Abstract
Background
Breast cancer survivors often experience many somatic and cognitive side effects resulting from their cancer diagnosis and treatment, including higher rates of pain, fatigue, and memory/concentration problems. Emotion regulation offers opportunities to either enhance or dampen physical health.
Purpose
In a secondary analysis of a double-blind randomized controlled trial (RCT) using a typhoid vaccine to assess factors associated with breast cancer survivors’ inflammatory responses, we assessed how two specific aspects of emotion regulation, mindfulness, and worry, corresponded to acute changes in focus problems, memory problems, and fatigue along with performance on pain sensitivity and cognitive tasks across two visits among breast cancer survivors.
Methods
Breast cancer survivors (N = 149) completed two 8.5-hr visits at a clinical research center. Survivors were randomized to either the vaccine/saline placebo or a placebo/vaccine sequence. Worry and mindfulness questionnaires provided data on trait-level emotion regulation abilities. Fatigue, memory problems, and focus difficulties were assessed via Likert scales six times—once before the injections and then every 90 min for 7.5 hr thereafter. Women also completed a pain sensitivity task and several cognitive tasks at each visit.
Results
Findings from this study showed that breast cancer survivors who worried more and were less mindful experienced subjective memory problems, focus problems, and cold pain sensitivity across two visits and irrespective of injection type. Lower mindfulness also corresponded to higher subjective fatigue and hot pain sensitivity and objective ratings. Emotion regulation skills did not predict objective pain sensitivity or cognitive problems.
Conclusion
Results from this study highlight the benefits of adaptive emotion regulation in helping mitigate symptoms associated with breast cancer survivorship.
Keywords: Mindfulness, Worry, Breast cancer, Pain, Fatigue
Survivors of breast cancer who were more worried and less mindful experienced greater subjective memory and focus problems and pain sensitivity.
Introduction
Survival rates from breast cancer continue to increase; however, breast cancer survivors commonly experience numerous deleterious health effects associated with diagnosis and treatment [1]. Several of these symptoms are somatic, including pain and fatigue. Epidemiological data suggest that cancer survivors have twice the likelihood of poor health and disability as individuals without a cancer history [2]. Nearly 30% of breast cancer survivors suffer from chronic pain 5 years after treatment [3], and cancer patients often endure pain and fatigue simultaneously [4]. In addition to self-reported pain, experimental pain sensitivity predicts the development of future chronic pain [5, 6]. For many survivors, pain persists after treatment completion, in part due to increased sensitivity of both the central and peripheral nervous systems. Pain sensitivity is also associated with pain catastrophizing and pain persistence among breast cancer survivors [7, 8]. Fatigue may also persist for years after cancer treatment [9] and can worsen other symptoms such as pain above and beyond oncological treatment [10].
In addition to the somatic symptoms experienced throughout survivorship, breast cancer survivors may also experience treatment-related difficulties with memory and focus. For example, breast cancer survivors had poorer executive function, working memory, and general cognitive function than women without a history of cancer [11, 12]. Across studies, research highlights that several aspects of cognitive function including memory, focus, and processing speed are impaired following chemotherapy and hormone-related therapies in breast cancer survivors [13]. Further, up to 67% of breast cancer survivors reported focus and/or memory problems after treatment completion [14–18]. Self-reported cognitive problems predict distress, fatigue, and poorer quality of life, and may reflect brain structure abnormalities (e.g., white matter lesions, hippocampal abnormalities) [19–22]. Indeed, self-report measures may detect subtle lapses in memory and concentration that neurocognitive tests are not sensitive enough to detect [15, 20]. While cancer-related cognitive difficulties dissipates over time for many survivors, a subgroup of women experience long-term impairments in memory, focusing, and executive function [23, 24].
Emotional health influences the experience and reporting of cognitive and somatic symptoms throughout survivorship. Emotion regulation, or the ability to intervene on one’s emotional experience, is an important consideration among breast cancer survivors [25]. Notably, the ability to adaptively regulate emotions can play a role in better adjustment following cancer treatment during survivorship [26]. The biobehavioral model of negative emotionality emphasizes the role that emotion regulation has in contributing to physical problems and long-term health problems [27]. Within this framework, emotion regulation can either enhance or suppress biological processes associated with the physical symptoms that commonly occur throughout cancer survivorship, leading to long-term physical difficulties that ultimately reduce quality of life and overall well-being.
Mindfulness, or the ability to view things from a detached, non-judgmental, and present-moment perspective, is one key emotion regulation strategy that may benefit survivors psychological and physical health throughout survivorship [28]. Indeed, greater mindfulness corresponds to better physical health among adults with and without chronic diseases [29–31]. In both physically health and disease populations, trait mindfulness corresponds to lower pain sensitivity and lower clinical pain more broadly [32, 33]. Among breast cancer patients and survivors, trait mindfulness is negatively correlated with pain sensitivity and fatigue [34]. Path analyses have also revealed low trait mindfulness as the strongest psychological correlate of fatigue among breast cancer survivors compared to anxiety, depression, and loneliness, and further, pathways between fatigue and mindfulness were both direct and indirect through these other variables [35]. Mindfulness is central to several contemporary treatment approaches to reduce distress among cancer survivors, highlighting its benefits for improving both psychological and physical health [36–38]. Regarding cognitive function, a core feature of mindfulness pertains to the ability to shift and sustain attention, thus enhancing attentional control and executive function [39]. Further, mindfulness training can enhance memory [40, 41].
In contrast, worry, or thinking repeatedly about things that may or may not happen in the future, is considered a maladaptive emotion regulation strategy [42]. Specifically, worry is used maladaptively to try to “solve” or “plan” real or perceived threats [43]. Research highlights that breast cancer survivors experience high rates of anxiety, including fears of cancer recurrence and more general worries associated with diagnosis, treatment, lifestyle changes, and changes in functioning [44]. These rates differ based on a number of treatment-related factors including stage, prognosis, disruptions to family, marital, financial, and occupational functioning; however, a 2020 meta-analysis demonstrated approximately 42% of breast cancer patients experienced anxiety—a staggering statistic that emphasizes the need to intervene on the anxiety experienced among these women [44]. Like their healthy counterparts, breast cancer survivors may worry about family, finances, and the future; yet in addition, they may worry about cancer recurrence, role changes because of their diagnosis and treatment, upcoming doctors’ appointments, testing, and ongoing symptom burden. Breast cancer survivors who report higher levels of worry have poorer self-rated health, as well as greater self-reported pain and fatigue [45, 46]. Worry also tracks cross-sectionally with poorer self-rated health in physically healthy adults [47]. Consistent with the perseverative cognition hypothesis, worry (along with rumination) heightens emotional intensity and creates distress by prolonging both objectively innocuous and threatening situations. Further, this heightened emotional intensity resulting from worry prolongs physiological reactivity to stress. Worry is a hallmark of anxiety among both healthy individuals and cancer patients [48, 49]. Consequently, the maladaptive process of worrying serves as an important risk factor for decreasing psychological and physical health throughout cancer treatment and survivorship.
Current Study
Understanding how emotion regulation skills influence symptom burden will help identify useful intervention targets for breast cancer survivors. Emotion regulation skills can be changed while, alternatively, other factors associated with cancer, such as treatment-related factors, are not as easily modified. This secondary analysis of a randomized controlled trial (RCT) [50]; assessed the relationship among two factors representing emotion regulation (mindfulness and worry) and acute changes of cognitive (memory and focus problems) and somatic (fatigue) symptoms across 2 day-long study visits in breast cancer survivors.
