Abstract
Purpose: The purpose of this article is to assist the pharmacist engaged in nutrition support therapy in staying current with pertinent literature. Methods: Several clinical pharmacists engaged in nutrition support therapy compiled a list of articles published in 2022 considered important to their clinical practice. The citation list was compiled into a spreadsheet where the author participants were asked to assess whether the article was considered important to nutrition support pharmacy practice. A culled list of publications was then identified whereby at least 5 out of the 8 author participants considered the article to be important. Guideline and consensus papers, important to practice but not ranked, were also included. Results: A total of 162 articles were identified; 8 from the primary literature were voted by the group to be of high importance. An additional 10 guidelines, position, recommendation, or consensus papers were also identified. The top-ranked articles from the primary literature were summarized and a narrative regarding its implications to pharmacy nutrition support practice were provided. Conclusion: We recommend that pharmacists engaged in nutrition support therapy be familiar with these articles as it pertains to their practice.
Keywords: fluid and electrolyte disorders, gastrointestinal disorders, intravenous therapy, nutrition, nutritional support, vitamins, pharmacists education
Introduction
Keeping up with the literature is an essential requirement for maintaining an evidence-based clinical practice. However, staying current, within a specialized field such as pharmacy nutrition support, is challenging. Many institutions have adopted an integrated practice model whereby the pharmacist provides pharmacotherapy services in addition to specialized services. Clinicians are held accountable for having expertise in numerous therapeutic areas that interface with their clinical practice. Because new knowledge for pharmacists engaged in nutrition support is often integrated within differing clinical practices and numerous journals, it is a daunting task for an individual to screen the abundance of journals to seek out those clinical studies, position papers, or clinical guidelines that may enhance or change their current clinical practice. For the past several years, as clinicians who practice in pharmacy nutrition support, it has been our intent to provide a yearly source of new literature important to pharmacists. This manuscript identifies and discusses significant articles that were published in 2022.
Methods
To assist pharmacists engaged in nutrition support in staying current with the most pertinent literature, the corresponding author (R.N.D.) invited 7 clinical pharmacists to participate in this project. All participants are board certified nutrition support pharmacists. Some authors have multiple board certifications including critical care, pharmacotherapy, and pediatrics. The duration of individual practice experience of the authors ranges from 8 years to more than 30 years post-training. Members of this authorship group have advanced practice roles with direct patient care responsibilities for prescribing parenteral nutrition (PN) and/or enteral nutrition (EN), laboratory analysis, and pharmacotherapy integrated with nutrition therapy (eg, fluid and electrolytes, vitamins, trace elements, prokinetic drugs, insulin, antidiarrheal, and laxative therapy), and some have administrative or supervisory roles with respect to nutrition support therapies. This authorship group has a broad range of practice experiences. Most authors are acute care-based, but some have long-term care (home PN and EN) responsibilities. Current practices of the group range from pediatrics to geriatrics. Some members have a clinical practice with a diverse patient population, whereas others are within a focused patient population (eg, pediatrics, oncology, trauma).
A modified Delphi approach was used to identify articles published from January 2022 to December 2022 that resulted in a change or affirmation of their current clinical practice or considered to be important for future clinical practice. Some authors collated papers from their personal journal subscriptions, organizational membership newsletters, or institutional-based journal clubs. Others included those articles retrieved from an online search strategy. This methodology for collection of articles has the limitation that the process for identifying significant articles lacked a consistent structured literature search strategy. As a result, it is possible that pertinent articles applicable to pharmacy nutrition support practice that were published in less common journals were not identified.
Only those articles available in print format were allowed for potential inclusion. Articles available only in preprint electronic format (with intention of print publication by the journal in 2023) were not evaluated. The citation list was compiled into a spreadsheet whereby the author participants were asked to denote whether the paper was considered important to pharmacy nutrition support practice. An abstract and complete citation of each paper was provided along with the electronic scoring spreadsheet to assist the author participants with the evaluation process. To ensure an independent voting process without influence from the other participant members, only the corresponding author was aware of others’ rankings. To prevent influence from the other authors on the corresponding author, dichotomous grading of all articles as important or not by the corresponding author was completed prior to review of the results from the other contributors. The votes were tallied. The article was considered most important if at least 5 out of 8 participants voted for a paper to be valuable. From this scoring system, a culled list of the most important articles was created.
