To the Editor:
The Editorial “Expression of Concern”1 further to the “Commentary”2 by Rothrock et al regarding our systematic review3 of clinical trials of ivermectin in COVID-19, raises issues to which we responded4 rapidly, following the allegations of unreliability made against one large clinical trial.5 The Editorial “Erratum” now posted with our original article3 contains hyperlinks not corresponding with the citations in the PDF version1; these point in error to irrelevant citations.
We have already responded to the criticisms made by Rothrock et al2 in a separate letter.6 Concerning the disputed study,5 the preprint server Research Square posted a notice that concerns expressed by undisclosed complainants were “under formal investigation.” Enquiry of the Egyptian Ministry of Education has been acknowledged but without substantive report; at this time, we have no further information.
The imbalance of covariates in the control group of Niaee7 would not normally be a ground for exclusion of a trial from a systematic review. If such imbalance was detected, then it would justify placing the trial at unclear or high risk of bias, but not for exclusion. As with Elgazzar,5 the Niaee7 study was included because the trial met the inclusion criteria of our review protocol.8 The substantive criticism is that the control group had fewer diagnoses confirmed by the polymerase chain reaction (PCR) test (inclusions being laboratory-confirmed by either PCR or computed tomography (CT) scan). However, all participants had severity attributed from CT, and severity is if anything biased toward slightly fewer severe cases in the control group, corresponding to a bias conservatively against ivermectin. Suspicion that lack of PCR confirmation might imply inclusion of non–COVID-19 cases in controls would likely produce a similarly conservative bias. The slightly lower SpO2 in the controls appears typical of what randomization in a small group might be expected to deliver, and the body mass index (BMI) distribution appears remarkably uniform across study arms, contrary to the complaint.2 Vital sign data do appear anomalous, but these are not critical to the mortality assessment, only to the bias assessment.
Subsequent to our article,3 further clinical trials of ivermectin in COVID-19 have (as expected) also been published. We offer 3 illustrations of mortality results derived with the addition of several new clinical trials, by Vallejos et al9 and Abd-Elsalam et al,10 the TOGETHER clinical trial11 and the recently published I-TECH study.12 The mortality data for the latter are, unusually, not reported in the main article but are available in the Supplementary Materials.13 The TOGETHER trial11 has been formally reported even more recently, and many questions have been raised by multiple parties. Although the Data Sharing Statement promises a complete deidentified patient data set “immediately on publication,” the individual patient data as demanded by Rothrock et al2 (among others) are not yet available. Here, we take data at face value from the article, but using all-cause mortality results from Table S6 of the Supplementary Materials (the mortality reported for our meta-analysis) as published on March 30, 2022 and advised by one Principal Investigator on April 3, 2022. From the Niaee7 study, we now use mortality stratified by severity, derived from raw data provided to Karale et al,14 but not available to us at the time of our original article.3
Figure 1 shows the results for the mortality outcome, subgrouped by disease severity, including all qualifying randomized trials. Figure 2 shows the consequence of deleting the disputed Elgazzar trial.5 We have no adequate basis for excluding Niaee7 but exhibit the results of doing so in Figure 3, which excludes both Elgazzar5 and Niaee.7
FIGURE 1.
