Abstract
We performed a quality improvement intervention to increase universal lipid screening in well-child visits (age 9 to 11 years): 12-month preintervention; phase 1 (8 months) with provider education, group monthly chart review with feedback, and electronic health record cues to order lipids; and phase 2 (16 months) with electronic health record cues and examination room phlebotomy. Outcomes were compared with clinics having no intervention. In phase 1, immediate treatment effect on the regression line for provider behavior (proportion of visits with lipids ordered) showed 34% increase in intervention and 7% decrease in comparison clinics; patient behavior (phlebotomy completed) showed 19% increase in intervention and 5% decrease in comparison clinics. At the beginning of phase 2, the intervention clinic had average 44% orders entered and 33% phlebotomy completed per well-child visit, and these proportions were maintained. Provider education and chart review with feedback were associated with the greatest gains in outcomes.
Keywords: cardiovascular, pediatrics, prevention, cholesterol
Introduction
Cardiovascular disease remains the leading cause of death in the United States, despite a 50% decrease since 1980.1 Statistical modeling suggests that half of the decrease was caused by a reduction in risk factors resulting from improved health-related behaviors.2 The clinical manifestations of atherosclerosis may not be apparent until adulthood, but the pathophysiologic processes begin early in childhood, and dyslipidemia in older children and teens is associated with elevated intima-media thickness in adults.3,4 In patients who have heterozygous familial hypercholesterolemia (HeFH; frequency, 1 in 250 people), 25% of girls and 50% of boys are predicted to have their first cardiovascular event by age 50 years, but only 25% of children with HeFH are diagnosed by middle age.5-7 Other childhood dyslipidemias that are more frequent than HeFH may involve environmental, disease, and genetic risk factors. Dyslipidemia may be present in 20% of US children aged 8 to 17 years.8 Universal screening is needed because the use of family history to determine the need for lipid screening has low sensitivity and specificity.9
In 2011, the National Heart, Lung, and Blood Institute (NHLBI) published guidelines to reduce cardiovascular risk in children, including the recommendation to screen all children aged 9 to 11 years with a nonfasting lipid panel.10 In 2014, the American Academy of Pediatrics incorporated the NHLBI recommendation into the Bright Futures screening guidelines for well-child care.11 Universal screening guidelines for children aged 9 to 11 years remained in the Bright Futures guidelines even after the United States Preventive Services Task Force published a statement that there was insufficient evidence to balance the benefits and harms of universal screening.5 Such evidence is difficult to obtain because the time between childhood screening and an adult cardiovascular event is too long to enable the use of classic study designs.12
Despite publication of the guidelines for childhood universal lipid screening, adherence to the guidelines has been low, and <10% of children aged 9 to 11 years are screened.13,14 The reasons for poor adherence to guidelines are multifactorial, including controversy about the evidence, lack of familiarity of health care providers with screening and treatment guidelines, and barriers to obtaining blood samples.15-17
We hypothesized that a quality improvement (QI) intervention would increase the frequency of universal lipid screening in well-child visits (WCV) in children aged 9 to 11 years. In this article, we report the results of a study to evaluate the effect of a 2-phase QI intervention on provider and patient adherence to the guideline to screen all children aged 9 to 11 years with a nonfasting lipid panel.
Methods
This study was performed from January 2014 to December 2016 at University Pediatric Clinic, an academic pediatric hospital–based clinic with 10 faculty members and 24 residents who provided 17 000 child visits per year. We compared the results at the intervention clinic with those of 27 primary care clinics (control) in the same health care system (24 community practices, 2 academic family practices, and 1 academic pediatric practice). None of the comparison clinics had implemented QI projects to address pediatric lipid screening during the study period. The university institutional review board reviewed and approved the study.
The preintervention period (defined as baseline) was from January to December 2014. The intervention was designed by faculty members who identified key drivers and several barriers to lipid screening in the clinic, including the barrier that patients had to go to a location outside the clinic for phlebotomy.
