Abstract
Background:
Augmented reality (AR)-assisted navigation system are currently good techniques for hepatectomy; however, its application and efficacy for laparoscopic pancreatoduodenectomy have not been reported. This study sought to focus on and evaluate the advantages of laparoscopic pancreatoduodenectomy guided by the AR-assisted navigation system in intraoperative and short-time outcomes.
Methods:
Eighty-two patients who underwent laparoscopic pancreatoduodenectomy from January 2018 to May 2022 were enrolled and divided into the AR and non-AR groups. Clinical baseline features, operation time, intraoperative blood loss, blood transfusion rate, perioperative complications, and mortality were analyzed.
Results:
AR-guided laparoscopic pancreaticoduodenectomy was performed in the AR group (n=41), whereas laparoscopic pancreatoduodenectomy was carried out routinely in the non-AR group (n=41). There was no significant difference in baseline data between the two groups (P>0.05); Although the operation time of the AR group was longer than that of the non-AR group (420.15±94.38 vs. 348.98±76.15, P<0.001), the AR group had a less intraoperative blood loss (219.51±167.03 vs. 312.20±195.51, P=0.023), lower blood transfusion rate (24.4 vs. 65.9%, P<0.001), lower occurrence rates of postoperative pancreatic fistula (12.2 vs. 46.3%, P=0.002) and bile leakage (0 vs. 14.6%, P=0.026), and shorter postoperative hospital stay (11.29±2.78 vs. 20.04±11.22, P<0.001) compared with the non-AR group.
Conclusion:
AR-guided laparoscopic pancreatoduodenectomy has significant advantages in identifying important vascular structures, minimizing intraoperative damage, and reducing postoperative complications, suggesting that it is a safe, feasible method with a bright future in the clinical setting.
Keywords: augmented reality, intraoperative navigation, laparoscopic pancreaticoduodenectomy, three-dimensional visualization
Introduction
Highlights
Laparoscopic pancreatoduodenectomy is an operation of great difficulty.
An augmented reality-assisted navigation system helps surgeons visualize the internal structure of organs, accurately identify the location of lesions, and the route of important vessels.
Augmented reality-guided laparoscopic pancreaticoduodenectomy is a novel technique to improve safety and accuracy, which is not restricted to decreasing the risk of bleeding, but is also evident among reducing the occurrence of postoperative pancreatic fistula and biliary leakage.
Pancreaticoduodenectomy, a complex surgical procedure commonly utilized for treating malignant tumors of the pancreatic head, ampulla, and distal bile duct, as well as for benign tumors, and trauma of the pancreatic head and duodenum1, involves the removal of the head of the pancreas, the duodenum, the gallbladder, and a portion of the bile duct2,3. Due to its complexity, the procedure presents a significant challenge to surgeons and is associated with a long learning curve4,5. In addition, the pancreas is a retroperitoneal organ with a complex anatomical structure and hidden location, as well as the close relationship with surrounding tissues, which poses additional difficulties during surgery. However, with the continuous advancements in medicine, the updating of surgical instruments and the gradual improvement of surgical level, laparoscopic pancreaticoduodenectomy (LPD) has gradually become routine. Compared to open surgery, laparoscopic surgery offers a wider field of vision and clearer sight, allowing for multiangle observation and magnification of anatomical structures6. Nevertheless, laparoscopic surgery presents unique challenges for surgeons, primarily due to the loss of the direct sense of touch, replaced by force feedback through minimally invasive instruments. As a result, surgeons must take extra time to ensure the safety and operability of the surgery due to the difficulty in distinguishing between hard and soft tissues (usually used for fibrotic localization) and feeling the pulsations (for vascular localization). Secondly, it is still difficult to understand the internal tissue structure of abdominal parenchymal organs and the route of blood vessels that are not exposed through laparoscopic images. Surgeons rely more on experience to roughly judge the location and anatomical structure of the lesions, which is challenging to ensure the safety and accuracy of the operation7.
In order to solve this problem, our team developed an augmented reality-assisted navigation system (AR-ANS) based on augmented reality (AR) technology. This system integrates a preoperative three-dimensional (3D) model with a laparoscopic surgery image in real time registration. By rendering the abdominal organs and blood vessels transparent, this system helps surgeons visualize the internal structure of organs, and accurately identify the location of lesions and the route of important vessels8,9. This enhanced visualization capability leads to improved surgical control. While AR-ANS has been preliminarily applied in liver surgery, it has not been extensively used in pancreatic surgery. Therefore, we conducted a retrospective cohort study to investigate the application value of AR navigation in LPD.
Methods
Study design and patient selection
This study was conducted in compliance with the Declaration of Helsinki and was approved by our institution. This retrospective study has been reported in line with the strengthening the reporting of cohort, cross-sectional and case-control studies in surgery (STROCSS) criteria10, Supplemental Digital Content 1,http://links.lww.com/JS9/A718.
Patients who were diagnosed with tumors of the pancreatic head, ampulla, and distal bile duct and underwent LPD at the Department of Hepatobiliary Surgery from January 2018 to May 2022 were retrospectively collected, then were divided into two groups: the AR group (41 cases) and the non-AR group (41 cases), based on whether AR-ANS was applied during the operation (Fig. 1). All surgical procedures were decided after taking definitive diagnosis, patients’ overall health situation, the risk, and the benefits into account, and were performed based on a preoperative 3D model operation simulation.
Figure 1.
