Abstract
Introduction: This systematic review addresses the effects of n-3 long-chain polyunsaturated fatty acids consumption on human neurodevelopment. It evaluates articles published between 2000 and 2022 investigating the cognitive outcomes during the period of neurodevelopment: from fetal development to adolescence. For the purpose of this review the terms LC PUFA and omega-3 fatty acid will be used interchangeably. Method: Data were sourced from several major databases including PubMed (MEDLINE), Web of Science, and ProQuest Central. Randomized controlled trials (RCTs), nonrandomized controlled trials, prospective or retrospective cohort studies, and observational studies investigating the effects of omega-3 fatty acid consumption from dietary supplements, multiple-nutrient supplement, or food questionnaire on neurodevelopment were considered. Study population was separated in three developmental phases: (1) in-utero, (2) lactation/infancy, and (3) childhood/adolescence. Each article was evaluated for several key factors such as study type, type/dosage of PUFAs, number of subjects, length of intervention, participant age range, population characteristics, outcome measure (both primary/cognitive and secondary/other), results, conclusion, and confounding variables/limitations. Results: A total of 88 articles were included in the review, 69 RCTs and 19 longitudinal or observational studies. The results indicate equivocal effect of intervention, with some short-term benefits observed in the areas of visual attention, working memory, executive function, and communication. Omega-3 supplement might have a short-term positive impact on neurodevelopment in all three phases. Supplementation is recommended throughout life, rather than only during the earliest developmental stage.
Keywords: omega-3s, LC PUFAs, cognition, neurodevelopment
Although LC PUFAs supplied during infancy and in-utero seldom have shown significant cognitive effects, it appears that they do not persist at long-term follow-up assessments.
Introduction
As the most lipid-dense organ of the body, after adipose tissue, the brain requires fatty acids for purposes of fluidity, function, and structure. 1 Importantly, neither omega-3 (n-3) nor omega-6 (n-6) long-chain polyunsaturated fatty acids (LC PUFAs) can be synthesized de novo in human cells, meaning that dietary sources are important throughout life, particularly during several key, sensitive periods. Fatty acids affect white matter tracts in terms of myelination and fiber integrity, thereby influencing neural signaling. 2 Among LC PUFAs, docosahexaenoic acid, (DHA; 22:6, n-3) has received the most extensive attention throughout extant research, since it has the highest concentration in the brain; however, studies have also addressed eicosapentaenoic acid (EPA), alpha-linolenic acid (ALA), arachidonic acid (AA/ARA), and the importance of LC PUFA ratios. The frontal lobe, where complex cognitive processes occur, has a high concentration of DHA, which has a significant role in membrane fluidity and signal transmission.3-5
Westernization and the move toward diets with higher percentages of processed foods and lower consumption levels of dietary n-3 sources have led to concerns about population LC PUFA levels. Indeed, estimates of this shift suggest that traditional diets with 1:1 ratios of n-6 to n-3 PUFAs have been replaced by ratios closer to 20:1. 6
Omega-3 Fatty Acid and In-Utero Cognitive Development
The prenatal period is the most frequently cited sensitive period during which n-3 LC-PUFAs are important to cognitive development. Based on epidemiological studies conducted in regions with high levels of fish intake, researchers identified a potential correlation between the maternal consumption of EPA and DHA and factors including gestational length and birth weight. 1
During the third trimester an upsurge of PUFA accretion, primary DHA, in fetal brain matter occurs at a rate of 70 mg per day due to an increased cellular synthesis.1,7 The accumulation of LC PUFAs is particularly important in the frontal lobe and hippocampus, areas associated with higher-order cognitive functioning. 8
DHA supplementation has been previously related to extended gestational length, increased birth weight, and neurodevelopment. 9 Given that the third trimester is a significant period during which PUFAs are deposited in the fetal brain, many studies have attempted to identify deficiencies in or the effects of supplementation on preterm infants. Recently, the attention on the role of DHA in fetal development has been increased, and, in connection with concerns about consuming mercury-containing fish during pregnancy, the consumption of prenatal fish-oil and other n-3 supplements have increased.
Omega-3 Fatty Acid and Cognitive Development During Infancy
Supplementation with n-3 LC PUFAs during the infancy phase of development has its foundation in the longstanding research on the advantages of breastfeeding for cognitive development, and questions regarding whether this is due to the composition of human milk (including DHA), or other demographic factors associated with the decision to breastfeed.10,11 Studies conducted during infancy have highlighted a positive correlation between PUFA supplementation and both cognitive and visual functions due to the PUFA accretion in fetal brain and retinal tissue, Supplementation studies during infancy have largely been focused on DHA, often in combination with EPA and AA, given their role in various neural processes including synapse maturation, processing speed, and the structure of the neuronal membrane.3,11
Omega-3 Fatty Acid and Cognitive Development During Childhood and Adolescence
While the third trimester of pregnancy and first 18 months of life are particularly significant phases of n-3 accretion in brain and retinal tissue, it is important to consider the continued process of cognitive development in early childhood and adolescence, and clarify the neurodevelopmental effects of LC PUFAs in this later phase. Researchers focusing on this age group are particularly driving attention to pathologies that affect cognitive development, including attention-deficit/hyperactivity disorder (ADHD), phenylketonuria, and autism spectrum disorder (ASD).
Review Objective
Omega-3 LC-PUFAs function as cell membrane components, tissue formation and neuroprotection has been largely proved. 12 However, omega-3 supplement effect on neurodevelopment addressed in a large number of studies and reviews have reported controversial conclusions. Therefore, the need of a comprehensive review to extrapolate and evaluate data from studies published in the last 2 decade.
In this review we summaries the finding on the effect of omega-3 supplementation on cognitive development across three neurodevelopmental periods: (1) in-utero, (2) lactation/infancy, and (3) childhood and adolescence. The objective is to evaluate the efficacy of omega-3 supplementation on neurodevelopment in different brain developmental phases from in-utero to adolescence.
Methodology
For the purposes of this comprehensive review, we searched PubMed (MEDLINE), Web of Science, and ProQuest Central, using filters for peer-reviewed articles published between 2000 and 2022. Search terms included: omega-3s; docosahexaenoic acid (DHA); cognitive function; macronutrients; healthy fats; polyunsaturated fatty acids; brain health; developmental outcomes; n-3 polyunsaturated fats; LC PUFAs; dietary fats; and brain function.
Study Selection
Both the authors (DS and AS) were involved in the literature search, screening for eligible studies, and review. After articles were identified, the authors thoroughly reviewed titles, abstracts, and full texts, and selected the included ones according to the inclusion/exclusion criteria. Duplicate were eliminated. To confirm the comprehensiveness of the literature included, the Authors also consulted the reference lists of all articles published in the last 5 years, as well as previous literature review and meta-analysis articles, and used the “cited by” function in a university library database-wide search; additional abstracts were reviewed, and appropriated articles were included.
Inclusion and Exclusion Criteria
The authors defined inclusion and exclusion criteria a priori and included the following study designs: randomized controlled trials (RCTs), nonrandomized controlled trials, prospective or retrospective cohort studies, case-control studies, and observational studies. Only articles written in English published between 2000 and 2022 were included. Studies investigating the effects of omega-3 fatty acid intake from dietary supplements or multiple-nutrient supplement on cognition, or studies analyzing dietary omega-3 intake through a food questionnaire were considered. The populations included were: (i) in-utero, (ii) lactation/infancy, and (iii) childhood and adolescence. Case studies and studies in which PUFAs supplementation was not a primary (or differentiable) independent variable were not considered in this review.
Data Extraction
Included articles were analyzed and data were extracted using a custom data extraction form developed by DS and AS. The outcome of interests for the in-utero population were: communication and language, attention tasks, and visual development. For the lactation/infancy were: communication, intelligence, working memory, attention, and problem solving. For the childhood and adolescence population were: attention and executive functioning, information processing, speeds and impulsivity, memory, and cognitive pathologies.
After publication analysis, we extracted the following categories: study type, LC PUFA dosage and composition, sample number, length of intervention, subjects age, and population characteristics. Study characteristics and outcomes were also extrapolated. We reported: primary and secondary outcome (eg cognitive tests result, memory function, learning test), summary of result and conclusion, study limitation and funding sources.
Results
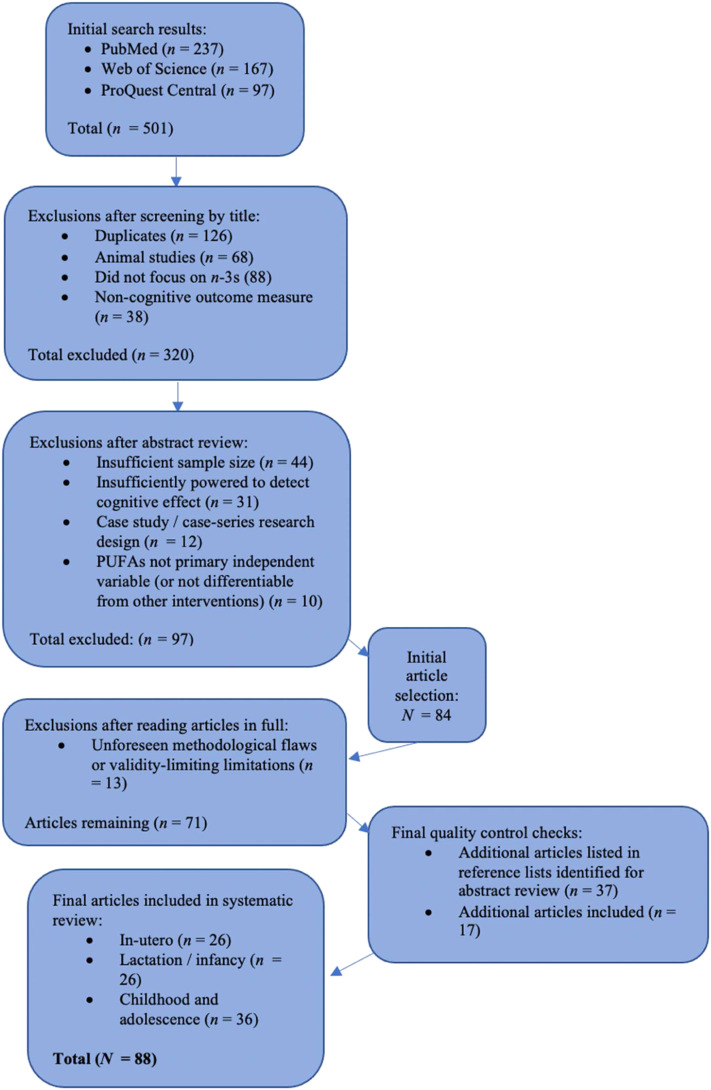
The literature search resulted in a total of 501 articles. Articles initially excluded were duplicates (n = 120), animal studies (n = 68), studies not focusing on omega-3 (88), studies with non-cognitive outcomes measure (n = 33). We reviewed the abstract of the remaining 181 articles and selected 81 pertaining to neurodevelopment to read in full. We narrowed these to a final set of 71 articles that met the inclusion criteria. To have a more comprehensive review, we expanded the search to the reference list of the included articles and reviewed an additional 34 abstracts of which 17 were included.
Of the 82 included articles, 26 investigated omega-3 prenatal supplementation (8 RCTs, 12 revision of RCTs, and 6 observational studies), 26 investigated omega-3 supplementation during infancy (19 RCT, 4 previous RCTs, and 3 observational studies), and 36 articles investigated omega-3 supplementation during childhood and adolescence (25 RCTs, 7 observational studies, and 4 secondary analyses of previous RCTs).
In-Utero Supplementation
To our knowledge, only two large scale (N > 500) studies have been conducted on humans to assess the cognitive outcomes of n-3 LC PUFA supplementation in-utero: the DOMInO trial, 13 and the POSTGRAD cohort study. 14 Study sample sizes for the remaining included publications ranged from 76 to 350.
The studies included in this review primarily assessed the effect of omega-3 DHA supplementation (n = 9). In the included studies DHA dosage levels ranged from 220 mg to 800 mg per day. Rees et al. also assessed for DHA levels, but via a food frequency questionnaire rather than a supplementation intervention. 15 In the remaining studies DHA was combined with EPA,16-19 and AA. 20 In terms of intervention lengths, the studies reviewed here featured gestational supplementation ranging from week 12 to week 22 of gestation. The studies used a broad range of cognitive outcome measures, with the Kaufman Assessment Battery for Children (K-ABC) appearing most frequently.
Importantly, while most studies cited academic and/or governmental funding sources, industry interests also appeared as financial contributors to several of the studies: a company that produces omega-3 supplements18,19,21; a dairy industry stakeholder 20 ; and a nutrition company. 15 Table 1 presents the study and population characteristics of the 26 studies included in this analysis of in-utero supplementation studies.22-32 Table 2 provides the instrumentation, results, and limitations information for the included studies.
Table 1.
In-Utero Supplementation Studies: Study Types and Participant Characteristics.
| Study type | Type/dosage of PUFAs | N | Length of intervention | Participant age range | Population characteristics | |
|---|---|---|---|---|---|---|
| Boucher et al. (2011) | Prospective, longitudinal | Fish eating community as setting; diet questionnaire | 153 | Non-intervention; 11-year follow-up | Mean = 11.3 | School-age Inuit children from Nunavik |
| Brei et al. (2017) | Follow-up of RCT | 1020 mg DHA+180 mg EPA+9 mg vitamin E | 130 | 15 weeks’ gestation through 4 months postpartum | In-utero/infancy at time of intervention; follow-up at 4-5 years | Healthy pregnant women |
| Campoy et al. (2011) | Follow-up study of the NUHEAL (Nutraceuticals for a Healthier Life) cohort | 500 mg DHA and 150 mg of EPA per day; 5-methyltetrahydro-folate (5-MTHF); both; or a placebo during pregnancy; infants in the intervention group received formula with .5% DHA and .4% AA | 270 in original study; 154 analyzed in follow-up | 20 weeks’ gestation through delivery; formula supplementation through 6 months | In-utero/infancy at time of intervention; follow-up at 6.5 years | Healthy pregnant women |
| Colombo et al. (2016) | RCT | 600 mg DHA per day | 350 randomized | Last two trimesters (14.5 weeks to delivery) | In-utero (follow-up at 4, 6, and 9 months) | n/s |
| Colombo et al. (2019) | RCT (follow-up) | 600 mg DHA per day | 78 in placebo and 83 in intervention completed 72-month follow-up | Last two trimesters (14.5 weeks to delivery) | 10 month-72-month follow-up | n/s |
| Daniels et al. (2004) | Observational | Fish intake measured by questionnaire | 7421 | Prenatal; non-intervention | Cognitive testing at 15 and 18 months | n/s |
| Dijck-Brouwer et al. (2005) | Observational | Umbilical artery and vein FA concentrations | 317 | Prenatal; non-intervention | Testing day 10-14 after birth | Born at 37-42 weeks’ gestation |
| Dunstan et al. (2008) | RCT | 1.1 g EPA and 2.2 g DHA per day | 98 | 20 weeks’ gestation through delivery | In-utero (follow-up at 2.5 years) | n/s |
| Escolano-Margarit et al. (2011) | RCT | 500 mg/d DHA +150 mg/d EPA), 400 mg/d 5-methyltetrahydrofolate, both, or placebo | 157 | 20 weeks’ gestation through delivery | In-utero (follow-up at 5.5 years) | Healthy pregnant women |
| Gould et al. (2014) | Secondary analysis of the DOMInO trial | 800 mg DHA/day | 185 | 20 weeks’ gestation through delivery | In-utero (follow-up at 27 ± 2 months) | 18-21 week-gestation singleton pregnancies; no fetal abnormalities |
| Gould et al. (2016) | Secondary analysis of the DOMInO trial | 800 mg DHA/day | 2399 randomized in original study; 646 in 4-year follow-up | 20 weeks’ gestation through delivery | In-utero (follow-ups at 18 months and 4 years) | 18-21 week-gestation singleton pregnancies; no fetal abnormalities |
| Helland et al. (2001) | RCT | 10 mL of cod liver oil (1183 mg of DHA, and 803 mg of EPA) per day | 590 recruited; 341 mothers completed intervention; 245 infants complied with follow-up request | 17-19 weeks’ gestation to 3 months after delivery | In-utero (follow-up at 6 and 9 months) | Healthy pregnant women |
| Helland et al. (2003) | RCT (follow-up—subgroup analysis) | Human milk containing 270% more DHA and 88% less AA than that of control group mothers | 76 | 18 weeks’ gestation to 3 months after delivery | In-utero and during lactation (follow-up at 4 years) | Healthy pregnant women; infants who were breastfed at 3 months of age |
| Helland et al. (2008) | RCT (follow-up) | 10 mL of cod liver oil (1183 mg of DHA, and 803 mg of EPA) per day | 590 recruited [. . . ] 84 tested for K-ABC at 4 years; 143 tested for K-ABV at 7 years | 17-19 weeks’ gestation to 3 months after delivery | In-utero (follow-up at 4 and 7 years) | Healthy pregnant women |
| Hibbeln et al. (2007) | Secondary analysis of Avon Longitudinal Study of Parents and Children | Food-frequency questionnaire | 11 875 | Non-intervention; seafood consumption at 32 weeks’ gestation assessed | In-utero; children assessed at 6, 18, 30, 42, and 81 months | n/s |
| Hurtado et al. (2015) | RCT | 400 mg DHA/day | 110 | 28th week of gestation and lactation period | In-utero and during lactation | Healthy; appropriate weight gain; no DHA supplementation during pregnancy |
| Makrides et al. (2010) | RCT (DOMInO trial) | 800 mg DHA/day | 2320 women evaluated for depressive symptoms; 694 children completed follow-up | 20 weeks’ gestation through delivery | In-utero; follow-up at 18 months | Singleton pregnancies; approached at > 21 weeks ‘gestation |
| Mendez et al. (2009) | Prospective cohort | Seafood consumption during pregnancy assessed | 392 | Non-intervention; results stratified by breastfeeding duration | Neurodevelopment assessed at age 4 | Full-term children; data on maternal diet during pregnancy |
| Mulder et al. (2018) | RCT (follow-up study) | 800 mg DHA/day | 98 in follow-up | 16 weeks’ gestation to delivery | In-utero; follow-up at 5-6 years | Follow-up only of singleton, term infants without neurological development affecting diseases |
| Oken et al. (2005) | Prospective cohort | Non-interventional; fish servings per week and hair mercury assessed | 135 | Non-intervention | In-utero; infants assessed at 6 months of age | Singleton pregnancy |
| Ostadrahimi et al. (2018) | RCT | 120 mg DHA and 180 mg EPA per day | 150 | 20 weeks’ gestation to 30 days postpartum | In-utero; follow-up at 4 and 6 months of age | Low-risk singleton pregnancy; follow-up of infants without major congenital deformities or metabolic disorders |
| Ramakrishnan et al. (2016) | RCT (follow-up of findings from the POSTGRAD study) | 400 mg DHA/day | 10 494 pregnant women randomized; 797 children included in follow-up | 18-22 weeks’ gestation through delivery | In-utero; follow-up at 5 years | n/s |
| Rees et al. (2019) | Observational | Non-intervention (food frequency questionnaire to estimate DHA levels) | 125 | Second and third trimester DHA levels assessed | In-utero; follow-up at 4.5 and 9 months | Single, healthy pregnancy; no significant health problems |
| Steer et al. (2013) | Secondary analysis of Avon Longitudinal Study of Parents and Children cohort | Maternal FAs in erythrocytes | 2839 | Non-intervention | In-utero; follow-up at 8 years | Pregnant women in Bristol; per original study |
| van Goor et al. (2010) | RCT | 220 mg per day of DHA, 220 mg of DHA and 220 mg AA per day, or a placebo | 119 | 17 weeks’ gestation through 20 weeks postpartum | In-utero; follow-up at 2 and 12 weeks postpartum | Healthy pregnant women |
| Vollet et al. (2007) | Secondary analysis of Upstate KIDS Study (birth cohort) | Non-intervention; self-reported consumption of fish oil before and during pregnancy | 5845 | Non-intervention | In-utero; children assessed from 4 months to 3 years of age | Mothers from the original study who had self-reported fish oil supplementation |
Table 2.
