Abstract
Research has shown that neoadjuvant immunotherapy may provide more significant clinical benefits to cancer patients undergoing surgery than adjuvant therapy. This study examines the development of neoadjuvant immunotherapy research using bibliometric analysis. As of 12 February 2023, articles on neoadjuvant immunotherapy in the Web of Science Core Collection were collected. Co-authorship and keyword co-occurrence analyses and visualizations were performed using VOSviewer, while CiteSpace was used to identify bursting keywords and references. The study analyzed a total of 1222 neoadjuvant immunotherapy publications. The top contributors to this field were the United States, China, and Italy, and the journal with the most publications was Frontiers in Oncology. Francesco Montorsi had the highest H-index. The most common keywords were ‘immunotherapy’ and ‘neoadjuvant therapy’. The study conducted a bibliometric analysis of over 20 years of neoadjuvant immunotherapy research, identifying the countries, institutions, authors, journals, and publications involved in this field. The findings provide a comprehensive overview of neoadjuvant immunotherapy research.
Keywords: bibliometrics, CiteSpace, frontiers, neoadjuvant immunotherapy, VOSviewer
Introduction
Highlights
This study presents the first bibliometric analysis in the field of neoadjuvant immunotherapy.
Burst detection revealed that applying neoadjuvant immunotherapy for urothelial carcinoma and radical cystectomy had become a hot topic.
The United States is the leading country in articles, researchers, and institutions researching neoadjuvant immunotherapy, with China following closely behind.
Neoadjuvant immunotherapy has emerged as a promising approach for treating various types of cancer, including melanoma1 and breast cancer2. It involves administering immunotherapeutic agents before surgical resection to enhance the host immune response and reduce the primary tumor size3. The use of neoadjuvant immunotherapy in cancer treatment has gained significant attention in recent years, and there is a growing body of literature on the subject. An in-depth bibliometric study of publications, countries, institutions, journals, authors, and keywords is still required.
Bibliometrics is a field of study that uses quantitative and statistical methods to analyze the production and dissemination of research literature. It involves collecting, organizing, and analyzing bibliographic data, such as the number of citations, co-authorship patterns, and publication venues4. Bibliometrics has several advantages, including the ability to identify and quantify the impact of research, provide evidence-based assessments of scientific productivity, and track the dissemination and influence of study over time. Bibliometrics can also support the identification of research trends, emerging fields, and collaborations and inform strategic planning and resource allocation in research organizations5. With the growing volume of scientific literature and the increasing importance of research impact, bibliometrics will continue to play a significant role in evaluating and assessing research.
This bibliometric study aims to provide a comprehensive overview of the current knowledge and understanding of neoadjuvant immunotherapy. This study addresses the following research question: What is the current knowledge and experience of neoadjuvant immunotherapy in cancer treatment? A thorough bibliometric analysis was conducted to assess the trends, findings, and gaps in the existing research on neoadjuvant immunotherapy. This research revealed that neoadjuvant immunotherapy had shown promising results in preclinical and clinical trials, with some studies demonstrating improved outcomes compared to standard adjuvant therapies. However, the literature also highlights the need for further research to fully understand the mechanisms underlying neoadjuvant immunotherapy’s efficacy and optimize treatment protocols. This study will fill the gap in the existing literature by synthesizing the present findings and trends in the field, providing a comprehensive overview of the current state of knowledge and understanding about neoadjuvant immunotherapy for researchers, clinicians, and policymakers.
Method
Data source and literature search strategy
Web of Science was selected as the primary database for this study due to its comprehensive coverage of over 12 000 academic journals and its frequent usage by researchers. When compared to other databases such as Scopus, Medline, and PubMed, Web of Science provides the most comprehensive and reliable bibliometric analysis6. The relevant articles in the Web of Science Core Collection (WoSCC) were searched and exported on 12 February 2023, using all database versions. All authors agreed on the search strategy following consultations with senior literature search experts, with the following criteria: (TS = (Neoadjuvant) AND (TS = (Immunotherapy) OR (TS = (Immunotherapies)). To facilitate further analysis of literature content, only regular articles written in English were included. A complete record and cited references were then extracted from relevant publications, saved in plain text format, for further research. To further compare the development patterns and frontier topics of neoadjuvant immunotherapy in different cancer types, we conducted an additional investigation on 25 April 2023, examining the publication status of neoadjuvant immunotherapy articles for the top 5 cancer types by frequency. The keyword frequency, search strategy, and the number of articles are displayed in Table S2, Supplemental Digital Content 1, http://links.lww.com/JS9/A537. The remaining limitations are consistent with the primary search strategy mentioned above.
Software for bibliometric analysis
This study used R version 4.0.17, VOSviewer8, and CiteSpace9 as the software tools for performing bibliometric analysis. We calculated the frequency of collaboration between countries using the Bibliometrix R package version 4.0.010. The number of publications, citations, and keyword frequency were calculated using VOSviewer. With the software’s embedded clustering algorithm, co-occurrence networks of essential keywords from scientific literature were constructed and visualized11. The co-authorship and co-occurrence analysis was the primary focus of this study. This tool was used to analyze country, institution, and author collaborations.
To identify highly cited references and keywords that experienced significant citation increases during a specific period, CiteSpace was used.
Through the online bibliometrics website (https://bibliometric.com/), we were able to visualize international collaborations between countries. The number of publications was analyzed using an exponential growth function in Excel12.
Result
Overview of Publication Status
As shown in Figure 1, 1222 conventional articles on neoadjuvant immunotherapy were included in this study. Figure 2 displays the annual and cumulative publication counts related to neoadjuvant immunotherapy. From 1 publication in 1990 to 10 publications in 2011, the cumulative number has steadily increased. Immune checkpoint inhibitors (ICIs) subsequently led to a rapid increase in publications over the following 11 years, reaching 1222 in 2022. An exponential growth function was then used to evaluate the relationship between cumulative publications and publication year, which matched the trend in the cumulative number of publications (R2=0.966). This strong correlation suggests that neoadjuvant immunotherapy has experienced significant growth and development, particularly in the era of ICIs.