Results
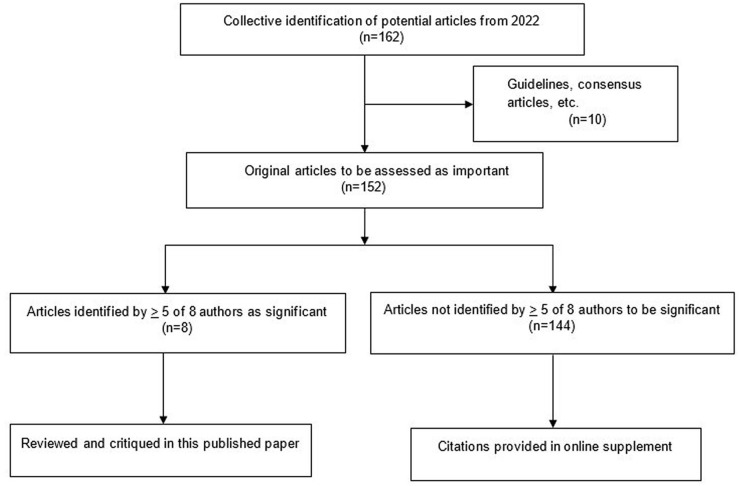
A total of 162 articles were collated for initial evaluation by the authorship group. Ten articles comprising guidelines, consensus, recommendation, and position papers were excluded from the ranking process.1 -10 An average of 18 articles (range: 11-25) from the primary literature were denoted as important by individual members of the author group. Eight papers from the primary literature (receiving 5 or more votes) were collectively identified as the most important by the author group.11 -18 Results from the sorting process are depicted in Figure 1.
Figure 1.
Flow diagram of identification and sorting of significant articles published in 2022 for pharmacy nutrition support practice.
Of the 18 total primary literature and guidelines/consensus articles, 6 were published in the Journal of Parenteral and Enteral Nutrition, 4 in Clinical Nutrition, 2 in Nutrition in Clinical Practice, 2 in New England Journal of Medicine, and 4 articles from assorted medical, critical care, pediatric, and pharmacy journals. The finalist publications from the primary literature are summarized in the discussion along with a narrative regarding their implications for pharmacy nutrition support practice. A list of guidelines, position, recommendation, and consensus articles are provided in Table 1. The remaining 144 citations from the primary literature not making the final selection are provided in an online supplement.
Table 1.
Guidelines, Position, Recommendation, and Consensus Articles (in Alphabetical Order by First Author).
| First author | Title |
|---|---|
| Adams et al 1 | Safe care transitions for patients receiving parenteral nutrition |
| Bechtold et al 2 | When is enteral nutrition indicated? |
| Berger et al 3 | ESPEN micronutrient guideline |
| Bischoff et al 4 | European guideline on obesity care in patients with gastrointestinal and liver diseases—Joint ESPEN/UEG guideline |
| Boullata et al 5 | Parenteral nutrition compatibility and stability: A comprehensive review |
| Cárdenas et al 6 | Nutritional care is a human right: Translating principles to clinical practice |
| Compher et al 7 | Guidelines for the provision of nutrition support therapy in the adult critically ill patient: The American Society for Parenteral and Enteral Nutrition |
| Donini et al 8 | Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement |
| Meek and Noble 9 | Policy statement: Breastfeeding and the use of human milk |
| Volkert et al 10 | ESPEN practical guideline: Clinical nutrition and hydration in geriatrics |
Discussion
The 8 finalist articles, listed in alphabetical order by the first author, are summarized and critiqued below.
Alvira-Arill et al 11 Comparison of catheter-related bloodstream infection rates in pediatric patients receiving parenteral nutrition with soybean oil-based intravenous fat emulsion versus a mixed oil fat emulsion.