The intervention focused on universal nonfasting lipid screening of children aged 9 to 11 years. The intervention was implemented in 2 phases: phase 1 (8 months; January to August 2015) included provider education and group monthly chart review with feedback (beginning in January 2015), and addition of electronic health record (EHR) cues to order lipids (beginning in February 2015); and phase 2 (16 months; September 2015 to December 2016) included the continuation of EHR cues and addition of phlebotomy in patient examination rooms in the clinic rather than an external laboratory. Provider education included a presentation of the NHLBI guidelines for universal screening to the faculty. Chart review occurred at monthly faculty meetings and included group review of a monthly report that included the names of patients aged 9 to 11 years who were seen for a WCV during the previous month, the name of the provider who saw the patient, lipid test orders and results, and medical record documentation by the provider about lipid screening. Providers reviewed results of all providers to promote transparency. The EHR cues to order lipids were added toward the end of age-appropriate WCV encounters, including EHR buttons for deferred, ordered today, or previous results. Clicking on previous results cued the provider to review the result in the EHR laboratory results section and confirm the review by classifying the result as normal, low, or high in the WCV encounter.
Implementation of in-room phlebotomy began with inservice training of all 10 medical assistants. Training included a lecture, practice drawing blood on artificial phlebotomy training arms (Male Multi-Venous IV Training Arm and Pedi Multi-Venous IV Training Arm, Laerdal Medical, Wappinger Falls, NY), and practicing on fellow medical assistants. After completing the in-service training, the medical assistants gained experience by performing in-clinic phlebotomy for older teens and progressed to providing phlebotomy for younger children.
We defined provider behavior as a provider entering a lipid panel order and patient behavior as a patient having phlebotomy for the lipid test. The study denominator was the number of eligible WCVs of patients aged 9 to 11 years; children with a previous lipid result at any age were excluded. For example, a child’s 11-year-old WCV was not included in the denominator if she had had a lipid panel performed previously.
We analyzed the proportion of WCVs with lipids ordered (provider behavior) and phlebotomy done (patient behavior) using interrupted time series.18 The immediate treatment effect was defined as the difference between the outcome (proportion of WCV with orders) value on the regression line for the first month after starting an intervention minus the outcome value on the regression line that would have occurred at the same time had the intervention not begun (ie, 1-month extrapolation of the preintervention regression line).18 To assess the effect of in-room phlebotomy, the proportions of phlebotomy completed per orders were compared before and after the implementation of in-room phlebotomy with χ2 test.
Results
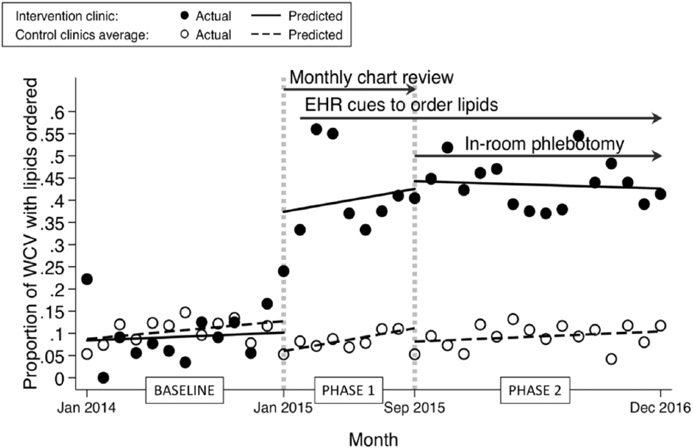
Lipid panels were ordered for 286 of the 895 eligible WCVs at the intervention clinic (32%) and 661 of the 6862 WCVs at the comparison clinics (10%; Table 1). In the first month of the baseline period, the interrupted time series of the proportion of WCVs with lipids ordered (provider behavior) showed no significant difference between intervention vs comparison clinics, with an average of 0.087 WCV with orders in the comparison clinics compared with an average of 0.084 in the intervention clinic (intervention minus control, −0.003 WCV with orders; CI [confidence interval] = −0.08 to +0.07; P = .93; Figure 1). The baseline effect was a nonsignificant increase of 0.002 orders/WCV/month in the intervention clinic and 0.003 orders/WCV/month in the control clinics (difference, −0.002; CI = −0.012 to +0.009; P = .72). The immediate treatment effect on the intervention regression line (interruption of the line) at the start of phase 1 showed an average of 0.34 orders/WCV (CI = 0.17 to 0.51; P < .001). Provider education and chart review with feedback at the beginning of phase 1 were associated with the greatest gains in outcomes. The average proportion of orders per WCV at the beginning of phase 2 in the intervention clinic was 44% compared with 8% in the comparison clinics, and these proportions did not change significantly throughout phase 2. There were no differences in trend (slope of the regression line) between intervention and control in all phases of the study (Figure 1).
