Abstract
The Toscana virus can cause neurological infection in adults. This study of 112 cases of acute meningitis which occurred during the summers of 1995, 1996, and 1997 demonstrated the presence of viral RNA in the cerebrospinal fluid of 56 patients. Their sequence analysis shows four variants of the Toscana virus.
Toscana virus, genus Phlebovirus, is an enveloped virion with a segmented negative-strand RNA genome consisting of three noncovalently closed, circular RNA species: small, medium, and large, coding, respectively, for the nucleocapsid protein and the nonstructural protein, for the envelope glycoproteins (G1 and G2), and for the large protein (1, 2, 8). The three serotypes, Toscana virus, Sicilian virus, and Naples virus, are present in the Mediterranean area and are the cause of sandfly fever. Toscana virus infection is characterized by aseptic meningitis or meningoencephalitis (9, 15). This neuropathic infection is more frequent during the summer, and it peaks in August, as it is correlated with the life cycle of its insect vectors (Phlebotomus perniciosus and Phlebotomus perfiliewi) (13). We report data regarding a molecular and epidemiological study of Toscana (TOS) virus detected by reverse transcriptase (RT)-PCR and sequencing in 56 of 112 cases of meningitis that occurred during the summers of 1995 to 1997.
A total of 112 patients (76 male and 36 female, aged 10 to 59 years) who were hospitalized showed typical neurological symptoms, such as fever, myalgia, severe frontal headache, vomiting, ocular pain, and neck rigidity, described as a “clinical picture” of aseptic meningitis or meningoencephalitis. At the time of hospitalization, blood and cerebrospinal fluid (CSF) were drawn from the patients. The laboratory data referring to the CSF showed cell counts of >103/mm3 and protein amounts of >50 mg/dl; the blood samples showed glucose concentrations of >60%. All the specimens gave negative results for bacteriological and mycological analysis. A total of 200 μl of the CSF and blood samples was analyzed by PCR and RT-PCR to detect the nucleic acids of neurotropic viruses such as herpes simplex virus types 1, 2, 6, 7, and 8, cytomegalovirus, Epstein-Barr virus, herpes zoster virus, enterovirus, Toscana virus, mumps virus.
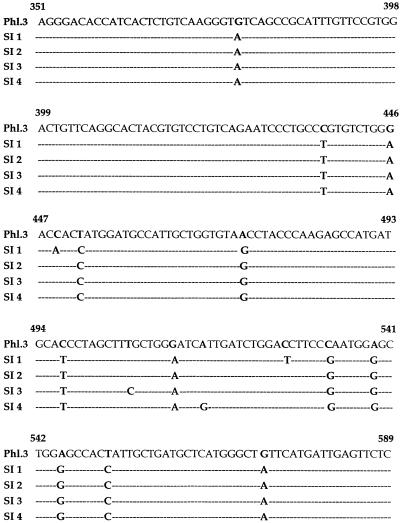
The RNA was extracted from a 200-μl aliquot of each sample by using the procedure described by Chomczynsky and Sacchi (3). The DNA extraction was performed on CSF and blood specimens by using the QIamp blood kit (Qiagen, Hilden, Germany). PCR was carried out according to the recommended guidelines, including three negative controls and one positive control in each assay. The blood samples were negative for all the neurotropic viruses, and the CSF samples were negative for all the DNA neurotropic viruses. The presence of enterovirus was demonstrated in six CSF samples. It was possible to detect the TOS virus genome by RT-PCR by using the TOS-specific primers (14) in 15 of 33 (45.4%) cases in the summer of 1995, in 23 of 35 (65.7%) cases in the summer of 1996, and in 18 of 38 (47.3%) cases in the summer of 1997. We have compared some epidemiological parameters referring to the 3 years considered in our study. The comparison of the mean age of the patients with acute meningitis did not show a marked difference among the three groups. Only two children, aged 10 and 11, tested positive for TOS virus, respectively, in the summers of 1996 and 1997. In the period from June to September, the seasonal distribution of TOS virus infection peaked in August during each of the 3 years considered in this study. In order to check the presence of TOS virus variants, PCR products were purified by using the QIA-quick spin PCR purification kit (Qiagen) and sequenced by dideoxy chain termination with a Sequenase kit by using sense and antisense primers in order to sequence both strands. The sequence of this short fragment compared with the TOS reference sequence of the ISS Phleb.3 strain (8) of the N gene showed (Fig. 1) 12 canonic point mutations, which are present in all the isolates. The variant named SI 1 shows only these point mutations; more nucleotide changes are present in the variant SI 2, with A→C and T→C mutations at positions 449 and 527. The variants SI 3 and SI 4 have a C→T point mutation at position 506 and an A→G point mutation at position 516. The analysis of the corresponding amino acid sequence showed that these point mutations do not result in amino acid changes.
FIG. 1.
Comparison of the nucleotide sequences of the SI 1, 2, 3, and 4 variants of Toscana virus with the reference sequence of the ISS Phleb.3 strain (Phl.3) (8).
Only the SI 4 variant, circulating in 1995, shows an amino acid substitution (Ile→Val), which does not involve a significant conformational change. This variant was the one circulating in Tuscany in 1993, since it presented the same sequence as that detected in a CSF sample collected in that year. This data is very interesting because it provides evidence for the presence of different variants of the TOS virus circulating in the same area, and it is not in agreement with the data published by Schwarz et al. (11), who, however, considered a small number of cases.
