Abstract
Objectives
This study aims to identify a new barrier to the use of the Mini‐Nutrition Assessment Short‐Form (MNA‐SF), which is a malnutrition assessment tool for the risk assessment of sarcopenia in a nourished population.
Methods
The MNA‐SF was completed, and individuals with a score of > 11 were considered nourished in this cross‐sectional feasibility study of a registry. Sarcopenia was assessed in 766 healthy, nourished adults (33.4% men, 64.9 ± 7.1 years) based on the European Working Group on Sarcopenia in Older People 2 (EWGSOP2).
Results
The MNA‐SF scores for non‐sarcopenia, pre‐, confirmed, and severe sarcopenia were 13.59 ± 0.69, 13.73 ± 0.60, 12.64 ± 0.74, and 12.5 ± 0.71, respectively. The higher MNA‐SF score association with pre‐sarcopenia [odds ratio (OR): 1.41, 95% confidence interval (CI): 1.06–1.86, P = 0.02], confirmed sarcopenia (OR = 0.25, 95% CI: 0.13–0.49, P < 0.001), and severe sarcopenia (OR = 0.20, 95% CI: 0.09–0.46, P < 0.001) was as significant as in the MNA‐SF categories. Individuals with a score = 13 (compared with 14), had a higher risk of confirmed sarcopenia (OR = 10.07, 95% CI: 1.92–52.71, P = 006) and severe sarcopenia (OR = 12.09, 95% CI: 1.24–117.50, P = 0.032). Individuals with a score of 12 had a higher risk of confirmed sarcopenia (OR = 30.94, 95% CI: 4.25–103.02, P < 0.001) and severe sarcopenia (OR = 35.90, 95% CI: 4.25–303.07, P = 0.001) compared with subjects with a score of 14. The models also showed that MNA‐SF < 13 could predict sarcopenia.
Conclusion
There was a significant association between MNA‐SF and confirmed and severe sarcopenia in nourished people. Sarcopenia assessment in people with MNA‐SF < 13 can be beneficial. Developing a tool to identify the risk of malnutrition and sarcopenia at the same time based on MNA‐SF can be considered.
Keywords: healthy aging, malnutrition, MNA‐SF, nutritional assessment, sarcopenia
There were significant differences between the Mini‐Nutrition Assessment Short‐Form (MNA‐SF) score and the incidence of sarcopenia, muscle strength, and performance, even in well‐nourished subjects. Although the results of the current study showed that MNA‐SF was not clinically significantly different from pre‐sarcopenic and non‐sarcopenic, this tool could predict confirmed and severe sarcopenia independent of other risk factors, even in a nourished population. Sarcopenia assessment in persons with MNA‐SF < 13 can be considered clinically beneficial for the early diagnosis of sarcopenia associated with malnutrition (MNA‐SF < 12), even in well‐fed subjects in all clinical settings.
1. INTRODUCTION
Sarcopenia is a condition that primarily affects the elderly, characterized by a progressive loss of skeletal muscle mass and function. 1 , 2 , 3 , 4 This condition has been linked to an increased risk of disability, falls, fall‐related injuries, hospitalization, limitations in independence, and mortality. 2 , 3 , 5 Despite a recent systematic review and meta‐analysis of 35 articles estimating the overall prevalence of sarcopenia to be 10% in men and women over 60 years of age, the prevalence may be higher due to the lack of consideration of pre‐sarcopenia in most studies. 4 , 6
Numerous studies have been conducted over the past decade to understand the impact of nutrition and dietary intake on sarcopenia. Although the association between nutritional health and sarcopenia requires further study, protein‐energy malnutrition is often considered a predictor of sarcopenia that occurs concurrently in many populations. 7 , 8 , 9 Evidence suggests that low energy intake, inadequate protein intake, and poor nutritional status are associated with accelerated muscle loss and impaired muscle function, thereby contributing to the development and progression of sarcopenia. 2 , 8 , 9 Therefore, malnutrition assessment has been identified as a suitable predictor method for sarcopenia. 2 , 8
Malnutrition assessment in the elderly is a challenge for health professionals. The Mini Nutritional Assessment (MNA) is a tool developed for the elderly population. 10 The MNA is one of the most commonly used nutrition screening and assessment tools, designed to perform a multidimensional assessment in four domains: anthropometry, general health, eating habits, and self‐perceived health and nutritional status. 10 , 11 However, the MNA incorporates a great domain of variables, thereby leading to the development of the Mini Nutritional Assessment‐Short Form (MNA‐SF), a quicker and easier tool to determine the risk of malnutrition in the elderly. 10 , 11 The MNA‐SF has a maximum score of 14 that can be used to identify patients who are malnourished or at risk of malnutrition. 10 , 11 There are considerable criteria linking MNA‐SF to sarcopenia risk by linking malnutrition and wasting. 4 , 7 , 8 , 9 , 10 , 11 Therefore, using the MNA‐SF to identify individuals at risk of malnutrition would also allow health professionals to identify those at risk for sarcopenia. 4 , 7 , 8 , 9 , 10 , 11
Previous reviews indicate that the MNA‐SF has the potential to identify individuals at risk of developing sarcopenia due to inadequate energy and protein intake, poor nutritional status, and impaired functional parameters. 4 , 7 , 8 , 9 , 10 , 11 However, it remains unclear whether a narrow range of MNA‐SF scores in individuals identified as nourished is associated with sarcopenia in individuals over 55 years old. Additionally, to reduce the confounding impact of malnutrition when assessing the impact of nutritional health, diet, and nutrients on sarcopenia, the exclusion of malnourished and those at risk of malnutrition can be useful. Therefore, this study aims to examine and report the relationship between the MNA‐SF and sarcopenia in a nourished population. The findings of this study can expand the possible use of this tool as a suitable predictor of both sarcopenia and malnutrition in nourished populations.
