Abstract
The aim was to investigate with very large-scale analyses whether there are underlying functional connectivity differences between humans that relate to food reward and whether these in turn are associated with being overweight. In 37 286 humans from the UK Biobank, resting-state functional connectivities of the orbitofrontal cortex (OFC), especially with the anterior cingulate cortex, were positively correlated with the liking for sweet foods (False Discovery Rate (FDR) P < 0.05). They were also positively correlated with the body mass index (BMI) (FDR P < 0.05). Moreover, in a sample of 502 492 people, the ‘liking for sweet foods’ was correlated with their BMI (r = 0.06, P < 10−125). In a cross-validation with 545 participants from the Human Connectome Project, a higher functional connectivity involving the OFC relative to other brain areas was associated with a high BMI (≥30) compared to a mid-BMI group (22–25; P = 6 × 10−5), and low OFC functional connectivity was associated with a low BMI (≤20.5; P < 0.024). It is proposed that a high BMI relates to increased efficacy of OFC food reward systems and a low BMI to decreased efficacy. This was found with no stimulation by food, so may be an underlying individual difference in brain connectivity that is related to food reward and BMI.
Keywords: orbitofrontal cortex, food reward, resting-state functional connectivity, anterior cingulate cortex, obesity, body mass index
Introduction
The primate including human orbitofrontal cortex (OFC) is involved in food reward, in that neurons in it respond to the sight, smell, taste and texture of food only when hunger is present and the food is rewarding, and functional magnetic resonance imaging (fMRI) activations in humans follow suit (Rolls et al., 1989; Critchley and Rolls, 1996; Kringelbach et al., 2003; Rolls, 2016b, 2019b; Rolls, 2020, 2021b). Further, activations of the OFC are linearly related to the subjective pleasantness of a food reward (de Araujo et al., 2003; Kringelbach et al., 2003; Grabenhorst and Rolls, 2008, 2009; Grabenhorst et al., 2010). It has been proposed that a higher sensitivity to the food reward value of the sight, smell and taste of food represented in the OFC could contribute to obesity (Rolls, 2011, 2016b). At the other end of the spectrum, it is much less clear about whether a decreased sensitivity of the OFC food reward system contributes to some individuals having a low body weight. This is especially the case for anorexia, to which many other factors may contribute (Kaye, 2008; Kaye et al., 2020). Much previous research on brain connectivity and obesity has focussed more on reduced functionality of brain systems related to executive function, rather than on an enhanced sensitivity of brain systems to food reward (Tregellas et al., 2011; Kullmann et al., 2012; Lips et al., 2014; Garcia-Garcia et al., 2015; Lowe et al., 2019; Donofry et al., 2020; Legget et al., 2021). Social and cognitive factors can operate by top-down biassing of the reward-related representations in the OFC, with their origin in the dorsolateral prefrontal cortex (de Araujo et al., 2005; Deco and Rolls, 2005; Grabenhorst and Rolls, 2008; Grabenhorst et al., 2008; Rolls et al., 2008; Grabenhorst and Rolls, 2010; Ge et al., 2012; Luo et al., 2013; Rolls, 2013, 2021a,b). For example, if a person is informed by another individual or by advertising that a food is in some way good or delicious, that can influence the eating behavior. One route by which this can happen is by top-down, cognitive and social, influences on the OFC food reward system, as described by (Rolls 2021b). In this context, a key aim of the present paper is to understand better the operation of food reward systems in the OFC and how they relate to body weight and body mass index (BMI).
To analyze whether there are differences in the functioning of the OFC that relate to whether individuals are overweight or underweight, in this investigation we measured the resting-state functional connectivity of the OFC and related this to food reward measured by the liking for sweet foods, and whether the participants were overweight or underweight, measured by their BMI. Functional connectivity is measured by the correlations between the blood oxygen level–dependent (BOLD) signal in different brain areas and reflects how strongly a pair of brain areas interacts. A high functional connectivity between brain areas provides an indication that there are strong influences between them, whether directly or indirectly. For example, a high functional connectivity of some brain areas may mean that they are more influenced by their inputs or that they influence their output regions more. Measurement of resting-state functional connectivity may provide a measure of whether the intrinsic brain circuitry is different in some individuals, even when it is not being used, for example, for food reward and the control of food intake.
Given this background, the first hypothesis that we wished to test was whether the resting-state functional connectivity of the OFC was higher in individuals who like sweet foods. The second hypothesis was whether those who like sweet foods have a higher BMI. The third hypothesis was whether a high functional connectivity of the OFC is associated with a higher BMI. There were also prior hypotheses about the other brain areas with which a high connectivity of the OFC would be of interest in relation to food reward and BMI. One is the anterior cingulate cortex, which has strong connections with the OFC, and is involved as a link from the reward-related OFC to output areas to perform actions to obtain rewards (Rolls, 2019a). This connectivity is highly correlated with sensation-seeking for reward (Wan et al., 2020). Another brain region is the insula, which has a strong connectivity with the OFC, and parts of which may be involved in taste and in autonomic output to rewards (such as salivation to the sight of food) (Rolls, 2016a).
A feature of the present investigation is that functional connectivity and its relation to obesity were investigated in a very large number of individuals in the UK Biobank, with cross-validation with the Human Connectome Project (HCP), in order to promote robustness of the findings. Small resting-state fMRI studies with fewer than 50–100 participants may report false-positive results due to statistical fluctuations (Poldrack et al., 2017; Gong et al., 2019; Jiao et al., 2020), and the present investigation helps to provide a quantitative assessment of not only whether effects are present but also of what their magnitude is to help guard against false-positive results in small studies. Another feature of the investigation is that brain function was being measured in the resting state without the sight or taste or food, to investigate whether there are differences in OFC functional connectivity even when food is not available. To our knowledge, this is the first time that discoveries of the type described here have been reported in large-scale studies, which are not subject to the vagaries of small-scale studies.
Methods
UK Biobank
Participants.
The dataset used for this investigation was selected from the September 2019 public data release from the UK Biobank, which includes a wide range of phenotypic information, as well as biological samples, for ∼500 000 participants. The UK Biobank sample used in these neuroimaging analyses included 37 286 participants [of whom 19 807 (53.12%%) were female; age range, 45–79 years]. The UK Biobank received ethical approval from the research ethics committee (REC reference 11/NW/0382). The present analyses were conducted under UK Biobank application number 1954. Written informed consent was obtained from each subject. The demographic characteristics of participants are summarized in Table 1.
Table 1.
Demographic characteristics of the 37 286 UK Biobank participants
| Characteristics | No. (%) |
|---|---|
| Age, mean (s.d.), years | 61.74 (7.44) |
| Female | 19 807 (53.12) |
| Townsend deprivation index, mean (s.d.), points | −1.90 (2.71) |
| Drinker status | |
| Prefer not to answer | 9 (0.02) |
| Never | 930 (2.49) |
| Previous | 792 (2.12) |
| Current | 35 544 (95.36) |
| Smoking status | |
| Never | 22 782 (61.12) |
| Previous | 12 179 (32.67) |
| Current | 2,314 (6.21) |
| Education qualifications | |
| College or university degree | 17 126 (46.56) |
| A levels/AS levels or equivalent | 4,847 (13.18) |
| O levels/GCSEs or equivalent | 7,101 (19.30) |
| CSEs or equivalent | 1,505 (4.09) |
| NVQ or HND or HNC or equivalent | 1,977 (5.37) |
| Other professional qualifications, e.g., nursing, teaching | 1,823 (4.96) |
| None of the above | 2,405 (6.54) |
| Liking for sweet foods, mean (s.d.), points | 6.03 (2.10) |
| BMI, mean (s.d.), kg/m2 | 26.49 (4.37) |
Analyses were performed for participants with both neuroimaging data and behavioral data concerning ‘liking for sweet foods’ (Field ID: 20732), which is liking for sweet foods measured on a 9-point scale from ‘extremely dislike’ to ‘extremely like’.
Imaging data collection and preprocessing.