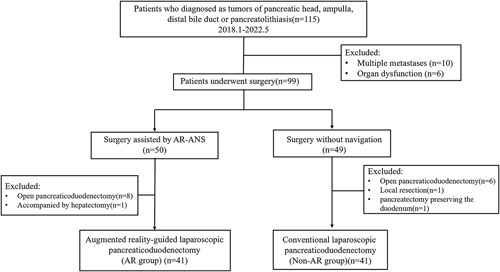
Flowchart of participant selection for the study.
All patients signed the informed consent for surgery, which was in line with the routine medical and nursing practice. The study was registered in the ClinicalTrials.gov database.
Inclusion criteria
Age greater than or equal to 18 years, both sex; Enhanced computed tomography (CT)/MRI results confirmed the presence of tumors in the head of the pancreas, distal bile duct, ampulla, or pancreatolithiasis; Participants underwent LPD; No contraindications such as severe heart, lung, kidney, and brain dysfunction or multiple metastases; The clinical data were complete.
Exclusion criteria
Participants with clinical contraindications such as severe heart, lung, brain, and renal insufficiency or multiple metastases, unable to tolerate surgical treatment; Open pancreatoduodenectomy, other surgical options or conservative treatment are adopted; Clinical data are missing.
Research method
All patients in the two groups completed preoperative examinations and excluded the relevant surgical contraindications. After adequate general anesthesia was received, the non-AR group underwent conventional LPD, and the AR group underwent AR-guide LPD. The resected specimens were biopsied and the diagnosis was confirmed by the experienced pathologist after the operation.
Constructing individual 3D model
All patients in the two groups underwent a 256-slice spiral CT after admission. The CT data, including plain scan, arterial phase, venous phase, and portal venous phase, were collected, saved in DICOM, and imported into the medical image 3D visualization system (MI-3DVS, Software Copyright No.: 2008SR18798)11–13. The liver, gallbladder, biliary tract, spleen, pancreas, pancreatic duct, duodenum, arterial system, and venous system were reconstructed and analyzed in a homogenous and standardized manner (Fig. 2A). The relationship between abdominal blood vessels and pancreatic lesions (Fig. 2B, C, D) was evaluated by an individualized 3D model, based on which the clinical diagnosis and surgical plan can be performed14,15.
Figure 2.
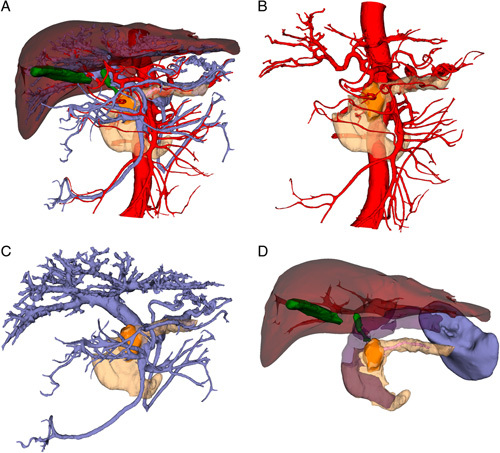
(A) Preoperative individual three-dimensional visualization model. (B) Relationship between pancreatic tumor and venous system. (C) The relationship between pancreatic tumor and arterial system. (D) Relationship between pancreatic tumor and pancreatic duct and surrounding organs.
Augmented reality navigation
The AR-ANS (software copyright number: 2018SR840555)16 was applied to collect and output the intraoperative real time 3D high-definition laparoscopic surgery video in Line-by Line format, Supplemental Digital Content 2, http://links.lww.com/JS9/A719. After being analyzed by the parser, the video, Supplemental Digital Content 2, http://links.lww.com/JS9/A719 was inputted into the laptop through the capture card. Consequently, the 3D visualization model was superimposed onto the 3D laparoscopic surgery image in AR-ANS in real time (Fig. 3). The color and transparency of celiac blood vessels, organs, and lesions in the model were modified in accordance with the needs of the operation, which helped the surgeon identify abdominal organs, ligate or protect key vessels, and separate pathological tissues17.
Figure 3.
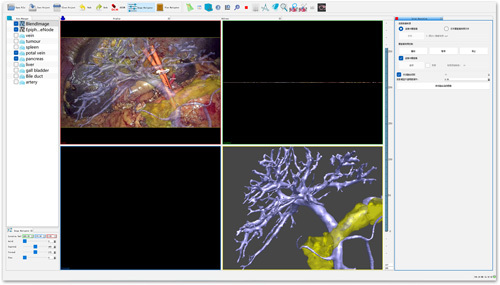
Operation page of augmented reality-assisted navigation system.
