Abstract
Background:
Post-traumatic related limb osteomyelitis (PTRLO) is a complex bone infection. Currently, there are no available microbial data on a national scale that can guide appropriate antibiotic selection, and explore the dynamic changes in dominant pathogens over time. This study aimed to conduct a comprehensive epidemiological analysis of PTRLO in China.
Methods:
The study was approved by the Institutional Research Board (IRB), and 3526 PTRLO patients were identified from 212 394 traumatic limb fracture patients at 21 hospitals between 1 January 2008 and 31 December 2017. A retrospective analysis was conducted to investigate the epidemiology of PTRLO, including changes in infection rate (IR), pathogens, infection risk factors and antibiotic resistance and sensitivity.
Results:
The IR of PTRLO increased gradually from 0.93 to 2.16% (Z=14.392, P<0.001). Monomicrobial infection (82.6%) was significantly higher than polymicrobial infection (17.4%) (P<0.001). The IR of Gram-positive (GP) and Gram-negative (GN) pathogens showed a significant increase from the lowest 0.41% to the highest 1.15% (GP) or 1.62% (GN), respectively. However, the longitudinal trend of GP vs. GN’s composition did not show any significance (Z=±1.1918, P>0.05). The most prevalent GP strains were Methicillin-sensitive Staphylococcus aureus (MSSA) (17.03%), Methicillin-resistant Staphylococcus aureus (MRSA) (10.46%), E. faecalis (5.19%) and S. epidermidis (4.87%). In contrast, the dominant strains GN strains were Pseudomonas Aeruginosa (10.92%), E. cloacae (10.34%), E. coli (9.47%), Acinetobacter Baumannii (7.92%) and Klebsiella Pneumoniae (3.33%). In general, the high-risk factors for polymicrobial infection include opened-fracture (odds ratio, 2.223), hypoproteinemia (odds ratio, 2.328), and multiple fractures (odds ratio, 1.465). It is important to note that the antibiotics resistance and sensitivity analysis of the pathogens may be influenced by complications or comorbidities.
Conclusions:
This study provides the latest data of PTRLO in China and offers trustworthy guidelines for clinical practice. (China Clinical Trials.gov number, ChiCTR1800017597).
Keywords: multiple-centre, osteomyelitis, post-traumatic related limb, risk factors, staphylococcus aureus
Introduction
Highlights
The lack of microbial data related to post-traumatic related limb osteomyelitis (PTRLO) in China poses a significant challenge in selecting appropriate antibiotics for the population.
We identified the prevalent microbial species responsible for PTRLO and determined the antibiotic susceptibility and resistance patterns of the predominant Gram-positive and Gram-negative bacteria.
Our study presents a clear picture of the epidemiology of PTRLO in a patient cohort in China and provides valuable insights for patient management and clinical decision-making.
Fracture-related infection (FRI) encompasses a range of infections, including those involving both operative and non-operative treatment of bone fractures 1,2. It is known that post-traumatic related limb osteomyelitis (PTRLO) constitutes a major proportion of FRI, and it is a complex bone infection that can cause significant morbidity, potentially leading to limb loss and imposing a heavy economic burden3,4. Effective management of this condition typically involves a prolonged course of therapy with intravenous antibiotic agents and surgery5, although oral antibiotic therapy has also recently shown excellent efficacy on complex orthopaedic infections during the first 6 weeks6. Accurate diagnosis of FRI is crucial, and the decision to use antibiotics should be based on factors such as microbial sensitivity and resistance, as well as the patient’s comorbidities and complications7,8.
Previous studies have reported single hospital-based data on PTRLO in Southwest and Southern China, indicating that the incidence of post-traumatic osteomyelitis was around 2–3%4,9,10. Staphylococcus aureus was found to be the most common pathogen, and the lower extremity (specifically the tibia and fibula) was the most frequent affected site of infection. These findings differ from those reported in the USA, UK and Middle East11–14. We have also noted that there is currently no microbial data available to guide appropriate antibiotic selection on a regional or national scale9,10,15. Furthermore, it is unclear whether the dominant pathogens causing infection remain consistent over time or change3,16. Recent studies, conducted in Northeast and East China with limited numbers of multicenters, have revealed the distribution of pathogenic bacteria on FRI. However, neither of these studies could provide significant evidence to reveal the relative risk factors among the infected populations, nor the dynamic changes of major pathogens during the past period11,17 . To address these gaps, we conducted a comprehensive 10-year retrospective multicenter study to examine dynamic changes in infection rates and identify risk factors for monomicrobial and polymicrobial infection. Our study utilized logistic regression to perform a univariate analysis of the data, identifying the current dominant microbial species in infection and mapping the antibiotic sensitivity and resistance of main Gram-positive and Gram-negative bacterial strains.
Material and methods
Study design and data collection
Geographic coverage of all participating hospitals
In order to ensure comprehensive geographic coverage, we conducted a multicenter study of PTRLO at 21 teaching hospitals (Fig. 1 A). These hospitals were carefully selected based on their reputation for providing the highest quality of healthcare in their respective regions (for a detailed list of these hospitals, please refer to the supplemental materials, Supplemental Digital Content 2, http://links.lww.com/JS9/A578). The study was approved by the Institutional Review Board (IRB), and all investigators provided written commitments to scientific integrity to ensure the accuracy of the data. The study was registered online at the Chinese Clinical Trials Registry in 2018 (ChiCTR1800017597). The work has been reported in line with the STROCSS criteria18, Supplemental Digital Content 1, http://links.lww.com/JS9/A576.
Figure 1.