Figure 1.
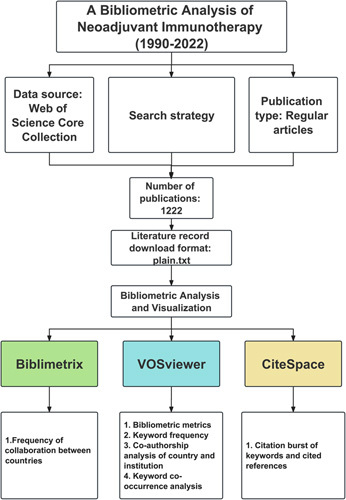
Flow-chart of the study.
Figure 2.

Number of publications per year and the cumulative number.
Analysis of national publication counts
National publication counts were analyzed to investigate the countries/regions contributing the most in this field. In Figure 3, the United States ranks first with 414 publications, followed by China (279), Italy (65), Germany (58), and Japan (52). The remaining countries/regions have fewer than 50 publications.
Figure 3.

Each country’s contribution to the neoadjuvant immunotherapy.
As part of our investigation, we visualized the collaborations among countries/regions in Figure S1, Supplemental Digital Content 2, http://links.lww.com/JS9/A538. According to the results, the US leads the way in neoadjuvant immunotherapy research. The most frequent collaborations are between the US and China and between the US and Italy (with a frequency of 47). The following are the most frequent collaborators are: Germany (frequency=43), France (frequency=35), and the UK (frequency=34). The collaborators from these countries are all from the US. Thirty-four countries have published five or more articles (34/56). We conducted a co-authorship analysis of all publications from the 34 countries above (Figure S2, Supplemental Digital Content 2, http://links.lww.com/JS9/A538) to investigate the collaboration between countries. The size of the circles in the clustering network and the time-overlapping network represent the number of publications. The color of the circles indicates the collaboration strength of the research groups in the clustering network. The colors of the circles represent the average publication year for each country in a specific area of research in the time-overlapping network. As shown in Figure S2A, Supplemental Digital Content 2, http://links.lww.com/JS9/A538, the 34 countries formed five clusters. The blue cluster, which includes most countries, has seven countries. In Figure S2B, Supplemental Digital Content 2, http://links.lww.com/JS9/A538, the US was an early pioneer in neoadjuvant immunotherapy, while Chinese researchers’ studies in this field are relatively recent.
Analysis of institution publications
To explore institutions’ contributions to neoadjuvant immunotherapy, the number of publications from various institutions was analyzed. Neoadjuvant immunotherapy research was conducted at approximately 2296 institutions worldwide. As shown in Figure 4, 14 research institutions are from the US, four from China, one from Italy, and one from the Netherlands. With 86 articles published, the University of Texas MD Anderson Cancer Center ranked first.
Figure 4.

The top 20 institutions with the most publications in the field of neoadjuvant immunotherapy.
To further investigate collaboration between institutions, we performed a co-authorship analysis of all publications. Supplementary Figure 3A, Supplemental Digital Content 2, http://links.lww.com/JS9/A538 shows that 68 institutions published at least 10 papers. These 68 institutions formed five clusters, with the red cluster being the largest, consisting of 21 institutions, mainly from China. Several American research institutions, led by Johns Hopkins University, contributed significantly to neoadjuvant immunotherapy’s early development. In contrast, multiple Chinese research institutions became more involved in neoadjuvant immunotherapy research after 2020.
Analysis of publication quantity and journal impact
The study included 1222 articles published in 405 journals. Table 1 lists the top 10 journals ranked by publication quantity and their latest 2022 impact factors (IF)13. Seven top 10 journals were in the first quartile (Q1) of the Journal Citation Reports (JCR). There are three publishers from the US, UK, and Switzerland, but only one publisher from China.
Table 1.
Top 10 journals in the field of neoadjuvant immunotherapy.
| Rank | Source | Article | Country | IF | H-index | JCR-c |
|---|---|---|---|---|---|---|
| 1 | Frontiers in Oncology | 54 | Switzerland | 5.738 | 53 | Q2 |
| 2 | The Journal for ImmunoTherapy of Cancer | 48 | UK | 12.469 | 46 | Q1 |
| 3 | Clinical Cancer Research | 43 | USA | 13.801 | 61 | Q1 |
| 4 | Cancers | 39 | Switzerland | 6.575 | 54 | Q1 |
| 5 | Frontiers in Immunology | 37 | Switzerland | 8.786 | 84 | Q1 |
| 6 | Oncoimmunology | 29 | USA | 7.723 | 26 | Q1 |
| 7 | BMC Cancer | 28 | UK | 4.638 | 31 | Q2 |
| 8 | Cancer Immunology Immunotherapy | 28 | USA | 6.63 | 30 | Q1 |
| 9 | Journal of Translational Medicine | 17 | UK | 8.44 | 46 | Q1 |
| 10 | Annals of Translational Medicine | 15 | China | 3.616 | 32 | Q3 |
Author impact analysis
A total of 10 825 authors participated in research on neoadjuvant immunotherapy. Table 2 shows Andrea Necchi to be the most productive author, with 18 articles published and a 39 H-index. Following closely behind were Francesco Montorsi (14 articles, H-index=110) and Alberto Briganti (13 articles, H-index=81).
Table 2.
Top 10 authors in the field of neoadjuvant immunotherapy.
| Rank | Author | Article | H-index |
|---|---|---|---|
| 1 | Andrea Necchi | 18 | 39 |
| 2 | Francesco Montorsi | 14 | 110 |
| 3 | Alberto Briganti | 13 | 81 |
| 4 | Paolo A. Ascierto | 12 | 83 |
| 5 | Christian Blank | 12 | 63 |
| 6 | Jennifer A. Wargo | 12 | 83 |
| 7 | Marco Bandini | 11 | 41 |
| 8 | Gallina Andrea | 11 | 51 |
| 9 | Lajos Pusztai | 11 | 74 |
| 10 | Daniele Raggi | 11 | 19 |
Researchers’ collaborative relationships are illustrated in Supplementary Figure 4A, Supplemental Digital Content 2, http://links.lww.com/JS9/A538. Circle size represents the number of publications, and color represents cluster. One hundred twenty-two authors with five or more articles were grouped into 14 clusters. Six clusters were located outside the more prominent community consisting of eight clusters. There was no collaboration between these six scattered clusters and the larger clusters, indicating that collaboration between research teams/laboratories conducting research related to neoadjuvant immunotherapy needs to be strengthened. The time-overlapping network of clustering results is shown in Supplementary Figure 4B, Supplemental Digital Content 2, http://links.lww.com/JS9/A538. We observed that researchers from China are forming a new research network on neoadjuvant immunotherapy. National and institutional collaborations are one of the future directions due to the inadequate collaboration between different research groups.