In-Utero Supplementation Studies: Instrumentation, Results, and Limitations.
| Outcome measures | Results | Conclusion | Confounding variables/limitations | Funding source | ||
|---|---|---|---|---|---|---|
| Primary/cognitive | Secondary/other measures | |||||
| Boucher et al. (2011) | Continuous visual recognition task | Digit span forward from Wechsler Intelligence Scales for Children, 4th edition; California Verbal Learning Test–Children’s Version | Higher levels of cord DHA concentration correlated with improved memory performance at 11 years of age | Prenatal fish consumption improves cognitive outcomes in school age, regardless of seafood contaminant amounts | Non-RCT | NIH/National Institute of Environmental Health Sciences; Northern Contaminants Program, Indian and Northern Affairs, Canada; the NIH/National Institute on Alcohol Abuse and Alcoholism; Joseph Young Sr Fund from the State of Michigan |
| Brei et al. (2017) | Child development inventory (CDI); assessment of mirror movements | No significant difference among groups on the CDI or in mirror movements | The results did not indicate either benefits or harms of a prenatal shift in n-3:n-6 ratio with supplementation | Small sample size; insufficient statistical power | Else Kroner-Fresenius Foundation, Bad Homburg; the European Union-funded Early Nutrition Programming Project consortium; German Ministry of Education and Research via the Competence Network Obesity | |
| Campoy et al. (2011) | Kaufman Assessment Battery for Children (K-ABC) | FAs in maternal blood at 20 and 30 weeks; FAs in cord blood | No significant differences among the four intervention groups in K-ABC scores; however, higher DHA in maternal erythrocytes did correlate with a higher mental processing composite score | Further research required on efficacy of supplementation beyond early childhood and optimal doses at different developmental stages | High attrition; disparities in groups’ representation of parental educational level and birth anthropometric measures | Commission of the European Community–specific Research and Technological Development Programme |
| Colombo et al. (2016) | Visual habituation (looks to habituation, look duration, sustained attention | Heart rate; task completion and fussiness | Prenatal DHA supplementation correlated with improved performance on attentional tasks during the first year of life | Results suggest attentional task benefits; further research required to determine whether prenatal supplementation benefits persist into early childhood | Failure to control for postnatal dietary DHA intake; exclusion of children born before 34 weeks’ gestation | Eunice Kennedy Shriver National Institute of Child Health and Development, the Office of Dietary Supplements, and the Kansas Intellectual and Developmental Disabilities Research Center |
| Colombo et al. (2019) | Cognitive and behavioral assessments | n/a | Maternal blood DHA during pregnancy correlated with higher verbal and full-scale IQ at 5-6 years, but this result disappeared after controlling for SES | No long-term positive effects of DHA supplementation | Both intervention and control groups may have achieved sufficient DHA levels; SES as a confounding factor | NIH |
| Daniels et al. (2004) | MacArthur Communicative Development Inventory; Denver Developmental Screening Test | Maternal prenatal fish intake and infant postnatal fish intake associated with higher development scores; threshold effect with more than 2 fish meals per week | While mercury levels increased with fish intake, these did not appear to affect development; pre- and postnatal fish consumption appears to have safe, cognitive benefits | Non-intervention; potential bias in food frequency questionnaires | Medical Research Council; Wellcome Trust; Department of Health, Department of the Environment, and DfEE; Nutricia; “other companies” | |
| Dijck-Brouwer et al. (2005) | Neonatal neurological examination technique as described by Prechtl | Obstetrical history | Infants who were neurologically abnormal tended to have lower umbilical vein DHA levels | Low levels of DHA, AA, and EFA have a negative impact on neurological optimality among neonates | Results do not imply causation; further research required | Numico Research BV |
| Dunstan et al. (2008) | Griffiths Mental Development Scales (GMDS) | Cord blood FA levels | Hand-eye coordination scores significantly positively correlated with EPA level in cord blood at 36 months, and inversely correlated with AA level at birth | Consistent with other findings on the benefit of n-3 LC PUFAs for visual development; supplementation is safe and warrants further study | Small sample size; potential type 1 error due to multiple comparisons | Raine Medical Research Foundation of Western Australia and the National Health and Medical Research Foundation of Australia |
| Escolano-Margarit et al. (2011) | Touwen examination | Maternal and neonatal LC-PUFA levels in plasma and erythrocyte PL | Likelihood of high neurological optimality score increased with cord blood DHA level | While supplementation did not result in higher neurological examination scores, DHA in cord blood does correlate with better NOS scores long-term | High attrition; neurological assessment performed by different people | Commission of the European Communities |
| Gould et al. (2014) | Distraction; working memory and inhibitory control (WMIC) | n/a | Neither attention nor working memory/inhibitory control measures differed between the intervention and control groups | Null findings consistent with other studies on well-nourished children born at term | Power to detect WMIC measure less than originally intended; larger sample may have changed result | University of Adelaide the National Health and Medical Research Council of Australia, and the NIH |
| Gould et al. (2016) | Bayley-III (cognitive composite and language composite scores), and differential ability scales (DAS) | Gestational age at birth, birth weight, birth length, and birth head circumference; maternal PPD; child allergic disease | Children in the supplementation scored higher on the Bayley-III, but only when their mothers had not completed further education; smoking appeared to nullify benefits in the non-smoking group | The results indicate the importance of assessing subgroups of supplemented populations to identify potential moderating variables | Powered to detect supplementation in overall sample rather than subgroups | Australian National Health and Medical Research Council |
| Helland, et al. (2001) | Novelty preference using the Fagan test | Gestational length and birth weight | Novelty preference test did not indicate any differences in cognitive function at either 6 or 9 months between the groups; high concentration of DHA in umbilical plasma did correlate with higher gestational age | Already high FA intake among population may have affected the results, and suggested conducting a similar study should be conducted with population with a low intake of DHA or among mothers who did not intend to breastfeed | May have been too early to determine a statistically significant difference in novelty preference; later/more subtle testing may be required | Peter Møller, Avd. Orkla ASA (omega-3 supplement industry) and “Aktieselskabet Freia Chocoladefabriks Medicinske Fond” |
| Helland et al. (2003) | K-ABC | Maternal dietary intake | Significantly higher scores on the mental processing composite of the K-ABC in children from the intervention group than those in the control group | Supplementation with LC PUFAs during pregnancy and lactation improves children’s intelligence at 4 years of age | Small subsample size | Peter Møller, avd. Orkla ASA (omega-3 supplement industry), Eckbos Legater, and Aktieselskabet Freia Chocolade-fabriks Medicinske Fond |
| Helland et al. (2008) | K-ABC | Body mass index | At 4 years of age, the cod liver oil group demonstrated higher scores; no significant difference at 7 years of age, excepting slightly higher scores for sequential processing in the intervention group, which was deemed insignificant | The positive effect of n-3 LC PUFA supplementation may be diluted by other factors like nutrients, drugs, social stimulation, and diseases by the age of 7 | Methods of cognitive testing may not be sufficiently sensitive | Peter Möller Department of Orkla ASA (omega-3 supplement industry), Johan Throne-Holst Foundation for Nutrition Research, Freia Chocolade Fabriks Medicinske Fond, the Research Council of Norway, and the Thematic Program on Perinatal Nutrition, Faculty of Medicine, University of Oslo |
| Hibbeln et al. (2007) | Scale developed by the ALSPAC (items from Denver Developmental Screening Test) | Strengths and Difficulties Questionnaire; Child Behavior Checklist; IQ | Low maternal seafood intake (less than 340g per week) increased the risk of children being in the lowest verbal intelligence quartile and having other suboptimum development outcomes | Advice to limit seafood could be problematic, as children whose mothers consumed more than 340g per week performed better | Social differences between groups may have been a confounding variable; benefits may be due to other aspects of good nutrition than seafood intake | Medical Research Council; Wellcome trust; University of Bristol; UK government departments; medical charities; “other sources” |
| Hurtado et al. (2015) | Bayley Scales of Infant Development, second edition (BSID-II); Mental Development Index (MDI) | Maternal dietary intake (assessed with food frequency questionnaire) FA levels in mother’s milk, plasma, placenta, and erythrocytes | No significant differences between intervention and control group | While the intervention increased both maternal and infant FA status, it did not produce expected neurodevelopment effects | Limited sample size/ability to evaluate neurodevelopment outcomes | Not reported; one author declared a conflict of interest as an employee of Lactalis Puleva (dairy industry) |
| Makrides et al. (2010) | Cognitive and Language Scales of the Bayley Scales of Infant and Toddler Development, Third Edition (BSID-III) | Edinburgh Postnatal Depression Scale (EPDS) to measure maternal depression | While mean cognitive scores of children did not differ between the groups, the intervention group did exhibit less cognitive development delays than the control group | Need cognitive outcomes at later ages to clarify longevity of effects; gender differences should be addressed in more detail | No assessment of dietary intake | Australian National Health and Medical Research Council |
| Mendez et al. (2009) | McCarthy Scales of Children’s Abilities (MCSA) | Maternal fish consumption more that 2-3 times per week correlated with significantly higher MCSA subscales, but only among children breastfed for less than 6 months | Moderately high fish intake during pregnancy appears to confer positive benefits among infants breastfed for less than 6 months | Further research on breastfeeding duration as a mechanism require | Spanish Ministry of Health; Instituto de Salud Carlos III; ‘Fundació La Caixa’; European Com mission | |
| Mulder et al. (2018) | K-ABC | Subject characteristics, dietary analysis, biochemical analysis | No differences in mean test performance or achieving scores in the upper quartile for performance on any of the tests assessed between groups; maternal DHA level associated with higher language/short-term memory scales | Despite having found differences in neurodevelopment test scores at 18 months of age, these benefits may not have lasted or may have been confounded by other variables by the time the children reached this age | Potentially insufficient design to detect DHA insufficiency; benefits may be confounded by other variables by this age | Canadian Institutes for Health Research |
| Oken et al. (2005) | Novelty preference on visual recognition memory (VRM) | Hair mercury assay | Higher fish intake was found to correlate with higher infant cognition levels; however, higher mercury levels were associated with lower cognition | Women should aim to consume low-mercury sources of fish during pregnancy for optimal infant cognition outcomes | Sample included many highly educated, white, and high SES mothers, so generalization should be undertaken with caution | Harvard Medical School; Harvard Pilgrim Health Care Foundation; National Institutes of Health |
| Ostadrahimi et al. (2018) | Age and stages questionnaire (ASQ-2) | Weight, height, and head circumference at birth | Of the five neurodevelopment domains assessed at each age, only the communication domain was found to have a positive correlation with fish oil supplementation, and only at 4 months of age | Further studies should be conducted with a broader population, particularly in contexts where consumption of fish tends to be low | Small sample size; dosage may have been inadequate, and cognitive measures may have been insufficiently sensitive | Research Vice-chancellor, Tabriz University of Medical Sciences |
| Ramakrishnan et al. (2016) | McCarthy Scales of Children’s Abilities (MSCA) | The parental scale of the Behavioral Assessment System for Children, Second Edition (BASC-2), and the Conners’ Kiddie Continuous Performance Test (K-CPT) | No significant differences on the MSCA subscales or mean general cognitive score. Higher DHA levels correlated with less effect of the home environment; children in the intervention group had fewer omissions on the K-CPT. | Among children with less stimulating home environments, DHA supplementation can have a particularly important role in cognitive development; DHA supplementation can have positive effects on measures of attention and executive functioning | Lack of data on child’s learning environment at follow-up | NIH and the March of Dimes Foundation |
| Rees et al. (2019) | Cognition, visual acuity, habituation, and visual attention | n/s | DHA levels were found to be significantly correlated with visual acuity, even after adjusting for birth weight. There were no differences in habituation levels or visual attention levels | Empirical support for DHA supplementation remains inconclusive | Women of high SES levels more likely to participate; higher breastfeeding rates than general population; use of an FFQ rather than intervention | The Biotechnology and Biological Sciences Research Council and Mead Johnson Nutrition (industry) |
| Steer et al. (2013) | Wechsler Intelligence Scale for Children | FADS genotype analysis | Higher AA and DHA levels associated with higher IQ | FADS genes may have a significant role in synthesis of these FAs, and should be assessed in further research | Selective dropout (of socially/economically disadvantaged participants) | National Oceanic and Atmospheric Administration; Commission of the European Communities’ Seventh Framework Programme NUTRIMENTHE; National Institute on Alcohol Abuse and Alcoholism |
| van Goor et al. (2010) | Standardized neonatal neurological examination at 2 weeks | Assessment of general movement quality at 2 and 12 weeks | Infants in the DHA group exhibited more abnormal movements than those in the DHA and AA and the control groups at 2 weeks; at 12 weeks, this result was repeated, but with a more significant difference between the groups | AA rather than DHA may be associated with general movement quality, and that DHA/AA balance during pregnancy is important to consider | Small sample size | FrieslandCampina, The Netherlands (dairy industry) |
| Vollet et al. (2007) | Ages and Stages Questionnaire (ASQ)–fine motor, gross motor, communication, personal–social functioning and problem solving domains | Maternal fish oil supplementation was related to lower risk of failing the problem solving domain of the ASQ in children up to 3 years | Prenatal fish oil supplementation may be beneficial | Limited information on dose/supplementation frequency; maternal reporting on ASQ may have introduced bias | Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development | |
Overall, the extant RCTs and RCT follow-up studies that feature in-utero n-3 LC PUFA supplementation have yielded mixed results. The largest study to date, the DOMInO trial (N = 2399; follow-up with 726 children), found that DHA supplementation during pregnancy had no significant effect on either maternal depression levels 6 months postpartum or the children’s mean cognitive composite or mean language composite scores on the Bayley Scales of Infant and Toddler Development, Third Edition (BSID-III). Importantly, these null results are at odds with those of previous epidemiological studies, which the authors noted may have overestimated effect sizes, and those of some smaller studies that have reported positive outcomes.