The multi-modal imaging was collected using a standard Siemens Skyra 3T running VD13A SP4, with a standard Siemens 32-channel RF receive head coil. The resting-state functional brain imaging data used in this study included 22 331 participants and were obtained and processed by the UK Biobank. After quality controls and removing some participants without behavioral data, 4227 participants remained in the neuroimaging analysis. The details of the image acquisition are provided on the UK Biobank website in the form of a protocol (http://biobank.ctsu.ox.ac.uk/crystal/refer.cgi?id=2367). All the quality checking and data preprocessing procedures were conducted by the UK Biobank, and the details of the preprocessing are available on the UK Biobank website (http://biobank.ctsu.ox.ac.uk/crystal/refer.cgi?id=1977) and elsewhere (Miller et al., 2016). Briefly, data preprocessing was carried out using FSL (FMRIB Software Library). All the data preprocessing procedures were performed by the UK Biobank team as described by Miller et al. (2016). The data preprocessing included correction for spatial and gradient distortions and head motion, intensity normalization and bias field removal, registration to the T1 weighted structural image, transformation to 2 mm Montreal Neurological Institute (MNI) space, and the FIX artifact removal procedure (Smith et al., 2013; Navarro Schroder et al., 2015). Finally, the head motion parameters were regressed out, and structured artifacts were removed by ICA + FIX processing (Independent Component Analysis followed by FMRIB’s ICA-based X-noiseifier; Griffanti et al., 2014; Salimi-Khorshidi et al., 2014). The data preprocessing pipeline developed by FMRIB (Oxford University Centre for Functional MRI of the Brain) used here has been widely used in resting-state fMRI studies (Navarro Schroder et al., 2015; Smith et al., 2015; Colclough et al., 2017; Vidaurre et al., 2018).
Statistical analyses.
Partial correlations were measured between the measure of ‘liking for sweet foods’ in the UK Biobank (Field ID: 20732) and the 94 × 94 Automated Anatomical Labeling 2 (AAL2) functional connectivity matrix across all the individuals, enabling statistically significant correlations to be identified in the 94 × 94 correlation matrix using FDR correction for multiple comparisons, with gender, age, education qualification, smoking status, drinker status, Townsend deprivation index, imaging center and head motion regressed out. The AAL2 atlas (Rolls et al., 2015) was used because it defines a number of different OFC areas, with the full list of AAL2 areas shown in Supplementary Table S1, and because it has already proved useful in a number of investigations about the OFC (Du et al., 2020; Hsu et al., 2020; Rolls et al., 2020; Wan et al., 2020).
Similarly, partial correlations were measured between the BMI measure calculated from the data in the UK Biobank and the 94 × 94 AAL2 functional connectivity matrix across all the individuals, enabling statistically significant correlations to be identified in the 94 × 94 correlation matrix using FDR correction (Benjamini and Hochberg, 1995) for multiple comparisons, with gender, age, education qualification, smoking status, drinker status, Townsend deprivation index, imaging center and head motion regressed out.
The hypothesis for both these analyses is to find functional connectivity links in the brain that are positively correlated with the liking or BMI, as it is hypothesized that brain areas such as the OFC involved in food reward have activations and functional connectivities that are higher for high reward (Rolls, 2016b; Rolls et al., 2020; Wan et al., 2020).
Human Connectome Project
Participants.
The dataset was selected from the March 2017 public data release from the HCP (N = 1200), WU-Minn Consortium. The sample included 1002 subjects (ages 22–37 years, 534 females) scanned on a 3T Siemens connectome-Skyra scanner. The WU-Minn HCP Consortium obtained full informed consent from all participants, and research procedures and ethical guidelines were followed in accordance with the Institutional Review Boards. The demographic characteristics of 545 participants in this study are summarized in Table 2.
Table 2.
Demographic characteristics of the 545 HCP participants
| Characteristics | No. (%) |
|---|---|
| Age, mean (SD), years | 28.69 (3.75) |
| Female | 310 (56.88) |
| Education, mean (SD), years | 15 (1.77) |
| Drinker status | |
| Prefer not to answer | 25 (4.59) |
| Never | 80 (14.68) |
| Previous | 255 (46.79) |
| Current | 185 (33.94) |
| Smoking status | |
| Never | 295 (54.13) |
| Previous | 160 (29.36) |
| Current | 90 (16.51) |
| BMI | |
| Low BMI (BMI ≤ 20.5) | 83 (15.23) |
| Mid BMI (22 < BMI < 25) | 256 (44.99) |
| High BMI (BMI ≥ 30) | 206 (36.20) |
The HCP participants were divided into three groups, using the principle that there should be >80 participants in each of a low, mid and high BMI group, with a gap of BMI between each group to facilitate comparison between the different groups. The BMI is defined as the weight (in kg)/height2 (in meters). Using these criteria, the high BMI group with BMI ≥30 contained 206 participants (111 females), the mid group with BMI between 22 and 25 contained 256 participants (137 females) and the low BMI group with BMI ≤20.5 contained 83 participants (62 females). These particular values for the BMI for the different groups were selected to have sufficient participants in each group for the analyses, to allow a gap between the BMIs of the groups to facilitate statistical comparison and to provide data from groups with low, mid and high BMIs from the population in the dataset. The collection and preprocessing of the data are provided on the HCP website (http://www.humanconnectome.org/), together with information about ethical permission and informed consent.
Data collection and preprocessing.
The data collection and preprocessing were performed by the HCP. The participants included in this sample were scanned on a 3T connectome-Skyra scanner (Siemens). Data preprocessing was carried out using FSL (FMRIB Software Library), FreeSurfer and the Connectome Workbench software. All the data preprocessing procedures were performed by the HCP as described in detail elsewhere (Glasser et al., 2013). The data preprocessing included correction for spatial and gradient distortions and head motion, intensity normalization and bias field removal, registration to the T1 weighted structural image, transformation to 2 mm MNI space and the FIX artifact removal procedure (Smith et al., 2013; Navarro Schroder et al., 2015). Finally, the head motion parameters were regressed out and structured artifacts were removed by ICA + FIX processing (Independent Component Analysis followed by FMRIB’s ICA-based X-noiseifier; Griffanti et al., 2014; Salimi-Khorshidi et al., 2014). The data preprocessing pipeline developed by FMRIB (Oxford University Centre for Functional MRI of the Brain) used here has been widely used in resting-state fMRI studies (Navarro Schroder et al., 2015; Smith et al., 2015; Colclough et al., 2017; Vidaurre et al., 2018). The first 20 volumes were discarded to suppress equilibration effects, and participants without the full 1200 time points in four resting-state runs were also removed from the following analysis. The resulting time courses were used for the construction and analysis of the functional connectivity brain network.
Construction of the whole-brain functional connectivity network.
After the preprocessing, the gray matter of the whole brain was divided into 94 regions using the AAL2 atlas (Rolls et al., 2015). This atlas was chosen because it has a useful parcellation of the OFC based on (Chiavaras and Petrides, 2000) and because there is evidence on the connectivity of each of the OFC divisions with each other and with other brain regions, using both direct connectivity (Hsu et al., 2020) and functional connectivity (Du et al., 2020). Based on the AAL2 atlas, the time series were extracted by determining the mean of the signals of all voxels within each region across 490 time points. The whole-brain functional network (94 × 94 regions with 4371 links) was established by calculating the Pearson’s correlation between the BOLD signal for all pairs of brain regions for each individual participant, followed by z transformation to improve normality (Finn et al., 2015; Rosenberg et al., 2016). The anatomical regions in the AAL2 atlas are shown in Supplementary Table S1. Age, gender, education years, drinking status, smoking status and head motion were regressed out in the analyses.
Statistical analyses.
t tests were performed to test for differences of the 94 × 94 functional connectivity matrix between the different groups, with age, gender, education years, drinking status, smoking status and head motion regressed out. This resulted in a single 94 × 94 t matrix for the t values of the differences between each of the functional connectivities for the difference between, for example, the high BMI group compared to the mid BMI group. To test whether the functional connectivities were different for the high BMI group and the mid BMI group, the difference of the mean OFC functional connectivity from the mean Other functional connectivity was calculated for each participant and then the difference of these was compared using a t-test across participants between the two BMI groups. This controlled for any possible difference of the mean functional connectivity between the three BMI groups, which was tested (and found to be present as shown in Figure 5). The OFC functional connectivities were those within the OFC region of interest (OFC ROI1, from Olfactory to OFClat in the AAL2 atlas) together with the connectivities of these OFC regions with other brain areas. The Other functional connectivities were those not involving areas in the OFC ROI1. A similar comparison was performed to test whether the OFC functional connectivities were different from all the Other functional connectivities when comparing the low BMI group to the mid BMI group. The OFC ROI2 was defined as AAL2 areas OFCant to OFClat (see Supplementary Table S1). These ROIs for the OFC areas were selected based on very extensive evidence from primate single neuron recording and human fMRI that these brain regions are activated by stimuli such as the sight, smell, taste and texture of food when they are rewarding and are subjectively pleasant (Rolls et al., 1989; Critchley and Rolls, 1996; Kringelbach et al., 2003; Rolls, 2014; 2015; Rolls, 2016b, 2019b; 2020; Rolls, 2021a,b), with ROI1 extended to include areas known to be connected with the OFC (Du et al., 2020; Hsu et al., 2020) that also had a high functional connectivity in the high BMI vs mid BMI groups in Figure 3.