PN remains a risk factor for catheter-related bloodstream infections (CR-BSIs) and inclusion of intravenous lipid emulsion (ILE) is thought to increase its incidence. This retrospective cohort study evaluated 1131 pediatric patients exposed to ILE in either the inpatient or outpatient setting and compared rates of CR-BSIs between those receiving a soybean oil-based ILE (SO-ILE) to those receiving a mixed-oil-ILE (MO-ILE). There were 743 patients exposed to SO-ILE and 450 patients exposed to MO-ILE. A significantly higher rate of CR-BSI was found among patients receiving SO-ILE. The total average rates of CR-BSI were 7.33/1000 days and 4.52/1000 days with SO-ILE and MO-ILE, respectively. The rate of CR-BSI with coagulase-negative Staphylococcus (CoNS) was also significantly higher in the SO-ILE group in comparison to the MO-ILE group [3.58/1000 days vs 1.39/1000 days, respectively]. Utilizing adjusted models, patients receiving MO-ILE were 73% less likely to have a CoNS CR-BSI and 40% less likely to have any CR-BSI than patients who received SO-ILE. The overall difference in CR-BSIs was primarily the result of the difference in infection with CoNS in patients who received MO-ILE instead of SO-ILE.
This study is limited by its retrospective nature, but the protocols in place for preventing CR-BSIs did not change throughout the study period. It was conducted in a single pediatric center with limited direct management of hematology/oncology patients and may not be applicable to other sites or all patient groups receiving PN. An increased incidence of infection was associated with receiving PN in the inpatient compared to the outpatient setting, which could be further explored. Most patients receiving PN in the outpatient setting received prophylactic ethanol lock therapy which may have impacted the rates of CR-BSI and is no longer routinely being used by most institutions due to high cost. The authors recommend further investigation of how the impact ILE source plays on the biology of CoNS and its potential promotion of biofilm formation as a risk factor to developing CR-BSIs.
Bloomfield et al 12 Early amino acids in extremely preterm infants and neurodisability at 2 years.
The intake of protein at doses exceeding the international standard 3.5 to 4 g/kg/day in extremely preterm infants within the first few days of life has been shown in observational studies to improve growth and neurodevelopment. This multi-center, parallel-group, double-blind, randomized, placebo-controlled trial conducted in New Zealand and Australia evaluated supplementation of parenteral amino acids in 434 infants weighing less than 1000 g and with an existing umbilical arterial catheter in place. Patients were enrolled within 24 hours after birth and randomized 1:1 to receive either amino acid 1 g/day (intervention) or 0.45% sodium chloride (placebo) infused continuously for 5 days through the umbilical arterial catheter. All patients received standard PN or EN therapy. Patients received a mean parenteral amino acid intake during the first 7 days after birth of 3.4 ± 0.6 g/kg/day in the intervention group and 2.6 ± 0.6 g/kg/day in the placebo group. There was no difference in survival without neurodisability at 2 years of age (corrected for gestational age at birth) between groups. Bayley-III composite scores for language were significantly different and favored the placebo group. Moderate-to-severe neurodisability and cognitive delay were more common in the intervention group, but neither were statistically significant.
This study is limited by the selection of preterm neonates at highest risk for severe illness due to the requirement of an umbilical artery catheter for administration of the intervention or placebo. Sicker patients tend to have delayed EN that would necessitate use of PN. Patients in the intervention group received parenteral amino acid within the currently recommended 3.5 to 4 g/kg/day for extremely low birth weight neonates. In a post hoc analysis, the investigators did find an increased incidence of refeeding syndrome in the intervention group. This is consistent with reports of neonatal refeeding syndrome associated with enhanced nutrition support in preterm neonates and inadequate phosphorus supplementation. 19 The authors commented that neurodevelopmental assessment at 2 years corrected age weakly correlates with outcomes later in life and therefore follow-up at 6 to 7 years of age in this study’s patient population is ongoing.
Finfer et al 13 Balanced multielectrolyte solution versus saline in critically ill adults.
Recent research suggests that balanced multi-electrolyte solutions (BMES) may reduce the risk of acute kidney injury (AKI) and death in critically ill patients versus 0.9% sodium chloride. However, the optimal IV fluid choice remains controversial. Plasma-Lyte 148 versus Saline (PLUS) is a double-blind, parallel-group, randomized trial aimed to compare Plasma-Lyte 148, a BMES, versus saline on 90-day mortality in critically ill adult patients. The total volume of crystalloids infused was based on the clinician’s judgment. A total of 4702 patients across 53 intensive care unit (ICU) sites in Australia and New Zealand who met criteria for IV fluid resuscitation were included in the final analysis. The median total volume of trial fluids infused in the first 7 days was similar between the saline and BMES groups (3.7 L vs 3.9 L). Serum chloride concentrations were consistently lower with BMES, but the difference did not translate to any changes in arterial blood pH or survival benefits at 90 days. Subgroup analysis failed to demonstrate any differences on mortality risk based on age, sex, presence of kidney injuries, sepsis, surgical cause for ICU admission, or acute physiology and chronic health evaluation (APACHE) II. For secondary endpoints, the incidence of AKI was similar between the 2 groups with comparable changes in the peak and pattern of serum creatinine concentration changes during the first 7 days after randomization. No difference was observed regarding receipt of new renal replacement therapy, vasopressor requirements, duration of mechanical ventilation, incidence of organ failure, and days alive outside the ICU and hospital.