The studies included in this review that did yield positive outcomes of supplementation focused on communication and language, attentional tasks, and visual development. However, results in those areas are also mixed. Further, most studies that included a long-term follow-up largely reported that, even when positive outcomes were identified at a short-term follow-up, these outcomes often failed to persist at longer-term assessments.
Communication and Language
Several studies that assessed cognitive measures identified positive outcomes of n-3 LC PUFA supplementation and communication and language. The results of an RCT conducted in Iran found prenatal fish oil supplementation to be beneficial for neurodevelopment, but only in the communication domain, among infants at 4 months. 33 Similarly, although Mulder et al. did not report childhood neurodevelopment benefits of supplementation in-utero, the Authors did find higher maternal DHA level to be correlated to higher language scores on the K-ABC at 5-6 years of age. 34 While Colombo et al. found maternal blood DHA to correlate with higher verbal IQ at 5-6 years, this was not the case after controlling for the socioeconomic status. 35
Attentional Tasks
One of the key findings for this cognitive developmental phase is the indication that prenatal supplementation with n-3 LC PUFAs has positive effects on infantile attention. Notably, the results of a large RCT (the Kansas University DHA Outcomes Study [KUDOS]) that assessed visual habituation among 4-, 6-, and 9-month-old infants whose mothers had been prenatally supplemented with DHA, indicate that maternal DHA supplementation correlated with improved performance on sustained attention and behavioral state tests during the first year of life. 36 In a secondary analysis of the DOMINO trial assessing the prenatal effects of DHA on working memory and inhibitory control (WMIC), no difference between the control and the treatment group were observed. 9
While the results of the study by Ramakrishnan et al. did not indicate an effect of supplementation on general cognitive scores or MSCA subscales, children in the intervention group did make fewer omissions on the Conners’ Kiddie Continuous Performance Test (K-CPT), which is used to measure attention. 14 The results of that study also indicate that n-3 LC PUFA supplementation attenuated the effect of home environment on cognition, suggesting the importance of DHA supplementation for children with less stimulating home environments.
Visual Development
In addition to its role in fetal brain tissue development, n-3 LC PUFAs play a significant role in retinal development; therefore, several studies have addressed prenatal n-3 supplementation in relation to visual acuity and cognitive-associated visual and attention tasks. Dunstan et al. found hand-eye coordination scores on the Griffiths Mental Development Scales (GMDS) to be correlated with cord blood n-3 LC PUFA levels. 17 In Hurtado et al. study there was not a correlation between supplementation with n-3 LC PUFAs during pregnancy or during lactation on neurodevelopment outcomes, or on visual acuity for the sample as a whole. 37 However, when stratification was done by gender, supplementation did cause visual acuity to improve for boys, but not for girls. Rees et al 15 conducted a visual acuity test and found that children whose mothers were in the moderate DHA intake group performed the best on this measure at 9 months, and that this finding was only significant in relation to third-trimester DHA levels. 15 However, the results did not indicate any significant difference in habituation and visual attention levels in relation to DHA levels.
Long-Term Effects
Although several studies found n-3 LC PUFA supplementation having beneficial effects during follow-up assessment conducted on younger children, reported controversial outcome with more negligible results. A follow-up to the Nutraceuticals for a Healthier Life (NUHEAL) cohort found that, at 6.5 years of age, prenatal DHA supplementation (500 mg DHA daily) did not correlate with K-ABC scores. 16 Similarly, a long-term follow-up to the KUDOS study revealed that, while prenatal DHA supplementation produced positive attention development outcomes during the first year of life, positive effects tended to dissipate at older ages. 35 For example, a significant correlation between supplementation and rule learning and flexibility was seen at 36 months, but not at older ages. Correlations between supplementation and attention and spatial memory that were observed at 24 and 36 months did not persist at older ages, and the significance of these findings decreased when the researchers controlled for sex and task variables. Also, while maternal blood DHA level during pregnancy correlated with higher IQ at 5-6 years of age, this finding disappeared entirely when the researchers controlled for SES. 35 The lack of long-term effects may have been due to the fact that both placebo and intervention groups obtained sufficient levels of LC PUFAs for cognitive development postnatally.
Similarly, although Mulder et al. found DHA insufficiency during gestation to be associated with risk of failing to achieve high neurodevelopmental test scores at 18 months of age, by 5.75 years of age, these results had dissipated. 34 However, maternal DHA status was related to child scores on some language and short-term memory scales of the K-ABC.
Helland at al. found that, while higher umbilical plasma DHA level did correlate with longer length of gestation, the groups did not show any cognitive differences at 6- or 9-month follow-ups. 18 While the original study by Helland et al. did not report a correlation between cod liver oil supplementation during pregnancy and lactation and scores on the Fagan test for novelty preference at 6 or 9 months of age, a follow-up study on the original cohort reveled that, at 4 years of age, the children in the cod liver oil group scored an average of 4.1 points higher on the mental processing composite of the K-ABC. 21 While this result suggests that n-3 LC PUFA supplementation improves the intelligence of children at the age of 4, the researchers were unable to discern whether this result should be attributed to supplementation during pregnancy, lactation, or both. In a second follow-up study, however, Helland et al. did not find a difference in intelligence scores for children at 7 years of age, excepting insignificantly higher sequential processing scores among members of the intervention group. 19 However, by this time, the effects of supplementation may have been diluted by other diet and lifestyle factors; parental education level was also identified as a significant confounding variable.
Confounding Variables
In some cases, demographic variables were found to potentially skewing study results. For example, a secondary analysis of the DOMInO trial was conducted to assess whether maternal smoking status, maternal education, and socioeconomic status mediated the results. The adverse effects of smoking tended to negate the positive effects of DHA supplementation during pregnancy, and 18-month Bayley-III cognitive composite scores were higher for children in the DHA supplementation group, but only when their mothers had not completed further education. 9
Supplementation During Infancy
In the 26 included studies assessing the effects of n-3 LC PUFA supplementation during infancy, the non-intervention studies featured analysis of FA levels in maternal human milk, cord levels, and supplementation via formula; supplementation studies featured maternal n-3 supplementation; supplemented human milk; supplemented formula; or direct supplementation to the infant independent of feeding. FA supplementation sources included fish oil, borage oil, and egg yolk, and various ratios of DHA, EPA, and AA (see Table 3 for more detail).38-50 Sample sizes ranged from 52 to 604, and intervention lengths ranged from 9 weeks to 1 year. Ten of these studies were wholly or partially funded by organizations with interests in infant formula manufacturing or nutritional supplements.
Table 3.
Supplementation during Infancy: Study Type and Participant Characteristics.
| Study type | Type/dosage of PUFAs | N | Length of intervention | Participant age range | Population characteristics | |
|---|---|---|---|---|---|---|
| Almaas et al. (2016) | RCT | .5 mL oil (DHA/AA or placebo) per 100 mL of human milk | 129 in original study; 92 participated in follow-up; 82 analyzed | 9 weeks | Infancy during supplementation; follow-up at 8 years | Very low birth weight (VLBW; > 1500g) |
| Auestad et al. (2003) | RCT; follow-up study | .43% ARA and .12% DHA from egg-phospholipid; .23% DHA from fish oil; or control | 157 in follow-up | 1 week after birth–1 year | Follow-up at 39 months | n/s |
| Birch et al. (2000) | RCT | Formula supplemented with .35% DHA or with .36% DHA and .72% AA | 56 | From first 5 days of life to 17 weeks | Infancy during supplementation; cognitive testing at 18 months | Term infants |
| Birch et al. (2007) | RCT | Formula supplemented with .35% DHA or with .36% DHA and .72% ARA | 52 | 17 weeks | Infancy during supplementation; follow-up at 4 years | Singleton infants with appropriate birth weights |
| Cheatham et al. (2011) | RCT | Lactating mothers supplemented with 1.5 g n-3 LC PUFA fish oil per day | 122 mothers randomized; 98 children tested at age 7 | First 4 months of lactation | Infancy during maternal supplementation; cognitive testing at 2.5 and 7 years | Women in 36th week of pregnancy with lower than median fish consumption (53 with high fish consumption also participated as a reference group) |
| Collins et al. (2015) | Follow-up of RCT | DHA rich tuna oil to achieve a human milk HA concentration of around 1% | 604 | 40 weeks from first enteral feed | Infancy during original study; 7 years at follow-up | Born <33 weeks’ gestation |
| Dalmeijer et al. (2016) | Observational cohort | Non-intervention (FA levels in human milk analyzed) | 227 non-breastfed children (control); 157 breastfed children | Non-interventional | Human milk samples collected when children were 3 months; school performance data gathered at 12 years | n/s |
| de Jong et al. (2010) | RCT | Breast feeding; standard formula; or formula supplemented with 0·45% AA and 0·30% DHA | 474 | 2 months | Infancy; follow-up at 9 years | n/s |
| de Jong et al. (2015) | RCT | Breast feeding; standard formula; or formula supplemented with 0·45% AA and 0·30% DHA | 235 | 2 months | Infancy; follow-up at 9 years | n/s |
| Drover et al. (2011) | RCT | Control (0% DHA), .32% DHA, .64% DHA, or .96% DHA | 181 | 12 months | Infancy; follow-up at 18 months | Children who had enrolled in the DIAMOND study |
| Fewtrell et al. (2004) | RCT | Unsupplemented or LC PUFA supplemented (borage oil and tuna fish oil) formula | 238 | To 9 months after term | Infancy; follow-up at 18 months | Preterm (<35 weeks, <2000 g birth weight) |
| Gale et al. (2010) | Prospective cohort | Breastfeeding or DHA-supplemented formula | 241 | Non-intervention; first 6 months | Infancy; follow-up at 4 years | Singleton live births to women in the Southampton Women’s Survey |
| Henriksen et al. (2008) | RCT | 31 mg AA and 32 mg DHA (supplemented human milk) | 141 randomized; 129 completed | From 1 week after birth to discharge from the hospital (an average of 9 weeks) | Infancy; follow-up at 6 months | Birth weights of <1500 g |
| Henriksen et al. (2016) | RCT (follow-up) | 31 mg AA and 32 mg DHA (supplemented human milk) | 98 children participated in 8-year follow-up | From 1 week after birth to discharge from the hospital (an average of 9 weeks) | Infancy; follow-up at 8 years | Birth weights of <1500 g |
| Isaacs et al. (2011) | RCT | .5% DHA from borage and fish oils (supplemented formula) | 107 | First 9 months after birth | Infancy; follow-up at 10 years | Preterm infants |
| Jacobson et al. (2008) | Observational | Cord and maternal plasma and milk | 109 | Non-intervention | Assessed at 6 and 11 months | Residents of Arctic Quebec |
| Jensen et al. (2005) | RCT | approximately200 mg DHA/day | 83 in DHA and 77 in control group participated to 30-month follow-up | 4 months | Assessed at 4, 8, 12, and 30 months | Women who planned to breastfeed exclusively |
| Jensen et al. (2010) | RCT | 200 mg DHA/day from algal oil | 60 in DHA and 59 in control at 5-year follow-up | 4 months | Follow-up at 5 years | Women who planned to breastfeed exclusively |
| Kalhoff et al. (2020) | RCT | Dietary intervention; complementary food with rapeseed oil, oily fish, or corn oil | 54 in rapeseed oil group; 48 in fish group; 58 in corn oil group | 5th to 7th month through 10th month | Infancy; assessed at 10 months of age | Healthy term infants; mothers willing to breastfeed |
| Lauritzen et al. (2005) | RCT | 4.5 g/day fish oil | 122 with reported low fish intake; 53 with reported high fish intake | 4 months | Infancy; assessed at 9 months, 1 and 2 years of age | Uncomplicated delivery; intention to breastfeed for at least 4 months |
| Lepping et al. (2018) | RCT | Control or formula with .64% of total fatty acids as ARA and .32%, .64%, or .96% DHA | 159; 42 enrolled at follow-up | 12 months | Infancy; follow-up at 9 years | Completed 12 months of formula feeding in parent trial; no serious health conditions |
| Liao et al. (2017) | RCT (follow-up of the DIAMOND clinical trial) | Formula with .64% of total fatty acids as AA and various concentrations of DHA (.32%, .64%, or .96%) | 69 | First 12 months of life | Infancy; follow-up at 5.5 years | Healthy, full-term, formula-fed, singleton-birth |
| Meldrum et al. (2012) | RCT | 250 mg DHA and 60 mg EPA per day (direct infant supplementation) | 287 | Birth–6 months | Infancy; assessment at 18 months | Allergic pregnant women (those who consumed more than 3 fish meals per week/1000 mg FO per day were excluded); term infants |
| Westerberg et al. (2011) | RCT | 32 mg DHA and 31 mg AA (supplemented human milk) | 92 | 1 week after birth to discharge from hospital | Infancy; assessment at 20 months | Very low birth weight infants (<1500 g) |
| Willatts et al. (2013) | RCT | Egg yolk (approximately 70% LC PUFAs) added to formula | 235 | First 4 months of life | Infancy; follow-up at 6 years | Healthy term singletons |
| van der Merwe et al. (2013) | RCT | Fish oil with 200 mg DHA and 300 mg EPA per day (administered to the infant before breastfeeding) | 172 | 3 months of age—9 months of age | Infancy; assessed at 1 year | All 3-month-old infants in the 16 largest villages of the West Kiang region of The Gambia |
The majority of studies were conducted in high-income countries where LC PUFA levels are often already sufficient, and numerous authors mentioned the possibility of a threshold/lack of deficiency as a potential explanation for their null results. The study by van der Merwe et al. is unique in having been conducted in a low-income country where infants were presumably more likely to suffer from adverse effects from n-3 LC PUFA deficiencies. 51 While no cognitive effects were reported, and maternal human milk FA levels were higher than anticipated, that study does highlight the issues with conducting research among affluent populations and in countries with high fish intake levels, as cognitive differences may be more detectable when deficiencies are present Table 4.
Table 4.
Supplementation during Infancy: Instrumentation and Results.