Fig. 5.
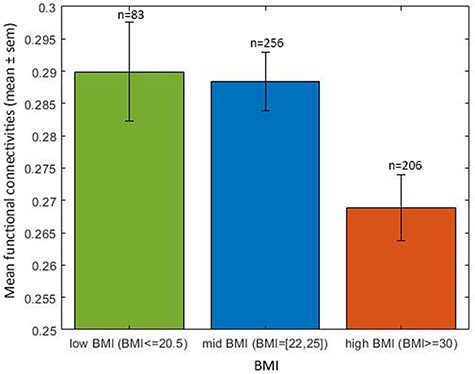
High BMI is associated with low mean functional connectivity. The relation between the mean FC across all cortical areas for the three groups with different BMI for the HCP. The high BMI group has a lower mean functional connectivity compared to the mid BMI group (t = −2.9, Cohen’s d = −0.13, df = 460, P = 4.3 × 10−3), with age, gender, education years, drinking status, smoking status and head motion regressed out. The numbers of participants in each group are shown.
Fig. 3.
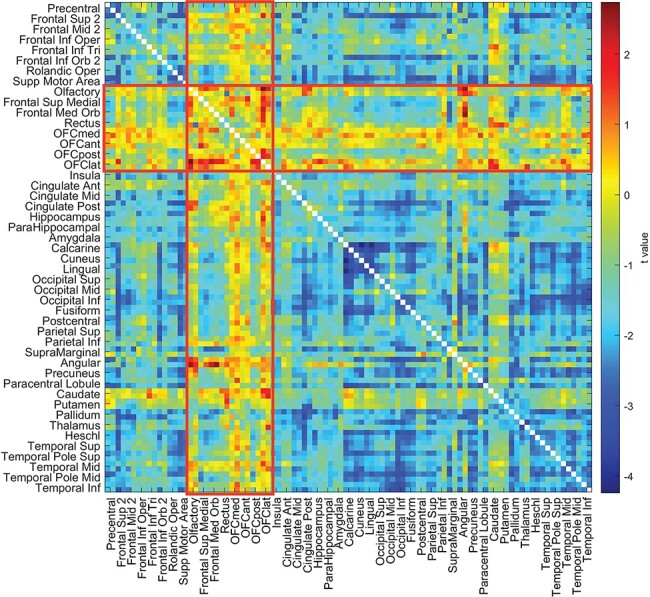
The resting-state functional connectivities involving the OFC are higher relative to other functional connectivities in the high BMI group than the mid BMI group in the HCPt dataset. For the HCP dataset, the t values for the resting-state functional connectivity differences for the high BMI minus the mid BMI participants are shown, with age, gender, education years, drinking status, smoking status and head motion regressed out. The areas referred to are olfactory (an area just posterior to the OFC in the AAL2 atlas) to the lateral OFC (OFClat) in the AAL2 atlas, and their connectivities with all other brain areas are within the red rectangles. The BMI groups and the numbers of participants in each are shown in Figure 5. The statistics are provided in the text and compared connectivities within the red rectangles for olfactory to OFClat with those outside the red rectangles.
Results
UK Biobank
Correlation between resting-state functional connectivity and the liking for sweet food.
The correlations between the ‘liking for sweet foods’ and the resting-state functional connectivities between all AAL2 brain areas are shown in Figure 1, with the top 50 correlated links in Table 3. From Figure 1, it is evident that there is a set of ventromedial prefrontal cortex (VMPFC) areas (Frontal_Med_Orb with Rectus that corresponds to the VMPFC and Frontal_Sup_Medial just superior to this) whose connectivity with the anterior and midcingulate cortex, and the insula, is correlated with the reported liking for sweet foods. The functional connectivities of the same VMPFC medial prefrontal areas with many other brain areas also had high correlations with the reported ‘liking for sweet foods’. This is confirmed by the links shown in Table 4, many of which involved VMPFC and related areas (Frontal_Med_Orb; together with Frontal_Sup_Medial just superior to this) with the anterior cingulate cortex. Other links that feature in the table involve the precuneus, parietal areas, including the angular, supramarginal and inferior parietal cortex, the middle frontal gyrus, and the temporal lobe.
Fig. 1.
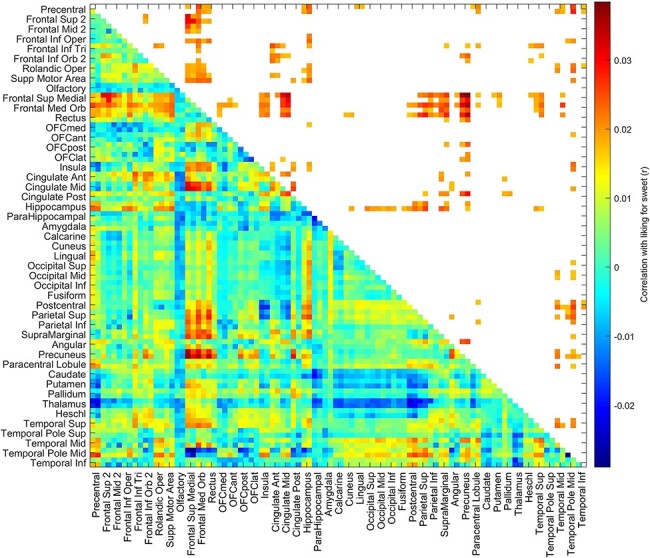
The liking for sweet foods is correlated with functional connectivities of the VMPFC (in this case gyrus rectus, FrontalMedOrb and FrontalSupMed from the AAL2 atlas). For the UK Biobank, the correlation between the ‘liking for sweet foods’ and the resting-state functional connectivities between all AAL2 brain areas. The lower left triangle shows the Pearson’s correlations with the 94 × 94 functional connectivities, and the upper right triangle shows those that were significantly higher after FDR correction (P < 0.05 with a two-tailed test). A list of the AAL2 areas and their abbreviations is provided in Supplementary Table S1. Frontal_Med_Orb with Rectus corresponds to the VMPFC and Frontal_Sup_Medial includes cortex just superior to this. The OFC areas are OFCmed to OFClat. There were 37 286 participants.
Table 3.