These results differ from the single-center Isotonic Solutions and Major Adverse Renal Events Trial (SMART), which showed that BMES reduced the composite outcome of death from any cause, new renal replacement therapy, or persistent renal dysfunction compared to saline. 20 Whether the pragmatic trial design in SMART or the conventional RCT approach in PLUS better represent real life scenario can be debated. The definitions and methods to quantify AKI are different between these 2 trials: PLUS used peak and rise in serum creatinine concentrations whereas SMART evaluated persistent renal dysfunction defined as creatinine concentrations exceeding 2× baseline. Another difference is that the median volume of crystalloid infused was one-third lower in SMART compared to PLUS. The BMES used differed with PLUS using exclusively Plasma-Lyte 148, whereas SMART included both Lactated Ringer’s and Plasma-Lyte A (similar to Plasma-Lyte 148). Solution differences include a notable lower sodium content (128-131 mEq/L vs 140 mEq/L), lactate versus acetate and gluconate, and the presence of calcium in Lactated Ringer’s solution. It is possible that these differences are accentuated by the total infusion volume which may influence clinical outcomes. In summary, whether use of BMES or saline is an independent factor affecting survival and renal outcomes of ICU patients remains unclear. Regardless of resuscitation fluid used, evaluation of therapy should be individualized and include monitoring of serum electrolytes, volume status, and other biochemical markers to best prevent and reverse AKI.
Flordelis Lasierra et al 14 Enteral nutrition in critically ill patients under vasoactive drug therapy: The NUTRIVAD study.
The administration of EN for critically ill patients receiving vasoactive drugs (VADs) remains controversial due to the risk of mesenteric ischemia or bowel necrosis, both associated with a high mortality. This prospective, observational study assessed the safety and tolerability of EN use in 200 medical and surgical critically ill adult patients requiring mechanical ventilation and VADs. Norepinephrine (NE) was used in all patients with an average overall dose of 0.29 mcg/kg/min. Dobutamine was added to NE in about 40% of patients with an average overall dose of 2.84 mcg/kg/min. Fourteen patients (7%) required three concurrent VADs. EN was started on average within 34 hours of ICU admission with mean energy delivery of 1159 kcal/day and mean protein delivery of 56 grams/day. A higher sequential organ failure assessment (SOFA) score, NE dose (>0.5 mcg/kg/min) and blood lactate concentration (>3 mmol/L) in the first 48 hours of ICU were independently associated with lower EN provision. While 77% of patients experienced EN-related adverse effects (high gastric residual volumes, abdominal distension, nausea, vomiting, diarrhea), only 1 patient (0.5%) experienced confirmed mesenteric ischemia. SOFA score was found to be a significant risk factor for greater EN-related complications requiring feeding interruption.
The authors concluded that EN use in the mechanically ventilated, critically ill patient requiring VAD treatment is feasible and safe after resuscitation/hemodynamic stabilization is achieved and with close monitoring for intestinal ischemia. The low incidence of confirmed intestinal ischemia (0.5%) in this study reflects other published results, except for those found in NUTRIREA-2 where intestinal ischemia was higher. 21 The difference may be due to the early and more aggressive feeding strategies used in the NUTRIREA-2 study. While limitations of this study include its small sample size, observational design, and lack of standardized feeding protocols amongst sites, this study does support safe use of EN for patients requiring VADs. It also suggests that full caloric and protein provision may not be feasible with EN alone in this patient population and may support use of SPN. Further trials using a similar study approach including clinical outcomes such as mortality and length of stay are needed.
Gao et al 15 Effect of early vs late supplemental parenteral nutrition in patients undergoing abdominal surgery: A randomized clinical trial.