| Outcome measures | Results | Conclusion | Confounding variables/limitations | Funding source | ||
|---|---|---|---|---|---|---|
| Primary/cognitive | Secondary/other | |||||
| Almaas et al. (2016) | Behavioral outcomes measured with the Strengths and Difficulties Questionnaire [SDQ)] parent report version | Cerebral white matter microstructure (measured by diffusion tensor imaging [DTI]) | No significant differences between the treatment and placebo groups on behavioral outcomes or cerebral white matter microstructure; the intervention group did exhibit higher fractional anisotropy in the corpus callosum | While the fractional anisotropy finding was non-significant, it may warrant additional research on this subject | Supplementation level may have been insufficient to produce long-term effects; confounding variables may have been present by age 8; exclusion of neurologically vulnerable groups | The Research Council of Norway, the South-Eastern Norway Regional Health Authority and the University of Oslo, Norway |
| Auestad et al. (2003) | Stanford-Binet Intelligence Scale Form L-M; the Peabody Picture Vocabulary Test-Revised (PPVT-R); and the Beery Visual-Motor Index test | Visual-motor function, red blood cell fatty acid levels, and the frequency of illnesses and hospitalizations since birth | At 39 months, the cognitive outcome measures did not differ among the intervention groups | Given that their previous results indicated lower vocabulary scores at 14 months among supplemented groups, the authors concluded that this follow-up confirmed that these results were either transient or anomalous, and that DHA + ARA supplementation in formula does support visual and cognitive development | Sample size | Ross Products Division, Abbott Laboratories, Columbus, Ohio |
| Birch et al. (2000) | BSID-II at 18 months | Blood fatty-acid composition, sweep visual evoked potential acuity, and forced-choice preferential looking acuity at 4 and 12 months | Mean increase of 7 points on the mental development index scale of the Bayley test for the DHA + AA group, and a trend toward more accelerated performance on the same subscale amount this group | The authors concluded that early dietary DHA supply correlated with improved performance on the MDI. | Forced-choice preferential looking acuity was not found to correlate with 18-month MDI scores, and may not have been an effective measure of cognitive development; BSID scores’ ability to predict school-age performance may have been limited as the sample did not include a range of developmental speeds (most were within normal range) | NIH and Mead Johnson Nutrition (infant formula manufacturer) |
| Birch et al. (2007) | Wechsler preschool and primary scale of intelligence | HOTV visual acuity | In visual acuity and IQ maturation, the supplemented groups had similar scores to the breast-fed group | DHA + ARA supplemented formula is a comparable alternative to breast feeding in terms of cognitive outcomes | Maternal variables associated with decision to breastfeed | NIH |
| Cheatham et al. (2011) | Infant Planning Test (means end problem solving); Woodcock Johnson Tests of Cognitive Abilities III (speed of cognitive processing); Stroop task (higher-order cognitive functioning) | Socio-emotional functioning (Strength and Difficulties Questionnaire) | No differences between groups for the processing speed measure; the prosocial score on the SDQ tended to be higher in the control group, which was significant when only boys’ results were included. Maternal LC PUFA intake level and maternal education level were significant predictors for speed of processing | The authors noted that lower language comprehension scores among boys in the fish oil group may have contributed to their lower prosocial scores, but did not discern why fish oil supplementation would have produced a negative effect on these measures | Sample size may have been too small to detect small effect size; results may not be generalizable given the typically high fish intake among Danish people; not powered to detect a potential inverted-U effect of FO supplementation | Food Technology Research and Development Programme, Denmark (FØTEK) |
| Collins et al. (2015) | Full Scale IQ of the Wechsler Abbreviated Scale of Intelligence | Subtests of: of Everyday Attention for Children; Rey Auditory Verbal Learning Test; Test of Visual Perceptual Skill; Wide Range Achievement Test. Conners 3rd Edition ADHD Index; Strengths and Difficulties Questionnaire | No effect of supplementation on IQ, attention, executive function, behavior, visual-spatial perceptual skills, educational progress, or quality of life at 7 years of age | No long-term benefit of this type of supplementation | Variations in maternal compliance/DHA concentrations in human milk; no teacher assessment of children’s cognitive function | National Health and Medical Research Council, Australia; Mead Johnson Nutrition |
| Dalmeijer et al. (2016) | Cito standardized school achievement test and teachers’ assessment of secondary school potential | n/a | No association between Cito score and LC PUFAs among boys; girls who received human milk with high amounts of n-3 LC PUFAs scored higher on the Cito test | While some of this effect can be explained by sociodemographic factors, high levels of PUFA content, particularly DHA, in human milk can produce cognitive benefits among girls at the age of 12 | Only one sample of human milk obtained; not known whether LC PUFA levels remained consistent throughout lactation; sample size sufficient to evaluate results based on sex, but not to have more than two groups | Netherlands Organization for Health Research and Development; Netherlands Organization for Scientific Research; Netherlands Asthma Fund; Netherlands Ministry of Spatial Planning, Housing, and the Environment; Netherlands Ministry of Health, Welfare, and Sport |
| de Jong et al. (2010) | Neurological examination according to Touwen | Obstetrical Optimality Score; maternal intelligence; social condition | Cognitive outcome measures (Neurological Optimality Score) did not differ between the two formula groups | Postnatal n-3 supplementation does not have an effect at 9 years of age | Attrition rates | Food Quality and Safety Priority of the Sixth Framework Programme for Research and Technical Development of the European Community |
| de Jong et al. (2015) | Minor neurological dysfunction | IQ; behavioral development | Among boys, those with minor neurological dysfunction were more likely to have had lower umbilical vein DHA levels | Higher levels of DHA at birth correlate with lower levels of neurological dysfunction at 9 years of age, only among boys | Attrition rates | Food Quality and Safety Priority of the Sixth Framework Programme for Research and Technical Development of the European Community |
| Drover et al. (2011) | Bayley Scales of Infant Development, 2nd edition (BSID II) | Fatty acid analysis | No significant between-group cognitive difference. When all intervention groups together were compared to the control group, supplemented children had better Mental Development Index scores | The results indicate a threshold effect at .32% concentration of DHA in formula, which is beneficial for cognitive development up to 18 months | There are several alternative explanations (other than increased DHA status) for these findings; further research is required | Mead Johnson Nutrition |
| Fewtrell et al. (2004) | Bayley Mental and Psychomotor Indexes | Growth; safety and tolerance outcomes | No overall difference between groups in neurodevelopment outcomes; only among boys, those in the LC PUFA group had significantly higher mental development index scores than those in the control group | The supplementation proved to be safe and to have mental development index improvement implications for boys, and growth implications for all children | Global outcome measure tests may not have been sufficiently subtle | H. J. Heinz Company Ltd |
| Gale et al. (2010) | Wechsler Pre-School and Primary Scale of Intelligence; Test of Visual-Perceptual Skills (Non-Motor); Developmental Neuropsychological Assessment | Dietary assessment; maternal and child characteristics | Higher total and verbal IQ scores for children who were breast fed or fed with DHA-fortified formula than those fed unfortified formula | These results may be explained by maternal or family characteristics | Women who took part in the survey tended to be better education with a higher SES | Medical Research Council and the Dunhill Medical Trust |
| Henriksen et al. (2008) | Ages and Stages Questionnaire (ASQ) | Event related potentials—recognition memory | Plasma DHA levels increased by 12% in the treatment group and decreased by 9% in the control group during the intervention; the intervention group scored higher on the problem solving subtest of the ASQ. The results of the event-related potentials testing showed a significant benefit on working memory in the intervention group | DHA and/or AA have cognitive benefits in terms of problem solving and working memory at 6 months of age | Low power for detecting ASQ differences; possibility of type 2 errors | Norwegian Foundation for Health and Rehabilitation; Johan Throne Holst Foundation for Nutrition Research; Freia Medical Research Foundation; Research Council of Norway; Thematic Program on Perinatal Nutrition, Faculty of Medicine, University of Oslo |
| Henriksen et al. (2016) | Wechsler Abbreviated Scale of Intelligence | Growth measures and blood biomarkers | While supplementation with DHA and AA produced lower levels of insulin-like growth factor-1 (IGF-1) at follow-up, it did not affect weight, length, or IQ; however, blood DHA levels at follow-up did correlated with higher IQ after correcting for birth weight and maternal education | The results are in alignment with those of previous studies, which did not find significant long-term effects of LC-PUFA supplementation, but do indicate that DHA should be supplied specifically throughout life | Number of participants inhibits subgroup analyses and many have increased the risk of type 2 errors | Research Council of Norway, the South-Eastern Norway Regional Health Authority, and the University of Oslo |
| Isaacs et al. (2011) | Wechsler Abbreviated Scale of Intelligence, the Neuropsychological Test for Children, the word pairs test from the Children’s Memory Scale (to assess association learning ,which is related to the hippocampus), the Weschler Individual Achievement Test, the Test of Everyday Attention for Children, and the Behavioural Assessment of the Dysexecutive Syndrome for Children | n/a | 10-year-old girls in the supplemented group demonstrated improved performance in reading and spelling measures of the Weschler Individual Achievement test | The results also indicate that both groups that received human milk scored higher on subscales of the Weschler Individual Achievement Test, again prompting the question of whether breastfeeding or PUFAs was the cause of the positive effect | Relatively small group numbers (low follow-up participation rate) | European Union Early Nutrition Programming Project, as part of the Sixth Framework Program; Heinz UK; two researchers received funding from infant feeding manufacturers |
| Jacobson et al. (2008) | Teller Visual Acuity Card Test; Fagan Test of Infant Intelligence (6 months); and the same as well as the Bayley Scales of Infant Development, 2nd edition at 11 months | Demographic control variables | Higher cord DHA correlated with longer gestation, better visual acuity and novelty preference scores, and better Bayley Scale scores, but higher maternal milk DHA did not have any significant effect | The results confirm the third trimester as the crucial period for DHA accretion | Less precise measurement techniques available for breastfeeding than for prenatal intake, which may have affected the results | National Institute of Environmental Health Sciences; Indian and Northern Affairs, Canada; Health Canada; Hydro-Quebec; Joseph Young, Sr, Fund of the State of Michigan |
| Jensen et al. (2005) | Bayley Scales of Infant Development | Gesell Developmental Inventory; Clinical Linguistic and Auditory Milestone Scale; Clinical Adaptive Test | Bayley Psychomotor Development Index scores were higher in the supplemented group at 30 months of age | No other advantages, and none identified at testing before 30 months | Inadequate sensitivity of motor function tests early in life may have affected the results | US Department of Agriculture/National Research Initiative; Martek Biosciences Corp; Mead-Johnson Nutritionals |
| Jensen et al. (2010) | Bailey-Lovie acuity chart, transient visual evoked potential, sweep visual evoked potential testing | No differences between DHA and control groups at 5 years of age, except on the on the Sustained Attention Subscale of the Leiter International Performance Scale (in which supplemented children performed significantly better | DHA supplementation during infancy can have long-term effects on sustained attention | Attrition | Martek Biosciences Corp; U.S. Department of Agriculture/ National Research Initiative | |
| Kalhoff et al. (2020) | Flash visual evoked potential (FVEP); Bayley Scales of Infant Development | No significant differences between intervention and control groups | This dietary intervention did not appear to influence either cognitive or visual development of infants at 10 months of age | Explorative evaluation of functional outcomes; sample size | Federal Ministry of Education and Research | |
| Lauritzen et al. (2005) | Willatts Infant Planning Test; MacArthur Communicative Development Inventory | No effect on problem solving ability; word comprehension inversely associated with DHA level at 4 months | There may be a small adverse effect of supplementation on early language development, which may be transient | Lower than suspected power for outcome measures; not all participants were breastfed for the entire study period | Danish Research and Development Program for Food and Technology; BASF Aktiengesellschaft | |
| Lepping et al. (2018) | Functional (fMRI, Flanker task), resting state (rsMRI), anatomic, and proton magnetic resonance spectroscopy (1H MRS) | Supplemented children showed more activity in anterior cingulate cortex (ACC) and parietal regions; greater connectivity between prefrontal and parietal regions in the .64% DHA group; greater white matter volume in ACC and parietal region for .32 and .64% groups | LCPUFA supplementation during infancy appears to have long-term effects on brain structure | Small sample size; some problems with children participating in the imaging studies | Mead Johnson Nutrition | |
| Liao et al. (2018) | Group differences in behavior and event-related potentials (ERPs) while performing a task requiring response inhibition (Go/No-Go) | n/a | No significant difference in either accuracy or reaction time between the dose levels; while the intervention groups collectively tended to have better reaction times than the control group, these results were not significant; participants in the intervention groups exhibited activation of a larger, synchronized neuronal network than the control group | Early LC PUFA supplementation can have an important programming effect on the brain during development | Smaller sample size/participants lost to follow-up | Mead Johnson Nutrition |
| Meldrum et al. (2012) | Bayley Scales of Infant and Toddler Development and the Child Behavior Checklist; Macarthur–Bates Communicative Development Inventory (language development) | Allergy outcomes | No effect on neurodevelopmental skills on Bayley Scales; more observed anxious/depressed behaviors among FO group; FO group performed better in language assessments, with a higher number of gestures | While the results do not fully support the hypothesis, further studies with larger sample sizes are warranted | The majority of participants correctly guessed group allocation—potential for bias; discrepant sample sizes for different assessment measures | National Health and Medical Research Council (NHMRC) of Australia |
| Westerberg et al. (2011) | Bayley MDI and Ages and Stages questionnaire | Free-play session to assess attention | No significant between-group differences on the Bayley scale or the ASQ; in the free-play sessions, the intervention group demonstrated more time sequences with a high level of attention and higher levels of sustained attention than the control group | The results do not fully support the hypothesis, and small sample size means that their significance is limited; further study is required to discern the impact of supplementation on attention | High level of follow-up attrition; lack of statistical power to detect small between-group differences | Norwegian Foundation for Health and Rehabilitation; Johan Throne Holst Foundation for Nutrition Research; Faculty of Medicine at the University of Oslo; Freia Medical Research Foundation; Research Council of Norway |
| Willatts et al. (2013) | IQ attention control (Day-Night Test), and speed of processing on the Matching Familiar Figures Test (MFFT) | The results did not indicate any difference between the two groups in terms of IQ scores. On the impulsivity/information processing efficiency test (the MFFT), response latencies of children in the intervention group were shorter | Supplementation with LC PUFAs does not appear to improve problem solving ability, but does improve its efficiency. The LC PUFAs in this study were derived from egg yolks (and therefore in phospholipid rather than triglyceride form), and DHA was at the lower end of the recommended amounts scale, which may have affected the result | Potential selection bias (children in follow-up had higher birth weight/several other advantageous demographic factors, making them less susceptible to developmental delays) | Numico Research, Friedrichsdorf, Germany | |
| van der Merwe et al. (2013) | Willatts’ 2-step Infant Planning Test, and a single-object task attention test | Anthropometric measures; gut integrity | There were no significant differences on either of these cognitive outcome measures, but the intervention did result in a significant increase in mid-upper arm circumference | Maternal human milk LC PUFA levels in The Gambia appeared, unexpectedly, to be sufficiently high, which may have affected the study results; further studies should address low-income settings | Sufficient maternal n-3 PUFA levels; intervention may have been more effective if infants who were not breastfed had been targeted | UK Medical Research Council, the Overseas Research Students Awards Scheme, and the Ernest Oppenheimer Memorial Trust |
In general, LC PUFA supplementation during the early neonatal phase is done by supplementing lactating mothers or providing infant supplementation through DHA-containing formula or supplemented human milk. Effects of direct LC PUFA supplementation has been tested by Meldrum et al. which supplemented infants between birth and 6 months with a high-dose DHA-enriched ethyl ester Fatty Oil (FO). 52 Van der Merwe et al. also administered FO to the infant before breastfeeding. 51 Both studies failed to show any difference between treated group and control, however Meldrum et al. reported some benefits to early communicative development Table 5.
Table 5.
Supplementation During Childhood and Adolescence: Study Type and Participant Characteristics.