2. MATERIALS AND METHODS
2.1. Participants and study design
This cross‐sectional study, aimed to determine the feasibility of a national registry for lifelong monitoring of sarcopenia in healthy, nourished individuals, adhering to the guidelines established in the Declaration of Helsinki. Approval was granted by Mashhad University of Medical Science, Iran: IR.MUMS. REC.1398.229, and written informed consent was obtained from the 1074 eligible registered subjects. 4 The study included a comprehensive assessment of nutritional, socioeconomic, anthropometric, clinical, and psychological factors, conducted via face‐to‐face interviews with registered experts in separate rooms. A cluster sampling technique was used to obtain the sample size from nursing homes and the community, which included both retired and employed populations. Inclusion criteria were age ≥ 55 years, residence in Mashhad, adequate dietary intake, and no confirmed mental disorder, whereas exclusion criteria were the presence of serious illnesses that could result in wasting. A full protocol and methodology, as well as inclusion and exclusion criteria, primary report, and procedures, have been published elsewhere. 4
2.2. Sarcopenia assessment
In this study, the established diagnostic setting was based on the European Working Group on Sarcopenia in Older People 2 (EWGSOP2). Muscle strength was measured using a hydraulic hand dynamometer (Saehan Hydraulic Hand Dynamometer 08‐010113 – made in Korea) and the four‐meter gait speed test was applied to evaluate muscular performance. Skeletal muscle mass (SMM) was measured using a bioelectric impedance analyzer (BIA) InBody‐270 made in Korea in 2018. To calculate the skeletal muscle mass index (SMMI), the SMM was divided by the square of the height in meters. The cutoff points for sarcopenia assessments were 2 , 3 , 4 :
(a) Muscle strength: grip strength < 27 kg for male patients and < 16 kg for female patients
(b) SMMI: < 7 kg/m2 for male patients and < 5.5 kg/m2 for female patients
(c) Muscle performance: gait speed ≤ 0.8 m/s for both sexes.
Based on the EWGSOP2 instructor, having (a), (b), and (c) are indicators of low muscle strength, mass, and performance that are the primary identifiers of sarcopenia. 2 , 3 People with criteria (a), (b), and (c) were categorized as having severe sarcopenia, (a) and (b) as having confirmed sarcopenia, and only (a) as having pre‐sarcopenia. 2 , 3 All the assessments were made under the supervision of an expert.
2.3. Malnutrition assessment
The main study was a feasibility study that targeted healthy nourished people. The key nutritional aim of the study was minimizing the risk factors of sarcopenia, which can impact the result, especially dietary intake. In order to minimize the potential confounding effects of malnutrition or individuals who were at risk for malnutrition, the study excluded such individuals as part of its eligibility criteria. Considering the population, range of questionaries, and structure of the national health care system in the region, choosing a suitable tool was a challenge. After conducting a systematic review of available tools, the MNA‐SF was selected to assess the malnutrition risk in older adults. 4 The questionnaire was administered during a face‐to‐face interview by a qualified Clinical Nutrition Registered Nutritionist and Dietitian (RDN) using validated instruments. 11 Individuals with an MNA score of < 12 were excluded from participation, and only individuals who were nourished, as categorized by a score of 12–14, were included in the analysis.