Clinical practice guidelines recommend initiation of SPN for patients not meeting nutritional requirements with EN alone after 7 days of hospitalization,7,22 but the specific timing of SPN is controversial. This multicenter, open-label randomized clinical trial investigated whether early SPN (E-SPN), initiated on day 3 after surgery, or late SPN (L-SPN), initiated on day 8 after surgery, affected the incidence of nosocomial infections in patients undergoing major elective abdominal surgery. Two hundred twenty-nine patients undergoing elective gastric, colorectal, hepatic, and pancreatic resections who were nutritionally at risk (defined by the Nutrition Risk Screening, NRS-2002), with an expected postoperative hospital stay ≥7 days and had received ≤30% of target energy from EN on day 2 after surgery were enrolled. As expected, patients in the E-SPN group received higher amounts of energy and protein than the L-SPN group, though both groups received lower than recommended amounts of protein (1.02 g/kg ideal body weight vs 0.48 g/kg ideal body weight, P < .001). The E-SPN group had fewer infectious complications (8.7% vs 18.4%, P = .04), driven mostly by pneumonia and abdominal infection. No differences were found in noninfectious complications, length of stay, or hospitalization costs.
These data indicate E-SPN may be beneficial in preventing infection in patients undergoing elective abdominal surgery who poorly tolerate EN. The fact that it was conducted in a relatively healthy (87% with no comorbidities), predominantly male, Chinese cohort may limit its applicability to other more heterogeneous and critically ill patient populations. Although all participants were defined as nutritionally at risk, the majority (80%) were assigned the minimum NRS-2002 score of 3 (out of 7) to meet this criterion. Also of note, length of stay (approximately 17 days) is longer than that reported in many other surgical studies. Additional data in other select patient populations is warranted to determine the appropriate role and timing for SPN.
Gundogan et al 16 Serum micronutrient levels in critically ill patients receiving continuous renal replacement therapy: A prospective, observational study.
Critically ill patients are at risk of micronutrient deficiencies due to increased losses, decreased intake and increased requirements due to severity of illness. Patients requiring continuous renal replacement therapy (CRRT) may be at an even higher risk of micronutrient deficiencies, as CRRT removes micronutrients easily due to their low molecular weight. 3 This prospective study evaluated serum concentrations of pyridoxine, folic acid, ascorbic acid, chromium, copper, selenium, and zinc in 50 critically ill patients receiving CRRT. Blood samples for micronutrient testing were taken 10 to 15 minutes prior to CRRT initiation, at 24 and 72 hours during CRRT, and 24 and 48 hours after CRRT cessation. All patients had at least 1 micronutrient concentration below the normal range at baseline, and 96% had ≥2 micronutrient levels below the normal range during CRRT. Ascorbic acid concentrations were well below normal at baseline, decreased after CRRT initiation and remained low 48 hours after CRRT discontinuation. Pyridoxine concentrations decreased from 24 to 72 hours on CRRT but normalized after 48 hours off CRRT. Folic acid was initially normal but decreased after 72 hours of CRRT and normalized after CRRT discontinuation. Copper was low-normal at all time points. Selenium and zinc concentrations were well below the normal range at all points and did not decrease further with CRRT. Chromium concentrations were elevated at all timepoints. No correlation between c-reactive protein (CRP) and baseline micronutrient levels was observed, although CRP was significantly elevated at all data points.
This study supports evidence that serum micronutrient concentrations are commonly low in critically ill patients and may worsen during CRRT. Certain clinical practices described in this report vary significantly from typical practice in the US and may limit interpretation of the results. Of note, CRRT was initiated late in the stay (hospital day 22, ICU day 14) and continued for a median of only 2 days. At initiation of CRRT, 70% of patients were not receiving any nutrition due to uncontrolled shock. Most patients received significantly less than recommended nutrition due to multiple organ system failure and 90% died. Patients likely to be at higher risk of micronutrient deficiencies, including those with chronic renal failure, chronic liver disease, and a history of gastric bypass were excluded from study entry. Thiamine, which has been shown to be depleted in ICU patients and during CRRT, was not evaluated. 3 More research evaluating micronutrient depletion in CRRT is warranted, specifically in patients receiving standard nutrition support therapy and receiving CRRT for longer durations. Because serum micronutrient concentrations are altered in the presence of inflammation, the utility of routine laboratory assessments to determine micronutrient status during critical illness is unclear. Further strategies to guide micronutrient management during CRRT are needed.