| Study type | Type/dosage of PUFAs | N | Length of intervention | Participant age range | Population Characteristics | |
|---|---|---|---|---|---|---|
| Åberg et al. (2009) | Highly powered observational | Food frequency questionnaire data | 3972 | Non-intervention; cognitive performance assessed 3 years after questionnaire data collected | 15 at time of questionnaire | 15-year-old males in western Sweden |
| Baumgartner et al. (2012) | 2-by-2 factorial trial | 420 mg DHA/80 mg EPA; iron; placebo; or combination | 321 | 8.5 months | 6-11 | Children with iron deficiency |
| Bos et al. (2015) | RCT | 650 mg EPA and 650 mg DHA | 40 | 16 weeks | 8-14 | Boys with ADHD |
| Brew et al. (2015) | Secondary analysis of the Childhood Asthma Prevention Study RCT | Tuna fish oil with 37% LC PUFAs (added to formula or food) | 239 | From the age of 6 months through age 5 | 8-14 years at follow-up | History of asthma |
| Cornu et al. (2018) | RCT | DHA and EPA (dosage in alignment with age group guidelines/previous studies) or placebo | 71 in treatment group; 77 in placebo | 3 months | 6-15 | Children with ADHD |
| Crippa et al. (2019) | RCT | 500 mg algal DHA/day | 50 | 6 months | 7-14 | Confirmed ADHD diagnosis; drug-naïve; no n-3 or n-5 supplement consumption in the previous 3 months |
| Dalton et al. (2009) | RCT | Fish-flour bread spread | 193 | 6 months | 7-9 | Students in grade 2 at a primary school serving a community with low socioeconomic status and of mixed ancestry from the Northern Cape Province of South Africa |
| Darcey et al. (2019) | Longitudinal | Food frequency questionnaire | 87 | Non-intervention | 13.3 ± 1.1 years | — |
| de Groot et al. (2012) | Observational | Non-intervention; fish consumption data | 700 | Non-intervention | 12-18 | Dutch high school students |
| Demmelmair et al. (2018) | RCT | Between 0 and 127 mg of DHA (from algal oil) per day | 109 | 6 months | 5-13 | Diagnosis of phenylketonuria (PKU) |
| Devlin et al. (2017) | RCT | 200 mg of DHA per day and 200 mg of ARA per day | 133 | 1 year | 12-24 months of age | Healthy term toddlers |
| Handeland et al. (2017) | RCT | Either fatty fish meals three times per week (90 g fish per serving), meat meals three times per week, or fish oil supplementation (158 mg EPA, 105 mg DHA, and 13 mg DPA | 426 | 14-15 years of age | 9th-graders at 8 participating schools | |
| Kean et al. (2017) | RCT | Green-lipped mussel extract | 144 | 14 weeks | 6-14 | DSM-IV ADHD rating score of greater than 15 |
| Keim et al. (2018) | RCT | 200 mg of DHA (from Schizochytrium species algal oil) and 200 mg of AA (from fungal Mortierella alpina oil) | 377 | 6 months | 12-24 months | Preterm children (born at less than 35 weeks’ gestation) |
| Kim et al. (2010) | Observational | Food frequency questionnaire | 10 837 | Non-intervention | 15 | Living in Västra Götaland region of Sweden |
| Kennedy et al. (2009) | RCT | 400 mg or 1000 mg DHA/day | 90 | 8 weeks | 10-12 | Healthy |
| Kirby et al. (2010) | RCT | 400 mg fish oil; 260 mg of which provided v-3 nutrients, including DHA (200 mg) and EPA (28 mg) | 450 | 16 weeks | 8-10 | Typically developing |
| Mazahery et al. (2019) | RCT | Vitamin D3 (2000 IU/day), DHA (722 mg/day), both, or placebo | 73 | 1 year | 2.5-8 | Medical diagnosis of ASD |
| Milte et al. (2012) | RCT | 4 capsules/day with either: EPA-rich fish oil (EPA 1109 mg; DHA 108 mg); DHA-rich fish oil (EPA 264 mg and DHA 1032 mg), or safflower oil (LA 1467 mg) | 90 | 4 months | 7-12 | Over 90th percentile score for ADHD symptoms |
| Milte et al. (2015) | RCT | EPA-rich fish oil, providing a total of 1109 mg EPA and 108 mg DHA or DHA-rich fish oil, providing 264 mg EPA and 1032 mg DHA | 87 | 4 months | 6-13 | Diagnosis of ADHD or parent-rated symptoms >90th percentile on the Conners’ Parent Rating Scale (CPRS; Conners, 2000) and parent-reported learning difficulties (described as literacy performance behind their year level at school) |
| Montgomery et al. (2013) | Observational | Non-intervention; whole blood fatty acid levels assessed | 493 | Non-intervention | 7-9 | Children from mainstream Oxfordshire schools with below average performance in reading |
| Øyen et al. (2018) | RCT | Herring or mackerel for lunch three times a week, while the control group received chicken, lamb, or beef; the meals included a mean concentration of .21 mg/g EPA + DHA in the meat group and 15.2 in the fish group | 218 | 16 weeks | 4-6 | Children at the 13 participating schools from ages 4-6 with sufficient Norwegian language skills for cognitive testing |
| Parletta et al. (2013) | RCT | 750 mg docosahexaenoic plus eicosapentaenoic acids, and 60 mg gamma linolenic acid/school day | 409 | 20 weeks; one way crossover (assessed at baseline, 20, and 40 weeks) | 3-13 | Indigenous Australian children |
| Portillo-Reyes et al. (2014) | RCT | 3 capsules with 60 mg of DHA and 90 mg of EPA | 59 | 3 months | 8-12 | Mild to moderate malnutrition |
| Raz et al. (2009) | RCT | 600 mg EFA/day | 73 | 7 weeks | 7-13 | ADHD diagnosis from a child psychiatrist |
| Richardson and Montgomery (2005) | RCT | Food supplement with n-3 and n-6 fatty acids | 117 | 3 months | 5-12 | Children with developmental coordination disorder (DCD) |
| Richardson et al. (2012) | RCT | 600 mg/day DHA (from algal oil) | 362 | 16 weeks | 7-9 | Children underperforming in reading |
| Ryan and Nelson (2008) | RCT | 400 mg DHA/day | 175 | 4 months | 4 | Healthy; normal developmental milestones; did not consume more than 3 oz of fish more than twice per week or take omega-3 supplements |
| Sheppard and Cheatham (2013) | Observational | Non-intervention (three 24-hour diet recalls | 70 | n/a | 7-9 | 7-9 years old, English speaking; no pervasive developmental issues |
| Sinn et al. (2008) | RCT | EPA (93 mg), DHA (29 mg) from 400 mg fish oil; alongside evening primrose oil | PUFA group = 129; placebo group = 104 | 15 weeks | 7-12 | Connors’ ADHD index score |
| Sørensen et al. (2015) | Secondary analysis of the Optimal Well-Being, Development and Health for Danish Children through a Healthy New Nordic Diet School Meal Study | 2 fish meals per week | 726 | 3 weeks | 8-11 | Danish second- and third-grade children |
| Widenhorn-Müller et al. (2014) | RCT | 600 mg EPA, 120 mg DHA | 95 | 16 weeks | Average age of participants at the beginning of treatment was 8.86 years old for boys and 9.10 for girls | 6-12 years of age, meeting DSM-IV criteria for the ADHD combined subtype (hyperactive–inattentive), the primarily inattentive or the hyperactive/impulsive subtype |
| van der Wurff et al. (2016) | Secondary analysis of the Food2Learn RCT | Non-intervention; Omega-3 index determined via finger prick blood sample | 266 | Non-intervention | 13-15 | Typically developing |
| van der Wurff et al. (2019) | RCT | 400 mg EPA + DHA per day in cohort I; 800 mg EPA + DHA per day in cohort II | 267 | 1 year | Mean = 14 | Omega-3 index ≤5% |
| Vesco et al. (2018) | Secondary analysis of two RCTs | 1.87 g n-3s daily (or two 50-minute psychoeducational psychotherapy sessions per week) | 95 | 12 weeks | 7-14 | Diagnosis of depressive, cyclothymic, or bipolar disorder |
| Voigt et al. (2011) | RCT | 345 mg DHA/day | 63 | 4 months | 6-12 | Children with ADHD receiving effective maintenance therapy |
Dalmeijer et al. assessed the potential role of PUFAs benefits during breastfeeding, comparing with a control non-breastfed cohort (at the time of the study, formulas did not include PUFAs in the Netherlands). In 3 months old infants the results indicated some correlation between DHA level in human milk and higher scores on the Cito school standardized test; however, this was only true of girls, which indicates that sex-specific differences in ability to metabolize n-3 LC PUFAs require further investigation. 53
Supplements in Very Low Birth Weight Infants
FA insufficiency during the third-trimester has been correlated to fetal brain development, premature birth, and very low birth weight (VLBW). Considering that VLBW children have increased risk of behavioral problems, Almaas et al. suggested that DHA supplement to VLBW infants would influence cerebral white matter and improve behavioral outcome. Results did not indicate significant differences between control and intervention groups for both the outcomes. 2
Henriksen et al. tested the effect of DHA and AA supplementation in 129 VLBW infants fed human milk. The follow-up lasted 8 years, and whereas no differences in growth or intelligence quotient (IQ) were found, blood DHA at 8 years was positively associated with IQ. 54
Specific Outcomes of Interest
A limitation of previous research on LC PUFA supplementation during infancy is the lack in assessing specific cognitive domains. More recent studies conducted over the past 2 decades have endeavored the use of subscales and specific measures to access the cognitive function effects of FA supplementation during infancy. As such, the results of this review are organized according to the following cognitive domains: Communication, Intelligence, Working Memory, Programming During Brain Development, Attention, Problem Solving Table 6.
Table 6.
Supplementation During Childhood and Adolescence: Instrumentation and Results.
| Outcome measure | Results | Conclusion | Confounding variables/limitations | Funding source | ||
|---|---|---|---|---|---|---|
| Primary/cognitive | Secondary/other | |||||
| Åberg et al. (2009) | Intelligence tests conducted as part of the Swedish military conscription examination | Compared to those who consumed fish meals less than once per week, those who consumed more than one fish meal per week had higher scores of combined intelligence 3 years later, as well as higher scores in verbal and visuospatial performance, even after accounting for education level | Fish intake has a significant effect on composite, visual, and visuospatial intelligence, measured 3 years later | Physical activity may have been a confounding variable; lack of information on diet other than fish | Swedish Society of Medicine; Department of Public Health at the Västra Götaland Region; Swedish Science Council | |
| Baumgartner et al. (2012) | Hopkins Verbal Learning Test (HVLT) and subscales of the Kaufman Assessment Battery for Children | DHA/EPA did not produce a benefit on any of the cognitive tests, and appeared to have a negative impact on working memory | n-3 supplementation does not appear to have cognitive benefits for children with iron deficiency | Study period/dosage, and previous level of FAs in the population may have affected the results | Unilever Research and Development; Medicor Foundation, Vaduz; Principality of Liechtenstein; North-West University | |
| Bos et al. (2015) | fMRI study with a traditional Go-NoGo paradigm | Parent-rated Child Behavior Checklist (CBCL) and Strengths and Weaknesses of ADHD symptoms and Normal behavior scale (SWAN); Essential Fatty Acids Questionnaire (EFAQ) | Supplementation improved parent-rated ADHD symptoms, but did not affect cognitive control or fMRI results | Omega-3 supplementation can complement other ADHD treatments | Some participants had changes to ADHD medication during the intervention period | Two authors are employees of Unilever |
| Brew et al. (2015) | National Assessment Program—Literacy and Numeracy (NAPLAN) scores | Supplementation did not improve long-term academic performance as measured by the NAPLAN; however, n-3 LC PUFA levels at 8 years of age did correlate with academic performance from between 8 and 14 years of age | Other variables (like maternal education and SES) may explain the correlation between LC PUFA levels at age 8 and test scores, in part because children’s n-3 levels by that time were food- rather than supplement-derived | High attrition rates for follow-up; potential consequent introduction of selection bias | Not industry funded (individual authors supported by various NHMRC/Swedish Research Council Grants); one author declared an advisory board role with Novartis | |
| Cornu et al. (20 180 | Attention-Deficit Hyperactivity Disorder Rating Scale version 4 | Alouette test; Test of Attentional Performance for Children; 48-item Conners Parent Rating Scale-Revised; Children’s Depression Inventory—CDI | ADHD rating scale score reduction was more significant in the placebo than intervention group | Supplementation does not appear to produce a positive effect on ADHD symptoms | Did not measure blood levels | URGO laboratories |
| Crippa et al. (2019) | ADHD rating scale IV Parent Version–Investigator | Conners’ Parent Rating Scale–R; Strengths and Difficulties Questionnaire (SDQ); Child Health Questionnaire; Clinical Global Impression—severity Scale; Amsterdam Neuropsychological Tasks | No differences between treatment groups identified for the primary outcome measure; DHA supplementation also produced a significant (though small) improvement in measures of focused attention | Small improvements in the CHQ Psychosocial summary and improved in parental ratings of emotional problems on SDQ indicate a limited potential benefit of n-3 supplementation among this population | Small sample size; underpowered to detect the effect of n-3 supplementation | Dietetic Metabolic Food srl. (prescription drug industry) |
| Dalton et al. (2009) | Hopkins Verbal Learning Test; Reading test; Spelling test | Biochemical analysis; blood samples | EPA and DHA significantly increased in the intervention group; as did scores on the Hopkins Verbal Learning Test Recognition, Discrimination Index, and Spelling test | Dietary omega-3 supplementation improves children’s memory and verbal learning | Mental performance may have been affected by other nutritional factors; another component of the fish flour than DHA may have produced the improvements | Department of Science and Technology Innovation Fund |
| Darcey et al. (2019) | Behavior Rating Inventory of Executive Function; Go/No-Go Task | Kauffman Brief Intelligence Questionnaire; Pubertal Development Scale; family socioeconomic status | Higher omega-3 intake levels associated with caregiver-assessed inhibitory control, as well as task-based impulse control | Results also indicate poorer cortical development (based on activation in the dorsal anterior cingulate), indicating the importance of omega-3s in adolescent development | Food-frequency questionnaire relies on diet recall | NIH/NIAAA |
| de Groot et al. (2012) | End term grades; Amsterdam Vocabulary Test; Youth Self-Report | More fish intake (up to the recommended amount) correlated with higher end-term grades and better vocabulary | Adolescents should be advised to eat fish twice a week | Non-intervention; portion size not accounted for | n/s | |
| Demmelmair et al. (2018) | Raven’s Progressive Matrices (RPM) | Latencies of visually evoked potentials; Lincoln-Oseretzky Motor Development Scale was used to test fine and gross motor skills; glycerophospholipid (GPL) fatty acids measured | While amount of DHA in GPL increased proportionally to treatment group, there were no differences in cognitive and neurological functions | The researchers also evaluated fatty acid desaturase genotypes, and found that they were associated with AA levels and with DHA levels when accounting for previous ALA levels as a precursor to DHA | Smaller than expected sample size; inconsistencies measuring the Phe level; differences in test procedures across included centers | Commission of the European Communities, the Sixth Framework Programme NUTRIMENTHE; Nutricia (medical nutrition industry) |
| Devlin et al. (2017) | Bayley Scales of Infant and Toddler Development 3rd Edition (Bayley-III) | Beery–Buktenica Developmental Test of Visual–Motor Integration; measures of attention during play | No significant effects of supplementation identified on the primary cognitive function and language development measures; boys in the supplement group exhibited fewer inattention episodes than those in the control group; positive association between ARA levels in red blood cell phosphatidylethanolamine and cognition, only among boys; both boys’ and girls’ ARA levels correlated with their language scores | Future research should further investigate the ideal DHA and ARA levels for this age group using more sensitive measures of cognition | Outcome measures may have been insufficiently sensitive | DSM Nutritional Products (supplement manufacturer) |
| Handeland et al. (2017) | Attention performance measured by the d2 test of attention | Performance in a Norwegian reading and spelling test named “Kartleggeren” was assessed but not reported, due to considerable ceiling effects in nearly all outcomes at pre and post intervention. Mental health status was measured with the Strengths and Difficulties Questionnaire (SDQ) | The results of the d2 test of attention indicate that, for total performance and processing speed, the fish meal group scores were significantly higher than the supplement and meat groups. While omission errors decreased in the meat group, this finding was not significant after controlling for dietary compliance | The benefit of omega-3s on speed of cognition was identified even in individuals with low levels of dietary compliance, which should prompt careful interpretation of the results | No pure placebo group; low dietary compliance; only one cognitive outcome | The Norwegian Seafood Research Fund |
| Kean et al. (2017) | Conners Parent Rating Scales (CPRS) | Test of Variables of Attention and Computerised Mental Performance Assessment System as the secondary outcome variables to assess cognition and hyperactivity/attention symptoms | The results did not indicate any correlation between treatment group and CPRS measures; however, subgroup analysis showed that participants with non-combined type participants with less severe symptoms showed improved CPRS hyperactivity scores, as well as those on behavior at home and learning abilities | Participants in the intervention groups demonstrated improved memory in cognitive tasks, indicating the potential benefit of n-3 supplementation on delayed working memory in particular | Variety in participant demographics | Pharmalink Pty Ltd. (industry) |
| Keim et al. (2018) | Bayley-III composite score | The Bayley-III language and motor composite scores and the IBQ-R and Early Childhood Behavior Questionnaire effortful control and activity level scores | The results did not indicate improvement on the basis of daily DHA and AA supplementation for this population | In all but the poorest groups and those with the lowest birth weight, some negative effects in early language ability were identified, suggesting that those children may not have been previously deficient in DHA/AA and the supplementation therefore upset their previous fatty acid balance in the brain | Smaller than planned sample size; underpowered to detect subgroup differences | US Health Resources and Services Administration Maternal and Child Health Field–initiated Innovative Research Studies Program; the March of Dimes, the Allen Foundation; Cures Within Reach; National Center for Advancing Translational Sciences/National Institutes of Health; Research Institute at Nationwide Children’s Hospital |
| Kim et al. (2010) | Academic grades | Participants who reported fish consumption more than once per week reported higher grades than those with lower levels of fish consumption | Fish consumption may correlate with higher academic achievement | Lack of more detailed dietary information | Swedish Society of Medicine, the Department of Public Health at the Västra Götaland Region | |
| Kennedy et al. (2009) | Internet Battery; Cognitive Drug Research (CDR) Battery | Those in the 400 mg DHA group performed better on the word recognition task, while those in the higher dosage group performed more slowly | The authors suggested that this findings was likely due to chance | Sample size; slight lack of power | Martek Biosciences | |
| Kirby et al. (2010) | Kaufman Brief Intelligence Test, Wechsler Individual Achievement Test Working Memory Test Battery for Children, Creature Counting for attention, the Matching Familiar Figures Task (MFFT), the Computerised Penmanship Evaluation Tool, the SNAP-IV ADHD rating scale, and the SDQ | On the MFFT (which measures impulsivity and visual perception), the supplemented group had a significantly higher number of first correct responses than the control group. The placebo group also demonstrated lower levels of parent-reported prosocial behavior | Supplementation could have had a protective effect against decline in prosocial functioning | Longer supplementation period may have been necessary; while statistically significant, correlations identified were weak | Seven Seas Ltd (supplement production) | |
| Mazahery et al. (2019) | Social responsiveness scale (SRS) and sensory processing measure (SPM) | Biochemical analysis | Of the four social functioning domains assessed in the SRS, improved social awareness was found to be significantly correlated with both the n-3 and n-3 + vitamin D interventions. The SPM results indicate possible correlations between the taste and smell domain with n-3 + vitamin D supplementation and between the balance and motion domain with n-3 supplementation | Noting that other studies have speculated on the benefit of n-3 and vitamin D supplementation in combination, given that they have shared/complementary nutrient functions, the authors noted this type of combined treatment to have a nonsignificant positive effect on social interaction and communication | Smaller than anticipated sample size | Massey University Strategic Innovation Fund, Massey University, New Zealand |