As part of the parent RCT, survivors completed two laboratory visits and received either a placebo or typhoid vaccine injection at each visit. Fatigue, focus, and memory were measured six times—once before the injection and then every 90 min for 7.5 hr thereafter. Survivors also completed a task measuring pain sensitivity (i.e., the hot–cold plate task) and several cognitive tasks, detailed below, across the day at each visit. We hypothesized that higher trait worry would correspond to more subjective fatigue, memory problems, and focus difficulties across the day compared to low levels of worry. Relatedly, we hypothesized that higher trait mindfulness would correspond to less subjective fatigue, focus problems, and memory problems across the day among survivors. We expected that mindfulness and worry would have contrasting effects on pain sensitivity and performance on the cognitive tasks: we hypothesized that worry would enhance pain sensitivity and lower performance on the cognitive tasks, while mindfulness would suppress pain sensitivity and contribute to enhanced cognitive performance. Given the parent study’s interests in examining the influence of injection type on inflammation across the day, we also assessed whether any differences in somatic and cognitive symptoms emerged based on injection type.
Methods
Participants and Procedures
Participants included breast cancer survivors (N = 149) ages 36–78, who had been diagnosed with Stage I–IIIA breast cancer 1–9 years after the completion of all primary cancer treatment except for longer-term hormonal therapies (tamoxifen, aromatase inhibitors). All of the women in this study were breast cancer survivors and had completed their primary cancer treatment, and subsequently, no women were currently receiving chemotherapy or radiation at their time of study participation. Participant demographics are presented in Table 1. The majority of survivors in this study were White and well educated. The primary recruitment sources were the James Cancer Hospital breast cancer clinics, with secondary recruitment through the Army of Women website. Exclusions included a prior history of any other malignancy except basal or squamous cell skin cancers, strokes, diabetes, anemia, current heart disease, or uncontrolled hypertension, liver disease, autoimmune and/or inflammatory diseases, a prior typhoid vaccination, any other vaccination within the past month, alcohol/drug abuse, smoking, and medical conditions that would have limited participation (e.g., cognitive dysfunction). Based on the parent trial’s examination of inflammatory responses to vaccine, medication exclusions included steroids, statins, and other medications with anti-inflammatory actions. Women who reported secondary cancers or a breast cancer recurrence we also excluded. Additional information regarding inclusion criteria and recruitment are available elsewhere [50]. We chose a typhoid vaccine because its inflammatory sequalae have been well characterized in multiple studies which have shown that a typhoid vaccination reliably elicits IL-6, IL-1 receptor antagonist (IL1Ra), and WBC responses within a few hours without inducing fever or notable discomfort, aside from mild injection site pain.
Table 1.
Participant demographics
| Mean (SD) or N (%) | Range | |
|---|---|---|
| Age | 56.8 (8.3) | 36.0–78.0 |
| Race | 137 (92.0%) | |
| White | 10 (6.7%) | |
| Black | 2 (1.3%) | |
| Mixed Race | ||
| BMI, kg/m2 | 27.9 (6.0) | 18.7–45.5 |
| <18.5 underweight | 0 (0%) | |
| 18.5-24.9 Healthy | 54 (36.2%) | |
| 25-29.9 Overweight | 50 (33.6%) | |
| >29.9 Obese | 45 (30.2%) | |
| Trunk fat, kg (DXA) | 15.7 (6.8) | 3.1–34.3 |
| Education | ||
| High school or less | 18 (12.1%) | |
| Some college | 25 (16.8%) | |
| College graduate | 52 (34.9%) | |
| Graduate school/professional training | 54 (36.2%) | |
| Years since treatment | 3.2 (2.3) | |
| Chemotherapy treatment | 100 (67.1%) | |
| Radiation treatment | 87 (58.4%) | |
| Current Hormone therapy | 121 (81.2%) | |
| Cancer stage | ||
| Stage I | 74 (49.7%) | |
| Stage II | 69 (46.3%) | |
| Stage III | 6 (4.0%) | |
| Any comorbidities | 18 (12.1%) | |
| PSWQ | 43.6 (12.8) | 17–76 |
| MAAS | 4.3 (0.9) | 1.4–6.0 |
SD standard deviation, BMI body mass index, PSWQ Penn State Worry Questionnaire total score, MAAS Mindful Attention & Awareness Scale total score. Comorbidities were measured using the Charlson Comorbidity Index.
All survivors received financial compensation ($25 for the screening visit; $250 for each of the two full-day study visits, totaling $525) for their participation and provided written informed consent. In the study’s double-blind crossover design, women were randomized to either the vaccine/placebo or the placebo/vaccine sequence at the first of two full-day study visits. Survivors first completed a screening appointment where they completed trait-level self-report questionnaires. The first full-day study visit occurred within 1 month of the screening appointment. Consistent with the parent trial’s goal of assessing inflammatory changes based on the vaccine challenge, during each day-long visit an intravenous catheter was inserted on admission, and baseline blood samples were drawn following a 20–30 min adaptation period. A nurse injected saline (the placebo) or Typhoid capsular polysaccharide vaccine (Typhim-Vi, Sanofi Pasteur) into the non-dominant deltoid muscle. Women received standardized meals. Subsequent blood draws occurred every 90 min for the next 7.5 hr. Women rated the intensity of physical symptoms (focus, memory, and fatigue) from 0 to 9 at each blood draw (for a total of 8 ratings across the day). Between ratings #6 and #7 (between approximately 1:45 and 3:00 pm), women completed a pain sensitivity task (see details below). They also completed cognitive tasks between ratings #6 and #8 (between approximately 1:45 and 5:00 pm). Data collection occurred between August 2013 and May 2021. Although the goal was 1 month between visits, the two ~ 9.5-hr sessions occurred 26–420 days apart (M = 46.87, SD = 47.48) because the COVID-19 pandemic delayed some sessions. A total of nine participants dropped out between Visit 1 and Visit 2. A detailed CONSORT diagram can be found elsewhere [50].
Emotion Regulation Measures
The Penn State Worry Questionnaire (PSWQ) is a 16-item measure assessing trait worry. Participants rate their responses on a 1–5 scale for what is typical for them, with higher scores indicating greater general worry; The PSWQ is considered the gold standard measure of worry [51] and validation studies have found PSWQ scores to be consistent across a 1-month period in the absence of participation in psychotherapy [51]. Internal consistency for the PSWQ in this study was excellent (α = 0.92).
The Mindful Attention and Awareness Scale (MAAS) is a 15-item measure of trait-level mindfulness validated in a cancer population; Higher scores indicate being more mindful [52]. Prior research in participants in the USA demonstrates that the MAAS has good test–retest reliability through 10 weeks [53]. The MAAS demonstrated excellent internal consistency in this study (α = 0.89).
Symptom Changes
Six times throughout the day, women rated their fatigue, memory problems, and focus difficulties on a Likert scale of 0–9 with higher ratings indicating that they were experiencing more of a given symptom. Each question asked women to report their symptoms at the current moment (e.g., how much fatigue are you currently experiencing?; how much difficulty are you currently having focusing?; how much difficulty are you currently having remembering things?)