Li et al 17 An analysis of nonnutritive calories from propofol, dextrose, and citrate among patients who are critically ill that are receiving continuous renal replacement therapy.
Dextrose-containing IV fluid or medication diluents (3.4 kcal/g), propofol (1.1 kcal/mL) and citrate (0.59 kcal/mmol) are common contributors of non-nutritive calories (NNC) in hospitalized patients and may contribute to overfeeding, especially in the ICU setting. This prospective audit conducted over the first 7 days of ICU admission in mechanically ventilated adult patients compared NNC intake in those receiving CRRT with citrate (n = 42) to those not receiving CRRT (n = 135). The majority were male medical ICU patients admitted with a pulmonary, neurological, cardiac, or sepsis diagnosis and receiving EN (only 4.5% received PN). Those patients receiving CRRT had a higher APACHE II score (P < .001) and need for vasopressor therapy (P = .039) with slightly higher admission weight (P = .044) and body mass index (P = .046), likely from fluid retention. The CRRT group received more (mean ± standard deviation) NNC calories as expected over the first 7 ICU days except day 1 (132.9 ± 21.2 kcal vs 52.9 ± 14.8 kcal; P = .068) since CRRT requires time to get implemented. The maximum NNC intakes for all 177 patients were 992 kcal of dextrose (ICU day 1), 331 kcal of citrate (ICU day 2), and 492 kcal of propofol (ICU day 4). There were no significant differences in hospital length of stay, 30-day and 90-day mortality between the CRRT and non-CRRT groups.
One concern from overfeeding an intubated ICU patient is the inability to wean from mechanical ventilation regardless of the use of CRRT. This trial did not attribute the NNC contribution specifically to duration of mechanical ventilation, but the similar hospital length of stay between groups indirectly suggests that NNC did not prolong mechanical ventilation. Whether the contribution of NNC influences overfeeding appears to also be dependent on their oral, EN or PN therapy as dextrose is the largest contributor followed by propofol and lastly, citrate (also dependent on CRRT). This study showed citrate contributed approximately 100 more kcal/day which would not significantly contribute to overfeeding. Each institution should evaluate if their citrate based CRRT therapy contains dextrose, as this could also contribute to NNC since this study used a citrate product that did not contain dextrose.
Sharif et al 18 Probiotics in critical illness: A systematic review and meta-analysis of randomized controlled trials.
Probiotics and synbiotics have been proposed to positively modulate the gut microbiome and decrease infectious complications, but the impact on clinical outcomes in critically ill patients has been inconsistent. In this systematic review and meta-analysis of randomized controlled trials comparing enteral probiotics or synbiotics to placebo or no treatment in critically ill adults or children, 65 trials included a total of 8483 patients (pediatric: 8 studies [n = 670]). Probiotic agents included in these trials contained a wide variety of organisms in differing combinations and dosages with the most common being Lacticaseibacillus rhamnosus GG (n = 3152). Probiotics and synbiotics were found to decrease ventilator-associated pneumonia, healthcare-associated pneumonia, and ICU as well as hospital length of stay. Probiotics had no effect on mortality, requirement for inotrope/vasopressor therapy, initiation of invasive mechanical ventilation, or frequency of diarrhea. Serious adverse events were documented in 18 trials with 2 trials reporting events (mesenteric ischemia [n = 9]; probiotic organism isolates from sterile sites or nonsterile sites [n = 15]).
This publication is the most comprehensive investigation of the safety and efficacy of probiotics or synbiotics in critically ill patients to date, including 35 additional studies since the last systematic review. While this investigation addresses an ongoing clinical controversy, there are some limitations. Definitive conclusions cannot be drawn due to the low or very low certainty of evidence and heterogeneity found within its systematic review format. Furthermore, there were variable outcome definitions used across studies and certain significant data were not consistently included, such as dose of probiotic and use of EN. While microbiome modulation in critical illness remains a promising area for further research, the role of probiotics and synbiotics is unclear at this time due to low certainty of evidence and significant heterogeneity of probiotic organisms and dosages studied.
Conclusion
With the large volume of publications pertinent to pharmacy nutrition support from a variety of journals, it is challenging to stay current with the literature. We have identified a select group of articles from 2022 that we consider important for pharmacists engaged in nutrition support. Although only those highest ranked articles from the primary literature were discussed, other publications may be important depending on the patient population and the role of the pharmacist at their respective institution.