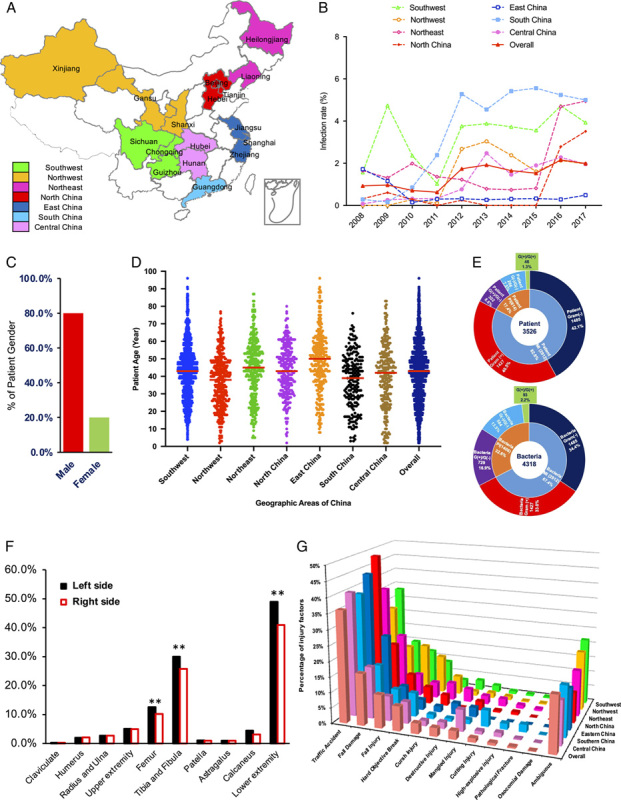
Geographic coverage of participants, dynamic changes of infection rate and related epidemiological data of post-traumatic related limb osteomyelitis (PTRLO). The geographical coverage of all participating hospitals (A), the infection rates (B) and affected gender (C) and age (D) of all regions; the distributions of all populations and pathogen (E) and affected limb sites (F) and the cause of injury (G).
Patient identification and data collection
This study focuses on patients who were admitted to hospitals for the first time or referred to them between 1 January 2008, and 31 December 2017, with FRIs. Out of the 212 394 patients with traumatic limb fractures investigated, only those who met strict screening criteria were included in the analysis. The inclusion and exclusion criteria can be found in the Supporting Information section. The study included patients of all age groups, and a total of 3526 patients were enroled in the retrospective epidemiological analysis to evaluate the changes in infection rates (IRs) and to identify risk factors for monomicrobial infections (MIs) vs. polymicrobial infections (PIs). The risk factors considered were ethnicity, sex, injury factors, age, primary visit or referral from other hospitals, injury type and complications or comorbidities. The analysis distinguished between MI of Gram-positive bacteria (MP), MI of Gram-negative bacteria (MN), PI of Gram-positive bacteria (PP), PI of Gram-negative bacteria (PN), and PI of both Gram-positive and Gram-negative bacteria (PP+PN).
To investigate antibiotic resistance, we analyzed bacterial cultivation and antibiotic susceptibility for 65 strains of Gram-positive (GP) bacteria and 98 strains of Gram-negative (GN) bacteria using the Clinical and Laboratory Standards Institute (CLSI) M100-S18-S27, 2008–2017 guidelines (Supplemental Table 1, Supplemental Digital Content 2, http://links.lww.com/JS9/A578). The analysis considered the risk factors mentioned above for MI vs. PI, MP vs. MN, PP vs. PN and PP+PN.
Statistical analysis
Statistical analyses were performed by using GraphPad Prism (GraphPad Software, LLC. Version 8.4.2), and SAS (SAS Institute, Version 9.4) software. The following tests were used: t-test for primary epidemiological data comparison, by Cochran–Armitage Trend Test for dynamic changes in infection rates, and Pearson’s χ2 test or Fisher’s exact test for antibiotic-susceptibility data of dominant pathogens with different complications or comorbidities. Univariate risk factors associated with MI, PI, MP, MN, PP and PN were evaluated using odds ratio. Variables with a p value less than 0.1 in the univariate analysis, along with other relevant variables, were included in multivariate logistic regression modelling using the forward method for variable selection. A P value less than 0.05 (P<0.05) was considered statistically significant.
Results
Geographic coverage of participants, dynamic changes in infection rate and related epidemiological data of PTRLO
A total 3526 patients were screened from 212 394 post-traumatic related limb fracture cross China. The patients included 1598 (45.32%) from the Southwest, 438 (12.42%) from the Northwest, 375 (10.64%) from the Central Area, 306 (8.68%) from the North, 319 (9.05%) from the South, 206 (5.84%) from the East and 284 (8.54%) from the Northeast of China, respectively (Fig. 1 A). The epidemiology data showed that the overall IR of PTRLO in China gradually increased from 0.93 to 2.16% over 10 years (Z=14.392, P<0.001), and the regional dynamic changes also increased dramatically: Southwest from 1.57 to 3.93% (Z=8.155, P<0.001), Northwest from 0.28 to 1.97% (Z=3.046, P=0.0023), Central from 0.08 to 1.96% (Z=8.2975, P<0.001), North from 0.32 to 3.92% (Z=2.9593, P=0.0031), South from 0.29 to 5.24% (Z=8.2961, P<0.0001) and Northeast from 1.65 to 4.95% (Z=9.0498, P<0.0001). Only East China showed a somewhat stable infection ratio between 1.72 and 0.49% (Z=1.3796, P=0.1677284) (Fig. 1 B). The patients were 79.98% males and 20.02% females with an average age of 42.2 years (95% CI, 26.2–58.2 years), and among them, 92.54% were over 18 years old, 3.49% were 14–18 years old and 3.97% were under 14 years old (Figs. 1 C, Dand Supplemental Table 2, Supplemental Digital Content 2, http://links.lww.com/JS9/A578). Out of the total number of patients (3526), 82.6% were found to have suffered from open fractures. Further analysis showed that 40.5% of these fractures were caused by GP bacteria or GN bacteria, which was significantly higher than the combined incidence rate of GP/GN (8.6%), GN/GN (7.5%) or GP/GN (1.3%) causing fractures (17.4%) (P<0.001) (Fig. 1 E). Rates of open fractures (67.35%) were significantly higher than closed fractures (22.04%) and ambiguous fractures (10.61%) (P<0.05). (Data is not shown). We found that the main sites of injury were in the lower extremity (89.97%) (tibia and fibula 55.74%; femur 22.77%; patella 1.95%; calcaneus 7.60%; astragalus 1.92%), which were significantly more common than in the upper extremity (10.03%) (radius and ulna 5.42%; humerus 4.17%) (P<0.01) (Fig. 1 F). Interestingly, our findings suggest that the left lower extremity is more susceptible to PTRLO than other anatomical sites (P<0.05) (Fig. 1 F). Further injury factor analysis indicates that traffic accidents are the most dominant factor throughout each region and the entire country (P<0.001) (Fig. 1 G).