The 50 most significant functional connectivity links positively correlated with the liking for sweet foods in the UKB (N = 37 286)
| Region_1 | Region_2 | r Value | P value | Region_1 | Region_2 | r Value | P value |
|---|---|---|---|---|---|---|---|
| Frontal_Sup_Medial_L | Precuneus_R | 0.04 | 6.10E-12 | Frontal_Med_Orb_L | SupraMarginal_R | 0.03 | 2.41E-06 |
| Frontal_Sup_Medial_L | Precuneus_L | 0.04 | 1.02E-10 | Cingulate_Mid_R | Cingulate_Post_L | 0.03 | 2.56E-06 |
| Frontal_Med_Orb_L | Precuneus_R | 0.04 | 2.43E-10 | Frontal_Mid_2_L | Frontal_Sup_Medial_L | 0.03 | 2.65E-06 |
| Rectus_L | Precuneus_R | 0.03 | 7.01E-10 | Postcentral_R | Temporal_Pole_Mid_R | 0.03 | 2.67E-06 |
| Frontal_Sup_Medial_L | Cingulate_Mid_R | 0.03 | 1.19E-09 | Angular_L | Precuneus_R | 0.03 | 2.95E-06 |
| Frontal_Sup_Medial_L | Cingulate_Mid_L | 0.03 | 1.01E-08 | Hippocampus_R | Parietal_Sup_R | 0.03 | 2.96E-06 |
| Frontal_Med_Orb_L | Precuneus_L | 0.03 | 1.20E-08 | Frontal_Sup_Medial_R | Precuneus_L | 0.03 | 2.96E-06 |
| Frontal_Sup_2_L | Frontal_Sup_Medial_L | 0.03 | 1.35E-08 | Frontal_Med_Orb_R | Precuneus_R | 0.03 | 3.42E-06 |
| Frontal_Sup_Medial_R | Cingulate_Mid_R | 0.03 | 1.80E-08 | Precentral_L | Frontal_Med_Orb_L | 0.03 | 3.53E-06 |
| Frontal_Sup_2_R | Frontal_Sup_Medial_L | 0.03 | 2.84E-08 | Frontal_Med_Orb_L | Parietal_Inf_L | 0.03 | 3.66E-06 |
| Frontal_Sup_Medial_R | Precuneus_R | 0.03 | 4.48E-08 | Frontal_Med_Orb_R | SupraMarginal_L | 0.03 | 3.76E-06 |
| Frontal_Sup_2_R | Frontal_Sup_Medial_R | 0.03 | 4.82E-08 | Precentral_L | Temporal_Pole_Mid_R | 0.03 | 4.72E-06 |
| Cingulate_Post_L | Precuneus_R | 0.03 | 7.75E-08 | Frontal_Med_Orb_L | Parietal_Sup_L | 0.02 | 6.66E-06 |
| Frontal_Med_Orb_L | Cingulate_Mid_R | 0.03 | 2.12E-07 | Frontal_Sup_Medial_L | Parietal_Inf_L | 0.02 | 6.84E-06 |
| Rectus_L | Parietal_Sup_R | 0.03 | 2.28E-07 | Frontal_Inf_Tri_L | Cingulate_Ant_L | 0.02 | 7.56E-06 |
| Precentral_R | Temporal_Pole_Mid_R | 0.03 | 3.67E-07 | Cingulate_Post_L | Precuneus_L | 0.02 | 8.29E-06 |
| Rectus_L | SupraMarginal_R | 0.03 | 5.22E-07 | Angular_L | Precuneus_L | 0.02 | 8.31E-06 |
| Rectus_L | Precuneus_L | 0.03 | 5.25E-07 | Frontal_Sup_2_R | Frontal_Med_Orb_L | 0.02 | 8.34E-06 |
| Frontal_Med_Orb_L | Parietal_Sup_R | 0.03 | 1.23E-06 | Rectus_L | Cingulate_Mid_L | 0.02 | 1.03E-05 |
| Frontal_Sup_Medial_L | SupraMarginal_R | 0.03 | 1.73E-06 | Hippocampus_R | SupraMarginal_L | 0.02 | 1.05E-05 |
| Rectus_L | Cingulate_Mid_R | 0.03 | 1.85E-06 | Parietal_Sup_R | Temporal_Pole_Mid_R | 0.02 | 1.11E-05 |
| Rectus_L | Parietal_Sup_L | 0.03 | 1.96E-06 | Frontal_Med_Orb_L | SupraMarginal_L | 0.02 | 1.15E-05 |
| Frontal_Med_Orb_L | Temporal_Mid_R | 0.03 | 2.05E-06 | OFCpost_L | Precuneus_R | 0.02 | 1.28E-05 |
| Frontal_Med_Orb_L | Cingulate_Mid_L | 0.03 | 2.07E-06 | Supp_Motor_Area_R | Frontal_Med_Orb_L | 0.02 | 1.54E-05 |
| Frontal_Sup_Medial_R | Cingulate_Mid_L | 0.03 | 2.16E-06 | Supp_Motor_Area_R | Hippocampus_R | 0.02 | 1.64E-05 |
The P values shown are uncorrected for multiple comparisons, and the value corresponding to FDR P < 0.05 is P = 5.0 × 10–3.
Table 4.
The 50 most significant functional connectivity links positively correlated with BMI in the UKB (N = 37 286)
| Region_1 | Region_2 | r Value | P value | Region_1 | Region_2 | r Value | P value |
|---|---|---|---|---|---|---|---|
| Supp_Motor_Area_R | Parietal_Inf_R | 0.06 | 3.60E-31 | Precentral_R | Temporal_Sup_R | 0.04 | 2.55E-14 |
| Frontal_Med_Orb_R | Caudate_L | 0.05 | 1.07E-21 | Frontal_Med_Orb_R | Angular_L | 0.04 | 2.74E-14 |
| Frontal_Inf_Tri_L | OFCant_L | 0.05 | 3.41E-21 | Frontal_Med_Orb_L | Angular_L | 0.04 | 8.74E-14 |
| Supp_Motor_Area_L | Parietal_Inf_R | 0.05 | 4.11E-20 | Olfactory_L | Frontal_Sup_Medial_L | 0.04 | 8.75E-14 |
| Frontal_Inf_Orb_2_R | OFCant_R | 0.05 | 1.12E-19 | Postcentral_R | Parietal_Inf_R | 0.04 | 1.14E-13 |
| Precentral_L | Parietal_Inf_R | 0.05 | 3.24E-19 | Cingulate_Ant_L | Angular_L | 0.04 | 1.75E-13 |
| Frontal_Inf_Orb_2_L | OFCant_L | 0.05 | 4.14E-18 | Olfactory_L | Angular_R | 0.04 | 2.22E-13 |
| Precentral_R | Parietal_Inf_R | 0.05 | 7.44E-18 | Frontal_Sup_2_L | Cingulate_Ant_L | 0.04 | 3.16E-13 |
| Frontal_Med_Orb_L | Caudate_L | 0.04 | 4.40E-17 | Frontal_Sup_Medial_R | Caudate_L | 0.04 | 3.32E-13 |
| Frontal_Sup_2_L | Frontal_Med_Orb_R | 0.04 | 5.93E-17 | Frontal_Inf_Oper_L | SupraMarginal_R | 0.04 | 3.35E-13 |
| Frontal_Sup_Medial_R | Cingulate_Ant_L | 0.04 | 1.91E-16 | Frontal_Sup_Medial_L | Cingulate_Ant_L | 0.04 | 5.37E-13 |
| Olfactory_L | Angular_L | 0.04 | 2.76E-16 | Precentral_R | Parietal_Inf_L | 0.04 | 5.39E-13 |
| Frontal_Med_Orb_R | Cingulate_Ant_R | 0.04 | 5.31E-16 | Parietal_Inf_R | Paracentral_Lobule_L | 0.04 | 8.03E-13 |
| Frontal_Inf_Orb_2_L | OFCant_R | 0.04 | 5.56E-16 | Frontal_Mid_2_L | Frontal_Med_Orb_R | 0.04 | 1.21E-12 |
| Postcentral_L | Parietal_Inf_R | 0.04 | 1.52E-15 | Frontal_Inf_Tri_R | Supp_Motor_Area_R | 0.04 | 2.14E-12 |
| Supp_Motor_Area_L | Cingulate_Ant_L | 0.04 | 3.93E-15 | Parietal_Sup_L | Parietal_Inf_R | 0.04 | 2.39E-12 |
| Olfactory_L | Frontal_Sup_Medial_R | 0.04 | 4.99E-15 | Rectus_L | OFCant_L | 0.04 | 3.92E-12 |
| Frontal_Med_Orb_R | Caudate_R | 0.04 | 5.00E-15 | Postcentral_R | Parietal_Inf_L | 0.04 | 4.73E-12 |
| Frontal_Sup_Medial_R | Cingulate_Ant_R | 0.04 | 6.16E-15 | Frontal_Mid_2_L | Frontal_Med_Orb_L | 0.04 | 4.99E-12 |
| Frontal_Med_Orb_R | Cingulate_Ant_L | 0.04 | 8.58E-15 | Frontal_Sup_2_R | Supp_Motor_Area_L | 0.04 | 5.17E-12 |
| Frontal_Med_Orb_L | Cingulate_Ant_L | 0.04 | 9.81E-15 | Frontal_Mid_2_L | Angular_L | 0.04 | 6.38E-12 |
| Frontal_Sup_2_L | Angular_L | 0.04 | 1.40E-14 | Frontal_Inf_Tri_L | OFCant_R | 0.04 | 8.19E-12 |
| Frontal_Inf_Oper_R | SupraMarginal_L | 0.04 | 1.82E-14 | Frontal_Sup_2_R | Frontal_Med_Orb_R | 0.04 | 1.10E-11 |
| SupraMarginal_L | SupraMarginal_R | 0.04 | 2.00E-14 | OFCant_L | Angular_L | 0.04 | 1.36E-11 |
| Frontal_Sup_Medial_R | OFCant_R | 0.04 | 2.44E-14 | Supp_Motor_Area_R | Parietal_Inf_L | 0.04 | 1.43E-11 |
The P values shown are uncorrected for multiple comparisons, and the value corresponding to FDR P < 0.05 is P = 6.1 × 10−3.