Supplemental Material
Supplemental material, sj-docx-1-hpx-10.1177_00185787231161515 for Significant Published Articles in 2022 for Pharmacy Nutrition Support Practice by Roland N. Dickerson, Angela L. Bingham, Todd W. Canada, Lingtak Neander Chan, M. Petrea Cober, Sarah V. Cogle, Anne M. Tucker and Vanessa J. Kumpf in Hospital Pharmacy
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Dickerson is a speaker for Abbott Nutrition Health Institute and Baxter Healthcare Corporation. Dr. Bingham is a speaker for Abbott Nutrition Health Institute. Dr. Canada is a consultant for Fresenius Kabi. Dr. Chan has no potential or perceived conflicts of interest. Dr. Cober is a consultant for B Braun, Fresenius Kabi, Wolters Kluwer and a speaker for Baxter Healthcare Corporation. Dr. Cogle is a speaker and consultant for Fresenius Kabi. Dr. Tucker is a consultant for Up to Date, Inc. Dr. Kumpf is a consultant for Takeda Pharmaceuticals and Fresenius Kabi.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Roland N. Dickerson  https://orcid.org/0000-0002-2086-6317
https://orcid.org/0000-0002-2086-6317
Todd W. Canada  https://orcid.org/0000-0002-2704-5749
https://orcid.org/0000-0002-2704-5749
M. Petrea Cober  https://orcid.org/0000-0002-4618-4998
https://orcid.org/0000-0002-4618-4998
Vanessa J. Kumpf  https://orcid.org/0000-0002-3390-3869
https://orcid.org/0000-0002-3390-3869
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Adams SC, Gura KM, Seres DS, et al. Safe care transitions for patients receiving parenteral nutrition. Nutr Clin Pract. 2022;37(3):493-508. doi: 10.1002/ncp.10861 [DOI] [PubMed] [Google Scholar]
- 2. Bechtold ML, Brown PM, Escuro A, et al. When is enteral nutrition indicated? JPEN J Parenter Enteral Nutr. 2022;46(7):1470-1496. doi: 10.1002/jpen.2364 [DOI] [PubMed] [Google Scholar]
- 3. Berger MM, Shenkin A, Schweinlin A, et al. ESPEN micronutrient guideline. Clin Nutr. 2022;41(6):1357-1424. doi: 10.1016/j.clnu.2022.02.015 [DOI] [PubMed] [Google Scholar]
- 4. Bischoff SC, Barazzoni R, Busetto L, et al. European guideline on obesity care in patients with gastrointestinal and liver diseases - joint ESPEN/UEG guideline. Clin Nutr. 2022;41(10):2364-2405. doi: 10.1016/j.clnu.2022.07.003 [DOI] [PubMed] [Google Scholar]
- 5. Boullata JI, Mirtallo JM, Sacks GS, et al. Parenteral nutrition compatibility and stability: a comprehensive review. JPEN J Parenter Enteral Nutr. 2022;46(2):273-299. doi: 10.1002/jpen.2306 [DOI] [PubMed] [Google Scholar]
- 6. Cárdenas D, Davisson Correia MIT, Hardy G, et al. Nutritional care is a human right: translating principles to clinical practice. Nutr Clin Pract. 2022;37(4):743-751. doi: 10.1002/ncp.10864 [DOI] [PubMed] [Google Scholar]
- 7. Compher C, Bingham AL, McCall M, et al. Guidelines for the provision of nutrition support therapy in the adult critically ill patient: the American Society for Parenteral and Enteral Nutrition. JPEN J Parenter Enteral Nutr. 2022;46(1):12-41. doi: 10.1002/jpen.2267 [DOI] [PubMed] [Google Scholar]
- 8. Donini LM, Busetto L, Bischoff SC, et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Clin Nutr. 2022;41(4):990-1000. doi: 10.1016/j.clnu.2021.11.014 [DOI] [PubMed] [Google Scholar]
- 9. Meek JY, Noble L. Policy statement: breastfeeding and the use of human milk. Pediatrics. 2022;150(1):e2022057988. doi: 10.1542/peds.2022-057988 [DOI] [PubMed] [Google Scholar]
- 10. Volkert D, Beck AM, Cederholm T, et al. ESPEN practical guideline: clinical nutrition and hydration in geriatrics. Clin Nutr. 2022;41(4):958-989. doi: 10.1016/j.clnu.2022.01.024 [DOI] [PubMed] [Google Scholar]