| Milte et al. (2012) | Wechsler Individual Achievement Test III (literacy); Conners Rating Scales (behavior) | Test of Everyday Attention for Children; computerized go/no-go task | DHA proportion was associated with improved scores on word reading measures and lower parent-rated oppositional behavior; DHA level associated with improved reading, spelling, ability to divide attention, parent-ratted oppositional behavior, hyperactivity, restlessness, and ADHD symptoms | Among children with ADHD, LC PUFA supplementation can improve literacy and behavior outcome measures | Smaller than intended sample size; not all children had a clinical ADHD diagnosis | South Australian Health Department; Australian Research Council Linkage Project; Novasel Australia |
| Milte et al. (2015) | Wechsler Individual Achievement Test–III and the Wechsler Scale of Children’s Intelligence–III for the primary outcome measures of literacy and behavior | A test battery from the TEA-ch was used to assess for attention and inhibition behaviors as a secondary outcome measure compared with erythrocyte PUFA status | Increased erythrocyte levels of EPA, DHA, and n-3 FAs overall (resulting in lower n-6 to n-3 ratios) correlated with improved overall literacy, attention, and parent-rated behavior measures | Contrary to previous findings on the importance of EPA over DH, results indicate DHA as an important factor in the positive results observed in this study | Small sample size | Australian Research Council Linkage Grant; Novasel Australia (industry) |
| Montgomery et al. (2013) | British Ability Scales (II); Connors’ rating scales | Lower levels of DHA were found to correspond to lower reading levels and inferior working memory performance; higher levels of oppositional behavior and emotional liability | The authors suggested that DHA supplementation may be particularly useful for children with learning differences | Other nutritional ingredients/components of fish may have produced the positive results; further study is required | Martek Biosciences | |
| Øyen et al. (2018) | Wechsler Pre-school and Primary Scale of Intelligence, 3rd edition (WPPSI-III) and the 9-Hole Peg Test (9-HPT) | Biological samples and caregiver questionnaires | Overall, there were no differences between the groups on the WPPSI-III, and a slightly better improvement, with the non-dominant hand, on the 9-HPT for the fish group. However, when researchers adjusted for dietary compliance, the WPPSI-III total raw score improved more in the fish than the control group: more specifically, fatty fish intake correlated with the Vocabulary, Block Design, and Symbol Search subtests of the outcome measure | Red blood cell DHA concentration was found to be a mediating factor for the WPPSI-III total raw score | Smaller than expected sample size; a longer intervention period may have yielded different results | The Norwegian Seafood Research Fund |
| Parletta et al. (2013) | (Draw-A-Person) non-verbal cognitive development; reading; and spelling | Conners Behaviour Rating Scales (completed by teachers) | Improvements in Draw-A-Person scores in treatment group, and in placebo group after switching to treatment | The intervention appeared to produce improvements in cognitive development, but not literacy | Low dosage; longer intervention may have produced more effects | Australian Research Council; Vifor Pharma |
| Portillo-Reyes et al. (2014) | Neuropsychological test battery | Anthropometric and academic measures; parent-completed food frequency questionnaire | Among more than 70% of malnourished children in the treatment group, improvements were seen in processing speed; perceptual integration; visual-motor coordination; attention; executive function | Clinically significant cognitive improvements of 3 months of supplementation among malnourished children | Homogenous group in terms of SES; serum and n-3 to n-6 ratios not assessed | n/s |
| Raz et al. (2009) | Conners’ Abbreviated Parent-Teacher questionnaire; DSM-IV questionnaire for ADHD; Continuous Performance Test | Blood tests; EFA deficiency questionnaire | Both the EFA and the 1000 mg vitamin C placebo appeared to improve ADHD symptoms | EFA supplementation does not appear to work beyond a placebo effect on improving ADHD symptoms | EFA amount or composition may have been less than optimal | No disclosures reported |
| Richardson and Montgomery (2005) | Movement Assessment Battery for Children; Wechsler Objective Reading Dimensions; Conners’ Teacher Rating Scales, Long Version | No effect on motor skills; significant improvements to reading, spelling, and behavior scores | FA supplementation may function as a treatment option for DCD | Optimal dosage information unknown; more studies required | Dyslexia Research Trust; Durham Local Education Authority | |
| Richardson et al. (2012) | Reading; working memory; parent- and teacher-rated behavior | No effect of supplementation on reading for the group overall; a subgroup of more significantly underperforming students showed significant reading improvements; parent-rated behavior improved | DHA supplementation offers potential for improvements to reading, particularly among severely underperforming children | Missing data from parent ratings may have caused positive bias | Martek Biosciences | |
| Ryan and Nelson (2008) | Leiter-R Test of Sustained Attention, Peabody Picture Vocabulary Test (PPVT), Day-Night Stroop Test, and Conners’ Kiddie Continuous Performance Test | Interaction between blood DHA levels and efficacy end points | While the results did not indicate significant treatment effects on the primary outcome measures, regression analysis did indicate a significant positive correlation between DHA level and higher PPVT scores | Since the PPVT tests for comprehension in English, and is associated with memory and cognitive function, these results suggest potential implications in those domains in particular | Ceiling effect reduced the sample size | n/s |
| Sheppard and Cheatham (2013) | Cambridge Neuropsychological Test Assessment Battery (specifically focused on executive function and working memory) | Spatial Working Memory (SWM); Spatial Span (SSP); Stockings of Cambridge (SOC); Intra-Extra Dimensional Set Shift | Participants with balanced n-6 to n-3 ratios demonstrated shorter initial processing times on the spatial working memory task, shorter mean planning times on the planning task, and better executive function scores overall | Balanced intake of n-3 and n-6 fatty acids is important, as when n-3 and n-6 intake were both low (low ratio) and when n-3 intake was high and n-6 intake was very high (high ratio), results were good | Small but sufficiently powered sample size; results require replication; only spatial memory tests included in this study | North Carolina Translational and Clinical Science Institute |
| Sinn et al. (2008) | ADHD-related symptoms on Conners’ Parent Rating Scales (CPRS)—Revised; cognitive test battery | Significant improvements in ability to shift and control attention among the supplementation group | Supplementation may yield minor improvements for this population | Not possible to detect meaningful cognitive outcomes on all tests; lack of intention-to-treat analysis | University of South Australia; Commonwealth Scientific and Industrial Research Organisation | |
| Sørensen et al. (2015) | d2-test of attention and Danish standard tests in reading and maths | Physical activity; dietary intake; biomarkers of PUFA status | Overall school performance, reading comprehension and blood EPA + DHA levels improved as a result of the intervention | Dietarily derived n-3 LC PUFAs have positive cognitive effects on school-aged children | No participant blinding; short study period | Nordea Foundation |
| Widenhorn-Müller et al. (2014) | Cognitive assessment: Hamburg Wechsler Intelligence Scales for Children–IV (particularly working memory and processing speed domains); “Test-batterie zur Aufmerksamkeitsprüfung für Kinder” (KITAP 6–10 y) and the “Testbatterie zur Aufmerksamkeitsprüfung” (TAP 10-18y) for attentional performance | Behavior assessment: FBB ADHS parent-rated and teacher-rated questionnaires; Child Behavior Checklist 4–18 y; Teacher's Report Form 5-18 y | While scores for parent-rated (DISYPS-II, CBCL) and teacher-rated (DISYPS-II, TRF) behavior did not change significantly, there was a significant improvement in working memory among the intervention group; there was a significant decrease of parent-rated thought problems in the placebo group, which may have been caused by the oleic acid in the placebo capsules | The authors suggested that the working memory improvement may be specific to a population with ADHD, but that future research is required to determine this | Small sample size; inability to determine whether the working memory finding was specific to children with ADHD | German Federal Ministry of Education and Research |
| van der Wurff et al. (2016) | Letter Digit Substitution Test (LDST), D2 test of attention, Digit Span Forward and Backward, Concept Shifting Test, and Stroop test | Questionnaires to assess covariates and fish consumption | On the LDST, results indicate that a higher omega-3 index was associated with better information processing speeds: each 1% higher the omega-3 index, the LDST score improved by 1.23 digits. On the D2 test of attention, participants with higher omega-3 levels had fewer errors of omission, which indicates less impulsivity | Overall, the results indicate a positive effect of omega-3 supplementation on select cognitive measures | Observational study; cannot prove causality | Food, Cognition and Behaviour from the Dutch Scientific Organisation |
| van der Wurff et al. (2019) | Letter digit substitution task (LDST), the D2 test of attention (D2), and the Digit Span backward and forward. After these group tests were administered, the Stroop Interference Test and the Concept Shifting Task were administered individually | Pubertal Development Scale; demographic questionnaires | The results did not indicate an effect of one year of krill oil supplementation on participants, or between O3I and neurocognitive test scores | However the authors noted a high level of non-compliance, and suggested that adherence difficulties may have contributed to the lack of demonstrated correlation between krill oil supplementation and neurocognitive benefits | High drop-out rate; low adherence | The Netherlands Organization for Scientific Research; AkerBiomarine (fishing and biotech with krill interests) |
| Vesco et al. (2018) | Behavior Rating Inventory of Executive Functioning (BRIEF) | Mood disorders and mood symptom severity assessed using K-SADS Depression (KDRS) and Mania Rating Scales (KMRS) (Geller et al., 2001), the Children’s Depression Rating Scale-Revised (CDRS-R), and Young Mania Rating Scale (YMRS) | Both intervention groups receiving n-3 supplementation (with and without PEP), showed a significant improvement in executive functioning, particularly in the areas of inhibition control, adaptability to emotions, and cognitive flexibility | The authors noted that, while this study indicates a potentially positive effect of n-3 supplementation on executive functioning treatment for mood disorders, further research should be conducted to assess for ideal supplementation amounts and to identify moderating effects | Potential parental bias on symptom ratings; short-term nature of follow-up; uncertainty as to whether results generalize to populations without mood disorders | National Institute of Mental Health; National Center for Research Resources |
| Voigt et al. (2001) | Test of Variables of Attention and Children’s Color Trails test (inattention measures); Child Behavior Checklist, Conners’ Rating Scale (parent-rated behavior scales | Plasma phospholipid DHA content | No statistically significant effect of supplementation on ADHD among this population | The treatment intervention did not appear to be efficacious in improving inattention or parent-rated behavior related to ADHD | Plasma phospholipid DHA content may not indicate DHA presence in brain or synapses | US Department of Agriculture; Martek Biosciences Corporation |
Communication
As addressed in the in-utero supplementation section, Ostadrahimi et al. conducted a study with a supplementation range including both in-utero and lactation periods, and found that—of five neurodevelopment domains assessed at each age—only the communication domain had a positive correlation with fish oil supplementation, and only at 4 months of age. 33 However, negative language results have also been reported. Cheatham et al. found language and prosocial scores to be lower, only among boys, in the fish oil supplementation group than in the control group. 3
While they did not find a significant effect of DHA supplementation during the first 9 months of life on overall cognitive function at 10 years of age, Isaacs et al. did find that girls in the supplemented group performed better on the reading and spelling measures of the Weschler Individual Achievement test. 55 However, both groups that received human milk scored higher on the Weschler subscales, again prompting the question of whether DHA or another factor associated with breastfeeding accounted for this correlation. While Meldrum et al. found that infants supplemented with FO exhibited higher gestural scores—important in relation to gesture as a precursor to language—the overall results of the study were inconclusive due to methodological issues including discrepant sample sizes across outcome measures and a high rate of correctly guessed group allocation, which may have introduced bias. 54
Intelligence
In an early study on DHA supplementation during infancy, Birch et al. found that supplementation with DHA and AA during infancy correlated with an increase of 7 points on the BSID-II mental development index (MDI). 7 The cognitive and motor subscales of the MDI were significantly higher among both the DHA and DHA + AA groups, and the language scores were higher, but not significantly so. Isaacs et al. found that, while there were no overall differences in cognitive outcomes between the groups, 10-year-old girls in the supplemented group demonstrated improved performance in reading and spelling measures of the Weschler Individual Achievement test. 55
While an 8-year follow-up study did not indicate any effect of supplementation during infancy on IQ, Henriksen et al. did find DHA level assessed at the time of the follow-up to correlate with higher IQ scores after correcting for maternal education and birth weight. 56 Willatts et al. did not find any difference in IQ scores among control or intervention (formula supplemented with egg yolk) group children at 6 years of age, but noted that the global testing measure may not be sufficiently sensitive to detect group differences. 11
Working Memory
Henriksen et al. reported a significant benefit of DHA and AA supplementation during infancy on working memory, tested using event related potentials. 56 They also reported that the test required infants to be relatively calm, and that agitated infants were excluded from the analyzed sample.
Programming During Brain Development
Liao et al. found that participants in the intervention groups exhibited activation of a larger, synchronized neuronal network than the control group, suggesting that early LC PUFA supplementation can have an important programming effect on the brain during development. 57
Attention
Westerberg et al. found that intervention group infants demonstrated more time sequences with a high level of attention during free play sessions than the control group, as well as higher levels of sustained attention among members of the intervention group. 58 Higher DHA levels at hospital discharge did correlate with better sustained attention levels and MDI scores, but due to loss to follow-up, the study’s ability to detect small between-group differences was limited, and the results may be attributable to chance. Willatts et al. did not find LC PUFA supplementation during infancy to affect scores on an attention control test. 11 However, the results did indicate an effect of supplementation on the speed of information processing, with shorter response latencies on the Matching Familiar Figures Test among members of the intervention group. Jensen et al. found that children whose mothers received modest DHA supplementation during breastfeeding for the first 4 months after delivery performed better on a test of sustained attention at 5 years of age. 59
Problem Solving
In a study supplementing infant with formula containing DHA and AA, Willatts et al. noted that supplementation with LC PUFAs did not improve problem solving ability, but did improve its efficiency. 11 In addition, Henriksen et al. found that supplementation significantly improved performance on the problem solving subset of the Ages and Stages Questionnaire. 56
Childhood and Adolescence
In the 36 studies60-95 described in tables 5 and 6 that met the inclusion criteria for this systematic review intervention lengths ranged from 3 weeks to 4.5 years. The main source of n-3 LC PUFA was primarily fish or algal oil. Subjects in the reviewed studies divided between healthy developing populations (n = 13) and those with clinical characteristics: history of asthma (n = 1), ADHD (n = 10), potential DHA deficiency (either as a result of preterm birth or low n-3 index; n = 2), ASD (n = 1), mood disorders (n = 1), or phenylketonuria (n = 1), iron deficiency (n = 1), malnutrition (n = 1); or below average reading level (n = 2) Figure 1.
Figure 1.
Literature search flow chart.
While significant attention has been given to the importance of LC PUFA level during gestation and the first year of life, less is known about its role in subsequent development. Specifically, the period from 12 months to 24 months remains a significant developmental period, which is also marked by dietary changes that can lead to deficiencies. Devlin et al. investigated the cognitive effect of DHA and ARA supplementation in toddler. The author reported no effects on cognitive and language composite, but did find red blood cell ARA level to correlate with improved language scores. 60 Another study conducted with toddlers did not indicate positive outcomes of DHA and AA supplementation, and found some negative effects of supplementation on language development among all but the lowest birth weight and lowest SES groups. 61
In a long-term study, Brew et al. attempted to discern whether FO supplementation from 6 months to 5 years of age correlated with children’s scores on the NAPLAN. 62 Similar to studies assessing in-utero supplementation’s effects on long-term cognitive outcomes, the results did not indicate that supplementation had any effect on scores. However, LC PUFA level at 8 years of age did correlate with test scores after correcting for birth weight, maternal age and education level. Similarly, in an adolescent population, Åberg et al. found that those who had consumed more than one fish meal per week at the age of 15 (assessed via questionnaire, had significantly higher cognitive performance levels in terms of composite, verbal, and visuospatial intelligence than those who had less, even after accounting for education level. 63
In one study investigating the effects of omega-3 supplementation on children’s learning and behavior, the authors reported equivocal results with a significant increase in DHA and EPA levels in both the placebo as well as the treatment group. Authors suggested that this may have been due to dietary changes based on increased awareness via information provided to participants’ parents on the purpose of the study. 64
Two important childhood and adolescence intervention studies—FINS-KIDS and FINS-TEENS—addressed the effect of dietarily supplied n-3 LC PUFAs from herring or mackerel meals three times per week, as opposed to meat meals three times per week. In the FINS-KIDS study, the meals included a mean concentration of .21 mg/g EPA + DHA in the meat group and 15.2 in the fish group, and caregivers filled out a food frequency questionnaire to provide information on non-intervention n-3 consumption. 65 After adjusting for dietary compliance, Øyen et al. found that the fish group demonstrated more WPPSI-III raw score improvement, as well as three subtests and the non-dominant hand 9-HPT test, but the overall results did not indicate improved general cognitive performance after intervention. Sheppard and Cheatham also addressed food-derived n-3s (specifically in relation to n-6: n-3 ratios) in terms of effect on cognitive function through an observational study using diet recalls and histories rather than a supplement intervention, and found that participants with balanced ratio scores scored higher on overall measures of executive function. 66 Importantly, the results indicate that when intake for both n-3 and n-6 was high (low ratio) cognitive abilities declined. In comparison to other studies, these results are important due to the focus on balance rather than high levels of supplementation, and may interact with/help explain the threshold findings offered in other studies.