Longitudinal observation of dominant microbial strains
We conducted a longitudinal observation of dominant microbial strains over a period of 10 years. To investigate dynamic changes in microbial strains, we divided pathogens into GP and GN groups and employed the Cochran–Armitage Trend Test for statistical analysis. Our results showed that the total number of bone infections significantly increased during the past 10 years (P<0.05) (Figs. 2 A, C, E). Interestingly, the IR of GP and GN significantly increased from the lowest 0.41% to the highest 1.15% or 1.62%, respectively (Figs. 2 A, 2 B). However, the trend of the composition of GP vs. GN did not significantly change (Z=±1.1918, P>0.05) (Fig. 2 B). Among GP infections (44.46% of whole infection), the top four dominant strains (37.55%) were MSSA (17.03%), MRSA (10.46%), E. faecalis (5.19%) and S. epidermidis (4.87%) (Figs. 2 C, 2 D). The 10-year dynamic change of S. epidermis infection rates decreased from the highest 10.42% (2009) to 3.20% (2017) (Z=−2.3356, P<0.05), but the infection rate of the other dominant GP strains remained unchanged (P>0.05). A similar trend was observed in GN infection (55.54%). The top five dominant strains of GN (41.98%) were Pseudomonas Aeruginosa (10.92%), E. cloacae (10.34%), E. coli (9.47%), Acinetobacter Baumannii (ABA) (7.92%) and Klebsiella Pneumoniae (KPN) (3.33%), and their 10-year trends in infection rate exhibited no change during this period (P>0.05) (Fig. 2 F).
Figure 2.
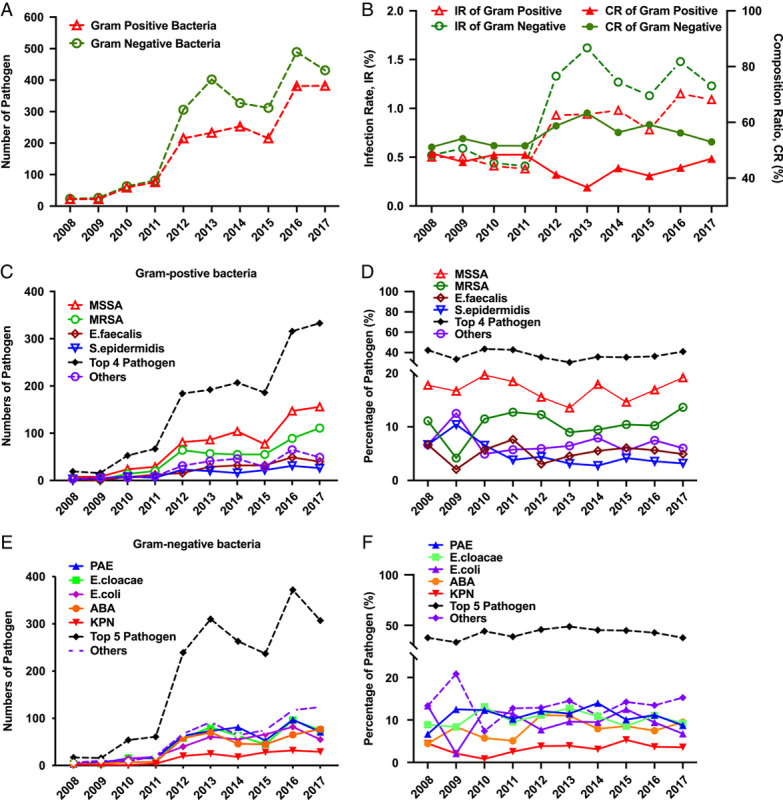
The total number of cases and infection rates of pathogens and the top dominant strains have been tracked over the past decade. The total number of pathogen (Gram postive and negative bacteria) (A), and infection rate (IR, in dotted lines) or composition ration (CR, in solid lines) (B) of over the past ten years. The dynamic changes of top four Gram-positive strains (MSSA, MRSA, E. faecalls, S. epidermidis) and others in number (C) and persentage (D). The dynamic changes of top five Gram-negative strains (PAE, E.cloacae, E. coil, ABA, KPN) and others in number (E) and persentage (F).
PTRLO-related risk factors
To understand whether the risk factors of PTRLO including (1) ethnicity, (2) sex, 3) injury factors, (4) age, (5) primary visiting or referred from other hospitals, (6) injury type and (7) complications or comorbidities had an influence on the type of infection and antibiotic resistance, we performed the logistic regression of univariate and multivariate analyses. We identified the relative risk factors for patients with MI vs. PI (Tables 1, 2), MP vs. MN (Supplemental Table 3, Supplemental Digital Content 2, http://links.lww.com/JS9/A578 and Supplemental Table 4, Supplemental Digital Content 2, http://links.lww.com/JS9/A578), PP vs. PN (Supplemental Table 5, Supplemental Digital Content 2, http://links.lww.com/JS9/A578 and Supplemental Table 6, Supplemental Digital Content 2, http://links.lww.com/JS9/A578), PP vs. PP+PN (Supplemental Table 7, Supplemental Digital Content 2, http://links.lww.com/JS9/A578 and Supplemental Table 8, Supplemental Digital Content 2, http://links.lww.com/JS9/A578) and PN vs. PP+PN (Supplemental Table 9, Supplemental Digital Content 2, http://links.lww.com/JS9/A578 and Supplemental Table 10, Supplemental Digital Content 2, http://links.lww.com/JS9/A578).
Table 1.
Univariate analysis of logistic regression was conducted to identify relative risk factors for patients with monomicrobial infection (MI) or polymicrobial infection (PI).