Research hotspot analysis
Most cited publications
It is possible to evaluate the most cited articles based on the frequency of citations in that field. In Supplementary Table 1, Supplemental Digital Content 9, http://links.lww.com/JS9/A545, we list the top 10 most cited publications, with all articles cited over 300 times. There was one article published in 2018 that has received the most citations: ‘Neoadjuvant PD-1 Blockade in Resectable Lung Cancer’14. The study reported that neoadjuvant therapy with nivolumab had few side effects, did not delay surgery, and induced complete pathological responses in 45% of resected tumors. The New England Journal of Medicine also published the second most cited publication. Schmid et al. found that in early triple-negative breast cancer (TNBC) patients, patients who received pembrolizumab plus neoadjuvant chemotherapy had a significantly higher rate of complete pathological response than who received placebo plus neoadjuvant chemotherapy15. These two studies provide strong evidence for the clinical application of neoadjuvant immunotherapy.
Analysis of citation bursts
The top 25 most cited references are illustrated in Supplementary Figure 5, Supplemental Digital Content 2, http://links.lww.com/JS9/A538. A burst is when a publication receives a significantly higher number of citations than usual, lasting at least two years9. The blue line represents the observation period from 1990 to 2022, while the red line indicates the burst time. The article ‘Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer’, published in the New England Journal of Medicine, has the highest citation burst value (citation burst=14.08) between 1990 and 202216. Additionally, 11 articles are still experiencing citation bursts, including ‘Durvalumab after Chemoradiotherapy in Stage III NonSmall-Cell Lung Cancer’, which has the highest burst value of 11.24. This article was published in the New England Journal of Medicine in 2017 and reports on the safety and efficacy of using Durvalumab after chemotherapy in stage III nonsmall-cell lung cancer17. The results show that the progression-free survival is significantly longer with Durvalumab than with a placebo, and the safety between the two groups is similar. Necchi et al. reported that neoadjuvant pembrolizumab therapy resulted in a pT0 status in 42% of patients and was safe in patients with muscle-invasive urothelial bladder carcinoma (MIBC). This study suggests that pembrolizumab may be a reliable neoadjuvant therapy for MIBC when restricted to patients with PD-L1-positive or high tumor mutation burden tumors18. In the future, such research topics may continue to be popular and become a potential frontier area of neoadjuvant immunotherapy research.
Frequency and clustering analysis of keywords
Among 2027 keywords, 54 met the threshold of 10 occurrences, and were analyzed. If these keywords had similar meanings, they were merged. Figure 5A shows the network visualization of these keywords. Keyword frequency is reflected in the size of the nodes, while relationship strength is reflected in the distance between them8. In order to reflect the critical themes in neoadjuvant immunotherapy research, the 54 keywords were grouped into six clusters. Closely related keywords were clustered together. Group 1, represented in red, focused on the application of neoadjuvant chemotherapy in different cancers, such as ‘breast cancer’, ‘colorectal cancer’, and ‘ovarian cancer’. Group 2, represented in green, focused on various treatment methods for bladder and urothelial carcinoma, with keywords such as ‘cisplatin’, ‘cystectomy’, ‘immunotherapy’, and ‘PD-1’. Some terms, such as ‘survival’, were also included in Group 2. Group 3, represented in blue, focused on the clinical application of neoadjuvant immunotherapy, with keywords such as ‘neoadjuvant immunotherapy’, ‘lung cancer’, ‘muscle-invasive bladder cancer’, and common ICIs. The yellow Group 4 focused on the clinical application of neoadjuvant chemoradiotherapy combined with immunotherapy, mainly involving ‘pancreatic cancer’, ‘prostate cancer’, ‘esophageal squamous cell carcinoma’, and ‘rectal cancer’. The purple Group 5 mainly included adjuvant and neoadjuvant therapy for renal cell carcinoma. The sixth light blue cluster mainly included ‘esophageal cancer’, ‘gastric cancer’, ‘chemotherapy’, ‘surgery’, and ‘radiotherapy’, which seemed to be related to traditional treatment methods. Figure 5B shows the visualization of the time-overlapping of keywords. Early-appearing keywords are displayed in blue, while recent keywords are displayed in red. Early research mainly focused on ‘chemotherapy’, ‘renal cell carcinoma’, and ‘breast cancer’. Recent studies have concentrated on topics such as ‘lung cancer’, ‘esophageal squamous cell carcinoma’, ‘complete pathologic response’, ‘tumor microenvironment’, and ‘immune checkpoint inhibitors’. Figure 5C displays the top 20 keywords sorted by frequency, with ‘immunotherapy’being the most frequently used keyword, appearing 403 times, followed by ‘neoadjuvant therapy’ (N=195) and ‘immune checkpoint inhibitors’ (N=96). ‘Neoadjuvant immunotherapy’ appeared 55 times, ranking ninth. Among the top 20 keywords, cancer types that appeared included ‘non-small cell lung cancer (NSCLC)’ (N=93), ‘breast cancer’ (N=66), ‘bladder cancer’ (N=41), ‘melanoma’ (N=38) and ‘triple-negative breast cancer’ (N=38).
Figure 5.

Research hotspots on neoadjuvant immunotherapy (A). keyword co-occurrence network; (B). time-overlapping co-occurrence analysis network of keywords; (C). a list of the 20 most frequently used keywords; (D). the 20 keywords with the strongest citation bursts).