2.4. Statical analysis
Statistical analyses were performed using IBM SPSS Statistics for Windows version 20.0 (IBM Co.). After determining normality, homogeneity, sensitivity, and specificity, linear regression and analysis of variance (ANOVA; F‐distribution) power of the study were calculated using R‐Studio. Single‐factor ANOVA with Tukey's Honestly Significant Difference (HSD) test was used for continuous variables and the chi‐square test for qualitative variables. Odds ratios (ORs; 95% confidence intervals [CIs]), expressed as OR, were determined using multinomial logistic regression. The figures were generated using SPSS or Chartel to improve the quality of the charts. The P value ˂ 0.05 was considered statistically significant.
3. RESULTS
Sarcopenia data from 766 nourished individuals over 55 years of age were used for the current study [73% (n = 559) without sarcopenia, 23.9% (n = 183) with pre‐sarcopenia, 1.8% (n = 14) with confirmed sarcopenia, and 1.3% (n = 10) with severe sarcopenia]. 4 The characteristic data of the population is in Figure 1. Data distribution among groups was normal: and data were homogeneous (P = 0.902). Among the 766 healthy older adults [33.4% male patients, age = 64.9 ± 7.12 (min = 55, max = 81) years] who participated in the study, the mean MNA‐SF score for non‐sarcopenia was 13.59 ± 0.69. This score for pre‐sarcopenia was 13.73 ± 0.6, for confirmed sarcopenia was 12.64 ± 0.74, and for severe sarcopenia was 12.5 ± 0.71. The differences between the mean scores of the wedge of all sarcopenia groups were significant (Table 1). In addition, there was a significant small correlation between the MNA‐SF score with muscle performance (R = 0.085, P = 0.018) and muscle strength (R = −0.186, P < 0.001), whereas there was no correlation between SMMI and MNA‐SF. Cluster 3D bar model of the MNA score by sarcopenia and muscle strength also showed a lower MNA score was correlated with reduced muscle strength and severity of sarcopenia (Figure 2).
FIGURE 1.
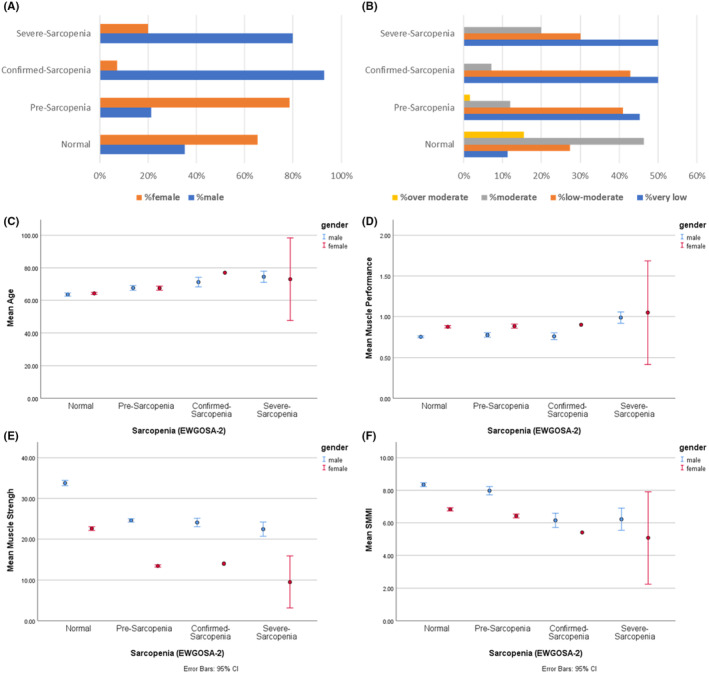
Main characteristics of the population including percentage of gender (A), percentage of current physical activity (B), age (C), muscle performance (D), muscle strength (E), and SMMI (F). Age (C), muscle performance (D), muscle strength (E), and SMMI (F) are stratified by gender. SMMI, skeletal muscle mass index.
TABLE 1.
The association of MNA‐SF score and sarcopenia.
| Total | Non‐sarcopenia (n = 559) | Pre‐sarcopenia (n = 183) | Confirmed sarcopenia (n = 14) | Severe sarcopenia (n = 10) | P value 1 | |
|---|---|---|---|---|---|---|
| MNA‐SF score | ||||||
| Descriptive | 13.59 ± 0.7 | 13.59 ± 0.69a,** | 13.73 ± 0.6b,** | 12.64 ± 0.74a,b,** | 12.5 ± 0.71a,b,** | < 0.001 |
| OR (95% CI) 2 | – | Ref | 1.41 (1.06–1.86)* | 0.25 (0.13–0.49)** | 0.2 (0.09–0.46)** | |
| OR (95% CI) 3 | – | Ref | 1.00 (1.00–1.00)** | 0.999 (0.998–0.999)** | 0.999 (0.999–1.000) | |
Variables sharing the same alphabet (a, b, c) are significantly different from each other. P value < 0.001 indicate with ** and P value < 0.05 indicate with * (bold).