Table 1.
Lipid Screening in Well-Child Visits in Patients Aged 9 to 11 Yearsa.
| Variable | Intervention Clinic |
Comparison Clinics |
||||
|---|---|---|---|---|---|---|
| Baseline | Phases 1 and 2 | Total | Baseline | Phases 1 and 2 | Total | |
| Unique patients | 275 | 547 | 822 | 2048 | 4247 | 6295 |
| Eligible well-child visits | 278 | 617 | 895 | 2068 | 4794 | 6862 |
| Lipid screening orders | 24 | 262 | 286 | 223 | 438 | 661 |
| Laboratory results | 11 | 181 | 192 | 98 | 245 | 343 |
Data reported as number during the 3-year study (January 2014 to December 2016). Baseline was the preintervention period (12 months); phase 1 (8 months) included provider education, group monthly chart review with feedback, and electronic health record cues to order lipids; and phase 2 (16 months) included electronic health record cues and examination room phlebotomy. The intervention clinic was an academic pediatric hospital–based clinic that had 10 faculty members and 24 residents; comparison clinics were 27 primary care clinics within the same health care system as the intervention clinic.
Figure 1.
Interrupted time series showing the relation between quality improvement interventions and lipids ordered (provider behavior) in well-child visits (WCVs) in patients aged 9 to 11 years. Baseline was before intervention (January to December 2014). During phase 1 (January to August 2015), the intervention clinic had provider education, group monthly chart review with feedback, and, starting 1 month into phase 1, electronic health record (EHR) cues to order lipids. During phase 2 (September 2015 to December 2016), the intervention clinic had continuing EHR cues and addition of in-room phlebotomy. Intervention clinic, filled circles; comparison (control) clinics, open circles.
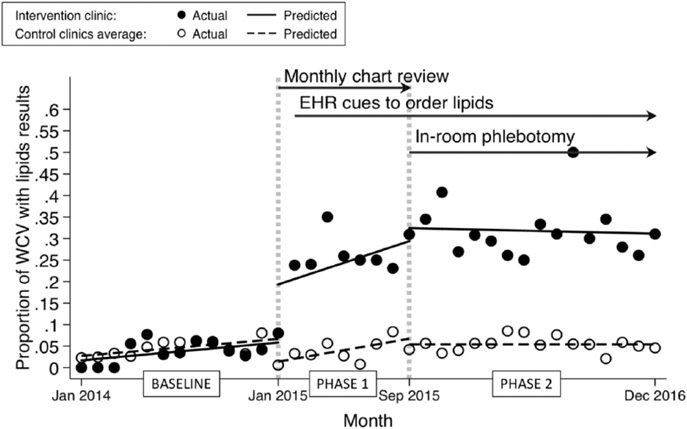
Interrupted time series analysis of completed phlebotomies (patient behavior) showed similar results, with a 19% immediate effect increase associated with the intervention at the beginning of phase 1 (CI: 0.1 to 0.3; P < .001) compared with a 5% decrease in the comparison clinics (Figure 2). There were no differences in trend (slope of the regression line) for patient behavior between intervention and comparison clinics throughout the study. The average proportion of phlebotomy completed per WCV at the intervention clinic at the beginning and throughout phase 2 was 33% compared with 5% at the comparison clinics, and these proportions did not change significantly throughout phase 2. In the intervention clinic, the proportion of orders that resulted in phlebotomy increased from baseline and phase 1 combined (52 phlebotomies in 95 orders [55%]) to phase 2 (140 phlebotomies in 191 orders [73%]) after implementation of in-room phlebotomy (33% increase; P = .002).