Augmented reality-guided laparoscopic pancreaticoduodenectomy
After the establishment of pneumoperitoneum, the abdominal cavity was routinely explored, especially to determine whether it occurred with ascites and tumor metastasis, with the assistance of AR-ANS guidance. AR surgery navigation required debugging, 3D model importing and rendering, surgical video conversion, Supplemental Digital Content 2, http://links.lww.com/JS9/A719, image positioning, and manual registration. Firstly, the 3D model was imported into the system for visualization and rendering, respectively, in which the liver, gallbladder, extrahepatic bile duct, duodenum, artery, vein, pancreas, pancreatic lesions, and spleen were given different colors. After that, the 3D model was displayed, hidden, enlarged, rotated, and transparent to ensure that the image was accurately superimposed and fused. The operation process in AR group17 involved the following steps: Guided by the edge of liver and gallbladder, the location of pancreas, abdominal aorta and portal vein (PV) was identified; Release the gastrocolic ligament and expose the pancreas; The position and course of the proper hepatic artery and gastroduodenal artery (GDA) were navigated with the common hepatic artery as the marker point, and the GDA was ligated freely (Fig. 4A, B); The pancreatic and hepatic portal arteries were used as markers to define the position of the gastrocolic trunk, the major PV, and the superior mesenteric artery (SMA), after which the gastrocolic trunk was exposed and ligated; navigation by AR-ANS allowed for the identification of the superior mesenteric vein (SMV) at the lower edge of the pancreatic neck, and the tunnel juxtaposition zone between the posterior pancreas and SMV was dissociated (Fig. 4C, D); The blood vessels and tissues surrounding the pancreatic head were isolated and ligated. The pancreas was hyalinized in the AR-ANS to highlight the location of the lesion and pancreatic duct inside the pancreatic parenchyma; After the pancreas was cut off from the pancreatic neck, the broken end of the pancreatic duct was carefully protected. A silicone tube with a suitable diameter was placed into the pancreatic duct for drainage, and the supporting tube of the pancreatic duct was stitched with absorbable suture; The uncinate process of the pancreas was isolated from the bottom up along the SMV (Fig. 4E, F). The lymph nodes and nerve tissues on the right side of SMA were dissected; After the removal of the gallbladder, stomach, and the common hepatic or common bile duct, the pancreaticojejunostomy, choledochojejunostomy, gastroenterostomy, and enterostomy were performed, respectively.
Figure 4.
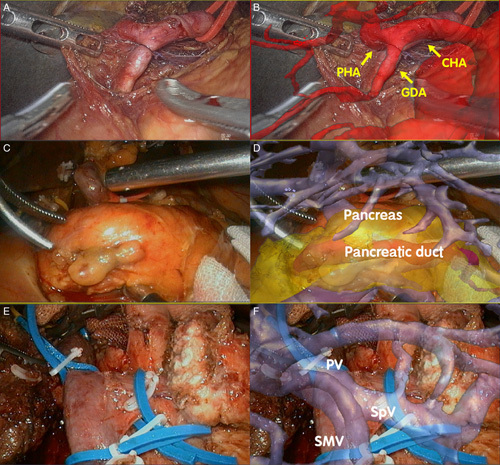
4A+4B: Real Time Registration and Navigation of Gastroduodenal Artery (GDA); Figure 4C+4D: Real Time Registration and Navigation of Pancreas, Pancreatic Duct and Pancreatic Dorsal Tunnel. 4E+4F: Real Time Registration and Navigation of Portal Vein (PV), Superior Mesenteric Vein (SMV) and Splenic Vein.
Data acquisition
The preoperative general data (age, sex, history of diabetes, preoperative biliary drainage, lesion location, lesion size, diameter of pancreatic duct, and bile duct), preoperative and postoperative examination indexes (hemoglobin , white blood cell, total bilirubin, direct bilirubin, serum amylase, and serum lipase), intraoperative data (operation duration, intraoperative blood loss, intraoperative blood transfusion, conversion to Laparotomy, gland texture, and pathological diagnosis), and the postoperative data (perioperative mortality, drainage tube indwelling time, postoperative hospital stay, and postoperative complications18–20) were collected. According to the Clavien–Dindo classification system21,22, all complications were graded.
Statistical analysis
SPSS 26.0 statistical software was used for data analysis. Continuous variables were expressed as mean ± SD (x ± SD), Categorical variables were described as frequencies (n) and proportion (%). The student’s t-test or Mann–Whitney U test was used for comparison of continuous variables as applicable, while the χ 2 test or Fisher’s exact test was used for comparison of categorical variables when appropriate. Univariate and multivariate logistic regression with odds ratios and 95% CI were conducted to identify independent risk factors associated with postoperative pancreatic fistula and bile leakage. Variables with a P value less than 0.1on univariate analysis were entered into multivariate analysis. P < 0.05 was considered as a statistical significant.
Results
Preoperative results
A total of 82 patients were enrolled in this study, including 41 patients in the AR group and 41 patients in the non-AR group. The difference of lesion location between the two groups was statistically significant (P<0.05). There was no significant difference in other baseline features between the two groups (P>0.05). The general information of the two groups is shown in Table 1.
Table 1.
Preoperative general data of AR group and non-AR group.