Abbreviations: CI, confidence interval; MNA‐SF, Mini Nutrition Assessment‐Short Form; OR, odds ratio; Ref, reference.
ANOVA; OR was calculated using multinomial logistic regression custom/stepwise interaction model.
Crude model.
Adjusted model for age, sex, and physical activity.
FIGURE 2.
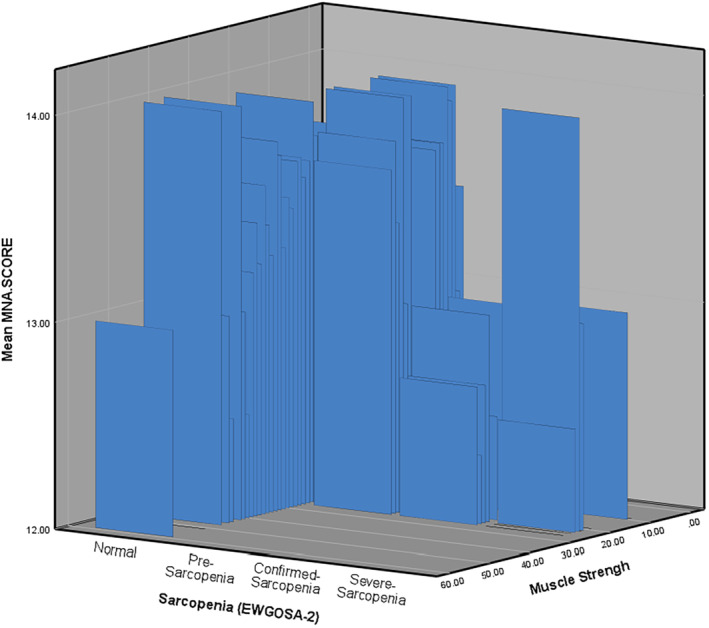
Cluster 3D bar model of the MNA score by sarcopenia classification (EWGSOP2) and muscle strength within a nourished population. EWGSOP2, European Working Group on Sarcopenia in Older People 2; MNA, Mini‐Nutrition Assessment.
The frequency of people with MNA‐SF scores 12, 13, and 14 showed a significant difference (P < 0.001) among groups (Table 2). The differences between non‐sarcopenia and all three stages of sarcopenia (pre‐sarcopenia, confirmed sarcopenia, and severe sarcopenia, P = 0.734) and non‐sarcopenia with pre‐sarcopenia (P = 0.730) were not significant. Nevertheless, there was a significant difference between non‐sarcopenia with confirmed sarcopenia and severe sarcopenia and pre‐sarcopenia with confirmed sarcopenia and severe sarcopenia (P < 0.001).
TABLE 2.
The association of MNA‐SF classification and sarcopenia.
| Total | Non‐sarcopenia (n = 559) | Pre‐sarcopenia (n = 183) | Confirmed sarcopenia (n = 14) | Severe sarcopenia (n = 10) | P value 1 | |
|---|---|---|---|---|---|---|
| Frequency of MNA‐SF scores | ||||||
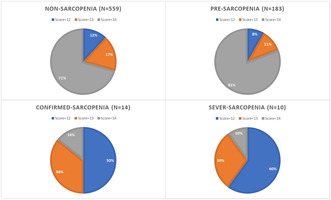
| Score = 12 | 94 (12.5%) | 66 (11.8%) | 15 (8.2%) | 7 (50%) | 6 (60%) | < 0.001 |
| Score = 13 | 126 (16.3%) | 98 (17.5%) | 20 (10.9%) | 5 (35.7%) | 3 (30%) | |
| Score = 14 | 546 (71.2%) | 395 (70.7%) | 148 (80.9%) | 2 (14.3%) | 1 (10%) | |
| OR (95% CI) 2 , 4 score = 12 | – | Ref | 0.607 (0.336–1.096) | 20.947 (4.259–103.020)** | 35.909 (4.255–303.074)* | |
| OR (95% CI) 2 , 4 score = 13 | – | Ref | 0.545 (0.325–0.913)* | 10.077 (1.926–52.714)* | 12.092 (1.244–117.505)* | |
| OR (95% CI) 2 , 5 | – | Ref | 1.40 (1.064–1.856)* | 0.25 (0.131–0.483)** | 0.20 (0.08–0.46)** | |
| OR (95% CI) 3 , 5 | – | Ref | 0.999 (0.998–0.999)** | 0.991 (0.987–0.995)** | 0.993 (0.989–0.997)** | |
Variables sharing the same alphabet (a, b, c) are significantly different from each other. P value < 0.001 indicate with ** and P value < 0.05 indicate with * (bold). Within‐group adjusted OR was not included for the “frequency of MNA‐SF scores” because of the small sample size of people with confirmed and severe‐sarcopenia. However, the OR of factors can indicate the effect of scores.