Comparison of neoadjuvant immunotherapy research across cancer types
To compare the progress of neoadjuvant immunotherapy development across different cancer types, we conducted a bibliometric analysis of the top five cancer types by keyword frequency. In summary, bibliometric analysis indicates that the research popularity of neoadjuvant immunotherapy varies among cancer types, with melanoma and NSCLC demonstrating the most significant growth in publications and citations. In comparison, bladder cancer, breast cancer, and TNBC have experienced relatively slower progress. This information is valuable for oncologists and researchers to identify areas of opportunity and focus their efforts on advancing neoadjuvant immunotherapy research across all cancer types. Table S3, Supplemental Digital Content 3, http://links.lww.com/JS9/A539 presents the top 10 countries, institutions, journals, and authors regarding publication volume for each cancer type. The United States is leading in neoadjuvant immunotherapy for breast, bladder, melanoma, and TNBC. China ranks first with 91 published articles on neoadjuvant immunotherapy for NSCLC. The University of Texas System has the highest number of publications in neoadjuvant immunotherapy research for NSCLC, breast cancer, and melanoma, consistent with the overall research findings. The journals with the most publications in NSCLC, breast cancer, bladder cancer, melanoma, and TNBC are Frontiers in Oncology, Clinical Cancer Research, Urologic Oncology: Seminars and Original Investigations, Journal of Translational Medicine, and Clinical Cancer Research, respectively. In NSCLC, the most active author is Boris Sepesi, with six published articles. Lajos Pusztai has published 11 articles on neoadjuvant immunotherapy for breast cancer and six on neoadjuvant immunotherapy for TNBC, making him the most active author in both fields. Andrea Necchi is the most active author in bladder cancer neoadjuvant immunotherapy research, with 15 articles published. In melanoma research, Paolo A. Ascierto leads the field with 11 articles.
To further understand the application of neoadjuvant immunotherapy across different cancer types, we analyzed the citation frequency of the included articles. Tables S4 through S8, Supplemental Digital Content 4, http://links.lww.com/JS9/A540, Supplemental Digital Content 5, http://links.lww.com/JS9/A541, Supplemental Digital Content 6, http://links.lww.com/JS9/A542, Supplemental Digital Content 7, http://links.lww.com/JS9/A543, Supplemental Digital Content 8, http://links.lww.com/JS9/A544 present the top 10 most cited articles in neoadjuvant therapy research for NSCLC, breast cancer, bladder cancer, melanoma, and TNBC.
Analysis of keywords bursts
In Figure 5D, we present the top 30 keywords with the strongest citation bursts lasting at least one year. The keywords ‘immunotherapy’ (1994–2009) received the most sustained attention. However, keywords such as ‘radical cystectomy’ (2020–2022), ‘ipilimumab’ (2020–2022), and ‘urothelial carcinoma’ (2020–2022) have recently been used, indicating that future research will focus on these keywords.
Discussion
Bibliometric methods were used to analyze the growth pattern of neoadjuvant immunotherapy-related research from 1990 to 2022. There are two stages of neoadjuvant immunotherapy-related research growth based on whether the number of publications exceeds 10 per year for two consecutive years. Before 2011, there was a slow growth stage, except for 12 publications in 2007. The number of publications in other years was at most 10. Since 2016, neoadjuvant immunotherapy-related research has entered a rapid growth stage, with more than 10 publications per year. By 2022, the annual publication volume has reached 1222, indicating that neoadjuvant immunotherapy-related research has entered a phase of rapid development. The potential reason may be that with the popularity of ICIs in cancer treatment, people began to realize that moving ICIs treatment forward may favor patients3,19. Therefore, research institutions have continued to increase their support for neoadjuvant immunotherapy-related research, and research funding has continued to increase, promoting the rapid development of this field.
A total of 1026 articles were published by the top 10 countries, accounting for 83.96% of all articles. The US and China dominate the number of publications among the 10 countries. Furthermore, US-centered international cooperation occupies eight positions in the 10 countries with the highest frequency of cooperation. The above findings confirm the US’s critical contributions and leading position in neoadjuvant immunotherapy research, which may result from the United States’ national economic conditions and high medical investment levels. This field will benefit from extensive international cooperation, which will improve the overall standard of research.
Fourteen of the top 20 institutions are in the US, similar to the distribution of publication numbers by country. Despite China’s second-place ranking in publication numbers, only four institutions make the top 20. Italy ranked third in the number of publications and has only one institution in the top 20, ranking 17th. In contrast, a Netherlands institution ranks sixth with 29 papers. These studies are mainly based on international cooperation, indicating that seeking extensive cooperation between institutions may be crucial to improving research competitiveness under economic or resource limitations.
A peer-reviewed journal is essential to academic publishing. Core journals often publish the necessary research in the field. Researchers can identify potential journals to submit their manuscripts to based on the number of journal publications in the neoadjuvant immunotherapy field. Frontiers in Oncology has the most publications, with 54. The journal with the highest impact factor is Clinical Cancer Research (IF 13.801), followed by The Journal for ImmunoTherapy of Cancer (IF 12.469). Impact factor and JCR are commonly used indicators to evaluate the influence of journals. JCR divides all journals into four quartiles (Q1–Q4) based on their IF. In the top 10 journals by paper count, Q1 journals account for 70%. Moreover, despite China’s significant contributions to neoadjuvant immunotherapy research, Asian publishers are underrepresented in the top 10 journals. There is a need to establish and develop internationally influential journals in Asia.
This study aims to answer a question regarding the research hotspots that were widely studied by researchers over a certain period of time. Citation count can serve as one of the indicators of the academic influence of a publication20. Highly cited publications often represent the fundamental themes in a research field. Research hotspots can be identified by calculating citation counts and identifying highly cited publications. In this study, the 10 most frequently cited publications were published between 2013 and 2020 and mainly focused on neoadjuvant immunotherapy’s mechanism and clinical application.