Correlation between the liking for sweet food and the BMI.
In a sample of 502 492 people in the UK Biobank, the ‘liking for sweet foods’ was significantly correlated with their BMI (r = 0.06, P < 10−125). (The larger number for this analysis is because there are data on BMI and the liking for sweet foods in more UK Biobank participants than had neuroimaging data. For the subset of 37 286 participants with neuroimaging data and with measurement for ‘liking for sweet food’, the correlation between ‘liking for sweet food and BMI was 0.06, P < 10−32.)
Correlation between resting-state functional connectivity and the BMI.
The correlations between the BMI and the resting-state functional connectivities between all AAL2 brain areas are shown in Figure 2, with the top 50 functional connectivity links positively correlated with BMI shown in Table 4. From Figure 2, it is evident that the key part of the VMPFC (Frontal_Med_Orb; together with Frontal_Sup_Medial just superior to this) has functional connectivity with the anterior cingulate cortex that is significantly correlated with the BMI. (This was confirmed in that the functional connectivities between areas FrontalSupMed to OFClat had higher correlations with BMI than did the connectivities not involving these brain regions, t = 50.6, Cohen’s d = 0.53, P < 10−325, df = 7756.) More details are provided by the links shown in Table 4, many of which involved VMPFC and related areas (Frontal_Med_Orb; together with Frontal_Sup_Medial just superior to this) with the anterior cingulate cortex. Other links that feature in the table involve the precuneus, parietal areas, including the angular, supramarginal and inferior parietal cortex, and the middle frontal gyrus. The positive links were selected for Table 4 and Figure 2, because the hypothesis is that high functional connectivities of the ventromedial prefrontal/OFC and related areas are related to a high BMI and because a high BMI is associated with a tendency for functional connectivities to be generally lower, as shown in Figures 2 and 5.
Fig. 2.

BMI is correlated with functional connectivities of the VMPFC and anterior OFC (OFCant from the AAL2 atlas). For the UK Biobandatak, the correlation between the BMI and the resting-state functional connectivities between all AAL2 brain areas. The lower left triangle shows the Pearson’s correlations with the 94 × 94 functional connectivities, and the upper right triangle shows those that were significantly higher after FDR correction (P < 0.05 with a two-tailed test). There were 37 286 participants.
Human Connectome Project
OFC functional connectivity is higher relative to other brain areas in the high BMI group compared to the mid BMI group.
The resting-state functional connectivity differences between the high BMI and mid-weight participants, with age, gender, education years, drinking status, smoking status and head motion regressed out, are shown in Figure 3. It appears that functional connectivity differences between the high and mid BMI groups have higher t values between the OFC ROI1 areas than between the other AAL2 areas. We tested this, whether ex hypothesi the functional connectivity between the OFC areas relative to the other areas was higher in the high BMI group than the mid BMI group, as shown by the t values of the differences. This was performed by taking for each participant the mean value of the OFC functional connectivities with themselves and all other brain areas, and the mean value of the functional connectivities of all the other brain areas with each other, and using a t-test to compare the differences of these two values across all participants in the high BMI group vs the mid BMI group. This showed that the OFC functional connectivities relative to the other functional connectivities were higher in the high than the mid BMI groups (t = 4.05, Cohen’s d = 0.10, df = 462, P = 6 × 10−5). This design involving a within-subject difference of the OFC from the other functional connectivities was useful, as it factors out any overall decrease of functional connectivity with a high BMI evident in Figure 5. For this analysis, the OFC ROI1 definition included all areas from Olf to OFClat in the AAL2 atlas (see Figure 3, in which these brain regions are outlined with red rectangles). This analysis thus confirms that the OFC functional connectivities are higher relative to the other functional connectivities in the high BMI group than in the mid BMI group.
OFC functional connectivity is lower relative to other brain areas in the low BMI group compared to the mid BMI group.
The resting-state functional connectivity differences between the low BMI and mid-weight participants are shown in Figure 4. This shows that at least some OFC regions had lower functional connectivities relative to other brain areas in the low BMI group than in the mid BMI group, with the main OFC areas involved from OFCant to OFClat. Using a statistical analysis similar to that used for Figure 3, it was found that these OFC functional connectivities are lower relative to the other functional connectivities in the low BMI group than in the mid BMI group (t = −2.27, Cohen’s d = −0.10, df = 340, P < 0.024). For this analysis, the OFC definition included all areas from OFCant to OFClat in the AAL2 atlas (see Figure 4, in which these brain regions are outlined with red rectangles). For this analysis, the low BMI group included 83 individuals with a BMI ≤20.5, to increase a little the separation of the BMI between the low and mid BMI groups (with 259 individuals).
Fig. 4.
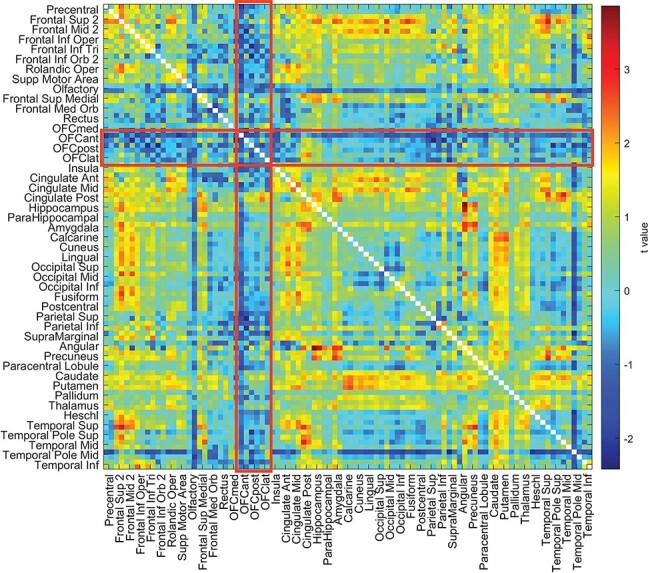
The resting-state functional connectivities involving the OFC are lower relative to other functional connectivities in the low BMI group than the mid BMI group in the HCP dataset. For the HCP dataset, the t values for the resting-state functional connectivity differences for the low BMI minus the mid BMI participants are shown, with age, gender, education, drinking frequency, smoking status and head motion regressed out. The areas referred to are anterior OFC (OFCant) to lateral OFC (OFClat) in the AAL2 atlas, and their connectivities with all other brain areas are within the red rectangles. The BMI groups and the numbers of participants in each are shown in Figure 5. The statistics are provided in the text and compared connectivities within the red rectangles for OFCant to OFClat with those outside the red rectangles.
It was noticeable that the functional connectivities of brain areas other than those related to the OFC were negatively correlated with the BMI in the HCP dataset, and indeed, it was found that the mean functional connectivity across the whole brain was lower in the high BMI group compared to the mid BMI group, as shown in Figure 5. It was because of what is shown in Figure 5 that it was useful in the HCP analyses to compare the functional connectivities of the OFC areas with the functional connectivities of other brain areas.