| Variables | AR group (41 cases) | Non-AR group (41 cases) | T (χ 2) value | P |
|---|---|---|---|---|
| Age, years, mean±SD | 61.05±12.12 | 61.71±10.41 | −0.264 | 0.792 |
| Sex, n (%) | 1.892 | 0.169 | ||
| Male | 23 (56.1) | 29 (70.7) | ||
| Female | 18 (43.9) | 12 (29.3) | ||
| Preoperative biliary drainage n (%) | 13 (31.7) | 6 (14.6) | 3.357 | 0.067 |
| Diabetes, n (%) | 10 (24.4) | 9 (22.0) | 0.069 | 0.794 |
| Hemoglobin, g/l, mean±SD | 118.22±15.46 | 117.37±17.93 | 0.121 | 0.231 |
| White blood cell, G/l, mean±SD | 6.55±3.66 | 7.51±2.13 | −1.452 | 0.150 |
| Total bilirubin, umol/l, mean±SD | 60.68±72.81 | 114.99±148.90 | 1.164 | 0.244 |
| Direct bilirubin, umol/l, mean±SD | 45.69±96.79 | 96.79±132.86 | 1.266 | 0.205 |
| Serum amylase, IU/l, mean±SD | 128.97±156.64 | 104.05±66.35 | 0.833 | 0.405 |
| Serum lipase, IU/l, mean±SD | 212.76±356.04 | 171.19±189.03 | 0.563 | 0.576 |
| Lesion location, n (%) | 10.254 | 0.017* | ||
| Distal bile duct | 5 (12.2) | 15 (36.6) | ||
| Head of pancreas | 23 (56.1) | 13 (31.7) | ||
| Ampulla | 5 (12.2) | 9 (22.0) | ||
| Duodenum | 8 (19.5) | 4 (9.8) | ||
| Size of lesion, mm, mean±SD | 26.50±14.108 | 24.57±19.496 | 0.505 | 0.615 |
| Diameter of pancreatic duct, mm, mean±SD | 4.77±3.44 | 4.34±3.43 | −0.446 | 0.656 |
| Diameter of bile duct, mm, mean±SD | 10.74±6.80 | 10.05±7.24 | −0.268 | 0.713 |
Statistically significant.
AR, Augmented reality.
Intraoperative results
The operation duration of the AR group was longer than that of the non-AR group (420.15±94.38 min vs. 348.98±76.15 min), but the intraoperative blood loss (219.51±167.03 ml vs. 312.20±195.51 ml) and intraoperative blood transfusion rate (24.4 vs. 65.9%) of the AR group were significantly less than those of the Non-AR group. Eight patients in the AR group and four patients in the non-AR group were converted to laparotomy, with no significant difference between the two groups (P>0.05). No vascular reconstruction was performed in both groups. In the AR group, postoperative pathology showed 28 patients of malignant tumors, six patients of benign tumors, six patients of inflammatory changes and one patients of pancreatic duct stone. In the non-AR group, there were 37 patients of malignant tumors, two patients of benign tumors and two patients of inflammatory changes, with no significant difference between the two groups. The intraoperative data of the two groups are shown in Table 2.
Table 2.
Surgical data of AR group and non-AR group.
| Variables | AR group (41 cases) | Non-AR group (41 cases) | T (χ 2) value | P |
|---|---|---|---|---|
| Operation duration, min, mean±SD | 420.15±94.38 | 348.98±76.15 | 3.758 | 0.000* |
| Intraoperative blood loss, ml, mean±SD | 219.51±167.03 | 312.20±195.51 | −2.320 | 0.023* |
| Intraoperative blood transfusion rate, n (%) | 10 (24.4) | 27 (65.9) | 14.233 | 0.000* |
| Conversion to laparotomy, n (%) | 1.562 | 0.211 | ||
| Yes | 8 (19.5) | 4 (9.8) | ||
| No | 33 (80.5) | 37 (90.2) | ||
| Gland texture | 0.467 | 0.494 | ||
| Soft | 24 (58.5) | 27 (65.9) | ||
| Firm | 17 (41.5) | 14 (43.1) | ||
| Pathological type, n (%) | 6.246 | 0.1 | ||
| Malignant tumor | 28 (68.3) | 37 (90.2) | ||
| Innocent tumor | 6 (14.6) | 2 (4.9) | ||
| Inflammatory change | 6 (14.6) | 2 (4.9) | ||
| Pancreatic duct stone | 1 (2.4) | 0 (0) |
Statistically significant.
AR, Augmented reality.
Postoperative results
Postoperative data of the two groups are presented in Table 3. The parameters such as hemoglobin, white blood cell, total bilirubin, direct bilirubin, blood amylase, and blood lipase between the two groups were not statistically significant(P>0.05).
Table 3.
Postoperative data of AR group and non-AR group.
| Variables | AR group (41 cases) | Non-AR group (41 cases) | t (χ 2) | P |
|---|---|---|---|---|
| Hemoglobin, g/l, mean±SD | 110.23±14.91 | 107.73±16.92 | 0.703 | 0.484 |
| White blood cell, G/l, mean±SD | 14.11±5.60 | 13.60±4.95 | 0.321 | 0.666 |
| Total bilirubin, umol/l, mean±SD | 58.01±69.12 | 105.32±118.88 | 1.908 | 0.056 |
| Direct bilirubin, umol/l, mean±SD | 44.41±53.92 | 85.17±106.86 | 1.700 | 0.089 |
| Serum amylase, IU/l, mean±SD | 261.37±232.62 | 253.49±183.82 | 0.139 | 0.890 |
| Serum lipase, IU/l, mean±SD | 252.75±270.95 | 288.71±281.92 | −0.481 | 0.632 |
| Complications, n (%) | ||||
| Pancreatic fistula | 11.7 | 0.002* | ||
| Without | 36 (87.8) | 22 (53.7) | ||
| Grade A (biochemical fistula) | 4 (9.8) | 12 (29.3) | ||
| Grade B | 1 (2.4) | 7 (17.1) | ||
| Grade C | 0 (0) | 0 (0) | ||
| Gastric emptying disorder | 1 (2.4) | 5 (12.2) | 2.877 | 0.090 |
| Bile leakage | 0 (0) | 6 (14.6) | 0.026* | |
| Abdominal bleeding | 4 (9.8) | 8 (19.5) | 1.562 | 0.211 |
| Abdominal cavity infection | 3 (7.3) | 7 (17.1) | 1.822 | 0.177 |
| Ascites | 2 (4.9) | 8 (19.5) | 2.847 | 0.092 |
| Pleural effusion | 9 (22) | 11 (26.8) | 0.265 | 0.607 |
| Perioperative mortality, n (%) | 0 (0) | 1 (2.4) | 1.012 | 0.314 |
| Postoperative hospital stay, days, mean±SD | 11.29±2.78 | 20.04±11.22 | 5.902 | 0.000* |
| Drainage tube indwelling time, days, mean±SD | 24.09±27.15 | 54.62±117.53 | −1.479 | 0.143 |
Statistically significant.