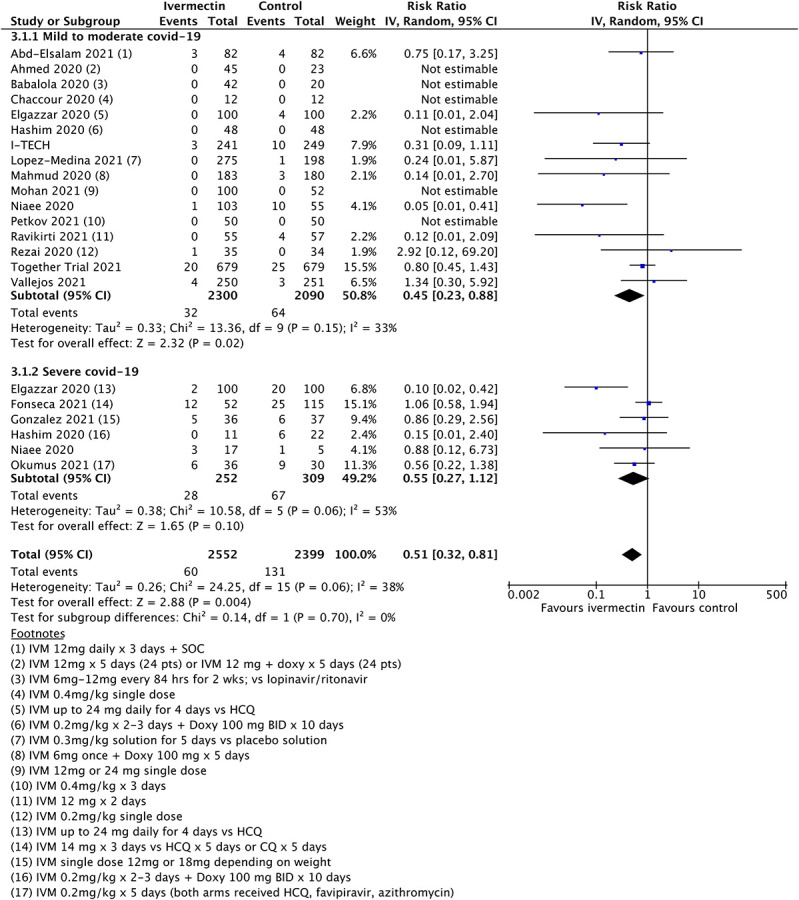
Updated mortality meta-analysis including all qualifying trials.
FIGURE 2.
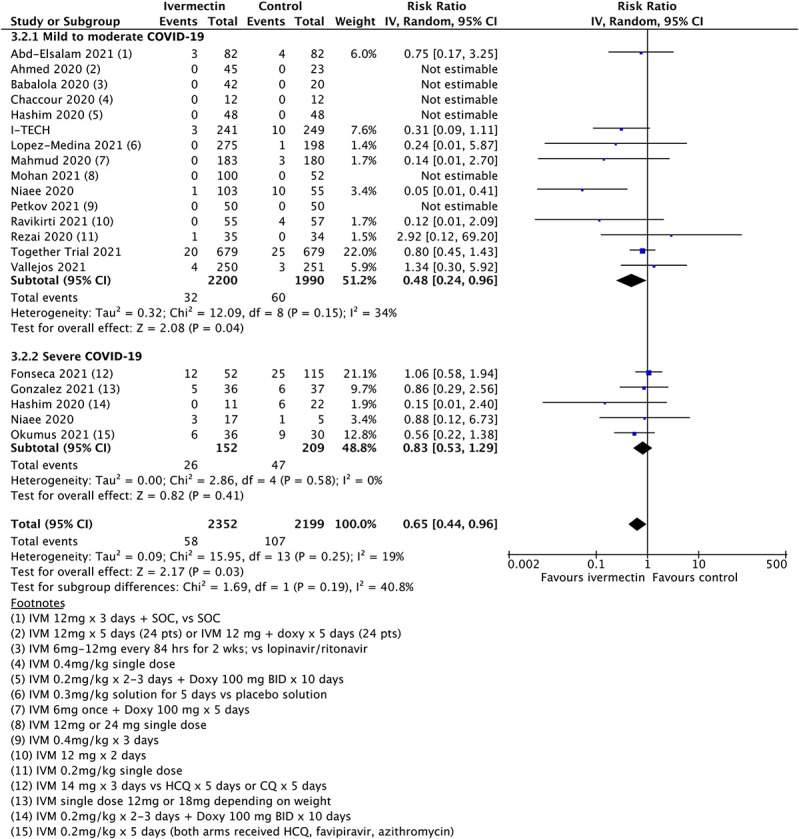
Updated mortality meta-analysis excluding Elgazzar.
FIGURE 3.