Discussion
The first key finding was that the resting-state functional connectivity between the VMPFC /OFC (VMPFC–OFC) and especially the anterior cingulate cortex was positively correlated with the liking for sweet foods (Figure 1). What is especially interesting about this is that the functional connectivity was measured in the resting state, when food was not present as a reward stimulus. There is much evidence that the OFC has neurons that respond to the reward value of the sight, smell taste and oral texture of food and that activations of the medial OFC in humans are related to the reward value of all of these sensory aspects of food (Rolls, 2015, 2016b, 2019b, 2021b; Rolls et al., 2020), but the evidence that the functional connectivity of these areas is higher in those who report a high liking for sweet foods provides an indication that these brain food reward systems may be more strongly connected in individuals who like sweet foods. The stronger connections in this food reward system in some individuals may, we suggest, be a factor that drives some individuals more toward eating foods, which may not be limited to sweet foods but could extend to other types of food. The connectivity of the VMPFC/medial OFC with the anterior cingulate cortex is of especial interest, for the cingulate cortex is involved in actions, and in particular in associating actions (using information received from the parietal cortex) with rewards (received from the OFC/VMPFC) (Rolls, 2019a). Consistent with this, there are strong connections between the OFC and anterior cingulate cortex (Du et al., 2020; Hsu et al., 2020), and it is possible to predict from the strength of these connections human sensation-seeking behavior, which involves being highly motivated to seek out rewards (Wan et al., 2020). Other areas with a high functional connectivity with the VMPFC in those who liked sweet included the precuneus (which is implicated in the sense of self; Cavanna and Trimble, 2006; Freton et al., 2014); parts of the parietal cortex implicated in language including the angular, supramarginal and inferior parietal areas (Rolls, 2021a); the middle frontal gyrus (involved in executive function) and the temporal lobes (involved in visual and semantic representations) (Rolls, 2021a). It was further of interest that the VMPFC–OFC area related to the liking for food extended into other cortex in the medial prefrontal cortex (FrontalSupMed in the AAL2 atlas), which is an area with strong connections with the VMPFC (Du et al., 2020; Hsu et al., 2020).
The second key finding was that this same extended VMPFC–OFC area has functional connectivity positively correlated with BMI (Figure 2). This is consistent with the hypothesis that a high sensitivity to food reward (in this case measured by the liking for sweet foods) in this extended VMPFC system is associated with a higher BMI. Indeed, we suggest that this provides evidence that high liking for food in this food reward circuitry over-drives food intake to result in being overweight. Again, it is of great interest that the association between the connectivity of this extended VMPFC–OFC system and BMI can be revealed even in the resting state when no food is present, providing an indication that increased connectivity in this brain system is related to increased liking for foods and increased BMI. Of course these findings are associations and do not prove causality, but it is a long-established hypothesis that too much sensitivity to the sensory stimuli that produce food reward is a factor in obesity (Rolls, 2014, 2016b).
These discoveries in the UK Biobank dataset were cross-validated in the HCP dataset but also extended as described next, in that in addition to measuring correlations between VMPFC/OFC functional connectivity (relative to other brain areas) and body weight (which were significant), we were able to go beyond correlations and examine separately the situations in groups with a high BMI from those with a low BMI, with the latter of interest in relation to whether underconnectivity of the same system is related to a low BMI produced potentially by less food reward and therefore eating. This latter hypothesis was supported.
The finding shown in Figure 3 from the HCP that the functional connectivities of the OFC are higher in the high BMI group relative to the other FCs is consistent with the hypothesis that increased efficacy in the OFC food reward system may be a driving factor that contributes to increased eating and a higher BMI.
The finding shown in Figure 4 from the HCP that the FCs of the OFC (and especially the part involving OFCant, OFCpost and OFClat) are lower in the low BMI group relative to the other FCs is consistent with the hypothesis that decreased efficacy in the OFC food reward system may be a driving factor that contributes to decreased eating and a lower BMI. Although this low BMI group was not selected to have a diagnosis of anorexia, the findings here do provide evidence from the HCP dataset that in at least low BMI individuals (BMI < 20.5), the OFC, involved in food reward, does have decreased efficacy. That could relate to the low BMI if these individuals find food less rewarding and eat less. Indeed, for the low BMI group, our hypothesis is that the low functional connectivities of the OFC regions are related to the low BMI because of lower food reward, with reasoning similar to that provided above.
The results in Figures 3 and 5 suggest that a high BMI may be associated on average with somewhat lower functional connectivities averaged across all brain regions. This was tested by comparing the mean FC across all brain areas with the BMI, and the mean functional connectivity was lower in the high BMI group compared to the mid BMI group, with a significant difference in the HCP (t = −2.9, Cohen’s d = −0.13, df = 460, P = 4.3 × 10−3) and in the UK Biobank (UKB) (t = −13.4, Cohen’s d = −0.10, df = 16 673, P = 1.1 × 10−40). Because there are some differences of mean functional connectivity in the groups with different BMIs in this investigation (Figure 5), it was an important part of the statistical design of this investigation that the functional connectivities of the OFC ROI were compared with all the other functional connectivities within the same individuals, as a direct comparison of the functional connectivities of the OFC ROIs between groups with different BMIs could be influenced by the difference in mean functional connectivity between the groups with different BMIs that are shown in Figure 5.
An interesting finding from the HCP investigation was that the functional connectivities of the OFC ROI with the anterior cingulate cortex were high relative to the FCs of the OFC with other brain regions. This is consistent with the hypothesis that food reward signals from the OFC provide an extra strong input to the anterior cingulate cortex, which provides a route to implementing actions to obtain the food reward (Rolls, 2019a, 2021a,b). Consistent with this, sensation-seeking for rewards is associated with a higher functional connectivity between the reward-related medial OFC and the anterior cingulate cortex (Wan et al., 2020). It has also been reported that those who binge eat compared to a control group show greater activation to food stimuli of the anterior cingulate cortex (Geliebter et al., 2016). The pregenual anterior cingulate cortex, which receives inputs from the medial OFC (Du et al., 2020; Hsu et al., 2020), shares with the medial OFC activation produced by many rewarding stimuli, including olfactory and food-related stimuli, and correlations with the subjective pleasantness of these stimuli (Kringelbach et al., 2003; Grabenhorst and Rolls, 2011; Rolls, 2014, 2019a,b).
Another interesting finding was that in the HCP the OFC ROI had a higher functional connectivity with the olfactory tubercle region in the high BMI group, which is part of the ventral striatum (Figures 3 and 5). The ventral striatum is a region activated by rewards and provides another route for the OFC to influence behavior (Rolls, 2017, 2019b, 2021a,b), as does the connectivity with the caudate nucleus and putamen also evident in Figures 3 and 4.
A further interesting finding in the HCP was that the OFC ROI had a higher functional connectivity with a number of other frontal cortex areas in the high BMI group, including the superior and middle frontal gyri and the superior medial prefrontal area (FrontalSupMed) (Figures 3 and 5). These are brain areas implicated in executive control and planning (Rosati, 2017; Shallice and Cipolotti, 2018; Fiske and Holmboe, 2019; Rolls, 2021a), and a hypothesis is that these prefrontal executive function areas receive a stronger food reward input from the OFC in high BMI individuals, which may influence the outcome of their plans.
Given the association described here between resting-state functional connectivity of the OFC and related regions and the liking for food and potentially thereby BMI, the differences between individuals might reflect genetic differences with some individuals more influenced by food reward, with individual variability in the value of different specific types of reward a driving factor in evolution by natural selection (Rolls, 2014). In a complementary way, top-down social or cognitive influences might bias the OFC reward systems and contribute to individual differences (Rolls, 2021b). For the findings from the UK Biobank, one of the covariates of no interest regressed out of the analysis was the Townsend deprivation index. The Townsend deprivation index reflects a range of measures of socioeconomic status, and so the results described here do not reflect what is measured by the Townsend deprivation index. Further research will of course be of interest to assess different contributions to the associations described here between functional connectivity, food reward and BMI.
In considering possible limitations, it is noted that with large datasets, the results can become highly significant. We therefore provide either r values or where t tests have been performed Cohen’s d values, in order to show the effect sizes. The effect sizes were in the range d = 0.1–0.53 (Figure 2). However, in fact, the large datasets used in this research are a great strength of this study, for they provide a firm foundation for research in this area that is not subject to the problems and possible false positives of small resting-state functional connectivity studies. We further note that the results described are quite selective, in that as shown in the top right parts of Figures 1 and 2, only a small proportion of the functional connectivities were significantly correlated after FDR correction for multiple comparisons with the liking for sweet food or with BMI.