Abbreviations: CI, confidence interval; MNA‐SF, Mini Nutrition Assessment‐Short Form; OR, odds ratio; Ref, reference.
ANOVA; OR was calculated using multinomial logistic regression custom/stepwise interaction model.
Crude model.
Adjusted model for age, sex, and physical activity.
Comparison to non‐sarcopenia people with an MNA score of 14.
The score shows how each increase in the level of score category can impact OR of having sarcopenia.
The OR of the MNA‐SF score compared with non‐sarcopenia for pre‐sarcopenia (OR = 1.41, 95% CI: 1.06–1.86, P = 0.024), confirmed sarcopenia (OR = 0.25, 95% CI: 0.13–0.49, P < 0.001), and severe sarcopenia (OR = 0.2, 95% CI: 0.09–0.46, P < 0.001) were significant. The result showed by increasing the MNA score, the OR of confirmed and severe sarcopenia 75% and 80% reduced, respectively (Table 1).
In comparison with normal people with an MNA‐SF score of 14, people with a score of 13 were at significantly higher risk of confirmed sarcopenia (OR = 10.077, 95% CI: 1.926–52.714) and severe‐sarcopenia (OR = 12.092, 95% CI: 1.244–117.505). The risk raised within people with a score of 12 to OR = 20.947 (95% CI: 4.259–103.020) for confirmed sarcopenia and OR = 35.909 (95% CI : 4.255–303.074) for severe sarcopenia in comparison with people with an MNA‐SF score of 14 (Table 2). In addition, a significant OR (95% CI) in the MNA‐SF scores categories confirmed the high OR of sarcopenia in people with an MNA‐SF score of 13 and 12 in comparison with 14 (Table 2).
The adjustment, which incorporated the most robust predictors of sarcopenia and nutritional health, and the limited range of the variable significantly restricted and influenced the statistics of the OR. Despite this, the Wald score validated the test. After adjusting the MNA‐SF score and frequency to age, gender, and physical activity, the findings showed that the OR of having sarcopenia based on the MNA‐SF is independent of the main risk factors of sarcopenia. In addition, people with lower MNA‐SF scores were more prevalent in the higher clinical levels of sarcopenia (Figure 3). Furthermore, the chi‐squared automatic interaction detector (CHAID) regression classification risk assessment also showed that an MNA score ≤ 12 could significantly predict the risk of having sarcopenia (adjusted P = 0.004).
FIGURE 3.
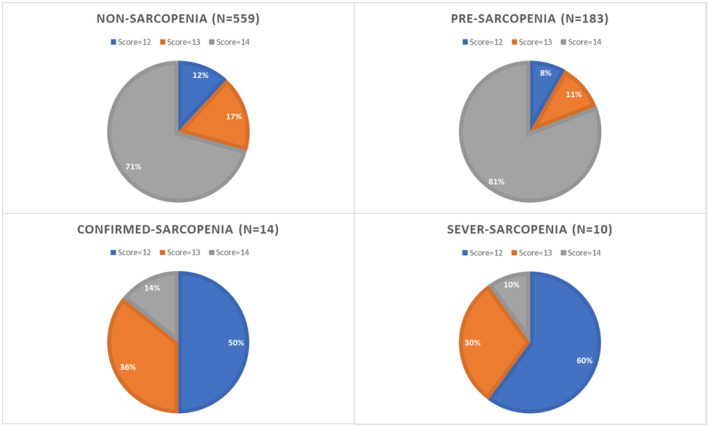
The pie chart of people with MNA‐SF scores of 12, 13, and 14 in each clinical status of sarcopenia within a nourished population. The score has a direct association with malnutrition. MNA‐SF, Mini‐Nutrition Assessment‐Short Form.
4. DISCUSSION
The results of the current study showed that although the MNA‐SF score was not clinically significantly different between pre‐ and non‐sarcopenia, this tool could be considered a possible tool for predicting confirmed and severe sarcopenia independent of other risk factors, even in a nourished population. Furthermore, the MNA‐SF score was correlated with muscle performance and strength in this healthy population which can confirm the hypothesis.