Hot/Cold Task
A thermal sensory analyzer was used to determine sensitivity to heat- and cold-induced pain (TECA AHP-1200DCP Advanced Medical Systems, Minneapolis, MN). The order of whether the hot or cold trial was administered first was randomized for each visit; the time between trials was approximately 45 min. During both trial types, a thermode plate was placed in full contact with the survivors’ hand. The temperature was increased or decreased, depending on the trial type, at a rate of 1°C/s. Women were asked to inform the research assistant when the pain became noticeable and were instructed that they could remove their hand at any time when the heat/cold became unbearable. Survivors reported their pain rating on a 0–100 scale every 30 s for the cold condition and every 15 s for the hot condition. The maximum thermode temperature was limited to 53°C to prevent tissue damage. The time each survivor kept their hand on the plate for either the hot or cold trial corresponded to the objective pain sensitivity for hot/cold, respectively. Survivors also reported their pain level using the 0–100 scale at the time they removed their hand from the plate; this rating corresponded to their subjective pain tolerance.
Cognitive and Memory Tasks
The Conners’ Continuous Performance Test (CPT) is widely used to measure attention, focus, and reaction time [54]. On this computerized task, survivors were told to click the space bar when any letter appeared except the letter “X”; They were instructed to refrain from clicking if they saw the letter “X”. The CPT yields several metrics of attention and focus including overall reaction time (the amount of time between the presentation of the stimulus and when the survivor responded), omissions (the number of times a target was presented but the survivor did not respond), and commission errors (the number of times the survivor responded but no target was presented). High omission errors correspond to inattention, while high commission errors index impulsivity.
The Hopkins Verbal Learning Task assessed verbal learning and verbal memory [55, 56]. Survivors were asked to immediately recall a presented word list, then to recall it after a 20-min delay. For the purposes of the current study, the total number of words recalled after the 20-min delay assessed delayed recall.
Covariates
Covariates were chosen a priori based on their theoretical and biological connections to physical symptoms associated with breast cancer diagnosis and treatment [57–59]. These covariates included age, cancer treatment, obesity, depressive symptoms, physical fitness, injection sequence, cancer stage, time since cancer treatment, receipt of hormone therapy (yes/no), and presence of medical comorbidities. Models for focus problems, memory problems, and cognitive outcomes additionally controlled for education level. The Charlson comorbidity index, originally developed with breast cancer patients, assessed physical comorbidities [60]. The Charlson assigns weights to 19 medical conditions with greater scores equal to a greater comorbidity burden. The Center for Epidemiological Studies Depression Scale assessed current depressive symptoms [61]. Central obesity was assessed using dual X-ray absorptiometry (DXA) (model DPX-NT/software version 5.60, GE Lunar, Madison, WI).
Statistical Methods
Changes in symptoms (fatigue, focus problems, memory problems) across the day for both visits were modeled using generalized estimating equations (GEE) with robust standard errors and an independent working correlation structure to account for within-subject correlations both within visits and between visits. Time since injection was used as a categorical variable in all models since changes in symptoms were not assumed to be linear. Vaccine effects on outcomes were tested first by modeling fatigue, focus problems, and memory problems across the day (including baseline) with effects of injection type, time since injection, and their interaction. The effects of worry and mindfulness on post-injection symptoms were then tested by modeling only the post-injection symptom measurements while controlling for pre-injection baseline symptom levels, to adjust for pre-injection outcome differences across levels of predictors (e.g., difference in baseline fatigue by worry or mindfulness). The three-way interaction of worry or mindfulness by injection type by time since injection, as well as all lower-order terms, were included in all models to test if effects of worry or mindfulness on symptoms differed between the placebo and vaccine visit. All interactions were retained in models regardless of their significance so as to properly reflect the study design. To assess whether worry or mindfulness were associated with pre-injection fatigue, focus problems, and memory problems, GEE with robust standard errors was also used due to each participant having two visits and thus two baseline symptom measurements.
GEE with robust standard errors and an independent working correlation structure was also used for outcomes measured only once per visit (pain sensitivity: temperature and pain ratings at pain threshold during hot and cold task; cognitive outcomes: HVLT: total number words correctly recalled; CPT: correct response speed, response speed consistency, missed targets, incorrect responses to non-targets). In these models the two-way interaction of worry or mindfulness by injection type was of primary interest.
Covariates were entered into all models. A two-sided significance level of α = 0.05 was used for all tests. Only participants who completed both full-day study visits were included in analysis. Analyses were performed in SAS version 9.4 (SAS institute, Cary, NC).
Results
Vaccine Effects on Symptoms
Post-injection changes in fatigue (p = .91), focus (p = .80), and memory problems (p = .81) did not differ by injection type (Fig. 1A–C). For fatigue and focus problems, mean levels at the end of the day were significantly higher than at baseline for both placebo (fatigue: B = 0.63, SE = 0.18, p = .0005; focus: B = 0.55, SE = 0.15, p = .0003) and vaccine visits (fatigue: B = 0.69, SE = 0.17, p < .0001; focus: B = 0.35, SE = 0.17, p = .04), but there was not a difference between injection types (ps > .3). There was not a significant change in memory problems from baseline to the end of the day at either visit (ps > .05).
Fig. 1.
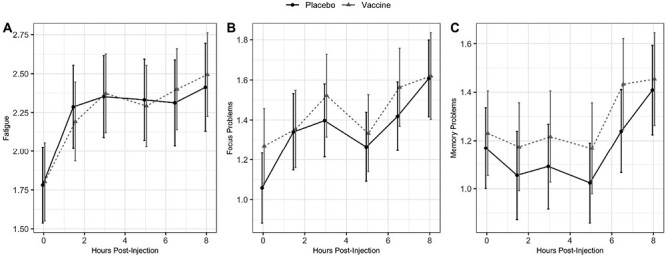
Trajectories of (A) fatigue, (B) focus problems, and (C) memory problems across the day, by injection type. Estimates from GEE models adjusting for age, cancer treatment, hormone treatment, obesity, depression, physical fitness, visit order, cancer stage, time since cancer treatment, and presence of comorbidities. Error bars represent 1 standard deviation above/below the mean
Effects of Worry and Mindfulness on Pre-injection Symptoms
Neither worry nor mindfulness were significantly associated with pre-injection levels of fatigue or memory problems (ps > .14). Higher worry was associated with lower pre-injection levels of focus problems (B = −0.21, SE = 0.10, p = .04), but there was no association between mindfulness and pre-injection focus (p = .14).
Effects of Worry and Mindfulness on Post-injection Symptoms
Higher worry and lower mindfulness were associated with higher average post-injection focus and memory problems averaged across injection types (main effect ps < .01, Fig. 2) after controlling for pre-injection outcome levels. Furthermore, consistent with study hypotheses, the mindfulness effect on memory problems was exaggerated during the vaccine visit compared to placebo visit (p = .03). A one standard deviation increase in mindfulness was associated with a 0.14 unit decrease in memory problems at the placebo visit compared to a 0.36 unit decrease at the vaccine visit, highlighting that greater mindfulness decreased memory problems throughout the vaccine visit. Higher mindfulness also corresponded to lower post-injection fatigue (main effect p < .001, Fig. 2) and this effect did not differ by injection type (p = .12). Worry did not significantly correspond to post-injection fatigue (ps > .09). Table 2 presents the coefficients for each of these tests.