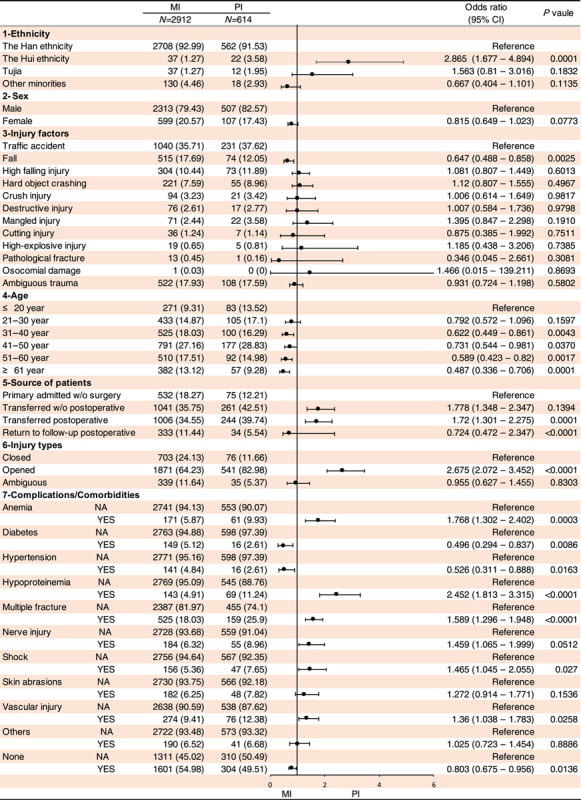
Table 2.
Multivariate analysis of logistic regression was conducted to identify the relative risk factors for patients with monomicrobial infection (MI) or polymicrobial infection (PI).
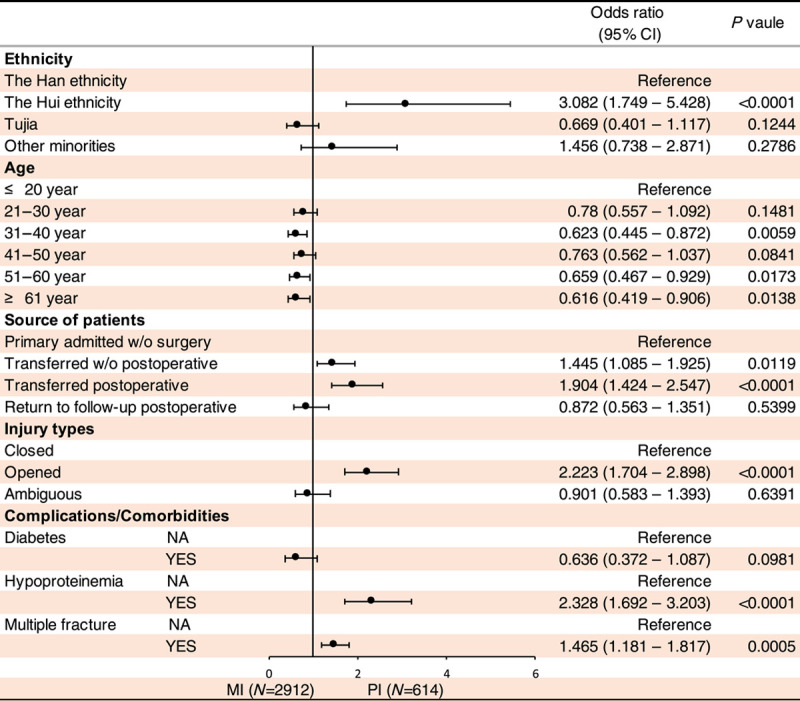
Our univariate and multivariate logistic regression analyses indicated that (1) the risk factors of PI (vs. MI) are the Hui ethnicity (odds ratio, 2.865; 95% CI, 1.677–4.894; P<0.001), patients transferred from other hospitals post-operation (odds ratio, 1.72; 95% CI, 1.301–2.275; P<0.001), open fracture (odds ratio, 2.675; 95% CI, 2.072–3.452; P<0.0001), anaemia (odds ratio, 1.768; 95% CI, 1.302–2.402; P<0.001), hypoproteinemia (odds ratio, 2.452; 95% CI, 1.813–3.315; P<0.0001), multiple fractures (odds ratio, 1.589; 95% CI, 1.296–1.948; P<0.0001), shock (odds ratio, 1.465; 95% CI, 1.045–2.055; P<0.05) and vascular injury (odds ratio, 1.36; 95% CI, 1.038–1.783; P<0.05) (Tables 1, 2). (2) In regard to MP vs. MN, several risk factors were identified. For MP, the Hui ethnicity (odds ratio, 0.453; 95% CI, 0.225–0.913; P<0.05), fall injury (odds ratio, 0.676; 95% CI, 0.536–0.582; P<0.001), transferred postoperative (odds ratio, 0.654; 95% CI, 0.52–0.823; P<0.001) and return to follow-up postoperative (odds ratio, 0.683; 95% CI, 0.511–0.913; P<0.05) were found to be significant risk factors. For MN, the significant risk factors were female (odds ratio, 0.683; 95% CI, 0.511–0.913; P<0.05), opened-fracture (odds ratio, 2.04, 95% CI,1.709–2.434; P<0.0001), anaemia (odds ratio, 1.415; 95% CI, 1.034–1.936; P<0.05), multiple fractures (odds ratio, 1.787; 95% CI, 1.472–2.171; P<0.0001), nerve injury (odds ratio, 1.535; 95% CI, 1.131–2.084; P<0.05), shock (odds ratio, 2.838; 95% CI, 1.971–4.082; P<0.0001), skin broken (odds ratio, 2.094; 95% CI, 1.521–2.881; P<0.0001) and vascular injury (odds ratio, 1.702; 95% CI, 1.317–2.199; P<0.0001) (Supplemental Table 3, Supplemental Digital Content 2, http://links.lww.com/JS9/A578 and Supplemental Table 4, Supplemental Digital Content 2, http://links.lww.com/JS9/A578).