AR, Augmented reality.
The pancreatic fistula rate (12.2 vs. 46.3%, P=0.002) in the AR group was substantially less than that in the non-AR group, among which clinical pancreatic fistula (grades B or C) occurred in one patient (2.4%) in the AR group and seven patients (17.1%) in the non-AR group, with no significant difference. There was no bile leakage in the AR group but six patients in the non-AR group after the operation (0 vs. 14.6%, P=0.026). Although operation duration, intraoperative blood transfusion, and AR-ANS were significantly associated with pancreatic fistula and AR-ANS assistance was significantly associated with bile leakage in univariable analysis, there was no significant independent factor according to the multivariate analysis (Supplemental Table 1, Supplemental Digital Content 3, http://links.lww.com/JS9/A720, 2, Supplemental Digital Content 4, http://links.lww.com/JS9/A721). The incidence of other postoperative complications and perioperative mortality were not significantly different between the two groups (P>0.05). All patients with postoperative complications were cured after treatment, except for one patient in the non-AR groups who died during the perioperative period because biliary-intestinal anastomotic leakage occurred after the operation, developed into a large amount of pleural effusion and abdominal cavity effusion, and finally, septic shock led to death. The postoperative hospital days in the AR group were 11.29 ±2.798 days, and those in the non-AR group were 20.04 ±11.22 days, with statistical significance (P<0.001). There was no significant difference in indwelling days of an abdominal drainage tube between the two groups (P>0.05).
Discussion
Based on the AR visualization method, the author team independently developed AR-ANS, which was initially applied to 3D laparoscopic hepatectomy for hepatocellular carcinoma23 and hepatolithiasis16. However, there are few reports on the application of AR navigation technology in pancreaticoduodenectomy at home and abroad. Okamoto24 and Onda25 found that the AR-based NS provided precise anatomical information, which allowed the surgeons to rapidly identify and perform early ligation of IPDA in pancreaticoduodenectomy; Tang26 and Marzano27 have found that AR navigation technology based on preoperative CT image data can not only assist surgeons to resect and reconstruct SMV in pancreaticoduodenectomy but also improve the negative margin rate of pancreatic cancer. Onda etc.28 reported that the novel short rigid scope, stereo-scope, and AR navigation in open pancreaticoduodenectomy can improve the efficiency, safety, and accuracy of the operations. However, the above researches are limited to open pancreaticoduodenectomy, and mainly consists of case reports or observation studies, without a comparison group. We built the first self-made AR-ANS for LPD and carried out a retrospective cohort analysis as a result. The preoperative surgical plan created using a 3D model was implemented in the AR group, resulting in the realization of the switch from indirect to direct surgical navigation. Under the background of minimally invasive surgery, AR-ANS allows the important structures such as tumors and blood vessels to be located before reached, without roughly identifying their positions by experience, which is conducive to depicting the anatomical plane or tumor margins and preventing damage to hidden structures29.
Pancreaticoduodenectomy, also known as the ‘Mount Qomolangma’ of surgical procedure, requires separation, and denudation of several abdominal blood vessels. The most significant advantage of AR-guided LPD is its ability to navigate the shape, direction, and adjacent position of GDA, PV, SMA, SMV, and tumor in real time by fusing and registering laparoscopic images with 3D visualization models, especially be of the great value in evaluating the course and branches of peripheral blood vessels with SMA and PV-SMV as the longitudinal axis: With the aid of AR-ANS, the GDA can be accurately located and severed using the common hepatic artery, pancreas, and PV as landmarks, and then the retropancreatic tunnel can be established. In some cases, the branches of SMA can replace the right hepatic artery or the common hepatic artery, and they may pass through the uncinate process mesangium or even the tumor(Fig. 5); therefore, the operator should be fully aware of the branches of SMA and the variant hepatic artery’ s course assisted with AR-ANS to avoid incorrect ligation; The gastrocolic trunk and its branches have fragile blood vessel walls, so knowing the variation of the gastrocolic trunk can help prevent tearing and bleeding of the vein wall when separating the fused fascia in front of the pancreatic head30; It is common in LPD that SMV is invaded, requiring resection, and reconstruction. The scope of the invaded area can be indicated via AR-ANS, which is beneficial for prediction, resection, and reconstruction31.
Figure 5.
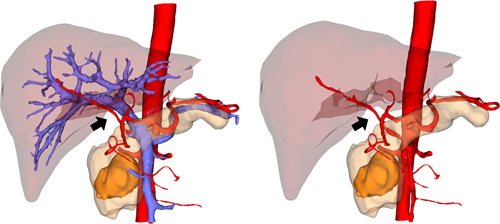
Vascular abnormalities---the branches of SMA replace the right hepatic artery.