Fig. 2.
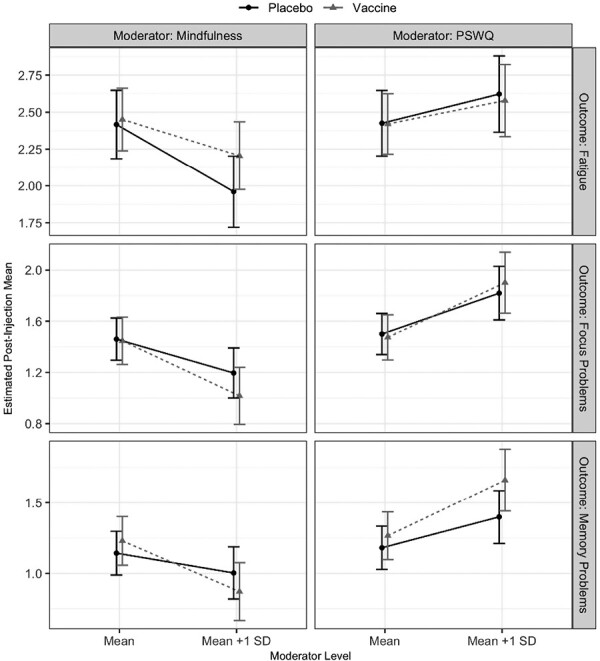
Average post-injection levels of fatigue, focus problems, and memory problems by injection type and levels of mindfulness (left column) and worry (right column). Estimates from GEE models adjusting for age, cancer treatment, hormone treatment, obesity, depression, physical fitness, visit order, cancer stage, time since cancer treatment, and presence of comorbidities. Error bars represent 1 standard deviation above/below the mean.
Table 2.
Estimated effects of mindfulness and worry on average post-injection fatigue, focus problems, and memory problems
| Outcome | Injection type | Change for +1 SD increase in mindfulness (95% CI) | p-value | Change for +1 SD increase in PSWQ (95% CI) | p-value |
|---|---|---|---|---|---|
| Fatigue | Placebo | −0.46 (−0.69, −0.22) | .0001 | 0.20 (−0.05, 0.44) | .12 |
| Vaccine | −0.25 (−0.48, −0.01) | .04 | 0.16 (−0.08, 0.39) | .19 | |
| Averaged | −0.35 (−0.54, −0.16) | .0004 | 0.18 (-0.03, 0.38) | .09 | |
| Focus | Placebo | −0.26 (−0.46, −0.07) | .008 | 0.32 (0.10, 0.54) | .004 |
| Vaccine | −0.43 (−0.67, −0.19) | .0004 | 0.43 (0.18, 0.68) | .0008 | |
| Averaged | −0.35 (−0.54, −0.16) | .0003 | 0.37 (0.17, 0.58) | .0004 | |
| Memory | Placebo | −0.14 (−0.35, 0.07) | .19 | 0.22 (0.04, 0.40) | .02 |
| Vaccine | −0.36 (−0.58, −0.14) | .001 | 0.39 (0.17, 0.62) | .0006 | |
| Averaged | −0.25 (−0.44, −0.06) | .01 | 0.31 (0.13, 0.49) | .0009 |
Models adjusted for: age, cancer treatment, hormone treatment, obesity, depression, physical fitness, visit order, cancer stage, time since cancer treatment, and presence of comorbidities.
Effect of Worry and Mindfulness on Pain Sensitivity
There were significant worry effects on pain ratings at the pain threshold during both the hot and cold tasks such that higher worry was associated with higher pain ratings averaged across injection type (ps < .001, Table 1). These effects were not different between the placebo and vaccine injections (ps > .20). Worry was not associated with temperature at pain threshold for either the hot or cold task (ps > .07). Averaged across injections, higher mindfulness was associated with lower pain ratings at the pain threshold during the cold task (p = .01) and higher temperature at pain threshold during the hot task (p = .009), but not with pain ratings during the hot task (p = .67). These effects did not differ by injection type (ps > .34). The effect of mindfulness on temperature at the pain threshold during the cold task was significantly different between the placebo and vaccine visits (p = .01). However, individual slope tests did not show significant mindfulness effects for either placebo or vaccine injections (ps > .4), highlighting that there was no differential impact of injection type on the relationship between higher mindfulness and lower pain ratings. Table 3 presents the coefficients for these effects.
Table 3.
Estimated effects of mindfulness and worry on post-injection pain sensitivity
| Outcome | Injection type | Change for +1 SD increase in mindfulness (95% CI) | p-value | Change for +1 SD increase in worry (95% CI) | p-value |
|---|---|---|---|---|---|
| Cold Pain Rating | Placebo | −3.19 (−6.47, 0.08) | .06 | 5.36 (2.45, 8.27) | .0003 |
| Vaccine | −4.26 (−7.26, −1.27) | .005 | 5.22 (2.22, 8.22) | .0007 | |
| Averaged | −3.73 (−6.66, −0.80) | .01 | 5.29 (2.46, 8.12) | .0003 | |
| Hot Pain Rating | Placebo | −0.50 (−3.79, 2.80) | .77 | 3.69 (0.59, 6.79) | .02 |
| Vaccine | −0.88 (−4.27, 2.50) | .61 | 5.81 (2.43, 9.19) | .0008 | |
| Averaged | −0.69 (−3.82, 2.44) | .67 | 4.75 (1.97, 7.53) | .0008 | |
| Cold Pain Temperature | Placebo | −0.39 (−1.29, 0.51) | .40 | 0.41 (−0.41, 1.24) | .33 |
| Vaccine | 0.28 (−0.56, 1.13) | .51 | 0.05 (−0.77, 0.88) | .90 | |
| Averaged | −0.05 (−0.89, 0.78) | .90 | 0.23 (−0.56, 1.02) | .56 | |
| Hot Pain Temperature | Placebo | 0.62 (0.22, 1.02) | .002 | −0.23 (−0.64, 0.18) | .26 |
| Vaccine | 0.50 (0.01, 0.98) | .046 | −0.34 (−0.70, 0.03) | .07 | |
| Averaged | 0.56 (0.14, 0.98) | .009 | −0.28 (−0.65, 0.08) | .13 |
Models adjusted for: age, cancer treatment, hormone treatment, obesity, depression, physical fitness, visit order, cancer stage, time since cancer treatment, and presence of comorbidities.
Effect of PSWQ and Mindfulness on Objective Cognitive Outcomes
There were no significant associations between worry or mindfulness and performance on any of the cognitive or memory-related tasks, either averaging across injection types or separately by injection type (ps > .10).
Sensitivity Analysis
Given the fact that several participants had significant delays in their Visit 2 scheduling due to the COVID-19 pandemic, a sensitivity analysis was conducted that excluded participants who experienced a delay between Visits 1 and 2 due to the COVID-19 pandemic. Analyses revealed that there was no effect of the time between Visits 1 and 2 on our pattern of results (ps > .05). Time since diagnosis was also explored as a potential covariate. Analyses revealed that time since diagnosis did not significantly alter the current pattern of results (ps > .05). Lastly, we examined whether chemotherapy or hormone therapy impacted this pattern of results. Chemotherapy (chemotherapy vs. no chemotherapy) or hormone therapy (hormone therapy vs. no hormone therapy), respectively, were entered into our models as moderator variables. Resulted highlighted no significant interaction effects (ps > .05).