In 2013, Klug et al. suggested that low-dose irradiation of human pancreatic cancer with new adjuvant local gamma rays can normalize abnormal blood vessels and effectively recruit tumor-specific T cells in the tumor area. The authors further verified T-cell-mediated antitumor immune response and extended survival in otherwise immune refractory spontaneous and xenotransplant mouse tumor models21. Although this study does not directly confirm that neoadjuvant immunotherapy provides a survival benefit for pancreatic cancer patients, it provides an essential theoretical basis for neoadjuvant immunotherapy and boosts the investigators’ confidence.
In 2016, Loi et al. analyzed tumor-infiltrating lymphocytes (TILs)’ clinical and molecular characteristics in residual lesions of TNBC after neoadjuvant chemotherapy. Their research aimed to develop a better understanding of the complex interactions between TILs and TNBC in order to improve treatment strategies. They explored a treatment strategy combining MEK inhibitors and PD-1/PD-L1 targeted immunotherapy in a mouse model. The authors pointed out that activating the Ras-MAPK pathway could promote immune escape in TNBC. Their findings suggest that a combined approach targeting MEK and PD-L1 might effectively counteract this immune escape, potentially leading to improved outcomes for TNBC patients22. In addition, Liu et al. used a mouse model of spontaneous metastatic breast cancer to demonstrate that neoadjuvant therapy was significantly more effective than adjuvant therapy23. These studies highlight the potential of neoadjuvant immunotherapy as a promising treatment strategy for TNBC patients, particularly when combined with targeted therapies such as MEK inhibitors. By building on these foundational findings, future research can continue to refine and optimize treatment approaches for TNBC, ultimately improving patient outcomes and quality of life.
In 2018, Forde et al. published an article titled ‘Neoadjuvant PD-1 Blockade in Resectable Lung Cancer’ in the New England Journal of Medicine, which had the highest number of citations in this research. In this study, the authors administered PD-1 inhibitor nivolumab to adults with previously untreated, operable early (stage I, II, or IIIA) NSCLC. The results showed that the side effects of neoadjuvant therapy with nivolumab were acceptable and unrelated to the delay of surgery. Nine of the 20 resected tumors showed a complete pathological response (45%)14. As higher complete response rates are associated with a better prognosis, this study’s results further increase the researchers’ confidence to conduct clinical studies on neoadjuvant immunotherapy. Subsequently, researchers have achieved breakthroughs in patients with glioma24, TNBC15, and colon cancer25, confirming the safety and effectiveness of neoadjuvant immunotherapy from multiple perspectives and cancer types. However, a clinical study conducted by Blank et al. suggests that although neoadjuvant immunotherapy seems promising, the current regimen (ipilimumab 3 mg/kg and nivolumab 1 mg/kg, two courses before and two courses after surgery) induced high toxicity in resectable stage III melanoma patients, with 9/10 patients experiencing one or more grade 3/4 adverse events. Therefore, further research is needed to maintain efficacy while reducing toxicity26. Overall, while the results from numerous studies demonstrate the potential of neoadjuvant immunotherapy in treating different cancers, continued research is necessary to refine treatment regimens and ensure the best possible outcomes for patients.
In 2020, Helmink reported a critical study emphasizing the importance of focusing on immune cells and structures beyond T cells to develop new biomarkers and immune-enhancing strategies. Helmink et al. found that B-cell biomarkers were differentially expressed in tumors of immune responders and nonresponders, indicating that B cells and their products may play a critical role in the antitumor immune response27. By integrating B-cell biomarkers and further exploring the roles and interactions of various immune cells within the tumor microenvironment, researchers may enhance the overall efficacy of cancer immunotherapy and contribute to developing more effective, personalized treatment strategies.
Since keywords reflect the core content of a study, co-occurrence analysis can identify high-frequency keywords that appear in different studies, thus helping researchers to quickly grasp research hotspots. In this study, the most frequent keywords were ‘immunotherapy’ and ‘neoadjuvant therapy’. The frequency of ‘neoadjuvant immunotherapy’ was 55 times. Furthermore, ‘biomarkers’ were another frequently appearing keyword. Studies have shown that biomarkers can predict the efficacy of ICIs, disease progression, and recurrences28–30. However, research on biomarkers related to neoadjuvant immunotherapy is rare. Since some patients may experience disease progression after neoadjuvant immunotherapy or lose the opportunity for surgery due to immune-related adverse events, screening for patient groups with potential benefits for neoadjuvant immunotherapy is critical31,32. Exploring biomarkers related to the efficacy of neoadjuvant immunotherapy through various methods can be an effective tool for maximizing individual treatment outcomes. In conclusion, co-occurrence analysis of high-frequency keywords in research studies can highlight focus areas, such as neoadjuvant immunotherapy and biomarkers. Expanding our understanding of biomarkers related to the efficacy of neoadjuvant immunotherapy is essential for optimizing treatment outcomes. Further research in this area can significantly enhance the effectiveness of personalized cancer treatments.
To further investigate the differences in neoadjuvant immunotherapy among various cancer types to guide clinical practice better. We analyzed the five cancer types with the highest frequency of keywords. The development of neoadjuvant immunotherapy for NSCLC, breast cancer, bladder cancer, melanoma, and TNBC was compared. The different levels of development of neoadjuvant immunotherapy research in these cancer types are discussed, as well as the reasons for these differences.
Neoadjuvant immunotherapy in NSCLC has shown promising results. For instance, PD-1 blockade has successfully improved patient outcomes, with studies such as the SAKK 16/14 trial investigating the addition of durvalumab to neoadjuvant chemotherapy in stage IIIA (N2) NSCLC patients33. Combined therapies, such as nivolumab and ipilimumab, have also been explored in resectable NSCLC, with positive outcomes34. Furthermore, compartmental analysis of T-cell clonal dynamics has helped identify pathologic responses to neoadjuvant PD-1 blockade, providing insights into treatment efficacy and resistance35. Despite these advancements, further research is needed to optimize treatment strategies and improve patient outcomes.