(3) Interestedly, no significantly differences were observed between PP vs. PN for all the aforementioned risk factors (refer to Supplemental Table 5, Supplemental Digital Content 2, http://links.lww.com/JS9/A578 and Supplemental Table 6, Supplemental Digital Content 2, http://links.lww.com/JS9/A578), as well as PP vs. PP+PN (refer to Supplemental Table 7, Supplemental Digital Content 2, http://links.lww.com/JS9/A578 and Supplemental Table 8, Supplemental Digital Content 2, http://links.lww.com/JS9/A578). However, multiple fractures (odds ratio, 0.528; 95% CI, 0.351–0.796; P<0.005) and nerve injury (odds ratio, 0.529; 95% CI, 0.28–0.997; P<0.05) were found to contribute to PN when compared with PP+PN (refer to Supplemental Table 9, Supplemental Digital Content 2, http://links.lww.com/JS9/A578 and Supplemental Table 10, Supplemental Digital Content 2, http://links.lww.com/JS9/A578).
Analysis of antibiotic resistance and sensitivity against the top 20 antibiotics for the dominant GP or GN pathogens under influences of complications or comorbidities
Our study was based on the annual renewal of CLSI, M100-S18-S27 between 2008 and 2017. We focused on the top four dominant GP strains: MSSA, MRSA, E. faecalis and S. epidermidis, which account for 84.45% of GP, while the remaining GP bacteria (15.55% of GP) were grouped together. We included a total of twenty available antibiotics and analyzed their effectiveness under the influence of anaemia, diabetes, hypertension, hypoproteinemia, multiple fractures, nerve injury, shock, broken skin and vascular injury. Our findings show that all GP pathogens were sensitive to Vancomycin, Tigecycline, Tetracycline, Teicoplanin and Linezolid. Additionally, we found that (1) MSSA is sensitive to Amoxicillin, Ciprofloxacin, Dalfopristin, Gentamicin, Levofloxacin, Moxifloxacin, Piperacillin, Rifampicin, SMZCo, Teicoplanin, but has primary resistance to Ampicillin, Penicillin and Azithromycin. (2) MRSA is sensitive to Dalfopristin, SMZCo, but is resistant to Amoxicillin, Ampicillin, Azithromycin, Erycin, Lincomycin and Penicillin. (3) Enterococcus faecalis is sensitive to Amoxicillin and mostly resistant to Azithromycin, Cefoxitin, Clindamycin, Lincomycin, Oxacillin, SMZCo. (4) S. epidermidis is sensitive to Dalfopristin and Gentamicin but mostly resistant to Ampicillin, Erycin, and Penicillin. (5) All other GP bacteria are susceptible to Dalfopristin, but mainly resistant to Oxacillin and Penicillin (Fig. 3). We further performed the statistical analysis and the statistically significant threshold was set at P less than 0.05 (Supplemental Fig. 1, Supplemental Digital Content 2, http://links.lww.com/JS9/A578).
Figure 3.
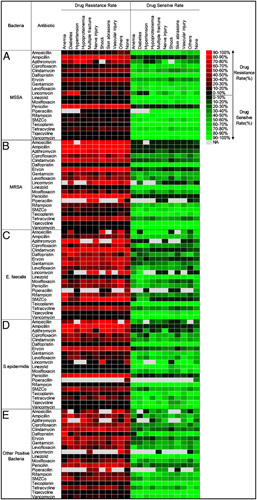
The statistical results of analyses comparing antibiotic resistance and sensitivity in Gram-positive bacteria across 20 commonly available antibiotics were evaluated in the presence of complications or comorbidities. The statistical results of those top bacteria are shown in A) MSSA; B) MRSA; C) E. faecalis; D) S.epidermidis; E) Other positive bacteria, respectively. NA means no data applied.
In this study, we observed that all PN cultures exhibited sensitivity to Amikacin and displayed resistance to Ampicillin and most SMZCo. Additionally, further analysis revealed specific antibiotic sensitivities and resistances for different GN as follows: (1) Pseudomonas Aeruginosa displayed sensitivity to Ciprofloxacin, Gentamicin, Levofloxacin, Piperacillin/Tazobactam and Tobramycin, but was resistant to Ampicillin/Sulbactam, Cefazolin, Cefotaxime, Cefotetan, Ceftriaxone and Cefuroxime. (2) Enterobacter Cloacae was found to be sensitive to Cefotetan, Ertapenem, and Piperacillin/Tazobactam, while mainly displaying resistance to Amoxicillin, Ampicillin/Sulbactam, Cefazolin, Cefotetan and Cefuroxime. (3) E. coli demonstrated sensitivity to Cefepime, Cefoperazone/ Sulbactam, Ciprofloxacin, Ertapenem, Levofloxacin and Piperacillin/Tazobactam, but resistance to Cefazolin, Cefotaxime and Cefuroxime. (4) Acinetobacter Baumannii was sensitive to Ertapenem but appeared to be resistant to all other tested antibiotics. (5) Klebsiella pneumoniae was susceptible to Cefotetan and Ertapenem, but largely resistant to Cefazolin and Cefotaxime. (6) Most other minority negative bacteria were found to be sensitive to Cefepime, Cefoperazone/ Sulbactam, Ertapenem and Piperacillin/Tazobactam, but were resistant to Cefazolin and Cefuroxime (Fig. 4). The statistical analysis results of GN infection can be found in Supplemental Figure 2, Supplemental Digital Content 2, http://links.lww.com/JS9/A578.
Figure 4.
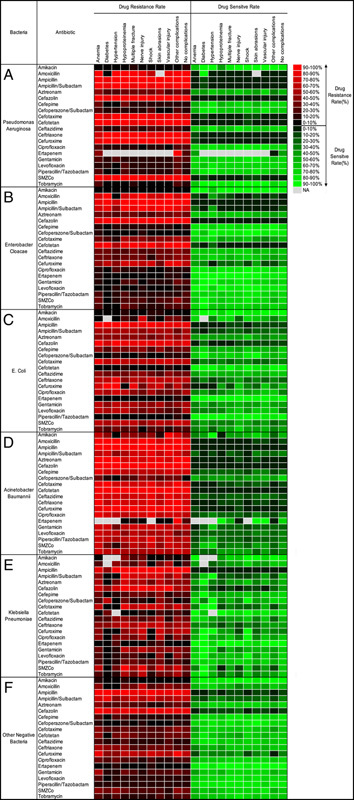
The statistical results of analyses comparing antibiotic resistance and sensitivity in Gram-negative bacteria across 20 commonly available antibiotics were evaluated in the presence of complications or comorbidities. The statistical results of the top bacteria are shown as follows: (A) Pseudomonas aeruginosa; (B) Enterobacter cloacae; (C) Escherichia coli; (D) Acinetobacter baumannii; (E) Klebsiella pneumoniae; (F) Other Gram-negative bacteria. "NA" indicates no data available.