Discussion
Emotion regulation plays an important role in adjusting to the psychological and physical demands associated with cancer treatment and survivorship [26]. This study examined the influence of emotion regulation strategies in altering somatic and cognitive symptoms across two visits in breast cancer survivors. Consistent with study hypotheses, higher worry, and lower mindfulness were each associated with greater increases in post-injection focus and memory problems across the day. These effects did not differ based on injection type, suggesting that emotion regulation strategies may be a potent predictor of symptoms irrespective of the vaccine challenge. Higher mindfulness also corresponded to lower fatigue across the day, which was not dependent upon injection type. Interestingly, worry did not predict post-injection fatigue across the day. In terms of pre-injection symptom levels, worry corresponded to lower levels of focus problems. Prior research and theory highlight that worry is associated with hyperfixation/hypervigilance as a means of attempting to control one’s worry and overall environment. This, therefore, contributes to a reduction in focus problems, as individuals experiencing worry and anxiety may be overly focused on stimuli around them [62]. Overall, these findings add to a growing body of literature examining symptom trajectories in breast cancer patients and survivors [63, 64]. Although our study assessed acute changes in 2 day-long study visits and these changes were not assessed longitudinally, these results highlight helpful avenues for future research. Translating the current findings into longitudinal research will allow us to better understand the long-term impact of emotion regulation on these physical and cognitive symptoms. To our knowledge, this is the first test of specific emotion regulation strategies in altering both objective assessment and subjective reporting of somatic and cognitive symptoms in breast cancer survivors.
Breast cancer survivors can experience heightened pain sensitivity compared to non-cancer, pain-free controls [65]. In this study, worry and mindfulness predicted subjective ratings of pain sensitivity on the Hot/Cold Plate Task. Again, as hypothesized, worry and mindfulness revealed contrasting effects, specifically regarding cold pain ratings on the task. Greater worry was associated with higher pain ratings for the cold task while higher mindfulness was related to lower pain ratings on both the hot and cold tasks. Injection type did not influence this pattern of results, highlighting a robust relationship between emotion regulation strategies and reported symptoms across the two visits. Interestingly, only higher mindfulness was significantly related to objective ratings of pain sensitivity, and only for the hot task. Although these results did not transfer to the hot task, women in this study, on average, endured the hot task for a shorter duration than the cold task. It is therefore possible that mindfulness helped survivors endure the more unpleasant hot task for longer. Further, prior research highlights genetic differences of hot versus cold pain sensitivity, which may have influenced the contrasting effects in this study [66].
The overall lack of significant relationships between emotion regulation and objective pain sensitivity may be explained in several ways. Prior findings emphasized that anxiety, which commonly co-occurs with worry, was more strongly associated with constant rather than intermittent pain [3]. Therefore, it is plausible that the transient effects of the Hot/Cold Task as well as the injections across the day were not salient enough to evoke psychological distress in this sample of survivors. Another plausible hypothesis is that emotion regulation may significantly alter perceptions of bodily sensations and experiences rather than objective pain sensitivity.
Emotion regulation strategies did not alter performance on either the cognitive or memory tasks. This contrasted with study hypotheses and is contradictory to the findings that both emotion regulation strategies related to self-reported symptoms across the day. It is possible that emotion regulation alters perceptions of difficulties rather than performance, which could also explain the lack of significant findings for objective metrics on the Hot/Cold Task. Indeed, affect science theory posits that emotion regulation strategies can alter health both directly (e.g., physiological responses) or indirectly (e.g., perceptions of pain, guiding medical decision-making, and health behaviors) [67]. Further, the Self-Regulatory Model of Illness Behavior highlights the importance of illness perceptions and emotional responses when managing a health threat such as cancer [68]. Within this model, poor self-regulation can increase distress and maladaptive coping behaviors, thus making a survivor more susceptible to lingering symptoms in treatment and survivorship. In terms of cognitive problems, prior research indicates that cognitive complaints may parallel neuropsychological test performance in some domains. Breast cancer survivors who reported more memory problems had lower scores on a standardized verbal memory task than those who reported fewer memory problems [69]. On the other hand, breast cancer survivors who just stopped endocrine therapy continued to report cognitive problems over the following year, despite improvement in objective neuropsychological test scores [70].
This study contributes to literature highlighting the importance of adaptive emotion regulation throughout cancer survivorship. These findings suggest that negative emotions, and the lack of ability to effectively regulate them such as using maladaptive strategies such as worry, can affect a woman’s experience during cancer survivorship and worsen their overall psychological and physical health. In contrast, understanding how mindfulness corresponds to acute improvements in physical and cognitive symptoms, such as those seen in this study, highlights the potential for these adaptive emotion regulation skills to serve as a protective factor for women throughout survivorship. Evidence-based interventions such as mindfulness based stress reduction are beneficial in offsetting some of the psychological and physical symptom consequences associated with breast cancer survivorship [38, 71]. Yoga, which often incorporates mindfulness into the practice, has also proven useful in reducing the physical, psychological, and biological consequences of cancer survivorship [72]. Barriers that limit cancer survivors use of these treatments include not having access to these interventions, medical appointment burden, physical health complications, and/or the affordability of specialized mental health services [73]. As a result, there is a clear need for brief, feasible, emotion regulation interventions to be widely disseminated to improve physical and psychological health among breast cancer survivors.
This study has several notable strengths. Using both self-reported symptoms as well as examining objective task data allows for a more comprehensive understanding of emotion regulation’s impact following a vaccine challenge. Further, having six measures of somatic and cognitive symptoms across the day allowed us to examine trajectories of change following the vaccine rather than looking at these symptoms at a single timepoint. The double-blind crossover design also allowed comparisons of emotion regulation skills and their impact on symptoms within the same woman. This study has several limitations. The parent study’s extensive exclusionary criteria produced a sample that was likely healthier than breast cancer survivors generally. Further, a more diverse sample would be useful in replicating these findings to increase generalizability. Although rates differ based on a number of contextual factors, a 2022 study found a larger proportion of Black (41.69%) and Hispanic (44.23%) physically healthy participants were categorized as having anxiety compared to White participants. Notably, individuals high in anxiety often worry more and are less mindful as compared to their non-anxious counterparts. Given our homogenous sample, we were unable to examine whether our results different based on belongingness to different racial, ethnic, or socioeconomic groups—a limitation of this study. This study was burdensome in terms of time and resources, as it required participants to spend nearly a full day in the Clinical Research Center, thus likely limiting who may have been able to participate. Future research would benefit from examining these questions in a way that can increase feasibility, particularly to marginalized and/or historically underrepresented groups as well as individuals experiencing significant contextual stressors that may prove to be a barrier to study engagement and participation. Lastly, future research would benefit from examining a stronger inflammatory stimulus (e.g., endotoxin) that produces greater somatic and cognitive symptoms to see if emotion regulation overrides the inflammatory effects [74, 75].
Findings from this study showed that breast cancer survivors who worried more and were less mindful experienced subjective memory problems, focus problems, and cold pain sensitivity across two visits than those who worried less and were more mindful. Lower mindfulness also corresponded to higher subjective fatigue and hot pain sensitivity. Results from this study highlight the benefits of adaptive emotion regulation in helping mitigate symptoms associated with breast cancer survivorship.