Neoadjuvant immunotherapy for breast cancer has emerged as a promising approach, particularly in aggressive subtypes like TNBC. Studies have shown that neoadjuvant immunotherapy can improve outcomes compared to adjuvant therapy, which helps eradicate metastatic disease23. Combining ICIs, such as pembrolizumab and durvalumab, with standard anthracycline-taxane-based chemotherapy has shown clinical promise36. Research also reveals correlations between immune biomarkers, such as PD-L1 expression, TILs, and response to neoadjuvant therapy37. These findings highlight the therapeutic potential of neoadjuvant immunotherapy and emphasize the need for further research to optimize treatment strategies.
Developing neoadjuvant immunotherapy in bladder cancer has centered around using ICIs, such as pembrolizumab and atezolizumab. The PURE-01 study demonstrated the preliminary activity of neoadjuvant pembrolizumab in patients with muscle-invasive bladder carcinoma38. At the same time, the NABUCCO trial investigated the preoperative combination of ipilimumab and nivolumab in locoregionally advanced urothelial cancer39. Although the FDA has approved atezolizumab for treating progressive advanced urothelial carcinoma, more research is needed to explore the full potential of neoadjuvant immunotherapy in this cancer type.
Neoadjuvant immunotherapy has shown significant promise in melanoma, particularly in combination therapies such as ipilimumab and nivolumab. The OpACIN-neo and OpACIN trials analyzed survival and biomarker data from neoadjuvant immunotherapy in stage III melanoma patients1. Additionally, immune circulation and tumor microenvironment monitoring has been investigated in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab40. While these studies provide valuable insights into treatment efficacy and patient stratification, further research is necessary to refine neoadjuvant immunotherapy approaches in melanoma.
TNBC, a particularly aggressive breast cancer subtype, has seen significant research into neoadjuvant immunotherapy. Studies such as the GeparNuevo trial have investigated the use of durvalumab in addition to anthracycline-taxane-based neoadjuvant therapy, with promising results36. Another area of interest has been the relationship between RAS/MAPK activation and TILs and the therapeutic cooperation between MEK inhibitors and PD-1/PD-L1 ICIs22. The role of PD-L1 expression and TILs in TNBC prognosis has also been investigated, highlighting the importance of understanding the tumor microenvironment in developing effective immunotherapies41.
Innovative approaches, such as transformable nanoparticle-enabled immunotherapy and anti-folate receptor alpha-directed antibody therapies, have been explored to enhance TNBC treatment42. Furthermore, neoadjuvant interferons are critical for effective PD-1-based immunotherapy in TNBC43. Lastly, the therapeutic cooperation between auranofin, a thioredoxin reductase inhibitor, and anti-PD-L1 antibodies has been examined for TNBC treatment44.
The development of neoadjuvant immunotherapy varies across cancer types, with melanoma and NSCLC demonstrating more advanced progress than bladder, breast, and TNBC. These disparities may include differences in tumor biology, the immune microenvironment, and the prevalence of specific cancer subtypes that respond well to immunotherapies.
Melanoma, in particular, has shown a high response rate to immunotherapies due to its high mutational burden and immunogenicity45. NSCLC also exhibits significant progress in neoadjuvant immunotherapy development, partly because of its high incidence and the presence of actionable molecular targets, such as PD-L146.
In contrast, bladder cancer, breast cancer, and TNBC have experienced a slower pace of development in neoadjuvant immunotherapy. The complex tumor biology and heterogeneity of breast cancer, including varying responses to treatment across subtypes, may partly explain this disparity. TNBC, a highly aggressive subtype of breast cancer, has shown promise in neoadjuvant immunotherapy research, but challenges remain in understanding and overcoming immune evasion mechanisms. Lastly, bladder cancer has a lower incidence than NSCLC and breast cancer, which may contribute to the slower progress in neoadjuvant immunotherapy research.
Neoadjuvant immunotherapy has demonstrated the potential to improve patient outcomes across various cancer types, including NSCLC, breast cancer, bladder cancer, melanoma, and TNBC. While melanoma and NSCLC have made significant progress, bladder cancer, breast cancer, and TNBC still require further investigation to optimize treatment strategies. Understanding the underlying reasons for disparities in neoadjuvant immunotherapy development can help guide future research, with the ultimate goal of providing personalized and effective treatment options for patients.
CiteSpace’s ‘burst detection’ method identifies keywords or cited references with significant changes over time9. Researchers can use keywords and cited references with burst features to explore hotspots of research. In this study, ‘urothelial carcinoma’, ‘ipilimumab’, and ‘radical cystectomy’ were keywords that continued to burst as of 2022. This suggests that neoadjuvant immunotherapy for urological system cancer may be a potential research hotspot in the future39,47–49. In addition, seven cited references continued to burst in 2022. Of these, three studies focused on the application of ICIs in neoadjuvant immunotherapy14,15,23, and two discussed the application of ICIs in adjuvant therapy50,51. One study by Necchi et al. is worth noting. This study showed that pembrolizumab could be a reliable neoadjuvant therapy for MIBC, limited to patients with PD-L1 positivity or high tumor mutation burden. This is consistent with the results of the burst detection of keywords18. In addition, Ayers et al. reported an IFN-γ-related mRNA profile that can predict the efficacy of ICIs52. Reck et al.53 pointed out that in advanced NSCLC patients, pembrolizumab significantly prolonged progression-free survival and overall survival with fewer adverse events compared to platinum-based chemotherapy when PD-L1 was expressed in at least 50% of tumor cells. In light of the burst detection results mentioned above, clinical trials of neoadjuvant immunotherapy and predictive biomarkers for therapeutic efficacy in different types of cancer may be research directions worth paying attention to soon.
There are some limitations to this study. First, it only includes articles written in English and recorded in the WoSCC database. Since WoSCC covers the vast majority of high-quality studies, this does not affect the overall trend of the results. Second, recently published high-quality studies may not have received the attention they deserved due to citation delays and need to be updated in subsequent studies. Nevertheless, this study will significantly assist relevant researchers in understanding the development, hotspots, trends, and frontiers of neoadjuvant immunotherapy and identifying areas that still require further research.