Figure 2.
Interrupted time series showing the relation between quality improvement interventions and phlebotomy done (patient behavior, lipid results obtained) in well-child visits (WCVs) in patients aged 9 to 11 years. Baseline was before intervention (January to December 2014). During phase 1 (January to August 2015), the intervention clinic had provider education, group monthly chart review with feedback, and, starting 1 month into phase 1, electronic health record (EHR) cues to order lipids. During phase 2 (September 2015 to December 2016), the intervention clinic had continuing EHR cues and addition of in-room phlebotomy. Intervention clinic, filled circles; comparison (control) clinics, open circles.
Discussion
This quality improvement intervention to increase universal screening of children for dyslipidemia resulted in sustained increases in the proportions of orders for lipid panels and completion of phlebotomy. Provider education and chart review with feedback were associated with the greatest gains in these measures. Although improvements were sustained, universal screening was not achieved.
The observation that providers entered orders in fewer than half of the visits despite education and feedback may be due to the lack of consensus regarding universal screening among experts,19-22 discomfort with managing abnormal results, inadequate referral resources for preventive cardiology, exercise, and nutrition support, and lack of clarity about the strength of association between child and adult cardiovascular risk. Patients may not follow through with provider orders because of the absence of cardiovascular symptoms in childhood and lack of public health programs to educate the public about cardiovascular risk for children. Aversion to phlebotomy by parents and children has not been reported as a barrier but was apparent during implementation; use of finger stick technology might reduce the barrier to phlebotomy and improve compliance.17 The 3-year span available for screening in patients aged 9 to 11 years makes 100% screening per WCV unlikely, because parents of younger children may elect to delay phlebotomy until the child is aged 11 years.
Limitations of the present study included the implementation of the study interventions only at 1 academic pediatric clinic that had a history of participation in QI projects, which may limit generalizability to other types of primary care practices. In addition, 2 faculty members retired from practice during the study, and the replacement providers did not participate fully in phase 1 education and feedback.
In summary, this QI intervention increased adherence to lipid screening guidelines for children aged 9 to 11 years, but there were persistent barriers to full participation. Creative modeling to assess the effect of childhood interventions on adult-onset disease may improve provider and parent awareness of the importance of addressing pediatric precursors of adult cardiovascular disease.23
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Roth GA, Dwyer-Lindgren L, Bertozzi-Villa A, et al. Trends and patterns of geographic variation in cardiovascular mortality among US counties, 1980-2014. JAMA. 2017;317:1976–1992. doi: 10.1001/jama.2017.4150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in US deaths from coronary disease, 1980-2000. N Engl J Med. 2007;356:2388–2398. doi: 10.1056/NEJMsa053935 [DOI] [PubMed] [Google Scholar]
- 3.Berenson GS, Srinivasan SR, Bao W, Newman WP 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302 [DOI] [PubMed] [Google Scholar]
- 4.McGill HC Jr, McMahan CA, Zieske AW, et al. Association of coronary heart disease risk factors with microscopic qualities of coronary atherosclerosis in youth. Circulation. 2000;102:374–379. doi: 10.1161/01.CIR.102.4.374 [DOI] [PubMed] [Google Scholar]
- 5.US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, et al. Screening for lipid disorders in children and adolescents: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:625–633. doi: 10.1001/jama.2016.9852 [DOI] [PubMed] [Google Scholar]