Discussion
The findings of this study show that PTRLO in 21 teaching hospitals is mainly focused on middle-aged males who suffer from open fractures on the left lower extremity, caused by traffic accidents. Previous publications have identified several risk factors that contribute to delayed wound closure, fracture non-union, or bone infection, such as segmental bone loss, increasing BMI and age19–21. The most recent review of fracture-related infection also confirms that fractures in the lower extremities have a higher risk of infection than upper extremity injuries1. During the 10-year study period, we observed an overall increase in the incidence rate of PTRLO, with a significant increase in the incidence rate of GP and GN pathogens. Our research also identified four dominant strains of GP and five dominant strains of GN that require special attention22, Among these dominant strains, S. aureus (including MSSA and MRSA) is highly toxic and capable of biofilm formation, potentially causing a higher rate of recurrence even after decades of dormancy23. Despite our selection of highly sensitive and resistant antibiotics for these pathogens, their limited pharmacokinetics may prevent them from killing the pathogens within staphylococcal abscess communities and the osteocyte lacuno-canalicular network. Therefore, we recommend exploring the use of conjugated antibiotics as a possible solution24,25.
The incidence rate of PTRLO in China has gradually increased from 0.93 to 2.16% between 2008 and 2017. The previous report on single-centre PTRLO cases also fell within this range and was consistent with other reports26. Analysis of patient characteristics led us to speculate that open fractures and patients transferred from other hospitals without surgery may be mainly responsible for the increasing IR, in line with previous studies that open fractures are inevitably contaminated and can compromise immune system function1,27. We recommend improving surgical debridement, irrigation and soft tissue management for open fractures in community hospitals and basic medical units, particularly early intervention for infection prevention28. Scholars have suggested that repeated debridement 24–48 h later may be an effective measure to reduce the infection rate29.
While the infection rate of GP and GN pathogens increased significantly over the past decade, the proportion and infection rate of each top pathogen remained relatively stable, except for S. epidermidis. S. aureus remains the most dominant pathogen, which is consistent with our previous publications9,30, despite research in Europe indicating a decline in the prevalence of MRSA over the past 10 years31,32. Moreover, it is crucial to note that the most common bacterial genus for PTRLO is GN, emphasizing the need for prevention measures.
This study utilized logistic regression to identify various risk factors for different target populations. Some previous studies have investigated risk factors for specific targets, such as older age, working in agriculture, open fracture Gustilo type III, and need for blood transfusion being risk factors for polymicrobial post-traumatic osteomyelitis33. Notably, Gustilo type III A–C leads to 30% higher infection rate of FRI as vascular damage leads to reduced systemic antibiotic perfusion26. However, the current study expands the scope of risk factors and target populations. The hope is that this study’s results will provide valuable insights for predicting the type of PTRLO in a timely manner, particularly during the early stages of the disease or for patients without access to antibiotic-susceptibility test results or with low-grade infections34.
However, one major limitation of this retrospective study is our inability to accurately collect more detailed information regarding patients’ living habits, geographical environment, medical history and health conditions to explore potential risk factors. Another significant limitation is the inability to establish whether the identified risk factors are indeed pathophysiological factors for different infection types or underlying biological mechanisms involved. Additionally, we did not investigate the dynamics of drug resistance/sensitivity rates of the main pathogens during the study period or compare the pathogenic strain’s drug resistance/sensitivity rates between the seven geographical regions in China. We intend to address these questions in future research.
In this study, we aimed to analyze the impact of complications or comorbidities on the antimicrobial resistance and sensitivity of dominant pathogens in PTRLO. We identified several antibacterial agents that showed resistance or susceptibility toward the pathogens and observed a significant difference in their resistance and sensitivity in patients with and without complications or comorbidities. Previous studies have reported that certain comorbidities, such as diabetes, systemic vascular disease and smoking, can increase the risk of bone infection35,36. However, our study is unique in that it highlights the impact of complications or comorbidities on the susceptibility and resistance of pathogens to antibiotics. This finding can be used to develop personalized drug selection strategies for dominant strains in patients with or without complications or comorbidities. This approach can help to prevent the emergence of multi-drug resistant bacteria.
In summary, this study presents the most recent epidemiological findings on PTRLO among patient populations in China, and the results can serve as a reliable reference for healthcare professionals in patient care and clinical practice. Despite some limitations, future research will focus on identifying the changing trends in antibiotic sensitivity and resistance to pathogens, as well as performing a comparative analysis between the seven regions in the past decade.
Ethical approval
Ethical Approval was approved by the Second Affiliated Hospital of Chongqing Medical University, and reference number is 2018 (13). Registration of Research was approved by the Chinese Clinical Trial Registry (ChiCTR-1800017597). (https://www.chictr.org.cn/showproj.aspx?proj=29755)
Source of funding
The International Postdoctoral Exchange Fellowship Program of Chongqing (2021JLPY004), the Fellowship of China Postdoctoral Science Foundation (2021M693758) and Natural Science Foundation Postdoctoral Science Foundation Project of Chongqing (cstc2021jcyj-bsh0019)
National Natural Science Foundation of China (NSFC) to Dr. Zhongliang Deng (81672230), Dr. Zhao Xie (81672160), Dr. Lei Liu (81874002), Dr. Yimin Chai (81572122)
Technological Innovation Projects of Southwest Hospital of China to Dr. Zhao Xie (SW2017ZDCX4105)
AOCPP-Bone Infection to Drs. Edward M. Schwarz, Stephen L. Kates, and Chao Xie.