Despite the established effects of energy‐protein malnutrition on sarcopenia, the use of nutritional monitoring tools to predict sarcopenia remains to be clarified. 7 , 8 , 9 All the studies that assessed the nutritional tool on sarcopenia, were included with mixed model of nourished and malnutrition subjects. There was also no study that concentrated on the healthy nourished subjects by the authors’ knowledge. Consequently, a clinical mixture of findings of the current study and other studies could be helpful. Although work on the MNA as one of the most common tools to predict malnutrition on sarcopenia is limited, in a study of non‐hospitalized older adults (2018) using a methodology similar to the current study, the MNA score was significantly lower in people with sarcopenia than in people without sarcopenia. 12 The results of the other studies also confirm the current association between the MNA long and short‐form scores with sarcopenia. 13 , 14 , 15 , 16 , 17 Nevertheless, in contrast with most of the studies, there is evidence that the presumed incidence of sarcopenia is not significantly related to the risk of malnutrition, using both the long and short forms of the MNA. 18 Despite the study that rejected the usability of the MNA 18 in sarcopenia, the majority of findings regarding the use of the MNA indicated the evaluated malnutrition based on the MNA is associated with sarcopenia. It is therefore, although most studies indicate the usability of this tool to predict the risk of sarcopenia in malnutrition and disease, the current study shows that the MNA‐SF can be used to predict sarcopenia risk in a nourished population. The clinical combination of the current and past studies can suggest sarcopenia assessment is better to take place in people with an MNA‐SF score ≤ 12. 7 , 8 , 9 , 13 , 14 , 15 , 16 , 17
The results of the current study show that the MNA‐SF score was associated with clinical indicators of sarcopenia in this population. The small but significant correlation of the MNA‐SF can be explained by the narrow confident interval of the score which was limited between 12 and 14 that could still show the direction of effect. Although the MNA‐SF score had a weak correlation only with muscle strength and power in the current study, there was no correlation between the score and SMMI. In other studies, the MNA score was associated with muscle mass and strength, confirming the current report. 12 , 15 , 17 The result of the studies showed that lower strength and performance are associated with a lower MNA score. Based on the findings regarding SMMI and MNA in another study, the insignificant association of SMMI with the MNA score in the current study can be explained by the triangle of malnutrition, muscle wasting, and sarcopenia. 2 , 3 , 7 , 8 , 9 Malnutrition is strongly associated with muscle wasting, and by including the nourished population, muscle wasting, and as the result reduced muscle mass and SMMI in the current population were not pronounced. Nevertheless, the MNA‐SF correlated negatively with muscle strength and power, indicating a reduction in muscle quality that can predict the first node of sarcopenia. Based on this finding, further studies are suggested to determine the correlation among the MNA‐SF score, sarcopenia, and their clinical indicators. Moreover, this study suggests sarcopenia may occur before malnutrition starts by impacting the strength and performance of muscles.
Several underlying biological mechanisms have been proposed to explain the relationship between the MNA tool and sarcopenia. First, malnutrition leads to a decrease in the availability of essential amino acids, which are necessary for protein synthesis and muscle growth. 19 , 20 Second, malnutrition can result in hormonal imbalances, such as reduced levels of insulin‐like growth factor‐1 (IGF‐1), which play a crucial role in muscle development and maintenance. 21 Third, malnutrition can lead to increased inflammation and oxidative stress, which can contribute to muscle wasting and dysfunction. 19 , 20 All these pathways can reduce the strength, mass, or performance of the muscle. Additionally, considering the structure of the MNA‐SF, this tool can be a proper instrument for general assessment of muscle wasting, strength loss, and malnutrition at the same time which is highly associated with sarcopenia. 10 , 11 , 20 , 22 Moreover, based on the findings of the current study, the MNA‐SF score over 12 which indicates lack of the risk of malnutrition cannot guarantee the lack of sarcopenia. This is where an MNA‐SF score of less than 13 can predict the risk of sarcopenia.
The main limitations and strengths of the current study are mentioned elsewhere. 4 The most important limitation of the current report was the exclusion of subjects with malnutrition at baseline. The greater range of the MNA score could improve the result. However, the inclusion of the undernourished and at‐risk‐of‐malnutrition (MNA < 12) population (n = 84) 4 improved the clinical efficiency of the current study in determining the usability of the MNA‐SF in nourished adults. The greatest strength of the study was related to the methodology and sensitivity of the main study. This study was conducted in a population that did not have any of the major clinical risk factors for sarcopenia, diseases, and elderly malnutrition, that increased its strength. Although, these studies can provide an important rationale for further investigations in the field of MNA and sarcopenia with the aim of improving this nutritional assessment tool.