Particularly among adolescents, compliance issues may introduce biases in studies that address these populations. Handeland et al. also found that, while dietary intake is preferable to supplementation, taste preferences may be a factor. 67 The results of the study by van der Wurff et al. did not indicate an effect of 1 year of krill oil supplementation on participants, or between n-3 index and neurocognitive test scores. 68 However, the authors noted a high level of non-compliance, and suggested that adherence difficulties may have contributed to the lack of a demonstrated correlation between krill oil supplementation and neurocognitive benefits.
Specific Outcomes of Interest
Given the prefrontal cortex development that occurs in childhood and adolescence, and the increasing ability to test for higher-order cognitive functions among older children, studies conducted during the later childhood and adolescence period in particular assessed for higher-order cognitive function, including: attention and executive functioning, information processing speeds and impulsivity, and memory.
Attention and Executive Functioning
Several studies found that DHA supplementation can have positive effects on attention and executive functioning during the childhood and adolescence periods, in both clinical and cognitive healthy populations. For instance, Vesco et al. studied a group of 7-14-year-olds with disorders that affect executive functioning, and found that n-3 supplementation may aid in executive functioning treatment for mood disorders. 69 In particular, executive functioning in the areas of inhibition control, adaptability to emotions, and cognitive flexibility improved more than in the areas of task initiation, planning, and organization. In the FINS-TEENS study, Handeland et al. found that total performance and processing speed improved in the fish meal group compared to meat and supplement groups; the authors noted that dietary intake appears preferable to supplementation. 67
Information Processing Speeds and Impulsivity
Van der Wurff et al. found that, on a letter digit substitution test, a higher omega-3 index was associated with better information processing speeds: each 1% higher the omega-3 index, the LDST score improved by 1.23 digits. 70 On the D2 test of attention, participants with higher omega-3 levels had fewer errors of omission, which indicates less impulsivity. While the overall results did not indicate an impact of fatty fish intake on general cognitive function, Øyen et al. did report a small beneficial effect on three subtests: processing speed subscale, coding and symbol search, and non-dominant hand 9-HPT, with 78% power to detect an effect size of .37. 65 Due to DHA’s role in neural communication, the dietary increases to n-3 intake in this study may indicate an interaction with processing speed, though the results are inconclusive and further studies using processing speed measures are indicated. Other studies have also reported an interaction between LC PUFAs and measures of impulsivity, primarily among the adolescent population. One study with a younger population (8-10 years of age) included the Matching Familiar Figures Test (MFFT) to measure visual attention and impulsivity, and found that the number of first correct responses increased with supplementation, although the study overall did not yield positive results. 64
Memory
Ryan and Nelson found that, while their results did not indicate significant treatment effects on the primary outcome measures, regression analysis did indicate a significant positive correlation between DHA level and higher PPVT scores (which tests for listening comprehension in English, and is associated with memory and cognitive function). 71 Sheppard and Cheatham similarly found a balanced n-3: n: 6 ratio correlated with better working memory scores. 66 Widenhorn-Müller et al. found DHA/EPA supplementation to improve working memory among a population with ADHD. 72
Cognitively Clinical Populations Studies
During the childhood and adolescence supplementation period, an increasing number of studies have addressed clinical rather than typically developing populations, assessing for the effect of LC PUFAs on pathologies including ADHD and ASD.
ADHD
Given the high concentration of DHA in the frontal areas of the brain associated with executive function, the correlation between n-3s and ADHD symptomatology have been of interest to various researchers. Crippa et al. tested the effect of DHA as an ADHD monotherapy. 73 The results showed no difference between treatment and intervention group on the primary outcome measure: ADHD rating scale IV. However, DHA supplementation did appear to produce small benefits to focused attention, parent-rated improvements to psychosocial functioning, and parent-identified reductions in emotional problems. Raz et al. did not find supplementation with EFA to produce any amelioration of ADHD symptoms beyond that of the placebo effect. 74
Kean et al. reported improved Conners’ Parent Rating Scale, behavior at home, and learning abilities scores as a result of supplementation with green-lipped mussel extract. 75 Further, participants in the intervention groups demonstrated improved memory in cognitive tasks, indicating the potential benefit of n-3 supplementation on delayed working memory in particular. Similarly, Milte et al. supplemented children with EPA,DHA, or linoleic acid for 4 months, and although outcome measures showed no significant differences between the three treatments, the Authors found that increased erythrocyte levels of EPA, DHA, and n-3 FAs overall (resulting in lower n-6 to n-3 ratios) correlated with improved overall literacy, attention, and parent-rated behavior measures.76,77
ASD
Due to insufficiencies of pharmacological treatments, there has been increasing interest in complementary and supplement-based treatments for ASD, including n-3 LC PUFAs, DHA and EPA. 78 1 study evaluating the efficacy of vitamin D, LC PUFA, or both, on core symptoms of ASD reported that improved social awareness and social communicative functioning was significantly correlated with both n-3 and n-3 + Vitamin D interventions. Authors suggested that either alone or in combination with Vitamin D, n-3s can have a positive effect on the core symptoms of ASD. 79
Discussion
This review evaluated the effect of omega-3 fatty acid supplementation on neurodevelopment in 3 different cognitive developmental phases: in-utero; lactation/infancy; and childhood and adolescence. Overall, the clinical studies reviewed reported equivocal results regarding cognitive function among the 3 populations.
Generally, researchers have reported beneficial effects on several specific cognitive domains, and at times with small effect sizes. The most consistent positive results include those pertaining to language/communication, executive functioning, attention, and memory.
Further, most research is conducted among populations that do not exhibit deficiencies in n-3 LC PUFA status, and numerous studies indicate the presence of a threshold or even a U-shaped curve in relation to n-3 consumption. It could appear that in populations that are not deficient in DHA or other n-3 LC PUFAs, supplementation has a negligible or at times detrimental effect. Few studies have addressed populations that are deficient in n-3s; 1 study conducted on a population of children with mild to moderate malnourishment resulted in improvements in an extensive number of domains on a neuropsychological test battery. 80
Although LC PUFAs supplied during infancy and in-utero seldom have shown significant cognitive effects, it appears that they do not persist at long-term follow-up assessments. This may indicate that other confounding variables such as nutritional and lifestyle factors can influence the outcomes in the intervening years. In dietary-based studies in particular, authors noted that higher levels of fish intake tend to correlate with higher parental education and socioeconomic status levels, which may confer their own benefits. However, while most studies have alluded to the superiority of dietarily-derived omega-3 sources, a recent study found no effect of supplementation with fatty fish or rapeseed oil from 5-10 months post-delivery on infants’ latencies of FVEP, Bayley’s MDI, or in PDI index. 48
Several studies across intervention periods yielded gender-dependent results, but further research on differences in how n-3 LC PUFAs are metabolized and synthesized according to sex is required to draw clear conclusions.
Overall, the authors of the majority of the studies with short interventions noted that it is important for n-3s to be supplied throughout life, rather than only during the earliest developmental stages.
The main strength of this systematic review is the fairly large number of papers analyzed and searched through multiple databases. Including reference lists in the search assured a comprehensive overview of the published studies. Also, To avoid bias, manuscripts were reviewed by two reviewers.
This review has also several limitations. Inconsistencies in LC PUFA source and type persist across the included studies, and contribute to the equivocality of the overall results. Studies have tested DHA in isolation, or in combination with EPA, ARA, and AA, derived from different sources and administered in a broad range of dosages. Further, some researchers have addressed an advantage of dietarily derived over supplement based n-3s, which may be due to bioavailability. For instance, LC PUFAs in fatty fish are bound to both phospholipids and triacylglycerol, whereas those in supplements are only bound to triacylglycerol. 67 Another source of bias could be that nearly all studies, even those funded by purely academic or governmental sources, reported that the intervention capsules/supplements were provided by a supplement or vitamin industry stakeholder.
Conclusion
Studies addressing n-3 LC PUFA supplementation at three key neurodevelopmental periods—in-utero, during the first year of life, and throughout childhood and adolescence—have reported some benefits in the areas of visual attention, working memory, executive function, and communication. However, effects are more often reported for specific cognitive domains rather than cognitive composite scores and do not tend to persist to long-term follow-up assessment. This may be due in part to the presence of confounding variables like other demographic and lifestyle characteristics and methodological difficulties (like lack of compliance, loss to follow-up, and a very broad range of outcome measures, dosages, and intervention types). Findings remain equivocal, and further research that is powered to detect small, subgroup effect sizes remains necessary.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Ayesha Sherzai1 https://orcid.org/0000-0003-0600-294X
References
- 1.Clandinin MT. Brain development and assessing the supply of polyunsaturated fatty acid. Lipids. 1999;34:131-137. [DOI] [PubMed] [Google Scholar]
- 2.Almaas AN, Tamnes CK, Nakstad B, et al. Diffusion tensor imaging and behavior in premature infants at 8 years of age, a randomized controlled trial with long-chain polyunsaturated fatty acids. Early Hum Dev. 2016;95:41-46. [DOI] [PubMed] [Google Scholar]
- 3.Cheatham CL, Nerhammer AS, Asserhøj M, Michaelsen KF, Lauritzen L. Fish oil supplementation during lactation: Effects on cognition and behavior at 7 years of age. Lipids. 2011;46:637-645. [DOI] [PubMed] [Google Scholar]
- 4.Li D, Wahlqvist ML, Sinclair AJ. Advances in n-3 polyunsaturated fatty acid nutrition. Asia Pac J Clin Nutr. 2019;28:1-5. [DOI] [PubMed] [Google Scholar]
- 5.Milte CM, Parletta N, Buckley JD, Coates AM, Young RM, Howe PRC. Increased erythrocyte eicosapentaenoic acid and docosahexaenoic acid are associated with improved attention and behavior in children with ADHD in a randomized controlled three-way crossover trial. J Atten Disord. 2015;19:954-964. [DOI] [PubMed] [Google Scholar]
- 6.Simopoulos A. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients. 2016;8:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez M. Tissue levels of polyunsaturated11 fatty acids during early human development. J Pediatr. 1992;120:S129-S138. [DOI] [PubMed] [Google Scholar]
- 8.Cao A, Yu L, Wang Y, Wang G, Lei G. Composition of long chain polyunsaturated fatty acids (LC-PUFAs) in different encephalic regions and its association with behavior in spontaneous hypertensive rat (SHR). Brain Res. 2013;1528:49-57. [DOI] [PubMed] [Google Scholar]
- 9.Gould JF, Anderson AJ, Yelland LN, Gibson RA, Makrides M. Maternal characteristics influence response to DHA during pregnancy. Prostagl Leukot Essent Fat Acids. 2016;108:5-12. [DOI] [PubMed] [Google Scholar]
- 10.Birch EE, Garfield S, Hoffman DR, Uauy R, Birch DG. A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants. Dev Med Child Neurol . 2000;42:174-181. [DOI] [PubMed] [Google Scholar]
- 11.Willatts P, Forsyth S, Agostoni C, Casaer P, Riva E, Boehm G. Effects of long-chain PUFA supplementation in infant formula on cognitive function in later childhood. Am J Clin Nutr. 2013;98:536S-542S. [DOI] [PubMed] [Google Scholar]
- 12.Hornstra G. Essential fatty acids in mothers and their neonates. Am J Clin Nutr. 2000;71:1262S-1269S. [DOI] [PubMed] [Google Scholar]
- 13.Makrides M, Gibson RA, McPhee AJ, et al. Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: a randomized controlled trial. JAMA. 2010;304:1675-1683. [DOI] [PubMed] [Google Scholar]
- 14.Ramakrishnan U, Gonzalez-Casanova I, Schnaas L, et al. Prenatal supplementation with DHA improves attention at 5 y of age: A randomized controlled trial. Am J Clin Nutr. 2016;104:1075-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rees A, Sirois S, Wearden A. Prenatal maternal docosahexaenoic acid intake and infant information processing at 4.5mo and 9mo: a longitudinal study. PLoS One. 2019;14:e0210984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campoy C, Escolano-Margarit MV, Ramos R, et al. Effects of prenatal fish-oil and 5-methyltetrahydrofolate supplementation on cognitive development of children at 6.5 y of age. Am J Clin Nutr . 2011;94:1880S-1888S. [DOI] [PubMed] [Google Scholar]
- 17.Dunstan JA, Simmer K, Dixon G, Prescott SL. Cognitive assessment of children at age 2½ years after maternal fish oil supplementation in pregnancy: A randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2008;93:F45. [DOI] [PubMed] [Google Scholar]
- 18.Helland IB, Saugstad OD, Smith L, et al. Similar effects on infants of n-3 and n-6 fatty acids supplementation to pregnant and lactating women. Pediatrics. 2001;108:e82-e82. [DOI] [PubMed] [Google Scholar]
- 19.Helland IB, Smith L, Blomen B, Saarem K, Saugstad OD, Drevon CA. Effect of supplementing pregnant and lactating mothers with n-3 very-long-chain fatty acids on children’s IQ and body mass index at 7 Years of age. Pediatrics. 2008;122:e472-e479. [DOI] [PubMed] [Google Scholar]
- 20.van Goor SA, Janneke Dijck-Brouwer DA, Doornbos B, et al. Supplementation of DHA but not DHA with arachidonic acid during pregnancy and lactation influences general movement quality in 12-week-old term infants. Br J Nutr. 2010;103:235-242. [DOI] [PubMed] [Google Scholar]
- 21.Helland IB, Smith L, Saarem K, Saugstad OD, Drevon CA. Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children’s IQ at 4 years of age. Pediatrics. 2003;111:e39-e44. [DOI] [PubMed] [Google Scholar]
- 22.Boucher O, Burden MJ, Muckle G, et al. Neurophysiologic and neurobehavioral evidence of beneficial effects of prenatal omega-3 fatty acid intake on memory function at school age. Am J Clin Nutr. 2011;93:1025-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brei C, Stecher L, Brunner S, et al. Impact of the n-6:n-3 long-chain PUFA ratio during pregnancy and lactation on offspring neurodevelopment: 5-year follow-up of a randomized controlled trial. Eur J Clin Nutr. 2017;71:1114-1120. [DOI] [PubMed] [Google Scholar]
- 24.Daniels JL, Longnecker MP, Rowland AS, Golding J, ALSPAC Study Team . University of Bristol Institute of Child Health. Fish intake during pregnancy and early cognitive development of offspring. Epidemiology. 2004;15:394-402. [DOI] [PubMed] [Google Scholar]
- 25.Dijck-Brouwer DAJ, Hadders-Algra M, Bouwstra H, et al. Lower fetal status of docosahexaenoic acid, arachidonic acid and essential fatty acids is associated with less favorable neonatal neurological condition. Prostagl Leukot Essent Fat Acids. 2005;72:21-28. [DOI] [PubMed] [Google Scholar]
- 26.Escolano-Margarit MV, Ramos R, Beyer J, et al. Prenatal DHA status and neurological outcome in children at age 5.5 years are positively associated. J Nutr. 2011;141:1216-1223. [DOI] [PubMed] [Google Scholar]
- 27.Gould JF, Makrides M, Colombo J, Smithers LG. Randomized controlled trial of maternal omega-3 long-chain PUFA supplementation during pregnancy and early childhood development of attention, working memory, and inhibitory control. Am J Clin Nutr. 2014;99:851-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hibbeln JR, Davis JM, Steer C, et al. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. The Lancet (British edition). 2007;369:578-585. [DOI] [PubMed] [Google Scholar]
- 29.Mendez MA, Torrent M, Julvez J, Ribas-Fitó N, Kogevinas M, Sunyer J. Maternal fish and other seafood intakes during pregnancy and child neurodevelopment at age 4 years. Publ Health Nutr. 2009;12:1702-1710. [DOI] [PubMed] [Google Scholar]