Author contribution
Conceptualization: C.X. Data curation: Y.R., C.X., Z.D. Investigation: Y.L.R., D.S., Z.D.Z., M.L., M.M.Y., Z.M.L., Y.L.Z., N.J., Z.P.H., J.Z., J.Y., W.F., Y.Z.W., W.R.Z., A.A., ZhiLin Xia, H.F.S., G.D.W., Y.F.Z., J.F., X.H.Z. Formal analysis: C.X., Z.D., Z.X., B.Y., Y.C., L.H. Methodology: Y.R., Z.L., Z.L., W.H., W.F., C.X. Project administration: C.X., Z.D., Z.X., B.Y., Y.C., L.H. Supervision: L.L., X.L., J.D.N., W.H., A.Q.P., K.G.S., Q.B., Q.Y., Y.L., X.B.T., X.H.S., Q.J., X.M.L., Y.S.H., G.Y.Y., S.Q.F., L.H., Y.M.C., B.Y., Z.X., Z.L.D., C.X. Validation: C.X., Z.D., Z.X., B.Y., Y.C., L.H. Writing—original draft: Y.R., C.X., Z.D. Writing—review and editing: Y.R., E.M.S., S.L.K., C.X.
Conflicts of interest disclosure
The authors have no conflict of interest to declare.
Guarantor
Drs. Chao Xie and Zhongliang Deng.
Data statement
The data to support the findings of this study are available upon request. For additional inquiries, kindly contact the corresponding author.
Provenance and peer review:
Not commissioned, externally peer-reviewed
Supplementary Material
Acknowledgements
The authors express their gratitude for the financial support. The authors extend their gratitude to the following individuals for their valuable contributions: Dr. Bin Peng and Dr. Lin Sun from the Department of Biostatistics, School of Public Health and Management, Chongqing Medical University, for providing assistance with statistical analysis, and Dr. Yunying Wang from the Department of Laboratory Microbiology, the Second Affiliated Hospital of Chongqing Medical University, for offering guidance on drug selection.
Footnotes
Y.R., L.L. and D.S. contributed equally to this article as co-first authors;
C.X., Z.D., Z.X., B.Y., Y.C., and L.H. contributed equally to this study as co-corresponding authors.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article
Published online 26 May 2023
Contributor Information
YouLiang Ren, Email: Youliang_Ren@urmc.rochester.edu.
Lei Liu, Email: liuinsistence@163.com.
Dong Sun, Email: sumersun07@126.com.
ZhengDong Zhang, Email: doctorzzd@vip.qq.com.
Meng Li, Email: lilionli@yahoo.com.
Xu Lan, Email: lzzyjw@sina.com.
JiangDong Ni, Email: Nijiangdong001@csu.edu.cn.
Wei Huang, Email: weihuang68@hotmail.com.
AQin Peng, Email: pengaqin@126.com.
YanLong Zhang, Email: zhangyanlongwf@163.com.
Nan Jiang, Email: hnxyjn@smu.edu.cn.
KeGuan Song, Email: songkeguan@sohu.com.
ZhiPeng Huang, Email: doctorhuang106@163.com.
Qing Bi, Email: biqing@hmc.edu.cn.
Jun Zhang, Email: spinezhangjun@aliyun.com.
Qun Yang, Email: yangqundl@hotmail.com.
Jun Yang, Email: worldsapart@126.com.
Yi Liu, Email: liuyiguyike@163.com.
Wei Fu, Email: 2240038958@qq.com.
XiaoBin Tian, Email: txbvip@163.com.
YuanZheng Wang, Email: wangyuanzheng1978@163.com.
WanRun Zhong, Email: jxxgzwr@126.com.
XingHua Song, Email: songxinghua19@163.com.
Abuduxukuer Abudurexiti, Email: 844585713@qq.com.
ZhiLin Xia, Email: zlxia1976@163.com.
Qing Jiang, Email: qingj@nju.edu.cn.
HongFei Shi, Email: dr.hfshi@gmail.com.
XiMing Liu, Email: gklxm@163.com.
GuoDong Wang, Email: gkwgd@163.com.
YunSheng Hu, Email: huys11@163.com.
YunFei Zhang, Email: 289936456@qq.com.
GuoYong Yin, Email: guoyong_yin2005nanjing@yahoo.com.
Jin Fan, Email: fanjin@njmu.edu.cn.
ShiQing Feng, Email: sqfeng@tmu.edu.cn.
XianHu Zhou, Email: zhouxh007@126.com.
ZhengDao Li, Email: jiddao@foxmail.com.
WenBin He, Email: 841298432@qq.com.
Edward M Schwarz, Email: Edward_schwarz@URMC.Rochester.edu.
Stephen L Kates, Email: Stephen.kates@vcuhealth.org.
Lei Huang, Email: huangleijst@126.com.
YiMin Chai, Email: ymchai@sjtu.edu.cn.
Zhao Xie, Email: xiezhao54981@163.com.
ZhongLiang Deng, Email: dengzl@cqmu.edu.cn.
Chao Xie, Email: chao_xie@urmc.rochester.edu.