5. CONCLUSION
There was a significant difference between the MNA‐SF score and the incidence of sarcopenia, muscle strength, and performance, even in nourished individuals. Investigating and developing a sarcopenia‐related nutritional monitoring tool based on the MNA‐SF can be helpful. Furthermore, sarcopenia assessment in people with an MNA‐SF score < 13 can be considered clinically beneficial to early diagnosis of sarcopenia along with malnutrition (MNA‐SF < 12) even in nourished individuals.
AUTHOR CONTRIBUTIONS
Study concept and design: Hosseini, Shadmand Foumani Moghadam, and Pezeshki. Manuscript writing: Shadmand Foumani Moghadam, Rezvani, and Shahraki Jazinaki. Conducting the study: Shadmand Foumani Moghadam, Rezvani, Rashidipour, and Shahraki Jazinaki. Data validation: Hosseini, Ghayour Mobarhan, and Mohammad Reza Shadmand Foumani Moghadam. Statistical analysis and interpretation of data: Shadmand Foumani Moghadam, and Ghayour Mobarhan. All authors approved the final version and agreed to publish the paper. Hosseini is responsible for the paper.
FUNDING INFORMATION
The grant was awarded through an internal grant from the Research Committee of the Varastegan Institute for Medical Sciences.
CONFLICT OF INTEREST STATEMENT
There is no conflict of interest to declare.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The Ethics Committee approval was obtained from Mashhad University of Medical Science, Iran: IR.MUMS.REC.1398.229 (http://ethics.research.ac.ir).
CONSENT FOR PUBLICATION
Not applicable.
ACKNOWLEDGMENTS
The authors would like to thank the “Varastegan Institute for Medical Sciences,” the “Welfare Organization of Khorasan Razavi Provence,” and the “Khorasan Razavi Retirement Association,” and all those who participated in this study.
Shadmand Foumani Moghadam MR, Shahraki Jazinaki M, Rashidipour M, et al. Mini Nutrition Assessment‐Short Form score is associated with sarcopenia even among nourished people – A result of a feasibility study of a registry. Aging Med. 2023;6:264‐271. doi: 10.1002/agm2.12257
Mohammad Reza Shadmand Foumani Moghadam and Mostafa Shahraki Jazinaki contributed equally to this work and are considered co‐first authors.
DATA AVAILABILITY STATEMENT
The data will be available through a proposal for further research.
REFERENCES
- 1. Traub J, Bergheim I, Eibisberger M, Stadlbauer V. Sarcopenia and liver cirrhosis—comparison of the European Working Group on Sarcopenia Criteria 2010 and 2019. Nutrients. 2020;12(2):547. doi: 10.3390/nu12020547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cruz‐Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019;393:2636‐2646. doi: 10.1016/S0140-6736(19)31138-9 [DOI] [PubMed] [Google Scholar]
- 3. Cruz‐Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16‐31. doi: 10.1093/ageing/afy169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shadmand Foumani Moghadam MR, Etemadi S, Bozzetti F, et al. Evaluation of pre‐sarcopenia and sarcopenia in a well‐nourished late‐middle‐aged population: a feasibility study of a registry. J Nutr Fast Health. 2022;10(4):258‐270. doi: 10.22038/jnfh.2022.68278.1405 [DOI] [Google Scholar]
- 5. Senior HE, Henwood TR, Beller EM, Mitchell GK, Keogh JW. Prevalence and risk factors of sarcopenia among adults living in nursing homes. Maturitas. 2015;82(4):418‐423. doi: 10.1016/j.maturitas.2015.08.006 [DOI] [PubMed] [Google Scholar]
- 6. Petermann‐Rocha F, Balntzi V, Gray SR, et al. Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta‐analysis. J Cachexia Sarcopenia Muscle. 2022;13(1):86‐99. doi: 10.1002/jcsm.12783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cruz‐Jentoft AJ, Landi F, Topinková E, Michel JP. Understanding sarcopenia as a geriatric syndrome. Curr Opin Clin Nutr Metab Care. 2010;13(1):1‐7. doi: 10.1097/MCO.0b013e328333c1c1 [DOI] [PubMed] [Google Scholar]