Finally, we emphasize that this investigation is a very large study, with 37 286 participants with functional neuroimaging, which results in robust findings of the type that are frequently absent in small-scale neuroimaging investigations. In fact, this may be the largest neuroimaging investigation yet performed of food reward systems in the brain and their relation to body weight/BMI. For example, activation studies to food presentation have typically involved <30 participants (Tang et al., 2012; Masterson et al., 2019), and previous functional connectivity studies related to eating or obesity have typically involved <100 participants (Tregellas et al., 2011; Kullmann et al., 2012; Lips et al., 2014; Garcia-Garcia et al., 2015; Donofry et al., 2020; Legget et al., 2021). Further, the results were cross-validated with HCP data. Moreover, the relation between the liking for food and high BMI was confirmed in an even larger investigation, involving 502 492 participants. The findings here are of special interest, for they establish an association between the resting-state functional connectivity of the extended VMPFC/OFC and the liking of the individual person for sweet foods and their BMI. A hypothesis is that the increased functional connectivity we describe here even when no food is present may be an individual difference that does influence how rewarding food is for an individual, and the increased body weight that may be related to higher eating of such foods. This hypothesis relates to the much broader hypothesis that a driving factor in evolution may be variation in the reward value of different specific types of reward in different individuals, which provides a fundamental basis of personality, that is, individual differences (Rolls, 2014). In the present case, the implication is that the variation in the connectivity of food reward systems in the brain may lead some individuals to like food more, which of course can be adaptive in some environments, and that this can in some environments, especially when food is highly palatable and readily available, be associated with a high body weight/BMI (Rolls, 2014, 2016b, 2021b).
Supplementary Material
Acknowledgements
Use of the UK Biobank (https://www.ukbiobank.ac.uk) and HCP (http://www.humanconnectome.org/) datasets is gratefully acknowledged. The HCP neuroimaging data were provided by the Human Connectome Project, WU-Minn Consortium (principal investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH institutes and centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University. Interesting discussions with Professor Walter Kaye (UCSD) about individuals with low BMI are warmly acknowledged by Edmund Rolls. ETR and RF can be considered as co-first authors.
Contributor Information
Edmund T Rolls, Department of Computer Science, University of Warwick, Coventry CV4 7AL, UK; Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai 200433, China; Oxford Centre for Computational Neuroscience, Oxford, UK.
Ruiqing Feng, Department of Computer Science, University of Warwick, Coventry CV4 7AL, UK.
Wei Cheng, Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai 200433, China.
Jianfeng Feng, Department of Computer Science, University of Warwick, Coventry CV4 7AL, UK; Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai 200433, China; Key Laboratory of Computational Neuroscience and Brain-Inspired Intelligence, Fudan University, Ministry of Education, Shanghai 200433, China.
Funding
J. Feng is supported by Shanghai Science and Technology Innovation Plan (nos. 15JC1400101 and 16JC1420402), the National Natural Science Foundation of China (nos. 71661167002 and 91630314), the 111 Project (no. B18015) and Shanghai Municipal Science and Technology Major Project (no. 2018SHZDZX01). W. Cheng is supported by grants from the National Natural Sciences Foundation of China (nos. 81701773 and 11771010), sponsored by Shanghai Sailing Program (no. 17YF1426200). W. Cheng is also sponsored by the Natural Science Foundation of Shanghai (no. 18ZR1404400). The funders took no part in the design of the implementation of this research. The funding sources took no part in the design of the implementation of the research.
Conflict of interest
The authors have no competing interests to declare.
Data and code availability statement
Data for the UK Biobank are available at https://www.ukbiobank.ac.uk/ and for the Human Connectome Project at https://www.humanconnectome.org/. Standard code functions available in Matlab and SPM were used.
Ethics statement
No data were collected as part of this research. Details about the ethical permission and written informed consent are available for the UK Biobank at https://www.ukbiobank.ac.uk and for the Human Connectome project at http://www.humanconnectome.org.
Supplementary data
Supplementary data are available at SCAN online.
References
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate - a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B-Statistical Methodology, 57, 289–300. [Google Scholar]
- Cavanna A.E., Trimble M.R. (2006). The precuneus: a review of its functional anatomy and behavioural correlates. Brain, 129, 564–83. [DOI] [PubMed] [Google Scholar]
- Chiavaras M.M., Petrides M. (2000). Orbitofrontal sulci of the human and macaque monkey. Journal of Comparative Neurology, 422, 35–54. [PubMed] [Google Scholar]
- Colclough G.L., Smith S.M., Nichols T.E., et al. (2017). The heritability of multi-modal connectivity in human brain activity. Elife, 6, e20178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley H.D., Rolls E.T. (1996). Hunger and satiety modify the responses of olfactory and visual neurons in the primate orbitofrontal cortex. Journal of Neurophysiology, 75, 1673–86. [DOI] [PubMed] [Google Scholar]
- de Araujo I.E.T., Rolls E.T., Kringelbach M.L., McGlone F., Phillips N. (2003). Taste-olfactory convergence, and the representation of the pleasantness of flavour, in the human brain. European Journal of Neuroscience, 18, 2059–68. [DOI] [PubMed] [Google Scholar]
- de Araujo I.E.T., Rolls E.T., Velazco M.I., Margot C., Cayeux I. (2005). Cognitive modulation of olfactory processing. Neuron, 46, 671–9. [DOI] [PubMed] [Google Scholar]
- Deco G., Rolls E.T. (2005). Neurodynamics of biased competition and co-operation for attention: a model with spiking neurons. Journal of Neurophysiology, 94, 295–313. [DOI] [PubMed] [Google Scholar]
- Donofry S.D., Stillman C.M., Erickson K.I. (2020). A review of the relationship between eating behavior, obesity and functional brain network organization. Social Cognitive and Affective Neuroscience, 15, 1157–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J., Rolls E.T., Cheng W., et al. (2020). Functional connectivity of the orbitofrontal cortex, anterior cingulate cortex, and inferior frontal gyrus in humans. Cortex, 123, 185–99. [DOI] [PubMed] [Google Scholar]
- Finn E.S., Shen X., Scheinost D., et al. (2015). Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nature Neuroscience, 18, 1664–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiske A., Holmboe K. (2019). Neural substrates of early executive function development. Developmental Review, 52, 42–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freton M., Lemogne C., Bergouignan L., Delaveau P., Lehericy S., Fossati P. (2014). The eye of the self: precuneus volume and visual perspective during autobiographical memory retrieval. Brain Structure and Function, 219, 959–68. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia I., Jurado M.A., Garolera M., et al. (2015). Functional network centrality in obesity: a resting-state and task fMRI study. Psychiatry Research, 233, 331–8. [DOI] [PubMed] [Google Scholar]
- Ge T., Feng J., Grabenhorst F., Rolls E.T. (2012). Componential granger causality, and its application to identifying the source and mechanisms of the top-down biased activation that controls attention to affective vs sensory processing. NeuroImage, 59, 1846–58. [DOI] [PubMed] [Google Scholar]
- Geliebter A., Benson L., Pantazatos S.P., Hirsch J., Carnell S. (2016). Greater anterior cingulate activation and connectivity in response to visual and auditory high-calorie food cues in binge eating: preliminary findings. Appetite, 96, 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser M.F., Sotiropoulos S.N., Wilson J.A., et al. (2013). The minimal preprocessing pipelines for the human connectome project. NeuroImage, 80, 105–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong W., Cheng F., Rolls E.T., et al. (2019). A powerful and efficient multivariate approach for voxel-level connectome-wide association studies. NeuroImage, 188, 628–41. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F., Rolls E.T., Bilderbeck A. (2008). How cognition modulates affective responses to taste and flavor: top down influences on the orbitofrontal and pregenual cingulate cortices. Cerebral Cortex, 18, 1549–59. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F., D’Souza A., Parris B.A., Rolls E.T., Passingham R.E. (2010). A common neural scale for the subjective pleasantness of different primary rewards. NeuroImage, 51, 1265–74. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F., Rolls E.T. (2008). Selective attention to affective value alters how the brain processes taste stimuli. European Journal of Neuroscience, 27, 723–9. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F., Rolls E.T. (2009). Different representations of relative and absolute value in the human brain. NeuroImage, 48, 258–68. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F., Rolls E.T. (2010). Attentional modulation of affective vs sensory processing: functional connectivity and a top-down biased activation theory of selective attention. Journal of Neurophysiology, 104, 1649–60. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F., Rolls E.T. (2011). Value, pleasure, and choice in the ventral prefrontal cortex. Trends in Cognitive Sciences, 15, 56–67. [DOI] [PubMed] [Google Scholar]