- 30.Oken E, Wright RO, Kleinman KP, et al. Maternal fish consumption, hair mercury, and infant cognition in a U.S. cohort. Environmental Health Perspectives. 2005;113:1376-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steer CD, Lattka E, Koletzko B, Golding J, Hibbeln JR. Maternal fatty acids in pregnancy, FADS polymorphisms, and child intelligence quotient at 8 y of age. Am J Clin Nutr. 2013;98:1575-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vollet K, Ghassabian A, Sundaram R, Chahal N, Yeung EH. Prenatal fish oil supplementation and early childhood development in the Upstate KIDS Study. J Dev Ori Health Dis. 2017;8:465-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ostadrahimi A, Ostadrahimi A, Salehi-Pourmehr H, et al. The effect of perinatal fish oil supplementation on neurodevelopment and growth of infants: A randomized controlled trial. Eur J Nutr. 2018;57:2387-2397. [DOI] [PubMed] [Google Scholar]
- 34.Mulder KA, Elango R, Innis SM. Fetal DHA inadequacy and the impact on child neurodevelopment: A follow-up of a randomised trial of maternal DHA supplementation in pregnancy. Br J Nutr. 2018;119:271-279. [DOI] [PubMed] [Google Scholar]
- 35.Colombo J, Shaddy DJ, Gustafson K, et al. The Kansas University DHA Outcomes Study (KUDOS) clinical trial: Long-term behavioral follow-up of the effects of prenatal DHA supplementation. Am J Clin Nutr . 2019;109:1380-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colombo J, Gustafson KM, Gajewski BJ, et al. Prenatal DHA supplementation and infant attention. Pediatr Res. 2016;80:656-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hurtado JA, Iznaola C, Peña M, et al. Effects of maternal Ω-3 supplementation on fatty acids and on visual and cognitive development. J Pediatr Gastroenterol Nutr . 2015;61:472-480. [DOI] [PubMed] [Google Scholar]
- 38.Auestad N, Scott DT, Janowsky JS, et al. Visual, cognitive, and language assessments at 39 months: A follow-up study of children fed formulas containing long-chain polyunsaturated fatty acids to 1 year of age. Pediatrics. 2003;112:e177-e183. [DOI] [PubMed] [Google Scholar]
- 39.Birch EE, Garfield S, Castañeda Y, Hughbanks-Wheaton D, Uauy R, Hoffman D. Visual acuity and cognitive outcomes at 4 years of age in a double-blind, randomized trial of long-chain polyunsaturated fatty acid-supplemented infant formula. Early Hum Dev. 2007;2006;83:279-284. [DOI] [PubMed] [Google Scholar]
- 40.Collins CT, Gibson RA, Anderson PJ, et al. Neurodevelopmental outcomes at 7 years’ corrected age in preterm infants who were fed high-dose docosahexaenoic acid to term equivalent: A follow-up of a randomised controlled trial. BMJ Open. 2015;5:e007314-e007314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Jong C, Kikkert HK, Fidler V, Hadders-Algra M. The Groningen LCPUFA study: No effect of postnatal long-chain polyunsaturated fatty acids in healthy term infants on neurological condition at 9 years. Br J Nutr. 2010;104:566-572. [DOI] [PubMed] [Google Scholar]
- 42.de Jong C, Kikkert HK, Seggers J, Boehm G, Decsi T, Hadders-Algra M. Neonatal fatty acid status and neurodevelopmental outcome at 9 years. Early Hum Dev. 2015;91:587-591. [DOI] [PubMed] [Google Scholar]
- 43.Drover J, Hoffman DR, Castañeda YS, Morale SE, Birch EE. Three randomized controlled trials of early long-chain polyunsaturated fatty acid supplementation on means-end problem solving in 9-month-olds. Child Dev. 2009;80:1376-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fewtrell MS, Abbott RA, Kennedy K, et al. Randomized, double-blind trial of long-chain polyunsaturated fatty acid supplementation with fish oil and borage oil in preterm infants. J Pediatr. 2004;144:471-479. [DOI] [PubMed] [Google Scholar]
- 45.Gale CR, Marriott LD, Martyn CN, et al. Breastfeeding, the use of docosahexaenoic acid-fortified formulas in infancy and neuropsychological function in childhood. Arch Dis Child. 2010;95:174-179. [DOI] [PubMed] [Google Scholar]
- 46.Jacobson JL, Jacobson SW, Muckle G, Kaplan-Estrin M, Ayotte P, Dewailly E. Beneficial effects of a polyunsaturated fatty acid on infant development: evidence from the Inuit of Arctic Quebec. J Pediatri. 2008;152:356-364.e1. [DOI] [PubMed] [Google Scholar]
- 47.Jensen CL, Voigt RG, Prager TC, et al. Effects of maternal docosahexaenoic acid intake on visual function and neurodevelopment in breastfed term infants. Am j clinic nutri. 2005;82:125. [DOI] [PubMed] [Google Scholar]
- 48.Kalhoff H, Mesch CM, Stimming M, et al. Effects of LC-PUFA supply via complementary food on infant development-a food based intervention (RCT) embedded in a total diet concept. Eur J Clin Nutr. 2020;74:682-690. [DOI] [PubMed] [Google Scholar]
- 49.Lauritzen L, Jørgensen MH, Olsen SF, Straarup EM, Michaelsen KF. Maternal fish oil supplementation in lactation: Effect on developmental outcome in breast-fed infants. Reproduction, Nutrition, development. 2005;45:535-547. [DOI] [PubMed] [Google Scholar]
- 50.Lepping RJ, Honea RA, Martin LE, et al. Long-chain polyunsaturated fatty acid supplementation in the first year of life affects brain function, structure, and metabolism at age nine years. Dev Psychobiol. 2019;61:5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van der Merwe, Liandré F, Moore SE, Fulford AJ, et al. Long-chain PUFA supplementation in rural African infants: A randomized controlled trial of effects on gut integrity, growth, and cognitive development. Am J Clin Nutr. 2013;97:45-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meldrum SJ, D’Vaz N, Simmer K, Dunstan JA, Hird K, Prescott SL. Effects of high-dose fish oil supplementation during early infancy on neurodevelopment and language: A randomised controlled trial. Br J Nutr. 2012;108:1-12. [DOI] [PubMed] [Google Scholar]
- 53.Dalmeijer GW, Wijga AH, Gehring U, et al. Fatty acid composition in breastfeeding and school performance in children aged 12 years. Eur J Nutr . 2016;55:2199-2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Henriksen C, Almaas AN, Westerberg AC, Drevon CA, Iversen PO, Nakstad B. Growth, metabolic markers, and cognition in 8-year old children born prematurely, follow-up of a randomized controlled trial with essential fatty acids. Eur J Pediatr. 2016;175:1165-1174. [DOI] [PubMed] [Google Scholar]
- 55.Isaacs EB, Ross S, Kennedy K, Weaver LT, Lucas A, Fewtrell MS. 10-year cognition in preterms after random assignment to fatty acid supplementation in infancy. Pediatri. 2011;128:e890-e898. [DOI] [PubMed] [Google Scholar]
- 56.Henriksen C, Haugholt K, Lindgren M, et al. Improved cognitive development among preterm infants attributable to early supplementation of human milk with docosahexaenoic acid and arachidonic acid. Pediatrics. 2008;121:1137-1145. [DOI] [PubMed] [Google Scholar]
- 57.Liao K, McCandliss BD, Carlson SE, et al. Event‐related potential differences in children supplemented with long‐chain polyunsaturated fatty acids during infancy. Dev Sci. 2017;20:e12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Westerberg AC, Schei R, Henriksen C, et al. Attention among very low birth weight infants following early supplementation with docosahexaenoic and arachidonic acid. Acta Paediatr. 2011;100:47-52. [DOI] [PubMed] [Google Scholar]
- 59.Jensen CL, Voigt RG, Llorente AM, et al. Effects of early maternal docosahexaenoic acid intake on neuropsychological status and visual acuity at five years of age of breast-fed term infants. J Pediatr. 2010;157:900-905. [DOI] [PubMed] [Google Scholar]
- 60.Devlin AM, Chau CMY, Dyer R, et al. Developmental outcomes at 24 Months of age in toddlers supplemented with arachidonic acid and docosahexaenoic acid: Results of a double blind randomized, controlled trial. Nutrients. 2017;9:975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Keim SA, Boone KM, Klebanoff MA, et al. Effect of docosahexaenoic acid supplementation vs placebo on developmental outcomes of toddlers born preterm: A randomized clinical trial. JAMA Pediatr. 2018;172:1126-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brew BK, Toelle BG, Webb KL, et al. Omega-3 supplementation during the first 5 years of life and later academic performance: A randomised controlled trial. Eur J Clin Nutr. 2015;69:419-424. [DOI] [PubMed] [Google Scholar]
- 63.Åberg MA, Åberg N, Brisman J, et al. Fish intake of Swedish male adolescents is a predictor of cognitive performance. Acta Pædiatrica. 2009;98:555-560. [DOI] [PubMed] [Google Scholar]
- 64.Kirby A, Woodward A, Jackson S, Wang Y, Crawford MA. A double-blind, placebo-controlled study investigating the effects of omega-3 supplementation in children aged 8–10 years from a mainstream school population. Res Dev Disabil. 2010;31:718-730. [DOI] [PubMed] [Google Scholar]
- 65.Øyen J, Kvestad I, Midtbø LK, et al. Fatty fish intake and cognitive function: FINS-KIDS, a randomized controlled trial in preschool children. BMC Med. 2018;16:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sheppard KW, Cheatham CL. Omega-6 to omega-3 fatty acid ratio and higher-order cognitive functions in 7- to 9-y-olds: A cross-sectional study. Am J clinic nutrition. 2013;98:659-667. [DOI] [PubMed] [Google Scholar]
- 67.Handeland K, Øyen J, Skotheim S, et al. Fatty fish intake and attention performance in 14-15 year old adolescents: FINS-TEENS - a randomized controlled trial. Nutr J. 2017;16:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van der Wurff, Inge SM, Von Schacky C, Bergeland T, et al. Effect of 1 year krill oil supplementation on cognitive achievement of Dutch adolescents: A double-blind randomized controlled trial. Nutrients. 2019;11:1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vesco AT, Young AS, Arnold LE, Fristad MA. Omega‐3 supplementation associated with improved parent‐rated executive function in youth with mood disorders: Secondary analyses of the omega 3 and therapy (OATS) trials. JCPP (J Child Psychol Psychiatry). 2018;59:628-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van der Wurff I, von Schacky C, Berge K, Zeegers M, Kirschner P, de Groot R. Association between blood omega-3 index and cognition in typically developing Dutch adolescents. Nutrients. 2016;8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ryan AS, Nelson EB. Assessing the effect of docosahexaenoic acid on cognitive functions in healthy, preschool children: A randomized, placebo-controlled, double-blind study. Clin Pediatr. 2008;47:355-362. [DOI] [PubMed] [Google Scholar]
- 72.Widenhorn-Müller K, Schwanda S, Scholz E, Spitzer M, Bode H. Effect of supplementation with long-chain ω-3 polyunsaturated fatty acids on behavior and cognition in children with attention deficit/hyperactivity disorder (ADHD): A randomized placebo-controlled intervention trial. Prostagl Leukot Essent Fat Acids. 2014;91:49-60. [DOI] [PubMed] [Google Scholar]
- 73.Crippa A, Crippa A, Tesei A, et al. Behavioral and cognitive effects of docosahexaenoic acid in drug-naïve children with attention-deficit/hyperactivity disorder: A randomized, placebo-controlled clinical trial. Eur Child Adolesc Psychiatr. 2019;28:571-583. [DOI] [PubMed] [Google Scholar]
- 74.Raz R, Carasso RL, Yehuda S. The influence of short-chain essential fatty acids on children with attention-deficit/hyperactivity disorder: A double-blind placebo-controlled study. J Child Adolesc Psychopharmacol. 2009;19:167-177. [DOI] [PubMed] [Google Scholar]
- 75.Kean JD, Kean JD, Sarris J, et al. Reduced inattention and hyperactivity and improved cognition after marine oil extract (PCSO-524®) supplementation in children and adolescents with clinical and subclinical symptoms of attention-deficit hyperactivity disorder (ADHD): A randomised, double-blind, placebo-controlled trial. Psychopharmacology. 2017;234:403-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Milte CM, Parletta N, Buckley JD, Coates AM, Young RM, Howe PRC. Eicosapentaenoic and docosahexaenoic acids, cognition, and behavior in children with attention-deficit/hyperactivity disorder: A randomized controlled trial. Nutrition. 2012;28:670-677. [DOI] [PubMed] [Google Scholar]
- 77.Milte CM, Parletta N, Buckley JD, Coates AM, Young RM, Howe PRC. Increased erythrocyte eicosapentaenoic acid and docosahexaenoic acid are associated with improved attention and behavior in children with ADHD in a randomized controlled three-way crossover trial. J Atten Disord. 2015;19:954-964. [DOI] [PubMed] [Google Scholar]
- 78.Demmelmair H, MacDonald A, Kotzaeridou U, et al. Determinants of plasma docosahexaenoic acid levels and their relationship to neurological and cognitive functions in PKU patients: A double blind randomized supplementation study. Nutrients. 2018;10:1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mazahery H, Conlon CA, Beck KL, et al. A randomised-controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of core symptoms of autism spectrum disorder in children. J Autism Dev Disord . 2019;49:1778-1794. [DOI] [PubMed] [Google Scholar]
- 80.Portillo-Reyes V, Pérez-García M, Loya-Méndez Y, Puente AE. Clinical significance of neuropsychological improvement after supplementation with omega-3 in 8–12 years old malnourished Mexican children: A randomized, double-blind, placebo and treatment clinical trial. Res Dev Disabil. 2014;35:861-870. [DOI] [PubMed] [Google Scholar]
- 81.Baumgartner J, Smuts CM, Malan L, et al. Effects of iron and n-3 fatty acid supplementation, alone and in combination, on cognition in school children: a randomized, double-blind, placebo-controlled intervention in South Africa. Am J Clin Nutr. 2012;96:1327-1338. [DOI] [PubMed] [Google Scholar]
- 82.Sørensen LB, Damsgaard CT, Dalskov S, et al. Diet-induced changes in iron and n-3 fatty acid status and associations with cognitive performance in 8–11-year-old Danish children: secondary analyses of the optimal well-being, development and health for Danish children through a healthy new nordic diet school meal study. Br J Nutr. 2015;114:1623-1637. [DOI] [PubMed] [Google Scholar]
- 83.Bos DJ, Oranje B, Veerhoek ES, et al. Reduced symptoms of inattention after dietary omega-3 fatty acid supplementation in boys with and without attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2015;40:2298-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cornu C, Mercier C, Ginhoux T, et al. A double-blind placebo-controlled randomised trial of omega-3 supplementation in children with moderate ADHD symptoms. Eur Child Adolesc Psychiatr. 2018;27:377-384. [DOI] [PubMed] [Google Scholar]
- 85.Dalton A, Wolmarans P, Witthuhn RC, van Stuijvenberg ME, Swanevelder SA, Smuts CM. A randomised control trial in schoolchildren showed improvement in cognitive function after consuming a bread spread, containing fish flour from a marine source. Prostagl Leukot Essent Fat Acids. 2009;80:143-149. [DOI] [PubMed] [Google Scholar]
- 86.Darcey VL, McQuaid GA, Fishbein DH, VanMeter JW. Dietary long-chain omega-3 fatty acids are related to impulse control and anterior cingulate function in adolescents. Front Neurosci. 2018;2019;12:1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de Groot RHM, Ouwehand C, Jolles J. Eating the right amount of fish: inverted U-shape association between fish consumption and cognitive performance and academic achievement in Dutch adolescents. Prostagl Leukot Essent Fat Acids. 2012;86:113-117. [DOI] [PubMed] [Google Scholar]
- 88.Kim J, Winkvist A, Aberg MA, et al. Fish consumption and school grades in Swedish adolescents: A study of the large general population. Acta Pædiatrica 2010;2009;99:72-77. [DOI] [PubMed] [Google Scholar]
- 89.Kennedy DO, Jackson PA, Elliott JM, et al. Cognitive and mood effects of 8 weeks’ supplementation with 400 mg or 1000 mg of the omega-3 essential fatty acid docosahexaenoic acid (DHA) in healthy children aged 10-12 years. Nutr Neurosci. 2009;12:48-56. [DOI] [PubMed] [Google Scholar]
- 90.Montgomery P, Burton JR, Sewell RP, Spreckelsen TF, Richardson AJ. Low blood long chain omega-3 fatty acids in UK children are associated with poor cognitive performance and behavior: A cross-sectional analysis from the DOLAB study. PLoS One. 2013;8:e66697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Parletta N, Cooper P, Gent DN, Petkov J, O’Dea K. Effects of fish oil supplementation on learning and behaviour of children from Australian Indigenous remote community schools: A randomised controlled trial. Prostagl Leukot Essent Fat Acids. 2013;89:71-79. [DOI] [PubMed] [Google Scholar]
- 92.Richardson AJ, Montgomery P. The Oxford-Durham study: a randomized, controlled trial of dietary supplementation with fatty acids in children with developmental coordination disorder. Pediatrics. 2005;115:1360-1366. [DOI] [PubMed] [Google Scholar]
- 93.Richardson AJ, Burton JR, Sewell RP, Spreckelsen TF, Montgomery P. Docosahexaenoic acid for reading, cognition and behavior in children aged 7–9 years: A randomized, controlled trial (the DOLAB study). PLoS One. 2012;7:e43909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sinn N, Bryan J, Wilson C. Cognitive effects of polyunsaturated fatty acids in children with attention deficit hyperactivity disorder symptoms: A randomised controlled trial. Prostagl Leukot Essent Fat Acids. 2008;78:311-326. [DOI] [PubMed] [Google Scholar]
- 95.Voigt RG, Llorente AM, Jensen CL, Fraley JK, Berretta MC, Heird WC. A randomized, double-blind, placebo-controlled trial of docosahexaenoic acid supplementation in children with attention-deficit/hyperactivity disorder. J Pediatr. 2001;139:189-196. [DOI] [PubMed] [Google Scholar]