Acknowledgments
Work on this project was supported by NIH grants R01CA186251, T32 CA229114, and TL1TR002735.
Contributor Information
Megan E Renna, School of Psychology, The University of Southern Mississippi, Hattiesburg, MS, USA.
Annelise A Madison, Department of Psychology, The Ohio State University, Columbus, OH, USA.
Juan Peng, Center for Biostatistics, The Ohio State University College of Medicine, Columbus, OH, USA.
Marcella Rosie Shrout, Department of Human Development and Family Studies, Purdue University, West Lafayette, IN, USA.
Maryam Lustberg, Yale Cancer Hospital, Yale School of Medicine, New Haven, CT, USA.
Bhuvaneswari Ramaswamy, James Cancer Hospital, The Ohio State University College of Medicine, Columbus, OH, USA.
Robert Wesolowski, James Cancer Hospital, The Ohio State University College of Medicine, Columbus, OH, USA.
Jeffrey B VanDeusen, James Cancer Hospital, The Ohio State University College of Medicine, Columbus, OH, USA.
Nicole O Williams, James Cancer Hospital, The Ohio State University College of Medicine, Columbus, OH, USA.
Sagar D Sardesai, James Cancer Hospital, The Ohio State University College of Medicine, Columbus, OH, USA.
Anne M Noonan, James Cancer Hospital, The Ohio State University College of Medicine, Columbus, OH, USA.
Raquel E Reinbolt, James Cancer Hospital, The Ohio State University College of Medicine, Columbus, OH, USA.
Daniel G Stover, James Cancer Hospital, The Ohio State University College of Medicine, Columbus, OH, USA.
Mathew Cherian, James Cancer Hospital, The Ohio State University College of Medicine, Columbus, OH, USA.
William B Malarkey, Department of Internal Medicine, The Ohio State University College of Medicine, Columbus, OH, USA.
Rebecca Andridge, Department of Biostatistics, The Ohio State University, Columbus, OH, USA.
Janice K Kiecolt-Glaser, Institute for Behavioral Medicine Research, The Ohio State University College of Medicine, Columbus, OH, USA.
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards Authors Megan E. Renna, Annelise A. Madison, Juan Peng, M. Rosie Shrout, Maryam Lustberg, Bhuvaneswari Ramaswamy, Robert Wesolowski, Jeffrey B. VanDeusen, Nicole O. Williams, Sagar D. Sardesai, Anne M. Noonan, Raquel E. Reinbolt, Daniel G. Stover, Mathew Cherian, William B. Malarkey, Rebecca Andridge, and Janice K. Kiecolt-Glaser declare that they have no conflict of interest. All procedures, including the informed consent process, were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
Authors’ Contributions Megan Renna (Conceptualization: Lead; Formal analysis: Equal; Investigation: Lead; Project administration: Supporting; Writing – original draft: Lead; Writing – review & editing: Lead), William Malarkey (Methodology: Supporting; Writing – review & editing: Supporting), Mathew Cherian (Data curation: Supporting; Writing – review & editing: Supporting), Daniel Stover (Data curation: Supporting; Writing – review & editing: Supporting), Raquel Reinbolt (Data curation: Supporting; Writing – review & editing: Supporting), Anne Noonan (Data curation: Supporting; Writing – review & editing: Supporting), Sagar Sardesai (Data curation: Supporting; Writing – review & editing: Supporting), Rebecca Andridge (Formal analysis: Equal; Writing – review & editing: Supporting), Nicole Williams (Data curation: Supporting; Writing – review & editing: Supporting), Robert Wesolowski (Data curation: Supporting; Writing – review & editing: Supporting), Bhuvaneswari Ramaswamy (Data curation: Supporting; Writing – review & editing: Supporting), Maryam Lustberg (Funding acquisition: Supporting; Resources: Supporting), M. Rosie Shrout (Conceptualization: Supporting; Writing – original draft: Supporting; Writing – review & editing: Supporting), Juan Peng (Formal analysis: Equal; Writing – review & editing: Supporting), Annelise A Madison (Conceptualization: Supporting; Writing – original draft: Supporting; Writing – review & editing: Supporting), Jeffrey VanDeusen (Data curation: Supporting; Writing – review & editing: Supporting), and Janice Kiecolt-Glaser (Conceptualization: Supporting; Funding acquisition: Lead; Methodology: Lead; Writing – review & editing: Supporting)
Transparency statement The study was pre-registered at clinicaltrials.gov (ClinicalTrials.gov Identifier: NCT02415387). The analysis plan was not formally pre-registered. De-identified data from this study are not available in a public archive. De-identified data from this study will be made available (as allowable according to institutional IRB standards) by emailing the corresponding author. Analytic code used to conduct the analyses presented in this study are not available in a public archive. They may be available by emailing the corresponding author. Materials used to conduct the study are not publicly available.
References
- 1. Maajani K, Jalali A, Alipour S, Khodadost M, Tohidinik HR, Yazdani K.. The global and regional survival rate of women with breast cancer: A systematic review and meta-analysis. Clin Breast Cancer. 2019; 19(3): 165–177. [DOI] [PubMed] [Google Scholar]
- 2. Hewitt, M, Rowland JH, Yancik R.. Cancer survivors in the United States: Age, health, and disability. J Gerontol Med Sci. 2003; 58(1):82–91. [DOI] [PubMed] [Google Scholar]
- 3. Sheridan D, Foo I, O'Shea H, et al. Long-term follow-up of pain and emotional characteristics of women after surgery for breast cancer. J Pain Symptom Manage. 2012; 44(4): 608–614. [DOI] [PubMed] [Google Scholar]
- 4. Laird BJ, Scott AC, Colvin LA, et al. Pain, depression, and fatigue as a symptom cluster in advanced cancer. J Pain Symptom Manage. 2011; 42(1): 1–11. [DOI] [PubMed] [Google Scholar]
- 5. Werner MU, et al. Prediction of postoperative pain: A systematic review of predictive experimental pain studies. J Am Soc Anesthesiol. 2010; 112(6): 1494–1502. [DOI] [PubMed] [Google Scholar]
- 6. Slade G, Diatchenko L, Bhalang K, et al. Influence of psychological factors on risk of temporomandibular disorders. J Dent Res. 2007; 86(11): 1120–1125. [DOI] [PubMed] [Google Scholar]
- 7. Manfuku M, Nishigami T, Mibu A, Tanaka K, Kitagaki K, Sumiyoshi K.. Comparison of central sensitization-related symptoms and health-related quality of life between breast cancer survivors with and without chronic pain and healthy controls. Breast Cancer. 2019; 26(6): 758–765. [DOI] [PubMed] [Google Scholar]
- 8. Feeney LR, Tormey SM, Harmon DC.. Breast cancer and chronic pain: A mixed methods review. Ir J Med Sci. 2018; 187(4): 877–885. [DOI] [PubMed] [Google Scholar]
- 9. Bower JE, Ganz PA, Desmond KA, et al. Fatigue in long‐term breast carcinoma survivors: A longitudinal investigation. Cancer. 2006; 106(4): 751–758. [DOI] [PubMed] [Google Scholar]
- 10. Hagen KB, Aas T, Kvaløy JT, Eriksen HR, Søiland H, Lind R.. Fatigue, anxiety and depression overrule the role of oncological treatment in predicting self-reported health complaints in women with breast cancer compared to healthy controls. Breast. 2016; 28: 100–106. [DOI] [PubMed] [Google Scholar]