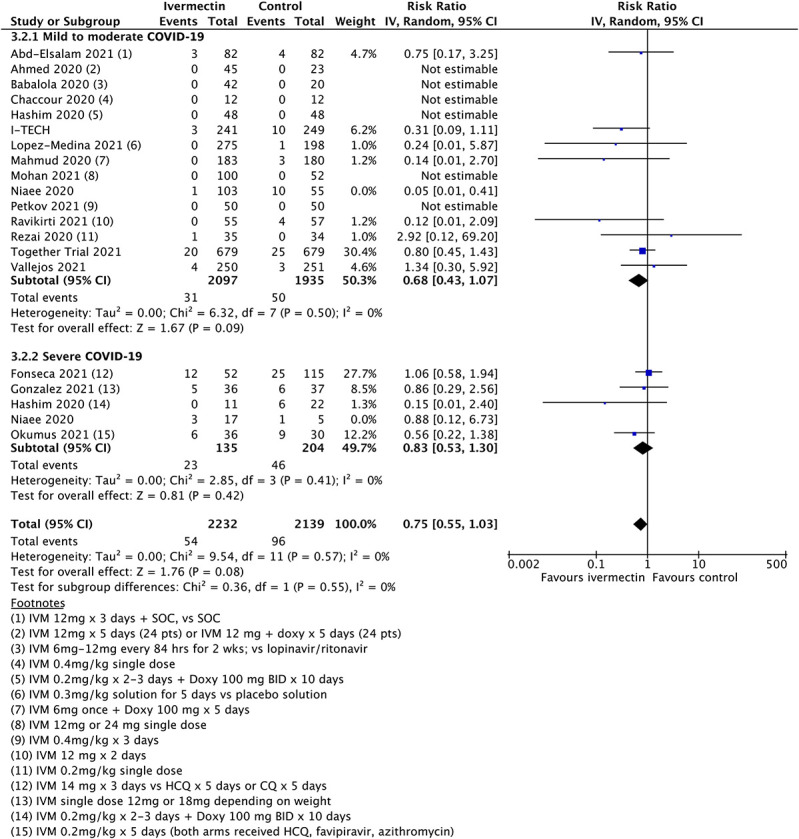
Updated mortality analysis with exclusions of both Elgazzar and Niaee.
The overall point estimates of mortality risk ratio vary from 0.51 (all studies) to 0.65 (Elgazzar excluded) to 0.75 (both Elgazzar and Niaee excluded). Only in the last case do orthodox 95% confidence intervals stray above unity, and by a very small amount. Above 95% CIs in Figures 2 and 3 are 0.96 and 1.03, respectively, in practical terms a minor difference. It is obvious that removal of study data will decrease the overall statistical power of any meta-analysis. However, even when significantly diluted by exclusion of disputed trials, meta-analysis continues to show an improved mortality outcome. Moreover, additional data from the later trials are broadly consistent with the original findings.
New data could justify full revision15 of a systematic review.3 As previously commented,6 there are 3 leading options for updating systematic reviews: (1) a living review continuously updated, (2) periodic review subject to criteria9 (typical intervals for specialist groups are 2–3 years), and (3) a Trial Sequential Analysis. We opted for the latter approach.
Subsequent to our article,3 Neil and Fenton16 confirmed by Bayesian hypothesis testing robust evidence of a mortality advantage under treatments including ivermectin. Their sensitivity analysis covered the exclusion of Elgazzar,5 the Niaee7 trial having been already disregarded, not on grounds of reliability, but simply because mortalities were not, at that time, reported7 by disease severity. Severity being a key component of the hypothesis of Neil and Fenton,16 data not stratified by severity were of no value. They showed explicitly that the removal of the disputed study5 (Niaee7 excluded by design) simply reduced the probability of a favorable risk reduction to around 0.77 or odds of 77:23 that ivermectin treatment offers a mortality benefit. Even under this significant reduction in participants, the conclusion of mortality benefit continued to hold.16
The Trial Sequential Analysis3 and the independent corroboration of Neil and Fenton16 by different methods provided good evidence that the conclusion of mortality advantage is robust. On the criteria of Garner et al15 and their decision flowchart (their Figure 1), the question “will the new studies change findings or credibility” could arguably be answered No, however given the controversies raised and the speed with which new data have arisen an updated review may be justified. This will be offered for publication in due course and include outcomes other than mortality.
Finally, we remark that we know of no clinicians using ivermectin in COVID-19 who would regard it as the sole therapeutic to be used in severe cases. In particular, corticosteroids are now recognized17 as critically important in late-stage disease. For seriously ill patients, it should be obvious that their survival probability will depend on many details of their management, not simply the use or nonuse of ivermectin.
Footnotes
The authors have no conflicts of interest to declare.