Conclusion
In recent years, there has been increasing attention paid to research related to neoadjuvant immunotherapy. The substantial increase in publications each year indicates the growing importance of this research field. This study identifies the top researchers and institutions worldwide involved in neoadjuvant immunotherapy research. Frontiers in Oncology is the most active journal, and Francesco Montorsi is the most influential author. Immunotherapy as a neoadjuvant treatment for urological cancer and the development of efficacy predictive biomarkers are hot topics. The long-term prognosis of patients receiving neoadjuvant immunotherapy may be a critical area for future research. Therefore, a comprehensive overview of the evolution and frontiers of the field is available to researchers and policymakers who are new to the area.
Ethical approval
No ethical approval and patient consent were required for all analyses were based on literature research.
Sources of funding
This research was partly supported by the National High Level Hospital Clinical Research Funding (2022-PUMCH-C-049).
Author contribution
X.L., Y.X., and X.S.: conceived and designed the study; S.J.: wrote the manuscript and participated in the design of the study; S.J., H.Z., and Y.L.: were responsible for analysis and network construction; L.Z.: and performed the data analysis and data interpretation. All authors contributed to the article and reviewed the submitted version.
Conflicts of interest disclosure
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Research registration Unique Identifying number (UIN)
Not required.
Guarantor
Yiyao Xu, Department of Liver Surgery, Peking Union Medical College (PUMC) Hospital, PUMC & Chinese Academy of Medical Sciences, Dongcheng, Beijing 100730, China. E-mail: xuyiyao@pumch.cn.
Xin Lu, Department of Liver Surgery, Peking Union Medical College (PUMC) Hospital, PUMC & Chinese Academy of Medical Sciences, Dongcheng, Beijing 100730, China. E-mail: luxin@pumch.cn.
Data availability statement
The data in this study is not sensitive in nature and is accessible in the public domain. The data is therefore available and not of a confidential nature. All the data used in the study have been included in the article/Supplementary Material.
Supplementary Material
Acknowledgements
The authors want to thank CiteSpace and VOSviewer for free access by researchers.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.lww.com/international-journal-of-surgery.
Published online 20 May 2023
Contributor Information
Shitao Jiang, Email: jiangshitao0910@gmail.com.
Yaoge Liu, Email: 2965327374@qq.com.
Han Zheng, Email: zheng_study357@126.com.
Lei Zhang, Email: zhanglei_tj@163.com.
Haitao Zhao, Email: zhaoht@pumch.cn.
Xinting Sang, Email: sangxt@pumch.cn.
Yiyao Xu, Email: xuyiyao@pumch.cn.
Xin Lu, Email: luxin@pumch.cn.
References
- 1. Rozeman E, Hoefsmit E, Reijers I, et al. Survival and biomarker analyses from the OpACIN-neo and OpACIN neoadjuvant immunotherapy trials in stage III melanoma. Nat Med 2021;27:256–263. [DOI] [PubMed] [Google Scholar]
- 2. Nanda R, Liu MC, Yau C, et al. Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: an analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial. JAMA Oncol 2020;6:676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science 2020;367:0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hicks D, Wouters P, Waltman L, et al. Bibliometrics: the Leiden Manifesto for research metrics. Nature 2015;520:429–431. [DOI] [PubMed] [Google Scholar]
- 5. Mukherjee D, Lim WM, Kumar S, et al. Guidelines for advancing theory and practice through bibliometric research. J Bus Res 2022;148:101–115. [Google Scholar]
- 6. Yeung AWK. Comparison between Scopus, Web of Science, PubMed and publishers for mislabelled review papers. Curr Sci 2019;116:1909–1914. [Google Scholar]
- 7. Ihaka R, Gentleman R. R: a language for data analysis and graphics. J Comput Graph Stat 1996;5:299–314. [Google Scholar]
- 8. Van Eck N, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010;84:523–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen C. CiteSpace II: detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inform Sci Technol 2006;57:359–377. [Google Scholar]
- 10. Aria M, Cuccurullo C. bibliometrix: an R-tool for comprehensive science mapping analysis. J Inform 2017;11:959–975. [Google Scholar]
- 11. Jiang S-T, Liu Y-G, Zhang L, et al. Immune-related adverse events: a bibliometric analysis. Front Immunol 2022;13:7703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang J, Yang X, Fang J, et al. Evolution of associating liver partition and portal vein ligation for staged hepatectomy from 2012 to 2021: a bibliometric analysis. Review. Int J Surg 2022;103:106648. [DOI] [PubMed] [Google Scholar]
- 13. Garfield E. Journal impact factor: a brief review. CMAJ 1999;161:979–980. [PMC free article] [PubMed] [Google Scholar]
- 14. Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. New Engl J Med 2018;378:1976–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early triple-negative breast cancer. New EnglJ Med 2020;382:810–821. [DOI] [PubMed] [Google Scholar]
- 16. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. New Engl J Med 2012;366:2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. New Engl J Med 2017;377:1919–1929. [DOI] [PubMed] [Google Scholar]
- 18. Necchi A, Anichini A, Raggi D, et al. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): an open-label, single-arm, phase II study. J Clin Oncol 2018;36:3353–3360. [DOI] [PubMed] [Google Scholar]
- 19. Maio M, Blank C, Necchi A, et al. Neoadjuvant immunotherapy is reshaping cancer management across multiple tumour types: the future is now!. Eur J Cancer 2021;152:155–164. [DOI] [PubMed] [Google Scholar]
- 20. Xu A-H, Sun Y-X. Research hotspots and effectiveness of repetitive transcranial magnetic stimulation in stroke rehabilitation. Neural Regen Res 2020;15:2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klug F, Prakash H, Huber PE, et al. Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 2013;24:589–602. [DOI] [PubMed] [Google Scholar]