- 11. Nguyen CM, Yamada TH, Beglinger LJ, Cavanaugh JE, Denburg NL, Schultz SK.. Cognitive features 10 or more years after successful breast cancer survival: Comparisons across types of cancer interventions. Psychooncology. 2013; 22(4): 862–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bernstein LJ, McCreath GA, Komeylian Z, Rich JB.. Cognitive impairment in breast cancer survivors treated with chemotherapy depends on control group type and cognitive domains assessed: A multilevel meta-analysis. Neurosci Biobehav Rev. 2017; 83: 417–428. [DOI] [PubMed] [Google Scholar]
- 13. Lange M, Joly F, Vardy J, et al. Cancer-related cognitive impairment: An update on state of the art, detection, and management strategies in cancer survivors. Ann Oncol. 2019; 30(12): 1925–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jean-Pierre P, Winters PC, Ahles TA, et al. Prevalence of self-reported memory problems in adult cancer survivors: A national cross-sectional study. J Oncol Pract. 2012; 8(1): 30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kohli S, Griggs JJ, Roscoe JA, et al. Self-reported cognitive impairment in patients with cancer. J Oncol Pract. 2007; 3(2): 54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wefel JS, Schagen SB.. Chemotherapy-related cognitive dysfunction. Curr Neurol Neurosci Rep. 2012; 12(3): 267–275. [DOI] [PubMed] [Google Scholar]
- 17. Schmidt ME, Wiskemann J, Steindorf K.. Quality of life, problems, and needs of disease-free breast cancer survivors 5 years after diagnosis. Qual Life Res. 2018; 27(8): 2077–2086. [DOI] [PubMed] [Google Scholar]
- 18. Merriman JD, Sereika SM, Brufsky AM, et al. Trajectories of self‐reported cognitive function in postmenopausal women during adjuvant systemic therapy for breast cancer. Psychooncology. 2017; 26(1): 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Castellon SA, Ganz PA, Bower JE, Petersen L, Abraham L, Greendale GA.. Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. J Clin Exp Neuropsychol. 2004; 26(7): 955–969. [DOI] [PubMed] [Google Scholar]
- 20. Shilling V, Jenkins V.. Self-reported cognitive problems in women receiving adjuvant therapy for breast cancer. Eur J Oncol Nurs. 2007; 11(1): 6–15. [DOI] [PubMed] [Google Scholar]
- 21. Ferguson RJ, McDonald BC, Saykin AJ, Ahles TA.. Brain structure and function differences in monozygotic twins: Possible effects of breast cancer chemotherapy. J Clin Oncol. 2007; 25(25): 3866–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Apple AC, Ryals AJ, Alpert KI, et al. Subtle hippocampal deformities in breast cancer survivors with reduced episodic memory and self-reported cognitive concerns. NeuroImage Clin. 2017; 14: 685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jim HS, Phillips KM, Chait S, et al. Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J Clin Oncol. 2012; 30(29): 3578–3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Joly F, Lange M, Dos Santos M, Vaz-Luis I, Di Meglio A.. Long-term fatigue and cognitive disorders in breast cancer survivors. Cancers. 2019; 11(12): 1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gross JJ. The emerging field of emotion regulation: An integrative review. Rev Gen Psychol. 1998; 2(3): 271–299. [Google Scholar]
- 26. Martins-Klein B, Bamonti PM, Owsiany M, Naik A, Moye J.. Age differences in cancer-related stress, spontaneous emotion regulation, and emotional distress. Aging Ment Health. 2021; 25(2): 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Renna ME. A review and novel theoretical model of how negative emotions influence inflammation: The critical role of emotion regulation. Brain Behav Immun Health. 2021; 18: 100397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kabat-Zinn, J. Full Catastrophe Living: The Program of the Stress Reduction Clinic at the University of Massachusetts Medical Center. New York, NY: Delta; 1990: 264–273. [Google Scholar]
- 29. Shiyko MP, Siembor B, Greene PB, Smyth J, Burkhalter JE.. Intra-individual study of mindfulness: Ecological momentary perspective in post-surgical lung cancer patients. J Behav Med. 2019; 42(1): 102–110. [DOI] [PubMed] [Google Scholar]
- 30. Mischkowski D, Stavish CM, Palacios-Barrios EE, Banker LA, Dildine TC, Atlas LY.. Dispositional mindfulness and acute heat pain: Comparing stimulus-evoked pain with summary pain assessment. Psychosom Med. 2021; 83(6): 539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sarenmalm EK, et al. Mindfulness and its efficacy for psychological and biological responses in women with breast cancer. Cancer Med. 2017; 6(5):1108–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McCracken LM, Gauntlett-Gilbert J, Vowles KE.. The role of mindfulness in a contextual cognitive-behavioral analysis of chronic pain-related suffering and disability. Pain. 2007; 131(1–2): 63–69. [DOI] [PubMed] [Google Scholar]
- 33. Schütze R, Rees C, Preece M, Schütze M.. Low mindfulness predicts pain catastrophizing in a fear-avoidance model of chronic pain. Pain. 2010; 148(1): 120–127. [DOI] [PubMed] [Google Scholar]
- 34. Zimmaro LA, Carson JW, Olsen MK, Sanders LL, Keefe FJ, Porter LS.. Greater mindfulness associated with lower pain, fatigue, and psychological distress in women with metastatic breast cancer. Psychooncology. 2020; 29(2):263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ikeuchi K, Ishiguro H, Nakamura Y, Izawa T, Shinkura N, Nin K.. The relation between mindfulness and the fatigue of women with breast cancer: Path analysis. Biopsychosoc Med. 2020; 14(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bower JE, Crosswell AD, Stanton AL, et al. Mindfulness meditation for younger breast cancer survivors: A randomized controlled trial. Cancer. 2015; 121(8): 1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boyle CC, Stanton AL, Ganz PA, Crespi CM, Bower JE.. Improvements in emotion regulation following mindfulness meditation: Effects on depressive symptoms and perceived stress in younger breast cancer survivors. J Consult Clin Psychol. 2017; 85(4): 397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cramer H, Lauche R, Paul A, Dobos G.. Mindfulness-based stress reduction for breast cancer—A systematic review and meta-analysis. Curr Oncol. 2012; 19(5): 343e343–343e352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yakobi O, Smilek D, Danckert J.. The effects of mindfulness meditation on attention, executive control and working memory in healthy adults: A meta-analysis of randomized controlled trials. Cognit Ther Res. 2021; 45(4): 543–560. [Google Scholar]
- 40. Jha AP, Stanley EA, Kiyonaga A, Wong L, Gelfand L.. Examining the protective effects of mindfulness training on working memory capacity and affective experience. Emotion. 2010; 10(1): 54–64. [DOI] [PubMed] [Google Scholar]
- 41. Jha AP, Denkova E, Zanesco AP, Witkin JE, Rooks J, Rogers SL.. Does mindfulness training help working memory ‘work’ better? Curr Opin Psychol. 2019; 28: 273–278. [DOI] [PubMed] [Google Scholar]
