peralta AR, et al. Task positive and default mode networks during a parametric working memory task in obstructive sleep apnea patients and healthy controls. Sleep 2011;34:293–301a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yaouhi K, Bertran F, Clochon P, Mezenge F, Denise P, Foret J, et al. A combined neuropsychological and brain imaging study of obstructive sleep apnea. Journal of sleep research 2009;18:36–48. [DOI] [PubMed] [Google Scholar]
- [22].Lundblad LC, Fatouleh RH, McKenzie DK, Macefield VG, Henderson LA. Brain stem activity changes associated with restored sympathetic drive following CPAP treatment in OSA subjects: a longitudinal investigation. J Neurophysiol 2015;114:893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Douglas RM, Miyasaka N, Takahashi K, Latuszek-Barrantes A, Haddad GG, Hetherington HP. Chronic intermittent but not constant hypoxia decreases NAA/Cr ratios in neonatal mouse hippocampus and thalamus. American journal of physiology Regulatory, integrative and comparative physiology 2007;292:R1254–9. [DOI] [PubMed] [Google Scholar]
- [24].Prilipko O, Huynh N, Thomason ME, Kushida CA, Guilleminault C. An fMRI study of cerebrovascular reactivity and perfusion in obstructive sleep apnea patients before and after CPAP treatment. Sleep medicine 2014;15:892–8. [DOI] [PubMed] [Google Scholar]
- [25].Jones BE. Arousal systems. Frontiers in bioscience : a journal and virtual library 2003;8:s438–51. [DOI] [PubMed] [Google Scholar]
- [26].Oppenheimer SM, Kedem G, Martin WM. Left-insular cortex lesions perturb cardiac autonomic tone in humans. Clinical autonomic research : official journal of the Clinical Autonomic Research Society 1996;6:131–40. [DOI] [PubMed] [Google Scholar]
- [27].Kumar R, Macey PM, Cross RL, Woo MA, Yan-Go FL, Harper RM. Neural alterations associated with anxiety symptoms in obstructive sleep apnea syndrome. Depression and anxiety 2009;26:480–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Park B, Palomares JA, Woo MA, Kang DW, Macey PM, Yan-Go FL, et al. Aberrant Insular Functional Network Integrity in Patients with Obstructive Sleep Apnea. Sleep 2016;39:989–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhang Q, Qin W, He X, Li Q, Chen B, Zhang Y, et al. Functional disconnection of the right anterior insula in obstructive sleep apnea. Sleep medicine 2015;16:1062–70. [DOI] [PubMed] [Google Scholar]
- [30].Wang X, Li J, Yuan Y, Wang M, Ding J, Zhang J, et al. Altered putamen functional connectivity is associated with anxiety disorder in Parkinson’s disease. Oncotarget 2017;8:81377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sforza E, Roche F. Sleep apnea syndrome and cognition. Frontiers in neurology 2012;3:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rosenzweig I, Williams SC, Morrell MJ. The impact of sleep and hypoxia on the brain: potential mechanisms for the effects of obstructive sleep apnea. Current opinion in pulmonary medicine 2014;20:565–71. [DOI] [PubMed] [Google Scholar]
- [33].Xiong Y, Zhou XJ, Nisi RA, Martin KR, Karaman MM, Cai K, et al. Brain white matter changes in CPAP-treated obstructive sleep apnea patients with residual sleepiness. J Magn Reson Imaging 2017;45:1371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].de Morree HM, Rutten G-J, Szabó BM, Sitskoorn MM, Kop WJ. Effects of insula resection on autonomic nervous system activity. Journal of neurosurgical anesthesiology 2016;28:153–8. [DOI] [PubMed] [Google Scholar]
- [35].Macey PM, Wu P, Kumar R, Ogren JA, Richardson HL, Woo MA, et al. Differential responses of the insular cortex gyri to autonomic challenges. Autonomic neuroscience : basic & clinical 2012;168:72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kumar R, Pham TT, Macey PM, Woo MA, Yan-Go FL, Harper RM. Abnormal myelin and axonal integrity in recently diagnosed patients with obstructive sleep apnea. Sleep 2014;37:723–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kumar R, Chavez AS, Macey PM, Woo MA, Yan-Go FL, Harper RM. Altered global and regional brain mean diffusivity in patients with obstructive sleep apnea. J Neurosci Res 2012;90:2043–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Macey PM, Kumar R, Woo MA, Valladares EM, Yan-Go FL, Harper RM. Brain structural changes in obstructive sleep apnea. Sleep 2008;31:967–77. [PMC free article] [PubMed] [Google Scholar]
- [39].Chuah LY, Chee MW. Functional neuroimaging of sleep deprived healthy volunteers and persons with sleep disorders: a brief review. Annals of the Academy of Medicine, Singapore 2008;37:689–94. [PubMed] [Google Scholar]
- [40].Aloia MS, Sweet LH, Jerskey BA, Zimmerman M, Arnedt JT, Millman RP. Treatment effects on brain activity during a working memory task in obstructive sleep apnea. Journal of sleep research 2009;18:404–10. [DOI] [PubMed] [Google Scholar]
- [41].Rosenzweig I, Glasser M, Crum WR, Kempton MJ, Milosevic M, McMillan A, et al. Changes in Neurocognitive Architecture in Patients with Obstructive Sleep Apnea Treated with Continuous Positive Airway Pressure. EBioMedicine 2016;7:221–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Huynh NT, Prilipko O, Kushida CA, Guilleminault C. Volumetric Brain Morphometry Changes in Patients with Obstructive Sleep Apnea Syndrome: Effects of CPAP Treatment and Literature Review. Frontiers in neurology 2014;5:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].O’Donoghue FJ, Wellard RM, Rochford PD, Dawson A, Barnes M, Ruehland WR, et al. Magnetic resonance spectroscopy and neurocognitive dysfunction in obstructive sleep apnea before and after CPAP treatment. Sleep 2012;35:41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ju G, Yoon IY, Lee SD, Kim YK, Yoon E, Kim JW. Modest changes in cerebral glucose metabolism in patients with sleep apnea syndrome after continuous positive airway pressure treatment. Respiration; international review of thoracic diseases 2012;84:212–8. [DOI] [PubMed] [Google Scholar]
- [45].Goldstein AN, Walker MP. The role of sleep in emotional brain function. Annual review of clinical psychology 2014;10:679–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Almendros I, Wang Y, Gozal D. The polymorphic and contradictory aspects of intermittent hypoxia. American journal of physiology Lung cellular and molecular physiology 2014;307:L129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Palomares JA, Tummala S, Wang DJ, Park B, Woo MA, Kang DW, et al. Water Exchange across the Blood-Brain Barrier in Obstructive Sleep Apnea: An MRI Diffusion-Weighted Pseudo-Continuous Arterial Spin Labeling Study. J Neuroimaging 2015;25:900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Abel T, Havekes R, Saletin JM, Walker MP. Sleep, plasticity and memory from molecules to whole-brain networks. Current biology : CB 2013;23:R774–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.