Although the operation duration for the AR group was significantly longer than that of the non-AR group, the intraoperative blood loss and blood transfusion rate of the AR group were much lower. This can be attributed to the time spent on preoperative preparation, registration, repeated fusion, and manual regulation for AR-guided LPD, which undoubtedly improves the safety and certainty of the operation. The considerably lower blood loss and transfusion rate in the AR group than in the non-AR group suggests better identification and protection of targeted blood vessels in the AR group surgery.
The Incidence of pancreatic fistula in the AR group is obviously lower than that in the non-AR group (P<0.001). There are many factors that may influence the occurrence of pancreatic fistula, including age, pathology, pancreatic duct size, pancreatic texture, surgical method, operation duration, pancreaticojejunostomy method, etc.32,33. In this study, we mainly consider three reasons: First, there is an association between the occurrence of postoperative pancreatic fistula and the amount of intraoperative blood loss. Relevant research shows that intraoperative blood loss >400 ml is an independent predictor of postoperative pancreatic fistula34. In this study, the lower intraoperative blood loss of the AR group may be one of the factors contributing to the difference between the two groups. Additionally, the operator can assess the position, quantity, and diameter of the pancreatic duct during operation, which is advantageous for the protection of the pancreatic duct35. Third, the pancreatic duct stump can be located using AR-ANS during the surgery, allowing a drainage tube to be inserted into the pancreatic duct and preventing the need to directly anastomose the pancreatic duct stump with the jejunum because the pancreatic duct cannot be found. Relevant studies have confirmed that placing a drainage tube in the pancreatic duct can reduce the incidence of pancreatic fistula36,37.
Additionally, we found that the incidence of bile leakage was significantly lower in the AR group than it was in the non-AR group. This is partly attributable to the fact that AR-guided LPD better protects blood vessels, ensuring a satisfied blood supply to bile ducts, facilitating the healing of biliary-intestinal anastomosis and reducing the risk of bile leakage. Secondly, pancreatic fistula and bile leakage are closely related and mutually causal. The drainage fluid of pancreatic fistula contains pancreatin, which is highly corrosive. Bile leakage will occur if pancreatin proceeds to erode the anastomosis. The results revealed that the average postoperative hospital stay in the AR group was significantly shorter than that in the non-AR group, which could be attributed to less intraoperative blood loss and postoperative complications.
Pancreas locates on the deep surface of the peritoneum, where its deformation and displacement during surgery are smaller than those of the liver, so the registration error is also smaller, making it conducive to the development of AR-guided LPD14,38. However, the pancreas is a flexible tissue. The pulling and squeezing of the pancreas during the operation and the breathing movement of the patient will still lead to the deformation of the pancreas39, causing the change of the registration point. Müller et al.40 performed the minimally invasive endopancreatic surgery by targeting the pancreatic lesions and tracking the pancreatic duct with a passive optical tracking system. Although, the above methods still fail to achieve the ideal real time fusion. Currently, our team uses the method of manual interaction to perform surgical registration, displaying laparoscopic images and preoperative 3D models on the same display screen. Surgeons can manually modify the position, size ratio and direction of the models so that the 3D models and organs, blood vessels in laparoscopy images can be superimposed correctly, effectively reducing the error of automatic registration. However, on the one hand, it takes much workforce to carry out manual-assisted registration navigation. On the other hand, the preparation, registration and fusion process, intraoperative verification, and strategic planning of AR navigation prolong the operation time to some extent. Therefore, our team is currently developing an AR automated navigation system with artificial intelligence that will improve the accuracy of image integration, shorten the time required for manual correction to improve registration efficiency and promote the continuity of intraoperative navigation, providing better auxiliary technology for LPD. Additionally, recent studies have indicated that indocyanine green fluorescence imaging technology shows great advantages in pancreatic tumor localization, lymph node imaging, and pancreatic leakage identification. In the future, we can apply AR navigation technology combined with indocyanine green molecular fluorescence technology to LPD, and continue to break through the difficulties in the field of pancreatic surgery.
Although this study provides a valuable intraoperative navigation technique for LPD and evaluates its safety and effectiveness, it still has limitations. In our study, there is a difference in the lesion position of baseline characteristics between two groups. Intuitively, we consider that the location of the lesion and its distance from the surgical margin are inherently associated with the long-term outcomes of the LPD. As a result, our study was designed to focus on the intraoperative efficacy and short-term outcome of the LPD, hoping to reduce the error caused by the lesion position to some extent. However, it is inevitable that the lesion position may exert impact on the difficulty of the surgery, which is associate with the results of the study. The other major drawback of this study is its retrospective nature, which has a strong inherent risk of selection bias. In addition, the small sample size and short follow-up time of the research group have affected the research results to some extent, and the long-term effect of AR-guided LPD remains to be studied. In the next step, we will expand the sample size, increase the follow-up time, and carry out multicenter clinical trials to further improve the evidence level of evidence-based medicine.
Conclusions
AR-guided laparoscopic pancreatoduodenectomy has significant advantages in identifying important vascular structures, minimizing intraoperative damage and reducing postoperative complications, suggested that it is a safe, feasible method, which has a bright future in the clinical setting.
Ethical approval
Ethical approval for this study (No. 2018-GDYK-003) was provided by the Ethical Committee of Zhujiang Hospital of Southern Medical University, Guangzhou, China on 2 January 2019.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Sources of funding
This work was supported by grant funding from the National Natural Science Foundation of China Mathematics Tianyuan Foundation (12026602); the National Key Research and Development Program (2016YFC0106500); the National Natural Science Foundation of China (81627805); the National Natural Science Foundation of China-Guangdong Union Foundation (U1401254); the National High-tech R&D Program of China (863 Program) (2006AA02Z346,2012AA021105); the Natural Science Foundation of Guangdong Province, China(6200171, 2022A1515010176); Science and Technology Program of Guangdong Province, China (2012A080203013); and Science and Technology Plan Project of Guangzhou (201604020144).