- 11. Alvira-Arill GR, Herrera OR, Tsang CCS, Wang J, Peters BM, Stultz JS. Comparison of catheter-related bloodstream infection rates in pediatric patients receiving parenteral nutrition with soybean oil-based intravenous fat emulsion versus a mixed oil fat emulsion. Pharmacotherapy. 2022;42(12):898-904. doi: 10.1002/phar.2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bloomfield FH, Jiang Y, Harding JE, Crowther CA, Cormack BE. Early amino acids in extremely preterm infants and neurodisability at 2 years. New Engl J Med. 2022;387(18):1661-1672. doi: 10.1056/NEJMoa2204886 [DOI] [PubMed] [Google Scholar]
- 13. Finfer S, Micallef S, Hammond N, et al. Balanced multielectrolyte solution versus saline in critically ill adults. New Engl J Med. 2022;386(9):815-826. doi: 10.1056/NEJMoa2114464 [DOI] [PubMed] [Google Scholar]
- 14. Flordelís Lasierra JL, Montejo González JC, López Delgado JC, et al. Enteral nutrition in critically ill patients under vasoactive drug therapy: the NUTRIVAD study. JPEN J Parenter Enteral Nutr. 2022;46(6):1420-1430. doi: 10.1002/jpen.2371 [DOI] [PubMed] [Google Scholar]
- 15. Gao X, Liu Y, Zhang L, et al. Effect of early vs late supplemental parenteral nutrition in patients undergoing abdominal surgery: a randomized clinical trial. JAMA Surg. 2022;157(5):384-393. doi: 10.1001/jamasurg.2022.0269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gundogan K, Yucesoy FS, Ozer NT, et al. Serum micronutrient levels in critically ill patients receiving continuous renal replacement therapy: a prospective, observational study. JPEN J Parenter Enteral Nutr. 2022;46(5):1141-1148. doi: 10.1002/jpen.2378 [DOI] [PubMed] [Google Scholar]
- 17. Li P, Huang Y, Wong A. An analysis of nonnutritive calories from propofol, dextrose, and citrate among patients who are critically ill that are receiving continuous renal replacement therapy. JPEN J Parenter Enteral Nutr. 2022;46(8):1883-1891. doi: 10.1002/jpen.2405 [DOI] [PubMed] [Google Scholar]
- 18. Sharif S, Greer A, Skorupski C, et al. Probiotics in critical illness: a systematic review and meta-analysis of randomized controlled trials. Crit Care Med. 2022;50(8):1175-1186. doi: 10.1097/CCM.0000000000005580 [DOI] [PubMed] [Google Scholar]
- 19. Bonsante F, Iacobelli S, Latorre G, et al. Initial amino acid intake influences phosphorus and calcium homeostasis in preterm infants—it is time to change the composition of the early parenteral nutrition. PLoS One. 2013;8(8):e72880. doi: 10.1371/journal.pone.0072880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Semler MW, Self WH, Wanderer JP, et al. Balanced crystalloids versus saline in critically ill adults. New Engl J Med. 2018;378(9):829-839. doi: 10.1056/NEJMoa1711584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reignier J, Boisrame-Helms J, Brisard L, et al. Enteral versus parenteral early nutrition in ventilated adults with shock: a randomised, controlled, multicentre, open-label, parallel-group study (NUTRIREA-2). Lancet. 2018;391(10116):133-143. doi: 10.1016/S0140-6736(17)32146-3 [DOI] [PubMed] [Google Scholar]
- 22. Weimann A, Braga M, Carli F, et al. ESPEN practical guideline: clinical nutrition in surgery. Clin Nutr. 2021;40(7):4745-4761. doi: 10.1016/j.clnu.2021.03.031 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-hpx-10.1177_00185787231161515 for Significant Published Articles in 2022 for Pharmacy Nutrition Support Practice by Roland N. Dickerson, Angela L. Bingham, Todd W. Canada, Lingtak Neander Chan, M. Petrea Cober, Sarah V. Cogle, Anne M. Tucker and Vanessa J. Kumpf in Hospital Pharmacy