It has been reported that the major outbreaks of Phlebovirus have occurred when a large population of nonimmune subjects have moved into an area where the virus is endemic (5–7, 10). The number of cases of acute meningitis in the summer was relatively high, considering the total number of cases of acute meningitis occurring during other periods of the year; in fact, 112 of 186 patients with meningitis were hospitalized during the months of June to September in 1995 to 1997. Nevertheless, in our study the number of tourists coming from areas where the virus is not endemic during the summer was low (only 11 of 56 cases of TOS virus meningitis); most of the patients with TOS meningitis were residing in the area of endemicity. The circulation of the virus variants was randomly distributed and could not be correlated with a particular group of patients. The relatively high number of cases of TOS virus meningitis in the resident population (45 of 56) could be due to the fact that natural TOS-specific immunity could not be long lasting or that the new variants of TOS virus modified important epitopes which could escape immunological surveillance. It should also be pointed out that, at the moment, a “gold standard” serological method, which would be sensitive enough to recognize the presence of a protective immune response, is not available (12). This is the first study which allowed a characterization of TOS virus variants detected in 56 clinical specimens. The presence of these variants is probably due to the nature of the virus genome and the cycle of the host-vector transmission. Further studies are in progress in order to analyze a larger region of the small segment or the medium segment (4). The molecular approach to this epidemiological study appeared to be very useful in detecting the variants of the TOS virus, which would not otherwise be recognized by classical serological methods.
REFERENCES
- 1.Accardi L, Grò M C, Di Bonito P, Giorgi C. Toscana virus genomic L segment: molecular cloning, strategy and amino acid sequence in comparison with other negative strand RNA viruses. Virus Res. 1993;27:119–131. doi: 10.1016/0168-1702(93)90076-y. [DOI] [PubMed] [Google Scholar]
- 2.Bishop D H. Bunyaviridae and their replication. I. Bunyaviridae. In: Fields B N, Knipe D M, Chanock R M, Melnick J L, Hirsh M S, Monath T P, Roizman B, editors. Virology. New York, N.Y: Raven Press; 1990. pp. 1155–1173. [Google Scholar]
- 3.Chomczynsky P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 4.Di Bonito P, Mochi S, Grò M C, Fortini D, Giorgi C. Organization of the M genomic segment of Toscana phlebovirus. J Gen Virol. 1997;78:77–81. doi: 10.1099/0022-1317-78-1-77. [DOI] [PubMed] [Google Scholar]
- 5.Eckhof-Donovan S, Schwarz T F, Baetmann M, Voit T, Lenard H G. Pappataci-fever as differential diagnosis of meningitis in childhood. Monatsschr Kinderheilkd. 1995;143:839–842. [Google Scholar]
- 6.Eitrem R, Niklasson B, Weiland O. Sandfly fever among Swedish tourists. Scand J Infect Dis. 1991;23:451–457. doi: 10.3109/00365549109075093. [DOI] [PubMed] [Google Scholar]
- 7.Eitrem R, Vene S, Niklasson B. Incidence of sandfly fever among Swedish United Nations soldiers on Cyprus during 1985. Am J Trop Med Hyg. 1990;43:207–211. doi: 10.4269/ajtmh.1990.43.207. [DOI] [PubMed] [Google Scholar]
- 8.Giorgi C, Accardi L, Nicoletti L, Grò M C, Takehara K, Hilditch C, Morikawa S, Bishop D H L. Sequences and coding strategies of the S RNAs of Toscana virus and Rift Valley fever virus compared to those of Punta Toro, Sicilian sandfly fever and Uukuniemi viruses. Virology. 1991;180:738–753. doi: 10.1016/0042-6822(91)90087-r. [DOI] [PubMed] [Google Scholar]
- 9.Nicoletti L, Verani P, Caciolli S. Central nervous system involvement during infection by Phlebovirus Toscana of residents in natural foci in central Italy (1977–1988) Am J Trop Med Hyg. 1991;45:429–434. doi: 10.4269/ajtmh.1991.45.429. [DOI] [PubMed] [Google Scholar]
- 10.Schwarz T F, Glich S, Jäger G. Travel-related Toscana virus infection. Lancet. 1993;ii:803. doi: 10.1016/0140-6736(93)91568-7. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 11.Schwarz T F, Jäger G, Gilch S, Nitshko H. Nested RT-PCR for detection of sandfly fever virus, serotype Toscana, in clinical specimens, with confirmation by nucleotide sequence analysis. Res Virol. 1995;146:355–362. doi: 10.1016/0923-2516(96)80598-x. [DOI] [PubMed] [Google Scholar]
- 12.Schwarz T F, Jäger G, Glich S, Pauli C. Serosurvey and laboratory diagnosis of imported sandfly fever virus, serotype Toscana, infection in Germany. Epidemiol Infect. 1995;114:501–510. doi: 10.1017/s0950268800052213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tesh R B. The genus Phlebovirus and its vectors. Annu Rev Entomol. 1988;33:169–181. doi: 10.1146/annurev.en.33.010188.001125. [DOI] [PubMed] [Google Scholar]
- 14.Valassina M, Cusi M G, Valensin P E. Rapid identification of Toscana virus by nested PCR during an outbreak in the Siena area of Italy. J Clin Microbiol. 1996;34:2500–2502. doi: 10.1128/jcm.34.10.2500-2502.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verani P, Nicoletti L, Ciufolini M G, Balducci M. Viruses transmitted by sandflies in Italy. Parassitologia. 1991;33:513–518. [PubMed] [Google Scholar]