- Griffanti L., Salimi-Khorshidi G., Beckmann C.F., et al. (2014). ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging. NeuroImage, 95, 232–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C.-C.H., Rolls E.T., Huang C.-C., et al. (2020). Connections of the human orbitofrontal cortex and inferior frontal gyrus. Cerebral Cortex, 30, 5830–43. [DOI] [PubMed] [Google Scholar]
- Jiao Z., Lai Y., Kang J., et al. (2020). Assessing study reproducibility through M2RI: a novel approach for large-scale high-throughput association studies. bioRxiv. 10.1101/2020.08.18.253740doi: 10.1101/2020.08.18.253740. [DOI] [Google Scholar]
- Kaye W. (2008). Neurobiology of anorexia and bulimia nervosa. Physiology and Behavior, 94, 121–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye W.H., Wierenga C.E., Bischoff-Grethe A., et al. (2020). Neural insensitivity to the effects of hunger in women remitted from anorexia nervosa. American Journal of Psychiatry, 177, 601–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach M.L., O’Doherty J., Rolls E.T., Andrews C. (2003). Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cerebral Cortex, 13, 1064–71. [DOI] [PubMed] [Google Scholar]
- Kullmann S., Heni M., Veit R., et al. (2012). The obese brain: association of body mass index and insulin sensitivity with resting state network functional connectivity. Human Brain Mapping, 33, 1052–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legget K.T., Wylie K.P., Cornier M.A., Berman B.D., Tregellas J.R. (2021). Altered between-network connectivity in individuals prone to obesity. Physiology and Behavior, 229, 113242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips M.A., Wijngaarden M.A., van der Grond J., et al. (2014). Resting-state functional connectivity of brain regions involved in cognitive control, motivation, and reward is enhanced in obese females. American Journal of Clinical Nutrition, 100, 524–31. [DOI] [PubMed] [Google Scholar]
- Lowe C.J., Reichelt A.C., Hall P.A. (2019). The prefrontal cortex and obesity: a health neuroscience perspective. Trends in Cognitive Sciences, 23, 349–61. [DOI] [PubMed] [Google Scholar]
- Luo Q., Ge T., Grabenhorst F., Feng J., Rolls E.T. (2013). Attention-dependent modulation of cortical taste circuits revealed by Granger causality with signal-dependent noise. PLoS Computational Biology, 9, e1003265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masterson T.D., Stein W.M., Beidler E., Bermudez M., English L.K., Keller K.L. (2019). Brain response to food brands correlates with increased intake from branded meals in children: an fMRI study. Brain Imaging and Behavior, 13, 1035–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K.L., Alfaro-Almagro F., Bangerter N.K., et al. (2016). Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nature Neuroscience, 19, 1523–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro Schroder T., Haak K.V., Zaragoza Jimenez N.I., Beckmann C.F., Doeller C.F. (2015). Functional topography of the human entorhinal cortex. Elife, 4, e06738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack R.A., Baker C.I., Durnez J., et al. (2017). Scanning the horizon: towards transparent and reproducible neuroimaging research. Nature Reviews: Neuroscience, 18, 115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls E.T., Sienkiewicz Z.J., Yaxley S. (1989). Hunger modulates the responses to gustatory stimuli of single neurons in the caudolateral orbitofrontal cortex of the macaque monkey. European Journal of Neuroscience, 1, 53–60. [DOI] [PubMed] [Google Scholar]
- Rolls E.T., Grabenhorst F., Margot C., da Silva M.A.A.P., Velazco M.I. (2008). Selective attention to affective value alters how the brain processes olfactory stimuli. Journal of Cognitive Neuroscience, 20, 1815–26. [DOI] [PubMed] [Google Scholar]
- Rolls E.T. (2011). Taste, olfactory, and food texture reward processing in the brain and obesity. International Journal of Obesity, 35, 550–61. [DOI] [PubMed] [Google Scholar]
- Rolls E.T. (2013). A biased activation theory of the cognitive and attentional modulation of emotion. Frontiers in Human Neuroscience, 7, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls E.T. (2014). Emotion and Decision-Making Explained. Oxford: Oxford University Press. [Google Scholar]
- Rolls E.T. (2015). Taste, olfactory, and food reward value processing in the brain. Progress in Neurobiology, 127–128, 64–90. [DOI] [PubMed] [Google Scholar]
- Rolls E.T., Joliot M., Tzourio-Mazoyer N. (2015). Implementation of a new parcellation of the orbitofrontal cortex in the Automated Anatomical Labeling atlas. NeuroImage, 122, 1–5. [DOI] [PubMed] [Google Scholar]
- Rolls E.T. (2016a). Functions of the anterior insula in taste, autonomic, and related functions. Brain and Cognition, 110, 4–19. [DOI] [PubMed] [Google Scholar]
- Rolls E.T. (2016b). Reward systems in the brain and nutrition. Annual Review of Nutrition, 36, 435–70. [DOI] [PubMed] [Google Scholar]
- Rolls E.T. (2017). The roles of the orbitofrontal cortex via the habenula in non-reward and depression, and in the responses of serotonin and dopamine neurons. Neuroscience and Biobehavioral Reviews, 75, 331–4. [DOI] [PubMed] [Google Scholar]
- Rolls E.T. (2019a). The cingulate cortex and limbic systems for emotion, action, and memory. Brain Structure and Function, 224, 3001–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls E.T. (2019b). The Orbitofrontal Cortex. Oxford: Oxford University Press. [Google Scholar]
- Rolls E.T. (2020). The texture and taste of food in the brain. Journal of Texture Studies, 51, 23–44. [DOI] [PubMed] [Google Scholar]
- Rolls E.T., Cheng W., Feng J. (2020). The orbitofrontal cortex: reward, emotion, and depression. Brain Communications, 2, fcaa196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls E.T. (2021a). Brain Computations: What and How. Oxford: Oxford University Press. [Google Scholar]
- Rolls E.T. (2021b). The orbitofrontal cortex, food reward, body weight, and obesity. Social Cognitive and Affective Neuroscience. 10.1093/scan/nsab044doi: 10.1093/scan/nsab044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati A.G. (2017). The evolution of primate executive function: from response control to strategic decision-making. In: Kaas, J.H., editor. Evolution of Nervous Systems, 2nd edn, Vol 3, Amsterdam: Elsevier, 423–37. [Google Scholar]
- Rosenberg M.D., Finn E.S., Scheinost D., et al. (2016). A neuromarker of sustained attention from whole-brain functional connectivity. Nature Neuroscience, 19, 165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi-Khorshidi G., Douaud G., Beckmann C.F., Glasser M.F., Griffanti L., Smith S.M. (2014). Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. NeuroImage, 90, 449–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T., Cipolotti L. (2018). The prefrontal cortex and neurological impairments of active thought. Annual Review of Psychology, 69, 157–80. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Beckmann C.F., Andersson J., et al. (2013). Resting-state fMRI in the human connectome project. NeuroImage, 80, 144–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Nichols T.E., Vidaurre D., et al. (2015). A positive-negative mode of population covariation links brain connectivity, demographics and behavior. Nature Neuroscience, 18, 1565–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D.W., Fellows L.K., Small D.M., Dagher A. (2012). Food and drug cues activate similar brain regions: a meta-analysis of functional MRI studies. Physiology and Behavior, 106, 317–24. [DOI] [PubMed] [Google Scholar]
- Tregellas J.R., Wylie K.P., Rojas D.C., et al. (2011). Altered default network activity in obesity. Obesity (Silver Spring), 19, 2316–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidaurre D., Abeysuriya R., Becker R., et al. (2018). Discovering dynamic brain networks from big data in rest and task. NeuroImage, 180, 646–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Z., Rolls E.T., Cheng W., Feng J. (2020). Sensation-seeking is related to functional connectivities of the medial orbitofrontal cortex with the anterior cingulate cortex. NeuroImage, 215, 116845. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data for the UK Biobank are available at https://www.ukbiobank.ac.uk/ and for the Human Connectome Project at https://www.humanconnectome.org/. Standard code functions available in Matlab and SPM were used.