All authors were members of the British Ivermectin Recommendation Development (BiRD) panel at the “Evidence to Decision” event convened on February 20, 2021. Mr Bryant and Dr Lawrie were members of the steering group and did not vote. Drs Fordham and Mitchell were ordinary members of the panel. BiRD continues as a public information activity managed by EbMCsquared, a nonprofit Community Interest Company. Dr Fordham is a member of the Health Advisory and Recovery Team (HART), an unincorporated membership association with no financial or material interests in ivermectin or any other medical product. This work is not a project of HART and is not funded in any way by them.
REFERENCES
- 1.Manu P. Expression of concern for Bryant A, Lawrie TA, Dowswell T, Fordham EJ, Mitchell S, Hill SR, Tham TC. Ivermectin for prevention and treatment of COVID-19 infection. Am J Ther. 2022;29:e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rothrock SG, Weber KD, Giordano PA, et al. Meta-analyses do not establish improved mortality with ivermectin use in COVID-19. “Commentary”. Am J Ther. 2021;29:e87–e94. [DOI] [PubMed] [Google Scholar]
- 3.Bryant A, Lawrie TA, Dowswell T, et al. Ivermectin for prevention and treatment of COVID-19 infection: a systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines. Am J Therap. 2021;28:e434–e460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryant A, Lawrie TA, Fordham EJ. Ivermectin for prevention and treatment of COVID-19 infection: a systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines. Am J Ther. 2021;2828:e434–e460, e573–e576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elgazzar A, Hany B, Youssef SA, et al. Efficacy and safety of ivermectin for treatment and prophylaxis of COVID-19 pandemic. Res Square. 2020. Preprint. doi: 10.21203/rs.3.rs-100956/v2. [DOI] [Google Scholar]
- 6.Bryant A, Lawrie TA, Fordham EJ, et al. Re: “Commentary” by Rothrock et al. “meta-analyses did not establish improved mortality with ivermectin use in COVID-19”. Am J Ther. 2021;29:e233–e237. [DOI] [PubMed] [Google Scholar]
- 7.Niaee MS, Namdar P, Allami A, et al. Ivermectin as an adjunct treatment for hospitalized adult COVID-19 patients: a randomized multi-center clinical trial. Asian Pac J Trop Med. 2021;14:266. [Google Scholar]
- 8.Bryant A, Lawrie T, Dowsell T, et al. Ivermectin for prevention and treatment of covid-19 (protocol). The evidence-based Medical Consultancy Ltd. 2021. Available at: https://tinyurl.com/cx7pnaxa. Accessed November 11, 2021.
- 9.Vallejos J, Zoni R, Bangher M, et al. Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial. BMC Infect Dis. 2021;21:635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abd-Elsalam S, Noor RA, Badawi R, et al. Clinical study evaluating the efficacy of ivermectin in COVID-19 treatment: a randomized controlled study. J Med Virol. 2021;93:5833–5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reis G, Silva EA, Silva DC, et al. Effect of early treatment with ivermectin among patients with covid-19. N Engl J Med. 2022;386:1721–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim SCL, Hor CP, Tay KH, et al. Efficacy of ivermectin treatment on disease progression among adults with mild to moderate COVID-19 and comorbidities: the I-TECH randomized clinical trial. JAMA Intern Med. 2022;182:426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim SCL, Hor CP, Tay KH, et al. Supplement 2. eTable 6: “Post-hoc analyses on clinical outcomes by vaccination status in primary analysis population”. JAMA Intern Med. 2022. Available at: https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2789362. Accessed March 1, 2022. [Google Scholar]
- 14.Karale S, Bansal V, Makadia J, et al. A meta-analysis of mortality, need for ICU admission, use of mechanical ventilation and adverse effects with ivermectin use in COVID-19 patients. medRxiv. 2021. Preprint. doi: 10.1101/2021.04.30.21256415. [DOI] [Google Scholar]
- 15.Garner P, Hopewell S, Chandler J, et al. When and how to update systematic reviews: consensus and checklist. BMJ. 2016;354:i3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neil M, Fenton N. Bayesian hypothesis testing and hierarchical modeling of ivermectin effectiveness. Am J Ther. 2021;28:e576–e579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horby P, Lim WS, Emberson J, et al. Effect of Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2020;384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]