- 8. Muneshige S, Kunihiro S. Malnutrition and sarcopenia. In: Farhan S, Aftab A, Muhammad A, eds. Combating Malnutrition Through Sustainable Approaches. IntechOpen:Ch. 6; 2022. doi: 10.5772/intechopen.104967 [DOI] [Google Scholar]
- 9. Shadmand Foumani Moghadam MR, Dahakzade F, Shariatmadar Tehrani N, Molavi SF, Kavoosi F, Hosseini Z. The high prevalence of malnutrition in the cancer patients admitted to Omid Hospital in Mashhad, Iran based on the PG‐SGA questionnaire (2020). J Nutr Fast Health. 2021;9(1):43‐49. doi: 10.22038/jnfh.2020.50776.1283 [DOI] [Google Scholar]
- 10. Cereda E. Mini nutritional assessment. Curr Opin Clin Nutr Metab Care. 2012;15(1):29‐41. doi: 10.1097/MCO.0b013e32834d7647 [DOI] [PubMed] [Google Scholar]
- 11. Mahdavi AM, Mahdavi R, Lotfipour M, Jafarabadi MA, Faramarzi E. Evaluation of the Iranian mini nutritional assessment short‐form in community‐dwelling elderly. Health Promot Perspect. 2015;5(2):98‐103. doi: 10.15171/hpp.2015.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liguori I, Curcio F, Russo G, et al. Risk of malnutrition evaluated by mini nutritional assessment and sarcopenia in noninstitutionalized elderly people. Nutr Clin Pract. 2018;33(6):879‐886. doi: 10.1002/ncp.10022 [DOI] [PubMed] [Google Scholar]
- 13. Velázquez‐Alva MC, Irigoyen‐Camacho ME, Zepeda‐Zepeda MA, Lazarevich I, Arrieta‐Cruz I, D'Hyver C. Sarcopenia, nutritional status and type 2 diabetes mellitus: a cross‐sectional study in a group of Mexican women residing in a nursing home. Nutr Diet. 2020;77(5):515‐522. doi: 10.1111/1747-0080.12551 [DOI] [PubMed] [Google Scholar]
- 14. Juby AG, Mager DR. A review of nutrition screening tools used to assess the malnutrition‐sarcopenia syndrome (MSS) in the older adult. Clin Nutr ESPEN. 2019;32:8‐15. doi: 10.1016/j.clnesp.2019.04.003 [DOI] [PubMed] [Google Scholar]
- 15. Tan VMH, Pang BWJ, Lau LK, et al. Malnutrition and sarcopenia in community‐dwelling adults in Singapore: Yishun health study. J Nutr Health Aging. 2021;25(3):374‐381. doi: 10.1007/s12603-020-1542-x [DOI] [PubMed] [Google Scholar]
- 16. Sri‐on J, Fusakul Y, Kredarunsooksree T, Paksopis T, Ruangsiri R. The prevalence and risk factors of sarcopenia among Thai community‐dwelling older adults as defined by the Asian Working Group for Sarcopenia (AWGS‐2019) criteria: a cross‐sectional study. BMC Geriatr. 2022;22(1):786. doi: 10.1186/s12877-022-03471-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saka B, Ozkaya H, Karisik E, et al. Malnutrition and sarcopenia are associated with increased mortality rate in nursing home residents: a prospective study. Eur Geriatr Med. 2016;7(3):232‐238. doi: 10.1016/j.eurger.2015.12.010 [DOI] [Google Scholar]
- 18. Lengelé L, Bruyère O, Beaudart C, Reginster JY, Locquet M. Malnutrition, assessed by the Global Leadership Initiative on Malnutrition (GLIM) criteria but not by the mini nutritional assessment (MNA), predicts the incidence of sarcopenia over a 5‐year in the SarcoPhAge cohort. Aging Clin Exp Res. 2021;33(6):1507‐1517. doi: 10.1007/s40520-021-01880-5 [DOI] [PubMed] [Google Scholar]
- 19. Remelli F, Volpato S. Sarcopenia and malnutrition: impact on the outcome in hospitalized patients. Geriatr Care. 2018;4(3). doi: 10.4081/gc.2018.7723 [DOI] [Google Scholar]
- 20. Azzolino D, Passarelli PC, De Angelis P, Piccirillo GB, D'addona A, Cesari M. Poor oral health as a determinant of malnutrition and sarcopenia. Nutrients. 2019;11(12):2898. doi: 10.3390/nu11122898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ascenzi F, Barberi L, Dobrowolny G, et al. Effects of IGF‐1 isoforms on muscle growth and sarcopenia. Aging Cell. 2019;18(3):e12954. doi: 10.1111/acel.12954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Isenring EA, Banks M, Ferguson M, Bauer JD. Beyond malnutrition screening: appropriate methods to guide nutrition care for aged care residents. J Acad Nutr Diet. 2012;112(3):376‐381. doi: 10.1016/j.jada.2011.09.038 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data will be available through a proposal for further research.