Author contribution
X.W. and D.W.: contributed equally to this work; C.F., X.W., D.W., N.X., J.Y.: conception and design; X.W., D.W., N.X., M.P., F.J., J.Y., C.F.: acquisition of data; X.W., D.W.: analysis and interpretation of data; X.W., D.W.: drafting of manuscript; C.F., J.Y., X.W., D.W., N.X.: critical revision of the manuscript for important intellectual content; C.F., J.Y., N.X., M.P., F.J.: administrative, technical, or material support; C.F., J.Y.: final approval of the version to be submitted. All the authors reviewed the manuscript for important intellectual content and approved the final manuscript.
Conflicts of interest disclosure
The authors declare that they have no conflicts of interest.
Research registration unique identifying number (UIN)
Name of the registry: ClinicalTrials.gov (https://clinicaltrials.gov/).
Unique Identifying number or registration ID:NCT03749005.
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://clinicaltrials.gov/ct2/show/NCT03749005?term=NCT03749005&draw=2&rank=1.
Guarantor
Chihua Fang.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Supplementary Material
Acknowledgements
None.
Footnotes
X.W. and D.W. contributed equally to this work as co-first authors.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.lww.com/international-journal-of-surgery.
Published online 19 June 2023
Contributor Information
Xiwen Wu, Email: 710991566@qq.com.
Dehui Wang, Email: 1769730919@qq.com.
Nan Xiang, Email: zjyyxn@126.com.
Mingxin Pan, Email: pmxwxy@sohu.com.
Fucang Jia, Email: fc.jia@siat.ac.cn.
Jian Yang, Email: yangjian486@126.com.
Chihua Fang, Email: fangchihua@smu.edu.cn.
References
- 1. Karim SAM, Abdulla KS, Abdulkarim QH, et al. The outcomes and complications of pancreaticoduodenectomy (Whipple procedure): cross sectional study. Int J Surg 2018;52:383–387. [DOI] [PubMed] [Google Scholar]
- 2. Liu S, Sheng JY, Zhang XW. Application status and progress of pancreaticoduodenetomy. Int J Surg 2022;49:365–371. [Google Scholar]
- 3. Wang M, Peng B, Liu J, et al. Practice patterns and perioperative outcomes of laparoscopic pancreaticoduodenectomy in china: a retrospective multicenter analysis of 1029 patients. Ann Surg 2021;273:145–153. [DOI] [PubMed] [Google Scholar]
- 4. Nagakawa Y, Nakamura Y, Honda G, et al. Learning curve and surgical factors influencing the surgical outcomes during the initial experience with laparoscopic pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci 2018;25:498–507. [DOI] [PubMed] [Google Scholar]
- 5. Zhang T, Zhao ZM, Gao YX, et al. The learning curve for a surgeon in robot-assisted laparoscopic pancreaticoduodenectomy: a retrospective study in a high volume pancreatic center. Surg Endosc 2019;33:2927–2933. [DOI] [PubMed] [Google Scholar]
- 6. Croome KP, Farnell MB, Que FG, et al. Total laparoscopic pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: oncologic advantages over open approaches? Ann Surg 2014;260:633–638; 638-640. [DOI] [PubMed] [Google Scholar]
- 7. Nicolau S, Soler L, Mutter D, et al. Augmented reality in laparoscopic surgical oncology. Surg Oncol 2011;20:189–201. [DOI] [PubMed] [Google Scholar]
- 8. Giannone F, Felli E, Cherkaoui Z, et al. Augmented reality and image-guided robotic liver surgery. Cancers (Basel) 2021;13:6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang WQ, Zhu W, Yang J, et al. Augmented reality navigation for stereoscopic laparoscopic anatomical hepatectomy of primary liver cancer: preliminary experience. Front Oncol 2021;11:663236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mathew G, Agha R, Albrecht J, et al. STROCSS 2021: strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg 2021;96:106165. [DOI] [PubMed] [Google Scholar]
- 11. Fang CH, An JY, Bruno A, et al. Consensus recommendations of three-dimensional visualization for diagnosis and management of liver diseases. Hepatol Int 2020;14:437–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fang CH, Zhang P, Qi XL. Digital and intelligent liver surgery in the new era: Prospects and dilemmas. EBioMedicine 2019;41:693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fang CH, Liu J, Fan YF, et al. Outcomes of hepatectomy for hepatolithiasis based on 3-dimensional reconstruction technique. J Am Coll Surg 2013;217:280–288. [DOI] [PubMed] [Google Scholar]
- 14. Pancreatic Surgery Group, Surgery Branch of Chinese Medical Association Digital Medical Branch of Chinese Medical Association Digital Intelligent Surgery Professional Committee of Chinese Research Hospital Association Pancreatic Diseases Professional Committee of Chinese Research Hospital Association. Chinese expert consensus on digital intelligent precise diagnosis and treatment of pancreatic surgical diseases (2022 edition). Chin J Surg 2022;60:881–887. [DOI] [PubMed] [Google Scholar]
- 15. Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc 1994;8:408–410. [DOI] [PubMed] [Google Scholar]