- 42. Aldao A, Nolen-Hoeksema S.. The influence of context on the implementation of adaptive emotion regulation strategies. Behav Res Ther. 2012; 50(7–8): 493–501. [DOI] [PubMed] [Google Scholar]
- 43. Gladstone G, Parker G.. What’s the use of worrying? Its function and its dysfunction. Aust N Z J Psychiatry. 2003; 37(3): 347–354. [DOI] [PubMed] [Google Scholar]
- 44. Hashemi S-M, Rafiemanesh H, Aghamohammadi T, et al. Prevalence of anxiety among breast cancer patients: A systematic review and meta-analysis. Breast Cancer. 2020; 27(2): 166–178. [DOI] [PubMed] [Google Scholar]
- 45. Renna ME, Rosie Shrout M, Madison AA, et al. Worry and rumination in breast cancer patients: Perseveration worsens self-rated health. J Behav Med. 2021; 44(2): 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Renna ME, et al. Distress disorder histories relate to greater physical symptoms among breast cancer patients and survivors: Findings across the cancer trajectory. Int J Behav Med. 2022; 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Verkuil B, Brosschot JF, Gebhardt WA, Thayer JF.. When worries make you sick: A review of perseverative cognition, the default stress response and somatic health. J Exp Psychopathol. 2010; 1(1): jep. 009110. [Google Scholar]
- 48. Brosschot JF, Gerin W, Thayer JF.. The perseverative cognition hypothesis: A review of worry, prolonged stress-related physiological activation, and health. J Psychosom Res. 2006; 60(2): 113–124. [DOI] [PubMed] [Google Scholar]
- 49. Deimling GT, Bowman KF, Sterns S, Wagner LJ, Kahana B.. Cancer‐related health worries and psychological distress among older adult, long‐term cancer survivors. Psychooncology. 2006; 15(4): 306–320. [DOI] [PubMed] [Google Scholar]
- 50. Kiecolt-Glaser JK, Renna M, Peng J, et al. Breast cancer survivors’ typhoid vaccine responses: Chemotherapy, obesity, and fitness make a difference. Brain Behav Immun. 2022; 103: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn state worry questionnaire. Behav Res. 1990; 28(6): 487–495. [DOI] [PubMed] [Google Scholar]
- 52. Carlson LE, Brown KW.. Validation of the Mindful Attention Awareness Scale in a cancer population. J Psychosom Res. 2005; 58(1): 29–33. [DOI] [PubMed] [Google Scholar]
- 53. Barnes S, Brown KW, Krusemark E, Campbell WK, Rogge RD.. The role of mindfulness in romantic relationship satisfaction and responses to relationship stress. J Marital Fam Ther. 2007; 33(4): 482–500. [DOI] [PubMed] [Google Scholar]
- 54. Conners KC. Conners’ Continuous Performance Test. North Tonawanda: Multi-Health Systems, Inc.; 2000. [Google Scholar]
- 55. Krabbe KS, Reichenberg A, Yirmiya R, Smed A, Pedersen BK, Bruunsgaard H.. Low-dose endotoxemia and human neuropsychological functions. Brain Behav Immun. 2005; 19(5): 453–460. [DOI] [PubMed] [Google Scholar]
- 56. Brandt J. The Hopkins Verbal Learning Test: Development of a new memory test with six equivalent forms. Clin Neuropsychol. 1991; 5(2): 125–142. [Google Scholar]
- 57. Stanton AL, Bernaards CA, Ganz PA.. The BCPT symptom scales: A measure of physical symptoms for women diagnosed with or at risk for breast cancer. J Natl Cancer Inst. 2005; 97(6): 448–456. [DOI] [PubMed] [Google Scholar]
- 58. Fu MR, Axelrod D, Guth AA, et al. Comorbidities and quality of life among breast cancer survivors: A prospective study. J Pers Med. 2015; 5(3): 229–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Partridge AH, Burstein HJ, Winer EP.. Side effects of chemotherapy and combined chemohormonal therapy in women with early-stage breast cancer. JNCI Monogr. 2001; 2001(30): 135–142. [DOI] [PubMed] [Google Scholar]
- 60. Charlson ME, Pompei P, Ales KL, MacKenzie CR.. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987; 40(5): 373–383. [DOI] [PubMed] [Google Scholar]
- 61. Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977; 1(3): 385–401. [Google Scholar]
- 62. Wittchen H-U, Hoyer J.. Generalized anxiety disorder: Nature and course. J Clin Psychiatry. 2001; 62: 15–21. [PubMed] [Google Scholar]
- 63. Merriman JD, et al. Predictors of the trajectories of self-reported attentional fatigue in women with breast cancer undergoing radiation therapy in Oncology nursing forum. In: Oncology nursing forum (Vol. 37). NIH Public Access; 2010:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bean HR, Diggens J, Ftanou M, Weihs KL, Stanton AL, Wiley JF.. Insomnia and fatigue symptom trajectories in breast cancer: A longitudinal cohort study. Behav Sleep Med. 2021; 19(6): 814–827. [DOI] [PubMed] [Google Scholar]
- 65. Rasmussen G, et al. Pain sensitivity and shoulder function among breast cancer survivors compared to matched controls: A case-control study. J Cancer Surviv. 2021; 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kim H. Genetic predictors for acute experimental cold and heat pain sensitivity in humans. J Med Genet. 2006; 43(8): e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. DeSteno D, Gross JJ, Kubzansky L.. Affective science and health: The importance of emotion and emotion regulation. Health Psychol. 2013; 32(5): 474–486. [DOI] [PubMed] [Google Scholar]
- 68. Leventhal H, Meyer D, Nerenz D.. The common sense representation of illness danger. Contrib Med Psychol. 1980; 2: 7–30. [Google Scholar]
- 69. Ganz PA, Kwan L, Castellon SA, et al. Cognitive complaints after breast cancer treatments: Examining the relationship with neuropsychological test performance. J Natl Cancer Inst. 2013; 105(11): 791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ribi K, Aldridge J, Phillips K-A, et al. ; BIG 1-98 Collaborative Group. Subjective cognitive complaints one year after ceasing adjuvant endocrine treatment for early-stage breast cancer. Br J Cancer. 2012; 106(10): 1618–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tatrow K, Montgomery GH.. Cognitive behavioral therapy techniques for distress and pain in breast cancer patients: A meta-analysis. J Behav Med. 2006; 29(1): 17–27. [DOI] [PubMed] [Google Scholar]
- 72. Kiecolt-Glaser JK, Bennett JM, Andridge R, et al. Yoga’s impact on inflammation, mood, and fatigue in breast cancer survivors: A randomized controlled trial. J Clin Oncol. 2014; 32(10):1040–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rankin NM, Butow PN, Thein T, et al. Everybody wants it done but nobody wants to do it: An exploration of the barrier and enablers of critical components towards creating a clinical pathway for anxiety and depression in cancer. BMC Health Serv Res. 2015; 15(1): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Grigoleit J-S, Kullmann JS, Wolf OT, et al. Dose-dependent effects of endotoxin on neurobehavioral functions in humans. PLoS One. 2011; 6(12): e28330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Thomson CA, McColl A, Graham GJ, Cavanagh J.. Sustained exposure to systemic endotoxin triggers chemokine induction in the brain followed by a rapid influx of leukocytes. J Neuroinflammation. 2020; 17(1): 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]