References
- 1. Moriarty TF, Metsemakers WJ, Morgenstern M, et al. Fracture-related infection. Nat Rev Dis Primers 2022;8:67. [DOI] [PubMed] [Google Scholar]
- 2. Metsemakers WJ, Morgenstern M, McNally MA, et al. Fracture-related infection: a consensus on definition from an international expert group. Injury 2018;49:505–10. [DOI] [PubMed] [Google Scholar]
- 3. Schmitt SK. Osteomyelitis. Infect Dis Clin North Am 2017;31:325–38. [DOI] [PubMed] [Google Scholar]
- 4. Jiang N, Wu HT, Lin QR, et al. Health care costs of post-traumatic osteomyelitis in China: current situation and influencing factors. J Surg Res 2020;247:356–63. [DOI] [PubMed] [Google Scholar]
- 5. Lew DP, Waldvogel FA. Osteomyelitis. Lancet 2004;364:369–379. [DOI] [PubMed] [Google Scholar]
- 6. Li HK, Rombach I, Zambellas R, et al. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med 2019;380:425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lazzarini L, Mader JT, Calhoun JH. Osteomyelitis in long bones. J Bone Jt Surg Am Vol 2004;86:2305–2318. [DOI] [PubMed] [Google Scholar]
- 8. Govaert GAM, Kuehl R, Atkins BL, et al. Diagnosing fracture-related infection: current concepts and recommendations. J Orthop Trauma 2020;34:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peng J, Ren Y, He W, et al. Epidemiological, clinical and microbiological characteristics of patients with post-traumatic osteomyelitis of limb fractures in Southwest China: a hospital-based study. J Bone Jt Infect 2017;2:149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang X, Yu S, Sun D, et al. Current data on extremities chronic osteomyelitis in southwest China: epidemiology, microbiology and therapeutic consequences. Sci Rep 2017;7:16251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang B, Xiao X, Zhang J, et al. Epidemiology and microbiology of fracture-related infection: a multicenter study in Northeast China. J Orthop Surg Res 2021;16:490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kremers HM, Nwojo ME, Ransom JE, et al. Huddleston PM, 3rd. Trends in the epidemiology of osteomyelitis: a population-based study, 1969 to 2009. J Bone Jt Surg Am Vol 2015;97:837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rodham P, Panteli M, Vun JSH, et al. Lower limb post-traumatic osteomyelitis: a systematic review of clinical outcomes. Eur J Orthop Surg Traumatol 2022;8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fily F, Ronat JB, Malou N, et al. Post-traumatic osteomyelitis in Middle East war-wounded civilians: resistance to first-line antibiotics in selected bacteria over the decade 2006-2016. BMC Infect Dis 2019;19:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ma X, Han S, Ma J, et al. Epidemiology, microbiology and therapeutic consequences of chronic osteomyelitis in northern China: A retrospective analysis of 255 Patients. Sci Rep 2018;8:14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hou Y, Bai L, Jiang N, et al. Screening of TNF-α gene polymorphisms in patients with extremity chronic osteomyelitis in China. Personal Med 2018;15:395–401. [DOI] [PubMed] [Google Scholar]
- 17. Ma T, Lyu J, Ma J, et al. Comparative analysis of pathogen distribution in patients with fracture-related infection and periprosthetic joint infection: a retrospective study. BMC Musculoskelet Disord 2023;24:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mathew G, Agha R, Albrecht J, et al. STROCSS 2021: Strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg 2021;96:106165. [DOI] [PubMed] [Google Scholar]
- 19. Cone R, Roszman A, Conway Y, et al. Risk factors for nonunion of distal femur fractures. J Orthop Trauma 2022;37:175–180. [DOI] [PubMed] [Google Scholar]
- 20. Shu HT, Yang VB, Badin D, et al. What factors are associated with delayed wound closure in open reduction and internal fixation of adult both-bone forearm fractures? Clin Orthop Relat Res 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Albright JA, Meghani O, Rebello E, et al. A comparison of the rates of postoperative infection following distal radius fixation between pediatric and young adult populations: an analysis of 32 368 patients. Hand (NY) 2022;23:15589447221142896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Masters EA, Ricciardi BF, Bentley KLM, et al. Skeletal infections: microbial pathogenesis, immunity and clinical management. Nat Rev Microbiol 2022;20:385–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kong P, Ren Y, Yang J, et al. Relapsed boyhood tibia polymicrobial osteomyelitis linked to dermatophytosis: a case report. BMC Surg 2022;22:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adjei-Sowah E, Peng Y, Weeks J, et al. Development of bisphosphonate-conjugated antibiotics to overcome pharmacodynamic limitations of local therapy: initial results with carbamate linked sitafloxacin and tedizolid. Antibiotics (Basel) 2021;10:732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ren Y, Xue T, Rainbolt J, et al. Efficacy of bisphosphonate-conjugated sitafloxacin in a murine model of S. aureus osteomyelitis: evidence of “Target & Release” kinetics and killing of bacteria within canaliculi. Front Cell Infect Microbiol 2022;12:910970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Papakostidis C, Kanakaris NK, Pretel J, et al. Prevalence of complications of open tibial shaft fractures stratified as per the Gustilo-Anderson classification. Injury 2011;42:1408–1415. [DOI] [PubMed] [Google Scholar]
- 27. Morgenstern M, Vallejo A, McNally MA, et al. The effect of local antibiotic prophylaxis when treating open limb fractures: A systematic review and meta-analysis. Bone Jt Res 2018;7:447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Metsemakers WJ, Onsea J, Neutjens E, et al. Prevention of fracture-related infection: a multidisciplinary care package. Int Orthop 2017;41:2457–69. [DOI] [PubMed] [Google Scholar]
- 29. Zalavras CG. Prevention of infection in open fractures. Infect Dis Clin North America 2017;31:339–52. [DOI] [PubMed] [Google Scholar]
- 30. Yang L, Feng J, Liu J, et al. Pathogen identification in 84 Patients with post-traumatic osteomyelitis after limb fractures. Ann Palliat Med 2020;9:451–458. [DOI] [PubMed] [Google Scholar]
- 31. Cassini A, Högberg LD, Plachouras D, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis 2019;19:56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev 2010;23:616–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jorge LS, Fucuta PS, Oliveira MGL, et al. Outcomes and risk factors for polymicrobial posttraumatic osteomyelitis. J Bone Jt Infect 2018;3:20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Birt MC, Anderson DW, Bruce Toby E, et al. Osteomyelitis: recent advances in pathophysiology and therapeutic strategies. J Orthop 2017;14:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kortram K, Bezstarosti H, Metsemakers WJ, et al. Risk factors for infectious complications after open fractures; a systematic review and meta-analysis. Int Orthop 2017;41:1965–82. [DOI] [PubMed] [Google Scholar]
- 36. Sangaletti R, Zanna L, Akkaya M, et al. Periprosthetic joint infection in patients with multiple arthroplasties. Bone Jt J 2023;105-b:294–300. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