- 16. Wu XW, Zeng N, Hu HY, et al. Preliminary exploration on the efficacy of augmented reality-guided hepatectomy for hepatolithiasis. J Am Coll Surg 2022;235:677–688. [DOI] [PubMed] [Google Scholar]
- 17. Xiang N, Yang J, Zhang P, et al. Augmented reality navigated 3D laparoscopic pancreaticoduodenectomy. Chin J Surg 2020;58:E12. [Google Scholar]
- 18. Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017;161:584–591. [DOI] [PubMed] [Google Scholar]
- 19. El NA, El SM, Hamed H, et al. Biliary leakage following pancreaticoduodenectomy: prevalence, risk factors and management. Hepatobiliary Pancreat Dis Int 2019;18:67–72. [DOI] [PubMed] [Google Scholar]
- 20. Koch M, Garden OJ, Padbury R, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 2011;149:680–688. [DOI] [PubMed] [Google Scholar]
- 21. Clavien PA, Barkun J, de Oliveira M-L, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187–196. [DOI] [PubMed] [Google Scholar]
- 22. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang P, Luo HL, Zhu W, et al. Real-time navigation for laparoscopic hepatectomy using image fusion of preoperative 3D surgical plan and intraoperative indocyanine green fluorescence imaging. Surg Endosc 2020;34:3449–3459. [DOI] [PubMed] [Google Scholar]
- 24. Okamoto T, Onda S, Yasuda J, et al. Navigation Surgery Using an Augmented Reality for Pancreatectomy. Dig Surg 2015;32:117–123. [DOI] [PubMed] [Google Scholar]
- 25. Onda S, Okamoto T, Kanehira M, et al. Identification of inferior pancreaticoduodenal artery during pancreaticoduodenectomy using augmented reality-based navigation system. J Hepatobiliary Pancreat Sci 2014;21:281–287. [DOI] [PubMed] [Google Scholar]
- 26. Tang R, Yang W, Hou YC, et al. Augmented reality-assisted pancreaticoduodenectomy with superior mesenteric vein resection and reconstruction. Gastroenterol Res Pract 2021;2021:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marzano E, Piardi T, Soler L, et al. Augmented reality-guided artery-first pancreatico-duodenectomy. J Gastrointest Surg 2013;17:1980–1983. [DOI] [PubMed] [Google Scholar]
- 28. Onda S, Okamoto T, Kanehira M, et al. Short rigid scope and stereo-scope designed specifically for open abdominal navigation surgery: clinical application for hepatobiliary and pancreatic surgery. J Hepatobiliary Pancreat Sci 2013;20:448–453. [DOI] [PubMed] [Google Scholar]
- 29. Sugimoto M, Yasuda H, Koda K, et al. Image overlay navigation by markerless surface registration in gastrointestinal, hepatobiliary and pancreatic surgery. J Hepatobiliary Pancreat Sci 2010;17:629–636. [DOI] [PubMed] [Google Scholar]
- 30. Wang W, Hua J. Common vascular variation and management strategies in pancreaticoduodenectomy. J Surg Concepts Pract 2022;27:34–38. [Google Scholar]
- 31. Tang R, Zhang XJ, Ning GC, et al. Application value of augmented reality technology in pancreatoduodenectomy. Chin J Dig Surg 2019;18:986–991. [Google Scholar]
- 32. Roberts KJ, Hodson J, Mehrzad H, et al. A preoperative predictive score of pancreatic fistula following pancreatoduodenectomy. HPB 2014;16:620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meng LW, Cai YQ, Li YB, et al. Analysis of risk factors of clinically relevant pancreatic fistula after laparoscopic pancreaticoduodenectomy. Chin J Laparoscopic Surg (Electronic Edition) 2017;10:355–360. [Google Scholar]
- 34. Trudeau MT, Casciani F, Maggino L, et al. The influence of intraoperative blood loss on fistula development following pancreatoduodenectomy. Ann Surg 2022;276:e527–e535. [DOI] [PubMed] [Google Scholar]
- 35. Akamatsu N, Sugawara Y, Komagome M, et al. Risk factors for postoperative pancreatic fistula after pancreaticoduodenectomy: the significance of the ratio of the main pancreatic duct to the pancreas body as a predictor of leakage. J Hepatobiliary Pancreat Sci 2010;17:322–328. [DOI] [PubMed] [Google Scholar]
- 36. Meng GX, Xing QZ, Yuan Q, et al. Internal compared with external drainage of pancreatic duct during pancreaticoduodenectomy: a retrospective study. Chin J Cancer Res 2014;26:277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oda T, Hashimoto S, Shimomura O, et al. Inter-anastomosis drainage tube between the pancreas and jejunum: a novel technique for preventing pancreatic fistula after pancreaticoduodenectomy. J Am Coll Surg 2015;221:e55–e60. [DOI] [PubMed] [Google Scholar]
- 38. Abe Y, Itano O, Kitago M, et al. Computer assisted surgery, preoperative planning and navigation for pancreatic cancer. J Hepatobiliary Pancreat Sci 2014;21:251–255. [DOI] [PubMed] [Google Scholar]
- 39. Bari H, Wadhwani S, Dasari BVM. Role of artificial intelligence in hepatobiliary and pancreatic surgery. World J Gastrointest Surg 2021;13:7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Muller PC, Haslebacher C, Steinemann DC, et al. Image-guided minimally invasive endopancreatic surgery using a computer-assisted navigation system. Surg Endosc 2021;35:1610–1617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