- 22. Loi S, Dushyanthen S, Beavis PA, et al. RAS/MAPK activation is associated with reduced tumor-infiltrating lymphocytes in triple-negative breast cancer: therapeutic cooperation between MEK and PD-1/PD-L1 immune checkpoint inhibitors. Clin Cancer Res 2016;22:1499–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu J, Blake SJ, Yong MC, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic DiseaseNeoadjuvant immunotherapy is effective against metastases. Cancer Discovery 2016;6:1382–1399. [DOI] [PubMed] [Google Scholar]
- 24. Cloughesy TF, Mochizuki AY, Orpilla JR, et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med 2019;25:477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chalabi M, Fanchi LF, Dijkstra KK, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med 2020;26:566–576. [DOI] [PubMed] [Google Scholar]
- 26. Blank CU, Rozeman EA, Fanchi LF, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med 2018;24:1655–1661. [DOI] [PubMed] [Google Scholar]
- 27. Helmink BA, Reddy SM, Gao J, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 2020;577:549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baltussen JC, Welters MJ, Verdegaal EM, et al. Predictive biomarkers for outcomes of immune checkpoint inhibitors (ICIs) in melanoma: a systematic review. Cancers 2021;13:6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bai R, Lv Z, Xu D, et al. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark Res 2020;8:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Camidge DR, Doebele RC, Kerr KM. Comparing and contrasting predictive biomarkers for immunotherapy and targeted therapy of NSCLC. Nat Rev Clin Oncol 2019;16:341–355. [DOI] [PubMed] [Google Scholar]
- 31. Liu J, O’Donnell JS, Yan J, et al. Timing of neoadjuvant immunotherapy in relation to surgery is crucial for outcome. Oncoimmunology 2019;8:e1581530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haanen J, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv119–iv142. [DOI] [PubMed] [Google Scholar]
- 33. Rothschild SI, Zippelius A, Eboulet EI, et al. SAKK 16/14: durvalumab in addition to neoadjuvant chemotherapy in patients with stage IIIA (N2) non–small-cell lung cancer—a multicenter single-arm phase II trial. J Clin Oncol 2021;39:2872–2880. [DOI] [PubMed] [Google Scholar]
- 34. Reuss JE, Anagnostou V, Cottrell TR, et al. Neoadjuvant nivolumab plus ipilimumab in resectable non-small cell lung cancer. J Immunother Cancer 2020;8:e001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang J, Ji Z, Caushi JX, et al. Compartmental analysis of T-cell clonal dynamics as a function of pathologic response to neoadjuvant PD-1 blockade in resectable non–small cell lung cancerT-cell dynamics during neoadjuvant Anti–PD-1 in NSCLC. Clin Cancer Res 2020;26:1327–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Loibl S, Untch M, Burchardi N, et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann Oncol 2019;30:1279–1288. [DOI] [PubMed] [Google Scholar]
- 37. Wimberly H, Brown JR, Schalper K, et al. Pd-L1 expression correlates with tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy in breast cancerpd-L1 and response to neoadjuvant therapy in breast cancer. Cancer Immunol Res 2015;3:326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Necchi A, Raggi D, Gallina A, et al. Updated results of PURE-01 with preliminary activity of neoadjuvant pembrolizumab in patients with muscle-invasive bladder carcinoma with variant histologies. Eur Urol 2020;77:439–446. [DOI] [PubMed] [Google Scholar]
- 39. van Dijk N, Gil-Jimenez A, Silina K, et al. Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: the NABUCCO trial. Nat Med 2020;26:1839–1844. [DOI] [PubMed] [Google Scholar]
- 40. Tarhini AA, Edington H, Butterfield LH, et al. Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PLoS One 2014;9:e87705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bassez A, Vos H, Van Dyck L, et al. A single-cell map of intratumoral changes during anti-PD1 treatment of patients with breast cancer. Nat Med 2021;27:820–832. [DOI] [PubMed] [Google Scholar]
- 42. Xu C, Yu Y, Sun Y, et al. Transformable nanoparticle‐enabled synergistic elicitation and promotion of immunogenic cell death for triple‐negative breast cancer immunotherapy. Advanced Functional Materials 2019;29:1905213. [Google Scholar]
- 43. Brockwell NK, Owen KL, Zanker D, et al. Neoadjuvant Interferons: critical for effective PD-1–based immunotherapy in tnbccombination immunotherapy for triple-negative breast cancer. Cancer Immunol Res 2017;5:871–884. [DOI] [PubMed] [Google Scholar]
- 44. Raninga PV, Lee AC, Sinha D, et al. Therapeutic cooperation between auranofin, a thioredoxin reductase inhibitor and anti‐PD‐L1 antibody for treatment of triple‐negative breast cancer. Int J Cancer 2020;146:123–136. [DOI] [PubMed] [Google Scholar]
- 45. Chan TA, Yarchoan M, Jaffee E, et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol 2019;30:44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang M, Herbst RS, Boshoff C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat Med 2021;27:1345–1356. [DOI] [PubMed] [Google Scholar]
- 47. Rouanne M, Bajorin DF, Hannan R, et al. Rationale and outcomes for neoadjuvant immunotherapy in urothelial carcinoma of the bladder. Eur Urol Oncol 2020;3:728–738. [DOI] [PubMed] [Google Scholar]
- 48. van Dorp J, Pipinikas C, Suelmann BB, et al. High-or low-dose preoperative ipilimumab plus nivolumab in stage III urothelial cancer: the phase 1B NABUCCO trial. Nat Med 2023;19:588–592. [DOI] [PubMed] [Google Scholar]
- 49. Basile G, Bandini M, Gibb EA, et al. Neoadjuvant pembrolizumab and radical cystectomy in patients with muscle-invasive urothelial bladder cancer: 3-year median follow-up update of PURE-01 trial. Clin Cancer Res 2022;28:5107–5114. [DOI] [PubMed] [Google Scholar]
- 50. Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. New Engl J Med 2017;377:1824–1835. [DOI] [PubMed] [Google Scholar]
- 51. Eggermont AM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. New Engl J Med 2018;378:1789–1801. [DOI] [PubMed] [Google Scholar]
- 52. Ayers M, Lunceford J, Nebozhyn M, et al. IFN-γ–related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest 2017;127:2930–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med 2016;375:1823–1833. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data in this study is not sensitive in nature and is accessible in the public domain. The data is therefore available and not of a confidential nature. All the data used in the study have been included in the article/Supplementary Material.