- 6.de Ferranti SD, Rodday AM, Mendelson MM, Wong JB, Leslie LK, Sheldrick RC. Prevalence of familial hypercholesterolemia in the 1999 to 2012 United States National Health and Nutrition Examination Surveys (NHANES). Circulation. 2016;133:1067–1072. doi: 10.1161/CIRCULATIONAHA.115.018791 [DOI] [PubMed] [Google Scholar]
- 7.Neil HA, Hammond T, Huxley R, Matthews DR, Humphries SE. Extent of underdiagnosis of familial hypercholesterolaemia in routine practice: prospective registry study. BMJ. 2000;321:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kit BK, Kuklina E, Carroll MD, Ostchega Y, Freedman DS, Ogden CL. Prevalence of and trends in dyslipidemia and blood pressure among US children and adolescents, 1999-2012. JAMA Pediatr. 2015;169:272–279. doi: 10.1001/jamapediatrics.2014.3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ritchie SK, Murphy EC, Ice C, et al. Universal versus targeted blood cholesterol screening among youth: the CARDIAC project. Pediatrics. 2010;126:260–265. doi: 10.1542/peds.2009-2546 [DOI] [PubMed] [Google Scholar]
- 10.National Heart, Lung, and Blood Institute. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents. Summary Report NIH Publication No.12-7486. Bethesda, MD: National Institutes of Health; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simon GR, Baker C, Barden GA 3rd, et al. 2014 Recommendations for pediatric preventive health care. Pediatrics. 2014;133:568–570. doi: 10.1542/peds.2013-4096 [DOI] [PubMed] [Google Scholar]
- 12.Urbina EM, de Ferranti SD. Lipid screening in children and adolescents. JAMA. 2016;316:589–591. doi: 10.1001/jama.2016.9671 [DOI] [PubMed] [Google Scholar]
- 13.Margolis KL, Greenspan LC, Trower NK, et al. Lipid screening in children and adolescents in community practice: 2007 to 2010. Circ Cardiovasc Qual Outcomes. 2014;7:718–726. doi: 10.1161/CIRCOUTCOMES.114.000842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valle CW, Binns HJ, Quadri-Sheriff M, Benuck I, Patel A. Physicians’ lack of adherence to National Heart, Lung, and Blood Institute Guidelines for pediatric lipid screening. Clin Pediatr (Phila). 2015;54:1200–1205. doi: 10.1177/0009922815576885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stipelman C, Young PC, Hemond J, Brown LL, Mihalopoulos NL. Dyslipidemia screening of 9- to 11-year-olds at well-child visits by Utah pediatricians. Clin Pediatr (Phila). 2017;56:1286–1290. doi: 10.1177/0009922816684601 [DOI] [PubMed] [Google Scholar]
- 16.Dixon DB, Kornblum AP, Steffen LM, Zhou X, Steinberger J. Implementation of lipid screening guidelines in children by primary pediatric providers. J Pediatr. 2014;164:572–576. doi: 10.1016/j.jpeds.2013.10.027 [DOI] [PubMed] [Google Scholar]
- 17.de Ferranti SD, Rodday AM, Parsons SK, et al. Cholesterol screening and treatment practices and preferences: a survey of United States pediatricians. J Pediatr. 2017;185:99–105.e2. doi: 10.1016/j.jpeds.2016.12.078 [DOI] [PubMed] [Google Scholar]
- 18.Linden A. Conducting interrupted time-series analysis for single- and multiple-group comparisons. Stata J. 2015;15: 480–500. [Google Scholar]
- 19.Resnicow K, Berenson G, Shea S, Srinivasan S, Strong W, Wynder EL. The case against “the case against childhood cholesterol screening.” JAMA. 1991;265:3003–3005. doi: 10.1001/jama.1991.03460220091039 [DOI] [PubMed] [Google Scholar]
- 20.McCrindle BW, Kwiterovich PO, McBride PE, Daniels SR, Kavey RE. Guidelines for lipid screening in children and adolescents: bringing evidence to the debate. Pediatrics. 2012;130:353–356. doi: 10.1542/peds.2012-1137 [DOI] [PubMed] [Google Scholar]
- 21.Newman TB, Pletcher MJ, Hully SB. Overly aggressive new guidelines for lipid screening in children: evidence of a broken process. Pediatrics. 2012;130:349–352. doi: 10.1542/peds.2012-0481 [DOI] [PubMed] [Google Scholar]
- 22.Benuck I, McBride PE. Universal lipid screening: response regarding implications for primary care practice. Pediatrics. 2013;131:e1386–e1387. doi: 10.1542/peds.2012-3818C [DOI] [PubMed] [Google Scholar]
- 23.Wise PH. The rebirth of pediatrics. Pediatrics. 2009;123:413–416. doi: 10.1542/peds.2008-3254 [DOI] [PubMed] [Google Scholar]